Abstract
Background:
Acute otitis media (AOM) is the most common indication for antibiotics in children. The associated organism can influence the likelihood of antibiotic benefit and optimal treatment. Nasopharyngeal polymerase chain reaction can effectively exclude the presence of organisms in middle-ear fluid. We explored the potential cost-effectiveness and reduction in antibiotics with nasopharyngeal rapid diagnostic testing (RDT) to direct AOM management.
Methods:
We developed 2 algorithms for AOM management based on nasopharyngeal bacterial otopathogens. The algorithms provide recommendations on prescribing strategy (ie, immediate, delayed, or observation) and antimicrobial agent. The primary outcome was the incremental cost-effectiveness ratio (ICER) expressed as cost per quality-adjusted life day (QALD) gained. We used a decision-analytic model to evaluate the cost-effectiveness of the RDT algorithms compared to usual care from a societal perspective and the potential reduction in annual antibiotics used.
Results:
An RDT algorithm that used immediate prescribing, delayed prescribing, and observation based on pathogen (RDT-DP) had an ICER of $1,336.15 per QALD compared with usual care. At an RDT cost of $278.56, the ICER for RDT-DP exceeded the willingness to pay threshold; however, if the RDT cost was <$212.10, the ICER was below the threshold. The use of RDT was estimated to reduced annual antibiotic use, including broad-spectrum antimicrobial use, by 55.7% ($4.7 million for RDT vs $10.5 million for usual care).
Conclusion:
The use of a nasopharyngeal RDT for AOM could be cost-effective and substantially reduce unnecessary antibiotic use. These iterative algorithms could be modified to guide management of AOM as pathogen epidemiology and resistance evolve.
Acute otitis media (AOM) is the most common reason antibiotics are prescribed to children in the United States; it affects >60% of children by 3 years of age. 1–3 Up to 85% of infections will self-resolve, 4,5 but most children with AOM (>95%) 6 are prescribed an antibiotic, resulting in substantial unnecessary antibiotic use. The overuse of antibiotics promotes the development of antibiotic-resistant organisms, which is increasingly common among otopathogens. 7,8 Additionally, >25% of children who are prescribed an antibiotic report an antibiotic-associated adverse drug event (ADE), 9 and antibiotic use increases the risk for Clostridioides difficile infection and may be associated with chronic diseases later in life. 10–12 Although AOM is typically described as a single entity, it is caused by several different pathogens including respiratory viruses, Streptococcus pneumoniae, Haemophiles influenzae, and Moraxella catarrhalis. 7,13,14 The associated otopathogen has important implications for management because each one has a different severity of infection and likelihood of resolving without an antibiotic (Table 1). 7,13,15–20 Additionally, the optimal antibiotic agent differs between otopathogens based on β-lactamase production. 7 Unfortunately, no clinical features can reliably distinguish between causative organisms, and otopathogens are not routinely tested for in clinical practice. Thus, national recommendations take a one-size-fits-most approach for AOM management. 21
Table 1.
Parameter Values Used in the Decision-Analytic Model
| Variable | Baseline | Low | High | Reference(s) |
|---|---|---|---|---|
| Probabilities | ||||
| Associated organism | Kaur et al
7
Kaur et al 26 Yatsyshina et al 27 Wald et al 29 Casey et al 55 Frost et al 56 |
|||
| Bacterial pathogen detected a | 0.88 | 0.8 | 1.0 | |
| Haemophiles influenzae | 0.37 | 0.21 | 0.60 | |
| Moraxella catarrhalis | 0.15 | 0.12 | 0.57 | |
| Streptococcus pneumoniae | 0.29 | 0.15 | 0.38 | |
| No organism present | 0.12 | 0.083 | 0.20 | |
| Prescription types, usual care | ||||
| Agent | ||||
| Amoxicillin | 0.566 | N/A | N/A | Frost et al
57
McGrath et al 58 |
| Broad-spectrum (non-amoxicillin) | 0.434 | N/A | N/A | Frost et al
57
McGrath et al 58 |
| Prescription type | ||||
| Immediate | 0.95 | N/A | N/A | Froom et al
6
Frost et al 57 Frost et al 59 Norlin et al 60 |
| Delayed | 0.025 | N/A | N/A | Calculated |
| Observation (no antibiotic) | 0.025 | N/A | N/A | Calculated |
| Cure and failure rates | ||||
| Delayed cure | 0.69 | 0.47 | 0.755 | Chao et al
32
Mas-Dalmau et al 34 McCormick et al 35 Siegel et al 36 Spurling et al 37 Hoberman et al 38 |
| Observation cure | 0.81 | 0.66 | 0.81 | Chao et al
32
Mas-Dalmau et al 34 McCormick et al 35 Siegel et al 36 Spurling et al 37 Hoberman et al 38 |
| Amoxicillin failure | 0.037 | 0.017 | 0.055 | Gerber et al
9
Frost et al 57 |
| Broad-spectrum antibiotic failure | 0.046 | 0.046 | 0.1129 | Gerber et al
9
Frost et al 56 Frost et al 57 |
| Adverse drug events and complications | ||||
| Parent reported | ||||
| Narrow-spectrum antibiotic (amoxicillin) | 0.251 | No range | No Range | Gerber et al 9 |
| Broad-spectrum antibiotic (amoxicillin-clavulanate) | 0.356 | No range | No Range | Gerber et al 9 |
| Requires office visit | 0.031 | No range | No Range | Gerber et al 9 |
| Mastoiditis, immediate treatment | 0.000018 | 0.000015 | 0.000021 | Shaikh et al 42 |
| Mastoiditis, delayed treatment | 0.000038 | 0.000032 | 0.000044 | Shaikh et al 42 |
| Costs | ||||
| Direct costs | ||||
| Drugsb | ||||
| Amoxicillin (90 mg/kg/day) | 10.97 | 10.91 | 11.03 | Redbook |
| Broad-spectrum antibiotic | 34.28 | 26.72 | 41.90 | Redbook, calculated |
| Diagnostic testing | ||||
| Swab kit + rapid PCR test | 278.56 | 41.42 | 286.51 | Fischer Scientific, CMS |
| Indirect costs | ||||
| Diapers (12 individual) | 4.20 | 2.40 | 6.24 | Target, Walmart Calculated |
| Barrier cream | 2.50 | 1.25 | 3.75 | Target, Walmart |
| Topical antifungal cream (Nystatin) | 6.42 | 3.21 | 9.63 | Redbook |
| Benadryl | 6.39 | 5.49 | 11.32 | Target,Walmart |
| Cost of work lost (per day) c | 239.68 | N/A | N/A | BLS |
| Hospitalization for mastoiditis | 7487.00 | 3744.00 | 11231.00 | Shaikh et al 42 |
| Antibiotic resistance (per treatment episode) | 13.00 | 3.00 | 95.00 | Michaelidis et al 41 |
| Office visit cost | ||||
| Insurance payer costs | 97.16 | N/A | N/A | CMS |
| Patient out-of-pocket costs | 24.29 | N/A | N/A | CMS |
| Disutility values | ||||
| Acute mastoiditis | 0.56 | 0.36 | 0.76 | Shaikh et al
42
Oh et al 43 Coco 44 |
| Acute otitis media | 0.21 | 0.01 | 0.41 | Shaikh et al
42
Oh et al 43 Coco 44 |
| ADE, amoxicillin | 0.092 | 0.063 | 0.12 | Gerber et al,
9
Shaikh et al 42 Oh et al 43 Coco 44 Calculated |
| ADE, broad-spectrum antibiotic | 0.127 | 0.09 | 0.17 | Gerber et al,
9
Shaikh et al 42 Oh et al 43 Coco 44 Calculated |
| Treatment failure d | 0.337 | 0.01 | 0.41 | Shaikh et al
42
Oh et al 43 Coco 44 |
Note. AOM, acute otitis media; ADE, adverse drug effect; CMS, Centers for Medicare and Medicaid Services; BLS, Bureau of Labor Statistics; PCR, polymerase chain reaction assay.
Includes pathogens listed below. Polymicrobial infection rates calculated from referenced studies.
The composite cost of broad-spectrum antibiotics was calculated from Redbook values for amoxicillin-clavulanate, cefdinir, and azithromycin based on US prescribing distribution of each drug.
Assumed that for each episode of AOM, one 8-hour work-day productivity was lost.
Disutility calculated as a total of disutility of another episode of AOM with ADE for broad-spectrum antibiotic use (does not include mastoiditis complication).
Ideally, clinicians would diagnose AOM using stringent criteria 21,22 ; they would prescribe antibiotics only for children who are likely to benefit, and they would use the narrowest-spectrum antibiotic needed to treat the infection. However, national treatment guidelines and antimicrobial stewardship programs have not resulted in a substantial reduction in antibiotic prescribing for AOM on a national scale. 23 For other infections, such as pharyngitis, the use of rapid diagnostic tests (RDTs) has significantly reduced unnecessary antibiotic use as has individualized care based on the organism(s) detected. 24,25 An RDT for AOM could prevent unnecessary antibiotic use for children while assuring that children likely to benefit from an antibiotic receive one. Additionally, it could ensure that the optimal antibiotic agent is prescribed. Although tympanocentesis is not routinely performed on children with AOM in clinical practice, organisms detected in the nasopharynx have a high negative predictive value (>92%) for organisms in the middle ear. Therefore, nasopharynx testing could effectively exclude the presence of organisms during AOM episodes. 26,27
We propose 2 diagnostic algorithms for the management of AOM in children using rapid nasopharyngeal polymerase chain reaction (PCR). We evaluated the cost-effectiveness and potential annual reduction in antibiotic use for each algorithm compared to usual care.
Methods
Design of the study
We used a decision analytic model to estimate the costs and utilities of 2 nasopharyngeal RDT-based management strategies for uncomplicated AOM in children compared to usual care (Fig. 1). We also estimated the reduction in annual antibiotic use with each strategy. In accordance with the recommendations for the conduct of cost-effectiveness analyses, we evaluated outcomes from a societal perspective, which included costs associated with loss of work by parents and antimicrobial resistance. 28
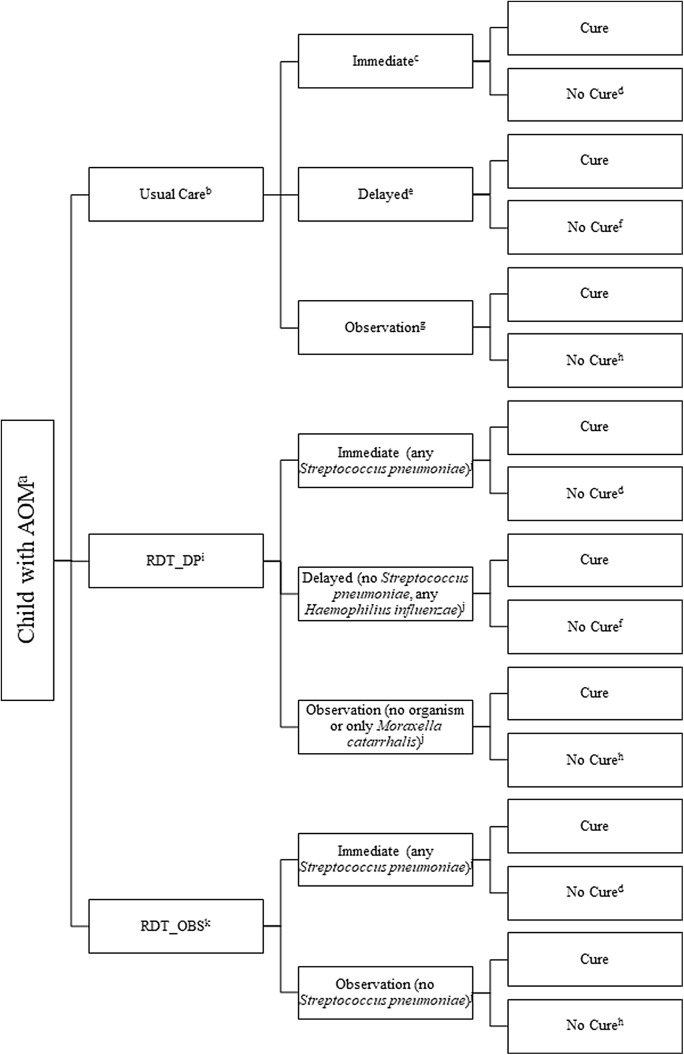
Fig. 1.
Flow diagram of usual care and rapid diagnostic testing algorithms for management of uncomplicated acute otitis media.
aAcute otitis media.
bBased on US prescribing rates.
cAntibiotic prescription to take right away.
dSignifies that the child requires a healthcare visit as well as antibiotic treatment, narrow or broad spectrum (first time or additional agent), and may additionally develop mastoiditis.
entibiotic prescription to take only if the child worsens or does not improve within 72 hours.
fSignifies that the child requires antibiotic treatment, narrow or broad-spectrum (first time or additional agent), fills the delays prescription, and may additionally develops mastoiditis.
gManagement with pain control only and no antibiotic prescription.
hSignifies that the child requires antibiotic treatment, narrow or broad spectrum (first time or additional agent), contacts clinician for a prescription, and may additionally develop mastoiditis.
iRapid diagnostic test with immediate prescribing, delayed prescribing or observation based on otopathogen(s).
jAny Streptococcus pneumoniae, no Moraxella catarrhalis or any β-lactamase–producing Haemophilius influenzae is initially treated with amoxicillin. Any Moraxella catarrhalis or β-lactamase–producing Haemophilius influenzae is treated with amoxicillin-clavulanate.
kRapid diagnostic test with immediate prescribing or observation (no delayed prescribing) based on otopathogen(s).
Comparator strategies
We used 2 nasopharyngeal PCR-based RDT algorithms to guide the AOM antibiotic prescribing strategy: (1) observation without an antibiotic, delayed prescription to fill and take if symptoms worsen or do not improve in 72 hours, or (2) an immediate amoxicillin or amoxicillin-clavulanate prescription to fill and take immediately (Fig. 1). 12 We assumed that the RDT would include testing for S. pneumoniae, H. influenzae, and M. catarrhalis, the 3 most common otopathogens, as well as sequences associated with β-lactamase production by H. influenzae. 7,13
The prescribing strategy was determined based on known severity and self-resolution rates of otopathogens, whereas the antibiotic agent was determined based on the likelihood of β-lactamase production by organism(s) detected (Supplementary Table 1 online). Because up to 40% of infections are polymicrobial, 26 we used a tier system to determine the optimal prescribing strategy and agent in these polymicrobial cases (Table 1). Proportions of AOM associated with each otopathogen were obtained from the literature. 7,26,27,29 The model was designed to be iterative so it could be updated as the proportion of infections caused by each otopathogen and resistance patterns change over time.
Decision model
We compared 3 treatment strategies in a hypothetical cohort of children aged 6 months–12 years with uncomplicated AOM. We defined uncomplicated AOM as AOM not associated with severe systemic symptoms, with recurrent disease requiring multiple antibiotic treatments, or with tympanic membrane perforation. We did not assess the cost-effectiveness for children with tympanostomy tubes, recurrent AOM, or other underlying medical conditions (eg, immunocompromised). In the primary analysis, we compared 3 strategies: (1) usual care; (2) the use of an RDT with immediate prescribing, delayed prescribing, or observation based on otopathogen(s) (RDT-DP); and (3) use of an RDT with immediate prescribing or observation (no delayed prescribing) based on otopathogen(s) (RDT-OBS). In the Supplementary Material, we also provide a comparative analysis of 2 additional AOM management strategies: (1) initial observation for all children, which is common in many European countries and (2) adherence to the 2013 American Academy of Pediatrics (AAP) guidelines (Supplementary Table 2). 21
Exploratory analyses were conducted to estimate costs associated with in-person versus phone or electronic follow-up for children managed by observation whose treatment failed. Given the high costs associated with in-person follow-up, we assumed in the final model that patients managed by observation whose treatment failed would primarily be prescribed an antibiotic via phone or electronic follow-up rather than an in-person office visit. We used a 30-day time horizon because most outcomes secondary to AOM occur during this time and because there are no significant differences in longer-term outcomes between placebo and antibiotic treatment. 30,31 Given the short time horizon, we did not discount costs or utilities. The outcomes considered within the model included cure (resolution of symptoms), clinical treatment failure (persistent symptoms after 3 days or worsening symptoms), and mastoiditis. We estimated clinical treatment failure rates for children managed with observation or a delayed prescription from prior clinical trials, 32–38 and we estimated the proportion of children that would qualify for initial watchful waiting with AAP guidelines using observation data and clinical trials. 39,40
The model and analyses were completed using Amua version 0.3.0 software.
Costs
We included direct and indirect costs including the costs of medications, follow-up office visits, diagnostic testing, ADE-associated costs (diapers, diphenhydramine, etc), mastoiditis, and lost productivity (Table 1). The cost of antimicrobial resistance was valued at $13 for every AOM episode that required antibiotics. 41 This cost included societal costs associated with growing antimicrobial resistance including increased hospitalizations from antimicrobial-resistant infections and the need for broader, non–first-line antibiotics in inpatient and outpatient settings. Because all strategies would have the same initial office visit cost these costs were not included in the model except in the cases of treatment failure. 21 Additional productivity losses were added for treatment failures. We did not include costs of antipyretics or analgesics because all children are expected to receive pain control, and clinical trials did not show a difference in analgesic use between children who received antibiotics or placebo. 39,42 We assumed that the test could be run on existing PCR platforms such as those used for rapid influenza and severe acute respiratory coronavirus virus 2 (SARS-CoV-2) testing, which are commonly used in emergency departments and outpatient clinics. Thus, we did include costs for additional capital investment. Finally, we assumed 100% uptake by health systems and providers. Because the model is iterative, capital costs and lower uptake could be incorporated into future analyses.
Quality of life
Quality of life was determined using quality-adjusted life days (QALD), with 0 representing death and 1 representing ideal health. Because the time horizon was 30 days, the maximum QALD for each child was 30. We estimated that each clinical treatment failure resulted in an additional 0.21 disutility. 42,43 We calculated a summary disutility value for each antibiotic agent based on associated ADE (Table 1). Finally, we estimated that mastoiditis resulted in an additional 0.56 disutility. 42,44
Statistical and sensitivity analysis
The primary outcome measured was the incremental cost-effectiveness ratio (ICER) expressed as cost per QALD. Secondary outcomes included (1) the cost at which an RDT would result in the ICER being below the willingness-to-pay threshold and (2) the estimated reduction in annual antibiotics used. We set the willingness-to-pay threshold at $274 per QALD. 45,46
We used deterministic and probabilistic sensitivity analyses to evaluate the model’s results. 47 For the 1-way deterministic sensitivity analyses, we evaluated the results by changing the variables over the range of estimated values (Table 1). For the probabilistic sensitivity analyses, the variables were entered as probability distributions based on their values and were varied simultaneously across 10,000 iterations. We used β distributions for probability and utilities, and we used normal distributions for cost variables. We used a 1-way sensitivity analysis to determine the cost at which a diagnostic test would result in an ICER below the willingness-to-pay threshold and at which point it would equal the cost of usual care.
The study was reviewed and approved by the Colorado Multiple Institutional Review Board.
Results
The results of the cost-effectiveness analysis are shown in Table 2. The costs of each strategy listed in order of least costly to most costly, were as follows: usual care ($334.88), RDT-DP ($418.34) and RDT-OBS ($439.27). The primary driver of cost in the RDT strategies was the cost of testing, whereas the primary driver of cost for usual care was ADEs. RDT-DP incurred lower costs than RDT-OBS because RDT-DP avoided an extra day of disutility and productivity loss because these patients could simply fill the delayed prescription rather than needing to recontact the provider for a prescription. Disutility was similar between strategies: 0.12 for usual care, 0.06 for RDT-DP, and 0.09 for RDT-OBS. RDT-DP had an ICER of $1,336.15 per QALD compared with usual care and strongly dominated RDT-OBS.
Table 2.
Cost-Effectiveness of Rapid Diagnostic Testing Algorithms for Management of Acute Otitis Media Compared to Usual Care
| Analytic Components | Usual Care | RDT-DP | RDT-OBS |
|---|---|---|---|
| Total costs | 334.88 | 418.34 | 439.27 |
| Total QALDs lost in 30 d | 0.12 | 0.06 | 0.09 |
| Total QALDs remaining in 30 d | 29.88 | 29.94 | 29.91 |
| ICER (total cost per QALD in 30-d time horizon) comparing the 2 rapid test algorithms with usual care a | Reference | 1,336.15 | Dominated a |
Note. RDT-DP, rapid diagnostic test with immediate prescribing, delayed prescribing or observation based on otopathogen(s) (Fig. 1); RDT-OBS, rapid diagnostic test with immediate prescribing or observation, no delayed prescribing, based on otopathogen(s) (Fig. 1); QALD, quality-adjusted life days; ICER, incremental cost effectiveness ratio.
RDT-DP had an ICER of $1,336.15 per QALD compared with usual care and strongly dominated RDT-OBS.
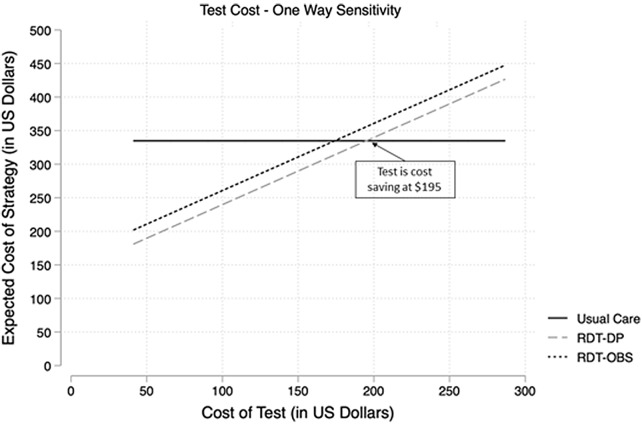
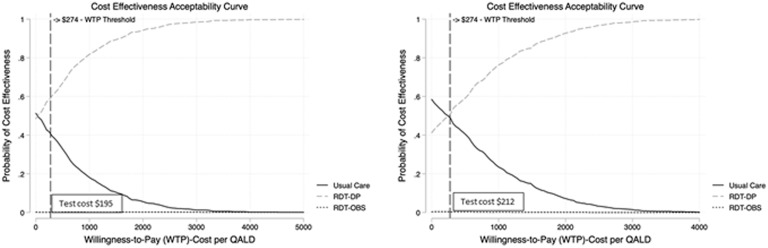
Using one-way sensitivity analyses, we sought to determine whether changes to the cost of the RDT affected the preferred strategy. At an RDT cost of $278.56, the ICER for RDT-DP exceeded the willingness-to-pay threshold. However, if the cost of the RDT was <$212.10, the ICER was below the willingness-to-pay threshold, and if the cost of the RDT was <$195.00, the RDT-DP was cost saving compared to usual care (Fig. 2). At a test cost of $195, RDT-DP was likely to be more cost-effective than usual care (Fig. 3).
Fig. 2.
One-way sensitivity analysis based on the cost of the rapid diagnostic test.
aRDT-DP, rapid diagnostic test with immediate prescribing, delayed prescribing or observation based on otopathogen(s).
bRDT-OBS, rapid diagnostic test with immediate prescribing or observation (no delayed prescribing) based on otopathogen(s).
Fig. 3.
Probabilistic sensitivity analysis at a rapid diagnostic test cost of US$212 and US$195.
aRDT-DP, rapid diagnostic test with immediate prescribing, delayed prescribing, or observation based on otopathogen(s).
bRDT-OBS, rapid diagnostic test with immediate prescribing or observation (no delayed prescribing) based on otopathogen(s).
Both RDT algorithms reduced predicted annual antibiotic use, including broad-spectrum antibiotic use, compared to usual care (Table 3). RDT-OBS resulted in the fewest antibiotic prescriptions taken (4.67 million, a 55.7% reduction) followed by RDT-DP (5.38 million, a 48.9% reduction) in comparison to usual care (10.5 million 2 ).
Table 3.
Estimated US Annual Antibiotic Use for Usual Care and Rapid Diagnostic Testing Algorithms
| Antibiotic Prescriptions | Usual Care a | RDT-DP | RDT-OBS |
|---|---|---|---|
| No. (%) | No. (%) | No. (%) | |
| Narrow spectrum, million b | 5.72 (54.3) | 3.50 (65.0) | 3.13 (67.0) |
| Broad spectrum, million c | 4.81 (45.7) | 1.89 (35.1) | 1.54 (33.0) |
| Total Antibiotics, million d | 10.53 | 5.38 | 4.67 |
Note. RDT-DP, rapid diagnostic test with immediate prescribing, delayed prescribing or observation based on otopathogen(s) (Fig. 1); RDT-OBS, rapid diagnostic test with immediate prescribing or observation (no delayed prescribing) based on otopathogen(s) (Fig. 1).
Based on US prescribing rates.
Amoxicillin.
Non-amoxicillin antibiotic.
Patients with clinical failure may have had >1 prescription for an antibiotic.
The use of initial observation for all children or complete adherence to the AAP guidelines would be cost saving compared to usual care or the use of RDT. Compared to initial observation for all children the ICER for RDT-DP ($44,789.7) exceeded the willingness-to-pay threshold (Supplementary Table 2).
Discussion
In this study, we have demonstrated that a nasopharyngeal RDT to guide management of AOM could be cost-effective compared to usual care if the cost of the RDT is <$212. RDT could also substantially reduce overall antibiotic use (57%) and broad-spectrum antibiotic use (68%). This approach has the potential to individualize care for AOM and to reduce antibiotic-associated morbidity and the development of antibiotic resistance among otopathogens, while assuring that children most likely to benefit from an antibiotic receive the appropriate agent.
Historically, a single first-line agent (amoxicillin) has been recommended for treatment of most children with AOM. 21 The increase in β-lactamase–producing organisms associated with AOM has prompted some to call for a change in the firstline agent to a broader-spectrum antibiotic (ie, amoxicillin-clavulanate) for most children. 48 Most children are currently prescribed an immediate antibiotic and 40% are prescribed a broad-spectrum antibiotic. 40 A change in guidelines that recommends first-line use of a broad-spectrum antibiotic would likely result in increased resistance, ADEs, and cost. 8,9,49 Fortunately, scientific advancement has rendered it feasible to identify the presence of organisms and resistance-associated genes quickly and reliably. A shift to an evidence-based RDT-guided therapy could reduce ambiguity around which bacterial pathogens are present, if treatment with immediate antibiotics is necessary, and reduce unnecessary costs and ADEs.
Previous studies have demonstrated that individualization of care based on otopathogens improved outcomes in pediatric AOM. 50 Unfortunately, these studies have relied on tympanocentesis, which has limited their generalizability and scalability because most clinicians are no longer trained in tympanocentesis and time constraints reduce its utility in routine practice. Although tympanocentesis more reliably detects otopathogens than nasopharyngeal testing, nasopharyngeal testing is likely to be more feasible in most care settings. In particular, nasopharyngeal testing can effectively exclude the presence of otopathogens in the middle-ear fluid >92% of the time. 26 In the minority of cases in which nasopharyngeal testing may not accurately detect pathogens that are present in the middle-ear fluid, the risk of complications from delayed antibiotic treatment is exceedingly low. 42 Approaches have been further limited using culture rather than PCR, which is expensive, time-consuming, and may yield less accurate results than PCR, particularly for fastidious organisms such as S. pneumoniae.
We previously demonstrated that the sensitivity of nasopharyngeal PCR for otopathogens compared to culture is >99%. 51 In addition, serum biomarkers have been suggested as a potential option for pathogen-directed therapy for AOM. 52 However, this would likely require capital investments from health systems, and it is not clear how antimicrobial resistance would be determined, though it is necessary to choose the appropriate agent, particularly for H. influenzae.
Finally, management of AOM should logically progress over time as AOM pathogens and resistance evolve. The use of an algorithm associated with an RDT would automatically help tailor management to local pathogen epidemiology and resistance patterns.
Although initial observation and complete adherence to the AAP guidelines are the most cost-effective approaches, they do not manifest in routine practice. Management guided by RDTs has improved care for other infections. For example, the use of RDTs, including molecular-based RDTs, to direct treatment of group A β-hemolytic streptococci (GAS) pharyngitis improved care by reducing unnecessary antibiotic use while assuring that patients likely to benefit were prescribed an antibiotic. 24,25 Similar to AOM, signs and symptoms of pharyngitis can be nonspecific, and accurately establishing the causative organism to appropriately direct antibiotic therapy using clinical criteria alone is difficult. 53 The use of an RDT could similarly guide management of AOM, albeit more complex than pharyngitis because multiple organisms are associated with AOM and antibiotic resistance is more prevalent among otopathogens than GAS.
Additional parallels exist between AOM and pharyngitis. Both conditions exhibit high carriage rates (eg, high carriage of M. catarrhalis in the nasopharynx). 26,54 In both scenarios, diagnostic testing is most effective at excluding the presence of organisms rather than predicting that organisms are causing disease. Thus, like pharyngitis, testing would still result in some overtreatment. Additionally, clinicians would similarly need to be able to select appropriate children for testing; thus, AOM diagnostic accuracy would remain important. Despite these challenges, the use of RDTs for GAS profoundly reduced unnecessary antibiotic use for pharyngitis. An AOM RDT has the potential to be a valuable stewardship tool that could be coupled with other stewardship interventions to reduce overall and/or broad-spectrum antibiotic use.
Several important practical aspects of a AOM RDT need to be addressed prior to implementation. First, at an estimated cost of $274, which aligns with commercially available respiratory viral molecular based tests, RDT is unlikely to be cost-effective using the current willingness-to-pay threshold. However, a modest reduction in price (23%, $212) meets the currently established threshold. Notably, this price is comparable to currently used molecular-based tests for GAS. The test would also need to have a quick turnaround time (optimally <30 minutes) to be useful in most outpatient settings. This turnaround time may be a challenge for smaller practices that typically send out samples for PCR testing. Given the complexity of AOM pathogenesis, clinicians would need to have an easy-to-use support tool to interpret RDT results to guide management. Finally, the test would ideally be used on currently available RDT platforms, such as those already used for SARS-CoV-2, influenza, or GAS testing to reduce the need for capital investment.
The strengths of this study include the creation of an iterative model for AOM that can easily be modified as epidemiology and resistance patterns change. We were able to address the polymicrobial nature of AOM by using an algorithm. We also included costs associated with antimicrobial resistance. In addition to evaluating the cost-effectiveness of RDT guided care, we determined the potential reduction in antibiotic use, which is important from antimicrobial stewardship and public health perspectives. We focused on a highly pragmatic aspect of care that included an estimated cost needed for an RDT to be cost-effective. To our knowledge, no prior research has evaluated the potential cost-effectiveness or reduction in antibiotic use with an RDT for AOM. We hope that this analysis will stimulate future studies and discussion on how we can potentially use an RDT to better guide appropriate management of AOM.
Our study also had several limitations. As with all cost-effectiveness evaluations, the results are subject to underlying assumptions. We assumed complete uptake of the algorithm and that all clinicians followed the algorithm. A formative study evaluating parent and clinician acceptability of potential RDT use for AOM would help to inform expected test uptake and antibiotic use with an RDT. We presumed no need for additional capital investment for testing given the widespread implementation of PCR-based platforms. Clinical settings without ready access to PCR-based RDTs, such as rural clinics or private practices, may not be able to adopt this strategy to guide management. We based antibiotic use with each prescribing strategy (ie, immediate antibiotic, delayed antibiotic, and observation) on pragmatic studies of fill rates with these strategies rather than calculating the likelihood of success of treatment by organism. Thus, we may have overestimated actual antibiotic use and costs. A call-in rather than in-person care strategy was assumed for all patients managed with observation whose treatment failed, which might not be appropriate for some patients. Additionally, we estimated a full day of lost productivity for patients whose clinical treatment with observation failed, which may have been an overestimate. Some variables were from single studies, and other variables had a wide range of values. Given the variation in variables, we completed sensitivity analyses to vary values throughout the range of estimates. Given the paucity of evidence on the risks of chronic medical conditions (eg, inflammatory bowel disease) associated with a single antibiotic course, we did not include costs and disutilities associated with chronic medical conditions. Finally, this study was designed to be exploratory to begin the discussion on how we move the field of AOM management forward. It was not designed to be a definitive study on best strategies for AOM management.
In conclusion, RDT could be a feasible mechanism to individualize care for AOM. In the era of increasing antimicrobial resistance, RDT should be explored as a potential patient-centered mechanism to improve care and reduce unnecessary antibiotic use.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Gerber Foundation or the National Institutes of Health.
Supplementary material
For supplementary material accompanying this paper visit http://doi.org/10.1017/ash.2023.127.
click here to view supplementary material
Financial support
In part, this research was supported by The Gerber Foundation. H.F. received salary support from the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (grant no. K23HD099925).
Conflicts of Interest
Drs. Frost and Sebastian are inventors on US provision patent 63/335,801 “Methods for Diagnosing and/or Treatment Otitis Media.” All other authors have no conflicts relevant to this article.
References
- 1. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA 2016;315:1864–1873. [DOI] [PubMed] [Google Scholar]
- 2. Hersh AL, Shapiro DJ, Pavia AT, Shah SS. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics 2011;128:1053–1061. [DOI] [PubMed] [Google Scholar]
- 3. Kaur R, Morris M, Pichichero ME. Epidemiology of acute otitis media in the postpneumococcal conjugate vaccine era. Pediatrics 2017;140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Glasziou PP, Del Mar CB, Sanders SL, Hayem M. Antibiotics for acute otitis media in children. Cochrane Database Syst Rev 2004:CD000219. [DOI] [PubMed]
- 5. Venekamp RP, Sanders SL, Glasziou PP, Del Mar CB, Rovers MM. Antibiotics for acute otitis media in children. Cochrane Database Syst Rev 2015:Cd000219. [DOI] [PubMed]
- 6. Froom J, Culpepper L, Green LA, et al. A cross-national study of acute otitis media: risk factors, severity, and treatment at initial visit. Report from the International Primary Care Network (IPCN) and the Ambulatory Sentinel Practice Network (ASPN). J Am Board Fam Pract 2001;14:406–417. [PubMed] [Google Scholar]
- 7. Kaur R, Fuji N, Pichichero ME. Dynamic changes in otopathogens colonizing the nasopharynx and causing acute otitis media in children after 13-valent (PCV13) pneumococcal conjugate vaccination during 2015–2019. Eur J Clin Microbiol Infect Dis 2022;41:37–44. [DOI] [PubMed] [Google Scholar]
- 8. Antibiotic resistance threats in the United States, 2019. Centers for Disease Control and Prevention website. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf. Published 2019. Accessed February 28, 2023.
- 9. Gerber JS, Ross RK, Bryan M, et al. Association of broad- vs narrow-spectrum antibiotics with treatment failure, adverse events, and quality of life in children with acute respiratory tract infections. JAMA 2017;318:2325–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miranda-Katz M, Parmar D, Dang R, Alabaster A, Greenhow TL. Epidemiology and risk factors for community associated Clostridioides difficile in children. J Pediatrics 2020;221:99–106. [DOI] [PubMed] [Google Scholar]
- 11. Horton DB, Scott FI, Haynes K, et al. Antibiotic exposure and juvenile idiopathic arthritis: a case–control study. Pediatrics 2015;136:e333–e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kronman MP, Zaoutis TE, Haynes K, Feng R, Coffin SE. Antibiotic exposure and IBD development among children: a population-based cohort study. Pediatrics 2012;130:e794–e803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Van Dyke MK, Pirçon JY, Cohen R, et al. Etiology of acute otitis media in children less than 5 years of age: a pooled analysis of 10 similarly designed observational studies. Pediatr Infect Dis J 2017;36:274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martin JM, Hoberman A, Shaikh N, et al. Changes over time in nasopharyngeal colonization in children under 2 years of age at the time of diagnosis of acute otitis media (1999–2014). Open Forum Infect Dis 2018;5:ofy036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Broides A, Dagan R, Greenberg D, Givon-Lavi N, Leibovitz E. Acute otitis media caused by Moraxella catarrhalis: epidemiologic and clinical characteristics. Clin Infect Dis 2009;49:1641–1647. [DOI] [PubMed] [Google Scholar]
- 16. Klein JO. Otitis media. Clin Infect Dis 1994;19:823–833. [DOI] [PubMed] [Google Scholar]
- 17. Leibovitz E, Satran R, Piglansky L, et al. Can acute otitis media caused by Haemophilus influenzae be distinguished from that caused by Streptococcus pneumoniae? Pediatr Infect Dis J 2003;22:509–515. [DOI] [PubMed] [Google Scholar]
- 18. Liu K, Kaur R, Almudevar A, Pichichero ME. Higher serum levels of interleukin 10 occur at onset of acute otitis media caused by Streptococcus pneumoniae compared to Haemophilus influenzae and Moraxella catarrhalis. Laryngoscope 2013;123:1500–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Palmu AA, Herva E, Savolainen H, Karma P, Mäkelä PH, Kilpi TM. Association of clinical signs and symptoms with bacterial findings in acute otitis media. Clin Infect Dis 2004;38:234–242. [DOI] [PubMed] [Google Scholar]
- 20. Polachek A, Greenberg D, Lavi-Givon N, et al. Relationship among peripheral leukocyte counts, etiologic agents, and clinical manifestations in acute otitis media. Pediatric Infect Dis J 2004;23:406–413. [DOI] [PubMed] [Google Scholar]
- 21. Lieberthal AS, Carroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics 2013;131:e964–e999. [DOI] [PubMed] [Google Scholar]
- 22. Shaikh N, Hoberman A, Rockette HE, Kurs-Lasky M. Development of an algorithm for the diagnosis of otitis media. Acad Pediatr 2012;12:214–218. [DOI] [PubMed] [Google Scholar]
- 23. King LM, Tsay SV, Hicks LA, Bizune D, Hersh AL, Fleming-Dutra K. Changes in outpatient antibiotic prescribing for acute respiratory illnesses, 2011 to 2018. Antimicrob Steward Healthc Epidemiol 2021;1:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luo R, Sickler J, Vahidnia F, Lee YC, Frogner B, Thompson M. Diagnosis and management of group a streptococcal pharyngitis in the United States, 2011–2015. BMC Infect Dis 2019;19:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maltezou HC, Tsagris V, Antoniadou A, et al. Evaluation of a rapid antigen detection test in the diagnosis of streptococcal pharyngitis in children and its impact on antibiotic prescription. J Antimicrob Chemother 2008;62:1407–1412. [DOI] [PubMed] [Google Scholar]
- 26. Kaur R, Czup K, Casey JR, Pichichero ME. Correlation of nasopharyngeal cultures prior to and at onset of acute otitis media with middle ear fluid cultures. BMC Infect Dis 2014;14:640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yatsyshina S, Mayanskiy N, Shipulina O, et al. Detection of respiratory pathogens in pediatric acute otitis media by PCR and comparison of findings in the middle ear and nasopharynx. Diagnost Microbiol Infect Dis 2016;85:125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA 2016;316:1093–1103. [DOI] [PubMed] [Google Scholar]
- 29. Wald ER, DeMuri GP. Antibiotic recommendations for acute otitis media and acute bacterial sinusitis: conundrum no more. Pediatr Infect Dis J 2018;37:1255–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mygind N, Meistrup-Larsen KI, Thomsen J, Thomsen VF, Josefsson K, Sørensen H. Penicillin in acute otitis media: a double-blind placebo-controlled trial. Clin Otolaryngol Allied Sci 1981;6:5–13. [DOI] [PubMed] [Google Scholar]
- 31. Le Saux N, Gaboury I, Baird M, et al. A randomized, double-blind, placebo-controlled noninferiority trial of amoxicillin for clinically diagnosed acute otitis media in children 6 months to 5 years of age. CMAJ 2005;172:335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chao JH, Kunkov S, Reyes LB, Lichten S, Crain EF. Comparison of two approaches to observation therapy for acute otitis media in the emergency department. Pediatrics 2008;121:e1352–e1356. [DOI] [PubMed] [Google Scholar]
- 33. Little P, Moore M, Kelly J, et al. Delayed antibiotic prescribing strategies for respiratory tract infections in primary care: pragmatic, factorial, randomised controlled trial. BMJ 2014;348:g1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mas-Dalmau G, Villanueva López C, Gorrotxategi P, et al. Delayed antibiotic prescription for children with respiratory infections: a randomized trial. Pediatrics 2021;147. [DOI] [PubMed] [Google Scholar]
- 35. McCormick DP, Chonmaitree T, Pittman C, et al. Nonsevere acute otitis media: a clinical trial comparing outcomes of watchful waiting versus immediate antibiotic treatment. Pediatrics 2005;115:1455–1465. [DOI] [PubMed] [Google Scholar]
- 36. Siegel RM, Kiely M, Bien JP, et al. Treatment of otitis media with observation and a safety-net antibiotic prescription. Pediatrics 2003;112:527–531. [DOI] [PubMed] [Google Scholar]
- 37. Spurling GK, Del Mar CB, Dooley L, Foxlee R, Farley R. Delayed antibiotic prescriptions for respiratory infections. Cochrane Database Syst Rev 2017;9:CD004417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stuart B, Hounkpatin H, Becque T, et al. Delayed antibiotic prescribing for respiratory tract infections: individual patient data meta-analysis. BMJ Clin Res 2021;373:n808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hoberman A, Paradise JL, Rockette HE, et al. Treatment of acute otitis media in children under 2 years of age. N Engl J Med 2011;364:105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McGrath LJ, Frost HM, Newland JG, et al. Utilization of nonguideline concordant antibiotic treatment following acute otitis media in children in the United States. Pharmacoepidemiol Drug Saf 2023;32:256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Michaelidis CI, Fine MJ, Lin CJ, et al. The hidden societal cost of antibiotic resistance per antibiotic prescribed in the United States: an exploratory analysis. BMC Infect Dis 2016;16:655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shaikh N, Dando EE, Dunleavy ML, et al. A cost-utility analysis of 5 strategies for the management of acute otitis media in children. J Pediatrics 2017;189:54–60.e3. [DOI] [PubMed] [Google Scholar]
- 43. Oh PI, Maerov P, Pritchard D, Knowles SR, Einarson TR, Shear NH. A cost-utility analysis of second-line antibiotics in the treatment of acute otitis media in children. Clin Therapeut 1996;18:160–182. [DOI] [PubMed] [Google Scholar]
- 44. Coco AS. Cost-effectiveness analysis of treatment options for acute otitis media. Ann Fam Med 2007;5:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Laupacis A, Feeny D, Detsky AS, Tugwell PX. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ 1992;146:473–481. [PMC free article] [PubMed] [Google Scholar]
- 46. Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA 2016;316:1093–1103. [DOI] [PubMed] [Google Scholar]
- 47. Briggs AH. Handling uncertainty in cost-effectiveness models. PharmacoEconomics 2000;17:479–500. [DOI] [PubMed] [Google Scholar]
- 48. Wald ER, DeMuri GP. Antibiotic recommendations for acute otitis media and acute bacterial sinusitis: conundrum no more. Pediatr Infect Dis J 2018;37:1255–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Butler AM, Brown DS, Durkin MJ, et al. Association of inappropriate outpatient pediatric antibiotic prescriptions with adverse drug events and healthcare expenditures. JAMA Netw Open 2022;5:e2214153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pichichero ME, Casey JR, Almudevar A. Reducing the frequency of acute otitis media by individualized care. Pediatr Infect Dis J 2013;32:473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Frost HM, Sebastian T, Keith A, et al. Reliability of nasopharyngeal PCR for the detection of otopathogens in children with uncomplicated acute otitis media. Open Forum Infect Dis 2021;8 suppl 1:S69–S70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pichichero ME, Morris MC, Almudevar A. Three innate cytokine biomarkers predict presence of acute otitis media and relevant otopathogens. Biomark Appl 2018. doi: 10.29011/2576-9588.100018. [DOI] [PMC free article] [PubMed]
- 53. Shaikh N, Swaminathan N, Hooper EG. Accuracy and precision of the signs and symptoms of streptococcal pharyngitis in children: a systematic review. J Pediatr 2012;160:487–93.e3. [DOI] [PubMed] [Google Scholar]
- 54. Shaikh N, Leonard E, Martin JM. Prevalence of streptococcal pharyngitis and streptococcal carriage in children: a meta-analysis. Pediatrics 2010;126:e557–e564. [DOI] [PubMed] [Google Scholar]
- 55. Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J 2010;29:304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Frost HM, Dominguez S, Parker S, et al. Clinical failure rates of amoxicillin for the treatment of acute otitis media in young children. Open Forum Infect Dis 2020;7 suppl 1:S682–S683. [Google Scholar]
- 57. Frost HM, Bizune D, Gerber JS, Hersh AL, Hicks LA, Tsay SV. Amoxicillin versus other antibiotic agents for the treatment of acute otitis media in children. J Pediatr 2022. ;251:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. McGrath L FH, Newland J, Sahrmann J, Ma YJ, Mobley-Butler A. Visualizing inappropriate antibiotic use following otitis media in children in the United States. International Society for Pharmacoepidemiology Annual Conference; August 24, 2021, held virtually.
- 59. Frost H, Monti J, Andersen L, et al. Improving delayed antibiotic prescribing for acute otitis media. Pediatrics 2021;147:e2020026062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Norlin C, Fleming-Dutra K, Mapp J, et al. A learning collaborative to improve antibiotic prescribing in primary care pediatric practices. Clin Pediatr (Phila) 2021;60:230–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit http://doi.org/10.1017/ash.2023.127.
click here to view supplementary material