Abstract
Background and Hypothesis
Voice atypicalities are potential markers of clinical features of schizophrenia (eg, negative symptoms). A recent meta-analysis identified an acoustic profile associated with schizophrenia (reduced pitch variability and increased pauses), but also highlighted shortcomings in the field: small sample sizes, little attention to the heterogeneity of the disorder, and to generalizing findings to diverse samples and languages.
Study Design
We provide a critical cumulative approach to vocal atypicalities in schizophrenia, where we conceptually and statistically build on previous studies. We aim at identifying a cross-linguistically reliable acoustic profile of schizophrenia and assessing sources of heterogeneity (symptomatology, pharmacotherapy, clinical and social characteristics). We relied on previous meta-analysis to build and analyze a large cross-linguistic dataset of audio recordings of 231 patients with schizophrenia and 238 matched controls (>4000 recordings in Danish, German, Mandarin and Japanese). We used multilevel Bayesian modeling, contrasting meta-analytically informed and skeptical inferences.
Study Results
We found only a minimal generalizable acoustic profile of schizophrenia (reduced pitch variability), while duration atypicalities replicated only in some languages. We identified reliable associations between acoustic profile and individual differences in clinical ratings of negative symptoms, medication, age and gender. However, these associations vary across languages.
Conclusions
The findings indicate that a strong cross-linguistically reliable acoustic profile of schizophrenia is unlikely. Rather, if we are to devise effective clinical applications able to target different ranges of patients, we need first to establish larger and more diverse cross-linguistic datasets, focus on individual differences, and build self-critical cumulative approaches.
Keywords: vocal analysis, psychosis, speech signal, digital phenotyping, prosody, negative symptoms
Introduction
From its very first definitions, schizophrenia has been associated with voice atypicalities,1,2 qualitatively described in terms of eg, poverty of speech, increased pauses, distinctive tone and intensity of voice. A recent systematic meta-analysis3 indicates a plausible acoustic profile associated with schizophrenia. In this study, we assess how generalizable that profile is within a large cross-linguistic dataset, as well as sources of heterogeneity in vocal patterns of patients with schizophrenia.
Atypical voice patterns are included amongst negative symptoms of schizophrenia, such as alogia and blunted affect, which are among the primary diagnostic criteria and prognostic indicators of the disorder (eg response to treatment and reduced likelihood of remission4–7). Vocal behavior may constitute a window into the underlying social and cognitive features of the disorder.8 For example, the social and cognitive impairments frequently reported in schizophrenia9–11 may be reflected in difficulties in speech fluency (eg increased pauses), or in controlling the voice to express affective and emotional contents and to mark relevant information.12,13 In other words, not only can the quantitative analysis of vocal behavior scaffold the current evaluation of negative symptoms,14–19 but also it could offer a more fine-grained perspective on its clinical, social, and cognitive dimensions, eg, social and cognitive functioning over time.8,18,20,21
However, while extensive literature exists on vocal atypicalities in schizophrenia, with studies spanning back to the 60s, the findings are often contradictory and difficult to interpret. A recent meta-analysis3 identified weak atypicalities in pitch variability—potentially related to flat affect– and stronger atypicalities in proportion of spoken time, speech rate, and pauses—potentially related to alogia and flat affect. The effects had large heterogeneity, and were modest compared to clinical judgments of vocal atypicalities.22 The studies were noted to have small sample sizes, high variability in methods and features analyzed, with little to no attention to the heterogeneity of the disorder23–25 and to the replicability and generalizability of previous results on diverse samples.20,25–27 Further, voice quality features—highlighted as important by speech pathologists and speech processing research28,29—had been largely neglected.
To assess the robustness and potential clinical impact of biobehavioral vocal markers of schizophrenia, we need to understand under which conditions they vary and under which they can be relied upon. We need to map which variations in clinical and socio-cognitive features might underlie voice atypicalities, and how stable they are across languages, time and recordings. For example, although vocal behavior has been shown to be influenced by linguistic and cultural factors, all studies of vocal markers of schizophrenia have investigated single monolingual samples. We need to assess how voice atypicalities relate to the development of the disorder, eg, whether they are a long-term consequence of chronicity or already present at illness onset, and whether they vary along with symptom severity, thus being potentially useful for tracking the development of the disorder and monitoring symptomatology over time.30–32 Another crucial issue is how antipsychotic drugs relate to these atypicalities and impact our ability to use them as biobehavioral markers. For example, effects of antipsychotic medication have been hypothesized to affect language in different ways, such as causing extrapyramidal motor symptoms or increasing negative symptoms by blocking dopamine receptors.33
In other words, there is a need for a more rigorous cumulative scientific approach to understand vocal and prosodic atypicalities in schizophrenia: the synthesis and integration of data across studies and laboratories, in order to assess which patterns might generalize across contexts and samples, identify possible sources of variation and improve estimations. In this work, we provide the first steps towards such an approach.
First, we made use of the recommendations developed in the previous meta-analysis3 and collected a large dataset of multiple audio-recordings of patients with schizophrenia and controls in four different languages and three language families (Germanic, Mandarin-Chinese, and Japonic). Such a setup provides a stronger basis for estimating the robustness of vocal atypicalities, within and between subjects and samples, as well as their variability. Further, this design allows us to explicitly compare results across different languages for the first time in schizophrenia, thus accounting for the natural differences in vocal patterns across languages.
Second, we provided a more systematic investigation of the acoustic features potentially associated with schizophrenia. While a previous meta-analysis3 identified relevant estimates for a limited set of features (n = 8), pertaining to pitch and rhythm, recent investigations into Parkinson’s disease and depression28,34 suggest that voice quality and articulatory features might have higher discriminative power compared to traditional acoustic features. In other words, they might be particularly involved in the mechanisms underlying the disorders. We therefore extended the acoustic features investigated to include them.
Third, we cumulatively build on previous findings by explicitly (but critically) including the meta-analytic findings in the statistical Bayesian analysis of the current study. This practice—referred to as “informed priors” or “posterior passing”35—allows us to directly estimate how well our results match previous findings and in which ways they deviate, but also potentially increases the precision of our estimates.
Fourth, we include a more comprehensive assessment of the patients’ symptomatology and clinical profile. Specifically, we model the relationships between acoustic features and pharmacotherapy, relevant clinical aspects, demographic and social features, relationships that have rarely been jointly investigated in previous studies.36
Finally, we rely on an open methodology: not only do we carefully describe the methodology used and test the robustness of the results to variations in the methodology; but we also use open-source software, extracting the features in a reproducible manner with openly available scripts.
By providing an initial consolidation and test of acoustic atypicalities in schizophrenia that systematically builds on and extends the previous literature, we aim to set the foundations for more critical theory development, more cumulative and holistic approaches to the understanding of the underlying mechanisms and potentially the development of applications that constructively support clinical practices.
Methods
Participants
We collected a Danish (DK), German (GE), Chinese (CH),and Japanese (JP) cross-linguistic dataset involving 231 participants with schizophrenia (105 DK, 61GE, 51CH, 14JP) and 238 matched controls (HC) (116DK, 62GE, 43CH, 17JP). The samples for the present study were collected in separate studies assessing mentalizing ability in patients with schizophrenia and healthy controls. Information on demographics, IQ, psychopathology, and social functioning is summarized in Table 1. Detailed information on each study is reported in the Supplementary Material (SM) - S1.
Table 1.
Demographic and Clinical Characteristics of Patients with Schizophrenia and Healthy Controls (HC)
| Corpus | Danish | German | Chinese | Japanese | ||||
|---|---|---|---|---|---|---|---|---|
| Diagnosis | SCZ N = 105 |
HC N = 116 |
SCZ N = 61 |
HC N = 62 |
SCZ N = 51 |
HC N = 43 |
SCZ N = 14 |
HC N = 17 |
| N. of recording | N = 900 | N = 989 | N = 612 | N = 609 | N = 401 | N = 340 | N = 121 | N = 144 |
| Age | 26.5 (8.82) |
26.4 (8.96) |
31.7 (9.92) |
33.2 (8.79) |
27.2 (7.25) | 29.7 (8.72) |
28,6 (6.71) | 41.9 (13.7) |
| Education | 12.9 (2.74) |
14.9 (2.61) |
12.1 (1.48) |
12.3 (1.11) |
12.7 (2.69) | 14.1 (2.37) |
11.9 (1.37) | 14.7 (2.93) |
| Sex (n. of females and %) | 45 (43%) |
50 (43%) |
22 (36%) |
24 (38%) |
23 (45%) |
19 (44%) |
4 (29%) |
11 (65%) |
| Verbal IQ | 89.13 (18.7) | 102.12 (16.1) | 112.0 (15.6) | 116.5 (16.9) | 96.3 (16.6) | 100.27 (14.7) | NA | NA |
| SANS total | 9.66 (4.41) |
NA | NA | NA | 7.61 (2.99) | NA | NA | NA |
| SAPS total | 10.32 (4.91) | NA | NA | NA | 7.23 (4.79) | NA | NA | NA |
| PANSS total | NA | NA | 54.65 (10.63) | NA | 75.79 (10.44) | NA | 58.86 (15.25) | NA |
| PANSS negative | NA | NA | 14.61 (4.19) | NA | 20.09 (5.24) | NA | 15.28 (3.95) | NA |
| PANSS positive | NA | NA | 11.31 (3.13) | NA | 18.51 (4.37) | NA | 12.86 (4.10) | NA |
| Illness duration (months) | 8.87 (6.68) |
NA | 54.38 (83.24) | NA | 63.13 (68.87) | NA | 83.86 (91.33) | NA |
| PSP | 56.01 (15.70) | 86.46 (6.72) | NA | NA | 54.09 (8.02) | NA | NA | NA |
Note: The table displays means and standard deviations of demographic (age, education and sex) and clinical information. Clinical symptoms were measured using the Scale for the Assessment of Negative Symptoms (SANS),37 the Scale for the Assessment of Positive Symptoms (SAPS),38 and the Positive and Negative Syndrome Scale (PANSS).39 Social functioning was measured using the Personal and Social Performance scale (PSP).40 NA = data not available; SCZ = patients with schizophrenia, HC = healthy controls.
Voice Recordings
The dataset included a total of 2034 recordings of individuals with schizophrenia (mean recording length = 17.3 s, sd = 15.6 s) and 2.082 recordings of control participants (recording length = 18.5 s, sd = 12.7 s). Voice recordings were collected using the Animated Triangles Task (ATT).41,42 The task is generally used to measure theory of mind (ToM) and involves 12 video clips representing an interaction between animated geometrical shapes (triangles). The participants were asked to provide an interpretation of what was going on in each animation and their answers were audio-recorded. A detailed description of the task and its validity for assessing speech production is included in Supplementary Material 2. Recording setting and experimental procedure are described in detail in Supplementary Material 3, and were kept constant across the different sites. All recordings were manually pre-processed to remove background noise and interviewer speech when present, and to ensure that all recordings analyzed had adequate audio quality. A full description of the process and of the extracted acoustic features is available in Supplementary Material 3.
Statistical Modeling
Analysis of Effect of Diagnosis on Acoustic Features
To estimate the differences between individuals with schizophrenia and HC in the different acoustic measures, we used Bayesian multilevel Gaussian regression models on the current data with each acoustic feature as outcome, and diagnosis (schizophrenia vs HC) and language (DK, GE, CH, and JP) as predictors. Within the same model, we separately assessed the effect of diagnosis for each language, and modeled varying effects of participants, ie, intercepts, separately for each group and language. For each acoustic feature, we built a first model with weakly informative priors, ie, expectations of no effects of diagnosis, thus conservatively regularizing the model parameters, reducing overfitting and leading to improved predictions.43 We then built a second model with an informed prior (when available), that is meta-analytic effect size (ES), and compared results across the two models. We aimed to assess whether the effects of diagnosis are robust across changes of priors, and whether the skeptical and informed priors led to more robust inference, that is, in lower estimated out-of-sample error—measured in terms of Leave-One-Out based stacking weights.44 To evaluate the potential role of reported biological sex (male vs female), age and level of intelligence, we built additional models, one per each moderator interacting with group separately in the 4 languages. We then reported the model estimates for the interaction, including credible (ie, Bayesian confidence) intervals (CIs) and evidence ratios (ERs), ie, evidence in favor of the effect observed against alternative hypotheses (see Supplementary Material 4). When ER was weak (below 10, that is, less than ten times as much evidence for the effect as for alternative hypotheses), we also calculated the ER in favor of the null hypothesis. Further details are presented in the Supplementary Material 4. In addition to the more traditional acoustic features included in the previous meta-analysis, we extracted 24 novel voice quality acoustic features including both spectral and glottal properties of voice,28 for a total of 32 including those of rhythm. Median and interquartile range (IQR) were calculated for each of these measures (see Supplementary Material 3). We then estimated the differences between individuals with schizophrenia and HC for all measures (see Supplementary Material 5). We report additional analyses in Supplementary Material 7 to assess the robustness of the findings: we repeat all analyses on audio segments of 6 s to control for recording length. The results generally support our main findings and we report here only qualitative divergences.
The Relationship Between Acoustic Features and Clinical Features, Pharmacotherapy and Social Functioning
To assess the relationship between acoustic features and clinical ratings, we built Bayesian multilevel regression models with each acoustic feature as outcome, and clinical features (one at a time) as ordinal predictors. We separately assessed the relationship between the different acoustic features and clinical ratings for each language, and modeled varying effects of participants, ie, intercepts, separately for each language. This analysis was performed on the schizophrenia group only (see Supplementary Material 4 for more details).
To assess the effect of medication, patients were divided into two categories based on the mechanism of action of antipsychotic medication,45 namely patients taking medication with (1) low D2R occupancy, ie, Clozapine, Olanzapine, Paliperidone, Quetiapine or (2) high D2R occupancy, ie, Aripiprazole, Amisulpride, Risperidone, Ziprasidone, Sertindole (see Supplementary Material 6). Antipsychotic dose was converted to chlorpromazine (CPZ) equivalents.46 We used Bayesian multilevel regression models, as described above, with each acoustic feature as outcome, and antipsychotic medication (high D2R, low D2R) and language (DK, CH, and GE) as predictors. For each acoustic feature we built a first model with weakly informative priors, that is expectations of no effects of medication, and we then built a second model with informed prior (relying on DeBoer et al., 33 see Supplementary Material 6). Our main aim was to assess the generalizability of previous findings on the effect of medication on speech production in schizophrenia, more than evaluating the causal pathways of medication on speech production, which would have required a larger sample and more detailed information on potential confounders (see discussion section). To assess the role of drug dosage, duration of illness (DUI) and social functioning (PSP scale), we built Bayesian multilevel regression models with each acoustic feature as outcome, and chlorpromazine equivalents, DUI and PSP scale score (one at a time) as predictors. We also compared acoustic patterns between patients with first-episode (FES) schizophrenia and chronic patients. To this aim, we used Bayesian multilevel regression models with each acoustic feature as outcome, illness onset (three groups: FES patients, chronic patients, and healthy controls) and language as predictors (see Supplementary Material 6 for more details).
The code used for the analysis and the features extraction are openly available (see SM8).
Results
Effect of Diagnosis
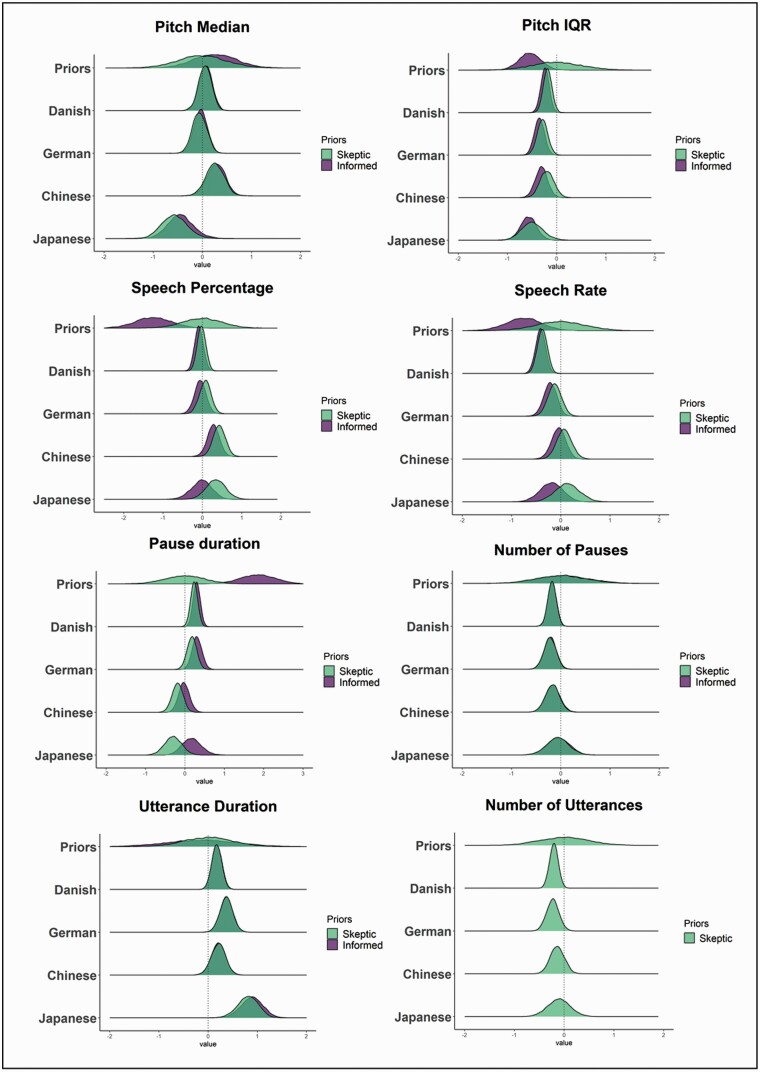
The detailed results and comparisons to meta-analytic effects are reported in Table 2 and Figure 1. We only partially replicated previous meta-analytic findings: only reduced pitch variability was found across all datasets, and with smaller effect sizes. Duration atypicalities (reduced speech rate, increased pause duration) were replicated only in the German and Danish corpora, and with smaller effect sizes. We also identified a new potential marker across languages: longer utterance duration. In agreement with the inconsistent replications, meta-analytically informed models were more robust and generalizable to new data (LOO weights for skeptic models above .75) only in about half of the models, indicating that meta-analytic findings were not fully representative of the current samples.
Table 2.
Estimated standardized mean difference (HC- schizophrenia) for the eight acoustic measures present in the meta-analysis, as estimated separately by the meta-analytically informed and the skeptical models
| Acoustic Features | Group (HC—SCZ) | Group * Biological sex (M-F) | Age | IQ |
|---|---|---|---|---|
| Pitch Median | MA priors = 0.25 (-0.72, 1.30) | |||
| Skeptical DK | 0.05 (-0.16 0.26) ER = 1.94 ER01 = 3.53 | 0.1 (-0.1 0.29) ER = 3.77 | 0.02 (0 0.03) ER = 32.33 | 0 (-0.01 0) ER = 2.09 ER01 = 119.29 |
| Skeptical GE | -0.06 (-0.29 0.17) ER = 1.98 ER01 = 3.09 | 0.04 (-0.3 0.38) ER = 1.43 ER01 = 3.26 | 0 (-0.02 0.01) ER = 2 ER01 = 51.01 | 0 (-0.01 0.01) ER = 1.11 ER01 = 87.94 |
| Skeptical CH | 0.25 (-0.05 0.55) ER = 11.05 | 0.28 (-0.01 0.57) ER = 16.76 | 0.02 (0 0.04) ER = 45.3 | 0.01 (0 0.02) ER = 4.11 |
| Skeptical JP | -0.57 (-0.98 -0.13) ER = 58.88 | 0.63 (0.18 1.08) ER = 77.12 | -0.01 (-0.08 0.04) ER = 1.04 ER01 = 11.37 | -0.01 (-0.86 0.82) ER = 1.01 ER01 = 0.93 |
| Informed DK | 0.07 (-0.15 0.28) ER = 2.3 ER01 = 3.35 | NA | NA | NA |
| Informed GE | -0.04 (-0.26 0.18) ER = 1.58 ER01 = 3.97 | NA | NA | NA |
| Informed CH | 0.28 (-0.02 0.57) ER = 15 | NA | NA | NA |
| Informed JP | -0.44 (-0.86 0) ER = 19.2 | NA | NA | NA |
| Stacking weight | Informed Model 1.00 | |||
| Pitch IQR | MA priors = -0.55 (- 1.06, 0.09) | |||
| Skeptical DK | -0.19 (-0.32 -0.05) ER = 62.29 | 0.16 (-0.1 0.41) ER = 5.29 | 0.01 (0 0.02) ER = 9 | 0 (-0.01 0) ER = 3.76 |
| Skeptical GE | -0.29 (-0.46 -0.11) ER = 343.83 | -0.03 (-0.34 0.28) ER = 1.29 ER01 = 3.72 | 0 (-0.01 0.02) ER = 1.83 ER01 = 59.83 | 0 (-0.01 0.01) ER = 1.46 ER01 = 128.11 |
| Skeptical CH | -0.2 (-0.43 0.04) ER = 11.02 | 0.03 (-0.4 0.46) ER = 1.25 ER01 = 1.33 | 0.01 (-0.01 0.03) ER = 5.49 | 0 (-0.01 0) ER = 3.22 |
| Skeptical JP | -0.5 (-0.85 -0.13) ER = 64.79 | 0.09 (-0.59 0.77) ER = 1.43 ER01 = 1.75 | 0.01 (-0.02 0.03) ER = 1.92 ER01 = 27.15 | 0 (-0.82 0.83) ER = 1.01 ER01 = 0.97 |
| Informed DK | -0.24 (-0.37 -0.1) ER = 832.33 | NA | NA | NA |
| Informed GE | -0.35 (-0.52 -0.19) ER = 9999 | NA | NA | NA |
| Informed CH | -0.31 (-0.52 -0.1) ER = 134.14 | NA | NA | NA |
| Informed JP | -0.57 (-0.84 -0.3) ER = 2499 | NA | NA | NA |
| Stacking weight | Informed Model 1.00 | |||
| Speech Rate | MA priors -0.75 (-1.51, 0.04) | |||
| Skeptical DK | -0.37 (-0.54 -0.22) ER = 9999 | 0.22 (-0.09 0.53) ER = 6.99 | 0 (-0.02 0.01) ER = 1.21 ER01 = 57.1 | 0 (-0.01 0.01) ER = 1.98 ER01 = 109.93 |
| Skeptical GE | -0.12 (-0.35 0.1) ER = 4.46 | 0.29 (-0.17 0.74) ER = 5.49 | 0.01 (0 0.03) ER = 11.95 | 0 (-0.01 0.01) ER = 1.09 ER01 = 105.22 |
| Skeptical CH | 0.07 (-0.18 0.32) ER = 2.09 ER01 = 2.94 | -0.1 (-0.56 0.36) ER = 1.8 ER01 = 2.94 | 0.01 (-0.01 0.03) ER = 3.14 | 0 (0 0.01) ER = 5.52 |
| Skeptical JP | 0.13 (-0.28 0.55) ER = 2.43 ER01 = 1.68 | -0.39 (-1.12 0.34) ER = 4.43 | 0.02 (-0.01 0.06) ER = 5.08 | 0 (-0.82 0.83) ER = 1.01 ER01 = 1 |
| Informed DK | -0.41 (-0.57 -0.25) ER = 9999 | NA | NA | NA |
| Informed GE | -0.21 (-0.43 0) ER = 19.58 | NA | NA | NA |
| Informed CH | -0.04 (-0.28 0.19) ER = 1.62 ER01 = 19.09 | NA | NA | NA |
| Informed JP | -0.19 (-0.58 0.19) ER = 3.77 | NA | NA | NA |
| Stacking weight | Skeptic Model 1.00 | |||
| Speech Percentage | MA priors -1.26 (-2.26, 0.25) | |||
| Skeptical DK | -0.02 (-0.19 0.15) ER = 1.4 ER01 = 4.7 | -0.04 (-0.36 0.28) ER = 1.35 ER01 = 3.67 | 0 (-0.01 0.02) ER = 1.69 ER01 = 46.55 | 0 (-0.01 0.01) ER = 1.51 ER01 = 114.89 |
| Skeptical GE | 0.07 (-0.18 0.3) ER = 2.24 ER01 = 2.94 | 0.25 (-0.23 0.73) ER = 3.99 | 0.02 (0 0.03) ER = 15.08 | 0 (0 0.01) ER = 7.16 |
| Skeptical CH | 0.43 (0.17 0.68) ER = 332.33 | -0.25 (-0.75 0.24) ER = 4.05 | 0.02 (0 0.04) ER = 10.64 | 0.01 (0 0.02) ER = 13.86 |
| Skeptical JP | 0.34 (-0.07 0.75) ER = 10.82 | -0.22 (-0.95 0.48) ER = 2.28 ER01 = 1.47 | 0.04 (0.01 0.07) ER = 43.05 | -0.01 (-0.82 0.8) ER = 1.02 ER01 = 1.02 |
| Informed DK | -0.09 (-0.26 0.09) ER = 3.87 | NA | NA | NA |
| Informed GE | -0.06 (-0.3 0.18) ER = 1.86 ER01 = 109.19 | NA | NA | NA |
| Informed CH | 0.28 (0.02 0.53) ER = 24.91 | NA | NA | NA |
| Informed JP | -0.03 (-0.46 0.39) ER = 1.13 ER01 = 62.41 | NA | NA | NA |
| Stacking weight | Skeptic Model 0.50 | |||
| Pause Number | MA priors 0.05 (-1.23, 1.13) | |||
| Skeptical DK | -0.18 (-0.31 -0.05) ER = 73.63 | 0.34 (0.07 0.6) ER = 57.14 | -0.01 (-0.02 0.01) ER = 5.33 | 0 (0 0.01) ER = 3.27 |
| Skeptical GE | -0.22 (-0.42 -0.02) ER = 32.22 | 0.15 (-0.24 0.55) ER = 2.78 ER01 = 2.43 | 0.01 (0 0.03) ER = 7.36 | 0 (-0.01 0.01) ER = 2.06 ER01 = 99.91 |
| Skeptical CH | -0.17 (-0.4 0.06) ER = 8.16 | -0.34 (-0.77 0.1) ER = 8.99 | 0 (-0.02 0.02) ER = 1.23 ER01 = 41.58 | 0 (-0.01 0) ER = 5.31 |
| Skeptical JP | -0.06 (-0.44 0.32) ER = 1.58 ER01 = 2.15 | -0.28 (-0.97 0.43) ER = 2.94 ER01 = 1.35 | 0.03 (0 0.06) ER = 20.1 | 0 (-0.82 0.81) ER = 1.02 ER01 = 1.03 |
| Informed DK | -0.18 (-0.31 -0.04) ER = 64.36 | NA | NA | NA |
| Informed GE | -0.21 (-0.41 -0.02) ER = 25.18 | NA | NA | NA |
| Informed CH | -0.16 (-0.4 0.08) ER = 6.82 | NA | NA | NA |
| Informed JP | -0.05 (-0.42 0.32) ER = 1.38 ER01 = 2.24 | NA | NA | NA |
| Stacking weight | Skeptic Model 1.00 | |||
| Pause duration | MA priors 1.89 (0.72, 3.21) | |||
| Skeptical DK | 0.23 (0.09 0.38) ER = 180.82 | -0.13 (-0.41 0.14) ER = 3.48 | 0 (-0.02 0.01) ER = 1.93 ER01 = 53.4 | 0 (-0.01 0.01) ER = 1.34 ER01 = 103.28 |
| Skeptical GE | 0.17 (-0.03 0.37) ER = 12.04 | -0.17 (-0.59 0.26) ER = 2.91 ER01 = 2.18 | -0.01 (-0.03 0) ER = 9.73 | 0 (-0.01 0) ER = 1.97 ER01 = 110.6 |
| Skeptical CH | -0.19 (-0.42 0.03) ER = 11.5 | 0.27 (-0.14 0.69) ER = 6.09 | -0.02 (-0.04 0) ER = 8.67 | 0 (-0.01 0) ER = 2.71 ER01 = 85.99 |
| Skeptical JP | -0.3 (-0.65 0.07) ER = 10.52 | 0.28 (-0.4 0.95) ER = 3.05 | -0.04 (-0.07 0) ER = 30.65 | 0 (-0.82 0.81) ER = 1.02 ER01 = 1.02 |
| Informed DK | 0.3 (0.16 0.44) ER = 4999 | NA | NA | NA |
| Informed GE | 0.31 (0.11 0.51) ER = 177.57 | NA | NA | NA |
| Informed CH | -0.02 (-0.24 0.21) ER = 1.36 ER01 = 4640.1 | NA | NA | NA |
| Informed JP | 0.17 (-0.21 0.58) ER = 3.08 | NA | NA | NA |
| Stacking weight | Informed Model 0.77 | |||
| Utterance Duration | MA priors −0.13 (−1.571 1.342) | |||
| Skeptical DK | 0.17 (0.02 0.33) ER = 29.12 | −0.34 (−0.67 −0.02) ER = 23.57 | 0.01 (−0.01 0.02) ER = 2.86 ER01 = 43.25 | −0.01 (−0.01 0) ER = 10.66 |
| Skeptical GE | 0.37 (0.15 0.58) ER = 332.33 | 0.1 (−0.35 0.55) ER = 1.77 ER01 = 2.42 | 0.01 (0 0.03) ER = 12.79 | 0 (0 0.01) ER = 3.5 |
| Skeptical CH | 0.21 (−0.03 0.44) ER = 12.39 | −0.17 (−0.56 0.23) ER = 3.29 | 0 (−0.01 0.01) ER = 2 ER01 = 64.92 | 0 (0 0.01) ER = 3.2 |
| Skeptical JP | 0.81 (0.45 1.15) ER = 1427.57 | −0.05 (−0.84 0.72) ER = 1.13 ER01 = 1.52 | 0.04 (0.01 0.07) ER = 42.29 | −0.01 (−0.84 0.82) ER = 1.01 ER01 = 0.96 |
| Informed DK | 0.18 (0.02 0.34) ER = 27.65 | NA | NA | NA |
| Informed GE | 0.37 (0.16 0.58) ER = 475.19 | NA | NA | NA |
| Informed CH | 0.21 (-0.02 0.44) ER = 13.99 | NA | NA | NA |
| Informed JP | 0.89 (0.51 1.25) ER = 4999 | NA | NA | NA |
| Stacking weight | Skeptic Model 1.00 | |||
| Number of Utterance | ||||
| Skeptical DK | −0.21 (−0.35 −0.07) ER = 171.41 | 0.37 (0.1 0.64) ER = 76.52 | −0.01 (−0.02 0.01) ER = 4.31 | 0 (0 0.01) ER = 3.39 |
| Skeptical GE | −0.23 (−0.43 −0.03) ER = 29.21 | 0.18 (−0.22 0.58) ER = 3.37 | 0.01 (0 0.03) ER = 8.36 | 0 (0 0.01) ER = 2.45 ER01 = 97.14 |
| Skeptical CH | −0.14 (−0.37 0.1) ER = 4.93 | −0.37 (−0.81 0.05) ER = 12.12 | 0 (−0.02 0.02) ER = 1 ER01 = 41.2 | 0 (−0.01 0) ER = 5.51 |
| Skeptical JP | −0.1 (−0.48 0.27) ER = 2.12 ER01 = 1.99 | −0.34 (−1.02 0.33) ER = 3.78 | 0.03 (0 0.06) ER = 11.5 | 0 (−0.83 0.83) ER = 0.98 ER01 = 1.07 |
| Total Duration | ||||
| Skeptical DK | −0.03 (−0.22 0.15) ER = 1.64 ER01 = 4.24 | 0.19 (−0.17 0.56) ER = 4.16 | 0 (−0.02 0.02) ER = 1.02 ER01 = 48.82 | 0 (0 0.01) ER = 2.19 ER01 = 141.8 |
| Skeptical GE | 0.01 (−0.19 0.22) ER = 1.17 ER01 = 3.93 | 0.14 (−0.27 0.53) ER = 2.56 ER01 = 2.56 | 0 (−0.01 0.02) ER = 1.92 ER01 = 45.72 | 0 (−0.01 0.01) ER = 1.2 ER01 = 110.36 |
| Skeptical CH | −0.21 (−0.43 0.02) ER = 13.06 | 0.54 (0.11 0.97) ER = 50.02 | 0 (−0.02 0.02) ER = 1.19 ER01 = 41.85 | 0 (−0.01 0.01) ER = 1.18 ER01 = 100.72 |
| Skeptical JP | −0.27 (−0.63 0.12) ER = 7.35 | −0.08 (−0.72 0.55) ER = 1.42 ER01 = 1.75 | −0.03 (−0.06 0) ER = 17.66 | 0 (−0.82 0.85) ER = 0.99 ER01 = 1.02 |
Note: The first column reports the main effect of the diagnostic group (across sex and age), respectively from the meta-analysis (MA), for the skeptical Danish (DK), German (GE), Chinese (CH) and Japanese (JP) models, and for the informed ones. The second column indicates the interaction between the effect of the diagnostic group and biological sex (male–female), that is, the difference in effect of group between the male and the female participants. The third column reports the interaction between the effect of diagnostic group and age, that is, the change in effect size as age increases by 1 standard deviation (SD). The fourth column reports the interaction between the effect of diagnostic group and level of intelligence (IQ), the change in effect size as IQ increases by 1 SD. ER indicates the evidence ratio for the difference, ER01 the evidence ratio for the null effect. Bold text indicates findings for which there is more than anecdotal evidence (evidence ratio above 10). SCZ, schizophrenia; HC, healthy controls. NA = not applicable.
Fig. 1.
Comparing meta-analysis, skeptical expectations and results. Each panel presents a separate acoustic measure, with the x-axis corresponding to standardized mean differences (schizophrenia—HC) equivalent to Hedges’ g, with estimates above 0 indicating higher scores for patients with schizophrenia. The overlap between the skeptical and meta-analytic posterior distributions suggests no real advantage in using meta-analytic informed priors compared to skeptic ones. For utterance number we only built the model with skeptic prior, since no meta-analytic prior was available.
Biological sex, age and level of intelligence of the participants also affected the group differences, although inconsistently across languages.
Our results are generally confirmed when only the 6-second segments of the audio recordings are analyzed, although with some differences: the results of the robustness analysis (see SM7) tend to be more consistent with the results of the meta-analysis3 (eg, reduced speech rate also in German, reduced speech percentage also in Danish, no more evidence of reduced pause duration in Chinese and Japanese). Interestingly, the robustness analysis also showed that patients with schizophrenia were more likely to give very short responses to the task than controls (total recording duration < 6 s, see SM7).
Novel Acoustic Features
The detailed results are reported in Table S5_A. Generally, we found some evidence for reduced formants median frequency and formants variability, and for increased median relative amplitude (H1H2) and reduced H1H2 variability. However, these findings are small and not robust across languages. Results for the other novel features are very uncertain or inconsistent across languages. As for more traditional features, we found that reported biological sex, age, and level of intelligence again affect several differences.
Effect of Symptoms
Detailed results and comparison with meta-analytic findings are reported in Table 3. Clinical features generally correlated with acoustic features, and these associations were in line with meta-analytic priors. However, we did not find reliable and robust associations across all languages. The associations are generally stronger for temporal-duration measures (lower speech percentage, increased pause duration and reduced speech rate are associated with higher flat affect, alogia and negative symptom severity), whereas pitch measures (median and IQR) are more weakly associated with clinical ratings. Most of the correlations are between small and moderate (5–16% of explained variance), and vary across languages and rating scales.
Table 3.
Estimated standardized relation between acoustic and clinical features. ER indicates the evidence ratio for the difference, ER01 the evidence ratio for the null effect.
| Rating scales | SANS | SAPS | SANS—Alogia | SANS—Flat affect | |
|---|---|---|---|---|---|
| Pitch Median | |||||
| MA priors | 0.096 [−0.158, 0.346] | −0.185 [−0.691, 0.316] | |||
| Skeptical DK | −0.01 (−0.19 0.15) ER = 1.22 ER01 = 6.52 | 0.1 (−0.05 0.25) ER = 6.87 | 0.11 (−0.05 0.27) ER = 6.77 | −0.04 (−0.19 0.11) ER = 2.01 ER01 = 6.14 | |
| Skeptical CH | −0.01 (−0.35 0.33) ER = 1.13 ER01 = 3.12 | −0.07 (−0.3 0.14) ER = 2.34 ER01 = 4.07 | −0.03 (−0.47 0.4) ER = 1.21 ER01 = 2.41 | −0.15 (−0.45 0.15) ER = 4.39 | |
| Informed DK | −0.02 (−0.19 0.16) ER = 1.32 ER01 = 3.89 | 0.1 (−0.04 0.25) ER = 8.39 | |||
| Informed CH | 0.04 (−0.26 0.34) ER = 1.54 ER01 = 2.16 | −0.08 (−0.3 0.13) ER = 2.89 ER01 = 4.52 | |||
| Stacking weight | Informed Model 1.00 | Informed Model 0.56 | |||
| Pitch IQR | |||||
| MA priors | −0.01 [−0.196, 0.144] | −0.027 [−0.686, 0.763] | −0.035 [−0.317, 0.22] | −0.106 [−0.262, −0.047] | |
| Skeptical DK | 0.01 (−0.02 0.05) ER = 2.91 ER01 = 24.69 | −0.01 (−0.03 0.02) ER = 1.64 ER01 = 33.01 | 0.01 (−0.02 0.04) ER = 1.69 ER01 = 31.04 | 0 (−0.03 0.02) ER = 1.4 ER01 = 39.65 | |
| Skeptical CH | −0.06 (−0.25 0.1) ER = 2.48 ER01 = 5.84 | −0.03 (−0.14 0.07) ER = 2.21 ER01 = 8.3 | −0.32 (−0.6 −0.1) ER = 124 | −0.26 (−0.42 −0.11) ER = 599 | |
| Informed DK | 0.01 (−0.02 0.05) ER = 2.95 ER01 = 11.54 | −0.01 (−0.03 0.02) ER = 1.62 ER01 = 51.25 | 0.01 (−0.02 0.04) ER = 1.66 ER01 = 20.87 | 0 (−0.03 0.02) ER = 1.45 ER01 = 16.74 | |
| Informed CH | −0.05 (−0.23 0.11) ER = 2.15 ER01 = 2.47 | −0.03 (−0.14 0.07) ER = 2.24 ER01 = 11.95 | −0.3 (−0.53 −0.1) ER = 141.86 | −0.24 (−0.39 −0.1) ER = 749 | |
| Stacking weight | Informed Model 1.00 | Skeptic Model 1.00 | Skeptic Model 1.00 | Informed Model 1.00 | |
| Speech percentage | |||||
| MA priors | −0.229 [−0.499, 0.035] | −0.413 [−0.723, −0.07] | −0.1 (−0.18 −0.01) ER = 34.29 | ||
| Skeptical DK | −0.12 (−0.23 −0.02) ER = 47.78 | 0.04 (−0.05 0.13) ER = 3.37 | −0.03 (−0.12 0.06) ER = 2.17 ER01 = 9.57 | −0.09 (−0.17 0) ER = 23.49 | |
| Skeptical CH | −0.02 (−0.3 0.24) ER = 1.15 ER01 = 3.88 | 0.07 (−0.1 0.24) ER = 3.17 | −0.11 (−0.46 0.21) ER = 2.48 ER01 = 2.76 | −0.06 (−0.3 0.17) ER = 2.03 ER01 = 4.01 | |
| Informed DK | −0.13 (−0.24 −0.02) ER = 51.63 | NA | −0.03 (−0.13 0.06) ER = 2.55 ER01 = 10.71 | −0.09 (−0.18 −0.01) ER = 31.97 | |
| Informed CH | −0.05 (−0.33 0.2) ER = 1.62 ER01 = 3.16 | NA | −0.18 (−0.54 0.14) ER = 4.28 | −0.11 (−0.34 0.12) ER = 3.46 | |
| Stacking weight | Informed Model 1.00 | Informed Model 0.88 | Informed Model 1.00 | ||
| Speech rate | |||||
| Skeptical DK | −0.18 (−0.28 −0.1) ER = 1999 | −0.02 (−0.09 0.06) ER = 1.91 ER01 = 13.81 | −0.13 (−0.22 −0.05) ER = 499 | −0.11 (−0.18 −0.04) ER = 332.33 | |
| Skeptical CH | −0.15 (−0.38 0.05) ER = 8.09 | 0.05 (−0.09 0.19) ER = 2.87 ER01 = 6.31 | −0.22 (−0.54 0.06) ER = 9.64 | −0.17 (−0.37 0.01) ER = 14.62 | |
| Pause duration | |||||
| MA priors | 0.302 [−0.199, 0.783] | ||||
| Skeptical DK | 0.06 (0.02 0.11) ER = 186.5 | 0 (−0.03 0.04) ER = 1.16 ER01 = 29.07 | 0.04 (0 0.08) ER = 24.1 | 0.03 (0 0.07) ER = 21.99 | |
| Skeptical CH | −0.03 (−0.22 0.16) ER = 1.52 ER01 = 5.35 | −0.02 (−0.14 0.11) ER = 1.43 ER01 = 8.44 | −0.02 (−0.29 0.25) ER = 1.17 ER01 = 4.15 | 0.01 (−0.17 0.2) ER = 1.21 ER01 = 5.81 | |
| Informed DK | 0.07 (0.02 0.11) ER = 221.22 | NA | NA | NA | |
| Informed CH | −0.03 (−0.23 0.18) ER = 1.42 ER01 = 6.85 | NA | NA | NA | |
| Stacking weight | Informed Model 1.00 | ||||
| Number of pauses | |||||
| Skeptical DK | −0.06 (−0.13 0.01) ER = 10.11 | 0.02 (−0.04 0.08) ER = 2.1 ER01 = 15.8 | −0.03 (−0.09 0.03) ER = 3.6 | −0.05 (−0.11 0) ER = 15.81 | |
| Skeptical CH | 0.08 (−0.14 0.3) ER = 2.86 ER01 = 3.72 | −0.15 (−0.3 −0.01) ER = 23.19 | 0.19 (−0.05 0.46) ER = 9.71 | 0.08 (−0.08 0.26) ER = 3.91 | |
| Duration of utterance | |||||
| Skeptical DK | −0.07 (−0.13 −0.01) ER = 67.97 | −0.05 (−0.09 0) ER = 16.7 | −0.08 (−0.14 −0.03) ER = 374 | −0.03 (−0.08 0.02) ER = 6.33 | |
| Skeptical CH | −0.1 (−0.28 0.06) ER = 5.97 | 0.1 (0 0.21) ER = 19.76 | −0.25 (−0.51 −0.04) ER = 35.14 | −0.18 (−0.34 −0.04) ER = 49.85 | |
| Number of utterances | |||||
| Skeptical DK | −0.06 (−0.13 0.01) ER = 13.89 | 0.01 (−0.05 0.07) ER = 1.43 ER01 = 18.21 | −0.04 (−0.1 0.02) ER = 6.63 | −0.06 (−0.11 0) ER = 20.82 | |
| Skeptical CH | 0.06 (−0.15 0.28) ER = 2.24 ER01 = 4.43 | −0.15 (−0.3 −0.02) ER = 27.99 | 0.15 (−0.09 0.41) ER = 5.74 | 0.06 (−0.11 0.24) ER = 2.71 ER01 = 4.99 | |
| Rating scales | PANSS Total |
PANSS
Negative |
PANSS
Positive |
PANSS Conversation |
PANSS
Blunted affect |
| Pitch Median | |||||
| MA priors | 0.096 [−0.158, 0.346] | −0.185 [−0.691, 0.316] | |||
| Skeptical CH | −0.3 (−0.79 0.16) ER = 5.97 | −0.14 (−0.46 0.17) ER = 3.31 | −0.24 (−0.6 0.11) ER = 6.73 | 0 (−0.25 0.26) ER = 1.1 ER01 = 3.97 | −0.07 (−0.37 0.22) ER = 1.85 ER01 = 3.26 |
| Skeptical JP | −0.43 (−1.03 0.18) ER = 7 | −0.4 (−1.01 0.23) ER = 6.36 | −0.51 (−1.11 0.08) ER = 11.66 | NA | NA |
| Skeptical GE | −0.1 (−0.48 0.28) ER = 1.92 ER01 = 2.45 | −0.03 (−0.34 0.27) ER = 1.29 ER01 = 3.41 | −0.34 (−0.71 −0.01) ER = 21.56 | −0.12 (−0.3 0.06) ER = 6.52 | 0.26 (−0.14 0.73) ER = 5.87 |
| Informed CH | NA | −0.1 (−0.4 0.2) ER = 2.32 ER01 = 1.83 | −0.26 (−0.62 0.1) ER = 7.72 | NA | NA |
| Informed JP | NA | −0.18 (−0.66 0.31) ER = 2.66 ER01 = 1.06 | −0.63 (−1.28 0.03) ER = 16.7 | NA | NA |
| Informed GE | NA | −0.02 (−0.3 0.25) ER = 1.18 ER01 = 2.37 | −0.36 (−0.74 −0.03) ER = 25.79 | NA | NA |
| Skeptic Model 1.00 | Skeptic Model 1.00 | ||||
| Pitch IQR | |||||
| MA priors | −0.091 [−0.34, 0.15] | −0.01 [−0.196, 0.144] | −0.027 [−0.686, 0.763] | 0 (−0.25 0.26) ER = 1.1 ER01 = 3.97 | −0.07 (−0.37 0.22) ER = 1.85 ER01 = 3.26 |
| Skeptical CH | 0.23 (−0.11 0.56) ER = 6.95 | −0.15 (−0.35 0.05) ER = 8.45 | 0.4 (0.24 0.57) ER = Inf | −0.1 (−0.24 0.03) ER = 9.45 | −0.07 (−0.26 0.11) ER = 2.7 ER01 = 5.13 |
| Skeptical JP | 0.04 (−0.43 0.53) ER = 1.26 ER01 = 2.21 | −0.08 (−0.61 0.45) ER = 1.5 ER01 = 1.84 | 0.06 (−0.44 0.56) ER = 1.44 ER01 = 2.01 | NA | NA |
| Skeptical GE | −0.16 (−0.33 0.01) ER = 16.39 | −0.27 (−0.44 −0.14) ER = 1999 | −0.01 (−0.17 0.16) ER = 1.29 ER01 = 5.93 | −0.04 (−0.13 0.04) ER = 4.15 | −0.1 (−0.34 0.08) ER = 4.43 |
| Informed CH | 0.18 (−0.14 0.48) ER = 5.4 | −0.13 (−0.3 0.05) ER = 7.08 | 0.41 (0.25 0.59) ER = 5999 | −0.1 (−0.24 0.03) ER = 8.85 | −0.08 (−0.25 0.08) ER = 3.62 |
| Informed JP | 0 (−0.43 0.43) ER = 1.05 ER01 = 1.71 | −0.05 (−0.39 0.29) ER = 1.42 ER01 = 1.18 | 0.06 (−0.5 0.63) ER = 1.38 ER01 = 2.72 | NA | NA |
| Informed GE | −0.15 (−0.32 0.01) ER = 15.57 | −0.25 (−0.39 −0.12) ER = 1199 | −0.02 (−0.19 0.15) ER = 1.36 ER01 = 8.98 | −0.05 (−0.13 0.03) ER = 4.63 | −0.1 (−0.29 0.06) ER = 5.3 |
| Informed Model 1.00 | Skeptic Model 1.00 | Skeptic Model 1.00 | Skeptic Model 1.00 | Informed Model 1.00 | |
| Speech percentage | |||||
| MA priors | 0.302 [−0.199, 0.783] | ||||
| Skeptical CH | 0.03 (−0.45 0.5) ER = 1.18 ER01 = 2.06 | −0.31 (−0.62 0) ER = 18.05 | 0.14 (−0.23 0.5) ER = 2.97 ER01 = 2.22 | −0.15 (−0.38 0.07) ER = 7.02 | −0.2 (−0.49 0.08) ER = 7.6 |
| Skeptical JP | 0.24 (−0.25 0.73) ER = 4.03 | 0.14 (−0.42 0.67) ER = 2.01 ER01 = 1.72 | 0.19 (−0.36 0.72) ER = 2.64 ER01 = 1.55 | NA | NA |
| Skeptical GE | −0.26 (−0.57 0.04) ER = 11.88 | −0.12 (−0.36 0.12) ER = 3.93 | −0.11 (−0.38 0.17) ER = 2.91 ER01 = 3.11 | −0.17 (−0.32 −0.03) ER = 36.97 | −0.17 (−0.5 0.13) ER = 5.63 |
| Informed CH | 0.03 (−0.45 0.53) ER = 1.18 ER01 = 2.11 | −0.32 (−0.63 −0.02) ER = 22.35 | NA | −0.19 (−0.42 0.03) ER = 11.88 | −0.26 (−0.48 −0.05) ER = 51.63 |
| Informed JP | 0.23 (−0.29 0.74) ER = 3.56 | 0 (−0.51 0.48) ER = 0.97 ER01 = 1.75 | NA | NA | NA |
| Informed GE | −0.26 (−0.57 0.04) ER = 11.42 | −0.14 (−0.38 0.08) ER = 5.51 | NA | −0.19 (−0.34 −0.04) ER = 56.14 | −0.23 (−0.51 −0.03) ER = 34.71 |
| Informed Model 1.00 | Informed Model 1.00 | Skeptic Model 1.00 | Informed Model 1.00 | ||
| Speech Rate | |||||
| Skeptical CH | −0.38 (−0.76 −0.01) ER = 20.28 | −0.16 (−0.41 0.08) ER = 6.48 | −0.01 (−0.28 0.27) ER = 1.13 ER01 = 3.78 | −0.13 (−0.32 0.06) ER = 6.8 | −0.11 (−0.35 0.11) ER = 3.74 |
| Skeptical JP | 0.26 (−0.28 0.8) ER = 3.98 | 0.15 (−0.46 0.74) ER = 2.05 ER01 = 1.44 | 0.3 (−0.28 0.84) ER = 4.45 | NA | NA |
| Skeptical GE | −0.39 (−0.69 −0.1) ER = 61.5 | −0.24 (−0.47 −0.02) ER = 23.9 | −0.12 (−0.4 0.15) ER = 3.22 | −0.18 (−0.34 −0.04) ER = 51.63 | −0.21 (−0.55 0.08) ER = 8.01 |
| Mean Pause Duration | |||||
| MA priors | 0.302 [−0.199, 0.783] | ||||
| Skeptical CH | 0.14 (−0.24 0.49) ER = 2.78 ER01 = 2.15 | 0.38 (0.22 0.54) ER = 1199 | −0.12 (−0.39 0.15) ER = 3.39 | 0.23 (0.1 0.36) ER = 351.94 | 0.26 (0.11 0.43) ER = 351.94 |
| Skeptical JP | −0.5 (−0.96 −0.01) ER = 20.98 | −0.42 (−0.96 0.13) ER = 8.93 | −0.49 (−1 0.02) ER = 16.05 | ||
| Skeptical GE | 0.11 (0 0.23) ER = 18.54 | 0.07 (−0.02 0.16) ER = 10.45 | 0.02 (−0.09 0.13) ER = 1.85 ER01 = 9 | 0.11 (0.04 0.17) ER = 299 | 0.11 (−0.01 0.28) ER = 15.48 |
| Informed CH | NA | 0.39 (0.24 0.55) ER = 5999 | NA | NA | NA |
| Informed JP | NA | −0.37 (−0.95 0.21) ER = 6.18 | NA | NA | NA |
| Informed GE | NA | 0.07 (−0.01 0.16) ER = 10.83 | NA | NA | NA |
| Informed Model 1.00 | |||||
| Pause Number | |||||
| Skeptical CH | −0.51 (−0.94 −0.08) ER = 33.09 | −0.32 (−0.62 −0.04) ER = 29.15 | 0.02 (−0.32 0.37) ER = 1.15 ER01 = 2.78 | −0.3 (−0.5 −0.09) ER = 92.75 | −0.25 (−0.52 0) ER = 19.91 |
| Skeptical JP | 0.24 (−0.26 0.73) ER = 3.94 | 0.14 (−0.41 0.67) ER = 2.07 ER01 = 1.79 | 0.33 (−0.2 0.81) ER = 6.23 | NA | NA |
| Skeptical GE | −0.2 (−0.39 −0.03) ER = 32.9 | −0.12 (−0.26 0.02) ER = 12.95 | −0.02 (−0.19 0.14) ER = 1.52 ER01 = 5.83 | −0.14 (−0.23 −0.06) ER = 271.73 | −0.15 (−0.39 0.03) ER = 11.35 |
| Utterance duration | |||||
| Skeptical CH | 0.3 (0 0.59) ER = 18.05 | 0.2 (0.02 0.38) ER = 29.61 | 0.1 (−0.12 0.32) ER = 3.63 | 0.09 (−0.03 0.22) ER = 8.51 | 0.19 (0.06 0.33) ER = 60.22 |
| Skeptical JP | 0.06 (−0.44 0.54) ER = 1.5 ER01 = 1.96 | −0.01 (−0.53 0.49) ER = 1.04 ER01 = 2.05 | 0.11 (−0.4 0.6) ER = 1.94 ER01 = 1.87 | NA | NA |
| Skeptical GE | −0.19 (−0.33 −0.07) ER = 110.11 | −0.17 (−0.29 −0.07) ER = 544.45 | −0.07 (−0.21 0.06) ER = 4.38 | −0.07 (−0.14 0) ER = 18.29 | −0.07 (−0.27 0.1) ER = 3.06 |
| Utterance Number | |||||
| Skeptical CH | −0.49 (−0.9 −0.07) ER = 34.71 | −0.34 (−0.62 −0.07) ER = 40.1 | 0.03 (−0.3 0.35) ER = 1.25 ER01 = 3.21 | −0.3 (−0.5 −0.11) ER = 145.34 | −0.24 (−0.5 −0.01) ER = 21.47 |
| Skeptical JP | 0.25 (−0.25 0.73) ER = 4.16 | 0.12 (−0.42 0.65) ER = 1.83 ER01 = 1.68 | 0.35 (−0.17 0.83) ER = 7.15 | NA | NA |
| Skeptical GE | −0.22 (−0.41 −0.03) ER = 39 | −0.14 (−0.29 0) ER = 20.66 | −0.03 (−0.21 0.14) ER = 1.68 ER01 = 5.22 | −0.15 (−0.25 −0.07) ER = 314.79 | −0.15 (−0.38 0.04) ER = 9.95 |
Note: ER, evidence ratio for the difference; ER01, evidence ratio for the null effect. The Scale for the Assessment of Negative Symptoms (SANS), the Scale for the Assessment of Positive Symptoms (SAPS), and the Positive and Negative Syndrome Scale (PANSS). Danish (DK), German (GE), Chinese (CH) and Japanese (JP) models. NA = not applicable.
Medication, duration of illness and social functioning (PSP)
We found that medication was related to vocal patterns, but inconsistently across languages. The more widespread patterns were that patients who use high D2R occupancy drugs, compared to patients who use low D2R occupancy drugs, show reduced pitch variability, higher number of pauses, longer utterance duration and higher speech rate. Further, higher drug dosage (CPZ equivalents) was associated with lower pitch median, lower speech rate, increased pause and utterance duration and reduced total number of words (see Table S6_B).
We generally found no difference between patients with FES and patients with chronic schizophrenia, and only weak evidence supporting a role of illness duration: acoustic atypicalities are present already at disease onset and not just associated with a longer and more severe course of the disorder (see Table S6_C). Finally, we found that increased speech percentage and reduced pause duration were associated with higher scores on PSP, although only in the Danish corpus (see Table S6_D).
Discussion
In the present article, we aimed at developing a critical, cumulative scientific approach to the understanding of vocal and prosodic atypicalities in schizophrenia. Relying on a previous meta-analysis3 of the field, we systematically assessed the generalizability of established and novel acoustic markers on a new large cross-linguistic dataset. We also assessed whether explicitly incorporating previous findings as informed priors would increase the generalizability of the results and provide additional insights on heterogeneity of the findings compared to previous literature.
Is There a Universal Generalizable Acoustic Profile of Schizophrenia?
Our study assessed the generalizability of findings across a heterogeneous dataset (4 different languages). In other words, we assessed whether previous findings would be shown in a new study applying analogous experimental and/or statistical procedures, ie, replication,47 “to populations with for instance a different language, age distribution, or other demographic and clinical characteristics”, ie, generalization.48 We only found a minimal generalizable acoustic profile of schizophrenia: reduced pitch variability and increased utterance duration, albeit with modest effect sizes. Given the heterogeneity of previous studies and uncertainty about publication bias reported in a previous meta-analysis,3 even these minimal cross-linguistically generalized findings are far from trivial.
One possible mechanism for the generalizable acoustic profile is a relation to negative symptoms, emotional and effort-related ones. Reduced pitch variability is related to monotone speech and flat affect,49–51 and increased utterance duration to lower energy and increased vocal effort.52,53 Further, the most promising of the novel features we investigated (eg, reduced formant frequency and increased H1H2, albeit not consistent across all languages) also fit this explanation.54–56 Reduced formant frequencies have been found to be associated with clinical ratings of blunted affect and alogia in schizophrenia,55 and a decrease in articulatory effort in patients with depression.28 Furthermore, many studies in patients with depression have shown that an increase in H1H2 can be considered one of the acoustic indicators of breathiness and associated with psychomotor retardation.57 However, we should be skeptical of any simplistic explanation as yet, since we did not find a cross-linguistically robust association between these acoustic features and clinical ratings of negative symptoms.
In particular, we argue that heterogeneity of the studies and samples has not been insufficiently accounted for, so far.
Source of Heterogeneity in the Voice Profiles of Patients With Schizophrenia
The second crucial contribution of this study is highlighting the importance and complexity of clinical, socio-demographic, contextual (eg speech task) and linguistic differences in assessing vocal markers of schizophrenia.
Clinical Heterogeneity
Individuals with schizophrenia present wildly heterogeneous constellations of clinical features: symptoms, onset and duration of the disorder, as well as medications.24,27 Moreover, some of the clinical ratings are based on the perceptual assessment of speech features, and accordingly, we should expect acoustic heterogeneity co-varying with clinical heterogeneity.36,54,55 Indeed, we found associations between acoustic parameters and clinical ratings, with duration aspects being more closely related to clinical ratings, in line with meta-analytic findings.3 Lower proportion of speech and reduced speech rate, as well as longer pause duration were generally associated with higher ratings of negative symptoms and, in particular, alogia and flat affect.
However, the acoustic atypicalities were generally smaller than differences in clinical ratings,33,43 and their relation to clinical ratings was often inconsistent across languages and rating scales. This might have several explanations. The first is that the acoustic features analyzed are only a subset of those actually used by clinicians to produce clinical ratings of alogia and blunted affect.15 Different approaches using larger sets of acoustic features and machine learning techniques could be required to better characterize the acoustic markers of clinical ratings.54–56 Second, the divergence between acoustic features and clinical ratings can be explained by the fact that these two signals have different temporal (ie, precision with respect to time) and spatial (ie, precision with respect to environmental changes) resolution, thus expecting high convergence between the two may not be realistic.49 Third, different clinical scales might not be fully overlapping in the symptoms they include and in their definition of the symptoms,58,59 and linguistic and cultural differences may also affect the frequency, expression and rating of symptoms, thus generating several inconsistencies.60,61
Different clinical profiles also often imply different medication profiles, and different medications can differentially impact vocal production.41 Indeed, D2R drug occupancy and medication dosage were shown to relate to acoustic patterns, albeit inconsistently across languages. This suggests that the field needs a more fine-grained assessment of the different medications involved in schizophrenia, its comorbidities and their impact on voice.62
Finally, we found no differences between patients with FES and chronic patients, and a limited role of illness duration on acoustic profiles: vocal atypicalities are already present at the onset of the disease and not only associated with a longer and more severe course of the disorder, thus having the potential for tracking its development and monitoring the symptomatology over time.
Socio-Demographic Heterogeneity
Another crucial source of heterogeneity in acoustic patterns is socio-demographic heterogeneity. Indeed, we found several reliable effects of sex and age on vocal atypicalities. However, the picture is currently very sparse, and more than identifying systematic effects, we rather recommend socio-demographic variables be taken explicitly into account. Promising progress has been made in normative modeling,63,64 which relies on large samples to provide expectations, accounting for clinical and socio-demographic features, and assess individual deviations from such expectations. This approach is particularly relevant in light of recent evidence showing how computational speech analysis may be prone to serious bias determined by socio-demographic factors, such as racial identity,65 as it may help to identify such potential bias. An alternative approach is to collect larger samples and use propensity scores66,67 to better match patients and controls and account for potential confounders. These approaches may also help to tackle the problem of selection bias, as most previous studies have used convenience samples with relatively homogeneous clinical and sociodemographic features (eg, chronic or FES patients), that may not be fully representative of the schizophrenia spectrum and its heterogeneity.
Linguistic Heterogeneity
Not least, albeit previously neglected, linguistic and cultural differences can also play a role. We modeled each language separately to account for the heterogeneity in the different corpora, thus allowing us to compare results across languages. In general, we found that atypical voice patterns were more similar within Germanic (Danish and German) and non-Germanic (Japanese and Chinese) language families. The most prominent results of the previous meta-analysis3 (reduced speech percentage and speech rate, longer pause duration) were not consistently found across all languages in our study. One possible source of this discrepancy is the amount of noise and bias present in previous studies. Meta-analyses are often afflicted by publication bias, and heterogeneous quality in the estimates being collated, which often results in consistent discrepancies with multi-lab replications.68 However, a complementary explanation is that quantitative vocal aspects may vary with the languages being studied, and that vocal atypicalities reported to be abnormal in schizophrenia may not be universally expressed in the same way. For instance, prosodic use of speech rate and pauses to create emphasis may differ across languages. Thus, it is possible that decreased prosodic emphasis is a universal feature of schizophrenia, but the acoustic ways in which this can be measured vary across languages. Vocal patterns must also be considered in relation to their cultural and linguistic context,8,69 and the generalizability across social and linguistic groups should be systematically tested in future studies.
Acoustic Heterogeneity and Speech Task
The use of a common standardized task (ie, the ATT41) allowed us to compare voice patterns across the different corpora. This is particularly important considering that a previous meta-analysis3 has shown that varying speech tasks may yield different outcomes, and recent studies further support this finding.51,55 Further, our task included repeated measurements and allowed us to model intra-speaker variability, which previous studies found to be relevant.70–72 Even by controlling for intra-speaker variability and keeping the task constant, we found large and important differences in voice patterns between the corpora. Further, we noticed that the patients produced very short responses (<6 s) much more frequently than controls, and when excluding these trials, we observed that results were more in line with meta-analytic findings.3 This suggests that acoustic patterns may be task-related (with the task also interacting with language) and not robust across different tasks.
Limitations and Future Perspectives
The large differences in terms of the clinical and socio-demographic features within our corpus is a limitation of the present study. For example, the sample size differs across languages, and the samples in the different languages are not exactly matched in terms of the relevant clinical features, such as medication or symptom profiles. Thus, larger sample sizes might be more representative than smaller ones, and partially different subpopulations might be investigated across languages. Further, even if we kept the task and the experimental procedure constant across sites, a more controlled acoustic setting, with high-quality headset microphones placed at a constant distance from the mouth, would have allowed to further reduce potential differences across the different recording settings. This variability, albeit limited, between our corpora in terms of these features, may have contributed to the differences in the main results; however, it also provided an opportunity to assess the generalizability of results across more varied conditions and thus provided more generalizable results. Moreover, although we collected a larger multilingual sample compared to previous studies,3,22 an ideal sample systematically representative of the schizophrenia spectrum and its clinical and sociodemographic variability would be even larger.64 Future efforts should thus be directed to build a large open cross-linguistic corpus of speech recordings of patients with schizophrenia able to capture linguistic, cultural, socio-economic and clinical variability. This multilingual corpus may represent an ideal benchmark dataset for testing the reliability and generalizability (eg, out of sample predictability) of voice analysis results in schizophrenia,73 and the necessary ground for assessing its clinical applicability.74 Not least, future studies should focus more on cross-diagnostic comparisons aimed at capturing symptom dimensions which extend over a single disorder,75,76 and implement longitudinal designs able to test more complex hypothesis on the interaction between antipsychotic medication type and dosage, clinical (eg, illness severity and duration) and sociodemographic (eg, gender differences77) characteristics, and speech production.
Another limitation is that we focused on single features as markers of schizophrenia, and did not vary the speech task. For example, the specific social context in which the speech task takes place, the social actors involved in it, and the communicative goal to be fulfilled can influence speech production and thus acoustic patterns. Speaking to a superior vs a peer, having a formal interview vs. an informal chat, all involve different prosodic patterns. Individual participants might perceive the experiment context differently from each other, some as more formal than others; and this is further complicated by cultural factors affecting how interactions with researchers are perceived and dealt with, and therefore the acoustic patterns. Further, linguistic and vocal patterns are inherently multidimensional, with different acoustic features interacting with each other, and cross-linguistic variations potentially affecting these interactions. Looking at single features could thus be reductive: future studies should focus more on examining patterns of shared variance across features,78–80 their relations with different speech tasks (in terms of social and cognitive demands),78,79 and with linguistic variability.8,80 However, this would require testing more fine-grained hypotheses on mechanisms relying on formal linguistic theories69 and on psychopathological functioning theories.
Conclusion
Overall, we found scarce evidence for a universal, distinctive vocal pattern that characterizes schizophrenia: vocal patterns are highly heterogeneous with different sources of heterogeneity interacting at different levels. These results raise some questions about the generalizability of previous findings and the possibility to cumulatively build on them.81 However, they also indicate where future attempts may be directed: a larger shared multilingual corpus representative of the heterogeneity of the schizophrenia spectrum, a more explicit focus on multidimensional acoustic patterns and their relations hips with speech tasks, and a self-correcting cumulative approach. These are the necessary conditions to develop effective clinical applications in the near future that can target different ranges of patients and address the issue of potential bias.
Supplementary Material
Contributor Information
Alberto Parola, Department of Linguistics, Cognitive Science and Semiotics, Aarhus University, Aarhus, Denmark; The Interacting Minds Center, Institute of Culture and Society, Aarhus University, Aarhus, Denmark; Department of Psychology, University of Turin, Turin, Italy.
Arndis Simonsen, The Interacting Minds Center, Institute of Culture and Society, Aarhus University, Aarhus, Denmark; Psychosis Research Unit, Department of Clinical Medicine, Aarhus University, Aarhus, Denmark.
Jessica Mary Lin, Department of Linguistics, Cognitive Science and Semiotics, Aarhus University, Aarhus, Denmark; The Interacting Minds Center, Institute of Culture and Society, Aarhus University, Aarhus, Denmark.
Yuan Zhou, Institute of Psychology, Chinese Academy of Sciences, Beijing, China.
Huiling Wang, Department of Psychiatry, Renmin Hospital of Wuhan University, Wuhan, China.
Shiho Ubukata, Department of Psychiatry, Kyoto University, Kyoto, Japan.
Katja Koelkebeck, LVR-Hospital Essen, Department of Psychiatry and Psychotherapy, Hospital and Institute of the University of Duisburg-Essen, Essen, Germany; Center for Translational Neuro- and Behavioral Sciences (C-TNBS), University Duisburg-Essen, Germany.
Vibeke Bliksted, The Interacting Minds Center, Institute of Culture and Society, Aarhus University, Aarhus, Denmark; Psychosis Research Unit, Department of Clinical Medicine, Aarhus University, Aarhus, Denmark.
Riccardo Fusaroli, Department of Linguistics, Cognitive Science and Semiotics, Aarhus University, Aarhus, Denmark; The Interacting Minds Center, Institute of Culture and Society, Aarhus University, Aarhus, Denmark; Linguistic Data Consortium, University of Pennsylvania, Philadelphia, USA.
Funding
A.P is supported by a Marie Skłodowska-Curie Actions—H2020-MSCA-IF-2018 grant (ID: 832518, Project: MOVES). A.S is supported by the Carlsberg Foundation. K. K has been supported by the Japan Society for the Promotion of Science (JSPS) (PE 07550). The project has been supported by seed funding from the Interacting Minds Center, Aarhus University.
Conflict of Interest
Riccardo Fusaroli has been a paid consultant on related but not overlapping topics for Roche. The other authors have no real or potential conflicts of interest that could have influenced the research.
References
- 1. Bleuler E, Aschaffenburg G.. Dementia Praecox or the Group of Schizophrenias. Deuticke, ed. New York, NY: International Universities Press; 1950. [Google Scholar]
- 2. Kraepelin E. Dementia Praecox and Paraphrenia, 1919. Barclay RM, transl. Huntington, NY: Robert E. Kreiger Publishing Co., Inc.; 1999. [Google Scholar]
- 3. Parola A, Simonsen A, Bliksted V, Fusaroli R.. Voice patterns in schizophrenia: a systematic review and Bayesian meta-analysis. Schizophr Res. 2020. doi: 10.1016/j.schres.2019.11.031 [DOI] [PubMed] [Google Scholar]
- 4. Couture SM, Granholm EL, Fish SC.. A path model investigation of neurocognition, theory of mind, social competence, negative symptoms and real-world functioning in schizophrenia. Schizophr Res. 2011;125(2–3):152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rabinowitz J, Levine SZ, Garibaldi G, Bugarski-Kirola D, Berardo CG, Kapur S.. Negative symptoms have greater impact on functioning than positive symptoms in schizophrenia: analysis of CATIE data. Schizophr Res. 2012;137(1–3):147–150. [DOI] [PubMed] [Google Scholar]
- 6. Häfner H, Löffler W, Maurer K, Hambrecht M, An Der Heiden W.. Depression, negative symptoms, social stagnation and social decline in the early course of schizophrenia. Acta Psychiatr Scand. 1999;100(2):105–118. [DOI] [PubMed] [Google Scholar]
- 7. Tandon R, Keshavan MS, Nasrallah Ha.. Schizophrenia, “just the facts” what we know in 2008. 2. Epidemiology and etiology. Schizophr Res. 2008;102(1–3):1–18. [DOI] [PubMed] [Google Scholar]
- 8. Palaniyappan L. More than a biomarker: could language be a biosocial marker of psychosis? NPJ Schizophr. 2021;7(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Parola A, Berardinelli L, Bosco FM.. Cognitive abilities and theory of mind in explaining communicative-pragmatic disorders in patients with schizophrenia. Psychiatry Res. 2018;260:144–151. [DOI] [PubMed] [Google Scholar]
- 10. Bambini V, Arcara G, Bechi M, Buonocore M, Cavallaro R, Bosia M.. The communicative impairment as a core feature of schizophrenia: frequency of pragmatic deficit, cognitive substrates, and relation with quality of life. Compr Psychiatry. 2016;71:106–120. [DOI] [PubMed] [Google Scholar]
- 11. Bliksted V, Fagerlund B, Weed E, Frith C, Videbech P.. Social cognition and neurocognitive deficits in first-episode schizophrenia. Schizophr Res. 2014;153(1–3):9–17. [DOI] [PubMed] [Google Scholar]
- 12. Cohen AS, Dinzeo TJ, Donovan NJ, Brown CE, Morrison SC.. Vocal acoustic analysis as a biometric indicator of information processing: implications for neurological and psychiatric disorders. Psychiatry Res. 2015;226(1):235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cohen AS, McGovern JE, Dinzeo TJ, Covington MA.. Speech deficits in serious mental illness: a cognitive resource issue? Schizophr Res. 2014;160(1–3):173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Insel TR. Digital phenotyping: technology for a new science of behavior. JAMA. 2017;318(13):1215–1216. [DOI] [PubMed] [Google Scholar]
- 15. Chandler C, Foltz PW, Cohen AS, et al. Machine learning for ambulatory applications of neuropsychological testing. Intell Med. 2020;1–2:100006. https://www.sciencedirect.com/science/article/pii/S2666521220300065 [Google Scholar]
- 16. Cohen AS, Cox CR, Masucci MD, et al. Digital phenotyping using multimodal data. Curr Behav Neurosci Reps. 2020;7(4):212–220. [Google Scholar]
- 17. Ben-Zeev D, Brian R, Wang R, et al. CrossCheck: integrating self-report, behavioral sensing, and smartphone use to identify digital indicators of psychotic relapse. Psychiatr Rehabil J. 2017;40(3):266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Corcoran CM, Cecchi GA.. Using language processing and speech analysis for the identification of psychosis and other disorders. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5(8):770–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Galatzer-Levy IR, Abbas A, Koesmahargyo V, et al. Facial and vocal markers of schizophrenia measured using remote smartphone assessments. medRxiv. 2020;1(646). doi: 10.1101/2020.12.02.20219741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hitczenko K, Mittal VA, Goldrick M.. Understanding language abnormalities and associated clinical markers in psychosis: the promise of computational methods. Schizophr Bull. 2021;47(2):344–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Corcoran R, Frith CD.. Autobiographical memory and theory of mind: evidence of a relationship in schizophrenia. Psychol Med. 2003;33(5):897–905. [DOI] [PubMed] [Google Scholar]
- 22. Cohen AS, Mitchell KR, Elvevåg B.. What do we really know about blunted vocal affect and alogia? A meta-analysis of objective assessments. Schizophr Res. 2014;159(2–3):533–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schnack HG. Improving individual predictions: machine learning approaches for detecting and attacking heterogeneity in schizophrenia (and other psychiatric diseases). Schizophr Res. 2019;214:34–42. [DOI] [PubMed] [Google Scholar]
- 24. Gratton C, Mittal VA.. Embracing the complexity of heterogeneity in schizophrenia: a new perspective from latent clinical-anatomical dimensions. Schizophr Bull. 2020;46(6):1337–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Honnorat N, Dong A, Meisenzahl-Lechner E, Koutsouleris N, Davatzikos C.. Neuroanatomical heterogeneity of schizophrenia revealed by semi-supervised machine learning methods. Schizophr Res. 2019;214:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fisher AJ, Medaglia JD, Jeronimus BF.. Lack of group-to-individual generalizability is a threat to human subjects research. Proc Natl Acad Sci USA. 2018;115(27):E6106–E6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dickinson D, Pratt DN, Giangrande EJ, et al. Attacking heterogeneity in schizophrenia by deriving clinical subgroups from widely available symptom data. Schizophr Bull. 2018;44(1):101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cummins N, Scherer S, Krajewski J, Schnieder S, Epps J, Quatieri TF.. A review of depression and suicide risk assessment using speech analysis. Speech Commun. 2015;71:10–49. [Google Scholar]
- 29. Arora S, Baghai-Ravary L, Tsanas A.. Developing a large scale population screening tool for the assessment of Parkinson’s disease using telephone-quality voice. J Acoust Soc Am. 2019;145(5):2871–2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ben-Zeev D, Buck B, Kopelovich S, Meller S.. A technology-assisted life of recovery from psychosis. NPJ Schizophr. 2019;5(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Arevian AC, Bone D, Malandrakis N, et al. Clinical state tracking in serious mental illness through computational analysis of speech. PLoS One. 2020;15(1):e0225695. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0225695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tan EJ, Meyer D, Neill E, Rossell SL.. Investigating the diagnostic utility of speech patterns in schizophrenia and their symptom associations. Schizophr Res. 2021;238:91–98. [DOI] [PubMed] [Google Scholar]
- 33. de Boer JN, Voppel AE, Brederoo SG, Wijnen FNK, Sommer IEC.. Language disturbances in schizophrenia: the relation with antipsychotic medication. NPJ Schizophr. 2020;6(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moro-Velazquez L, Gomez-Garcia JA, Arias-Londoño JD, Dehak N, Godino-Llorente JI.. Advances in Parkinson’s disease detection and assessment using voice and speech: a review of the articulatory and phonatory aspects. Biomed Signal Process Control. 2021;66:102418. [Google Scholar]
- 35. Brand CO, Ounsley JP, Van der Post DJ, Morgan TJH.. Cumulative science via bayesian posterior passing. Meta-Psychology. 2019;(3):1–16. doi: 10.15626/mp.2017.840 [DOI] [Google Scholar]
- 36. Oomen PP, de Boer JN, Brederoo SG, et al. Characterizing speech heterogeneity in schizophrenia-spectrum disorders. J Psychopathol Clin Sci. 2022;131(2):172–181. [DOI] [PubMed] [Google Scholar]
- 37. Andreasen NC. Scale for the Assessment of Negative Symptom (SAPS). Iowa City, Iowa: University of Iowa; 1984. [Google Scholar]
- 38. Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, Iowa: University of Iowa; 1984. [Google Scholar]
- 39. Kay SR, Fiszbein A, Opler LA.. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(1):261–276. [DOI] [PubMed] [Google Scholar]
- 40. Nasrallah H, Morosini P, Gagnon DD.. Reliability, validity and ability to detect change of the Personal and Social Performance scale in patients with stable schizophrenia. Psychiatry Res. 2008;161(2):213–224. [DOI] [PubMed] [Google Scholar]
- 41. Abell F, Happé F, Frith U.. Do triangles play tricks? Attribution of mental states to animated shapes in normal and abnormal development. Cogn Dev. 2000. doi: 10.1016/S0885-2014(00)00014-9 [DOI] [Google Scholar]
- 42. Castelli F, Happé F, Frith U, Frith C.. Movement and mind: a functional imaging study of perception and interpretation of complex intentional movement patterns. Neuroimage. 2000;12(3):314–325. [DOI] [PubMed] [Google Scholar]
- 43. Gelman A, Vehtari A, Simpson D, et al. Bayesian workflow. arXiv Prepr arXiv201101808. Published online November 3, 2020. doi: 10.48550/arxiv.2011.01808 [DOI] [Google Scholar]
- 44. Yao Y, Vehtari A, Simpson D, Gelman A.. Using stacking to average Bayesian predictive distributions (with discussion). 2018;13(3):917–1007. doi: 10.1214/17-BA1091 [DOI] [Google Scholar]
- 45. Aringhieri S, Carli M, Kolachalam S, et al. Molecular targets of atypical antipsychotics: from mechanism of action to clinical differences. Pharmacol Ther. 2018;192:20–41. [DOI] [PubMed] [Google Scholar]
- 46. Leucht S, Samara M, Heres S, Patel MX, Woods SW, Davis JM.. Dose equivalents for second-generation antipsychotics: the minimum effective dose method. Schizophr Bull. 2014;40(2):314–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Goodman SN, Fanelli D, Ioannidis JPA.. What does research reproducibility mean? Sci Transl Med. 2016;8(341):96–102. doi: 10.1126/SCITRANSLMED.AAF5027 [DOI] [PubMed] [Google Scholar]
- 48. Vandenbroucke JP, Von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4(10):e297. https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.0040297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cohen AS, Schwartz E, Le TP, et al. Digital phenotyping of negative symptoms: the relationship to clinician ratings. Schizophr Bull. 2021;47(1):44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Alpert M, Shaw RJ, Pouget ER, Lim KO.. A comparison of clinical ratings with vocal acoustic measures of flat affect and alogia. J Psychiatr Res. 2002;36(5):347–353. [DOI] [PubMed] [Google Scholar]
- 51. Compton MT, Lunden A, Cleary SD, et al. The aprosody of schizophrenia: computationally derived acoustic phonetic underpinnings of monotone speech. Schizophr Res. 2018;197:392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Beechey T, Buchholz JM, Keidser G.. Measuring communication difficulty through effortful speech production during conversation. Speech Commun. 2018;100:18–29. [Google Scholar]
- 53. Traunmüller H, Eriksson A.. Acoustic effects of variation in vocal effort by men, women, and children. J Acoust Soc Am. 2000;107(6):3438. [DOI] [PubMed] [Google Scholar]
- 54. De Boer JN, Voppel AE, Brederoo SG, et al. Acoustic speech markers for schizophrenia-spectrum disorders: a diagnostic and symptom-recognition tool. Psychol Med. 2021:1–11. doi: 10.1017/S0033291721002804. https://www.cambridge.org/core/journals/psychological-medicine/article/acoustic-speech-markers-for-schizophreniaspectrum-disorders-a-diagnostic-and-symptomrecognition-tool/CD60278BD0F09390E8987CB5AB8A887F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cohen AS, Cox CR, Le TP, et al. Using machine learning of computerized vocal expression to measure blunted vocal affect and alogia. NPJ Schizophr. 2020;6(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tahir, Y., Yang, Z., Chakraborty, D., Thalmann, N., Thalmann, D., Maniam, Y., ... & Dauwels, J.. Non-verbal speech cues as objective measures for negative symptoms in patients with schizophrenia. PLoS One. 2019;14(4):e0214314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Asiaee M, Vahedian-azimi A, Atashi SS, Keramatfar A, Nourbakhsh M.. Voice quality evaluation in patients with COVID-19: an acoustic analysis. J Voice. 2020. doi: 10.1016/J.JVOICE.2020.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fried EI. The 52 symptoms of major depression: lack of content overlap among seven common depression scales. J Affect Disord. 2017;208:191–197. [DOI] [PubMed] [Google Scholar]
- 59. Micoulaud-Franchi J, Quiles C, Batail J.. Making psychiatric semiology great again: a semiologic, not nosologic challenge. L’encephale. 2018;44(4):343–353. doi: 10.1016/j.encep.2018.01.007 [DOI] [PubMed] [Google Scholar]
- 60. Khan A, Yavorsky C, Liechti S, et al. A rasch model to test the cross-cultural validity in the positive and negative syndrome scale (PANSS) across six geo-cultural groups. BMC Psychol. 2013;1(1):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Aggarwal NK, Tao H, Xu K, Stefanovics E, Zhening L, Rosenheck RA.. Comparing the PANSS in Chinese and American inpatients: cross-cultural psychiatric analyses of instrument translation and implementation. Schizophr Res. 2011;132(2–3):146–152. [DOI] [PubMed] [Google Scholar]
- 62. Fusaroli M, Simonsen A, Borrie S, Low DM, Parola A, Raschi E, ... & Fusaroli R.. Identifying medications underlying communication atypicalities in psychotic and affective disorders: A pharmacosurveillance study within the FDA Adverse Event Reporting System. medRxiv. 2022. [DOI] [PubMed] [Google Scholar]
- 63. Marquand AF, Kia SM, Zabihi M, Wolfers T, Buitelaar JK, Beckmann CF.. Conceptualizing mental disorders as deviations from normative functioning. Mol Psychiatry. 2019;24(10):1415–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Marquand AF, Rezek I, Buitelaar J, Beckmann CF.. Understanding heterogeneity in clinical cohorts using normative models: beyond case-control studies. Biol Psychiatry. 2016;80(7):552–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hitczenko K, Cowan HR, Goldrick M, Mittal VA.. Racial and ethnic biases in computational approaches to psychopathology. Schizophr Bull. 2022;48(2):285–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ali MS, Prieto-Alhambra D, Lopes LC, et al. Propensity score methods in health technology assessment: principles, extended applications, and recent advances. Front Pharmacol. 2019;10:973. https://www.frontiersin.org/articles/10.3389/fphar.2019.00973/full?&utm_source=Email_to_authors_&utm_medium=Email&utm_content=T1_11.5e1_author&utm_campaign=Email_publication&field=&journalName=Frontiers_in_Pharmacology&id=466792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gooden TE, Gardner M, Wang J, et al. The risk of mental illness in people living with HIV in the UK: a propensity score-matched cohort study. Lancet HIV. 2022;9(3):e172–e181. [DOI] [PubMed] [Google Scholar]
- 68. Kvarven A, Strømland E, Johannesson M.. Comparing meta-analyses and preregistered multiple-laboratory replication projects. Nat Hum Behav. 2020;4(4):423–434. [DOI] [PubMed] [Google Scholar]
- 69. Çokal D, Zimmerer V, Turkington D, et al. Disturbing the rhythm of thought: speech pausing patterns in schizophrenia, with and without formal thought disorder. PLoS One. 2019;14(5):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dellwo V, Leemann A, Kolly M-J.. Rhythmic variability between speakers: articulatory, prosodic, and linguistic factors. J Acoust Soc Am. 2015;137(3):1513–1528. [DOI] [PubMed] [Google Scholar]
- 71. Kanber E, Lavan N, McGettigan C.. Highly accurate and robust identity perception from personally familiar voices. J Exp Psychol Gen. 2022;151(4):897–911. doi: 10.1037/XGE0001112 [DOI] [PubMed] [Google Scholar]
- 72. Kreiman J, Park SJ, Keating PA, Alwan A.. The Relationship Between Acoustic and Perceived Intraspeaker Variability in Voice Quality. In: Sixteenth Annual Conference of the International Speech Communication Association; INTERSPEECH, 2015:2357–2360. [Google Scholar]
- 73. Rocca R, Yarkoni T.. Putting psychology to the test: rethinking model evaluation through benchmarking and prediction. 2021;4(3): 1–24. doi: 10.1177/25152459211026864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Loth E, Ahmad J, Chatham C, et al. The meaning of significant mean group differences for biomarker discovery. PLoS Comput Biol. 2021;17(11):e1009477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Stein F, Buckenmayer E, Brosch K, et al. Dimensions of formal thought disorder and their relation to gray- and white matter brain structure in affective and psychotic disorders. Schizophr Bull. 2022;48(4):902–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tang SX, Hänsel K, Cong Y, et al. Latent factors of language disturbance and relationships to quantitative speech features. medRxiv. Published online April 1, 2022. doi: 10.1101/2022.03.31.22273263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Brand BA, Haveman YRA, De Beer F, De Boer JN, Dazzan P, Sommer IEC.. Antipsychotic medication for women with schizophrenia spectrum disorders. Psychol Med. 2022;52(4):649–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Parola A, Gabbatore I, Berardinelli L, Salvini R, Bosco FM.. Multimodal assessment of communicative-pragmatic features in schizophrenia: a machine learning approach. NPJ Schizophr. 2021;7(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Parola A, Salvini R, Gabbatore I, Colle L, Berardinelli L, Bosco FM.. Pragmatics, Theory of Mind and executive functions in schizophrenia: disentangling the puzzle using machine learning. PLoS One. 2020; 15(3):e0229603. doi: 10.1371/journal.pone.0229603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lau JCY, Patel S, Kang X, et al. Cross-linguistic patterns of speech prosodic differences in autism: a machine learning study. Pegoraro C, ed. PLoS One. 2022;17(6):e0269637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Parola A, Lin JM, Simonsen A, et al. Speech disturbances in schizophrenia: assessing cross-linguistic generalizability of NLP automated measures of coherence. Schizophr Res. 2022. doi: 10.1016/J.SCHRES.2022.07.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.