Abstract
Objectives:
Incarcerated persons in the United States have a high burden of hepatitis C virus (HCV) infection. This study assessed the impact of a statewide effort in Vermont to treat HCV in this group.
Methods:
We performed a retrospective, observational cohort study of all HCV-infected persons who were imprisoned in Vermont during the 19-month study period (December 2018–June 2020). The cascade of care comprised opt-out HCV screening, full access to direct-acting antiviral treatment (without hepatic fibrosis-based treatment restrictions), HCV specialist involvement, and medication-assisted treatment for patients with opioid use disorder. The primary outcome was sustained virologic response at 12 weeks after treatment completion (SVR12).
Results:
The study included 217 HCV-infected patients; the median age was 35 years (range, 18-73 years), 89% were male, 76% had opioid use disorder, 67% had a psychiatric comorbidity, and 9% had cirrhosis. Of the 217 patients, 98% had a liver fibrosis assessment, 59% started direct-acting antiviral treatment, 55% completed direct-acting antiviral treatment, and 51% achieved documented SVR12. Of the 129 HCV-infected persons who started direct-acting antiviral treatment, 92% completed therapy and 86% achieved documented SVR12. Psychiatric comorbidity was not significantly associated with achieving SVR12 (odds ratio = 0.67; 95% CI, 0.27-1.65; P = .38), nor was receiving medication-assisted treatment for patients with opioid use disorder (odds ratio = 1.45; 95% CI, 0.62-2.56; P = .45).
Conclusions:
This study reports the highest SVR12 rate achieved in a state incarcerated population to date. HCV treatment in incarcerated populations is a practical and efficacious strategy that should serve a foundational role in HCV elimination.
Keywords: hepatitis C virus, incarceration, prison, prison health, care cascade
Although incarcerated persons historically face more barriers than the general population to accessing medical care, paradoxically in many ways they make an ideal hepatitis C virus (HCV) treatment population.1-4 The estimated prevalence of HCV infection among incarcerated persons is 9.6% to 41.1%,2,5-9 far above the nonincarcerated US population prevalence of 1.0% to 1.5%.1,10 They have high rates of HCV transmission both within prison and surrounding communities, making treatment an important public health intervention.11-13 Incarcerated persons are available for daily direct observed therapy, which may mitigate the risk of treatment noncompliance that often negatively affects outpatient HCV treatment in other difficult-to-access populations.11,14 In addition, health care for incarcerated persons is paid for by the government, obviating the need for health insurance coverage and pre-approval of HCV therapeutics. Furthermore, in the era of HCV direct-acting antiviral (DAA) treatment, patients who complete guideline-directed therapy can expect a >95% chance of achieving HCV cure with minimal side effects. 15
Despite these factors, HCV care for patients in prison has been historically suboptimal, as elucidated from previous data on HCV care cascades in incarcerated populations. The HCV care cascade refers to the chain of steps, starting with screening, that lead to cure of HCV infection, with assessment of what proportion of the study population achieves each step. 16 In a study of persons who were incarcerated in Wisconsin from 2011 to 2015, of 3126 patients with HCV infection (defined as a positive HCV RNA viral load), only 328 (10.5%) received HCV treatment and only 186 (6.0%) achieved a sustained virologic response (SVR12), 16 defined as undetectable serum HCV RNA 12 weeks after treatment completion. 17 Another study assessed the HCV care cascade at a post-incarceration transition clinic in New York City during 2009-2014 and found that of 84 patients with HCV infection, 8 (9.5%) were treated and only 5 (6.0%) achieved SVR12. 18 Each of these studies was performed before DAA availability, when antiviral regimens included long-acting interferon, were not robust, and were associated with substantial adverse side effects. In more recent data from the DAA era, in the New York City jail system during 2014-2017, of 269 patients with HCV infection who started DAA treatment, 172 (64%) achieved documented SVR12, although there was a 16% subsequent reinfection rate, and the population-level SVR12 among all incarcerated HCV-infected patients was 3.2%.19,20 International data on treatment of HCV infection among incarcerated persons are sparse, although 1 study assessed the HCV care cascade in prisons in Quebec, Canada, during 2017-2018 and found that 2 of 16 incarcerated HCV-infected patients achieved SVR12. 21 A more recent study from Taiwan with opt-in screening found that of 276 people in jail identified as having positive HCV serology (HCV exposure), 191 (69%) had HCV infection and 165 (60%) achieved SVR12 with DAA treatment. 22
In aggregate, these studies suggest the presence of several important barriers to successful HCV treatment among incarcerated persons. These barriers include high rates of psychiatric disorders and substance use disorders, lack of medication-assisted treatment (MAT) for opioid use disorder (OUD), costs of medications, variable and sometimes unpredictable lengths of incarceration, requirements to go to a facility outside incarceration for HCV care, limited knowledge among health care staff, confidentiality concerns, health insurance requirements for the presence of advanced liver fibrosis, opt-in–only HCV screening systems, and inadequate post-incarceration transition-of-care resources.16,18,19,21,23-25 Given the long-term morbidities of HCV infection and that a large proportion of HCV transmission is driven by persons who are in and out of the justice system and incarceration, overcoming these barriers is a public health necessity.2,12
Little is known about the impact on HCV treatment outcomes among incarcerated persons of universal opt-out HCV screening and access to DAA treatment (regardless of the extent of hepatic fibrosis) guided by HCV specialists, coupled with voluntary provision of MAT for OUD. To address this issue, a statewide program in Vermont was launched to incorporate each of these policies into HCV management in Vermont prisons. We hypothesized that the SVR12 rates achieved by such a program would be higher than SVR12 rates reported in other studies of incarcerated HCV-infected patients. To test this hypothesis, we examined HCV treatment outcomes in this population.
Methods
Study Design, Location, Population, and Data Collection
We conducted a retrospective observational cohort study on the entire population of incarcerated persons in Vermont. The study period was from December 2018 through June 2020 (19 months), during which the incarcerated population in Vermont was approximately 1500 persons per year. Medical care for these persons was administered through Centurion Managed Care through a contractual agreement with the Vermont Department of Corrections.
We used de-identified patient-level data on each incarcerated HCV-infected patient during the study period. Data were not available after incarceration.
The study population comprised all incarcerated HCV-infected persons during the study period as documented by detection of serum HCV RNA, regardless of whether they started treatment, completed treatment while incarcerated, or were released early. We collected data on the following patient characteristics: age, sex, OUD status, MAT status, psychiatric disorder diagnoses, drug and alcohol use, HIV and hepatitis B virus coinfection, cirrhosis status, presence of other comorbid conditions, and detainer status (“detainer” refers to an incarcerated person awaiting sentencing, and the length of incarceration of such is unknown and unpredictable).
The study protocol was approved by the Vermont Department of Corrections, the administrative body with jurisdiction over the health care data of incarcerated persons in Vermont, and it was determined to be exempt from University of Vermont Medical Center Institutional Review Board oversight.
HCV Management
Policies about HCV testing and treatment were governed by Centurion Managed Care, and Centurion Managed Care was responsible for their administration. Before December 2018 (before the study period), Centurion Managed Care generally required a hepatic fibrosis score of F2 (ie, intermediate stage) or higher for an incarcerated person to qualify for HCV treatment in Vermont, but all fibrosis restrictions were dropped at that time. Centurion Managed Care contracted with University of Vermont Medical Center to have an infectious disease specialist provide consultation for the care of HCV-infected patients. Centurion Managed Care did have a policy during the analysis period, designed to minimize partial treatment, that required a planned incarceration length of ≥1 year to qualify for DAA treatment, unless the patient had a hepatic fibrosis score of ≥F2, in which case DAA treatment was offered independent of incarceration length.
HCV Screening and Treatment
All incarcerated persons during the study period were offered opt-out screening for HCV with a serologic test for detection of HCV antibody. Determination of detectable serum HCV RNA (HCV infection) and HCV genotype was recommended for all patients with a positive HCV antibody test result (HCV-exposed patients). Screening for hepatitis B virus and HIV coinfection was also recommended. For incarcerated HCV-infected persons, initial hepatic fibrosis assessment was performed via medical history review, signs and symptoms of cirrhosis, and Fibrosis-4 (FIB-4) score.26,27 We used the FIB-4 score, which has a predictive value of 90% to 95% for the absence of advanced hepatic fibrosis/cirrhosis with scores <1.45, for ease of obtaining results in a short period.26,27 Patients whose hepatic fibrosis status was unknown and who had a FIB-4 score ≥1.45 were offered transient elastography, a noninvasive test that measures liver stiffness and provides an estimate of the extent of hepatic fibrosis. 28 New HCV diagnoses were assessed by a Centurion Managed Care clinician and, if deemed to be acute, a 6-month waiting period was put into place to assess for spontaneous viral clearance; patients who achieved spontaneous viral clearance were counted as successfully completing the HCV care cascade, but they were not included in the SVR12 group. All HCV-infected patients for whom incarceration was expected to last ≥1 year were offered DAA treatment. If incarceration was expected to be <1 year, DAA treatment was offered to those with at least F2 hepatic fibrosis. Patients with lesser degrees of hepatic fibrosis (F0-F1) who were expected to be incarcerated for <1 year received work-up along the care cascade in anticipation of community-based DAA treatment and referral upon discharge.
The choice of DAA treatment was directed by the guidelines of the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America. 29 When appropriate, fixed-dose combination elbasvir/grazoprevir and sofosbuvir/velpatasvir were used, given the lower cost in this setting relative to other DAA medications. We examined HCV resistance-associated substitutions as recommended by guidelines on a case-by-case basis. 29 For all patients with OUD, MAT with buprenorphine/naloxone was offered. For DAA-treated patients who were discharged to the community before treatment completion, the remainder of the DAA medication was provided upon discharge, along with a referral to an HCV specialist and primary health professional. Costs of DAA medications (but not other aspects of the HCV care cascade) were recorded.
Outcomes
The primary outcome was documented SVR12. Additional outcomes were assessed at each major step along the HCV care cascade, which included the percentage of HCV-infected patients who (1) were assessed for liver fibrosis, (2) initiated DAA treatment, and (3) completed documented DAA treatment. When dropout from any step occurred, we investigated the cause. The DAA treatment adherence rate was recorded during daily direct observed therapy. Our assessment of SVR12 was per protocol (in the population of patients who received ≥1 dose of DAA medication). Patients without documented completion of DAA treatment were assumed to have stopped it; patients without a documented SVR12 were assumed to have failed therapy, even if they had completed DAA treatment.
Psychiatric disorders have been shown to negatively impact treatment adherence and success in various diseases; conversely, among people with OUD, MAT has been shown to have a positive impact.30,31 To our knowledge, the impact of psychiatric disorders on DAA outcomes among incarcerated persons is unknown. We therefore sought to determine the extent to which psychiatric disorders were associated with SVR12.
Statistical Analysis
We used descriptive statistics for the HCV care cascade. We calculated the association between demographic variables and SVR12 as odds ratios (ORs) and 95% CIs, with significance defined as P < .05. All statistical analysis was carried out using SAS version 9.4 (SAS Institute, Inc).
Results
We identified 236 incarcerated persons with a positive HCV antibody test (HCV exposed) result during the study period. Of this group, 217 persons had detectable serum HCV RNA (HCV infected), of whom 27 (12%) were not known to be previously infected. The median age was 35 years (range, 18-73 [IQR, 30-43]) (Table 1). Twenty patients (9%) had cirrhosis, 1 had HBV coinfection, and none had HIV coinfection. OUD was present in 164 (76%) persons; of these, 146 (89%) were receiving MAT. Of the 217 HCV-infected persons, 145 (67%) had a coexisting psychiatric diagnosis.
Table 1.
Characteristics of the study population of incarcerated HCV-infected persons (n = 217), Vermont, December 2018–June 2020 a
| Characteristic | No. (%) b |
|---|---|
| Median age (range) [IQR], y | 35 (18-73) [30-43] |
| Male | 193 (89) |
| Detainer status c | 71 (33) |
| Cirrhosis | 20 (9) |
| Hepatitis B virus infection d | 1 (<1) |
| HIV | 0 |
| Opioid use disorder | 164 (76) |
| Medication-assisted treatment for opioid use disorder | |
| Yes | 146 (89) |
| No | 18 (11) |
| Alcohol use disorder | 31 (14) |
| Polysubstance use | 29 (13) |
| Psychiatric disorder | 145 (67) |
| Depression | 63 (43) |
| Anxiety | 54 (37) |
| Posttraumatic stress disorder | 39 (27) |
| Personality disorder | 25 (17) |
| Bipolar disorder | 20 (14) |
| Attention deficit hyperactivity disorder | 17 (12) |
| Behavior disorder | 8 (6) |
| Other | 18 (12) |
| >1 Psychiatric disorder | 79 (54) |
| Cardiovascular disease | 30 (14) |
| Pulmonary disease | 30 (14) |
| Gastroesophageal reflux disease | 35 (16) |
| Chronic kidney disease | 1 (<1) |
| Diabetes | 4 (2) |
Abbreviation: HCV, hepatitis C virus.
Data obtained through a retrospective, observational cohort study of all HCV-infected persons who were imprisoned in Vermont during the study period. HCV infection determined by detectable serum HCV RNA.
All values are number (percentage) unless otherwise indicated.
A detainer is an incarcerated person awaiting sentencing; the length of incarceration of such is unknown and unpredictable.
Serum hepatitis B virus surface antigen positive.
HCV Treatment
Among the 217 HCV-infected patients, the most common HCV genotype was genotype 1a, occurring in 101 patients, and 157 patients had a FIB-4 score <1.45 (Table 2). Of 56 patients with FIB-4 scores ≥1.45, 37 (66%) received transient elastography; of the remaining 19 patients, transient elastography was not performed because of patient refusal (13 patients) or release from incarceration before completion (6 patients). Of the 217 HCV-infected patients, 12 had subsequent spontaneous HCV clearance; thus, 205 were eligible for DAA treatment. DAA treatment was initiated in 129 (63%) patients (Figure). Reasons for not receiving therapy included patient refusal (n = 38), early release (n = 23), and spontaneous viral clearance (n = 9). Of patients receiving DAA treatment, the most common regimens were velpatasvir/sofosbuvir (44%) and grazoprevir/elbasvir (43%). The mean medication adherence rate was 99%. The overall cost of DAA treatment (not including other nonpharmaceutical components of HCV care) during the study period was $2 773 710, or $24 988 per documented SVR12 achieved.
Table 2.
Virological profiles, hepatic fibrosis assessments, and direct-acting antiviral regimens used in the study population of incarcerated HCV-infected persons (n = 217), Vermont, December 2018–June 2020 a
| Profile | No. (% b ) |
|---|---|
| Hepatitis C virus genotype | |
| Genotype 1a | 101 (47) |
| NS5A+ | 5 (2) |
| Genotype 1b | 8 (4) |
| NS5A+ | 1 (<1) |
| Genotype 2 | 26 (12) |
| Genotype 3 | 41 (19) |
| Genotype 4 | 0 |
| Genotype 5 | 0 |
| Genotype 6 | 1 (<1) |
| Mixed | 6 (3) |
| Unknown | 28 (13) |
| Fibrosis-4 score c | 213 (98) |
| <1.45 | 157 (74) |
| ≥1.45 | 56 (26) |
| Got a fibroscan | 37 (66) |
| Did not get a fibroscan | 19 (34) |
| Refused | 13 (68) |
| Released | 6 (32) |
| Fibrosis scores per transient elastography (n = 37) | |
| F0 | 10 (27) |
| F1 | 1 (3) |
| F2 | 4 (11) |
| F3 | 8 (22) |
| F4 | 14 (38) |
| Direct-acting antiviral regimen (n = 129) | |
| Velpatasvir/sofosbuvir | 57 (44) |
| Grazoprevir/elbasvir | 56 (43) |
| Glecaprevir/pibrentasvir | 15 (12) |
| Ledipasvir/sofosbuvir | 1 (1) |
Abbreviations: HCV, hepatitis C virus; NS5A, nonstructural protein 5A.
Data obtained through a retrospective, observational cohort study of all HCV-infected persons who were imprisoned in Vermont during the study period. HCV infection determined by detectable serum HCV RNA.
Some categories may not add to 100% because of rounding.
The Fibrosis-4 (FIB4) score estimates hepatic fibrosis. A score of <1.45 has a predictive value of 90% to 95% for the absence of advanced hepatic fibrosis/cirrhosis.
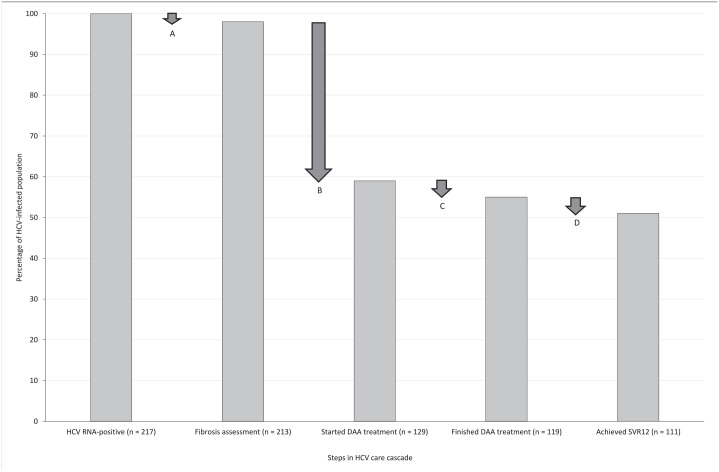
Figure.
Major steps along the hepatitis C virus (HCV) care cascade in the study population of incarcerated HCV-infected persons (n = 217), Vermont, December 2018–June 2020. Data were obtained through a retrospective, observational cohort study of all HCV-infected persons who were imprisoned in Vermont during the study period. HCV infection determined by detectable serum HCV RNA. At Step A, drop-off was 4 patients: 3 with spontaneous HCV clearance and 1 from early release. At Step B, drop-off was 84 patients: 38 from patient refusal, 23 from early release, 14 who were detainers (ie, incarcerated persons awaiting sentencing), and 9 with spontaneous HCV clearance. At Step C, drop-off was 10 patients: 8 from early release and 2 from patient refusal. At Step D, drop-off was 8 patients: 6 from early release and 2 from patient refusal. Abbreviations: DAA, direct-acting antiviral; SVR12, sustained virologic response at 12 weeks post-treatment.
HCV Care Cascade Outcomes
Of the 217 HCV-infected patients, 213 (98%) had a fibrosis assessment, 129 (59%) started DAA treatment, 119 (55%) had documented completion of DAA treatment, and 111 (51%) had documented SVR12 (Figure). Of the 129 patients who started DAA treatment, 111 (86%) had documented SVR12. Twelve patients had spontaneous viral clearance without DAA treatment; thus, of the initial 217 HCV-infected patients, 123 (57%) achieved presumed HCV elimination.
Patient Characteristics Associated With SVR12 Outcomes
Contrary to expectations, in our study group, the presence of a history of psychiatric comorbidity (compared with its absence) was not significantly associated with starting DAA treatment (OR = 1.56; 95% CI, 0.92-2.64; P = .10), completing DAA treatment (OR = 0.21; 95% CI, 0.03-1.67; P = .14), or achieving SVR12 (OR = 0.67; 95% CI, 0.27-1.65; P = .38). Similarly, among patients with OUD, the use of MAT (compared with none) was not significantly associated with starting DAA treatment (OR = 1.37; 95% CI, 0.51-3.65; P = .52) or achieving SVR12 (OR = 1.45; 95% CI, 0.62-2.56; P = .45).
Discussion
This study reports on outcomes of a novel statewide management plan of HCV treatment among incarcerated persons and demonstrates that it is possible to achieve high SVR12 rates among this traditionally difficult-to-access population. During the 19-month study period, documented SVR12 was 51% for the entire population of HCV-infected patients; including patients with spontaneous HCV clearance, 57% of the cohort achieved presumed HCV elimination. Treatment adherence was nearly universal (99%). Among the group that initiated treatment, SVR12 was documented in 86% of patients, and we found no instances of treatment failure. It should be emphasized that we do not know the outcomes of patients who were released early, specifically among patients who completed treatment during incarceration and patients in whom treatment had not been completed by the time of release (but who were provided at that time with the necessary doses for treatment completion). We conservatively assumed that all such cases were treatment failures, but given expected SVR12 rates of >95%, 32 it is plausible that some treatment successes were missed and the reported SVR12 rates are underestimations.
Our documented SVR12 rates compare favorably with prior literature on the HCV care cascade among incarcerated persons, which demonstrated population-level SVR12 rates ranging from 6.0% to 12.5%,16,18,21 although data in the DAA era are limited. Limitations to higher SVR12 rates in these prior studies included risk-based or on-demand, opt-in screening rather than opt-out screening in our study, and used restrictions to treatment access based upon hepatic fibrosis stage, which was not the case in our population. A study in New York City demonstrated a documented SVR12 rate of 66% in an incarcerated or recently incarcerated population that started DAA treatment (compared with 86% in our study), although in a separate study this same group demonstrated that in their total study population (incarcerated people in the New York City jail system), of 4665 HCV-infected patients, population-level SVR12 was 3.2%. 20
The outcomes achieved in the HCV care cascade reported here compare favorably with those reported in nonincarcerated populations, in which population-level SVR12 rates vary from 4% to 43%.33-35 A potential explanation for the high SVR12 rate reported in our study is the use of direct observed therapy, with resultant DAA compliance rates (99%) that are competitive with those achieved in clinical trials. 29 In addition, with the costs of HCV care and DAA treatment covered by a government-contracted health plan and no need for prior authorization, access to treatment is likely increased. The direct cost of DAA medications in our study was $2 773 710, or $24 988 per documented SVR12 achieved. This substantial cost suggests that other states pursuing similar programs will need adequate legislative and financial support.
The most substantial barriers to completed HCV cure in our cohort were length of incarceration <1 year, detainer status, early release from incarceration, and refusal of care. Although all patients released before cure were referred to a primary care provider and HCV specialist upon prison discharge, follow-up rates are unknown and would be a worthwhile area for future investigation. Incarcerated persons with a detainer status remain a particular challenge, given unclear lengths of stay in the prison system. As demonstrated by others, a post-incarceration clinic may improve SVR12 rates in this population. 19
Refusal of care among incarcerated persons may be a challenging fix given well-documented suspicion of medical care in this group.36,37 Data from incarcerated populations with HIV (another potentially stigmatizing disease) suggest that medical engagement is enhanced by (1) systemic factors such as the quality of the patient–health care professional relationship and continuity between the penal system and the community; (2) social factors such as rates of substance use disorder and treatment, family and social supports, and how stigma and confidentiality are managed; and (3) individual factors such as mental illness, substance dependency, and resilience.38,39 We also posit that improved treatment access and education on treatment benefit would be helpful and decrease rates of refusal. Importantly, we did not find that psychiatric comorbidities and substance use were significantly associated with lower SVR12 rates, suggesting these conditions did not represent barriers to effective treatment.
Limitations
Our study had several limitations. First, Vermont is a small rural state, and our findings may not be generalizable to larger incarcerated groups or states with a different composition of rural and urban areas. That said, data on incarcerated persons in small rural states are lacking, and our study demonstrates the feasibility of mass treatment in such a setting. Second, we had no data available on outcomes of HCV-infected persons who initiated DAA treatment while incarcerated but left the penal system before completion of HCV care; thus, our outcomes are likely underestimations. Third, we did not have data on reinfection rates in this cohort, which have previously been reported as high as 16%. 19 Reinfection rates would be an important area for future study.
Conclusion
Incarcerated persons are a historically vulnerable population and can be challenging to access for care. However, our findings demonstrate that high rates of SVR12 can be achieved in a system that uses a coordinated, proactive approach to HCV screening and treatment. Although our study, to our knowledge, reports the highest SVR12 rate achieved in a state incarcerated population to date, we hypothesize that further improvements in HCV treatment outcomes should be attainable through improved systematic linkage to care upon return to the community, particularly for patients with short incarceration periods, and improved methods of engaging incarcerated persons in health care. The model of care we reported here supports the concept that HCV treatment in correctional settings is a feasible and highly effective strategy that should serve as a cornerstone in the effort to eradicate HCV for all.
Footnotes
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: At the time of this study, health care for incarcerated persons in Vermont was contracted from the Vermont Department of Corrections to Centurion Managed Care, which contracted with the University of Vermont Medical Center to provide consultation from its Infectious Diseases Division for incarcerated persons in Vermont regarding hepatitis C virus, hepatitis B virus, and HIV care. A.J.H. fulfilled this role during the study period but did not receive any direct compensation for this work. The contract ended on July 1, 2020.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Andrew J. Hale, MD  https://orcid.org/0000-0001-7038-1353
https://orcid.org/0000-0001-7038-1353
References
- 1. Edlin BR, Eckhardt BJ, Shu MA, Holmberg SD, Swan T. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology. 2015;62(5):1353-1363. doi: 10.1002/hep.27978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Spaulding AC, Anderson EJ, Khan MA, Taborda-Vidarte CA, Phillips JA. HIV and HCV in U.S. prisons and jails: the correctional facility as a bellwether over time for the community’s infections. AIDS Rev. 2017;19(3):134-147. [PubMed] [Google Scholar]
- 3. Hennessey KA, Kim AA, Griffin V, Collins NT, Weinbaum CM, Sabin K. Prevalence of infection with hepatitis B and C viruses and co-infection with HIV in three jails: a case for viral hepatitis prevention in jails in the United States. J Urban Health. 2009;86(1):93-105. doi: 10.1007/s11524-008-9305-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kohli A, Shaffer A, Sherman A, Kottilil S. Treatment of hepatitis C: a systematic review. JAMA. 2014;312(6):631-640. doi: 10.1001/jama.2014.7085 [DOI] [PubMed] [Google Scholar]
- 5. Abe CM, Aguwa M, Zhao M, Sullivan J, Porsa E, Nijhawan AE. Hepatitis C virus infection in the Dallas County Jail: implications for screening, prevention, and linkage to care. Public Health Rep. 2019;134(6):626-633. doi: 10.1177/0033354919874081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vescio MF, Longo B, Babudieri S, et al. Correlates of hepatitis C virus seropositivity in prison inmates: a meta-analysis. J Epidemiol Community Health. 2008;62(4):305-313. doi: 10.1136/jech.2006.051599 [DOI] [PubMed] [Google Scholar]
- 7. Varan AK, Mercer DW, Stein MS, Spaulding AC. Hepatitis C seroprevalence among prison inmates since 2001: still high but declining. Public Health Rep. 2014;129(2):187-195. doi: 10.1177/003335491412900213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rice J, Cervantes L, Lucey MR. Hepatitis C viral infection in incarcerated patients. Clin Liver Dis (Hoboken). 2012;1(3):84-86. doi: 10.1002/cld.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rice JP, Burnett D, Tsotsis H, et al. Comparison of hepatitis C virus treatment between incarcerated and community patients. Hepatology. 2012;56(4):1252-1260. doi: 10.1002/hep.25770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293-300. doi: 10.7326/M13-1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Redman JS, Sterling RK. Treating HCV in a captive audience: eradication efforts in the prison microenvironment. Am J Gastroenterol. 2018;113(11):1585-1587. doi: 10.1038/s41395-018-0201-x [DOI] [PubMed] [Google Scholar]
- 12. He T, Li K, Roberts MS, et al. Prevention of hepatitis C by screening and treatment in U.S. prisons. Ann Intern Med. 2016;164(2):84-92. doi: 10.7326/M15-0617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bui J, Wendt M, Bakos A. Understanding and addressing health disparities and health needs of justice-involved populations. Public Health Rep. 2019;134(1 suppl):3S-7S. doi: 10.1177/0033354918813089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosenthal ES, Silk R, Mathur P, et al. Concurrent initiation of hepatitis C and opioid use disorder treatment in people who inject drugs. Clin Infect Dis. 2020;71(7):1715-1722. doi: 10.1093/cid/ciaa105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Holmes JA, Rutledge SM, Chung RT. Direct-acting antiviral treatment for hepatitis C. Lancet. 2019;393(10179):1392-1394. doi: 10.1016/S0140-6736(18)32326-2 [DOI] [PubMed] [Google Scholar]
- 16. Hochstatter KR, Stockman LJ, Holzmacher R, et al. The continuum of hepatitis C care for criminal justice involved adults in the DAA era: a retrospective cohort study demonstrating limited treatment uptake and inconsistent linkage to community-based care. Health Justice. 2017;5(1):10. doi: 10.1186/s40352-017-0055-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen J, Florian J, Carter W, et al. Earlier sustained virologic response end points for regulatory approval and dose selection of hepatitis C therapies. Gastroenterology. 2013;144(7):1450-1455.e2. doi: 10.1053/j.gastro.2013.02.039 [DOI] [PubMed] [Google Scholar]
- 18. Hawks L, Norton BL, Cunningham CO, Fox AD. The hepatitis C virus treatment cascade at an urban postincarceration transitions clinic. J Viral Hepat. 2016;23(6):473-478. doi: 10.1111/jvh.12512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chan J, Schwartz J, Kaba F, et al. Outcomes of hepatitis C virus treatment in the New York City jail population: successes and challenges facing scale up of care. Open Forum Infect Dis. 2020;7(7):ofaa263. doi: 10.1093/ofid/ofaa263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chan J, Kaba F, Schwartz J, et al. The hepatitis C virus care cascade in the New York City jail system during the direct acting antiviral treatment era, 2014-2017. EClinicalMedicine. 2020;27:100567. doi: 10.1016/j.eclinm.2020.100567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kronfli N, Dussault C, Klein MB, Lebouché B, Sebastiani G, Cox J. The hepatitis C virus cascade of care in a Quebec provincial prison: a retrospective cohort study. CMAJ Open. 2019;7(4):e674-e679. doi: 10.9778/cmajo.20190068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang TH, Fang YJ, Hsu SJ, et al. Microelimination of chronic hepatitis C by universal screening plus direct-acting antivirals for incarcerated persons in Taiwan. Open Forum Infect Dis. 2020;7(8):ofaa301. doi: 10.1093/ofid/ofaa301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Crowley D, Van Hout MC, Lambert JS, Kelly E, Murphy C, Cullen W. Barriers and facilitators to hepatitis C (HCV) screening and treatment—a description of prisoners’ perspective. Harm Reduct J. 2018;15(1):62. doi: 10.1186/s12954-018-0269-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grebely J, Haire B, Taylor LE, et al. Excluding people who use drugs or alcohol from access to hepatitis C treatments—is this fair, given the available data? J Hepatol. 2015;63(4):779-782. doi: 10.1016/j.jhep.2015.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nguyen JT, Rich JD, Brockmann BW, Vohr F, Spaulding A, Montague BT. A budget impact analysis of newly available hepatitis C therapeutics and the financial burden on a state correctional system. J Urban Health. 2015;92(4):635-649. doi: 10.1007/s11524-015-9953-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. 2007;46(1):32-36. doi: 10.1002/hep.21669 [DOI] [PubMed] [Google Scholar]
- 27. Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317-1325. doi: 10.1002/hep.21178 [DOI] [PubMed] [Google Scholar]
- 28. Tapper EB, Lok AS. Use of liver imaging and biopsy in clinical practice. N Engl J Med. 2017;377(8):756-768. doi: 10.1056/NEJMra1610570 [DOI] [PubMed] [Google Scholar]
- 29. American Association for the Study of Liver Diseases–Infectious Diseases Society of America. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Accessed February 12, 2021. https://www.hcvguidelines.org [DOI] [PMC free article] [PubMed]
- 30. Norton BL, Akiyama MJ, Zamor PJ, Litwin AH. Treatment of chronic hepatitis C in patients receiving opioid agonist therapy: a review of best practice. Infect Dis Clin North Am. 2018;32(2):347-370. doi: 10.1016/j.idc.2018.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rifai MA, Gleason OC, Sabouni D. Psychiatric care of the patient with hepatitis C: a review of the literature. Prim Care Companion J Clin Psychiatry. 2010;12(6):PCC.09r00877. doi: 10.4088/PCC.09r00877whi [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Loo N, Hanysak B, Mann J, et al. Real-world observational experience with direct-acting antivirals for hepatitis C: baseline resistance, efficacy, and need for long-term surveillance. Medicine (Baltimore). 2019;98(26):e16254. doi: 10.1097/MD.0000000000016254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brady JE, Vellozzi C, Hariri S, et al. Hepatitis C care cascade among persons born 1945-1965: 3 medical centers. Am J Manag Care. 2018;24(9):421-427. [PubMed] [Google Scholar]
- 34. Norton BL, Southern WN, Steinman M, et al. No differences in achieving hepatitis C virus care milestones between patients identified by birth cohort or risk-based screening. Clin Gastroenterol Hepatol. 2016;14(9):1356-1360. doi: 10.1016/j.cgh.2016.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Isenhour C, Hariri S, Vellozzi C. Monitoring the hepatitis C care cascade using administrative claims data. Am J Manag Care. 2018;24(5):232-238. [PMC free article] [PubMed] [Google Scholar]
- 36. Valera P, Boyas JF, Bernal C, Chiongbian VB, Chang Y, Shelton RC. A validation of the group-based medical mistrust scale in formerly incarcerated Black and Latino men. Am J Mens Health. 2018;12(4):844-850. doi: 10.1177/1557988316645152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Binswanger IA, Nowels C, Corsi KF, et al. “From the prison door right to the sidewalk, everything went downhill,” a qualitative study of the health experiences of recently released inmates. Int J Law Psychiatry. 2011;34(4):249-255. doi: 10.1016/j.ijlp.2011.07.002 [DOI] [PubMed] [Google Scholar]
- 38. Remien RH, Bauman LJ, Mantell JE, et al. Barriers and facilitators to engagement of vulnerable populations in HIV primary care in New York City. J Acquir Immune Defic Syndr. 2015;69 suppl 1(0 1):S16-S24. doi: 10.1097/QAI.0000000000000577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Haley DF, Golin CE, Farel CE, et al. Multilevel challenges to engagement in HIV care after prison release: a theory-informed qualitative study comparing prisoners’ perspectives before and after community reentry. BMC Public Health. 2014;14:1253. doi: 10.1186/1471-2458-14-1253 [DOI] [PMC free article] [PubMed] [Google Scholar]