Abstract
Background
Approximately 13.8% and 6.1% of coronavirus disease 2019 (COVID-19) patients require hospitalization and sometimes intensive care unit (ICU) admission, respectively. There is no biomarker to predict which of these patients will develop an aggressive stage that we could improve their quality of life and healthcare management. Our main goal is to include new markers for the classification of COVID-19 patients.
Methods
Two tubes of peripheral blood were collected from a total of 66 (n = 34 mild and n = 32 severe) samples (mean age 52 years). Cytometry analysis was performed using a 15-parameter panel included in the Maxpar® Human Monocyte/Macrophage Phenotyping Panel Kit. Cytometry by time-of-flight mass spectrometry (CyTOF) panel was performed in combination with genetic analysis using TaqMan® probes for ACE2 (rs2285666), MX1 (rs469390), and TMPRSS2 (rs2070788) variants. GemStone™ and OMIQ software were used for cytometry analysis.
Results
The frequency of CD163+/CD206- population of transitional monocytes (T-Mo) was decreased in the mild group compared to that of the severe one, while T-Mo CD163-/CD206- were increased in the mild group compared to that of the severe one. In addition, we also found differences in CD11b expression in CD14dim monocytes in the severe group, with decreased levels in the female group (p = 0.0412). When comparing mild and severe disease, we also found that CD45- [p = 0.014; odds ratio (OR) = 0.286, 95% CI 0.104–0.787] and CD14dim/CD33+ (p = 0.014; OR = 0.286, 95% CI 0.104–0.787) monocytes were the best options as biomarkers to discriminate between these patient groups. CD33 was also indicated as a good biomarker for patient stratification by the analysis of GemStone™ software. Among genetic markers, we found that G carriers of TMPRSS2 (rs2070788) have an increased risk (p = 0.02; OR = 3.37, 95% CI 1.18–9.60) of severe COVID-19 compared to those with A/A genotype. This strength is further increased when combined with CD45-, T-Mo CD163+/CD206-, and C14dim/CD33+.
Conclusions
Here, we report the interesting role of TMPRSS2, CD45-, CD163/CD206, and CD33 in COVID-19 aggressiveness. This strength is reinforced for aggressiveness biomarkers when TMPRSS2 and CD45-, TMPRSS2 and CD163/CD206, and TMPRSS2 and CD14dim/CD33+ are combined.
Keywords: biomarkers, cytometry, COVID-19, SNPs, CyTOF
Graphical Abstract
Graphical abstract representing the workflow of the present project and summarizing the main techniques applied to the analysis of samples.
1. Introduction
Since its emergence in Wuhan in December 2019, the virus responsible for coronavirus disease 2019 (COVID-19), severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread globally and become a world-threatening disease (1). According to the World Health Organization (WHO) report of 7 December 2022, the pandemic has exceeded 640 million cases and 6.6 million deaths worldwide (2). Since the onset of the pandemic, the search for biomarkers to correctly classify patients has been one of the major challenges for experts in this disease. Several biomarkers such as C-reactive protein (CRP), serum ferritin, D-dimer, and interleukin-6 (IL-6) have been studied for prognostic assessment of patients with COVID-19 pneumonia or simply for patient management (3). New biomarkers such as presepsin as a soluble CD14 subtype in sepsis patients have been continuously included (4).
The role of cytokines and chemokines has been associated with disease severity and clinical COVID-19 outcomes during all pandemic, suggesting that these molecules are the most promising biomarkers for patient management. Some inflammatory biomarkers have been reported to be significantly associated with an increased risk of developing severe COVID-19, such as procalcitonin (PCT), serum ferritin, CRP, IL-6, or erythrocyte sedimentation rate (ESR) (4). In addition, technologies combining flow and mass cytometry have improved multiple single-cell immune profiling in COVID-19 patients by revealing changes in both innate and adaptive immune cell subpopulations and their correlation with disease severity. There are reports of reduced frequencies in cell populations of severe COVID-19 patients in monocytes [particularly CD14lo CD16hi nonclassical monocytes (NC-Mo)], dendritic cells (DCs), and natural killer (NK) cells (5).
Due to the important role of the immune system and the immunological mechanisms that correlate with disease progression, we focus on the analysis using cytometry by time-of-flight mass spectrometry (CyTOF). This is a method that allows the simultaneous analysis of more than 40 cell markers without spectral overlap (5, 6).
There are controversial data regarding the best immunologic marker for COVID-19 monitoring. Recently, it has been reported that severe disease can be distinguished from moderate disease by systemic loss and dysfunction of M1-like pro-inflammatory monocytes, conventional DCs, and plasmacytoid dendritic cells (pDCs) (7). Moreover, in pediatric patients, it has been described that serum soluble CD25 and soluble CD163 levels have been described to be upregulated in the serum of SARS-CoV-2 patients (8), as well as increased levels of CD11c+. A CD16hi population has been identified during the acute phase of infection and at disease severity in non-human primates (9).
There are also proteins such as the angiotensin-converting enzyme 2 (ACE2) whose high expression pattern is associated with an increased risk of severe infection and complications in COVID-19. In addition, a positive correlation of the upregulation of CD61 and CD163 with ACE2 expression has been found (10). The SARS-CoV-2 spike (S) protein binds to ACE2, which acts as an entry receptor. Once the receptor binds, the S protein is expressed and cleaved by the transmembrane protease serine 2 (encoded by TMPRSS2) (11). Therefore, these two genes have also come into focus as COVID-19 biomarkers, and the use of single-nucleotide polymorphisms (SNPs) as noninvasive biomarkers is readily available in routine clinical practice. For example, rs2285666 (G870A) has been suggested as the best option for the ACE2 polymorphism that modulates susceptibility to SARS-CoV-2 infection (12). Similarly, the TMPRSS2 gene has been reported to have several variants associated with susceptibility to COVID-19, mainly rs2070788, rs734056, rs12329760, rs2276205, and rs3787950 (13). Furthermore, rs2070788 is highlighted as a factor affecting COVID-19 severity. MX dynamin-like GTPase (MX1) has been highlighted as a critical responder in SARS-CoV-2 infection. Its expression is increased in COVID-19 patients and is strikingly associated with the increase in viral load (14). In addition, increased basal MX1 levels have been reported to correlate with SARS-CoV-2 infection, helping to identify the patient’s predisposition to severe disease (15).
Clinically, nearly 80% of COVID-19 cases are asymptomatic or have a mild form of the disease. However, approximately 13.8% and 6.1% are severe and critical, respectively, requiring hospitalization and even intensive care in the life-threatening cases (16). Our main objective is to demonstrate the role of various noninvasive biomarkers, especially cytometric and genetic biomarkers, that could predict or anticipate the most severe outcomes among these patients.
2. Materials and methods
2.1. Patients
A total of 66 (n = 34 mild and n = 32 severe) patients with a mean age of 52 years were recruited between 2020 and 2021. All clinical data [ferritin, D-dimer, CRP, troponin, lactate dehydrogenase (LDH)], symptoms (fever, anosmia, asthenia, dyspnea, long COVID, etc.), and intensive care unit (ICU) clinical follow-up (need for assisted ventilation, pneumonia, etc.) were included in the report; further details are described in Table 1 . The following inclusion variables were taken into account for the severe group: (i) hypoxia with peripheral oxygen saturation (SpO2) ≤93% or partial pressure of oxygen/inspired oxygen fraction (PaO2/FiO2) >300 mmHg; (ii) respiratory rate (RR) ≥30 breaths/min; or (iii) ICU admission. The inclusion criteria for the mild group were (i) SpO2 >93%; (ii) the presence of nonspecific symptoms such as fever, fatigue, cough, or muscle pain, without hospitalization; or (iii) imperceptible symptoms during infection. In all patients, SARS-CoV-2 infection was confirmed by positive reverse transcription polymerase chain reaction (RT-PCR) or by positive IgM antibody test and at the same timeline after COVID-19 recovery. These inclusion criteria were revised periodically. For sample collection, only severe samples were collected in the ICU during the patient’s hospitalization, and the remaining groups were collected in primary assistance all collected post COVID-19 infection. Two tubes of peripheral blood were collected in ethylenediaminetetraacetic acid (EDTA) anticoagulant from each patient.
Table 1.
Descriptive characteristic of the samples.
| Variants | Patients |
|---|---|
| Age (years) | |
| <55 | 40 (60.6%) |
| ≥55 | 26 (39.4%) |
| Sex | |
| Male | 30 (45.5%) |
| Female | 36 (55.5%) |
| Aggressiveness | |
| Mild | 34 (51.5%) |
| Severe | 32 (48.5%) |
| Flu Vaccine | |
| No | 44 (66.7%) |
| Yes | 21 (31.8%) |
| Missing | 1 (1.5%) |
| Long COVID | |
| No | 62 (93.9%) |
| Yes | 1 (1.5%) |
| Missing | 3 (4.5%) |
| Pneumonia | |
| No | 33 (50%) |
| Yes | 31 (47%) |
| Missing | 2 (3%) |
| Systemic inflammatory response | |
| No | 45 (68.2%) |
| Yes | 18 (27.3%) |
| Missing | 3(4.5%) |
Long COVID, individuals with confirmed SARS CoV-2 infection with symptoms that last for at least 2 months and cannot be explained by an alternative diagnosis.
Mo_Classical: Classical Monocytes.
Mo_Transitional: Transitional Monocytes.
Mo_Nonclassical Non classical Monocytes.
The study protocol was approved by the Research Ethics Committee of Granada (CEI-Granada) with internal code 1329-N-21. Written informed consent was obtained from all participants in accordance with the tenets of the Declaration of Helsinki.
2.2. Cytometry analysis
2.2.1. Whole-blood sample processing
For flow cytometry analysis, blood samples were processed within 3 h after collection. Blood cells were fixed by withdrawing 700 μl of blood and adding 1 ml of Proteomic Stabilizer PROT1 (Smart Tube Inc., San Carlos, CA, USA) and incubating for 10 min at room temperature (RT). The blood was then frozen and stored at -80°C until staining.
After thawing at 4°C on a roller, samples were diluted in 13 ml of Thaw-Lysis Buffer 1X (Smart Tube Inc., San Carlos, CA, USA), filtered through a 100-µm pluriStrainer (pluriSelect Life Science, Leipzig, DE), and lysed in a roller for 10 min at RT. Cells were then pelleted, and leukocytes were washed with Maxpar® Cell Staining Buffer (CST) (Fluidigm, San Francisco, CA, USA) and resuspended in 2 ml of CST. Blood samples were counted, aliquoting the same number of cells for each sample, 2.2 * 106 cells/sample in this protocol. Then, all samples were pooled in a single tube and 1 µl of Fc block (BD Biosciences, Franklin Lakes, NJ, USA) was added and incubated for 10 min at RT. We then stained the surface antigens with the previously thawed surface antibody cocktail and incubated it at 4°C for 30 min. We then washed with CST and fixed with 1 ml of paraformaldehyde (PFA) 2% [stock 16% formaldehyde solution (ThermoScientific, Rockford, IL, USA)] and incubated for 10 min at RT. Then, we stained the DNA with iridium (Ir) solution and incubated overnight at 4°C with 1:2,000 Ir (125 μM) in Fix and Perm buffer. Samples were frozen and stored at -80°C until collection.
2.2.3. Data acquisition
Cells stained for mass cytometry were thawed. Each sample was phenotyped at baseline using a 15-parameter monocyte and macrophage CyTOF panel ( Table 2 ). For CyTOF acquisition, samples were washed in Maxpar® Cell Acquisition Buffer (CAS). Prior to acquisition, 1 million cells/ml were resuspended in CAS containing EQ beads (1:10) and double filtered through 35-µm cell strainer cap tubes. Samples were acquired at a rate of 250–300 events per second on a Helios® Mass Cytometer (Fluidigm, San Francisco, CA, USA).
Table 2.
Antibody information.
| Maxpar® Human Monocyte/Macrophage Phenotyping Panel Kit | |||
|---|---|---|---|
| Target | Clone | Source | Isotope |
| CD19 | HIB19 | Fluidigm | 142Nd |
| CD11b | ICRF44 | Fluidigm | 144Nd |
| CD7 | CD7-6B7 | Fluidigm | 147Sm |
| CD66 | CD66a-B1.1 | Fluidigm | 149Sm |
| CD36 | 5-271 | Fluidigm | 152Sm |
| CD163 | GHI/61 | Fluidigm | 154Sm |
| CD45 | HI30 | Fluidigm | 156Gd |
| CD11c | Bu15 | Fluidigm | 159Tb |
| CD14 | M5E2 | Fluidigm | 160Gd |
| CD16 | 3G8 | Fluidigm | 165Ho |
| CD38 | HIT2 | Fluidigm | 167Er |
| CD206 | 15-2 | Fluidigm | 168Er |
| CD33 | WM53 | Fluidigm | 169Tm |
| CD3 | UCHT1 | Fluidigm | 170Er |
| HLA-DR | L243 | Fluidigm | 174Yb |
2.2.4. Data analysis
Raw data were normalized using MATLAB R2021a (MathWorks, Natick, MA, USA). We used GemStone™ version 2.0.45 software (Verity Software House, Topsham, ME, USA) and FlowJo version 10.8.1 (BD Biosciences, Franklin Lakes, NJ, USA) to analyze and clean CyTOF data. The normalized Flow Cytometry Standard File (FCS) files were transferred to GemStone™ software, which performs a standardized, automated, and unsupervised quality check (bead removal and selection of high-quality singletons). The software then analyzed different populations of leukocytes. Dimensionality reduction, clustering algorithm, and heatmap were performed using OMIQ data analysis software (OMIQ, Inc., Santa Clara, CA, USA). In addition, the Cen-se’ algorithm identifies related events based on measurement; we selected them and plotted them in a bivariate graph. Cen-se’ allows us to assess how accurately our model identifies these cells in our cell types. We can also examine cells that do not yet belong to any cell type and find out which measurements identify them (17).
2.3. Genetic analysis
Blood samples for genetic analysis were processed in the following 4–6 h after collection according to a protocol that depended on the subsequent analysis. For genotyping analysis, plasma was collected by centrifugation at 1,400g and 4°C for 10 min. After separation, plasma samples were frozen at -80°C until subsequent analysis.
The DNA extraction protocol was performed according to the manufacturer’s protocol of the Real Blood DNA Kit (Real life-science solutions, Valencia, Spain). All samples were standardized to 20 ng/μl using the Nanodrop 2000/2000c (ThermoFisher, Waltham, MA, USA) and had values between 1.8 and 2.0 A280/260. DNA genotyping was performed using the TaqMan® Genotyping Master Mix (Applied Biosystems, Foster City, CA, USA), which contains all essential components (except probes, templates, and water) for polymerase chain reaction (PCR). Allelic discrimination assays were performed in a 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Results were analyzed using SDS software version 2.4 (Applied Biosystems, Foster City, CA, USA).
The selection of SNPs was performed according to The National Center for Biotechnology Information website in the most relevant data according to COVID-19 and genetic markers. In addition, only those SNPs with an allelic frequency greater than 20% in the minor allele (MAF) in the Caucasian population were selected from the Ensembl database (18). Finally, we selected ACE2 (rs2285666), MX1 (rs469390), and TMPRSS2 (rs2070788) for the present analysis; see details of the probes in Supplementary Table S1 .
2.4. Statistical analysis
Continuous flow cytometry variables were transformed into categorical variables using a binning strategy. Thus, flow cytometry variables were divided into two groups according to higher or lower expression based on the median value of the total number of patients. Categorical variables were then analyzed by chi-square test (χ2). Logistic regression analysis (either binary or multiple) was used to assess which of the genetic and/or flow cytometric factors might be determinant of COVID-19 risk. Odds ratios (ORs) and 95% confidence intervals (95% CIs) were calculated with p-value <0.05 as the criterion for significance. All analyses were performed with SPSS version 22 statistical package (IBM Corporation, Armonk, NY, USA) and GraphPad Prism version 8.2.1 (GraphPad Software, USA). The heatmap was created using the packages tidyr version 1.2.0, ggplot2 version 3.3.6, and forcats version 0.5.1 of R version 4.1.3. Before generating the heatmap, the samples were normalized using the min-max normalization method.
3. Results
3.1. Cytometry analysis
3.1.1. General population description
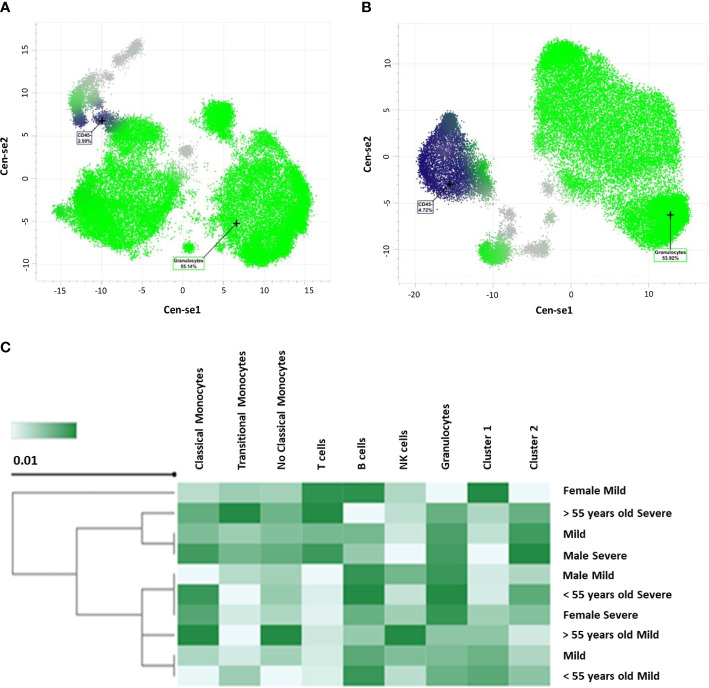
When performing a descriptive analysis of cytometry parameters in 66 patients (n = 34 mild and n = 32 severe) with a mean age of 52 years, we were able to summarize these markers as follows; see details in Table 3 . As can be seen, CD45- has a statistically significant value (p = 0.014) between mild and severe patients. Comparable results are reported for CD45- when comparing Cen-se’ algorithm performed with GemStone™ software. Cen-se’ algorithm also showed that we can easily differentiate between severe ( Figure 1A ) and mild patients ( Figure 1B ) in the granulocyte population ( Figure 1 ). A summary of the two populations is shown in a heatmap ( Figure 1C ).
Table 3.
Analysis of cytometry variables in the main peripheral blood cell populations.
| Variants | Mild | Severe | OR | 95% CI | p-value |
|---|---|---|---|---|---|
| CD3+ | |||||
| <22.51 | 16 | 17 | 0.784 | 0.298-2.063 | 0.622 |
| ≥22.51 | 18 | 15 | |||
| Granulocytes | |||||
| <54.41 | 18 | 15 | 1.275 | 0.485-3.354 | 0.622 |
| ≥54.41 | 16 | 17 | |||
| B Cells | |||||
| <2.78 | 15 | 17 | 0.697 | 0.264-1.837 | 0.464 |
| ≥2.78 | 19 | 15 | |||
| NK Cells | |||||
| <3.15 | 15 | 17 | 0.697 | 0.264-1.837 | 0.464 |
| ≥3.15 | 19 | 15 | |||
| CD45- | |||||
| <2.46 | 12 | 21 | 0.286 | 0.104-0.787 | 0.014 |
| ≥2.46 | 22 | 11 | |||
| Mo_Classical | |||||
| <4.82 | 17 | 16 | 1 | 0.381-2.626 | 1 |
| ≥4.82 | 17 | 16 | |||
| Mo_Transitional | |||||
| <0.07 | 14 | 17 | 0.618 | 0.233-1.636 | 0.331 |
| ≥0.07 | 20 | 15 | |||
| Mo_Nonclassical | |||||
| <0.18 | 15 | 17 | 0.697 | 0.264-1.837 | 0.464 |
| ≥0.18 | 19 | 15 | |||
| CD14dim | |||||
| <1.23 | 16 | 16 | 0.889 | 0.338-2.336 | 0.811 |
| ≥1.23 | 18 | 16 | |||
In bold statistically significant values.
Figure 1.
CD45- represented by Cen-se’ algorithm with GemStone™ software. (A) Severe population of patients. (B) Mild population of samples. (C) Heatmap representation of both populations.
3.1.2. Comparisons between severe and mild group of patients
CD163+/CD206- frequencies were decreased in the mild group compared to that of the severe group in transitional monocytes (T-Mo), while CD163-/CD206- frequencies were increased in the mild group compared to those in the severe group; see details in Figure 2 . No differences were observed between the frequencies of T-Mo in CD163+/CD206+ and CD163-/CD206+ ( Supplementary Figure S1 ). In addition, we found differences in the expression of CD11b in CD14dim-Mo in the severe group. These differences were decreased in the female (F in the figure) group (p = 0.0412) when clustering was performed in severe patients. Just to clarify, CD14dim-Mo is a monocyte population classified by GemStone™ software as an independent comparison with the classical monocyte (C-Mo) population ( Supplementary Figure S2 ).
Figure 2.
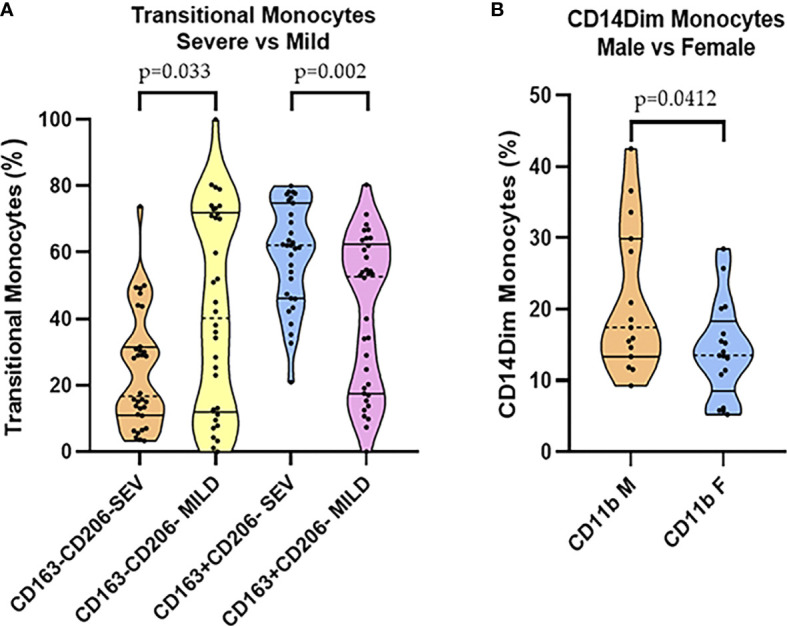
(A) CD163+/CD206- and CD163-/CD206- transitional monocyte comparisons in severe and mild groups. (B) CD11b CD14dim monocytes in male vs. Female comparisons among the severe group.
3.1.3. Age comparisons
In the severe group comparisons, by age, patients ≥55 years old had an increase in some markers in the monocyte group compared with those in patients <55 years. For instance, the expression of CD33 was higher in NC-Mo, and this difference was also observed when total CD14dim-Mo was analyzed in those older than 55 years ( Figures 3A, B ). The expression of Human Leukocyte Antigen – DR isotype (HLA-DR) in C-Mo was also higher in the ≥55 group ( Figure 3C ). Surprisingly, T-Mo frequencies of CD163+/CD206- were increased in the youngest, whereas CD163-/CD206- were increased in the older ( Figure 3D ). When we compared the mild groups by age, we also found that T-Mo CD38 and CD11b were good biomarkers for stratifying patients, as shown in Figure 3E .
Figure 3.
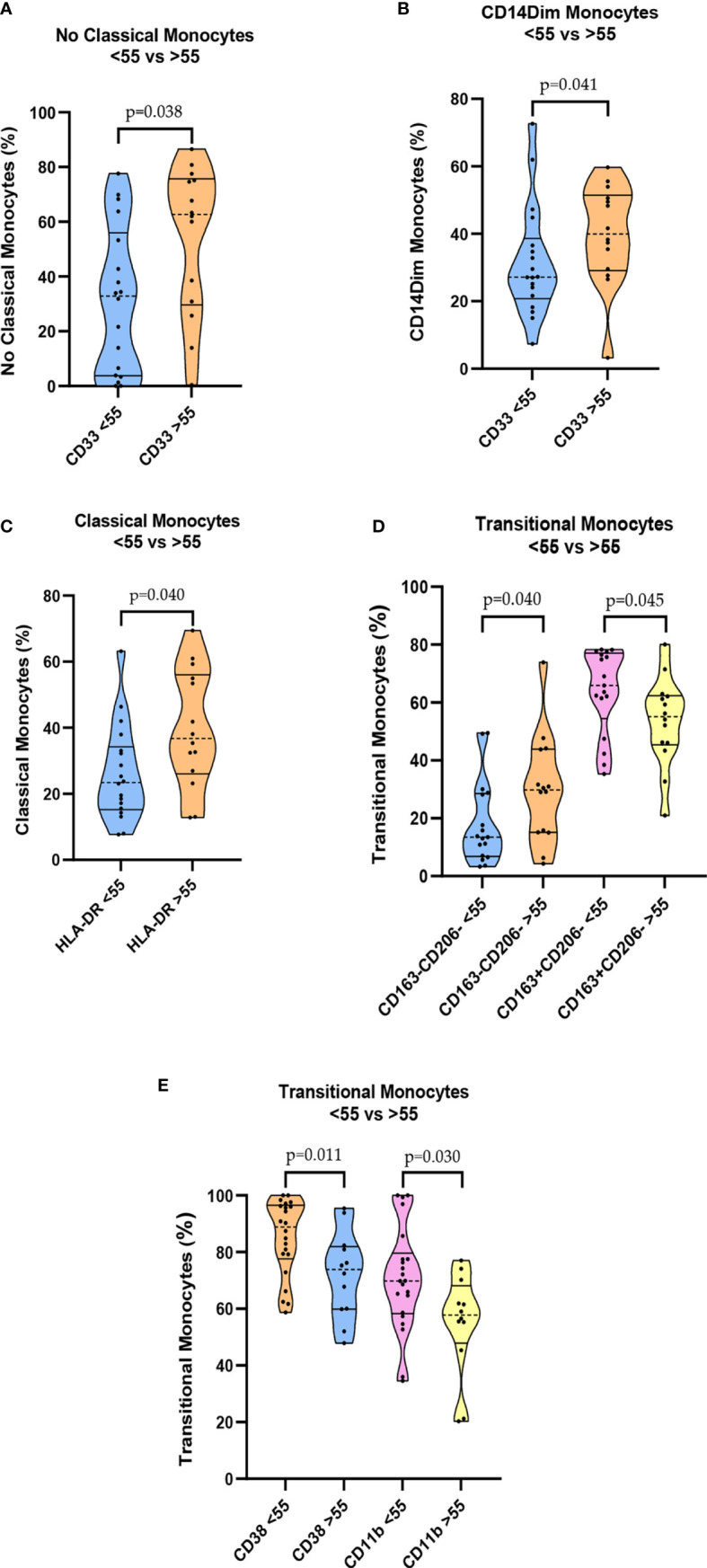
Cytometry analysis using age comparisons. Among the severe group: (A) Levels of CD33 NC-Mo cells. (B) Levels of CD33 CD14dim monocytes. (C) Levels of HLA-DR classical monocytes. (D) Levels of CD163+/CD206- T-Mo cells. Among the mild group: (E) Levels of CD38 T-Mo and CD11b T-Mo.
3.1.4. Gender comparisons
In the mild COVID group, we found a difference in NK cell expression. The frequencies were decreased in the female group compared to those in the male group (p = 0.030) ( Figure 4A ). The expression of CD11b was also higher in C-Mo (p = 0.043) ( Supplementary Figure S3 ) and CD163+/CD206+ NC-Mo in the men (p = 0.004) ( Figure 4A ). The expression of HLA-DR in CD14dim-Mo was lower (p = 0.009) and the frequencies in T-Mo of CD163+/CD206- were decreased in the men (p = 0.033) ( Figure 4B ). A heatmap of these data is provided in Supplementary Figure S4 .
Figure 4.
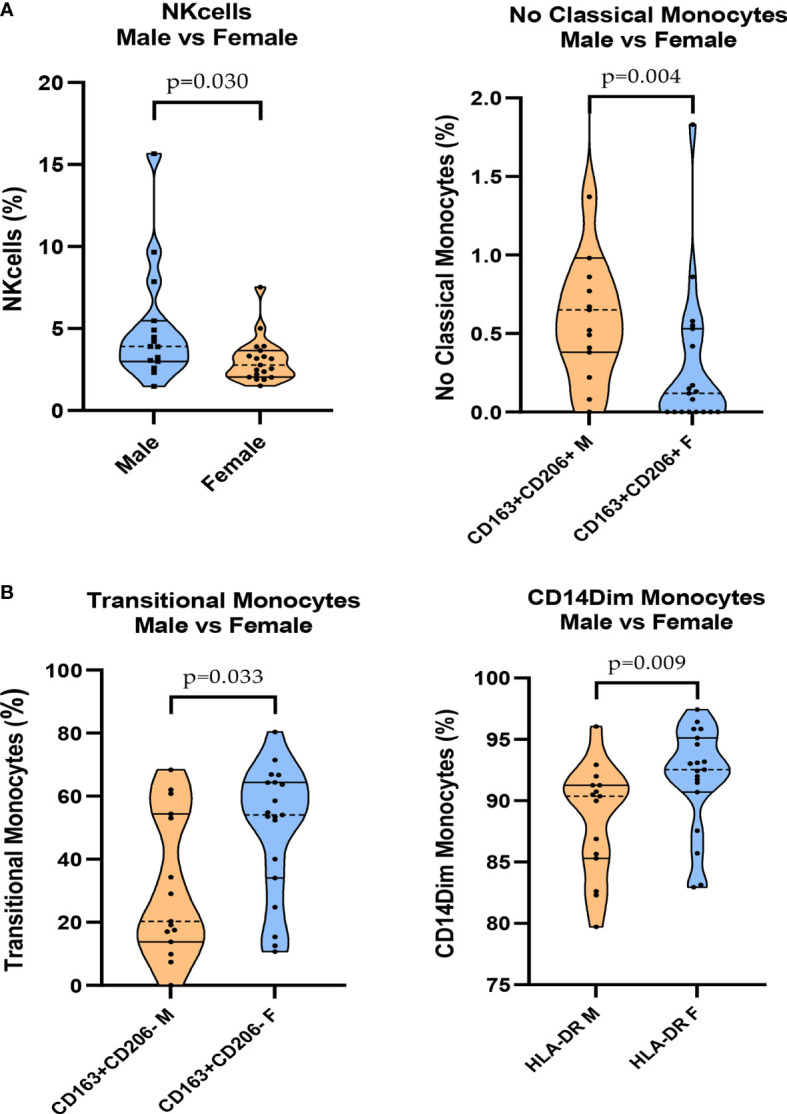
Mild group comparisons between men and women in cytometry analysis. (A) Levels of NK cells and CD163+/CD206+ NC-Mo cells. (B) Levels of CD163+/CD206- transitional monocytes and HLA-DR CD14dim monocytes.
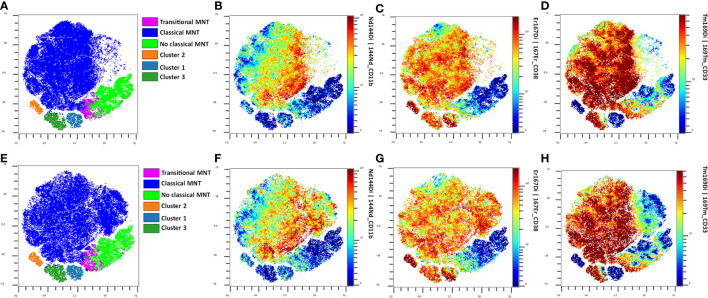
In summary, when comparing mild vs. severe COVID-19 patients, we found that CD45- (p = 0.014; OR = 0.286, 95% CI 0.104–0.787); T-Mo CD163+/CD206- (p = 0.049; OR = 2.692, 95% CI 0.995–7.284), and CD14dim/CD33+ (p = 0.014; OR = 0.286, 95% CI 0.104–0.787) are the best options as biomarkers to discriminate between these populations. We confirm the role of CD38 and CD11b as good biomarkers for patient stratification by analysis with GemStone™ software; see Figures 3E and 5 . When compared with C-Mo, there is a clear difference. This difference becomes even greater when CD33 is included, although we found no significant changes in T-Mo CD33+ populations between severe and mild patients older or younger than 55 years. FlowSOM supports flow cytometry data in a self-organizing map (SOM) as an unsupervised clustering and dimensionality reduction technique, training a discretized representation of the input space. FlowSOM can be used either as a starting point for analysis or after manual gating for easy visualization of the results. Thus, it also provides information about subpopulations that may have been missed during the original manual gating (19).
Figure 5.
FlowSOM plots from the analysis of monocytes in mild patients. (A) FlowSOM plot showing the data from mild patients older than 55 years. (B) FlowSOM plot from mild patients under 55 years. (C) CD11b plot from mild patients older than 55 years. (D) CD11b plot from mild patients under 55 years. (E) CD38 plot from mild patients older than 55 years. (F) CD38 plot from mild patients under 55 years. (G) CD33 plot from mild patients older than 55 years. (H) CD33 plot from mild patients under 55 years.
3.2. Genetic analysis
We found that G carriers (TMPRSS2 in rs2070788) have an increased risk (p = 0.02; OR = 3.37, 95% CI 1.18–9.60) of having severe COVID-19 compared to those with AA. In addition, it should be considered that other significances could not be observed due to the limited sample size for the present genetic analysis.
Interestingly, when combining genetic analysis with cytometry, we found three combinations with significant values, all in the TMPRSS2 gene. Alone in the dominant model, it shows significant values comparing mild vs. severe patients ( Table 4 ); now, this strength is reinforced when combining with CD45-, T-Mo CD163+/CD206- and CD14dim/CD33+; details in Table 5 .
Table 4.
Genetic and COVID-19 aggressiveness comparisons.
| Variants | Mild | Severe | p-value | OR | 95% CI |
|---|---|---|---|---|---|
| MX1_rs469390_Dom | |||||
| AA | 11 | 9 | 0.70 | 1.22 | 0.42-3.50 |
| AG + GG | 23 | 23 | |||
| MX1_rs469390_Rec | |||||
| GG | 9 | 5 | 0.28 | 1.94 | 0.57-6.59 |
| AG + AA | 25 | 27 | |||
| ACE2_rs2285666_Dom | |||||
| CC | 26 | 27 | 0.42 | 0.62 | 0.17-2.08 |
| CT + TT | 8 | 5 | |||
| ACE2_rs2285666_Rec | |||||
| CT + CC | 31 | 30 | 0.69 | 1.45 | 0.22-9.30 |
| TT | 2 | 3 | |||
| TMPRSS2_rs2070788_Dom | |||||
| AG + GG | 16 | 24 | 0.02 | 3.37 | 1.18-9.60 |
| AA | 18 | 8 | |||
| TMPRSS2_rs2070788_Rec | |||||
| AG + AA | 30 | 25 | 0.27 | 0.47 | 0.12-1.81 |
| GG | 4 | 7 | |||
Dom, dominant model; Rec, recessive model; OR, odds ratio; CI, confidence interval.
In bold statistically significant values.
Table 5.
TMPRSS2 and cytometry comparisons in COVID-19 aggressiveness.
| Mild vs. Severe | |||||
|---|---|---|---|---|---|
| Variants | OR | 95% CI | p-value | Omnibus test | R2 Nagelkerke |
| (Step/Block/Model) * | |||||
| TMPRSS2_rs2070788_Dom | 3.2 | 1.07-9.51 | 0.036 | 0.005 | 0.201 |
| CD45- | 0.3 | 0.10-0.85 | 0.025 | ||
| TMPRSS2_rs2070788_Dom | 5.28 | 1.57-17.68 | 0.007 | 0.002 | 0.228 |
| T-Mo CD163+/CD206- | 4.34 | 1.34-13.96 | 0.014 | ||
| TMPRSS2_rs2070788_Dom | 4.42 | 1.38-14.10 | 0.012 | 0.001 | 0.242 |
| CD14dim/CD33+ | 0.22 | 0.07-0.679 | 0.008 | ||
*All explanatory variables were added in one block and therefore have only one step. This means that the p-values are the same for the step, block, and model.
Dom, dominant model.
In bold statistically significant values.
4. Discussion
Although COVID-19 is a recent pathology, much of the research is focused on finding biomarkers for efficient diagnosis or patient stratification. There are data on the predictive ability of mortality in hospitalized patients using a score classification combining peripheral capillary oxygen saturation, albumin, D-dimer, and age (20). Others, focusing on neuroendocrine biomarkers such as copeptin, found an increase in severe cases (21). However, there are currently no conclusive data from clinical practice.
Here, we found the role of TMPRSS2 (rs2070788) G allele carriers as an interesting and simple way to classify severe COVID-19 patients. This is not the first time that the role of this marker has been suggested, as it was previously mentioned by Akin et al. (22), who identified high levels of soluble ACE2 as well as the TMPRSS2 rs2070788 non-AA genotype and low aldosterone/renin ratio as independent factors for disease severity. TMPRSS2 is the major host protease that enables cell entry of several coronaviruses and is highly expressed in lung and bronchial tissues. SARS-CoV-2 is known to utilize ACE2 as a cleavage site for S-peak protein with the help of TMPRSS2 (23). In contrast, in the present study, we found no differences between the severity of COVID-19 and the MX1 or ACE2 genes. MX1 encodes a protein with antiviral activity against RNA and DNA viruses. Several studies have shown a high expression in COVID-19 patients, but we did not find a correlation (24). ACE2 and especially several SNPs such as rs4646994 and rs2285666 have been suggested to correlate with COVID-19 susceptibility and/or disease severity, but controversial data on these two SNPs have been reported (25). Alimoradi et al. (26) and the present data suggest that there is no association between rs2285666 and COVID-19 severity.
In addition, the present analysis also exploited the power of CyTOF technology, which is currently one of the most powerful tools for immune phenotyping, allowing simultaneous and high-throughput quantification of more than 40 parameters at the single-cell level (5, 6). There are still few studies using this technology to characterize immune cell responses against SARS-CoV-2, as we do here (27). We focus on the role of monocytes, particularly the CD163-/CD206- and CD163+/CD206- populations, as the main markers for discriminating between severe and mild patients. A study conducted by Trombetta et al. (7) also suggests increased expression patterns of CD163 and CD206 as immunoregulatory markers in COVID-19. Elevated CD163 levels were positively correlated with ACE2 expression, and CD163 indirectly contributes to the anti-inflammatory response. In addition, higher ACE2 protein expression was found in severe COVID-19 disease, correlating with disease severity (10). CD163, due to its high expression in macrophages formed in response to tissue damage, is a potential inflammation biomarker and a therapeutic target (9). Here, we confirm its position in severe COVID-19 patients.
Here, we also reported the role of CD33 C-Mo associated with severe patients when compared with age. Previous data have shown that (CD33- HLA-DMA- CD14+) C-Mo and (CLEC10A- S100A9lo) pDCs are involved in viral persistence and in the innate immune response against SARS-CoV-2 infection. Wang et al. (28) suggested that the enrichment of (CD33- HLA-DMA- CD14+) C-Mo may attenuate antigen presentation and antiviral immune response, which may also explain the present data in severe patients. Alberca et al. (29) also proposed a combination of the cell markers CD11b+ CD33+ HLA-DR-CD14+ and CD11b+ CD33+ HLA-DR- CD66b+ as novel severity biomarkers for COVID-19. In the present study, we confirmed the presence of CD33+ CD11b+ cell markers in the blood of patients with severe disease. In addition, CD11b+ (macrophages and neutrophils) has also been described with high levels of cell infiltration in the lungs (9), which is common in severe patients. In addition, we found that CD14dim/CD33+ are good options as biomarkers for stratification of mild/severe COVID-19. Similarly, it has been previously reported that upregulation of C-Mo and, in particular, higher numbers of CD14+ CD33+ HLA-DR cells and S100A8/9/12 expressing C-Mo are present in severe/critical COVID-19 and sepsis (30). In the acute phase, Fahlberg et al. (9) showed a robust migration of CD16-expressing monocytes into the lung and described two subsets of interstitial macrophages (HLA-DR+CD206-) directly associated with plasma IL-6 levels. Furthermore, alveolar macrophages in acute lung injury with alveolar type II cell hyperplasia showed a characteristic phenotype (CD68, CD11c, CD14, CD205, CD206, CD123/IL3AR, and PD-L1) (31). As in our analysis in the severe population, we also found CD206 cell markers, in addition to those previously mentioned, in combination with CD80, which has been classified as an inflammatory monocyte subset not typically seen in healthy controls (32).
Moreover, CD45- seems to be a good marker between mild and severe patients. Similar results were previously reported by Jin et al. (33) who suggested CD45 as a useful tool to discriminate between severe and non-severe cases. Furthermore, recent publications performed in healthy vs. COVID-19 patients reported that CD45 expression on leukocytes is altered in COVID-19 patients. This event is explained by the changes in signal transduction from binding to Toll-like receptor 4, changes in leukocyte subtypes, or maturation of cells during the infection process (34). It is reinforced by the important role that CD45 plays in autoimmune and oncological events, but also in viral infections (33).
According to gender, we found differences in NK and monocyte populations (C-Mo, T-Mo, and NC-Mo). In several studies, patient characteristics such as male sex, advanced age, and the presence of comorbidities have been associated with an increased risk of severe COVID-19 and ICU admission (35). However, this is the first time that differences in immunologic markers between men and women have been reported.
5. Conclusions
This analysis indicates the relevant role of several markers such as TMPRSS2, CD45-, CD163/CD206, and CD33 for COVID-19 aggressiveness. The optimal classification of severe or more aggressive COVID-19 patients could help clinicians to offer different stratifications and follow-up. This is relevant considering that the opposite has been described in other infectious diseases, where CD163 is decreased in C-Mo and T-Mo. Here, for the first time, we report a combination of markers that can be performed in blood and will help in this classification, although we must consider that one of the challenges of the present project is due to the limited size of the population. However, the use of high-throughput analysis such as CyTOF provides a lot of novel information in the present data.
Data availability statement
The data presented in the study are deposited in the European Genome-Phenome Archive, accession number EGAD00010002445.
Ethics statement
The studies involving human participants were reviewed and approved by 1329-N-21. The patients/participants provided their written informed consent to participate in this study.
Author contributions
VA-R, SC-L, FM-B and PP-Q performed the experiments and data analysis. OS and JD-C performed cytometry analysis. LM-G and MA-C collected the related papers and drafted the manuscript. LM-G and MA-C participated in the design of the article. LM-G and MA-C wrote, and final proofed the manuscript. AA-M and MR-R performed the immunological revision and support of the article. SM-D, GL-T, SM-E and AC-V collected all the samples and updated clinical recording. LM-G and MA-C designed and supervised the study. SC-L and FM-B have statistical support. SM-D, CE-B, LM-G and MA-C obtained financial support. All authors contributed to the article and approved the submitted version.
Acknowledgments
We want to thank all donors, nursery and clinicians to make this study possible. Present published results are a part of the PhD thesis of the candidate Silvia Martinez Diz in the Biochemistry and Molecular Biology Doctoral Program of the University of Granada. The authors would also like to thank Pfizer for sponsoring the construction of the GENYO building and supporting its sustainability. Genyo research Centre and its maintenance are supported by the Regional Ministry of Economy and Knowledge, the Regional Ministry of Health, the University of Granada and the pharmaceutical company Pfizer.
Glossary
| ACE2 | angiotensin-converting enzyme 2 |
| CAS | Cell Acquisition Buffer |
| CEI | ethics committee |
| CI | confidence interval |
| C-Mo | classical monocytes |
| COVID-19 | coronavirus disease 2019 |
| CRP | C-reactive protein |
| CST | Cell Staining Buffer |
| EDTA | ethylenediaminetetraacetic acid |
| ESR | erythrocyte sedimentation rate |
| FiO2 | inspired oxygen fraction |
| ICU | intensive care unit |
| IL-6 | interleukin-6 |
| LDH | lactate dehydrogenase |
| MAF | minor allele frequency |
| MX1 | MX dynamin-like GTPase 1 |
| NC-Mo | nonclassical monocytes |
| OR | odds ratio |
| PaO2/FiO2 | partial pressure of oxygen/inspired oxygen fraction |
| PCR | polymerase chain reaction |
| PCT | procalcitonin |
| pDC | plasmacytoid dendritic cell |
| PFA | paraformaldehyde |
| RR | respiratory rate |
| RT | room temperature |
| RT-PCR | reverse transcription polymerase chain reaction |
| S | SARS-CoV-2 spike |
| SNP | single-nucleotide polymorphism |
| SpO2 | peripheral oxygen saturation |
| T-Mo | transitional monocytes |
| TMPRSS2 | transmembrane protease serine 2 |
| WHO | World Health Organization |
Funding Statement
This project is partially funded by “Desarrollo e Innovación (I+D+i) en Biomedicina y en Ciencias de la Salud en Andalucía, ´ FEDER”, internal code PECOVID-0006-2020, by Consejería de ´ Salud, Junta de Andalucía and ´ “Uso de la metodologíá NGS y CYTOF para caracterizar a los pacientes con COVID-19”, internal code CV20-36740, by Secretaria General Universidades, Investigación y Tecnología, Junta de Andalucía
Conflict of interest
Author CE-B is employed by LORGEN G.P.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1094644/full#supplementary-material
References
- 1. Aydin S, Benk IG, Geckil AA. May viral load detected in saliva in the early stages of infection be a prognostic indicator in COVID-19 patients? J Virol Methods (2021) 294. doi: 10.1016/j.jviromet.2021.114198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weekly epidemiological update on COVID-19 - 7 December 2022. Available at: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19—7-december-2022 (Accessed December 12, 2022).
- 3. Malik P, Patel U, Mehta D, Patel N, Kelkar R, Akrmah M, et al. Biomarkers and outcomes of COVID-19 hospitalisations: Systematic review and meta-analysis. BMJ Evid Based Med (2021) 26:107–8. doi: 10.1136/bmjebm-2020-111536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Assal HH, Abdelrahman SM, Abdelbasset MAA, Abdelaziz M, Sabry IM, Shaban MM. Presepsin as a novel biomarker in predicting in-hospital mortality in patients with COVID-19 pneumonia. Int J Infect Dis (2022) 118:155–63. doi: 10.1016/j.ijid.2022.02.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tian Y, Carpp LN, Miller HER, Zager M, Newell EW, Gottardo R. Single-cell immunology of SARS-CoV-2 infection. Nat Biotechnol (2022) 40:30–41. doi: 10.1038/s41587-021-01131-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Geanon D, Lee B, Gonzalez-Kozlova E, Kelly G, Handler D, Upadhyaya B, et al. A streamlined whole blood CyTOF workflow defines a circulating immune cell signature of COVID-19. Cytometry Part A (2021) 99:446–61. doi: 10.1002/cyto.a.24317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Trombetta AC, Farias GB, Gomes AMC, Godinho-Santos A, Rosmaninho P, Conceição CM, et al. Severe COVID-19 recovery is associated with timely acquisition of a myeloid cell immune-regulatory phenotype. Front Immunol (2021) 12:691725. doi: 10.3389/fimmu.2021.691725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mostafa GA, Ibrahim HM, al Sayed Shehab A, Gendy YGel, Aly DMM, Shousha GAH. Up-regulated serum levels of soluble CD25 and soluble CD163 in pediatric patients with SARS-CoV-2. Eur J Pediatr (2022) 181:2299–309. doi: 10.1007/s00431-022-04398-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fahlberg MD, Blair R v., Doyle-Meyers LA, Midkiff CC, Zenere G, Russell-Lodrigue KE, et al. Cellular events of acute, resolving or progressive COVID-19 in SARS-CoV-2 infected non-human primates. Nat Commun (2020) 11. doi: 10.1038/s41467-020-19967-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gheware A, Ray A, Rana D, Bajpai P, Nambirajan A, Arulselvi S, et al. ACE2 protein expression in lung tissues of severe COVID-19 infection. Sci Rep (2022) 12. doi: 10.1038/s41598-022-07918-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Irham LM, Chou WH, Calkins MJ, Adikusuma W, Hsieh SL, Chang WC. Genetic variants that influence SARS-CoV-2 receptor TMPRSS2 expression among population cohorts from multiple continents. Biochem Biophys Res Commun (2020) 529:263–9. doi: 10.1016/j.bbrc.2020.05.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martínez-Gómez LE, Herrera-López B, Martinez-Armenta C, Ortega-Peña S, Camacho-Rea M del C, Suarez-Ahedo C, et al. ACE and ACE2 gene variants are associated with severe outcomes of COVID-19 in men. Front Immunol (2022) 13:812940. doi: 10.3389/fimmu.2022.812940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pandey RK, Srivastava A, Singh PP, Chaubey G. Genetic association of TMPRSS2 rs2070788 polymorphism with COVID-19 case fatality rate among Indian populations. Infection Genet Evol (2022) 98. doi: 10.1016/j.meegid.2022.105206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Caccuri F, Bugatti A, Zani A, de Palma A, di Silvestre D, Manocha E, et al. Sars-cov-2 infection remodels the phenotype and promotes angiogenesis of primary human lung endothelial cells. Microorganisms (2021) 9. doi: 10.3390/microorganisms9071438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maras JS, Sharma S, Bhat A, Rooge S, Aggrawal R, Gupta E, et al. Multi-omics analysis of respiratory specimen characterizes baseline molecular determinants associated with SARS-CoV-2 outcome. iScience (2021) 24. doi: 10.1016/j.isci.2021.102823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Danwang C, Endomba FT, Nkeck JR, Wouna DLA, Robert A, Noubiap JJ. A meta-analysis of potential biomarkers associated with severity of coronavirus disease 2019 (COVID-19). biomark Res (2020) 8. doi: 10.1186/s40364-020-00217-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bagwell CB, Bray CM, Herbert DJ, Hill BL, Inokuma MS, et al. Improving the t-SNE algorithms for cytometry and other technologies: Cen-se′ mapping. J Biom Biostat (2019) 10:430. doi: 10.4172/2155-6180.1000430 [DOI] [Google Scholar]
- 18. Cunningham F, Allen JE, Allen J, Alvarez-Jarreta J, Amode MR, Armean IM, et al. Ensembl 2022. Nucleic Acids Res (2022) 50:D988–95. doi: 10.1093/nar/gkab1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Gassen S, Callebaut B, van Helden MJ, Lambrecht BN, Demeester P, Dhaene T, et al. FlowSOM: Using self-organizing maps for visualization and interpretation of cytometry data. Cytometry Part A (2015) 87:636–45. doi: 10.1002/cyto.a.22625 [DOI] [PubMed] [Google Scholar]
- 20. Surme S, Tuncer G, Bayramlar OF, Copur B, Zerdali E, Nakir IY, et al. Novel biomarker-based score (SAD-60) for predicting mortality in patients with COVID-19 pneumonia: A multicenter retrospective cohort of 1013 patients. biomark Med (2022) 16:577–88. doi: 10.2217/bmm-2021-1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hammad R, Elshafei A, Khidr EG, El-Husseiny AA, Gomaa MH, Kotb HG, et al. Copeptin: A neuroendocrine biomarker of COVID-19 severity. biomark Med (2022) 16:589–97. doi: 10.2217/bmm-2021-1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Akin S, Schriek P, van Nieuwkoop C, Neuman RI, Meynaar I, van Helden EJ, et al. A low aldosterone/renin ratio and high soluble ACE2 associate with COVID-19 severity. J Hypertens (2022) 40:606–14. doi: 10.1097/HJH.0000000000003054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schönfelder K, Breuckmann K, Elsner C, Dittmer U, Fistera D, Herbstreit F, et al. Transmembrane serine protease 2 polymorphisms and susceptibility to severe acute respiratory syndrome coronavirus type 2 infection: A German case-control study. Front Genet (2021) 12:667231. doi: 10.3389/fgene.2021.667231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bizzotto J, Sanchis P, Abbate M, Lage-Vickers S, Lavignolle R, Toro A, et al. SARS-CoV-2 infection boosts MX1 antiviral effector in COVID-19 patients. iScience (2020) 23. doi: 10.1016/j.isci.2020.101585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mahmood ZS, Fadhil HY, Abdul Hussein TA, Ad’hiah AH. Severity of coronavirus disease 19: Profile of inflammatory markers and ACE (rs4646994) and ACE2 (rs2285666) gene polymorphisms in Iraqi patients. Meta Gene (2022) 31:101014. doi: 10.1016/j.mgene.2022.101014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alimoradi N, Sharqi M, Firouzabadi D, Sadeghi MM, Moezzi MI, Firouzabadi N. SNPs of ACE1 (rs4343) and ACE2 (rs2285666) genes are linked to SARS-CoV-2 infection but not with the severity of disease. Virol J (2022) 19. doi: 10.1186/s12985-022-01782-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shi W, Liu X, Cao Q, Ma P, Le W, Xie L, et al. High-dimensional single-cell analysis reveals the immune characteristics of COVID-19. Am J Physiol Lung Cell Mol Physiol (2021) 320:L84–98. doi: 10.1152/AJPLUNG.00355.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang X, Bai H, Ma J, Qin H, Zeng Q, Hu F, et al. Identification of distinct immune cell subsets associated with asymptomatic infection, disease severity, and viral persistence in COVID-19 patients. Front Immunol (2022) 13:812514. doi: 10.3389/fimmu.2022.812514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alberca RW, Andrade MM de S, Branco ACCC, Pietrobon AJ, Pereira NZ, Fernandes IG, et al. Frequencies of CD33+CD11b+HLA-DR–CD14–CD66b+ and CD33+CD11b+HLA-DR–CD14+CD66b– cells in peripheral blood as severity immune biomarkers in COVID-19. Front Med (Lausanne) (2020) 7:580677. doi: 10.3389/fmed.2020.580677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ahern DJ, Ai Z, Ainsworth M, Allan C, Allcock A, Angus B, et al. A blood atlas of COVID-19 defines hallmarks of disease severity and specificity. Cell (2022) 185:916–938.e58. doi: 10.1016/j.cell.2022.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Doglioni C, Ravaglia C, Chilosi M, Rossi G, Dubini A, Pedica F, et al. Covid-19 interstitial pneumonia: Histological and immunohistochemical features on cryobiopsies. Respiration (2021) 100:488–98. doi: 10.1159/000514822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang D, Guo R, Lei L, Liu H, Wang Y, Wang Y, et al. Frontline science: COVID-19 infection induces readily detectable morphologic and inflammation-related phenotypic changes in peripheral blood monocytes. J Leukoc Biol (2021) 109:13–22. doi: 10.1002/JLB.4HI0720-470R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jin M, Shi N, Wang M, Shi C, Lu S, Chang Q, et al. CD45: A critical regulator in immune cells to predict severe and non-severe COVID-19 patients. Aging (2020) 12:19867–79. doi: 10.18632/aging.103941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ahmed MGT, Limmer A, Sucker C, Fares KM, Mohamed SAB, Othman AH, et al. Differential regulation of CD45 expression on granulocytes, lymphocytes, and monocytes in COVID-19. J Clin Med (2022) 11. doi: 10.3390/jcm11144219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cekmen N, Ersoy Z, Günay Y, Ghavam A, Tufan MS, Şahin İ. Evaluation of coronavirus diseases (COVID-19) in terms of epidemiological and clinical features, comorbidities, diagnostic methods, treatment, and mortality. J Educ Health Promot (2022) 11:236. doi: 10.4103/jehp.jehp_1328_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in the study are deposited in the European Genome-Phenome Archive, accession number EGAD00010002445.