Abstract
The gut microbiome is considered an endocrine organ that can influence distant organs and associated biological pathways. Recent advances suggest that gut microbial homeostasis is essential for reproductive health and that perturbations in the gut microbiota can lead to reproductive pathologies. This review provides an updated overview of the relationship between the gut microbiome and female reproductive diseases. Specifically, we highlight the most recent findings on the gut microbiome in gynecological pathologies including polycystic ovarian syndrome (PCOS), endometriosis, and endometrial cancer. Most studies revealed associations between altered gut microbial compositions and these reproductive diseases, though few have suggested cause-effect relationships. Future studies should focus on determining the molecular mechanisms underlying associations between gut microbiota and reproductive diseases. Understanding this bidirectional relationship could lead to the development of novel and effective strategies to prevent, diagnose and treat female reproductive organ-related diseases.
Keywords: Microbiome, reproduction, infertility, endometriosis, PCOS
Introduction
The gut microbiome – comprised of the bacteria, viruses, fungi, and protozoa living commensally, symbiotically, or pathogenically in the digestive tract – has co-evolved with its hosts for millennia. These microbes, combined with their genomic pool constitute the microbiome and participate in essential host activities such as digestion, immune cell maturation, and detoxification (Liang et al. 2018). Conversely, the perturbations in the microbiome disturb host energy homeostasis leading to the development of several diseases (Chassaing et al. 2012; Nicholson et al. 2012; Liang et al. 2018). Advances in the next generation sequencing technologies and ‘omics’ tools over the past two decades have allowed comprehensive identification of the gut microbiota composition and the identification of microbial taxa associated with human diseases including female reproductive tract pathologies (Tremellen & Pearce 2012; Franasiak & Scott Jr 2015; Green et al. 2015). The functions of the female reproductive tract are regulated by the endocrine system in a well-coordinated manner, which if disrupted, may lead to several disorders. Considered an extended endocrine organ, the gut microbiome acts as an important regulator of female reproductive health and associated diseases. In this review, we focus on associations and some causal connections between gut microbiota and polycystic ovarian syndrome (PCOS), endometriosis, and gynecologic cancers. Additionally, we review current knowledge regarding the use of pre/probiotics to manage some of these conditions.
The human gut microbiome:
The human body harbors numerous microorganisms that reside in various tissues including the mouth, skin, vagina, and gut. Of these sites, the human gut is particularly enriched in microorganisms than the rest of the body (Quigley 2013). A total of all the microorganisms present inside the gastrointestinal (GI) tract is referred to as “gut microbiota” which constitutes bacteria, fungi, viruses, protozoans, and archaea that co-evolved in an intricate and mutually beneficial relationship with the host (Backhed et al. 2005). The most dominant bacterial phyla in the gut include Firmicutes and Bacteroidetes, which constitute around 90% of the gut microbiome (Arumugam et al. 2011). About 200 genera belong to Firmicutes in the human gut and are dominated by the genus Clostridium (95%). The Bacteroidetes phylum is dominated by Bacteroides and Prevotella genera, while the Phylum Actinobacteria is proportionally less abundant and is mainly represented by the Bifidobacterium genus (Rinninella et al. 2019).
The microbial diversities are usually measured in terms of alpha and beta diversities, which represent the ‘within sample’ and ‘between samples’ diversities, respectively. Alpha diversity is the measure of the species richness (number) or evenness (distribution) or both in a sample. Alpha diversity is measured as the “Shannon index”, which is a quantitative measure of both the species abundance and evenness within a sample. On the other hand, beta diversity measures the variability in the microbial composition among different samples. These alpha and beta diversity indices identify broad differences in the microbiome compositions. Alterations in the ratio of this bacterial flora and their load, particularly involving the loss of beneficial microbes can lead to “dysbiosis of the gut microbiota” resulting in the development of various pathogenic diseases (Elias-Oliveira et al. 2020; Fan et al. 2021; Qi et al. 2021; Singh et al. 2021).
Gut microbiome-estrogen axis:
The gut microbiome is known to influence the hormone levels in the host including estrogen levels in females (Flores et al. 2012). This functional link between gut microbiota and estrogen was first noted three decades ago when Adlercreutz et al. found that antibiotic supplementation decreased estrogen levels in women (Adlercreutz et al. 1984). The gut microbiota principally regulates the estrogen level by secretion of β-glucuronidase (gmGUS), an enzyme that converts conjugated estrogen into deconjugated estrogen in the GI tract facilitating it to bind to estrogen receptors, resulting in subsequent signaling and physiological downstream effects (Figure 1). A decreased β-glucuronidase activity as a result of gut microbial dysbiosis can result in reduced deconjugation of estrogen and a decrease in the level of circulating estrogens (Flores et al. 2012). This further alters the activation of estrogen receptors leading to pathologies such as obesity and cardiovascular diseases. On the other hand, increased β-glucuronidase activity can result in elevated estrogens levels leading to pathologies, such as endometriosis and cancer (Plottel & Blaser 2011). Thus, optimal gmGUS activity is essential for maintaining estrogen levels in females.
Figure 1:

Schematic representation of the modulation of uterine functions through the estrogen-gut microbiome axis. The secretion of β-glucuronidase by gut bacteria converts conjugated estrogen into deconjugated estrogen in the GI tract. The deconjugated estrogen is reabsorbed by the gut and translocated into the bloodstream, facilitating estrogen entry into the uterus, wherein estrogen exerts its downstream action.
Another mechanism by which the gut microbiome might influence sex-steroid hormone levels in females is by producing short-chain fatty acids (SCFAs). SCFAs are the primary by-products of bacterial anaerobic fermentation of dietary fibers in the intestine. Acetate (C2), propionate (C3), and butyrate (C4) are the most abundant SCFAs that are produced by gut microbes. Importantly, butyrate has shown to regulate the synthesis of P4 (progesterone) and E2 (estradiol) in porcine granulosa cells (PGCs) via the cAMP signaling pathway (Lu et al. 2017). In this interesting in-vitro study, the PGCs were treated with lower concentrations of butyric acid stimulate the progesterone secretion, however, higher butyrate concentrations significantly inhibited the progesterone secretion (Lu et al. 2017). Another study by Liu et al. showed that gut derived butyrate contributes to nonalcoholic fatty liver disease in pre-menopause women due to estrogen deficiency (Liu et al. 2022). These studies highlight the plausible mechanism by which the dietary constituents and microbiota-derived metabolites may contribute to the regulation of estrogen and progesterone level in females, however, the underlying molecular mechanism is not clear yet. Nonetheless, the significance of gut microbiota in female reproductive pathologies is now well established, including PCOS, endometriosis, and other reproductive tract conditions.
Gut Microbiota and PCOS:
PCOS is a heterogeneous endocrine, neuroendocrine, and metabolic disorder that leads to difficult pregnancies or infertility (Tu et al. 2020; Wei et al. 2021) in 6.5% to 8.0% of reproductive-age women. The main characteristics of PCOS are hyperandrogenism, oligo/amenorrhoea, and polycystic ovarian morphology (Lindheim et al., 2016). Although genetic, lifestyle, and intrauterine factors have been suggested, the general etiology of PCOS is unclear. However, the gut microbiome contributes to several additional characteristics of PCOS such as obesity, insulin resistance, and low-grade inflammation (Lindheim et al. 2016; He & Li 2020; Lüll et al. 2021; Rizk & Thackray 2021).
Gut microbiome in PCOS patients:
Several investigators have compared the gut microbial compositions in stool samples from people with and without PCOS. Lull et al. identified four genera that differed between 102 patients with PCOS (n=102) and 201 age- and body mass index (BMI)-matched healthy controls (n=201). The abundance of two genera from Clostridiales, Ruminococcaceae UCG-002, and Clostridiales Family XIII AD3011, were correlated with several PCOS-related markers such as cystic ovarian morphology and higher testosterone levels. Moreover, patients with PCOS and pre-diabetes had significantly lower alpha diversity (Shannon index) and higher abundance of the Dorea and Bacteroides (Ruminococcus torques group and Lachnospiraceae UCG-004) genera than PCOS patients with normal glucose tolerance (Lüll et al. 2021). Liang et al. reported that gamma-aminobutyric acid-producing bacteria, including Parabacteroides distasonis and Bacteroides fragilis, were increased in PCOS patients, whereas Escherichia coli showed a positive relationship with serum Luteinizing Hormone (LH) levels and Luteinizing Hormone: Follicle Stimulating Hormone (FSH) ratios (Liang et al. 2021). As the higher level of LH is associated with PCOS condition (Zarei et al. 2021). Lindheim et al. found that the stool microbiome of PCOS patients (n=25) showed lower diversity and different phylogenetic composition than that of healthy controls (n=25). The authors did not observe any significant differences in any taxa with a relative abundance >1%. However, when assessing rare taxa, the relative abundances of bacteria from the phylum Tenericutes, specifically the order ML615J-28, and the family S24-7 (phylum Bacteroidetes) were significantly lower and associated with reproductive parameters in PCOS patients. Additionally, PCOS patients showed alterations in some, but not all, markers of gut barrier function and endotoxemia (Lindheim et al. 2017). Finally, Qi et al. examined 43 healthy controls (n=43) and 50 PCOS patients (n=50) and found that Bacteroides vulgatus was elevated in the gut microbiota of individuals with PCOS (Qi et al. 2019). Bacteroides vulgatus are gram-negative anaerobic bacteria inhabiting the distal human gut and are typically non-pathogenic in healthy individuals (Takaishi et al. 2008). These bacteria deconjugate the bile acids synthesized in the liver, such as glycodeoxycholic acid and tauroursodeoxycholic acid (Qi et al. 2019). Qi et al. reported a negative correlation between the abundances of B. vulgatus and glycodeoxycholic acid and tauroursodeoxycholic acid in PCOS patients. Collectively, these studies revealed that PCOS is associated with alterations in the gut microbiome, but no cause-effect relationships have been determined.
While most investigators have examined the microbiota in stool, only a few have examined the bacterial composition in saliva. For example, one study of the salivary microbiome revealed that PCOS patients (n=24) had fewer bacteria from the phylum Actinobacteria than healthy controls (n=20). PCOS patients also exhibited a borderline significant shift in bacterial community composition in unweighted UniFrac analysis (Lindheim et al. 2016). UniFrac, one of the distance metrics used to measure the beta diversity, collects the phylogenetic information to compare microbial communities in different samples (Lozupone et al. 2006). It measures the evolutionary distance among sets of taxa in a phylogenetic tree as a fraction of the branch length of the tree (Lozupone & Knight 2005). An unweighted UniFrac analysis can be used to ascertain the incidence of variation among the samples, however, weighted UniFrac can additionally quantify the variation occurring in different lineages (Ito et al. 2019).
The alpha diversity and weighted UniFrac analysis were unchanged between PCOS patients and controls. No differences were observed at any taxonomic level (Lindheim et al. 2016). The authors also noted altered gut microbiota in stool samples, though the findings were not identical. One group used 16S rRNA sequencing to examine fecal microbial diversity profiles of healthy women (n=48), women with polycystic ovarian morphology (PCOM) (n=42), and women diagnosed with PCOS by the Rotterdam criteria (n=73). Patients with PCOS had lower microbial diversity than healthy controls, and those with PCOM had an alpha diversity that was intermediate between that of healthy control and PCOS groups (Torres et al. 2018). Regression analysis showed that hyperandrogenism, total testosterone, and hirsutism were negatively correlated with alpha diversity. (Torres et al. 2018).
Several investigators have examined associations between fecal bacteria (gut microbiota) and PCOS in patients with and without obesity. For example, Liu et al. examined the gut microbiome in 33 patients with PCOS (12 non-obese and 21 obese) and 15 healthy controls (9 non-obese and 6 obese) and found that the co-abundance groups (CAGs) increased in the PCOS patients. CAGs are the clustering of bacterial species based on the SparCC (Sparse Correlations for Compositional data) correlation coefficients of their relative abundance (Liu et al. 2017). SparCC is a mathematical approach to estimating correlation values from compositional data (Friedman & Alm 2012). Additionally, abundances of Bacteroides, Escherichia/Shigella, and Streptococcus were negatively correlated with Ghrelin expression and positively correlated with testosterone and BMI (Liu et al. 2017). Therefore, the downregulation of Ghrelin is associated with PCOS (Ibrahim & Alobaidi 2021). Liang et al. analyzed data from 20 patients with PCOS (10 lean and 10 overweight) and 20 healthy controls (10 lean and 10 overweight) and reported that the intake of dietary fiber and vitamin D was significantly decreased in the PCOS group (Kim et al. 2021). Dietary fiber plays a crucial role in the composition of the gut microbiota where it acts as a prebiotic to support beneficial gut bacteria (probiotics) and suppress harmful bacteria (Kim et al. 2021). Future studies should further investigate the link between diet, PCOS, and the gut microbiome. Table 1A summarizes the list of studies that highlighted the gut microbiome changes in patients with PCOS.
Table 1.
The main findings of the human and rodent studies in association with gut microbial alterations in, A. PCOS; B. Endometriosis; and C. cervical and ovarian cancer.
| Disease | Strain | Main findings | References |
|---|---|---|---|
| Human studies | |||
| PCOS | ↑ Actinobacteria and ↓ Bacteroidetes in PCOS women. | (Jobira et al. 2020) | |
| PCOS | ↑ Clostridiales, Ruminococcaceae UCG-002, and Clostridiales Family XIII AD3011 in PCOS group. | (Lüll et al. 2021) | |
| PCOS | ↓ Alpha diversity, altered microbiota patterns in girls with PCOS. | Garcia-Beltran et al. 2021 | |
| PCOS | ↑ Parabacteroides distasonis and Bacteroides fragilis in PCOS patients. | (Liang et al. 2021) | |
| PCOS | ↓ Diversity and a dissimilar phylogenetic composition in PCOS group. | (Lindheim et al. 2017) | |
| PCOS | ↑ Bacteroides vulgatus in PCOS group. | (Qi et al. 2019) | |
| PCOS | ↓ Actinobacteria are in the PCOS group. | (Lindheim et al. 2016) | |
| PCOS | ↓ Microbial diversity in PCOS women. | (Torres et al. 2018) | |
| PCOS | ↑ Co-abundance groups (CAGs) in PCOS group. | (Liu et al. 2017) | |
| Rodent studies | |||
| PCOS | Sprague-Dawley rats | ↓ Lactobacillus, Ruminococcus, and Clostridium and ↑ Prevotella in PCOS rats. | (Guo et al. 2016) |
| PCOS | C57BL/6N mice | ↓ alpha diversity after 5 weeks of letrozole treatment in letrozole-treated mice. | (Kelley et al. 2016) |
| Human Studies | |||
| Endometriosis | ↑ Escherichia and Shigella in the endometriosis group. | (Ata et al. 2019) | |
| Endometriosis | No differences in the gut microbiomes. | (Perrotta et al. 2020) | |
| Endometriosis | ↑ Bacteroidia and Clostridia in endometriosis patients. | (Svensson et al. 2021) | |
| Endometriosis | Low alpha diversity and high Firmicutes to Bacteroidetes ratio in endometriosis group. | (Shan et al. 2021) | |
| Rodent studies | |||
| Endometriosis | C57BL/6N mice | Low alpha and beta diversity in mice with endometriosis. | (Ni et al. 2020) |
| Endometriosis | C57BL/6N mice | No difference in the diversity. | (Hantschel et al. 2019) |
| Endometriosis | C57BL/6N mice | ↑ Firmicutes-to-Bacteroidetes ratio and ↑ Bifidobacterium in endometriosis group. | (Yuan et al. 2018) |
| Endometriosis | C57BL/6N mice | ↑ Bacteriodetes and few Firmicutes in the endometriosis group. | (Chadchan et al. 2019) |
| Human studies | |||
| Cervical cancer | ↑ Abundance of Prevotella in early cervical cancer patients. | (Kang et al. 2020) | |
| Cervical cancer | ↑ Abundance of Prevotella, Porphyromonas, and Dialister and ↓ abundance of Acteroides, Alistipes, and Lachnospiracea in cervical cancer patients. | (Sims et al. 2019) | |
| Cervical cancer | ↑ Bacteroidetes and ↓ Firmicutes in cancer patients. | (Wang et al. 2019) | |
| Ovarian cancer | ↓ phylogenetic diversity in patients with the primary platinum-resistant disease. | (Jacobson et al. 2021) | |
Two studies have investigated the effects of treatments on the fecal microbiome of patients with PCOS. First, when non-diabetic PCOS patients with HIV taking antiretroviral therapy (n=23) were treated with metformin, the abundance of gut anti-inflammatory bacteria such as butyrate-producing species and the protective Akkermansia muciniphila increased in the gut (Ouyang et al. 2020). By producing short-chain fatty acids (SCFAs), they protect the gut epithelial barrier and reduce inflammation levels in patients with HIV (PWH) receiving antiretroviral therapy (ART). Second, Garcia-Beltran et al. examined the gut microbiota composition of non-obese females with PCOS (age 15.8 years; BMI 25 kg/m2) who were randomized to receive treatment with either an oral contraceptive (n=15) or with spironolactone pioglitazone-metformin (n=15). The authors reported that adolescent girls with PCOS had decreased alpha diversity, an altered microbiota pattern, and a taxonomic profile with more abundant Family XI and less abundant family Prevotellaceae, genus Prevotella, and genus Senegalimassilia than in healthy controls. Additionally, Family XI abundance showed a positive relationship to hepato-visceral fat (Garcia-Beltran et al., 2021). Treatment with spironolactone pioglitazone-metformin treatment, but not with oral contraceptives, normalized the abundance of Family XI. Prevotellaceae, Prevotella, and Senegalimassilia abundance remained unchanged after either treatment (Garcia-Beltran et al. 2021). More work is needed to determine whether these microbiome changes reflect the direct effects of the treatments or the resolution of PCOS.
Gut microbiota in rodent models of PCOS:
To address whether associations between altered gut bacteria and PCOS in humans reflect cause/effect relationships, several researchers have turned to rodent models of this disease. The most common methods of inducing PCOS in mice and rats are to treat them with Letrozole or dehydroepiandrosterone (DHEA). The abundances of Lactobacillus, Ruminococcus, and Clostridium were lower and Prevotella was higher in rats with Letrozole-induced PCOS than in control rats (Guo et al. 2016). Similar results were observed in the Letrozole-induced PCOS mouse model (Kelley et al. 2016). However, Guo et al. took their work one step further by transplanting fecal microbiota from healthy rats into rats with PCOS. This treatment improved the estrous cycles in all of the rats (n=8) and the ovarian functions were normalized. Interestingly, transfer of just Lactobacillus improved the estrous cycle in 75% of the rats, suggesting a single genus might have a profound impact on the estrous cycle in PCOS patients. In both cases, estrous cycle improvements were coupled with decreasing androgen biosynthesis and normalized ovarian morphology. Moreover, the composition of the gut microbiota was restored in both of the treated groups, with levels of Lactobacillus and Clostridium increasing and Prevotella decreasing. These results indicate that dysbiosis of the gut microbiota contributes to the pathogenesis of PCOS in rats (Guo et al. 2016), highlighting the need for investigations into probiotic-based treatment strategies for women with PCOS. The data also highlighted the significance of FMT and Lactobacillus transplantation for the treatments of PCOS.
In a different approach, Qi et al. transplanted feces from women with PCOS to B. vulgatus-colonized recipient mice and noted increased disruption of ovarian functions, insulin resistance, altered bile acid metabolism, reduced interleukin-22 secretion, and infertility (Qi et al. 2019). These features of PCOS were reversed when the mice were treated with glycodeoxycholic acid, which induced intestinal group 3 innate lymphoid cell IL-22 secretion through GATA binding protein 3 (Qi et al. 2019). Whether a similar treatment would be effective in humans has not been determined. The summary of the rodent studies is presented in Table 1A.
Gut Microbiota and Endometriosis:
Gut microbiota and endometriosis in humans:
Endometriosis is a disease in which cells from the epithelial lining of the uterus (the endometrium) implant and proliferate on peritoneal surfaces in the abdomen. Endometriosis affects approximately 196 million people worldwide (Zondervan et al. 2020). Nearly half of these patients experience chronic pelvic pain, and 30–50% are infertile (Missmer et al. 2004; Smolarz et al. 2021). Currently available treatments such as hormonal therapy and surgical excision have adverse side effects and do not prevent recurrences (Abbott et al. 2003). Endometriosis is thought to be caused by retrograde menstruation. However, whereas 90% of menstruating people experience retrograde menstruation, only 10% develop endometriosis (Mehedintu et al. 2014). The American Society for Reproductive Medicine (ASRM) and American Fertility Society (AFS) have classified endometriosis as stage I-IV depending on the size and number of lesions and presence of adhesions and ovarian vs. peritoneal involvement. Presence of the ovarian cysts and adhesions are assigned with higher stages but they don’t correlate with the severity of the pain (Zondervan et al. 2016). This has led many researchers to explore other factors that may contribute to the development of this disease.
Recent evidence suggests that endometriosis is associated with gut microbial dysbiosis, though the data are far from consistent across studies. For example, in one study, patients with stage three or stage four (moderate to severe) endometriosis (n=14) were more likely than healthy participants (n=14) to have abundant Shigella/Escherichia in their colonic microbiota (Ata et al. 2019). In contrast, another study identified no differences between the gut microbiomes of patients with endometriosis (n=35) and healthy controls (n=24) during the proliferative and secretory phases of the menstrual cycle (Perrotta et al. 2020). However, this study did not specify the stages of endometriosis. A larger study conducted on human stool samples suggested that both alpha (the microbial diversity of a single sample) and beta (a measure of similarity or dissimilarity between two communities) diversities, as well as the Firmicutes-to-Bacteroidetes ratio (Svensson et al. 2021), were higher in the stool samples of controls (n=198) than in patients with endometriosis (n=66) (Svensson et al. 2021). This study appears to contradict another study in which those with stage three or stage four endometriosis (n=12) had lower alpha diversity of gut microbiota and a higher Firmicutes-to-Bacteroidetes ratio than healthy controls (Shan et al. 2021). In the largest study to date, Svensson et al. reported the abundance of 12 bacteria belonging to the classes Bacilli, Bacteroidia, Clostridia, Coriobacteriia, and gammaproteobacteria, that differed significantly between stool samples from endometriosis patients (n=66) and those from matched healthy controls (n=198) (Svensson et al. 2021). Two bacteria from class Bacteroidia (Bacteroides and Parabacteroides) and two belonging to class Clostridia (Oscillospira and Coprococccus) were present in higher abundances in endometriosis patients, whereas two bacterial species from the Bacteroidia (Paraprevotella and one unidentified) and Clostridia (Lachnospira and one unidentified) classes were at lower abundances in the stool samples of endometriosis patients. The variability of findings across these studies points to the need for larger studies in which potential confounders (e.g., stage of endometriosis, age, race/ethnicity, other health conditions, medication, and diet) are assessed. Moreover, to date, no causal relationships between gut microbiota and endometriosis have been established in humans. Table 1B summarizes the studies that analyzed the gut microbiome changes in patients with endometriosis.
Gut microbiota and endometriosis in rodents:
To address some of the limitations of human experiments, some researchers have turned to rodent models of endometriosis. In these models, autologous or donor uterine tissue is injected into the peritoneal space or sutured to a peritoneal surface, resulting in endometriotic lesions that grow to up to 50 mm3 within 21 days (Chadchan et al. 2019, 2021).
As with human studies, the rodent studies of the gut microbiome thus far have reached somewhat divergent conclusions. For example, Ni et al. examined mouse fecal samples and found that the diversity (alpha and beta) and abundance of the gut microbiota were lower in mice with endometriosis than in mice without endometriosis (Ni et al. 2020). In contrast, another study conducted on feces reported no difference in the alpha- and beta-diversity between mice with and without endometriosis (Hantschel et al. 2019). Additionally, In another study, dysbiosis of the gut microbiota was observed 42 days after endometriosis induction, with an elevated Firmicutes-to-Bacteroidetes ratio and elevated abundance of Bifidobacterium, a commonly used probiotic (Yuan et al. 2018). This elevated Firmicutes-to-Bacteroidetes ratio was similar to the finding of (Shan et al. 2021). In a study of rats, those with endometriosis had an elevated Firmicutes-to-Bacteroidetes ratio and decreased abundance of Ruminococcaceae in stool samples (Cao et al. 2020). In contrast, we showed that the feces of mice with endometriosis had more Bacteriodetes and fewer Firmicutes than mice without endometriosis. Additionally, we reported that mice treated with antibiotics had reduced Firmicutes and Bacteroidetes in their feces and developed significantly smaller endometriotic lesions than vehicle-treated mice (Chadchan et al. 2019). The summary of the rodent studies is presented in Table 1B.
One mechanism by which mammalian gut bacteria affect host physiology is by processing otherwise indigestible nutrients into biologically active metabolites (Forbes et al. 2016; Kho & Lal 2018; Li et al. 2018), including short-chain fatty acids. Ni et al. conducted an advanced metabolomics analysis revealing higher abundances of chenodeoxycholic and ursodeoxycholic acids and lower abundances of alpha-linolenic acid and 12, 13s-epoxy-9z, 11, 15z-octadecatrienoic acid in feces of mice with endometriosis than in feces of mice without endometriosis (Ni et al. 2021). Moreover, they noted that exogenous supplementation of alpha-linolenic acid restored the abundance of Firmicutes and Bacteroidetes, improved the intestinal wall barrier, reduced abdominal inflammation, and reduced the abundance of lipopolysaccharide in mice with endometriosis (Ni et al. 2021).
Although the aforementioned studies identified the altered gut microbiota in endometriosis, it is not clear whether altered gut microbiota itself affects disease progression. Toward this, our group demonstrated that altered gut microbiota, in fact, drives the endometriotic lesion growth in mice (Chadchan et al. 2021). Moreover, we found feces derived from mice with endometriosis have fewer short-chain fatty acids, specifically n-butyrate compared to feces from mice without endometriosis. Consistent with this, treatment with n-butyrate attenuated the endometriotic lesion growth in mice and a pre-clinical mouse model. Molecular mechanistic studies found that n-butyrate acts via G-protein–coupled receptors (GPCRs), histone deacetylases (HDACs), and a GTPase activating protein, RAP1GAP to inhibit human endometriotic cell survival and lesion growth (Chadchan et al. 2021). Interestingly, multiple reports found HDAC overexpression in endometriotic lesions (Colón-Díaz et al. 2012; Gujral et al. 2020). Importantly, SCFAs are also known to play crucial roles in immune cell regulation by regulation of Treg cell expansion (Blander et al. 2017). This may serve as another possible mechanism driving the development of endometriosis, as it is known that alteration in the Treg cell population promotes inflammation and angiogenesis, facilitating the attachment and growth of endometrial implants (Tanaka et al. 2017). In conclusion, across all the studied species, endometriosis is generally associated with an imbalance in the Firmicutes to Bacteroidetes ratio, suggesting that dysbiosis of the gut microbiota is linked with endometriosis pathophysiology. Importantly, supplementation of n-butyrate in form of analogs or engineered bacteria with n-butyrate overproduction could be used as an effective treatment regime for patients with endometriosis (Chadchan et al. 2021).
Although mouse studies are beginning to suggest mechanisms by which the gut microbiota affects endometriosis, we thus far lack definitive cause/effect relationships between gut microbiota and endometriosis in humans. the relevance of specific gut-derived metabolites in female reproductive health still needs to be determined. This is a promising area of research, as it could lead to simple diet interventions to reduce the burden of endometriosis.
Gut Microbiome in Gynecologic Cancers:
The most common gynecological cancer is endometrial, affecting 66,570 women in the USA with 12,940 deaths predicted in the year 2021, according to the American Cancer Society. The next most common gynecologic cancer is cervical cancer, which is also the fourth most common female malignancy in the world (Small Jr et al. 2017; Zhang et al. 2020). The third most common gynecologic cancer is ovarian cancer, which accounts for 2.5% of all female malignancies. However, 80% of patients are diagnosed with advanced-stage ovarian cancer, so the disease accounts for 5% of all cancer deaths (Zhang et al. 2020). Thus far, only a few studies have examined relationships between the gut microbiome and gynecologic cancers. We highlight a few examples below.
In endometrial cancer, estrogen plays an important role (Popli et al. 2020) in modulating the inflammatory response (Baker et al. 2017). Given that the gut microbiota can metabolize estrogen and thereby alter the circulating estrogen concentration (Baker et al. 2017), gut bacteria could influence the development of endometrial cancer. However, no studies have thus far provided a direct link between gut bacteria and endometrial cancer. In one study, investigators examined the effect of Urolithin A, a gut-derived bacterial metabolite from Ellagic acid, on the endometrial cancer cell line, Ishikawa. This treatment disrupted Rac1 and Pak1 activity, caused actin depolymerization, and decreased cell migration (Alauddin et al. 2020).
Wang et al. found that the gut microbial composition differed significantly between stool samples of patients with cervical cancer (n=8) and those of healthy controls (n=5). Specifically, cancer patients had more Bacteroidetes and fewer Firmicutes than healthy controls (Wang et al. 2019). Another study revealed that Prevotella, Porphyromonas, and Dialister were significantly more abundant, and Acteroides, Alistipes, and Lachnospiracea were less abundant in the gut microbiomes of cervical cancer patients (n=42) than in those of matched healthy controls (n=46) (Sims et al. 2019). Similarly, Kang et al. reported that Prevotella was more abundant in fecal samples of early cervical cancer patients than in those from healthy controls (Kang et al. 2020).
Only one study has examined the gut microbiota composition in ovarian cancer patients, revealing that patients with primary platinum-resistant disease had lower phylogenetic diversity than platinum-sensitive patients (Jacobson et al. 2021). Table 1C provides a summary of the studies that investigated the association of gut microbiome alterations in women with cervical and ovarian cancer.
Unlike other types of cancer, a link between gut microbiota and gynecological cancers is not widely investigated and there is much room for exploration to identify connections between the gut microbiome and ovarian as well as other gynecologic cancers.
Few studies explored the role of the uterine, cervical, and vaginal microbiome in patients with endometriosis and infertility, albeit found differential microbial patterns (Table 2). One possible reason for this variance could be due to study design differences and a lack of defined normal uterine microbiome. The healthy vaginal microbiota exhibits fewer different bacteria than the gut microbiota, i.e., low taxonomic diversity which is typically dominated by Lactobacillus species (Ravel et al., 2011). It is worth noting that, the continuous change of the microbiota distribution was identified along the reproductive tract (Chen et al., 2017, Wei et al., 2020). However, enrichment of Gardnerella was seen in both endometriosis and infertility (Wee et al. 2018; Ata et al. 2019). Further, the study of vaginal microbiota in healthy controls (n=18) and endometriosis patients (n=16) also found a significant enrichment of Atopobium as well as Gardnerella, while Lactobacillus was found lower in patients with endometriosis (Lu et al., 2022). Similarly, the decrease in the Lactobacillus level of vaginal microbiome was highlighted in the patient with endometriosis (Wei et al., 2020). Furthermore, the level of Anaerococcus genus in vaginal samples can be utilized to predict the revised American Society for Reproductive Medicine (rASRM) stages of endometriosis (Perrotta et al. 2020), which showed a positive correlation with advanced stages of endometriosis. In addition, the analysis of vaginal microbiome of patients with Endometriosis/Adenomyosis associated with Chronic Pelvic Pain Syndrome (CPPS) displayed significantly higher alpha diversity, as well as higher counts of Clostridium butyricum, Clostridium disporicum, Alloscardovia omnicolens, and Veillonella montpellierensis when compared to either CPPS patients without Endometriosis/Adenomyosis or women without CPPS (Chao et al., 2021). In contrast, Hernandes et al. showed that there was no difference in the vaginal mucus microbiome of control and deep infiltrating endometriosis patients (Hernandes et al., 2020). Analysis of the cervical microbiome by Chang et al. revealed that significantly reduced richness and diversity were detected in endometriosis patients with more severe clinical symptoms when compared to control patients (Chang et al., 2022). The presence of L. crispatus was found to be associated with a higher rate of insemination success and fertility in two of the studies (Campisciano et al. 2017; Amato et al. 2020). It is interesting to note that the gut and the vaginal microbiome are in dynamic crosstalk with each other. In fact, the vaginal microbiome is known to have evolved from the translocation of species from the gut or by mother-to-child transfer at the time of delivery (Amabebe & Anumba 2020).
Table 2.
Study design, and results of recent literature describing the studies of uterine, cervical, and vaginal microbiota in association with endometriosis and infertility.
| Human | |||
|---|---|---|---|
| Disease | Microbiome | Major findings | Reference |
| Endometriosis | Gastrointestinal (GI) and urogenital (UG) microbiomes. | Identification of Clostridiales_Incertae_Sedis_XI Anaerococcus as a characteristic biomarker in AMEM patients. | (Chen et al. 2021). |
| Endometriosis | Gut and vaginal microbiome. | Strong positive association between the GI/UG bacteria and the concentrations of urinary estrogen and its metabolites in the P-EOSIS group. | (Le et al. 2021) |
| Endometriosis | vaginal, cervical, and gut microbiome. | Complete absence of Atopobium in the vaginal and cervical microbiota of the stage 3/4 endometriosis women. Enrichment of Gardnerella, Streptococcus, Escherichia, Shigella, and Ureoplasma in the cervical microbiome of patients in stage 3/4 endometriosis. | (Ata et al. 2019) |
| Endometriosis | Endometrial and vaginal microbiome. | The occurrence of a non-Lactobacillus-dominated microbiota in a receptive endometrium was correlated with a significant decrease in implantation rate. | (Moreno et al. 2016) |
| Endometriosis | Endometrial microbiome | Enrichment in Actinobacteria phylum, Oxalobacteraceae and Streptococcaceae families, and Tepidimonas genus in endometriosis group. | (Wessels et al. 2021) |
| Endometriosis | Vaginal microbiome | Significant enrichment of Gardnerella and Atopobium and reduction in Lactobacillus spp. in patients with endometriosis | (Lu et al. 2022) |
| Endometriosis | Gut and vaginal microbiome | Higher OTU (operational taxonomic unit) of Anaerococcus genus in vaginal samples with advanced stages of endometriosis | (Perrotta et al. 2020) |
| Endometriosis/Adenomyosis with CPPS | Vaginal microbiome | Higher alpha diversity, as well as higher counts of Clostridium butyricum, Clostridium disporicum, Alloscardovia omnicolens, and Veillonella montpellierensis in Endometriosis/Adenomyosis patient with chronic Pelvic pain syndrome (CPPS) when compared to either CPPS patients without EM/AM or women without CPPS. | (Chao et al. 2021) |
| Endometriosis | Cervical Microbiome | Reduced richness and diversity of cervical microbiome were detected in endometriosis patients with more severe clinical symptoms | (Chang et al. 2022) |
| Infertility | Cervical-vaginal microbiome | Differential presence of L. iners, L. crispatus, and L. gasseri in idiopathic infertile women. | (Campisciano et al. 2017) |
| Infertility | Vaginal, cervical, and endometrial microbiome. | Increased Ureaplasma and Gardnerella vagina in the cervix of Infertile women respectively. | (Wee et al. 2018) |
| Infertility | Vaginal and seminal microbiome. | L. crispatus correlated with a higher rate of intrauterine insemination success. | (Amato et al. 2020) |
The gut microbiome alterations are linked to several other female reproductive disorders such as pre-term birth and Intra-uterine growth retardation (IUGR). Dahls et.al. found that mothers delivering prematurely have lower alpha diversity in the gut than the term deliveries (Dahl et al. 2017). Interestingly, the gut microbiota composition in preterm neonates also differs significantly from that of born at full-term (Hiltunen et al. 2021). Additionally, a study analyzing the gut microbiota profiles in IUGR and normal birth weight piglets demonstrated that the metabolome profile in the IUGR piglets was significantly altered in comparison with controls (Zhang et al. 2019).
General Conclusions, Limitations, and Future Perspectives:
Humans acquire a unique, dynamic ecosystem of gut microbiota in early life and maintain this metabolically active “organ” throughout their existence (Gagliardi et al. 2018). Recent advances well-established that gut microbiome perturbations are linked to several female reproductive tract disorders, commonly endometriosis, PCOS, gynecological cancers, and infertility (Figure 2). (Ata et al. 2019; Lüll et al. 2021; Svensson et al. 2021). However, most studies merely report snapshots of the perturbed microbial diversity in reproductive disorders. Nonetheless, these alterations are disease-specific in nature, thus offering a unique opportunity for non-invasive based early detection of reproductive tract pathologies. Emerging findings well established that the gut microbiota affects host physiology through the production of metabolites including short-chain fatty acids. However, limited data are available on how the gut microbiota or -derived metabolites regulate reproductive function and dysfunctions. Moreover, it is not clear how gut microbiota might influence the peritoneal microenvironment, which could influence pathogenic manifestations of these diseases. Thus, efforts toward identifying novel treatment strategies by modulating bacteria via dietary intervention, microbial supplementation, or FMT are of high clinical relevance. However, a significant knowledge gap exists on the mechanisms by which specific bacterial species or groups of species may drive or influence reproductive tract function and dysfunctions. Importantly, additional studies are needed to determine whether dysbiosis in the gut bacteria causes these diseases or is itself a consequence of disease progression. Decoding the microbe–microbe-host interactions is key to linking gut microbiota alterations with microbial regulation of host processes and to optimally realizing the potential of gut microbiota in combating these diseases. Importantly, large, and longitudinal integrative studies are much needed to identify all the microbial species, including bacteria, fungi, and viruses that are altered in reproductive pathologies. Although much remains to be unearthed, the data thus far provided tantalizing hints that modifying the gut microbiome could be a valuable avenue for treating many female reproductive tract pathologies. Importantly, these studies indicate that the gut microbiome and derived metabolites represent a new frontier in the management of reproductive tract diseases.
Figure 2:
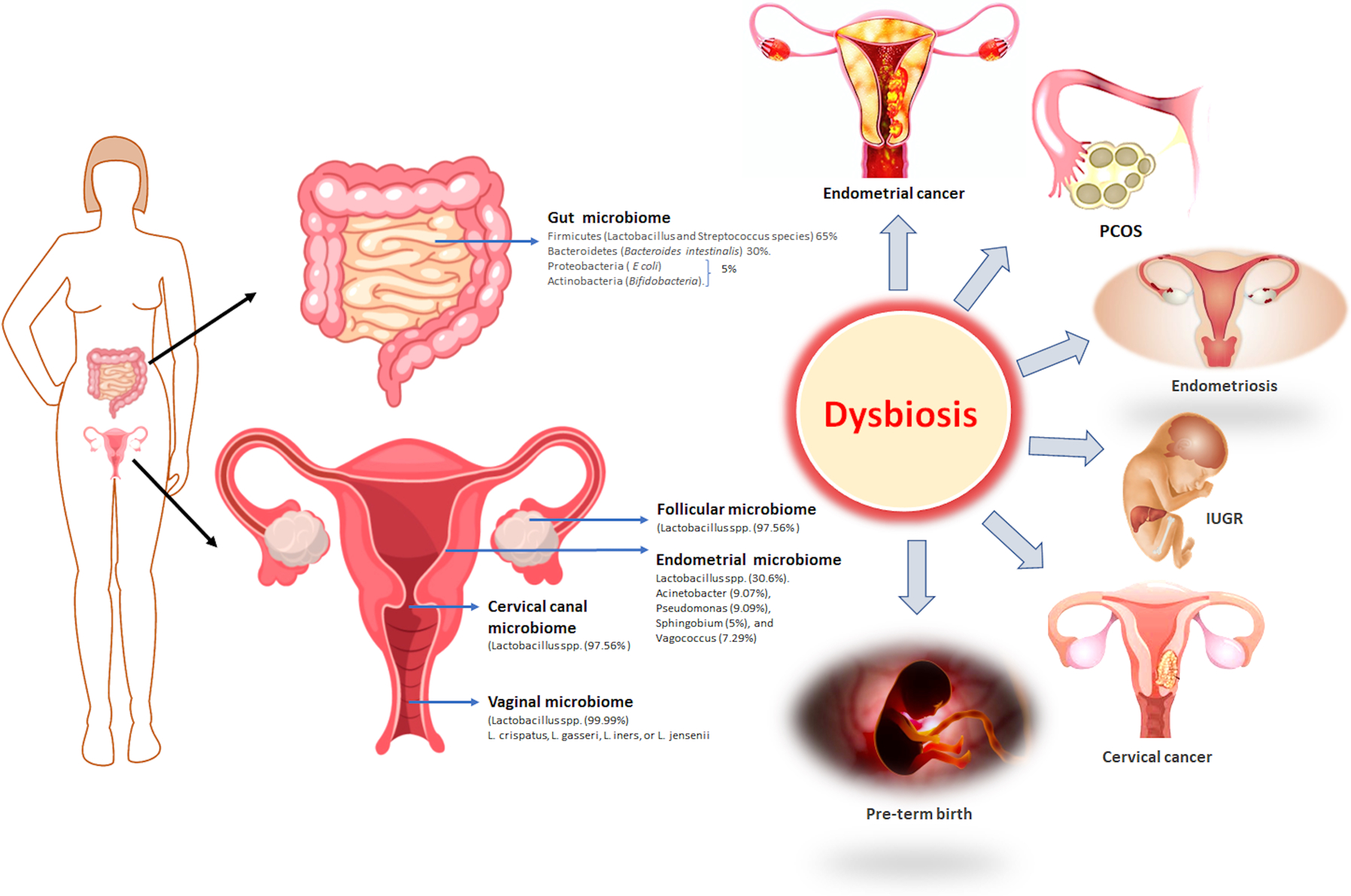
Representative description of the composition of the reproductive microbiome, and its association with the diseases.
Acknowledgments:
We thank Drs. Deborah J. Frank and Reilly Woodson (Department of Obstetrics and Gynecology, Washington University), Himanshi Bhatia, and Jennifer Brazill (InPrint, Washington University) for assistance with manuscript editing.
Funding:
RK received the funding from National Institutes of Health/National Institute of Child Health and Human Development (grants R00HD080742 and R01HD065435) and a Research Scholar Grant from the American Cancer Society.
Footnotes
Declaration of interest:
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
References
- Abbott JA, Hawe J, Clayton RD & Garry R 2003. The effects and effectiveness of laparoscopic excision of endometriosis: a prospective study with 2–5 year follow-up. Human Reproduction 18 1922–1927. [DOI] [PubMed] [Google Scholar]
- Adlercreutz H, Pulkkinen MO, Hämäläinen EK & Korpela JT 1984. Studies on the role of intestinal bacteria in metabolism of synthetic and natural steroid hormones. Journal of Steroid Biochemistry 20 217–229. [DOI] [PubMed] [Google Scholar]
- Alauddin M, Okumura T, Rajaxavier J, Khozooei S, Pöschel S, Takeda S, Singh Y, Brucker SY, Wallwiener D & Koch A 2020. Gut bacterial metabolite urolithin a decreases actin polymerization and migration in cancer cells. Molecular Nutrition & Food Research 64 1900390. [DOI] [PubMed] [Google Scholar]
- Amabebe E & Anumba DOC 2020. Female gut and genital tract microbiota-induced crosstalk and differential effects of short-chain fatty acids on immune sequelae. Frontiers in Immunology 2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato V, Papaleo E, Pasciuta R, Viganò P, Ferrarese R, Clementi N, Sanchez AM, Quaranta L, Burioni R & Ambrosi A 2020. Differential composition of vaginal microbiome, but not of seminal microbiome, is associated with successful intrauterine insemination in couples with idiopathic infertility: a prospective observational study. In Open Forum Infectious Diseases, p ofz525. Oxford University Press US. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam M, Raes J, Pelletier E, Le Paslier D & Yamada T 2011. Enterotypes of the human gut microbiome. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ata B, Yildiz S, Turkgeldi E, Brocal VP, Dinleyici EC, Moya A & Urman B 2019. The endobiota study: comparison of vaginal, cervical and gut microbiota between women with stage 3/4 endometriosis and healthy controls. Scientific Reports 9 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhed F, Ley RE, Sonnenburg JL, Peterson DA & Gordon JI 2005. Host-bacterial mutualism in the human intestine. Science 307 1915–1920. [DOI] [PubMed] [Google Scholar]
- Baker JM, Al-Nakkash L & Herbst-Kralovetz MM 2017. Estrogen–gut microbiome axis: physiological and clinical implications. Maturitas 103 45–53. [DOI] [PubMed] [Google Scholar]
- Blander JM, Longman RS, Iliev ID, Sonnenberg GF & Artis D 2017. Regulation of inflammation by microbiota interactions with the host. Nature Immunology 18 851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisciano G, Florian F, D’Eustacchio A, Stanković D, Ricci G, De Seta F & Comar M 2017. Subclinical alteration of the cervical–vaginal microbiome in women with idiopathic infertility. Journal of Cellular Physiology 232 1681–1688. [DOI] [PubMed] [Google Scholar]
- Cao Y, Jiang C, Jia Y, Xu D & Yu Y 2020. Letrozole and the traditional Chinese medicine, Shaofu Zhuyu decoction, reduce endometriotic disease progression in rats: A potential role for gut microbiota. Evidence-Based Complementary and Alternative Medicine 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadchan SB, Cheng M, Parnell LA, Yin Y, Schriefer A, Mysorekar IU & Kommagani R 2019. Antibiotic therapy with metronidazole reduces endometriosis disease progression in mice: a potential role for gut microbiota. Human Reproduction 34 1106–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadchan SB, Popli P, Ambati CR, Tycksen E, Han SJ, Bulun SE, Putluri N, Biest SW & Kommagani R 2021. Gut microbiota–derived short-chain fatty acids protect against the progression of endometriosis. Life Science Alliance 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CY, Chiang AJ, Lai MT, Yan MJ, Tseng CC, Lo LC, Wan L, Li CJ, Tsui KH, Chen CM, Hwang T, Tsai FJ & Sheu JJ 2022. A More Diverse Cervical Microbiome Associates with Better Clinical Outcomes in Patients with Endometriosis: A Pilot Study. Biomedicines, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao X, Liu Y, Fan Q, Shi H, Wang S & Lang J 2021. The role of the vaginal microbiome in distinguishing female chronic pelvic pain caused by endometriosis/adenomyosis. Ann Transl Med, 9, 771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Song X, Wei W, Zhong H, Dai J, Lan Z, Li F, Yu X, Feng Q, Wang Z, Xie H, Chen X, Zeng C, Wen B, Zeng L, Du H, Tang H, Xu C, Xia Y, Xia H, Yang H, Wang J, Wang J, Madsen L, Brix S, Kristiansen K, Xu X, Li J, Wu R & Jia H 2017. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat Commun, 8, 875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassaing B, Aitken JD, Gewirtz AT & Vijay-Kumar M 2012. Gut microbiota drives metabolic disease in immunologically altered mice. Advances in Immunology 116 93–112. [DOI] [PubMed] [Google Scholar]
- Colón-Díaz M, Báez-Vega P, García M, Ruiz A, Monteiro JB, Fourquet J, Bayona M, Alvarez-Garriga C, Achille A & Seto E 2012. HDAC1 and HDAC2 are differentially expressed in endometriosis. Reproductive Sciences 19 483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl C, Stanislawski M, Iszatt N, Mandal S, Lozupone C, Clemente JC, Knight R, Stigum H & Eggesbø M 2017. Gut microbiome of mothers delivering prematurely shows reduced diversity and lower relative abundance of Bifidobacterium and Streptococcus. PloS One 12 e0184336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias-Oliveira J, Leite JA, Pereira ÍS, Guimarães JB, Manso GM da C, Silva JS, Tostes RC & Carlos D 2020. NLR and intestinal dysbiosis-associated inflammatory illness: drivers or dampers? Frontiers in Immunology 11 1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Jin Y, Chen G, Ma X & Zhang L 2021. Gut microbiota dysbiosis drives the development of colorectal cancer. Digestion 102 508–515. [DOI] [PubMed] [Google Scholar]
- Flores R, Shi J, Fuhrman B, Xu X, Veenstra TD, Gail MH, Gajer P, Ravel J & Goedert JJ 2012. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. Journal of Translational Medicine 10 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes JD, Van Domselaar G & Bernstein CN 2016. The gut microbiota in immune-mediated inflammatory diseases. Frontiers in Microbiology 7 1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franasiak JM & Scott RT Jr 2015. Reproductive tract microbiome in assisted reproductive technologies. Fertility and Sterility 104 1364–1371. [DOI] [PubMed] [Google Scholar]
- Friedman J & Alm EJ 2012. Inferring correlation networks from genomic survey data. [DOI] [PMC free article] [PubMed]
- Gagliardi A, Totino V, Cacciotti F, Iebba V, Neroni B, Bonfiglio G, Trancassini M, Passariello C, Pantanella F & Schippa S 2018. Rebuilding the gut microbiota ecosystem. International Journal of Environmental Research and Public Health 15 1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Beltran C, Malpique R, Carbonetto B, González-Torres P, Henares D, Brotons P, Muñoz-Almagro C, López-Bermejo A, de Zegher F & Ibáñez L 2021. Gut microbiota in adolescent girls with polycystic ovary syndrome: Effects of randomized treatments. Pediatric Obesity 16 e12734. [DOI] [PubMed] [Google Scholar]
- Green KA, Zarek SM & Catherino WH 2015. Gynecologic health and disease in relation to the microbiome of the female reproductive tract. Fertility and Sterility 104 1351–1357. [DOI] [PubMed] [Google Scholar]
- Gujral P, Mahajan V, Lissaman AC & Ponnampalam AP 2020. Histone acetylation and the role of histone deacetylases in normal cyclic endometrium. Reproductive Biology and Endocrinology 18 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Qi Y, Yang X, Zhao L, Wen S, Liu Y & Tang L 2016. Association between polycystic ovary syndrome and gut microbiota. PloS One 11 e0153196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantschel J, Weis S, Schäfer K-H, Menger MD, Kohl M, Egert M & Laschke MW 2019. Effect of endometriosis on the fecal bacteriota composition of mice during the acute phase of lesion formation. PLoS One 14 e0226835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandes C, Silveira P, Rodrigues Sereia AF, Christoff AP, Mendes H, Valter De Oliveira LF & Podgaec S 2020. Microbiome Profile of Deep Endometriosis Patients: Comparison of Vaginal Fluid, Endometrium and Lesion. Diagnostics (Basel), 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F & Li Y 2020. Role of gut microbiota in the development of insulin resistance and the mechanism underlying polycystic ovary syndrome: a review. Journal of Ovarian Research 13 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltunen H, Collado MC, Ollila H, Kolari T, Tölkkö S, Isolauri E, Salminen S & Rautava S 2021. Spontaneous preterm delivery is reflected in both early neonatal and maternal gut microbiota. Pediatric Research 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MK & Alobaidi AHA 2021. Evaluation of the Role of Ghrelin and Leptin as Biochemical Markers in Female with Polycystic Ovarian Syndrome. Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Inflammatory and Anti-Allergy Agents) 20 373–379. [DOI] [PubMed] [Google Scholar]
- Ito M, Watanabe K, Maruyama T, Mori T, Niwa K, Chow S & Takeyama H 2019. Enrichment of bacteria and alginate lyase genes potentially involved in brown alga degradation in the gut of marine gastropods. Scientific Reports 9 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson D, Moore K, Gunderson C, Rowland M, Austin R, Honap TP, Xu J, Warinner C, Sankaranarayanan K & Lewis CM Jr 2021. Shifts in gut and vaginal microbiomes are associated with cancer recurrence time in women with ovarian cancer. PeerJ 9 e11574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang G-U, Jung D-R, Lee YH, Jeon SY, Han HS, Chong GO & Shin J-H 2020. Dynamics of Fecal microbiota with and without invasive cervical cancer and its application in early diagnosis. Cancers 12 3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley ST, Skarra DV, Rivera AJ & Thackray VG 2016. The gut microbiome is altered in a letrozole-induced mouse model of polycystic ovary syndrome. PloS One 11 e0146509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kho ZY & Lal SK 2018. The human gut microbiome–a potential controller of wellness and disease. Frontiers in Microbiology 1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MC, Lee S, Park JK, Park J, Lee D, Park J, Kim B-Y, Cho MS, Kim T-Y & Park HY 2021. Effects of ID-HWS1000 on the Perception of Bowel Activity and Microbiome in Subjects with Functional Constipation: A Randomized, Double-Blind Placebo-Controlled Study. Journal of Medicinal Food 24 883–893. [DOI] [PubMed] [Google Scholar]
- Li Z, Quan G, Jiang X, Yang Y, Ding X, Zhang D, Wang X, Hardwidge PR, Ren W & Zhu G 2018. Effects of metabolites derived from gut microbiota and hosts on pathogens. Frontiers in Cellular and Infection Microbiology 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D, Leung RK-K, Guan W & Au WW 2018. Involvement of gut microbiome in human health and disease: brief overview, knowledge gaps and research opportunities. Gut Pathogens 10 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, Di N, Li L & Yang D 2021. Gut microbiota alterations reveal potential gut–brain axis changes in polycystic ovary syndrome. Journal of Endocrinological Investigation 44 1727–1737. [DOI] [PubMed] [Google Scholar]
- Lindheim L, Bashir M, Münzker J, Trummer C, Zachhuber V, Pieber TR, Gorkiewicz G & Obermayer-Pietsch B 2016. The salivary microbiome in polycystic ovary syndrome (PCOS) and its association with disease-related parameters: a pilot study. Frontiers in Microbiology 7 1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindheim L, Bashir M, Münzker J, Trummer C, Zachhuber V, Leber B, Horvath A, Pieber TR, Gorkiewicz G & Stadlbauer V 2017. Alterations in gut microbiome composition and barrier function are associated with reproductive and metabolic defects in women with polycystic ovary syndrome (PCOS): a pilot study. PloS One 12 e0168390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Zhang C, Shi Y, Zhang F, Li L, Wang X, Ling Y, Fu H, Dong W & Shen J 2017. Dysbiosis of gut microbiota associated with clinical parameters in polycystic ovary syndrome. Frontiers in Microbiology 8 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Fu Q, Li T, Shao K, Zhu X, Cong Y & Zhao X 2022. Gut microbiota and butyrate contribute to nonalcoholic fatty liver disease in premenopause due to estrogen deficiency. Plos One 17 e0262855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C & Knight R 2005. UniFrac: a new phylogenetic method for comparing microbial communities. Applied and Environmental Microbiology 71 8228–8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C, Hamady M & Knight R 2006. UniFrac–an online tool for comparing microbial community diversity in a phylogenetic context. BMC Bioinformatics 7 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F, Wei J, Zhong Y, Feng Y, Ma B, Xiong Y, Wei K, Tan B & Chen T 2022. Antibiotic Therapy and Vaginal Microbiota Transplantation Reduce Endometriosis Disease Progression in Female Mice via NF-kappaB Signaling Pathway. Front Med (Lausanne), 9, 831115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu N, Li M, Lei H, Jiang X, Tu W, Lu Y & Xia D 2017. Butyric acid regulates progesterone and estradiol secretion via cAMP signaling pathway in porcine granulosa cells. The Journal of Steroid Biochemistry and Molecular Biology 172 89–97. [DOI] [PubMed] [Google Scholar]
- Lüll K, Arffman RK, Sola-Leyva A, Molina NM, Aasmets O, Herzig K-H, Plaza-Díaz J, Franks S, Morin-Papunen L & Tapanainen JS 2021. The gut microbiome in polycystic ovary syndrome and its association with metabolic traits. The Journal of Clinical Endocrinology & Metabolism 106 858–871. [DOI] [PubMed] [Google Scholar]
- Mehedintu C, Plotogea MN, Ionescu S & Antonovici M 2014. Endometriosis still a challenge. Journal of Medicine and Life 7 349. [PMC free article] [PubMed] [Google Scholar]
- Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM & Hunter DJ 2004. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. American Journal of Epidemiology 160 784–796. [DOI] [PubMed] [Google Scholar]
- Ni Z, Sun S, Bi Y, Ding J, Cheng W, Yu J, Zhou L, Li M & Yu C 2020. Correlation of fecal metabolomics and gut microbiota in mice with endometriosis. American Journal of Reproductive Immunology 84 e13307. [DOI] [PubMed] [Google Scholar]
- Ni Z, Ding J, Zhao Q, Cheng W, Yu J, Zhou L, Sun S & Yu C 2021. Alpha-linolenic acid regulates the gut microbiota and the inflammatory environment in a mouse model of endometriosis. American Journal of Reproductive Immunology 86 e13471. [DOI] [PubMed] [Google Scholar]
- Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W & Pettersson S 2012. Host-gut microbiota metabolic interactions. Science 336 1262–1267. [DOI] [PubMed] [Google Scholar]
- Ouyang J, Lin J, Isnard S, Fombuena B, Peng X, Marette A, Routy B, Messaoudene M, Chen Y & Routy J-P 2020. The bacterium Akkermansia muciniphila: a sentinel for gut permeability and its relevance to HIV-related inflammation. Frontiers in Immunology 11 645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotta AR, Borrelli GM, Martins CO, Kallas EG, Sanabani SS, Griffith LG, Alm EJ & Abrao MS 2020. The vaginal microbiome as a tool to predict rASRM stage of disease in endometriosis: a pilot study. Reproductive Sciences 27 1064–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plottel CS & Blaser MJ 2011. Microbiome and malignancy. Cell Host & Microbe 10 324–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popli P, Richters MM, Chadchan SB, Kim TH, Tycksen E, Griffith O, Thaker PH, Griffith M & Kommagani R 2020. Splicing factor SF3B1 promotes endometrial cancer progression via regulating KSR2 RNA maturation. Cell Death & Disease 11 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Yun C, Sun L, Xia J, Wu Q, Wang Y, Wang L, Zhang Y, Liang X & Wang L 2019. Gut microbiota–bile acid–interleukin-22 axis orchestrates polycystic ovary syndrome. Nature Medicine 25 1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Yun C, Pang Y & Qiao J 2021. The impact of the gut microbiota on the reproductive and metabolic endocrine system. Gut Microbes 13 1894070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley EMM 2013. Gut bacteria in health and disease. Gastroenterology & Hepatology 9 560. [PMC free article] [PubMed] [Google Scholar]
- Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, Mcculle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L & Forney LJ 2011. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A, 108 Suppl 1, 4680–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A & Mele MC 2019. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 7 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizk MG & Thackray VG 2021. Intersection of polycystic ovary syndrome and the gut microbiome. Journal of the Endocrine Society 5 bvaa177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan J, Ni Z, Cheng W, Zhou L, Zhai D, Sun S & Yu C 2021. Gut microbiota imbalance and its correlations with hormone and inflammatory factors in patients with stage 3/4 endometriosis. Archives of Gynecology and Obstetrics 304 1363–1373. [DOI] [PubMed] [Google Scholar]
- Sims TT, Colbert LE, Zheng J, Medrano AYD, Hoffman KL, Ramondetta L, Jazaeri A, Jhingran A, Schmeler KM & Daniel CR 2019. Gut microbial diversity and genus-level differences identified in cervical cancer patients versus healthy controls. Gynecologic Oncology 155 237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Zogg H, Wei L, Bartlett A, Ghoshal UC, Rajender S & Ro S 2021. Gut microbial dysbiosis in the pathogenesis of gastrointestinal dysmotility and metabolic disorders. Journal of Neurogastroenterology and Motility 27 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small W Jr, Bacon MA, Bajaj A, Chuang LT, Fisher BJ, Harkenrider MM, Jhingran A, Kitchener HC, Mileshkin LR & Viswanathan AN 2017. Cervical cancer: a global health crisis. Cancer 123 2404–2412. [DOI] [PubMed] [Google Scholar]
- Smolarz B, Szyłło K & Romanowicz H 2021. Endometriosis: Epidemiology, Classification, Pathogenesis, Treatment and Genetics (Review of Literature). International Journal of Molecular Sciences 22 10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson A, Brunkwall L, Roth B, Orho-Melander M & Ohlsson B 2021. Associations between endometriosis and gut microbiota. Reproductive Sciences 28 2367–2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaishi H, Matsuki T, Nakazawa A, Takada T, Kado S, Asahara T, Kamada N, Sakuraba A, Yajima T & Higuchi H 2008. Imbalance in intestinal microflora constitution could be involved in the pathogenesis of inflammatory bowel disease. International Journal of Medical Microbiology 298 463–472. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Mori T, Ito F, Koshiba A, Takaoka O, Kataoka H, Maeda E, Okimura H, Mori T & Kitawaki J 2017. Exacerbation of endometriosis due to regulatory T-cell dysfunction. The Journal of Clinical Endocrinology & Metabolism 102 3206–3217. [DOI] [PubMed] [Google Scholar]
- Torres PJ, Siakowska M, Banaszewska B, Pawelczyk L, Duleba AJ, Kelley ST & Thackray VG 2018. Gut microbial diversity in women with polycystic ovary syndrome correlates with hyperandrogenism. The Journal of Clinical Endocrinology & Metabolism 103 1502–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremellen K & Pearce K 2012. Dysbiosis of Gut Microbiota (DOGMA)–a novel theory for the development of Polycystic Ovarian Syndrome. Medical Hypotheses 79 104–112. [DOI] [PubMed] [Google Scholar]
- Tu Y, Zheng G, Ding G, Wu Y, Xi J, Ge Y, Gu H, Wang Y, Sheng J & Liu X 2020. Comparative analysis of lower genital tract microbiome between PCOS and healthy women. Frontiers in Physiology 1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Wang Q, Zhao J, Gong L, Zhang Y, Wang X & Yuan Z 2019. Altered diversity and composition of the gut microbiome in patients with cervical cancer. AMB Express 9 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee BA, Thomas M, Sweeney EL, Frentiu FD, Samios M, Ravel J, Gajer P, Myers G, Timms P & Allan JA 2018. A retrospective pilot study to determine whether the reproductive tract microbiota differs between women with a history of infertility and fertile women. Australian and New Zealand Journal of Obstetrics and Gynaecology 58 341–348. [DOI] [PubMed] [Google Scholar]
- Wei C-H, Chang R, Wan YH, Hung Y-M & Wei JC-C 2021. Endometriosis and New-Onset Coronary Artery Disease in Taiwan: A Nationwide Population-Based Study. Frontiers in Medicine 8 846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Zhang X, Tang H, Zeng L & Wu R 2020. Microbiota composition and distribution along the female reproductive tract of women with endometriosis. Ann Clin Microbiol Antimicrob, 19, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Li D, Zhang Z, Sun H, An M & Wang G 2018. Endometriosis induces gut microbiota alterations in mice. Human Reproduction 33 607–616. [DOI] [PubMed] [Google Scholar]
- Zarei E, Binabaj MM, Zadeh FM, Bakhshandeh Nosrat S, Veghari G & Mansourian A 2021. Kisspeptin levels in relation to sex hormone profile among PCOS patients. Irish Journal of Medical Science (1971-) 1–6. [DOI] [PubMed] [Google Scholar]
- Zhang W, Ma C, Xie P, Zhu Q, Wang X, Yin Y & Kong X 2019. Gut microbiota of newborn piglets with intrauterine growth restriction have lower diversity and different taxonomic abundances. Journal of Applied Microbiology 127 354–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Liu Y, Zhou X, Zhao R & Wang H 2020. Applications of CRISPR-Cas9 in gynecological cancer research. Clinical Genetics 97 827–834. [DOI] [PubMed] [Google Scholar]
- Zondervan KT, Rahmioglu N, Morris AP, Nyholt DR, Montgomery GW, Becker CM & Missmer SA 2016. Beyond endometriosis GWAS: From Genomics to Phenomics to the Patient Europe PMC Funders Group. Semin. Reprod. Med 34 242–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zondervan KT, Becker CM & Missmer SA 2020. Endometriosis. New England Journal of Medicine 382 1244–1256. (doi: 10.1056/NEJMra1810764) [DOI] [PubMed] [Google Scholar]


