Abstract
A 47‐year‐old Japanese man was admitted with dyspnoea on exertion (DOE), skin rash and myalgia. Clinical findings of Gottron's sign and mechanic's hands were observed, with increased serum levels of Krebs von den Lungen‐6, surfactant protein‐D, creatine kinase, and anti‐EJ on laboratory tests. In both lungs, chest computed tomography revealed diffuse reticular opacities and lower lobe predominance. The patient was diagnosed with anti‐synthetase syndrome (ASS) and associated interstitial lung disease. Despite repeated administration of high‐dose intravenous corticosteroids, cyclophosphamide and immunoglobulin, his skin rash, myalgia, and DOE followed a relapsing and remitting course. He was then given rituximab therapy. This was initially successful, but disease activity increased approximately 12 months after starting rituximab therapy. Finally, in addition to prednisolone and cyclosporine A, we administered baricitinib. There has been no relapse of the disease in the 12 months since he began baricitinib treatment.
Keywords: anti‐synthetase syndrome, baricitinib, interstitial lung disease
Interstitial lung disease (ILD) in patients with anti‐synthetase syndrome (ASS) is often a severe and progressive disease that requires a lengthy treatment course involving multiple agents. We present a case of refractory ASS‐ILD successfully treated with baricitinib after the failure of several other treatments. Bariticinib shows promise as a salvage treatment for patients with refractory ASS‐ILD.
INTRODUCTION
Anti‐synthetase syndrome (ASS) is characterized by the presence of anti‐aminoacyl transfer RNA synthetase (ARS) antibodies, polymyositis (PM)/dermatomyositis (DM), interstitial lung disease (ILD), arthritis, and mechanic's hands. Importantly, ILD is associated with higher morbidity and mortality. 1 Thus, ILD in ASS is often a severe and progressive disease that requires a lengthy treatment course involving multiple agents. Despite some therapeutic studies and case series, there has been little evidence of the efficacy of baricitinib acting on the Janus kinase (JAK) pathway in addition to conventional immunosuppressants.
CASE REPORT
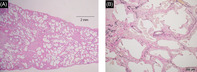
A 47‐year‐old man was admitted to our hospital with 2 months of dyspnoea on exertion (DOE), skin rash and myalgia. Physical examination revealed Gottron's sign, mechanic's hands, a heliotrope rash with facial erythema and poikiloderma on the neck, abdomen and back. He also had proximal lower extremity weakness, fatigue, myalgia and inflammatory arthropathy. Laboratory data from samples taken on admission revealed high levels of serum Krebs von den Lungen‐6 (KL‐6, 725 U/mL) and creatine kinase (CK, 1326 IU/L), as well as anti‐ARS antibodies (anti‐EJ antibody). Chest high‐resolution computed tomography (HRCT) revealed diffuse reticulation and ground glass opacities (GGO), with traction bronchiolectasis primarily in the left lower lobe (Figure 1A). Examination of the patient's bronchoalveolar lavage fluid showed a high total cell count of 4.18 × 105/mL, lymphocyte (8.7%), neutrophil (1.2%) and macrophage (83.1%) proportions in the normal CD4/CD8 ratio of 0.2. Lung biopsy specimens were taken from the right superior segment and the posterior basal segment using video‐assisted thoracic surgery. These demonstrated a temporally uniform thickening of the alveolar septa due to fibrosis, with mild inflammatory cell infiltration (Figure 2A,B). Consequently, he was diagnosed with ASS and ILD with fibrotic non‐specific interstitial pneumonia.
FIGURE 1.
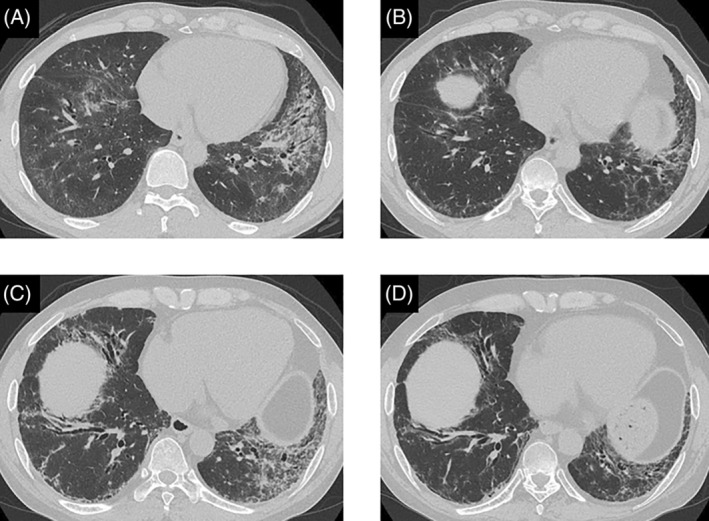
High‐resolution computed tomography (HRCT) images showing changes in the chest of the patient. (A) At initial presentation, HRCT revealed diffuse reticulation and ground glass opacities (GGO) with traction bronchiolectasis primarily in the left lower lobe. (B) Eighteen months after treatment initiation, the reticular opacities in the bilateral lower lobes had deteriorated. (C) After 12 months of treatment with rituximab, chest HRCT revealed reticular opacities admixed with GGO and marked traction bronchiolectasis in the bilateral lower lobes. (D) Twelve months after the start of baricitinib therapy, chest HRCT revealed GGO and reticulation.
FIGURE 2.
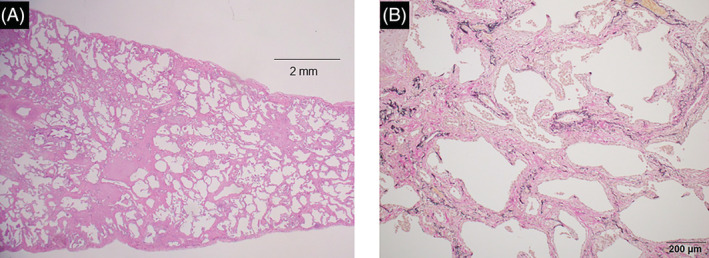
Histopathological findings from the patient's lung biopsy specimens obtained by video‐assisted thoracoscopic surgery. (A) Low‐magnification microscopy revealed a temporally uniform thickening of the alveolar septa by fibrosis (haematoxylin–eosin stain used). The bar represents 2 mm. (B) The fibrosis was collagenous with collapsed alveoli and mild chronic inflammation (elastic van Gieson was used). The bar represents 200 μm.
He was treated with intravenous methylprednisolone (mPSL pulse, 1000 mg/day for 3 consecutive days), followed by prednisolone (PSL, 30 mg/day) and tacrolimus (TAC, 3–4 mg/day) at the initial visit. Following this initial treatment, the GGO seen on his chest HRCT in the bilateral lung fields resolved. Six months after this initial admission, he experienced a recurrence of his cutaneous symptoms and worsening proximal lower extremity weakness with fatigue and myalgia. Despite starting a second course of treatment with high‐dose intravenous immunoglobulin (IVIG, 0.4 g/kg/day for 5 consecutive days), his myalgia worsened quickly. Intravenous cyclophosphamide (IVCY, 500 mg/m2, every 4 weeks for six cycles) treatment was commenced 12 months after the initial admission. He was administered IVIG and mPSL pulse therapy after his CT scans showed gradual deterioration and worsening of his DOE (Figure 1B). Despite these treatments, disease activity increased. Thirty months after initial treatments, we then gave rituximab (RTX, 700 mg/day) intravenously once a week for 3 weeks. This was effective for some time, but the patient returned 12 months later with refractory ILD and a rash that included facial erythema, poikilodermatous changes and inflammatory polyarthropathy (Figure 1C).
Finally, we decided to administer baricitinib in addition to PSL and cyclosporine A (CsA). After 1 month of baricitinib treatment, the patient's clinical symptoms of rash, myalgia and lower extremities weakness had significantly improved. He did not exhibit or complain about any serious adverse effects. The patient's clinical condition was stable after 12 months of PSL (15 mg/day), CsA (200 mg/day) and baricitinib (4 mg/day) combination therapy (Tables 1 and 2). His GGO and reticulation on his chest HRCT scan improved (Figure 1D). The patient reported no serious side effects.
TABLE 1.
List of treatment regimens.
| Initial treatments | mPSL pulse ⇒ PSL + TAC |
|---|---|
| After 6 months from initial treatments | IVIG ⇒ PSL + TAC |
| After 12 months from initial treatments | IVIG + IVCY (every 4 weeks for six cycles) ⇒ PSL + CsA |
| After 18 and 24 months from initial treatments | mPSL pulse + IVIG ⇒ PSL + CsA |
| After 30 months from initial treatments | mPSL pulse + RTX (weekly for three cycles) ⇒ PSL + CsA |
| After 42 months from initial treatments | mPSL pulse ⇒ PSL + CsA + Baricitinib |
TABLE 2.
Changes in the patient's clinical manifestations.
| Initial visit, mPSL pulse | After 6 m, IVIG | After 12 m, IVIG + IVCY | After 18 m, IVIG + mPSL pulse | After 24 m, IVIG + mPSL pulse | After 30 m, RTX + mPSL pulse | After 36 m | After 42 m, Baricitinib + mPSL pulse | After 54 m | |
|---|---|---|---|---|---|---|---|---|---|
| mMRC | 2 | 0 | 1 | 2 | 2 | 3 | 2 | 2 | 1 |
| Myalgia | + | + | ++ | +++ | + | ++ | + | ++ | − |
| 6MWD (m) | 540 | 644 | 649 | 616 | ND | 561 | ND | 583 | 614 |
| Lowest SpO2 (%) | 87 | 89 | 90 | 89 | ND | 84 | ND | 86 | 86 |
| %FVC (%) | 97.9 | 86.5 | 100.5 | 83 | ND | 69.9 | 75.3 | 70.7 | 69.9 |
| %DLco (%) | 67.3 | 70 | 67.5 | 50.7 | ND | 33.4 | 45.8 | 46.3 | 37.4 |
| PaO2 (Torr) | 98.1 | 98.5 | 102.4 | 90 | ND | 92.4 | 96.5 | 96.4 | 87.1 |
| KL‐6 (U/mL) | 725 | 643 | 1034 | 2790 | 2987 | 1938 | 840 | 1175 | 715 |
| SP‐D (ng/mL) | 256 | 171 | 272 | 745 | 571 | 525 | 250 | 308 | 170 |
| CK (U/L) | 1326 | 745 | 1170 | 2634 | 1523 | 2759 | 353 | 1297 | 93 |
| Aldolase (U/L) | 22.4 | 19.9 | 34.8 | 26.2 | 14.2 | 22.8 | 17.6 | 22.9 | 7.6 |
Abbreviations: 6MWD, 6‐minute walking distance; CK, creatine kinase; DLco, diffusing capacity for carbon monoxide; FVC, forced vital capacity; IVCY, intravenous cyclophosphamide; IVIG, intravenous immunoglobulin; KL‐6, Krebs von den Lungen‐6; m, months; mMRC, modified Medical Research Council; mPSL, methylprednisolone; ND, not determined; RTX, rituxiab; SP‐D, surfactant protein‐D; SpO2, percutaneous oxygen saturation; −,none; +, mild; ++, moderate; +++, severe.
DISCUSSION
We have presented a case of refractory ASS‐ILD in which disease activity was suppressed over a 12‐month period by baricitinib therapy combined with a conventional immunosuppression. Most patients with ASS‐ILD require long‐term treatment with a combination of corticosteroids and immunosuppressive drugs such as calcineurin inhibitors. Despite aggressive conventional treatments, some patients do not fully respond to these treatments and experience frequent relapses. Moreover, this case could be diagnosed without surgical lung biopsy. The decision for invasive lung tissue sampling needs to be judged based on individual cases.
JAK/‐signal transducers and activators of transcription (STAT) inhibitors block multiple cytokines, including type I interferon (IFN‐1), interleukin (IL)‐6, IL‐12 and IL‐23. 2 The pathogenesis of DM is linked to IFN‐1 and the JAK/STAT signal pathways. 2 Chen et al. have effectively treated patients with anti‐melanoma differentiation‐associated gene‐5 antibody‐positive DM associated with ILD using tofacitinib, a JAK1/3 inhibitor. 3 The %FVC, DLco, and chest HRCT findings in the tofacitinib group of the aforementioned study all improved over a 6‐month period. 3 Therefore, activation of the JAK/STAT pathway plays an important role in the progression of ILD. It was on this basis that we inferred that baricitinib might be a rescue option for patients with refractory ASS‐ILD after the failure of conventional treatment. Papadopoulou et al. have already demonstrated the efficacy of baricitinib (a JAK1/2 inhibitor) in a refractory juvenile case of DM, as well as identifying a relationship between clinical response and downregulation of IFN‐1 gene expression and STAT1 phosphorylation in peripheral blood mononucleated cells during baricitinib therapy. 4 More recently, Delvino et al. have reported baricitinib to be effective in the treatment of refractory skin, muscle and joint symptoms in refractory adult DM. 5
In our case, baricitinib induced a rapid and significant improvement in the clinical manifestations of ASS‐ILD. Baricitinib was well tolerated without significant adverse event. Although baricitinib was currently used for patients with COVID‐19 pneumonitis, clinicians should be aware of dose adjustment based on renal function, serious infection and thromboembolism. This case of refractory ASS‐ILD was successfully treated with baricitinib after the failure of several other treatments. Bariticinib shows promise as a salvage treatment for patients with refractory ASS‐ILD.
AUTHOR CONTRIBUTION
Keishi Sugino, Hirotaka Ono, Mikako Saito, Masahiro Ando and Eiyasu Tsuboi contributed to the study conception and design. Keishi Sugino, Hirotaka Ono and Mikako Saito analysed and interpreted the clinical data. Keishi Sugino, Hirotaka Ono, Mikako Saito, Masahiro Ando and Eiyasu Tsuboi drafted the manuscript. All authors read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
None declared.
ETHICS STATEMENT
The authors declare that appropriate written informed consent was obtained for the publication of this manuscript and accompanying images.
Sugino K, Ono H, Saito M, Ando M, Tsuboi E. Successful baricitinib treatment of refractory anti‐synthetase syndrome associated with interstitial lung disease. Respirology Case Reports. 2023;11:e01129. 10.1002/rcr2.1129
Associate Editor: Yet Hong Khor
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Lega J‐C, Fabien N, Reynaud Q, Durieu I, Durupt S, Dutertre M, et al. The clinical phenotype associated with myositis‐specific and associated autoantibodies: a meta‐analysis revisiting the so‐called antisynthetase syndrome. Autoimmun Rev. 2014;13:883–91. [DOI] [PubMed] [Google Scholar]
- 2. You H, Xu D, Zhao J, Li J, Wang Q, Tian X, et al. JAK inhibitors: prospects in connective tissue diseases. Clinic Rev Allerg Immunol. 2020;59:334–51. [DOI] [PubMed] [Google Scholar]
- 3. Chen Z, Wang X, Ye S. Tofacitinib in amyopathic dermatomyositis‐associate interstitial lung disease. N Engl J Med. 2019;381:291–3. [DOI] [PubMed] [Google Scholar]
- 4. Papadopoulou C, Hong Y, Omoyinmi E, Brogan PA, Eleftheriou D. Janus kinase 1/2 inhibition with baricitinib in the treatment of juvenile dermatomyositis. Brain. 2019;142:e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Delvino P, Bartoletti A, Monti S, Biglia A, Montecucco C, Carducci M, et al. Successful treatment with baricitinib in a patient with refractory cutaneous dermatomyositis. Rheumatology. 2020;59:125–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


