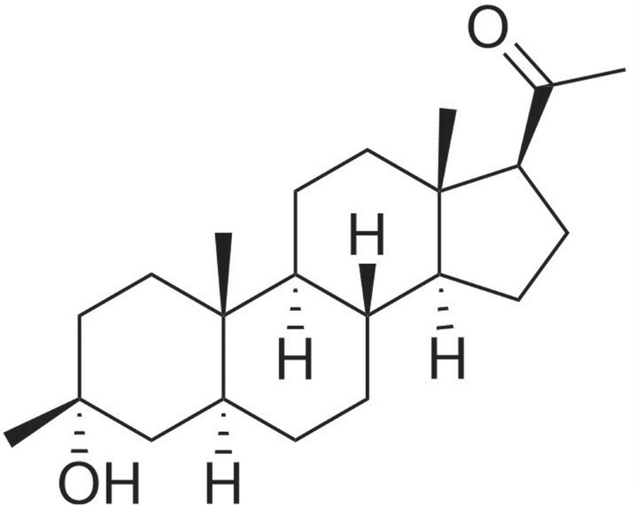
STRUCTURE: Ganaxolone is a 3β-methylated synthetic analog of the natural neurosteroid allopregnanolone, a metabolite of progesterone. It is also called 3α-hydroxy-3β-methyl-5α-pregnan-20-one and has a molecular formula C22H36O2, with a molecular weight of 332.5 g/mol. The 3β-methyl substitution prevents rapid metabolism and thus increases its stability (terminal half-life: 34 h). Ganaxolone is a white crystalline powder that is insoluble in water but soluble in organic solvents.
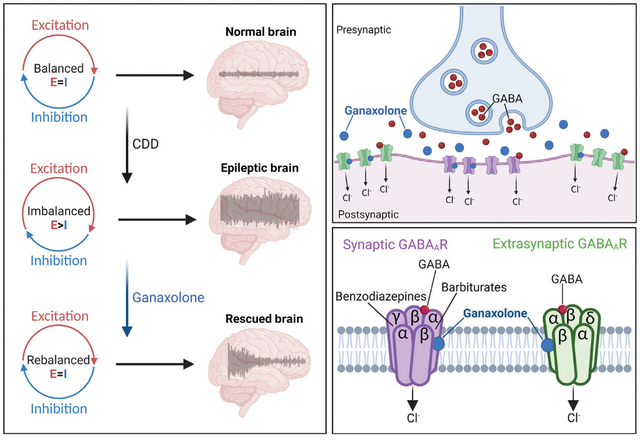
MECHANISM OF ACTION: γ-Aminobutyric acid (GABA) is the primary inhibitory neurotransmitter in the central nervous system. Its principal role is to lower neuronal excitability, mainly via acting on the GABA receptor A subtype (GABAAR), a ligand-gated ion channel receptor. Upon activation by GABA released from the presynaptic neurons, GABAAR on the postsynaptic cell selectively allows the influx of negatively charged ions (mainly chloride anion, Cl−) into the neurons, resulting in decreased neuronal excitability. GABAAR has long been implicated in neurological conditions, including seizures; it is the primary target for a number of antiseizure drugs (ASDs). Ganaxolone is a small molecule that has been identified as a positive allosteric modulator for GABAAR and demonstrated to significantly reduce the frequency of major motor seizures associated with the cyclin-dependent kinase-like 5 (CDKL5) deficiency disorder (CDD) in clinical trials. The mechanism of action of ganaxolone in the management of seizures is not fully understood; however, its antiseizure action is likely attributed to its potentiation of GABAAR activation. Ganaxolone is able to augment the activities of both synaptic GABAAR (containing α, β, and γ subunits) and extrasynaptic GABAAR (containing α, β, and δ subunits) through a binding site distinct from benzodiazepines or barbiturates (binding sites on GABAAR are indicated). By contrast, these two groups of conventional ASDs only act on the synaptic GABAAR. Ganaxolone’s capability of activating GABAAR on both synaptic and extrasynaptic sites leads to enhanced and persistent suppression of neuronal excitability during seizures. This unique feature differentiates ganaxolone from other GABA-potentiating ASDs, such as benzodiazepines or barbiturates.
NAME:
Ganaxolone; commercially available under the brand name ZTALMY.
DRUG CLASS:
First-in-class positive allosteric modulator that targets both synaptic and extrasynaptic GABA A receptor (GABAAR).
CLINICAL USE:
Indicated for the treatment of seizures associated with CDKL5 CDD in patients 2 years of age and older. Ganaxolone is administered as an oral suspension three times daily. CDD is a rare genetic disorder (one in 40 000–60 000 newborns) characterized by infantile-onset epilepsy and severe neurodevelopmental delay. It is caused by mutations in the CDKL5 gene, which encodes a protein essential for normal brain functions. Seizures in CDD patients are usually severe and difficult to control.
DEVELOPED BY:
Originally developed by CoCensys Inc. and acquired by Purdue Pharma in 1999. Marinus Pharmaceuticals acquired the compound from Purdue Pharma in 2004.
ADVERSE EFFECTS:
Somnolence, pyrexia, salivary hypersecretion, and seasonal allergy. Avoid concomitant use with strong or moderate CYP3A4 inducers as they will decrease ganaxolone exposure.
TIMELINE:
2018–2021, Phase 3 trials, NCT03572933
March 18, 2022, FDA approval for ZTALMY® (ganaxolone).
2023–2024, Phase 3 trials, NCT05249556
Acknowledgments
J.J. is supported by the National Institutes of Health (NIH)/National Institute of Neurological Disorders and Stroke (NINDS) grants R01NS100947, R21NS109687, and R61NS124923.
Footnotes
Declaration of interests
The authors declare no conflict of interest.
Literature
- 1.Lattanzi S et al. (2021) Ganaxolone treatment for epilepsy patients: from pharmacology to place in therapy. Expert. Rev. Neurother. 21, 1317–1332 [DOI] [PubMed] [Google Scholar]
- 2.Knight EMP et al. (2022) Safety and efficacy of ganaxolone in patients with CDKL5 deficiency disorder: results from the double-blind phase of a randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 21, 417–427 [DOI] [PubMed] [Google Scholar]
- 3.Saporito MS et al. (2019) Intravenously administered ganaxolone blocks diazepam-resistant lithium-pilocarpine–induced status epilepticus in rats: comparison with allopregnanolone. J. Pharmacol. Exp. Ther. 368, 326–337 [DOI] [PubMed] [Google Scholar]
- 4.Yawno T et al. (2017) Ganaxolone: a new treatment for neonatal seizures. Front. Cell. Neurosci. 11, 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noebels JL et al. (2012) Jasper’s Basic Mechanisms of the Epilepsies. (4th edn), National Center for Biotechnology Information; [PubMed] [Google Scholar]
- 6.Galanopoulou A (2008) GABAA receptors in normal development and seizures: friends or foes? Curr. Neuropharmacol. 6, 1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chuang S et al. (2018) Genetic and molecular regulation of extrasynaptic GABA-A receptors in the brain: therapeutic insights for epilepsy. J. Pharmacol. Exp. Ther. 364, 180–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenfield LJJ (2013) Molecular mechanisms of antiseizure drug activity at GABAA receptors. Seizure 22, 589–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jakimiec M et al. (2020) CDKL5 deficiency disorder-a complex epileptic encephalopathy. Brain Sci. 10, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sills GJ and Rogawski MA (2020) Mechanisms of action of currently used antiseizure drugs. Neuropharmacology 168, 107966. [DOI] [PubMed] [Google Scholar]


