Abstract
Tumor metastasis depends on the dynamic balance of the actomyosin cytoskeleton. As a key component of actomyosin filaments, non-muscle myosin-IIA disassembly contributes to tumor cell spreading and migration. However, its regulatory mechanism in tumor migration and invasion is poorly understood. Here, we found that oncoprotein hepatitis B X-interacting protein (HBXIP) blocked the myosin-IIA assemble state promoting breast cancer cell migration. Mechanistically, mass spectrometry analysis, co-immunoprecipitation assay and GST-pull down assay proved that HBXIP directly interacted with the assembly-competent domain (ACD) of non-muscle heavy chain myosin-IIA (NMHC-IIA). The interaction was enhanced by NMHC-IIA S1916 phosphorylation via HBXIP-recruited protein kinase PKCβII. Moreover, HBXIP induced the transcription of PRKCB, encoding PKCβII, by coactivating Sp1, and triggered PKCβII kinase activity. Interestingly, RNA sequencing and mouse metastasis model indicated that the anti-hyperlipidemic drug bezafibrate (BZF) suppressed breast cancer metastasis via inhibiting PKCβII-mediated NMHC-IIA phosphorylation in vitro and in vivo. We reveal a novel mechanism by which HBXIP promotes myosin-IIA disassembly via interacting and phosphorylating NMHC-IIA, and BZF can serve as an effective anti-metastatic drug in breast cancer.
KEY WORDS: Breast cancer metastasis, Actomyosin cytoskeleton, HBXIP, Myosin-IIA, NMHC-IIA, Phosphorylation, PKCβII, Bezafibrate
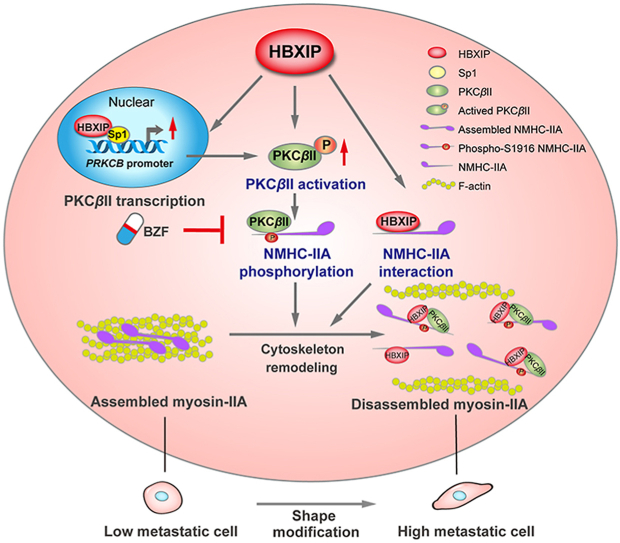
Graphical abstract
This study reveals a novel mechanism by which HBXIP promotes myosin-IIA disassembly via interacting and phosphorylating NMHC-IIA, and the anti-hyperlipidemic drug bezafibrate can serve as an effective anti-metastatic drug in breast cancer.
1. Introduction
Cell motility is a key function of individual tumor cells to escape the primary tumor, relying on the spatial arrangement of the actomyosin cytoskeletal components1. The make-up of the cytoskeleton consists of many pieces of key proteins that fit together to form a polymer. Unlike the concept of “skeleton”, the cytoskeleton system is an adaptive and dynamic balanced state in which polymer elements and regulatory proteins are in a constant state of flux. The polymerization and depolymerization of actomyosin cytoskeleton proteins remodel cell morphologies and control the formation of protrusions such as lamellipodia and filopodia2. Thus, the coordination of the myosin-IIA–actin network plays a critical role in cell motility. However, the spatiotemporal organization of myosin–IIA rearrangement and actin filament incorporation in cancer migration remain to be extensively explored.
Myosin-IIA is an omnipresent molecular motor protein that binds and contracts actin filaments. In addition to its manifold cellular functions, myosin-IIA has contractile properties as a key switch of invasive and metastatic behavior3,4. Two myosin heavy chains (NMHC-IIA), encoded by MYH9, compose the main structure of a hexameric myosin-IIA. Each NMHC-IIA contains a motor region carrying an actin-binding site and an ATPase-binding site, two IQ domains respectively binding with the essential light chains and the regulatory light chains, and a coiled-coil tail in charge of myosin-IIA assembly into filaments. The assembly-competent domain (ACD) near the C-terminal end of NMHC-IIA contains a 29 amino acid-region and is the functional domain at the helm of the organization of myosin-IIA monomers into filaments, which is needed for myosin-IIA activity and contains phosphorylation sites5.
Nevertheless, recently, the tumor promoter role of NMHC-IIA has been increasingly reported. The aberrant expression of NMHC-IIA relates to numerous malignant carcinomas, such as breast cancer, gastric cancer, colorectal cancer, esophageal squamous cancer, non-small cell lung cancer and bladder cancer6, 7, 8, 9, 10. Moreover, myosin-IIA activity is associated with therapy resistance in melanoma, nasopharyngeal carcinoma and hepatocellular carcinoma11, 12, 13. Consequently, the fundamental oncogenic function of NMHC-IIA, particularly during breast cancer metastasis, should be additionally clarified. As a conserved 18-kDa protein, hepatitis B X-interacting protein (HBXIP, also known as LAMTOR5) behaves as an oncoprotein in breast cancer and participates in multiple biological protumor processes including carcinogenesis, proliferation, migration, drug resistance and lipid metabolism reprogramming14, 15, 16. Aberrant expression of HBXIP is related to poor survival rate and prognosis in breast cancer patients. HBXIP has been reported as a regulator that signals amino acid levels to mTORC1, acting as a role of guanine nucleotide exchange factor in the Rag GTPases17. In addition, HBXIP has been confirmed to coactivate several transcription factors, such as E2F1, Sp1, LXR, and c-Myb, governing tumor-related genes transcription to facilitate the growth and metastasis of breast cancer16,18, 19, 20. Moreover, HBXIP can regulate the posttranslational modifications of several oncoproteins by recruiting related enzymes, involving O-linked glycosylation, acetylation, and ubiquitination21, 22, 23. In our previous study, HBXIP promotes the filopodia protrusion formation by upregulating the expression of Capn4 and reorganizing actin dynamics in breast cancer metastasis, which implied the reorganization function of HBXIP in actomyosin cytoskeleton system15. However, it is still unclear whether HBXIP participates in the management of myosin-IIA activity and actomyosin fiber assembly in breast cancer migration.
The regulatory mechanism of myosin-IIA assembly in physiologically relevant processes is accompanied by with heavy chain phosphorylation. Most phosphorylation sites of the NMHC-IIA are located at the C-terminal end, among the coiled coil and tailpiece regions24. In addition, biochemical and cellular studies have verified that molecular interactions between the C-terminal end of NMHC-IIA molecules and several regulatory proteins are essential to the assembly of the myosin hexamers and myosin-IIA filament formation25. Few studies have illustrated the relationship between phosphorylation and interaction with proteins in the molecular details of myosin-IIA filament disassembly, while both localize on the same key domain of the myosin-IIA molecule.
In this paper, we uncovered a novel mechanism by which HBXIP facilitates cell migration and invasion by inhibiting the myosin-IIA fiber assembly. Our findings indicate that the oncoprotein HBXIP directly interacts with the cytoskeletal protein NMHC-IIA to recruit the protein kinase βII (PKCβII) promoting the phosphorylation of NMHC-IIA S1916 and resulting in an unassembled myosin-IIA state. Therapeutically, we found that BZF depresses the HBXIP/PKCβII/NMHC-IIA axis and blocks breast cancer migration by inhibiting PKCβII expression.
2. Materials and methods
2.1. Cell lines and cell culture
The cell lines used, as well as the culture conditions, are presented in Supporting Information Table S1. Briefly, the cell lines were incubated in Roswell Park Memorial Institute-1640 medium (RPMI-1640, Gibco, Grand Island, NY, USA) or Dulbecco's modified Eagle's medium (Gibco) with 10% (v/v) fetal bovine serum (FBS, Gibco). pCMV-HBXIP plasmids were stably transfected into MCF-7 cells. MDA-MB-231 and LM-MCF-7 cells stably expressing ZsGreen-tagged sh-HBXIP were developed by lentiviral infection. To obtain positive single and mixed clones, the cells were selected in G418 (Invitrogen, Carlsbad, CA, USA) or puromycin (Invitrogen) for 2 weeks and expanded for further selection. The MCF-7-OE-HBXIP #5, LM-MCF-7-sh-HBXIP #21 and MDA-MB-231-sh-HBXIP #17 cells were used in subsequent experiments. Lipofectamine 3000 reagent (Invitrogen) was used.
2.2. Reagents
The reagents used in this study were LY333531 (MedChem Express, Monmouth Junction, NJ, USA) and bezafibrate (BZF) (Jiangsu Wan Gao Pharmaceutical Co., China).
2.3. Studies in humans and animals
The tissue microarray (TMA, Catalog # CC08-21-004) containing 50 breast cancer tissues, 40 breast carcinoma metastatic to lymph nodes and 10 normal breast tissues was purchased form Xi'an Aomei Biotechnology (China). The TMA contains detailed clinic pathological information, including patients age at diagnosis, sex, tumor node metastasis stage, histology grade, clinical stage, and pathological diagnosis. The information of the TMA was listed in Supporting Information Table S2. 44 cases of human breast cancer samples were collected from Tianjin Medical University Cancer Institute & Hospital, China. The clinical characteristics are presented in Supporting Information Table S3. All samples were approved by the Ethics Committee of the Hospital for providing tissues. Before patient samples were collected, written informed consent was obtained.
BALB/c nude female mice (45–48 days old) were purchased from the Experimental Animal Center of Peking (Beijing, China). For intravenous tail-vein injections, 5 × 105 MCF-7, LM-MCF-7 or MCF-7-OE-HBXIP tumor cells were digested and resuspended in 100 μL PBS. Enrolled mice were randomly assigned to groups without stratification (6–8 mice/group). Oral gavages of saline or BZF (25 mg/kg/day in saline)/LY333531 (1 mg/kg/day in saline) commenced daily at 10 days after injection. Mice were culled 55 days after intravenous tail-vein injection. Lung and liver metastasis nodes were embedded in paraffin (Leica, Westlar, Germany) or OCT (Sakura Finetek, Torrance, California, USA) and sectioned into 5-μm slides after euthanization. Paraffin-embedded sections were stained with H&E staining or IHC staining. The number of metastatic foci in lung and liver tissues counted from 5 different fields of H&E staining slides. Frozen sections were stained with immunofluorescence staining. All animal protocols used in this study were approved by the Institute Research Ethics Committee of Nankai University.
2.4. Plasmids and siRNAs
Plasmids pcDNA3.1(+), pcDNA3.1(+)-HBXIP, pCMV-tag2B, pCMV-tag2B-HBXIP, pGL3-Basic, pRL-TK, pMG.2G, psPAX, pCDH-GFP-HBXIP, pLVX-shRNA2-HBXIP, and pLVX-shRNA2-NC were kept in our lab. pcDNA-PKCβII-WT, pcDNA-PKCβII-DN, pcDNA-PKCβII-ΔNPS, and pcDNA-PKCβII-CAT were purified and provided ex gratia by the Key Laboratory of Glycoconjugate Research Ministry of Health, Fudan University, Shanghai, China. pENTER-MYH9 was provided ex gratia by the Cancer Center, Southern Medical University, Guangzhou, China. To generate the pGL3-PRKCB, the PRKCB gene promoter region was amplified from the genomic DNA of MCF-7 cells by PCR and cloned into the pGL3-Basic vector. All siRNAs and the control siRNAs were purchased from Ribo Bio (Guangzhou, China). All related primers and siRNA sequences are listed in Supporting Information Table S4.
2.5. RNA sequencing, RNA extraction, reverse transcription, and RT-qPCR
The cells were transfected or treated with plasmids or BZF for 48 h. Each group of cells or tissues was subjected to TRIzol (Invitrogen). RNA sequencing was accomplished by HuaDa Gene (Shenzhen, China). Complementary DNA was extracted with the Transcriptor First Strand cDNA Synthesis Kit (TransGene Biotech, China). RT-qPCR was performed with three replications using SYBR Premix ExTaq (TaKaRa, Shiga, Japan). Primer sequences of the related genes are listed in Table S4.
2.6. Mass spectrometry
The GST-tagged HBXIP plasmid was transformed into Escherichia coli BL-21 cells and purified with the anti-GST affinity gel. The GST peptide-eluted protein was separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel. The band at approximately 200 kD was excised from the gel and endured mass spectrometry. Interaction peptides and proteins were identified with the database search. The mass spectrometry procedure and analysis were performed by Prof. Qi's lab from the Hong Kong University of Science and Technology (Hong Kong, China).
2.7. Immunofluorescence staining assay (IF)
Cells and tissues were treated for immunofluorescence staining as previously described26. The primary antibodies were rabbit polyclonal anti-NMHC-IIA antibody, mouse monoclonal anti-flag tag antibody (Sigma–Aldrich, St. Louis, MO, USA), and mouse monoclonal anti-phosphorylated PKCβII (Santa Cruz Biotechnology, Dallas, TX, USA), and the secondary antibodies were goat anti-rabbit conjugated rhodamine TRITC antibody (Sigma–Aldrich) and rabbit anti-mouse conjugated FITC antibody (Sigma–Aldrich). The antibody dilution ratios are provided in Supporting Information Table S5.
2.8. Triton X-insoluble cytoskeletal ghost assay
Triton X-insoluble cytoskeletal ghost assay was performed as described to examine the influence of treatment on myosin-IIA assembly27. Western blotting analysis was probed by rabbit polyclonal anti-NMHC-IIA or mouse polyclonal anti-β-actin, and densitometry was performed using ImageJ software.
2.9. Co-immunoprecipitation assay
After 48 h of transfection with the appropriate plasmids, the cells were collected and lysed in a lysis buffer (10% glycerin (w/v), 1 mmol/L EDTA, 50 mmol/L pH 7.5 Tris-HCl, 0.5% Triton X-100 (v/v), 150 mmol/L NaCl, 1 mmol/L PMSF). The products were interacted with Flag M2 affinity gel (Sigma–Aldrich) at 4 °C for 4 h. To explore the endogenous interaction proteins in MCF-7 or LM-MCF-7 cells, protein G-Sepharose (Santa Cruz Biotechnology) was incubated with the negative control IgG, or anti-NMHC-IIA, anti-PKCβII and anti-HBXIP, then were incubated with the cell lysate. After washing with the washing buffer for eight times, the affiliative proteins were washed out from the gel with washing buffer and then separated by SDS-PAGE and immunoblotting.
2.10. Dual-luciferase report gene assay (DLR)
The pGL3-Basic vector containing the wild-type or mutant PRKCB promoter along with pRL-TK and other plasmids were transfected into the cells. After 48 h of transfection, the cells were incubated and analyzed using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA).
2.11. Chromatin immunoprecipitation assay (ChIP)
The EpiQuik ChIP Kit (EpiGentek Group, Farmingdale, NY, USA) was used to perform the ChIP assay coupled with RT-qPCR. HBXIP antibodies, as well as a negative control antibody IgG and a positive control antibody anti-RNA polymerase II immunoprecipitated with protein–DNA complexes. Amplification of the soluble fraction before immunoprecipitation was collected as an input control.
2.12. Western blotting analysis
Western blotting analysis was performed with standard protocols28. All primary antibodies used, and dilution ratios are provided in Table S5. The polyvinylidene fluoride membranes with bands was photographed by Glyco Band-Scan Software Version 4.50 (http://www.Glyco.com).
2.13. Transwell assay and wound healing assay
To detect the migration and invasion abilities of cells, Transwell assays (Corning, Grand Island, NY, USA) and wound healing assays were performed. Then, 100 μL medium without serum suspended with cells was seeded into the upper chamber, and 450 μL medium with 20% FBS was filled with the bottom chamber. Migrated cells were stained with crystal violet and then photographed. Cells in 5 random fields were counted and quantified.
For wound healing assays, cells were cultured for growth in 6-well plates in FBS-withdrawn RPMI-1640, and scratches were generated by pipette tip. Migration of cells was quantified by photographs taken at 0, 12 and 24 h after wounding.
2.14. Immunohistochemistry staining assay (IHC)
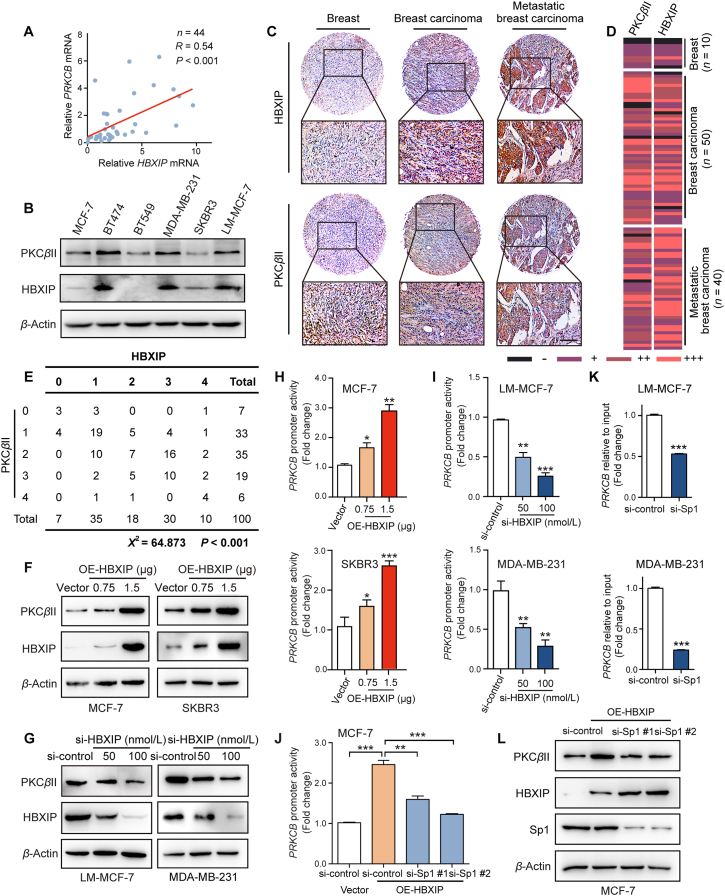
HBXIP, PKCβII and pi-PKCβII expression level in metastasis tumor and breast cancer tissues from TMA were examined by IHC staining method as standard protocol21. According to the intensity and area of HBXIP and PKCβII staining, tissues were divided into four groups (Scores: 0 = negative/–; 1 = low/+; 2 = moderate/++; 3 = high/+++). According to the staining level of HBXIP and PKCβII, the heatmap and χ2 table were produced by using final scores.
2.15. Statistical analysis
The statistical significance was quantified by averaging three independent replicates ± standard deviation (SD) via two-tailed Student's t test, one-way analysis of variance or two-way analysis of variance (ANOVA) symbolized by ∗∗∗P < 0.001, ∗∗P < 0.01, ∗P < 0.05, respectively, and not significant (ns). The association between HBXIP and breast cancer metastasis or PKCβII expression in breast cancer TMAs was statistically analyzed by Pearson chi-square independence test. The mRNA-seq data set used is from The Cancer Genome Atlas (TCGA; https://tcga-data.nci.nih.gov/tcga/). The multigene correlation map is displayed by the R software package pheatmap. The datasets used are GSE125989, GSE43837 and GSE42568 from the Gene Expression Omnius (GEO) database (https://www.ncbi.nlm.nih.gov/geo/), and the data format is MINIML. Gene Set Enrichment Analysis (GSEA) was analyzed using GSEA software (http://software.broadinstitute.org/gsea/). R Foundation (2020) Version 4.0.3 (The R Project for Statistical Computing, Vienna, Austria), GraphPad Prism 9 (GraphPad, Inc., La Jolla, CA, USA), and SPSS 22.0 (SPSS, Inc., Chicago, IL, USA) achieved to statistically analyzed all the above analysis.
3. Results
3.1. HBXIP contributes to myosin-IIA disassembly and breast cancer migration
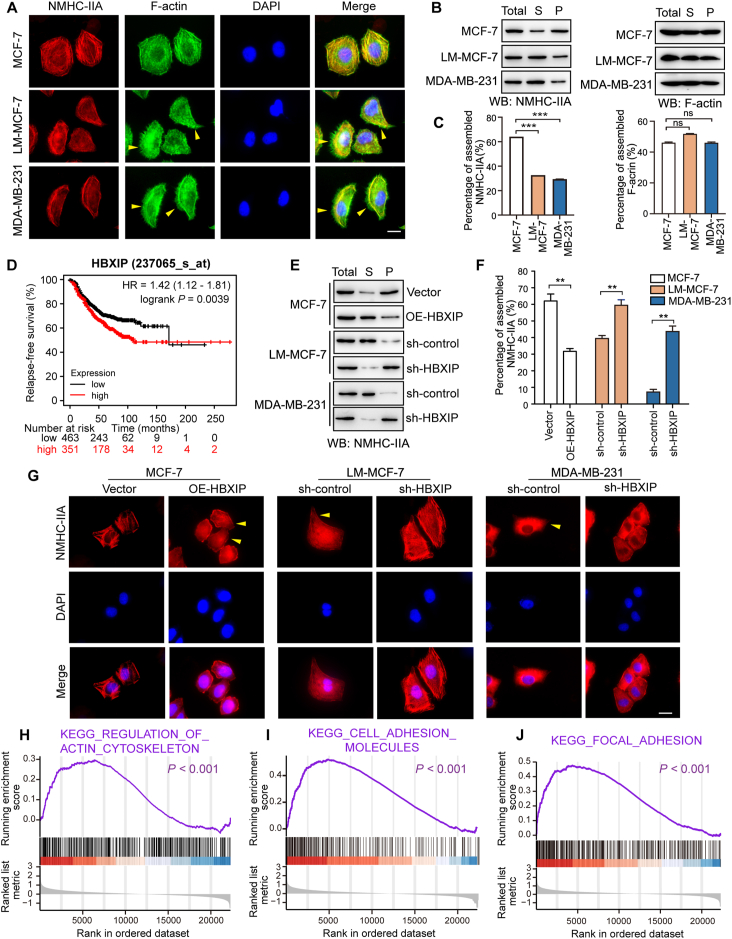
Tumor metastasis is always accompanied by the formation of protrusions, which are reorganized by the actomyosin cytoskeleton. The actomyosin cytoskeleton, a contractile structure, is mainly formed by bipolar arrays of non-muscle myosin-IIA and actin filaments. These filaments organize into plasma membrane protrusions and precede invasion, pathfinding and cell adhesion during metastasis29. Previous studies have shown that protrusion relies on contractile actomyosin bundles, where the force is produced through sliding of bipolar myosin-IIA filaments along actin filaments30. At the cell-scale, protrusions localized at the edge require assembly of actin filaments, while at the cell rear, myosin-IIA activity plays a direct role in actin network disassembly31. Here, we are wondering how the actomyosin cytoskeleton organizes in breast cancer cells with different metastatic abilities. Immunofluorescence staining showed the assembly and disassembly of myosin-IIA and F-actin fibers in three breast cancer cell lines, MCF-7, LM-MCF7 (MCF-7-derived mouse lung metastasis cells)32 and MDA-MB-231 (highly metastatic breast cancer cell line). Compared with MCF-7 cells, highly metastatic cell lines displayed an elongated and highly multipolar morphology with filopodia on the edge marked by F-actin and lost the high contractility phenotype of assembled myosin-IIA fiber bundles marked by NMHC-IIA (Fig. 1A). Because assembled cytoskeletal proteins were insoluble in Triton X-100 solution33, Triton X-insoluble cytoskeletal ghost assay was performed (Fig. 1B and C). Western blotting assays showed that in highly metastatic cells, less assembled myosin-IIA remained in the pellet of cell lysis solution, which implied that the percentage of myosin-IIA assembly contributed to breast cancer migration. However, there was no significant difference of the percentage of assembled actin filaments among these three breast cancer cell lines. These results indicate that assembly of actin filaments is a spatial locational change, and the disassembly of myosin-IIA might control actin filament assembly on the edge of cells and be positively related to the migration ability of breast cancer cells.
Figure 1.
HBXIP contributes to myosin-IIA disassembly and breast cancer migration. (A) The spatial distribution patterns of NMHC-IIA (red), F-actin (green) and nuclei (DAPI) were detected by IF. The filopodia were highlighted with yellow arrows. Scale bar, 20 μm. (B, C) Western blotting (WB) analysis showed Triton X-insoluble fractions of these cells with an anti-NMHC-IIA antibody and anti-β-actin antibody (B) and quantification of the intensity relative to the total lysate group (C). Total, total lysis; S, supernatant; P, pellet. (D) Kaplan–Meier plots of the disease-free survival rates of 814 metastatic breast cancer patients stratified according to the HBXIP mRNA levels from the TCGA dataset. (E, F) WB analysis showed Triton X-insoluble fractions of stably transfected cells with an anti-NMHC-IIA antibody (E) and quantification of the intensity relative to the total lysate group (F). (G) The spatial distribution patterns of NMHC-IIA (red) and nuclei (DAPI) were detected by IF. The elongated leading edges were highlighted with yellow arrows. Scale bar, 20 μm. (H–J) GSEA of breast cancer metastasis patients grouped by the mean expression value of HBXIP expression. Columns with error bars symbolize the average of three independent replicates ±SD. Three experiments with consistent results tendency were repeated by two-tailed Student's t test. ∗∗∗P < 0.001; ∗∗P < 0.01; ns, not significant.
The studies have revealed that HBXIP can behave as an oncoprotein in breast cancer and participates in the tumorigenesis and development of breast cancer21,34. The Kaplan–Meier plotter data showed that in 814 breast cancer patients with lymph node metastasis, and higher level of HBXIP was associated with shorter relapse-free survival, further implying the relationship of HBXIP and breast cancer metastasis (Fig. 1D). In addition, the level of HBXIP was determined by IHC assays in a TMA containing 50 breast cancer tissues, 40 breast carcinoma metastatic to lymph nodes and 10 normal breast tissues (Table S2). The data show that HBXIP was highly expressed in metastatic tissues, compared with the normal tissues and the in situ carcinoma tissues (Pearson chi-square independence test, χ2 = 12.235, P < 0.05, Supporting Information Fig. S1A–S1C). Besides, there was a robustly positive relationship between higher level of HBXIP and worse histology grade (Pearson chi-square independence test, χ2 = 15.989, P < 0.05, Fig. S1D). Next, we reverified the expression of HBXIP in three breast cell lines, MCF-7, LM-MCF7 and MDA-MB-231. Western blotting assays showed that the expression of HBXIP was positively correlated with cell metastatic ability (Fig. S1E). Furthermore, we generated stably HBXIP-overexpressing MCF-7 cells, as well as stably HBXIP-knockdown MDA-MB-231 and LM-MCF-7 cells (Fig. S1F–S1H). Trans-well assays proved that the expression of HBXIP in these three cell lines was positively associated with cell migration (Fig. S1I). These results were consistent with our previous findings21. We have reported that HBXIP can reorganize actin filament spatial arrangement by upregulating the expression of Capn4 and promote filopodia protrusion formation, which implies the association between HBXIP and actomyosin cytoskeleton reorganization15. Thus, we next tried to investigate whether HBXIP was also involved in the regulation of myosin-IIA assembly.
As expected, the overexpression of HBXIP promoted the disassembly state of NMHC-IIA and transformed the epithelial appearance into an aggressively elongated and highly multipolar phenotype in MCF-7 cells. In contrast, the HBXIP knockdown blocked NMHC-IIA disassembly and reversed cytoskeletal morphology into the epithelial phenotype in MDA-MB-231 and LM-MCF-7 cells (Fig. 1E–G). These results further explain our previous observations15, suggesting that HBXIP could robustly remodel the actomyosin cytoskeleton by suppressing myosin-IIA assembly and reorganizing actin dynamics, resulting in aggressively phenotypic transformation and the formation of filopodia on the edge.
Next, we downloaded and divided the raw data of 140 cases of stage IV breast cancer patients (GSE12647, https://www.ncbi.nlm.nih.gov/geo/) into two groups depending on the mean expression value of HBXIP. And then, we analyzed the DEGs between these two groups. GSEA showed a significant enrichment in cytoskeleton-related pathways, including “Cell adhesion molecules”, “Focal adhesion” and “Regulation of actin cytoskeleton” (Fig. 1H–J). These data actively demonstrate that HBXIP promotes aggressive mesenchymal morphology by reconstructing the actomyosin cytoskeleton system in breast cancer migration.
3.2. HBXIP directly binds with NMHC-IIA, promoting the disassembly of myosin-IIA
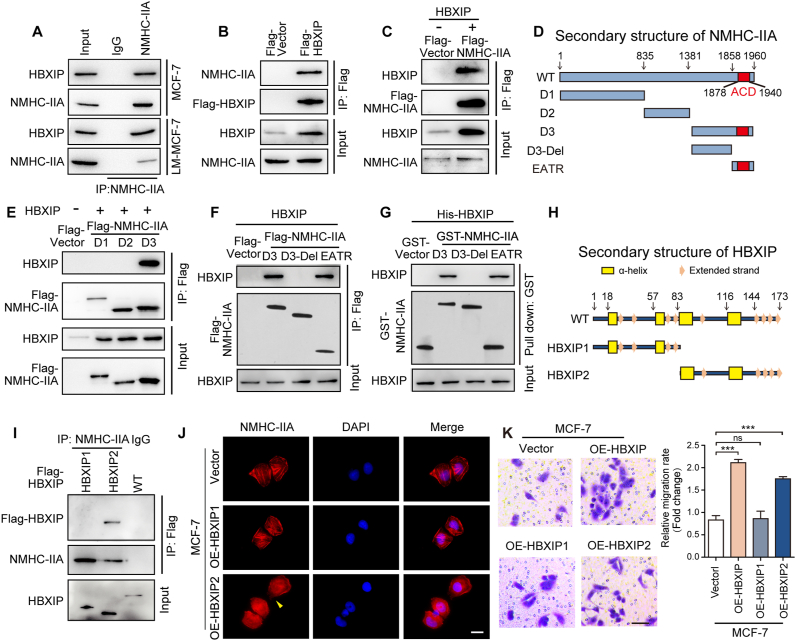
As the mechanism of HBXIP-mediated actin dynamic regulation is clearly understood, we focused on the details of myosin-IIA disassembly modification in response to HBXIP. Myosin-IIA disassembly can be mediated by interactions between NMHC-IIA and regulatory proteins, such as S100A435 and S100P36. To explore whether HBXIP directly bound to NMHC-IIA in the same manner as these proteins, mass spectrometric analysis was examined and showed that there were three peptides that matched the sequences of the human NMHC-IIA (Supporting Information Fig. S2A and S2B). Co-IP assays further identified that the interaction between endogenous or exogenous HBXIP and NMHC-IIA (Fig. 2A–C). In accordance with the secondary structure of NMHC-IIA, we constructed different mutants of NMHC-IIA fragments (Fig. 2D). Compared with other mutants, D3 (aa 1382–1960) showed obvious binding with HBXIP (Fig. 2E). According to the secondary structure of D3, including an ACD domain, a region responsible for myosin assembly and involving cell migration, the other two fragments of D3, D3-Del and EATR (extending ACD and tail region) were constructed (Fig. 2D). Only the deletion of the ACD domain NMHC-IIA could not bind to HBXIP (Fig. 2F). GST pull-down assays using recombinant His-HBXIP, and the three C-terminal fragments of NMHCII-A confirmed the interaction between two proteins is direct (Fig. 2G). These data suggest that HBXIP directly binds to the C-terminal fragment (aa 1858–1960) of NMHC-IIA in vivo and in vitro.
Figure 2.
HBXIP directly binds with NMHC-IIA, promoting myosin-IIA filament disassembly. (A) Co-IP analysis of the interaction between endogenous HBXIP and NMHC-IIA. (B) Co-IP analysis of exogenous Flag-HBXIP. pCMV-vector and pCMV-HBXIP were transiently transfected into HEK-293T cells. (C) Co-IP analysis of exogenous Flag-NMHC-IIA. pENTER-vector and pENTER-NMHC-IIA, along with pcDNA-vector and pcDNA-HBXIP were transiently transfected into HEK-293T cells. (D) The diagram shows the 5 separate segments of NMHC-IIA constructed amino acids 1–835 (D1), 836–1381 (D2), 1382–1960 (D3), 1382–1857 (D3-Del) and 1858–1960 (EATR). (E) Co-IP analysis of the interaction between Flag-D3 and HBXIP. pcDNA-vector and pcDNA-HBXIP, as well as pCMV-vector and pCMV-D1/D2/D3, were transiently transfected into HEK-293T cells. (F) Co-IP analysis of the interaction among Flag-D3, Flag-EATR and HBXIP. pcDNA-vector and pcDNA-HBXIP, as well as pCMV-vector and pCMV-D3/D3-Del/EATR were transiently transfected into HEK-293T cells. (G) GST pull-down of GST-D3/D3-Del/EATR with His-HBXIP. (H) The diagram shows the 2 separate segments of HBXIP constructed amino acids 1–83 (HBXIP1) and 84–173 (HBXIP2). (I) Co-IP analysis of the interaction between Flag-HBXIP2 and NMHC-IIA. pCMV-vector and pCMV-HBXIP1/HBXIP2/HBXIP were transiently transfected into HEK-293T cells. (J) The spatial distribution patterns of NMHC-IIA (red) and nuclei (DAPI) were detected in the stably expressing cells. The elongated leading edges were highlighted with yellow arrows. Scale bar, 20 μm. (K) Representative images of the indicated cells determined by Transwell invasion assay and cell counting results of Transwell invasion assay. Scale bar, 50 μm. Columns with error bars symbolize the average of five different fields ±SD. Three experiments with consistent results tendency were repeated by two-tailed Student's t test. ∗∗∗P < 0.001; ns, not significant.
Next, to map the crucial region of HBXIP response for the interaction with NMHC-IIA, we divided full-length HBXIP into two fragments (Fig. 2H). Co-IP assays showed that the HBXIP2 (aa 83–173) fragment bound to NMHC-IIA, but the HBXIP1 (aa 1–83) fragment could not (Fig. 2I).
Then, we observed the effects of different HBXIP fragments on the cytoskeletal phenotype. The data show that stable MCF-7-OE-HBXIP2 cells exhibited a similar pattern to MCF-7-OE-HBXIP cells (Fig. 2J). Importantly, we contrasted the effects between wild type HBXIP and its fragments on cell migration using a Trans-well assay, and we found that only HBXIP2 improved the migration of MCF-7 cells, similar to the wild type HBXIP (Fig. 2K).
The results above indicate that the 83–173 amido acid fragment of HBXIP blocks the myosin-II assembly through directly interacting with the NMHC-IIA C-terminal 1858–1960 amido acid fragment.
3.3. HBXIP enhances PKCβII-mediated NMHC-IIA phosphorylation fastening the interaction between HBXIP and NMHC-IIA
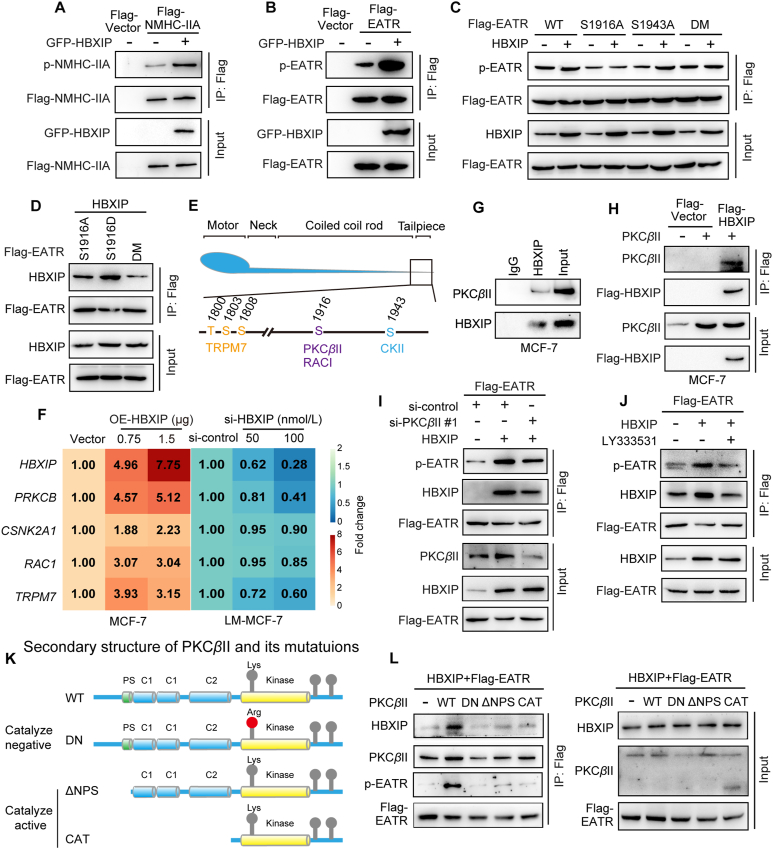
There are accumulating articles reporting that heavy chain phosphorylation mediates oligomerization and assembly of myosin-IIA filaments. Most NMHC-IIA phosphorylation sites are located at the C-terminal end among the coiled coil and tailpiece regions. While the HBXIP binding domain of NMHC-IIA mainly overlaps the phosphorylation site-rich domain, endogenous phosphorylation experiments of NMHC-IIA were carried out. Our result indicate that the overexpression of HBXIP promoted the phosphorylation of whole length NMHC-IIA and its C-terminal end EATR domain, which implied that upregulation of the NMHC-IIA phosphorylation was another way that HBXIP accelerated NMHC-IIA assembly (Fig. 3A and B, and Supporting Information Fig. S3A and S3B). The prediction of Group-based Prediction System, Version 2.0 (GPS 2.0; http://gps.biocuckoo.org/) showed that the fragment of EATR contained two phosphorylation sites, S1916 and S1943, which were reported to regulate the assembly of myosin-IIA filaments24,37. To identify the HBXIP-promoted phosphorylation sites of EATR, the S1916 and S1943 sites were mutated to alanine (A, mimics of phosphorylation deficiency). The phosphorylation level of EATR was no longer heightened by HBXIP in S1916A mutation and double mutation (DM) (Fig. 3C and Fig. S3C), suggesting that S1916 was the major phosphorylation site of NMHC-IIA regulated by HBXIP. Interestingly, we discovered that NMHC-IIA-S1916 was highly conserved during evolution in multiple species (Fig. S3D).
Figure 3.
HBXIP enhances PKCβII-mediated NMHC-IIA phosphorylation fastening the interaction between HBXIP and NMHC-IIA. (A) Co-IP analysis of the phosphorylation level of Flag-NMHC-IIA was detected using an anti-phospho-serine antibody. pENTER-vector and pENTER-NMHC-IIA, along with GFP-HBXIP were transiently transfected into HEK-293T cells. (B) Co-IP analysis of the phosphorylation level of Flag-EATR. pCMV-EATR with GFP-HBXIP were transiently transfected into HEK-293T cells. (C) Co-IP analysis of the phosphorylation level of Flag-EATR. pCMV-EATR-WT, pCMV-EATR-S1916A, pCMV-EATR-S1943A and pCMV-EATR-DM together with pcDNA-HBXIP or pcDNA-vector were transiently transfected into HEK-293T cells. (D) Co-IP analysis of the interaction between Flag-S1916A/S1916D/DM and HBXIP. pCMV-EATR-S1916A, pCMV-EATR-S1916D and pCMV-EATR-DM together with pcDNA-HBXIP were transiently transfected into HEK-293T cells. (E) Schematic diagram of phosphorylation sites and kinases of NMHC-IIA. (F) Fold change in mRNA levels of potential kinases determined by RT-qPCR assays. pCMV-vector and pCMV-HBXIP or si-control and si-HBXIP were dose-dependently transfected into the cells. (G, H) Co-IP analysis of the interaction between endogenous or exogenous HBXIP and PKCβII in MCF-7 cells. (I) Co-IP analysis of the phosphorylation level of Flag-EATR. pCMV-EATR together with pcDNA-HBXIP or pcDNA-vector, along with si-control or si-PKCβII #1 were transiently transfected into HEK-293T cells. (J) Co-IP analysis of the phosphorylation level of Flag-EATR. Before treatment with 10 μmol/L LY333531 for 24 h, pCMV-EATR along with pcDNA-HBXIP or pcDNA-vector were transfected into HEK-293T cells. (K) The diagram shows the 3 separate segments of PKCβII constructed amino acids 2–673 (DN), 30–673 (ΔNPS) and 329–673 (CAT). (L) Co-IP analysis of interaction between EATR, PKCβII and HBXIP. pcDNA-PKCβII-WT, pcDNA-PKCβII-DN, pcDNA-PKCβII-ΔNPS and pcDNA-PKCβII-CAT along with pcDNA-HBXIP and pCMV-EATR were transiently transfected into HEK-293T cells. Three experiments with consistent results tendency were repeated.
To evaluate whether the phosphorylation of EATR could affect the binding ability with HBXIP, the S1916 residue was mutated to aspartic acid (D, mimic of hyperphosphorylation). The mutant of S1916A and the DM of EATR, compared with the S1916D mutant, markedly less interacted with HBXIP (Fig. 3D and Fig. S3E), supporting that S1916 phosphorylation strengthened the affinity between NMHC-IIA and HBXIP.
Due to the absence of kinase activity of HBXIP, it is essential to recruit and activate tyrosine kinase on the NMHC-IIA for S1916 phosphorylation. According to the previous research, PKCβII, RAC1, CKII, and TRPM7 could serve as the candidate kinases to phosphorylate the C terminal of NMHC-IIA24,38, 39, 40 (Fig. 3E). And all these kinases controlled the disassembly of myosin-IIA. Therefore, RT-qPCR assays were performed to screen the effect of these kinases responding to HBXIP. Only the expression of PRKCB, which encoded PKCβII, was positively correlated with HBXIP, implying that PKCβII might be the major kinase mediated by HBXIP (Fig. 3F). Co-IP was applied to determine our hypothesis. Fig. 3G and H shows that PKCβII (in its exogenous or endogenous form) could bind with HBXIP in the MCF-7 cell line. Then, we designed two si-RNA fragments to silencing PKCβII expression. The interference efficiency of two PKCβII siRNAs is shown in Fig. S3F. Silencing or inhibiting of PKCβII with si-PKCβII or LY333531 (a PKCβ specific inhibitor) blocked the HBXIP-mediated hyperphosphorylation of NMHC-IIA (Fig. 3I and J, and Fig. S3G and S3H). And the Triton X-insoluble cytoskeletal ghost assay was followed. The assembly of myosin-IIA fibers were found in both si-PKCβII #1 and si-PKCβII #2 treated LM-MCF-7 cells, compared with si-control treated group (Fig. S3I). Co-IP assays showed that the interaction between NMHC-IIA and PKCβII was induced or suppressed upon the exogeneous transfection or depletion of HBXIP in MCF-7 and LM-MCF-7 breast cancer cell lines, respectively (Fig. S3J and S3K). These results indicate that protein kinase PKCβII is recruited by HBXIP to enhance the phosphorylation of NMHC-IIA.
Furthermore, we constructed an exogenous overexpression plasmid of PKCβII (pcDNA-PKCβII-WT) and three kinase activity mutation plasmids: kinase activity-inhibiting mutation (pcDNA-PKCβII-DN, dominant negative) and kinase activity-promoting mutation (pcDNA-PKCβII-ΔNPS and pcDNA-PKCβII-CAT) (Fig. 3K). Only when wild type PKCβII was overexpressed did hyperphosphorylated-EATR interact with high amounts of HBXIP (Fig. 3L and Fig. S3L), which implied that the N-terminal regulatory domain of PKCβII was also crucial to the phosphorylation of EATR and the interaction between EATR and HBXIP. Thus, we conclude that HBXIP posttranslationally affects the phosphorylation level of NMHC-IIA at the S1916 site via protein kinase PKCβII, which fastens the binding ability between HBXIP and NMHC-IIA.
3.4. HBXIP increases the transcriptional activity of the PRCKB promoter via Sp1
PKCβII, a multifunctional serin/threonine protein kinase, relates to a number of cytoskeleton-associated proteins that contribute to localized cytoskeletal reorganization41. However, the regulatory mechanism of PKCβII in breast cancer remains unclear. First, we analyzed the mRNA level of PKCβII and HBXIP from TCGA sequencing data provided by Chou's group using oncomine online platform (https://www.oncomine.org/resource/login.html). We observed that the mRNA level of HBXIP and PRKCB was increased in invasive ductal breast carcinoma stoma tissue, compared with normal breast tissue (Supporting Information Fig. S4A and S4B). Then, we examined with the mRNA levels of HBXIP and PRKCB in 44 breast cancer tissues by RT-qPCR experiments, which showed that the mRNA level of PRKCB was positively linked with HBXIP (Fig. 4A). The patients' information is listed in Table S3. Moreover, Fig. 4B reveals a positive relationship between HBXIP and PKCβII in six different breast cancer cell lines, MCF-7, BT474, BT549, MDA-MB-231, SKBR3 and LM-MCF-7. We used the previous consecutive section TMAs and confirmed the PKCβII expression in clinical samples by IHC staining. According to the intensity and area of HBXIP and PKCβII staining, the heatmap of staining levels were produced (Fig. 4C and D). The positive association between HBXIP and PKCβII expression in breast cancer tissues was statistically analyzed by Pearson chi-square independence test (χ2 = 64.873, P < 0.001) (Fig. 4E). The RT-qPCR assay and Western blotting assay demonstrated that overexpression of HBXIP affected the mRNA and the protein levels of PKCβII in a dose-dependent manner in MCF-7 and SKBR3 breast cancer cell lines (Fig. S4C and S4D, and Fig. 4F). And vice versa, silencing HBXIP dose-dependently decreased PKCβII mRNA and protein levels in LM-MCF-7 and MDA-MB-231 cell lines (Fig. S4E and S4F, and Fig. 4G). Since HBXIP can act as a transcriptional coactivator, the DLR showed that HBXIP could promote the activity of the full-length PRKCB promoter (−957 to +88) in breast cancer cells (Fig. 4H and I). These binding results were confirmed by ChIP assay followed RT-qPCR assay (Fig. S4G). Thus, the results above indicate that HBXIP transcriptionally upregulates the expression of PKCβII.
Figure 4.
HBXIP increases the transcriptional activity of the PRCKB promoter via Sp1. (A) Correlation of HBXIP and PRKCB mRNA levels in 44 clinical breast ductal carcinoma tissues examined by RT-qPCR assay. (B) WB analysis exhibited the expression levels of PKCβII and HBXIP in various cell lines, respectively. (C) IHC staining of HBXIP and PKCβII in 100 individual breast cancer TMAs, containing breast cancer tissues (n = 50), breast carcinoma metastatic to lymph nodes (n = 40) and normal breast tissues (n = 10). Scale bar, 50 μm. (D) The heatmap of staining level of HBXIP and PKCβII. According to the intensity and area of HBXIP and PKCβII staining, tissues were divided into four groups (Scores: 0 = negative/–; 1 = low/+; 2 = moderate/++; 3 = high/+++). (E) The association between HBXIP and PKCβII expression in TMAs was statistically analyzed by Pearson chi-square independence test (χ2 = 64.873, P < 0.001). (F) WB analysis exhibited the expression levels of PKCβII and HBXIP in MCF-7 and SKBR3 cells transfected with pcDNA-HBXIP and pcDNA-vector in a dose-dependent manner. (G) WB analysis exhibited the expression levels of PKCβII and HBXIP in LM-MCF-7 and MDA-MB-231 cells transfected with si-control and si-HBXIP in a dose-dependent manner. (H) PRKCB promoter activity was detected by DLR gene assay. pCMV-vector and pCMV-HBXIP were transiently transfected into MCF-7 and SKBR3 cells for 24 h. (I) PRKCB promoter activity was detected by DLR gene assay. Si-control and si-HBXIP were transiently transfected into LM-MCF-7 and MDA-MB-231 cells for 24 h. (J) PRKCB promoter activity was detected by DLR gene assay. Si-control and si-Sp1 #1 or si-Sp1 #2 were transiently transfected into MCF-7 or MCF-7-OE-HBXIP cells for 24 h. (K) ChIP analysis of the PRKCB promoter followed by RT-qPCR in LM-MCF-7 and MDA-MB-231 cells transfected with si-control and si-Sp1 #2 immunoprecipitated by negative control IgG or anti-HBXIP antibody. (L) WB analysis exhibited the expression levels of PKCβII, HBXIP and Sp1 in MCF-7 or MCF-7-OE-HBXIP cells together with si-control, si-Sp1 #1 or si-Sp1 #2. Columns with error bars symbolize the average of three independent replicates ±SD. Three experiments with consistent results tendency were repeated by two-tailed Student's t test or one-way ANOVA. ∗∗∗P < 0.001; ∗∗P < 0.01; ∗P < 0.05.
Our laboratory has detailed that HBXIP serves as a transcriptional coactivator of Sp119. Thus, we hypothesized that the transcription factor Sp1 might be related to the HBXIP-mediated promotion of PRKCB promoter activity. According to a previous report, Sp1 promotes PRKCB promoter activity at two sites (−94 and −63), and none of these two sites is dispensable42,43. We mutated both two sites (Sp1-M) using site-directed mutagenesis (Fig. S4H). Mutation variably decreased PRKCB promoter activity, and HBXIP overexpression or silencing did not alter mutation promoter activity (Fig. S4I and S4J). Two Sp1 siRNAs were designed, and the interference efficiency of the siRNAs is shown in Fig. S4K. The same results were found when Sp1 was knocked down by two Sp1 siRNAs in MCF-7-OE-HBXIP and SKBR3-OE-HBXIP cells (Fig. 4J and Fig. S4L). In addition, ChIP confirmed that knocking down of Sp1 by siRNAs triggered weaker affinity between HBXIP and the PRKCB promoter (Fig. 4K and Fig. S4M). Furthermore, in Sp1-silenced MCF-7 cells, the overexpression of HBXIP did not upregulate the protein level of PKCβII (Fig. 4L). Taken together, HBXIP stimulates PRKCB transcription and expression by coactivating the transcription factor Sp1.
3.5. The kinase activity and cytoplasmic localization of PKCβII can be enhanced by HBXIP
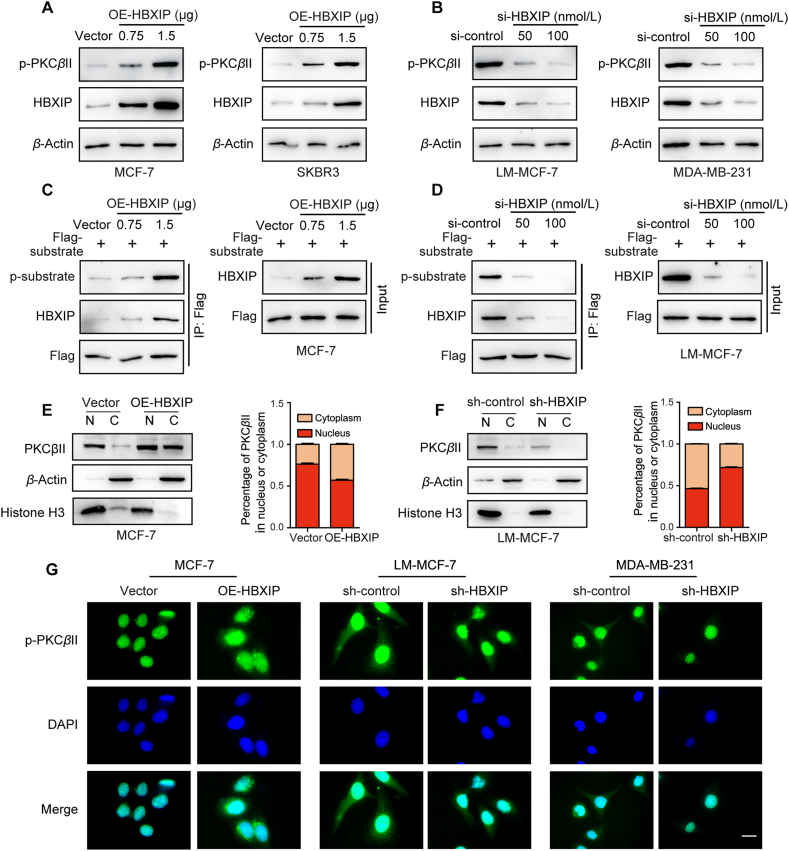
The kinase activity of PKCβII also affects phosphorylation regulation and the phosphorylation level at S660 of PKCβII could represent the activity of PKCβII. The phosphorylation level was dose-dependently upregulated when HBXIP was overexpressed and vice versa (Fig. 5A and B, and Supporting Information Fig. S5A–S5D). Next, we constructed the PKCβII specific substrate expression plasmid, and the in vivo phosphorylation assay was performed in MCF-7 and LM-MCF-7 breast cancer cells (Fig. 5C and D). We found that the phosphorylation level of the substrate was upregulated or decreased by HBXIP overexpression or silencing in breast cancer cell lines. These results reveal that HBXIP stimulates the PKCβII phosphorylation and self-activation.
Figure 5.
The kinase activity and cytoplasmic localization of PKCβII can be enhanced by HBXIP. (A) WB analysis demonstrated the levels of phosphorylated PKCβII and HBXIP incubated with anti-S660-phosphorylation-specific PKCβII or anti-HBXIP antibodies in MCF-7 and SKBR3 cells transiently transfected with pcDNA-HBXIP and pcDNA-vector in a dose-dependent manner. (B) WB analysis demonstrated the levels of phosphorylated PKCβII and HBXIP incubated with anti-S660-phosphorylation-specific PKCβII or anti-HBXIP antibodies in LM-MCF-7 and MDA-MB-231 cells transiently transfected with si-HBXIP and si-control in a dose-dependent manner. (C) Co-IP analysis of the PKCβII specific substrate phosphorylation level. pCMV-PKCβII-substrate together with pcDNA-HBXIP or pcDNA-vector were transiently transfected into MCF-7 cells. (D) Co-IP analysis of the PKCβII specific substrate phosphorylation level. pCMV-PKCβII-substrate together with si-HBXIP or si-control were transiently transfected into LM-MCF-7 cells. (E, F) Nuclear-cytoplasmic translocation of PKCβII was determined by WB analysis after isolation of nuclear and cytosolic proteins from the indicated stable cells. Separation of the nucleus (N) and the cytoplasm (C) was marked by histone-3 and β-actin. (G) The spatial distribution patterns of p-PKCβII (green) and nuclei (DAPI) were detected in the indicated stable cells by IF. Scale bar, 20 μm. Three experiments with consistent results tendency were repeated.
Previous studies have shown that different breast cancer subtypes have different subcellular localizations of PKCβII44. Interestingly, a nucleoplasm separation assay showed that HBXIP promoted the cytoplasmic localization of PKCβII (Fig. 5E and F). In keeping with this finding, we visualized the localization of active PKCβII by IF. Activated PKCβII had more cytoplasmic localization in cells overexpressing HBXIP than in HBXIP knockdown cells (Fig. 5G). Overall, these data support that HBXIP enhances PKCβII kinase activity by activating the phosphorylation level at S660 and cytoplasmic localization.
3.6. HBXIP/PKCβII/NMHC-IIA axis increases the disassembly of myosin-IIA filaments to promote breast cancer migration in vivo and in vitro
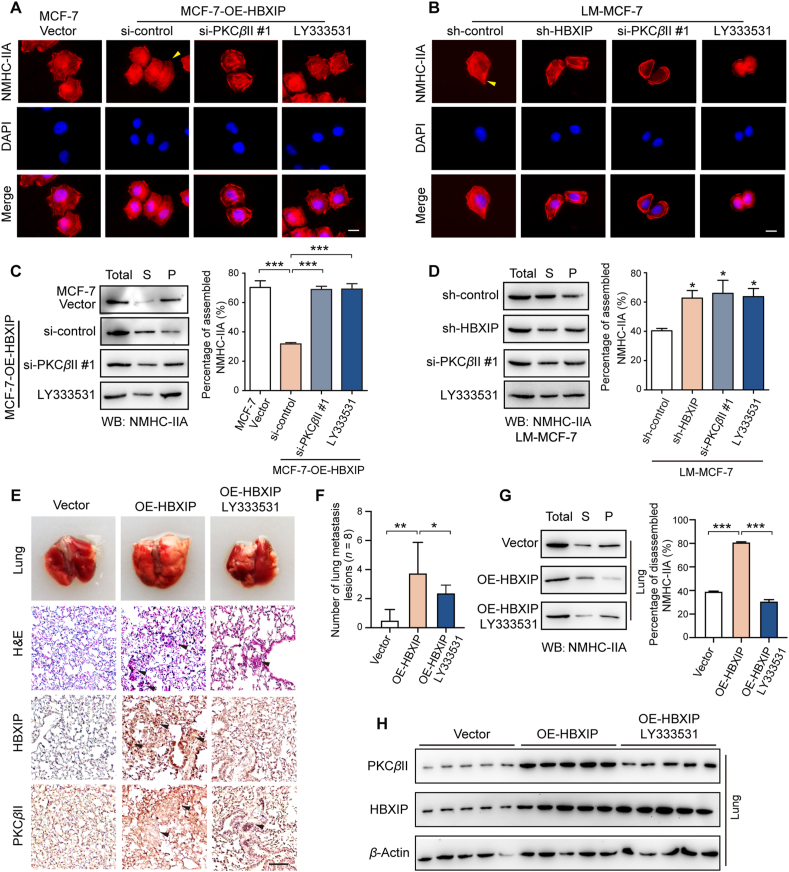
To verify that the HBXIP/PKCβII/NMHC-IIA axis is critical to the regulation of myosin-IIA filament disassembly, an IF was utilized in MCF-7 cell lines and MCF-7-OE-HBXIP cell lines, respectively transfected with si-control, si-PKCβII and LY333531, as well as LM-MCF-7-sh-HBXIP cell lines and LM-MCF-7 cell lines expressing si-control, si-PKCβII and LY333531 (Fig. 6A and B). As predicted, silencing PKCβII or inhibiting PKCβII kinase activity reversed the elongated and highly multipolar morphology with filopodia phenotypic change driven by HBXIP. In LM-MCF-7 cells, silencing HBXIP or PKCβII caused the cell fibers to assemble and lose the highly metastatic phenotype compared to the control group. Then, a Triton X-insoluble cytoskeletal ghost assay showed that the inhibition of PKCβII via siRNA or inhibitor LY333531 could rescue the amount of NMHC-IIA in the sediment (Fig. 6C and D). The transfection efficiency was verified, as shown in Supporting Information Fig. S6A and S6B. To explore the biological significance of the HBXIP/PKCβII/NMHC-IIA axis in breast cancer cell migration, Trans-well assays and would healing assays proved that silencing or inhibition of PKCβII in MCF-7-OE-HBXIP or LM-MCF-7 cell lines decreased the ability of cell migration (Fig. S6C–S6F). In vivo metastasis animal experiments were performed in female BALB/c nude mice. MCF-7 cells or MCF-7-OE-HBXIP cells were transplanted into mice by tail-vein injection (n = 8). Oral gavages of saline or LY333531 (25 mg/kg in saline) commenced daily in MCF-OE-HBXIP group at 10 days after injection. Mice were culled for 55 days after intravenous tail-vein injection. The image and H&E staining of lung metastasis nodes indicated that the overexpression of HBXIP increased the formation of metastasis nodes, while the inhibition of PKCβII activity by LY333531 reversed the HBXIP-promoted metastasis (Fig. 6E and F). Triton X-insoluble cytoskeletal ghost assay exhibited that the HBXIP-mediated myosin-IIA disassembly was reversed by the inhibition of PKCβII (Fig. 6G). Meanwhile, the expression of HBXIP and PKCβII in lung metastasis tumor were confirmed by IHC staining and Western blotting assays, which indicated the success of treatment (Fig. 6E and H). Taken together, myosin-IIA disassembly increased by the HBXIP/PKCβII/NMHC-IIA axis contributes to the migration of breast cancer cells.
Figure 6.
HBXIP/PKCβII/NMHC-IIA axis increases the disassembly of myosin-IIA filaments to promote breast cancer cell migration in vivo and in vitro. (A, B) The spatial distribution patterns of NMHC-IIA (red) and nuclei (DAPI) were detected in the indicated stable cells transiently treated with si-PKCβII or 10 μmol/L LY333531 by IF. The elongated leading edges are highlighted with yellow arrows. Scale bar, 20 μm. (C, D) WB analysis showed Triton X-insoluble fractions of the stable cells transiently treated with si-PKCβII or 10 μmol/L LY333531 (left panel) and quantification of the relation to the total lysate group (right panel). Total, total lysis; S, supernatant; P, pellet. (E) H&E and IHC staining of metastatic foci in lung paraffin sections from tail vein-injected BALB/c nude mice (n = 8) with MCF-7 or MCF-7-OE-HBXIP cells for 55 days treated with saline or LY333531. Black arrows indicate metastatic foci. Scale bar, 50 μm. (F) The quantification of metastatic foci in mouse lung. 5 different fields were selected from H&E staining slides. (G) WB analysis demonstrated Triton X-insoluble fractions of metastatic foci from the lungs (left panel) and quantification of the relation to the total lysate group (right panel). (H) WB analysis exhibited the expression of PKCβII and HBXIP in metastatic foci from the lungs. Columns with error bars symbolize the average of three independent replicates ±SD. Three experiments with consistent results tendency were repeated by two-tailed Student's t test. ∗∗∗P < 0.001; ∗∗P < 0.01; ∗P < 0.05.
3.7. Bezafibrate suppresses breast cancer metastasis by inhibiting the PKCβII/NMHC-IIA axis
After identifying the necessity of phosphorylated-NMHC-IIA and HBXIP/PKCβII/NMHC-IIA axis in breast cancer metastasis, we tried to discovery a potential target drug to inhibit the axis and myosin-IIA fiber disassembly. Therefore, we selected several classic drugs probably targeting the HBXIP/PKCβII/NMHC-IIA axis. The classic antiestrogen drug tamoxifen can inhibit the expression of ER-α-responsive genes by antagonizing estrogen by interacting with ER-α and recruiting corepressors45. However, breast cancer growth inhibition mediated by high concentrations of Tamoxifen may act independent of ERs by inhibiting PKC activity46. Accumulating studies uncover that the antitype 2 diabetic drug, Metformin can exert suppressor effects on various cancers47. AMPK activated by Metformin induces atypical PKC-ι/λ activation48. To test the efficiency of Tamoxifen and Metformin on the HBXIP/PKCβII/NMHC-IIA axis in breast cancer cells, we treated cells with metformin and tamoxifen. We found that treatment with metformin or tamoxifen did not dose-dependently affect the expression of HBXIP or PKCβII in MDA-MB-231 cells (Supporting Information Fig. S7A). The Co-IP assay showed that metformin did not affect the binding between NMHC-IIA and HBXIP or PKCβII (Fig. S7B). These results rule out that metformin or tamoxifen could not regulate the HBXIP/PKCβII/NMHC-IIA axis and myosin-IIA fiber assembly.
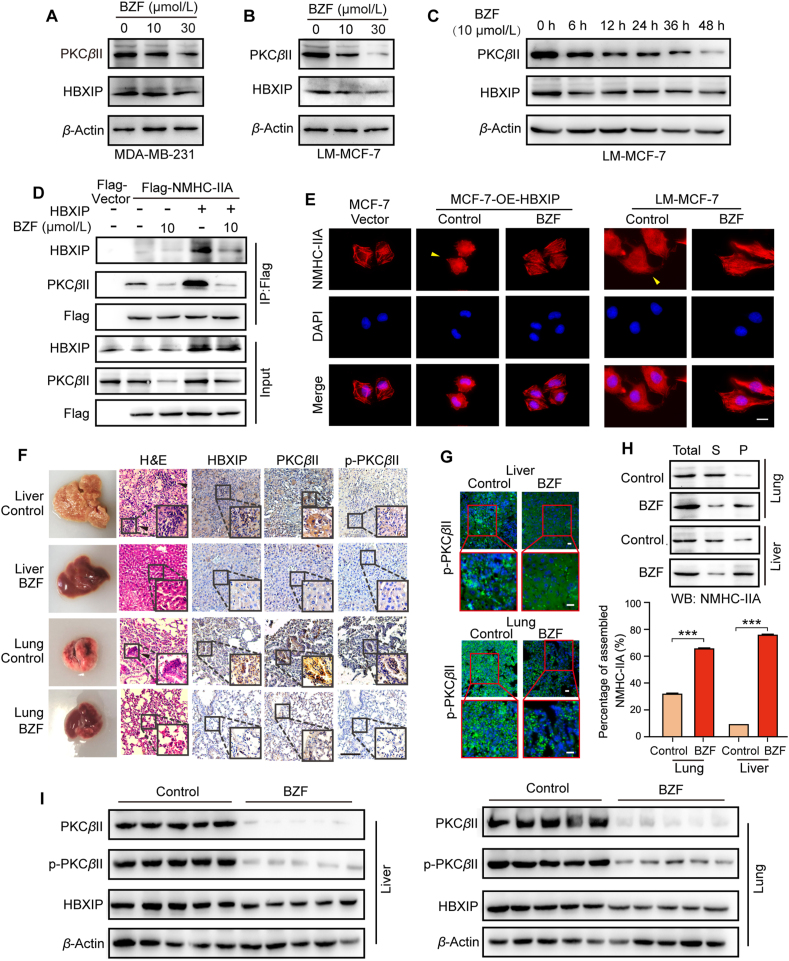
Also, the available small-molecule inhibitors of PKCβII, such as LY3335311 (also called ruboxistaurin), always have more than one target, off-target and side effects frequently occur. Thus, a novel drug inhibiting the HBXIP/PKCβII/NMHC-IIA axis is needed. Bezafibrate (BZF) is the first pan-PPAR activator used in the treatment of hypertriglyceridemia in human patients almost without side effects. It has been reported that some PPAR pathway genes contribute to pancreatic cancer susceptibility, particularly MED1, PRKCA, and PRKCB, which implied the anticancer function of fibrates49. Recently, fibrates have shown that enhanced antitumor immunity during PD-1 blockade50. However, it is still unclear whether they inhibit tumor metastasis. Strikingly, we found that BZF dose-dependently downregulated the expression of PKCβII but not HBXIP in MDA-MB-231 and LM-MCF-7 cells (Fig. 7A and B, and Fig. S7C and S7D). Here, we first report that BZF is involved in the HBXIP/PKCβII/NMHC-IIA axis in breast cancer migration. We further examined the effects of BZF at various time points. Our data show that the expression of PKCβII was time-dependently decreased when the LM-MCF-7 and MCF-7 cells were treated with 10 μmol/L BZF (Fig. 7C, Fig. S7E and S7F). Co-IP assay showed that BZF indeed affected the binding between NMHC-IIA and HBXIP or PKCβII (Fig. 7D). In support of these findings, IF and Triton X-insoluble cytoskeletal ghost assay demonstrated that BZF inhibited the disassembly of myosin-IIA caused by HBXIP overexpression (Fig. 7E, and Fig. S7G and S7H). Moreover, a wound healing assay proved that treatment with BZF decreased the ability of cell migration (Fig. S7I). The results above indicate that BZF represses the PKCβII/NMHC-IIA axis and myosin-IIA disassembly by inhibiting PKCβII expression to block the migration in breast cancer cells.
Figure 7.
Bezafibrate suppresses breast cancer metastasis by inhibiting PKCβII/NMHC-IIA axis. (A, B) WB analysis exhibited the expression of PKCβII and HBXIP in MDA-MB-231 cells or LM-MCF-7 cells dose-dependently treated with BZF for 48 h. (C) WB analysis exhibited the expression of PKCβII and HBXIP in LM-MCF-7 cells time-dependently treated with 10 μmol/L BZF. (D) Co-IP analysis of exogenous Flag-HBXIP. pENTER or pENTER-NMHC-IIA was transiently transfected into HEK-293T cells along with 10 μmol/L BZF. (E) The spatial distribution patterns of NMHC-IIA (red) and nuclei (DAPI) were detected in the indicated stable cells treated with 10 μmol/L BZF for 48 h. The elongated leading edges were highlighted with yellow arrows. Scale bar, 20 μm. (F) H&E and IHC staining of metastatic foci in liver and lung paraffin sections from BALB/c nude mice (n = 5) tail vein injected with LM-MCF-7 cells for 55 days treated with saline or BZF. Metastatic foci were highlighted with black arrows. Scale bar, 50 μm. (G) The spatial distribution patterns of p-PKCβII (green) and nuclear (DAPI) were detected in the metastatic foci frozen sections from the livers and lungs. Scale bar, 20 μm. (H) WB analysis demonstrated Triton X-insoluble fractions of metastatic foci from the lungs and livers. Total, total lysis; S, supernatant; P, pellet. (I) WB analysis exhibited the expression of PKCβII and HBXIP in metastatic foci from the livers and lungs. Columns with error bars symbolize the average of three independent replicates ±SD. Three experiments with consistent results tendency were repeated by two-tailed Student's t test. ∗∗∗P < 0.001.
We further constructed the animal model of BALB/c nude mice tail vein injected with LM-MCF-7. Interestingly, BZF treatment decreased the formation of metastatic nudes in lung and liver in mice (Fig. 7F, and Fig. S7J). IHC showed that HBXIP, PKCβII and phosphorylated PKCβII were positively stained in metastatic foci, and the staining intensity was weaker in the BZF-treated group (Fig. 7F). Next, IF demonstrated the expression and spatial distribution patterns of phosphorylated PKCβII (green). Consistent with the previous data, BZF treatment attenuated the distribution of phosphorylated PKCβII-positive cells in lung and liver metastatic foci (Fig. 7G). A Triton X-insoluble cytoskeletal ghost assay verified that the disassembled state of metastatic foci was blocked after BZF treatment (Fig. 7H). Meanwhile, Western blotting assays displayed similar protein expression changes as above in metastatic foci (Fig. 7I). Thus, we first observe that BZF suppresses breast cancer migration and metastasis in vivo and in vitro by inhibiting the PKCβII/NMHC-IIA axis and myosin-IIA filament disassembly.
3.8. Inhibition of PKCβII via bezafibrate or overexpression of dominant-negative mutant of PKCβII is negatively associated with cancer pathways
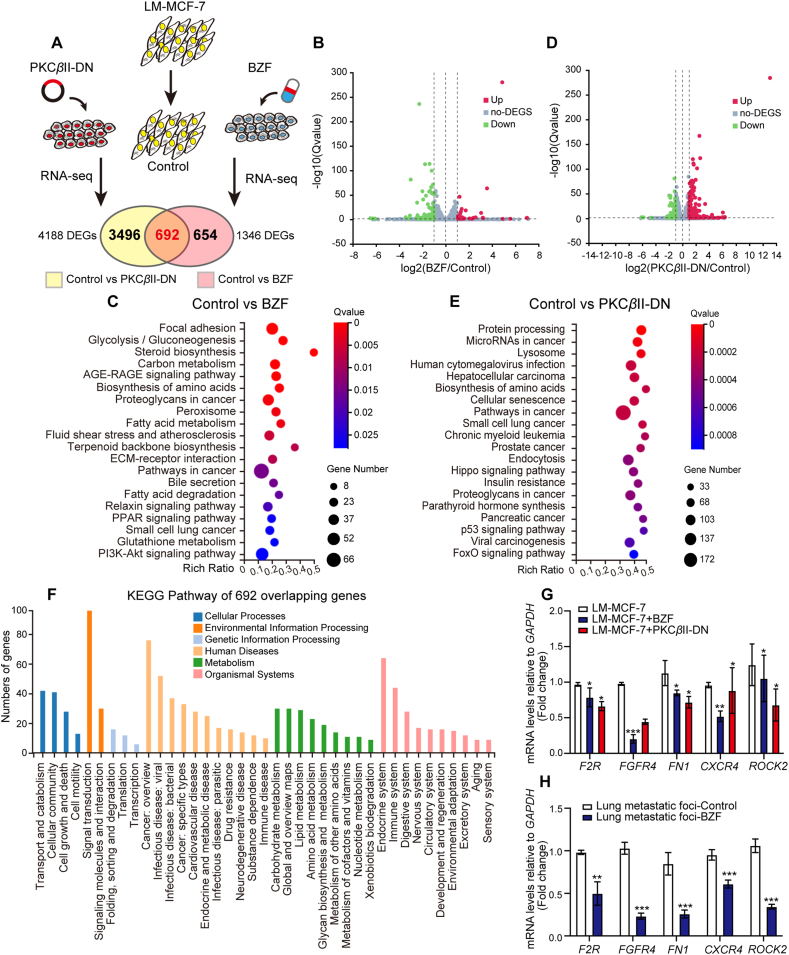
To clearly define the positive character of PKCβII in carcinoma and its downstream genes, we completed genome-wide RNA-seq to sequence the transcription of 432,117 genes in LM-MCF-7 cells after treatment with 10 μmol/L BZF or overexpression of the dominant negative plasmid of PKCβII (pcDNA-PKCβII-DN) for 48 h to inhibit PKCβII activity (Fig. 8A). We defined 4188 DEGs in the LM-MCF-7 control group compared with the PKCβII-DN expressing group and 1346 DEGs in the LM-MCF-7 control group compared with the BZF group. The volcano plot following KEGG pathway analysis (https://www.genome.jp/kegg/) summarized the RNA sequencing DEG results among the two kinds of treatments, which showed that the DEGs upon PKCβII inhibition were subjected to various biological processes, such as pathways in cancer, biosynthesis of amino acids and proteoglycans in cancer (Fig. 8B–E). Subsequently, we overlapped the two groups of DEGs and performed KEGG pathway analysis of 692 overlapping genes, showing the biological and physiological functions of these genes (Fig. 8F). Ultimately, RT-qPCR experiments were performed to identify the mRNA levels of F2R, FGFR4, FN1, CXCR4 and ROCK2, which were the top different oncogenes in 692 overlapping DEGs, in LM-MCF-7 cell lines or metastatic foci in the lungs (Fig. 8G and H). Conclusively, our work validates that BZF attenuates the activation of PKCβII, which is involved in breast cancer myosin-IIA fiber assembly and weakens breast tumor cell migration. This work also strengthens the tumor promoter status of HBXIP, and its downstream PKCβII signaling may be an effective therapeutic option.
Figure 8.
Inhibition of PKCβII via bezafibrate or overexpression of a dominant-negative mutant of PKCβII is negatively associated with cancer pathways. (A) The experimental scenario of the RNA sequencing of the LM-MCF-7 cell line. The 107 cells were treated with plasmid pcDNA-PKCβII-DN (10 μg) or BZF (10 μmol/L) for 48 h. (B, C) Volcano plot and KEGG pathway analysis of DEGs between the control group and BZF-treated group. (D, E) Volcano plot and KEGG pathway analysis of DEGs between the control group and PKCβII-DN-expressing group. (F) KEGG pathway analysis of 692 overlapping DEGs. (G) Fold change in mRNA levels of 5 oncogenes determined by RT-qPCR assays. LM-MCF-7 cells were treated with 10 μmol/L BZF or transfected with the plasmid pcDNA-PKCβII-DN. (H) Fold changes in mRNA levels of 5 oncogenes in lung metastatic foci from BALB/c nude mice performed by RT-qPCR assays. Columns with error bars symbolize the average of three independent replicates ±SD. Three experiments with consistent results tendency were repeated by two-tailed Student's t test. ∗∗∗P < 0.001; ∗∗P < 0.01; ∗P < 0.05.
4. Discussion
Over the past decades, the significant decrease in the fatality rate of breast cancer patients cannot be divorced from the early detection, as well as the advancement of more curative effects on adjuvant and targeted therapies. Nevertheless, it is unfortunate that the principal causes of treatment failure, local recurrence, and distant metastasis puzzle numerous patients with breast cancer after radical mastectomy and therapies51,52. Thus, there is a crisis looming to explore the in-depth molecular mechanism of metastatic tumors and efficacious drug targets for therapeutic intervention dealing with breast cancer.
During the progression of tumor migration and invasion, the contraction and spreading of tumor cells are important. Migrating cells often display invasive morphologies through the formation of protrusions53, while the fundamental regulatory mechanism remains unclear. As a critical ingredient of actomyosin fiber, myosin-IIA is indispensable for transforming chemical energy into kinesthetic energy and protrusion formation, which is necessary for cellular functions involving cell division, adhesion and migration. During the progression of myosin-IIA filament assembly, the myosin-IIA motors slide along actin filaments and produce the contractile force, which blocks the spreading of cells. Here, we found that highly metastatic cells endured a significantly different actomyosin fiber structure and a mesenchymal appearance with filopodia-rich elongated morphology. The disassembly of myosin-IIA in the body of highly metastatic cells was accompanied by the assembly of actin filaments on the filopodia edge. We could make a metaphor that a cell with assembled myosin-IIA bundles is like a car without releasing the parking brake. The progress of filopodia formation is like pressing the accelerator of the car. Only if the two parts cooperate with each other well, can the car (cell) obtain the ability to move forward. There must be a controller handling the progress of easing off the brake and hitting the gas to stimulate migration. Combined with our previous findings15, we found that HBXIP restructures the dynamic balance of the actomyosin system by promoting the disassembly of myosin-IIA (releasing the brake) and releasing actin fibers (pressing the accelerator), which form filopodia edges.
Non-muscle myosin heavy chain IIA (NMHC-IIA) contributes to cell metastatic behavior by reorganizing the cytoskeleton and cell contractility via myosin-IIA disassembly. Recently, the functions of NMHC-IIA in tumorigenesis and drug resistance have been unveiled. Fang's group unveiled that NMHC-IIA promoted GSK3β ubiquitination reversing FOXO1-mediated NPC invasion, metastasis and stemness. Activation of the NMHC-IIA/TRAF6/GSK3β axis increased the expression of its downstream gene β-catenin expression and its nuclear localization inducing EMT13,54. Natural-derived small-molecule J13 restrained the interaction between NMHC-IIA and actin molecular motor via prompting heat-shock protein A9 (HSPA9) attached to NMHC-IIA, resulting in excessive mitochondrial fission4. NMHC-IIA directly targeted by ruscogenin, an effective steroidal sapogenin of Radix Ophiopogon Japonicus, and mediated Toll-like receptor 4 (TLR4) interactions contributing to the development of acute lung injury55. Silencing of NMHC-IIA serves as a substitute clinical pattern to restrict cancer growth, invasion, migration and alleviate sorafenib resistance in HCC12. These articles offer a different perspective about the oncogene NMHC-IIA, which is contradictory to a previous study showing that NMHC-IIA serves as a tumor suppressor by modulating p53 stability in SCC56. It is ambiguous that NMHC-IIA attaches great importance to tumorigenesis and cancer development, but no study has focused on the function of myosin filaments in migration. Our results unveil the importance of myosin-IIA filament polymerization in releasing actin filaments and forming filopodia edges.
It has been reported that myosin-IIA disassembly is mediated by interactions between NMHC-IIA and regulatory proteins, such as lethal giant larvae, myosin binding protein H, S100A4 and S100P25,35,36,57. Our mass spectra results unveiled that HBXIP acted as a potential interactor of NMHC-IIA. The ACD, a prominent region in the NMHC-IIA C-terminus for myosin-II filament assembly, is near the coiled coil tail58. Apart from ACD, the tailpiece near the C-terminus is also pivotal for filament assembly, which is a region subjected to the NMHC-IIA isoform59. Our data indicate that HBXIP interacts with the extended-ACD region of the NMHC-IIA C-terminus and blocks the assembly of myosin-IIA stacks.
There are accumulating articles reporting that heavy chain phosphorylation mediates oligomerization and assembly of myosin-II filaments. Near the heavy chain C-terminal end, most myosin-II heavy chain phosphorylation sites remain in the tailpiece and coiled regions. Additionally, the HBXIP-binding domain of NMHC-IIA overlaps with the phosphorylation site-rich domain. Previous work has shown that phosphorylation of Ser 1943 of NMHC-IIA modulates assembly and localization of myosin-IIA filaments in vivo60. Moreover, in mammary epithelial cells, TGF-β-mediated epithelial–mesenchymal transition upregulates NMHC-IIA S1943 phosphorylation, and S1943A robustly decreases the invasion of breast tumor cells61. However, we found that HBXIP engages in the upregulation of NMHC-IIA phosphorylation at S1916, not S1943. We uncovered that the phosphorylation mimetic mutant S1916D of NMHC-IIA had a higher affinity for HBXIP. These data elucidate the underlying mechanism by which the interaction of HBXIP and NMHC-IIA mediated by S1916 phosphorylation affects the formation of myosin-IIA fibers and cell migration.
How might HBXIP regulate phosphorylation of NMHC-IIA? According to our data, protein kinase PKCβII was recruited by HBXIP to catalyze the phosphorylation of NMHC-IIA. Interestingly, we determined that the transcription factor coactivator HBXIP transcriptionally activates PKCβII. In addition, HBXIP has been confirmed to coactivate several transcription factors, such as E2F1, LXR and c-Myb, governing tumor-related genes transcription to accelerate the growth and metastasis of breast cancer16,62,63. Given that PRKCB promoter activity is subject to the transcription factor Sp143, a mutation in the two Sp1 binding sites was constructed and lost the response to HBXIP modification. These findings indicate that HBXIP coactivates with Sp1, promoting the transcription of PRKCB. In addition, silencing HBXIP or inhibiting the activity of protein kinase PKCβII changed cell mesenchymal-like features and myosin-IIA filament formation. This supports an interesting model wherein HBXIP hijacks the protein kinase PKCβII to phosphorylate NMHC-IIA and disassemble myosin-IIA filaments, resulting in the promotion of breast cancer cell migration.
To further explore the clinical practicability of the HBXIP/PKCβII/NMHC-IIA axis, we selected several drugs and luckily found that the pan-PPAR activator BZF robustly decreased the expression of PKCβII, as well as the binding between HBXIP and NMHC-IIA. The PPAR pathway genes contribute to pancreatic cancer susceptibility, particularly MED1, PRKCA, and PRKCB49. According to our results, treatment with the antihyperlipidemic drug BZF in breast cancer cells negatively regulated the disassembly of myosin-IIA. Moreover, our animal experiment first revealed that BZF treatment blocked LM-MCF-7 cells metastasis to the lung or liver. Our genome-wide RNA-seq results revealed a high association between BZF treatment and the suppression of cancer development. The differentially expressed genes of BZF treatment and inhibition of PKCβII are positively related to cell motility and cancer development. These discoveries demonstrate the PKCβII as a future anti-cancerous target, further contributing to the antimetastatic activity of BZF.
5. Conclusions
We identify a brand-new mechanism of myosin-II filament assembly regulation in breast cancer metastasis. We unveil that the convergence of the oncoprotein HBXIP and cytoskeletal protein NMHC-IIA in modulating myosin-IIA filament assembly to promote breast cancer migration. Additionally, HBXIP stimulates NMHC-IIA phosphorylation by recruiting kinase PKCβII. Meanwhile, hyperphosphorylation of NMHC-IIA at S1916 fastens its interaction with HBXIP. The antihypertriglyceridemic drug BZF inhibits the PKCβII/NMHC-IIA axis and blocks breast cancer migration in vivo and in vitro. These data shed light on the important role of the HBXIP/PKCβII/NMHC-IIA axis in promoting breast cancer metastasis and indicate that the NMHC-IIA phosphorylation-mediated disassemble state of myosin-IIA filaments is a potential therapeutic target for human breast cancer.
Acknowledgments
This study was supported by the grants from National Natural Science Foundation of China (82072929, 82072943, and 31870752, China). We thank Prof. Jianxin Gu and Prof. Weiyi Fang for providing plasmids.
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2022.11.025.
Contributor Information
Weiying Zhang, Email: zhwybao@nankai.edu.cn.
Lihong Ye, Email: yelihong@nankai.edu.cn.
Author contributions
Weiying Zhang and Lihong Ye conceived the project, designed the research, and edited the manuscript. Lu Zhang planned the experiments. Lu Zhang, Xiaolei Zhou, and Bowen Liu performed the experiments, analyzed the data, and prepared the manuscript. Lu Zhang, Xuhe Shi, Xianmeng Li, Feifei Xu, Xueli Fu, Xue Wang, Huimin Sun, Qianqian Li, and Kai Ye participated in the experiments. All authors contributed with productive discussions and knowledge to the final version of this manuscript.
Conflicts of interest
The authors declared no conflict of interest.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Kawska A., Carvalho K., Manzi J., Boujemaa-Paterski R., Blanchoin L., Martiel J.L., et al. How actin network dynamics control the onset of actin-based motility. Proc Natl Acad Sci U S A. 2012;109:14440–14445. doi: 10.1073/pnas.1117096109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bisaria A., Hayer A., Garbett D., Cohen D., Meyer T. Membrane-proximal F-actin restricts local membrane protrusions and directs cell migration. Science. 2020;368:1205–1210. doi: 10.1126/science.aay7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vicente-Manzanares M., Ma X., Adelstein R.S., Horwitz A.R. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol. 2009;10:778–790. doi: 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qian Y., Zhao M., Han Q., Wang J., Liao L., Yang H., et al. Pharmacologically targeting molecular motor promotes mitochondrial fission for anti-cancer. Acta Pharm Sin B. 2021;11:1853–1866. doi: 10.1016/j.apsb.2021.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakasawa T., Takahashi M., Matsuzawa F., Aikawa S., Togashi Y., Saitoh T., et al. Critical regions for assembly of vertebrate nonmuscle myosin II. Biochemistry. 2005;44:174–183. doi: 10.1021/bi048807h. [DOI] [PubMed] [Google Scholar]
- 6.Betapudi V., Licate L.S., Egelhoff T.T. Distinct roles of nonmuscle myosin II isoforms in the regulation of MDA-MB-231 breast cancer cell spreading and migration. Cancer Res. 2006;66:4725–4733. doi: 10.1158/0008-5472.CAN-05-4236. [DOI] [PubMed] [Google Scholar]
- 7.Liu D., Zhang L., Shen Z., Tan F., Hu Y., Yu J., et al. Clinicopathological significance of NMIIA overexpression in human gastric cancer. Int J Mol Sci. 2012;13:15291–15304. doi: 10.3390/ijms131115291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang B., Qi X., Liu J., Zhou R., Lin C., Shangguan J., et al. MYH9 promotes growth and metastasis via activation of MAPK/AKT signaling in colorectal cancer. J Cancer. 2019;10:874–884. doi: 10.7150/jca.27635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xia Z.K., Yuan Y.C., Yin N., Yin B.L., Tan Z.P., Hu Y.R. Nonmuscle myosin IIA is associated with poor prognosis of esophageal squamous cancer. Dis Esophagus. 2012;25:427–436. doi: 10.1111/j.1442-2050.2011.01261.x. [DOI] [PubMed] [Google Scholar]
- 10.Xiong D., Ye Y.L., Chen M.K., Qin Z.K., Li M.Z., Zhang H., et al. Non-muscle myosin II is an independent predictor of overall survival for cystectomy candidates with early-stage bladder cancer. Oncol Rep. 2012;28:1625–1632. doi: 10.3892/or.2012.1965. [DOI] [PubMed] [Google Scholar]
- 11.Orgaz J.L., Crosas-Molist E., Sadok A., Perdrix-Rosell A., Maiques O., Rodriguez-Hernandez I., et al. Myosin II reactivation and cytoskeletal remodeling as a hallmark and a vulnerability in melanoma therapy resistance. Cancer Cell. 2020;37:85–103. doi: 10.1016/j.ccell.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin X., Li A.M., Li Y.H., Luo R.C., Zou Y.J., Liu Y.Y., et al. Silencing MYH9 blocks HBx-induced GSK3β ubiquitination and degradation to inhibit tumor stemness in hepatocellular carcinoma. Signal Transduct Targeted Ther. 2020;5:13. doi: 10.1038/s41392-020-0111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y., Jiang Q., Liu X., Lin X., Tang Z., Liu C., et al. Cinobufotalin powerfully reversed EBV-miR-BART22-induced cisplatin resistance via stimulating MAP2K4 to antagonize non-muscle myosin heavy chain IIA/glycogen synthase 3β/β-catenin signaling pathway. EBioMedicine. 2019;48:386–404. doi: 10.1016/j.ebiom.2019.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L., Fang R., Liu B., Shi H., Wang Y., Zhang W., et al. Deacetylation of tumor-suppressor MST1 in Hippo pathway induces its degradation through HBXIP-elevated HDAC6 in promotion of breast cancer growth. Oncogene. 2016;35:4048–4057. doi: 10.1038/onc.2015.476. [DOI] [PubMed] [Google Scholar]
- 15.Li Y., Zhang Z., Zhou X., Li L., Liu Q., Wang Z., et al. The oncoprotein HBXIP enhances migration of breast cancer cells through increasing filopodia formation involving MEKK2/ERK1/2/Capn4 signaling. Cancer Lett. 2014;355:288–296. doi: 10.1016/j.canlet.2014.09.047. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y., Li H., Zhang Y., Li L., Fang R., Li Y., et al. Oncoprotein HBXIP modulates abnormal lipid metabolism and growth of breast cancer cells by activating the LXRs/SREBP-1c/FAS signaling cascade. Cancer Res. 2016;76:4696–4707. doi: 10.1158/0008-5472.CAN-15-1734. [DOI] [PubMed] [Google Scholar]
- 17.Bar-Peled L., Schweitzer L.D., Zoncu R., Sabatini D.M. Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell. 2012;150:1196–1208. doi: 10.1016/j.cell.2012.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu F., You X., Liu F., Shen X., Yao Y., Ye L., et al. The oncoprotein HBXIP up-regulates Skp2 via activating transcription factor E2F1 to promote proliferation of breast cancer cells. Cancer Lett. 2013;333:124–132. doi: 10.1016/j.canlet.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 19.Yue L., Li L., Liu F., Hu N., Zhang W., Bai X., et al. The oncoprotein HBXIP activates transcriptional coregulatory protein LMO4 via Sp1 to promote proliferation of breast cancer cells. Carcinogenesis. 2013;34:927–935. doi: 10.1093/carcin/bgs399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y., Cai X., Zhang S., Cui M., Liu F., Sun B., et al. HBXIP up-regulates ACSL1 through activating transcriptional factor Sp1 in breast cancer. Biochem Biophys Res Commun. 2017;484:565–571. doi: 10.1016/j.bbrc.2017.01.126. [DOI] [PubMed] [Google Scholar]
- 21.Fang R., Xu F., Shi H., Wu Y., Cao C., Li H., et al. LAMTOR5 raises abnormal initiation of O-glycosylation in breast cancer metastasis via modulating GALNT1 activity. Oncogene. 2020;39:2290–2304. doi: 10.1038/s41388-019-1146-2. [DOI] [PubMed] [Google Scholar]
- 22.Liu B., Wang T., Wang H., Zhang L., Xu F., Fang R., et al. Oncoprotein HBXIP enhances HOXB13 acetylation and co-activates HOXB13 to confer tamoxifen resistance in breast cancer. J Hematol Oncol. 2018;11:26. doi: 10.1186/s13045-018-0577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Y., Wang X., Xu F., Zhang L., Wang T., Fu X., et al. The regulation of acetylation and stability of HMGA2 via the HBXIP-activated Akt–PCAF pathway in promotion of esophageal squamous cell carcinoma growth. Nucleic Acids Res. 2020;48:4858–4876. doi: 10.1093/nar/gkaa232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pasapera A.M., Plotnikov S.V., Fischer R.S., Case L.B., Egelhoff T.T., Waterman C.M. Rac1-dependent phosphorylation and focal adhesion recruitment of myosin IIA regulates migration and mechanosensing. Curr Biol. 2015;25:175–186. doi: 10.1016/j.cub.2014.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hosono Y., Usukura J., Yamaguchi T., Yanagisawa K., Suzuki M., Takahashi T. MYBPH inhibits NM IIA assembly via direct interaction with NMHC IIA and reduces cell motility. Biochem Biophys Res Commun. 2012;428:173–178. doi: 10.1016/j.bbrc.2012.10.036. [DOI] [PubMed] [Google Scholar]
- 26.Li W., Wu J., Kim S.Y., Zhao M., Hearn S.A., Zhang M.Q., et al. Chd5 orchestrates chromatin remodelling during sperm development. Nat Commun. 2014;5:3812. doi: 10.1038/ncomms4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Breckenridge M.T., Dulyaninova N.G., Egelhoff T.T. Multiple regulatory steps control mammalian nonmuscle myosin II assembly in live cells. Mol Biol Cell. 2009;20:338–347. doi: 10.1091/mbc.E08-04-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang S., Zhang J., An Y., Zeng X., Qin Z., Zhao Y., et al. Multi-omics approaches identify and as candidate autophagic regulators and druggable targets in invasive breast carcinoma. Acta Pharm Sin B. 2021;11:1227–1245. doi: 10.1016/j.apsb.2020.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Svitkina T. The actin cytoskeleton and actin-based motility. Cold Spring Harbor Perspect Biol. 2018;10 doi: 10.1101/cshperspect.a018267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pollard T.D., Cooper J.A. Actin, a central player in cell shape and movement. Science. 2009;326:1208–1212. doi: 10.1126/science.1175862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson C.A., Tsuchida M.A., Allen G.M., Barnhart E.L., Applegate K.T., Yam P.T., et al. Myosin II contributes to cell-scale actin network treadmilling through network disassembly. Nature. 2010;465:373–377. doi: 10.1038/nature08994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li H., Wang Z., Jiang M., Fang R.P., Shi H., Shen Y., et al. The oncoprotein HBXIP promotes human breast cancer growth through down-regulating p53 via miR-18b/MDM2 and pAKT/MDM2 pathways. Acta Pharmacol Sin. 2018;39:1787–1796. doi: 10.1038/s41401-018-0034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mylvaganam S., Riedl M., Vega A., Collins R.F., Jaqaman K., Grinstein S., et al. Stabilization of endothelial receptor arrays by a polarized spectrin cytoskeleton facilitates rolling and adhesion of leukocytes. Cell Rep. 2020;31 doi: 10.1016/j.celrep.2020.107798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li H., Liu Q., Wang Z., Fang R., Shen Y., Cai X., et al. The oncoprotein HBXIP modulates the feedback loop of MDM2/p53 to enhance the growth of breast cancer. J Biol Chem. 2015;290:22649–22661. doi: 10.1074/jbc.M115.658468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ford H.L., Silver D.L., Kachar B., Sellers J.R., Zain S.B. Effect of Mts1 on the structure and activity of nonmuscle myosin II. Biochemistry. 1997;36:16321–16327. doi: 10.1021/bi971182l. [DOI] [PubMed] [Google Scholar]
- 36.Du M., Wang G., Ismail T.M., Gross S., Fernig D.G., Barraclough R., et al. S100P dissociates myosin IIA filaments and focal adhesion sites to reduce cell adhesion and enhance cell migration. J Biol Chem. 2012;287:15330–15344. doi: 10.1074/jbc.M112.349787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Norwood Toro L.E., Wang Y., Condeelis J.S., Jones J.G., Backer J.M., Bresnick A.R. Myosin-IIA heavy chain phosphorylation on S1943 regulates tumor metastasis. Exp Cell Res. 2018;370:273–282. doi: 10.1016/j.yexcr.2018.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ludowyke R.I., Elgundi Z., Kranenburg T., Stehn J.R., Schmitz-Peiffer C., Hughes W.E., et al. Phosphorylation of nonmuscle myosin heavy chain IIA on Ser1917 is mediated by protein kinase C beta II and coincides with the onset of stimulated degranulation of RBL-2H3 mast cells. J Immunol. 2006;177:1492–1499. doi: 10.4049/jimmunol.177.3.1492. [DOI] [PubMed] [Google Scholar]
- 39.Kriajevska M., Bronstein I.B., Scott D.J., Tarabykina S., Fischer-Larsen M., Issinger O., et al. Metastasis-associated protein Mts1 (S100A4) inhibits CK2-mediated phosphorylation and self-assembly of the heavy chain of nonmuscle myosin. Biochim Biophys Acta. 2000;1498:252–263. doi: 10.1016/s0167-4889(00)00100-2. [DOI] [PubMed] [Google Scholar]
- 40.Li M., Jiang J., Yue L. Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J Gen Physiol. 2006;127:525–537. doi: 10.1085/jgp.200609502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parker P.J., Murray-Rust J. PKC at a glance. J Cell Sci. 2004;117:131–132. doi: 10.1242/jcs.00982. [DOI] [PubMed] [Google Scholar]
- 42.Quan T., Fisher G.J. Cloning and characterization of the human protein kinase C-eta promoter. J Biol Chem. 1999;274:28566–28574. doi: 10.1074/jbc.274.40.28566. [DOI] [PubMed] [Google Scholar]
- 43.Hagiwara K., Ito H., Murate T., Miyata Y., Ohashi H., Nagai H. PROX1 overexpression inhibits protein kinase C beta II transcription through promoter DNA methylation. Genes Chromosomes Cancer. 2012;51:1024–1036. doi: 10.1002/gcc.21985. [DOI] [PubMed] [Google Scholar]
- 44.Gökmen-Polar Y., Mehta R., Tuzmen S., Mousses S., Thorat M.A., Sanders K.L., et al. Differential subcellular expression of protein kinase C betaII in breast cancer: correlation with breast cancer subtypes. Breast Cancer Res Treat. 2010;124:327–335. doi: 10.1007/s10549-010-0733-2. [DOI] [PubMed] [Google Scholar]
- 45.Alluri P.G., Asangani I.A., Chinnaiyan A.M. BETs abet Tam-R in ER-positive breast cancer. Cell Res. 2014;24:899–900. doi: 10.1038/cr.2014.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gundimeda U., Chen Z.H., Gopalakrishna R. Tamoxifen modulates protein kinase C via oxidative stress in estrogen receptor-negative breast cancer cells. J Biol Chem. 1996;271:13504–13514. doi: 10.1074/jbc.271.23.13504. [DOI] [PubMed] [Google Scholar]
- 47.Vancura A., Bu P., Bhagwat M., Zeng J., Vancurova I. Metformin as an anticancer agent. Trends Pharmacol Sci. 2018;39:867–878. doi: 10.1016/j.tips.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He L., Sabet A., Djedjos S., Miller R., Sun X., Hussain M.A., et al. Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell. 2009;137:635–646. doi: 10.1016/j.cell.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu X., Qian D., Liu H., Abbruzzese J.L., Luo S., Walsh K.M., et al. Genetic variants of the peroxisome proliferator-activated receptor (PPAR) signaling pathway genes and risk of pancreatic cancer. Mol Carcinog. 2020;59:930–939. doi: 10.1002/mc.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chowdhury P.S., Chamoto K., Kumar A., Honjo T. PPAR-induced fatty acid oxidation in t cells increases the number of tumor-reactive CD8 T cells and facilitates anti-PD-1 therapy. Cancer Immunol Res. 2018;6:1375–1387. doi: 10.1158/2326-6066.CIR-18-0095. [DOI] [PubMed] [Google Scholar]
- 51.Guan X. Cancer metastases: challenges and opportunities. Acta Pharm Sin B. 2015;5:402–418. doi: 10.1016/j.apsb.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou M., Zuo Q., Huang Y., Li L. Immunogenic hydrogel toolkit disturbing residual tumor “seeds” and pre-metastatic “soil” for inhibition of postoperative tumor recurrence and metastasis. Acta Pharm Sin B. 2022;12:3383–3397. doi: 10.1016/j.apsb.2022.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ridley A.J., Schwartz M.A., Burridge K., Firtel R.A., Ginsberg M.H., Borisy G., et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 54.Li Y., Liu X., Lin X., Zhao M., Xiao Y., Liu C., et al. Chemical compound cinobufotalin potently induces FOXO1-stimulated cisplatin sensitivity by antagonizing its binding partner MYH9. Signal Transduct Targeted Ther. 2019;4:48. doi: 10.1038/s41392-019-0084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu Y., Yu X., Wang Y., Huang Y., Tang J., Gong S., et al. Ruscogenin alleviates LPS-triggered pulmonary endothelial barrier dysfunction through targeting NMMHC IIA to modulate TLR4 signaling. Acta Pharm Sin B. 2022;12:1198–1212. doi: 10.1016/j.apsb.2021.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schramek D., Sendoel A., Segal J.P., Beronja S., Heller E., Oristian D., et al. Direct in vivo RNAi screen unveils myosin IIa as a tumor suppressor of squamous cell carcinomas. Science. 2014;343:309–313. doi: 10.1126/science.1248627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vasioukhin V. Lethal giant puzzle of Lgl. Dev Neurosci. 2006;28:13–24. doi: 10.1159/000090749. [DOI] [PubMed] [Google Scholar]
- 58.Sohn R.L., Vikstrom K.L., Strauss M., Cohen C., Szent-Gyorgyi A.G., Leinwand L.A. A 29 residue region of the sarcomeric myosin rod is necessary for filament formation. J Mol Biol. 1997;266:317–330. doi: 10.1006/jmbi.1996.0790. [DOI] [PubMed] [Google Scholar]
- 59.Dulyaninova N.G., Bresnick A.R. The heavy chain has its day: regulation of myosin-II assembly. BioArchitecture. 2013;3:77–85. doi: 10.4161/bioa.26133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang W., Gunst S.J. Non-muscle (NM) myosin heavy chain phosphorylation regulates the formation of NM myosin filaments, adhesome assembly and smooth muscle contraction. J Physiol. 2017;595:4279–4300. doi: 10.1113/JP273906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beach J.R., Hussey G.S., Miller T.E., Chaudhury A., Patel P., Monslow J., et al. Myosin II isoform switching mediates invasiveness after TGF-β-induced epithelial–mesenchymal transition. Proc Natl Acad Sci U S A. 2011;108:17991–17996. doi: 10.1073/pnas.1106499108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu B.W., Wang T.J., Li L.L., Zhang L., Liu Y.X., Feng J.Y., et al. Oncoprotein HBXIP induces PKM2 via transcription factor E2F1 to promote cell proliferation in ER-positive breast cancer. Acta Pharmacol Sin. 2019;40:530–538. doi: 10.1038/s41401-018-0015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Y., Fang R., Cui M., Zhang W., Bai X., Wang H., et al. The oncoprotein HBXIP up-regulates YAP through activation of transcription factor c-Myb to promote growth of liver cancer. Cancer Lett. 2017;385:234–242. doi: 10.1016/j.canlet.2016.10.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.