Abstract
Drug resistance presents one of the major causes for the failure of cancer chemotherapy. Cancer stem-like cells (CSCs), a population of self-renewal cells with high tumorigenicity and innate chemoresistance, can survive conventional chemotherapy and generate increased resistance. Here, we develop a lipid-polymer hybrid nanoparticle for co-delivery and cell-distinct release of the differentiation-inducing agent, all-trans retinoic acid and the chemotherapeutic drug, doxorubicin to overcome the CSC-associated chemoresistance. The hybrid nanoparticles achieve differential release of the combined drugs in the CSCs and bulk tumor cells by responding to their specific intracellular signal variation. In the hypoxic CSCs, ATRA is released to induce differentiation of the CSCs, and in the differentiating CSCs with decreased chemoresistance, DOX is released upon elevation of reactive oxygen species to cause subsequent cell death. In the bulk tumor cells, the drugs are released synchronously upon the hypoxic and oxidative conditions to exert potent anticancer effect. This cell-distinct drug release enhances the synergistic therapeutic efficacy of ATRA and DOX with different anticancer mechanism. We show that treatment with the hybrid nanoparticle efficiently inhibit the tumor growth and metastasis of the CSC-enriched triple negative breast cancer in the mouse models.
Key words: Drug delivery, Lipid-polymer hybrid nanoparticle, Chemotherapeutic resistance, Cancer stem-like cell, Differentiation therapy
Graphical abstract
This study developed an easy-to-fabricate lipid-polymer hybrid nanoparticle for co-delivery and cell-distinct release of the differentiation-inducing agent ATRA, and the chemotherapeutic drug DOX, to overcome the CSC-associated chemoresistance.
1. Introduction
Chemotherapy is the mainstay of cancer treatment in clinic by traditionally using “cytotoxic” drugs. However, drug resistance is a major obstacle as a significant proportion of cancer cells counteract the effect of chemotherapeutic drugs leading to cancer relapse and poor prognosis1,2. Cancer stem-like cells (CSCs), also known as tumor-initiating cells, have been identified and suggested to be a predominant factor for therapeutic resistance and cancer recurrence, which is attributed to their capacity to self-renew and give rise to heterogeneous lineages of descendants3, 4, 5. CSCs can go and lie dormant, pump drugs outside the cells by overexpressed ATP-binding cassette transporters, possess active DNA-repair and reactive oxygen species (ROS)-scavenging capacities, and avoid apoptosis6,7. Accumulating evidence shows that malignant tumors with higher invasiveness, proneness to relapse and poorer prognosis are closely correlated with increased quantity of CSCs in the tumors8,9, particularly triple-negative breast cancer (TNBC) as the most aggressive subtype of breast cancer with a high proportion of the CSC populations, which lacks targeted therapeutic options and is mainly treated by chemotherapy10,11. Unfortunately, most traditional chemotherapeutic drugs acting merely on fast-growing cancer cells cannot eliminate the relatively quiescent and insensitive CSCs, but enhance the stem cell-related phenotype with enrichment of CSCs12, 13, 14.
A number of CSC-targeted therapeutic approaches have been developed to eradicate CSCs by blocking cell surface biomarkers and inhibiting or reversing abnormal stemness signaling pathways15,16. Since these strategies might impair normal stem cells that share many characteristics of CSCs, differentiation therapy offers promise as a relatively safe therapeutic alternative, in which malignant cells are encouraged to differentiate into more mature forms with reduced tumorigenicity using differentiation-inducing agents17,18. The great success of differentiation therapy is that acute promyelocytic leukemia is now highly curable by combinatorial application of all-trans retinoic acid (ATRA) and arsenic19,20. Moreover, induction of differentiation can reduce the vitality and tumorigenicity of CSCs in a host of solid tumors including TNBC21, glioblastoma22 and pancreatic cancer23. Notably, differentiation therapy renders CSCs to transform into terminally-differentiated cancer cells with increased chemosensitivity, which can be efficiently decimated by cytotoxic drugs, such as doxorubicin (DOX)21, paclitaxel24 and cisplatin25. Despite these advances, strategies to potentiate the combined effect of differentiation-inducing agents and chemotherapeutic drugs with two different anticancer mechanisms still remain elusive. We have recently reported a cell-differentiation-regulated nanoparticle that enables differential release of ATRA and CPT within CSCs and bulk tumor cells in response to each specific biosignal26. The enhanced synergistic activity to inhibit the CSC-enriched tumor growth and relapse was validated. However, this design is limited by relatively inflexible drug structure and inconvenient dosage adjustment.
To this end, we herein report an easy-to-fabricate lipid-polymer hybrid nanoparticle for co-delivery and cell-distinct release of the differentiation-inducing agent, ATRA and the chemotherapeutic drug, DOX27, 28, 29, which can potentiate their synergistic effects on eliminating both CSCs and bulk tumor cells concurrently to overcome the CSC-derived chemoresistance (Fig. 1). The hybrid nanoparticle has a core–shell structure composed of liposomal membrane and polymeric nanoparticle core (Fig. 1A). ATRA and DOX are encapsulated in the outer lipid membrane and inner polymeric nanoparticle, respectively. This designed system has a variety of advantages: (1) the proportion of two drugs can be readily adjusted by changing the ratio of drug-loaded liposome and nanoparticle as needed; (2) co-encapsulation in a single nanocarrier can unify the differential pharmacokinetic and biodistribution of drug cargos and maintain a fixed synergistic drug ratio after intravascular administration; (3) the application of spatial drug-loading approach is conducive to sequential release of ATRA encapsulated in the outer lipid membrane and DOX loaded in the inner nanoparticle core27, 28, 29; (4) the formulation can be generalized for co-delivery of different combinatorial drugs on demand due to the drug loading via physical encapsulation rather than chemical conjugation associated with specific drug molecule structure. To further modulate the drug release patterns within the targeted cells precisely for enhanced synergistic effect, the characteristic physiological and biological properties of CSCs and bulk tumor cells are leveraged as specific triggers to obtain ATRA and DOX co-loaded bioresponsive nanoparticles consisting of hypoxia-sensitive liposomal shell and ROS-degradable dextran nanoparticle core (denoted as ATRA/hL@DOX/rDNPs).
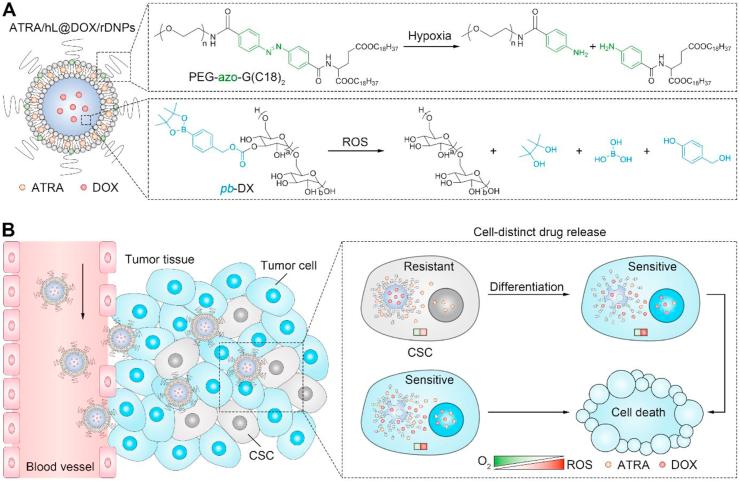
Figure 1.
Schematic illustration of lipid-polymer hybrid nanoparticle with cell-distinct drug release for combined differentiation therapy and chemotherapy. (A) Schematic illustration of structure of ATRA/hL@DOX/rDNPs. ATRA is loaded in the lipid-bilayered membrane containing a hypoxia-labile lipid, PEG-azo-G (C18)2, while DOX is encapsulated in the polymeric nanoparticle core composed of a ROS-degradable dextran, pb-DX (B) Schematic illustration of ATRA/hL@ DOX/rDNPs with differential release of ATRA and DOX within CSCs and bulk tumor cells for enhanced combination differentiation therapy and chemotherapy in the treatment of the CSC-derived resistant tumor.
ATRA/hL@DOX/rDNPs can efficiently preserve the drug ratio in a steady state different from the traditional mixture of free drugs with a changing ratio in the blood after intravascular administration (Fig. 1B). Hypoxia is one of the hallmarks in a majority of malignant tumors30,31, and CSCs are identified to be located in the hypoxic niche that contributes to maintaining their stemness32,33. After accumulation in the tumor tissue, ATRA is promptly released from ATRA/hL@DOX/rDNPs once internalized by CSCs, since degradation of a synthetic lipid, PEG-azo-G (C18)2 causes dissociation of the liposomal shell by hypoxia-triggered cleavage of the azobenzene linker34,35. In contrast, DOX is stably encapsulated in the nanoparticle core prepared by ROS-responsive phenylboronic ester-modified dextran (pb-DX)36 due to the low intracellular ROS concentration of CSCs that inherently have high levels of free radical scavengers37,38. This differential drug release within the undifferentiated CSCs not only enable ATRA to more efficiently produce differentiation-inducing activity, but also reduces the likelihood of prematurely released DOX being pumped out of CSCs or inducing upregulation of stemness-associated properties. The released ATRA promotes differentiation of CSCs, resulting in decreased stemness and increased chemosensitivity. During the differentiation process, the mitochondrial biogenesis is accelerated, leading to increased production of mitochondrial superoxide and elevation of intracellular ROS level39 that, in turn, boosts the differentiation40,41. DOX is timely released in response to increased level of ROS, and can be effective for eliminating the differentiated cells with dramatically reduced chemoresistance. Compared with CSCs, bulk tumor cells exhibit higher levels of ROS in general38. Accordingly, ATRA/hL@DOX/rDNPs release ATRA and DOX rapidly and synchronously after internalization by bulk tumor cells, which supports potent synergistic effect against these cells. We show that ATRA/hL@DOX/rDNPs with cell-distinct drug release property efficiently suppress the growth and metastasis of the CSC-enriched TNBC tumors in the mouse models.
2. Materials and methods
2.1. Materials
Dextran (DX) (10 kDa), carboxymethyl DX (10 kDa), poly (lactic-co-glycolic acid) (PLGA) (lactide:glycolide = 50:50, 0.67 dL/g) and ATRA were purchased from Aladdin Bio-Chem Technology Co., Ltd. (Shanghai, China). PEG-DSPE was purchased from A.V.T. Pharmaceutical Co., Ltd (Shanghai, China). DOX·HCl was purchased from Meilun Biotechnology Co., Ltd. (Dalian, China). FITC-labeled murine stem cell antigen-1 (Sca-1) antibody was purchased from Thermo Fisher Scientific Inc. (Waltham, MA, USA). ALDEFLUOR kit was purchased from STEMCELL Technologies Inc (Waltham, MA, USA). Rabbit anti-mouse Sox2 antibodies were purchased from Servicebio Technology Co., Ltd (Wuhan, China).
2.2. Preparation and characterization of ATRA/hL@DOX/rDNPs
ATRA/hLs were prepared by thin-film dispersion method. ATRA (3 mg), phospholipid (27 mg), cholesterol (5 mg) and PEG-azo-G (C18)2 (13 mg) were dissolved in chloroform (5 mL). A lipid film was formed after removal of chloroform by rotary evaporator (Eyela, OSB-2100, Tokyo, Japan), followed by hydration. The suspension was dispersed by probe-type ultrasonicator (Scientz, JY92-IIN, Ningbo, China). After membrane filtration, ATRA/hLs were obtained. The non-responsive ATRA-loaded liposomes (ATRA/Ls) were prepared using the same method except that PEG-azo-G (C18)2 was replaced by PEG-DSPE.
DOX/rDNPs were prepared by single emulsion method. DOX·HCl (3 mg) and pb-DX (30 mg) were dissolved in dimethyl sulfoxide (1 mL), followed by the addition of triethylamine (2 μL). The above solution was added dropwise to the polyvinyl alcohol solution (1%, 4 mL). The suspension was stirred for 30 min, and then dialyzed against water to remove dimethyl sulfoxide. After membrane filtration, DOX/rDNPs were obtained. DOX-loaded PLGA nanoparticles (DOX/PNPs) and DOX-loaded hypoxia-responsive dextran nanoparticles (DOX/hDNPs) were prepared using the same method except that pb-DX was replaced by PLGA and synthetic hypoxia-sensitive nitroimidazole-modified dextran (n-DX), respectively.
ATRA/hL@DOX/rDNPs were prepared by repeatedly extruding the mixture of ATRA/hLs and DOX/rDNPs through membrane filters by an extruder (AVESTIN, LF-1, Ottawa, ON, Canada). ATRA/L@DOX/PNPs were prepared by co-extrusion of ATRA/Ls and DOX/PNPs, while ATRA/hL@DOX/hDNPs were prepared by co-extrusion of ATRA/hLs and DOX/hDNPs.
The particle size and zeta potential of nanoparticles were determined by zetasizer (Malvern, ZS90, Malvern, UK). The morphology of ATRA/hL@DOX/rDNPs was examined by transmission electronic image (TEM) (Hitachi, HT7700, Tokyo, Japan). For assessment of the ROS-responsiveness, rDNPs were incubated with different concentrations of hydrogen peroxide (H2O2) over time, and the absorbance was determined at the wavelength of 350 nm by microplate reader (Tecan, Infinite M1000 Pro, Männedorf, Swiss). For stability evaluation, ATRA/hL@DOX/rDNPs were incubated with bovine serum albumin (BSA) (10%, w/v) over time, and the particle size was determined by zetasizer.
2.3. Drug release
For determination of the in vitro drug release, the nanoparticles (1 mL) were added into a dialysis bag (MWCO: 14 KDa), which was further immersed into the release medium composed of PBS (pH 7.4, 50 mL) containing Tween 80 (1%) under stirring at 37 °C. For the establishment of a hypoxic environment, nicotinamide adenine dinucleotide phosphate reduced form was added into the release medium at a final concentration of 100 μmol/L, and the release medium was degassed by nitrogen bubbling and maintained in a hypoxic condition. For the establishment of a peroxidative condition, H2O2 was added into the release medium at a pre-determined concentration. The release medium (600 μL) was collected at pre-determined time intervals, and the same volume of fresh medium was supplemented. The concentrations of ATRA and DOX in the release medium were quantified by high performance liquid chromatography (HPLC) (Shimadzu, LC-20, Kyoto, Japan) and microplate reader, respectively.
2.4. Animals and CSC-enriched breast tumor models
Sprague–Dawley rats (female, 250 g) were purchased from the Experimental Animal Center of Nanjing Qinglongshan. BALB/c mice (female, 18 g) were purchased from the Comparative Medicine Centre of Yangzhou University (Yangzhou, China). All the animals were treated according to the Guide for Care and Use of Laboratory Animals, approved by the Animal Experimentation Ethics Committee of China Pharmaceutical University.
To establish the orthotopic mouse model of CSC-enriched breast tumor, the murine mouse TNBC (4T1) tumorsphere cells (TCs) (5 × 105 cell/mouse) were inoculated into the mammary fat pad of the mice. The length and width of the tumor were measured by a vernier caliper, and the volume of tumor was calculated as length × width2/2.
To establish the mouse model of CSC-enriched breast cancer metastasis, the 4T1 or luciferase-tagged 4T1 (4T1-Luc) TCs (1 × 105 cell/mouse) were intravenously injected into the mice. The tumor metastasis could be examined by the bioluminescent signal from the 4T1-Luc cells. D-Luciferin sodium salt (150 mg/kg) was intraperitoneally injected into the mice, and after 15 min, the mice were imaged by in vivo imaging system (IVIS) (PerkinElmer, Spectrum, Waltham, MA, USA). The metastatic foci in the lung could also be observed by the Bouin staining.
2.5. Pharmacokinetics
ATRA/hL@DOX/rDNPs and the physical mixture of ATRA and DOX were injected into the rats via tail vein, respectively. The dosages of ATRA and DOX were 4 and 2.5 mg/kg, respectively. At pre-determined time interval, the blood was sampled and centrifuged. The drugs were extracted from the plasma supernatant by adding chloroform/methanol (5/1, v/v) and vortex. After centrifugation, the organic phase was collected and evaporated. The drug residue was re-dissolved in methanol. The concentrations of ATRA and DOX were determined by HPLC (Shimadzu) and microplate reader (Tecan), respectively.
2.6. Tumor targeting
To evaluate the tumor targeting ability of the hybrid nanoparticles, the fluorescent dye, DiR was loaded in the polymeric nanoparticle core to obtain hL@DiR/rDNPs. hL@DiR/rDNPs were intravenously injected into the orthotopic CSC-enriched 4T1 tumor-bearing mice at DiR dosage of 50 nmol/kg. After 48 h, the mice were euthanized. Different tissues that include heart, liver, spleen, lung, kidney and tumor were harvested and imaged by IVIS (PerkinElmer). The fluorescent intensities of the DiR signal in the tissues were quantified by Living Image Software.
To estimate the targeting capacity of the hybrid nanoparticles to the metastatic lesion, the 4T1 TCs were intravenously injected into the mice, and after 7 days, hL@DiR/rDNPs were intravenously injected into the mice at DiR dosage of 50 nmol/kg. After 48 h, the mice were euthanized. The lung tissues were harvested and imaged by IVIS (PerkinElmer). The fluorescent intensities of the DiR signal in the lungs were quantified by Living Image Software.
2.7. Inhibition of primary CSC-enriched breast tumor growth
The orthotopic CSC-enriched 4T1 tumor-bearing mice were treated by intravenous injection of different drug-loaded nanoparticles five times every two days at the dosages of 4 mg/kg ATRA and 2.5 mg/kg DOX. The tumor volumes and mouse body weights were measured. On Day 22, the mice were euthanized. The tumor was harvested and weighed. The tumor inhibition ratio was calculated as in Eq. (1):
| (1) |
where W is the tumor weight of the nanoparticle-treated group and W0 is the tumor weight of the saline-treated group. Different tissues that include heart, liver, spleen, lung, kidney and tumor were histologically examined by the hematoxylin and eosin (H&E) staining. The expression of Sox2 in the tumors was assessed by the immunohistochemistry (IHC) staining. The stained tissue sections were observed by microscope (Nikon, Ts2R, Tokyo, Japan).
2.8. Inhibition of CSC-enriched breast tumor metastasis
The mice were intravenously injected with the 4T1-Luc TCs (1 × 105 cell/mouse). After 3 days, the mice were treated by intravenous injection of different drug-loaded nanoparticles five times every two days at the dosages of 4 mg/kg ATRA and 2.5 mg/kg DOX. At pre-determined time interval, the tumor metastasis was detected by the bioluminescent signal from the 4T1-Luc TCs. The mice were intraperitoneally injected with D-Luciferin sodium salt (150 mg/kg), and after 15 min, the mice were imaged by IVIS (PerkinElmer). The bioluminescent intensities of the mice were quantified by Living Image Software. The mouse body weights were measured. On Day 14, the mice were euthanized. Different tissues that include heart, liver, spleen, lung, kidney and tumor were harvested. The lung tissues were stained by the Bouin's solution and photographed. The metastatic foci in the lung tissues were counted. Different tissues were histologically examined by the H&E staining, and the stained tissue sections were observed by microscope (Nikon). The proportions of the area of the metastatic lesion to that of the lung tissue in the H&E-stained images were determined by ImageJ software. The expression of Sox2 in the metastatic foci was assessed by the IHC staining, and the stained tissue sections were observed by microscope (Nikon).
2.9. Cardiotoxicty evaluation
The mice were treated by intravenous injection of saline, the free DOX solution (2.5 mg/kg) and ATRA/hL@DOX/rDNPs (2.5 and 5 mg/kg) five times every two days. On Day 10, the blood was collected, and the levels of creatine kinase (CK) and cardiac troponin (cTn) in the serum were determined using the corresonding assay kits (Jiancheng Bioengineering, Nanjing, China) according to the manufacturers’ protocols.
2.10. Statistical analysis
The data are shown as means ± standard deviation (SD). The statistical analyses were conducted using the GraphPad Prism software. Two-tailed Student's t test was applied for analysis of statistical difference between two groups. One-way analysis of variance (ANOVA) was applied for analysis of statistical difference among multiple groups. Two-sided likelihood ratio test was applied for analysis of statistical difference in the in vitro limiting dilution assay.
3. Results and discussion
3.1. Preparation and characterization of hybrid nanoparticles
The oxidation-responsive pb-DX was synthesized by conjugating the phenylboronic pinacol ester on the polysaccharide backbone of DX (Supporting Information Scheme S1A). Based on the modification at the hydroxyls increasing the hydrophobicity of DX, DOX/rDNPs could be prepared from pb-DX using standard single emulsion method36. The obtained DOX/rDNPs had a hydrodynamic diameter of ∼91 nm determined by dynamic light scattering (DLS) (Supporting Information Fig. S1). To confirm the oxidation-sensitivity, we first monitored the changes in the turbidities of rDNPs in phosphate-buffered solution (PBS, pH 7.4) with or without H2O2 over time (Fig. 2A). rDNPs in the H2O2-free PBS did not show any noticeable turbidity change, while the turbidity showed substantial decrease in the presence of H2O2, which dropped to 56% and 7% of initial turbidity after incubation with 0.1 and 1 mmol/L H2O2 within 4 h, respectively. The rDNP suspension became transparent after incubation with 1 mmol/L H2O2 for 2 h (Supporting Information Fig. S2). This is because phenylboronic pinacol ester of pb-DX is oxidized to phenols, followed by a quinone methide arrangement and subsequent exposure of the hydroxyl groups36, resulting in recovery of the hydrophilicity of DX and consequential collapse of nanoparticles. By comparison, no obvious variation in the turbidity of PNPs was observed after incubation with H2O2 (Supporting Information Fig. S3). Furthermore, the release profiles of DOX from DOX/rDNPs were determined under oxidative conditions (Fig. 2B). As expected, DOX/rDNPs revealed an accelerating release rate of DOX as the concentration of H2O2 increased due to the responsive degradation of rDNPs.
Figure 2.
Characterization of ATRA/hL@DOX/rDNPs. (A) Changes in the turbidities of the rDNP PBS solution (pH 7.4) containing different concentrations of H2O2 over time. Data are shown as mean ± SD (n = 3). (B) Release profiles of DOX from DOX/rDNPs in the presence of different concentrations of H2O2. Data are shown as mean ± SD (n = 3). (C) Release profiles of ATRA from ATRA/hLs under normoxic and hypoxic conditions. Data are shown as mean ± SD (n = 3). (D) Particle sizes and zeta potentials of DOX/rDNPs, ATRA/hLs and ATRA/hL@DOX/rDNPs. Data are shown as mean ± SD (n = 3). (E) Representative histogram of size distribution of ATRA/hL@DOX/rDNPs measured by DLS. Inset: representative TEM image of ATRA/hL@DOX/rDNPs. Scale bar = 100 nm. (F) Change in the particle sizes of ATRA/hL@DOX/rDNPs without (w/o) and with (w/) of BSA over time. Data are shown as mean ± SD (n = 3). (G–I) Release profiles of ATRA and DOX from ATRA/hL@DOX/rDNPs (G), ATRA/hL@DOX/hDNPs (H) and ATRA/L@DOX/PNPs (I) under different conditions. Data are shown as mean ± SD (n = 3).
ATRA/hLs containing hypoxia-degradable PEG-azo-G (C18)2 (Scheme S1B) were prepared and had a hydrodynamic diameter of ∼106 nm (Supporting Information Fig. S4A). The azobenzene linker in PEG-azo-G (C18)2 (Fig. S4B) can degrade under hypoxic condition along with the separation between hydrophilic head and hydrophobic tail that leads to the dissociation of liposome42. The drug release study showed that the release rate of ATRA from ATRA/hLs under hypoxia was higher than that under normoxia (Fig. 2C), confirming that the hypoxia-responsive disintegration of hLs can dramatically promote the release of ATRA.
ATRA/hL@DOX/rDNPs were engineered by integration of DOX/rDNPs and ATRA/hLs via repeated extrusion27,43,44. The obtained ATRA/hL@DOX/rDNPs exhibited a slightly increased particle size than DOX/rDNPs and an approximate zeta potential with ATRA/hLs (Fig. 2D), suggesting that the hybrid nanoparticles are formed by the liposomal membrane coated on the DX nanoparticle. The TEM image further evidenced the core–shell structure of ATRA/hL@DOX/rDNPs with a diameter of ∼108 nm (Fig. 2E and Fig. S4C). Meanwhile, the drug ratio could be easily adjusted by altering the amounts of ATRA/hLs and DOX/rDNPs on demand. Two control hybrid nanoparticles were prepared (Supporting Information Table S1): one was non-responsive ATRA/L@DOX/PNPs consisting of ATRA-loaded traditional liposomal membrane and DOX-loaded PNPs, and the other was hypoxia-responsive ATRA/hL@DOX/hDNPs composed of ATRA/hL and DOX/hDNPs prepared from n-DX (Scheme S1C and Supporting Information Fig. S5)45. In addition, the particle size of ATRA/hL@DOX/rDNPs had no significant change in the presence of BSA (Fig. 2F), indicating that ATRA/hL@DOX/rDNPs are highly stable against serum proteins.
Next, the release profiles of ATRA and DOX from ATRA/hL@DOX/rDNPs were determined simultaneously after successive incubation under the hypoxic and oxidative conditions (Fig. 2G), which mimics the biosignal variation within CSCs during differentiation. ATRA/hL@DOX/rDNPs were found to release ATRA rapidly but DOX slowly under hypoxia, while the release rate of DOX dramatically increased once exposure to H2O2. By comparison, both of ATRA and DOX were swiftly released from ATRA/hL@DOX/hDNPs under the hypoxic condition (Fig. 2H), which was due to hypoxia-triggered degradation of both PEG-azo-G (C18)2 and n-DX causing the collapse of the whole hybrid nanoparticles. In addition, the non-responsive ATRA/L@DOX/PNPs displayed much slower release of both drugs under similar conditions (Fig. 2I). DOX released more slowly than ATRA is mainly ascribed to the slower diffusivity of the internally-encapsulated DOX through two spatial barriers, polymeric matrix and lipid bilayer.
3.2. In vitro combination antitumor activity
The serum-free suspension culture method was applied to enrich the breast CSC population from the 4T1 cells and human TNBC (HCC1937 and SUM159PT) cells. The cells formed tumorspheres under the serum-free suspension culture condition. The cytotoxicity study showed that the 4T1 TCs were highly resistant to free DOX than the adherent cells (ACs) (Fig. 3A). The 4T1 TCs also evinced significantly higher colony-formation ability as demonstrated by the in vitro limiting dilution assay (Fig. 3B). Moreover, the 4T1 TCs had a higher in vivo tumorigenicity, which could form tumors at a relatively lower cell density compared with the 4T1 ACs after implantation into the mice (Fig. 3C). The recognizable biomarkers associated with the phenotypes of breast CSCs were further assayed, including Sca-146 and aldehyde dehydrogenase (ALDH)47. The proportions of Sca-1+ and ALDH+ cell subpopulations in the 4T1 TCs were significantly higher than that in the 4T1 ACs (Supporting Information Fig. S6A and S6B). Both HCC1937 and SUM159PT TCs also showed significant elevation of the expression of Sox2, a typical stemness-associated transcription factor (Fig. S6C and S6D). These results indicate that the obtained TCs are enriched with a significant proportion of cells that harbor the breast CSC characteristics, such as high drug resistance, self-renewal and tumorigenicity.
Figure 3.
In vitro anticancer effect of DOX combined with ATRA. (A) Viabilities of the 4T1 TCs and ACs after treatment with DOX for 96 h. Data are shown as mean ± SD (n = 6). (B) In vitro limiting dilution analysis on the 4T1 TCs and ACs (n = 12). ∗∗∗P < 0.001. (C) In vivo tumorigenesis evaluation of the 4T1 TCs and ACs (n = 6). The tumor formation rates were determined after implantation with the 4T1 TCs and ACs at different densities. (D, E) Proportions of the Sca-1+ (D) and ALDH+ (E) cell subpopulations in the 4T1 TCs after treatment with ATRA for 96 h. Data are shown as mean ± SD (n = 3). ∗∗P < 0.01, ∗∗∗P < 0.001. (F) Viabilities of the 4T1 TCs after treatment with DOX combined with ATRA at different drug ratios. The 4T1 TCs were co-treated with ATRA and DOX for 48 h. Data are shown as mean ± SD (n = 6). (G) Viabilities of the 4T1 TCs after treatment with DOX combined with ATRA at different schedules. The 4T1 TCs were pre-treated with ATRA for 48 h, and then co-treated with ATRA and DOX (3/1, mol/mol) for another 48 h. Data are shown as mean ± SD (n = 6).
The potential of ATRA to induce differentiation of the breast CSCs was evaluated. The variation in the percentages of both Sca-1+ and ALDH+ cell subpopulations was determined after the 4T1 TCs were treated with ATRA. The ATRA treatment brought about a significant reduction in the proportion of the Sca-1+ and ALDH+ cell subpopulation (Fig. 3D and E), suggesting that ATRA can efficiently promote differentiation of breast CSCs and decrease their stemness. Notably, both mitochondrial content and ROS level within the ATRA-treated 4T1 TCs were significantly higher than that in the untreated counterparts (Supporting Information Fig. S7), indicating that the differentiating breast CSCs induced by ATRA present downregulation of antioxidant defense systems accompanied by enhancement of the mitochondrial biogenesis and elevation of the ROS level. This variation of intracellular ROS level of CSCs from undifferentiated to differentiating states lays a foundation for temporally-controlled release of DOX from the hybrid nanoparticles. The synergistic antitumor effect of ATRA and DOX was assessed at the aspects of drug ratio and administration schedule. The addition of ATRA markedly increased the cytotoxicity of DOX against the 4T1 TCs in a concentration-dependent manner (Fig. 3F). The optimal ratio of ATRA and DOX was determined as 3/1 (mol/mol), which had a combination index of 0.69 indicating a synergism of two drugs to eliminate the breast CSCs. Note that pre-treatment with ATRA substantially potentiated the combined anti-proliferative activity of DOX and ATRA on the 4T1 TCs (Fig. 3G). The data confirm that the breast CSCs pre-treated with ATRA exhibit significantly reduced resistance and increased sensitivity to DOX compared with that co-treated simultaneously.
We further monitored the intracellular amounts of ATRA and DOX within the 4T1 TCs after incubation with the drug mixed solution or ATRA/hL@DOX/rDNPs over time (Fig. 4A). The drug ratio changed constantly within the cells incubated with the drug mixture. In contrast, the cells revealed a steady intracellular drug proportion as the loading ratio of ATRA and DOX after incubation with ATRA/hL@DOX/rDNPs, indicating that the hybrid nanoparticles maintain a fixed intracellular drug ratio after internalization by the CSCs, which supports a maximal synergistic cytotoxic effect on the CSCs. In addition, the enhancement of the anti-CSC activity of the combined drugs delivered by ATRA/hL@DOX/rDNPs was investigated. ATRA/hL@DOX/rDNPs had a superior effect on reducing the self-renewal capacity of the 4T1 TCs (Fig. 4B) and destructing the 4T1 tumorspheres (Fig. 4C and Supporting Information Fig. S8). Treatment with ATRA/hL@DOX/rDNPs also led to significant reduction in both Sca-1+ and ALDH+ cell subpopulation of the 4T1 TCs (Fig. 4D and E). The results suggest that ATRA/hL@DOX/rDNPs are capable of eliminating the breast cancer cells with the CSC characteristics.
Figure 4.
In vitro anticancer effect of ATRA/hL@DOX/rDNPs. (A) Changes in the intracellular amounts of ATRA and DOX within the 4T1 TCs over time after incubation with ATRA/hL@DOX/rDNPs and free drug mixture. Data are shown as mean ± SD (n = 3). (B) In vitro limiting dilution analysis on the 4T1 TCs after different treatments for 96 h (n = 12). Ctrl: medium; (1): ATRA/hL@rDNPs; (2): hL@DOX/rDNPs; (3): ATRA/L@DOX/PNPs; (4): ATRA/hL@DOX/hDNPs; (5): ATRA/hL@DOX/rDNPs. (C) Changes in the numbers of the 4T1 tumorspheres over time after different treatments. Data are shown as mean ± SD (n = 6). (D, E) Proportions of the Sca-1+ (D) and ALDH+ (E) cell subpopulations in the 4T1 TCs after different treatments for 96 h. Data are shown as mean ± SD (n = 3). ∗∗P < 0.01, ∗∗∗P < 0.001. (F–I) Viabilities of the 4T1 ACs (F) and TCs (G), HCC1937 TCs (H) and SUM159PT TCs (I) after different treatments for 96 h. Data are shown as mean ± SD (n = 6).
The viabilities of the 4T1 ACs and TCs were assayed after treatment with different drug-loaded hybrid nanoparticles. All the drug-loaded nanoparticles showed high cytotoxic effect on the 4T1 ACs except ATRA/hL@rDNPs (Fig. 4F). However, no obvious difference in the anti-proliferative activity among hL@DOX/rDNPs, ATRA/hL@DOX/hDNPs and ATRA/hL @DOX/rDNPs. By comparison, ATRA/hL@DOX/rDNPs exhibited a greater effect on killing the 4T1 TCs cells than either ATRA/L@DOX/PNPs or ATRA/hL@DOX/hDNPs (Fig. 4G). ATRA/L@DOX/PNPs had a sustainable but inefficient release of two drugs that limited their synergistic anticancer effects. ATRA/hL@DOX/hDNPs could respond to the tumor hypoxia and release both drugs rapidly and synchronously, whereas presented lower cytotoxic effect than ATRA/hL@DOX/rDNPs on the 4T1 TCs, indicating that cell-distinct release of drug combination is crucial for the nanoparticles to strengthening the synergistic efficacy on eliminating the CSC-enriched cancer cells. In addition, ATRA/hL@DOX/rDNPs also showed the strongest anti-proliferative activities against both HCC1937 and SUM159PT TCs (Fig. 4H and I).
3.3. In vivo tumor targeting and antitumor efficacy on primary tumor mouse model
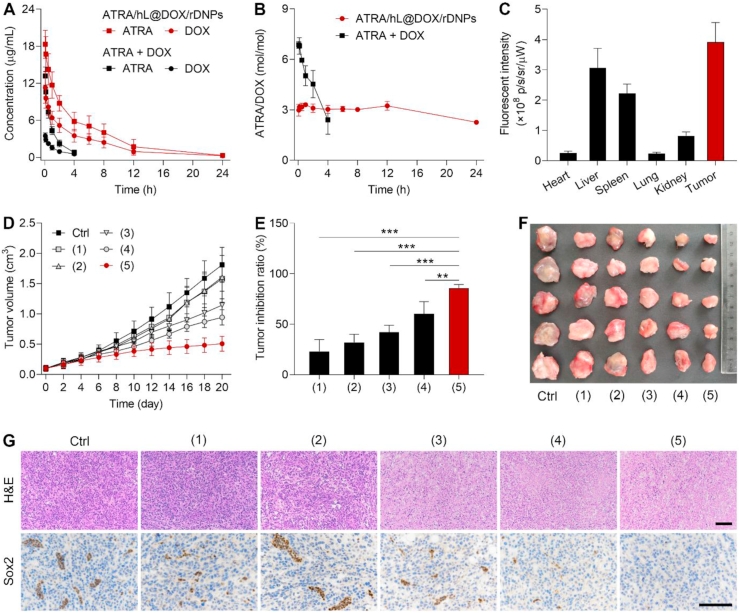
We monitored the pharmacokinetic behaviors of ATRA and DOX after intravenous injection of drug mixture or ATRA/hL@DOX/rDNPs in rats (Fig. 5A). As expected, ATRA/hL@DOX/rDNPs increased the drug concentration and prolonged the circulation time in the blood compared with the drug cocktail. More importantly, the drug ratio in the blood varied constantly over time after administration of the drug mixture, while remained as the administration drug dosage of ATRA/hL@DOX/rDNPs in a steady state within 12 h (Fig. 5B). The tumor targeting of the hybrid nanoparticles was evaluated on the orthotopic breast CSC-enriched tumor mouse model established by implantation of the 4T1 TCs. The near-infrared fluorescent dye, DiR labeled hybrid nanoparticles (hL@DiR/rDNPs) were intravenously injected into the tumor-bearing mice, and different tissues including tumors were harvested and imaged using IVIS. The fluorescent intensity of the DiR signal was observed to be highest in the tumor compared with major normal organs (Supporting Information Fig. S9), as confirmed by the quantitative analysis results (Fig. 5C). These data suggest that the hybrid nanoparticles can enhance the blood persistence of two encapsulated drugs with a stationary drug ratio and show increased accumulation in the primary breast tumor enriched with CSCs.
Figure 5.
In vivo therapeutic efficacy of ATRA/hL@DOX/rDNPs on the orthotopic mouse model of CSC-enriched breast tumor. (A, B) Changes in the individual concentrations (A) and drug ratios (B) of ATRA and DOX in the blood of the rats within 24 h after intravenous injection of ATRA/hL@DOX/rDNPs and free drug mixture. Data are shown as mean ± SD (n = 3). (C) Intensities of the fluorescent signals in different tissues harvested from the tumor-bearing mice after intravenous injection of hL@DiR/rDNPs for 48 h. Data are shown as mean ± SD (n = 3). (D) Changes in the tumor sizes of the tumor-bearing mice within 20 days after different treatments. Ctrl: saline; (1): ATRA/hL@rDNPs; (2): hL@DOX/rDNPs; (3): ATRA/L@DOX/PNPs; (4): ATRA/hL@DOX/hDNPs; (5): ATRA/hL@DOX/rDNPs. Data are shown as mean ± SD (n = 5). (E) Tumor inhibition ratios on Day 22 after different treatments. Data are shown as mean ± SD (n = 5). ∗∗P < 0.01, ∗∗∗P < 0.001. (F) Images of the tumors harvested from the mice on Day 22 after different treatments (n = 5). (G) Representative H&E− and IHC-stained images of the tumors harvested on Day 22 after different treatments. Scale bar = 100 μm.
The in vivo combination anticancer efficacy of ATRA/hL@DOX/rDNPs was evaluated on the orthotopic mouse model of CSC-enriched breast tumor. The tumor growth was monitored after different treatments (Fig. 5D). Treatment with ATRA/hL@DOX/rDNPs resulted in superior effect on suppressing the tumor growth. The tumor inhibition ratio of ATRA/hL@DOX/rDNPs was determined to be significantly higher than that of either ATRA/L@DOX/PNPs or ATRA/hL@DOX/hDNPs (Fig. 5E). The smallest size of the tumors was observable after the ATRA/hL@DOX/rDNP treatment (Fig. 5F). The treated tumor was further histologically examined using the H&E staining48 (Fig. 5G). Substantial remission of a large majority of the tumor cells was visualized in the tumor tissue treated with ATRA/hL@DOX/rDNPs. Furthermore, the IHC examination was employed to assess the change in intratumoral expression of the typical stemness-related transcription factor, Sox2 (Fig. 5G)49. The downregulation of the Sox2 expression was observed in the ATRA/hL@DOX/rDNP-treated tumor. In addition, treatment with ATRA/hL@DOX/rDNPs did not cause any noticeable variation in the body weight of the mice (Supporting Information Fig. S10) or pathological change of the major normal organs at the studied period of time (Supporting Information Fig. S11). Moreover, the nanoparticle-mediated delivery of DOX reduced its cardiotoxicity, as evidenced by non-significant changes of both CK and cTn levels (the cardiotoxicity indices) in the serum of the mice after treatments (Supporting Information Fig. S12). The results indicate that cell-specifically differential release of ATRA and DOX accomplished by hL/rDNPs plays a crucial role in enhancing their synergistic efficacy on suppressing the growth of the CSC-associated resistant breast tumor.
3.4. In vivo tumor targeting and antitumor efficacy on metastatic tumor mouse model
The tumor-targeting of the hybrid nanoparticles to the metastatic lesions of breast cancer was investigated on the mouse model of CSC-enriched breast tumor metastasis. The mice were intravenously injected with the 4T1 TCs, and after 7 days, hL@DiR/rDNPs were intravenously injected into the mice. For a comparison, hL@DiR/rDNPs were intravenously injected into the normal mice. The lungs are the first sites of breast cancer metastasis. After 48 h, the lungs were collected and imaged by IVIS. The fluorescent images showed that the lung harvested from the mouse model of breast cancer metastasis showed a strong DiR fluorescent signal (Supporting Information Fig. S13A), which was significantly higher than that in the lung withdrawn from the normal mice (Fig. S13B). The results indicate that the hybrid nanoparticles possess favorable targeting capability not only to the primary breast tumor but also to the metastatic lung lesion.
The in vivo synergistic anti-metastatic efficacy of ATRA/hL@DOX/rDNPs was further evaluated on the mouse model of CSC-enriched breast cancer metastasis. The mice were intravenously injected with the 4T1-Luc TCs, and the cancer metastasis was assessed by monitoring the bioluminescent signal from the 4T1-Luc TCs using IVIS50. The established mouse models were treated with different drug-loaded nanoparticles, and the changes of the bioluminescent signals were detected over time. All the mice treated with the drug-loaded nanoparticles were observed and determined to show lower bioluminescent intensities than the mice treated with saline as control (Fig. 6A and Supporting Information Fig. S14), suggesting that the drug-loaded nanoparticles could inhibit the distal metastasis of breast cancer. However, ATRA/L@DOX/PNPs did not show a higher anti-metastatic activity in vivo than DOX/rDNPs, indicating that the sustained but inefficient drug release property of ATRA/L@DOX/PNPs greatly curbs the synergistic effect of ATRA and DOX. In contrast, treatment with ATRA/hL@DOX/hDNPs resulted in a preferable effect on suppressing the breast cancer metastasis, confirming that the synchronic rapid release of both drugs rendered by ATRA/hL@DOX/hDNPs in response to the tumor hypoxia promotes their combination anticancer activities. Inspiringly, ATRA/hL@DOX/rDNPs with the cell-distinct drug release feature produced more efficient control of CSC-enriched breast cancer metastasis than ATRA/hL@DOX/hDNPs.
Figure 6.
In vivo anti-metastatic efficacy of ATRA/hL@DOX/rDNPs on the mouse model of CSC-enriched breast tumor metastasis. (A) Changes in the individual bioluminescent intensities from the 4T1-Luc TCs within 14 days after different treatments (n = 4). Ctrl: saline; (1): ATRA/hL@rDNPs; (2): hL@DOX/rDNPs; (3): ATRA/L@DOX/PNPs; (4): ATRA/hL@DOX/hDNPs; (5): ATRA/hL@DOX/rDNPs. (B) Images of the lungs harvested from the mice on Day 14 after different treatments (n = 4). The lungs were stained by the Bouin's solution. (C) Numbers of metastatic foci in the lungs harvested on Day 14 after different treatments. Data are shown as mean ± SD (n = 4). ∗P < 0.05, ∗∗∗P < 0.001. (D) Representative H&E− and IHC-stained images of the lungs and metastatic foci on Day 14 after different treatments. Scale bars = 500 μm (H&E) and 100 μm (IHC). (E) Changes in the body weights of the mice within 14 days after different treatments. Data are shown as mean ± SD (n = 4).
The lungs were harvested after treatment and stained using the Bouin's solution. The superficial metastatic foci on the lungs could be obviously distinguished from the lung tissues and easily detected through the pale color51. As shown in the stained lung images, treatment with ATRA/hL@DOX/rDNPs resulted in the fewest metastatic foci in the lung tissues (Fig. 6B). The metastatic lung foci of the mice treated with ATRA/hL@DOX/rDNPs was significantly lower in quantity than that treated with other drug-loaded nanoparticles (Fig. 6C). Subsequently, the lung tissues were histologically examined using the H&E staining (Fig. 6D). Large tumor lesions were observed in the lung tissue of the saline-treated mice. Encouragingly, the lung tissue harvested from the mice treated with ATRA/hL@DOX/rDNPs exhibited the smallest and fewest metastatic lesions (Supporting Information Fig. S15). The expression of Sox2 in the lung metastase was further assessed using the IHC staining (Fig. 6D). Lower expression of Sox2 was observed after treatment with ATRA/hL@DOX/rDNPs. In addition, treatment with ATRA/hL@DOX/rDNPs did not cause any noticeable change in the body weight of the mice (Fig. 6E). No significant pathological variation was examined in other normal organs, including heart, liver, spleen and kidney (Supporting Information Fig. S16). Collectively, ATRA/hL@DOX/rDNPs enables ATRA and DOX to generate more potent synergistic effect on inhibiting the metastasis of the CSC-enriched breast cancer.
4. Conclusion
The combination of differentiation therapy and chemotherapy, with the indicated differentiation-inducing agents, elevates the sensitivity of the tumor to the chemotherapeutic drugs in preclinical and clinical studies. Differentiation therapy induces the highly-tumorigenic CSCs to differentiate into their descendant cells with reduced stemness and resistance, which significantly promotes the tumor chemosensitivity and enhances the chemotherapeutic efficacy. Considering that the differentiation-inducing agents and chemotherapeutic drugs have individual pharmacokinetic property and anticancer mechanism, it is a challenge to design the optimized drug combinations that potentiate their synergistic anticancer effect. In this study, we have developed the lipid-polymer hybrid nanoparticle, ATRA/hL@DOX/rDNPs to unify the pharmacokinetics of the differentiation-inducing agent, ATRA and chemotherapeutic drug, DOX and modulate their release profiles within the CSCs and bulk tumor cells for enhanced combination treatment of the CSC-related resistant tumor. ATRA and DOX were encapsulated in the liposomal membrane and polymeric nanoparticle core of the hybrid nanoparticle, respectively. The drug ratio could be readily adjusted by changing the amounts of ATRA/hLs and DOX/rDNPs on demand. ATRA/hL@DOX/rDNPs prolonged the circulation time of both drug cargos and preserved the fixed synergistic drug ratio during the circulation. Furthermore, ATRA/hL@DOX/rDNPs could differentially release two drugs within the CSCs in the undifferentiated and differentiating states and bulk tumor cells in response to each characteristic biosignal. This cell-distinct drug release significantly enhanced the synergistic efficacy of the combined drugs with different anticancer mechanisms on eliminating the CSCs and bulk tumor cells. We showed that treatment with ATRA/hL@DOX/rDNPs effectively inhibited the tumor growth and metastasis of the CSC-enriched TNBC on the mouse models. Taken together, the hybrid nanoparticles with controlled cell-distinct drug release for co-delivery of differentiation-inducing agent and chemotherapeutic drug can be a promising nanotherapeutic strategy to treat the CSC-derived resistant tumors.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (82273876, 81971730, 81673381, 82104090), the Fok Ying-Tong Education Foundation for Young Teachers in the Higher Education Institutions of China (171028), the Project of State Key Laboratory of Natural Medicines of China Pharmaceutical University (SKLNMZZ202024, China), the Natural Science Foundation of Jiangsu Province (BK20210425, China) and the Postdoctoral Research Funding of Jiangsu Province (2021K051A, China). We acknowledge the Public platform of State Key Laboratory of Natural Medicines at China Pharmaceutical University for the use of analytical instrumentation facilities.
Author contributions
Ran Mo conceived and supervised the project. Shiyang Shen, Teng Li and Ran Mo designed the experiments, analyzed the data, and wrote the manuscript. Shiyang Shen, Teng Li, Jinyi Fan, Quanlin Shao, He Dong and Xiao Xu performed the experiments. All the authors have read and approved the final manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2022.11.009.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Vasan N., Baselga J., Hyman D.M. A view on drug resistance in cancer. Nature. 2019;575:299–309. doi: 10.1038/s41586-019-1730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shibue T., Weinberg R.A. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14:611–629. doi: 10.1038/nrclinonc.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarke M.F. Clinical and therapeutic implications of cancer stem cells. N Engl J Med. 2019;380:2237–2245. doi: 10.1056/NEJMra1804280. [DOI] [PubMed] [Google Scholar]
- 4.Batlle E., Clevers H. Cancer stem cells revisited. Nat Med. 2017;23:1124–1134. doi: 10.1038/nm.4409. [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Lytle N.K., Barber A.G., Reya T. Stem cell fate in cancer growth, progression and therapy resistance. Nat Rev Cancer. 2018;18:669–680. doi: 10.1038/s41568-018-0056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Mayea Y., Mir C., Masson F., Paciucci R., Lleonart M.E. Insights into new mechanisms and models of cancer stem cell multidrug resistance. Semin Cancer Biol. 2020;60:166–180. doi: 10.1016/j.semcancer.2019.07.022. [DOI] [PubMed] [Google Scholar]
- 8.Pece S., Tosoni D., Confalonieri S., Mazzarol G., Vecchi M., Ronzoni S., et al. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell. 2010;140:62–73. doi: 10.1016/j.cell.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Hurt E.M., Kawasaki B.T., Klarmann G.J., Thomas S.B., Farrar W.L. CD44+ CD24‒ prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. Br J Cancer. 2008;98:756–765. doi: 10.1038/sj.bjc.6604242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hua Z., White J., Zhou J. Cancer stem cells in TNBC. Semin Cancer Biol. 2021;82:26–34. doi: 10.1016/j.semcancer.2021.06.015. [DOI] [PubMed] [Google Scholar]
- 11.Li Z., Ding Y., Liu J., Wang J., Mo F., Wang Y., et al. Depletion of tumor associated macrophages enhances local and systemic platelet-mediated anti-PD-1 delivery for post-surgery tumor recurrence treatment. Nat Commun. 2022;13:1845. doi: 10.1038/s41467-022-29388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samanta D., Gilkes D.M., Chaturvedi P., Xiang L., Semenza G.L. Hypoxia-inducible factors are required for chemotherapy resistance of breast cancer stem cells. Proc Natl Acad Sci U S A. 2014;111:E5429–E5438. doi: 10.1073/pnas.1421438111. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Zhuang X., Zhang W., Chen Y., Han X., Li J., Zhang Y., et al. Doxorubicin-enriched, ALDH(br) mouse breast cancer stem cells are treatable to oncolytic Doxorubicin-enriched, ALDH(br) mouse breast cancer stem cells are treatable to oncolytic herpes simplex virus type 1. BMC Cancer. 2012;12:549. doi: 10.1186/1471-2407-12-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang S., Zhang H., Ghia E.M., Huang J., Wu L., Zhang J., et al. Inhibition of chemotherapy resistant breast cancer stem cells by a ROR1 specific antibody. Proc Natl Acad Sci U S A. 2019;116:1370–1377. doi: 10.1073/pnas.1816262116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saygin C., Matei D., Majeti R., Reizes O., Lathia J.D. Targeting cancer stemness in the clinic: from hype to hope. Cell Stem Cell. 2019;24:25–40. doi: 10.1016/j.stem.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 16.Ramos E.K., Hoffmann A.D., Gerson S.L., Liu H. New opportunities and challenges to defeat cancer stem cells. Trends Cancer. 2017;3:780–796. doi: 10.1016/j.trecan.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thé H de. Differentiation therapy revisited. Nat Rev Cancer. 2018;18:117–127. doi: 10.1038/nrc.2017.103. [DOI] [PubMed] [Google Scholar]
- 18.Arima Y., Nobusue H., Saya H. Targeting of cancer stem cells by differentiation therapy. Cancer Sci. 2020;111:2689–2695. doi: 10.1111/cas.14504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z., Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood. 2008;111:2505–2515. doi: 10.1182/blood-2007-07-102798. [DOI] [PubMed] [Google Scholar]
- 20.Zheng H., Jiang H., Hu S., Liao N., Shen D., Tian X., et al. Arsenic combined with all-trans retinoic acid for pediatric acute promyelocytic leukemia: report from the CCLG-APL2016 protocol study. J Clin Oncol. 2021;39:3161–3170. doi: 10.1200/JCO.20.03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merino V.F., Nguyen N., Jin K., Sadik H., Cho S., Korangath P., et al. Combined treatment with epigenetic, differentiating, and chemotherapeutic agents cooperatively targets tumoriInitiating cells in triple-negative breast cancer. Cancer Res. 2016;76:2013–2024. doi: 10.1158/0008-5472.CAN-15-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goranci-Buzhala G., Mariappan A., Ricci-Vitiani L., Josipovic N., Pacioni S., Gottardo M., et al. Cilium induction triggers differentiation of glioma stem cells. Cell Rep. 2021;36 doi: 10.1016/j.celrep.2021.109656. [DOI] [PubMed] [Google Scholar]
- 23.Marta H.-V., Tze-Kiong E., Luis B. Retinoic acid reduces stem cell-like features in retinoic acid reduces stem cell-like features in pancreatic cancer cells. Pancreas. 2015;44:918–924. doi: 10.1097/MPA.0000000000000373. [DOI] [PubMed] [Google Scholar]
- 24.Karmakar S., Banik N.L., Ray S.K. Combination of all-trans retinoic acid and paclitaxel-induced differentiation and apoptosis in human glioblastoma U87MG xenografts in nude mice. Cancer. 2008;112:596–607. doi: 10.1002/cncr.23223. [DOI] [PubMed] [Google Scholar]
- 25.Moro M., Bertolini G., Pastorino U., Roz L., Sozzi G. Combination treatment with all-trans retinoic acid prevents cisplatin-induced enrichment of CD133+ Tumor-Initiating Cells and Reveals heterogeneity of cancer stem cell compartment in lung cancer. J Thorac Oncol. 2015;10:1027–1036. doi: 10.1097/JTO.0000000000000563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen S., Xu X., Lin S., Zhang Y., Liu H., Zhang C., et al. A nanotherapeutic strategy to overcome chemotherapeutic resistance of cancer stem-like cells. Nat Nanotechnol. 2021;16:104–113. doi: 10.1038/s41565-020-00793-0. [DOI] [PubMed] [Google Scholar]
- 27.Sengupta S., Eavarone D., Capila I., Zhao G., Watson N., Kiziltepe T., et al. Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature. 2005;436:568–572. doi: 10.1038/nature03794. [DOI] [PubMed] [Google Scholar]
- 28.Wang Z., Ho P.C. Self-assembled core-shell vascular-targeted nanocapsules for temporal antivasculature and anticancer activities. Small. 2010;6:2576–2583. doi: 10.1002/smll.201001122. [DOI] [PubMed] [Google Scholar]
- 29.Meng H., Wang M., Liu H., Liu X., Situ A., Wu B., et al. Use of a lipid-coated mesoporous silica nanoparticle platform for synergistic gemcitabine and paclitaxel delivery to human pancreatic cancer in mice. ACS Nano. 2015;9:3540–3557. doi: 10.1021/acsnano.5b00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petrova V., Annicchiarico-Petruzzelli M., Melino G., Amelio I. The hypoxic tumour microenvironment. Oncogenesis. 2018;7:10. doi: 10.1038/s41389-017-0011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding Y., Wang Y., Hu Q. Recent advances in overcoming barriers to cell-based delivery systems for cancer immunotherapy. Exploration. 2022;2 doi: 10.1002/EXP.20210106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohyeldin A., Garzón-Muvdi T., Quiñones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 33.Plaks V., Kong N., Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16:225–238. doi: 10.1016/j.stem.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perche F., Biswas S., Wang T., Zhu L., Torchilin V.P. Hypoxia-targeted siRNA delivery. Angew Chem Int Ed. 2014;53:3362–3366. doi: 10.1002/anie.201308368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng S., Ouyang B., Xin Y., Zhao W., Shen S., Zhan M., et al. Hypoxia-degradable and long-circulating zwitterionic phosphorylcholine-based nanogel for enhanced tumor drug delivery. Acta Pharm Sin B. 2021;11:560–571. doi: 10.1016/j.apsb.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Broaders K.E., Grandhe S., Fréchet J.M.J. A biocompatible oxidation-triggered carrier polymer with potential in therapeutics. J Am Chem Soc. 2011;133:756–758. doi: 10.1021/ja110468v. [DOI] [PubMed] [Google Scholar]
- 37.Diehn M., Cho R.W., Lobo N.A., Kalisky T., Dorie M.J., Kulp A.N., et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi X., Zhang Y., Zheng J., Pan J. Reactive oxygen species in cancer stem cells. Antioxid Redox Signal. 2012;16:1215–1228. doi: 10.1089/ars.2012.4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye X., Li Q., Wang G., Sun F., Huang G., Bian X., et al. Mitochondrial and energy metabolism-related properties as novel indicators of lung cancer stem cells. Int J Cancer. 2011;129:820–831. doi: 10.1002/ijc.25944. [DOI] [PubMed] [Google Scholar]
- 40.Owusu-Ansah E., Banerjee U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature. 2009;461:537–541. doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sato A., Okada M., Shibuya K., Watanabe E., Seino S., Narita Y., et al. Pivotal role for ROS activation of p38 MAPK in the control of differentiation and tumor-initiating capacity of glioma-initiating cells. Stem Cell Res. 2014;12:119–131. doi: 10.1016/j.scr.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 42.Yang G., Phua S.Z.F., Lim W.Q., Zhang R., Feng L., Liu G., et al. A hypoxia-responsive albumin-based nanosystem for deep tumor penetration and excellent therapeutic efficacy. Adv Mater. 2019;31 doi: 10.1002/adma.201901513. [DOI] [PubMed] [Google Scholar]
- 43.Hu C.-M.J., Fang R.H., Wang K.-C., Luk B.T., Thamphiwatana S., Dehaini D., et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;526:118–121. doi: 10.1038/nature15373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang H., Le Q.V., Shim G., Oh Y.K., Shin Y.K. Molecular engineering of antibodies for site-specific conjugation to lipid polydopamine hybrid nanoparticles. Acta Pharm Sin B. 2020;10:2212–2226. doi: 10.1016/j.apsb.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thambi T., Deepagan V.G., Yoon H.Y., Han H.S., Kim S.-H., Son S., et al. Hypoxia-responsive polymeric nanoparticles for tumor-targeted drug delivery. Biomaterials. 2014;35:1735–1743. doi: 10.1016/j.biomaterials.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 46.Holmes C., Stanford W.L. Concise review: stem cell antigen-1: expression, function, and enigma. Stem Cell. 2007;25:1339–1347. doi: 10.1634/stemcells.2006-0644. [DOI] [PubMed] [Google Scholar]
- 47.Ginestier C., Hur M.H., Charafe-Jauffret E., Monville F., Dutcher J., Brown M., et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang T., Ma Y., Xu X., Ji Q., Feng M., Cheng C., et al. Enzyme-instructed hybrid nanogel/nanofiber oligopeptide hydrogel for localized protein delivery. Acta Pharm Sin B. 2021;11:2070–2079. doi: 10.1016/j.apsb.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oliphant M.U.J., Vincent M.Y., Galbraith M.D., Pandey A., Zaberezhnyy V., Rudra P., et al. Six 2 mediates late-stage metastasis via direct regulation of Sox 2 and induction of a cancer stem cell program. Cancer Res. 2019;79:720–734. doi: 10.1158/0008-5472.CAN-18-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zou J., Zhu X., Xiang D., Zhang Y., Li J., Su Z., et al. LIX1-like protein promotes liver cancer progression via miR-21-3p-mediated inhibition of fructose-1,6-bisphosphatase. Acta Pharm Sin B. 2021;11:1578–1591. doi: 10.1016/j.apsb.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li D., Wang Y., Li C., Wang Q., Sun B., Zhang H., et al. Cancer-specific calcium nanoregulator suppressing the generation and circulation of circulating tumor cell clusters for enhanced anti-metastasis combinational chemotherapy. Acta Pharm Sin B. 2021;11:3262–3271. doi: 10.1016/j.apsb.2021.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.