Abstract
BACKGROUND:
The role of antisecretory drugs for the prevention of upper gastrointestinal bleeding in patients using anticoagulants is unclear. We investigated this question in a systematic review and meta-analysis.
METHODS:
We searched Embase, PubMed, Web of Science, Scopus, the Cochrane Library, and clinicaltrials.gov thru April 2021 for controlled randomized trials and observational studies evaluating the association of proton pump inhibitors (PPIs) or H2-receptor antagonists with overt upper gastrointestinal bleeding in patients using anticoagulants. Independent duplicate review, data extraction, and risk of bias assessment were performed. Observational studies were included only if they provided results controlled for at least 2 variables. Meta-analyses were performed using random effects models.
RESULTS:
Six observational studies and 1 randomized trial were included. All but 1 study had low risk of bias. None of the studies excluded patients with concomitant aspirin or nonsteroidal anti-inflammatory drug use. For PPIs, the pooled relative risk of upper gastrointestinal bleeding was 0.67 (95% confidence interval 0.61, 0.74) with low statistical heterogeneity (I2 = 15%). Individual studies showed greater treatment effect in patients with higher risk for upper gastrointestinal bleeding (eg, nonsteroidal anti-inflammatory drug or aspirin use, elevated bleeding risk score). A single observational study evaluating the association of H2-receptor antagonists with upper gastrointestinal bleeding found a relative risk of 0.69 (95% confidence interval 0.24–2.02).
CONCLUSIONS:
Evidence drawn mostly from observational studies with low risk of bias demonstrate that PPIs reduce upper gastrointestinal bleeding in patients prescribed oral anticoagulants. The benefit appears to be most clearcut and substantial in patients with elevated risk of upper gastrointestinal bleeding.
Keywords: Meta-analyses, Peptic ulcer disease, Stomach and duodenum, Upper gastrointestinal bleeding
INTRODUCTION
Increasing numbers of patients in the United States are treated with oral anticoagulants, driven by expanded use of direct oral anticoagulants (DOACs) and new indications, such as coronary artery disease.1–3 It is well known that anticoagulants increase the risk of major bleeding. The single-most common anatomic site of major bleeding is the gastrointestinal tract, which accounts for roughly 40% of bleeding with oral anticoagulation.4–6 Despite this, uncertainty exists regarding therapies to reduce upper gastrointestinal bleeding.
Proton pump inhibitors (PPIs) reduce the risk of upper gastrointestinal bleeding for patients using aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs) by 79% in pooled estimates.7 However, the utility of antisecretory therapy in patients on anticoagulants remains unclear because these patients have been excluded from most studies of PPI gastroprotection. On the one hand, antisecretory drugs might not have the same benefits for patients taking anticoagulants because aspirin and other NSAIDs directly cause mucosal injury, whereas anticoagulants do not.8 On the other hand, anticoagulants increase the risk of clinically significant hemorrhage by systemic and, in the case of DOACs, local effects, in patients with preexisting or incident mucosal breaks, providing a plausible rationale for PPI gastroprotection.9,10 In patients taking anticoagulants, acid peptic diseases, including peptic ulcer disease, erosive gastritis, and erosive esophagitis, for which PPIs would have salutary effects, are the most common sources of upper gastrointestinal bleeding.11,12
Herein, we perform a systematic review and meta-analysis of the association of antisecretory therapy, both PPIs and H2-receptor antagonists (H2RAs), with upper gastrointestinal bleeding. We specifically included observational studies because of the limited number of randomized controlled trials (RCTs) on this subject. By synthesizing the full extent of available data, we sought to clarify the role of antisecretory therapy in preventing one of the most common major complications for patients taking anticoagulants.
METHODS
Study Selection
This review was prospectively registered with PROSPERO (CRD42020136610).13 The search strategy (Supplement 1, available online) was designed with a health librarian and iteratively refined to capture all known relevant studies. To summarize, it combined terms associated with anticoagulants AND anti-secretory drugs AND gastrointestinal hemorrhage. We searched Embase, PubMed, Web of Science, Scopus, the Cochrane Library, and clinicaltrials. gov from inception to April 7, 2021 with no language, date, or other search restrictions. We also performed forward and backward citation tracking for all included articles and conferred with 3 topic experts to identify additional relevant studies.
Our specific research question was, in adults prescribed oral anticoagulants, how effective is antisecretory therapy in reducing overt upper gastrointestinal bleeding in either RCTs or observational studies? Studies were eligible if they included patients aged 18 or older using warfarin or a DOAC, at any dose, including as part of a subgroup analysis. There were no eligibility criteria related to use of aspirin, P2Y12 inhibitors, or NSAIDs. Comparisons of PPI or H2RA to placebo or no therapy, or comparison of PPI to H2RA, were required. Studies that relied on administrative/claims data to establish a diagnosis of upper gastrointestinal bleeding were eligible. Observational studies were excluded if they did not control for at least 2 confounding variables (eg, concomitant use of aspirin, NSAIDs, prior history of peptic ulcer or upper gastrointestinal bleeding); case-control studies that matched on 1 confounding variable were only required to control for 1 additional confounding variable. Additional exclusion criteria were use of antisecretory agent for treatment of acute upper gastrointestinal bleeding, short-term treatment to prevent rebleeding after an upper gastrointestinal bleeding, treatment of Helicobacter pylori infection, bleeding prevention after endoscopic interventions (eg, endoscopic mucosal resection), or prevention of stress ulcers in hospitalized patients; and failure to specify whether gastrointestinal bleeding was from the upper or lower gastrointestinal tract. Only articles written in English were included.
Titles and abstracts were independently reviewed by 2 of 3 authors (AF, JJG, DH), and articles deemed potentially relevant were retrieved in full text. All full text again underwent independent dual review to determine study eligibility. Discrepancies in study selection were resolved through discussion, with adjudication from a third author (JEK) when necessary. Covidence systematic review software (Veritas Health Innovation) was used for screening and study tracking.
Data Extraction, Quality Assessment, and Synthesis of Results
Two authors (AF, JJG) independently performed data extraction using standardized forms, and discrepancies were resolved by a third author (DH). We extracted information on study design, participant characteristics, confounders included in multivariable analyses, definition of upper gastrointestinal bleeding, and association of antisecretory drug with upper gastrointestinal bleeding. Two authors (AF, JJG) independently assessed methodological quality with disagreements resolved by a third author (JK). Case-control and cohort studies were assessed using the Newcastle-Ottawa Scale.14 RCTs were assessed using the Cochrane Risk of Bias (RoB 2) tool.15
For observational studies, the adjusted values for relative effects are presented as incidence rate ratio (IRR), hazard ratio (HR), or relative risk (RR) based on each study’s multivariable analysis. When possible, adjusted absolute treatment differences are presented as risk differences, with number needed to treat (NNT) calculated when bounds of the 95% confidence interval (CI) for risk difference did not cross zero.
Lanas et al16 included separate RR estimates for patients on PPI with anticoagulation and no PPI with anticoagulation, both relative to patients on no PPI without anticoagulation; the authors supplied the RR and CI for PPI use in patients taking anticoagulants. Lee et al17 provided estimates only for individual anticoagulants; therefore, the pooled estimate and CI of the effect of PPI across warfarin and all DOACs were calculated using weighted averages in a fixed effects meta-analysis using RevMan 5.1 (Cochrane Collaboration). For the single RCT identified, Stata 16.1 (StataCorp) was used to calculate overall overt bleeding outcomes within each anticoagulated subgroup (presented as RRs),18 and absolute risk differences between PPI and placebo groups for each gastrointestinal bleeding definition were calculated using standard methods after extrapolating the person-years of follow-up.19
Meta-analysis was performed using random effects models. The I2 statistic was used to explore study heterogeneity. The effect size was calculated as RR. For studies that reported HRs or IRRs, those values were used as approximations of the RR. For Lee et al,17 we used the pooled estimate calculated from patients using all types of anticoagulants. For Moayeddi et al,18 the RRs calculated for each of the anticoagulated subgroups were included separately. A sensitivity analysis was done excluding the RCT because the study only included participants not felt to require PPI therapy, and the study design was distinct from the observational studies. With fewer than 10 studies, we did not test for funnel plot asymmetry.20
We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist (Supplement 2, available online).21 Study data and materials are available on request.
RESULTS
The search strategy identified 2654 records after removing duplicates. Following screening of titles and abstracts, 354 were reviewed in full text (Figure 1). Of these, 262 did not meet inclusion criteria, 67 were not available in full text, 10 were non-English publications, and 8 were duplicates, leaving 7 studies that were eligible and included in this review.
Figure 1.
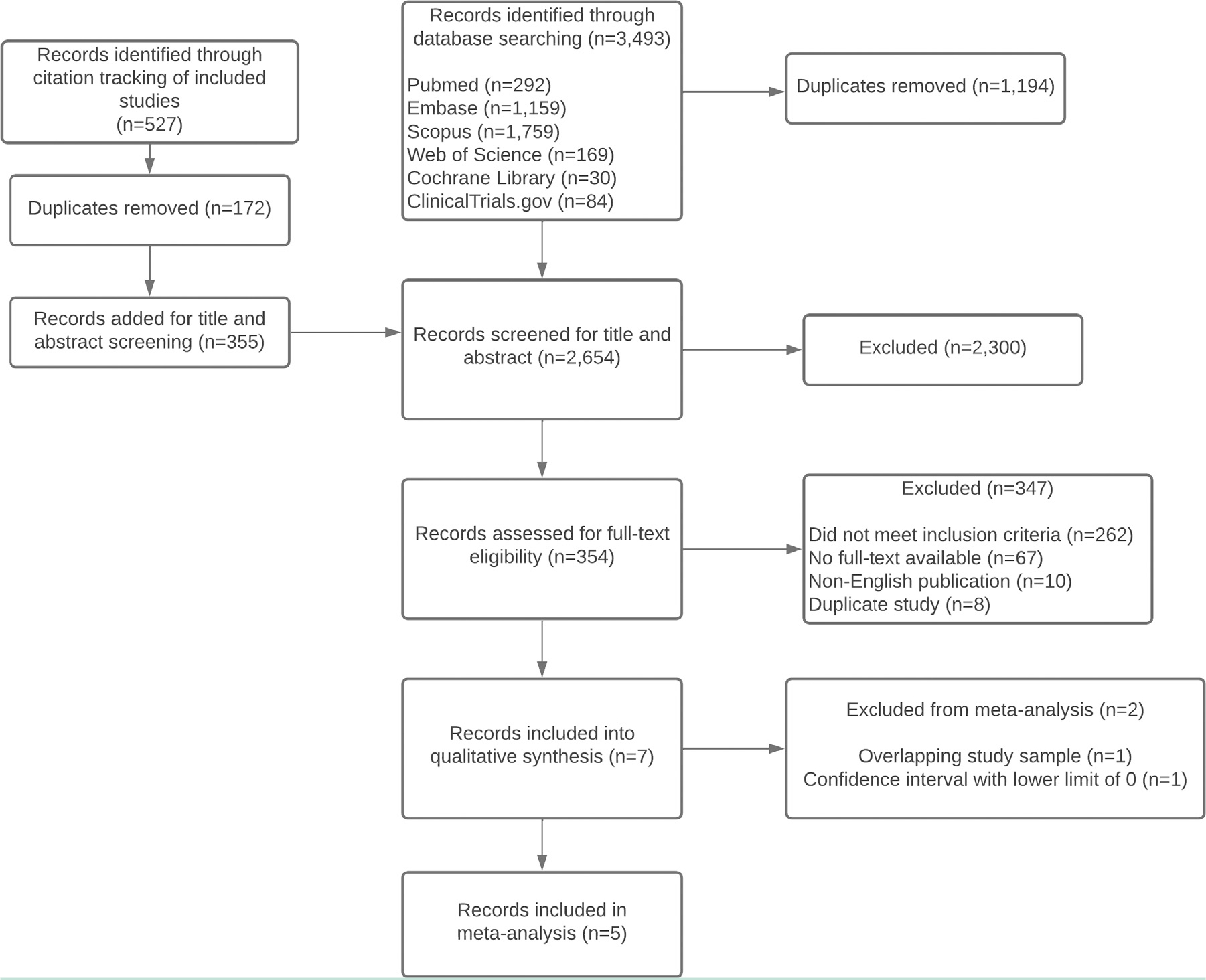
PRISMA flow diagram. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta Analyses.
Study Characteristics
The 7 eligible studies were published between 2011 and 2021 and included 2 case-control studies,16,22 4 retrospective cohort studies,17,23–25 and 1 RCT,18 all of which evaluated PPIs as the antisecretory drug. A single study additionally evaluated H2RAs (Lin et al22). All the observational studies compared active use of the antisecretory drug to nonuse, while the RCT compared active use to a placebo. The methodologic characteristics are summarized in Table 1.
Table 1.
Methodologic Characteristics of Included Studies
| Study | Lanas 201516 | Lee 202117 | Lin 201122 | Maruyama 201825 | Moayyedi 201918 | Ray 201624 | Ray 201823 |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Primary study design | Case-control study with prospective case ascertainment | Retrospective cohort study | Retrospective, population-based, nested case-control study | Retrospective cohort study | 3 × 2 partial factorial double-blind trial, with simultaneous randomization to 1 of 3 antithrombotic drug regimens, and to PPI vs placebo | Retrospective cohort study | Retrospective cohort study |
| Anticoagulants | Not stated | Warfarin, rivaroxaban, dabigatran, apixaban, edoxaban; DOACs included both regular and reduced dosing | Warfarin | Dabigatran, rivaroxaban, or apixaban | Group 1: rivaroxaban 2.5 mg twice daily with aspirin 100 mg/d Group2: rivaroxaban 5 mg twice daily* | Warfarin | Apixaban, dabigatran, rivaroxaban, warfarin |
| Antisecretory agent | Any PPI | Any prescription PPI | Current use of any PPI (initiated at least 1 month before index date) Current use of any H2RA (initiated at least 1 month before index date) | Any PPI | Pantoprazole 40 mg/d | Any prescription PPI | Any prescription PPI |
| Comparator | No PPI | No PPI | No PPI (in prior year); no H2RA (in prior year) | No PPI | Placebo | No PPI | No PPI |
| Country, time period | Spain, 2009 to mid- 2013 | Korea, with patients initiating anticoagulant between January 2010 and April 2018 | UK, January 1, 2000 to December 31, 2007 | Japan, prescribed anticoagulant between April 2011 and November 2015 | 580 centers in 33 countries, randomization March 2013 through May 2016 | United States, Tennessee Medicaid 1996–2011 and 5% national Medicare Sample 2011–2013 | United States, January 1, 2011 to September 30, 2015 |
| Source population | A network of general hospitals integrated within the Spanish Association of Gastroenterology and the Biomedical Investigation Network Center of hepatic and digestive diseases | Korean Health Insurance Review and Assessment database, which includes the entire South Korean population | THIN database: UK primary care record database with >3 million patients | Single institution | — | Patients receiving Tennessee Medicaid and the 5% National Medicare Sample | Medicare beneficiaries |
| Inclusion and exclusion criteria | Inclusion: Age 20–90 years Exclusion: liver disease, coagulation disorders or malignancy within the previous 5 years; bleeding caused by gastroesophageal or intestinal varices, GI cancer, Mallory-Weiss lesions, associated coagulopathy, and esophagitis; patients with unreliable sources of information; patients refusing participation; patients with inhospital bleeding. |
Inclusion: Atrial fibrillation, initiated oral anticoagulant between January 2010 and April 2018, with a prior history of upper GI bleeding before oral anticoagulant initiation Exclusion: Patients prescribed an oral anticoagulant within 1 year before the study period (January 2009-December 2009), patients <20 years old, diagnosis of valvular atrial fibrillation, pulmonary embolism, deep vein thrombosis, end-stage renal disease, or joint replacement |
Inclusion: Age 40–84 years, enrolled with primary care provider for 2 years, prescription data for 1 year Exclusion for source population: Diagnosis of cancer, esophageal varices, Mallory-Weiss disease, alcohol abuse, liver disease, or coagulopathy For cases, no exclusion criteria could be met in the 2 months after the recorded upper GI bleed, and no discharge from the hospital in the month prior to the upper GI bleed |
Inclusion: Age ≥18 years, prescribed anticoagulant for nonvalvular atrial fibrillation Exclusion: None stated. |
Inclusion: Stable atherosclerotic cardiovascular or peripheral arterial disease with no clinical need for PPI. Patients with coronary artery disease <65 years old additionally required to have arterial disease involving 2 vascular beds or 2 additional risk factors. Exclusions: high risk of bleeding from any site, severe heart failure, significant renal impairment, need for dual antiplatelet or anticoagulant therapy, known hypersensitivity to any of the study drugs. |
Inclusion: Age ≥30 years, first prescription for warfarin filled during study period, complete demographic information, full pharmacy benefits, ≥1 outpatient visit and ≥1 filled prescription in past year Exclusion: Prescription of oral anticoagulant in preceding year, end-stage renal disease, serious GI illness predisposing to bleeding, or bleeding-related hospitalization in past year. |
Inclusion: Age ≥30 years, initiated oral anticoagulant during study period, complete demographic information in Medicare files, full pharmacy benefits, ≥1 outpatient visit and ≥1 filled prescription in past year. Exclusion: Prescription of oral anticoagulant in preceding year, end-stage renal disease, serious GI illness predisposing to bleeding, or bleeding-related hospitalization in past year. |
| Entire study sample analyzed versus subset who were anticoagulated | Subset | Entire study sample | Subset | Entire study sample | Subset | Entire study sample | Entire study sample |
| Case definition for upper gastrointestinal bleeding | Hospitalization for GI bleeding (hematemesis, melena, hematochezia, or red blood per rectum). Classified as upper GI bleeding if witnessed hematemesis by hospital staff or either blood in the stomach or a lesion with stigmata of bleeding at upper endoscopy. | Occurrence of an ICD-10 code for upper GI bleeding during an admission that lasted at least 3 days. | Bleeding in stomach or duodenum | Overt “actionable” bleeding, defined as Bleeding Academic Research Consortium (BARC) types 2–5. Anatomic sites considered upper GI bleeding not stated. | Two definitions were examined:† a: Overt bleeding of gastroduodenal origin (hematemesis or melena) with actively bleeding gastroduodenal lesion (peptic ulcer or neoplasia) confirmed by endoscopy or radiography b: Overt upper gastrointestinal bleeding of unknown origin (hematemesis with or without melena thought to be related to upper gastrointestinal tract) |
Hospitalization for upper gastrointestinal bleeding that was potentially preventable by PPI, including bleeding related to esophagitis, peptic ulcer disease, and gastritis. | Hospitalization for upper gastrointestinal bleeding that was potentially preventable by PPI, including bleeding related to esophagitis, peptic ulcer disease, and gastritis. |
| Newcastle Ottawa Scale for observational studies† | |||||||
| Selection, max 4 | 4 | 4 | 4 | 1 | n/a | 4 | 4 |
| Comparability, max 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| Exposure (for case-control studies)/Outcome (for cohort studies), max 3 | 2 | 3 | 3 | 1 | 3 | 3 | |
| Cochrane Risk of Bias for RCTs (max 6) | — | — | — | — | Low risk of bias in all 6 categories | — | — |
DOAC = direct-acting oral anticoagulant; GI = gastrointestinal; H2RA = histamine-2 receptor antagonist; PPI = proton pump inhibitor; RCT = randomized controlled trial.
Participants were also randomized to an aspirin-only group; however, this arm of the trial did not meet the inclusion criteria for the systematic review so data are not presented.
The trial’s primary efficacy outcome was defined as a composite of upper GI clinical events, which also included occult bleeding (drop in the hemoglobin of ≥2 g/dL), symptomatic gastroduodenal ulcers with at least 3 days of gastrointestinal pain, or ≥5 gastroduodenal erosions (confirmed by endoscopy) with at least 3 days of GI pain, upper GI obstruction, or perforation. Data are presented here only for the 2 components of the composite endpoint that included upper GI bleeding.
All 6 observational studies controlled for age, gender, and NSAIDs, in some cases only if prescribed but not over-the-counter (Table 2). Five studies controlled for use of over-the-counter or prescription aspirin and P2Y12 inhibitors. Five controlled for history of peptic ulcer disease or upper gastrointestinal bleeding, whereas in another study it was required for study inclusion.
Table 2.
Select Patient Factors Used as Matching Variables or Covariates in Observational Studies Examining Association of Antisecretory Use with Upper GI Bleeding
| Lanas 201516 | Lee 202117 | Lin 201122 | Maruyama 201825,* | Ray 201624 | Ray 201823 | |
|---|---|---|---|---|---|---|
|
| ||||||
| Age | Y† | Y | Y† | Y | Y | Y |
| Gender | Y† | Y | Y† | Y | Y | Y |
| Smoking | Y | — | Y | — | Y | Y |
| Alcohol use or abuse | Y | — | Y | — | Y | Y |
| History of coronary artery disease | — | Y | —‡ | — | Y | Y |
| History of cerebrovascular disease | — | Y | — | Y | Y | Y |
| History of peptic ulcer disease or upper GI bleeding | Y | — (patient inclusion requirement) | Y | Y | Y | Y |
| History of abdominal/epigastric pain or dyspepsia | Y | — | — | — | Y | Y |
| Kidney function/renal failure | — | Y | — | Y | Y | Y |
| Anemia | — | — | — | — | Y | Y |
| PPI | Y | — | Y§ | — | — | — |
| H2RA | Y | — | Y§ | Y | Y | Y |
| Specific anticoagulant if >1 included in study sample | — | Y | — | Y | — | Y |
| Aspirin | Y (OTC or prescription) | Y (not stated if OTC or prescription) | Yes (prescription only) | Y (not stated if OTC or prescription) | Y (only for OTC aspirin if associated with a prescription in TN Medicaid sample but not for Medicare sample) | — |
| NSAID | Y (OTC or prescription) | Y | Yes (prescription only) | Y (not stated if OTC or prescription) | Y (all prescription NSAIDs, as well as OTC NSAIDs if associated with a prescription in TN Medicaid sample but not Medicare sample) | Y (prescription only) |
| P2Y12 inhibitors | Y | Y | — | Y | Y | Y |
| Corticosteroids | — | — | Y | Y | Y | Y |
| COX2-selective NSAID | Y | — | — | — | Y | Y |
| SSRI | — | — | Y | — | Y | Y |
| Aldosterone antagonists | — | — | — | — | — | — |
| Calendar year | Y† | — | Y | — | Y | Y |
| Number of primary care visits | — | — | Y | — | — | — |
COX = cyclooxygenase; GI = gastrointestinal; H2RA = histamine-2 receptor antagonist, NSAID = nonsteroidal anti-inflammatory drug, OTC = over the counter, PPI = proton pump inhibitor; SSRI = selective serotonin reuptake inhibitor
Covariates were included in a forward stepwise regression but not necessarily in the final model.
[Indicates matching variable in case-control study.
Cardiovascular disease was not included in the final model because it was not associated with upper GI bleeding after adjusting for antithrombotic drugs.
Both PPI and H2RA were simultaneously included in logistic regression models to estimate the effect of each.
Moayyedi et al18 conducted a 3 × 2 partial factorial double-blind trial (the Cardiovascular Outcomes for People Using Anticoagulation Strategies [COMPASS] trial) that evaluated PPI gastroprotection with pantoprazole 40 mg/d versus placebo in 3 antithrombotic treatment groups (rivaroxaban 2.5 mg twice daily with aspirin 100 mg/d, rivaroxaban 5 mg twice daily alone, or aspirin 100 mg/d alone). The trial included patients with stable atherosclerotic cardiovascular or peripheral arterial disease. Importantly, only patients without a clinical need for long-term PPI were included in the randomization for antisecretory therapy. The primary efficacy outcome for the effect of antisecretory therapy was a composite of upper gastrointestinal clinical events: symptomatic gastroduodenal ulcers or gastroduodenal erosions, overt upper gastrointestinal bleeding, occult bleeding, perforation, or obstruction. In keeping with the aims of this review, data were only extracted for overt upper gastrointestinal bleeding outcomes, for the 2 antithrombotic arms assigned rivaroxaban with or without aspirin.
On the Newcastle Ottawa Scale for study quality, the study by Lanas et al16 received 8 of 9 possible points, the study by Maruyama et al25 received only 4 of 9 points, and the remaining observational studies17,22–24 received the maximum number of points (Table 1). The trial by Moayyedi et al18 was low risk for bias on all 6 dimensions of the Cochrane Risk of Bias tool.
Association of Antisecretory Use with Upper GI Bleeding in Individual Studies
The results of the studies are summarized in Table 3 and below. In the case control study by Lanas et al,16 among the subset of anticoagulated patients, PPI use was associated with an adjusted RR of 0.47 (95% CI 0.18, 1.29). In the case-control study by Lin et al,22 among patients using warfarin, PPI use was associated with an adjusted RR for upper gastrointestinal bleeding of 0.48 (95% CI 0.22, 1.04). In the cohort study by Maruyama et al,25 among patients using dabigatran, rivaroxaban, or apixaban, none of 9 upper gastrointestinal bleeding events occurred in patients using a PPI, resulting in an adjusted HR of 0 (95% CI 0, 2 × 10134).
Table 3.
Association of Antisecretory Use with Upper GI Bleeding
| Observational studies | Number of participants prescribed anticoagulants or person-years at risk | Rate of upper gastrointestinal bleeding events (antisecretory exposed/antisecretory unexposed), unadjusted unless otherwise stated | Adjusted association of upper gastrointestinal bleeding events with antisecretory use versus no antisecretory (HR/RR/IRR with 95% CI) | Adjusted annual risk difference with antisecretory use in cohort studies and randomized trials (% with 95% CI) |
|---|---|---|---|---|
|
| ||||
| Case control studies | ||||
| Lanas 201516 | 73 cases, 30 controls | — | PPI: RR 0.47 (0.18–1.29)*,† | — |
| Lin 201122 | 113 cases using warfarin, 636 controls | — | PPI: RR 0.48 (0.22, 1.04)* H2RA: RR 0.69 (0.24, 2.02)* |
— |
| Cohort studies | ||||
| Lee 202117 | 42,048 participants; 16,988 on PPI at baseline; 48,477 person-years | Any anticoagulant: Data not provided Warfarin: 1.15 per 100 person-years with PPI / 1.96 per 100 person-years without PPI Rivaroxaban: 1.12 per 100 person-years with PPI / 2.44 per 100 person-years without PPI Dabigatran: 0.94 per 100 person-years with PPI / 1.69 per 100 person-years without PPI Apixaban: 1.24 per 100 person-years with PPI / 1.81 per 100 person-years without PPI Edoxaban: 1.39 per 100 person-years with PPI / 1.22 per 100 person-years without PPI |
PPI: HR 0.67 (0.57, 0.78)‡ PPI: HR 0.67 (0.55, 0.82)* PPI: HR 0.55 (0.38, 0.79)* PPI: HR 0.71 (0.40, 1.27)* PPI: HR 0.72 (0.44, 1.15)* PPI: HR 1.04 (0.53, 2.06)* |
§ |
| Maruyama 201825 | 658 participants; 313 on PPI; 1342.2 person-years | 0 with PPI / 9 without PPI | PPI: HR 0(0, 2 × 10134)* | § |
| Ray 201624 | 97,430 participants; 14,658 person-years with PPI cotherapy, 52,407 person-years without co-therapy | 123.5 per 10,000 person-years with PPI / 119.1 per 10,000 person-years without PPI | PPI: HR 0.76 (0.63, 0.91)* | −0.29% (−0.44%, −0.10%) |
| Ray 201823 | 1,643,123 participants; 264,447 person-years with PPI co-therapy; 754,389 person-years without PPI co-therapy | Any anticoagulant (primary analysis; adjusted‖): 76 per 10,000 person-years with PPI / 115 per 10,000 person-years without PPI Apixaban (adjusted): 48.5 per 10,000 person-years with PPI / 72.9 per 10,000 person-years without PPI Dabigatran (adjusted): 59.2 per 10,000 person-years with PPI / 120.4 per 10,000 person-years without PPI Rivaroxaban (adjusted): 108.3 per 10,000 person-years with PPI / 143.8 per person-years without PPI Warfarin (adjusted): 74.0 per 10,000 person-years with PPI / 113.3 per 1,000 person-years without PPI |
PPI: IRR 0.66 (0.62, 0.69)* PPI: IRR 0.66 (0.52, 0.85)* PPI: IRR 0.49 (0.41, 0.59)* PPI: IRR 0.75 (0.68, 0.84)* PPI: IRR 0.65 (0.62, 0.69)* |
−0.40% (−0.44%, −0.35%) −0.24% (−0.38%, −0.11%) −0.61% (0.75%, −0.47%) −0.36% (−0.49%, −0.22%) −0.39% (−0.44%, −0.34%) |
| Randomized trials | ||||
| Moayyedi 201918 | Group 1 (rivaroxaban with aspirin): 2945 randomized to pantoprazole; 2933 randomized to placebo | Composite outcome of all overt upper gastrointestinal bleeding (combining definitions a and b): Definition a: Overt bleeding of gastroduodenal origin confirmed by endoscopy or radiography: 0.09% per year with pantoprazole / 0.12% per year without pantoprazole Definition b: Overt upper gastrointestinal bleeding of unknown origin: 0.25% per year with pantoprazole / 0.18% per year without pantoprazole |
RR 1.11 (0.66,1.86) HR 0.73 (0.29, 1.80) HR 1.37 (0.72, 2.61) |
0.1% (−0.4%, 0.6%) −0.03% (−0.12, 0.06) 0.07% (−0.07, 0.21) |
| Group 2 (rivaroxaban alone): 2918 randomized to pantoprazole; 2941 randomized to placebo | Composite outcome of all overt upper gastrointestinal bleeding¶ Definition a: Overt bleeding of gastroduodenal origin confirmed by endoscopy or radiography: 0.03% per year with pantoprazole / 0.14% per year without pantoprazole Definition b: Overt upper gastrointestinal bleeding of unknown origin: 0.21% per year with pantoprazole / 0.14% per year without pantoprazole |
RR 0.88 (0.49, 1.58) HR 0.25 (0.07, 0.89) HR 1.52 (0.73, 3.15) |
−0.1% (−0.5, 0.4%) −0.11% (−0.19, −0.02) 0.07% (−0.06, 0.20) |
|
CI = confidence interval; GI = gastrointestinal; HR = hazard ratio; IRR = incident rate ratio; PPI = proton pump inhibitor; H2RA = H2-receptor antagonist.
Results based on adjusted analysis
Data supplied by author.
Pooled PPI effect estimated using weighted average for the individual anticoagulants.
Adjusted differences not reported and unable to be calculated using reported data.
All results in this cell were adjusted with a time-dependent Poisson regression model in the original analysis.
In the cohort study by Lee et al,17 among patients with atrial fibrillation and a history of upper gastrointestinal bleeding, the pooled estimate of PPI effect across warfarin and DOACs was HR 0.67 (95% CI 0.57, 0.78) with low heterogeneity (I2 = 0%, Supplement 3, available online). Subgroups of patients using different anticoagulants had modestly different point estimates but with widely overlapping CIs. Annual absolute risk differences exceeded 0.5% (NNT <200) for all anticoagulants except edoxaban.
In the cohort study by Ray et al24 in 2016, among patients using warfarin, the adjusted HR with PPI use was 0.76 (95% CI 0.63, 0.91) with adjusted annual risk difference of −0.29% (95% CI −0.44%, −0.10%) and NNT of 334 (95% CI 250, 1000). The reduction in upper gastrointestinal bleeding was greater (P = .01) in patients using antiplatelet drugs or NSAIDs (adjusted HR = 0.55, 95% CI 0.39, 0.77; adjusted annual risk difference = −1.3%, 95% CI −1.7%, −0.7%; NNT 77, 95% CI 59 to 143) than in nonusers (adjusted HR 0.86, 95% CI 0.70, 1.06; adjusted annual risk difference −0.1%, 95% CI −0.3%, 0.06%). The magnitude of effect was similar across patients prescribed NSAIDs, aspirin, and other antiplatelet drugs.
In the cohort study by Ray et al23 in 2018 including patients using warfarin or DOACs, the adjusted IRR for PPI use was 0.66 (95% CI 0.62, 0.69), with an annual risk difference of −0.40% (95% CI −0.44%, −0.35%) and an NNT of 250 (95% CI 225, 286). PPIs were also associated with reduced upper gastrointestinal bleeding in separate analyses of patients using each individual anticoagulant. The treatment effect increased with increasing gastrointestinal bleeding risk (based on an internally derived risk score calculated using ~85 variables). At the lowest decile of risk, no treatment effect was identified: IRR 0.98 (95% CI 0.79, 1.20), annual risk difference 0% (95% CI −0.1%, 0.1%). In all other deciles, the relative risk reduction was significant and similar and in the range of 30%−50%. However, the annual absolute risk reduction was 0.2% (NNT = 500) in the third to sixth deciles and rose to 0.5% (NNT = 200) in the eighth decile and 1.2% (NNT = 84) in the highest decile of risk.
In the COMPASS trial, for the pooled estimate of overall upper gastrointestinal bleeding, the RR for pantoprazole (vs placebo) in the rivaroxaban plus aspirin arm was 1.11 (95% CI 0.66, 1.86), with an annual risk difference of 0.1% (95% CI −0.4%, 0.6%), and in the rivaroxaban alone arm was 0.88 (95% CI 0.49, 1.58), with an annual risk difference of −0.1% (95% CI −0.5%, 0.4%).18 In the rivaroxaban alone arm, the HR for pantoprazole was 0.25 (95% CI 0.07, 0.89), with an annual NNT = 909 (95% CI 526, 909) for 1 of the 2 definitions of upper gastrointestinal bleeding—confirmed gastroduodenal bleeding. In both antithrombotic arms, there were low annual rates of confirmed gastroduodenal bleeding (range 0.03%–0.14%) and overt upper gastrointestinal bleeding of unknown origin (0.14%–0.25%).
In the single observational study evaluating H2RAs, the RR was 0.69 (95% CI 0.24, 2.02).22
Pooled Estimate of Effect for PPIs
The study by Maruyama et al25 was not included in the meta-analysis because the HR and its lower 95% CI were 0, which precluded conversion to a defined logarithmic value. The study by Ray et al24 from 2016 was not included because the study sample overlapped with the sample in Ray et al23 from 2018. The latter study was preferentially included because it had the larger sample size. As depicted in Figure 1, the pooled RR was 0.67 (95% CI 0.61, 0.74) with low statistical heterogeneity (I2 = 15%, P = 0.32). Ray et al23 contributed the greatest weight to the pooled estimate (67.5%). In the sensitivity analysis excluding the trial by Moayyedi et al,18 the pooled RR was similar (0.66, 95% CI 0.57, 0.78; Supplement 4, available online).
DISCUSSION
In this systematic review and meta-analysis, PPIs were associated with a reduced likelihood of upper gastrointestinal bleeding in patients taking anticoagulants with a RR of 0.67 (95% CI 0.61, 0.74). Effect sizes were relatively consistent in populations that used warfarin, DOACs, or both.
Each individual observational study showed a reduction in upper gastrointestinal bleeding with PPI co-therapy, with estimates of relative risk reduction ranging from 24% to 100%, although the upper bound of the 95% CI exceeded 1 in the 3 smallest studies.16,22,25 The benefit of PPI co-therapy appeared to be most clearcut and substantial in patients at increased risk for upper gastrointestinal bleeding. For example, Ray et al24 from 2016, showed a significantly greater treatment effect in patients using anticoagulants plus aspirin or NSAIDs (HR = 0.55) compared those not taking aspirin or NSAIDs (HR = 0.86), with an NNT of 77 in the higher-risk group. Ray et al23 from 2018, found that PPIs provided benefit in all patients other than those in the lowest decile of gastrointestinal bleeding risk. Although relative risk reduction was similar across the other 90% of patients, given a low baseline risk in most deciles, the annual absolute risk reduction exceeded 0.5% (and NNT <200) only in the 30% of patients at highest risk. Collectively, these findings suggest that PPI therapy is appropriate for patients at increased risk due to factors such use of NSAIDs or aspirin, with NNT <200 for the prevention of a potentially life-threatening event, in support of existing guidelines for such patients.26,27
Only 1 RCT met inclusion criteria, the COMPASS trial, in which participants treated with low-dose rivaroxaban either with or without aspirin, were randomized to pantoprazole or placebo. PPIs were not associated with a reduced likelihood of overt upper gastrointestinal bleeding in either antithrombotic arm.18 We believe the findings of the COMPASS trial are consistent with the results of the observational studies previously discussed. COMPASS enrolled only “participants with no clinical need for a PPI.” Thus, patients in this trial were considered by investigators to have a low risk for upper gastrointestinal bleeding and did not represent the population who would typically be candidates for co-therapy in real-world clinical practice. In fact, in the COMPASS trial, the incidence of gastroduodenal bleeding for participants assigned rivaroxaban without pantoprazole (0.14% per year) was one-tenth the incidence in the largest available observational study (1.44% per year).23 The results in observational studies indicate such low-risk patients would be expected to have minimal if any benefit from PPI therapy, as shown in the COMPASS trial. In addition, risk of bleeding in the COMPASS trial may have been reduced given the low doses of anticoagulant—either rivaroxaban 5 mg twice daily or 2.5 mg twice daily (in combination with aspirin); 20 mg/d is the standard dose for atrial fibrillation. Although some have concluded from the COMPASS trial that PPIs are not effective for the prevention of upper gastrointestinal bleeding in patients taking anticoagulants,28 these features of the trial raise questions about its generalizability to patients taking anticoagulants in clinical practice.
Limitations
Our study has several limitations. First, the results of the review are susceptible to the biases of the constituent studies. However, all but 1 of the studies were low risk for bias. The observational studies are particularly susceptible to misclassification of PPI, aspirin, and NSAID use, which would tend to bias estimates of PPIs’ effect toward the null. Second, the studies had heterogenous study populations and designs. However, the consistency of the negative association between PPIs and upper gastrointestinal bleeding in the observational studies and low statistical heterogeneity in the meta-analysis is reassuring. Third, some relevant studies may have been overlooked. To minimize this risk, we involved a health librarian to design a comprehensive search strategy.
Moving forward, it is unlikely that traditional RCTs will be done in patients taking anticoagulants and at increased risk for upper gastrointestinal bleeding for ethical reasons because guidelines already recommend PPI gastroprotection for certain high-risk groups.26,27,29 Future observational and quasi-experimental studies can still be informative. Research should focus on better characterizing individual risk of upper gastrointestinal bleeding. To our knowledge, no validated risk score exists for this purpose. Given the multidisciplinary nature of anticoagulation care, it is important that recommendations about PPI use be incorporated broadly into relevant guidance statements.30,31
CONCLUSION
In conclusion, our systematic review and meta-analysis suggest that PPIs reduce the risk of upper gastrointestinal bleeding in patients taking anticoagulants, but that the absolute benefits vary substantially depending on baseline risk. For patients taking anticoagulants without additional risk factors for upper gastrointestinal bleeding, the benefit from PPIs is likely minimal. We believe that future guideline panels should incorporate our results and include a recommendation for PPI co-therapy in patients taking oral anticoagulants who have elevated baseline risk for upper gastrointestinal bleeding, including those using concomitant antiplatelet therapy or NSAIDs.
Supplementary Material
Figure 2.
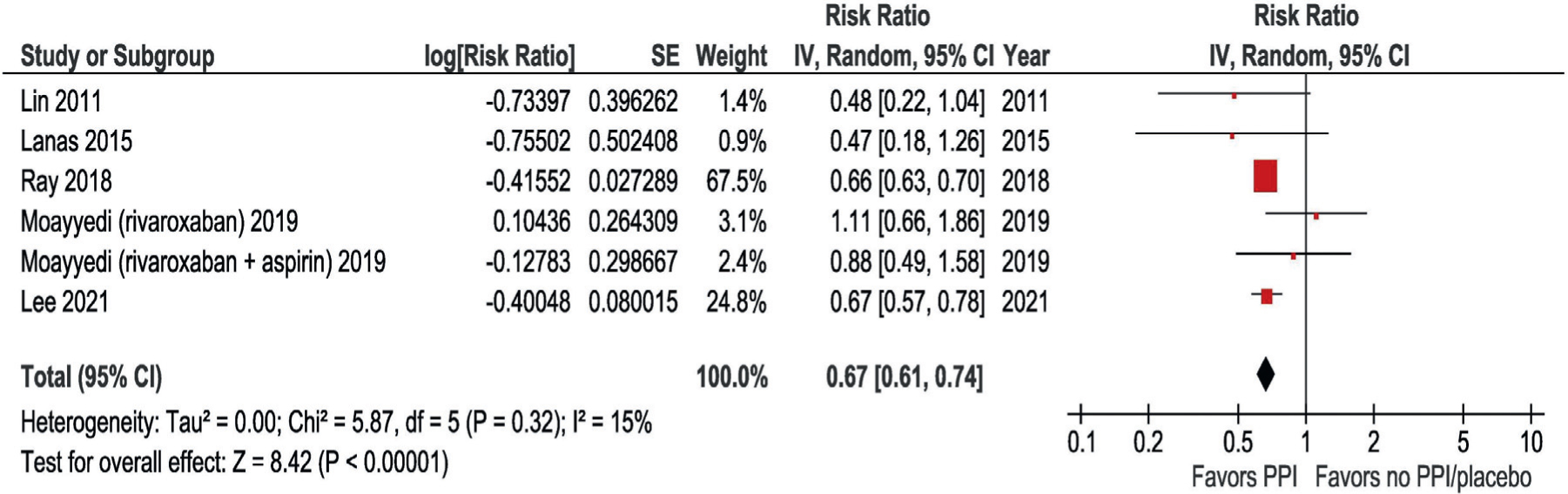
Analysis of the association between PPI use and risk of upper gastrointestinal bleeding in meta-analysis. The study by Maruyama et al25 was not included because the confidence interval could not be estimated. The study by Ray et al24 from 2016 was not included because its study sample overlapped with Ray et al23 from 2018. For the trial by Moayyedi et al18, the subgroups of patients treated with rivaroxaban alone and rivaroxaban plus aspirin were included separately. PPI = proton pump inhibitor.
CLINICAL SIGNIFICANCE.
Meta-analysis showed that for patients who use anticoagulants, proton pump inhibitors were associated with a reduction in upper gastrointestinal bleeding (relative risk 0.67, 95% confidence interval 0.61, 0.74).
The benefits of proton pump inhibitors vary substantially depending on baseline risk of upper gastrointestinal bleeding and are greatest in patients who use nonsteroidal anti-inflammatory drugs or antiplatelet drugs in addition to anticoagulants.
ACKNOWLEDGMENTS
We are grateful to Whitney Townsend for her assistance with conducting the search, and to Dr Alan Barkun, Dr Luis Garcia Rodriguez, and Dr Grigorios Leontiadis for helping to identify relevant studies. All authors approved the final manuscript for submission.
Funding:
JEK received funding from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant, K23DK 11879. SDS, LL, Y-XY, and JEK received funding for study 1 I01HX002693-01 from the Veterans Affairs Health System.
Footnotes
Conflicts of Interest: GB reports consulting fees from Pfizer/Bristol-Myers Squibb, Janssen and AcelisConnected Health. NS reports consulting fees from Alexion Pharmaceuticals. LL reports consulting fees from Phathom Pharmaceuticals. JEK, AF, JJG, DH, SDS, Y-XY, and JS report none.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amjmed.2022.05.031.
References
- 1.Barnes GD, Lucas E, Alexander GC, Goldberger ZD. National trends in ambulatory oral anticoagulant use. Am J Med 2015;128:1300–1305. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ho KH, van Hove M, Leng G. Trends in anticoagulant prescribing: a review of local policies in English primary care. BMC Health Serv Res 2020;20:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith M, Wakam G, Wakefield T, Obi A. New trends in anticoagulation therapy. Surg Clin North Am 2018;98:219–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamberts M, Staerk L, Olesen JB, et al. Major bleeding complications and persistence with oral anticoagulation in non-valvular atrial fibrillation: contemporary findings in real-life Danish patients. J Am Heart Assoc Cardiovasc Cerebrovasc Dis 2017;6:e004517. 10.1161/JAHA.116.004517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodrıguez LAG, Lin KJ, Hernández-Dıaz S, Johansson S. Risk of upper gastrointestinal bleeding with low-dose acetylsalicylic acid alone and in combination with clopidogrel and other medications. Circulation 2011;123:1108–15. [DOI] [PubMed] [Google Scholar]
- 6.Hippisley-Cox J, Coupland C. Predicting risk of upper gastrointestinal bleed and intracranial bleed with anticoagulants: cohort study to derive and validate the QBleed scores. BMJ 2014;349:g4606. 10.1136/bmj.g4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scally B, Emberson JR, Spata E, et al. Effects of gastroprotectant drugs for the prevention and treatment of peptic ulcer disease and its complications: a meta-analysis of randomised trials. Lancet Gastroenterol Hepatol 2018;3:231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan FK, Leung W. Peptic-ulcer disease. Lancet 2002;360:933–41. [DOI] [PubMed] [Google Scholar]
- 9.Desai J, Kolb JM, Weitz JI, Aisenberg J. Gastrointestinal bleeding with the new oral anticoagulants – defining the issues and the management strategies. Thromb Haemost 2013;110:205–12. [DOI] [PubMed] [Google Scholar]
- 10.Vaduganathan M, Bhatt DL. Gastrointestinal bleeding with oral anticoagulation: understanding the scope of the problem. Clin Gastroenterol Hepatol 2017;15:691–3. [DOI] [PubMed] [Google Scholar]
- 11.Thomopoulos KC, Mimidis KP, Theocharis GJ, Gatopoulou AG, Kartalis GN, Nikolopoulou VN. Acute upper gastrointestinal bleeding in patients on long-term oral anticoagulation therapy: Endoscopic findings, clinical management and outcome. World J Gastroenterol 2005;11:1365–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pourafkari L, Ghaffari S, Zamani N, et al. Upper gastrointestinal bleeding in the setting of excessive warfarin anticoagulation: Risk factors, and clinical outcome. Cor Vasa 2017;59:e128–33. [Google Scholar]
- 13.Kurlander J, Fisher A, Gonzalez J, et al. Review protocol: The effect of anti-secretory agents on upper gastrointestinal bleeding risk for patients using oral anticoagulants: a systematic review. Available at: https://www-crd-york-ac-uk.proxy.lib.umich.edu/prospero/display_record.php?ID=CRD42020136610. Accessed January 14, 2021.
- 14.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed May 15, 2019.
- 15.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 16.Lanas Á, Carrera-Lasfuentes P, Arguedas Y, et al. Risk of upper and lower gastrointestinal bleeding in patients taking nonsteroidal anti-inflammatory drugs, antiplatelet agents, or anticoagulants. Clin Gastroenterol Hepatol 2015;13:906–912.e2. [DOI] [PubMed] [Google Scholar]
- 17.Lee SR., Kwon S, Choi EK. et al. Proton Pump Inhibitor Co-Therapy in Patients with Atrial Fibrillation Treated with Oral Anticoagulants and a Prior History of Upper Gastrointestinal Tract Bleeding. Cardiovasc Drugs Ther (2021). 10.1007/s10557-021-07170-6. Accessed December 15, 2021. [DOI] [PubMed] [Google Scholar]
- 18.Moayyedi P, Eikelboom JW, Bosch J, et al. Pantoprazole to prevent gastroduodenal events in patients receiving rivaroxaban and/or aspirin in a randomized, double-blind, placebo-controlled trial. Gastroenterology 2019;157:403–412.e5. [DOI] [PubMed] [Google Scholar]
- 19.Confidence Interval for a Rate Difference. Available at: https://sphweb.bumc.bu.edu/otlt/MPH-Modules/PH717-QuantCore/PH717_ComparingFrequencies/PH717_ComparingFrequencies12.html. Accessed July 12, 2021.
- 20.Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed Hoboken, NJ: Wiley-Blackwell; 2019:1–728. [Google Scholar]
- 21.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin KJ, Hernández-Dıaz S, García Rodríguez LA. Acid suppressants reduce risk of gastrointestinal bleeding in patients on antithrombotic or anti-inflammatory therapy. Gastroenterology 2011;141:71–9. [DOI] [PubMed] [Google Scholar]
- 23.Ray WA, Chung CP, Murray KT, et al. Association of oral anticoagulants and proton pump inhibitor cotherapy with hospitalization for upper gastrointestinal tract bleeding. JAMA 2018;320:2221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ray WA, Chung CP, Murray KT, et al. Association of proton pump inhibitors with reduced risk of warfarin-related serious upper gastrointestinal bleeding. Gastroenterology 2016;151:1105–12 [e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maruyama K, Yamamoto T, Aoyagi H, et al. Difference between the upper and the lower gastrointestinal bleeding in patients taking nonvitamin K oral anticoagulants. BioMed Res Int 2018;2018:7123607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumbhani DJ, Cannon CP, Beavers CJ, et al. 2020. ACC Expert Consensus Decision Pathway for anticoagulant and antiplatelet therapy in patients with atrial fibrillation or venous thromboembolism undergoing percutaneous coronary intervention or with atherosclerotic cardiovascular disease. J Am Coll Cardiol. 77(5):629–658. 10.1016/j.jacc.2020.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Lanza FL, Chan FKL, Quigley EMM. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol 2009;104:728–38. [DOI] [PubMed] [Google Scholar]
- 28.Lee TC, McDonald EG. Deprescribing proton pump inhibitors: overcoming resistance. JAMA Intern Med 2020;180(4):571–3. 10.1001/jamainternmed.2020.0040. [DOI] [PubMed] [Google Scholar]
- 29.Barkun AN, Almadi M, Kuipers EJ, et al. Management of nonvariceal upper gastrointestinal bleeding: guideline recommendations from the International Consensus Group. Ann Intern Med 2019;171(11):805–22. 10.7326/M19-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomaselli GF, Mahaffey KW, Cuker A, et al. 2020 ACC Expert Consensus Decision Pathway on management of bleeding in patients on oral anticoagulants. J Am Coll Cardiol 2020;76:594–622. [DOI] [PubMed] [Google Scholar]
- 31.Frost J, Campos-Outcalt D, Hoelting D, et al. Pharmacologic management of newly detected atrial fibrillation updated clinical practice guideline; Updated Clinical Practice Guideline; 2017. Available at: https://www.aafp.org/family-physician/patient-care/clinical-recommendations/all-clinical-recommendations/atrial-fibrillation.html. Accessed October 26, 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


