Abstract
Re-aligning eating patterns with biological rhythm can reduce the burden of metabolic syndrome in older adults with overweight or obesity. Time-restricted eating (TRE) has been shown to result in weight loss and improved cardiometabolic health while being less challenging than counting calories. The New York Time-Restricted EATing study (NY-TREAT) is a two-arm, randomized clinical trial (RCT) that aims to examine the efficacy and sustainability of TRE (eating window ≤10 h/day) vs. a habitual prolonged eating window (HABIT, ≥14 h/day) in metabolically unhealthy midlife adults (50–75 years) with overweight or obesity and prediabetes or type 2 diabetes (T2D). Our primary hypothesis is that the TRE will result in greater weight loss compared to HABIT at 3 months. The efficacy of the TRE intervention on body weight, fat mass, energy expenditure, and glucose is tested at 3 months, and the sustainability of its effect is measured at 12 months, with ambulatory assessments of sleep and physical activity (ActiGraph), eating pattern (smartphone application), and interstitial glucose (continuous glucose monitoring). The RCT also includes state-of-the-art measurements of body fat (quantitative magnetic resonance), total energy expenditure (doubly-labelled water), insulin secretion, insulin resistance, and glucose tolerance. Adherence to self-monitoring and reduced eating window are monitored remotely in real-time. This RCT will provide further insight into the effects of TRE on cardiometabolic health in individuals with high metabolic risk. Sixty-two participants will be enrolled, and with estimated 30% attrition, 42 participants will return at 12 months. This protocol describes the design, interventions, methods, and expected outcomes.
Clinical trial registration:
IRB:
AAAS7791.
Keywords: Glucose, Continuous glucose monitoring system, Meal timing, Time-restricted eating, Prediabetes, Diabetes, Doubly labelled water
1. Introduction
Weight loss with caloric restriction can prevent the progression of prediabetes to type 2 diabetes (T2D) if untreated [1] and decrease the risk of cardiovascular disease (CVD) [2–7]. However, because caloric restriction is difficult to sustain long-term, alternative lifestyle interventions have been proposed, including reducing the duration of the daily eating window, or time-restricted eating (TRE) [8–30].
The majority of adults in the United States have an eating window that exceeds 15 h/day [11,31,32], a pattern often associated with obesity [33–35]. The temporal aspect of meal intake is an important target to decrease CVD risk [36–39]. Studies in mice [9,10] and humans [11–30,33–35] demonstrated that TRE could result in increased sleep satisfaction [11], improved insulin sensitivity, and reduced inflammation and oxidative stress, independent of weight loss [16].
Despite these intriguing findings, previous TRE clinical trials have limitations: small scale and short duration [16,17], targeting healthy individuals [11–14] or only men [12–17,25,30], severe time restriction [11,16,29], no account of habitual temporal eating patterns [16,18–20], no monitoring of adherence [12,13,15,19–21,24,27,30], or lack of randomization [16,21,23,25,27], with questionable clinical translation. Results from these studies are inconsistent, with some showing metabolic benefits [17,18,21,23,25], while others do not [11,19,24,26,27]. Therefore, further exploration of TRE with more rigorous study design and accurate methods to measure health outcomes is warranted.
Mobile health interventions allow real-time monitoring of behavior in an ambulatory setting [40–44]. Our objective is to test the efficacy of a 10-h TRE intervention, compared to habitual prolonged eating window (>14 h, HABIT), on CVD risk in metabolically unhealthy adults.
Enrollment began in May 2021 and will end in the Spring of 2024 for study completion in 2025.
We hypothesize that TRE will result in greater loss of fat mass (FM) and body weight compared to HABIT, and that these effects will be mediated by a decrease in total daily energy intake (EI) (Fig. 1).
Fig. 1.
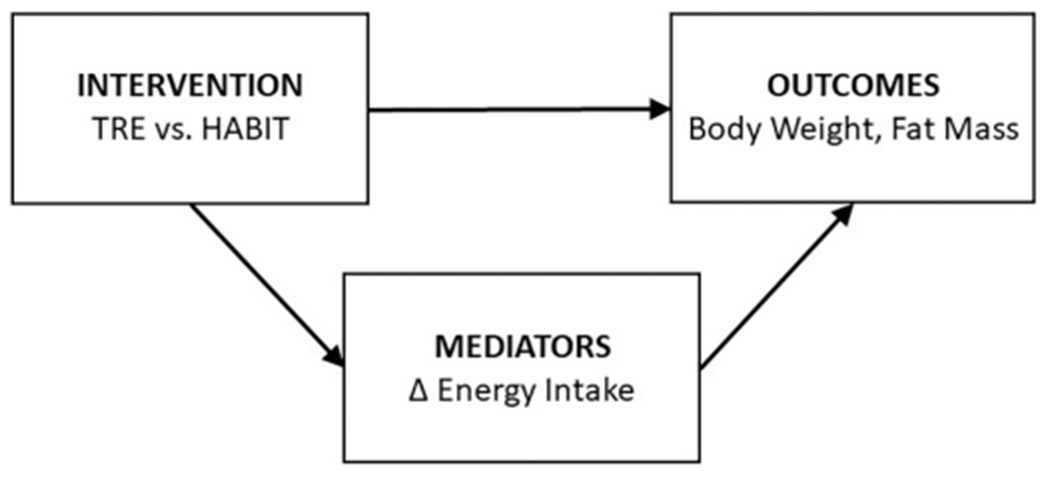
Working model.
The effect of TRE on fat mass and glucose will be mediated by decreased energy intake - assessed by doubly labelled water.
Secondary outcomes include glucose levels, glycemic variability (GV), insulin resistance (assessed by the Matsuda Index and HOMA-IR), blood pressure, sleep duration and quality, and inflammation markers [45].
2. Study design
2.1. Study participants
The study is approved by the Columbia University Institutional Review Board (IRB). Participants will be men and women with overweight or obesity and prediabetes or T2D, of any racial or ethnic groups, aged 50 to 75 years, who have a prolonged eating window of ≥14 h/day (Table 1).
Table 1.
Inclusion and exclusion criteria.
| Inclusion Criteria | Exclusion criteria |
|---|---|
| Age: 50–75 years old | Sleep disorder, e.g. known obstructive sleep apnea (OSA), severe OSA with apnea-hypopnea index >30 events/h, significant daytime symptoms of OSA, periodic limb movements of sleep, narcolepsy, severe insomnia (a score ≥ 15 on Insomnia Severity Index) |
| BMI ≥25 and ≤ 45 kg/m2 with a diagnosis of prediabetes or T2D | Current shift work or recent shift work in the last 6 months |
| Fasting glucose ≥100 mg/dL, and/or HbA1c ≥5.7% and < 7.5% | Travel more than one time zone during the intervention |
| Treatment with metformin and/or diet-controlled if T2D | Significant organ system dysfunction/disease or evidence of active illness |
| Two or more of the following metabolic syndrome criteria: - Diagnosis of hypertension on stable medication regimen - Diagnosis of dyslipidemia on stable regimen - HDL cholesterol men <40 mg/dL and women <50 mg/dL - Waist circumference men: >102 cm (>40 40 in); women >88 cm (>35 in) |
Use of dietary supplements and/or medications known to affect sleep, circadian rhythms, or metabolic Function |
| In possession of a smartphone | History of seizure disorder |
| Habitual prolonged eating window (≥14 h) | Habitual eating window <14 h |
| ≥70% of days with logging adherence (2 or more log entries/day separated by at least 5 h), assessed during 2-week remote screening | Previous bariatric surgery or use of weight loss medication |
| Sleep duration ≥6 h, with habitual self-reported wake-up time > 5:00 am and < 11:00 am, and average self-reported bedtime before 2:00 am | History of or current significant food intake or psychiatric disorder (BDI score ≥ 29; BAI score ≥ 26) |
| Subject habitually eats breakfast | Excessive alcohol (women: >14 drinks/week; men: >21 drinks/week), smoking tobacco, or using illegal or recreational drugs |
| Weight stability within 5% for the 3 months preceding screening | Severe food allergies |
| Lives in the New York City metro area | Anemia (hemoglobin <10 g/dl and hematocrit <30%) |
| English fluency, since the smartphone application has not been translated | Unwilling/unable to provide informed Consent |
BAI = Beck’s Anxiety Inventory; BDI = Beck’s Depression Inventory; BMI = Body Mass Index; HbA1c = glycated hemoglobin; HDL = High-Density Lipoprotein; OSA = Obstructive Sleep Apnea; T2D = Type 2 Diabetes.
2.2. Recruitment
Potential participants, identified through flyers and brochures, websites, social media, community centers, health care provider referrals, and/or electronic medical records, are screened over the phone for inclusion/exclusion criteria (Table 1). Detailed information about the study is provided via Zoom, phone, or email with a link to a short descriptive YouTube video [46].
Eligible participants are instructed remotely to download the study application (app) on their smartphone and attend a 10-min coaching session on how to use the app, i.e. how to self-monitor in real-time by taking photos and/or logging their daily meals, snacks, and beverages during the 2-week remote screening phase. At completion of the 2-week remote screening, individuals with a prolonged daily eating window (≥14 h) [11,31,32] and at least 70% adherence to logging [31] are eligible for enrollment and proceed to sign the informed consent form prior to remotely completing the Beck Anxiety Inventory (BAI) and Beck Depression Inventory (BDI-II) questionnaires [47].
Individuals with adequate scores in the BAI and BDI-II questionnaires (Supplemental Table 1) are scheduled for an in-person screening visit at the Clinical Research Center (CRC) for medical history, physical examination, and fasted blood tests, including glycated hemoglobin (HbA1c), blood count, metabolic panel, fasting glucose, creatinine, lipids, liver profile, and thyroid stimulating hormone.
2.3. Intervention and study phases
This is a one-to-one randomized controlled prospective trial of 12-month duration (Fig. 2) taking place at Columbia University Irving Medical Center. After completion of the screening, enrolled participants undergo a 2-week baseline assessment, prior to being randomized to TRE or HABIT. Participants randomized to HABIT follow their daily habitual eating window. Those randomized to TRE will reduce their daily eating duration to a self-selected 10-h window, beginning within three hours of wake-up time and ending at least three hours before bedtime. Participants randomized to TRE will continue to follow their 10-h window for the duration of the study. At the end of months 3 and 12, a repeat 2-week assessment is done.
Fig. 2.
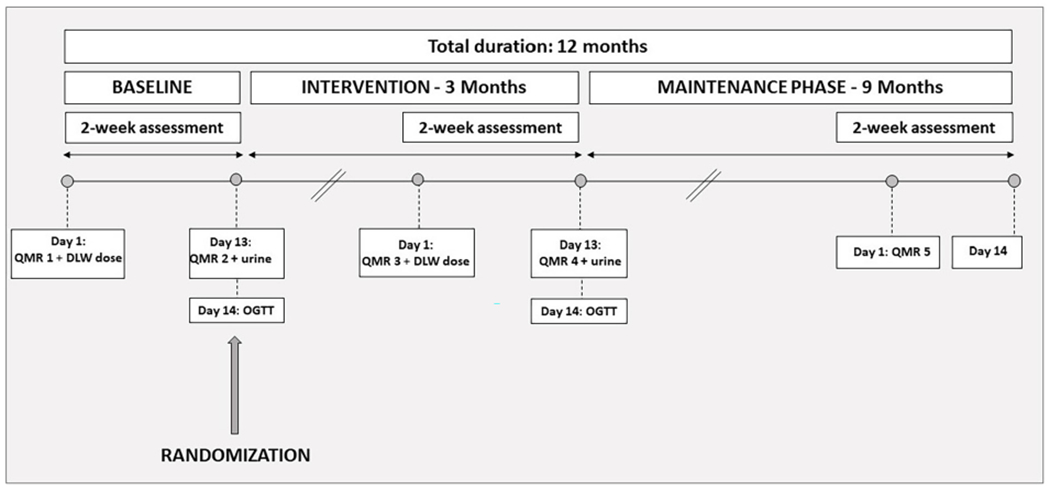
Study timeline.
After screening, participants are enrolled for a total duration of 12 months. They complete a baseline period of two weeks before randomization. Upon randomization, subjects enter the 12-month intervention, with a repeat 2-week assessment at the end of 3 months, and at 12 months. DLW = Doubly Labelled Water. OGTT = Oral Glucose Tolerance Test. QMR = Quantitative Magnetic Resonance.
Subjects randomized to TRE have the option to gradually transition from their baseline eating window to their self-selected TRE window over the course of the first two weeks (Supplemental Document 1). TRE participants receive daily push notifications 1-h before the start and 1-h before the end of their eating window.
Participants remain on their habitual medications, metformin included, during the entire duration of the study. Any change and/or adjustment made by the participants’ providers is recorded.
Transportation by car service is offered to ensure timely arrival to the CRC. In addition, participants receive compensation for their time and effort following completion of each study period. The compensation schedule is the same for both groups.
2.3.1. Two-week baseline assessment
After enrollment, fasted participants visit the CRC on Day-1 for measurements of weight, height, waist circumference, and body composition via quantitative magnetic resonance (QMR) (Fig. 3); participants receive of a weight-based dose of doubly labelled water (DLW) for ingestion, are fitted with the continuous glucose monitoring sensor (CGM, Abbott Freestyle Libre Pro, Abbott Park, IL, USA) and Actigraph-GT3X (ActiGraph LLC, Pensacola, FL, USA), and complete the following questionnaires: Insomnia Severity Index (ISI) [48], Pittsburgh Sleep Quality Index (PSQI) [49] to assess self-reported sleep quality, Morningness-Eveningness questionnaire [50] to determine daily sleep-wake habits, visual analog scales (VAS) [51] to rate appetite and hunger, Berlin Questionnaire [52] to identify sleep apnea, and International Physical Activity Questionnaire (IPAQ) [53] to assess typical weekly physical activity levels (Supplemental Table 1).
Fig. 3.
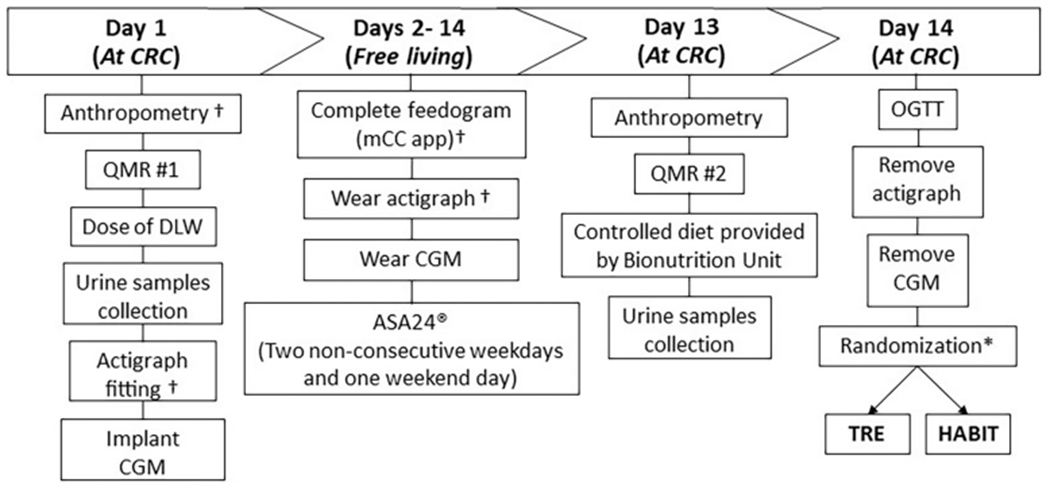
Timetable of study procedures during the 2-week baseline assessment.
* = All assessments are repeated during the last 2 weeks of 3 months, except randomization.
† = Assessments completed at 12 months. QMR = Quantitative Magnetic Resonance, DLW = Doubly Labelled Water, CGM = Continuous Glucose Monitoring, mCC = myCircadianClock, ASA24 = Automated Self-Administered 24-h, OGTT = Oral Glucose Tolerance Test, TRE = Time-Restricted Eating.
For the following 12 days, participants follow a free-living routine at home and return to the CRC on Day-13 for a repeat QMR, to provide urine samples, and to eat a controlled eucaloric diet (Supplemental Fig. 1a and b) calculated with the Mifflin-St. Jeor equation [54,55]. The macronutrient composition of the diet is: 56–59% carbohydrates, 14–17% protein, and 26–28% fat.
The timing of the meals and composition of the diet on Day-13 are controlled and identical for both groups, followed by a 10-h overnight fast at baseline to mimic their habitual short overnight fast, and a 14-h overnight fast at 3 months, prior to the oral glucose tolerance test (OGTT) the next day. Breakfast and lunch are administered under staff supervision. Dinner and an evening snack are provided to the participants in an isothermal bag with preparation instructions provided by the research nutritionist. Participants log all the provided meals using the myCircadianClock (mCC) app and return containers, which are inspected for completion of meals upon return.
On Day-14, fasted participants report to the CRC at 7:30 am for a 2-h OGTT. At the completion of the OGTT, sensors are collected, and participants are randomized to HABIT or TRE.
2.3.2. Initial intervention: 3-month phase
During the following 3 months, participants in both groups continue logging daily meals, snacks, and beverages on the mCC app. However, those randomized to TRE follow their personalized 10-h eating window, and those randomized to HABIT follow their habitual eating schedule. Baseline assessments are repeated at the end of 3 months.
2.3.3. Sustainability of the intervention: 9-month phase
Participants remain in their assigned TRE or HABIT group for the remainder of the study. During the 9-month phase they are only asked to use the mCC app for the first ten days of each month. At 12 months, participants return to the CRC for a final 2-week assessment with the mCC app, ASA24, actigraphy, and fasting blood draw, but without DLW, CGM, and OGTT. All enrolled participants are blinded to ActiGraph, QMR, DLW, and CGM data. HbA1c results are shared after baseline, 3-month, and 12-month assessments.
3. Methods
3.1. Anthropometric measurements
Weight and height are obtained in triplicate on calibrated instruments on Day-1 and Day-13 at baseline and 3 months, and on Day-1 at 12 months. Prior to these measurements, participants must void, remove garments and jewelry, and change into a hospital gown and slippers.
Body weight is measured to the nearest 0.1 kg (Ohaus Champ General Purpose Bench Scale, Ohaus Corp., Pine Brook, NJ, USA) and height to the nearest 1 mm using a stadiometer (Holtain Ltd., Crymych, UK). Waist circumference is measured at baseline, 3 months, and 12 months in triplicate; the study physician locates the lowest point of the costal margins on the mid-axillary lines and the highest point of the iliac crests and marks the midpoint with a washable marker, bilaterally. The abdominal circumference is measured by aligning the two marks using the Gulick II measurement tape (Country Technology, Inc., Gays Mills, WI); results are recorded to the nearest 0.1 cm.
3.2. Body composition
Quantitative magnetic resonance (QMR, EchoMRI 2020, Echo Medical Systems, Houston TX, USA) is a noninvasive measure of body FM and is standardized to detect at little as 50-g change in FM [56,57]. This system generates a low magnetic field at 0.0068 Tesla and employs magnetic resonance relaxation analysis for measuring live body composition in terms of fat tissue, lean tissue, total body water, and free water. QMR is performed under fasting condition in duplicate by a trained technologist on Day-1 and Day-13 at baseline and 3 months, and Day-1 at 12 months. Fat-free mass (FFM) is calculated by subtracting FM from body weight.
3.3. Energy expenditure
Doubly labelled water (DLW) is the gold-standard method to assess free-living total energy expenditure (TEE) [58]. On Day-1 of the 2-week assessment at baseline and 3 months, a urine specimen is collected prior to the participant drinking a weight-based dose of DLW containing 1.8 g/kg of total body water (TBW) of 10APE 18O labelled water and 0.12 g/kg TBW of 99.9 APE 2H labelled water [58,59]. Three timed urine samples are obtained at 1, 3, and 4 h after administration of the DLW dose on Day-1. On Day-13, two additional urine samples are obtained at times matching the 3-h and 4-h post-dose samples collected on Day-1. TEE measures the differential rates of elimination of stable isotope tracers 2H (deuterium) and 18O. Stable isotopes in tap water vary across geographic locations [60], thus, participants are instructed to limit travel to <200 miles from the New York City area for 2 weeks prior to and during each 2-week ambulatory period.
For each 2-week period, EI is calculated from the sum of TEE and changes in FM and FFM, measured by QMR. This is computed from the regression (slope, grams per day) of calibrated body weight obtained on Day-1 and Day-13 and changes in FM and FFM [58,61]. Long-term changes in energy balance will be estimated using QMR and DLW data collected at baseline and 3 months.
3.4. Eating patterns
The mCC app was developed and validated to monitor the daily temporal pattern of caloric intake in free-living conditions, via time-stamped photos of meals and beverages in the JPEG format [11,62]. Participants are instructed to use the in-app camera to take a photo of all meals and beverages prior to consumption in real time. A text entry option is enabled if subjects are unable to obtain a photo. The meal images and text entries are transferred to a remote server immediately after data submission. These entries are thoroughly reviewed by two independent trained research staff and compared with the ASA24® dietary recalls and glucose excursions from CGM (Sections 4.6. and 4.8.).
The app automatically sends reminders one hour prior to the start and the end of the eating window for participants in TRE.
3.5. Adherence
Self-monitoring and tailored feedback using smartphone technology increase intervention adherence [11]. Logging adherence is defined as the number of days with at least 2 daily entries separated by a minimum of 5 h. Adherence to the eating window is defined as logging all eating occasions (EO) within the self-selected 10-h window (±15 min, for TRE). Research staff monitor log entries daily and send personalized push notifications biweekly for the three 2-week ambulatory assessments, and weekly in the 3- and 9-month phases, to reinforce logging and eating window adherence. Additional contact by email or phone can occur as needed. The research staff keeps track of all contacts with participants.
3.6. Dietary recall
The ASA24® is a web-based, self-administered diet recall to assess energy, macronutrient, and micronutrient intake [63]. On Day-1 of the baseline assessment, participants receive detailed instructions along with a practice session under staff supervision. All participants are asked to complete 24-h recalls on two non-consecutive weekdays and one weekend day during each of the three 2-week ambulatory assessments (baseline, 3 months, and 12 months) and during months 6 and 9, and receive reminders to complete the dietary recalls from the research staff. Participants are asked to complete an additional recall if missing data are reported, there is implausibly low or high caloric intake, or there is a discrepancy of >500 kcal between the three recalls.
3.7. Physical activity and sleep
Participants wear the ActiGraph-GT3X on their non-dominant wrist during each 2-week assessment period to obtain non-invasive measures of sleep and physical activity [64] and complete a sleep log to record wake-up time and in-bed times (Supplemental Document 2) as a backup measure. Sleep data include in-bed time, sleep onset time, wake time, out-of-bed time, total sleep time, sleep onset latency, sleep efficiency, total minutes in bed, wake time after sleep onset, total awakenings after sleep onset, average time per awakening, movement index, fragmentation index, and sleep fragmentation index. Sleep quality, timing, and duration are also assessed with the Pittsburgh Sleep Quality Index (PSQI), the Insomnia Severity Index (ISI), the Berlin Questionnaire, and the Morningness-Eveningness questionnaire, administered at baseline, 3 months, and 12 months.
Physical activity data by actigraphy include daily/hourly kcals, metabolic equivalent of task (METs), amount and percent of the time in sedentary, light, moderate, vigorous, and very vigorous activity. Subjective estimation of activity levels is also assessed by the IPAQ Questionnaire, administered at the beginning of each 2-week assessment period.
3.8. Continuous glucose monitoring (CGM)
The CGM sensor (Abbott Freestyle Libre) is worn on the back of the non-dominant arm during the 2-week assessment periods at baseline and 3 months; it does not require calibration by finger sticks [65]. CGM glucose data are downloaded from LibreView software [65]. The EasyGV 8.6 software [66] is used to calculate mean amplitude of glycemic excursions (MAGE), which is the mean of blood glucose values, ignoring excursions of 1 standard deviation (SD) or less, and the largest amplitude of glycemic excursion (LAGE), which is the difference between the maximum and minimum blood sugar levels of a day.
3.9. Oral glucose tolerance test (OGTT)
To assess glucose tolerance and β-cell function, participants undergo a 2-h 75 g OGTT on Day-14 at baseline and 3 months. Participants on metformin remain on their medication for the duration of the ambulatory testing period. On the morning of Day-14, an intravenous catheter is inserted in an antecubital vein by a research nurse. Blood samples at –15 and 0 min are obtained immediately before 75 g glucose drink at 8:00 am, and again at 15, 30, 60, 90, and 120 min after the drink, before being centrifuged, aliquoted, and stored at −80C.
3.10. Visual analog scales (VAS)
At baseline and 3 months, sleep satisfaction, energy level, hunger, desire to eat, craving and fullness rating, and gastrointestinal symptoms are assessed by a 150 mm VAS before each meal and snack, and after each meal on Day-13; the same VAS are repeated under fasting condition on Day-1 at baseline, 3 months, and 12 months (Supplemental Document 3). VAS are anchored at each extremity with ‘not at all’ to ‘extremely’. A survey is administered on Day-1 at 3 and 12 months to assess participants’ acceptance of TRE, difficulty using the study app or adhering to intervention, and willingness to pursue TRE after study completion.
3.11. Biomarker assays
Biomarkers will be assessed at the Columbia Biomarker Core Laboratory and include: fasting blood glucose, HbA1C, total cholesterol, low-density lipoprotein, high-density lipoprotein, triglycerides, β-hydroxylbutyrate, glycerol, free fatty acids, insulin, C-peptide, leptin, adiponectin, high sensitivity C-reactive protein, interleukin-6, tumor necrosis factor-α, total Receptor for Advanced Glycation End products, and 8-Isoprostane (Methods for biomarker assays are provided in Supplemental Table 2).
3.12. Statistical analyses
Primary and secondary outcomes are shown in Supplemental Table 3.
Randomization.
Individuals (n = 62) will be randomized to TRE or HABIT in a 1:1 ratio, stratified by overweight/obesity, gender (men or women), and age (≤60 or > 60). Permuted blocks with a block size of 2 will be used for the stratified randomization using the Microsoft Structured Query Language Server (MS-SQL) database, version T-SQL, to house the study data; random selection is performed using the T-SQL internal random number generator, using the current time as a seed.
Sample size justification.
The study is powered for the primary outcome: change in body weight. Based on our pilot study [11], assuming a standard deviation of 3.39 in each group, with 26 subjects per group, we will have 93% power to detect a weight difference of 3.27 kg weight loss between TRE and HABIT. The Type I error rate was controlled at 0.05. Based on our pilot study [31], we estimated that approximately 500 individuals need to be phone screened, 200 will enter remote screening, 130 will complete the remote screening, of which 60% will have a prolonged eating window. Assuming an estimated attrition rate of 30% over the 12-month intervention, 62 participants will be enrolled with the goal of retaining 52 participants at 3 months and 42 participants at 12 months.
Calculations.
We will calculate total under the curve (tAUC) and incremental under the curve (iAUC) area with the trapezoid method for glucose, insulin, and C-peptide [67]; the β insulinogenic index [II30min = 0.007 x insulin30min (pmol/L) – insulin0min (pmol/L)]; insulin secretion rate by C-peptide deconvolution using a two-compartment model [68]; insulin resistance by the Matsuda Index, calculated as: 10,000/([fasting insulin (mU/mL) x fasting glucose (mmol/L)] x [mean OGTT insulin (mU/mL) x mean OGTT glucose (mmol/ L)]), and HOMA-IR, calculated as [fasting insulin (mU/ mL) x fasting glucose (mmol/L)]/22.5 [69–71].
Data analysis plans.
The treatment effect will be assessed using a linear mixed-effects model with weight as the outcome variable, treatment group (TRE, HABIT), and time (baseline, 3 months, 12 months) as main predictors, and a treatment-by-time interaction. The random effect is included in the model to account for within-subject correlation. A significant treatment-by-time interaction confirms the hypothesis. Treatment effect on other outcomes will be analyzed similarly. To assess how the treatment affects change in body weight or FM from 0 to 3 months and from 0 to 12 months, a linear mixed model with change of FM (or body weight) from baseline as the outcome with similar analyses will be used.
The Preacher and Hayes’ Bootstrap Method will be used to assess the mediating effect of EI and sleep duration on insulin resistance. Specifically, we consider linear models:
HOMA-IR change = β0 + β1*E intake change+β2*Group+ε;
EI change = ϒ0 + ϒ1*Group+ε HOMA-IR change = β0 + β1*Sleep duration change+β2*Group+ε;
Change in sleep duration = ϒ0 + ϒ1*Group+ε; where Group = 0 if assigned to HABIT and Group = 1 if assigned to TRE. The hypothesis H0: β1* ϒ1 = 0 will be tested by the bootstrap method.
The treatment effect on glucose metabolism will be assessed formally using a linear mixed-effects model with either glucose tAUC or iAUC as the outcome variable, treatment group (1 = TRE, 0 = HABIT), and time (0 = baseline, 1 = 3 months, 2 = 12 months) as main predictors and a treatment-by-time interaction. The random effect is included in the model to account for within-subject correlation. A significant treatment-by-time interaction confirms the hypothesis.
Adherence will be calculated as the number and percentage of days meeting definition of logging adherence, and, for the TRE group, number of days meeting the target eating window and mean reduction of the duration of the eating window.
Various repeated measure linear models will be run to test the association of adherence on weight loss (and FM loss) at 3 and 12 months. Analysis will begin with a linear mixed-effects model with treatment, time, and their interaction, and then move on by controlling for the stratifying variables and baseline variables. These controlling variables will be dropped if not significant.
Handling of missing data.
To take full advantage of the strengths of the randomized design, it is necessary to maintain the full sample size in both intervention arms (TRE and HABIT). This will allow to adhere to the intention-to-treat principle. Missing data from dropouts will be handled using multiple imputation [72]. For the secondary completers-only, linear mixed models will be used, which do not require balanced groups at random assumption.
4. Adverse events
There are minimal risks associated with the intervention and sensor use, and subjects are made aware of any of the following potential risks before consent: the ActiGraph can cause mild skin irritation from the wrist strap; CGM could cause skin irritation, pain or discomfort, bruising, scarring, allergic reactions to the sensor, local infection, sensor breakage with fragments retained under the skin; participants may experience claustrophobia during the QMR; the IV catheter insertion for the OGTT can result in bruising, inflammation, or infection.
The study staff ensures that potential participants understand these risks before enrollment and are given instructions to communicate with the study staff if any adverse event occurs, for immediate assessment by the study physician and sensor, assessment, or intervention discontinuation, as applicable.
5. Study limitations and potential pitfalls
Older individuals may not be able to navigate the smartphone app easily, but the screening process helps select individuals able to effectively operate the app. In an attempt to minimize study burden, the study design includes three 2-week ambulatory assessments and two half-day metabolic studies, and the TRE intervention is delivered primarily via the smartphone app. Monetary compensation is implemented to enhance retention. Should drop-outs be higher than expected, recruitment will continue to ensure 26 subjects per group at 3-month completion.
Some participants may find the TRE intervention difficult. Frequent contact after randomization and reassessment for goal setting specific to the timing of the eating window will be prioritized, within the limits defined by the protocol. Prior evidence suggests a 10-h eating window is feasible [31]. While duration of the eating window and overnight fast is fully controlled in the TRE group, participants self-select their eating window, which should increase compliance. Prior to the OGTT, the overnight fast is controlled and identical in both groups, with a fasting duration of 10 h at baseline and 14 h at 3 months, to obtain between-group consistency before testing glucose tolerance the following morning, and avoid overestimating any improvements in insulin sensitivity [16].
Physical activity is an important component of energy balance and fitness, which are predictors of weight maintenance after weight loss. Although selected participants are expected to be relatively sedentary, and it is unlikely that participants will increase their physical activity levels in response to the intervention, physical activity changes could be a confounder. Physical activity is measured and added as a covariate in the analysis.
The mCC app is available only in the English language and outcomes may not be generalizable to non-English speaking communities.
Dietary recall often results in underestimation of intake [73,74]. To limit this issue, recalls are completed on 3 non-consecutive days, following a thorough supervised ASA24 recall on Day-1 of the ambulatory assessment period.
Due to the nature of the behavioral intervention, participants and study staff are not blinded to randomization assignment, however, participants are blinded to body sensors data. Research staff obtaining anthropometries, QMR, and DLW, laboratory technicians, and statistician are blinded to group assignment.
6. Future studies
In-depth modeling of functional data of sleep, physical activity, meal pattern, and CGM-glucose could be done in future analyses and used to generate a predictive model of glucose control based on diet composition and meal timing. Blood samples will be stored for future metabolomics and proteomics analyses, including bile acids, a metabolic biomarker particularly affected by the fasting/feeding cycle. Future TRE interventions could target patients with other types of chronic conditions such as gastroesophageal reflux, younger individuals, and other ethnic groups, and/or implement TRE via eHealth in a clinical setting.
7. Conclusions
Calorie restriction, the recommended first approach to treat obesity and associated CVD, is resource-intensive to implement and difficult to sustain over time. TRE offers a potentially low-cost and sustainable approach to treating obesity. Daily energy intake is restricted within a consistent interval of 8 to 10 h, usually without explicitly attempting to modify diet composition or reduce calories. The rationale for TRE is based on the role of circadian rhythms in metabolism, as chronic circadian rhythm disruption increases the risk of obesity and metabolic diseases, hence, restricting the eating window sustains circadian rhythms and improves metabolism.
Past TRE trials have had mixed results, perhaps due to confounding effects of study design and subject selection. The NY-TREAT randomized trial provides an innovative approach by targeting metabolically unhealthy individuals with measured prolonged baseline eating windows and assessing their energy expenditure and body composition using state-of-the-art, highly precise methods. Therefore, this trial will clarify the role of TRE on energy balance in individuals with overweight and obesity.
Supplementary Material
Funding
This work is supported by the National Institute on Aging (NIA) 1R01AG065569-02A1, the National Center for Advancing Translational Sciences (NCATS) UL1TR001873 and the NY NORC 5P30DK026687-42. Santos-Báez is supported by the NIHNational Heart, Lung, and Blood Institute (NHLBI) T32 HL07343. Popp is supported by R01NR018916. Panda is supported by DK124484, DK129668, and DK118278. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations:
- ALT
Alanine Transaminase
- ASA24®
Automated Self-Administered 24-h Dietary Assessment Tool
- AST
Aspartate Aminotransferase
- AUC
Area Under the Curve
- BAI
Beck Anxiety Inventory
- BDI
Beck’s Depression Inventory
- BMP
Basic Metabolic Panel
- CGM
Continuous Glucose Monitoring
- CPAP
Continuous Positive Airway Pressure
- CRC
Clinical Research Center
- CVD
Cardiovascular Disease
- DLW
Doubly Labelled Water
- EDTA
Ethylenediaminetetraacetic Acid
- EI
Energy Intake
- EE
Energy Expenditure
- ES
Energy Stores
- eGFR
Estimated Glomerular Filtration Rate
- EMR
Electronic Medical Records
- FG
Fasting Glucose
- FM
Fat Mass
- FFM
Fat-Free Mass
- HABIT
Habitual Eating
- HbA1c
Glycated Hemoglobin
- HDL
High-Density Lipoprotein
- HOMA-IR
Homeostatic Model Assessment for Insulin Resistance
- hsCRP
High Sensitivity C-Reactive Protein
- IPAQ
International Physical Activity Questionnaire
- ISI
Insomnia Severity Index
- ITT
Intention-To-Treat
- LDL
Low-Density Lipoprotein
- mCC
myCircadianClock
- MRI
Magnetic Resonance Imaging
- MVPA
Moderate-to-Vigorous Physical Activity
- NIA
National Institute on Aging
- NHLBI
National Heart, Lung, and Blood Institute
- NIA
National Institute of Aging
- NCATS
National Center for Advancing Translational Sciences
- OGTT
Oral Glucose Tolerance Test
- OSA
Obstructive Sleep Apnea
- PSQI
Pittsburgh Sleep Quality Index
- QMR
Quantitative Magnetic Resonance
- RAGE
Receptor for Advanced Glycation End Product
- T2D
Type 2 Diabetes
- TEE
Total Energy Expenditure
- TG
Triglycerides
- TNF
Tumor Necrosis Factor
- TRE
Time-Restricted Eating
- TSH
Thyroid Stimulating Hormone
- VAS
Visual Analog Scales
Footnotes
CRediT authorship contribution statement
Leinys S. Santos-Báez: Visualization, Writing – original draft. Alison Garbarini: Writing – review & editing. Delaney Shaw: Writing – review & editing. Collin J. Popp: Writing – review & editing. Emily N. C. Manoogian: Visualization, Writing – review & editing. Satchidananda Panda: Visualization, Writing – review & editing. Blandine Laferrère: Funding acquisition, Conceptualization, Methodology, Visualization, Supervision, Resources, Project administration, Writing – review & editing.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
CJP is a Sports Nutrition Consultant for Renaissance Periodization, LLC.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cct.2022.106872.
Data availability
No data was used for the research described in the article.
References
- [1].CDC, National Diabetes Statistics Report, 2017 2017 The National Diabetes Statistics Report is a periodic publication of the Centersfor Disease Control and Prevention (CDC) that Provides Updated Statistics About Diabetes in the United States for a Scientific Audience. It Includesinformation on Prevalence and Incidence of Diabetes, Prediabetes, Risk Factors for Complications, Acute and Long-Term Complications, Deaths, and Costs. These Data can Help Focus Efforts to Prevent and Control Diabetes Across the United States. T, Available from, https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf, 2017. [Google Scholar]
- [2].Pi-Sunyer FX, Medical hazards of obesity, Ann. Intern. Med 119 (7 Pt 2) (1993) 655–660. [DOI] [PubMed] [Google Scholar]
- [3].Liou T, Pi-Sunyer FX, Laferrère B, Physical disability and obesity, Nutr. Rev 63 (10) (2005) 321–331. [DOI] [PubMed] [Google Scholar]
- [4].CDC, Promoting Preventative Services for Adults 50–64: Community and Clinical Partnerships, Available from, https://www.cdc.gov/aging/pdf/promoting-preventive-services.pdf, 2009.
- [5].Rao VN, Zhao D, Allison MA, Guallar E, Sharma K, Criqui MH, Cushman M, Blumenthal RS, Michos ED, Adiposity and incident heart failure and its subtypes: MESA (multi-ethnic study of atherosclerosis), JACC (2018), 10.1016/j.jchf.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hirode G, Wong RJ, Trends in the prevalence of metabolic syndrome in the United States, 2011-2016, JAMA. 323 (24) (2020) 2526–2528, 10.1001/jama.2020.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nathan DM, Barrett-Connor E, Crandall JP, Edelstein SL, Goldberg RB, Horton ES, Knowler WC, et al. , Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the diabetes prevention program outcomes Study, Lancet Diabetes Endocrinol. 3 (11) (2015) 866–875, 10.1016/s2213-8587(15)00291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sumamo EHC, Korownyk C, et al. , Lifestyle Interventions for Four Conditions: Type 2 Diabetes, Metabolic Syndrome, Breast Cancer, and Prostate Cancer [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US) 2011. [updated 2011 May 26.], May 26.:[Available from, https://www.ncbi.nlm.nih.gov/books/NBK254022/, 2011. [PubMed] [Google Scholar]
- [9].Chaix A, Zarrinpar A, Miu P, Panda S, Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges, Cell Metab. 20 (6) (2014) 991–1005, 10.1016/j.cmet.2014.ll.001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Belkacemi L, Selselet-Attou G, Louchami K, Sener A, Malaisse WJ, Intermittent fasting modulation of the diabetic syndrome in sand rats. II. In vivo investigations, Int. J. Mol. Med. 26 (5) (2010) 759–765. Epub 2010/09/30, 10.3892/ijmm_00000523 . [DOI] [PubMed] [Google Scholar]
- [11].Gill S, Panda S, A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits, Cell Metab. 22 (5) (2015) 789–798. Epub 09/24, 10.1016/j.cmet.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Moro T, Tinsley G, Bianco A, Marcolin G, Pacelli QF, Battaglia G, Palma A, Gentil P, Neri M, Paoli A, Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males, J. Transl. Med 14 (1) (2016) 290. Epub 2016/10/16, 10.1186/s12967-016-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tinsley GM, Forsse JS, Butler NK, Paoli A, Bane AA, La Bounty PM, Morgan GB, Grandjean PW, Time-restricted feeding in young men performing resistance training: a randomized controlled trial, Eur. J. Sport Sci 17 (2) (2017) 200–207, 10.1080/17461391.2016.1223173. [DOI] [PubMed] [Google Scholar]
- [14].Matthew J McAllister BLP, Renteria Liliana I, Waldman Hunter S.,. Time-restricted feeding improves markers of cardiometabolic health in physically active college-age men: a 4-week randomized pre-post pilot study. Nutr. Res 75(0271–5317):32–43. doi: 10.1016/j.nutres.2019.12.001.; [DOI] [PubMed] [Google Scholar]
- [15].LeCheminant JD, Christenson E, Bailey BW, Tucker LA, Restricting night-time eating reduces daily energy intake in healthy young men: a short-term cross-over study, Br. J. Nutr 110 (11) (2013) 2108–2113. Epub 2013/05/25, 10.1017/S0007114513001359. [DOI] [PubMed] [Google Scholar]
- [16].Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM, Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes, Cell Metab. 27 (6) (2018), 10.1016/j.cmet.2018.04.010, 1212–21.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hutchison AT, Regmi P, Manoogian ENC, Fleischer JG, Wittert GA, Panda S, Heilbronn LK, Time-restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: a randomized crossover trial, Obesity (Silver Spring) 27 (5) (2019) 724–732. Epub 2019/04/20. [DOI] [PubMed] [Google Scholar]
- [18].Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, Peterson CM, Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans, Nutrients. 11 (6) (2019), 10.3390/null061234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gabel K, Hoddy KK, Haggerty N, Song J, Kroeger CM, Trepanowski JF, Panda S, Varady KA, Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: a pilot study, Nutr. Healthy Aging. 4 (4) (2018) 345–353, 10.3233/NHA-170036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gabel K, Hoddy KK, Burgess HJ, Varady KA, Effect of 8-h time-restricted feeding on sleep quality and duration in adults with obesity, Appl. Physiol. Nutr. Metab 44 (8) (2019) 903–906. Epub 2019/02/26. [DOI] [PubMed] [Google Scholar]
- [21].Antoni R, Robertson TM, Robertson MD, Johnston JD, A pilot feasibility study exploring the effects of a moderate time-restricted feeding intervention on energy intake, adiposity and metabolic physiology in free-living human subjects, J. Nutr. Sci 7 (2018), 10.1017/jns.2018.13. [DOI] [Google Scholar]
- [22].Wilkinson MJ, Manoogian ENC, Zadourian A, Lo H, Fakhouri S, Shoghi AWX, Fleischer JG, Navlakha S, Panda S, PR. T, Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome, Cell Metab. 31 (1) (2020) 92–104, 10.1016/j.cmet.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chow LSM, Alvear A, C. EN, Fleischer JG, Thor H, Dietsche K, Wang Q, Hodges JS, Esch N, Malaeb S, Time restricted eating effects on body composition and metabolic measures in humans who are overweight: a feasibility study, Obesity (Silver Spring) 28 (5) (2020) 860–869. Epub April 9, 2020, 10.1002/oby.22756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Anton SD, Lee SA, Donahoo WT, McLaren C, Manini T, Leeuwenburgh C, Pahor M, The effects of time restricted feeding on overweight, older adults: a pilot study, Nutrients. 11 (7) (2019), 1500, 10.3390/null071500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Parr EB, Devlin BL, Radford BE, Hawley JA, A delayed morning and earlier evening time-restricted feeding protocol for improving glycemic control and dietary adherence in men with overweight/obesity: a randomized controlled trial, Nutrients. 12 (2) (2020) 505. 10.3390/nu12020505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Parr EB, Devlin BL, Lim KHC, Moresi LNZ, Geils C, Brennan L, Hawley JA, Time-restricted eating as a nutrition strategy for individuals with type 2 diabetes: a feasibility study, Nutrients. 12 (11) (2020), 3228, 10.3390/nul2113228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lowe DA, Wu N, Rohdin-Bibby L, Moore AH, Kelly N, Liu YE, Philip E, Vittinghoff E, Heymsfield SB, Olgin JE, Shepherd JA, Weiss EJ, Effects of time-restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity: the TREAT randomized clinical trial, JAMA Intern. Med 180 (11) (2020) 1491–1499, 10.1001/jamainternmed.2020.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cioffi I, Evangelista A, Ponzo V, Ciccone G, Soldati L, Santarpia L, Contaldo F, Pasanisi F, Ghigo E, Bo S, Intermittent versus continuous energy restriction on weight loss and cardiometabolic outcomes: a systematic review and meta-analysis of randomized controlled trials, J. Transl. Med 16 (1) (2018) 371, 10.1186/s12967-018-1748-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Stote KS, Baer DJ, Spears K, Paul DR, Harris GK, Rumpler WV, Strycula P, Najjar SS, Ferrucci L, Ingram DK, Longo DL, Mattson MP, A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults, Am. J. Clin. Nutr 85 (4) (2007) 981–988. Epub 2007/04/07, 10.1093/ajcn/85.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Falak Zeb XW, Chen Lijun, Fatima Sadia, Haq Ijaz-Ul, Chen Aochang, Majeed Fatima, Feng Qing, Li Min, Effect of time-restricted feeding on metabolic risk and circadian rhythm associated with gut microbiome in healthy males, Br. J. Nutr 123 (11) (2020) 1216–1226. Epub 06 January 2020, 10.1017/S0007114519003428. [DOI] [PubMed] [Google Scholar]
- [31].Prasad M, Fine K, Gee A, Nair N, Popp CJ, Cheng B, Manoogian ENC, Panda S, Laferrère B, A smartphone intervention to promote time restricted eating reduces body weight and blood pressure in adults with overweight and obesity: a pilot study, Nutrients. 13 (7) (2021), 2148, 10.3390/nu13072148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Popp CJ, Curran M, Wang C, Prasad M, Fine K, Gee A, Nair N, Perdomo K, Chen S, Hu L, St-Jules DE, Manoogian ENC, Panda S, Sevick MA, Laferrère B, Temporal eating patterns and eating windows among adults with overweight or obesity, Nutrients. 13 (12) (2021), 4485. 10.3390/nu13124485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].McHill AW, Phillips AJ, Czeisler CA, Keating L, Yee K, Barger LK, Garaulet M, Scheer FA, Klerman EB, Later circadian timing of food intake is associated with increased body fat, Am. J. Clin. Nutr 106 (5) (2017) 1213–1219. Epub 2017/09/08, 10.3945/ajcn.117.161588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Karatzi K, Moschonis G, Choupi E, Manios Y, Healthy Growth Study g. Late-night overeating is associated with smaller breakfast, breakfast skipping, and obesity in children: The Healthy Growth Study, Nutrition 33 (2017) 141–144. Epub 2016/07/28, 10.1016/j.nut.2016.05.010. [DOI] [PubMed] [Google Scholar]
- [35].Gupta NJ, Kumar V, Panda S, A camera-phone based study reveals erratic eating pattern and disrupted daily eating-fasting cycle among adults in India, PLoS One 12 (3) (2017), 10.1371/journal.pone.0172852 e0172852. Epub 2017/03/07.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Regmi Prashant, Heilbronn LK, Time-restricted eating: benefits, mechanisms, and challenges in translation, iScience 23 (6) (2020), 10.1016/j.isci.2020.101161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mattson MP, Longo VD, Harvie M, Impact of intermittent fasting on health and disease processes, Ageing Res. Rev 39 (2017) 46–58, 10.1016/j.arr.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Van Cauter E, Polonsky KS, Scheen AJ, Roles of circadian rhythmicity and sleep in human glucose regulation*, Endocr. Rev 18 (5) (1997) 716–738, 10.1210/edrv.18.5.0317. [DOI] [PubMed] [Google Scholar]
- [39].Morgan L, Hampton S, Gibbs M, Arendt J, Circadian aspects of postprandial metabolism, Chronobiol. Int 20 (5) (2003) 795–808. [DOI] [PubMed] [Google Scholar]
- [40].Murray E, Burns J, See TS, Lai R, Nazareth I, Interactive health communication applications for people with chronic disease, Cochrane Database Syst. Rev (4) (2005), 10.1002/14651858.CD004274.pub4. CD004274.. [DOI] [PubMed] [Google Scholar]
- [41].Pal K, Eastwood SV, Michie S, Farmer AJ, Barnard ML, Peacock R, Wood B, Inniss JD, Murray E, Computer-based diabetes self-management interventions for adults with type 2 diabetes mellitus, Cochrane Database Syst. Rev (3) (2013), 10.1002/14651858.CD008776.pub2. CD008776.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Khadjesari Z, Murray E, Hewitt C, Hartley S, Godfrey C, Can stand-alone computer-based interventions reduce alcohol consumption? A systematic review, Addiction. 106 (2) (2011) 267–282, 10.1111/j.1360-0443.2010.03214.x. [DOI] [PubMed] [Google Scholar]
- [43].Foster C, Richards J, Thorogood M, Hillsdon M, Remote and web 2.0 interventions for promoting physical activity, Cochrane Database Syst. Rev 9 (2013), 10.1002/14651858.CD010395.pub2. CD010395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Villinger K, Wahl DR, Boeing H, Schupp HT, Renner B, The effectiveness of app-based mobile interventions on nutrition behaviours and nutrition-related health outcomes: a systematic review and meta-analysis, Obes. Rev 20 (10) (2019) 1465–1484. Epub 2019/07/30, 10.llll/obr.12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ninoska D, Peterson KRM, Nackers Lisa M., Medina Kristen E., Milsom Vanessa A., Perri Michael G., Dietary self-monitoring and long-term success with weight management, Obesity (Silver Spring) 22 (9) (2014) 1962–1967 (Epub 2014 Jun 13;). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Study N-T, New York TREAT Study Video. YouTube, 2021. [Google Scholar]
- [47].Piotrowski C, The status of the Beck inventories (BDI, BAI) in psychology training and practice: a major shift in clinical acceptance, J. Appl. Biobehav. Res 23 (3) (2017), e12112, 10.llll/jabr.12112. [DOI] [Google Scholar]
- [48].Morin CM, Belleville G, Bélanger L, Ivers H, The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response , Sleep. 34 (5) (2011) 601–608, 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Daniel J, Buysse CFR, Monk Timothy H., Berman Susan R., Kupfer David J., The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research, Psychiatry Res. 28 (2) (1989) 193–213, 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- [50].Horne JAOO, A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms, Int. J. Chronobiol 4 (2) (1976) 97–110. [PubMed] [Google Scholar]
- [51].Douglas SM, Leidy HJ, Novel methodological considerations regarding the use of visual analog scale (VAS) appetite questionnaires in tightly controlled feeding trials, Curr. Develop. Nutr 3 (6) (2019), 10.1093/cdn/nzz061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP, Using the Berlin questionnaire to identify patients at risk for the sleep apnea syndrome, Ann. Intern. Med 131 (7) (1999) 485–491, 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- [53].Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P, International physical activity questionnaire: 12-country reliability and validity, Med. Sci. Sports Exerc 35 (8) (2003) 1381–1395, 10.1249/01.Mss.0000078924.61453.Fb.. [DOI] [PubMed] [Google Scholar]
- [54].Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO, A new predictive equation for resting energy expenditure in healthy individuals, Am. J. Clin. Nutr 51 (2) (1990) 241–247, 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- [55].Mikael Karlsson EO, Wulf Becker, Brita Karlström, Tommy Cederholm, Per Sjögren, Ability to predict resting energy expenditure with six equations compared to indirect calorimetry in octogenarian men, Exp. Gerontol 92 (2017) 52–55, 10.1016/j.exger.2017.03.013. [DOI] [PubMed] [Google Scholar]
- [56].Wells J, Ruto A, Treleaven P, Whole-body three-dimensional photonic scanning: a new technique for obesity research and clinical practice, Int. J. Obes 32 (2022) 232–238, 10.1038/sj.ijo.0803727. [DOI] [PubMed] [Google Scholar]
- [57].Gallagher D, Thornton JC, He Q, Wang J, Yu W, Bradstreet TE, Burke J, Heymsfield SB, Rivas VM, Kaufman R, Quantitative magnetic resonance fat measurements in humans correlate with established methods but are biased, Obesity (Silver Spring) 18 (10) (2010) 2047–2054. Epub 2010/05/08, 10.1038/oby201097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ravelli MN, Schoeller DA, An objective measure of energy intake using the principle of energy balance, Int. J. Obes 45 (2021) 725–732, 10.1038/S41366-021-00738-0. [DOI] [PubMed] [Google Scholar]
- [59].Schoeller DA, Energy expenditure from doubly labeled water: some fundamental considerations in humans, Am. J. Clin. Nutr 38 (6) (1983) 999–1005, 10.1093/ajcn/38.6.999. [DOI] [PubMed] [Google Scholar]
- [60].Bowen GJ, Ehleringer JR, Chesson LA, Stange E, Cerling TE, Stable isotope ratios of tap water in the contiguous United States, Water Resour. Res 43 (3) (2007), 10.1029/2006WR005186. [DOI] [Google Scholar]
- [61].Tasali E, Wroblewski K, Kahn E, Kilkus J, Schoeller DA, Effect of sleep extension on objectively assessed energy intake among adults with overweight in real-life settings: a randomized clinical trial, JAMA Intern. Med 182 (4) (2022) 365–374, 10.1001/jamainternmed.2021.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Manoogian ENC, Wei-Shatzel J, Panda S, Assessing temporal eating pattern in free living humans through the myCircadianClock app, Int. J. Obes (2022), 10.1038/s41366-021-01038-3. Epub 2022/01/09.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Subar AF, Kirkpatrick SI, Mittl B, Zimmerman TP, Thompson FE, Bingley C, Willis G, Islam NG, Baranowski T, McNutt S, The automated self-administered 24-hour dietary recall (ASA24): a resource for researchers, clinicians and educators from the National Cancer Institute, J. Acad. Nutr. Diet 112 (8) (2012) 1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Chen KY, Bassett DRJ, The technology of accelerometry-based activity monitors: current and future, Med. Sci. Sports Exerc 37 (11) (2005) S490–S500, 10.1249/01.mss.0000185571.49104.82.. [DOI] [PubMed] [Google Scholar]
- [65].Blum A, Freestyle libre glucose monitoring system, Clin. Diabetes 36 (2) (2018) 203–204, 10.2337/cd17-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Easy GV, Easy GV Allows You to Calculate 10 Different Measures of Glycaemic Variability (GV) from Continuos Glucose Monitoring Data Using a Simple Interface [cited 2022 May 19, 2022] Available from, https://www.phc.ox.ac.uk/research/resources/easygv, 2022.
- [67].Tai MM, A mathematical model for the determination of Total area under glucose tolerance and other metabolic curves, Diabetes Care 17 (2) (1994) 152–154, 10.2337/diacare172152. [DOI] [PubMed] [Google Scholar]
- [68].Van Cauter E, Blackman JD, Roland D, Spire JP, Refetoff S, Polonsky KS, Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep, J. Clin. Invest 88 (3) (1991) 934–942, 10.1172/JCU15396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC, Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man, Diabetologia. 28 (7) (1985) 412–419, 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- [70].Caumo A, Perseghin G, Brunani A, Luzi L, New insights on the simultaneous assessment of insulin sensitivity and β-cell function with the HOMA2 method, Diabetes Care 29 (12) (2006) 2733–2734, 10.2337/dc06-0070. [DOI] [PubMed] [Google Scholar]
- [71].Wallace TM, Levy JC, Matthews DR, Use and abuse of HOMA modeling, Diabetes Care 27 (6) (2004) 1487–1495, 10.2337/diacare.2761487. [DOI] [PubMed] [Google Scholar]
- [72].Little R, Rubin D, Statistical Analysis with Missing Data, 2nd ed., New York John Wiley & Sons, 2002. [Google Scholar]
- [73].Caan B, Ballard-Barbash R, Slattery ML, Pinsky JL, Iber FL, Mateski DJ, Marshall JR, Paskett ED, Shike M, Weissfeld JL, Schatzkin A, ElaineLanza., Low energy reporting may increase in intervention participants enrolled in dietary intervention trials, J. Am. Diet. Assoc 104 (3) (2004) 357–366. Epub 20 April 2004, 10.1016/j.jada.2003.12.023. [DOI] [PubMed] [Google Scholar]
- [74].Johansson G, Wikman Å, Åhrén A, Hallmans G, Johansson I, Underreporting of energy intake in repeated 24-hour recalls related to gender, age, weight status, day of interview, educational level, reported food intake, smoking habits and area of living, Public Health Nutr. 4 (4) (2001) 919–927, 10.1079/phn2001124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.


