Abstract
The treatment of traumatic wounds with exposed bone or tendons is often challenging. An induced membrane (IM) is used to reconstruct bone defects, as it provides an effective and sufficient blood supply for bone and soft‐tissue reconstruction. This study explored a novel two‐stage strategy for wound management, consisting of initial wound coverage with polymethyl methacrylate (PMMA) and an autologous split‐thickness skin graft under the IM. Fifty inpatients were enrolled from December 2016 to December 2019. Each patient underwent reconstruction according to a two‐stage process. In the first stage, the defect area was thoroughly debrided, and the freshly treated wound was then covered using PMMA cement. After 4‐6 weeks, during the second stage, the PMMA cement was removed to reveal an IM covering the exposed bone and tendon. An autologous split‐thickness skin graft was then performed. Haematoxylin and eosin (H&E) staining and immunohistochemical analysis of vascular endothelial growth factor (VEGF), CD31 and CD34 were used to evaluate the IM and compare it with the normal periosteal membrane (PM). The psychological status and the Lower Extremity Function Scale (LEFS) as well as any complications were recorded at follow‐up. We found that all skin grafts survived and evidenced no necrosis or infection. H&E staining revealed vascularised tissue in the IM, and immunohistochemistry showed a larger number of VEGF‐, CD31‐ and CD34‐positive cells in the IM than in the normal PM. The duration of healing in the group was 5.40 ± 1.32 months with a mean number of debridement procedures of 1.92 ± 0.60. There were two patients with reulceration in the group. The self‐rating anxiety scale scores ranged from 35 to 60 (mean 48.02 ± 8.12). Postoperatively, the LEFS score was 50.10 ± 9.77. Finally, our strategy for the management of a non‐healing wound in the lower extremities, consisting of an IM in combination with skin grafting, was effective, especially in cases in which bony structures were exposed in the elderly. The morbidity rate was low.
Keywords: induced membrane, non‐healing wound, skin grafting, VEGF
1. INTRODUCTION
Victims of high‐speed vehicular accidents often present with large degloving injuries, compound fractures and severe damage to soft tissues. 1 , 2 These wounds are frequently left open and require repeated debridement, resulting in large soft‐tissue defects. Traumatic wounds with exposed bone or tendon heal poorly unless aggressive debridement or free‐flap microsurgery is performed, which poses a substantial reconstructive challenge. 3 Moreover, the use of these techniques is, in practice, frequently barred by the magnitude of the tissue defect and/or the paucity of a donor site. In addition, free‐flap operations are complex, technically demanding, costly and time‐consuming, with significant rates of complications, donor‐site morbidities and failure. 4 , 5 , 6 Furthermore, the patient's general condition may be too poor to tolerate prolonged free flap surgery, and a donor site may not be available for an effective flap.
Before the development of microsurgical techniques, the treatment of severe lower‐extremity wounds often consisted of skin grafting. 7 In complex wounds, skin grafting is usually inadequate because of the difficulty of healing over exposed bone, leading to high rates of osteomyelitis and amputation. 8 Chen et al 9 developed a method for managing these wounds in stages, using an artificial dermis and skin grafting technique, and tested its feasibility in 17 wounds in 15 patients. The authors concluded that in the management of a non‐healing wound, specifically in the lower extremity, a staged approach using an artificial dermis followed by a skin graft is effective, especially in cases involving exposed bony structures. However, the disadvantages included a risk of immune rejection, prolonged hospitalisation and an increased surgical risk.
Masquelet's induced membrane (IM) technique, which uses a two‐stage procedure to surgically reconstruct segmental bone defects, is relatively new. 10 , 11 In this approach, the second surgery is performed only after the polymethyl methacrylate (PMMA) spacer has elicited a local foreign‐body response and an autologous foreign‐body IM, the key component of the procedure, has formed around the spacer. The presence of pro‐angiogenic factors in association with the IM, including vascular endothelial growth factor (VEGF), angiotensin II and fibroblast growth factor 2, has been documented. 12 , 13 Recently, Tang et al 14 reported elevated levels of the anti‐angiogenic Notch signalling components DLL4 and NOTCH1 in more mature membranes. Vessel density in the IM increases during the first few weeks to months after the first surgery, which is evidence that an IM can provide an effective and sufficient blood supply for soft‐tissue and bone reconstruction.
Here we present a two‐stage strategy for wound management, comprising initial coverage of a fresh wound with PMMA, followed by grafting with an autologous split‐thickness skin graft under the IM. The experience gained from the treatment of 50 patients using this technique with a 3‐year follow‐up is discussed herein.
2. MATERIALS AND METHODS
2.1. Patient materials
Fifty patients (35 males, 15 females) ranging in age from 60 to 78 years (mean: 68.54 ± 5.49 years) were treated from December 2016 to December 2019. All wounds were located in the lower extremities, and all involved exposure of the underlying bony or tendon structures. Car accidents were the most common cause of injuries (35 patients), followed by crush injuries (7 patients) and falls (8 patients). General information on the patients is presented in Table 1. All experimental procedures were approved by the ethics committee of our hospital and were conducted in accordance with the Declaration of Helsinki. The requirement for informed consent was waived as the data were analysed anonymously and personal identifiers were completely removed.
TABLE 1.
General information of the 50 patients
| No. | Age (year) | Sex | Cause | Location | Size of defect (cm × cm) | Reason for not performing flap |
|---|---|---|---|---|---|---|
| 1 | 68 | Female | Car accident | Lateral calf with exposed bone | 5 × 4 | Varicose veins of lower extremities |
| 2 | 76 | Male | Car accident | Dorsum of foot with exposed tendon | 6.5 × 5 | Cirrhosis |
| 3 | 65 | Male | Crush injury | Heel with exposed bone | 10 × 5 | Arteriosclerosis and poor pulmonary function |
| 4 | 64 | Male | Car accident | Medial calf with exposed bone | 7 × 3 | Lower extremity arteriosclerosis |
| 5 | 72 | Female | Crush injury | Achilles tendon exposed | 3 × 4 | Emphysema |
| 6 | 74 | Male | Car accident | Dorsum of foot with exposed tendon | 7 × 8 | Varicose veins of lower extremities |
| 7 | 66 | Male | Car accident | Anterior ankle with exposed tendon | 5 × 4 | Emphysema |
| 8 | 66 | Female | Fell down | Dorsum of foot with exposed bone | 10 × 4 | Scars around the wound |
| 9 | 64 | Male | Car accident | Dorsum of foot with exposed tendon | 8 × 4 | Varicose veins of lower extremities |
| 10 | 61 | Male | Fell down | Dorsum of foot with exposed bone | 6 × 5 | Cirrhosis |
| 11 | 72 | Male | Car accident | Medial and lateral malleolus |
12 × 6 6 × 5 |
Lower extremity arteriosclerosis |
| 12 | 62 | Male | Car accident | Medial calf with exposed bone | 4 × 5 | Scars around the wound |
| 13 | 68 | Female | Car accident | Dorsum of foot with exposed tendon | 5 × 6 | Varicose veins of lower extremities |
| 14 | 66 | Male | Crush injury | Dorsum of foot with exposed bone | 8 × 4 | Emphysema |
| 15 | 76 | Male | Car accident | Medial foot with exposed bone | 10 × 5 | Malnutrition |
| 16 | 62 | Female | Car accident | Dorsum of foot with exposed tendon | 9 × 4 | Varicose veins of lower extremities |
| 17 | 71 | Male | Fell down | Medial calf with exposed bone | 4 × 5 | Malnutrition |
| 18 | 66 | Female | Car accident | Dorsum of foot with exposed tendon | 6 × 7 | Emphysema |
| 19 | 77 | Male | Crush injury | Anterior ankle with exposed tendon | 7 × 8 | Cirrhosis |
| 20 | 60 | Male | Car accident | Medial calf with exposed bone | 3 × 4 | Varicose veins of lower extremities |
| 21 | 75 | Female | Car accident | Achilles tendon exposed | 2 × 4 | Pleural effusion |
| 22 | 60 | Male | Tractor accident | Lateral knee | 10 × 4 | Degloving injury of the whole lower extremity |
| 23 | 78 | Male | Car accident | Medial foot with exposed bone | 2 × 6 | Scars around the wound |
| 24 | 70 | Male | Fell down | Dorsum of foot with exposed bone | 6 × 6 | Varicose veins of lower extremities |
| 25 | 63 | Female | Car accident | Anterior ankle with exposed tendon | 5 × 5 | Malnutrition |
| 26 | 62 | Male | Car accident | Heel with exposed bone | 4 × 5 | Varicose veins of lower extremities |
| 27 | 77 | Female | Car accident | Medial foot with exposed bone | 3 × 7 | Scars around the wound |
| 28 | 67 | Male | Fell down | Dorsum of foot with exposed tendon | 6 × 6 | Cirrhosis |
| 29 | 64 | Male | Car accident | Lateral knee | 7 × 7 | Degloving injury of the whole lower extremity |
| 30 | 65 | Female | Car accident | Medial calf with exposed bone | 10 × 3 | Vertebral fractures, pleural effusion |
| 31 | 74 | Male | Crush injury | Anterior ankle with exposed tendon | 2 × 3 | Coronary heart disease |
| 32 | 60 | Male | Car accident | Heel with exposed bone | 5 × 6 | Vertebral fractures, pleural effusion |
| 33 | 68 | Male | Car accident | Dorsum of foot with exposed tendon | 7 × 6 | Varicose veins of lower extremities |
| 34 | 78 | Male | Car accident | Achilles tendon exposed | 2 × 4 | Renal insufficiency |
| 35 | 72 | Female | Car accident | Dorsum of foot with exposed bone | 4 × 3 | Scars around the wound |
| 36 | 61 | Male | Car accident | Lateral calf with exposed bone | 5 × 6 | Degloving injury of the whole lower extremity |
| 37 | 66 | Male | Fell down | Lateral knee | 7 × 4 | Poor skin condition around the wound |
| 38 | 65 | Female | Car accident | Heel with exposed bone | 4 × 4 | Renal insufficiency |
| 39 | 68 | Male | Car accident | Dorsum of foot with exposed bone | 3 × 7 | Poor skin condition around the wound |
| 40 | 74 | Female | Car accident | Dorsum of foot with exposed tendon | 5 × 6 | Varicose veins of lower extremities |
| 41 | 65 | Male | Crush injury | Lateral calf with exposed bone | 3 × 4 | Coronary heart disease |
| 42 | 70 | Male | Car accident | Achilles tendon exposed | 2 × 4 | Renal insufficiency |
| 43 | 68 | Male | Car accident | Dorsum of foot with exposed bone | 6 × 5 | Poor skin condition around the wound |
| 44 | 75 | Female | Fell down | Lateral knee | 4 × 5 | Degloving injury of the whole lower extremity |
| 45 | 65 | Male | Car accident | Dorsum of foot with exposed tendon | 8 × 5 | Renal insufficiency |
| 46 | 73 | Male | Car accident | Lateral calf with exposed bone | 4 × 5 | Coronary heart disease |
| 47 | 68 | Male | Crush injury | Anterior ankle with exposed tendon | 3 × 7 | Varicose veins of lower extremities |
| 48 | 63 | Female | Car accident | Medial calf with exposed bone | 5 × 6 | Scars around the wound |
| 49 | 77 | Male | Car accident | Dorsum of foot with exposed tendon | 5 × 5 | Renal insufficiency |
| 50 | 77 | Male | Fell down | Achilles tendon exposed | 4.5 × 3 | Varicose veins of lower extremities |
2.2. Surgical procedure
Our reconstruction strategy is based on a two‐stage process. During the first stage, the entire defect area is thoroughly inspected and aggressively debrided. Surgical debridement is performed in all patients, in addition to the removal of devitalised tissues. For wounds without periosteal coverage, the bony or tendon surface is lightly abraded (Figure 1A). The exposed bone is decorticalised (Figure 1B), and fluid and tissue samples are sent for microscopy, culture, and antibiotic sensitivity testing, with or without histology as indicated. In patients with fractures, the appropriate fixation method is chosen. The suitability of the implant is evaluated and possibly revised. Internal or external methods of fixation are appropriate, as long as the construction is stable enough to minimise disturbance of the cement spacer and IM formation without risking infection. If only bone or tendon is exposed, external fixation (cast or brace) after surgery may suffice. The freshly treated wound is covered with PMMA bone cement (Figure 1C) loaded with antibiotics (vancomycin). The amount of cement used will depend on the size of the wound but should be larger than the actual wound area. The cement is anchored tightly before it solidifies by stapling along the wound edges. Antibiotics against most gram‐positive variants are administered intravenously to all patients for 3‐5 days postoperatively. The patient can then be discharged after wound covering.
FIGURE 1.
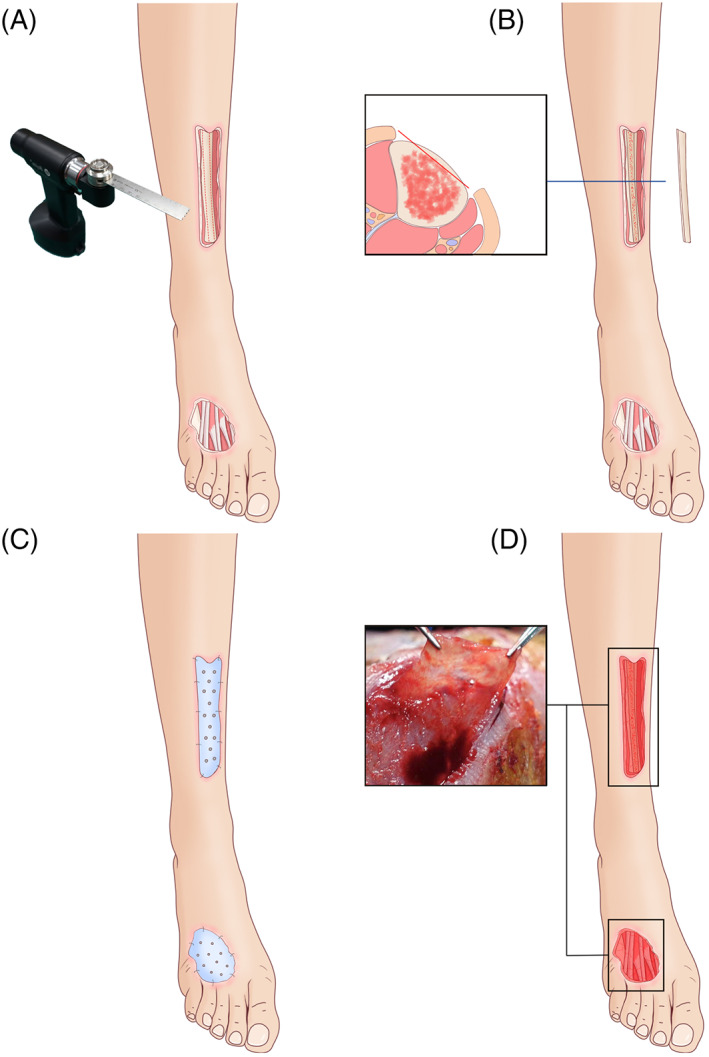
Schematic diagram of the induced membrane (IM) procedure. A, Thorough debridement of the bone and soft tissue; B, decorticalisation of the exposed bone; C, the freshly treated wound covered with polymethyl methacrylate bone cement; D, the exposed bone and tendon covered with the IM
After 4‐6 weeks, during the second stage, the PMMA cement is removed and the wound base lightly abraded to determine the adequacy of revascularisation; light blood seepage is usually observed in a mature wound bed. At this stage, the IM should cover the exposed bone and tendon (Figure 1D), forming a mature IM. After confirmation that neither the IM nor the wound is infected, a 1 × 1 cm piece of IM is removed for laboratory testing. The remaining IM is removed as well and fixed in 10% paraformaldehyde for pathological examination. In this study, normal periosteal membrane (PM) was harvested from adult patients with fractures as a control. An autologous split‐thickness piece of skin (0.15‐0.20‐mm thick) is harvested using an electric dermatome to cover the resultant wound above the IM. The grafted wound is managed routinely, with the graft anchored to the wound base with sutures or staples and covered with a piece of petrolatum gauze. Finally, the surgical area is closed using pressure. After 6‐7 days, the wound is uncovered to ascertain the survival of the skin graft. The patient is observed for the next 6‐12 months to ensure proper recovery from the procedure and healing of the injuries.
The antibiotic therapy was first managed empirically and then modified according to the results of an antibiogram from the intraoperative culture. All patients were regularly tracked by our multidisciplinary team until 36 months.
2.3. Histology and immunohistochemistry
Histological sections of the IM and normal PM were stained with haematoxylin and eosin (H&E) and analysed by two clinical pathologists, who assessed IM vascularisation via immunohistochemistry (IHC) detection of VEGF, CD31 and CD34. Paraffin sections were prepared by sequential washes (100% xylene, 20 minutes thrice; 100% alcohol, 5 minutes; 95% alcohol, 5 minutes; 85% alcohol, 5 minutes; 75% alcohol, 5 minutes; distilled water, 5 minutes), followed by antigen retrieval and incubation first with the primary antibody (VEGF, CD31 or CD34) and then with the secondary antibody (anti‐rabbit). Finally, the IHC sections were stained with DAB and haematoxylin, dehydrated with alcohol and examined by microscopy at ×400 magnification (DMIL‐LED, LEICA, Wetzlar, Germany). Positive immunostaining of the cells was determined quantitatively.
2.4. Clinical observational indicators
The primary outcomes were the healing rate. Healing was determined to be complete epithelialisation of the surgical wound at two consecutive clinic visits. Non‐healing was defined as no significant reduction in the wound size or no significant decrease or worsening of secretions, with no need for or refusal for major amputation. The secondary outcomes included the duration of healing (the number of weeks from the initial surgical intervention to the date of complete healing), frequency of debridement procedures, and reulceration (the appearance of a new ulcer on the same or contralateral limb during the follow‐up).
The psychological status of the patients was determined using the self‐rating anxiety scale (SAS), 15 which was administered at the last follow‐up. The SAS has a total possible score of 80 points. Patients with a score <50 are regarded as normal, those scoring 50‐59 as slightly anxious or depressed, those scoring 60‐69 points as moderately anxious or depressed, and those scoring >70 points as severely anxious or depressed.
The functional recovery was determined 12 months after IM and autologous skin grafting by using the Lower Extremity Function Scale (LEFS) survey, 16 including the determination of internal consistency, reliability, construct validity, sensitivity to change, and clinical application. The LEFS is a validated scoring system that can track changes in lower limb function on a numerical scale from 0 to 80.
Patients were also monitored for the following complications: skin graft necrosis, infections, blisters, chronic ulcers and hypertrophic scarring at the donor site.
3. RESULTS
All wounds healed successfully. The smallest wound was approximately 2 × 3 cm and the largest, 12 × 6 cm (Table 1). The reasons for not performing a flap are presented in Table 1. A mature IM developed in 48 of 50 patients, and all 48 were lightly revascularised 4‐6 weeks postoperatively. The wounds of the remaining 2 patients did not form a mature IM. In those cases, after a second complete debridement followed by bone decortication and replacement of the PMMA cement, a mature IM appeared after 4‐6 weeks.
3.1. Gross histology
An intensely fibrous, cell‐rich, vascularised tissue was seen in the IM by H&E staining (Figure 2), whereas in the normal PM, there were few vessel‐like structures.
FIGURE 2.
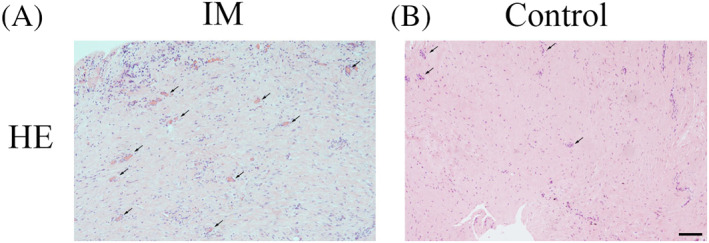
Haematoxylin and eosin (HE) staining of the induced membrane (IM) and the control periosteal membrane. A, The intensely fibrous, cell‐rich, vascularised tissue of the IM. B, Very few vessel‐like structures seen in the normal periosteum. Black arrows indicate new blood vessels. Scale bar: 500 μm
3.2. Vascularisation
Vascularisation in the IM and PM was assessed by IHC to determine the percentages of VEGF‐, CD31‐ and CD34‐positive cells, containing brown or yellow particles. As shown in Figure 3, the positive cells were mainly scattered and formed tube‐like vascular structures in the IM. The qualitative results indicated significantly higher numbers of VEGF‐, CD31‐ and CD34‐positive cells in the IM than in the PM.
FIGURE 3.
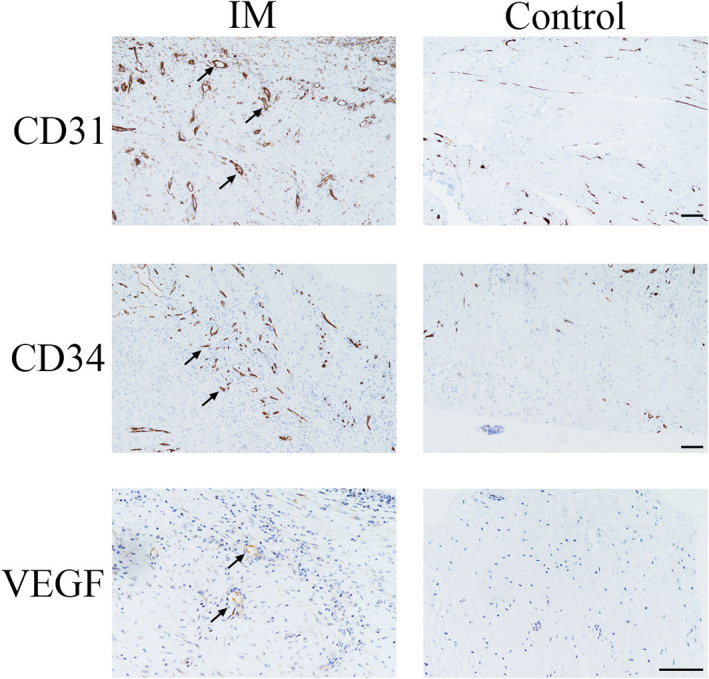
Immunohistochemical analyses of vascular endothelial growth factor (VEGF), CD31 and CD34 protein levels in the induced membrane (IM) and control. Black arrows indicate positive cells. Scale bar: 500 μm
3.3. Clinical outcomes
At the final follow‐up, all wounds were well healed. The duration of healing in the group was 5.4 ± 1.32 months, with a mean number of debridement procedures of 1.92 ± 0.60. There were two patients with reulceration in the group, in whom the wound was located on the heel. Both of them healed after multiple dressing changes. According to the SAS, all patients had comparable levels of severe anxiety and depression preoperatively, with SAS scores ranging from 35 to 60 (mean 48.02 ± 8.12). Postoperatively, the LEFS score was 57.83 ± 11.89.
3.4. Case report
3.4.1. Case 1
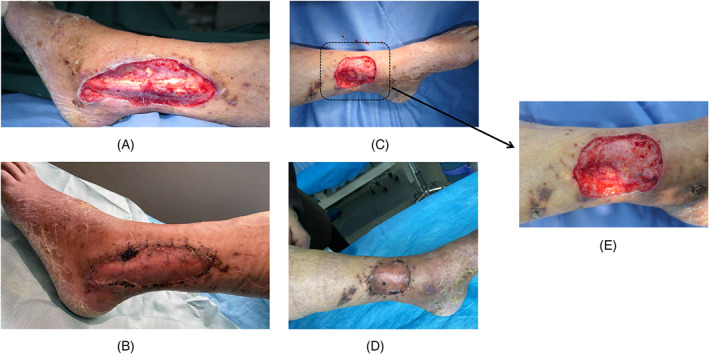
A 65‐year‐old male suffered a machine‐related crush injury, resulting in a soft‐tissue defect of the lateral malleolus and heel, as well as an exposed calcaneus (Figure 4A,B). The involved heel area was approximately 10 × 5 cm. No fracture in either the calcaneus or ankle joint was seen by X‐ray (Figure 4C). An ultrasound of the blood vessels in the lower extremities revealed varicose veins with thrombosis as well as arteriosclerosis with atherosclerotic plaque. A pulmonary function test indicated that the patient's lung function was poor. Thus, in this patient, a free flap was not the best choice. Instead, after thorough debridement, the wound with exposed bone was covered with PMMA cement (Figure 4D).
FIGURE 4.
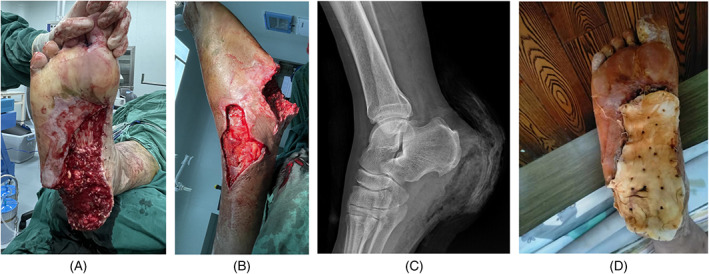
Case 1. A 65‐year‐old male with a machine‐related crush injury. A and B, Soft‐tissue defect in the lateral malleolus, exposing the calcaneus and plantar fascia; C, absence of fracture in the calcaneus and ankle joint, as seen on X‐ray; D, the wound with exposed bone covered with polymethyl methacrylate cement
Five weeks later, the PMMA cement was removed, revealing an IM over the wound that was rich in blood vessels with freshly oozing blood and an elastic and moist surface (Figure 5A). An autologous partial‐thickness skin graft was used to cover the IM, followed by bandage compression (Figure 5B). At the 1‐month (Figure 5C) and 6‐month (Figure 5D) follow‐up examinations, good wound healing was observed. The patient was very satisfied with the outcome.
FIGURE 5.
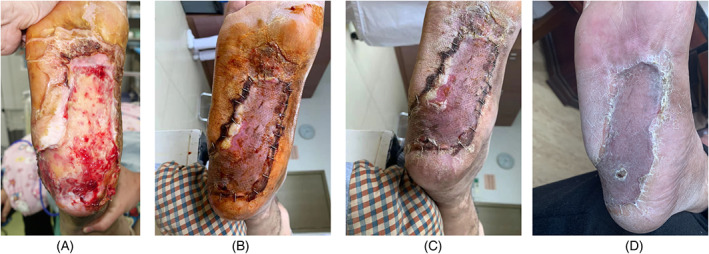
Case 1. A, Removal of the polymethyl methacrylate cement revealing the induced membrane (IM) covering the wound; B, an autologous partial‐thickness skin graft used to cover the IM; C and D, confirmation of good wound healing at the 1‐month and 6‐month follow‐up exams
3.4.2. Case 2
A 72‐year‐old male suffered a skin contusion of the left ankle during a car accident (Figure 6A,B). The injured area also had extensive sulfuric acid burns. Emergency debridement of the wounds revealed two skin defects, one at the lateral malleolus (12 × 6 cm) that included an exposed tendon and another at the medial malleolus (6 × 5 cm) with exposed bone (Figure 6C,D). Due to the poor local conditions at the burn site and the patient's lower‐extremity arteriosclerosis, a pedicled or random flap was, in our opinion, contraindicated. Thus, the wound with the exposed bone was thoroughly debrided and then covered with PMMA cement (Figure 6E,F).
FIGURE 6.
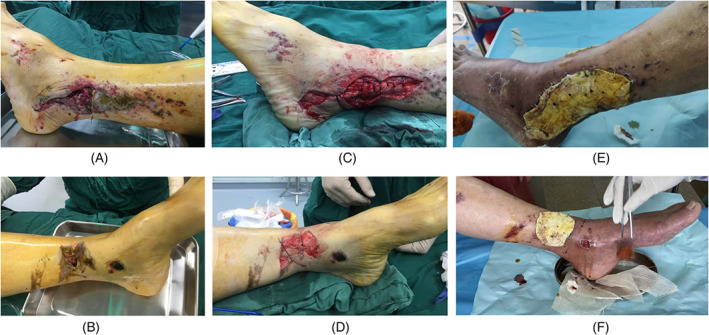
Case 2. A 72‐year‐old male with a crush injury from a heavy object. A and B, Skin contusion of the left ankle; C, skin defect with exposed tendon at the lateral malleolus; D, skin defect with exposed bone at the medial malleolus; E and F, after thorough debridement, the wound with exposed bone was covered with polymethyl methacrylate cement
Four weeks later, after removal of the bone cement, the IM was clearly visible (Figure 7A,C,E). An autologous partial‐thickness skin graft was then used to treat the newly freshened wound. Three months later, the patient's ankle had healed well, and the patient was satisfied with the outcome (Figure 7B,D).
FIGURE 7.
Case 2. A, C, and E, Removal of the bone cement clearly revealing the induced membrane; B and D, proper ankle healing and patient satisfaction with the outcome
3.4.3. Case 3
Following a tractor accident, a 60‐year‐old male suffered an extensive skin degloving injury of the right lower extremity, from the thigh down to the ankle, involving the deep fascia and muscle plane and exposing the tibia and fibula (Figure 8A,B), as well as an open fracture of the right ankle joint. During emergency surgery of the right lower limb, the necrotic tissue and skin were completely removed during thorough wound debridement. A split‐thickness skin piece harvested from the left thigh was used as the skin graft. The exposed bone in a skin defect (10 × 4 cm) on the lateral side of the knee was covered with PMMA cement (Figure 8C,D).
FIGURE 8.
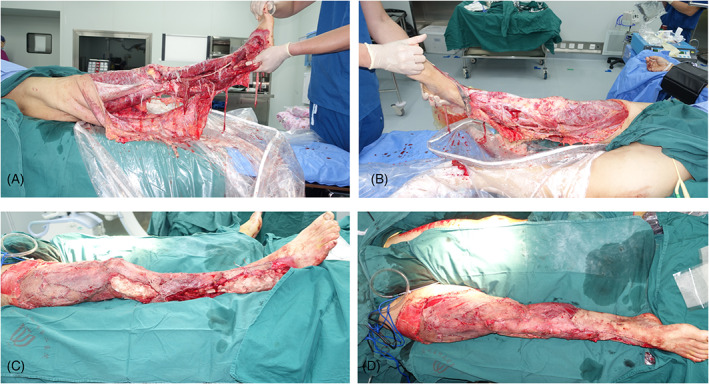
Case 3. A 60‐year‐old man injured in a tractor accident. A and B, Skin degloving injury of the right lower extremity; C and D, the wounds on the exposed bone of the knee and right lateral malleolus covered with polymethyl methacrylate cement
Two weeks later, exudate was still present on the wound surface, indicating that the debridement had not been sufficiently thorough. Thus, a second debridement and replacement of the bone cement were performed (Figure 9A). Six weeks later, the cement was removed, revealing an IM rich in new blood vessels on the exposed bone surface (Figure 9B,C). An autologous partial‐thickness skin graft was used to cover the IM, followed by bandage compression. At the last follow‐up, the inner thigh skin graft area was still viable (Figure 9D). All wounds healed well after the skin graft (Figure 10).
FIGURE 9.
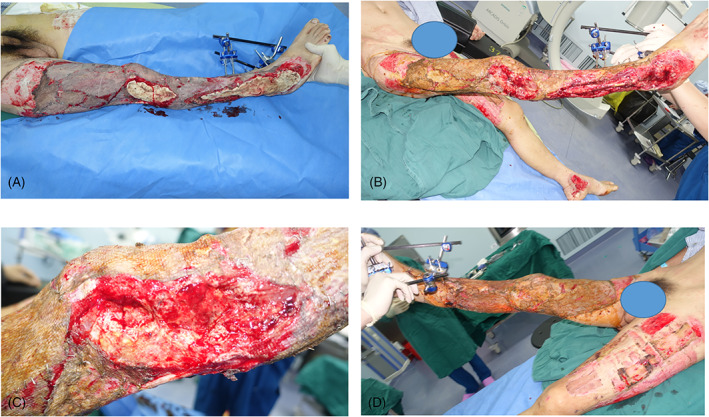
Case 3. Induced membrane (IM) harvest. A, Second debridement and replacement of the bone cement; B and C, removal of the cement revealing an IM rich in new blood vessels; D, complete survival of the skin graft on the inner thigh
FIGURE 10.
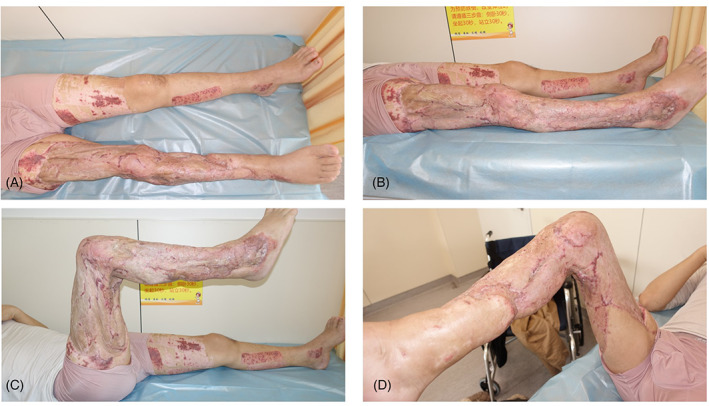
Case 3. Proper healing of all wounds after skin grafting of 1‐year fellow. A and B, anterior‐posterior (AP) view and lateral view of the wound. C and D, Functional activity of lower extremity
4. DISCUSSION
The treatment of non‐healing wounds with exposed bone or tendon is a surgical challenge, 17 , 18 although appropriate treatment has long been the focus of considerable research, and the goals (early irrigation and debridement followed by early soft‐tissue coverage) have remained essentially the same. Free tissue transfer allows the greatest surface coverage and the most flexibility in terms of flap placement; thus, it is still the gold standard for wound coverage. Moreover, good long‐term results with respect to limb or joint function have been reported. 19 However, free‐tissue transfer is a lengthy and expensive procedure that requires specialised practitioners as well as postoperative monitoring in the intensive care unit. 4 , 5 Parrett et al 20 reviewed the changing treatment protocols used by their institution to treat traumatic wounds with exposed bone or tendon. Among the changes in practice was a trend of less reconstruction, such that the use of free flaps has become less common, closure and skin graft procedures are intentionally delayed, and closures are frequently supported by the use of a vacuum‐assisted closure sponge. 21
Less‐invasive treatments with equally efficacious results, such as artificial dermis and skin grafting 9 and a skin graft with perifascial areolar tissue, 22 are appealing but pose risks of immune rejection and prolonged hospitalisation. The use of a composite tissue transfer, while preferred, may not be possible because of the lack of a graft donor site and/or the patient's poor physical status. In their animal studies, Koga et al 23 showed that the periosteum is an essential tissue component of a wound bed for an exposed bone requiring a skin graft. In the absence of a periosteal covering, the result will be poor and the application of a full‐thickness skin graft over the bony or tendon surface will be required. Neo‐vascularisation induced by decorticating the bone or drilling multiple holes into the medullary cavity, for instance, is unreliable and the extent of vascularised bed growth unpredictable, with an inadequate bed possibly causing severe disabilities due to the need to resect functional bone or tendon postoperatively. Although negative pressure wound therapy is a well‐established method to promote wound healing and its efficacy is well accepted by many clinicians, it is often used as a bridging device for the closure of a skin graft or skin flap. 24 , 25 Therefore, the formation of vascularised granulation tissue on the exposed areas may be slower, which can be a burden on patients.
In this study, the angiogenic potential of the IM surrounding the PMMA cement 26 was exploited in combination with skin grafting to repair exposed open wounds in patients with injuries resulting in exposed bone or tendon wounds with a periosteal defect. The outcomes ranged from good to excellent in nearly all patients. The advantage of our approach is that, because the wound surface is covered by bone cement, the patient can be discharged from the hospital before IM formation, thus reducing the length and cost of hospitalisation. The IM is an initially avascular bed that supports the survival of the skin graft, and it differs from the PM. VEGF 27 and CD31, 28 produced by platelets, stimulate the growth of vascular endothelial cells, 29 thereby accelerating revascularisation and enhancing the blood supply to ischemic flaps, thus increasing their survival and, in turn, wound coverage. 30 , 31 IHC showed the greater vascularisation potential of the IM compared with the PM due to the higher‐level expression of VEGF, CD31 and CD34. IM vascularisation has also been demonstrated in other human and animal studies. 32 , 33
Sufficient debridement is a prerequisite for wound coverage, 34 including radical excision of non‐viable tissues and those of doubtful viability. The infection of granulation tissue must be avoided as it can result in poor adhesion despite sufficient surgical debridement and other measures during surgery. Bone or tendon must be excised until healthy, bright, bleeding tissue is seen. An abundant blood supply to both the soft tissue and bone is an important factor in the formation of mature IM. In two of our patients, a second more thorough debridement and bone decortication were required for mature IM formation. Niikura et al 32 reported histologically confirmed IM formation, with rich vascularity, slight inflammation, a foreign‐body reaction and fibrosis, in all of their patients, as well as less IM vascularisation in patients with than in those without free flap surgery. This observation provides further evidence of the importance of a rich blood supply in the soft tissues for the formation of a mature IM. However, the amount of time that should be allowed for IM formation is a matter of debate. Although basic research studies suggest 4‐8 weeks, 35 , 36 , 37 due to patient‐related circumstances, the second surgery in the Masquelet technique is often substantially delayed, ranging from 4 to 96 weeks. 38 A clear correlation between IM maturation and a worse outcome for bone reconstruction has yet to be demonstrated. Similar studies of the effect on wound coverage are also lacking. In our method, the PMMA spacer is removed 4‐6 weeks postoperatively, at which time the IM is mature, as shown by the survival of all subsequent skin grafts. Whether a shorter or longer waiting time results in a more mature IM is unknown, but emerging clinical evidence suggests that time alone is not a reliable measure of IM maturity. 39 , 40
The SAS score showed good psychological status in the patients after the repair, and good functional recovery was achieved by combining an IM with skin grafting. Similar to our technique, Shang et al 41 used an artificial dermis combined with autologous split‐thickness skin grafting. A higher SAS score and greater functional recovery were obtained in those patients compared with patients who underwent autologous intermediate‐thickness skin grafting. The LEFS has been widely validated in the orthopaedic literature following hip and knee arthroplasty, as well as other reconstructive procedures. In addition, it boasts a high test‐retest and inter‐rater reliability; it is also more sensitive at detecting change than other tests of functional outcome, such as the SF‐36. Current literatures reported that the LEFS after lower extremity wound reconstruction ranged from 35 to 65. 42 , 43 Our reported LEFS was 57.83. The patient was mostly satisfied.
This is a rare report of a method combining an IM with autologous split‐thickness skin grafting to cover a non‐healing wound with exposed bone or tendon. Liu et al 44 had used this technology in the treatment of diabetic foot ulcer (DFU) and achieved good results. They provided preliminary information on IM formation followed by PMMA implantation in the management of DFUs when revascularization is not feasible. Nonetheless, free skin flaps remain the method of choice for these traumatic wounds, and the indications for our approach should be carefully considered based on the following: First, the patient's general condition is poor, and a long operation to achieve free skin flap transplantation would not be tolerated. Second, the condition of the flap donor site is too poor (infected or scarred) for a flap to be provided. Third, in addition to multiple injuries, the patient has diseases of other systems that delay wound treatment; in such cases, bone cement can be used to temporarily cover the wound. Fourth, the arterial condition of the flap graft area is a contraindication for the procedure. Likewise, this technique may not be applicable in weight‐bearing areas of the foot and in locations with high joint mobility.
A limitation of our study was the small sample size. Thus, although the clinical outcome of our patients was good, the results remain to be confirmed in a larger series with a control group (visual analogue scale (VAS) or dressing group). In addition, our study was conducted at a single centre. Prospective multi‐centre investigations are needed to confirm the adaptability of our approach. The signalling pathways and mechanisms involved in IM maturation will be explored further via in vivo and vitro experiments.
5. CONCLUSION
This study demonstrated a good‐to‐excellent clinical outcome and low morbidity rate using a novel approach for the management of non‐healing wounds involving the lower extremities, especially in cases in which bony structures were exposed in the elderly. The method is based on the use of an IM membrane and skin grafting.
FUNDING INFORMATION
This work is supported by a grant from Wenzhou Science and Technology Bureau Foundation (Grant No. Y20210046).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Cai L, Hong Z, Zhang Y, et al. Management of wounds with exposed bone structures using an induced‐membrane followed by polymethyl methacrylate and second‐stage skin grafting in the elderly with a 3‐year follow‐up. Int Wound J. 2023;20(4):1020‐1032. doi: 10.1111/iwj.13955
Funding information Wenzhou Science and Technology Bureau Foundation, Grant/Award Number: Y20210046
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Ali M, Reda FM, Abbassi H, Issaoui H, Gargouri M, Razanabola F. Management of a severe degloving injury with a type 2 open tibia fracture using negative pressure wound therapy with instillation and dwell time. Wounds. 2020;32(12):E110‐E113. [PubMed] [Google Scholar]
- 2. Messner J, Harwood P, Johnson L, Itte V, Bourke G, Foster P. Lower limb paediatric trauma with bone and soft tissue loss: ortho‐plastic management and outcome in a major trauma Centre. Injury. 2020;51(7):1576‐1583. [DOI] [PubMed] [Google Scholar]
- 3. Kozak GM, Hsu JY, Broach RB, et al. Comparative effectiveness analysis of complex lower extremity reconstruction: outcomes and costs for biologically based, local tissue rearrangement, and free flap reconstruction. Plast Reconstr Surg. 2020;145(3):608e‐616e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Suominen S, Asko‐Seljavaara S. Free flap failures. Microsurgery. 1995;16(6):396‐399. [DOI] [PubMed] [Google Scholar]
- 5. Maffi TR, Tran NV. Free‐tissue transfer experience at a county hospital. J Reconstr Microsurg. 2001;17(6):431‐433. [DOI] [PubMed] [Google Scholar]
- 6. Heinz TR, Cowper PA, Levin LS. Microsurgery costs and outcome. Plast Reconstr Surg. 1999;104(1):89‐96. [PubMed] [Google Scholar]
- 7. Aldea PA, Shaw WW. The evolution of the surgical management of severe lower extremity trauma. Clin Plast Surg. 1986;13(4):549‐569. [PubMed] [Google Scholar]
- 8. Friedrich JB, Katolik LI, Hanel DP. Reconstruction of soft‐tissue injury associated with lower extremity fracture. J Am Acad Orthop Surg. 2011;19(2):81‐90. [DOI] [PubMed] [Google Scholar]
- 9. Chen X, Chen H, Zhang G. Management of wounds with exposed bone structures using an artificial dermis and skin grafting technique. Clin Plast Surg. 2012;39(1):69‐75. [DOI] [PubMed] [Google Scholar]
- 10. Rohilla R, Sharma PK, Wadhwani J, das J, Singh R, Beniwal D. Prospective randomized comparison of bone transport versus Masquelet technique in infected gap nonunion of tibia. Arch Orthop Trauma Surg. 2021;142:1923‐1932. [DOI] [PubMed] [Google Scholar]
- 11. Wang P, Wu Y, Rui Y, Wang J, Liu J, Ma Y. Masquelet technique for reconstructing bone defects in open lower limb fracture: analysis of the relationship between bone defect and bone graft. Injury. 2021;52(4):988‐995. [DOI] [PubMed] [Google Scholar]
- 12. Coris JGF, Rahal SC, Fonseca‐Alves CE, et al. Effect of low‐level laser therapy on the membrane induced by the Masquelet technique at an orthotopic site in rabbits. Acta Cir Bras. 2021;36(10):e361003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang X, Wei F, Luo F, Huang K, Xie Z. Induction of granulation tissue for the secretion of growth factors and the promotion of bone defect repair. J Orthop Surg Res. 2015;10:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tang Q, Jin H, Tong M, et al. Inhibition of Dll4/Notch1 pathway promotes angiogenesis of Masquelet's induced membrane in rats. Exp Mol Med. 2018;50(4):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Olatunji BO, Deacon BJ, Abramowitz JS, Tolin DF. Dimensionality of somatic complaints: factor structure and psychometric properties of the Self‐Rating Anxiety Scale. J Anxiety Disord. 2006;20(5):543‐561. [DOI] [PubMed] [Google Scholar]
- 16. Binkley JM, Stratford PW, Lott SA, Riddle DL. The Lower Extremity Functional Scale (LEFS): scale development, measurement properties, and clinical application. North American Orthopaedic Rehabilitation Research Network. Phys Ther. 1999;79(4):371‐383. [PubMed] [Google Scholar]
- 17. Dissemond J. Non‐healing surgical wound with exposed bone. J Wound Care. 2020;29(sup10a):S11‐S13. [DOI] [PubMed] [Google Scholar]
- 18. Zhao Y, Wang M, Liang F, Li J. Recent strategies for enhancing the therapeutic efficacy of stem cells in wound healing. Stem Cell Res Ther. 2021;12(1):588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cigna E, Mardini S, Chen HC, et al. An improved method of supercharged transposed latissimus dorsi flap with the skin paddle for the management of a complicated lumbosacral defect. Eur Rev Med Pharmacol Sci. 2015;19(6):921‐926. [PubMed] [Google Scholar]
- 20. Parrett BM, Matros E, Pribaz JJ, Orgill DP. Lower extremity trauma: trends in the management of soft‐tissue reconstruction of open tibia‐fibula fractures. Plast Reconstr Surg. 2006;117(4):1315‐1322. [DOI] [PubMed] [Google Scholar]
- 21. Klein DS, Yingling JM, Patel P, Capo JT. Vacuum‐assisted therapy for combined volar‐dorsal soft‐tissue defects of the hand: a case report. Adv Skin Wound Care. 2022;35(1):57‐61. [DOI] [PubMed] [Google Scholar]
- 22. Abe Y, Hashimoto I, Ishida S, Mineda K, Yoshimoto S. The perifascial areolar tissue and negative pressure wound therapy for one‐stage skin grafting on exposed bone and tendon. J Med Invest. 2018;65(1.2):96‐102. [DOI] [PubMed] [Google Scholar]
- 23. Koga Y, Komuro Y, Yamato M, et al. Recovery course of full‐thickness skin defects with exposed bone: an evaluation by a quantitative examination of new blood vessels. J Surg Res. 2007;137(1):30‐37. [DOI] [PubMed] [Google Scholar]
- 24. McNamara SA, Hirt PA, Weigelt MA, et al. Traditional and advanced therapeutic modalities for wounds in the paediatric population: an evidence‐based review. J Wound Care. 2020;29(6):321‐334. [DOI] [PubMed] [Google Scholar]
- 25. Apelqvist J, Willy C, Fagerdahl AM, et al. EWMA document: negative pressure wound therapy. J Wound Care. 2017;26(sup3):S1‐S154. [DOI] [PubMed] [Google Scholar]
- 26. Wang W, Zuo R, Long H, et al. Advances in the Masquelet technique: myeloid‐derived suppressor cells promote angiogenesis in PMMA‐induced membranes. Acta Biomater. 2020;108:223‐236. [DOI] [PubMed] [Google Scholar]
- 27. Fei L, Zhang J, Niu H, Yuan C, Ma X. Effects of rosuvastatin and MiR‐126 on myocardial injury induced by acute myocardial infarction in rats: role of vascular endothelial growth factor A (VEGF‐A). Med Sci Monit. 2016;22:2324‐2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lertkiatmongkol P, Liao D, Mei H, Hu Y, Newman PJ. Endothelial functions of platelet/endothelial cell adhesion molecule‐1 (CD31). Curr Opin Hematol. 2016;23(3):253‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Melincovici CS, Bosca AB, Susman S, et al. Vascular endothelial growth factor (VEGF) ‐ key factor in normal and pathological angiogenesis. Rom J Morphol Embryol. 2018;59(2):455‐467. [PubMed] [Google Scholar]
- 30. Gao Y, Gao B, Zhu H, et al. Adipose‐derived stem cells embedded in platelet‐rich plasma scaffolds improve the texture of skin grafts in a rat full‐thickness wound model. Burns. 2020;46(2):377‐385. [DOI] [PubMed] [Google Scholar]
- 31. Zhang F, Oswald TM, Lin L, Wang S, Lin S, Lineaweaver WC. Improvement of full‐thickness skin graft survival by application of vascular endothelial growth factor in rats. Ann Plast Surg. 2008;60(5):589‐593. [DOI] [PubMed] [Google Scholar]
- 32. Niikura T, Jimbo N, Komatsu M, et al. Histological analysis of induced membranes in patients whose bone defects were treated with the Masquelet technique to identify factors affecting the vascularity of induced membranes. J Orthop Surg Res. 2021;16(1):248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xie J, Liu D, Wang H, et al. Effects of topical mechanical stability on the formation of Masquelet membrane in a rabbit radial defect model. Sci Rep. 2020;10(1):18939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Piwnica‐Worms W, Azoury SC, Kozak G, et al. Flap reconstruction for deep sternal wound infections: factors influencing morbidity and mortality. Ann Thorac Surg. 2020;109(5):1584‐1590. [DOI] [PubMed] [Google Scholar]
- 35. Roddy E, DeBaun MR, Daoud‐Gray A, Yang YP, Gardner MJ. Treatment of critical‐sized bone defects: clinical and tissue engineering perspectives. Eur J Orthop Surg Traumatol. 2018;28(3):351‐362. [DOI] [PubMed] [Google Scholar]
- 36. Karger C, Kishi T, Schneider L, Fitoussi F, Masquelet AC, French Society of Orthopaedic Surgery and Traumatology (SoFCOT) . Treatment of posttraumatic bone defects by the induced membrane technique. Orthop Traumatol Surg Res. 2012;98(1):97‐102. [DOI] [PubMed] [Google Scholar]
- 37. Taylor BC, French BG, Fowler TT, Russell J, Poka A. Induced membrane technique for reconstruction to manage bone loss. J Am Acad Orthop Surg. 2012;20(3):142‐150. [DOI] [PubMed] [Google Scholar]
- 38. Morelli I, Drago L, George DA, Gallazzi E, Scarponi S, Romanò CL. Masquelet technique: myth or reality? A systematic review and meta‐analysis. Injury. 2016;47(suppl 6):S68‐S76. [DOI] [PubMed] [Google Scholar]
- 39. Gindraux F, Loisel F, Bourgeois M, et al. Induced membrane maintains its osteogenic properties even when the second stage of Masquelet's technique is performed later. Eur J Trauma Emerg Surg. 2020;46(2):301‐312. [DOI] [PubMed] [Google Scholar]
- 40. Durand M, Barbier L, Mathieu L, et al. Towards understanding therapeutic failures in masquelet surgery: first evidence that defective induced membrane properties are associated with clinical failures. J Clin Med. 2020;9(2):450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shang F, Lu YH, Gao J, Hou Q. Comparison of therapeutic effects between artificial dermis combined with autologous split‐thickness skin grafting and autologous intermediate‐thickness skin grafting alone in severely burned patients: a prospective randomised study. Int Wound J. 2021;18(1):24‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ciudad P, Kaciulyte J, Torto FL, et al. The profunda artery perforator free flap for lower extremity reconstruction. Microsurgery. 2022;42(1):13‐21. [DOI] [PubMed] [Google Scholar]
- 43. Falola RA, Lakhiani C, Green J, et al. Assessment of function after free tissue transfer to the lower extremity for chronic wounds using the lower extremity functional scale. J Reconstr Microsurg. 2018;34(5):327‐333. [DOI] [PubMed] [Google Scholar]
- 44. Liu C, You JX, Chen YX, et al. Effect of induced membrane formation followed by polymethylmethacrylate implantation on diabetic foot ulcer healing when revascularization is not feasible. J Diabetes Res. 2019;2019:2429136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


