Abstract
Exposure to certain chemicals in the environment may contribute to the risk of developing cancer. Although cancer risk from environmental chemical exposure among general populations is considered low compared to that in occupational settings, many people may nevertheless be chronically exposed to relatively low levels of environmental chemicals which vary by such various factors as residential area, lifestyle, and dietary habits. It is therefore necessary to assess population-specific exposure levels and examine their association with cancer risk. Here, we reviewed epidemiological evidence on cancer risk and exposure to dichlorodiphenyltrichloroethane (DDT), hexachlorocyclohexane (HCH), polychlorinated biphenyls (PCBs), per- and polyfluoroalkyl substances (PFASs), cadmium, arsenic, and acrylamide. Japanese are widely exposed to these chemicals, mainly through the diet, and an association with increased cancer risk is suspected. Epidemiological evidence from Japanese studies to date does not support a positive association between blood concentrations of DDT, HCH, PCBs, and PFASs and risk of breast or prostate cancer. We established assessment methods for dietary intake of cadmium, arsenic, and acrylamide using a food frequency questionnaire. Overall, dietary intakes of cadmium, arsenic, and acrylamide were not significantly associated with increased risk of total cancer and major cancer sites in the Japan Public Health Center-based Prospective Study. However, statistically significant positive associations were observed between dietary cadmium intake and risk of estrogen receptor-positive breast cancer among postmenopausal women, and dietary arsenic intake and risk of lung cancer among male smokers. In addition, studies using biomarkers as exposure assessment revealed statistically significant positive associations between urinary cadmium concentration and risk of breast cancer, and between ratio of hemoglobin adducts of acrylamide and glycidamide and risk of breast cancer. Epidemiological studies of general populations in Japan are limited and further evidence is required. In particular, studies of the association of organochlorine and organofluorine compounds with risk of cancer sites other than breast and prostate cancer are warranted, as are large prospective studies of the association between biomarkers of exposure and risk of cancer.
Keywords: Environmental chemicals, Dichlorodiphenyltrichloroethane, Hexachlorocyclohexane, Polychlorinated biphenyls, Per- and polyfluoroalkyl substances, Cadmium, Arsenic, Acrylamide, Cancer risk, Epidemiological study
Background
Environmental factors play an important role in the causation of a majority of human cancers. “Environmental factors” are generally recognized as everything that is not specifically genetic in origin, including tobacco smoking, alcohol consumption, diet and nutrition, infectious agents, radiation, sunlight, exposure to environmental chemicals, and so on. In Japan, the greatest contributing factors to cancer in 2015 were infectious agents and active tobacco smoking, followed by alcohol drinking [1]. Although attributable fraction might be smaller than for infectious agents and tobacco smoking, exposure to certain chemicals in the environment, at home, and at work may contribute to the risk of developing cancer [2]. Environmental chemicals refer to chemical compounds or chemical elements present in air, water, food, soil, dust, or other environmental media such as consumer products. The International Agency for Research on Cancer (IARC) has recognized a number of well-known environment pollutants as posing a carcinogenic hazard in humans, including indoor and outdoor air pollutants, contamination of drinking water by arsenic, and contaminants of soil and food such as dioxin and polychlorinated biphenyls (PCBs) [3–6]. In addition, a large number of environmental chemicals are suspected to be carcinogenic in humans, and further evidence is required [7].
Since the amounts of chemicals in air, water, food, and soil are typically much lower than those in the work environment, cancer risk from environmental chemical exposures among general populations is thought to be low compared to the risk in occupational settings. Nevertheless, even though exposure is likely low, large numbers of people are affected, in accordance with residential area, lifestyle, and dietary habits. It is therefore necessary to assess population-specific exposure levels and to examine their association with cancer risk.
Here, we review epidemiological studies among Japanese, focusing on environmental chemicals to which Japanese are widely exposed, mainly through diet, and are suspected of being associated with increased risk of cancer. This evidence is presented as ‘epidemiological evidence from general populations in Japan’. In addition, we briefly introduce epidemiological evidence commonly seen in Japanese exposed to chemical-contaminated foods or living in polluted areas as background information.
Organochlorine pesticides
Dichlorodiphenyltrichloroethane (DDT)
Background and overview
One abundant organochlorine contaminant is DDT. Introduced as an insecticide in the 1940s, DDT came into widespread use for insect control in forestry and agriculture and for vector control after World War II. Although most developed countries had banned its use by the early 1980s, some countries still use it for malaria control. Technical-grade DDT predominantly contains p,pʹ-DDT and smaller amounts of other compounds such as o,pʹ-DDT, p,pʹ-DDD (dichlorodiphenyldichloroethane) and o,pʹ-DDE (dichlorodiphenyldichloroethylene) [8, 9]. Being both highly lipophilic and resistant to degradation, it is bioaccumulated in the lipid component of biological systems through the food chain [8, 9], and human exposure to DDT and to its metabolite DDE still occurs, mainly through the diet. Indeed, it was widely used in Japan following World War II until the beginning of the 1980s, and residue is still detected in the blood among Japanese [10].
DDT is known as an endocrine disruptor which modulates receptor-mediated effects that can operate in humans [11–13]. Estrogenic effects of o,pʹ-DDT and p,pʹ-DDT, such as binding and activation of estrogen receptor, have been consistently seen across numerous experimental studies [12, 13]. DDT and its metabolites antagonize the androgen receptor, with p,pʹ-DDE being the most potent, and this effect is seen consistently across non-human experimental studies in vivo and in cells from a variety of species, including humans [11]. In addition, there is strong mechanistic evidence that DDT exerts immunosuppression and induces oxidative stress [8]. Accordingly, breast cancer is the most studied cancer site, and more than 40 epidemiological studies have been reported [14]. Interestingly, however, these have shown no association overall between p,pʹ-DDE or p,pʹ-DDT concentration in blood or adipose tissue and breast cancer risk [14]. For other cancer sites, positive associations have been suggested for liver and testicular cancer and non-Hodgkin lymphoma [15, 16]. IARC evaluated the evidence regarding the carcinogenicity of DDT in humans as limited [8, 17] but, considered together with sufficient evidence for the carcinogenicity of DDT in experimental animals, gave an overall evaluation of probably carcinogenic to humans (Group 2A) in 2015 (Table 1) [8, 17].
Table 1.
Evaluation of carcinogenic risks to humans by the International Agency for Research on Cancer
| Agent | Overall evaluation | Tumour sites (or types) for which there is sufficient evidence in humans | Other sites with limited evidence in humans |
|---|---|---|---|
| Dichlorodiphenyltrichloroethane | Group 2A | Liver, testis, non-Hodgkin lymphoma | |
| Lindane, the γ-isomer of hexachlorocyclohexane | Group 1 | Non-Hodgkin lymphoma | |
| Polychlorinated biphenyls | Group 1 | Malignant melanoma | Breast, non-Hodgkin lymphoma |
| Perfluorooctanoic acid | Group 2B | Testis, kidney | |
| Cadmium and cadmium compounds | Group 1 | Lung | Prostate, kidney |
| Arsenic and inorganic arsenic compounds | Group 1 | Lung, skin, urinary bladder | Kidney, liver, prostate |
| Acrylamide | Group 2A |
Epidemiological evidence from general populations in Japan
Among epidemiological studies in Japanese, we reported two studies for breast cancer and one for prostate cancer (Table 2) [18–20]. In a nested case–control study within the Japan Public Health Center-based Prospective Study (JPHC Study), measurement of plasma samples from 139 breast cancer cases and 278 controls collected from 1990–1994 found no statistically significant association for p,pʹ-DDT and p,pʹ-DDE (Table 2) [18]. A stratified analysis by menopausal status showed positive associations in premenopausal but not postmenopausal women, albeit without statistical significance. The second study for breast cancer was a hospital-based case–control study in Nagano Prefecture, Japan, which included 403 pairs recruited from 2001–2005 [19]. No statistically significant association was found for o,pʹ-DDT, p,pʹ-DDT, or p,pʹ-DDE regardless of menopausal status or hormone receptor subtype (Table 2). These findings are in general agreement with a majority of previous studies and meta-analyses including more than 40 studies [14, 21]. This suggests that exposure to DDT during adult life within the range detectable in general populations is not associated with risk of breast cancer. Of interest, however, Cohn et al. reported that higher concentration of p,pʹ-DDT during pregnancy was significantly associated with increased risk of breast cancer before age 50 years, with the effect substantially stronger in women exposed before puberty, and strongest for exposure in utero or infancy [22]. On this basis, the possible importance of early-life exposure to DDT has been proposed.
Table 2.
Summary of epidemiological studies on the association of organochlorines and cancer risk among Japanese
| Reference | Study period | Outcome | Design | Study subjects | Exposure assessment | Exposure | Exposure level (median or range among control group) | Category | Odds ratios | 95% confidence interval | P for trend | Confounding factors | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Definition | Age range | Number of subjects | ||||||||||||
| Iwasaki et al. [18] |
Enrolment 1990–1994, follow-up to 2002 |
Breast cancer |
Nested case– control study |
Cases: identified from local hospitals, population-based cancer registries and death certificates, Controls: cohort members matched by age, public health centre area, area, date of blood collection, time of day of blood collection, fasting time at blood collection and menopausal status |
40–69 years old at enrolment | 139 cases and 278 controls | Plasma samples measured by a gas-chromatography isotope-dilution massspectrometry (GC-IDMS) | p,pʹ-DDT (ng/mL) | 0.50 | Quartile 1 | 1 | 0.97 | Adjusted for age at menarche, menopausal status and age at menopause, number of births, age at first birth, height, body mass index, and alcohol consumption | |
| 0.89 | Quartile 2 | 0.65 | (0.32–1.32) | |||||||||||
| 1.36 | Quartile 3 | 0.56 | (0.26–1.22) | |||||||||||
| 2.24 | Quartile 4 | 0.99 | (0.47–2.08) | |||||||||||
| p,pʹ-DDE (ng/mL) | 2.50 | Quartile 1 | 1 | 0.25 | ||||||||||
| 4.83 | Quartile 2 | 1.01 | (0.47–2.19) | |||||||||||
| 7.58 | Quartile 3 | 1.24 | (0.60–2.53) | |||||||||||
| 14.41 | Quartile 4 | 1.48 | (0.70–3.13) | |||||||||||
| β-HCH (ng/mL) | Not detected | Quartile 1 | 1 | 0.34 | ||||||||||
| Not detected | Quartile 2 | 1 | ||||||||||||
| 0.69 | Quartile 3 | 0.84 | (0.45–1.57) | |||||||||||
| 1.38 | Quartile 4 | 0.74 | (0.39–1.39) | |||||||||||
| Itoh et al. [19] | Enrolment 2001–2005 | Breast cancer | Hospital-based case–control | Cases: histopathologically confirmed, Controls: medical check-up examinees matched by age and area of residence | 20–74 years old | 403 cases and 403 controls | Serum samples measured by gas chromatography-isotope-dilution high resolution mass spectrometry (GC-ID-HRMS) | o,pʹ-DDT (ng/g lipid) | 0.90 | Quartile 1 | 1 | 0.48 | Adjusted for total lipid concentration in serum, bodymass index, menopausal status and age at menopause, smoking status, fish consumption, vegetable consumption, family history of breast cancer in a first-degree relative, age at first childbirth, parity, age at menarche, history of breast cancer screening, and history of breast feeding | |
| 1.3 | Quartile 2 | 0.57 | (0.25–1.29) | |||||||||||
| 2.0 | Quartile 3 | 1.13 | (0.53–2.38) | |||||||||||
| 4.1 | Quartile 4 | 0.67 | (0.30–1.52) | |||||||||||
| p,pʹ-DDT (ng/g lipid) | 5.6 | Quartile 1 | 1 | 0.33 | ||||||||||
| 8.5 | Quartile 2 | 0.58 | (0.27–1.25) | |||||||||||
| 12.0 | Quartile 3 | 0.99 | (0.47–2.07) | |||||||||||
| 23.0 | Quartile 4 | 0.58 | (0.27–1.25) | |||||||||||
| p,pʹ-DDE (ng/g lipid) | 160 | Quartile 1 | 1 | 0.46 | ||||||||||
| 300 | Quartile 2 | 0.47 | (0.24–0.92) | |||||||||||
| 490 | Quartile 3 | 0.99 | (0.48–2.02) | |||||||||||
| 1100 | Quartile 4 | 1.02 | (0.46–2.26) | |||||||||||
| β-HCH (ng/g lipid) | 26 | Quartile 1 | 1 | 0.63 | ||||||||||
| 52 | Quartile 2 | 0.81 | (0.39–1.72) | |||||||||||
| 82 | Quartile 3 | 0.72 | (0.31–1.69) | |||||||||||
| 160 | Quartile 4 | 1.04 | (0.43–2.52) | |||||||||||
| Total PCBs (ng/g lipid) | 110 | Quartile 1 | 1 | 0.008 | ||||||||||
| 160 | Quartile 2 | 0.79 | (0.36–1.72) | |||||||||||
| 200 | Quartile 3 | 0.57 | (0.28–1.15) | |||||||||||
| 290 | Quartile 4 | 0.33 | (0.14–0.78) | |||||||||||
| Sawada et al. [20] |
Enrolment 1990–1994, follow-up to 2005 |
Prostate cancer |
Nested case– control study |
Cases: identified from local hospitals, population-based cancer registries and death certificates, Controls: cohort members matched by age, public health centre area, area, date of blood collection, time of day of blood collection, and fasting time at blood collection |
40–69 years old at enrolment | 201 cases and 402 controls | Plasma samples measured by gas chromatography-isotope-dilution high resolution mass spectrometry (GC-ID-HRMS) | o,pʹ-DDT (ng/g lipid) | < 2.5 | Quartile 1 | 1 | 0.61 | Adjusted for smoking status, alcohol intake, marital status, body mass index, and intake of green tea and miso soup | |
| 2.5–4.2 | Quartile 2 | 1.39 | (0.79–2.44) | |||||||||||
| 4.3–7.6 | Quartile 3 | 1.29 | (0.71–2.34) | |||||||||||
| 7.7 | Quartile 4 | 1.04 | (0.54–2.03) | |||||||||||
| p,pʹ-DDT (ng/g lipid) | < 24 | Quartile 1 | 1 | 0.45 | ||||||||||
| 24–40 | Quartile 2 | 1.51 | (0.87–2.63) | |||||||||||
| 41–63 | Quartile 3 | 0.92 | (0.50–1.70) | |||||||||||
| 64 | Quartile 4 | 1.00 | (0.52–1.92) | |||||||||||
| p,pʹ-DDE (ng/g lipid) | < 560 | Quartile 1 | 1 | 0.65 | ||||||||||
| 560–939 | Quartile 2 | 1.00 | (0.60–1.66) | |||||||||||
| 940–1599 | Quartile 3 | 0.89 | (0.52–1.53) | |||||||||||
| 1600 | Quartile 4 | 0.90 | (0.52–1.54) | |||||||||||
| β-HCH (ng/g lipid) | < 200 | Quartile 1 | 1 | 0.05 | ||||||||||
| 200–319 | Quartile 2 | 0.89 | (0.52–1.50) | |||||||||||
| 320–519 | Quartile 3 | 0.85 | (0.50–1.46) | |||||||||||
| 520 | Quartile 4 | 0.56 | (0.30–1.01) | |||||||||||
| Total PCBs (ng/g lipid) | < 319 | Quartile 1 | 1 | 0.90 | ||||||||||
| 319–447 | Quartile 2 | 1.06 | (0.63–1.79) | |||||||||||
| 448–668 | Quartile 3 | 0.84 | (0.49–1.46) | |||||||||||
| 669 | Quartile 4 | 0.97 | (0.51–1.87) | |||||||||||
DDT Dichlorodiphenyltrichloroethane, DDE Dichlorodiphenyldichloroethylene, HCH Hexachlorocyclohexane, PCBs Polychlorinated biphenyls
We also conducted a nested case–control study of prostate cancer within the JPHC Study, using plasma samples collected from 201 cases and 402 matched controls between 1990 and 1994 [20]. No statistically significant association was seen for o,pʹ-DDT, p,pʹ-DDT, or p,pʹ-DDE regardless of stage at diagnosis of prostate cancer (localized or advanced) (Table 2). This result is consistent with a meta-analysis based on studies that measured serum DDT concentration in the general population—although a positive association was found in studies based on occupational exposure, it was not statistically significant [23]. Similarly to the case with breast cancer, this indicates no elevated risk of prostate cancer within the range detectable among general populations.
Hexachlorocyclohexane (HCH)
Background and overview
Lindane, the γ-isomer of HCH, has been extensively used for insect control in agriculture and for treatment of human ectoparasites. Technical-grade HCH, containing approximately 60–70% α-HCH, 5–12% β-HCH, 10–40% γ-HCH, 6–10% δ-HCH, and 3–4% ε-HCH, has reportedly been used as an insecticide although only γ-HCH has insecticidal properties [8]. Technical-grade HCH was banned for production and use in the United States in 1976, but may still be used in other countries in small quantities [24]. Although worldwide use of HCH declines, it has been measured in food, air, surface water, groundwater, sediment, soil, fish, wildlife, and humans as a consequence of biological persistence. Relatively many epidemiological studies have provided consistent evidence of a positive association between mostly occupational exposure to lindane and the risk of non-Hodgkin lymphoma [15, 16], and it was consequently evaluated as carcinogenic to humans (Group 1) in 2015 (Table 1) [8, 17]. Meanwhile, several epidemiological studies of the association of lindane or HCH isomers (β-HCH) measured in blood with the risk of breast, prostate, or testicular cancer reported inconsistent results [8, 23]. Although the blood concentration of β-HCH might be a surrogate marker of exposure to lindane, exposure to β-HCH can occur through the diet and through contact with other environment media. The blood concentration of β-HCH might therefore not reflect exposure to lindane, and this is likely one reason for these inconsistent findings.
Epidemiological evidence from general populations in Japan
We measured β-HCH concentration in plasma or serum samples in a nested case–control study for breast or prostate cancer within the JPHC Study and in a hospital-based case–control study in Nagano Prefecture, Japan, as described above (Table 2) [18–20]. Overall, β-HCH concentration was not significantly associated with increased risk of breast cancer in either study [18, 19], while an inverse association was found for prostate cancer, albeit without statistical significance [20].
PCBs
Background and overview
PCBs are a class of aromatic compounds comprising 209 congeners, each containing one to ten chlorine atoms attached to a biphenyl nucleus. They were predominantly used as dielectric fluids (in transformers and electric capacitors) and as additives for pesticides, flame retardants, insulators, paints, glues and printing inks [5, 25]. Similarly to organochlorine pesticides, they are now classified as persistent organic pollutants (POPs) and are ubiquitously present in the environment worldwide due to persistence and bioaccumulation, though their production was banned in most countries by the 1980s. Exposure in the general population today is mainly from eating contaminated foods or breathing contaminated air [5, 25].
PCBs or their metabolites have been shown to exert genotoxic effects, immune suppression, inflammatory responses, and endocrine effects via a number of mechanisms [5, 26–28]. Low-chlorinated PCBs involved in oxidative metabolism may produce oxidative stress and genotoxicity [28]. Meanwhile, highly chlorinated PCBs activate aryl hydrocarbon receptor (AhR) and the constitutive androstane and pregnane xenobiotic receptor (CAR/PXR). In particular, 12 PCB congeners that have a strong affinity for the AhR are referred to as “dioxin-like PCBs”. Activation of the AhR is one of the key events linked to carcinogenesis mediated by dioxin-like compounds [27]. In addition, they interact with nuclear steroid hormone receptor and can act as estrogen agonists or antagonists [26].
Based on studies documenting an increased risk of cutaneous malignant melanoma—mostly occupational cohort studies—PCBs were classified by IARC in 2013 as carcinogenic to humans (Group 1) (Table 1) [5, 29]. Positive associations were suggested for non-Hodgkin lymphoma and breast cancer, but evaluation found limited evidence for the carcinogenicity of PCBs in humans. Notable epidemiological evidence in Japan came from a cohort study of Yusho patients, who experienced a mass food poisoning incident from cooking oil accidentally contaminated with PCBs, polychlorinated dibenzofurans (PCDFs), and other dioxin-related compounds in 1968: a recent 50-year follow-up study of these patients showed a significant increase in standardized mortality ratio for all cancers, and for lung cancer in men and liver cancer in women [30]. Blood concentrations of some PCB congeners were approximately 3–4 times higher than in the normal control group even 35 years after exposure [31]. In addition to studies in subjects with relatively high exposure levels, future studies should also clarify the effect of exposure to PCBs on cancer risk at blood concentrations within the range detectable among general populations in Japan.
Epidemiological evidence from general populations in Japan
We have reported two studies in general populations in Japan (Table 2) [19, 20]. In the hospital-based case–control study in Nagano Prefecture, mentioned above, measurement of 41 PCB peaks showed a significant association of serum lipid-adjusted concentration of total PCBs with decreased risk of breast cancer (Table 2) [19]. An inverse association was observed regardless of hormone receptor subtype or menopausal status, and half of 34 individual congeners were associated with decreased risk of breast cancer. Moreover, selected PCB congeners were categorized into group 1A (weak phenobarbital inducers, estrogenic, not persistent), group 1B (weak phenobarbital inducers, persistent), group 2A (potentially antiestrogenic and immunotoxic, dioxin-like, moderately persistent), group 2B (potentially antiestrogenic and immunotoxic, limited dioxin activity, persistent), and group 3 (phenobarbital, cytochrome P450 1A [CYP1A] and cytochrome P450 2B [CYP2B] inducers, biologically persistent) according to their structural, biological and pharmacokinetics properties as proposed by Wolff et al. [26, 32]. Statistically significant inverse associations were observed for groups 1B, 2B, and 3, whereas no statistically significant association was seen for group 1A or 2A. Eventually, we did not observe a positive association between the serum concentration of PCBs and risk of breast cancer among Japanese women. According to a meta-analysis based on case–control or cohort studies using measured PCB concentration in biological samples, a statistically significant positive association was not found for total PCBs, but was found for groups 2 and 3 [33]. This is inconsistent with our Japanese study, which showed an overall inverse association. The reason for this discrepancy is unclear but might be partly explained by the difference in concentrations of individual congeners and their composition across studies.
The second study was the nested case–control study of prostate cancer within the JPHC Study mentioned above [20]. We measured 41 PCB peaks and found no statistically significant association of prostate cancer risk with total PCBs, individual PCBs, or for PCBs grouped according to the definition of Wolff et al. (Table 2) [32]. In addition, no statistically significant differences were seen for total PCBs according to stage at diagnosis (localized or advanced). Although few previous studies are available, their findings are inconsistent [34, 35]. For example, a nested case–control study within a population-based cohort from Norway found no statistically significant association for total PCBs and most individual PCBs, but did find a significant inverse association for PCB 44 [34]. In contrast, a case-cohort study within the Korean Cancer Prevention Study-II showed statistically significant positive associations for moderately or highly chlorinated PCBs, total PCBs, and group 3 by the definition of Wolff et al. [32, 35].
Per- and polyfluoroalkyl substances (PFASs)
Background and overview
PFASs are synthetic organofluorine chemical compounds that have been used since the 1950’s in a variety of industrial and commercial applications, such as firefighting foams, non-stick cookware, waterproof clothing, cosmetics, and paper coating used in food packaging [36, 37]. Due to extreme resistance to biodegradation, they are highly persistent in the environment and are classified as POPs. They can bioaccumulate in humans with serum elimination half-lives ranging from about 3 to 8 years and have been detected in blood among most populations as consequence of their widespread use [38, 39]. The major sources of exposure for most of the general population are contamination of drinking water; food, mainly seafood, including transfer of PFASs from food packaging; some consumer products; and household dust [36, 40, 41]. The production, use, import and export of perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA), and perfluorohexane sulfonate (PFHxS) have been controlled by the Stockholm Convention on Persistent Organic Pollutants since 2009, 2019, and 2022, respectively.
Of the thousands of PFASs currently in use, the two most studied are PFOS and PFOA, owing to their relatively higher environmental levels compared to other PFASs. At this point, only PFOA has been evaluated for carcinogenicity by IARC, which classified it as a possible human carcinogen (Group 2B) based on limited evidence for testicular and kidney cancer from human and animal studies, in addition to moderate evidence for carcinogenic mechanisms (Table 1) [42, 43]. This evaluation referred to epidemiological studies showing positive associations with the risk of testicular and kidney cancer among highly exposed subjects working or living near PFAS production plants [44–46]. Regarding the mechanism of PFOA carcinogenesis, some studies indicated that its induction of oxidative stress may induce indirect DNA damage, but that direct genotoxicity is unlikely [42]. Animal studies indicated a potential role of peroxisome proliferator-activated receptor alpha (PPARα) activation, which is a crucial factor in the regulation of lipid metabolism and inflammation [47]. Moreover, endocrine disrupting properties have been shown, and several studies have suggested estrogenic and anti-androgenic activities [48, 49].
Epidemiological evidence from general populations, including Japanese evidence
In addition to epidemiological studies among highly exposed populations, a population-based prospective cohort study in the US found a statistically significant positive association of prediagnostic serum PFOA concentration, which was comparable with the general population, with the risk of renal cell carcinoma [50]. Moreover, an increasing number of studies have examined associations with the risk of breast cancer, given that a potential mechanism of carcinogenicity is their endocrine disrupting properties [21]. Findings have been inconsistent, however: Mancini et al. reported a statistically significant positive association between serum PFOS concentration and risk of hormone receptor-positive breast cancer among non-occupationally exposed postmenopausal French women [51] while Hurley et al. observed no overall significant association between serum PFASs concentration and risk of breast cancer in a nested case–control study within the California Teachers Study, despite a statistically significant inverse association of serum perfluoroundecanoic acid (PFUnDA) and PFHxS concentration with risk of hormone receptor-negative breast cancer [52].
Recently, we measured serum concentrations of 20 PFAS congeners using samples from a hospital-based case–control study in Nagano Prefecture, Japan, and found a statistically significant inverse association of 17 of 20 PFAS congeners and risk of breast cancer [53]. Table 3 shows the results for total PFOS (n-PFOS, 1 m-PFOS, 3 m-PFOS, 4 m-PFOS, 5 m-PFOS, and 6 m-PFOS) and total PFOA (n-PFOA and 6 m-PFOA). Although we distinguished branched PFAS isomers from linear isomers (the prefix “n-” indicates a linear isomer, whereas a prefix starting with a number indicates a branched isomer), similar associations were observed (data not shown). Since only a few epidemiological studies have been reported to date, further accumulation of evidence is desirable [21].
Table 3.
Summary results for the association of per- and polyfluoroalkyl substances (PFASs) with cancer risk among Japanese
| Reference | Study period | Outcome | Design | Study subjects | Exposure assessment | Exposure | Exposure level (median or range among control group) | Category | Odds ratios | 95% confidence interval | P for trend | Confounding factors | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Definition | Age range | Number of subjects | ||||||||||||
| Itoh et al. [53] | Enrolment 2001–2005 | Breast cancer | Hospital-based case–control | Cases: histopathologically confirmed, Controls: medical check-up examinees matched by age and area of residence | 20–74 years old | 401 cases and 401 controls | Serum samples measured by in-port arylation gas chromatography-negative chemical ionization-mass spectrometry (GC-NCI-MS) | Total PFOS (ng/mL) | 7.63 | Quartile 1 | 1 | 0.0001 | Adjusted for body mass index, height, menopausal status and age at menopause, age at first childbirth, family history of breast cancer, smoking status, strenuous physical activity in the past five years, moderate physical activity in the past five years, age at menarche, number of births, breastfeeding duration, alcohol intake, isoflavone intake, education level, serum total concentrations of polychlorinated biphenyls, fish and shellfish intake, vegetable intake, and calendar year of blood sampling | |
| 12.2 | Quartile 2 | 0.38 | (0.18–0.82) | |||||||||||
| 16.27 | Quartile 3 | 0.31 | (0.14–0.69) | |||||||||||
| 24.67 | Quartile 4 | 0.15 | (0.06–0.39) | |||||||||||
| Total PFOA (ng/mL) | 3.18 | Quartile 1 | 1 | 0.001 | ||||||||||
| 4.71 | Quartile 2 | 0.37 | (0.19–0.73) | |||||||||||
| 6.46 | Quartile 3 | 0.39 | (0.18–0.84) | |||||||||||
| 9.31 | Quartile 4 | 0.20 | (0.08–0.51) | |||||||||||
| PFOS Perfluorooctane sulfonate, PFOA Perfluorooctanic acid | ||||||||||||||
Cadmium
Background and overview
Cadmium is found at low concentrations in the Earth's crust, mainly as the sulfide in zinc-containing mineral deposits, and is widely but sparsely distributed through natural activities, such as volcanic activity, weathering and erosion, and river transport. It has also been widely dispersed into the environment through mining, smelting and refining of nonferrous metals, industrial emissions, and incineration of municipal waste (especially cadmium-containing batteries and plastics). Other than occupational exposure, the major source of cadmium exposure in general populations is foods, in addition to tobacco smoking, since cadmium contained in soil and water can be taken up by certain crops and aquatic organisms and accumulate in the food chain [54].
Although a well-known health effect of chronic exposure to cadmium is renal dysfunction, IARC classified cadmium and cadmium compounds as carcinogenic to humans (Group 1) based on sufficient evidence for an increase in lung cancer risk, mostly from studies in occupational settings (Table 1) [3, 55]. Moreover, positive associations have been suggested for kidney and prostate cancer. Several mechanisms that potentially contribute to cadmium-induced carcinogenesis have been identified [3, 56–60]: cadmium alters DNA repair and tumor-suppressor proteins, leading to chromosomal damage and genomic instability [58, 59]; induces alterations in epigenetic and signal transduction processes, which may contribute to the deregulation of cell growth; and shows estrogenic properties in both in vitro and in vivo studies [56, 57].
Studies in cadmium-polluted areas in Japan showed that urinary excretion concentration of β2-microglobulin as a marker of cadmium toxicity was significantly associated with increased risk of cancer mortality, but the small number of cancer deaths did not allow further detailed analysis [61]. A study of the cadmium-polluted Jinzu River basin in Toyama, Japan, however, revealed significantly increased mortality risk for cancer from total, renal, and uterus among exposed women with proteinuria, glucosuria, and glucoproteinuria based on a review of historical records [62]. Meanwhile, the effect of cadmium exposure on cancer occurrence among Japanese in non-polluted areas has been of particular interest considering that mean cadmium level via food in a general population in Japan (26 µg/ day) [63] is higher than that in general populations in China (10 µg/day) [64] and Sweden (15 µg/day) [65].
Epidemiological evidence from general populations in Japan
We therefore developed a practical method for assessing dietary cadmium intake using a food frequency questionnaire (FFQ) used for the JPHC Study, and evaluated the validity of the FFQ in estimating intake by comparing 24-h urinary excretion of cadmium with dietary cadmium intake estimated from the FFQ [66]. Spearman correlation coefficients between these variables were 0.38 in men and 0.45 in women, indicating that the FFQ is reasonably valid for assessing cadmium intake in epidemiological studies. We then examined the association of dietary cadmium intake with risk of total cancer and site-specific cancers using data from the 5-year follow-up survey of the JPHC Study [66]. No statistically significant association was observed for total cancer or site-specific cancer in 9 years of follow-up data for 90,383 Japanese men and women aged 45–74 years (Fig. 1). In contrast, a cohort study in general populations living in non-cadmium-polluted areas (Ishikawa and Chiba Prefecture) found that urinary cadmium concentration was significantly associated with increased risk of total cancer and pancreatic cancer mortality for women but not men, based on 19-year follow-up of 1107 men and 1697 women [67].
Fig. 1.
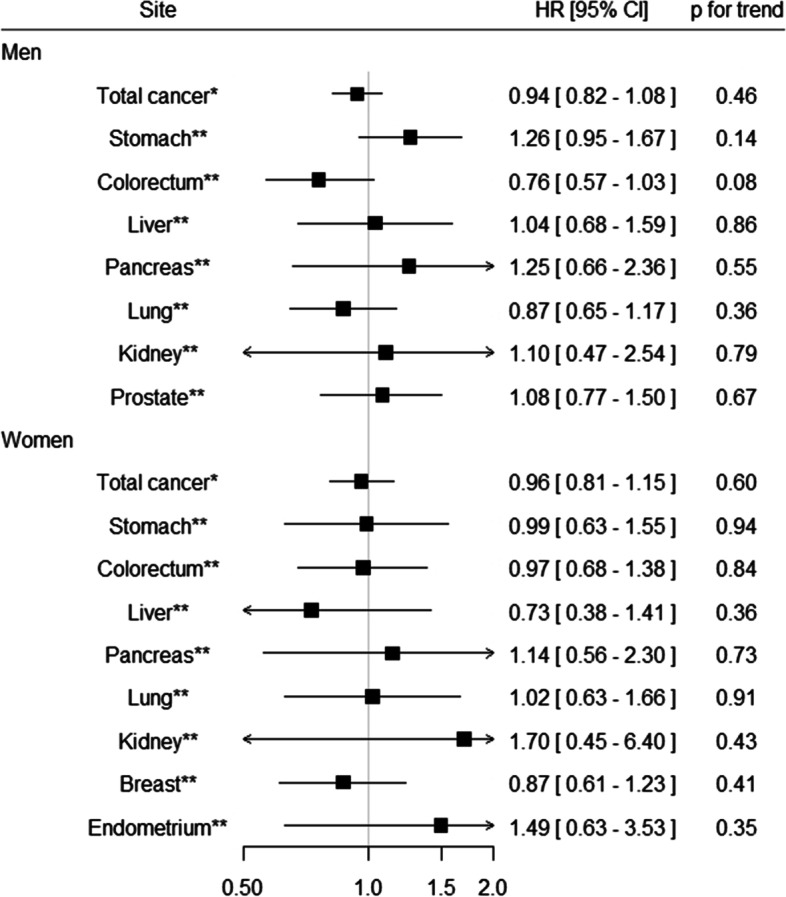
Summary results for the association between dietary cadmium intake and cancer risk in the JPHC Study
* Quartile category
** Tertile category
Data from Sawada et al. [66]. Adjusted HRs (95% CIs) for the highest versus lowest category are shown
CI, confidence interval; HR, hazard ratio; JPHC Study, Japan Public Health Center-based Prospective Study
The estrogenic properties of cadmium have focused attention on their role in the etiology of breast cancer [68]. As described, dietary cadmium intake was not associated with the risk of breast cancer in the JPHC Study (Fig. 1 and Table 4) [66]. We also investigated the association between dietary cadmium intake and risk of breast cancer in a hospital-based case–control study in Nagano Prefecture, Japan. Although no statistically significant association was observed for breast cancer overall (Table 4), higher cadmium intake was significantly associated with increased risk of estrogen receptor-positive breast cancer among postmenopausal women [69]. Adjusted OR (95% CI) for the highest versus lowest tertile was 1.94 (1.04–3.63) and the trend test was also statistically significant (p = 0.032). Furthermore, a hospital-based case–control study in Gifu Prefecture, Japan measured urinary cadmium concentration using spot urine samples collected from 153 breast cancer cases and 431 matched control subjects [70]. A statistically significant positive association was found between urinary cadmium concentration and risk of breast cancer (Table 4). Adjusted ORs (95% CI) for the highest versus lowest tertile were 6.05 (2.90–12.62) for all subjects and 4.60 (2.67–10.2) among never smokers. Considering the findings from studies using urinary biomarkers [68, 70], additional evidence from prospective studies with a large sample size are warranted.
Table 4.
Summary results for the association between cadmium and breast cancer risk among Japanese
| Reference | Study period | Design | Study subjects | Exposure assessment | Exposure | Exposure level (median or range among control group) | Category | Relative risk | 95% confidence interval | P for trend | Confounding factors | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Definition | Age range | Number of subjects | |||||||||||
| Sawada et al. [66] |
Enrolment 1995–1998, follow-up to 2006 |
Population-based cohort | Residents in 10 public health center areas in Japan | 45–74 years old at enrolment | 402 breast cancer cases among 48,351 women | Food frequency questionnaire | Dietary intake (ug/day) | 19.2 | Tertile 1 | 1 | 0.41 | Adjusted for age, area, body mass index, smoking status, frequency of alcohol intake, leisure-time physical activity, intake of meat, soybean, vegetable, and fruit, menopausal status, and use of exogenous female hormones | |
| 24.9 | Tertile 2 | 1.16 | (0.87–1.54) | ||||||||||
| 32.3 | Tertile 3 | 0.87 | (0.61–1.23) | ||||||||||
| Itoh et al. [69] | Enrolment 2001–2005 | Hospital-based case–control | Cases: histopathologically confirmed, Controls: medical check-up examinees matched by age and area of residence | 20–74 years old | 390 cases and 390 controls | Food frequnecuy questionnaire | Dietary intake (ug/day) | 21.4 | Tertile 1 | 1 | 0.39 | Adjusted for menopausal status, moderate physical activity in the past five years, smoking status, family history of breast cancer, number of births, isoflavone intake, vegetable intake, and total energy intake | |
| 26.2 | Tertile 2 | 1.19 | (0.79–1.79) | ||||||||||
| 31.5 | Tertile 3 | 1.23 | (0.76–2.00) | ||||||||||
| Nagata et al. [70] | Enrolment 2000–2002 | Hospital-based case–control | Cases: histopathologically confirmed, Controls: examinees of breast cancer mass screening matched by age, menopausal status, and period of urine sampling | 25–70 + years old | 153 cases and 431 controls | Spot urine samples measured by flameless atomic absorption spectrometry | Urinary cadmium (ug/g creatinine) | < 1.674 | Tertile 1 | 1 | < 0.01 | Adjusted for age, years of education, age at menarche, number of births, age at first birth, body mass index, smoking status, alcohol intake, and family history of breast cancer among first-degree relatives | |
| 1.674–2.620 | Tertile 2 | 2.25 | (1.17–4.37) | ||||||||||
| > 2.620 | Tertile 3 | 6.05 | (2.90–12.62) |
Arsenic
Background and overview
Arsenic is used in industrial processes such as nonferrous smelting, wood preservation, glass manufacturing, production and application of arsenic-based pesticides, and electronics. Inhalation is the primary route of exposure to arsenic in the workplace [3]. Arsenic is widely distributed throughout the Earth’s crust, and is a known contaminant of many groundwater sources worldwide via dissolution or desorption of minerals. In addition to naturally occurring groundwater contamination, it can also occur as a consequence of mining activities, use of arsenic-based pesticides and herbicides, industrial processes, and irrigation. In countries where inorganic arsenic is present at high levels in the groundwater, drinking water is major route of exposure, whereas food is usually the major contributor in areas where arsenic levels are not naturally high [71].
Regarding the health effects of long-term exposure, IARC classified arsenic and inorganic arsenic compounds as carcinogenic to humans (Group 1) based on epidemiological studies which showed increased risk of lung, skin, and urinary bladder cancer due to exposure to arsenic through inhalation in the workplace or drinking water contaminated with high levels of inorganic arsenic (Table 1) [3, 55]. Moreover, a positive association has been observed between exposure to arsenic and inorganic arsenic compounds and risk of kidney, liver, and prostate cancer. Several mechanisms by which arsenic and inorganic arsenic compounds induce carcinogenesis have been proposed, including induction of oxidative DNA damage and DNA-repair inhibition, aneuploidy, gene amplification, and epigenetic alterations leading to altered gene expression and genomic instability [3, 60].
In Japan, a historical cohort study was conducted in an arsenic-polluted area in which well water was polluted by liquid waste containing inorganic arsenic from a dye factory [72]. Results showed a significant increase in mortality for lung and urinary tract cancer among residents who drank well water containing a high concentration of arsenic (≥ 1 ppm). Meanwhile, the Japanese Water Supply Law and Ordinance restricts arsenic concentration in drinking water to less than 0.01 mg/L, and most Japanese are therefore exposed to arsenic via foods, particularly seafood and seaweeds [73]. Although the arsenic in seafood and seaweeds is usually in the form of organic compounds, which are known to have low toxicity, arsenosugars detected in seaweeds are metabolized to dimethylarsinic acid in humans, which is more toxic than arsenosugars [74, 75]. In addition, the edible seaweed hijiki (Hizikia fusiforme) contains inorganic arsenic, and concerns over a potential effect on cancer risk have been raised given its relatively common consumption among seaweeds by Japanese [76].
Epidemiological evidence from general populations in Japan
We developed a validated method for estimating dietary arsenic intake based on the FFQ used in the JPHC Study [73]. From a validation study in a subsample of subjects in the JPHC Study which compared intake between the FFQ and dietary records (DRs), Spearman’s rank correlation coefficients for arsenic and inorganic arsenic were 0.30 and 0.33 in men and 0.15 and 0.19 in women, respectively. The contributions of seafood, hijiki, seaweeds, rice, and vegetables to total arsenic intake were 32%, 28%, 20%, 16%, and 1%, respectively, while the contributions of hijiki, rice, seaweeds, seafood, vegetables, and fruits to inorganic arsenic intake were 50%, 35%, 5%, 4%, 3%, and 2%, respectively. We investigated the association of dietary arsenic intake with risk of total and site-specific cancer based on 10.9 years of follow-up data for 90,378 Japanese men and women aged 45–74 years in the JPHC Study [73]. Overall, we found no statistically significant association between total arsenic and inorganic arsenic intake and risk of total cancer for both men and women, respectively (Fig. 2 (a) and (b)).
Fig. 2.
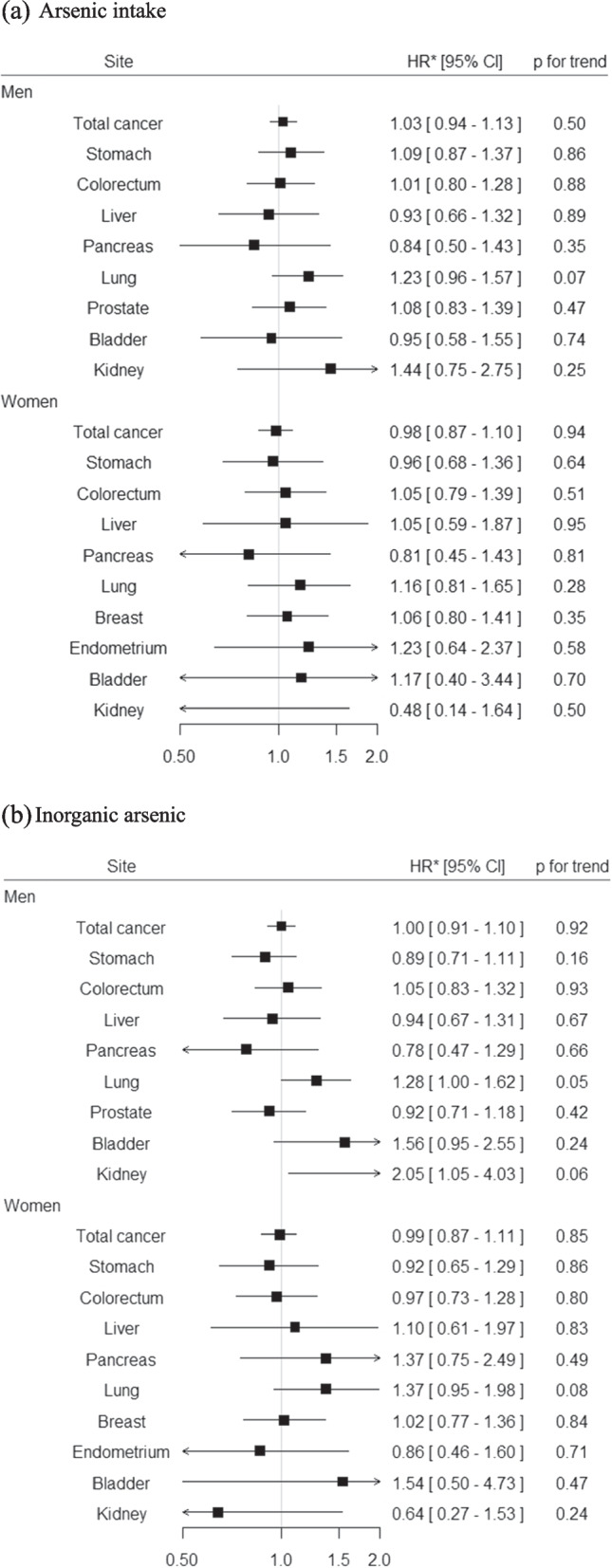
Summary results for the association between dietary arsenic intake and cancer risk in the JPHC Study. a Arsenic intake (b) Inorganic arsenic
Data from Sawada et al. [73]
*Adjusted HRs (95% CIs) for the highest versus lowest quartile category are shown
CI, confidence interval; HR, hazard ratio; JPHC Study, Japan Public Health Center-based Prospective Study
For site-specific cancers, no statistically significant association was observed for total arsenic intake among both men and women (Fig. 2 (a)) [73]. Meanwhile, we found positive associations of inorganic arsenic intake with risk of lung cancer for both men and women, and risk of kidney cancer for men (Fig. 2 (b)). Of interest, subgroup analysis by smoking status revealed a statistically significant interaction of total arsenic intake and smoking status in relation to the risk of lung cancer among men: a statistically significant positive association was found for ever smokers while a statistically significant inverse association was seen for never smokers (data not shown). A similar result was observed for inorganic arsenic intake among men, although without statistical significance. On the other hand, a positive association was found for never smokers but a test for interaction was not statistically significant among women. This observed interaction among men is consistent with studies characterized by a high level of arsenic exposure [72, 77, 78]. On the other hand, the reason for the discrepant results between men and women is less clear, although possible explanations include the small number of smokers and relatively low validity of estimated arsenic intake from the FFQ among women.
Acrylamide
Background and overview
Acrylamide is a chemical compound produced industrially mainly as a precursor to polyacrylamides, which have a variety of uses such as water-soluble thickeners and flocculation agents. In 1994, IARC classified acrylamide as probably carcinogenic to humans (Group 2A) based on sufficient evidence in experimental animals for the carcinogenicity of acrylamide (Table 1) [79]. Mechanistic evidence has shown that acrylamide and its metabolite glycidamide form DNA and hemoglobin adducts and that acrylamide induces gene mutation and chromosomal aberrations [80, 81]. In addition to acrylamide-induced genotoxicity, hormonal pathways have been hypothesized, particularly given that tumorgenicity by acrylamide was found in rat endocrine and mammary gland [80, 81].
Prior to 2002, exposure to acrylamide was thought to primarily occur in occupational settings or through tobacco smoke. The discovery in 2002 that some cooked foods contain acrylamide, however, has raised concerns about potential health effects in the general population [82]. The major pathway by which acrylamide is formed in foods is through the Maillard reaction during food cooking at temperatures higher than > 120 °C [82]. The content of acrylamide in foods varies widely, depending on the food matrix and food processing method. As a consequence of different dietary habits and cooking methods across countries, dietary exposure to acrylamide and foods contributing to acrylamide intake might differ across populations, which in turn indicates the necessity of population-specific studies to assess dietary exposure level and its association with cancer risk.
Epidemiological evidence from general populations in Japan
We established a validated method for estimating dietary acrylamide intake from the FFQ used for the JPHC Study [83]. Deattenuated correlation coefficients for energy-adjusted dietary acrylamide intake between DRs and the FFQ ranged from 0.37 to 0.54, which indicates its suitability for use in epidemiological studies. Mean acrylamide intake from DRs was about 7 µg/day, and 0.12 µg/kg body weight/day, which was less than in European populations (12 to 48 µg/day in 27 areas of 10 European countries within the European Prospective Investigation into Cancer and Nutrition (EPIC) study) [84, 85]. The main contributing food groups from DRs in the JPHC Study were beverages, confectioneries, vegetables, potatoes and starches, and cereals [83]. In contrast, the primary contributing foods in European countries were potato-based foods (eg, fried potato), wheat-based products (eg, biscuits), and coffee [84, 85].
We examined the association of dietary acrylamide intake with risk of site-specific cancers using data from the 5-year follow-up survey of the JPHC Study [86–93]. No statistically significant association was observed for any cancer site (Fig. 3). These findings are in general agreement with recent meta-analyses [94, 95]. Meanwhile, we recently measured hemoglobin adducts of acrylamide and glycidamide (HbAA and HbGA) in erythrocytes collected from 125 breast cancer cases and 250 controls in a nested case–control study within the JPHC Study [96]. We found no statistically significant positive association for either HbAA or HbGA, but a positive association between HbGA/HbAA ratio and risk of breast cancer. Adjusted OR (95% CI) for the highest versus lowest tertile was 2.19 (1.11–4.31) and the trend test was also statistically significant (p = 0.027). Given that acrylamide is primarily metabolized by phase I enzymes such as cytochrome P450 2E1 (CYP2E1) to the epoxide metabolite, glycidamide, which is likely to play an important role in the carcinogenicity [80, 81], HbGA/HbAA ratio reflects individual differences in susceptibility to acrylamide exposure and might be a biomarker for acrylamide-related genotoxic exposure [97].
Fig. 3.
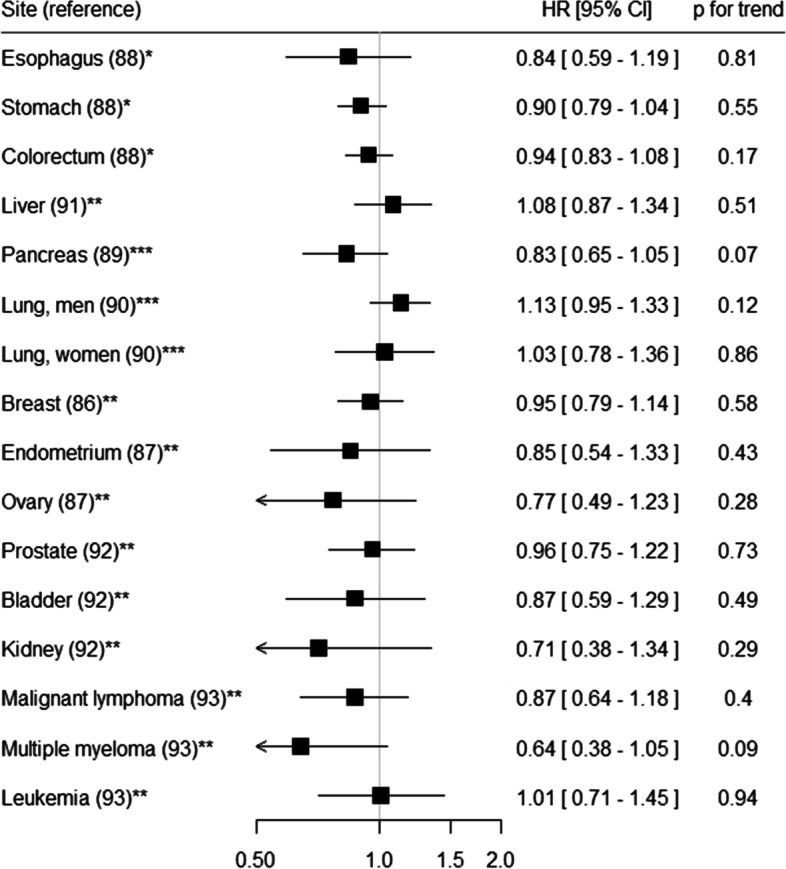
Summary results for the association between dietary acrylamide intake and cancer risk in the JPHC Study
* Quintile category
** Tertile category
*** Quartile category
Adjusted HRs (95% CIs) for the highest versus lowest category are shown
CI, confidence interval; HR, hazard ratio; JPHC Study, Japan Public Health Center-based Prospective Study
Conclusions
We reviewed epidemiological studies of DDT, HCH, PCBs, PFASs, cadmium, arsenic, and acrylamide, to which Japanese populations are widely exposed. DDT, HCH, PCBs, and PFASs were detected in blood samples from general populations in Japan. Epidemiological evidence did not support positive associations between blood concentration of DDT, HCH, PCBs, and PFASs and risk of breast and prostate cancer. We established assessment methods for the dietary intake of cadmium, arsenic, and acrylamide using an FFQ. Overall, dietary intake of cadmium, arsenic, and acrylamide was not significantly associated with increased risk of total cancer and major cancer sites in the JPHC Study. However, a statistically significant positive association was observed between dietary cadmium intake and risk of estrogen receptor-positive breast cancer among postmenopausal women, and between dietary arsenic intake and risk of lung cancer among male smokers. In addition, studies using biomarkers as exposure assessment revealed statistically significant positive associations between urinary cadmium concentration and risk of breast cancer, and between HbGA/HbAA ratio and risk of breast cancer. Since the number of epidemiological studies in general populations in Japan is limited, further accumulation of evidence is required, with particular focus on the following: the association of organochlorine and organofluorine compounds with risk of cancer sites other than breast and prostate cancer; and large prospective studies of the association between biomarkers of exposure and risk of cancer. For the latter, collaboration among relevant researchers to ensure that epidemiological studies incorporate precise biologic sample-based exposure assessment will be essential.
Acknowledgements
We thank members of the National Cancer Center Institute for Cancer Control for their support and helpful discussions.
Abbreviations
- AhR
Aryl hydrocarbon receptor
- CAR
Constitutive androstane receptor
- CI
Confidence interval
- CYP1A
Cytochrome P450 1A
- CYP2B
Cytochrome P450 2B
- CYP2E1
Cytochrome P450 2E1
- DDD
Dichlorodiphenyldichloroethane
- DDE
Dichlorodiphenyldichloroethylene
- DRs
Dietary records
- DDT
dichlorodiphenyltrichloroethane
- EPIC
European Prospective Investigation into Cancer and Nutrition
- FFQ
Food frequency questionnaire
- HbAA
Hemoglobin adducts of acrylamide
- HbGA
Hemoglobin adducts of glycidamide
- HCH
Hexachlorocyclohexane
- IARC
International Agency for Research on Cancer
- JPHC Study
Japan Public Health Center-based Prospective Study
- OR
Odds ratio
- PCBs
Polychlorinated biphenyls
- PCDFs
Polychlorinated dibenzofurans
- PFASs
Per- and polyfluoroalkyl substances
- PFHxS
Perfluorohexane sulfonate
- PFOA
Perfluorooctanoic acid
- PFOS
Perfluorooctane sulfonate
- PFUnDA
Perfluoroundecanoic acid
- PHC
Public health center
- POPs
Persistent organic pollutants
- PPARα
Peroxisome proliferator-activated receptor alpha
- PXR
Pregnane xenobiotic receptor
Authors’ contributions
Motoki Iwasaki drafted the manuscript and contributed to the discussion. Hiroaki Itoh, Norie Sawada, and Shoichiro Tsugane reviewed and edited the manuscript, and contributed to the discussion. All authors read and approved the final manuscript.
Funding
This work was supported by JSPS KAKENHI Grant Number 20K10512.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have declared no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Inoue M, Hirabayashi M, Abe SK, Katanoda K, Sawada N, Lin Y, et al. Burden of cancer attributable to modifiable factors in Japan in 2015. Glob Health Med. 2022;4(1):26–36. doi: 10.35772/ghm.2021.01037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wild CP, Weiderpass E, Stewart BW, editors. World Cancer Report: Cancer Research for Cancer Prevention. Lyon, France: International Agency for Research on Cancer. 2020.
- 3.IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, vol. 100C: Arsenic, Metals, Fibres, and Dusts. 2012. [PMC free article] [PubMed]
- 4.IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, vol. 100F: Chemical Agents and Related Occupations. 2012. [PMC free article] [PubMed]
- 5.IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, vol. 107: Polychlorinated Biphenyls and Polybrominated Biphenyls. 2015. [PubMed]
- 6.IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, vol. 109: Outdoor Air Pollution. 2016.
- 7.IARC Monographs Priorities Group Advisory Group recommendations on priorities for the IARC Monographs. Lancet Oncol. 2019;20(6):763–764. doi: 10.1016/S1470-2045(19)30246-3. [DOI] [PubMed] [Google Scholar]
- 8.IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, vol. 113: DDT, Lindane, and 2,4-D. 2016.
- 9.Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological profile for DDT, DDE, and DDD. 2022. [PubMed]
- 10.Hanaoka T, Takahashi Y, Kobayashi M, Sasaki S, Usuda M, Okubo S, et al. Residuals of beta-hexachlorocyclohexane, dichlorodiphenyltrichloroethane, and hexachlorobenzene in serum, and relations with consumption of dietary components in rural residents in Japan. Sci Total Environ. 2002;286(1–3):119–127. doi: 10.1016/S0048-9697(01)00969-X. [DOI] [PubMed] [Google Scholar]
- 11.Kelce WR, Stone CR, Laws SC, Gray LE, Kemppainen JA, Wilson EM. Persistent DDT metabolite p, p'-DDE is a potent androgen receptor antagonist. Nature. 1995;375(6532):581–585. doi: 10.1038/375581a0. [DOI] [PubMed] [Google Scholar]
- 12.Legler J, Zeinstra LM, Schuitemaker F, Lanser PH, Bogerd J, Brouwer A, et al. Comparison of in vivo and in vitro reporter gene assays for short-term screening of estrogenic activity. Environ Sci Technol. 2002;36(20):4410–4415. doi: 10.1021/es010323a. [DOI] [PubMed] [Google Scholar]
- 13.Scippo ML, Argiris C, Van De Weerdt C, Muller M, Willemsen P, Martial J, et al. Recombinant human estrogen, androgen and progesterone receptors for detection of potential endocrine disruptors. Anal Bioanal Chem. 2004;378(3):664–669. doi: 10.1007/s00216-003-2251-0. [DOI] [PubMed] [Google Scholar]
- 14.Ingber SZ, Buser MC, Pohl HR, Abadin HG, Murray HE, Scinicariello F. DDT/DDE and breast cancer: a meta-analysis. Regul Toxicol Pharmacol. 2013;67(3):421–433. doi: 10.1016/j.yrtph.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schinasi L, Leon ME. Non-Hodgkin lymphoma and occupational exposure to agricultural pesticide chemical groups and active ingredients: a systematic review and meta-analysis. Int J Environ Res Public Health. 2014;11(4):4449–4527. doi: 10.3390/ijerph110404449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Odutola MK, Benke G, Fritschi L, Giles GG, van Leeuwen MT, Vajdic CM. A systematic review and meta-analysis of occupational exposures and risk of follicular lymphoma. Environ Res. 2021;197:110887. doi: 10.1016/j.envres.2021.110887. [DOI] [PubMed] [Google Scholar]
- 17.Loomis D, Guyton K, Grosse Y, El Ghissasi F, Bouvard V, Benbrahim-Tallaa L, et al. Carcinogenicity of lindane, DDT, and 2,4-dichlorophenoxyacetic acid. Lancet Oncol. 2015;16(8):891–892. doi: 10.1016/S1470-2045(15)00081-9. [DOI] [PubMed] [Google Scholar]
- 18.Iwasaki M, Inoue M, Sasazuki S, Kurahashi N, Itoh H, Usuda M, et al. Plasma organochlorine levels and subsequent risk of breast cancer among Japanese women: a nested case-control study. Sci Total Environ. 2008;402(2–3):176–183. doi: 10.1016/j.scitotenv.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Itoh H, Iwasaki M, Hanaoka T, Kasuga Y, Yokoyama S, Onuma H, et al. Serum organochlorines and breast cancer risk in Japanese women: a case-control study. Cancer Causes Control. 2009;20(5):567–580. doi: 10.1007/s10552-008-9265-z. [DOI] [PubMed] [Google Scholar]
- 20.Sawada N, Iwasaki M, Inoue M, Itoh H, Sasazuki S, Yamaji T, et al. Plasma organochlorines and subsequent risk of prostate cancer in Japanese men: a nested case-control study. Environ Health Perspect. 2010;118(5):659–665. doi: 10.1289/ehp.0901214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wan MLY, Co VA, El-Nezami H. Endocrine disrupting chemicals and breast cancer: a systematic review of epidemiological studies. Crit Rev Food Sci Nutr. 2022;62(24):6549–6576. doi: 10.1080/10408398.2021.1903382. [DOI] [PubMed] [Google Scholar]
- 22.Cohn BA, Wolff MS, Cirillo PM, Sholtz RI. DDT and breast cancer in young women: new data on the significance of age at exposure. Environ Health Perspect. 2007;115(10):1406–1414. doi: 10.1289/ehp.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis-Mikhael AM, Olmedo-Requena R, Martinez-Ruiz V, Bueno-Cavanillas A, Jimenez-Moleon JJ. Organochlorine pesticides and prostate cancer, Is there an association? A meta-analysis of epidemiological evidence. Cancer Causes Control. 2015;26(10):1375–1392. doi: 10.1007/s10552-015-0643-z. [DOI] [PubMed] [Google Scholar]
- 24.Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological profile for alpha-, beta-, gamma-, and delta-hexachlorocyclohexane. 2005.
- 25.Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Polychlorinated Biphenyls (PCBs). 2010. [PubMed]
- 26.Wolff MS, Toniolo PG. Environmental organochlorine exposure as a potential etiologic factor in breast cancer. Environ Health Perspect. 1995;103 Suppl 7(Suppl 7):141–5. doi: 10.1289/ehp.95103s7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faust D, Vondracek J, Krcmar P, Smerdova L, Prochazkova J, Hruba E, et al. AhR-mediated changes in global gene expression in rat liver progenitor cells. Arch Toxicol. 2013;87(4):681–698. doi: 10.1007/s00204-012-0979-z. [DOI] [PubMed] [Google Scholar]
- 28.Ludewig G, Robertson LW. Polychlorinated biphenyls (PCBs) as initiating agents in hepatocellular carcinoma. Cancer Lett. 2013;334(1):46–55. doi: 10.1016/j.canlet.2012.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lauby-Secretan B, Loomis D, Grosse Y, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, et al. Carcinogenicity of polychlorinated biphenyls and polybrominated biphenyls. Lancet Oncol. 2013;14(4):287–288. doi: 10.1016/S1470-2045(13)70104-9. [DOI] [PubMed] [Google Scholar]
- 30.Onozuka D, Nakamura Y, Tsuji G, Furue M. Mortality in Yusho patients exposed to polychlorinated biphenyls and polychlorinated dibenzofurans: a 50-year retrospective cohort study. Environ Health. 2020;19(1):119. doi: 10.1186/s12940-020-00680-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Todaka T, Hori T, Hirakawa H, Kajiwara J, Yasutake D, Onozuka D, et al. Concentrations of polychlorinated biphenyls in blood of Yusho patients over 35 years after the incident. Chemosphere. 2009;74(7):902–909. doi: 10.1016/j.chemosphere.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 32.Wolff MS, Camann D, Gammon M, Stellman SD. Proposed PCB congener groupings for epidemiological studies. Environ Health Perspect. 1997;105(1):13–14. doi: 10.1289/ehp.9710513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J, Huang Y, Wang X, Lin K, Wu K. Environmental Polychlorinated Biphenyl Exposure and Breast Cancer Risk: A Meta-Analysis of Observational Studies. PLoS ONE. 2015;10(11):e0142513. doi: 10.1371/journal.pone.0142513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koutros S, Langseth H, Grimsrud TK, Barr DB, Vermeulen R, Portengen L, et al. Prediagnostic Serum Organochlorine Concentrations and Metastatic Prostate Cancer: A Nested Case-Control Study in the Norwegian Janus Serum Bank Cohort. Environ Health Perspect. 2015;123(9):867–872. doi: 10.1289/ehp.1408245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim JE, Nam C, Yang J, Rha KH, Lim KM, Jee SH. Serum persistent organic pollutants (POPs) and prostate cancer risk: A case-cohort study. Int J Hyg Environ Health. 2017;220(5):849–856. doi: 10.1016/j.ijheh.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 36.Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Perfluoroalkyls. 2021. [PubMed]
- 37.Gaines LGT. Historical and current usage of per- and polyfluoroalkyl substances (PFAS): A literature review. Am J Ind Med. 2022. [DOI] [PubMed]
- 38.Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, et al. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect. 2007;115(9):1298–1305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Fletcher T, Mucs D, Scott K, Lindh CH, Tallving P, et al. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup Environ Med. 2018;75(1):46–51. doi: 10.1136/oemed-2017-104651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Domingo JL, Nadal M. Human exposure to per- and polyfluoroalkyl substances (PFAS) through drinking water: A review of the recent scientific literature. Environ Res. 2019;177:108648. doi: 10.1016/j.envres.2019.108648. [DOI] [PubMed] [Google Scholar]
- 41.Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol. 2019;29(2):131–147. doi: 10.1038/s41370-018-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, vol. 110: Some Chemicals Used as Solvents and in Polymer Manufacture. 2016.
- 43.Benbrahim-Tallaa L, Lauby-Secretan B, Loomis D, Guyton KZ, Grosse Y, El Ghissassi F, et al. Carcinogenicity of perfluorooctanoic acid, tetrafluoroethylene, dichloromethane, 1,2-dichloropropane, and 1,3-propane sultone. Lancet Oncol. 2014;15(9):924–925. doi: 10.1016/S1470-2045(14)70316-X. [DOI] [PubMed] [Google Scholar]
- 44.Steenland K, Woskie S. Cohort mortality study of workers exposed to perfluorooctanoic acid. Am J Epidemiol. 2012;176(10):909–917. doi: 10.1093/aje/kws171. [DOI] [PubMed] [Google Scholar]
- 45.Barry V, Winquist A, Steenland K. Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant. Environ Health Perspect. 2013;121(11–12):1313–1318. doi: 10.1289/ehp.1306615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vieira VM, Hoffman K, Shin HM, Weinberg JM, Webster TF, Fletcher T. Perfluorooctanoic acid exposure and cancer outcomes in a contaminated community: a geographic analysis. Environ Health Perspect. 2013;121(3):318–323. doi: 10.1289/ehp.1205829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kennedy GL, Jr, Butenhoff JL, Olsen GW, O'Connor JC, Seacat AM, Perkins RG, et al. The toxicology of perfluorooctanoate. Crit Rev Toxicol. 2004;34(4):351–384. doi: 10.1080/10408440490464705. [DOI] [PubMed] [Google Scholar]
- 48.Du G, Huang H, Hu J, Qin Y, Wu D, Song L, et al. Endocrine-related effects of perfluorooctanoic acid (PFOA) in zebrafish, H295R steroidogenesis and receptor reporter gene assays. Chemosphere. 2013;91(8):1099–1106. doi: 10.1016/j.chemosphere.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 49.Zhao B, Li L, Liu J, Li H, Zhang C, Han P, et al. Exposure to perfluorooctane sulfonate in utero reduces testosterone production in rat fetal Leydig cells. PLoS ONE. 2014;9(1):e78888. doi: 10.1371/journal.pone.0078888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shearer JJ, Callahan CL, Calafat AM, Huang WY, Jones RR, Sabbisetti VS, et al. Serum Concentrations of Per- and Polyfluoroalkyl Substances and Risk of Renal Cell Carcinoma. J Natl Cancer Inst. 2021;113(5):580–587. doi: 10.1093/jnci/djaa143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mancini FR, Cano-Sancho G, Gambaretti J, Marchand P, Boutron-Ruault MC, Severi G, et al. Perfluorinated alkylated substances serum concentration and breast cancer risk: Evidence from a nested case-control study in the French E3N cohort. Int J Cancer. 2020;146(4):917–928. doi: 10.1002/ijc.32357. [DOI] [PubMed] [Google Scholar]
- 52.Hurley S, Goldberg D, Wang M, Park JS, Petreas M, Bernstein L, et al. Breast cancer risk and serum levels of per- and poly-fluoroalkyl substances: a case-control study nested in the California Teachers Study. Environ Health. 2018;17(1):83. doi: 10.1186/s12940-018-0426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Itoh H, Harada KH, Kasuga Y, Yokoyama S, Onuma H, Nishimura H, et al. Serum perfluoroalkyl substances and breast cancer risk in Japanese women: A case-control study. Sci Total Environ. 2021;800:149316. doi: 10.1016/j.scitotenv.2021.149316. [DOI] [PubMed] [Google Scholar]
- 54.WHO. Safety evaluation of certain food additives and contaminants in food. Prepared by the Seventy-third meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Geneva, World Health Organization (WHO Food Additives Series, No 64); pp 305–380. 2011.
- 55.Straif K, Benbrahim-Tallaa L, Baan R, Grosse Y, Secretan B, El Ghissassi F, et al. A review of human carcinogens–Part C: metals, arsenic, dusts, and fibres. Lancet Oncol. 2009;10(5):453–454. doi: 10.1016/S1470-2045(09)70134-2. [DOI] [PubMed] [Google Scholar]
- 56.Stoica A, Katzenellenbogen BS, Martin MB. Activation of estrogen receptor-alpha by the heavy metal cadmium. Mol Endocrinol. 2000;14(4):545–553. doi: 10.1210/mend.14.4.0441. [DOI] [PubMed] [Google Scholar]
- 57.Johnson MD, Kenney N, Stoica A, Hilakivi-Clarke L, Singh B, Chepko G, et al. Cadmium mimics the in vivo effects of estrogen in the uterus and mammary gland. Nat Med. 2003;9(8):1081–1084. doi: 10.1038/nm902. [DOI] [PubMed] [Google Scholar]
- 58.Giaginis C, Gatzidou E, Theocharis S. DNA repair systems as targets of cadmium toxicity. Toxicol Appl Pharmacol. 2006;213(3):282–290. doi: 10.1016/j.taap.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 59.Schwerdtle T, Ebert F, Thuy C, Richter C, Mullenders LH, Hartwig A. Genotoxicity of soluble and particulate cadmium compounds: impact on oxidative DNA damage and nucleotide excision repair. Chem Res Toxicol. 2010;23(2):432–442. doi: 10.1021/tx900444w. [DOI] [PubMed] [Google Scholar]
- 60.Zhu Y, Costa M. Metals and molecular carcinogenesis. Carcinogenesis. 2020;41(9):1161–1172. doi: 10.1093/carcin/bgaa076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arisawa K, Uemura H, Hiyoshi M, Dakeshita S, Kitayama A, Saito H, et al. Cause-specific mortality and cancer incidence rates in relation to urinary beta2-microglobulin: 23-year follow-up study in a cadmium-polluted area. Toxicol Lett. 2007;173(3):168–174. doi: 10.1016/j.toxlet.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 62.Nishijo M, Nakagawa H, Suwazono Y, Nogawa K, Sakurai M, Ishizaki M, et al. Cancer Mortality in Residents of the Cadmium-Polluted Jinzu River Basin in Toyama, Japan. Toxics. 2018;6(2). [DOI] [PMC free article] [PubMed]
- 63.Watanabe T, Zhang ZW, Moon CS, Shimbo S, Nakatsuka H, Matsuda-Inoguchi N, et al. Cadmium exposure of women in general populations in Japan during 1991–1997 compared with 1977–1981. Int Arch Occup Environ Health. 2000;73(1):26–34. doi: 10.1007/PL00007934. [DOI] [PubMed] [Google Scholar]
- 64.Zhang ZW, Moon CS, Watanabe T, Shimbo S, He FS, Wu YQ, et al. Background exposure of urban populations to lead and cadmium: comparison between China and Japan. Int Arch Occup Environ Health. 1997;69(4):273–281. doi: 10.1007/s004200050147. [DOI] [PubMed] [Google Scholar]
- 65.Akesson A, Julin B, Wolk A. Long-term dietary cadmium intake and postmenopausal endometrial cancer incidence: a population-based prospective cohort study. Cancer Res. 2008;68(15):6435–6441. doi: 10.1158/0008-5472.CAN-08-0329. [DOI] [PubMed] [Google Scholar]
- 66.Sawada N, Iwasaki M, Inoue M, Takachi R, Sasazuki S, Yamaji T, et al. Long-term dietary cadmium intake and cancer incidence. Epidemiology. 2012;23(3):368–376. doi: 10.1097/EDE.0b013e31824d063c. [DOI] [PubMed] [Google Scholar]
- 67.Watanabe Y, Nogawa K, Nishijo M, Sakurai M, Ishizaki M, Morikawa Y, et al. Relationship between cancer mortality and environmental cadmium exposure in the general Japanese population in cadmium non-polluted areas. Int J Hyg Environ Health. 2020;223(1):65–70. doi: 10.1016/j.ijheh.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 68.Florez-Garcia VA, Guevara-Romero EC, Hawkins MM, Bautista LE, Jenson TE, Yu J, et al. Cadmium exposure and risk of breast cancer: A meta-analysis. Environ Res. 2022;219:115109. doi: 10.1016/j.envres.2022.115109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Itoh H, Iwasaki M, Sawada N, Takachi R, Kasuga Y, Yokoyama S, et al. Dietary cadmium intake and breast cancer risk in Japanese women: a case-control study. Int J Hyg Environ Health. 2014;217(1):70–77. doi: 10.1016/j.ijheh.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 70.Nagata C, Nagao Y, Nakamura K, Wada K, Tamai Y, Tsuji M, et al. Cadmium exposure and the risk of breast cancer in Japanese women. Breast Cancer Res Treat. 2013;138(1):235–239. doi: 10.1007/s10549-013-2414-4. [DOI] [PubMed] [Google Scholar]
- 71.International Programme on Chemical Safety (IPCS). Arsenic and arsenic compounds, 2nd ed. Geneva, World Health Organization, International Programme on Chemical Safety (Environmental Health Criteria 224). 2001.
- 72.Tsuda T, Babazono A, Yamamoto E, Kurumatani N, Mino Y, Ogawa T, et al. Ingested arsenic and internal cancer: a historical cohort study followed for 33 years. Am J Epidemiol. 1995;141(3):198–209. doi: 10.1093/oxfordjournals.aje.a117421. [DOI] [PubMed] [Google Scholar]
- 73.Sawada N, Iwasaki M, Inoue M, Takachi R, Sasazuki S, Yamaji T, et al. Dietary arsenic intake and subsequent risk of cancer: the Japan Public Health Center-based (JPHC) Prospective Study. Cancer Causes Control. 2013;24(7):1403–1415. doi: 10.1007/s10552-013-0220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kaise T O-OY, Ochi T, Okubo T, Hanaoka K, Irgolic KJ, Sakurai T, Matsubara C. Toxicological study of organic arsenic compound in marine algae using mammalian cell culture technique. J Food Hyg Soc Jpn. 1996;37:135–141.
- 75.Wei C, Li W, Zhang C, Van Hulle M, Cornelis R, Zhang X. Safety evaluation of organoarsenical species in edible Porphyra from the China Sea. J Agric Food Chem. 2003;51(17):5176–5182. doi: 10.1021/jf026117j. [DOI] [PubMed] [Google Scholar]
- 76.Nakamura Y, Narukawa T, Yoshinaga J. Cancer risk to Japanese population from the consumption of inorganic arsenic in cooked hijiki. J Agric Food Chem. 2008;56(7):2536–2540. doi: 10.1021/jf0731797. [DOI] [PubMed] [Google Scholar]
- 77.Chen CL, Hsu LI, Chiou HY, Hsueh YM, Chen SY, Wu MM, et al. Ingested arsenic, cigarette smoking, and lung cancer risk: a follow-up study in arseniasis-endemic areas in Taiwan. JAMA. 2004;292(24):2984–2990. doi: 10.1001/jama.292.24.2984. [DOI] [PubMed] [Google Scholar]
- 78.Mostafa MG, McDonald JC, Cherry NM. Lung cancer and exposure to arsenic in rural Bangladesh. Occup Environ Med. 2008;65(11):765–768. doi: 10.1136/oem.2007.037895. [DOI] [PubMed] [Google Scholar]
- 79.IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, vol. 60: Some Industrial Chemicals. 1994.
- 80.Besaratinia A, Pfeifer GP. A review of mechanisms of acrylamide carcinogenicity. Carcinogenesis. 2007;28(3):519–528. doi: 10.1093/carcin/bgm006. [DOI] [PubMed] [Google Scholar]
- 81.Hogervorst JG, Baars BJ, Schouten LJ, Konings EJ, Goldbohm RA, van den Brandt PA. The carcinogenicity of dietary acrylamide intake: a comparative discussion of epidemiological and experimental animal research. Crit Rev Toxicol. 2010;40(6):485–512. doi: 10.3109/10408440903524254. [DOI] [PubMed] [Google Scholar]
- 82.Tareke E, Rydberg P, Karlsson P, Eriksson S, Tornqvist M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J Agric Food Chem. 2002;50(17):4998–5006. doi: 10.1021/jf020302f. [DOI] [PubMed] [Google Scholar]
- 83.Kotemori A, Ishihara J, Nakadate M, Sawada N, Iwasaki M, Sobue T, et al. Validity of a Self-administered Food Frequency Questionnaire for the Estimation of Acrylamide Intake in the Japanese Population: The JPHC FFQ Validation Study. J Epidemiol. 2018;28(12):482–487. doi: 10.2188/jea.JE20170186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Konings EJ, Hogervorst JG, van Rooij L, Schouten LJ, Sizoo EA, van Egmond HP, et al. Validation of a database on acrylamide for use in epidemiological studies. Eur J Clin Nutr. 2010;64(5):534–540. doi: 10.1038/ejcn.2010.17. [DOI] [PubMed] [Google Scholar]
- 85.Freisling H, Moskal A, Ferrari P, Nicolas G, Knaze V, Clavel-Chapelon F, et al. Dietary acrylamide intake of adults in the European Prospective Investigation into Cancer and Nutrition differs greatly according to geographical region. Eur J Nutr. 2013;52(4):1369–1380. doi: 10.1007/s00394-012-0446-x. [DOI] [PubMed] [Google Scholar]
- 86.Kotemori A, Ishihara J, Zha L, Liu R, Sawada N, Iwasaki M, et al. Dietary acrylamide intake and risk of breast cancer: The Japan Public Health Center-based Prospective Study. Cancer Sci. 2018;109(3):843–853. doi: 10.1111/cas.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kotemori A, Ishihara J, Zha L, Liu R, Sawada N, Iwasaki M, et al. Dietary acrylamide intake and the risk of endometrial or ovarian cancers in Japanese women. Cancer Sci. 2018;109(10):3316–3325. doi: 10.1111/cas.13757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu R, Sobue T, Kitamura T, Kitamura Y, Ishihara J, Kotemori A, et al. Dietary Acrylamide Intake and Risk of Esophageal, Gastric, and Colorectal Cancer: The Japan Public Health Center-Based Prospective Study. Cancer Epidemiol Biomarkers Prev. 2019;28(9):1461–1468. doi: 10.1158/1055-9965.EPI-18-1259. [DOI] [PubMed] [Google Scholar]
- 89.Kito K, Ishihara J, Kotemori A, Zha L, Liu R, Sawada N, et al. Dietary Acrylamide Intake and the Risk of Pancreatic Cancer: The Japan Public Health Center-Based Prospective Study. Nutrients. 2020;12(11). [DOI] [PMC free article] [PubMed]
- 90.Liu R, Zha L, Sobue T, Kitamura T, Ishihara J, Kotemori A, et al. Dietary Acrylamide Intake and Risk of Lung Cancer: The Japan Public Health Center Based Prospective Study. Nutrients. 2020;12(8). [DOI] [PMC free article] [PubMed]
- 91.Zha L, Sobue T, Kitamura T, Kitamura Y, Ishihara J, Kotemori A, et al. Dietary Acrylamide Intake and the Risk of Liver Cancer: The Japan Public Health Center-Based Prospective Study. Nutrients. 2020;12(9). [DOI] [PMC free article] [PubMed]
- 92.Ikeda S, Sobue T, Kitamura T, Ishihara J, Kotemori A, Zha L, et al. Dietary Acrylamide Intake and the Risks of Renal Cell, Prostate, and Bladder Cancers: A Japan Public Health Center-Based Prospective Study. Nutrients. 2021;13(3). [DOI] [PMC free article] [PubMed]
- 93.Zha L, Liu R, Sobue T, Kitamura T, Ishihara J, Kotemori A, et al. Dietary Acrylamide Intake and the Risk of Hematological Malignancies: The Japan Public Health Center-Based Prospective Study. Nutrients. 2021;13(2). [DOI] [PMC free article] [PubMed]
- 94.Benisi-Kohansal S, Salari-Moghaddam A, Seyed Rohani Z, Esmaillzadeh A. Dietary acrylamide intake and risk of women's cancers: a systematic review and meta-analysis of prospective cohort studies. Br J Nutr. 2021;126(9):1355–1363. doi: 10.1017/S0007114520005255. [DOI] [PubMed] [Google Scholar]
- 95.Filippini T, Halldorsson TI, Capitao C, Martins R, Giannakou K, Hogervorst J, et al. Dietary Acrylamide Exposure and Risk of Site-Specific Cancer: A Systematic Review and Dose-Response Meta-Analysis of Epidemiological Studies. Front Nutr. 2022;9:875607. doi: 10.3389/fnut.2022.875607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Narii N, Kito K, Sobue T, Zha L, Kitamura T, Matsui Y, et al. Acrylamide and glycidamide hemoglobin adduct levels and breast cancer risk in Japanese women: A nested case-control study in the JPHC. Cancer Epidemiol Biomarkers Prev. 2023;32(3):415–21. [DOI] [PubMed]
- 97.Duale N, Bjellaas T, Alexander J, Becher G, Haugen M, Paulsen JE, et al. Biomarkers of human exposure to acrylamide and relation to polymorphisms in metabolizing genes. Toxicol Sci. 2009;108(1):90–99. doi: 10.1093/toxsci/kfn269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


