Key Points
Question
Does 4-factor prothrombin complex concentrate (4F-PCC) reduce 24-hour blood product consumption in patients with trauma at risk of massive transfusion?
Findings
In this superiority randomized trial involving 324 patients, there was no difference in total 24-hour blood product consumption among patients treated with 4F-PCC (12 U) vs placebo (11 U). More thromboembolic events occurred in the 4F-PCC group.
Meaning
These findings do not support the administration of 4F-PCC in patients with trauma at risk of massive transfusion.
Abstract
Importance
Optimal transfusion strategies in traumatic hemorrhage are unknown. Reports suggest a beneficial effect of 4-factor prothrombin complex concentrate (4F-PCC) on blood product consumption.
Objective
To investigate the efficacy and safety of 4F-PCC administration in patients at risk of massive transfusion.
Design, Setting, and Participants
Double-blind, randomized, placebo-controlled superiority trial in 12 French designated level I trauma centers from December 29, 2017, to August 31, 2021, involving consecutive patients with trauma at risk of massive transfusion. Follow-up was completed on August 31, 2021.
Interventions
Intravenous administration of 1 mL/kg of 4F-PCC (25 IU of factor IX/kg) vs 1 mL/kg of saline solution (placebo). Patients, investigators, and data analysts were blinded to treatment assignment. All patients received early ratio-based transfusion (packed red blood cells:fresh frozen plasma ratio of 1:1 to 2:1) and were treated according to European traumatic hemorrhage guidelines.
Main Outcomes and Measures
The primary outcome was 24-hour all blood product consumption (efficacy); arterial or venous thromboembolic events were a secondary outcome (safety).
Results
Of 4313 patients with the highest trauma level activation, 350 were eligible for emergency inclusion, 327 were randomized, and 324 were analyzed (164 in the 4F-PCC group and 160 in the placebo group). The median (IQR) age of participants was 39 (27-56) years, Injury Severity Score was 36 (26-50 [major trauma]), and admission blood lactate level was 4.6 (2.8-7.4) mmol/L; prehospital arterial systolic blood pressure was less than 90 mm Hg in 179 of 324 patients (59%), 233 patients (73%) were men, and 226 (69%) required expedient hemorrhage control. There was no statistically or clinically significant between-group difference in median (IQR) total 24-hour blood product consumption (12 [5-19] U in the 4F-PCC group vs 11 [6-19] U in the placebo group; absolute difference, 0.2 U [95% CI, −2.99 to 3.33]; P = .72). In the 4F-PCC group, 56 patients (35%) presented with at least 1 thromboembolic event vs 37 patients (24%) in the placebo group (absolute difference, 11% [95% CI, 1%-21%]; relative risk, 1.48 [95% CI, 1.04-2.10]; P = .03).
Conclusions and Relevance
Among patients with trauma at risk of massive transfusion, there was no significant reduction of 24-hour blood product consumption after administration of 4F-PCC, but thromboembolic events were more common. These findings do not support systematic use of 4F-PCC in patients at risk of massive transfusion.
Trial Registration
ClinicalTrials.gov Identifier: NCT03218722
This randomized clinical trial examines whether systematic 4-factor prothrombin complex concentrate administration combined with a ratio-based transfusion protocol is superior to ratio-based transfusion alone in reducing 24-hour total blood product consumption in patients at risk of massive transfusion.
Introduction
Severe bleeding in patients with trauma remains a challenge.1,2 Use of tranexamic acid (TXA), reduction of fluid expansion, high-ratio blood product transfusion, and expedient hemorrhage control have improved some patient outcomes,3 but mortality among patients with trauma and bleeding remains high due in part to trauma-induced coagulopathy.4,5 Trials of fixed ratio-based blood product transfusion,6 viscoelastic testing (VET)7 to guide a tailored combination of blood products, and a hybrid of the 2 approaches8 have provided a management evidence base for the trauma community, but no strategy has proven superior to another.
All 3 strategies transfuse coagulation factors as soon as possible. Observational studies provide evidence that early administration of 4-factor prothrombin complex concentrate (4F-PCC; human coagulation factors II, VII, IX, and X and proteins S and C) to boost thrombin generation reduces blood product consumption,9,10 but concern about a potential increase in thromboembolic events remains. Recent observational studies indicate that combining 4F-PCC administration with fresh frozen plasma (FFP) reduces blood product consumption and mortality without an increase in thromboembolic events.11,12 Given the absence of high-level evidence, a randomized clinical trial was justified to explore the efficacy and safety of systematic 4F-PCC administration in patients with trauma. We therefore designed this multisite trial to test the hypothesis that systematic 4F-PCC administration combined with a ratio-based transfusion protocol is superior to ratio-based transfusion alone in reducing 24-hour total blood product consumption in patients at risk of massive transfusion.
Methods
Design and Setting
This superiority, randomized, placebo-controlled clinical trial took place in 12 level I academic trauma centers in France. Trial recruitment lasted from December 29, 2017, to August 4, 2021. Follow-up ended on August 31, 2021. The trial protocol and statistical analysis plan were registered under trial number NCT03218722 before first patient inclusion and published before the conclusion of enrollment (see Supplement 1 for trial protocol and CONSORT checklist).13 No major change to the protocol or outcome occurred after the start of the trial.
Ethical Review of the Study
The institutional review board (Comité de Protection des Personnes Sud-Ouest et Outre-Mer 2, Toulouse, France) approved the study on May 4, 2017, and the French Agency for the Safety of Health Products (Agence Nationale de Sécurité du Médicament et des Produits de Santé, Saint Denis, France) approved the study on April 21, 2017, in accordance with Good Clinical Practice guidelines, French regulation, and the Declaration of Helsinki. Each patient provided written informed consent before inclusion. If the patient could not consent, informed agreement was sought from the next of kin. If no surrogate was available, emergency inclusion was authorized by the institutional review board and the consent was obtained as soon as appropriate.
Patients
All adult patients (≥18 years) with trauma directly admitted from the injury scene to one of the participating centers with the highest trauma level activation (grade A)14 were considered for potential enrollment. The protocol included patients at risk of massive transfusion. At risk was defined as transfusion of at least 1 U of packed red blood cell concentrate (PRBC) during prehospital care or within 1 hour of admission and an Assessment of Blood Consumption (ABC) score of at least 215 or clinical assessment of the attending physician of risk of massive transfusion; massive transfusion was defined as administration of at least 3 PRBC within the hour of admission or at least 10 PRBC within the first 24 hours. Acute traumatic coagulopathy was defined as prothrombin time ratio (PTr; prothrombin time/laboratory normal reference value) greater than 1.2; severe acute traumatic coagulopathy was defined as PTr greater than 1.5.16,17
Exclusion criteria were traumatic cardiac arrest before randomization, patients with devastating injuries expected to die within the first hour of admission, secondary admission from another health care facility, preinjury treatment with anticoagulants, known pregnancy, known hypersensitivity to 4F-PCC or its excipients, known preinjury terminal condition, patient under guardianship, any inclusion in another trial within the last 30 days, and, according to the French law, patients without health insurance.
Randomization and Blinding
The reference time for each study measure was arrival in the trauma bay (admission). Randomization was performed within 1 hour of admission. Patients were randomized in blocks of random size from 2 to 6, stratified by center, according to a randomization sequence generated by a statistician not involved in patient recruitment or outcome assessment. The coordinating pharmacy in Grenoble, France, created a set of sealed envelopes for the pharmacy of each investigating site to ensure concealment. The pharmacy at each site provided a set of 4 sequentially numbered envelopes to the clinician investigators. A clinician investigator at each site enrolled the patient and picked a sealed envelope following the predetermined randomization sequence. The clinician conveyed patient name, weight, and study number to a trained nurse not involved in the resuscitation and subsequent care of the patient who unsealed the envelope and prepared either 4F-PCC or placebo in a protected dedicated space according to the assignment specified in the randomization envelope. The clinician investigator and all team members involved in the care of the patient remained blinded to the treatment assignment. The site and coordinating pharmacy remained unblinded to treatment assignment. Unblinding was possible by the coordinating pharmacy for safety reasons. All personnel involved in data handling, monitoring, and statistical analysis remained blinded for the duration of the trial.
Trial Intervention
The active intervention was 4F-PCC (Kanokad, Laboratoire Français du Biomédicament) at a dose of 25 IU of factor IX per kg (1 mL/kg). The placebo was 1 mL/kg of 0.9% saline solution. Both were stored in a designated and closed refrigerator at 2 °C to 8 °C and administered in opaque syringes according to randomization by the unblinded nurse at a speed of 120 mL/h with syringe pumps as soon as possible after admission.
Resuscitation Management
On admission, patients in both groups were treated according to European recommendations with restricted fluid expansion and early transfusion of blood products with a PRBC:FFP ratio between 1:1 and 2:1. TXA was administered intravenously within 3 hours after injury at a loading dose of 1 g followed by 1 g over 8 hours.18 The source of bleeding was identified and treated as soon as possible.19 Fibrinogen concentrate was administered in the case of fibrinogen concentration less than 1.5 g/L20 or viscoelastic criteria showing a functional deficiency.21 Platelets received transfusion as needed to maintain platelet count higher than 50 ×109/L at all times.19 Blood samples at regular intervals monitored hemostasis parameters (PTr, fibrinogen concentration, and VET if available; see Supplement 1 for details).
Outcomes
The primary outcome was the total number of all blood product units (RBC, FFP, and platelet concentrate) consumed within the first 24 hours after arrival in the trauma bay. Secondary outcomes were individual blood component units consumed within the first 24 hours; time to PTr less than 1.5 (severe acute traumatic coagulopathy); time to hemorrhage control; 24-hour and 28-day mortality; number of intensive care unit–free days (calendar days not in critical care unit), ventilator-free days (calendar days without need for ventilator support), and hospital-free days through day 28 (calendar days not in hospital); hospitalization status at 28 days; and Glasgow Outcome Scale-Extended score in patients with brain injury on admission computed tomography (CT) scan (Abbreviated Injury Scale score >2).
Safety
Arterial or venous thromboembolic events (pulmonary embolus, clinically manifest vein thrombosis, stroke, myocardial infarction, mesenteric ischemia, extremity ischemia) were recorded through day 28. Surveillance was passive to reduce radiation exposure from systematic CT scanning. Venous echography was left to the discretion of the attending physician. Every clinical suspicion of a thromboembolic event was confirmed by ultrasonography and/or contrast-enhanced CT.
Sample Size Estimation
Based on 1-year data from the Northern French Alps Registry,14 mean (SD) total blood product consumption was estimated at 12 (10) U of blood products (PRBC, FFP, and platelet concentrates) in the first 24 hours. The study group considered a reduction by 3 U per 24 hours or a decrease of 25% in 24 hours to be clinically significant. Assuming a nonnormal distribution (log-normal) of blood product consumption, nQuery software calculated the probability of observing a reduction in blood product consumption in the 4F-PCC vs placebo group of 59%. Accordingly, a bilateral Mann-Whitney test estimated the sample size of 162 patients in each group to obtain an 80% power with a risk of α error of .05, for a total sample size of 324 patients (nQuery, Sample Size, and Power Calculation, “Statsols” software, Statistical Solutions Ltd). To compensate for potential loss of follow-up and premature activation of the inclusion procedure without randomization (eg, erroneous assessment of inclusion criteria, erroneous handover, arrival in cardiac arrest), 350 randomization envelopes were prepared. No interim analysis was planned.
Statistical Analysis
All data handling and analysis were performed by independent statisticians (see the statistical analysis plan in Supplement 2). Results were expressed as mean and SD for normally distributed variables (tested with a Shapiro-Wilk test), median and IQR for nonnormally distributed variables, and numbers and percentages. After complete patient enrollment, but before data analyses, we performed a Mann-Whitney test to confirm that time spent in the study, up to 24 hours, did not differ between the 2 groups to prevent survivor bias and to ensure the same exposure time to receive blood products (primary outcome). The primary analysis included all randomized patients, except those who withdrew consent. No missing outcomes were imputed. Patients who died within the first 24 hours after arrival in the trauma bay were included in the transfusion analysis and their actual blood product consumption was taken into account. Mann-Whitney tests were used to compare continuous variables because variables were nonnormally distributed. Categorical variables were compared with a χ2 test. If the χ2 test was not applicable the Fisher exact test was used. The number of thromboembolic events at 28 days was recorded as a binary variable and compared using χ2 testing. Glasgow Outcome Scale-Extended score at hospital discharge was compared with a Fisher exact test. A mixed-effects linear regression model with time as a dependent variable and independent variables of PTr, group assignment, and a time × group interaction variable analyzed the time course of PTr in both groups (from admission to 24 hours). To account for patients with a PTr greater than 1.5 within the first 24 hours and a PTr greater than 1.5 and death within the first 24 hours, we used a competing-risks regression to analyze time to PTr less than 1.5 between the 2 groups. We also performed a post hoc subgroup analysis exploring an association between thromboembolic events in patients with vs without coagulopathy (PTr >1.2).
We repeated all analyses in the per-protocol population (ie, patients who received the study intervention within the first hour after arrival in the trauma bay) and analyzed outcomes in a prespecified subgroup of patients who required massive transfusion.
All tests were 2-sided and a P value less than .05 was considered significant. Professional statisticians from Grenoble Alpes University Hospital who were blinded to treatment randomization performed all calculations using Stata, version 15 (StataCorp).
Results
Among 4313 patients with the highest level of trauma activation admitted to the 12 participating centers, 350 patients were eligible for inclusion, 327 patients were randomized, and 324 were analyzed (160 in the placebo group and 164 in the 4F-PPC group; Figure 1). A total of 308 patients (95%) received the study intervention (4F-PCC or placebo) within the first hour of admission (159 in the 4F-PPC group and 149 in the placebo group; per-protocol population).
Figure 1. Flow of Participants in a Study of Administration of 4-Factor Prothrombin Complex Concentrate (4F-PCC) in Patients With Trauma at Risk of Transfusion.
aHighest level of trauma activation corresponds to patients with a Glasgow Outcome Scale score less than 9, systolic arterial blood pressure less than 90 mm Hg, and/or acute respiratory distress on arrival at the trauma bay.
bRandomization was stratified by center.
Among the 324 randomized patients, 233 (73%) were men and the median (IQR) age was 39 (26.5-56) years, median (IQR) Injury Severity Score was 36 (26-50), prehospital systolic arterial blood pressure less than 90 mm Hg occurred in 179 patients (59%), and median (IQR) plasma lactate concentration on admission was 4.6 (2.8-7.4) mmol/L. The 2 groups generally shared similar characteristics (Table 1), including need for expedient surgical or radiological hemorrhage control (115 in the 4F-PCC group and 111 in the placebo group), although in the placebo vs the 4F-PCC group, a higher percentage of patients received TXA (86% vs 76%) and a higher median (IQR) total dose of fibrinogen concentrate was given (3 [3-6] g vs 3 [3-7.5] g). Time spent in the study up to 24 hours was not different between the 2 groups (eTable 1 in Supplement 3).
Table 1. Patient Characteristics in a Study of Administration of 4-Factor Prothrombin Complex Concentrate (4F-PCC) in Patients With Trauma at Risk of Transfusion.
| Characteristic | Median (IQR) [total No.] | |
|---|---|---|
| 4F-PCC (n = 164) | Placebo (n = 160) | |
| Age, y | 39.5 (26-55.5) | 39 (27-57) |
| Sex, No. (%) | ||
| Women | 47 (29) | 44 (27) |
| Men | 117 (71) | 116 (73) |
| Trauma, No. (%) | ||
| Blunt | 135 (82) | 125 (78) |
| Penetrating | 29 (18) | 35 (22) |
| Prehospital | ||
| Heart rate, /min | 113 (90-131) [151] | 114 (90-130) [155] |
| Systolic arterial blood pressure, mm Hg | 101 (80-121) [151] | 90 (74-111) [152] |
| Glasgow Outcome Scale scorea | 14 (9-15) [160] | 14 (8-15) [153] |
| Tranexamic acid infused | 125 (76) | 138 (86) |
| Intubated | 78 (48) | 77 (48) |
| Time from injury to arrival in the trauma bay, min | 105 (80-132) [148] | 100 (75-132) [148] |
| Admission | ||
| Heart rate, /min | 119 (95-132) [162] | 115 (90-130) [158] |
| Systolic arterial blood pressure, mm Hg | 89 (70-115) [160] | 90 (70-110) [156] |
| Assessment of Blood Consumption scoreb | 2 (1-2) [161] | 2 (1-2) [147] |
| Assessment of Blood Consumption score ≥2, No. (%) | 84 (52) | 78 (53) |
| Time from arrival to beginning of treatment, min | 35 (25-45) [154] | 30 (15-50) [150] |
| Hemoglobin, g/dLc | 10.5 (8.7-12.0) [160] | 9.9 (8.2-11.6) [155] |
| Lactate, mmol/Lc | 4.5 (2.7-7.1) [132] | 4.7 (2.9-7.5) [129] |
| Platelet count, ×109/L | 214 (181-266) [132] | 204 (150-245) [125] |
| Fibrinogen, g/Lc | 1.7 (1.2-2.2) [134] | 1.8 (1.2-2.2) [128] |
| Fibrinogen ≤1.5 g/L, No. (%) [No.] | 49 (37) [134] | 47 (37) [128] |
| PTrc,d | 1.3 (1.15-1.51) [142] | 1.3 (1.16-1.53) [130] |
| PTr >1.2, No. (%) [No.] | 93 (65) [142] | 89 (68) [130] |
| PTr >1.5, No. (%) [No.] | 36 (25) [142] | 34 (26) [130] |
| Thromboelastometry coagulation time, sc,e | 73 (66-86) [31] | 74 (66-95) [34] |
| Thromboelastometry coagulation time ≥80 s, No. (%) [No.] | 11 (35) [31] | 13 (38) [34] |
| AIS Head score >2, No. (%) [No.]f | 55 (35) [156] | 50 (34) [149] |
| ISSg | 34 (25-50) [156] | 38 (29-50) [149] |
| ISS ≥15, No. (%) [No.] | 143 (92) [156] | 144 (97) [149] |
| Revised trauma scoreh | 6.8 (5.8-7.6) [160] | 6.6 (5.7-7.6) [153] |
| Resuscitation indicators, No. (%) i | ||
| Need for hemostasis control procedure (surgical or radiological) | 115 (70) | 111 (69) |
| Transfusion of ≥3 U of RBCs within the first hour | 67 (42) | 60 (38) |
| Transfusion of ≥10 U of RBCs within the first 24 h | 42 (26) | 43 (28) |
| Fibrinogen concentrate treatment | 141 (86) | 129 (81) |
| Total dose of fibrinogen concentrate, median (IQR), g | 3 (3-7.5) | 3 (3-6) |
| Time from arrival to transfusion of FFP, min | 73 (56-105) [122] | 91 (59-142) [130] |
Abbreviations: FFP, fresh frozen plasma; RBC, red blood cell.
The Glasgow Outcome Scale measures level of consciousness based on eye, verbal, and motor responses. Total scores range from 3 to 15, with higher scores indicating greater disability.
The Assessment of Blood Consumption score uses pulse rate, systolic blood pressure, ultrasonography, and mechanism of injury to predict need for massive transfusion. Scores range from 0 to 4, with higher scores indicating greater likelihood of requiring massive transfusion. Patients with a score of less than 2 (n = 162) were enrolled in the trial as physician overrides, which was defined as a score of less than 2 and attending physician determination that a massive transfusion was needed.
Laboratory reference ranges are as follows: hemoglobin, 12-17 g/dL; lactate, <1 mmol/L; platelet count, 150-450 ×109/L; fibrinogen, 2-4 g/L; PTr, 0.8-1.2; and thromboelastometry coagulation time, 31-63 s.
Prothrombin time ratio (PTr) is the ratio between the prothrombin time of the patient and the prothrombin time reference value of the laboratory. A PTr higher than 1.2 indicates posttraumatic coagulopathy and a PTr higher than 1.5 indicates severe posttraumatic coagulopathy.
Thromboelastometry coagulation time is the time before the initiation of the clot measured with thromboelastometry using activators of the extrinsic pathway. A time of more than 80 seconds indicates PTr greater than 1.2.
The Abbreviated Injury Scale (AIS) Head score assesses head injury on a scale of 0 to 6, with 0 indicating no injury and 6 indicating a fatal injury.
The Injury Severity Score (ISS) represents an overall assessment of bodily injury calculated as the sum of squares of the highest injury scores for body parts. It ranges from a total score of 0 to 75, with higher scores indicating greater injury. A score greater than 15 indicates major trauma.
Based on the Glasgow Outcome Scale score, systolic blood pressure, and respiratory rate, the revised trauma score range was 0 to 7.8, with higher scores associated with better survival probability.
Includes observations made after randomization.
There was no clinically or statistically significant between-group difference in median (IQR) 24-hour total blood product consumption in the 4F-PCC group vs the placebo group (12 [5-19] U vs 11 [ 6-19] U; absolute difference, 0.2 [95% CI, −2.99 to 3.33] U; P = .72) or consumption of individual components (RBC, FFP, platelets) (Figure 2A and Table 2). There was a non–statistically significant difference in time to correction of PTr in the 4F-PCC group (mixed-effects linear regression model P = .14) (Figure 2B) and no difference between the groups in time to PTr less than 1.5 among patients with severe coagulopathy (competing risks subhazard ratio, 1.08 [95% CI, 0.92-1.28]; P = .33; eFigure 1 in Supplement 3). There were no between-group differences in secondary outcomes (Table 2).
Figure 2. Transfusion-Related Secondary Outcomes by Treatment Group.
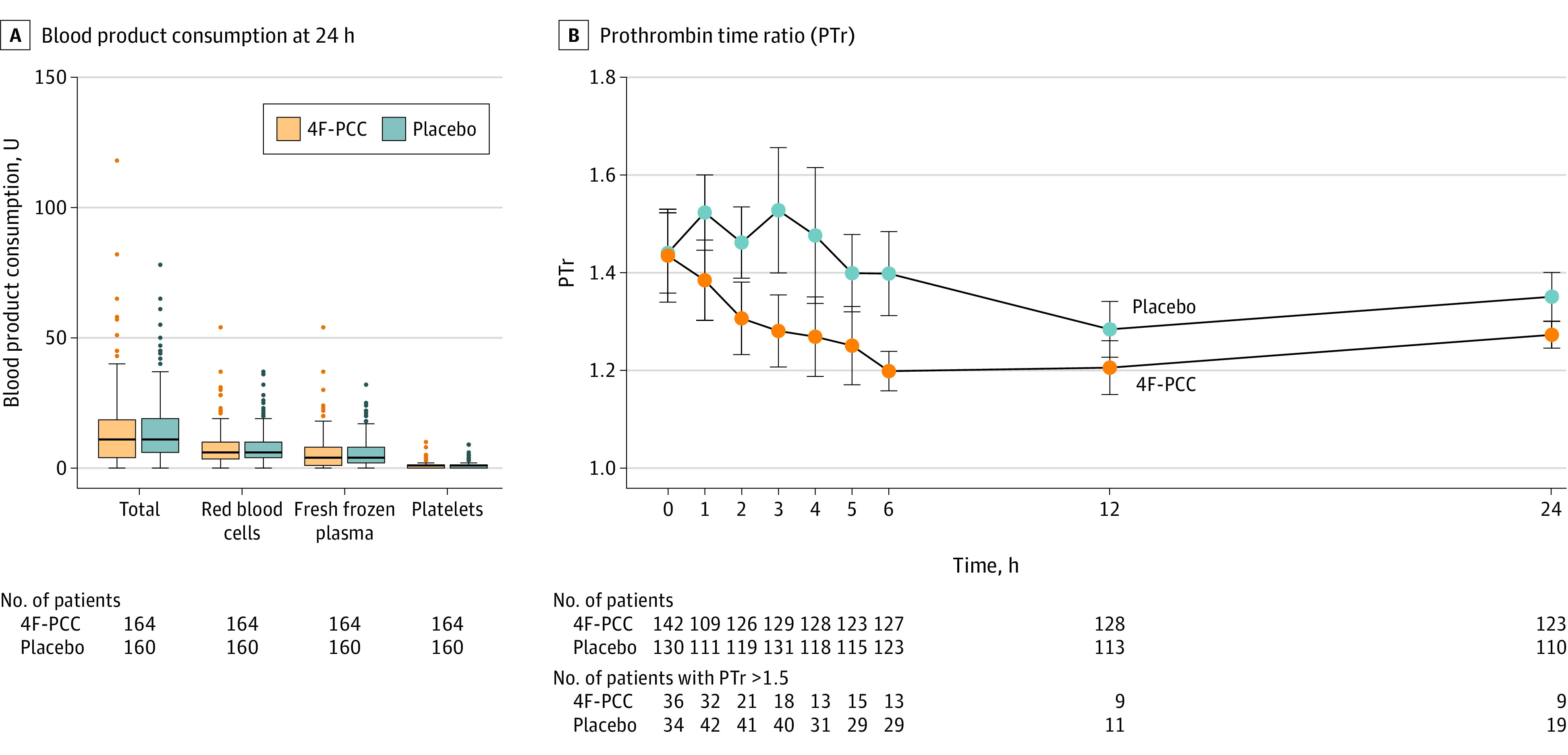
A, Lines within box indicate median values, lower and upper box edges are 25th and 75th percentile values, whiskers extend to ±1.5 times the interquartile range, and points outside are the most extreme values. Total blood product consumption at 24 hours (primary outcome) was not different between the 2 groups (median [IQR], 12 [5-19] U in the 4F-PCC group vs 11 [6-19] U in the placebo group; absolute difference, 0.2 [95% CI, −2.99 to 3.33] U; P = .72). Individual product consumption (secondary outcome) was also not different between the 2 groups (see Table 2 for data). Mann-Whitney tests were applied for all comparisons. B, Mean time course and 95% CI (bars) of prothrombin time ratio (PTr) between the groups. PTr is the ratio between the prothrombin time of the patient and the prothrombin time reference value of the laboratory. A PTr higher than 1.2 indicates posttraumatic coagulopathy and a PTr higher than 1.5 indicates severe posttraumatic coagulopathy. A mixed-effects linear regression model found no between-group difference (P = .14). 4F-PCC indicates 4-factor prothrombin complex concentrate.
Table 2. Trial Outcomes by Treatment Group.
| Outcome | No. (%) | Absolute difference (95% CI), %a | P valueb | |
|---|---|---|---|---|
| 4F-PCC (n = 164) | Placebo (n = 160) | |||
| Primary outcome | ||||
| Total blood product consumption, median (IQR), U | 12 (5 to 19) | 11 (6 to 19) | 0.2 (−2.99 to 3.33) | .72 |
| Secondary outcomes | ||||
| Red blood cell consumption, median (IQR), Uc | 6 (3.5 to 10) | 6 (4 to 10) | −0.3 (−1.8 to 1.3) | .93 |
| Fresh frozen plasma consumption, median (IQR), Ud | 4 (1 to 8) | 4 (2 to 8) | 0.1 (−1.3 to 1.5) | .56 |
| Platelet concentrate consumption, median (IQR), Ue | 1 (0 to 1) | 1 (0 to 1) | 0.0 (−0.3 to 0.3) | .83 |
| Time to PTr <1.5, median (IQR) [No.], minf | 0 (0 to 60) [154] | 0 (0 to 60) [145] | −8.5 (−48.9 to 32.0) | .86 |
| Mortality | ||||
| 24-h | 18 (11) | 20 (13) | −2 (−9 to 5) | .67 |
| 28-d | 26 (17) | 30 (21) | −3 (−12 to 5) | .48 |
| Time to achieve anatomic hemostasis, median (IQR) [No.], ming | 300 (203 to 423) [131] | 288 (210 to 404) [128] | 22 (−73.3 to 73.8) | .96 |
| Hospital-free days through day 28, median (IQR) | 6.5 (0 to 22.5) | 7 (0 to 22) | −0.15 (−1.65 to 1.35) | .78 |
| Ventilator-free days through day 28, median (IQR) | 4 (0.5 to 7) | 4 (0 to 8) | 0.33 (−1.0 to 1.6) | .51 |
| ICU-free days through day 28, median (IQR) | 6.5 (0 to 22.5) | 7 (0 to 22) | 1.22 (−5.93 to 8.37) | .78 |
| Disposition at day 28 | .81 | |||
| Remained hospitalized | 44 (33) | 44 (35) | 0 (−10 to 10) | |
| Intensive care unit | 37 (28) | 28 (23) | 5 (−5 to 16) | |
| Home | 31 (23) | 29 (23) | −3 (−12 to 6) | |
| Died | 26 (17) | 30 (21) | −3 (−12 to 5) | |
| Rehabilitation | 19 (14) | 22 (18) | −2 (−14 to 9) | |
| Other | 2 (2) | 1 (1) | 1 (−2 to 3) | |
| Unknown | 5 (3) | 6 (4) | ||
| Glasgow Outcome Scale-Extended score, median (IQR) [No.]h | 3 (3 to 4) [36] | 3 (3 to 5) [27] | −0.5 (−1.91 to 0.91) | .45 |
Abbreviations: 4F-PCC, 4-factor prothrombin complex concentrate; ICU, intensive care unit.
Absolute differences are mean (95% bootstrapped CI) for continuous variables; differences for continuous and categorical variables and are not adjusted.
Mann-Whitney or χ2 tests were applied for all comparisons, except for the Glasgow Outcome Scale-Extended, for which a Fisher exact test was applied.
One unit of packed red blood cells is approximately 300 mL.
One unit of fresh frozen plasma is approximately 300 mL.
One unit of platelet concentrate is approximately 500 mL.
Prothrombin time ratio (PTr) is the ratio between the prothrombin time of the patient and the prothrombin time reference value of the laboratory. A PTr >1.2 indicates posttraumatic coagulopathy and a PTr >1.5 indicates severe posttraumatic coagulopathy. Patients with a PTr >1.5 within the first 24 hours and patients with a PTr >1.5 and who died within the first 24 hours were omitted.
Anatomic hemostasis in the operating room was defined as an objective assessment by the surgeon indicating that bleeding within the surgical field was controlled and no further hemostatic interventions were anticipated. In the interventional radiology suite, anatomic hemostasis was defined as achieving resolution of contrast blush after embolization.
The Glasgow Outcome Scale-Extended is a broad assessment of neurological outcome, with scores ranging from 0 (dead) to 7 (full recovery). Scores of 2 to 5 indicate residual neurological disability. This score was obtained only from discharged patients who had a head injury.
Findings were qualitatively similar in patients who received the study intervention (4F-PCC or placebo) within the first hour after admission (per-protocol population; eTables 2-4 and eFigure 2 in Supplement 3). The subgroup analysis of patients requiring a massive transfusion yielded results identical to the main analysis (eTables 5-7 and eFigure 3 in Supplement 3).
The number of patients with at least 1 thromboembolic event was greater among those who received 4F-PCC vs placebo (56 [35%] vs 37 [24%]; absolute difference, 11% [95% CI, 1%-21%]; relative risk, 1.48 [95% CI, 1.04-2.10]; P = .03) (Table 3). There were 63 total thromboembolic events in the 4F-PCC group and 46 in the placebo group. A post hoc analysis of 266 patients (139 in the 4F-PCC group and 127 in the placebo group) exploring an association between coagulopathy and thromboembolic events revealed a higher percentage of thromboembolic events in patients with a PTr greater than 1.2 who received 4F-PCC (31/90 [34%]) vs placebo (19/87 [22%]) (P = .06). In patients with a PTr less than 1.2, the percentage of patients with thromboembolic events was comparable between the 4F-PCC and placebo groups (6/49 [33%] vs 13/40 [33%]; P = .99).
Table 3. Thromboembolic Events by Treatment Group.
| Thromboembolic event | No. (%) | Absolute difference (95% CI), %a | Relative risk (95% CI) | P valueb | |
|---|---|---|---|---|---|
| 4F-PCC (n = 164) | Placebo (n = 160) | ||||
| Patients with at least 1 thromboembolic event, No. (%) [No.] | 56 (35) [161] | 37 (24) [157] | 11 (1 to 21) | 1.48 (1.04 to 2.10) | .03 |
| Superficial venous thrombosis | 5 (3.1) | 1 (0.6) | 2 (−1 to 5) | ||
| Deep venous thrombosis | 27 (16.8) | 23 (14.6) | 2 (−6 to 10) | ||
| Pulmonary embolism | 20 (12.4) | 17 (10.8) | 2 (−5 to 9) | ||
| Strokec | 2 (1.2) | 0 | 1 (−1 to 3) | ||
| Otherd | 9 (5.6) | 5 (3.2) | 2 (−2 to 7) | ||
Abbreviation: 4F-PCC, 4-factor prothrombin complex concentrate.
Absolute differences were not adjusted.
χ2 test was used for the comparison.
Stroke was diagnosed using cerebral contrast-enhanced computed tomography.
Other includes extremity ischemia (n = 11), thrombosis of venous surgical anastomosis (n = 2), and mesenteric infarction (n = 1). There were no incidents of myocardial infarction in either group.
Discussion
In this randomized, double-blind, placebo-controlled superiority trial investigating the efficacy and safety of administering 4F-PCC with a ratio-based transfusion strategy in patients at risk of massive transfusion, there was no reduction in 24-hour blood product consumption or any differences in secondary outcomes compared with placebo. The trial detected a statistically significantly higher risk of thromboembolic events in the 4F-PCC group.
Previous physiological, observational, and interventional evidence has suggested a benefit of 4F-PCC administration through a mechanism of boosting factor concentration and thrombin generation.9,10 The idea underlying the current trial’s design was to generate a thrombin burst to reduce blood product use in patients with trauma at risk of massive transfusion. However, trauma-induced coagulopathy is a complex hemostatic disorder, involving interactions between vessel wall, platelet, coagulation factor, and fibrinolysis factors.22,23 In response to tissue injury and shock, procoagulant factors levels decrease. In other consumptive coagulopathies, natural inhibitors of coagulation, such as antithrombin levels, are also decreased. This results in a new hemostatic balance and may explain why thrombin generation capacity is preserved despite PTr greater than 1.5 after trauma.24 The results of this trial support this observation, indicating no incremental benefit of 4F-PCC even in patients at risk of massive transfusion.
Three previous studies are comparable to the present trial: 2 observational efforts exploring the effect of an FFP-only strategy vs FFP plus 4F-PCC11,12 and a single-center open-label trial comparing a factor combination with fibrinogen concentrate, factor XIII, and 4F-PCC vs FFP7 observed a reduction of blood product consumption with 4F-PCC administration. The trial was stopped prematurely because of increased rates of massive transfusion and organ failure in the control group. In contrast to these efforts, the current trial included a higher percentage of patients in shock (59% with prehospital arterial systolic blood pressure <90 mm Hg vs 35%, 45%, and 30%, respectively, in previous trials). Our trial recruited patients with more severe trauma as measured by higher median Injury Severity Scores, more frequent hemorrhage control interventions, higher lactate, lower hemoglobin, and a high percentage of patients with PTr greater than 1.2. The contrast in clinical profiles and design may explain the divergent results. The clinical profiles explored by our trial and its design are more likely to test the effect of hemostatic therapies in patients with trauma and bleeding and aligns with other recent trauma hemorrhage trials.6,8,25 Our protocol administered a dose of 25 IU of factor IX/kg, which is very comparable to 20 IU in the previous trial7; no data are available for the observational studies.11,12 Initial studies of 4F-PCC administered doses between 20 to 35 IU/kg.10 Therefore, we believe these differences do not have a significant impact on the results.
Despite these previous results, safety concerns about increased thromboembolic events were raised from the start.26,27 In addition, detection and reporting remain heterogeneous across studies and suggest systematic underreporting.28 The observational efforts reported thromboembolic risk to be around 5%,11,12 while the trial reported 8% in the interventional group vs 18% in the control group.7 We demonstrated a higher overall relative risk of 1.48 despite more TXA and fibrinogen concentrate administration in the control group, increasing the safety concern of 4F-PCC use. The post hoc analysis suggests this effect appears amplified in patients with posttraumatic coagulopathy, which is possibly explained by providing supplemental substrate for already excessive thrombin generation.
Limitations
This study has several limitations. First, the study drug was administered in combination with FFP without prior VET. The combination of 4F-PCC and FFP without VET may expose patients without coagulopathy to the risk of coagulation factor “overdosing.” However, the rationale to use VET to guide 4F-PCC remains questionable. VET thresholds that are used to trigger 4F-PCC administration are neither standardized nor consensual, and the investigators decided against this approach to conduct a pragmatic trial with a high external validity because VET is still not widely available in many centers across the world. Pragmatic inclusion criteria facilitated recruitment of patients with severe bleeding who might potentially benefit from 4F-PCC, enabling an elevated level of generalizability and external applicability. Second, the main end point, 24-hour blood product use, may appear as an inappropriate surrogate for a patient-centered outcome, such as mortality. However, reduction of blood product consumption remains a meaningful clinical goal, because robust evidence points toward a consistent association of an increase in organ failure with increasing amounts of blood product use.29 Third, despite randomization, the delay to FFP administration was longer in the placebo group. Fourth, the definition of the prespecified “massive transfusion” subgroup could be influenced by the intervention; given the clinical importance of this subgroup, the investigators decided to report this result. Fifth, the composition of other commercially available 4F-PCC may differ from the one used in the present study, so their use might generate a dissimilar outcome, and the results of the trial cannot be applied to pediatric patients with trauma.
Conclusions
This randomized, double-blind clinical trial found no beneficial effect of adding 4F-PCC to a ratio-based transfusion strategy in patients with severe trauma at risk of massive transfusion, and possible harm from a higher rate of thromboembolic events, particularly in patients with an increased PTr. The findings do not support systematic 4F-PCC use in patients with trauma at risk of massive transfusion.
Section Editor: Christopher Seymour, MD, Associate Editor, JAMA (christopher.seymour@jamanetwork.org).
Trial Protocol
Statistical Analysis Plan
eTable 1. Time Spent in the Study in the First 24 Hours
eTable 2. Primary Outcome in Patients Who Received the Study Intervention Within the First Hour After Arrival in the Trauma Bay (per-protocol population)
eTable 3. Secondary Outcomes in Patients Who Received the Study Intervention Within the First Hour After Arrival in the Trauma Bay (per-protocol population)
eTable 4. Thromboembolic Events in Patients Who Received the Study Intervention Within the First Hour After Arrival in the Trauma Bay (per-protocol population)
eTable 5. Primary Outcome in the Subgroup of Patients With a Massive Transfusion
eTable 6. Secondary Outcomes in the Subgroup of Patients With a Massive Transfusion
eTable 7. Thromboembolic Events in the Subgroup of Patients With a Massive Transfusion
eFigure 1. Analysis of Time to PTr <1.5 Using Competing Risk Regression Model by Treatment Group in the Intention-to-Treat Population
eFigure 2. Time Course of Prothrombin Time Ratio (PTr) in Patients Who Received the Study Intervention Within the First Hour After Arrival in the Trauma Bay (per-protocol population)
eFigure 3. Time Course of Prothrombin Time Ratio (PTr) in the Subgroup of Patients With a Massive Transfusion
Nonauthor collaborators
Data Sharing Statement
References
- 1.Oyeniyi BT, Fox EE, Scerbo M, Tomasek JS, Wade CE, Holcomb JB. Trends in 1029 trauma deaths at a level 1 trauma center: impact of a bleeding control bundle of care. Injury. 2017;48(1):5-12. doi: 10.1016/j.injury.2016.10.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holcomb JB, Moore EE, Sperry JL, et al. Evidence-based and clinically relevant outcomes for hemorrhage control trauma trials. Ann Surg. 2021;273(3):395-401. doi: 10.1097/SLA.0000000000004563 [DOI] [PubMed] [Google Scholar]
- 3.Cole E, Weaver A, Gall L, et al. A decade of damage control resuscitation: new transfusion practice, new survivors, new directions. Ann Surg. 2021;273(6):1215-1220. doi: 10.1097/SLA.0000000000003657 [DOI] [PubMed] [Google Scholar]
- 4.James A, Abback PS, Pasquier P, et al. ; Traumabase Group . The conundrum of the definition of haemorrhagic shock: a pragmatic exploration based on a scoping review, experts’ survey and a cohort analysis. Eur J Trauma Emerg Surg. 2022;48(6):4639-4649. doi: 10.1007/s00068-022-01998-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crombie N, Doughty HA, Bishop JRB, et al. ; RePHILL collaborative group . Resuscitation with blood products in patients with trauma-related haemorrhagic shock receiving prehospital care (RePHILL): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Haematol. 2022;9(4):e250-e261. doi: 10.1016/S2352-3026(22)00040-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holcomb JB, Tilley BC, Baraniuk S, et al. ; PROPPR Study Group . Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471-482. doi: 10.1001/jama.2015.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Innerhofer P, Fries D, Mittermayr M, et al. Reversal of trauma-induced coagulopathy using first-line coagulation factor concentrates or fresh frozen plasma (RETIC): a single-centre, parallel-group, open-label, randomised trial. Lancet Haematol. 2017;4(6):e258-e271. doi: 10.1016/S2352-3026(17)30077-7 [DOI] [PubMed] [Google Scholar]
- 8.Baksaas-Aasen K, Gall LS, Stensballe J, et al. Viscoelastic haemostatic assay augmented protocols for major trauma haemorrhage (ITACTIC): a randomized, controlled trial. Intensive Care Med. 2021;47(1):49-59. doi: 10.1007/s00134-020-06266-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schöchl H, Nienaber U, Hofer G, et al. Goal-directed coagulation management of major trauma patients using thromboelastometry (ROTEM)-guided administration of fibrinogen concentrate and prothrombin complex concentrate. Crit Care. 2010;14(2):R55. doi: 10.1186/cc8948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schöchl H, Nienaber U, Maegele M, et al. Transfusion in trauma: thromboelastometry-guided coagulation factor concentrate-based therapy versus standard fresh frozen plasma-based therapy. Crit Care. 2011;15(2):R83. doi: 10.1186/cc10078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jehan F, Aziz H, OʼKeeffe T, et al. The role of four-factor prothrombin complex concentrate in coagulopathy of trauma: a propensity matched analysis. J Trauma Acute Care Surg. 2018;85(1):18-24. doi: 10.1097/TA.0000000000001938 [DOI] [PubMed] [Google Scholar]
- 12.Zeeshan M, Hamidi M, Feinstein AJ, et al. Four-factor prothrombin complex concentrate is associated with improved survival in trauma-related hemorrhage: A nationwide propensity-matched analysis. J Trauma Acute Care Surg. 2019;87(2):274-281. doi: 10.1097/TA.0000000000002262 [DOI] [PubMed] [Google Scholar]
- 13.Bouzat P, Bosson JL, David JS, Riou B, Duranteau J, Payen JF; PROCOAG study group . Four-factor prothrombin complex concentrate to reduce allogenic blood product transfusion in patients with major trauma, the PROCOAG trial: study protocol for a randomized multicenter double-blind superiority study. Trials. 2021;22(1):634. doi: 10.1186/s13063-021-05524-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouzat P, Ageron FX, Brun J, et al. ; TRENAU group . A regional trauma system to optimize the pre-hospital triage of trauma patients. Crit Care. 2015;19(1):111. doi: 10.1186/s13054-015-0835-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nunez TC, Voskresensky IV, Dossett LA, Shinall R, Dutton WD, Cotton BA. Early prediction of massive transfusion in trauma: simple as ABC (assessment of blood consumption)? J Trauma. 2009;66(2):346-352. doi: 10.1097/TA.0b013e3181961c35 [DOI] [PubMed] [Google Scholar]
- 16.Frith D, Goslings JC, Gaarder C, et al. Definition and drivers of acute traumatic coagulopathy: clinical and experimental investigations. J Thromb Haemost. 2010;8(9):1919-1925. doi: 10.1111/j.1538-7836.2010.03945.x [DOI] [PubMed] [Google Scholar]
- 17.Baksaas-Aasen K, Van Dieren S, Balvers K, et al. ; TACTIC/INTRN collaborators . Data-driven development of ROTEM and TEG algorithms for the management of trauma hemorrhage: a prospective observational multicenter study. Ann Surg. 2019;270(6):1178-1185. doi: 10.1097/SLA.0000000000002825 [DOI] [PubMed] [Google Scholar]
- 18.Roberts I, Shakur H, Afolabi A, et al. ; CRASH-2 collaborators . The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. Lancet. 2011;377(9771):1096-1101. doi: 10.1016/S0140-6736(11)60278-X [DOI] [PubMed] [Google Scholar]
- 19.Rossaint R, Bouillon B, Cerny V, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fourth edition. Crit Care. 2016;20:100. doi: 10.1186/s13054-016-1265-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouzat P, Ageron FX, Charbit J, et al. Modelling the association between fibrinogen concentration on admission and mortality in patients with massive transfusion after severe trauma: an analysis of a large regional database. Scand J Trauma Resusc Emerg Med. 2018;26(1):55. doi: 10.1186/s13049-018-0523-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bouzat P, Guerin R, Boussat B, et al. Diagnostic performance of thromboelastometry in trauma-induced coagulopathy: a comparison between two level I trauma centres using two different devices. Eur J Trauma Emerg Surg. 2021;47(2):343-351. doi: 10.1007/s00068-019-01165-7 [DOI] [PubMed] [Google Scholar]
- 22.Torres Filho I. Hemorrhagic shock and the microvasculature. In: Terjung R, ed. Comprehensive Physiology. John Wiley & Sons, Inc; 2017:61-101. doi: 10.1002/cphy.c170006 [DOI] [PubMed] [Google Scholar]
- 23.Kleinveld DJB, Hamada SR, Sandroni C. Trauma-induced coagulopathy. Intensive Care Med. 2022;48(11):1642-1645. doi: 10.1007/s00134-022-06834-7 [DOI] [PubMed] [Google Scholar]
- 24.Dunbar NM, Chandler WL. Thrombin generation in trauma patients. Transfusion. 2009;49(12):2652-2660. doi: 10.1111/j.1537-2995.2009.02335.x [DOI] [PubMed] [Google Scholar]
- 25.Pusateri AE, Moore EE, Moore HB, et al. Association of prehospital plasma transfusion with survival in trauma patients with hemorrhagic shock when transport times are longer than 20 minutes: a post hoc analysis of the PAMPer and COMBAT clinical trials. JAMA Surg. 2020;155(2):e195085. doi: 10.1001/jamasurg.2019.5085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dickneite G, Pragst I. Prothrombin complex concentrate vs fresh frozen plasma for reversal of dilutional coagulopathy in a porcine trauma model. Br J Anaesth. 2009;102(3):345-354. doi: 10.1093/bja/aen391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghosh S, Krege W, Doerr B, et al. Evaluation of the prothrombotic potential of four-factor prothrombin complex concentrate (4F-PCC) in animal models. PLoS One. 2021;16(10):e0258192. doi: 10.1371/journal.pone.0258192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He LX, Xie JY, Lv J, et al. Quality evaluation of clinical practice guidelines for thromboprophylaxis in orthopaedic trauma based on AGREE II and AGREE-REX: a systematic review protocol. BMJ Open. 2022;12(11):e059181. doi: 10.1136/bmjopen-2021-059181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sims CA, Holena D, Kim P, et al. Effect of low-dose supplementation of arginine vasopressin on need for blood product transfusions in patients with trauma and hemorrhagic shock: a randomized clinical trial. JAMA Surg. 2019;154(11):994-1003. doi: 10.1001/jamasurg.2019.2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eTable 1. Time Spent in the Study in the First 24 Hours
eTable 2. Primary Outcome in Patients Who Received the Study Intervention Within the First Hour After Arrival in the Trauma Bay (per-protocol population)
eTable 3. Secondary Outcomes in Patients Who Received the Study Intervention Within the First Hour After Arrival in the Trauma Bay (per-protocol population)
eTable 4. Thromboembolic Events in Patients Who Received the Study Intervention Within the First Hour After Arrival in the Trauma Bay (per-protocol population)
eTable 5. Primary Outcome in the Subgroup of Patients With a Massive Transfusion
eTable 6. Secondary Outcomes in the Subgroup of Patients With a Massive Transfusion
eTable 7. Thromboembolic Events in the Subgroup of Patients With a Massive Transfusion
eFigure 1. Analysis of Time to PTr <1.5 Using Competing Risk Regression Model by Treatment Group in the Intention-to-Treat Population
eFigure 2. Time Course of Prothrombin Time Ratio (PTr) in Patients Who Received the Study Intervention Within the First Hour After Arrival in the Trauma Bay (per-protocol population)
eFigure 3. Time Course of Prothrombin Time Ratio (PTr) in the Subgroup of Patients With a Massive Transfusion
Nonauthor collaborators
Data Sharing Statement



