Abstract
Demographic factors are fundamental in shaping infectious disease dynamics. Aspects of populations that create structure, like age and sex, can affect patterns of transmission, infection intensity and population outcomes. However, studies rarely link these processes from individual to population-scale effects. Moreover, the mechanisms underlying demographic differences in disease are frequently unclear. Here, we explore sex-biased infections for a multi-host fungal disease of bats, white-nose syndrome, and link disease-associated mortality between sexes, the distortion of sex ratios and the potential mechanisms underlying sex differences in infection. We collected data on host traits, infection intensity and survival of five bat species at 42 sites across seven years. We found females were more infected than males for all five species. Females also had lower apparent survival over winter and accounted for a smaller proportion of populations over time. Notably, female-biased infections were evident by early hibernation and likely driven by sex-based differences in autumn mating behaviour. Male bats were more active during autumn which likely reduced replication of the cool-growing fungus. Higher disease impacts in female bats may have cascading effects on bat populations beyond the hibernation season by limiting recruitment and increasing the risk of Allee effects.
Keywords: sex-biased infection, emerging infectious disease, white-nose syndrome, bats, Allee effects, fungal disease
1. Introduction
Emerging infectious diseases are a serious threat to wildlife health [1,2]. Population structure can shape epidemics by influencing spatial spread, outbreak size and host impacts [2–4]. Elements of populations that create structure such as classes of individuals of specific ages, sexes or breeding stages can have profound effects on disease dynamics [3,5–9]. Sex is an especially important factor because sex-biases in infections can contribute to differential transmission within populations due to behaviour [10–15], amplify outbreaks due to seasonal changes in susceptibility [16–19] and modify population impacts through disproportionate mortality [20–22]. Differences in infection and mortality can also modulate virulence evolution through sex-specific immune responses that affect pathogen replication and growth [23,24]. As such, determining patterns and mechanisms of sex-biases in infections will improve efforts to minimize outbreaks and manage impacts.
Behavioural and physiological traits are two mechanisms that can produce sex-biases in transmission, susceptibility, infection intensity or disease-induced mortality [25–27]. In most systems, males have an elevated risk of disease compared to females due to territory defense and promiscuous mating, which enhances contacts with conspecifics or increases pathogen susceptibility due to physiological stress [9,25,28]. Sex hormones can also have strong effects on host immunity [29], such that testosterone suppresses immune responses while estrogens enhance it, resulting in weaker immune responses in males and increased susceptibility to pathogen infection [25,30,31]. As such, the majority of empirical studies find that infections are typically male-biased [28,32,33], although this generalization is sometimes reversed [34–40] or weak [41] and may be linked to seasonal reproductive stress associated with pregnancy, parturition or parental care [16,17]. Thus, it is likely that the effects of sex-biased infections may be highly pronounced in disease systems where host reproductive strategies differ seasonally among sexes [19].
White-nose syndrome (WNS) is a highly seasonal fungal disease of bats that has caused population collapse, with declines exceeding 95% in many populations of multiple species [42–46]. WNS is caused by the fungal pathogen, Pseudogymnoascus destructans, which invades the epidermal tissue of bats during hibernation, and disrupts bat homeostasis, causing water and electrolyte imbalances that increase arousals and deplete stored fat [47–54]. Transmission of P. destructans occurs through host-to-host contacts and contact between hosts and contaminated environmental reservoirs inside winter hibernation sites [55–59]. Pseudogymnoascus destructans has a temperature growth range of 0–20°C and optimal growth between 12 and 16°C [60]. This limits on-host growth to the periods when bats are in torpor and they lower their body temperature to the ambient temperatures of their hibernation sites [60–62]. The restricted growth of P. destructans above 20°C drives the seasonal patterns of WNS, with infection only occurring during winter hibernation and mortality peaking during late winter [56,59,63].
Temperate bat species have seasonal sex-biased differences in behaviours that may influence their exposure, susceptibility and mortality from WNS [64–69]. Bats mate in autumn swarms, and females store sperm over winter, delaying ovulation and giving birth at maternity colonies in spring [70]. Male reproductive energy expenditures are highest during autumn when they aggregate at hibernacula (subterranean sites where bats spend the winter) and mate indiscriminately with females [71]. Since autumn swarm coincides with the seasonal transmission of P. destructans [63], breeding stress among males as well as exposure to the environmental reservoirs [56,58] could increase their susceptibility to infection. Female energy expenditures are greatest during pregnancy and lactation in spring and summer when hosts typically clear infection [72,73] and transmission of P. destructans is low [63], suggesting females may be the less infected sex. However, female bats may spend more time torpid during winter than males to conserve energy for spring reproduction [66] which could influence the growth of P. destructans [60]. Due to the differences in reproductive investment and seasonal energy expenditure, sex-biased traits have the potential to affect exposure (e.g. contacting P. destructans from other hosts or in the environment) and pathogen growth (e.g. time spent at torpid temperatures that permit fungal growth). Thus, we predict sex-specific behaviours and the highly seasonal pattern of WNS may drive sex differences in infection, mortality and population impacts. Here, we examined differences in P. destructans infection between females and males across five bat species in 42 sites across 7 years. We then assessed the consequences of sex differences in infection at both the individual and population level. Lastly, we explored autumn activity patterns between sexes as a potential mechanism of sex-biased infections.
2. Materials and methods
(a) . Study sites and sampling design
We sampled bats at hibernacula in Illinois, Massachusetts, Michigan, New York, Vermont, Virginia and Wisconsin between 2011 and 2021 during a seasonal epizootic. Generally, bats were sampled at two time points: early winter (between November and December) and again in late winter (between March and April). During site visits, we walked each section of the hibernacula and counted all the bats of each species and resighted any bats that were previously banded. We sampled up to 20 bats of each species during each visit when possible and stratified sample collection throughout hibernacula to obtain a sample reflective of the spatial distribution of bats at each site. Bat species included little brown (Myotis lucifugus), northern long-eared (Myotis septentrionalis), tricolored (Perimyotis subflavus), big brown (Eptesicus fuscus) and Indiana (Myotis sodalis) bats. The animal sampling protocols were approved by the Virginia Tech IACUC protocol 17-180. We followed the field decontamination procedures outlined by the United States Fish and Wildlife Service Decontamination Guidelines as well as the recommendations provided by individual states.
(b) . Pseudogymnoascus destructans sample collection and quantification
For every bat we sampled, we determined species and biological sex based on external morphology and attached an aluminium-lipped band (2.4, 2.9 and 4.2 mm; Porzana, Icklesham, UK) to the wing for individual identification and resighting. We collected an epidermal swab from individuals to quantify infection prevalence (the fraction of individuals positive for P. destructans) and severity (the quantity of P. destructans on infected bats) using a standardized swabbing technique [45,63]. We placed swabs in RNAlater for storage before testing. We extracted DNA and tested for P. destructans presence and quantity by qPCR using protocols developed specifically for this fungal pathogen that included a standard growth curve [63,74].
(c) . Activity data collection
At three sites in Wisconsin in 2020, we installed radio frequency identification (RFID) systems, consisting of a passive antenna and an automated data logger (IS10001; Biomark, Boise, ID) at the entrances of each hibernaculum (2–4 per site). The systems at each entrance were equipped with a solar or direct power source to run continuously. During autumn swarm, we captured little brown bats swarming near hibernacula and injected 12.5 mm PIT tags (Biomark APT12; Biomark, Boise, ID). Each RFID system was programmed to record each time a unique individual passed through an entrance with a 1 min delay to avoid duplicate detections of tags. We scored a detection as an active bat and used it to characterize the activity of females and males.
(d) . Statistical analyses
(i) . Infection
We used generalized (GLMM) and linear (LMM) mixed effect models [75] to compare differences in P. destructans prevalence and infection severity between males and females of each of the five species. We defined prevalence as the fraction of bats testing positive for P. destructans on qPCR (0|1), and infection severity as the log10-transformed quantity of fungal DNA (ng) on positive bats (log10 fungal loads) which is a well-established metric of infection severity [43,76,77]. First, we performed a preliminary analysis of the effects of sex on disease in early hibernation using a GLMM with a binomial distribution for prevalence and a LMM with a Gaussian distribution for fungal loads with each including sex as a fixed effect and species as a random effect. We then estimated differences in prevalence between sexes using a GLMM with a binomial distribution and sex and species as fixed effects (Model 1, electronic supplementary material, appendix S1.1) and compared additive and interactive versions of the model using Akaike information criterion (AIC; electronic supplementary material, appendix S1.2). For prevalence models, we only used data from early hibernation as every bat was infected at the end of hibernation.
We used two datasets to examine changes in fungal loads over winter (between early and late hibernation) between sexes. First, to estimate population-level differences in fungal loads, we used a LMM with fixed additive effects of species and sex and date as a fixed effect interacting with each term (Model 2, electronic supplementary material, appendix S2.1). Fungal loads are well-correlated with mortality [43,76,78], and bats that begin hibernation with higher fungal loads are more likely to die of their infections before the end of winter [79], potentially resulting in survival biases in population-level analyses [43]. Therefore, in a second analysis, we also explored whether growth rates of P. destructans on individual female and male bats that were recaptured over winter clearly differed [43]. For example, if one sex had higher fungal loads than the other and subsequently died of their infections, these highly infected bats would be omitted from the late hibernation population-level sample [77]. By comparing the difference between late and early hibernation fungal loads on individual recaptured bats, we can directly assess whether female and male bats have different growth rates and better identify the timing of transmission differences that may underlie sex-biases in infection. Therefore, we compared the over winter increase in fungal loads on recaptured individuals (the log10 difference between late hibernation and early hibernation loads) using a LMM with sex as a fixed effect and site as a random effect (Model S1A, electronic supplementary material, appendix S5.1.1). Lastly, to explore differences in sex-based ecology that could lead to differences in infection between sexes, we also examined models including a priori hypothesized effects of roosting temperature and body mass on early hibernation prevalence and fungal loads but found no clear improvements over models with sex and species using AIC (electronic supplementary material, appendices S5.2 and S5.3). We used site as a random effect for all infection analyses and confirmed the performance of our infection models using fivefold cross-validation (see electronic supplementary material for description).
(ii) . Individual survival
We examined differences between sexes in over winter survival using data from individual little brown bats, which were abundant enough to obtain reasonable sample sizes. We compared the probability of observing bats in late hibernation (March) that we observed in early hibernation (November). Bats affected by WNS often emerge prematurely (mid-winter) and die on the landscape [80], enabling the use of recapture as an estimate of apparent survival between sampling dates [79,81]. We used a mixed effects model with site as a random effect with a binomial distribution and a logit link to quantify how sex affected the probability of a bat being resighted over winter (Model 3A; electronic supplementary material, appendix S3.1).
(iii) . Population-level impact of disease
We estimated the proportion of female little brown bats sampled at each visit from a core set of sites (n = 14) that were sampled in consecutive years to evaluate how female proportions were changing over time. We estimated the probability of sampling a female versus a male (1|0) over time using a generalized linear mixed model with a binomial distribution and a logit link with continuous years since P. destructans invasion as a fixed effect, and site as a random effect (Model 3B; electronic supplementary material, appendix S3.2).
(iv) . Radio frequency identification activity
To explore differences in autumn activity of female and male bats, we used a generalized linear mixed model with a binomial distribution and a logit link with sex and site as fixed effects (we did not include site as a random effect due to the small number of sites) and individual bat identification as a random effect (Model 4; electronic supplementary material, appendix S4.1). For our response variable, we treated each bat as a series of binomial trials where it could be detected (=1) or not detected (=0) on a given night throughout autumn swarm that any RFID system was operational at an entrance, as determined by the detection of a programmed test tag. We determined the period of autumn swarm to be between the dates in which more than 95% of all individual detections occurred (beginning of swarm; 19 August) and all individual detections concluded (end of swarm; 01 October). Results were qualitatively similar if we included all dates in which any bat was detected from early August to November. Since bats are nocturnal and daily activity patterns span 2 calendar days, we treated detections that occurred between hours 0 : 00–07 : 00 as a detection during the previous night for consistency with bat ecology. We used fivefold cross-validation to check the performance of our model (see electronic supplementary material for description).
3. Results
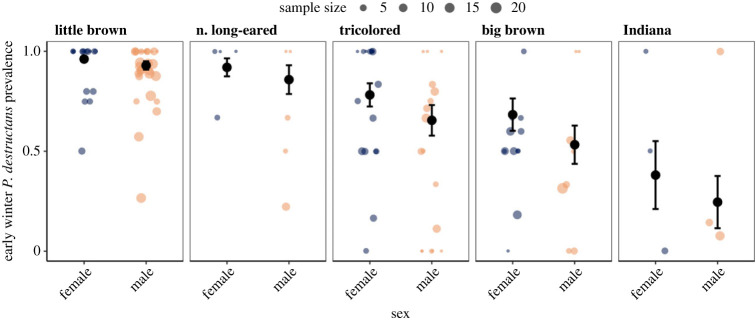
We examined the effects of sex on P. destructans infections of five bat species during winter (electronic supplementary material, table S1). We sampled a total of 665 females and 1071 males across all species, sites, years and seasons (electronic supplementary material, table S2A–D). On average, females had higher P. destructans prevalence than male bats in early winter (GLMM with site and species as random effects; female coef ± s.e.: 0.630 ± 0.221, p = 0.0043) and suffered from higher fungal loads (LMM with site and species as random effects; female coef ± s.e.: 0.487 ± 0.086, p < 0.0001). The model predicting early winter P. destructans prevalence, including species and an additive effect of sex, was better supported over the interactive version (ΔAIC = −3.11; electronic supplementary material, appendices S1.2–1.3), suggesting female infections are generally higher across all bat species in our study (GLMM with site as random effect; female coef ± s.e.: 0.637 ± 0.223, p = 0.0044; figure 1; electronic supplementary material, appendix S1.1).
Figure 1.
Early winter P. destructans prevalence by sex for five bat species. The coloured points show the proportion of individuals that were infected at a given site and year. The size of coloured points is scaled to the number of bats sampled during the site visit. The black circles show model-predicted prevalence with s.e. bars. Sample sizes are provided in the electronic supplementary material, table S2A.
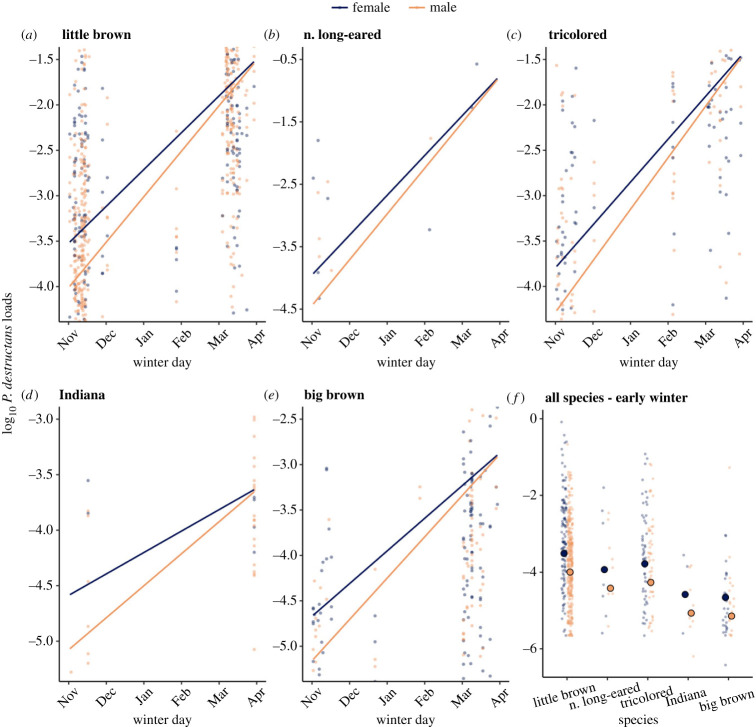
The differences in infection prevalence during early winter aligned with higher fungal loads on female bats during the hibernation season. Across species, females began hibernation with higher fungal loads than males (LMM with site as random effect; female coef ± s.e.: 0.490 ± 0.093, p < 0.0001; figure 2; electronic supplementary material, appendix S2.1). Fungal loads appeared to become more similar between females and males by the end of winter (smaller date slope for females (=0.405) versus males (=0.501); figure 2; electronic supplementary material, appendix S2.4). However, this may be an artefact of higher female mortality (figure 3; electronic supplementary material, appendix S3.1) such that individuals with higher fungal loads in early hibernation are less likely to survive to late winter, resulting in only bats with low early hibernation infections surviving to be sampled in late hibernation [77,79]. To assess whether mortality bias might be responsible for the apparent similarity of infections between sexes in late hibernation, we used data on differences in fungal loads of recaptured individual little brown bats sampled in both early and late winter. We found that the change in fungal loads over winter did not differ between sexes in the absence of selective mortality (LMM with site as a random effect; female coef ± s.e.: 0.090 ± 0.118, p = 0.448; electronic supplementary material, figure S1A and appendix S5.1.1).
Figure 2.
(a–e) Change in P. destructans infection severity (fungal loads) over winter by sex for each of the five host species. A point represents an individual bat, and females and males are shown in blue and orange, respectively. Lines represent model-predicted fungal loads over time. Sample sizes by species are provided in the electronic supplementary material, table S2A. (f) Fungal loads in early winter by sex for each host species. Larger circles outlined in black show estimated fungal loads on 1 November extracted from model predictions used in (a–e) to visualize the differences in early winter infections across species.
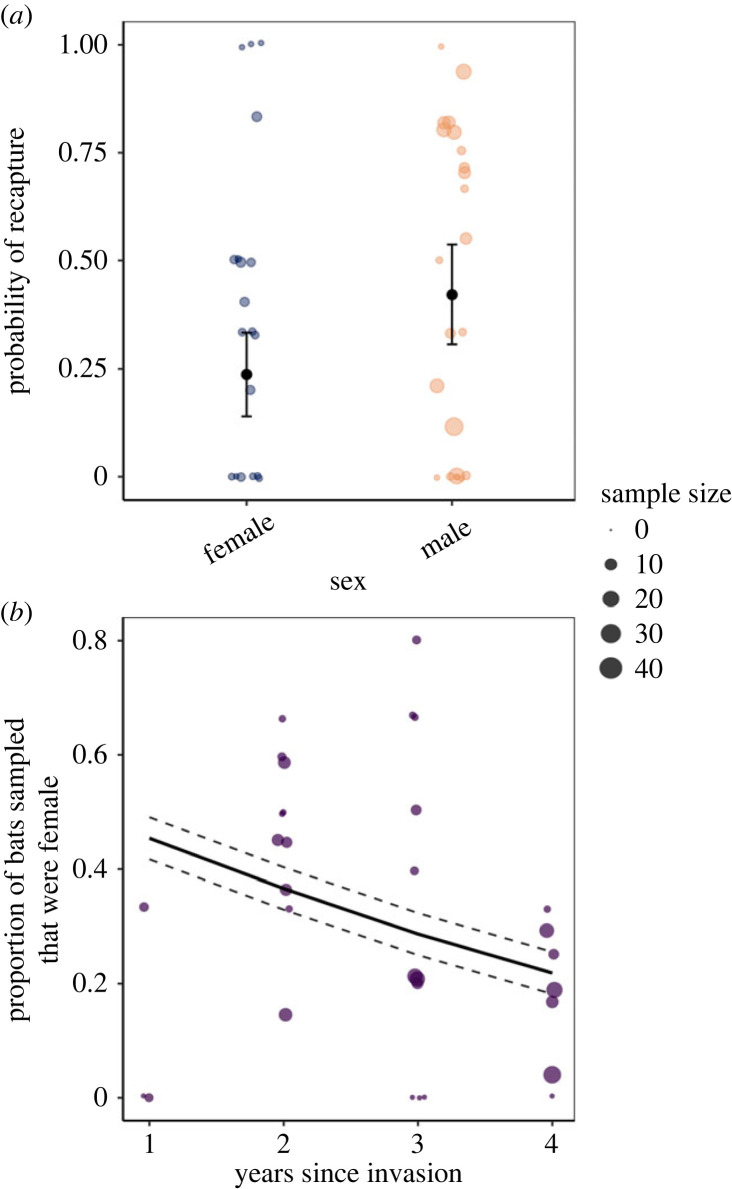
Figure 3.
(a) Recapture probability of little brown bats by sex. Blue and orange points show the observed probability of recapture from early (November) and late (March) winter from a given site and year for females and males, respectively. Black points and vertical bars represent the estimated mean probability of recapture and s.e. Sample sizes are provided in the electronic supplementary material, table S2B. (b) Proportional change in females with time since P. destructans invasion. Points show the proportion of all sampled individuals that were female at the same sites sampled over time since the invasion of P. destructans. The solid black line shows the model-predicted proportion of females and dashed lines show the s.e. around the model estimates. The size of points in both panels is scaled to the number of bats sampled during the site visit. Sample sizes are provided in the electronic supplementary material, table S2C.
We did not find support that hibernation temperatures or body mass significantly improved models of prevalence and fungal loads that already included sex (ΔAIC < 2; electronic supplementary material, appendix S5.2 and S5.3) and hibernation temperatures of male and female bats broadly overlapped (electronic supplementary material, figure S2 and appendix S5.2).
At an individual scale, using data from little brown bats that were banded in early hibernation (electronic supplementary material, table S2B), we found clear support that female little brown bats were less likely to be recaptured in late winter than males (GLMM with site as random effect; female coef ± s.e.: −0.855 ± 0.380, p = 0.0240; figure 3a; electronic supplementary material, appendix S3.1). At a population level, the proportion of females sampled in the same populations at the end of winter (electronic supplementary material, table S2C) decreased with time since WNS invasion (GLMM with site as random effect; years since invasion coef ± s.e.: −0.363 ± 0.173, p = 0.0365; figure 3b; electronic supplementary material, appendix S3.2). Collectively, these data suggest that females have higher, more severe infections (figures 1 and 2) and correspondingly experience higher mortality during winter (figure 3).
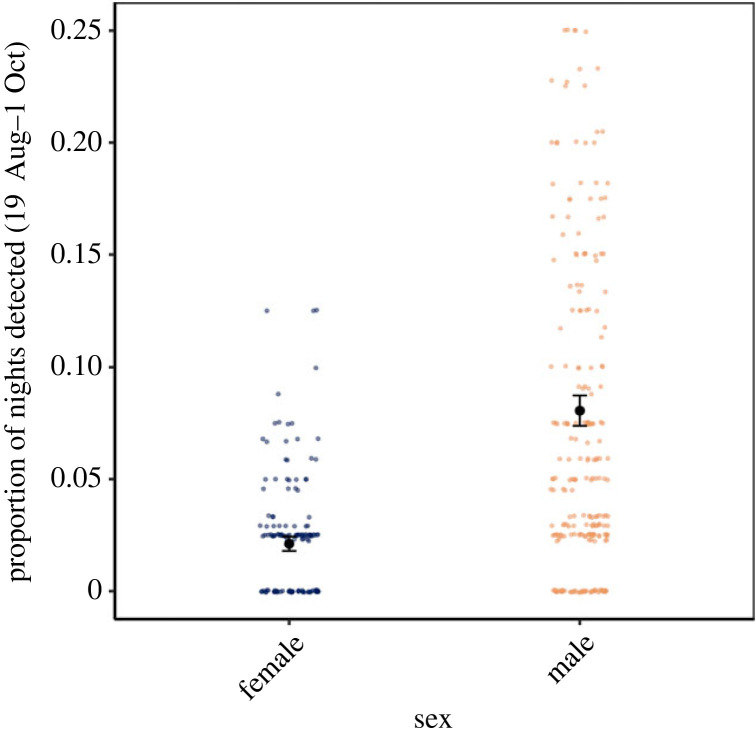
Females begin hibernation with more severe infections than male bats and differential activity between sexes may influence infections. We found that the probability of a female being active on a given night was fourfold lower than males during autumn. (GLMM with individual bat as random effect; female coef ± s.e.: −1.401 ± 0.171, p < 0.0001; figure 4; electronic supplementary material, table S2D and appendix S4.1).
Figure 4.
Differences in autumn activity of female and male little brown bats at three hibernacula. Each data point represents the proportion of nights an individual bat was active at a site. The proportion of nights active was calculated as the number of nights an individual was detected at least once on the RFID systems divided by the total number of nights the RFID systems at each site were operating throughout autumn swarm. Points at 0 represent tagged bats that were never detected on a reader. Black points show model-predicted activity by sex with s.e. denoted with vertical bars. Sample sizes are provided in the electronic supplementary material, table S2D.
4. Discussion
Our results demonstrate that sex differences in infection have an important role in disease dynamics. Across all species, females experienced higher infection prevalence (figure 1) and fungal loads by the beginning of winter hibernation (figure 2). In little brown bats, females were also less likely to be recaptured over winter (figure 3a), as we would expect if more severe infections resulted in higher mortality among females [43,56,76,77,79]. Finally, female-biased mortality associated with time since disease arrival likely contributed to population-level reductions in females over time (figure 3b). Higher impacts to female bats may mask even more severe long-term impacts from WNS [46,82] as a disproportionate loss of recruiting females may be expected to have more negative population-level consequences than male-biased declines.
We consistently observed higher pathogen prevalence and intensity in females than males across bat species (figures 1 and 2). In other vertebrates, males maximize fitness at the cost of greater exposure and susceptibility to pathogens [33,83–85], in part through higher testosterone concentrations which often correlate negatively with immune defense [86–88]. Given male bats undergo their most substantial investment in reproduction during autumn swarm compared to females that invest relatively little towards reproduction throughout autumn and over winter [70], we expected infection to be higher in males. However, we found the opposite pattern with females having more severe disease. Drivers of higher female infections likely include sex-based physiological differences [25]. Temperate female bats use fat stores acquired in autumn more slowly than males suggesting a more substantial constraint on energy expenditure compared to males throughout the autumn to spring period [66,67]. Therefore, different physiological strategies (e.g. torpor patterns) between female and male bats could be shaping sex-biased infections.
Differences in sex-based physiology and behaviour were supported by our data showing that the proportion of nights males were active during autumn was substantially higher than females (figure 4). Differences in autumn activity patterns are likely motivated by differences in reproductive energy allocation between sexes. For males, their fitness is enhanced by remaining active so they can maximize mating opportunities with females. However, female bats, which store sperm and delay ovulation until spring, may prefer to conserve energetic resources during fall and use torpor more extensively [67]. As bats arrive to contaminated hibernacula [56,58] for autumn swarm and are exposed to the pathogen, torpor use in autumn may permit pathogen growth as bats reduce their body temperatures to be within the thermal range for P. destructans replication. The relationship between sex-based activity and fungal intensity may arise through two potential pathways associated with torpor expression. First, less active females may provide favourable conditions for pathogen growth for a longer period prior to hibernation compared to males, thus resulting in the more severe female infections. Second, more active males may be able to inhibit pathogen growth through greater euthermia compared to females, as euthermic mammals mount more robust immune defenses than torpid or hibernating mammals [89,90]. Our activity estimates, which are consistent with other studies [65], suggest that vast differences between male and female bats' energy use strategies during the mating period likely contribute to differences in infection.
We were less likely to recapture female little brown bats over winter than males, suggesting greater mortality in females (figure 3a). Further, the sex ratio shifted to be more male-biased as WNS progressed (figure 3b), suggesting that fungal infection may be driving female-specific mortality. Generally, infections increase on bats over winter before reaching a threshold at which bats die from their infections [43], and this bias in survival of the least infected individuals in early hibernation (electronic supplementary material, figure S1B and appendix S5.1.3) could explain why male and female infections become more similar at the end of hibernation (figure 2). On average, female bats began hibernation with higher fungal loads than males (figure 1) but have similar fungal growth rates (electronic supplementary material, figure S1A and appendix S5.1) suggesting that females reach high infection levels that result in mid-winter mortality, thus, are removed from the sample population by late winter. This relationship between high early hibernation fungal loads and mortality is further supported by previous studies that directly link early hibernation fungal loads with mortality and impacts [43,79]. Our findings are supported by previous work demonstrating that WNS severity is positively correlated with mortality, which is mechanistically linked by more frequent arousals [43,47,53,77,81]. Several lines of evidence also suggest that females did not leave hibernation sites early and survive elsewhere. In our study region, emergence from hibernation in healthy populations does not begin until four to six weeks after our sampling [91]. Given that the proportion of females declined with WNS progression (figure 3b), our results suggest that disease-associated mortality may be contributing to the reduction in overwinter recaptures of females. Previous studies on sex differences in survival associated with WNS have found contrasting results. One study observed lower female survival in naturally infected bats [68] whereas another reported higher female survival in experimental infections where each sex was inoculated with the same dose of P. destructans at identical times [92]. Our results suggest that differences in disease outcomes between females and males may originate from differences in pathogen growth during autumn. Therefore, inoculating both sexes with the same dose simultaneously in the experimental infection may have eliminated the underlying difference in early winter infections between female and males.
We provide the first spatio-temporally broad evidence from the field that sex-based survival of bats affected by WNS likely scales to population-level impacts. In the first year after P. destructans invasion, the mean proportion of females in late hibernation was 45%, but this percentage declined to 21% after 4 years of WNS (figure 3b). The reduction in the percent of females with time since pathogen invasion differs from previous studies of healthy bat populations that show relatively consistent interannual proportions of females [93–96]. This suggests that a comparable decline in females is not typical in non-diseased populations. Losing females at higher rates than males will likely affect how bat populations respond to WNS. First, reducing the number of females could limit the recruitment potential of bat populations and is likely to be especially critical for imperiled populations that remain small and vulnerable to Allee effects. Many temperate bat species, including little brown bats, form maternity colonies during summer when they cooperatively rear offspring and larger colony sizes can allow females to expend less energy [97]. If birth and offspring survival rates decline with density, as shown in other temperate bat species [98], the disproportionate loss of females could further impact recruitment. Second, distorted sex ratios may negatively affect reproductive success during autumn. In other taxa, a significant increase in male to female sex ratios resulted in increased stress to females from exacerbated pressure for males to mate with limited females, negatively affecting female fecundity [99,100].
Our results include impacts to female bats up to 4 years after the arrival of WNS, which includes the epidemic phase of the disease, when the majority of mortality occurs [46]. Females are likely under strong selection pressure to evolve mechanisms of survival given their increased mortality and will need to adapt for populations to rebound [101]. Future work focusing on the effects of female infection and mortality biases on bat population persistence and recovery could benefit conservation efforts, especially as the negative effects are likely to compound over time if sex ratios become increasingly distorted.
We find a novel example in which female-biased infections may contribute to population-level impacts of an emerging disease. Our results provide a clear, yet less frequently observed, instance of an emerging pathogen that consistently impacts females more than males regardless of host species. We describe a new mechanistic explanation to female-biased infections that links temperature-dependent fungal growth to sex-specific seasonal physiology. Ultimately, disparate infections among demographic classes of hosts are fundamental for understanding and managing emerging infectious diseases, and cross-scale analyses can provide insights into the important consequences of demographic biases on disease systems.
Acknowledgements
We thank Steffany Yamada for data curation support, Skylar Hopkins for analysis support, Rick Reynolds, Carl Herzog, and University of Wisconsin Milwaukee Field Station for field and logistical support, and the many landowners for site access.
Ethics
All bat handling procedures were reviewed and approved by Virginia Tech Institute for Animal Care and Use Committee protocol 17–180.
Data accessibility
The datasets supporting this article have been deposited in the Dryad Digital Repository [102]. Exact site locations are not disclosed to protect endangered species and landowners.
The supporting materials including the appendix, tables, and figures are provided in the electronic supplementary material [103].
Authors' contributions
M.J.K.: conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing—original draft and writing—review and editing; J.R.H.: conceptualization, data curation, funding acquisition, investigation, methodology, project administration, resources, visualization and writing—review and editing; J.P.W.: investigation and writing—review and editing; H.M.K.: investigation and writing—review and editing; J.A.R.: investigation; A.E.L.: data curation, resources and writing—review and editing; T.E.R.: resources and writing—review and editing; J.E.D.: investigation, resources and writing—review and editing; W.H.S.: investigation and resources; K.L.P.: investigation and writing—review and editing; J.T.F.: funding acquisition, investigation and writing—review and editing; A.M.K.: funding acquisition, investigation and writing—review and editing; K.E.L.: conceptualization, data curation, funding acquisition, investigation, methodology, project administration, resources, supervision, visualization, writing—original draft and writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
The authors declare no competing interests.
Funding
The research was funded by NSF grants DEB-1115895 to A.M.K. and J.T.F., DEB-1911853 to K.E.L., J.R.H., A.M.K. and J.T.F., the USFWS (grant no. F17AP00591) to K.E.L., and by Virginia Tech Institute for Critical Technology and Applied Science to M.J.K.
Disclaimer
Any use of trade, firm or product names is for descriptive purposes only and does not imply endorsement by the US Government.
References
- 1.Daszak P, Cunningham AA, Hyatt AD. 2000. Emerging infectious diseases of wildlife: threats to biodiversity and human health. Science 287, 443-449. ( 10.1126/science.287.5452.443) [DOI] [PubMed] [Google Scholar]
- 2.De Castro F, Bolker B. 2004. Mechanisms of disease-induced extinction. Ecol. Lett. 8, 117-126. ( 10.1111/j.1461-0248.2004.00693.x) [DOI] [Google Scholar]
- 3.Silk MJ, Hodgson DJ, Rozins C, Croft DP, Delahay RJ, Boots M, McDonald RA. 2019. Integrating social behaviour, demography and disease dynamics in network models: applications to disease management in declining wildlife populations. Phil. Trans. R. Soc. B 374, 20180211. ( 10.1098/rstb.2018.0211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lloyd-Smith JO, Schreiber SJ, Kopp PE, Getz WM. 2005. Superspreading and the effect of individual variation on disease emergence. Nature 438, 355-359. ( 10.1038/nature04153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.VanderWaal KL, Ezenwa VO. 2016. Heterogeneity in pathogen transmission: mechanisms and methodology. Funct. Ecol. 30, 1606-1622. ( 10.1111/1365-2435.12645) [DOI] [Google Scholar]
- 6.Rogers W, Brandell EE, Cross PC. 2022. Epidemiological differences between sexes affect management efficacy in simulated chronic wasting disease systems. J. Appl. Ecol. 59, 1122-1133. ( 10.1111/1365-2664.14125) [DOI] [Google Scholar]
- 7.Silk MJ, Croft DP, Delahay RJ, Hodgson DJ, Boots M, Weber N, McDonald RA. 2017. Using social network measures in wildlife disease ecology, epidemiology, and management. Bioscience 67, 245-257. ( 10.1093/biosci/biw175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorsich EE, Ezenwa VO, Cross PC, Bengis RG, Jolles AE. 2015. Context-dependent survival, fecundity and predicted population-level consequences of brucellosis in African buffalo. J. Anim. Ecol. 84, 999-1009. ( 10.1111/1365-2656.12356) [DOI] [PubMed] [Google Scholar]
- 9.Perkins SE, Ferrari MF, Hudson PJ. 2008. The effects of social structure and sex-biased transmission on macroparasite infection. Parasitology 135, 1561-1569. ( 10.1017/S0031182008000449) [DOI] [PubMed] [Google Scholar]
- 10.Rogers LM, Delahay R, Cheeseman CL, Langton S, Smith GC, Clifton-Hadley RS. 1998. Movement of badgers (Meles meles) in a high-density population: individual, population and disease effects. Proc. R. Soc. Lond. Ser. B 265, 1269-1276. ( 10.1098/rspb.1998.0429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham J, Smith GC, Delahay RJ, Bailey T, McDonald RA, Hodgson D. 2013. Multi-state modelling reveals sex-dependent transmission, progression and severity of tuberculosis in wild badgers. Epidemiol. Infect. 141, 1429-1436. ( 10.1017/S0950268812003019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrari N, Cattadori IM, Nespereira J, Rizzoli A, Hudson PJ. 2003. The role of host sex in parasite dynamics: field experiments on the yellow-necked mouse Apodemus flavicollis. Ecol. Lett. 7, 88-94. ( 10.1046/j.1461-0248.2003.00552.x) [DOI] [Google Scholar]
- 13.Grear DA, Luong LT, Hudson PJ. 2012. Sex-biased transmission of a complex life-cycle parasite: why males matter. Oikos 121, 1446-1453. ( 10.1111/j.1600-0706.2012.20358.x) [DOI] [Google Scholar]
- 14.Stephenson JF. 2019. Parasite-induced plasticity in host social behaviour depends on sex and susceptibility. Biol. Lett. 15, 20190557. ( 10.1098/rsbl.2019.0557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ezenwa VO, Snider MH. 2016. Reciprocal relationships between behaviour and parasites suggest that negative feedback may drive flexibility in male reproductive behaviour. Proc. R. Soc. B 283, 20160423. ( 10.1098/rspb.2016.0423) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sweeny AR, Corripio-Miyar Y, Bal X, Hayward AD, Pilkington JG, McNeilly TN, Nussey DH, Kenyon F. 2022. Longitudinal dynamics of co-infecting gastrointestinal parasites in a wild sheep population. Parasitology 149, 593-604. ( 10.1017/S0031182022000324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cattadori IM, Boag B, Bjørnstad ON, Cornell SJ, Hudson PJ. 2005. Peak shift and epidemiology in a seasonal host-nematode system. Proc. R. Soc. B 272, 1163-1169. ( 10.1098/rspb.2004.3050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altizer S, Davis AK, Cook KC, Cherry JJ. 2004. Age, sex, and season affect the risk of mycoplasmal conjunctivitis in a southeastern house finch population. Can. J. Zool. 82, 755-763. ( 10.1139/z04-050) [DOI] [Google Scholar]
- 19.Altizer S, Dobson A, Hosseini P, Hudson P, Pascual M, Rohani P. 2006. Seasonality and the dynamics of infectious diseases. Ecol. Lett. 9, 467-484. ( 10.1111/j.1461-0248.2005.00879.x) [DOI] [PubMed] [Google Scholar]
- 20.Miller MR, White A, Wilson K, Boots M. 2007. The population dynamical implications of male-biased parasitism in different mating systems. PLoS ONE 2, e624. ( 10.1371/journal.pone.0000624) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosa GM, Bosch J, Martel A, Pasmans F, Rebelo R, Griffiths RA, Garner TWJ. 2019. Sex-biased disease dynamics increase extinction risk by impairing population recovery. Anim. Conserv. 22, 579-588. ( 10.1111/acv.12502) [DOI] [Google Scholar]
- 22.Härkönen T, Harding K, Rasmussen TD, Teilmann J, Dietz R. 2007. Age- and sex-specific mortality patterns in an emerging wildlife epidemic: the phocine distemper in European harbour seals. PLoS ONE 2, e887. ( 10.1371/journal.pone.0000887) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Úbeda F, Jansen VAA. 2016. The evolution of sex-specific virulence in infectious diseases. Nat. Commun. 7, 13849. ( 10.1038/ncomms13849) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall MD, Mideo N. 2018. Linking sex differences to the evolution of infectious disease life-histories. Phil. Trans. R. Soc. B 373, 20170431. ( 10.1098/rstb.2017.0431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guerra-Silveira F, Abad-Franch F. 2013. Sex bias in infectious disease epidemiology: patterns and processes. PLoS ONE 8, e62390. ( 10.1371/journal.pone.0062390) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDonald JL, Smith GC, McDonald RA, Delahay RJ, Hodgson D. 2014. Mortality trajectory analysis reveals the drivers of sex-specific epidemiology in natural wildlife–disease interactions. Proc. R. Soc. B 281, 20140526. ( 10.1098/rspb.2014.0526) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zuk M. 2009. The sicker sex. PLoS Pathog. 5, e1000267-e. ( 10.1371/journal.ppat.1000267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuk M, McKean KA. 1996. Sex differences in parasite infections: patterns and processes. Int. J. Parasitol. 26, 1009-1024. ( 10.1016/S0020-7519(96)80001-4) [DOI] [PubMed] [Google Scholar]
- 29.Ezenwa VO, Stefan Ekernas L, Creel S. 2012. Unravelling complex associations between testosterone and parasite infection in the wild. Funct. Ecol. 26, 123-133. ( 10.1111/j.1365-2435.2011.01919.x) [DOI] [Google Scholar]
- 30.Foo YZ, Nakagawa S, Rhodes G, Simmons LW. 2017. The effects of sex hormones on immune function: a meta-analysis. Biol. Rev. 92, 551-571. ( 10.1111/brv.12243) [DOI] [PubMed] [Google Scholar]
- 31.Klein SL. 2004. Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunol. 26, 247-264. ( 10.1111/j.0141-9838.2004.00710.x) [DOI] [PubMed] [Google Scholar]
- 32.Klein SL. 2000. The effects of hormones on sex differences in infection: from genes to behavior. Neurosci. Biobehav. Rev. 24, 627-638. ( 10.1016/S0149-7634(00)00027-0) [DOI] [PubMed] [Google Scholar]
- 33.Klein SL. 2000. Hormones and mating system affect sex and species differences in immune function among vertebrates. Behav. Processes 51, 149-166. ( 10.1016/S0376-6357(00)00125-X) [DOI] [PubMed] [Google Scholar]
- 34.Morales-Montor J, et al. 2004. Host gender in parasitic infections of mammals: an evaluation of the female host supremacy paradigm. J. Parasitol. 90, 531-546. ( 10.1645/GE-113R3) [DOI] [PubMed] [Google Scholar]
- 35.Christe P, et al. 2007. Host sex and ectoparasites choice: preference for, and higher survival on female hosts. J. Anim. Ecol. 76, 703-710. ( 10.1111/j.1365-2656.2007.01255.x) [DOI] [PubMed] [Google Scholar]
- 36.Sanchez A, Devevey G, Bize P. 2011. Female-biased infection and transmission of the gastrointestinal nematode Trichuris arvicolae infecting the common vole, Microtus arvalis. Int. J. Parasitol. 41, 1397-1402. ( 10.1016/j.ijpara.2011.09.004) [DOI] [PubMed] [Google Scholar]
- 37.Kiffner C, Stanko M, Morand S, Khokhlova IS, Shenbrot GI, Laudisoit A, Leirs H, Hawlena H, Krasnov BR. 2013. Sex-biased parasitism is not universal: evidence from rodent-flea associations from three biomes. Oecologia 173, 1009-1022. ( 10.1007/s00442-013-2664-1) [DOI] [PubMed] [Google Scholar]
- 38.Hillegass MA, Waterman JM, Roth JD. 2008. The influence of sex and sociality on parasite loads in an African ground squirrel. Behav. Ecol. 19, 1006-1011. ( 10.1093/beheco/arn070) [DOI] [Google Scholar]
- 39.McCurdy DG, Shutler D, Mullie A, Forbes MR. 1998. Sex-biased parasitism of avian hosts: relations to blood parasite taxon and mating system. Oikos 82, 303-312. ( 10.2307/3546970) [DOI] [Google Scholar]
- 40.Duneau D, Luijckx P, Ruder LF, Ebert D. 2012. Sex-specific effects of a parasite evolving in a female-biased host population. BMC Biol. 10, 104. ( 10.1186/1741-7007-10-104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelly CD, Stoehr AM, Nunn C, Smyth KN, Prokop ZM. 2018. Sexual dimorphism in immunity across animals: a meta-analysis. Ecol. Lett. 21, 1885-1894. ( 10.1111/ele.13164) [DOI] [PubMed] [Google Scholar]
- 42.Frick WF, Pollock JF, Hicks AC, Langwig KE, Reynolds DS, Turner GG, Butchkoski CM, Kunz TH. 2010. An emerging disease causes regional population collapse of a common North American bat species. Science 329, 679-682. ( 10.1126/science.1188594) [DOI] [PubMed] [Google Scholar]
- 43.Langwig KE, Frick WF, Hoyt JR, Parise KL, Drees KP, Kunz TH, Foster JT, Kilpatrick AM. 2016. Drivers of variation in species impacts for a multi-host fungal disease of bats. Phil. Trans. R. Soc. B 371, 20150456. ( 10.1098/rstb.2015.0456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Langwig KE, Frick WF, Bried JT, Hicks AC, Kunz TH, Marm Kilpatrick A. 2012. Sociality, density-dependence and microclimates determine the persistence of populations suffering from a novel fungal disease, white-nose syndrome. Ecol. Lett. 15, 1050-1057. ( 10.1111/j.1461-0248.2012.01829.x) [DOI] [PubMed] [Google Scholar]
- 45.Frick WF, et al. 2015. Disease alters macroecological patterns of North American bats. Glob. Ecol. Biogeogr. 24, 741-749. ( 10.1111/geb.12290) [DOI] [Google Scholar]
- 46.Hoyt JR, Kilpatrick AM, Langwig KE. 2021. Ecology and impacts of white-nose syndrome on bats. Nat. Rev. Microbiol. 19, 196-210. ( 10.1038/s41579-020-00493-5) [DOI] [PubMed] [Google Scholar]
- 47.Warnecke L, Turner JM, Bollinger TK, Lorch JM, Misra V, Cryan PM, Wibbelt G, Blehert DS, Willis CKR. 2012. Inoculation of bats with European Geomyces destructans supports the novel pathogen hypothesis for the origin of white-nose syndrome. Proc. Natl Acad. Sci. USA 109, 6999-7003. ( 10.1073/pnas.1200374109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Warnecke L, Turner JM, Bollinger TK, Misra V, Cryan PM, Blehert DS, Wibbelt G, Willis CKR. 2013. Pathophysiology of white-nose syndrome in bats: a mechanistic model linking wing damage to mortality. Biol. Lett. 9, 20130177. ( 10.1098/rsbl.2013.0177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verant ML, Meteyer CU, Speakman JR, Cryan PM, Lorch JM, Blehert DS. 2014. White-nose syndrome initiates a cascade of physiologic disturbances in the hibernating bat host. BMC Physiol. 14, 1. ( 10.1186/s12899-014-0010-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cryan PM, Meteyer CU, Boyles JG, Blehert DS. 2010. Wing pathology of white-nose syndrome in bats suggests life-threatening disruption of physiology. BMC Biol. 8, 135. ( 10.1186/1741-7007-8-135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McGuire LP, Mayberry HW, Willis CKR. 2017. White-nose syndrome increases torpid metabolic rate and evaporative water loss in hibernating bats. Am. J. Physiol. 313, R680-R686. ( 10.1152/ajpregu.00058.2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilcox A, Warnecke L, Turner JM, McGuire LP, Jameson JW, Misra V, Bollinger TC, Willis CKR. 2014. Behaviour of hibernating little brown bats experimentally inoculated with the pathogen that causes white-nose syndrome. Anim. Behav. 88, 157-164. ( 10.1016/j.anbehav.2013.11.026) [DOI] [Google Scholar]
- 53.Reeder DM, Frank CL, Turner GG, Meteyer CU, Kurta A. 2012. Frequent arousal from hibernation linked to severity of infection and mortality in bats with white-nose syndrome. PLoS ONE 7, 38920. ( 10.1371/journal.pone.0038920) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lilley TM, Johnson JS, Ruokolainen L, Rogers EJ, Wilson CA, Schell SM, Field KA, Reeder DAM. 2016. White-nose syndrome survivors do not exhibit frequent arousals associated with Pseudogymnoascus destructans infection. Front. Zool. 13, 1-8. ( 10.1186/s12983-016-0143-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lorch JM, et al. 2011. Experimental infection of bats with Geomyces destructans causes white-nose syndrome. Nature 480, 376-378. ( 10.1038/nature10590) [DOI] [PubMed] [Google Scholar]
- 56.Hoyt JR, et al. 2020. Environmental reservoir dynamics predict global infection patterns and population impacts for the fungal disease white-nose syndrome. Proc. Natl Acad. Sci. USA 117, 7255-7262. ( 10.1073/pnas.1914794117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoyt JR, et al. 2018. Cryptic connections illuminate pathogen transmission within community networks. Nature 563, 710-713. ( 10.1038/s41586-018-0720-z) [DOI] [PubMed] [Google Scholar]
- 58.Laggan NA, et al. 2022. Host abundance and heterogeneity in infectiousness determine extent of the environmental reservoir. bioRxiv. 2022.09.20.508714.
- 59.Langwig KE, et al. 2021. Mobility and infectiousness in the spatial spread of an emerging fungal pathogen. J. Anim. Ecol. 90, 1134-1141. ( 10.1111/1365-2656.13439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Verant ML, Boyles JG, Waldrep W, Wibbelt G, Blehert DS. 2012. Temperature-dependent growth of Geomyces destructans, the fungus that causes bat white-nose syndrome. PLoS ONE 7, 46280. ( 10.1371/journal.pone.0046280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perry RW. 2013. A review of factors affecting cave climates for hibernating bats in temperate North America. Environ. Rev. 21, 28-39. ( 10.1139/er-2012-0042) [DOI] [Google Scholar]
- 62.Webb PI, Speakman JR, Racey PA. 1996. How hot is a hibernaculum? A review of the temperatures at which bats hibernate. Can. J. Zool. 74, 761-765. ( 10.1139/z96-087) [DOI] [Google Scholar]
- 63.Langwig KE, et al. 2015. Host and pathogen ecology drive the seasonal dynamics of a fungal disease, white-nose syndrome. Proc. R. Soc. B 282, 20142335. ( 10.1098/rspb.2014.2335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burns LE. 2014. Dynamics of autumn swarming and population structure of little brown and north myotis bats (Myotis lucifugus and M. Septentrionalis). Thesis, Dalhousie University, Halifax, Nova Scotia, Canada. [Google Scholar]
- 65.Burns LE, Broders HG. 2015. Maximizing mating opportunities: higher autumn swarming activity in male versus female Myotis bats. J. Mammal. 96, 1326-1336. ( 10.1093/jmammal/gyv141) [DOI] [Google Scholar]
- 66.Czenze ZJ, Jonasson KA, Willis CKR. 2017. Thrifty females, frisky males: winter energetics of hibernating bats from a cold climate. Physiol. Biochem. Zool. 90, 502-511. ( 10.1086/692623) [DOI] [PubMed] [Google Scholar]
- 67.Jonasson KA, Willis CKR. 2011. Changes in body condition of hibernating bats support the thrifty female hypothesis and predict consequences for populations with white-nose syndrome. PLoS ONE 6, e21061. ( 10.1371/journal.pone.0021061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grieneisen LE, Brownlee-Bouboulis SA, Johnson JS, Reeder DM. 2015. Sex and hibernaculum temperature predict survivorship in white-nose syndrome affected little brown myotis (Myotis lucifugus). R. Soc. Open Sci. 2, 140470. ( 10.1098/rsos.140470) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jonasson KA, Willis CKR. 2012. Hibernation energetics of free-ranging little brown bats. J. Exp. Biol. 215, 2141-2149. ( 10.1242/jeb.066514) [DOI] [PubMed] [Google Scholar]
- 70.Racey P, Entwistle A, Crichton E, Krutzsch P. 2000. Reproductive biology of bats. London, UK: Academic Press. [Google Scholar]
- 71.Thomas DW, Brock Fenton M, Barclay RMR. 1979. Social behavior of the little brown bat, Myotis lucifugus. Behav. Ecol. Sociobiol. 6, 129-136. ( 10.1007/BF00292559) [DOI] [Google Scholar]
- 72.Kurta A, Kunz TH. 1987. Size of bats at birth and maternal investment during pregnancy. Symp. Zool. Soc. Lond. 57, 79-106. [Google Scholar]
- 73.Wimsatt WA. 1945. Notes on breeding behavior, pregnancy, and parturition in some vespertilionid bats of the Eastern United States. J. Mammal. 26, 23. ( 10.2307/1375029) [DOI] [Google Scholar]
- 74.Muller LK, Lorch JM, Lindner DL, O'Connor M, Gargas A, Blehert DS. 2013. Bat white-nose syndrome: a real-time TaqMan polymerase chain reaction test targeting the intergenic spacer region of Geomyces destructans. Mycologia 105, 253-259. ( 10.3852/12-242) [DOI] [PubMed] [Google Scholar]
- 75.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1-48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 76.McGuire LP, et al. 2016. White-nose syndrome disease severity and a comparison of diagnostic methods. EcoHealth 13, 60-71. ( 10.1007/s10393-016-1107-y) [DOI] [PubMed] [Google Scholar]
- 77.Langwig KE, Hoyt JR, Parise KL, Frick WF, Foster JT, Kilpatrick AM. 2017. Resistance in persisting bat populations after white-nose syndrome invasion. Phil. Trans. R. Soc. B 372, 20160044. ( 10.1098/rstb.2016.0044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hopkins SR, Fleming-Davies AE, Belden LK, Wojdak JM. 2020. Systematic review of modeling assumptions and empirical evidence: does parasite transmission increase nonlinearly with host density? Methods Ecol. Evol. 11, 476-486. ( 10.1111/2041-210x.13361) [DOI] [Google Scholar]
- 79.Hopkins SR, Hoyt JR, White JP, Kaarakka HM, Redell JA, DePue JE, Scullon WH, Kilpatrick AM, Langwig KE. 2021. Continued preference for suboptimal habitat reduces bat survival with white-nose syndrome. Nat. Commun. 12, 166. ( 10.1038/s41467-020-20416-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Frick WF, Puechmaille SJ. 2016. Willis CKR. White-nose syndrome in bats. In Bats in the anthropocene: conservation of bats in a changing world (eds Voigt CC, Kingston T), pp. 245-262. Cham, Switzerland: Springer International Publishing. [Google Scholar]
- 81.Hoyt JR, Langwig KE, White JP, Kaarakka HM, Redell JA, Parise KL, Frick WF, Foster JT, Kilpatrick AM. 2019. Field trial of a probiotic bacteria to protect bats from white-nose syndrome. Sci. Rep. 9, 9158. ( 10.1038/s41598-019-45453-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maslo B, Valent M, Gumbs JF, Frick WF. 2015. Conservation implications of ameliorating survival of little brown bats with white-nose syndrome. Ecol. Appl. 25, 1832-1840. ( 10.1890/14-2472.1) [DOI] [PubMed] [Google Scholar]
- 83.Luong LT, Perkins SE, Grear DA, Rizzoli A, Hudson PJ. 2010. The relative importance of host characteristics and co-infection in generating variation in Heligmosomoides polygyrus fecundity. Parasitology 137, 1003-1012. ( 10.1017/S0031182009991892) [DOI] [PubMed] [Google Scholar]
- 84.Jenkins HE, Cox DR, Delahay RJ. 2012. Direction of association between bite wounds and Mycobacterium bovis infection in badgers: implications for transmission. PLoS ONE 7, e45584. ( 10.1371/journal.pone.0045584) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Delahay RJ, Walker NJ, Forrester GJ, Harmsen B, Riordan P, Macdonald DW, Newman C, Cheeseman CL. 2006. Demographic correlates of bite wounding in Eurasian badgers, Meles meles L., in stable and perturbed populations. Anim. Behav. 71, 1047-1055. ( 10.1016/j.anbehav.2005.07.018) [DOI] [Google Scholar]
- 86.Folstad I, Karter AJ. 1992. Parasites, bright males, and the immunocompetence handicap. Am. Nat. 139, 603-622. ( 10.1086/285346) [DOI] [Google Scholar]
- 87.Edler R, Goymann W, Schwabl I, Friedl TWP. 2011. Experimentally elevated testosterone levels enhance courtship behaviour and territoriality but depress acquired immune response in Red Bishops Euplectes orix. Ibis. 153, 46-58. ( 10.1111/j.1474-919X.2010.01075.x) [DOI] [Google Scholar]
- 88.Roberts M, Peters A. 2009. Is testosterone immunosuppressive in a condition-dependent manner? An experimental test in blue tits. J. Exp. Biol. 212, 1811-1818. ( 10.1242/jeb.031047) [DOI] [PubMed] [Google Scholar]
- 89.Prendergast BJ, Freeman DA, Zucker I, Nelson RJ. 2002. Periodic arousal from hibernation is necessary for initiation of immune responses in ground squirrels. Am. J. Physiol. 282, R1054-R1062. ( 10.1152/ajpregu.00562.2001) [DOI] [PubMed] [Google Scholar]
- 90.Bouma HR, Carey HV, Kroese FGM. 2010. Hibernation: the immune system at rest? J. Leukoc. Biol. 88, 619-624. ( 10.1189/jlb.0310174) [DOI] [PubMed] [Google Scholar]
- 91.Meyer GA, Senulis JA, Reinartz JA. 2016. Effects of temperature and availability of insect prey on bat emergence from hibernation in spring. J. Mammal.. 97, 1623-1633. ( 10.1093/jmammal/gyw126) [DOI] [Google Scholar]
- 92.Johnson JS, et al. 2014. Host, pathogen, and environmental characteristics predict white-nose syndrome mortality in captive little brown myotis (Myotis lucifugus). PLoS ONE 9, e112502. ( 10.1371/journal.pone.0112502) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Davis WH, Hitchcock HB. 1965. Biology and migration of the bat, Myotis lucifugus, in New England. J. Mammal. 46, 296-313. ( 10.2307/1377850) [DOI] [Google Scholar]
- 94.Mohr CE. 1945. Sex ratios of bats in Pennsylvania. Proc. Pennsylvania Acad.Sci. 19, 65-69. [Google Scholar]
- 95.Tinkle DW, Milstead WW. 1960. Sex ratios and population density in hibernating Myotis. Am. Midland Naturalist 63, 327-334. ( 10.2307/2422796) [DOI] [Google Scholar]
- 96.Lynda MSL, William C. 2007. Sex ratio variation of Myotis velifer (Chiroptera: Vespertilionidae) in Oklahoma. Southwestern Naturalist 52, 67-74. ( 10.1894/0038-4909(2007)52[67:SRVOMV]2.0.CO;2) [DOI] [Google Scholar]
- 97.Willis CKR, Brigham RM. 2007. Social thermoregulation exerts more influence than microclimate on forest roost preferences by a cavity-dwelling bat. Behav. Ecol. Sociobiol. 62, 97-108. ( 10.1007/s00265-007-0442-y) [DOI] [Google Scholar]
- 98.Linton DM, Macdonald DW. 2018. Spring weather conditions influence breeding phenology and reproductive success in sympatric bat populations. J. Anim. Ecol. 87, 1080-1090. ( 10.1111/1365-2656.12832) [DOI] [PubMed] [Google Scholar]
- 99.Le Galliard JF, Fitze Patrick S, Ferrière R, Clobert J. 2005. Sex ratio bias, male aggression, and population collapse in lizards. Proc. Natl Acad. Sci. USA 102, 18 231-18 236. ( 10.1073/pnas.0505172102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Johanos TC, Becker BL, Baker JD, Ragen TJ, Gilmartin WG, Gerrodette T. 2010. Impacts of sex ratio reduction on male aggression in the Critically Endangered Hawaiian monk seal Monachus schauinslandi. Endangered Species Res. 11, 123-132. ( 10.3354/esr00259) [DOI] [Google Scholar]
- 101.Grimaudo AT, Hoyt JR, Yamada SA, Herzog CJ, Bennett AB, Langwig KE. 2022. Host traits and environment interact to determine persistence of bat populations impacted by white-nose syndrome. Ecol. Lett. 25, 483-497. ( 10.1111/ele.13942) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kailing MJ, et al. 2023. Data for: Sex-biased infections scale to population impacts for an emerging wildlife disease. Dryad Digital Respository. ( 10.5061/dryad.12jm63z2j) [DOI] [PMC free article] [PubMed]
- 103.Kailing MJ, et al. 2023. Supplementary materials for: Sex-biased infections scale to population impacts for an emerging wildlife disease. Figshare. ( 10.6084/m9.figshare.c.6456166) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Kailing MJ, et al. 2023. Data for: Sex-biased infections scale to population impacts for an emerging wildlife disease. Dryad Digital Respository. ( 10.5061/dryad.12jm63z2j) [DOI] [PMC free article] [PubMed]
- Kailing MJ, et al. 2023. Supplementary materials for: Sex-biased infections scale to population impacts for an emerging wildlife disease. Figshare. ( 10.6084/m9.figshare.c.6456166) [DOI] [PMC free article] [PubMed]
Data Availability Statement
The datasets supporting this article have been deposited in the Dryad Digital Repository [102]. Exact site locations are not disclosed to protect endangered species and landowners.
The supporting materials including the appendix, tables, and figures are provided in the electronic supplementary material [103].