Summary
In South Africa, maternal mortality from cardiovascular disease remains high. The recent Saving Mothers report 2017–2019 from the Confidential Enquiries into Maternal Deaths revealed that indirect maternal death from medical and surgical disorders is the fourth commonest cause of maternal death, accounting for 16.9% of deaths, with cardiac disease accounting for one-third of this.
The burden of rheumatic heart disease (RHD) is a significant contributor to maternal morbidity and mortality. The true burden is unknown due to limited data. The natural history of RHD confers additional risk as many cases may remain undiagnosed, with first presentation occurring during pregnancy. This undiagnosed subset of women may be the result of poor accessibility to healthcare facilities and primary healthcare interventions for acute rheumatic fever. RHD causes progressive damage to the heart valves, especially the left-sided valves, which eventually require surgical correction with mechanical prosthetic valves.
Keywords: bio-prosthetic heart valves, mechanical heart valves, pregnancy, warfarin, heparin, rheumatic heart disease
Anticoagulation in pregnant women with mechanical heart valves involves a delicate balance between maternal and foetal benefit. To date, a paucity of large, randomised, controlled trials in pregnancy, and an abundance of meta-analyses with widespread heterogeneity have provided little guidance and consensus among the experts. In the hope of achieving equipoise, various regimens are reported in the literature.
Mechanical heart valves are prone to thromboembolic complications (TEC), which require lifelong anticoagulation to prevent adverse events such as stuck valves, stroke and even death.1-3 The risk of TEC is dependent on the drug and anticoagulation regimen used. The commonest anticoagulants used in the general population are the vitamin K antagonists (VKA).4 In the absence of contra-indications, they remain an effective, cheap, easily administered option for persons with prosthetic valves.4
Herein follows a narrative appraisal of the current body of evidence with recommended guidelines for management in these women.
Rheumatic heart disease (RHD) and its sequelae are an important cause of cardiovascular pathology. The prevalence of RHD is unknown due to a paucity of studies and a lack of prospective data.5,6
In sub-Saharan Africa (SSA), RHD contributes to 30% of cardiac disease in pregnancy and it is the commonest cause of valvular heart disease.5-7 RHD is associated with maternal mortality rates of up to 34% and significant foetal loss.5 RHD, although extremely rare in high-income countries, continues to affect marginalised populations because of poor access to healthcare facilities, limited access to primary healthcare prevention strategies for acute rheumatic fever and delays in diagnosis due to a lack of skill on the part of healthcare workers or failure to follow up by those affected.7
RHD causes slow, progressive damage to the heart valves, especially the left-sided valves, which eventually require surgical correction with prosthetic valves. It has been estimated that over 280 000 prosthetic heart valves are replaced worldwide each year, half of which are mechanical.8
The modified World Health Organisation classification of maternal cardiovascular risk places prosthetic valves as category III. These women are therefore at significant risk for maternal morbidity and mortality, with reported rates for maternal adverse events of 19 to 27%. They require a multidisciplinary team approach in an expert centre for pregnancy and cardiac disease.9
Prosthetic heart valves
There are two main types of valves, namely mechanical (MHV) and bio-prosthetic valves (BPV).8,10 The type of valve used has to be carefully selected based on various factors such as age, haemodynamic performance, prevention of prosthesis– patient mismatch, durability, implantability, patient preference and a patient’s desire for fertility.10 The American College of Cardiology/American Heart Association (ACC/AHA) places more emphasis on patient preference than on age.11
In women desiring fertility and needing valve surgery, the benefits and risks of mechanical versus bio-prosthetic valves should be discussed.9,12 MHVs have the benefit of being more durable, with less chance of re-operation but with the need for lifelong anticoagulation with warfarin and international normalised ratio (INR) monitoring.8,13
In low- to middle-income countries (LMIC) such as South Africa, BPVs may not be a sustainable or accessible option due to socio-economic factors.14 BPVs, although significantly less thrombogenic, are prone to degeneration, especially in the young and during pregnancy, therefore needing re-operation.13,14 This unknown proportion of women are therefore generally offered MHV replacement and placed on lifelong anticoagulation with warfarin.3
This drug has been shown to be a highly effective option in the prevention of complications associated with MHV. Although this confers significant maternal benefit, it has been shown to be associated with deleterious consequences and complications to the developing embryo, with additional risk translated to the growing foetus.15
Pregnancy physiology
Pregnancy is a hypercoagulable state induced by the physiological changes to the coagulation and fibrinolytic systems.1,2 This adaptive mechanism is to prevent post-partum haemorrhage. There is an increase in fibrinogen levels and coagulation factors with a decrease in protein C and a relative protein S resistance, important natural anticoagulants.15 However, the natural anticoagulant antithrombin III (ATIII) remains normal. Changes in the fibrinolytic system lead to impaired fibrinolysis.
Pregnant women with prosthetic valves are therefore more at risk for thrombosis, stuck valves and TEC than the general population. The use of anticoagulation, patient compliance to regimens, strict mean targets set for therapy, as well as increased monitoring in pregnancy are warranted.
What drugs are available for anticoagulation in pregnant women with MHVs?
The most commonly used drugs for anticoagulation in pregnancy are the VKA and heparins, administered intravenously as unfractionated heparin (IV UFH) or subcutaneously as low-molecular-weight heparin (LMWH).16 Subcutaneous unfractionated heparin is rarely used.
Direct oral anticoagulants (DOAC) are considered a preferred treatment option in patients with non-valvular atrial fibrillation, deep-vein thrombosis and pulmonary embolus in the general population.17 A 2021 updated systematic review and metaanalysis by Bitar et al. showed a significant reduction in systemic embolism and risk of intracranial haemorrhage in patients with non-valvular AF and valvular heart disease when compared to warfarin.18 This analysis was not applicable in more severe mitral stenosis and in MHVs. Furthermore, pre-clinical and in vitro studies evaluating its use in patients with MHVs were not found to be promising.19 The ACC/AHA and European Society of Cardiology (ESC) advocate the use of warfarin in MHVs until more evidence becomes available. Evidence on its safety and efficacy in pregnancy and breastfeeding has not been demonstrated.20
The 2020 International Society on Thrombosis and Hemostasis congress concluded that based on their available data, the risk of embryopathy with DOAC was lower than with warfarin.21 Therefore, elective termination of pregnancy for fear of embryo toxicity should not be offered. Instead, closer pregnancy monitoring, pharmacovigilance and reporting of adverse pregnancy outcomes were suggested as management options. They further elucidated that the data are deficient and hence the use of DOAC should be avoided in pregnancy.
Historically, the use of LMWH had limited use in the anticoagulation of pregnant women with prosthetic valves. In early 2000, a controversy followed after Aventis Pharmaceuticals Inc and the Federal Drug Administration issued black-box warnings implicating LMWH in catastrophic TEC and maternal death.4 This recommendation was based on very small studies and small post-marketing reports. The systematic review by Chan et al. in 2000 recognised only three regimens at the time, namely VKA, sequential therapy and UFH.16
However, contemporary practice and recent consensus statements support the use of dose-adjusted LMWH, with vigilant anti-Xa monitoring to achieve peak levels four hours post drug administration, of between 0.8 and 1.2 U/ml. There is emerging evidence that trough levels should be maintained at 0.6 U/ml.9,22
LMWH has been shown to offer superior benefit to the foetus since it does not cross the placenta. However, the maternal risk for TEC is greater when compared to warfarin. In the first trimester there is generally an increase in renal plasma flow with a resultant increase in renal clearance.9 The increased renal clearance may necessitate much higher starting doses than the typical 1 mg/kg. Beresheim et al. conducted a small study that showed that the overall mean dose required to achieve therapeutic peak levels is 1.3 mg/kg.23
Generally, the first trimester may be associated with transitions from warfarin to heparin to lower the risk of embryopathy. Moreover, this practice is supported from data from the Registry of Pregnancy and Cardiac Disease (ROPAC), which showed higher rates of maternal adverse events during the first trimester compared to the rest of pregnancy.3,4 Therefore, these factors may place women at increased risk of TEC in the first trimester.
Pharmacology: mode of action
UFH binds to the enzyme inhibitor antithrombin III, the activated ATIII then inactivates thrombin and anti-factor Xa (Fig. 1).24 LMWH has a shorter glycosaminoglycan pentasaccharide structure compared to UFH and therefore only inhibits factor Xa.
Fig. 1.

Difference in mode of action of UFH and LMWH.
The therapeutic effects of UFH are monitored with activated partial thromboplastin time (aPTT) versus LMWH in which anti-Xa levels are used for monitoring. UFH has a half-life of one to two hours in comparison to LMWH, which has a half-life four times longer. This short half-life makes it the drug of choice prior to planned delivery. Furthermore, protamine sulphate can be used to reverse the effects of UFH. Protamine sulphate only partially reverses the effects of LMWH by about 70%.25
The main adverse events associated with UFH are heparininduced thrombocytopenia (HIT) and osteoporosis.24 These effects are uncommon with LMWH. HIT is a clinicopathological syndrome that occurs when heparin Ig G antibodies bind to the heparin/platelet 4 complexes, activating platelets and producing a hypercoagulable state. The immunological response also causes a consumptive coagulopathy, with the resultant thrombocytopenia. Treatment entails the use of an alternate anticoagulant class such as fondupurinax, a synthetic anti-Xa inhibitor.
The most common VKA used in South Africa is Coumarin, trade name warfarin (Fig. 2) Warfarin competitively inhibits the vitamin K epoxide reductase complex 1 (VKORC1). This results in a reduction of the vitamin K reserves needed for the hepatic synthesis of the vitamin K-dependent clotting factors II, VII, IX and X, as well as coagulation regulatory protein C and protein S, natural anticoagulants.3
Fig. 2.

Mode of action of warfarin.
Warfarin may be pro-thrombotic when first initiated.26 Hence the drug is generally bridged with either LMWH or UFH. Absorption is rapid and complete, with an onset of action in 24 to 72 hours, peak plasma concentration after four hours and peak therapeutic effect seen on day five or seven after initiation. The drug is highly protein bound, metabolised by the liver and excreted by the kidneys. The probability of bleeding increases with certain polymorphic variants of the cytochrome P450 enzyme,27 as well as genomic variants in VKORC that reduce warfarin clearance.28,29 Warfarin is associated with bleeding that can be mild, such as skin bruising, epistaxis and bleeding gums, to severe intracranial or gastrointestinal bleeding.
Rare cases of purple-toe syndrome and skin necrosis can occur due to the acquired protein C deficiency. This may be exacerbated in individuals with an inherent protein C deficiency.26 Calciphylaxis or calcium uraemic arteriolopathy is another rare adverse event that can occur with end-stage renal disease.
Regimens: one size does not fit all, why?
A delicate balance between maternal benefit and risk versus foetal benefit and risk should be considered. Current evidence recognises inherent risks and benefits to each regimen for both mother and foetus, with VKA having greater maternal benefit and the heparins favouring the foetus.
Common regimens used include:
VKA throughout pregnancy (ideally low-dose < 5 mg/day).
Sequential therapy with switching to IV UFH heparin or dose-adjusted LMWH in the first trimester and transitioning back to warfarin in the second (from 13 weeks) and early third trimester, followed by bridging to UFH at 36 weeks in preparation for planned delivery.
Switching to dose-adjusted LMWH in the first trimester, continuing LMWH (with strict anti-Xa monitoring) in the second and third trimester, with bridging to IV UFH at 36 hours prior to planned delivery.
UFH throughout pregnancy (rarely used).
Role of low-dose warfarin
The meta-analysis by Steinberg et al. showed more live births and fewer foetal anomalies with low-dose VKA < 5 mg versus VKA > 5 mg.1 Their results support the recommendation by the ACC/AHA for the use of low-dose warfarin < 5 mg in the background of optimal INR levels over sequential therapy with either UFH or LMWH in the first trimester.11
This was supported by a meta-analysis by Xu et al. in 2015 as well as the ESC guidelines that recommend that women on low-dose warfarin can be counselled on continuing warfarin in the first trimester if the dosage is < 5 mg.9,30 They do however clarify that the mother should be aware that the risk of embryopathy and foetopathy still exists and they should therefore be given all options, shared decision making should be offered, with full informed consent if low-dose warfarin is to be continued in the first trimester.9
Similarly, D’Souza et al. found a twice as high rate of live births and a five times lower rate of adverse events with warfarin doses < 5 mg.31 However, they felt this data should be interpreted with caution based on the very small numbers; 10 studies (312 pregnancies) on < 5 mg and five studies (121 pregnancies) on > 5 mg.
Recent data from the ROPAC share a similar sentiment.24,25 They considered the studies to be unclear on whether the adverse events were related to the anticoagulation or not. Also, with increasing pregnancy, the dose of VKA may increase due to the pharmacodynamic changes that occur with advancing pregnancy. It is prudent to note that the safety of first-trimester low-dose VKA < 5 mg remains unconfirmed and requires more study. Therefore, it is imperative that all options, risks and benefits are discussed with the mother.
Role of low-dose aspirin
Another gap in the literature is the recent advocacy of aspirin (acetylsalicylic acid, ASA) in the second and third trimesters in women with prosthetic valves.32 ROPAC reported only a 6.1% concomitant use of ASA.33 The addition of low-dose ASA after the first trimester is supported by the American College of Clinical Pharmacy, the ACC and AHA.11,32 The data are deficient on the safety and its effect on the mother and foetus. More prospective data are needed to guide the professional bodies.
What do the international guidelines say?
A review of the latest international guidelines from the American College of Chest Physicians (ACCP), European Society of Cardiology (ESC) and the ACC/AHA revealed the following consensus statements based on expert opinion (Fig. 3). All the experts are in agreement that pregnant women with MHVs are at high risk of morbidity, with maternal mortality rates of > 1%. These risks are commonly associated with poor compliance, inadequate monitoring and incorrect dosing or administration. Women should be counselled adequately and they should understand the inherent risks and benefits to each regimen. The guideline recommendations are based on consensus statements from expert opinion or based on small retrospective studies or registries. Overall data in pregnancy are limited and there is an urgent need for more prospective studies to guide current practice. The authors present the following regimens for anticoagulation in pregnant women with MHV, currently practiced at Inkosi Albert Luthuli Central Hospital (Fig. 4). These regimens are based on the available evidence, recent international guidelines and a long history of clinical experience and management of pregnant women with MHV.
Fig. 3.

What the international guidelines say.
Systematic reviews and meta-analysis
A recent systematic review and meta-analysis by D’Souza et al. in 2016 reviewed 1 786 published articles, which correlated to about 2 468 pregnancies and 1 874 women.31 Only 46 titles were eligible and included in the final analysis.31 This was mainly due to widespread heterogeneity and strict inclusion criteria. The authors further noted that a serious limitation of the metaanalysis was the high degree of bias between studies.31
In this analysis, almost 30% of the studies reported the older ball-and-cage type valves.31 Contemporary practice has advocated a move away from the highly thrombogenic ball-andcage valves to the newer single tilting or bi-leaflet type.34 The Starr Edwards ball-in-a-cage valves are now obsolete and production was stopped in 2007.34 It is to be noted that over half a million of these valves were implanted from 1960 to 2007, with about 300 000 implanted in the last seven years of its production.34 The main problem with this valve was the formation of pannus and the inability to accurately measure gradients due to its unique non-central flow.
The meta-analysis further failed to risk-stratify patients in terms of valve type, primary valvular disease and valve position. Only 26% of studies highlighted whether the primary valvular pathology was rheumatic or congenital in origin.33 Few studies used UFH and LMWH.33 This reflects a deficiency in the literature, as the heparins confer a safety benefit to the foetus since it does not cross the placenta.
D’Souza et al. reported a TEC risk in pregnant women on VKA as only 2.7% (1.4–4.0).31 This risk increased to 5.8 % (3.8– 7.7) in women using sequential therapy, 8.7% (3.9–13.4) in women on LMWH and 11.1% (2.8–19.6) in women on UFH.33 With VKAs, sequential treatment and LMWH, maternal mortality occurred in 0.9% (0.4–1.4), 2.0% (0.8–3.1) and 2.9% (0.2–5.7), respectively, live births in 64.5% (48.8–80.2), 79.9% (74.3–85.6) and 92.0% (86.1–98.0), respectively, and anticoagulant-related foetal/neonatal adverse events (embryopathy or foetopathy) in 2.0% (0.3–3.7), 1.4% (0.3–2.5) and 0%, respectively.31
Steinberg et al. conducted a systematic review and metaanalysis in 2016 with the goal of including studies using adjusted doses for UFH and LMWH, dependent on aPTT and anti-Xa targets, respectively.1 It is now well known that the use of LMWH without monitoring can lead to catastrophic TEC.1 Furthermore they wanted to reflect a more contemporary population and excluded studies with > 10% use of the older, more thrombogenic ball-and-cage prostheses.1
They further attempted to elucidate whether foetal risk with warfarin is dose dependent.1 Previous studies with low-dose warfarin < 5 mg showed warfarin to be less teratogenic in comparison to higher doses. Furthermore, they found no significant difference in foetal risk observed between women taking < 5 mg daily warfarin and those on a LMWH regimen (risk-adjusted ratio: 0.9; 95% CI: 0.3–2.4).1 In addition, they only included studies where the valve position was documented, and excluded the right-sided tricuspid and pulmonary valves.1 However, they did not capture data on primary valve pathology and the presence of RHD, which is of relevance in Africa and LMIC.
The meta-analysis focused on studies that shared a very similar geographical cluster and this may inadvertently have affected outcome data. Adverse events could not be stratified according to trimester as this information was not readily available. This is important as bridging with heparins normally occurs in the first trimester and in the two weeks prior to planned delivery (Fig. 4).
Fig. 4.
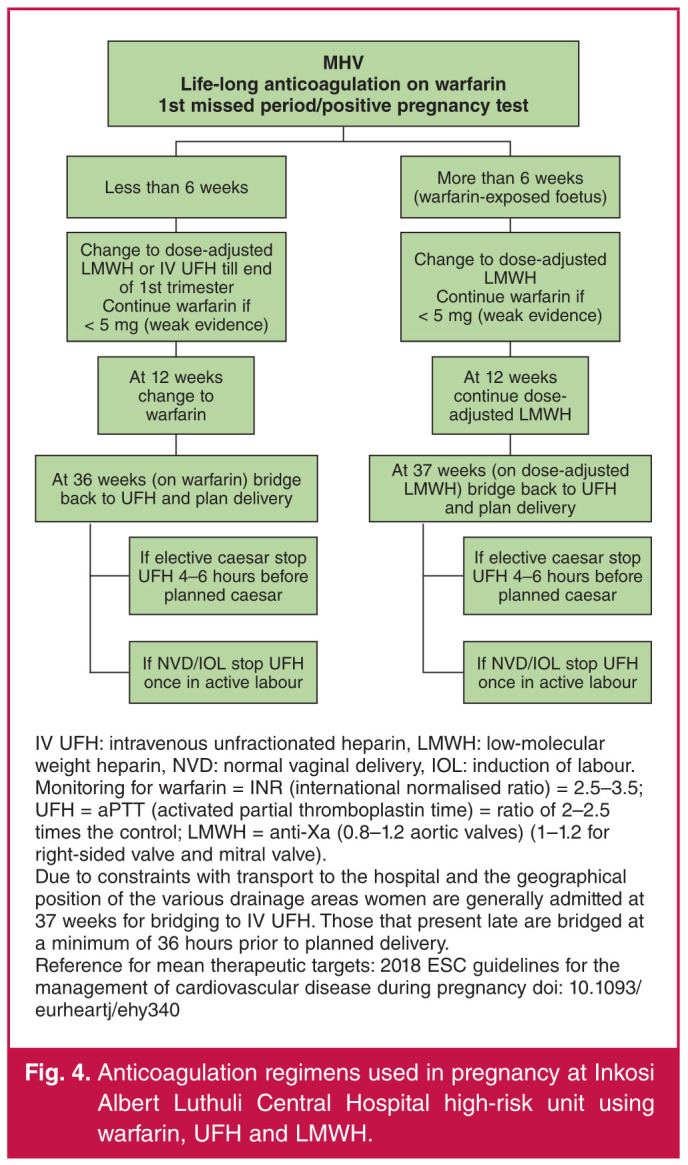
Anticoagulation regimens used in pregnancy at Inkosi Albert Luthuli Central Hospital high-risk unit using warfarin, UFH and LMWH.
A variability of 44% (95% CI: 18–82%) for averaged risk estimates of maternal composite and 81% (95% CI: 67–90%) for averaged risk estimates for foetal composite outcome was reported. This variability was the result of a significant degree of heterogeneity between studies. Of interest is that this metaanalysis reported a much lower risk of adverse maternal outcome with dose-adjusted LMWH than previously reported.
Both these meta-analyses showed that VKA had the fewest maternal complications but also the fewest live births. In contrast, LMWH was associated with more live births but an increase in maternal complications.
Author recommendations
The authors’ current practice is in line with some of the discussions above, and is as summarised in Figs 5 and 6.
Fig. 5.

Author recommendations for anticoagulation in pregnant women with mechanical heart valves at Inkosi Albert Luthuli Central Durban, South Africa.
Fig. 6.

Complications that can occur in the antenatal and peripartum periods.
A basic unit protocol for anticoagulation in pregnant women with MHV provides a good basis to direct management and provide women with the best available options. These recommendations can be adjusted to suit patient preferences if safe to do so, and to guide treatment in cases where patient risk may be complicated by other co-morbid conditions. The unit protocol is an effective tool but is not limited; instead, individual patient preferences after full counselling, past medical history, the background history of the valvular pathology and complications associated with valve replacement (such as history of stuck valves or TEC in the past) are all considered before a regimen is chosen.
Conclusion
It is evident that there are significant gaps in the literature. The current body of evidence is lacking and requires closer scrutiny to provide these women with the best evidence-based approach. However, there is a wealth of knowledge in the form of expert opinion dealing with these cases. This highlights the need to incorporate scientific research into our clinical practice through formal studies or audits, retrospective reviews and/or prospective ongoing collection of new data.
Pregnant women with prosthetic valves represent a vulnerable group at risk for significant morbidity and mortality. When deciding on anticoagulation regimens, the balance between maternal and foetal benefit needs to be questioned throughout the management of these women. Furthermore, all risks and benefits must be carefully explained to ensure shared decision making.
The above guideline recommendations are based on consensus statements from expert opinion or based on small retrospective studies or registries. Overall data in pregnancy are limited and there is an urgent need for more prospective studies to guide current practice.
A basic unit protocol for anticoagulation in pregnant women with MHV provides a good basis to direct management and provide women with the best available options. These recommendations can be adjusted to suit patient preferences if safe to do so, and to guide treatment in cases where patient risk may be complicated by other co-morbid conditions. The unit protocol is an effective tool but is not limited; instead, individual patient preferences after full counselling, past medical history, the background history of the valvular pathology and complications associated with valve replacement (such as history of stuck valves or TEC in the past) are all considered before a regimen is chosen.
Conclusion
It is evident that there are significant gaps in the literature. The current body of evidence is lacking and requires closer scrutiny to provide these women with the best evidence-based approach. However, there is a wealth of knowledge in the form of expert opinion dealing with these cases. This highlights the need to incorporate scientific research into our clinical practice through formal studies or audits, retrospective reviews and/or prospective ongoing collection of new data.
Pregnant women with prosthetic valves represent a vulnerable group at risk for significant morbidity and mortality. When deciding on anticoagulation regimens, the balance between maternal and foetal benefit needs to be questioned throughout the management of these women. Furthermore, all risks and benefits must be carefully explained to ensure shared decision making.
The above guideline recommendations are based on consensus statements from expert opinion or based on small retrospective studies or registries. Overall data in pregnancy are limited and there is an urgent need for more prospective studies to guide current practice.
Key message.
|
Contributor Information
M Jenneker, Email: jennekerm@ukzn.ac.za.
H Ramnarain, Department of Obstetrics and Gynaecology, School of Clinical Medicine, College of Health Sciences, University of KwaZulu-Natal, Durban, South Africa.
H Sebitloane, Department of Obstetrics and Gynaecology, School of Clinical Medicine, College of Health Sciences, University of KwaZulu-Natal, Durban, South Africa.
M Jenneker, Email: jennekerm@ukzn.ac.za.
References
- 1.Steinberg ZL, Dominguez-Islas CP, Otto CM, Stout KK. Maternal and fetal outcomes of anticoagulation in pregnant women with mechanical heart valves. J Am Coll Cardiol. 2017;69:22. doi: 10.1016/j.jacc.2017.03.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kujovich JL. Hormones and pregnancy: thromboembolic risks for women. Br J Haematol. 2004;126:443–454. doi: 10.1111/j.1365-2141.2004.05041.x. [DOI] [PubMed] [Google Scholar]
- 3.Leiria TLL, Lopes RD, Williams JD, Katz JN, Kalil RAK, Alexander JH. Antithrombotic therapies in patients with prosthetic heart valves: guidelines translated for the clinician. J Thromb Thrombolysis. 2011;31(4):514–522. doi: 10.1007/s11239-011-0574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ginsberg JS, Chan WS, Bates SM, Kaatz SK. Anticoagulation of pregnant women with mechanical hearts valves. Arch Intern Med. 2003;163:694–698. doi: 10.1001/archinte.163.6.694. [DOI] [PubMed] [Google Scholar]
- 5.Mocoumbi AO, Jamal KKF, Mbakwem A, Shung-King M, Sliwa K. The Pan- African Society of Cardiology position paper on reproductive healthcare for women with rheumatic heart disease. Cardiovasc J Afr. 2018;29:1–9. doi: 10.5830/CVJA-2018-044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sliwa K, Libhaber E, Elliot C, Momberg Z. et al. Spectrum of cardiac disease in maternity in low resource cohort in South Africa. Heart. 2014;100:1967–1974. doi: 10.1136/heartjnl-2014-306199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lumsden R, Barasa F, Park LP. et al. High burden of cardiac disease in pregnancy at a national referral hospital in western Kenya. Glob Heart. 2020;15:1. doi: 10.5334/gh.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pibarot P, Dumesnil JG. Prosthetic heart valves selection of the optimal prosthesis and long-term management. Circulation. 2009;119:1034–1048. doi: 10.1161/CIRCULATIONAHA.108.778886. [DOI] [PubMed] [Google Scholar]
- 9.ESC Guidelines for the management of cardiovascular diseases during pregnancy: Taskforce for the management of cardiovascular disease in pregnancy. Eur Heart J. 2018;39:3165–3241. doi: 10.1093/eurheartj/ehy340. [DOI] [PubMed] [Google Scholar]
- 10.Jeejeebhoy F. Prosthetic heart valves and management during pregnancy. Can Fam Phys. 2009;55:155–157. [PMC free article] [PubMed] [Google Scholar]
- 11.Otto C, Nishimura RA, Bonow R. et al. ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e72–e227. doi: 10.1161/CIR.0000000000000923. [DOI] [PubMed] [Google Scholar]
- 12.Steinberg Z, Stout K, Cheng E, Krieger E, Easterling T. Anticoagulation management for pregnant women with mechanical heart valves. University of Washington Obstetric Consensus Conference, September 2018 [Google Scholar]
- 13.Pradhan A, Gupta V, Vishwakarma P. Prosthetic heart valves and pregnancy: challenges and solutions. Indian J Cardivasc Dis Women. 2018;3:115–125. [Google Scholar]
- 14.Scherman J, Mangayi R, Humal P, Pennel T. et al. Isolated mechanical aortic valve replacememt in rheumatic patients in a low- to middlencome country. J Thorac Cardiovasc Surg. 2019;157:886–893. doi: 10.1016/j.jtcvs.2018.06.083. [DOI] [PubMed] [Google Scholar]
- 15.Alshawabkeh L, Economy KE, Valente AM. Anticoagulation during pregnancy. Evolving strategies with a focus on mechanical valves. J Am Coll Cardiol. 2016;68(16):1804–1813. doi: 10.1016/j.jacc.2016.06.076. [DOI] [PubMed] [Google Scholar]
- 16.Chan WS, Anand S, Ginsberg JS. Anticoagulation of pregnant women with mechanical heart valves: a systematic review of the literature. Arch Int Med. 2000;160:191–196. doi: 10.1001/archinte.160.2.191. [DOI] [PubMed] [Google Scholar]
- 17.Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom- Lundqvist C. et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 18.Bitar YdSL, Duraes AR, Roever L, Gomes Neto M, Lins-Kusterer L, Bocchi EA. Comparison of the direct oral anticoagulants and warfarin in patients with atrial fibrillation and valvular heart disease: updated systematic review and meta-analysis of randomized controlled trials. Front Cardiovasc Med. 2021;8:712585. doi: 10.3389/fcvm.2021.712585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maegdefessel L, Linde T, Krapiec F, Hamilton K, Steinseifer U, van Ryn J. et al. comparison of dabigatran, unfractionated heparin, and low-molecular-weight heparin in preventing thrombus formation on mechanical heart valves. In vitro. Thromb Res. 2010;126:e196–200. doi: 10.1016/j.thromres.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Lamejjer H, Aalberts JJ, van Veldhuisen DJ, Meijer K, Pieper PG. Efficacy and safety of direct oral anticoagulants during pregnancy; a systematic literature review. J Thrombres. 2018;169:123–127. doi: 10.1016/j.thromres.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 21.Beyer-Westendorf J, Naue C, Tittl L, Martin S. Outcome of DOAC exposure during pregnancy (…and the problem of event reporting…). Res Pract Thromb Haemost. 2020;4(Suppl 1) [Google Scholar]
- 22.Goland S, Schwartzenberg S, Fan J, Kozak N, Khatri N, Elkayam U. Monitoring of anti-Xa in pregnant patients with mechanical prosthetic valves receiving low molecular-weight heparin: Peak or trough levels? J Cardiovasc Pharmacol Ther. 2014;19:451–456. doi: 10.1177/1074248414524302. [DOI] [PubMed] [Google Scholar]
- 23.Beresheim M, Wilkie J, Nerenberg KA. et al. A case series of LMWH use in pregnancy: should trough anti Xa levels guide dosing? Thromb Res. 2014;134:1234–1240. doi: 10.1016/j.thromres.2014.09.033. [DOI] [PubMed] [Google Scholar]
- 24.Hirsh J, Anand SS, Halperin JL, Fuster V. Mechanism of action and pharmacology of unfractionated heparin. Arterioscler Thromb Vasc Biol. 2001;21:1094–1096. doi: 10.1161/hq0701.093686. [DOI] [PubMed] [Google Scholar]
- 25.Lauer BR, Nelson RA, Adamski JH, Gibbons J, Janko MR, Ravi G. et al. Protamine sulfate for the reversal of enoxaparin associated hemorrhage beyond 12 hours. Am J Emerg Med. 2019;37(1):174.e5–174.e6. doi: 10.1016/j.ajem.2018.09.043. [DOI] [PubMed] [Google Scholar]
- 26.Wali M, Latif MT, Lockwood M, Saeyeldin A, Borz-Bab C. Warfarininduced skin necrosis despite enoxaparin bridging therapy. Cureus. 2022;14(1):e20883. doi: 10.7759/cureus.20883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwarz UI, Ritchie MD, Bradford Y. et al. Genetic determinants of response to warfarin during initial anticoagulation. N Engl J Med. 2008;358:999–1008. doi: 10.1056/NEJMoa0708078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rieder MJ, Reiner AP, Gage BF. et al. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med. 2005;352:2285–2293. doi: 10.1056/NEJMoa044503. [DOI] [PubMed] [Google Scholar]
- 29.Cadamuro J, Dieplinger B, Felder T. et al. Genetic determinants of acenocoumarol and phenprocoumon maintenance dose requirements. Eur J Clin Pharmacol. 2010;66:253–260. doi: 10.1007/s00228-009-0768-7. [DOI] [PubMed] [Google Scholar]
- 30.Xu Z, Fan J, Luo X, Zhang WB, Ma J, Lin YB. et al. Anticoagulation regimens during pregnancy in patients with mechanical heart valves: a systematic review and meta-analysis. Can J Cardiol. 2016;32(10):1248.e1–1248.e9. doi: 10.1016/j.cjca.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 31.D’Souza R, Ostro J, Shah PS. et al. Anticogulation for pregnant women with mechanical heart valves: a systematic review and meta-analysis. Euroheart J. 2017;38:1509–1516. doi: 10.1093/eurheartj/ehx032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guyatt GH, Akl EA, Crowther M, Schunemann HJ, Gutterman DD, Lewis SZ. Introduction to the ninth edition: Antithrombotic therapy and prevention of thrombosis. 9th ed. American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):48S–52SS. doi: 10.1378/chest.11-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Hagen IM, Roos-Hesselink JW, Ruys TP. et al. Pregnancy in women with a mechanical heart valve: data of the European Society of Cardiology Registry of Pregnancy and Cardiac Disease (ROPAC). Circulation. 2015;132:132–142. doi: 10.1161/CIRCULATIONAHA.115.015242. [DOI] [PubMed] [Google Scholar]
- 34.Chan WS, Anand S, Ginsberg JS. Anticoagulation of pregnant women with mechanical heart valves: a systematic review of the literature. Arch Int Med. 2000;160:191–196. doi: 10.1001/archinte.160.2.191. [DOI] [PubMed] [Google Scholar]


