Abstract
As the demand for poultry meat continues to rise, industry production is constantly challenged with obtaining consumer needs. Integrators have answered this increasing demand by improving the growth rate of broilers allowing for increased production efficiently. The resulting broiler produces higher yields and a larger quantity of fresh poultry to satisfy consumer needs. However, this increase in efficiency has cost integrators as new quality issues continue to manifest through global production. Therefore, the objective of the current experiment was to evaluate the effect of genetic strain (standard and high yielding) and target weight on meat quality attributes such as pH, water holding capacity (WHC), and tenderness, alongside meat quality defects such as breast and tender myopathies. In the current study, 1,800 broilers from 4 commercial strains (2 high breast yielding (HY) and 2 standard yielding (SY) were raised sex separate to evaluate meat quality trends over time at 6 previously defined market weights. Birds were processed at weights ranging from 2,043 to 4,313 g in 454 g increments. HY strains produced higher breast and tender yields than those of SY strains (P < 0.05). There was an increase in breast and tender yield as target weight increased (P < 0.05) for both HY and SY strains. Differences were observed between strains for all fillet dimensions (P < 0.05); however, these measurements increased as target weight increased as expected. Woody breast (WB) had a higher severity (P < 0.05) in HY strains over SY strains, for both males and females. Differences were observed in white striping (WS; P < 0.05) for females in both strains, but no differences were observed in males. A main effect of target was noticed for both WB and WS (P < 0.05), expressing increased severity as target weight increased. Shear values were influenced more by target weight (P < 0.05), but inconsistent differences were observed between HY and SY groups. Meullenet-Owens Razor Shear (MORS) energy values increased slightly as target weights increased (P < 0.05) from 2,951 to 4,313 g in both males and females, but differences were minor and inconsistent with the smaller carcass weights. The MORS peak counts generally increased as target weight increased for both sexes. While strain had minimal effects on meat quality attributes, processing weight had a greater influence on quality, specifically muscle myopathies, WHC, and shear properties.
Key words: meat quality, myopathy, tender myopathy, broiler strain, texture
INTRODUCTION
Recent trends suggest that poultry meat continues to flourish in popularity among consumers due to affordability, availability, and nutritional qualities. In 2021, consumption of poultry meat was at an all-time high of 113.4 pounds (∼51 kg) per capita, and approximately 41.9 billion pounds (∼19 billion kg) of chicken was marketed on a ready-to-cook basis in the United States (National Chicken Council, 2023a,b). As present in most agriculture systems, the poultry industry is very much a consumer driven market with various segments to satisfy consumer needs. The growth potential of modern broilers provides the industry a facet to target each of these various markets for harvest while maximizing production efficiency. Market segments are then met as birds are processed at various weights and ages. By targeting processing size, efficiency and quality can be optimized. Generally, small bird markets utilize birds ranging from 35 to 40 d of age, as portion sizes align with the needs of the fast-food segment (Brewer et al., 2012a). Whereas big bird markets use broilers ranging from 45 to 60 d of age for tray pack and heavy debone markets. However, further efficiency can be obtained as these larger fillets can be portioned for use in the fast-food segment. Varying market segments generate the need to assess a wide range of processing ages in order to understand quality differences that may fluctuate over time. Continual increase in poultry meat demand provides a strong influence on market size impacts on final quality and production efficiency.
Genetic advancements have been made to maximize meat production while minimizing costs to provide a sustainable protein source to a variety of consumers, regardless of economic status. Selection practices and improved nutritional programs have allowed integrators to produce heavier, higher-yielding broilers faster than ever. While increased breast yield (BY) has been beneficial to meeting consumer demand, the industry has seen a multitude of adverse effects that muscle myopathies present alongside the faster growth rates of modern broilers (Kuttappan et al., 2012a; Kuttappan et al., 2016; Cai et al., 2018). Woody breast (WB), white striping (WS), and spaghetti meat (SM) in breast fillets have become more prevalent as a continuous push for a higher-yielding, faster-growing bird is utilized in commercial practice (Kuttappan et al., 2012a; Petracci et al., 2019). This, in turn, creates acceptability issues at the consumer level in appearance, taste, and texture (Kuttappan et al., 2012b).
There are several factors that influence changes in meat quality, as well as impact the incidence and severity of muscle myopathies. These factors can include, but are not limited to, sex, strain, age, and final live weight (Kuttappan et al., 2017; Mallmann, 2019; Zhang et al., 2021). Meat quality, more specifically eating quality, from a consumer standpoint can involve many aspects including appearance, juiciness, flavor, and texture. These factors can be affected by a combination of pH, color, water holding capacity (WHC), tenderness, and cook-loss which can ultimately determine product acceptability (Barbut, 1997; Fletcher, 2002). Due to these numerous factors, it is important to understand how sex, strain, and age affect meat quality over time so that the industry can optimize yields while limiting downgrades due to these quality issues.
The objective of this study was to evaluate the effect of strain (standard and high yielding) and target weight on meat quality attributes such as pH, WHC, and texture, alongside meat quality defects such as breast and tender myopathies. By evaluating these attributes over time, variation in meat quality can be observed as well as how these factors affect the eating quality of poultry products. Myopathy trends and their associated quality differences were also established over time to better understand when these myopathies develop and how they progress.
MATERIALS AND METHODS
All animal handling procedures were approved by the Institutional Animal Care and Use Committee at the University of Arkansas (Protocol #: 20016).
Animal Husbandry
Approximately 1,800 broiler chicks were obtained from a local primary breeder hatchery on day-of-hatch. Four common commercial strain crosses were chosen representing 2 standard yielding (SYA and SYB) and 2 high yielding (HYA and HYB). Chicks were sexed, vaccinated, and then packaged for transport by hatchery personnel and transported to the University of Arkansas Broiler Research Farm. Upon arrival, 12 chicks were group weighed and placed in individual pens according to a complete random experimental design. The house consisted of 144 floor pens (0.9 × 1.2 m, 0.08 m2 per bird) top dressed with pine shavings, outfitted with a nipple drinker water line and an individual feeder. At 42 d of age, birds were culled via random selection to 10 per pen to meet stocking density requirements. Feed and water were provided ad libitum throughout the trial and diets were provided in 4 feeding phases: starter (D0–D14), finisher (D14–D28), withdrawal I (D28–D42), and withdrawal II (D42 to end of trial). A common diet was formulated to meet or exceed nutritional requirements based on primary breeder recommendations for each growing type. An experimental lighting schedule was utilized with 24L:0D from d 0 to 1, 23L:1D from d 1 to 7, and 18L:6D d 7 for the remainder of the trial. Temperature was to 32°C at day of placement and maintained on a curve to decrease by 2°C every week until the conclusion of the trial. Daily assessment of bird well-being, house conditions, feed level, water line function, and mortality were collected twice a day.
Processing
Birds were processed at 6 different target weights in 454 g increments ranging from 2,043 to 4,313 g. Whole pens selected at random for processing (on a given day) were weighed 24 h prior to being processed. Predetermined processing days for a given target weight for males and females were estimated based on previous lab data and broiler guides. At day of processing, all birds per pen were harvested consisting of 3 replicate pens of a given sex, strain, and target weight (n = 30). Due to unforeseen circumstances, females were only processed at 5 of the 6 established target weights. Prior to processing, a 10-h feed withdrawal period was utilized before catching 10 birds per pen which were transported to the pilot processing plant (<0.5 mile). Broilers were processed using an inline processing system at the University of Arkansas pilot processing plant according to procedures described by Mehaffey et al. (2006). Briefly, birds were hung on shackles, stunned (10 mA of DC current for approximately 15 s), exsanguinated, scalded (53.8°C for approximately 2 min) and defeathered, eviscerated, and chilled using a 2-stage immersion chill system (prechill: 12°C for 15 min; chill: 1°C for 2.5 h with manual agitation). The total dwell time of 2.75 h was intentional to keep the dwell time and debone time (3 h postmortem) consistent throughout the trial accounting for large carcasses at the end of the trial. Tanks were then drained, and carcasses were weighed and deboned into subsequent parts at 3 h postmortem. Parts included breast, tenders, wings, leg quarter, and rack (i.e., cage and breast skin) which were weighed post debone. Following part weight collections, Pectoralis major (breast) and Pectoralis minor (tender) muscles were collected and subjected to further analysis. Breast fillets were scored for WB, WS, and SM. Tenders were scored for the presence of woody-like tender (WT) and tender feathering (TF). Breast fillets were also measured for fillet footprint dimension, WHC (drip and cook loss method), color, pH, and objective texture using Meullenet Owens Razor Shear (MORS).
Fillet Dimensions
Measurements of whole breast fillets were made to determine fillet dimensions as described by Mallmann (2019). Fillets were placed dorsal side down and 3 measurements were recorded. Length, width, and thickness were measured using a calibrated digital caliper (Model 500-764-10* IP67, Mitutoyo, Aurora, IL). Thickness was determined by measuring the depth of each left fillet at the thickest portion of the cranial region. Length was measured across the left side of fillet from cranial to caudal region at longest part. Finally, width was measured across the fillet at one-third the total length calculated once length had been recorded.
Muscle Myopathies
Breast fillets were scored for WB, WS, and SM myopathies. Intact butterflies were scored for woodiness or hardness of the muscle based on a scoring system developed by Tijare et al. (2016). The scale ranged from 0 to 3 in 0.5 increments. A score of 0 to 0.5 was categorized as normal with the fillet being flexible through the cranial, medial, and caudal regions. A score of 1 to 1.5 was considered mild, displaying hardness primarily in the cranial region of the fillet. A score of 2 to 3 was considered moderate and severe, respectively, with a fillet having qualities of being inflexible and having a rubber-like, hard consistency throughout all regions, though moderate WB has some flexibility in the middle region and a severe WB is rigid throughout the fillet. Fillets were also scored for WS using a scoring system ranging from 0 to 3 in 0.5 increments according to Kuttappan et al. (2012a). A score of 0 to 0.5 was categorized as normal displaying none to slight amounts of striping in the cranial shoulder. A score of 1 to 1.5 is considered moderate displaying striping <1 mm thick. A score of 2 to 3 is severe displaying heavy striping 1 to 2 mm thick or larger. Presence (1) or absence (0) of the SM myopathy was also recorded as described by Mallmann (2019).
Tenders were scored for hardness (WT), and separation/gaping between fibers and is referred to as “feathering” (TF), representing common industry terminology. For WT tender, the scoring system used was set from 0 to 2 with 0.5 increments using a scale described by Maynard (2020). Tenders that exhibiting no or minimal palpable hardness were given a score of 0 or 0.5, respectively, (normal/slight). Tenders exhibiting moderate hardness were given a score 1 or 1.5. Tenders exhibiting severe hardness throughout were given a score of 2; a score of 2 was generally associated with atrophied muscle as a result of green muscle condition. Tenders were evaluated for feathering (TF) using a scale similar to Soglia et al. (2019) with modifications to account for more severe fraying/soft texture typically observed by our lab (Maynard, 2020). The scoring system ranged from 0 to 2 with 0.5 increments. A tender receiving a score of 0 or 0.5 had no fraying (normal) or up to 2 splits in muscle (slight), respectively; a score of 1 or 1.5 had moderate fraying, >2 splits in muscle, and a score of 2 had severe fraying or gaping of the muscle fibers and a soft texture. All myopathy scoring procedures were completed by 1 single trained personnel to maintain consistency throughout the trial.
Drip Loss
After muscle myopathies were scored, all fillets (individually labeled) were placed on white storage trays (45.7 × 66 cm), side by side approximately 2 cm apart. Once tray was full (number of fillets varied on each tray depending on size of fillets at each processing day). Trays were then entirely covered with plastic overlay to minimize surface drying effects, and stored overnight in a 4°C walk-in cooler. After 24 h, butterfly fillets were individually patted dry with absorbent paper and weighed. Drip loss was determined for individual butterfly fillets by calculating weight loss (after 24 h storage) as a percentage of deboned butterfly fillet weight.
Color
At 24 h postmortem, color measurements were made on the left, dorsal side of each intact butterfly. Measurements were made using a calibrated CM-400 Chroma Meter (Konica Minolta., Ramsey, NJ), set with a 2-degree observer and utilizing a D65 reflectance. Three separate L*, a*, and b* measurement values were collected at 3 locations of the fillet consisting of the cranial, medial, and caudal regions (Brewer et al., 2012a,b; Kuttappan et al., 2013a). Color evaluation was based on Internatioal Commission of Illumination (CIE) models that include L*, a*, and b*. The L* value resprented the lightness of the product ranging from 0 to 100 (black to white), while a* and b* values ranged from -120 to +120. The a* value is related to the redness expressed in the product ranging from green (negative values) to red (positive values). The b* value is the degree of yellowness in the product, ranging from blue (negative values) to yellow (positive values) (Yam and Papadakis, 2004). Measurements were then recorded and averaged for each fillet in regards to L* (lightness), a* (redness), and b* (yellowness) of the product.
pH
Muscle pH was determined using a spear tip probe (Model 205, Testo Instruments, West Chester, PA). pH was assessed by inserting the probe near the wing joint of the left side of the fillet (Brewer et al., 2012a,b; Kuttappan et al., 2013a). After a value was displayed for 3 s without fluctuation, results were recorded.
Fillets were separated into right and left halves where the left side was discarded. The right side of the breast was retagged, weighed, and placed into vacuum sealed bags to be stored at −20°C until texture analysis could be completed (<4 wk).
Thaw Loss and Cook Loss
Fillets were removed from the freezer and allowed to thaw for approximately 48 h in a 4°C walk-in cooler. Samples were removed from vacuum sealed bags and weighed to calculate thaw loss as a percent weight prior to freezing. Fillets were placed (6 to 8 per pan depending on size) on wire cooking racks in (65 × 395 × 290 mm) cooking pans lined with aluminum foil. Samples were arranged in pans based on precook weights to help minimize variation in sample size and produce more uniform cooking. Samples were cooked according to the methods described by Sams (1990) and modified by Mehaffey et al. (2006). Eight pans were placed in a forced air convection oven (Model E101-E, Duke Manufacturing Company, St. Louis, MO) per cook. Fillets were cooked at 176°C until they reached an internal temperature of 76°C, as recorded by a calibrated thermometer (Model HT1000 thermometer, Cooper Instruments, Concord, Canada). Once cooked, fillets were allowed to cool to room temperature on white storage trays covered loosely with aluminum foil, before reweighing (to determine cooking loss percentage). Fillets were then wrapped individually in aluminum foil and placed in a cooler held at 4°C overnight (approximately 24 h) until texture analysis.
Texture Analysis
At 24 h post cook, samples were objectively analyzed for texture/tenderness using the MORS as described by Cavitt et al. (2004). A texture analyzer (Model TA-XT2 Plus, Texture Technologies, Scarsdale, NY) was used and set to the following parameters after calibration: speed set at 5mm/s, trigger force set at 5 g, and depth set to 20 mm. Force (F, N), energy (E, N.mm), and peak counts (PC) were determined, and shears were completed in the cranial (top 1/3) region of each right breast fillet. Three shears were collected in the cranial region and averaged for each fillet. The MORS blade was changed every 100 shears to prevent dulling (Cavitt et al., 2004).
Statistical Analysis
The final analysis consisted of a complete random design with 3 replications. Pen served as the experimental unit. Factors assessed were strain and target weight in a 2-way ANOVA using JMP Pro 15.2. The random design utilized a factorial arrangement of treatments for each sex. Male data was allocated to a factorial treatment of 4 × 6 consisting of 4 strains (SYA, SYB, HYA, HYB) and 6 target weights (2,043, 2,497, 2,951, 3,405, 3,859, 4,313). Female data was allocated to a factorial treatment of 4 × 5 consisting of the same strains and 5 target weights (2,043, 2,497, 2,951, 3,405, 4,313), due to a scheduling conflict. Statistical significance was set at P ≤ 0.05. Where appropriate, main effect means were separated using a Tukey's HSD test. For chi-square analysis, myopathy scores were pooled together on a whole number basis. Whole number pools then placed scores in 1 of 3 categories. A score of 0 or 0.5 was set to 0, or the absence of a myopathy. A score range of 1 to 1.5 was considered to be mild or moderate in occurrence with a score of 1. Lastly, a score range of 2 to 3 was considered severe and resembled a score of 2. Myopathies were then analyzed as percent incidence for each of the 3 previously listed categories and considered on a pen basis.
RESULTS AND DISCUSSION
Body Weight and Yields
Results for final live weight are presented in Tables 1 and 2. For all results presented herein, 4 commercial strains were compared for each analysis (2 high-yielding [HY] strains and 2 standard-yielding [SY] strains). Although target weight and final weight were not equal for each planned target weight category, final weight increased linearly (P < 0.05) as target weight increased, which was expected. For the remainder of this discussion, target weight will be used to describe the influence of weight on various attributes. For males and females, differences were observed due to strain and target weight for live weight (P < 0.05), but no strain × target weight interaction (P > 0.05) was noted. For males, SY strains had higher live weights (P < 0.05) when compared to HY strains. For females, SYA, SYB, and HYB exhibited higher live weight (P < 0.05) than HYA. Previous researchers have determined that strain has a significant influence on body weight as SY strains produce higher live weights than HY strains, which are supportive of the results in the current study (Young et al., 2001; Mehaffey et al., 2006; Brewer et al., 2012a,b; Maynard, 2020).
Table 1.
Effect of strain1 and target weight on carcass and parts yield of male broilers.
|
Treatment | |||||||
|---|---|---|---|---|---|---|---|
| Live wt. (g) | Carcass | Wing | Breast | Tender | Leg quarter | Rack | |
| Strain | |||||||
| SYA | 3,473a | 77.43b | 9.86a | 26.86c | 5.31b | 30.32a | 26.98ab |
| SYB | 3,393a | 76.84c | 9.87a | 26.96c | 5.37b | 29.93a | 27.22a |
| HYA | 3,211b | 78.56a | 9.59b | 29.83a | 5.67a | 28.49c | 25.72c |
| HYB | 3,276b | 77.67b | 9.73ab | 28.30b | 5.56a | 29.01b | 26.68b |
| SEM | 29 | 0.14 | 0.04 | 0.18 | 0.04 | 0.13 | 0.14 |
| Target weight (g) | |||||||
| 2,043 | 2,074f | 75.81c | 10.25a | 25.86d | 5.28d | 29.69a | 28.02a |
| 2,497 | 2,566e | 77.30b | 9.93b | 26.72cd | 5.21d | 28.83b | 28.52a |
| 2,951 | 2,955d | 78.00ab | 9.61c | 27.44c | 5.35cd | 29.30ab | 27.29b |
| 3,405 | 3,579c | 78.04ab | 9.58c | 28.74b | 5.50bc | 29.21ab | 26.51c |
| 3,859 | 4,233b | 78.14a | 9.62c | 29.42ab | 5.67b | 29.70a | 24.96d |
| 4,313 | 4,624a | 78.43a | 9.57c | 29.74a | 5.85a | 29.88a | 24.61d |
| SEM | 35 | 0.18 | 0.05 | 0.22 | 0.04 | 0.16 | 0.17 |
| P-values | |||||||
| Strain | <0.001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Target weight | <0.001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0003 | <0.0001 |
| Interaction | 0.1763 | 0.3475 | 0.6654 | 0.3185 | 0.1520 | 0.5194 | 0.8299 |
Abbreviations: HYA, high yielding A, HYB, high yielding B; SYA, standard yielding A, SYB, standard yielding B.
Means without a common superscript were determined to be significantly different to be significantly different (P < 0.05) by a Tukey's HSD test.
Table 2.
Effect of strain1 and target weight on carcass and parts yield of female broilers.
|
Treatment | |||||||
|---|---|---|---|---|---|---|---|
| Live wt. (g) | Carcass | Wing | Breast | Tender | Leg quarter | Rack | |
| Strain | |||||||
| SYA | 3,138a | 78.73bc | 9.71a | 27.31c | 5.78b | 29.18a | 26.95a |
| SYB | 3,109a | 78.17c | 9.64a | 27.50c | 5.86b | 28.49b | 27.29a |
| HYA | 3,008b | 79.78a | 9.38b | 30.42a | 6.02a | 27.49c | 25.57b |
| HYB | 3,125a | 78.89b | 9.46b | 29.17b | 6.02a | 27.45c | 26.79a |
| SEM | 26 | 0.17 | 0.04 | 0.18 | 0.04 | 0.14 | 0.14 |
| Target weight (g) | |||||||
| 2,043 | 1,885e | 77.04d | 10.14a | 25.71e | 5.47d | 28.90a | 28.36a |
| 2,497 | 2,463d | 79.39b | 9.69b | 27.02d | 5.67c | 28.44ab | 27.40b |
| 2,951 | 2,955c | 78.99bc | 9.52bc | 28.67c | 6.00b | 28.11b | 26.80c |
| 3,405 | 3,593b | 78.55c | 9.39c | 30.37b | 6.25a | 28.14b | 25.16d |
| 4,313 | 4,579a | 80.50a | 8.99d | 31.26a | 6.20a | 27.18c | 25.51d |
| SEM | 29 | 0.19 | 0.04 | 0.20 | 0.04 | 0.15 | 0.16 |
| P-values | |||||||
| Strain | 0.0046 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Target weight | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Interaction | 0.0577 | 0.3780 | 0.0273 | 0.8046 | 0.0238 | 0.4182 | 0.3332 |
Abbreviations: HYA, high yielding A, HYB, high yielding B; SYA, standard yielding A, SYB, standard yielding B.
Means without a common superscript were determined to be significantly different (P < 0.05) by a Tukey's HSD test.
Carcass yield data from this experiment are reported in Tables 1 and 2. As expected, there were significant differences (P < 0.05) between strain and target weight for both sexes, but no interaction (P > 0.05) was observed. Focusing on strictly white meat yield, HY strains expressed higher white meat yields (P < 0.05) than the SY strains, as anticipated. The differences in yield present here among strains have also previously been reported (Smith and Presti, 1998; Corzo et al., 2005; Mehaffey et al., 2006; Maynard, 2020). Corzo et al. (2005) compared a HY strain to 2 multipurpose strains and reported increased white meat yield (breast and tenders) for the HY strain when compared to both multipurpose strains. These results were expected as genetic selection for higher white meat yield was present in these specified strains. For the HY strains, HYA had a higher breast meat yield (P < 0.05) than HYB for both sexes, however there were no differences (P > 0.05) for tender yield of either sex.
For overall carcass yield (P < 0.05), HYA had the greatest yield and SYB was the lowest, with SYA and HYB being intermediate (P > 0.05) for males. For females, HYA was higher (P < 0.05) than SYB, however no differences were observed between SYA and HYB or SYA and SYB (P > 0.05). The lack of difference in carcass yield between SYA and HYB can be attributed to variation between growth rate at the cost of genetic profile. Although target weight was the main focus of interest in the current experiment, a significant difference in processing weight could explain this variation in final carcass yield for all target weights. Standard yielding strains had a higher leg quarter yield (P < 0.05) than both high yielding strains for both males and females. This difference can be attributed to the genetic selection of HY strains to possess greater white meat yield. The current study supports findings of Zuidhof et al. (2014) who compared 1957 and 1978 commercial lines with a 2005 higher breast yielding strain. The authors found that leg yields were lower in the high breast yielding strain.
With target weight as the main effect, there were differences (P < 0.05) for all calculated yields (carcass, wing, breast, tender, leg quarter, and rack) for both sexes, as expected. For white meat yield (breast and tenders), differences were present among 2,043 g and 4,313 g weights (P < 0.05), showing an increase in yield as target weight increased. These findings are supportive of previous research that reported an increase in breast meat yield as body weight increased (Young et al., 2001; Mehaffey et al., 2006; Kuttappan et al., 2017; Maynard, 2020).
Fillet Dimensions
Fillet dimension results are reported in Tables 3 and 4 for males and females, respectively. Results in this experiment express that strain had an impact on width and thickness of breast fillets for males (P < 0.05). These findings are consistent with results from previous studies conducted by Scheuermann et al. (2003), Mehaffey et al. (2006), and Brewer et al. (2012a,b). SYA was significantly wider (P < 0.05) than HYB. High-yielding strain A was thicker (P < 0.05) than SYB with no differences in thickness (P > 0.05) between HYB and SYA. These results could be attributed to the unique characteristics and conformational differences of genetic selection.
Table 3.
Effect of strain1 and target weight on breast fillet dimensions of male broilers.
| Treatment | Dimensions |
||
|---|---|---|---|
| Strain | Length2 | Width3 | Thickness4 |
| SYA | 172.87 | 165.67a | 34.61b |
| SYB | 173.51 | 163.33ab | 32.86c |
| HYA | 171.42 | 164.29ab | 36.50a |
| HYB | 170.83 | 161.99b | 34.20b |
| SEM | 0.80 | 0.92 | 0.34 |
| Target weight (g) | |||
| 2,043 | 145.87f | 135.80f | 27.99e |
| 2,497 | 158.01e | 148.95e | 29.86d |
| 2,951 | 164.83d | 157.09d | 31.97c |
| 3,405 | 177.01c | 166.99c | 36.14b |
| 3,859 | 190.06b | 184.31b | 39.89a |
| 4,313 | 197.15a | 189.77a | 41.40a |
| SEM | 0.98 | 1.13 | 0.42 |
| P-values | |||
| Strain | 0.0780 | 0.0468 | <0.0001 |
| Target weight | <0.0001 | <0.0001 | <0.0001 |
| Interaction | 0.5896 | 0.3287 | 0.0429 |
Abbreviations: HYA, high yielding A, HYB, high yielding B; SYA, standard yielding A, SYB, standard yielding B.
Measured in mm at the top of the fillet in the cranial region to the tip of the fillet in the caudal region.
Measured in mm at 1/3 the caudal end of the fillet.
Measured in mm at the thickest part of the cranial region of the fillet.
Means without a common superscript were determined to be significantly different (P < 0.05) by a Tukey's HSD test.
Table 4.
Effect of strain1 and target weight on breast fillet dimensions of female broilers.
| Treatment | Dimensions |
||
|---|---|---|---|
| Strain | Length2 | Width3 | Thickness4 |
| SYA | 168.90a | 167.83 | 33.16b |
| SYB | 169.85a | 166.13 | 32.63b |
| HYA | 166.57b | 164.42 | 34.80a |
| HYB | 169.80a | 167.35 | 35.96a |
| SEM | 0.60 | 1.07 | 0.39 |
| Target weight (g) | |||
| 2043 | 140.11e | 135.30e | 27.07e |
| 2497 | 155.02d | 152.69d | 29.76d |
| 2951 | 166.12c | 163.13c | 32.52c |
| 3405 | 182.31b | 179.44b | 37.88b |
| 4313 | 200.35a | 201.61a | 43.46a |
| SEM | 0.67 | 1.19 | 0.43 |
| P-values | |||
| Strain | 0.0010 | 0.1244 | <0.0001 |
| Target weight | <0.0001 | <0.0001 | <0.0001 |
| Interaction | 0.0803 | 0.9504 | 0.1186 |
Abbreviations: HYA, high yielding A, HYB, high yielding B; SYA, standard yielding A, SYB, standard yielding B.
Measured in mm at the top of the fillet in the cranial region to the tip of the fillet in the caudal region.
Measured in mm at 1/3 the caudal end of the fillet.
Measured in mm at the thickest part of the cranial region of the fillet.
Means without a common superscript were determined to be significantly different (P < 0.05) by a Tukey's HSD test.
For females, there were differences (P < 0.05) in strain for both length and thickness. High-yielding strain A had the shortest fillet length (P < 0.05) between all others, with no differences among the other 3 strains (P > 0.05). For thickness, results showed that HY strains were thicker than those of the SY stains (P < 0.05). As discussed in a previous study by Lubritz (1997), fillet thickness has 7 times more impact on fillet yield than length or width; these current findings support this conclusion, as the HY strains had significantly thicker fillets for than those of the SY strains.
With the respective difference in products from the small and big bird market, fillet dimensions play an important role in obtaining the ideal product for a consumer. As small bird markets are predominantly used for fast-food restaurants, fillets are expected to be smaller in size than that of the heavy bird debone market. In order to meet demand in whole muscle products, SY strains are typically used to provide a smaller fillet footprint, in particular with thickness that accurately matches a product such as a sandwich that requires minimal mechanical portioning. Thus, in big bird markets, the product is often portioned to obtain specifications needed (e.g., specific size or shape, thickness, etc.). Further processing can not only add value to a product but can also be targeted to produce a final product that is altered to better fit customer specifications. For this reason, HY strains generally produce thicker fillets to target the needs of further processing.
Between target weights, and as expected, there were differences for all 3 measurements (width, length, and thickness), regardless of sex (P < 0.05, Tables 3 and 4). However, an interaction of strain × target weight for thickness of male breast fillets was observed (P < 0.05). In this case, SYA had a smaller thickness at TW 4313g than both HYA and HYB (data not shown). The results of the current experiment were contrary to the findings of Mehaffey et al. (2006) who reported that differences in fillet measurements had no correlation to body size or BY. A possible explanation between these 2 experiments is that different strain variants and genetic advancements over a decade apart may be present from those used in the study by Mehaffey et al. (2006). Another major difference between Mehaffey et al. (2006) and the current study could be the narrow range of bird age targeted (6–7 wk), whereas the current study evaluated a much greater variation in bird size/age (5–10 wk).
There were significant differences between all 6 target weights for males, with the heaviest target weight, 4,313 g, having the greatest measurement values for all dimensions collected. This trend was also consistent for the 5 female target weights. There were significant correlations between all 3 fillet dimensions measurements (thickness, length, width) and live weight (males: r = 0.92, r = 0.99, r = 0.97, P < 0.001, respectively; females: r = 0.94, r = 0.98, and r = 0.97, P < 0.001, respectively) concluding that as target weight increased, fillet dimensions increased (Tables 5 and 6). Similarly, Scheuermann et al. (2003) reported high correlations of body weight to length, width, and depth (r = 0.77, 0.71, and 0.59, respectively) in broilers at 57 d; the higher correlations observed in this study are likely due to the broad range of data relative to the previously reported study.
Table 5.
Male correlations of various meat quality traits and associated myopathies.
| LW | BY | TY | WS | WB | SM | WT | TF | Thickness3 | Length1 | Width2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LW | • | • | • | • | • | • | • | • | • | • | |
| BY | 0.66*** | • | • | • | • | • | • | • | • | • | |
| TY | 0.61*** | 0.79*** | • | • | • | • | • | • | • | • | |
| WS | 0.80*** | 0.74*** | 0.47*** | • | • | • | • | • | • | • | |
| WB | 0.77*** | 0.94*** | 0.60*** | 0.90*** | • | • | • | • | • | • | |
| SM | -0.55*** | -0.26* | -0.36** | -0.38** | -0.32** | • | • | • | • | • | |
| WT | 0.65*** | 0.65*** | 0.63*** | 0.66*** | 0.70*** | -0.38** | • | • | • | • | |
| TF | 0.02 | 0.20 | 0.02 | 0.15 | 0.25 | 0.12 | 0.11 | • | • | • | |
| Thickness | 0.92*** | 0.84*** | 0.69*** | 0.87*** | 0.89*** | -0.46*** | 0.75*** | 0.13 | • | • | |
| Length | 0.99*** | 0.71*** | 0.67*** | 0.79*** | 0.76*** | -0.52*** | 0.65*** | 0.01 | 0.91*** | • | |
| Width | 0.97*** | 0.72*** | 0.68*** | 0.79*** | 0.75*** | -0.50*** | 0.66*** | 0.02 | 0.92*** | 0.98*** |
Abbreviations: BY, breast yield; FT, feathered tender; LW, live weight; SM, spaghetti meat; TY, tender yield; WB, woody breast; WS, white striping; WT, woody-like tender.
*P < 0.05, **P < 0.01, ***P < 0.001. Significance was determined at P < 0.05 using Spearman p correlation values.
Measured in mm at the top of the fillet in the cranial region to the tip of the fillet in the caudal region.
Measured in mm at 1/3 the caudal end of the fillet.
Measured in mm at the thickest part of the cranial region of the fillet.
Table 6.
Female correlations of various meat quality traits and associated myopathies.
| LW | BY | TY | WS | WB | SM | WT | TF | Thickness3 | Length1 | Width2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LW | • | • | • | • | • | • | • | • | • | • | |
| BY | 0.79*** | • | • | • | • | • | • | • | • | • | |
| TY | 0.78*** | 0.87*** | • | • | • | • | • | • | • | • | |
| WS | 0.85*** | 0.86*** | 0.76*** | • | • | • | • | • | • | • | |
| WB | 0.82** | 0.91*** | 0.78*** | 0.89*** | • | • | • | • | • | • | |
| SM | -0.45** | -0.17 | -0.21 | -0.17 | -0.21 | • | • | • | • | • | |
| WT | 0.84*** | 0.81*** | 0.76*** | 0.80*** | 0.83*** | -0.34** | • | • | • | • | |
| TF | 0.19 | 0.29* | 0.25 | 0.30* | 0.35** | 0.16 | 0.30* | • | • | • | |
| Thickness | 0.94*** | 0.92*** | 0.83*** | 0.91*** | 0.92*** | -0.29* | 0.87*** | 0.27* | • | • | |
| Length | 0.98*** | 0.79*** | 0.80*** | 0.83*** | 0.80*** | -0.45** | 0.81*** | 0.15 | 0.92*** | • | |
| Width | 0.97*** | 0.82*** | 0.80*** | 0.84*** | 0.82*** | -0.39*** | 0.83*** | 0.18 | 0.94*** | 0.96*** |
Abbreviations: BY, breast yield; FT, feathered tender; LW, live weight; SM, spaghetti meat; TY, tender yield; WB, woody breast; WS, white striping; WT, woody-like tender.
*P < 0.05, **P < 0.001, ***P < 0.0001. Significance was determined at P < 0.05 using Spearman p correlation values.
Measured in mm at the top of the fillet in the cranial region to the tip of the fillet in the caudal region.
Measured in mm at 1/3 the caudal end of the fillet.
Measured in mm at the thickest part of the cranial region of the fillet.
Breast Meat Myopathies
Meat quality from a producer and consumer prospective can be measured by multiple aspects. These factors may include, but are not limited to, appearance, specifically color, juiciness, pertaining to WHC, and eating texture. All myopathies reported in this study can impact these quality traits. Recently, there has been an increase in breast meat myopathies such as WB, WS, and SM (Kuttappan et al., 2016; Tijare et al., 2016; Baldi et al., 2018; Petracci et al., 2019) resulting in incidence rates as high as 35 percent (Kuttappan et al., 2016; Tijare et al., 2016). Previous researchers have suggested that the increase of these myopathies in modern broiler strains has been associated with a faster growth rate and a higher breast yielding broiler to meet current consumer demands (Kuttappan et al., 2016; Petracci et al. 2019). These myopathies are creating a challenge for the industry, resulting in economic loss due to downgrades and condemnations of edible product (Mudalal et al., 2014b; Petracci et al., 2019; Tasoniero et al., 2020).
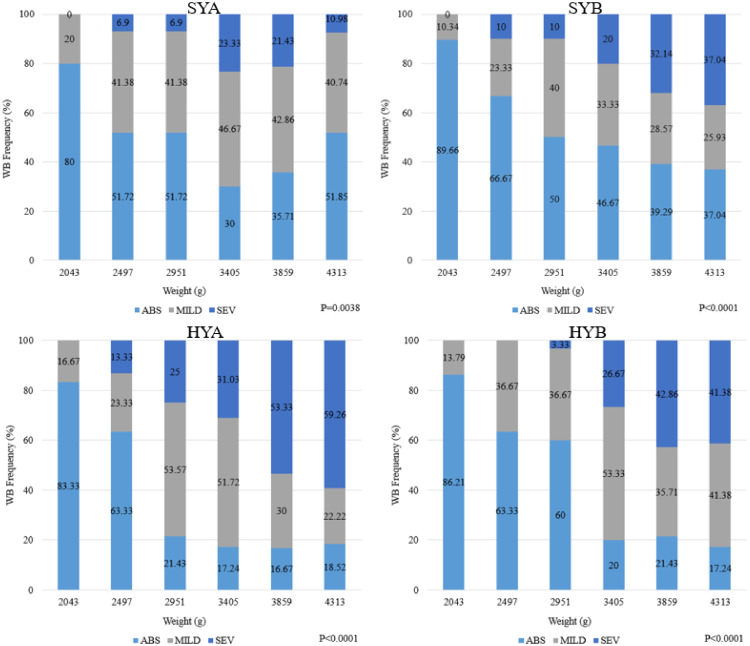
Results for this experiment expressed differences in the WB myopathy due to strain (P < 0.05) and target weight in both males and females (P < 0.05; Tables 7 and 8). However, an interaction between strain × target weight was also observed for males (P < 0.05, Tables 7 and 9). The HYA and SYA had higher WB mean scores than SYA at the highest target weight while other strains were similar to each other at all other target weights (P < 0.05, Table 9). For females, a trend was noted as HYA and HYB strains were higher for WB mean score than SYA and SYB (P < 0.05, Table 8). These results are supportive of findings by Zhang et al. (2021) who found variability in WB among various common and myopathy-selected broiler strains. Furthermore, as target weight increased, the mean WB score and the incidence of severe WB increased (P < 0.05; Figure 1, Figure 2 for males and females, respectively). For example, SYB males had an incidence of 10% for severe WB at 2,497 g and a 37.04% incidence at 4,313 g (Figure 1). The same trend was observed for females, SYB had a 0% incidence of severe WB at 2,497 g, however, expressed an incidence of 21.43% at a target weight of 4,313 g (Figure 2). This is supportive of results from Tijare et al. (2016) and Kuttappan et al. (2017), who reported a higher score and greater incidence rate for severe WB at 9 wk of age compared to those processed at 6 wk of age.
Table 7.
Effect of strain1 and target weight on breast and tender myopathies of male broilers.
| Treatment | WT2 | TF3 | WB4 | WS5 | SM6 |
|---|---|---|---|---|---|
| Strain | |||||
| SYA | 0.43a | 0.25ab | 0.91b | 0.85 | 0.09 |
| SYB | 0.23b | 0.18b | 0.93b | 0.83 | 0.08 |
| HYA | 0.47a | 0.23ab | 1.23a | 0.95 | 0.14 |
| HYB | 0.31b | 0.27a | 1.04b | 0.82 | 0.07 |
| SEM | 0.03 | 0.02 | 0.05 | 0.04 | 0.02 |
| Target weight (g) | |||||
| 2,043 | 0.20b | 0.19ab | 0.46c | 0.40c | 0.11abc |
| 2,497 | 0.14b | 0.23ab | 0.74b | 0.65b | 0.21a |
| 2,951 | 0.09b | 0.24ab | 0.91b | 0.81b | 0.17ab |
| 3,405 | 0.56a | 0.30a | 1.27a | 1.05a | 0.06bc |
| 3,859 | 0.54a | 0.26ab | 1.42a | 1.19a | 0.03c |
| 4,313 | 0.63a | 0.18b | 1.38a | 1.07a | 0.00c |
| SEM | 0.04 | 0.03 | 0.06 | 0.05 | 0.03 |
| P-values | |||||
| Strain | <0.0001 | 0.0440 | 0.0002 | 0.0759 | 0.3007 |
| Target weight (g) | <0.0001 | 0.0336 | <0.0001 | <0.0001 | <0.0001 |
| Interaction | 0.5462 | 0.0019 | 0.0104 | 0.3437 | 0.5005 |
Abbreviations: HYA, high yielding A, HYB, high yielding B; SYA, standard yielding A, SYB, standard yielding B.
Woody-like tender. Scored on a numeric scale from 0 to 2 with 0.5 increments.
Feathered tender. Scored on a numeric scale from 0 to 2 with 0.5 increments.
Woody breast. Scored on a numeric scale from 0 to 3 with 0.5 increments.
White striping. Scored on a numeric scale from 0 to 3 with 0.5 increments.
Spaghetti meat. Absence or presence of fraying throughout the Pectoralis major.
Means without a common superscript were determined to be significantly different (P < 0.05) by a Tukey's HSD test.
Table 8.
Effect of strain1 and target weight on breast and tender myopathies of female broilers.
| Treatment | WT2 | TF3 | WB4 | WS5 | SM6 |
|---|---|---|---|---|---|
| Strain | |||||
| SYA | 0.34ab | 0.29 | 0.56b | 0.71b | 0.16ab |
| SYB | 0.27b | 0.26 | 0.61b | 0.76ab | 0.09b |
| HYA | 0.39a | 0.32 | 0.97a | 0.87a | 0.29a |
| HYB | 0.33ab | 0.32 | 0.84a | 0.84ab | 0.14b |
| SEM | 0.03 | 0.03 | 0.05 | 0.04 | 0.03 |
| Target weight (g) | |||||
| 2,043 | 0.12c | 0.24 | 0.32d | 0.38c | 0.19ab |
| 2,497 | 0.11c | 0.27 | 0.51cd | 0.69b | 0.32a |
| 2,951 | 0.36b | 0.36 | 0.68c | 0.79b | 0.20ab |
| 3,405 | 0.48a | 0.33 | 0.97b | 0.97a | 0.10b |
| 4,313 | 0.60a | 0.28 | 1.24a | 1.13a | 0.04b |
| SEM | 0.03 | 0.04 | 0.05 | 0.04 | 0.04 |
| P-values | |||||
| Strain | 0.0278 | 0.4372 | <0.0001 | 0.0393 | 0.0025 |
| Target weight (g) | <0.0001 | 0.1285 | <0.0001 | <0.0001 | <0.0001 |
| Interaction | 0.5668 | 0.8730 | 0.0880 | 0.5342 | 0.6624 |
Abbreviations: HYA, high yielding A, HYB, high yielding B; SYA, standard yielding A, SYB, standard yielding B.
Woody-like tender. Scored on a numeric scale from 0 to 2 with 0.5 increments.
Feathered tender. Scored on a numeric scale from 0 to 2 with 0.5 increments.
Woody breast. Scored on a numeric scale from 0 to 3 with 0.5 increments.
White striping. Scored on a numeric scale from 0 to 3 with 0.5 increments.
Spaghetti meat. Absence or presence of fraying throughout the Pectoralis major.
Means without a common superscript were determined to be significantly different (P < 0.05) by a Tukey's HSD test.
Table 9.
Effect of strain1 and target weight on WB for male broilers.
| Treatment | Strain |
|||
|---|---|---|---|---|
| SYA1 | SYB1 | HYA1 | HYB1 | |
| Target weight (g) | ||||
| 2,043 | 0.52efg | 0.37g | 0.50efg | 0.43fg |
| 2,497 | 0.89bcdefg | 0.70cdefg | 0.80bcdefg | 0.57defg |
| 2,951 | 0.86bcdefg | 0.80bcdefg | 1.28abc | 0.70cdefg |
| 3,405 | 1.23abcd | 1.10abcdef | 1.41ab | 1.33abc |
| 3,859 | 1.13abcde | 1.27abc | 1.72a | 1.58a |
| 4,313 | 0.85bcdefg | 1.33abc | 1.70a | 1.61a |
| SEM | 0.13 | |||
| P-value | ||||
| Interaction | 0.0104 | |||
Abbreviations: HYA, high yielding A, HYB, high yielding B; SYA, standard yielding A, SYB, standard yielding B.
Means without a common superscript were determined to be significantly different (P < 0.05) by a Tukey's HSD test.
Figure 1.
Woody breast (WB) incidence rate of males. Abbreviations: HYA, high yielding A; HYB, high yielding B; SYA, standard yielding A; SYB, standard yielding B.
Figure 2.
Woody breast (WB) incidence rate of females. Abbreviations: HYA, high yielding A; HYB, high yielding B; SYA, standard yielding A; SYB, standard yielding B.
For both males and females, a higher WB incidence was observed in HY strains than in SY strains. For example, male SYA had an incidence rate of 23.33% severe WB at 3,405g, whereas HYA had an incidence of 31.03% at the same target weight (Figure 1). Similar results were observed in females, as SYA had a 3.33% incidence of severe WB at a target weight of 3,405g, whereas HYA had 44.38% at the same target weight (Figure 2). The results of Livingston et al. (2018) confirm these results as the authors found that HY strains tend to express a higher mean score and incidence of all myopathies when compared to SY strains.
Furthermore, thickness was highly correlated to WB score (r = 0.89 for males and r = 0.92 for females; Tables 5 and 6). These results suggest that as fillet thickness increases, the probability of WB occurrence increases. This idea supports that myopathies are associated with heavier and thicker fillets (Mudalal et al., 2014c; Dalle Zotte et al., 2017; Kuttappan et al., 2017; Mallmann et al., 2020). Length and width of breast fillets were also highly correlated WB (males, r = 0.76 and r = 0.75, respectively; females, r = 0.80 and r = 0.82, respectively).
Results for WS in the current experiment are reported in Tables 7 and 8. White striping is another myopathy generating quality issues and economic loss for the industry (Kuttappan et al., 2016). It is a condition distinguished by the occurrence of white striations, seen parallel to the muscle fibers on breast fillets, tenders, and even muscles of the thigh (Kuttappan et al., 2009; 2012b; 2013a). White striping is primarily considered an issue in terms of appearance for consumers but does slightly alter the nutritional quality of fresh poultry (Kuttappan et al., 2012b). In the present study, there were differences among strain for WS score (P < 0.05) in females but lacked differences (P > 0.05) in males. However, target weight had an impact on WS (P < 0.05), regardless of sex, where WS score increased as target weight increased. It has been reported that WS, as well as WB, is associated with breast fillets that are heavier and thicker (Kuttappan et al., 2013a; Mallmann, 2019). In the current experiment, correlation between WS and BY support this claim as WS increased alongside BY (males, r = 0.74 and r = 0.87 for WS to BY and thickness, respectively; females: r = 0.86 and r = 0.83 for WS to BY and thickness, respectively; Tables 5 and 6).
For females, WS score for HYA was higher (P < 0.05) than SYA, with HYB and SYB being intermediate. Previous studies by Kuttappan et al. (2012a) and Petracci et al. (2014) both reported that straight run HY strains exhibit a higher degree of WS when compared to SY strains. It has been hypothesized that HY strains have a greater occurrence of myopathies, at the cost of increased yield, resulting in increased muscle fiber myodegeneration (Sihvo et al., 2014). Speculation has been made that WS results from attempted muscle fiber damage repair with infiltration of excess fat and collagen. This may explain the altered nutritional (increased fat and collagen content and decreased protein levels) properties present in affected fillets, as well as the decrease in functionality for further processing (Kuttappan et al., 2012a,b; 2013b; Petracci et al, 2013; Mudalal et al., 2014a; Vignale et al., 2017). Therefore, the higher occurrence and severity of WS in HY strains was expected.
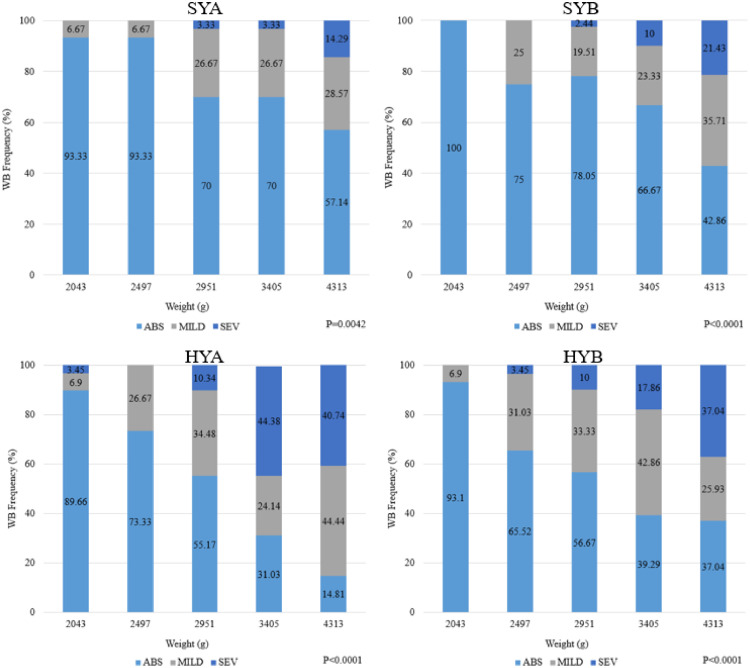
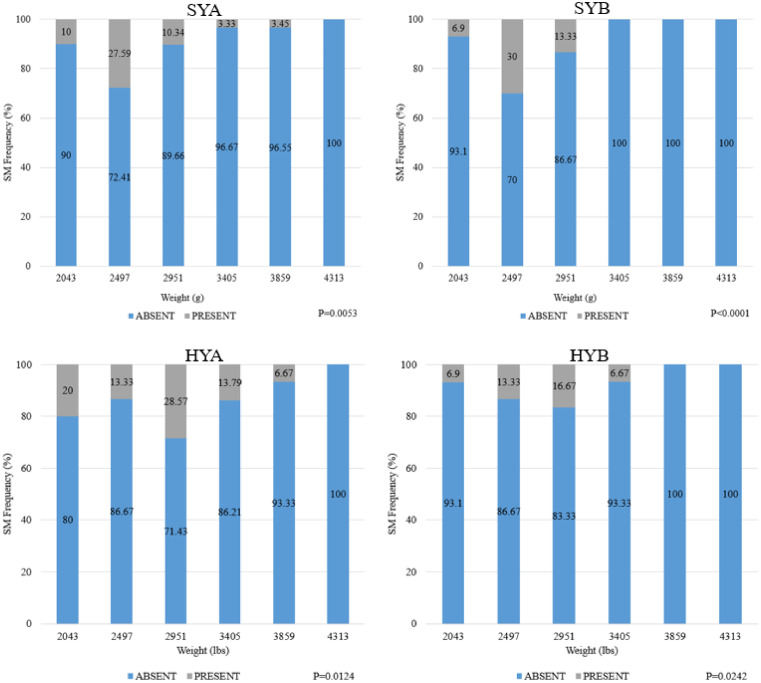
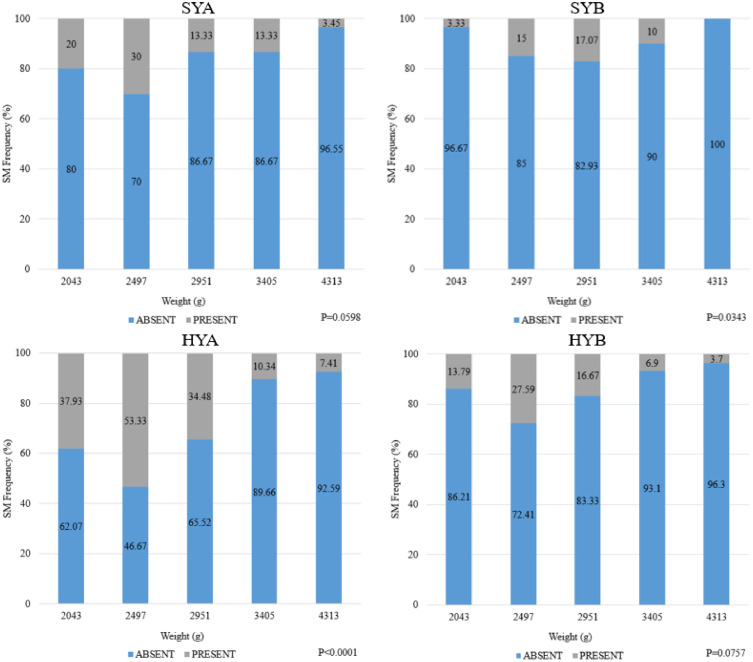
Results for strain and target weight effects on SM are shown in Tables 7 and 8. Spaghetti meat is described as exhibiting an altered structural integrity of the breast fillet by displaying separation of the fiber bundles in the Pectoralis major (Baldi et al., 2018; Petracci et al., 2019). There were no differences between strain (P > 0.05), but target weight was significant (P < 0.05) for males. There was a general decrease in mean score of SM as target weight increased, especially from 2497g to 4313g. Spaghetti meat of female breast fillets was impacted by both strain and target weight (P < 0.05). HYA had the greatest mean SM score while HYB and SYB had the lowest (P < 0.05). The greatest amount of SM was observed at a target weight of 2,497 g and the lowest at 4,313 g for both sexes. Previous findings by Mallmann (2019) reported a peak in the SM myopathy at 7 wk of age followed by a decrease thereafter (9 and 10 wk) though the authors had fewer age intervals than in the current study. An earlier peak of the myopathy was present in the current study. However, this could be explained by strain variation and the utilization of targeting a weight rather than an age. A direct effect of sex seemed to be present as females possessed a higher degree of severity when compared to males, which agrees with the findings of Mallmann (2019). The incidence of SM is shown in Figure 3, Figure 4 for male and females, respectively. For males, SYA had the highest incidence of SM present at 2,497 g (27.59%; Figure 3). At 2,043 g there was a 10% incidence and a 10.34% at 2,951 g. For SYB, the greatest incidence (30%) was observed at 2,951 g. At 2,043 g there was only a 6.9% incidence but at 2951g there was a 13.33% incidence of SM. For HYA males, there was a 20% incidence at 2,043 g, 13.33% incidence at 2,497 g, and a 28.57% incidence at 2,951 g. HYB males had a 6.9% incidence at 2,043 g, 13.33% incidence at 2,497 g, and a 16.67% incidence at 2,951 g. For females, SYA had the greatest incidence of the myopathy at lighter weights with 20% incidence at 2,043 g and 30% at 2,497 g (Figure 4). SYB had the greatest incidence at 2,497 g (15%) and 2,951 g (17.07%). For HYA females, a 37.93% incidence was observed at a target weight of 2,043 g, 53.33% at 2,497 g, and 34.48% at 2,951 g. HYB females had the greatest incidence at 2,497 g with 27.59% incidence followed by 16.67% at 2,951 g and 13.79% at 2,043 g.
Figure 3.
Spaghetti meat (SM) incidence rate of males. Abbreviations: HYA, high yielding A; HYB, high yielding B; SYA, standard yielding A; SYB, standard yielding B.
Figure 4.
Spaghetti meat (SM) incidence rate of females. Abbreviations: HYA, high yielding A; HYB, high yielding B; SYA, standard yielding A; SYB, standard yielding B.
While limited research is available regarding the SM myopathy, Baldi et al. (2018) noted that SM has similar histologic features to WB and WS myopathies including fiber degeneration, increased fat, and increased connective tissue. According to Bilgili (2015), it is possible that the altered integrity of the muscle is due to the immaturity of the newly deposited collagen, which is the major component of connective tissue in muscle. The SM myopathy is concerning issue in the poultry industry with the visually unappealing appearance of the raw fillet to consumers (Baldi et al., 2018; Petracci et al., 2019). Petracci et al. (2019) reported that altered meat appearance consisting of disintegrated meat structure is motivation for the consumer to reject a breast fillet, resulting in downgrades or even product loss. Rejection of the product can result in severe economic losses for the industry providing reason to better understand the epitome of this myopathy.
Tender Myopathies
Recently, processors have noticed issues similar to WB, WS, and SM affecting the Pectoralis minor or tender. On occasion, tenders exhibit a variation in hardness, similar to WB fillets. This will be referred to as WT in this study. However, it is unknown at this time if there are similar histologic features between the 2 myopathies. While little research is currently available regarding this issue, it is possible that WT could have the same altered texture properties seen in WB, which could create potential issues for processors. TF is similar to the SM myopathy, having separation of muscle fibers of the Pectoralis minor. The TF condition has been previously described by Soglia et al. (2019) but was referred to as gaping due to the similar characteristics between this condition and the defect of “gaping” that affects fish fillets. The term “feathering” is commonly used in industry in the U.S. to describe this condition (Maynard, 2020). The separation of the fibers results in an undesirable appearance and often result in downgrades of the product which in turn creates economic loss for the industry (Soglia et al., 2019).
Results from the current study are reported in Tables 7 and 8. Both strain and target weight had an impact on hardness of raw tenders for males (P < 0.05). SYA and HYA expressed higher scores (P < 0.05) than SYB and HYB. For females, both strain and target weight expressed differences in WT score (P < 0.05). Similar to the trend seen in males, there was an increase in WT score as target weight increased (P < 0.05). HYA expressed higher WT scores (P < 0.05) than SYB, while HYB and SYA were intermediate (P > 0.05) for females. As target weight increased, WT score also increased in both males and females (P < 0.05) similar to the trend observed with WB. Woody-like tender scores were moderately (males; Table 5) and highly (females; Table 6) correlated to live weight, BY, tender yield, along with WS and WB.
Strain and target weight also had an impact on male TF score for this study (P < 0.05), complemented by an interaction of strain × target weight (P < 0.05). However, results were inconsistent with target weight as a main effect. While significant differences were observed, no trend over time was apparent. HYB expressed higher TF (P < 0.05) than SYB, while HYA and SYA scores were intermediate. Furthermore, there were no differences in TF for female broilers with strain nor target weight (P > 0.05) as a main effect. It is likely that more handling in industry due to travel paths, transfers, time on conveyor belts, as well as further processing procedures may all lead to the increase of feathering of tenders in an industry setting. Probably assumption can be made that less handling occurs with tenders in a research setting, thus providing an inconsistent trend in TF. However, more research is needed to determine the true cause of this myopathy. In the experiment by Soglia et al. (2019), an incidence rate of 18.6 percent was reported on a total of 8,600 chickens from 43 flocks processed in a commercial facility ranging from 42 to 54 d of age. The high incidence rate reported in the study by Soglia et al. (2019) accompanied with the results of reduced meat quality attributes (lower pH, lighter color, impaired WHC) provides a basis for the need to research this emerging issue.
Breast Meat pH
Muscle pH has been well established as an indicator for several quality issues observed in poultry meat. Results in this experiment indicated that target weight had an impact for males and females on 24 h postmortem pH (P < 0.05; Tables 10 and 11). However, there was no consistent pattern observed as an increase or decrease in pH values as target weight increased. Previous research suggests that larger birds bred for HY progress through rigor at a slower rate than birds with smaller yields (Berri et al, 2001). Cooper and Fletcher (1997) reported a slower decline in pH associated with heavier flocks. However, Cavitt et al. (2005) also reported no significant difference in pH (at 24 h postmortem) due to body size (in the range of 2.24–2.88 kg). In this study, as birds increased in weight over the study period, it was expected that chilling rate would significantly decrease and may impact pH. However, results from this study suggest that post rigor pH is not impacted by bird size, but this may be due to the sampling time of 24 h postmortem which indicates more of an extent of pH decline rather than a rate of decline. Similar results were observed in a study conducted by Lopez et al. (2011) who reported there were no differences among strains in respect to ultimate pH. Furthermore, pH values in this this study were within the acceptable normal range of 5.6 to 6.0 (Barbut, 1997; Qiao et al., 2001).
Table 10.
Effect of strain1 and target weight on meat quality attributes of male broilers.
| Treatment | pH2 | L*3 | a*3 | b*3 | Drip loss % | Thaw loss % | Cook loss % | Total loss %4 |
|---|---|---|---|---|---|---|---|---|
| Strain | ||||||||
| SYA | 5.98 | 56.22a | 2.34 | 9.54a | 1.07 | 6.86 | 24.52 | 31.19 |
| SYB | 5.96 | 55.29b | 2.18 | 9.05ab | 1.07 | 6.71 | 23.08 | 29.72 |
| HYA | 6.00 | 55.63ab | 2.22 | 8.86b | 1.04 | 6.85 | 22.68 | 29.68 |
| HYB | 5.96 | 55.53ab | 2.37 | 9.62a | 0.82 | 6.99 | 24.13 | 30.48 |
| SEM | 0.01 | 0.20 | 0.08 | 0.15 | 0.09 | 0.28 | 0.54 | 0.68 |
| Target weight (g) | ||||||||
| 2,043 | 5.99a | 54.91b | 2.40ab | 8.58b | 1.19a | 7.91b | 22.40b | 30.31b |
| 2,497 | 5.91b | 55.25b | 2.10ab | 8.51b | 1.21a | 8.23b | 25.66a | 33.86a |
| 2,951 | 5.98a | 55.22b | 1.98b | 9.13ab | 1.09ab | 8.48ab | 25.30a | 32.84ab |
| 3,405 | 5.97ab | 56.37a | 2.33ab | 9.89a | 1.00ab | 9.71a | 23.43ab | 33.09ab |
| 3,859 | 6.00a | 56.43a | 2.45a | 9.71a | 0.83ab | 3.47c | 23.38ab | 26.76c |
| 4,313 | 5.99a | 55.84ab | 2.43a | 9.78a | 0.67b | 3.32c | 21.44b | 24.75c |
| SEM | 0.02 | 0.24 | 0.01 | 0.19 | 0.11 | 0.34 | 0.66 | 0.84 |
| P-values | ||||||||
| Strain | 0.1317 | 0.0140 | 0.3065 | 0.0017 | 0.1473 | 0.9127 | 0.0677 | 0.3587 |
| Target weight | 0.0015 | <0.0001 | 0.0076 | <0.0001 | 0.0056 | <0.0001 | 0.0002 | <0.0001 |
| Interaction | 0.5659 | 0.5996 | 0.1236 | 0.7818 | 0.7291 | 0.3104 | 0.8888 | 0.5445 |
Abbreviations: HYA, high yielding A, HYB, high yielding B; SYA, standard yielding A, SYB, standard yielding B.
pH was collected 24 h postmortem.
Color measurements were collected 24 h postmortem on the dorsal side of the fillet.
Total loss was determined as the combination of cook and thaw loss.
Means without a common superscript were determined to be significantly different (P < 0.05) by a Tukey's HSD test.
Table 11.
Effect of strain1 and target weight on meat quality attributes of female broilers.
| Treatment | pH2 | L*3 | a*3 | b*3 | Drip loss % | Thaw loss % | Cook loss % | Total loss %4 |
|---|---|---|---|---|---|---|---|---|
| Strain | ||||||||
| SYA | 5.89 | 57.12a | 2.40 | 10.10ab | 0.96 | 7.51 | 23.26 | 30.30 |
| SYB | 5.89 | 55.78b | 2.72 | 9.70b | 1.29 | 7.49 | 21.85 | 29.09 |
| HYA | 5.90 | 56.55ab | 2.57 | 9.80b | 1.21 | 7.93 | 22.84 | 30.11 |
| HYB | 5.86 | 56.47ab | 2.48 | 10.48a | 1.10 | 8.16 | 22.81 | 31.04 |
| SEM | 0.01 | 0.29 | 0.09 | 0.14 | 0.09 | 0.25 | 0.59 | 0.57 |
| Target weight (g) | ||||||||
| 2,043 | 5.92a | 55.39c | 2.31b | 9.12d | 1.44a | 8.02b | 23.71ab | 31.73b |
| 2,497 | 5.89a | 56.05bc | 2.38b | 9.61cd | 1.36a | 10.34a | 25.78a | 34.61a |
| 2,951 | 5.94a | 56.61abc | 2.54b | 9.94bc | 1.25ab | 10.59a | 21.92bc | 32.58ab |
| 3,405 | 5.94a | 56.75ab | 2.46b | 10.53ab | 0.93bc | 4.80c | 21.56bc | 26.31c |
| 4,313 | 5.73b | 57.58a | 2.99a | 10.91a | 0.72c | 5.10c | 20.24c | 25.44c |
| SEM | 0.02 | 0.32 | 0.10 | 0.16 | 0.10 | 0.27 | 0.67 | 0.64 |
| P-values | ||||||||
| Strain | 0.1012 | 0.0207 | 0.0629 | 0.0019 | 0.0603 | 0.1717 | 0.2519 | 0.1299 |
| Target weight | <0.0001 | 0.0004 | 0.0003 | <0.0001 | <0.0001 | <0.0001 | 0.0002 | <0.0001 |
| Interaction | 0.1934 | 0.3405 | 0.1666 | 0.5759 | 0.9631 | 0.0898 | 0.5718 | 0.1008 |
Abbreviations: HYA, high yielding A, HYB, high yielding B; SYA, standard yielding A, SYB, standard yielding B.
pH was collected 24 h postmortem.
Color measurements were collected 24 h postmortem on the dorsal side of the fillet.
Total loss was determined as the combination of cook and thaw loss.
Means without a common superscript were determined to be significantly different (P < 0.05) by a Tukey's HSD test.
There were no differences observed for pH in either sex with strain as a main effect (P > 0.05) in this study. There have been mixed results in the literature. Mehaffey et al. (2006) reported some variation in pH measured early postmortem (2 and 4 h postmortem) among broiler strains. In the current study, pH was measured at 24 h postmortem which would result in an overall lower pH than early postmortem, as pH continually declines as muscle progresses through the stages of rigor (Dransfield and Sosnicki, 1999). The results of the current study are supported by Berri et al. (2001) who reported similar pH at 24 h postmortem in breast meat from 2 broiler strains selected for high body weight and BY even when body weight differed. In contrast, Santiago et al. (2005) reported differences due to strain where HY broilers had lower muscle pH at 24 h PM than SY broilers. Differences between the current study and previous literature is likely due to 24 h sampling, sampling of pH over multiple weeks (representing differing body weights), and genetic profile (1–2 decades difference between studies), and myopathy presence which can impact pH (Mudalal et al., 2014c).
Breast Meat Color
Meat color plays an important role in the acceptability of the product by consumer, both during selection of raw meat in grocery stores and after being prepared at home before consumption (Fletcher, 1999). Aside from consumer acceptability based on appearance, color has been connected to the functionality of meat during processing as a measure of WHC pH values, texture, and the shelf-life of a product (Allen et al., 1998). Differences in meat color and pH have been shown to be related to preslaughter conditions, such as heat stress and variances in struggling when hung (Froning et al., 1978), myoglobin content (Brewer, 2004), and muscle myopathies (Aguirre et al., 2018).
Results for color are reported in Tables 10 and 11. Target weight had a significant effect on all 3 color measurements (L*, a*, and b*) for both sexes. For males, heavy target weights (3,405 g and 3,859 g) had a higher L* value (P < 0.05) than that of standard/lighter target weights (2,043 g, 2,497 g, and 2,951 g), indicating a lighter breast fillet color associated with larger birds (r = 0.45 P < 0.05). For females, differences were observed between the lightest (2,043 g) and heaviest body weights (4,313 g; P < 0.05), with the 4,313 g having a greater L* value than 2,043 g (all other TW L* values were intermediate). This increase may partly be due to an increased severity of WB at various target weights in both male and females. It has been reported that as target weight increases, WB incidence and severity increases to include lighter colored fillets and obtain higher pH values (Aguirre et al., 2018). In previous research, it has been established that L* values and pH have a significant relationship (r = -0.79, P < 0.01; Barbut, 1997). However, in this study, there limited correlation between pH and L* value (for females, r = -0.33, P < 0.001, Table 12; for males, P > 0.05, Table 13), likely because pH values in this study were considered normal. In previous studies reporting high correlation between pH and L* value, meat quality issue such as pale, soft, and exudative meat was included. Other researchers have reported an increase in L* value with increasing age which support the general trend for increasing L* with body weight in this study (Janisch et al., 2011; Schneider et al., 2012; Badar et al., 2021).
Table 12.
Correlations of various meat quality parameters for male broilers.
| LW | BY | TY | Drip | pH | L* | a* | b* | Thaw | Cook | Total | MF | ME | MPC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LW | • | • | • | • | • | • | • | • | • | • | • | • | • | |
| BY | 0.66*** | • | • | • | • | • | • | • | • | • | • | • | • | |
| TY | 0.61*** | 0.79** | • | • | • | • | • | • | • | • | • | • | • | |
| Drip | -0.42** | -0.23 | -0.35** | • | • | • | • | • | • | • | • | • | • | |
| pH | 0.15 | 0.26* | 0.23 | -0.13 | • | • | • | • | • | • | • | • | • | |
| L* | 0.45*** | 0.37** | 0.30* | -0.10 | -0.12 | • | • | • | • | • | • | • | • | |
| a* | -0.08 | 0.21 | 0.26* | -0.09 | -0.09 | 0.28* | • | • | • | • | • | • | • | |
| b* | 0.65*** | 0.45*** | 0.40** | -0.16 | 0.03 | 0.61*** | 0.46*** | • | • | • | • | • | • | |
| Thaw | -0.50*** | -0.32** | -0.50*** | 0.52*** | -0.28* | - | -0.10 | -0.15 | • | • | • | • | • | |
| Cook | -0.20 | -0.20 | -0.23 | 0.29* | -0.31** | 0.15 | -0.13 | 0.08 | 0.30* | • | • | • | • | |
| Total | -0.49*** | -0.36** | -0.51*** | 0.51*** | -0.31** | 0.03 | -0.18 | -0.06 | 0.80*** | 0.72*** | • | • | • | |
| M F | 0.21 | 0.14 | 0.22 | -0.29* | -0.25* | 0.08 | 0.04 | 0.19 | -0.15 | 0.24* | -0.01 | • | • | |
| M E | 0.36* | 0.29* | 0.38** | -0.45*** | -0.03 | 0.29* | 0.21 | 0.38** | -0.29* | -0.03 | -0.24* | 0.75*** | • | |
| M PC | 0.60*** | 0.53*** | 0.46*** | -0.15 | 0.21 | 0.60*** | 0.15 | 0.56*** | -0.20 | -0.07 | -0.20 | 0.02 | 0.25* |
Abbreviations: BW, Body weight; BY, breast yield; FT, feathered tender; MF, MORS force; ME, MORS energy; MPC, MORS peak counts; TY, tender yield; WT, woody-like tender.
*P < 0.05, **P < 0.001, ***P < 0.0001.
Table 13.
Correlations of various meat quality parameters for female broilers.
| LW | BY | TY | Drip | pH | L* | a* | b* | Thaw | Cook | Total | MF | ME | MPC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LW | • | • | • | • | • | • | • | • | • | • | • | • | • | |
| BY | 0.79*** | • | • | • | • | • | • | • | • | • | • | • | • | |
| TY | 0.78*** | 0.87*** | • | • | • | • | • | • | • | • | • | • | • | |
| Drip | -0.59*** | -0.45** | -0.47*** | • | • | • | • | • | • | • | • | • | • | |
| pH | -0.39* | -0.24 | -0.18 | 0.37** | • | • | • | • | • | • | • | • | • | |
| L* | 0.51*** | 0.41** | 0.36* | -0.31* | -0.33** | • | • | • | • | • | • | • | • | |
| a* | 0.47** | 0.41** | 0.30* | -0.09 | -0.26* | -0.11 | • | • | • | • | • | • | • | |
| b* | 0.80*** | 0.68*** | 0.68*** | -0.39** | -0.37** | 0.53*** | 0.40** | • | • | • | • | • | • | |
| Thaw | -0.58*** | -0.45** | -0.49*** | 0.59*** | 0.15 | -0.15 | -0.19 | -0.43** | • | • | • | • | • | |
| Cook | -0.47** | -0.49*** | -0.54*** | 0.41**- | 0.23 | -0.17 | -0.42** | -0.33* | 0.31* | • | • | • | • | |
| Total | -0.68*** | -0.56*** | -0.63*** | 0.64*** | 0.28* | -0.25 | -0.38** | -0.50*** | 0.82*** | 0.72*** | • | • | • | |
| M F | 0.08 | -0.07 | -0.13 | -0.12 | -0.36** | 0.13 | -0.16 | 0.03 | 0.08 | 0.29* | 0.18 | • | • | |
| M E | 0.18 | 0.02 | -0.04 | -0.21 | -0.37** | 0.15 | -0.19 | 0.10 | -0.10 | 0.35** | 0.09 | 0.89*** | • | |
| M PC | 0.46** | 0.37* | 0.51*** | -0.15 | 0.15 | 0.28* | 0.09 | 0.45** | -0.12 | -0.12 | -0.22 | -0.10 | -0.06 |
Abbreviations: BW, Body weight; BY, breast yield; FT, feathered tender; MF, MORS force; ME, MORS energy; MPC, MORS peak counts; TY, tender yield; WT, woody-like tender.
*P < 0.05, **P < 0.001, ***P < 0.0001.
Additionally, a* values had minor changes over the smallest to largest TW, but overall, the differences were very limited and inconsistent. Previous literature has indicated mixed results where some studies reported a decrease in redness (a* value) as birds increase in age (Janisch et al., 2011; Schneider et al., 2012; Badar et al., 2021) and other studies report no change (Ngoka et al., 1982; Smith et al., 2002) or an increase in redness (Froning et al., 1968). This suggests that other factors may also play a role in changes in redness of fillets such as stress, season, or diet. There was also a narrow range of values in the current study suggesting that age has little impact on the redness of fillets. In terms of target weight, b* values were impacted and increased (P < 0.05) in value as target weight increased, regardless of sex which is supported by others (Schneider et al., 2012; Badar et al., 2021). With strain as a main effect, there were differences in L* and b* values but lacked differences in a* values for both sexes. Other researchers have reported differences in color due to strain (Janisch et al., 2011; Petracci et al., 2013).
Water Holding Capacity
Water holding capacity is related to the loss of water from meat during processing, storage, and cooking and thus influences the juiciness/tenderness of a product when consumed (Jeffrey, 1983). Results in this experiment exhibited that strain had no impact on drip loss, thaw loss, cook loss, or total loss (P > 0.05; Tables 10 and 11) for both sexes. However, target weight influenced drip loss, cook loss, thaw loss, and total loss for both males and females (P < 0.05). In general, as target weight increased drip loss values decreased for males and females. The results from the current study are supportive of findings from previous studies (Dadgar et al., 2011; Janisch et al.,2011; Schneider et al., 2012). Fanatico et al. (2007), who reported a decrease in drip loss as breast weight increased (r = -0.73) using slow growing and fast growing broilers. For the current study, a correlation of r = -0.44 (Table 13) was observed for drip loss and breast weight for females with no significant correlation being noted for males. The lower correlation in the current may be a result of using all fast growing broilers.
For thaw loss, a general decrease was expressed as target weight increased (Tables 10 and 11). For males, thaw loss was lower (P < 0.05) for 3,859 g and 4,313 g weights compared to the lighter target weights such as 2,043 g (3.47 and 3.32 < 7.91, respectively). For females, thaw loss was also lower (P < 0.05) at 3,405 and 4,313 g compared to lighter target weights, 2,043 g, (4.80 and 5.10 < 8.02, respectively).
For cook loss, generally, cook loss decreased as TW increased (Tables 10 and 11). For males, cook loss percentage decreased as TW increased and broilers 4,313 g were lower (P < 0.05) than broilers 2,951 g and 2,497 g where those 3,405 g and 3,859 g were intermediate. For females, cook loss also decreased (P < 0.05) as TW increased where 4,313 g was lower (P < 0.05) than 2,497 g and 2,043 g and the 2,951 g and 3,405 g were intermediate. Contrary to the current study, Fanatico et al. (2007) reported lighter breast fillets had less thaw loss (0.63%) and less cook loss (13.37%) than heavier breast fillets (1.52 and 22.1%, respectively). Mehaffey et al. (2006) also reported lighter breast fillets to have less cook loss than heavier fillets at various deboning times. The main difference between the current study and Mehaffey et al. (2006) could be due the vast sampling times presented in the current experiment. The greater cook loss values observed in lighter target weights can also be attributed to the ability of the meat to expel free water in smaller breast fillets faster than larger breast fillets during cooking because changes in muscle fiber surface area. Previous literature has shown mixed results; some studies have shown an increase in cook loss with fillet weight or age (of broiler) and then a decrease in cook loss with increasing age as well (Janisch et al., 2011; Schneider et al., 2012). Differences in this study and previous studies may be attributed to different cooking methods, processing conditions, etc.
Total loss was determined as a combination of thaw and cook loss and it was apparent that total loss generally decreased (P < 0.05) from the first processing weight to the last processing weight for both sexes (males: 30.31–24.75; females: 31.73–25.44), following similar patterns of thaw and cook loss when analyzed separately. The current experiments findings for total loss are confirmed by those in Fanatico et al. (2007), who reported a greater total loss percentage (total loss calculated from the cooked weight as a percentage of the raw weight at time of deboning) for slow-growing birds (37.52% TL) than fast-growing birds (33.12% TL). However, it is important to note differences in method used to calculate total loss as the current study determined total loss as the combination of cook and thaw loss.
Texture
Consumer acceptability of poultry meat during consumption is dependent upon the tenderness and texture of the meat (Fletcher, 2002). The main driving factors that affect tenderness and texture of poultry meat are deboning time and muscle myopathies (Cavitt et al., 2005; Aguirre et al., 2018). In this study, the MORS method was used in the cranial (top one-third of the breast) region to determine instrumental tenderness values of breast fillets. The effect of both strain and target weight were evaluated on the energy (E), force (F), and PC values in the cranial region of both male and female fillets. Results for relative instrumental tenderness are reported in Tables 14 and 15.
Table 14.
Effect of strain1 and target weight on MORS properties of male broiler breast fillets.
| Treatment | Shear parameter |
||
|---|---|---|---|
| Strain | CR2 MORS F3 | CR2 MORS E3 | CR2 MORS PC3 |
| SYA | 12.62 | 182.46 | 9.1 |
| SYB | 12.86 | 182.71 | 8.69 |
| HYA | 12.7 | 182.19 | 8.9 |
| HYB | 13.03 | 186.69 | 8.88 |
| SEM | 0.18 | 2.57 | 0.21 |
| Target weight (g) | |||
| 2,043 | 12.32 | 181.43ab | 7.73b |
| 2,497 | 13.11 | 179.09b | 8.23b |
| 2,951 | 12.78 | 178.27b | 8.10b |
| 3,405 | 12.53 | 181.77ab | 10.10a |
| 3,859 | 12.94 | 187.70ab | 9.84a |
| 4,313 | 13.12 | 192.94a | 9.36a |
| SEM | 0.22 | 3.14 | 0.25 |
| P-values | |||
| Strain | 0.3802 | 0.5635 | 0.5706 |
| Target weight | 0.0685 | 0.0126 | <0.0001 |
| Interaction | 0.203 | 0.0972 | 0.6129 |
Abbreviations: HYA, high yielding A, HYB, high yielding B; SYA, standard yielding A, SYB, standard yielding B.
Cranial region—measurement taken in the top 1/3 of the Pectoralis major.
Meullenet-Owens Razor Shear (MORS) F = force; E = energy; PC = peak counts.
Means without a common superscript were determined to be significantly different (P < 0.05) by a Tukey's HSD test.
Table 15.
Effect of strain1 and target weight on MORS properties of female broiler breast fillets.
| Treatment | Shear parameter |
||
|---|---|---|---|
| Strain | CR2 MORS F3 | CR2 MORS E3 | CR2 MORS PC3 |
| SYA | 13.33 | 189.22 | 8.34 |
| SYB | 13.26 | 183.26 | 8.32 |
| HYA | 12.98 | 183.86 | 7.96 |
| HYB | 13.42 | 189.04 | 8.58 |
| SEM | 0.18 | 2.47 | 0.19 |
| Target weight (g) | |||
| 2,043 | 12.83b | 182.66ab | 7.49b |
| 2,497 | 14.02a | 191.14a | 7.78b |
| 2,951 | 12.78b | 179.68b | 8.95a |
| 3,405 | 13.14b | 186.99ab | 9.05a |
| 4,313 | 13.36ab | 191.26a | 8.25ab |
| SEM | 0.21 | 2.77 | 0.21 |
| P-values | |||
| Strain | 0.3395 | 0.1821 | 0.1577 |
| Target weight | 0.0007 | 0.0163 | <0.0001 |
| Interaction | 0.3356 | 0.4359 | 0.4508 |
Abbreviations: HYA, high yielding A, HYB, high yielding B; SYA, standard yielding A, SYB, standard yielding B.
Cranial region- Measurement taken in the top 1/3 of the Pectoralis major.
Meullenet-Owens Razor Shear (MORS) F = force; E = energy; PC = peak counts.
Means without a common superscript were determined to be significantly different (P < 0.05) by a Tukey's HSD test.
Strain, as a main effect, expressed no differences (P > 0.05) among force, energy, or PC for either sex. These results are contradictive of the findings from Mehaffey et al. (2006) who reported differences between 5 different commercial strains at 6 or 7 wk of age. The results also differ from the findings of Maynard (2020) who reported strain to have significant impact on MORSE, MORSF, and MORS PC. Differences in strain type used, as well as bird size used, in these studies may explain why MORS values were not impacted in the current study.
With target weight as a main effect, significant differences were observed for MORSF, MORSE, and MORS-PC for females, but only for E and PC for males. For males, differences (P < 0.05) were observed among target weights for MORSE with 4,313 g requiring a higher amount of energy compared to fillets at the 2,497 g and 2,951 g weight (192.94–179.09 and 178.27, respectively). The results from the current study are supportive of the findings of Yang et al. (2016) who reported MORSE and MORSF to increase as broiler age increased, which inherently increases weight. Differences (P < 0.05) among target weight for MORS-PC were observed with 4,313, 3,859, and 3,405 g having a larger number of peaks (P < 0.05) than the 3 lighter weights (9.36 at 4,313 and 7.73 at 2,043 g). These results are supportive of the findings from Solo (2016) who reported a greater number of PC at 63 and 70 d of age when compared to 45 d of age. While limited research is currently available regarding PC, some literature suggests that PCs are highly correlated to the WB condition. Sun et al. (2016) first reported severe WB fillets to have higher PC than fillets that exhibited only moderate WB and Sun et al. (2021) reported higher PC in severe WB compared to normal breast. In a recent study by Bowker and Zhuang (2019), the authors reported that as the severity of WB increases, PC of the fillet also increased (r = 0.65). Results from the current study are supportive of those findings as MORS-PC were highly correlated with the WB myopathy (r = 0.70 for males; r = 0.43 for females; data not shown). A hypothesized theory for PC differences is that excessive amounts of connective tissue observed in WB could result in increased resistance to shearing resulting in higher PC values (Bowker and Zhuang, 2019).
For females, significant differences among target weights for MORSE and MORSF were observed; however, the results were inconsistent across weights and did not display a general increase in E or F as target weight increased. The results of Cavitt et al. (2005) also lacked differences in MORSE or MORSF among small and large birds for either sex at a 3 h debone time. Variation among the current results and results reported in previous literature could be due to larger variation in bird size evaluated or the incidence of myopathies in the current study. For females, a similar trend was observed with differences (P < 0.05) in PC among target weights. In general, PC increased as target weight increased. Specific to females, the 2,043 g weight had significantly less peaks than the 3,405 g weight (7.49 and 9.05, respectively). The increase in PC with increasing weights may be due to increasing severity of WB as TW increases.
CONCLUSIONS
In conclusion, results of this study suggest strain and target weight have a significant impact on meat quality. As expected, there were greater yields from high-yielding (HY) strains than from those of standard-yielding (SY) strains, specifically in terms of white-meat (breast and tenders) and overall carcass yield. While minor strain impacts were observed, target weight had the greatest influence on quality attributes throughout the study. Target weight had a significant impact on color, water-holding capacity, and shear properties, which can ultimately affect quality, tenderness, and overall consumer acceptability. As target weight increased, L* a*, and b* values generally increased. Water-holding capacity was also significantly impacted by target weight as decreases in drip loss, cook loss, and thaw loss percentages were observed over time. Aside from yields, a strain effect was observed predominately throughout muscle myopathies. Greater incidences of myopathies, such as WB and SM, were observed in HY strains than in SY which can lead to decreased functionality. Woody breast and WS muscle myopathies generally increased in severity and occurrence as target weight increased. Instrumental tenderness was also impacted by target weight. MORSE was different for both sexes among all target weights and generally increased as target weight increased for males. MORS-PC generally increased as target weight increased for both sexes. Overall, while strain had some effects on meat quality attributes, processing weight (i.e., target weight) had a greater influence on quality, specifically muscle myopathies, WHC, and shear values.
ACKNOWLEDGMENTS
The authors are appreciative of the support of Cobb-Vantress Inc. (Siloam Springs, AR) for providing financial support for this project and the University of Arkansas Division of Agriculture (Fayetteville, AR) throughout the duration of this project for data collection and interpretation, facility use, and overview of financial responsibilities.
DISCLOSURES
Casey M. Owens reports financial support was provided by Cobb-Vantress Inc. via grant to University of Arkansas Division of Agriculture
REFERENCES
- Aguirre M.E., Owens C.M., Miller R.K., Alvarado C.Z. Descriptive sensory and instrumental texture profile analysis of woody breast in marinated chicken. Poult. Sci. 2018;97:1456–1461. doi: 10.3382/ps/pex428. [DOI] [PubMed] [Google Scholar]
- Allen C.D., Fletcher D.L., Northcutt J.K., Russell S.M. The relationship of broiler breast color to meat quality and shelf-life. Poult. Sci. 1998;77:361–366. doi: 10.1093/ps/77.2.361. [DOI] [PubMed] [Google Scholar]
- Badar I.H., Jaspal M.H., Yar M.K., Ijaz M., A.Khalique L.Zhang, Manzoor A., Ali S., Rahman A., Husnain F. Effect of strain and slaughter age on production performance, meat quality and processing characteristics of broilers reared under tropical climate conditions. Eur. Poult. Sci. 2021;85:1–17. [Google Scholar]
- Barbut S. Problem of pale soft exudative meat in broiler chickens. Br. Poult. Sci. 1997;38:355–358. doi: 10.1080/00071669708418002. [DOI] [PubMed] [Google Scholar]
- Berri C., Wacrenier N., Millet N., Le Bihan-Duval E. Effect of selection for improved body composition on muscle and meat characteristics of broilers from experimental and commercial lines. Poult. Sci. 2001;80:833–838. doi: 10.1093/ps/80.7.833. [DOI] [PubMed] [Google Scholar]
- Baldi G., Soglia F., Mazzoni M., Sirri F., Canonico L., Babini E., Laghi L., Cavani C. Implications of white striping and spaghetti meat abnormalities on meat quality and histological features in broilers. Animal. 2018;12:164–173. doi: 10.1017/S1751731117001069. [DOI] [PubMed] [Google Scholar]
- Bilgili, S. F. 2015. Broiler chicken myopathies: IV stringy/mushy breast. Worthwhile Operational Guidelines and Suggestion. Accessed Jan. 2023. https://mailchi.mp/a79c36424697/funphy9bdh
- Brewer S. Irradiation effects on meat color- review. Meat science. 2004;68:1–17. doi: 10.1016/j.meatsci.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Brewer V.B., Emmert J.L., Meullenet J.F., Owens C.M. Small bird programs: effect of phase-feeding, strain, sex, and debone time on meat quality of broilers. Poult. Sci. 2012;91:499–504. doi: 10.3382/ps.2011-01706. [DOI] [PubMed] [Google Scholar]
- Brewer V.B., Kuttappan V.A., Emmert J.L., Meullenet J.F., Owens C.M. Big-bird programs: effect of strain, sex, and debone time on meat quality of broilers. Poult. Sci. 2012;91:248–254. doi: 10.3382/ps.2011-01705. [DOI] [PubMed] [Google Scholar]
- Bowker B., Zhuang H. Detection of razor shear force differences in broiler breast meat due to the woody breast condition depends on measurement technique and meat state. Poult. Sci. 2019;98:6170–6176. doi: 10.3382/ps/pez334. [DOI] [PubMed] [Google Scholar]
- Cai K., Shao W., Chen X., Campbell Y.L., Nair M.N., Suman S.P., Beach C.M., Guyton M.C., Shilling M.W. Meat quality traits and proteome profile of woody broiler breast (pectoralis major) meat. Poult. Sci. 2018;97:337–346. doi: 10.3382/ps/pex284. [DOI] [PubMed] [Google Scholar]
- Cavitt L.C., Meullenet J.F., Gandhapuneni R.K., Youm G.W., Owens C.M. Rigor development and meat quality of large and small broilers and the use of allo-kramer shear, needle puncture, and razor blade shear to measure texture. Poult. Sci. 2005;84:113–118. doi: 10.1093/ps/84.1.113. [DOI] [PubMed] [Google Scholar]
- Cavitt L.C., Youm G.W., Meullenet J.F., Owens C.M., Xiong R. Prediction of poultry meat tenderness using razor blade shear, allo-kramer shear, and sarcomere length. J. Food Sci. 2004;69:11–15. [Google Scholar]
- Cooper M.A., Fletcher D.L. A comparison of broilers from “heavy” and “light” flocks on breast muscle rigor development, pH and shear. Poult. Sci. 1997;76(Suppl. 1):48. (Abstr.) [Google Scholar]
- Corzo A., Kidd M.T., Burnham D.J., Miller E.R., Branton S.L., Gonzalez-Esquerra R. Dietary amino acid density effects on growth and carcass of broilers differing in strain cross and sex. J. Appl. Poult. Res. 2005;14:1–9. [Google Scholar]
- Dadgar S., Lee E.S., Leer T.L.V., Crowe T.G., Classen H.L., Shand P.J. Effect of acute cold exposure, age, sex, and lairage on broiler breast meat quality. Poult. Sci. 2011;90:444–457. doi: 10.3382/ps.2010-00840. [DOI] [PubMed] [Google Scholar]
- Dalle Zotte A., Tasoniero G., Puolanne E., Remignon H., Cecchinatio M., Catelli E., Cullere M. Effect of “Wooden Breast” apperance on poultry meat quality, histological traits and lesions characterization. Czech J. Anim. Sci. 2017;62:51–57. [Google Scholar]
- Dransfield E., Sosnicki A.A. Relationship between muscle growth and poultry meat quality. Poult. Sci. 1999;78:743–746. doi: 10.1093/ps/78.5.743. [DOI] [PubMed] [Google Scholar]
- Fanatico A.C., Pillai P.B., Emmert J.L., Owens C.M. Meat quality of slow and fast-growing chicken genotypes fed low-nutrients or standard diets and raised indoors or with outdoor access. Poult. Sci. 2007;86:2245–2255. doi: 10.1093/ps/86.10.2245. [DOI] [PubMed] [Google Scholar]
- Fletcher D.L. Broiler breast meat color variation, pH, and texture. Poult. Sci. 1999;78:1323–1327. doi: 10.1093/ps/78.9.1323. [DOI] [PubMed] [Google Scholar]
- Fletcher D.L. Poultry meat quality. Worlds. Poult. Sci. J. 2002;58:131–145. [Google Scholar]
- Froning G.W., Babji A.S., Mather F.B. The effect of preslaughter temperature, stress, stuggle, and anesthetization on color and textural characteristics of turkey muscle. Poult. Sci. 1978;57:630–633. [Google Scholar]
- Froning G.W., Daddario J., Hartung T.E. Color and myoglobin concentration in turkey meat as affected by age, sex and strain. Poult. Sci. 1968;47:1827–1835. [Google Scholar]
- Janisch S., Krischek C., Wicke M. Color values and other meat quality characteristics of breast muscles collected from 3 broiler genetic lines slaughtered at 2 ages. Poult. Sci. 2011;90:1774–1781. doi: 10.3382/ps.2010-01073. [DOI] [PubMed] [Google Scholar]
- Jeffrey A.B. Principles of water-holding applied to meat technology. J. Sci. Food. Agric. 1983;34:1020–1021. [Google Scholar]
- Kuttappan V.A., Brewer V.B., Apple J.K., Waldroup P.W., Owens C.M. Influence of growth rate on the occurrence of white striping in broiler breast fillets. Poult. Sci. 2012;91:2677–2685. doi: 10.3382/ps.2012-02259. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Brewer V.B., Clark F.D., McKee S.R., Meullenet J.F., Emmert J.L., Owens C.M. Effect of white striping on the histological and meat quality characteristics of broiler fillets. Poult. Sci. 2009;88:136–137. (Abstr.) [Google Scholar]
- Kuttappan V.A., Brewer V.B., Mauromoustakos A., McKee S.R., Emmert J.L., Meullenet J.F., Owens C.M. Estimation of factors associated with the occurrence of white striping in broiler breast fillets. Poult. Sci. 2013;92:811–819. doi: 10.3382/ps.2012-02506. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Hargis B.M., Owens C.M. Review: white striping and woody breast myopathies in the modern poultry industry: a review. Poult. Sci. 2016;95:2724–2733. doi: 10.3382/ps/pew216. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Lee Y.S., Erf G.F., Meullenet J.F., McKee S.R., Owens C.M. Consumer acceptance of visual appearance of broiler breast meat with varying degrees of white striping. Poult. Sci. 2012;91:1240–1247. doi: 10.3382/ps.2011-01947. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Owens C.M., Coon C., Hargis B.M., Vazquez-Anon M. Incidence of broiler breast myopathies at 2 different ages and its impact on selected raw meat quality parameters. Poult. Sci. 2017;96:3005–3009. doi: 10.3382/ps/pex072. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Shivaprasad H.L., Shaw D.P., Valentine B.A., Hargis B.M., Clark F.D., McKee S.R., Owens C.M. Pathological changes associated with white striping in broiler breast muscles. Poult. Sci. 2013;92:331–338. doi: 10.3382/ps.2012-02646. [DOI] [PubMed] [Google Scholar]
- Livingston M.L., Landon L.C., Barnes H.J., Brake J. White striping and wooden breast myopathies of broiler breast muscle is affected by time-limited feeding, genetic background, and egg storage. Poult. Sci. 2018;98:217–226. doi: 10.3382/ps/pey333. [DOI] [PubMed] [Google Scholar]
- Lopez K.P., Schilling M.W., Corzo A. Broiler genetic strain and sex effects on meat characteristics. Poult. Sci. 2011;90:1105–1111. doi: 10.3382/ps.2010-01154. [DOI] [PubMed] [Google Scholar]
- Lubritz D.L. A statistical model for white meat yield in broilers. J. Appl. Poult. Res. 1997;6:253–259. [Google Scholar]
- Mallmann, B. 2019. Incidence of wooden breast myopathy by bird age, gender, and strain.
- Mallmann B., Tellez G., Mauromoustakos A., Coon C.N., Owens C.M. Fillet dimensions and meat quality attributes associated with woody breast in broilers. MMB. 2020;34:1–9. [Google Scholar]
- Maynard C.J. University of Arkansas; Fayetteville: 2020. Characterization of Growth Patterns and Meat Quality Characteristics of Four Commercial Broiler Strains in Small Bird and Large Bird Programs in the United States. Thesis. [Google Scholar]
- Mehaffey J.M., Pradhan S.P., Meullenet J.F., Emmert J.L., McKee S.R, Owens C.M. Meat quality evaluation of minimally aged broiler breast fillets from five commercial genetic strains. Poult. Sci. 2006;85:902–908. doi: 10.1093/ps/85.5.902. [DOI] [PubMed] [Google Scholar]
- Mudalal S., Babini E., Cavani C., Petracci M. Quantity and functionality of protein fractions in chicken breast fillets affected by white striping. Poult. Sci. 2014;93:2108–2116. doi: 10.3382/ps.2014-03911. [DOI] [PubMed] [Google Scholar]
- Mudalal S., Lorenzi M., Soglia F., Cavani C., Petracci M. Implications of white striping and spaghetti meat abnormalities on meat quality and histological features in broilers. Animal. 2014;12:164–173. doi: 10.1017/S1751731117001069. [DOI] [PubMed] [Google Scholar]
- Mudalal S., Lorenzi M., Soglia F., Cavani C., Petracci M. Implications of white striping and wooden breast abnormalities on quality traits of raw and marinated chicken meat. Animal. 2014;9:728–734. doi: 10.1017/S175173111400295X. [DOI] [PubMed] [Google Scholar]
- National Chicken Council. 2023a. Per Capita Consumption of Poultry and Livestock, 1960 to Forecast 2023, in Pounds. Accessed Jan. 2023. https://www.nationalchickencouncil.org/statistic/per-capita-consumption-poultry.
- National Chicken Council. 2023b. Broiler Chicken Industry Key Facts 2021. Accessed Jan. 2023. https://www.nationalchickencouncil.org/statistic/broiler-industry-key-facts.
- Ngoka D.A., Froning G.W., Lowry S.R., Babji A.S. Effects of sex, age, preslaughter factors and holding conditions on the quality characteristics and technical composition of turkey breast muscles. Poult. Sci. 1982;61:1996–2003. [Google Scholar]
- Petracci M., Mudalal S., Babini E., Cavani C. Effect of white striping on chemical composition and nutritional value of chicken breast meat. Ital. J. Anim. Sci. 2014;13:1. [Google Scholar]
- Petracci M., Mudalal S., Bonfiglio A., Cavani C. Occurrence of white striping under commercial conditions and its impact on breast meat quality in broiler chickens. Poult. Sci. 2013;92:1670–1675. doi: 10.3382/ps.2012-03001. [DOI] [PubMed] [Google Scholar]
- Petracci M., Soglia F., Madruga M., Carvalho L., Ida E., Estévez M. Wooden-breast, white striping, and spaghetti meat: causes, consequences and consumer perception of emerging broiler meat abnormalities. Compr. Rev. Food Sci. Food Saf. 2019;18:565–583. doi: 10.1111/1541-4337.12431. [DOI] [PubMed] [Google Scholar]
- Qiao M., Fletcher D.L., Smith D.P., Northcutt J.K. The effect of broiler breast meat color on pH, moisture, water-holding capacity, and emulsification capacity. Poult. Sci. 2001;80:676–680. doi: 10.1093/ps/80.5.676. [DOI] [PubMed] [Google Scholar]
- Sams A.R. Electrical stimulation and high-temperature conditioning of broiler carcasses. Poult. Sci. 1990;69:1781–1786. [Google Scholar]
- Santiago H.L., Denbow D.M., Emmerson D.A., Denbow C., Graham P., Hohenboken W. Effects of strain, plane of nutrition, and age at slaughter on performance and meat quality traits of broilers. Poult. Sci. 2005;84(E-Suppl. 1):128. (Abstr.) [Google Scholar]
- Scheuermann G.N., Bilgili S.F., Hess J.B., Mulvaney D.R. Breast muscle development in commercial broiler chickens. Poult. Sci. 2003;82:1648–1658. doi: 10.1093/ps/82.10.1648. [DOI] [PubMed] [Google Scholar]
- Schneider B.L., Renema R.A., Betti M., Carney V.L., Zuidhof M.J. Effect of holding temperature, shackling, sex, and age on broiler breast meat quality. Poult. Sci. 2012;91:468–477. doi: 10.3382/ps.2010-00952. [DOI] [PubMed] [Google Scholar]
- Sihvo H.K., Immonen K., Puolanne E. Myodegeneration with fibrosis and regeneration in the pectoralis major muscle of broilers. Vet. Pathol. 2014;51:619–623. doi: 10.1177/0300985813497488. [DOI] [PubMed] [Google Scholar]
- Smith D.P., Lyon C.E., Lyon B.G. The effect of age, dietary carbohydrate source, and feed withdrawal on broiler breast fillet color. Poult. Sci. 2002;81:1584–1588. doi: 10.1093/ps/81.10.1584. [DOI] [PubMed] [Google Scholar]
- Smith E.R., Presti G.M. Influence of broiler strain cross and dietary protein on the performance of broilers. Poult. Sci. 1998;77:276–281. doi: 10.1093/ps/77.2.276. [DOI] [PubMed] [Google Scholar]
- Soglia F., Silva A.K., Tappi S., Liao L.M., Rocculi P., Laghi L., Petracci M. Gaping of the pectoralis minor muscles: magnitude and characterization of an emerging quality issue in broilers. Poult. Sci. 2019;98:6194–6204. doi: 10.3382/ps/pez418. [DOI] [PubMed] [Google Scholar]
- Solo, J. L. 2016. Meat Quality and Sensory Analysis of Broiler Breast Fillets With Woody Breast Muscle Myopathy. Thesis: University of Arkansas, Fayetteville.
- Sun X., Giampietro-Ganeco A., Mueller A., Maynard C. J., Caldas-Cueva J. P. and Owens C. M., Meat quality traits and Blunt Meullenet-Owens Razor Shear characteristics of broiler breast fillets affected by woody breast condition and post-cooking meat temperature, Poultry Science, 100 (8), 2021,101212. [DOI] [PMC free article] [PubMed]
- Sun X., Yang F.L., Solo J.L., Mallmann B.A., Coon C.N., Owens C.M. Using peak counts in shear data to detect woody breast in cooked broiler fillets. Poult. Sci. 2016;95:52. (Abstr.) [Google Scholar]
- Tasoniero G., Zhuang H., Gamble G.R., Bowker B.C. Effect of spaghetti meat abnormality on broiler chicken breast meat composition and technological quality. Poult. Sci. 2020;99:1724–1733. doi: 10.1016/j.psj.2019.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tijare V.V., Yang F.L., Kuttappan V.A., Alvarado C.Z., Coon C.N., Owens C.M. Meat quality of broiler breast fillets with white striping and woody breast muscle myopathies. Poult. Sci. 2016;95:2167–2173. doi: 10.3382/ps/pew129. [DOI] [PubMed] [Google Scholar]
- Vignale K., Caldas J.V., England J.A., Boonsinchai N., Magnuson A., Pollock E.D., Owens C.M., Coon C.N. Effect of white striping myopathy on breast muscle protein turnover and gene expression in broilers. Poult. Sci. 2017;96:886–893. doi: 10.3382/ps/pew315. [DOI] [PubMed] [Google Scholar]
- Yam K.L., Papadakis S.E. A simple digital imaging method for measuring and analyzing color of food surfaces. J. Food Eng. 2004;61:137–142. [Google Scholar]
- Yang, F.L., J. K. Apple, and C. M. Owens. 2016. Evaluating breast meat texture and meat qualities of broilers at various ages and gender using the Meullenet-Owens Razor Shear method.
- Young L.L., Northcutt J.K., Buhr R.J., Lyon C.E., Ware G.O. Effects of age, sex, and duration of postmortem aging on percentage yield of parts from broiler chicken carcasses. Poult. Sci. 2001;80:376–379. doi: 10.1093/ps/80.3.376. [DOI] [PubMed] [Google Scholar]
- Zhang X., Virellia To K., Jarvis T.R., Campbell Y.L., Hendrix J.D., Suman S.P., Li S., Antonelo D.S., Zhai W., Chen J., Zhu H., Schilling M.W. Broiler genetics influences proteome profiles of normal and woody breast muscle. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuidhof M.J., Schneider B.L., Carney V.L., Korver D.R., Robinson F.E. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 2005. Poult. Sci. 2014;93:2970–2982. doi: 10.3382/ps.2014-04291. [DOI] [PMC free article] [PubMed] [Google Scholar]