Abstract
We summarize the most important advances in RNA delivery and nanomedicine. We describe lipid nanoparticle-based RNA therapeutics and the impacts on the development of novel drugs. The fundamental properties of the key RNA members are described. We introduced recent advances in the nanoparticles to deliver RNA to defined targets, with a focus on lipid nanoparticles (LNPs). We review recent advances in biomedical therapy based on RNA drug delivery and state-of-the-art RNA application platforms, including the treatment of different types of cancer. This review presents an overview of current LNPs based RNA therapies in cancer treatment and provides deep insight into the development of future nanomedicines sophisticatedly combining the unparalleled functions of RNA therapeutics and nanotechnology.
Key words: Antisense oligonucleotides, siRNA, miRNA, mRNA, Cancer treatment, Nanomedicine, RNA therapy, Lipid nanoparticles
Graphical abstract
This review presents an overview of current nanoparticles-based RNA therapies in cancer and provides deep insight into the development of future nanomedicines sophisticatedly combining unparalleled functions of RNA therapeutics and nanotechnology.
1. Introduction
Given the recent developments in scientific and medical technologies, the use of biological molecules as therapeutic agents has become an increasingly popular research topic. Among the identified biological molecules, RNA stands out owing to its unique biological functions and specific characteristics1. RNA was originally considered simple, intermediate transmission products that arise during DNA transcription. However, increasing research has reviewed the various roles of RNA and has indicated that they are co-related to most biochemical pathways2. One of the most exciting discoveries was using RNA as therapeutic molecules, which led to intense scientific research to develop therapeutic RNA and a constant stream of discoveries and outcomes in this field in recent years3.
Recently many RNA-based drugs have become the rising stars in pharmacotherapy. However, there are some important challenges. One of the largest obstacles in the clinical use of RNA-based drugs is to deliver RNA in vitro or in vivo4. To develop clinically effective RNA-based drugs, some difficulties must be overcome. The first is the robust defense system of most human cells that keeps exogenous RNA outside cell membranes, as naked RNA are large, negatively charged molecules5. RNA intrusion can also trigger the immune system. Different levels of inflammatory responses may occur when introducing certain RNA into the human system6. Furthermore, naked RNA is unstable and must be guarded against its tendency to degrade during the delivery process. Thus, most RNA-based drugs require protection to maintain their RNA integrity7. Ground-breaking drug delivery nanoplatforms are being rapidly developed, which has led to remarkable progress in RNA-based therapies in the previous five years. For example, several nanostructured vehicles for RNA delivery were designed, manufactured, and tested to guarantee RNA delivery efficacy and protection. Nanotechnology-based systems were also developed to overcome or bypass the barriers in the human body, thus enabling RNA-based drugs to perform their biochemical functions against their intended targets8. The merging of nanoscience and bioactive molecule discoveries has clearly led to striking advances in the development of a new era of RNA-based drugs, and this will lead to a revolution in future pharmacological treatments of cancer and other diseases.
2. The introduction of various RNA therapeutics
RNA therapies can enable targeted nucleic acid sequence delivery to edit specific genetic anomalies or mutations (e.g., down-regulation, augmentation, or correction)9. Given their reportedly outstanding therapeutic effects, RNA has become a favorable treatment agent for several diseases. The advantages of RNA therapies include the following: (i) the possibility for patient-specific treatments with relatively low cost and high safety levels. (ii) the same type of encoded RNA may perform different cell regulating functions when applied in different therapies. (iii) the RNA sequence design and synthesis are relatively simple. Most importantly, when compared with DNA therapies, RNA therapies keep the host genome intact, as they do not need to enter the nuclear membrane to initiate cytoplasmic protein translation10. Prompted by the above characteristics, more customized therapies with improved accuracy have been developed for different diseases (Table 1).
Table 1.
Customized RNA therapies developed for different types of chronic diseases.
| Disease | RNA | Delivery | Status | Company |
|---|---|---|---|---|
| Macular degeneration | Aptamer (RNA) | Intravitreal | FDA approval in 2014 | Bausch + Lomb |
| Spinal muscular atrophy | ASO | Intrathecal | FDA approval in 2016 | Ionis |
| Duchenne muscular dystrophy | ASO | Intravenous | FDA approval in 2016 | Sarepta |
| Polyneuropathy | siRNA | Intravenous | FDA approval in 2018 | Alnylam |
| Familial amyloid polyneuropathy | ASO | Subcutaneous | FDA approval in 2018 | Ionis |
| Acute hepatic porphyria | siRNA | Subcutaneous | FDA approval in 2019 | Alnylam |
| Primary hyperoxaluria type 1 | siRNA | Subcutaneous | FDA approval in 2020 | Alnylam |
| Familial chylomicronemia syndrome | ASO | Subcutaneous | EU approval in 2019 | Ionis |
| COVID-19 | mRNA | Intramuscular | FDA approval | Moderna |
| Brain cancer | Aptamer (RNA) | Intravenous | Phase I/II | NOXXON |
| Solid tumors | mRNA | Intravenous | Phase I/II | BioNTech |
| COVID-19 | mRNA | Intramuscular | FDA approved | BioNTech and Pfizer |
| Advanced melanoma | mRNA | Intratumoral | Phase I/II | BioNTech/Sanofi/Genmab |
| Generalized myasthenia gravis | mRNA | Intravenous | Phase I/II | Cartesian |
| Cystic fibrosis | mRNA | Inhalation | Phase I/II | Translate Bio |
| Solid tumors/lymphoma/advanced ovarian carcinoma | mRNA | Intratumoral | Phase I/II | Moderna |
| Solid tumors | mRNA | Intratumoral | Phase I | Moderna |
| Solid tumors | mRNA | Intratumoral | Phase I | Moderna |
| Chikungunya infection | mRNA | Intravenous | Phase I | Moderna |
| Zika | mRNA | Intramuscular | Phase I | Moderna |
| Cancer | mRNA | Intravenous | Phase I | Moderna |
| Advanced melanoma | mRNA | Intravenous | Phase I | BioNTech |
| Solid tumors | mRNA | Intratumoral | Phase I | CureVac |
| Rabies | mRNA | Intramuscular | Phase I | CureVac |
| Non-small cell lung cancer | mRNA | Intradermal | Phase I | CureVac |
| Urea disorder | mRNA | Intravenously | Phase I | Arcturus |
| Tissue repair | miRNA | Intradermal | Phase I | Mirage (Viridian) |
| Methylmalonic aciduria | mRNA | Intravenous | Phase I/II | Moderna |
| Blood cancers | Cobomarsen (MRG-106) | Intravenous/subcutaneous | Phase II | MiRagen (Viridian) |
| Keloids | miRNA | Intradermal | Phase II | Mirage (Viridian) |
| Cytomegalovirus infection | mRNA | Intramuscular | Phase II | Moderna |
| Cancer | mRNA | Intramuscular | Phase II | Moderna |
| Ischemic heart disease | mRNA | Epicardial | Phase II | Moderna/AstraZeneca |
| Diabetic nephropathy | Aptamer (RNA) | Intravenous/Subcutaneous | Phase II | NOXXON |
| Lung and pancreatic cancer | mRNA | Intravenous | Phase II | Poseida |
| COVID-19 | mRNA | Intramuscular | Phase III | CureVac |
Common RNA therapy approaches can be categorized into coding and noncoding. A major part of the coding RNA approach works on gene stimulation, as it triggers coded-protein antigen synthesis. It affects antibody and cytotoxic lymphocyte production, which consequently induces correlated immunity. In contrast, other small percentages of coding RNA modulate the production and activation of constitutive and functional proteins that can be used for protein supplementation therapy11. The noncoding RNA (ncRNA) approach works on gene silencing, as it silences single or multiple related genes to inhibit the production of encoded proteins.
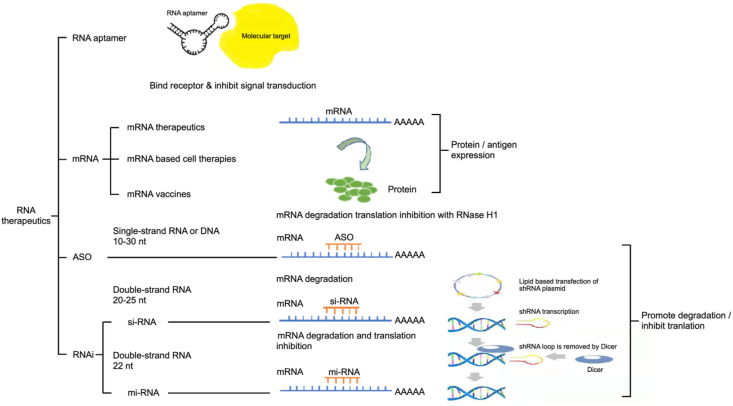
Various types of RNA have been used in RNA therapies, and in the following sections, the primary RNA therapeutics are described and discussed (Fig. 1).
Figure 1.
Types of RNA used in RNA therapies: RNA Aptamer, mRNA, ASO, and RNAi.
2.1. mRNA
Messenger RNA (mRNA) is a type of coding RNA, and each is a single-stranded structured nucleotide sequence produced during complementary DNA transcription. Specifically, three ribonucleotides form one codon, a series of organized codons form one nucleotide sequence, and the nucleotide sequence forms a strand of mRNA, ranging from 300 to 5000 kDa. During the transport of genetic information, mRNA functions as an intermediate agent for protein synthesis between nuclear DNA and the cytoplasm12. As mentioned earlier, mRNA can initiate the coding process without crossing the nuclear barrier, and importantly, they do not insert into the host genome. Furthermore, most mRNA naturally degrades in cells after translation and makes them safe to use in patients. Therefore, mRNA applied as a therapeutic agent is superior to other agents in high efficacy and guaranteed safety with each dose. Other advantages of mRNA therapies that make them efficient and desirable for future development include a fast pesticide effect, cost-effectiveness, and the possibility of in vitro production13.
However, the development of mRNA as therapeutics has been limited by their relative size, stability, biological activity, immunogenicity, and translation and delivery efficiency. In order to solve these obstacles limiting the clinical applications of mRNA, their modifications, including nucleotide substitutions and sequence optimizations, are widely investigated. So far, some mRNA, after the processing of architectonic stabilization and chemical treatments, exhibit significantly improved sensitivity to enzymatic degradation and host immunity14.
mRNA-based therapies have also been investigated in relation to liver regeneration. Injecting mRNA can trigger the high proliferation of hepatocytes, which can induce restoration of liver function and accelerate tissue regeneration18. Furthermore, vascular endothelial growth factor (VEGF) A-encoding mRNA has been explored as a therapeutic for type 2 diabetes mellitus. Research has shown that with upregulated VEGFA expression, skin blood flow subsequently improves, suggesting a potential clinical use regarding angiogenesis15, 16, 17, 18, 19.
2.2. RNA interference
ncRNA is a non-protein-coding transcript and is implicated in gene regulation and RNA processing20. ncRNA can function in gene silencing, DNA imprinting, and de-methylation and are generally classified into small regulatory ncRNA and long ncRNA. Small ncRNA named small interfering RNA (siRNA) or microRNA (miRNA) is produced during double-strand RNA (dsRNA) cleavage, and these can interfere with targeted mRNA transcription during translation21,22.
RNA-induced silencing complex (RISC) is a multi-protein complex made by the sequence-specific silencing of cognate genes, and it functions as a core intermediate for mRNA degradation and translational inhibition. RNA interference (RNAi) in the RISC can be triggered by siRNA, shRNA, miRNA, and long ncRNA. Briefly, in the process of mRNA degradation and translational inhibition, an endoribonuclease called dicer breaks long dsRNA and complex hairpin precursors into several shorter duplexes or siRNA. Then, the siRNA is loaded onto RISC, and once this incorporation has finished, ds-siRNA splits into passenger and guide strands. The RISC then activates and catalyzes the guide strand to bind with the target sequences, whereas former passenger strands are degraded and released. Cellular nucleases then cleave and degrade the bound mRNA. The expression of the target gene is inhibited at the end of this process23. Unlike siRNA, most short hairpin RNA (shRNA) applications are viral vector-based and face additional challenges. First, the shRNA sequence naturally forms a tight hairpin structure. shRNA-mediated gene silencing in cells could be impaired under low dicer levels, as they are mostly transcribed by RNA polymerase III or modified polymerase II. shRNA activation is also promoter-dependent, which means its pathway needs the interaction of chromosomal DNA to maintain its function24.
Inclisiran, a product of Novartis, is a first-in-class siRNA for cholesterol (CHO)-lowering, in which the therapeutic siRNA is chemically linked to triantennary N-acetylgalactosamine carbohydrates25, 26, 27, 28, 29. Recently, Ionis Pharmaceuticals and its subsidiary Akcea Therapeutics jointly announced its antisense oligonucleotide (ASO) drug Vupanorsen (AKCEA-ANGPTL3-LRx). Hypertriglyceridemia, diabetes, and nonalcoholic steatohepatitis are serious risk factors for cardiovascular disease. It has the potential to reduce the risk of diabetes and cardiovascular disease30.
miRNA is single-stranded RNA with hairpin loop structures that contain a duplex of approximately 22 nucleotides. During miRNA synthesis, the encoded gene is first transcribed into a primary-miRNA by RNA polymerases II and III, which forms a hairpin loop structure and is processed into precursor miRNA (pre-miRNA) by the Drosha-DiGeorge critical region 8 complex. Finally, specific dicers cleave the pre-miRNA to form a mature functional miRNA. The ss-miRNA is then included into the RISC, and thus the guide strand is greatly maintained with the capability to bind the corresponding mRNA. Compared with siRNA, miRNA complementarily binds to the target sequence, which leads to hundreds of possibilities for miRNA and mRNA sequence combinations31. With a precise match, the miRNA can regulate target mRNA degradation by inducing the cleavage of endo-nucleo-lytic. However, the imperfect match could adjust translation, resulting in the suppression of mRNA expression.
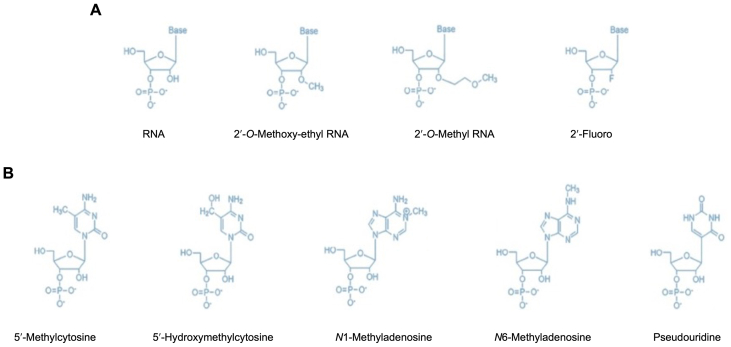
Among all the types of introduced RNA, most of them are not stable, especially in vivo. Besides the package of lipid nanoparticles (LNPs) or other carriers, post-modification of RNA is very important to stabilize the RNA (Fig. 2). The modifications enhance the resistance of the RNA to nuclease digestion and delivery of the RNA to the cell, whether the RNA is delivered alone or in combination with a transfection agent or nanocarriers. The activity of the RNA in the cell is better maintained with modifications.
Figure 2.
The post-synthetic modifications of RNA for the delivery of the RNA to a mammalian cell. (A) Molecular structure of 2′ position of the ribose sugar ring including RNA base, 2′-O-methoxy-ethyl RNA base, 2′-O-methyl RNA base, and 2′-Fluoro bases. (B) Molecular structure of modification at nitrogen bases, including 5′-methylcytosine, 5′-hydroxymethylcytosine, N1-methyladenosine, N6-methyladenosine, and pseudouridine.
3. The review of various delivery platforms
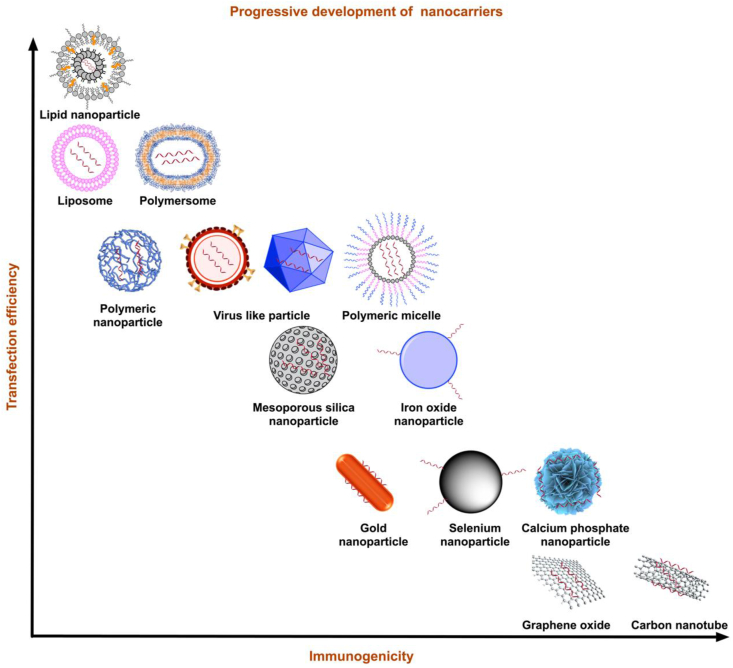
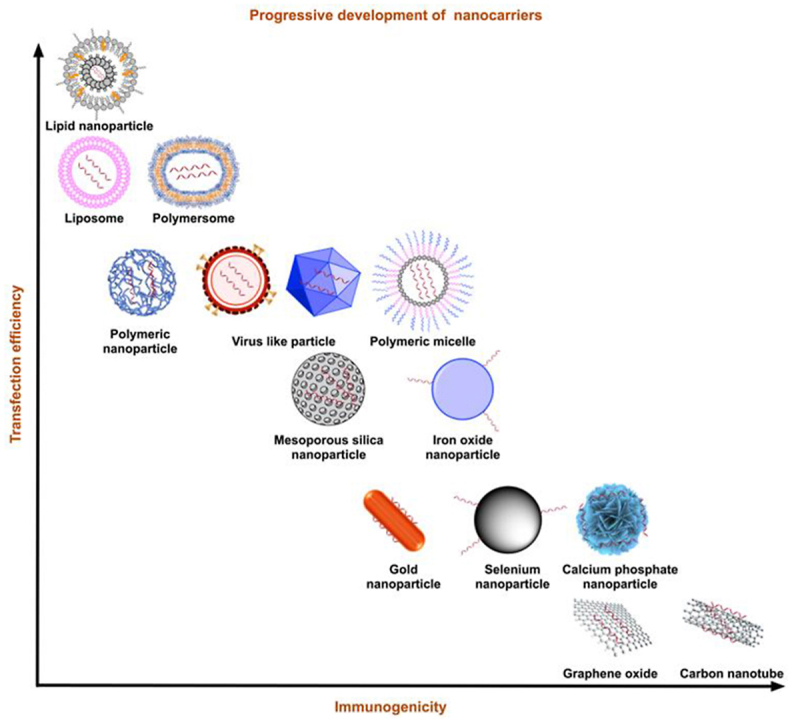
Safe delivery is critical for RNA therapy as RNA degradation quickly occurs. An efficient delivery platform is a key to guaranteeing efficient delivery of therapeutic RNA. Ideal delivery platforms should protect RNA from degradation while compensating for their inherent hydrophilicity and electron negativity as they traverse the cell membrane32. Additionally, the following features for RNA carriers are essential and must be considered: high loading efficacy, low toxicity, and low immunity33. Recent investigations have shown that nanostructured platforms are excellent candidates for RNA delivery34. These nanocarriers are stable and can spread easily in organs. Here, we have summarized recent advances in nanotechnology with a primary focus on LNPs (Fig. 3 and Table 2).
Figure 3.
Types of nanocarriers. Summary of nanocarriers developed in the last decade by transfection efficiency and immunogenicity. With balanced transfection efficiency and reduced immunogenicity, LNPs have the potential to evolve into the most promising nanocarriers.
Table 2.
Comparison of different types of carriers for RNAi molecules.
| Carrier | Mechanism | Key characteristic | Disadvantage | Advantage |
|---|---|---|---|---|
| Viral vectors | Bind to the cell receptors High possibility that it can integrated into the genome of host |
1) Higher efficiency than LNPs 2) Transfect targeting cells naturally and efficiently |
1) High toxicity 2) Genome modification |
1) Improved efficiency 2) Biodegradable, low immunogenicity 3) Improve the specificity 4) Low toxicity, good biocompatibility, high security 5) Good production, low cost |
| LNPs | Conjugate or complexion | 1) Good stability 2) Ability to protect the medicine from degradation 3) No immunogenicity 4) Easy to be stored after freeze-dried |
1) Low efficiency 2) Stability |
|
| Polymer-based nanoparticles | Combined with the skeleton phosphate or connected to the adapter body | 1) Higher thermodynamic stability 2) Higher dynamic stability |
Low transfection efficiency | |
| Inorganic nanoparticles | MSNs, carbon nanotubes, QDs, gold nanoparticles | 1) High surface area 2) Large pore volume 3) Strong surface absorption |
Low efficiency | |
| Exosome-mimetic nanovesicles | 1) High yield 2) Efficient loading 3) Low toxicity |
Preparation is not as simple as LNPs |
3.1. Fate of nanocarriers
The size and surface properties of nanocarriers should be the primary considerations during the designing process. The size of nanocarriers can be optimized. On the one hand, they should be small enough to escape the phagocytosis of macrophages, mainly present in the reticuloendothelial systems, such as the liver and spleen. On the other hand, their size is desired to be large enough to prevent themselves from extravasating out of the capillaries. Extensive research has determined that the optimal nanocarrier size is less than 100 nm35. In addition, nanocarrier surface modifications can influence the stability and destiny of nanoparticles in vivo. Modifying hydrophilic polymers [e.g., polyethylene glycol (PEG)] on the surface of nanocarriers can prevent opsonization and subsequent phagocytosis to achieve a long cycle for nanocarriers in vivo36. In addition, the ability to interact with the target cell can be enhanced by introducing a positively charged or targeted ligand on the surface of the nanocarrier, which enhances its interaction with a negatively charged target cell or a target cell with a specific receptor protein37.
The reduction of nonspecific uptake should also be considered during designing nano-drug delivery systems (DDSs). It is critical for the delivery of chemotherapeutics, as specific delivery to the target cells can minimize the side effects. Targeted drug delivery mainly includes passive and active targeting strategies38. Passive targeting considers the characteristics of blood vessels in tumor tissues, and it can allow nanocarriers to infiltrate them. It has been demonstrated that liposomes with an average diameter of around 400 nm are favorable for extravasation, and those <200 nm are more likely to be taken up by tumor cells39. To date, researchers have reported five types of endocytosis40.
After interacting with the target cell, the nanocarrier is transferred to early endosomes (EEs)41. The internalization of nanoformulations is just the first step in transporting therapeutic drugs. Studies have shown the mechanism of delivering nanocarriers to EEs. Notably, a drug endocytosed through the same pathway can be delivered to different EEs, and nanocarriers taken up via different signaling pathways can still be classified into the same EEs42.
It has been demonstrated that only 1%–2% of liposomes can escape the endosomal pathway43. Recent research on the endocytosis pathway indicates that clathrin-mediated endocytosis (CME), fast endophilin-mediated endocytosis (FEME), and clathrin-independent carrier/glycosylphosphatidylinositol-anchored protein-enriched early endocytic compartment endocytosis (CLIC/GEEC) are all involved for nanocarriers with diameters <200 nm, which means that these pathways are unlikely to internalize nanoparticles >200 nm44. Notably, the surfaces of nanocarriers are quickly adsorbed by serum proteins (e.g., vitronectin) to form protein crowns after being re-suspended in cell media or injections in vivo45. To provide data standardization, guidelines fall into 3 categories: (i) experimental methods, (ii) material characterization, and (iii) biological characterization46. However, there is very little data available on regulating different uptake pathways in various tissues within the physiological environment and how they improve the transport of nano-DDSs to target tissues.
3.2. LNPs
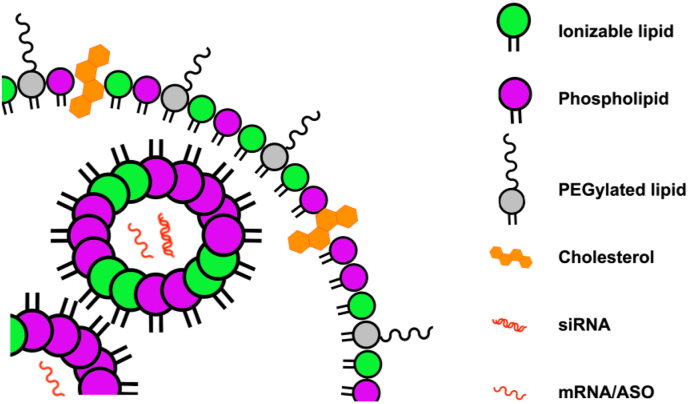
LNPs, non-viral vectors prepared from multiple lipids, have been applied for the precise delivery of RNA therapeutics. Since 1989, 1,2-di-O-octadecenyl-3-trimethylammonium-propane (DOTMA) and 1,2-dioleoyl-3-trimethyl ammonium propane (DOTAP) have become the two most widely used cation lipids. The structure of synthetic lipids is different from that of classical lipids, as the former consist of ester-connected glycerin heads and hydrocarbon tails. For example, DOTMA containing two unsaturated aliphatic hydrocarbon tails that are linked to a quaternary amine through ether groups exhibits the brilliant ability to deliver anionic RNA molecules47. This structure makes synthetic lipids more efficient at forming spherical liposomes or lipid complexes to load genetic molecules. In comparison with conventional liposomes consisting of a lipid bilayer, hybrid nanoparticles prepared from lipids and functional polymers exhibit higher stability, effectively avoiding premature leakage of the loaded cargo molecules48. If the long-chain molecule PEG is modified on the surface of the LPNs, the nanocarriers can circulate for a longer time in vivo. Recently, siRNA-loaded DOTAP/poly(lactic-co-glycolic acid) (PLGA) systems were employed to deliver genetic molecules to the lungs for severe lung disease treatment49. Generally, typical LNPs consist of four main components: cation lipids, CHO, phospholipids (auxiliary lipids), and PEG-modified lipids, which can increase the loading ability of RNA through positive and negative charges attraction, enhance nanoparticles stability, promote endosome escape, and prevent nanoparticles aggregation, respectively50. PEG-modified LNPs can reduce the protein opsonization effect and increase the blood circulation time in vivo owing to the PEGylated shell, leading to a stealth effect arising from the hydrophilic steric hindrance. However, the biocompatibility and long-term safety of PEG-modified nanocarriers remained unclear. Reduced transfection efficacy and cellular uptake are two disadvantages of PEG-modified LNPs. Researchers tried to solve these problems by modifying pH-responsive cleavable PEG on the surface of nanoparticles (Fig. 4)51. An important feature of this method is sequential targeting, including both passive and active targeting processes. First, the PEG-modified nanocarriers passively target the tumor sites. Then, pH-triggered PEGylation shedding occurs when the nanocarriers are in the tumor acidic microenvironment. Subsequently, the exposed ligands perform active targeting into the tumor cells. This design is sophisticated for cancer treatment as the microenvironment in tumor sites is always acidic. Many studies have used amino acid-derived peptides instead of PEG to endow nanocarriers with the same properties. For example, Nogueira et al.52 chose poly-sarcosine as a substitute for PEG, consisting of N-methylated glycine as the repeat units. To comprehensive study of the effects of PEG lipid hydrophobic domain on LNP formation, cell internalizations, intracellular translocation, and in vivo delivery, researchers have synthesized a majority of PEG lipids with dendritic structures. Nanoformulations 50–100 nm in diameter were prepared to investigate the effects of hydrophobic domain lengths on LNPs. The loading efficiency of siRNA was up to 90%. Only the PEG lipids containing first- and second-generation dendritic structures could be utilized for the effective delivery of siRNA in vitro and in vivo53.
Figure 4.
The structure of LNPs. LNPs are comprised of 4 main lipid components, including phospholipids (purple), PEGylated lipids (gray), ionizable lipids (green), and cholesterol (orange) encapsulating nucleic acid cargo including different types of RNA.
Biodegradable LNPs have been fabricated for systematic codelivery of clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 to enable effective and precise gene editing. Michael addition reaction was employed to synthesize novel lipids containing biodegradable disulfide bonds in the hydrophobic tails using amines, acrylates, or acrylamides as the reagents54. Notably, different linkers, including hydroxylamine, hydrazine, and ethanolamine, were chosen for the synthesis of new ionizable lipids, which exhibited outstanding ability to deliver siRNA into leukocytes. The transfection of leukocytes is reportedly difficult, so an anti-integrin beta 7 monoclonal antibody was added to the LNPs as a leukocyte-specific targeting ligand55.
Recently, ionizable lipid-like molecules containing branched tails were also reported, which were recognized as the next-generation lipids for the development of LNPs56. The ionic lipid-like molecules were prepared through the highly efficient reactions between amine-containing linkers and isodecyl acrylate-containing hydrophobic tails. The obtained ionic lipids worked with CHO, C14-PEG2000, and dioleoyl phosphatidylethanolamine (DOPE) to afford LNPs that were approximately 124 nm before loading mRNA. The loaded DDSs were compared with the LNPs using heptatriaconta-6,9,28,31-tetraen-19-yl 4-(dimethylamino)butanoate (DLin-MC3-DMA) as the ionizable lipid were prepared, and their loading performances were carefully compared. Unfortunately, for longer RNA (>100 nucleotides), the LNP constitutes had to be revised57. Researchers substituted CHO with CHO derivatives in LNPs to achieve their goals. The de-convolution of the morphology, size and internal structures of LNPs are able to be regulated by alternating the type and ratio of ionizable lipids, phospholipids, CHO, and PEG lipids. CHO, a primary component in LNPs, plays a pivotal role in controlling the final morphology of LNPs and affecting the efficiency of genes. Eygeris et al.58 prepared LNPs using natural phytosterols as the building blocks with varying degrees of crystallinity and rigidity.
Generally, LNPs with small sizes exhibit better tissue penetration. Thus, the diameter of LNP-based nanoformulations should be rationally controlled for biomedical applications. Microfluidic technology is a versatile and efficient mixing method and makes the preparation of small thermodynamically stable LNPs feasible59. However, small-size LNPs can be dissociated in the presence of serum, albumins, and other biological fluids, which means they have poor stability and weak intracellular trafficking60. To overcome this problem, Sato et al.61 investigated the influence of hydrophobic lengths and shapes on the stability of the LNPs. They found that the low stability of small-size LNPs was attributed to the diffusion of lipids within the nanostructures and proteins adsorption on the surfaces. Inspired by the findings, auxiliary lipids were replaced by lecithin, and 22 nm small LNPs were formed. A series of experiments were carried out on the properties of higher molecular-weight scaffolds with 18 or more carbons. To evaluate the networking of amino lipids in the delivery of siRNA in LNPs formulations, Anderluzzi et al.62 synthesized four lipids that were used to produce LNPs, which displayed similar size and comparable biophysical functions. Notably, in vitro antigen expression does not reflect immune response in vivo, indicating that in vitro assays are insufficient. This highlights the approach to caution and caution required to enter the clinical phase. Researchers should better understand the factors that control the correlations between in vitro and in vivo assays, as the final therapeutic efficacy of LNP vectors can vary in the two cases. For example, LNPs prepared from ionizable lipids containing saturated hydrocarbon tails were found to improve mRNA delivery63.
LNPs have also been used to deliver mRNA for cancer and coronavirus therapy64,65. After the RNAi drug patisiran was approved by the U.S. Food and Drug Administration (FDA), significant progress was made in the research of LNP carriers, much of which is dedicated to using natural substances in nanocarriers. The experience gained from developing coronavirus disease 2019 (COVID-19) vaccines has led to the reorganization and improvement of nanocarriers66. Current progress in designing suitable DDSs in academia and industry will ensure the future development of effective RNA therapies.
Advanced LNPs with ionizable lipid components can help to break the mutual restriction between transfection efficiency and cytotoxicity and further produce a series of highly biocompatible LNPs that can be loaded with various RNA67. At the same time, the combination of automated high-throughput screening and modern synthesis technology can shorten the evaluation time of the LNPs library, which will make it possible to respond quickly and positively to future crises similar to the COVID-19 pandemic68. These positive developments provide strong support for applying LNPs-based RNA delivery vectors (Table 3).
Table 3.
LNP vectors in clinical trial.
| Disease | Type of RNA | Target | Vector | Clinicaltrial | Status |
|---|---|---|---|---|---|
| Elevated LDL-cholesterol | siRNA | PCSK9 | LNPs | NCT01437059 | Phase I |
| Solid tumors | siRNA | KSP and VEGF | LNPs | NCT01158079 | Phase I |
| Hepatitis B | siRNA | HBV antigen | LNPs | NCT02631096 | Phase II |
| Solid tumors | siRNA | PLK1 | LNPs | NCT01437007 | Phase III |
| Hepatic fibrosis | siRNA | HSP47 | LNPs | NCT02227459 | Phase II |
| Hepatocellular carcinoma | siRNA | MYC | LNPs | NCT02314052 | Phase II |
| Hematological and solid tumors | siRNA | MYC | LNPs | NCT02110563 | Phase I |
| CMV infection | mRNA | Pentamer and T cell antigen | LNPs | NCT03382405 | Phase I |
| Zika | mRNA | prM and E | LNPs | NCT04064905 | Phase I |
| OTC deficiency | mRNA | OTC | LNPs | NCT03375047 | Phase I/II |
| COVID-19 | mRNA | S-protein | LNPs | NCT04283461 | Phase I |
| Solid tumors, lymphomas and ovarian cancer | mRNA | OX40L | LNPs | NCT03323398 | Phase I/II |
| Solid tumors and lymphomas | mRNA | OX40L | LNPs | NCT03739931 | Phase I |
Liposomes have been widely used to deliver both small and macromolecular therapeutic agents69, 70, 71. High encapsulation efficiency requires a higher amount of cationic lipids, which leads to a rise in the number of positive charges on the surface and subsequent toxicity72. To solve these problems, researchers used ionizable lipids containing amino head groups with the acid dissociation constant (pKa) below 773. Low pKa makes ionizable lipids neutral at physiological pH (7.4) but positively charged at an acidic pH (<6.0), and as a result, LNPs have a higher nucleic acid encapsulation efficiency under acidic pH. Ionizable and other auxiliary lipids also interact with the negatively charged endosomal membrane, causing membrane disruption and endosomal escape of nucleic acids74. Therefore, the ionizable amino lipids and auxiliary lipids constitute the lipid component of LNP preparations (Fig. 2). The lipid composition of the LNPs and liposome preparations and the chemical structures of the recently developed novel ionizable amino lipids are depicted in Fig. 3.
3.3. Limitations of LNPs
The clinical development of LNP technology in chemotherapy and nucleic acid therapy has confirmed the promising potential of lipid carriers in treating a range of diseases75. While several concerns faced by LNPs greatly limit their clinic applications. Compared to pre-clinical trials, only a small number of products are successfully approved, which suggest that the application of these nanoparticle-based therapies still faces many challenges when translating from animal model to human clinical trials. Besides, drug leaking is still the bottleneck of LNPs for clinical use, and the stability of nanoparticles is constantly in need of improvement76.
4. The summary of biomedical applications
4.1. Cancer treatment
According to the data released by the National Cancer Center of China in 2022, cancer is a leading public health challenge and has become one of the primary causes of death in China77. The most common and conventional cancer treatments include surgery, chemotherapy, radiotherapy, immunotherapy, and proton therapy, and to date. In recent years, the continuing improvement in conventional cancer treatments has significantly reduced the death rate78. Nevertheless, patients face various unsolved healthcare challenges, such as high levels of toxicity, long-term complications, and several other complex factors79.
Dysregulated gene expression remains a major hallmark of cancer, and consequently, altering the activity of cancer-related genes has been a research focus80. RNA plays a crucial role in gene expression. Thus, developing cancer treatments by altering RNA activity could be promising81. Nevertheless, nucleases can quickly degrade naked therapeutic RNA in the serum. Moreover, the cell membrane prevents therapeutic RNA from crossing due to the anionic charges on their surfaces. The remarkable progress in nanocarrier development has led to advances in DDSs for in vivo delivery of therapeutic RNA82,83. Here we introduced RNA therapies in several types of cancers with a focus on LNPs-based nanocarriers.
4.2. Lung cancer
Lung cancer is the most lethal cancer type and causes the number death of cancer worldwide, with an increased rate of 11.4% for new cases annually84. Lung cancers can be divided into non-small-cell lung carcinoma (NSCLC) and small-cell lung carcinoma (SCLC)85. The heterogeneity and adaptability of lung cancer limit the success of current therapies86. Thus, there is great interest in RNA-based therapeutics with the potential to fight cancer.
Nanoparticles are advantageous owing to their high surface area and because the construction of therapeutic RNA nanoparticles can be easily realized. In early-phase research of liposomal DDSs, Zhao et al.87 fabricated lipid-polycation-hyaluronic acid nanoparticles for VEGF siRNA delivery in a human lung cancer mouse model. These nanoparticles exhibited satisfactory antitumor outcomes through the activation of adenosine monophosphate-activated protein kinase and inhibition of rapamycin, and these functions were equivalent to those of the anticancer drug metformin. Moreover, mesoporous silica nanoparticles (MSNPs) are considered another powerful DDSs. Dilnawaz et al.88 developed DDSs for lung cancer therapy that co-delivered anticancer drugs (e.g., etoposide or docetaxel) with survivin siRNA by the MSNPs. They suggested that this system has a significant apoptotic effect when using high-dose co-deliver drugs in vitro. Nascimento et al.89 reported polyethylenimine (PEI) functionalized siRNA/MSNPs immobilized on electro-spun nanofibers. Another cancer treatment strategy is to disrupt cancer cell proliferation. In recent decades, although researchers have identified several siRNA that can suppress the growth of cancer cells, most of them are often associated with adverse side effects. Consequently, developing siRNA DDSs with low levels of systemic side effects is a field that requires further research.
4.3. Glioblastoma
Glioblastoma is one of the most common and aggressive brain tumors and shows poor treatment response, with a <2-year average survival rate. Glioblastoma pathogenesis is mainly associated with the BBB, as it creates a formidable obstacle when treating glioblastoma90.
To increase the efficiency and safety, Kong et al.91 utilized PEI-modified gold nanoparticles as carriers to deliver siRNA to glioblastoma, the delivery efficiency and therapeutic results were further promoted by conjugated Arg-Gly-Asp peptides as the targeting agent on the surface. In another study, Zheng et al.92 employed a polymeric vehicle to encapsulate siRNA through the combination of hydrophobic interactions, hydrogen bonds, and electrostatic interactions. Heterogeneous inheritance and epigenetic aberrations in glioblastomas make it exceptionally difficult to use conventional drugs. RNAi, however, is a promising strategy for glioblastoma treatment. A combination of RNAi using nanoparticles could inhibit tumor growth and increase the survival rate of patients. To treat glioblastoma specifically, Sukumar et al.93 chose a direct nose-to-brain transport pathway for the delivery of miRNA using hybrid polymeric nanoparticles, which exhibited dramatically different results from the commonly used subcutaneous administration. New therapies and challenges specific to glioblastoma require further investigation for practical applications.
4.4. Pancreatic cancer
Pancreatic cancer is one of the most aggressive cancer. Previous reports have demonstrated that oncogenic Kirsten ras (KRAS) mutations are involved in the pathogenesis of most pancreatic cancers94. Consequently, KRAS mutations have become a primary research target for treating pancreatic cancers95.
There are recent reports about the application of RNA therapies for pancreatic cancer. For example, Zeng et al.96 prepared DDSs consisting of siRNA and PEG-b-PLL on account of the extraordinary sequence specificity of RNAi to direct arsenic-induced apoptosis and KRAS silencing. Uchida et al.97 developed a novel nano-micelle composed of PEG-polycation block copolymer-CHO as an mRNA vehicle for treating pancreatic cancer. The mRNA nano-micelle efficiently expressed proteins in the tumor sites. The liposome-based RNA DDSs offered advantages over viral-based RNA DDSs, which were characterized by rapid blood circulation clearance and low efficiency. Kamerkar et al.98 engineered exosomes that could recognize oncogenic KRAS specifically as carriers for siRNA or shRNA. They found a significant increase in the overall survival in mouse models, suggesting that this newly developed exosome therapy could suppress pancreatic cancers. Han et al.99 targeted activated pancreatic stellate cells to construct a tumor microenvironment-responsive nanosystem.
4.5. Liver cancer
In 2020, liver cancer became the second most lethal cancer type worldwide. Patients with 5-year survival only ∼18%84. Primary liver cancer has been categorized into two main forms: hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma, which constitute 75%–85% and 10%–15% of all cases100. It is usually caused by chronic liver damage, and the common inducing factors include alcohol abuse, nonalcoholic fatty liver disease, and hepatitis virus infection. Several small-molecule drugs for the treatment of HCC have failed, and RNA-based drug development has shown promising results for liver cancer therapy101.
Dendrimers are an excellent example of a powerful vector application. Driven by electrostatic interactions, Khan et al.102 used dendritic molecules to efficiently complex with negatively charged siRNA under an acidic microenvironment. They utilized an alkyl to generate amine-rich ionizable dendrimer cores and demonstrated the siRNA could be specifically delivered to target cells, such as endothelial cells and hepatocytes. The main challenge when applying dendrimers in clinical use is overcoming tissue damage during delivery by lowering their toxicity and improving potency.
4.6. Prostate cancer
Prostate cancer is the second most common cause of death in males globally, and its case numbers are similar to those for lung cancer84. Conventional prostate cancer therapies include surgical prostate removal, hormone therapy, and radiotherapy. These therapies have exhibited remarkable clinical efficacy. However, the life quality of patients receiving these traditional therapies is impacted seriously by multiple side effects, such as those from surgical or chemical castration103. While prostate cancer can be induced by various risk factors, such as race, age, family history, and geneticity. The clinical management of prostate cancer is difficult owing to our relatively limited understanding of related genes. However, as RNA therapeutics can be used to silence the target genes, RNAi becomes an unparalleled therapeutic modality in the treatment of prostate cancer104.
There have been extensive efforts to develop RNA delivery systems for prostate cancer therapy. For example, Hasan et al.105 prepared siRNA/PLGA nanocarriers with a siRNA encapsulation efficiency of up to 32%–46% through the soft lithography method. Furthermore, Chen et al.106 developed nanoplexes with the functional group thiol–ene. These complexes showed a remarkable ability for hydrolytic degradation, which allowed the click functionalization of thiol–ene releasing interleukin-8 siRNA. In addition, researchers have now designed novel carriers with abundant stimuli-responsive functions, which are widely used for the construction of RNA-based nanomedicines. Xu et al.107 proposed a multifunctional envelope-type siRNA nanoparticle. Owing to these and previous efforts, future research may be able to focus on specific genes to promote the efficacy of targeted therapies.
4.7. LNPs-based therapy targeting T cells
LNPs-based therapy targeting T cells have been developed for combination with surgery, chemotherapy, radiotherapy, and targeted pathway inhibition, as these are known to fight aggressive diseases by triggering the immune reactions of patients108. Various forms of cancer immunotherapy have emerged recently, and research into tumor-infecting viruses, cell therapy, cancer vaccines, immunogenic cell death, immune checkpoints, targeted antibodies, cytokines, and immune factor adjuvants has seen great progress. Recent work showed that by injecting T cell-targeted LNPs containing the mRNA needed to reprogram T lymphocytes, the researchers could target T cells to treat heart disease109. The efficacy of many RNAi drugs has been proven, and they have now entered clinical trials, such as Patisiran and ENVISION in phase III trials110. The vast strides in RNA-based therapies for cancer have elevated the interest in RNA-based immunotherapies as a typical immunotherapy method and for their ability to elucidate the RNA in anticancer immune functions for different cancer types111. Therefore, this section further discusses and summarizes current RNA-based immunotherapies for cancers.
Vaccines are an effective therapeutic option widely used to protect and treat infectious diseases like measles, polio, and even COVID-19112. Current vaccines are usually designed to trigger antigen-specific cells by presenting tumor-associated antigens to antigen-presenting cells. In 1999, the first effective self-replicating RNA vector was constructed by Ying et al.113 to improve the immunogenicity of nucleic acid vaccines. They found that the treated mice had a positive survival rate after receiving the RNA vector-enhanced nucleic acid vaccine, which indicated that RNA could be an excellent candidate for novel cancer vaccines. After the first successful attempt, developments in the field of RNA-based vaccines have become extremely rapid, especially in recent years. One of the notable applications was reported by Sahin et al.114, who implemented an RNA-based poly-neoepitope to mobilize immunity against a spectrum of cancer-specific mutations through individualized mutanome vaccines. Clinical evidence indicated that this personalized RNA vaccine had positive effects against melanoma.
5. The prospects, challenges, and opportunities
Developmental landmarks have been achieved in drug delivery, cancer therapy, and vaccine in new RNA-based technologies. The current state-of-the-art research direction is translating from experimental use to clinical application of RNA therapeutics. Novel nanocarriers as drug delivery tools have unique advantages when applying nanoplatforms for RNA-based technologies. Structural stability of nanoplatforms during administration can effectively prevent bioactive molecules from degradation. Most importantly, Therapeutic RNA combing with delivering nanocarriers have high biocompatibility, allowing effective constituent of drugs to overcome biological barriers and targeting specific cells and tissues. In addition, because of its biocompatibility and stability, patients under treatment can alleviate undesired inflammation in tissue or organs, thus achieving a better therapeutic effect. These unique advantages have demonstrated the bright future of nanotechnology-assisted therapies. Different fields (e.g., biologists, chemists, material engineers, or physicians) have devoted enormous efforts to better rationally design, development, and experiment of RNA targeting nanoplatforms with special characteristics. We believe mRNA therapeutics will be continuously explored for the development of new vaccines, whereas miRNA-based therapeutics show brilliant potential in wound healing. However, great opportunities also come with enormous challenges, especially in the development of ideal therapeutic agents for the optimization of these nanocarriers and in the achievement of the intended goal. These unsolved challenges are the biggest obstacle to stop the broad applications of these vectors as genetic molecules carriers. Finding proper methods to reduce the bioaccumulation and stop the aggregation in the body fluids for nanocarriers becomes important since the structural stability of nanocarriers makes it more easily to accumulate in the circulation system. Besides, nonspecific adsorption of drugs in circulation makes the balance of stability and activity fragile and challenging to achieve. Take the development and application of carbonaceous nanomaterials, inorganic nanoparticles, and LNPs as examples, even though they already stand out in biocompatibility and bio-stability, especially for LNPs, which have enhanced biocompatibility and represent the most advanced and promising nanocarriers, their potential toxicity and carcinogenicity have remained unsolved. Other than the above holdback, more research is needed to clear the doubt between active dosage and DNA damage.
Despite the challenges, new trends of continuously exploring the application of RNA-based healthcare materials will never stop the steps. Artificial intelligence (AI) approaches applying to investigating potential RNA therapeutic agents in future research directions might bring new opportunities for biomedical applications and revolute the current pharmacological therapies. AI approaches are outstanding in several aspects. First, AI approaches are made by large, organized, structured datasets. The designed algorithms with high sensitivity allowed AI tools to process, integrate, interpret, and find patterns of datasets. Thus, they have the potential to level up medical diagnosis, detection of genetic disorders and mutations, infectious disease characteristics, and treatment accuracy, all cost-effectively and significantly. Additionally, AI tool shows complexities and variability due to the application of multiple algorithms, further enhances the problem solute capability and results in reliability, which could also contribute to analyzing RNA-related mechanisms such as RNA structure, predicting alterations such as RNA folding, and performing fast and accurate in complex gene expression patterns recognition. The above advantages gave the AI tool particular suitability to design RNA therapeutics for silencing and correcting to develop patient-specific gene mutation inhibitors and perform genome editing.
Recently, with enormous growth in cancer-related RNA therapeutics research, AI tools have been proven useful in RNA-based cancer treatments. Thus, we believe the upcoming request and interest in RNA-based research will ensure the continuous development of RNA therapeutics and the improvement of nanotechnology-assisted RNA delivery, such as LNPs, in the coming decades. We hope this review will offer new views and motivate scientists in all fields during the ongoing revolution in patient pharmacotherapy and personalized medicine.
Acknowledgments
Dr. Xingcai Zhang acknowledges the support from Harvard/MIT (USA). All others acknowledge the support of the National Key Research and Development Program of China (No. 2019YFC1315701), National Natural Science Foundation of China (No. 22005343), Cancer Hospital, Chinese Academy of Medical Sciences, Shenzhen Center/Shenzhen Cancer Hospital Research Project (No. SZ2020ZD004, China), Shenzhen Science and Technology Program (No. KCXFZ20201221173008022, China), Sanming Project of Medicine in Shenzhen (Nos. SZSM201812062 and SZSM201612097, China), and Shenzhen Key Medical Discipline Construction Fund (No. SZXK075, China). The authors would like to thank Gaozheng Zhou for helping draw some of the figures.
Author contributions
Xingcai Zhang and Yingli Sun conceived the idea. Xingcai Zhang, Yingli Sun, and Luo Hai performed the major literature search and wrote the original draft. Xingcai Zhang, Yingli Sun, Guocan Yu, and Yibo Gao revised and edited the manuscript. All authors read and approved the final manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Xingcai Zhang, Email: xingcai@mit.edu.
Guocan Yu, Email: guocanyu@mail.tsinghua.edu.cn.
Yingli Sun, Email: scaroll99@hotmail.com.
References
- 1.Manning K.S., Cooper T.A. The roles of RNA processing in translating genotype to phenotype. Nat Rev Mol Cell Biol. 2017;18:102–114. doi: 10.1038/nrm.2016.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen L.L. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat Rev Mol Cell Biol. 2020;21:475–490. doi: 10.1038/s41580-020-0243-y. [DOI] [PubMed] [Google Scholar]
- 3.Yu A.M., Choi Y.H., Tu M.J. RNA drugs and RNA targets for small molecules: principles, progress, and challenges. Pharmacol Rev. 2020;72:862–898. doi: 10.1124/pr.120.019554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paunovska K., Loughrey D., Dahlman J.E. Drug delivery systems for RNA therapeutics. Nat Rev Genet. 2022;23:265–280. doi: 10.1038/s41576-021-00439-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dowdy S.F. Overcoming cellular barriers for RNA therapeutics. Nat Biotechnol. 2017;35:222–229. doi: 10.1038/nbt.3802. [DOI] [PubMed] [Google Scholar]
- 6.Li I., Chen Y.G. Emerging roles of circular RNAs in innate immunity. Curr Opin Immunol. 2021;68:107–115. doi: 10.1016/j.coi.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li M.S., Ma Z.Z., Peng M., Li L., Yin M., Yan S., et al. A gene and drug co-delivery application helps to solve the short life disadvantage of RNA drug. Nano Today. 2022;43 [Google Scholar]
- 8.Agrawal M., Saraf S., Saraf S., Dubey S.K., Puri A., Patel R.J., et al. Recent strategies and advances in the fabrication of nano lipid carriers and their application towards brain targeting. J Control Release. 2020;321:372–415. doi: 10.1016/j.jconrel.2020.02.020. [DOI] [PubMed] [Google Scholar]
- 9.Phylactou L.A. Ribozyme and peptide-nucleic acid-based gene therapy. Adv Drug Deliv Rev. 2000;44:97–108. doi: 10.1016/s0169-409x(00)00088-0. [DOI] [PubMed] [Google Scholar]
- 10.Damase T.R., Sukhovershin R., Boada C., Taraballi F., Pettigrew R.I., Cooke J.P. The limitless future of RNA therapeutics. Front Bioeng Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.628137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbieri I., Kouzarides T. Role of RNA modifications in cancer. Nat Rev Cancer. 2020;20:303–322. doi: 10.1038/s41568-020-0253-2. [DOI] [PubMed] [Google Scholar]
- 12.Gao M.S., Zhang Q.Y., Feng X.H., Liu J.Z. Synthetic modified messenger RNA for therapeutic applications. Acta Biomater. 2021;131:1–15. doi: 10.1016/j.actbio.2021.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang W., Xiang H., Ke L., Guo J., Yang X. Application of quantitative and engineering biology in mRNA gene therapy. Chin Sci Bull. 2021;66:329–340. [Google Scholar]
- 14.Wadhwa A., Aljabbari A., Lokras A., Foged C., Thakur A. Opportunities and challenges in the delivery of mRNA-based vaccines. Pharmaceutics. 2020;12:102. doi: 10.3390/pharmaceutics12020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei G., Cao J., Huang P., An P., Badlani D., Vaid K.A., et al. Synthetic human ABCB4 mRNA therapy rescues severe liver disease phenotype in a BALB/c.Abcb4‒/‒ mouse model of PFIC3. J Hepatol. 2021;74:1416–1428. doi: 10.1016/j.jhep.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gan L.M., Lagerström-Fermér M., Carlsson L.G., Arfvidsson C., Egnell A.C., Rudvik A., et al. Intradermal delivery of modified mRNA encoding VEGF-A in patients with type 2 diabetes. Nat Commun. 2019;10:871. doi: 10.1038/s41467-019-08852-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winkle M., El-Daly S.M., Fabbri M., Calin G.A. Noncoding RNA therapeutics—challenges and potential solutions. Nat Rev Drug Discov. 2021;20:629–651. doi: 10.1038/s41573-021-00219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y., Liu X., Lin C.W., Jia X.H., Zhu H.M., Song J., et al. Noncoding RNAs regulate alternative splicing in cancer. J Exp Clin Cancer Res. 2021;40:11. doi: 10.1186/s13046-020-01798-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lam J.K.W., Chow M.Y.T., Zhang Y., Leung S.W.S. siRNA versus miRNA as therapeutics for gene silencing. Mol Ther Nucleic Acids. 2015;4:e252. doi: 10.1038/mtna.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwakawa H.O., Tomari Y. Life of RISC: formation, action, and degradation of RNA-induced silencing complex. Mol Cell. 2022;82:30–43. doi: 10.1016/j.molcel.2021.11.026. [DOI] [PubMed] [Google Scholar]
- 21.Snøve O., Jr., Rossi J.J. Expressing short hairpin RNAs in vivo. Nat Methods. 2006;3:689–695. doi: 10.1038/nmeth927. [DOI] [PubMed] [Google Scholar]
- 22.Ray K.K., Landmesser U., Leiter L.A., Kallend D., Dufour R., Karakas M., et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N Engl J Med. 2017;376:1430–1440. doi: 10.1056/NEJMoa1615758. [DOI] [PubMed] [Google Scholar]
- 23.Kosmas C.E., Estrella A.M., Sourlas A., Pantou D. Inclisiran in dyslipidemia. Drug Today. 2021;57:311–319. doi: 10.1358/dot.2021.57.5.3277083. [DOI] [PubMed] [Google Scholar]
- 24.Lamb Y.N. Inclisiran: first approval. Drugs. 2021;81:389–395. doi: 10.1007/s40265-021-01473-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright R.S., Ray K.K., Raal F.J., Kallend D.G., Jaros M., Koenig W., et al. Pooled patient-level analysis of inclisiran trials in patients with familial hypercholesterolemia or atherosclerosis. J Am Coll Cardiol. 2021;77:1182–1193. doi: 10.1016/j.jacc.2020.12.058. [DOI] [PubMed] [Google Scholar]
- 26.Basu D., Goldberg I.J. Regulation of lipoprotein lipase-mediated lipolysis of triglycerides. Curr Opin Lipidol. 2020;31:154–160. doi: 10.1097/MOL.0000000000000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foss-Freitas M.C., Akinci B., Neidert A., Bartlett V.J., Hurh E., Karwatowska-Prokopczuk E., et al. Selective targeting of angiopoietin-like 3 (ANGPTL3) with vupanorsen for the treatment of patients with familial partial lipodystrophy (FPLD): results of a proof-of-concept study. Lipids Health Dis. 2021;20:174. doi: 10.1186/s12944-021-01589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaudet D., Karwatowska-Prokopczuk E., Baum S.J., Hurh E., Kingsbury J., Bartlett V.J., et al. Vupanorsen, an N-acetyl galactosamine-conjugated antisense drug to ANGPTL3 mRNA, lowers triglycerides and atherogenic lipoproteins in patients with diabetes, hepatic steatosis, and hypertriglyceridaemia. Eur Heart J. 2020;41:3936–3945. doi: 10.1093/eurheartj/ehaa689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bejar N., Tat T.T., Kiss D.L. RNA therapeutics: the next generation of drugs for cardiovascular diseases. Curr Atheroscler Rep. 2022;24:307–321. doi: 10.1007/s11883-022-01007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graham M.J., Lee R.G., Brandt T.A., Tai L.J., Fu W., Peralta R., et al. Cardiovascular and metabolic effects of ANGPTL3 antisense oligonucleotides. N Engl J Med. 2017;377:222–232. doi: 10.1056/NEJMoa1701329. [DOI] [PubMed] [Google Scholar]
- 31.Mohr A.M., Mott J.L. Overview of microRNA biology. Semin Liver Dis. 2015;35:3–11. doi: 10.1055/s-0034-1397344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hou X.C., Zaks T., Langer R., Dong Y.Z. Lipid nanoparticles for mRNA delivery. Nat Rev Mater. 2021;6:1078–1094. doi: 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kozlowska A.K., Florczak A., Smialek M., Dondajewska E., Mackiewicz A., Kortylewski M., et al. Functionalized bioengineered spider silk spheres improve nuclease resistance and activity of oligonucleotide therapeutics providing a strategy for cancer treatment. Acta Biomater. 2017;59:221–233. doi: 10.1016/j.actbio.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang M., Zhao J.Z., Jiang H., Wang X.M. Tumor-targeted nano-delivery system of therapeutic RNA. Mater Horiz. 2022;9:1111–1140. doi: 10.1039/d1mh01969d. [DOI] [PubMed] [Google Scholar]
- 35.Sykes E.A., Chen J., Zheng G., Chan W.C.W. Investigating the impact of nanoparticle size on active and passive tumor targeting efficiency. ACS Nano. 2014;8:5696–5706. doi: 10.1021/nn500299p. [DOI] [PubMed] [Google Scholar]
- 36.Bhattacharjee S. Molecular descriptors as a facile tool toward designing surface-functionalized nanoparticles for drug delivery. Mol Pharm. 2022;19:1168–1175. doi: 10.1021/acs.molpharmaceut.1c00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saadat M., Zahednezhad F., Zakeri-Milani P., Heidari H.R., Shahbazi-Mojarrad J., Valizadeh H. Drug targeting strategies based on charge dependent uptake of nanoparticles into cancer cells. J Pharm Pharm Sci. 2019;22:191–220. doi: 10.18433/jpps30318. [DOI] [PubMed] [Google Scholar]
- 38.Raj S., Khurana S., Choudhari R., Kesari K.K., Kamal M.A., Garg N., et al. Specific targeting cancer cells with nanoparticles and drug delivery in cancer therapy. Semin Cancer Biol. 2021;69:166–177. doi: 10.1016/j.semcancer.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Rennick J.J., Johnston A.P.R., Parton R.G. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat Nanotechnol. 2021;16:266–276. doi: 10.1038/s41565-021-00858-8. [DOI] [PubMed] [Google Scholar]
- 40.Fan W., Xia D., Zhu Q., Hu L., Gan Y. Intracellular transport of nanocarriers across the intestinal epithelium. Drug Discov Today. 2016;21:856–863. doi: 10.1016/j.drudis.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 41.Jovic M., Sharma M., Rahajeng J., Caplan S. The early endosome: a busy sorting station for proteins at the crossroads. Histol Histopathol. 2010;25:99–112. doi: 10.14670/hh-25.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumari S., Mg S., Mayor S. Endocytosis unplugged: multiple ways to enter the cell. Cell Res. 2010;20:256–275. doi: 10.1038/cr.2010.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guevara M.L., Persano F., Persano S. Advances in lipid nanoparticles for mRNA-based cancer immunotherapy. Front Chem. 2020;8 doi: 10.3389/fchem.2020.589959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iversen T.G., Skotland T., Sandvig K. Endocytosis and intracellular transport of nanoparticles: present knowledge and need for future studies. Nano Today. 2011;6:176–185. [Google Scholar]
- 45.Yu Q.H., Zhao L.X., Guo C.C., Yan B., Su G.X. Regulating protein corona formation and dynamic protein exchange by controlling nanoparticle hydrophobicity. Front Bioeng Biotechnol. 2020;8:210. doi: 10.3389/fbioe.2020.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faria M., Björnmalm M., Thurecht K.J., Kent S.J., Parton R.G., Kavallaris M., et al. Minimum information reporting in bio–nano experimental literature. Nat Nanotechnol. 2018;13:777–785. doi: 10.1038/s41565-018-0246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin B., Sainlos M., Aissaoui A., Oudrhiri N., Hauchecorne M., Vigneron J.P., et al. The design of cationic lipids for gene delivery. Curr Pharm Des. 2005;11:375–394. doi: 10.2174/1381612053382133. [DOI] [PubMed] [Google Scholar]
- 48.Hadinoto K., Sundaresan A., Cheow W.S. Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review. Eur J Pharm Biopharm. 2013;85:427–443. doi: 10.1016/j.ejpb.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 49.Thanki K., van Eetvelde D., Geyer A., Fraire J., Hendrix R., Van Eygen H., et al. Mechanistic profiling of the release kinetics of siRNA from lipidoid-polymer hybrid nanoparticles in vitro and in vivo after pulmonary administration. J Control Release. 2019;310:82–93. doi: 10.1016/j.jconrel.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 50.Veiga N., Diesendruck Y., Peer D. Targeted lipid nanoparticles for RNA therapeutics and immunomodulation in leukocytes. Adv Drug Deliv Rev. 2020;159:364–376. doi: 10.1016/j.addr.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 51.Li Y., Lin J., Cai Z., Wang P., Luo Q., Yao C., et al. Tumor microenvironment-activated self-recognizing nanodrug through directly tailored assembly of small-molecules for targeted synergistic chemotherapy. J Control Release. 2020;321:222–235. doi: 10.1016/j.jconrel.2020.02.025. [DOI] [PubMed] [Google Scholar]
- 52.Nogueira S.S., Schlegel A., Maxeiner K., Weber B., Barz M., Schroer M.A., et al. Polysarcosine-functionalized lipid nanoparticles for therapeutic mRNA delivery. ACS Appl Nano Mater. 2020;3:10634–10645. [Google Scholar]
- 53.Zhou K., Johnson L.T., Xiong H., Barrios S., Minnig J.T., Yan Y., et al. Hydrophobic domain structure of linear-dendritic poly(ethylene glycol) lipids affects RNA delivery of lipid nanoparticles. Mol Pharm. 2020;17:1575–1585. doi: 10.1021/acs.molpharmaceut.9b01288. [DOI] [PubMed] [Google Scholar]
- 54.Liu J., Chang J., Jiang Y., Meng X., Sun T., Mao L., et al. Fast and efficient CRISPR/Cas9 genome editing in vivo enabled by bioreducible lipid and messenger RNA nanoparticles. Adv Mater. 2019;31 doi: 10.1002/adma.201902575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramishetti S., Hazan-Halevy I., Palakuri R., Chatterjee S., Naidu Gonna S., Dammes N., et al. A combinatorial library of lipid nanoparticles for RNA delivery to leukocytes. Adv Mater. 2020;32 doi: 10.1002/adma.201906128. [DOI] [PubMed] [Google Scholar]
- 56.Hajj K.A., Melamed J.R., Chaudhary N., Lamson N.G., Ball R.L., Yerneni S.S., et al. A potent branched-tail lipid nanoparticle enables multiplexed mRNA delivery and gene editing in vivo. Nano Lett. 2020;20:5167–5175. doi: 10.1021/acs.nanolett.0c00596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patel S., Ashwanikumar N., Robinson E., Xia Y., Mihai C., Griffith J.P., et al. Naturally-occurring cholesterol analogues in lipid nanoparticles induce polymorphic shape and enhance intracellular delivery of mRNA. Nat Commun. 2020;11:983. doi: 10.1038/s41467-020-14527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eygeris Y., Patel S., Jozic A., Sahay G. Deconvoluting lipid nanoparticle structure for messenger RNA delivery. Nano Lett. 2020;20:4543–4549. doi: 10.1021/acs.nanolett.0c01386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wei W., Sun J., Guo X.Y., Chen X., Wang R., Qiu C., et al. Microfluidic-based holonomic constraints of siRNA in the kernel of lipid/polymer hybrid nanoassemblies for improving stable and safe in vivo delivery. ACS Appl Mater Interfaces. 2020;12:14839–14854. doi: 10.1021/acsami.9b22781. [DOI] [PubMed] [Google Scholar]
- 60.Tanaka H., Sakurai Y., Anindita J., Akita H. Development of lipid-like materials for RNA delivery based on intracellular environment-responsive membrane destabilization and spontaneous collapse. Adv Drug Deliv Rev. 2020;154–155:210–226. doi: 10.1016/j.addr.2020.07.001. [DOI] [PubMed] [Google Scholar]
- 61.Sato Y., Okabe N., Note Y., Hashiba K., Maeki M., Tokeshi M., et al. Hydrophobic scaffolds of pH-sensitive cationic lipids contribute to miscibility with phospholipids and improve the efficiency of delivering short interfering RNA by small-sized lipid nanoparticles. Acta Biomater. 2020;102:341–350. doi: 10.1016/j.actbio.2019.11.022. [DOI] [PubMed] [Google Scholar]
- 62.Anderluzzi G., Lou G., Gallorini S., Brazzoli M., Johnson R., O'Hagan D.T., et al. Investigating the impact of delivery system design on the efficacy of self-amplifying RNA vaccines. Vaccines. 2020;8:212. doi: 10.3390/vaccines8020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miao L., Lin J.Q., Huang Y.X., Li L.X., Delcassian D., Ge Y.F., et al. Synergistic lipid compositions for albumin receptor mediated delivery of mRNA to the liver. Nat Commun. 2020;11:2424. doi: 10.1038/s41467-020-16248-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen C.Y., Tran D.M., Cavedon A., Cai X., Rajendran R., Lyle M.J., et al. Treatment of hemophilia a using factor VIII messenger RNA lipid nanoparticles. Mol Ther Nucleic Acids. 2020;20:534–544. doi: 10.1016/j.omtn.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao P., Hou X., Yan J., Du S., Xue Y., Li W., et al. Long-term storage of lipid-like nanoparticles for mRNA delivery. Bioact Mater. 2020;5:358–363. doi: 10.1016/j.bioactmat.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weiss C., Carriere M., Fusco L., Capua I., Regla-Nava J.A., Pasquali M., et al. Toward nanotechnology-enabled approaches against the COVID-19 pandemic. ACS Nano. 2020;14:6383–6406. doi: 10.1021/acsnano.0c03697. [DOI] [PubMed] [Google Scholar]
- 67.Billingsley M.M., Singh N., Ravikumar P., Zhang R., June C.H., Mitchell M.J., et al. Ionizable lipid nanoparticle-mediated mRNA delivery for human CAR T cell engineering. Nano Lett. 2020;20:1578–1589. doi: 10.1021/acs.nanolett.9b04246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cui L.L., Pereira S., Sonzini S., van Pelt S., Romanelli S.M., Liang L.H., et al. Development of a high-throughput platform for screening lipid nanoparticles for mRNA delivery. Nanoscale. 2022;14:1480–1491. doi: 10.1039/d1nr06858j. [DOI] [PubMed] [Google Scholar]
- 69.Hai L., Zhang A.M., Wu X., Cheng H., He D.G., Wang T.Z., et al. Liposome-stabilized black phosphorus for photothermal drug delivery and oxygen self-enriched photodynamic therapy. ACS Appl Nano Mater. 2020;3:563–575. [Google Scholar]
- 70.Large D.E., Abdelmessih R.G., Fink E.A., Auguste D.T. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv Drug Deliv Rev. 2021;176 doi: 10.1016/j.addr.2021.113851. [DOI] [PubMed] [Google Scholar]
- 71.Shen S.Y., Lin S.Q., Chen Y.Y., Zhang Y., He Y.J., Xu X., et al. Combating cancer stem-like cell-derived resistance to anticancer protein by liposome-mediated acclimatization strategy. Nano Lett. 2022;22:2419–2428. doi: 10.1021/acs.nanolett.2c00004. [DOI] [PubMed] [Google Scholar]
- 72.Knudsen K.B., Northeved H., Kumar P.E.K., Permin A., Gjetting T., Andresen T.L., et al. In vivo toxicity of cationic micelles and liposomes. Nanomedicine. 2015;11:467–477. doi: 10.1016/j.nano.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 73.Jayaraman M., Ansell S.M., Mui B.L., Tam Y.K., Chen J., Du X., et al. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew Chem Int Ed. 2012;51:8529–8533. doi: 10.1002/anie.201203263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hafez I.M., Maurer N., Cullis P.R. On the mechanism whereby cationic lipids promote intracellular delivery of polynucleic acids. Gene Ther. 2001;8:1188–1196. doi: 10.1038/sj.gt.3301506. [DOI] [PubMed] [Google Scholar]
- 75.Tenchov R., Bird R., Curtze A.E., Zhou Q.Q. Lipid nanoparticles-from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano. 2021;15:16982–17015. doi: 10.1021/acsnano.1c04996. [DOI] [PubMed] [Google Scholar]
- 76.Granot Y., Peer D. Delivering the right message: challenges and opportunities in lipid nanoparticles-mediated modified mRNA therapeutics—an innate immune system standpoint. Semin Immunol. 2017;34:68–77. doi: 10.1016/j.smim.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 77.Zheng R.S., Zhang S.W., Zeng H.M., Wang S.M., Sun K.X., Chen R., et al. Cancer incidence and mortality in China, 2016. J Nat Cancer Cent. 2022;2:1–9. doi: 10.1016/j.jncc.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.GBD Causes Death Collaborators Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980‒2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou J., Rao L., Yu G.C., Cook T.R., Chen X.Y., Huang F.H. Supramolecular cancer nanotheranostics. Chem Soc Rev. 2021;50:2839–2891. doi: 10.1039/d0cs00011f. [DOI] [PubMed] [Google Scholar]
- 80.Liu Y., Ao X., Jia Y., Li X.G., Wang Y., Wang J.X. The FOXO family of transcription factors: key molecular players in gastric cancer. J Mol Med. 2022;100:997–1015. doi: 10.1007/s00109-022-02219-x. [DOI] [PubMed] [Google Scholar]
- 81.Goodall G.J., Wickramasinghe V.O. RNA in cancer. Nat Rev Cancer. 2021;21:22–36. doi: 10.1038/s41568-020-00306-0. [DOI] [PubMed] [Google Scholar]
- 82.Ferdows B.E., Patel D.N., Chen W., Huang X.G., Kong N., Tao W. RNA cancer nanomedicine: nanotechnology-mediated RNA therapy. Nanoscale. 2022;14:4448–4455. doi: 10.1039/d1nr06991h. [DOI] [PubMed] [Google Scholar]
- 83.Jia X.N., Lv M.M., Fei Y.W., Dong Q., Wang H., Liu Q., et al. Facile one-step synthesis of NIR-responsive siRNA-inorganic hybrid nanoplatform for imaging-guided photothermal and gene synergistic therapy. Biomaterials. 2022;282 doi: 10.1016/j.biomaterials.2022.121404. [DOI] [PubMed] [Google Scholar]
- 84.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 85.Wang X.S., Chen H.Y., Zeng X.W., Guo W.P., Jin Y., Wang S., et al. Efficient lung cancer-targeted drug delivery via a nanoparticle/MSC system. Acta Pharm Sin B. 2019;9:167–176. doi: 10.1016/j.apsb.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maynard A., McCoach C.E., Rotow J.K., Harris L., Haderk F., Kerr D.L., et al. Therapy-induced evolution of human lung cancer revealed by single-cell RNA sequencing. Cell. 2020;182:1232–1251. doi: 10.1016/j.cell.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao Y., Wang W., Guo S., Wang Y., Miao L., Xiong Y., et al. PolyMetformin combines carrier and anticancer activities for in vivo siRNA delivery. Nat Commun. 2016;7 doi: 10.1038/ncomms11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dilnawaz F., Sahoo S.K. Augmented anticancer efficacy by si-RNA complexed drug-loaded mesoporous silica nanoparticles in lung cancer therapy. ACS Appl Nano Mater. 2018;1:730–740. [Google Scholar]
- 89.Nascimento A.V., Singh A., Bousbaa H., Ferreira D., Sarmento B., Amiji M.M. Overcoming cisplatin resistance in non-small cell lung cancer with Mad2 silencing siRNA delivered systemically using EGFR-targeted chitosan nanoparticles. Acta Biomater. 2017;47:71–80. doi: 10.1016/j.actbio.2016.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chan M.H., Huang W.T., Satpathy A., Su T.Y., Hsiao M.C., Liu R.S. Progress and viewpoints of multifunctional composite nanomaterials for glioblastoma theranostics. Pharmaceutics. 2022;14:456. doi: 10.3390/pharmaceutics14020456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kong L., Qiu J., Sun W., Yang J., Shen M., Wang L., et al. Multifunctional PEI-entrapped gold nanoparticles enable efficient delivery of therapeutic siRNA into glioblastoma cells. Biomater Sci. 2017;5:258–266. doi: 10.1039/c6bm00708b. [DOI] [PubMed] [Google Scholar]
- 92.Zheng M., Liu Y., Wang Y., Zhang D., Zou Y., Ruan W., et al. ROS-responsive polymeric siRNA nanomedicine stabilized by triple interactions for the robust glioblastoma combinational RNAi therapy. Adv Mater. 2019;31 doi: 10.1002/adma.201903277. [DOI] [PubMed] [Google Scholar]
- 93.Sukumar U.K., Bose R.J.C., Malhotra M., Babikir H.A., Afjei R., Robinson E., et al. Intranasal delivery of targeted polyfunctional gold-iron oxide nanoparticles loaded with therapeutic microRNAs for combined theranostic multimodality imaging and presensitization of glioblastoma to temozolomide. Biomaterials. 2019;218 doi: 10.1016/j.biomaterials.2019.119342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kidger A.M., Saville M.K., Rushworth L.K., Davidson J., Stellzig J., Ono M., et al. Suppression of mutant Kirsten-RAS (KRAS(G12D))-driven pancreatic carcinogenesis by dual-specificity MAP kinase phosphatases 5 and 6. Oncogene. 2022;41:2811–2823. doi: 10.1038/s41388-022-02302-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bannoura S.F., Uddin M.H., Nagasaka M., Fazili F., Al-Hallak M.N., Philip P.A., et al. Targeting KRAS in pancreatic cancer: new drugs on the horizon. Cancer Metastasis Rev. 2021;40:819–835. doi: 10.1007/s10555-021-09990-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zeng L., Li J., Wang Y., Qian C., Chen Y., Zhang Q., et al. Combination of siRNA-directed Kras oncogene silencing and arsenic-induced apoptosis using a nanomedicine strategy for the effective treatment of pancreatic cancer. Nanomedicine. 2014;10:463–472. doi: 10.1016/j.nano.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 97.Uchida S., Kinoh H., Ishii T., Matsui A., Tockary T.A., Takeda K.M., et al. Systemic delivery of messenger RNA for the treatment of pancreatic cancer using polyplex nanomicelles with a cholesterol moiety. Biomaterials. 2016;82:221–228. doi: 10.1016/j.biomaterials.2015.12.031. [DOI] [PubMed] [Google Scholar]
- 98.Kamerkar S., LeBleu V.S., Sugimoto H., Yang S., Ruivo C.F., Melo S.A., et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498–503. doi: 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Han X., Li Y., Xu Y., Zhao X., Zhang Y., Yang X., et al. Reversal of pancreatic desmoplasia by re-educating stellate cells with a tumour microenvironment-activated nanosystem. Nat Commun. 2018;9:3390. doi: 10.1038/s41467-018-05906-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen W.Q., Chiang C.L., Dawson L.A. Efficacy and safety of radiotherapy for primary liver cancer. Chin Clin Oncol. 2021;10:9. doi: 10.21037/cco-20-89. [DOI] [PubMed] [Google Scholar]
- 101.Gu Y., Wang Y.Y., He L.Y., Zhang J.H., Zhu X.X., Liu N.A., et al. Circular RNA circIPO11 drives self-renewal of liver cancer initiating cells via Hedgehog signaling. Mol Cancer. 2021;20:132. doi: 10.1186/s12943-021-01435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Khan O.F., Zaia E.W., Yin H., Bogorad R.L., Pelet J.M., Webber M.J., et al. Ionizable amphiphilic dendrimer-based nanomaterials with alkyl-chain-substituted amines for tunable siRNA delivery to the liver endothelium in vivo. Angew Chem Int Ed. 2014;53:14397–14401. doi: 10.1002/anie.201408221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ju G.Q., Zhu Y.J., Du T., Cao W.L., Lin J.H., Li C., et al. MiR-197 Inhibitor loaded AbCD133@MSNs@GNR affects the development of prostate cancer through targeting ITGAV. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.646884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Poddar A., Pyreddy S., Carraro F., Dhakal S., Rassell A., Field M.R., et al. ZIF-C for targeted RNA interference and CRISPR/Cas9 based gene editing in prostate cancer. Chem Commun. 2020;56:15406–15409. doi: 10.1039/d0cc06241c. [DOI] [PubMed] [Google Scholar]
- 105.Hasan W., Chu K., Gullapalli A., Dunn S.S., Enlow E.M., Luft J.C., et al. Delivery of multiple siRNAs using lipid-coated PLGA nanoparticles for treatment of prostate cancer. Nano Lett. 2012;12:287–292. doi: 10.1021/nl2035354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen C.K., Law W.C., Aalinkeel R., Nair B., Kopwitthaya A., Mahajan S.D., et al. Well-defined degradable cationic polylactide as nanocarrier for the delivery of siRNA to silence angiogenesis in prostate cancer. Adv Healthc Mater. 2012;1:751–761. doi: 10.1002/adhm.201200094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xu X.D., Wu J., Liu Y.L., Saw P.E., Tao W., Yu M., et al. Multifunctional envelope-type siRNA delivery nanoparticle platform for prostate cancer therapy. ACS Nano. 2017;11:2618–2627. doi: 10.1021/acsnano.6b07195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yong S.B., Ramishetti S., Goldsmith M., Diesendruck Y., Hazan-Halevy I., Chatterjee S., et al. Dual-targeted lipid nanotherapeutic boost for chemo-immunotherapy of cancer. Adv Mater. 2022;34 doi: 10.1002/adma.202106350. [DOI] [PubMed] [Google Scholar]
- 109.Rurik J.G., Tombacz I., Yadegari A., Fernandez P.O.M., Shewale S.V., Li L., et al. CAR T cells produced in vivo to treat cardiac injury. Science. 2022;375:91–96. doi: 10.1126/science.abm0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang M.M., Bahal R., Rasmussen T.P., Manautou J.E., Zhong X.B. The growth of siRNA-based therapeutics: updated clinical studies. Biochem Pharmacol. 2021;189 doi: 10.1016/j.bcp.2021.114432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pastor F., Berraondo P., Etxeberria I., Frederick J., Sahin U., Gilboa E., et al. An RNA toolbox for cancer immunotherapy. Nat Rev Drug Discov. 2018;17:751–767. doi: 10.1038/nrd.2018.132. [DOI] [PubMed] [Google Scholar]
- 112.Rana M.S., Ikram A., Salman M., Usman M., Umair M. Negative impact of the COVID-19 pandemic on routine childhood immunization: experience from Pakistan. Nat Rev Immunol. 2021;21:689–690. doi: 10.1038/s41577-021-00627-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ying H., Zaks T.Z., Wang R.F., Irvine K.R., Kammula U.S., Marincola F.M., et al. Cancer therapy using a self-replicating RNA vaccine. Nat Med. 1999;5:823–827. doi: 10.1038/10548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sahin U., Derhovanessian E., Miller M., Kloke B.P., Simon P., Lower M., et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547:222–226. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]