Abstract
Proprioceptive information makes us able to perceive the position of our joints from an internal point of view. In the certain cases, proprioceptive information has to be stored in short-term memory, for example, during the learning of new motor skills or the assessment of proprioceptive accuracy. However, there are contradictory findings about the modality-specific storage of proprioceptive information in working memory. In this preregistered study, we applied the interference paradigm, assessing proprioceptive memory capacity in the subdominant elbow joint for 35 young individuals in five different experimental conditions: (a) without competing task/interference (baseline condition), (b) with motor interference, (c) with spatial interference, (d) with visual interference, and (e) with verbal interference. Proprioceptive span was lower in the verbal and spatial interference condition than in the baseline condition, whereas no significant differences were found for the motor and visual conditions. These results indicate that individuals use verbal and spatial strategies to encode proprioceptive information in short-term memory, and, in contrast to our expectation, the motor subsystem of working memory is not substantially involved in this process.
Keywords: Proprioception, proprioceptive accuracy, proprioceptive memory, working memory, short-term memory, interference
Introduction
Certain types of mechanoreceptors, located in our locomotor system (i.e., muscles, joints, and ligaments) make us able to perceive the position and movement of our body, and to sense force and heaviness (Proske & Gandevia, 2012). This ability, called proprioception, plays a prominent role in movement regulation, along with other sensory modalities, such as vision and tactile sensation (Goodman & Tremblay, 2018; Veilleux & Proteau, 2011). Motor control typically relies on online proprioceptive feedback (Goodman & Tremblay, 2018). Sometimes, however, this information has to be stored in short-term memory (Goble, 2010). For example, the learning of new motor sequences in sports or everyday activities may require the ability to store and recall proprioceptive information for short term.
While teaching new motor skills, instructors often show the correct movement by grabbing athletes’ body parts, and moving them in the desired pattern (Chiyohara et al., 2020). This way of teaching proved to be effective: learning a movement trajectory by presenting it with passively moving the arm is more effective than learning by relying purely on visual presentation (Wong et al., 2012). In order to effectively execute the desired movement, one has to accurately perceive the proprioceptively presented joint positions, store them in short-term memory, and reproduce the entire sequence by active motion. When movement sequences are complex (containing several joint positions), one’s ability to store proprioceptive information in memory may limit the quality of movement reproduction and eventually motor learning. Despite its practical and theoretical importance, the exact mechanism of this process, that is, how proprioceptive information is stored in short-term memory, has gained little research attention to date.
The storage of proprioceptive information is necessary for most of the tests that measure proprioceptive accuracy, that is, the acuity of perception of the position of the joints. For example, in the ipsilateral version of the Joint Position Reproduction (JPR) test, the limb of participants is set to a target position, then moved away from it, and participants are asked to replicate the target position as accurately as possible. To do so, the target position needs to be stored in short-term memory (Goble, 2010). Cognitive factors, such as attentional load (Boisgontier et al., 2012; Yasuda et al., 2014) and working memory capacity (Goble et al., 2011) were proven to influence the outcome of the task, which supports the idea that short-term memory is involved in the process. Change in accuracy may also help to evaluate the feasibility an intervention (Isaac et al., 2007), and can be used for sport selection (Han et al., 2015) and injury prevention (Cameron et al., 2003). To be able to draw valid and reliable conclusions, it is important to explore the factors that could influence the outcome of the JPR test.
Based on interference studies, one can consider the capacity of four modality-specific subsystems belonging to working memory as possible moderating factors. These subsystems store and reproduce verbal (e.g., word lists), spatial (e.g., sequences of spatial positions), visual (e.g., complex figures), or motor (e.g., body-related movements) information (Baddeley & Logie, 1999; Klauer & Zhao, 2004; Smyth et al., 1988). The involvement of these subsystems in a certain task is typically studied with the so-called interference paradigm. The general assumption is that if the simultaneous use of two modalities does not disrupt each other’s retention, then the dual task activates two separate and independent subsystems of the working memory (Baddeley, 1992). Spatial task (e.g., repeatedly pointing to spatial positions) substantially disrupts the spatial memory performance (i.e., interference occurs) but does not influence the verbal, motor, or visual memory performance, and vice versa: verbal, motor, and visual tasks do not disrupt spatial memory (Baddeley, 1992; Della Sala et al., 1999; Smyth et al., 1988). In a similar vein, motor tasks do not disrupt the retention of verbal and spatial information (Smyth et al., 1988). The existence of the independent visual and spatial subsystem is further supported by correlation studies where capacity measures of the two modality were not associated (Horváth et al., 2020; Ichikawa, 1983). However, the position of the motor subsystem is less understood. A motor task does not disrupt the retention of verbal and spatial information (Smyth et al., 1988), but verbal tasks can disrupt the storage of movements (Moreau, 2013; Smyth et al., 1988), indicating that verbal strategies may play a role in the storage of motor information. Also, mental rotation performance (that requires visual short-term memory) is influenced by motor tasks (Moreau, 2012), indicating a further interference.
The question of which modality-specific subsystem of the short-term memory stores proprioceptive information is investigated scarcely, and studies have resulted in equivocal or even contradictory results. Goble and colleagues (2012) found that cerebral palsy patients could improve their accuracy in the JPR task if joint position were presented for a longer time (15 s), compared with short time presentation (2 s). As the magnitude of this improvement showed a positive relationship with the spatial memory span (assessed with the Corsi task) of the patients, the authors concluded that spatial memory plays an inherent role in storing joint position-related proprioceptive information. However, this conclusion was not supported by the findings of another study. Horváth and colleagues (2020) investigated the association between proprioceptive span (i.e., the maximal number of proprioceptively determined joint positions that one can store in short-term memory) and verbal or spatial short-term memory span (assessed with the digit span task and the Corsi task, respectively) in a sample of university students. Proprioceptive span proved to be independent of verbal and spatial spans, which suggests that proprioceptive information might be stored in another subsystem.
The primary goal of the present study was to explore the modality-specific storage of proprioceptive information in short-term memory. Our hypothesis was that people store a series of proprioceptively determined joint position in the same way as visually observed movement sequences, that is, in a motor form (Smyth et al., 1988; Smyth & Pendleton, 1990). Thus, executing a motor task while encoding sequences of joint positions should lead to a decreased performance, whereas other tasks (verbal, spatial, visual) would not impact it. For this purpose, we adapted and modified the task used by Horváth and colleagues (2020) to assess proprioceptive short-term memory span in a within-subject research design with five different experimental conditions: (a) without competing task/interference (baseline condition), (b) with motor interference, (c) with verbal interference, (d) with spatial interference, and (e) with visual interference.
Method
Our sample size, hypothesis, study design, and analyses were preregistered (available at https://osf.io/qx9me). The raw data and statistical analysis are also publicly available (https://osf.io/yvu97/).
Participants
We used the G*Power (version: 3.1.9.4) software to a priori determine the sample size. Based on previous similar (interference) studies (Moreau, 2013; Smyth et al., 1988), effect size was set to large (partial eta square = 0.14). To achieve an alpha of .05 and a power of .95, the required minimum sample size is 31 for a repeated measures ANOVA with five levels. Based on this, our priori decision was to stop when N = 35 is reached. Overall, 35 undergraduate students of the Eötvös Loránd University completed the measurements (25 women, 33 right-handed). Participants were at least 18 years old (mean age was 21.2 ± 3.05), without severe injury or disability of the elbow joint. In average, participants spend 2.9 ± 2.5 hr/week with sporting activity (e.g., running, callisthenics). They received partial course credit for the participation. The experiment was approved by the Research Ethics Committee of the Faculty of Education and Psychology of the Eötvös Loránd University (approval number: 2019/302-2). Every participant signed the informed consent before the experiment. All tasks were performed in accordance with the relevant guidelines and regulations. Participants had to confirm that they had not consumed any psychoactive drug (e.g., alcohol) before the experiment, otherwise they could not conduct the measurements.
Capacity measurement
To measure proprioceptive memory span, we adapted and modified the task developed by Horváth and colleagues (2020) for assessing the ability to memorise and reproduce sequences of elbow joint positions. For the assessment, a custom-made motor-driven device (proprioceptor, see Figure 1) was used, which enabled us to accurately set (with a precision of ±0.5°) and measure (±0.1°) the angle of the elbow joint. The speed of the motion was set to 30°/s in this experiment. The device (see Figure 1) consisted of a support surface for the elbow joint and a handle with a button. This button enables the participant to give a signal. The distance of the handle from the support surface was adjustable according to the length of the participant’s forearm. Quasi-random sequences of different lengths were composed from nine possible target positions (30°, 45°, 60°, 75°, 90°, 105°, 120°, 135°, and 150°, where the higher values refer to the bigger extension of the elbow joint). Every target position was presented only once in a sequence (until the length reached 10 positions). The starting position of the trials was always the same, that is, an almost fully extended elbow (160°). From there, the device started to move the elbow joint of the participant, then stopped the movement and kept the arm for 4 s in every target position. Target positions were presented directly after each other without returning to the starting position. After the presentation of an entire sequence, the proprioceptor moved back the elbow joint to the starting position; from this position, participants were asked to replicate the whole sequence by actively moving their arm and pressing a button at every target position. The measurement started with three 2-position practice sequences; than the assessment started with 3-position sequences. If one correctly reproduced two sequences of a given length out of a maximum of three attempts, the number of presented positions increased by one in the next sequence. However, if sequences of the given length were reproduced incorrectly twice, the task ended. The capacity score was determined by the number of elements in the longest, at least two times correctly reproduced sequence. The given sequence was considered correct if (a) the movement pattern was correct (no more or fewer positions were reproduced, and no movement was performed to the opposite direction) and (b) the difference between the target and the reproduced position was less than 30° in each case. We assessed proprioceptive memory capacity with respect to the subdominant elbow joint of the participants.
Figure 1.
The motor-driven proprioceptor used for the measurements.
Procedure
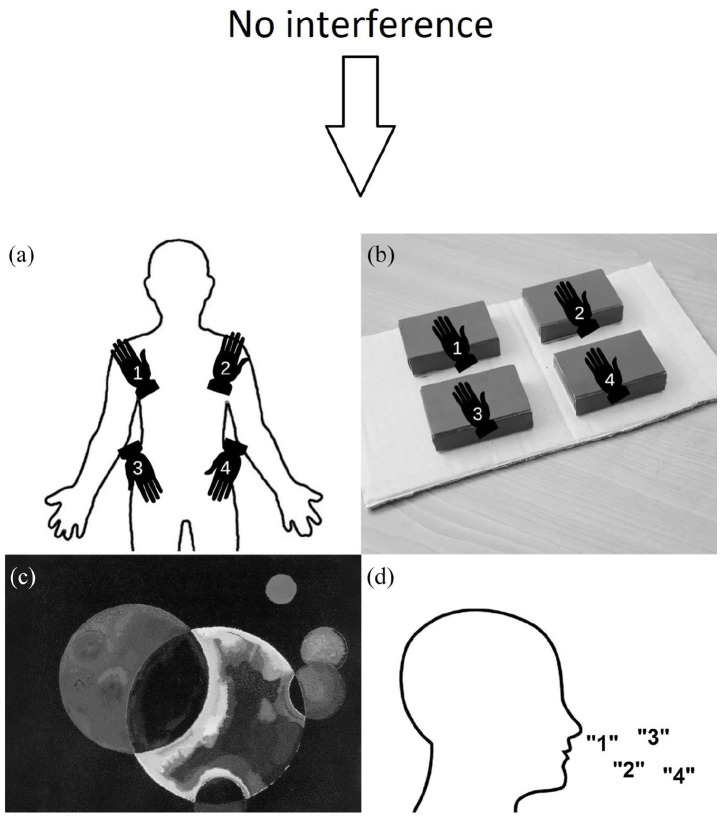
Every participant performed the proprioceptive memory capacity measurement in five different conditions: (a) no interference (baseline), (b) motor interference, (c) spatial interference, (d) visual interference, and (e) verbal interference (see Figure 2). The competing tasks were administered during the presentation phase of the proprioceptive measurement only. The no interference (baseline) condition was administered first, followed by the remaining four conditions in a randomised order.
Figure 2.
Illustration of the experimental procedure. Following the baseline capacity measurement without an interfering task, participants conducted the four experimental conditions in random order. (a) Motor interference: repeated touching of four body parts, (b) spatial interference: repeated touching of four objects with closed eyes, (c) visual interference: looking at abstract images, and (d) verbal interference: repeated counting from one to four.
Baseline
During the baseline measurement, participants had no competing task, so they could fully concentrate on the proprioceptive memory task. Thus, this task measured the memory span of participants.
Motor interference
Motor interference task was adapted from Smyth and colleagues (1988). Participants had to repeatedly touch their body parts with their dominant hand in the following order: left shoulder, right shoulder, left hip, and right hip. This was presented by the experimenter at a speed of approximately 4 touch/s, and participants were instructed to keep that speed.
Spatial interference
This task was adapted from Smyth and colleagues (1988). Participants had to repeatedly touch spatial positions, represented by rectangular boxes (width: 3.5 cm, length: 5 cm, height: 1.5 cm), aligned in a square layout, with 2.5 cm space between them, with their dominant hand and eyes closed. Participants had to touch the top of the boxes. This was presented by the experimenter with approximately a 4 box/s speed, and participants were asked to try to keep that rhythm.
Visual interference
The visual interference task was adapted from Della Sala and colleagues (1999). Participants had to watch abstract pictures on a laptop screen (e.g., pictures of Wassily Kandinsky or Jackson Pollock). The sight of the tested arm was blocked by a specific eye-mask. Each picture was presented for 5 s.
Verbal interference
The verbal interference task was adapted from Baddeley and colleagues (1975). Participants had to repeatedly count from one to four aloud. The task was presented by the experimenter with approximately a four digit per second speed, and they were asked to keep that speed.
Statistical analysis
Statistical analysis was conducted in the JAMOVI (version: 1.6) software (The jamovi project, 2021). The assumptions of repeated ANOVA were not met, as the Shapiro–Wilk test, indicated a significant deviation from normal distribution in every condition (p < .05) for the variables and for the residuals in the model too. Thus, to compare the experimental conditions, we used repeated measures Friedman test with five levels. Durbin–Conover test was used for the post hoc analysis with p < .05 as accepted level of significance.
Results
Descriptive statistics of the investigated variables are presented in Table 1.
Table 1.
Descriptive statistics of participants’ proprioceptive span in the five conditions.
| Condition | Median | M | SD | Minimum | Maximum |
|---|---|---|---|---|---|
| Baseline | 6 | 5.69 | 1.08 | 3 | 8 |
| Motor | 5 | 5.26 | 1.70 | 3 | 10 |
| Spatial | 5 | 4.97 | 1.36 | 3 | 9 |
| Visual | 5 | 5.31 | 1.35 | 3 | 8 |
| Verbal | 4 | 4.83 | 1.65 | 3 | 9 |
Hypothesis testing
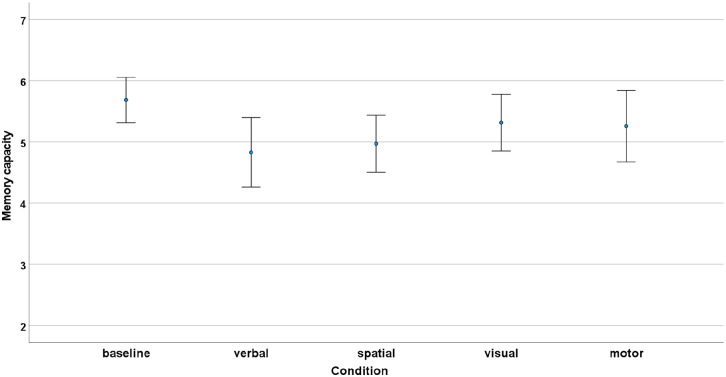
The repeated measures Friedman test indicated a significant difference between the conditions (χ2 = 13.3, p = .01). The Durbin–Conover test showed that proprioceptive span was significantly lower in the verbal condition than in the baseline condition (p < .001), and also significantly lower in the spatial condition than in the baseline condition (p = .006). No more significant differences were found in the post hoc analysis (Figure 3).
Figure 3.
Mean capacity scores in the different experimental conditions. Error bars represent 95% confidence intervals.
To explore whether the results apply when testing the hypothesis with a parametric statistical method, we also ran a repeated measures ANOVA. The results of the test confirmed the previous results: a significant effect of condition (F(4,136) = 3.40, p = .011, η2 = .042) was found, with significantly lower memory performance in the verbal (ptukey = .007), and spatial condition (ptukey = .015) than in the baseline condition, with no other significant differences (p > .05).
Discussion
In this study, we investigated the modality-specific storage of proprioceptive information in short-term memory by measuring participants’ ability to reproduce sequences of proprioceptively determined joint positions (i.e., proprioceptive span) while executing a competing verbal, visual, spatial, or motor task. We hypothesised that proprioceptive information is stored in a motor form, thus we predicted that motor interference would decrease proprioceptive span, while verbal, visual, and spatial interference would not. In contrast to our expectation, our findings show that competing verbal and spatial tasks had a negative impact on proprioceptive span, whereas no visual and motor interference effects were revealed. Overall, these results suggest that people typically use verbal and/or spatial strategies when they need to store a series of proprioceptively determined spatial position. The presence of spatial interference is in line with previous results (Rosenbaum et al., 1999; Weigelt et al., 2007) showing that when people have to reproduce a position, they are more likely to recall the spatial location than the body posture.
The results of Goble and colleagues (2012), which showed a positive association between spatial memory span and improvement in proprioceptive accuracy for longer presentation time of joint positions in patients with cerebral palsy, is partly in accordance with this conclusion. In contrast, proprioceptive memory capacity did not correlate either with spatial or verbal memory capacity in the study of Horváth and colleagues (2020). These differences can be explained by multiple reasons. One possible cause behind the equivocal results may be the difference between the investigated samples. It was shown that motor expertise influences processing in working-memory. In relation with cognitive control, athletes of an open-skill sport show better ability than athletes from a closed-skill sport, and the two groups also differed in brain-signal variability (Wang et al., 2020). There are also differences between different sports in various abilities; for example in a recent meta-analysis, combat sport athletes were found to have better spatial abilities than participants in other sports (e.g., ball sports, runners) (Voyer & Jansen, 2017). In relation with the topic of modality-specific storage, Moreau (2013) found that verbal interference disrupts the storage of visually observed movements for non-athletes, while motor interference affect that of elite athletes. Consequently, elite-athletes appear to store visually observed movements in a motor form, whereas non-athletes favour verbal strategies (Moreau, 2013). Also, interference between motor and mental rotation task was found in elite athletes but not in non-athletes, indicating that the former group utilised motor strategies for mental rotation (Moreau, 2012). Based on these findings, the involvement of motor short-term memory in the storage of proprioceptively determined joint position sequences may also depend on the motor expertise of the participants. The previous contradictory findings (Horváth et al., 2020), namely the independence of proprioceptive, verbal, and spatial spans, may be explained by participants’ intense physical activity (8.0 ± 3.4 hr/week), which indicates a higher level of motor expertise. Thus, the results of the current study may be specific to people who do less intense, recreational level physical activity (2.9 ± 2.47 hr/week). To test the effect of motor expertise on the storage of proprioceptive information, it would be valuable to compare how the different interference tasks influence memory capacity in samples that differ in motor expertise. For example, by comparing professional dancers/athletes with physically non-active individuals and people with a motor disorder (e.g., cerebral palsy).There are other, most importantly, methodological differences between the studies: Goble and colleagues (2012) used a correlational design, but the involvement of verbal, motor, and visual short-term memory was not tested. Horváth and colleagues (2020) also conducted a correlational study, however, they did not test the involvement of visual and motor short-term memory. The present study applied an experimental (interference) design, and all known modality-specific subsystems (i.e., motor, spatial, visual, and verbal) were tested. It is also important to note that there is no association between proprioceptive span (the maximal number of joint positions one can retain is short-term memory) and proprioceptive accuracy (the ability to store one joint position as accurately as possible) (Horváth et al., 2020). It is possible that different mechanisms are responsible for the storage of a single joint position (as in the study of Goble and colleagues, 2012) and for the storage of a maximal number of joint positions (as in the Horváth and colleagues [2020] and the present study). Table 2 summarises the most pivotal differences between these studies.
Table 2.
Summary of the differences between the articles investigating the modality-specific storage of proprioceptive information.
| Study | Goble et al. (2012) | Horváth et al. (2020) | Present study |
|---|---|---|---|
| Assessed variable | Proprioceptive accuracy | Proprioceptive span | Proprioceptive span |
| Sample | Cerebral palsy patients | University students (sporting 8.0 ± 4.0 hr/week) | University students (sporting 2.9 ± 2.5 hr /week) |
| Study design | Correlational | Correlational | interference-based |
| Subsystem found to be involved | Spatial | – | spatial, verbal |
| Subsystem not found to be involved | – | Verbal, spatial | motor, visual |
| Subsystem not investigated | Verbal, motor, visual | Motor, visual | – |
From a theoretical point of view, our findings also do not support the idea that people use the motor subsystem in short-term memory to store proprioceptive stimuli. This can be explained by the two-step process of motor learning. Conscious awareness and voluntary motor control are involved in the first stage only (Gentile, 1998; Lusardi & Bowers, 2013). In this initial phase, the use of the verbal modality (i.e., in the form of secondary representation, perhaps also supported by the spatial module of short-term memory) appears sufficient. In the second phase, patterns of movements are stored in procedural memory and executed automatically (Gentile, 1998; Lusardi & Bowers, 2013), which does not require a modality-specific subsystem of short-term memory.
Our findings may have important practical consequences related to the field of athletic training too. When teaching new motor sequences with proprioceptive presentation, it is important to consider that there are individual differences in the capacity to store joint positions in working memory. Thus, the ideal number of the presented joint positions depends on the individual. As the process utilises the verbal and spatial systems, it could be helpful to find the appropriate verbal labels and spatial strategies.
The validity of the interference tasks used in this study is very important. For example, one might argue that the visual interference task (i.e., viewing abstract pictures) does not require a response, thus maybe the participants were not paying attention to the task. However, the validity of this task is shown by Della Sala and colleagues (1999), who found that it disrupts visual, but not spatial short-term memory performance. Also, the spatial memory task (repeated touching of four objects) has a motor, and not only spatial component, which could be the reason that it interferes with the proprioceptive memory performance. According to the findings of Smyth and colleagues (1988), this spatial interference task disrupts only spatial, but not motor memory. In a similar vein, the verbal and interference tasks were also previously validated and widely used (Baddeley et al., 1975; Smyth et al., 1988).
Our study is not without limitations. We assessed the proprioceptive memory capacity only in the non-dominant hand; it cannot be excluded that proprioceptive sequences are stored differently in the case of the dominant hand. Also, as mentioned before, the results might be specific to the studied population (university students with comparatively low level of physical activity). Furthermore, we used a relatively liberal decision criterion (<30°) with respect to the acceptable difference between the target and the reproduced position in the measurement of proprioceptive memory span.
The strength of this study is that all known short-term memory subsystems (motor, spatial, visual, verbal) were investigated with a unitary experimental (interference) design. Further studies may be required to explore the effect of motor expertise on the storage of proprioceptive information. It would be also valuable to test how the capacity to store proprioceptive information can be improved, and how it affects motor learning.
Footnotes
Author contributions: All authors took part in the conceptualisation of the study. Á.H. wrote the first draft of the manuscript. E.F., A.R., and F.K. wrote sections of the manuscript. F.K. supervised the project. All the authors reviewed the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported by the ÚNKP-20-3 New National Excellence Program of the Ministry for Innovation and Technology from the source of the National Research, Development and Innovation Fund (ÚNKP-20-3-II-ELTE-163).
ORCID iD: Áron Horváth  https://orcid.org/0000-0001-9403-2086
https://orcid.org/0000-0001-9403-2086
Data accessibility statement:

The data from the present experiment are publicly available at the Open Science Framework website: https://osf.io/qx9me.
References
- Baddeley A. D. (1992). Working memory. Science, 255(5044), 556–559. 10.1126/science.1736359 [DOI] [PubMed] [Google Scholar]
- Baddeley A. D., Logie R. H. (1999). Working memory: The multiple-component model. In Miyake A., Shah P. (Eds.), Models of working memory: Mechanisms of active maintenance and executive control (pp. 28–61). Cambridge University Press. 10.1017/CBO9781139174909.005 [DOI] [Google Scholar]
- Baddeley A. D., Thomson N., Buchanan M. (1975). Word length and the structure of short-term memory. Journal of Verbal Learning and Verbal Behavior, 14(6), 575–589. 10.1016/S0022-5371(75)80045-4 [DOI] [Google Scholar]
- Boisgontier M. P., Olivier I., Chenu O., Nougier V. (2012). Presbypropria: The effects of physiological ageing on proprioceptive control. Age, 34(5), 1179–1194. 10.1007/s11357-011-9300-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron M., Adams R., Maher C. (2003). Motor control and strength as predictors of hamstring injury in elite players of Australian football. Physical Therapy in Sport, 4(4), 159–166. 10.1016/S1466-853X(03)00053-1 [DOI] [Google Scholar]
- Chiyohara S., Furukawa J., Noda T., Morimoto J., Imamizu H. (2020). Passive training with upper extremity exoskeleton robot affects proprioceptive acuity and performance of motor learning. Scientific Reports, 10(1), 11820. 10.1038/s41598-020-68711-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Sala S., Gray C., Baddeley A., Allamano N., Wilson L. (1999). Pattern span: A tool for unwelding visuo–spatial memory. Neuropsychologia, 37(10), 1189–1199. 10.1016/S0028-3932(98)00159-6 [DOI] [PubMed] [Google Scholar]
- Gentile A. M. (1998). Movement science: Implicit and explicit processes during acquisition of functional skills. Scandinavian Journal of Occupational Therapy, 5(1), 7–16. 10.3109/11038129809035723 [DOI] [Google Scholar]
- Goble D. J. (2010). Proprioceptive acuity assessment via joint position matching: From basic science to general practice. Physical Therapy, 90(8), 1176–1184. 10.2522/ptj.20090399 [DOI] [PubMed] [Google Scholar]
- Goble D. J., Aaron M. B., Warschausky S., Kaufman J. N., Hurvitz E. A. (2012). The influence of spatial working memory on ipsilateral remembered proprioceptive matching in adults with cerebral palsy. Experimental Brain Research, 223(2), 259–269. 10.1007/s00221-012-3256-8 [DOI] [PubMed] [Google Scholar]
- Goble D. J., Mousigian M. A., Brown S. H. (2011). Compromised encoding of proprioceptively determined joint angles in older adults: The role of working memory and attentional load. Experimental Brain Research, 216(1), 35–40. 10.1007/s00221-011-2904-8 [DOI] [PubMed] [Google Scholar]
- Goodman R., Tremblay L. (2018). Using proprioception to control ongoing actions: Dominance of vision or altered proprioceptive weighing? Experimental Brain Research, 236(7), 1897–1910. 10.1007/s00221-018-5258-7 [DOI] [PubMed] [Google Scholar]
- Han J., Waddington G., Anson J., Adams R. (2015). Level of competitive success achieved by elite athletes and multi-joint proprioceptive ability. Journal of Science & Medicine in Sport, 18(1), 77–81. [DOI] [PubMed] [Google Scholar]
- Horváth Á., Ragó A., Ferentzi E., Körmendi J., Köteles F. (2020). Short-term retention of proprioceptive information. Quarterly Journal of Experimental Psychology, 73(12), 2148–2157. 10.1177/1747021820957147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa S. (1983). Verbal memory span, visual memory span, and their correlations with cognitive tasks. Japanese Psychological Research, 25(4), 173–180. [Google Scholar]
- Isaac S. M., Barker K. L., Danial I. N., Beard D. J., Dodd C. A., Murray D. W. (2007). Does arthroplasty type influence knee joint proprioception? A longitudinal prospective study comparing total and unicompartmental arthroplasty. The Knee, 14(3), 212–217. 10.1016/j.knee.2007.01.001 [DOI] [PubMed] [Google Scholar]
- The jamovi project. (2021). jamovi (Version 1.6) [Computer software]. https://www.jamovi.org
- Klauer K. C., Zhao Z. (2004). Double dissociations in visual and spatial short-term memory. Journal of Experimental Psychology: General, 133(3), 355–381. 10.1037/0096-3445.133.3.355 [DOI] [PubMed] [Google Scholar]
- Lusardi M. M., Bowers D. M. (2013). Motor control, motor learning, and neural plasticity in orthotic and prosthetic rehabilitation. In Lusardi M., Jorge M., Nielsen C. (Eds.), Orthotics & prosthetics in rehabilitation (pp. 38–53). St. Louis, MO: Elsevier/Saunders. [Google Scholar]
- Moreau D. (2012). The role of motor processes in three-dimensional mental rotation: Shaping cognitive processing via sensorimotor experience. Learning and Individual Differences, 22(3), 354–359. 10.1016/j.lindif.2012.02.003 [DOI] [Google Scholar]
- Moreau D. (2013). Motor expertise modulates movement processing in working memory. Acta Psychologica, 142(3), 356–361. 10.1016/j.actpsy.2013.01.011 [DOI] [PubMed] [Google Scholar]
- Proske U., Gandevia S. C. (2012). The proprioceptive senses: Their roles in signaling body shape, body position and movement, and muscle force. Physiological Reviews, 92(4), 1651–1697. 10.1152/physrev.00048.2011 [DOI] [PubMed] [Google Scholar]
- Rosenbaum D. A., Meulenbroek R. G. J., Vaughan J. (1999). Remembered positions: Stored locations or stored postures? Experimental Brain Research, 124, 503–512. 10.1007/s002210050646 [DOI] [PubMed] [Google Scholar]
- Smyth M. M., Pearson N. A., Pendleton L. R. (1988). Movement and working memory: Patterns and positions in space. The Quarterly Journal of Experimental Psychology Section A, 40(3), 497–514. 10.1080/02724988843000041 [DOI] [PubMed] [Google Scholar]
- Smyth M. M., Pendleton L. R. (1990). Space and movement in working memory. The Quarterly Journal of Experimental Psychology Section A, 42(2), 291–304. 10.1080/14640749008401223 [DOI] [PubMed] [Google Scholar]
- Veilleux L.-N., Proteau L. (2011). Congruent visual and proprioceptive information results in a better encoding of initial hand position. Experimental Brain Research, 214(2), 215–224. 10.1007/s00221-011-2822-9 [DOI] [PubMed] [Google Scholar]
- Voyer D., Jansen P. (2017). Motor expertise and performance in spatial tasks: A meta-analysis. Human Movement Science, 54, 110–124. 10.1016/j.humov.2017.04.004 [DOI] [PubMed] [Google Scholar]
- Wang C.-H., Liang W.-K., Moreau D. (2020). Differential modulation of brain signal variability during cognitive control in athletes with different domains of expertise. Neuroscience, 425, 267–279. 10.1016/j.neuroscience.2019.11.003 [DOI] [PubMed] [Google Scholar]
- Weigelt M., Cohen R., Rosenbaum D. A. (2007). Returning home: Location memory versus posture memory in object manipulation. Experimental Brain Research, 179(2), 191–198. 10.1007/s00221-006-0780-4 [DOI] [PubMed] [Google Scholar]
- Wong J. D., Kistemaker D. A., Chin A., Gribble P. L. (2012). Can proprioceptive training improve motor learning? Journal of Neurophysiology, 108(12), 3313–3321. 10.1152/jn.00122.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda K., Sato Y., Iimura N., Iwata H. (2014). Allocation of attentional resources toward a secondary cognitive task leads to compromised ankle proprioceptive performance in healthy young adults. Rehabilitation Research and Practice, 2014, 170304. 10.1155/2014/170304 [DOI] [PMC free article] [PubMed] [Google Scholar]