Abstract
Background
People with intellectual disabilities (ID) experience high rates of lifestyle related morbidities, in part due to lack of access to tailored health promotion programmes. This study aimed to assess the feasibility and preliminary efficacy of a tailored healthy lifestyle intervention, Get Healthy!
Methods
Get Healthy! is a 12-week physical activity and healthy eating programme designed to address lifestyle-related risks for adults with mild-moderate ID. The feasibility pilot was designed to assess subjective participant experience and programme feasibility across: recruitment and screening, retention, session attendance and engagement, adverse events, and practicality and reliability of outcome procedures. Exploratory programme efficacy was assessed across the following measures: anthropometry (body mass index, weight, waist circumference), cardiovascular fitness, physical strength, dietary intake, healthy literacy, and quality of life.
Results
Six participants with moderate ID and two carer participants completed the feasibility trial, representing a 100% retention rate. Qualitative data indicated the programme was well received. Participants with ID attended 75% of sessions offered and displayed a high level of engagement in sessions attended (91% mean engagement score). While most data collection procedures were feasible to implement, several measures were either not feasible for our participants, or required a higher level of support to implement than was provided in the existing trial protocol. Participants with ID displayed decreases in mean waist circumference between baseline and endpoint (95% CI: − 3.20, − 0.17 cm) and some improvements in measures of cardiovascular fitness and physical strength. No changes in weight, body mass index, or objectively measured knowledge of nutrition and exercise or quality of life were detected from baseline to programme endpoint. Dietary intake results were mixed.
Discussion
The Get Healthy! programme was feasible to implement and well received by participants with moderate ID and their carers. Exploratory efficacy data indicates the programme has potential to positively impact important cardiometabolic risk factors such as waist circumference, cardiovascular fitness, and physical strength. Several of the proposed data collection instruments will require modification or replacement prior to use in a sufficiently powered efficacy trial.
Trial registration
ACTRN: ACTRN12618000349246. Registered March 8th 2018—retrospectively registered, https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=374497 UTN: U1111-1209–3132.
Keywords: Intellectual disability; Aging, Physical activity; Exercise; Nutrition; Protocol; Photographic food record; Accelerometry; Intervention; Health promotion
Key messages regarding feasibility
There was uncertainty regarding the feasibility of implementing the Get Healthy! group programme for adults with mild-moderate ID, and whether the selected outcome measures could be reliably administered to the population.
The Get Healthy! programme was feasible to implement, however, several outcome measures required a greater level of training/support to administer than was provided in the feasibility protocol, and a small number were too complex for the participants with moderate ID.
The Get Healthy! programme will be feasible to administer in a sufficiently powered trial; however, several screening and outcome measures will require modification prior to trial commencement.
Background
Despite significant advances in longevity and quality of life, people with intellectual disabilities (ID) continue to experience poorer health outcomes than the general population [1]. The term ‘intellectual disability’ is used to describe any person who experiences ‘significant limitations both in intellectual functioning and in adaptive behaviours, as expressed in conceptual, social and practical adaptive skills. The disability presents or originated during the developmental period before the age of 18 years’ [2]. Many causes of premature mortality in this population are linked to potentially preventable conditions [3]. Lifestyle risks including poor diet quality [4], low levels of physical activity [5], and high rates of sedentary behaviour [6], are prevalent across age groups. People with ID are more likely than the general population to be overweight or obese and have high rates of type 2 diabetes and lipid abnormalities [7, 8]. Common prescribing of high cardiometabolic liability psychotropics in this population [9] further exacerbates risk. Health status, quality of life and health expenditure are all negatively impacted by this high prevalence of lifestyle-related diseases [10, 11].
Tackling lifestyle-related behaviour has been identified as a priority area for improving health outcomes for people with ID [12]. However, people with ID still have low levels of engagement in health promotion initiatives and preventative screenings [1]. Financial, physical, social and disability related barriers limit this population’s ability to access health promotion programmes available to the general population [13]. The limited and inconsistent ID health training received by the medical and allied health workforce [14, 15] means that many care providers lack confidence tailoring health promotion practices to the unique needs of this group.
There is also a lack of clarity regarding the essential components of lifestyle change interventions most likely to improve health outcomes. Evidence for the efficacy of general population healthy lifestyle programmes is robust [16]; however, these programmes are not necessarily generalisable to people with ID. Results from ID population-specific interventions reported in the literature are limited and have had mixed results. Weight loss for adults with ID, for example, has been inconsistently reported across interventions, but appears to be most likely in the context of multi-modal interventions encompassing physical activity, dietary and behaviour change components—see [17] for a review. Methodological weaknesses, use of varied outcome measures and differing population characteristics (i.e. level and cause of ID, age-group, gender, living arrangements) across studies limits comparison of findings [18]. A meta-analysis of randomised-controlled healthy lifestyle trials for adults with ID showed statistically significant improvements in waist circumference only [18].
Further trials are needed to clarify the core components of interventions that will promote engagement and positive lifestyle change in this population. The primary aim of this study is to assess the feasibility of implementing a tailored healthy lifestyle programme, Get Healthy! with adults with mild-moderate ID. The secondary study aim is to explore potential programme efficacy. Results from the feasibility pilot will be used to refine the programme content and data collection protocol prior to undertaking a sufficiently powered efficacy trial.
Background to the ‘Get Healthy!’ programme
Get Healthy! is a 12-week multi-modal lifestyle intervention programme focusing on physical activity and healthy eating for adults (40 + years) with mild to moderate ID, however is suitable for all adults with ID. The programme was developed by a consortium of topic experts in the fields of nutrition, ID, ageing, exercise physiology, nursing, psychiatry, and psychology. A series of focus groups with adults with ID and their paid carers [19] contributed consumer input to the programme design. Table 1 summarises the setting, structure and content of the programme, and lists all behaviour-change techniques used in the programme delivery.
Table 1.
‘GET HEALTHY!’ Summary of program structure and content
| Program setting: Lifestyle Clinic in tertiary research institute | ||
|---|---|---|
| Program structure: 12-week small group program consisting of three face-to-face contact hours per week (1x one hour nutrition session per week plus 2x one hour physical activity sessions held on non-consecutive days). Physical activity sessions were delivered by a practicing Accredited Exercise Physiologist. Nutrition sessions were delivered by Accredited Practicing Dietitians. All instructors had previous experience working with people with intellectual disability | ||
| Program Content | ||
| Physical Activity | Healthy Eating | |
|
10% didactic information Topics covered: What it means to be healthy; Consequences of obesity; Physical activity and screen time guidelines; Appropriate goal setting; Planning for maintenance and self-management; Barriers to Physical activity and how to address them 40% aerobic exercise 30% strength-based exercise 20% balance-based exercise |
90% didactic content Topics covered: The five food groups; Discretionary foods and healthy snacks; Healthy drinks; Portion size and mindful eating; Eating out choices 10% practical food related outings/preparation |
|
| CALO-RE Behavior-change techniques useda | ||
| 1. Information provision (general) | ✓ | ✓ |
| 2. Information provision (to the individual) | ✓ | ✓ |
| 3. Information provision (others’ approval) | ✓ | |
| 4. Information provision (others’ behavior) | ✓ | ✓ |
| 5. Goal setting (behavior) | ✓ | ✓ |
| 6. Goal Setting (outcome) | ✓ | |
| 7. Action Planning | ✓ | ✓ |
| 8. Identifying barriers/Problem resolution | ✓ | |
| 9. Setting graded tasks | ✓ | ✓ |
| 10. Review of behavioral goals | ✓ | |
| 11. Review of outcome goals | ✓ | ✓ |
| 12. Effort or progress contingent rewards | ✓ | ✓ |
| 13. Successful behavior contingent rewards | ✓ | ✓ |
| 14. Shaping | ||
| 15. Generalization of target behavior | ||
| 16. Self-monitoring of behaviour | ||
| 17. Self-monitoring of behavioral outcome | ||
| 18. Focus on past success | ✓ | ✓ |
| 19. Provide feedback on performance | ✓ | ✓ |
| 20. Informing when and where to perform the behavior | ✓ | ✓ |
| 21. Instruction on how to perform the behavior | ✓ | ✓ |
| 22. Demonstrate behavior | ✓ | ✓ |
| 23. Training to use prompts | ||
| 24. Environmental restructuring | ✓ | |
| 25. Agreement of behavioral contract | ✓ | ✓ |
| 26. Prompt practice | ✓ | |
| 27. Use of follow-up prompts | ||
| 28. Facilitate social comparison | ✓ | |
| 29. Plan social support | ||
| 30. Prompt identification as role model | ✓ | ✓ |
| 31. Prompt anticipated regret | ||
| 32. Fear arousal | ✓ | |
| 33. Prompt self talk | ✓ | |
| 34. Prompt use of imagery | ✓ | |
| 35. Relapse prevention | ||
| 36. Stress management | ||
| 37. Motivational interviewing | ✓ | |
| 38. Time management | ||
| 39. Communication skills training | ✓ | |
| 40. Stimulate anticipation of future rewards | ✓ | |
aCALO-RE Taxonomy of Behavior Change. (PDF Download Available). Available from: https://www.researchgate.net/publication/274512164_CALO-RE_Taxonomy_of_Behavior_Change_Techniques [accessed Mar 29 2018]
Methods
The full feasibility pilot protocol has been published elsewhere [20]. Methodology is summarised below for convenience.
Recruitment
Participants were recruited through disability service providers proximal to the healthy lifestyle centre where the intervention was delivered in metropolitan NSW, Australia. Adults who were identified by carers/disability organisations as having mild to moderate ID and concerns about cardiometabolic health were eligible to participate in the programme. The participants’ main carers were also invited to participate either independently in the full programme (carer-protocol A) or as a support person to the enrolled participants with ID (carer-protocol B). Participants who were non-ambulatory, had severe-profound ID, or who were not cleared by their general practitioner (GP) to participate due to either high physical or psychiatric risk, were excluded.
Consent
Written informed consent was obtained from all participants prior to trial commencement. For participants who lacked capacity to consent (~ 70%), written consent was provided by their legal guardian/carer as required by law. All participants with ID also obtained a signed medical clearance from their GP prior to enrollment. The study was conducted in accordance with the ethics approval granted by the UNSW HREC (Approval number: HC17471).
Data analysis
Programme feasibility was assessed across the domains of recruitment and screening, retention, adverse events, session attendance and session engagement. Every session the programme facilitators recorded attendance and scored attendees based on their level of engagement in the session (0 = did not attend, 1 = participated minimally, 2 = participated moderately well to very well). At the completion of the intervention combined scores for every session attended were used to categorise participants into high (75–100%), medium (50–74%) or low engagement (< 50%) groups. Subjective participant experience was gathered in audio-recorded semi-structured exit interviews with all participants. Qualitative data from exit interviews was transcribed and thematically organised using the software programme NVivo (version 11.0.0).
All outcome measures included in the trial are listed in Table 2. The Statistical Package for Social Science (SPSS) was used to analyse percentages, score means and/or frequencies where relevant. Acknowledging the small sample, we used 95% confidence intervals to reported outcomes in order to provide a clinically relevant indication of the direction of the effect being measured.
Table 2.
Clinical outcome measurements/procedures used in the ‘Get Healthy!’ feasibility trial
| Dimension measured | Procedure details |
|---|---|
| Body mass index (BMI) | BMI = weight/height (kg/m2) [22] |
| Waist circumference | Measured at the midpoint between the iliac crest and the lowest rib, in full expiration, to the nearest 0.1 cm while the person is standing [23] |
| Blood pressure | To be measured using sphygmomanometer while the participant is seated and has rested for at least 5 min prior [24] |
| Cardiovascular fitness |
YMCA sub-maximal ergometer test 12 min duration [25]: - Minutes/stages performed - Peak heart rate (%APMHR) - Peak workload achieved |
| Physical activity level and sedentary behaviour |
Subjective data: - International Physical Activity Questionnaire-proxy respondent (IPAQ-pr) proxy report [26] Objective data: - Waist-based GTX3 actigraph accelerometer to be worn for a period of 3–5 days in each data collection period [27] |
| Physical Strength |
- 30-s modified push-up test [28] - Medicine ball throw/chest pass [29] - 10 RM testing [30] - 30-s sit-to-stand test [31] |
| Quality of life | Personal Wellbeing Index-Intellectual Disability (PWI-ID) [32] |
| Dietary intake |
- 3-day photographic food record [33] - Proxy-assisted 24-h recall [34] |
| Healthy literacy | Nutrition and Activity Knowledge Scale for Use with People with an Intellectual Disability (NAKS) questionnaire [35] |
Food intake data was calculated from photographic food and drink records at baseline and endpoint. Data was interpreted and analysed by two Accredited Practicing Dietitians using Foodworks® (version 9) nutrition analysis software (Xyris Software, 2018). Days with less than three meals captured were removed prior to analysis. A Healthy Eating Index for Australian Adults (HEIFA) [21] was then applied to determine overall diet quality.
Results
Six participants with ID and two carer participants completed the full screening process and were enrolled in the trial.
Participant demographics
Table 3 summarises demographics of participants with ID.
Table 3.
Demographics of participants with ID
| Age (years) | Gender | Level of ID | Mobility status | Type of residence | Co-morbidities |
|---|---|---|---|---|---|
|
Mean: 46 SD: 13 Range: 28–62 a |
Male (n = 4) Female (n = 2) |
Moderate b (n = 6) |
Able to ambulate independently (n = 5) Ambulate with cane (n = 1) |
Group disability housing (n = 4) With family (n = 1) Independently (n = 1) |
Obese (n = 3) Overweight (n = 2) Autism (n = 1) Impaired glucose tolerance (n = 1) Ventricular septal defect, and valvular heart anomalies (n = 1) |
aFour of the six participants were aged 40 years and over. A further two participants below this age bracket were included because they expressed an interest in improving cardiometabolic fitness
bLevel of ID was based on the assessment of the research team delivering the ‘Get Healthy!’ intervention
Carer participant demographics
Due to competing time commitments and variations in work schedules no family members or paid carers were able to enrol in the full programme (carer-option A). Two paid carers enrolled in the option B participation pathway. This participation pathway involved attending sessions in a support capacity as able. Both enrolled carer participants were female, over 18 years of age, and employed as paid disability staff. They supported several of the participants with ID in residential and day care settings. On average, these carers attended approximately 50% of the available sessions. Since carer protocol B did not include collection of outcome measures data, all efficacy data reported below pertains to the participants with ID only Table 4.
Table 4.
Feasibility of outcome measures
|
OUTCOME MEASURE |
Measure completed reliably and fully at both time points | Incomplete data returned or problems with measure validity noted | Identified problems with administration and or validity of measure |
|---|---|---|---|
| Anthropometric measures | X | ||
| Measures of cardiovascular fitness | X | ||
| Measures of physical strength (excluding 10RM) | X | ||
| Knowledge Scale for Use with People with an Intellectual Disability (NAKS) questionnaire | X | While all participants completed this measure, wide variability in baseline to endpoint scores raise questions about instrument reliability for our cohort: For example, one participant scored 13 at baseline but went on to score a significantly lower score of 5 at endpoint. Since it is unlikely that participants would ‘lose’ this amount of knowledge in a 12-week time frame it is possible that scores reflect guess-work rather than change in knowledge | |
| The Personal Wellbeing Index- Intellectual Disability (PWI-ID) | X | While all participants agreed to undertake the measure significant differences in pre-testing scores from baseline to endpoint raise concerns about instrument validity in our cohort: At baseline two participants were unable to complete step two of the pre-testing process. We were therefore unable to administer the measure to them. However, at endpoint, the same two participants were able to complete the full pre-testing protocol and the 11-point scale. The extremely high scores these participants recorded on the measure at endpoint (100 and 92.9 respectively) raise questions about the reliability of their responses, however. At baseline the remaining four participants pre-testing scores indicated that they were unable to complete the 11-point scale, however, at endpoint they were all able to appropriately answer the pre-testing questions and thus had the 11-point scale administered to them | |
| 24 hour food recall | X | While this form was handed to each participant and the support worker who attended the session with them, no completed or partially completed forms were returned at baseline or endpoint: Participants were unable to independently recall what they had eaten at previous meals, and family members and carers did not complete the form on their behalf | |
| Food photography | X | Only two participants provided photographic data at both the pre-and post-program data collection periods. While the two participants captured three full days at baseline, neither reached the target of a three complete photographic records at endpoint (capturing 1 and 2 days only). One participant declined to undertake this task at both time-points (reason was not stated). The remaining three participants either did not take photos despite agreeing to undertake the task, took incomplete days of records or took photos in which they had blocked the camera lens with their hand or clothes | |
| Accelerometer data | X | One participant (baseline) and two participants (endpoint) did not meet the minimum wear time of at least six hours on three out of the five wear days that was stipulated in our protocol. One participant who had a co-occurring diagnosis of autism, struggled with wearing the device due to sensory issues (stated he dislikes the feel of the device around his waist) | |
| IPAQ-proxy | X | While this form was handed to each participant and the support worker who attended the session with them, only two of the forms were returned at baseline or endpoint, and these were insufficiently completed to provide meaningful data | |
| Physical Strength: 10RM strength testing | X | We were unable to reliably establish participants’ rate of perceived exertion in the pre-testing phase of the protocol and thus were unable to administer this outcome measure. Inability to establish perceived rate of exertion was related to difficulties participants experienced using even a modified scale to rate their level of exertion. For example, participants, both in cases where the weight used was extremely light and in cases where the weight used was so heavy the participants could not attempt the task, reported the exercise as “easy”. Without this baseline measurement, all participants commenced the program on the lowest weight available and the decision to increase weight was based on technique alone and experienced Exercise Physiologist decision making |
Feasibility outcomes
Recruitment and screening
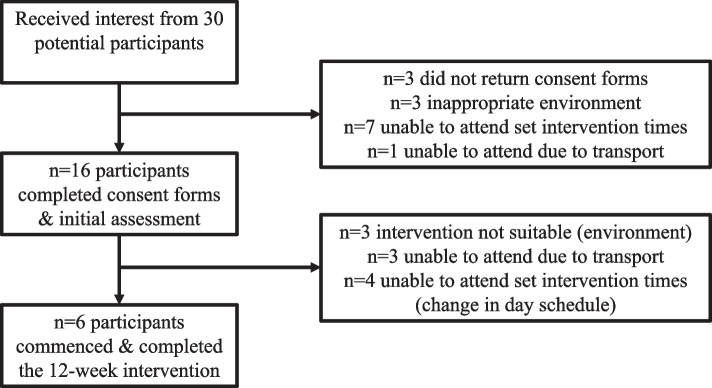
Recruitment was completed between July 2017 and February 2018. Thirty people with ID expressed an initial interest in participating in the trial programme. Of these, 14 were either unable to complete the consent form, or unable to determine a suitable time to attend the initial assessment. Sixteen participants completed the consent form and participated in the initial assessment. Ten participants dropped out during this screening process. Reasons for drop-out during the screening process included:
Scheduling and/or transport problems (n = 7)
Having a level of ID (severe to profound) that meant the person was unable to participate in the group learning structure of the ‘Get Healthy!’ programme (n = 3).
GPs screened each of the remaining six participants and provided signed consent for their participation in the feasibility trial (Fig. 1). Recruitment was ceased in February 2018 (6 months) in accordance with the funding allocated.
Fig. 1.
Consort diagram of participant recruitment
Retention rate
All six participants with ID who completed the full screening process and enrolled in the study went on to complete the programme, representing a 100% retention rate.
Attendance and session engagement
On average, participants with ID attended 75% of sessions offered as part of the programme. Attendance rates at physical activity and nutrition sessions were similar (74% and 76% respectively). The top reason participants missed scheduled sessions was to attend medical, allied health or dental appointments that had been arranged prior to study enrolment. Mean participant engagement scores across all sessions attended was ‘high’ (91%); however, participants were significantly more engaged in the physical activity sessions compared to the nutrition sessions (respective mean engagement scores of 99% versus 77%).
Outcome measure feasibility
Table 4 summarises the feasibility of all outcome measures according to whether they were.
-
(i)
Reliably administered to all participants at both baseline and endpoint, or.
-
(ii)
Either unable to be administered or administered but returned incomplete or unreliable data sets.
For all outcome measures where problems with data reliability or completeness were noted, specific issues of concern are listed. No adverse events were experienced by any participants.
Clinical outcomes
Anthropometric measures
Table 5 lists the groups’ mean baseline and endpoint anthropometric data. There was a decrease in the groups mean waist circumference (WC) from baseline to endpoint (95% CI: − 3.20, − 0.17 cm). Individually, one participant gained 0.5 cm in WC during the intervention, while all five other participants displayed reductions in WC (− 0.4 cm; − 2.2 cm; − 2.4 cm; − 3.4 cm; − 2.2 cm). There was no clinically significant change in the groups mean weight (95% CI: − 1.6, 1.9) or BMI (95% CI: − 0.80, 0.90) from intervention baseline to endpoint, with three participants displaying a non-significant increase in BMI post intervention, and three participants displaying a non-significant decrease.
Table 5.
Anthropometric means (± SD) at intervention baseline and endpoint
| N | Baseline mean (± SD) | Endpoint mean (± SD) | |
|---|---|---|---|
| Weight (kg) | 6 | 79.87 (11.78) | 80.00 (10.27) |
| BMI (kg/m2) | 6 | 32.86 (7.53) | 32.88 (7.12) |
| Waist Circumference (cm) | 6 | 108.32 (16.02) | 106.63 (15.50) |
Cardiovascular fitness (CV fitness)
Table 6 lists the groups mean CV data at baseline and endpoint. The mean number of minutes participants were able to undertake the activity increased from baseline to endpoint (95% CI: 2.44, 7.73). Similarly, the mean number of stages participants were able to perform increased from baseline to endpoint (95% CI: 1.16, 3.24 stages). The peak workload participants were able to achieve also increased from baseline to endpoint (95% CI: 49.17, 64.98). While none of the six participants were able to complete the full protocol at baseline, three participants were able to complete the protocol at endpoint. There were numerical improvements for mean YMCA Peak HR and APMHR from baseline to endpoint.
Table 6.
Cardiovascular fitness-means (± SD) at intervention baseline and endpoint
| Baseline mean (± SD) N = 6 |
Endpoint mean (± SD) N = 5 |
|
|---|---|---|
| YMCA minutes completed | 2.81 (2.19) | 7.80 (1.64) |
| YMCA stages completed | .50 (.84) | 2.6 (.55) |
| YMCA peak heart rate | 125.83 (25.93) | 129.60 (22.53) |
| YMCA % APMHR | 71.47 (13.30) | 74.74 (13.68) |
| YMCA peak workload | 70.83 (43.06) | 120.00 (57.00) |
Physical strength
All physical strength parameters showed numerical improvements across the intervention The mean improvement in the Sit To Stand (STS) exercise of 2.93 (95% CI: − 0.18, 7.00) from baseline to endpoint is promising, given that an improvement of STS = > 2 reps may be clinically significant [36] particularly in relation to falls risk Table 7.
Table 7.
Physical strength-means (± SD) at intervention baseline and endpoint
| N | Baseline mean (± SD) | N | Endpoint mean (± SD) | |
|---|---|---|---|---|
| 30sec push-ups | 5 | 16.20 (3.56) | 6 | 16.83 (2.48) |
| 5kg medicine ball chest pass (m) | 6 | 2.49 (0.43) | 6 | 2.74 (0.48) |
| 30sec sit to stand | 5 | 12.40 (2.88) | 6 | 15.33 (2.73) |
Structured aerobic exercise conducted throughout intervention
Cycling duration—session 1 started at 9.0 (± 2.0) min (n = 4) increased to 13.4 (± 1.4) min by session 24 (48.9% increase) (n = 6). Similarly, this is reflected by the distance cycled during each session—session 1 started at 2.7(± 1.3) km (n = 4), which increased to 5.6 (± 1.1) km by session 24 (107.4% increase) (n = 6).
Accelerometer data
Five participants (baseline) and four participants (endpoint) had sufficient accelerometer wear-time to meet the threshold for data analysis set in our protocol. Their results are summarised in Table 8 and Fig. 1.
Table 8.
Objective physical activity data—means (± SD) at intervention baseline and endpoint
| Baseline mean (± SD) N = 5 |
Endpoint mean (± SD) N = 4 |
|
|---|---|---|
| Sedentary | 643.94 ± 198.07 | 652.74 ± 128.57 |
| Light | 108.02 ± 78.72 | 73.42 ± 27.08 |
| Moderate | 24.96 ± 13.38 | 29.73 ± 10.21 |
| Vigorous | 0.60 ± 0.97 | 0.36 ± 0.25 |
| MVPA | 25.56 ± 12.98 | 20.06 ± 17.42 |
Total MVPA across the week (pre) —105.35 ± 47.11 min (n = 5) vs. post-intervention 133.48 ± 73.21 min (n = 4).Those meeting the PA guidelines (150 min of moderate PA) pre: 1, increased to 2 post-intervention.
Food intake
Only two participants (E and A) completed the food photography task to a sufficient extent to allow for a preliminary analysis to be undertaken. Key nutrition baseline and endpoint data for these participants are summarised in Table 9. Both participants decreased their total fat and saturated fat intake from baseline to endpoint. Wholegrain intake improved at endpoint; however, results for refined grain intake were mixed. While participant E’s HEIFA score increased from baseline to endpoint, indicating better overall diet quality, Participant A’s score decreased over the same period. Overall, average daily energy was lower for both participants at endpoint, along with most macronutrient and many micronutrients. It is unclear if these patterns reflect real changes in diet quality or the fact that both participants recorded fewer complete days of data at endpoint compared with baseline.
Table 9.
Food intake at intervention baseline and endpoint
| Participant ID | A | E | ||
|---|---|---|---|---|
| Timepoint | Baseline | Endpoint | Baseline | Endpoint |
| HEIFA scorea | 43 | 38 | 43.75 | 47 |
| Whole grain | 1.86928 | 2.588864 | 3.010851 | 3.972731 |
| Refined grain | 2.805117 | 2.017218 | 5.401268 | 5.543118 |
| Energy DF (kj) | 6973.163 | 5663.742 | 8558.675 | 4794.232 |
| Protein(g) | 91.17966 | 72.96273 | 92.15803 | 44.85995 |
| Carbs available (g) | 180.365 | 134.8632 | 245.0325 | 191.0486 |
| Total fat (g) | 57.93966 | 51.95127 | 72.11869 | 17.68252 |
| Saturated fat (g) | 20.43112 | 19.24836 | 30.19545 | 7.440686 |
| Dietary fibre (g) | 27.31206 | 22.57688 | 20.64223 | 19.43015 |
aHeifa score is out of 100 possible points- higher scores indicate better diet quality and correlates to greater adherence to national dietary guidelines
Health literacy
Results from this trial showed no difference in mean NAKS scores from baseline (15.17) to Endpoint (13.17). Two of the participants recorded higher scores at endpoint while the remaining three participants recorded lower scores at endpoint. As identified in Table 3, there were concerns about the reliability of these data. All six participants completed the NAKS questionnaire pre- and post-intervention (n = 6).
Quality of life
Only three of the six participants passed the baseline pre-testing phase for the PWI-ID measure. Matched pre-post intervention data for these three participants shows no significant change in mean quality of life scores (baseline mean 88.2 vs. endpoint mean of 83.3). One of the three participants showed an increased score at endpoint, while the other two recorded decreased scores. As identified in Table 3, there were concerns about the reliability of these data.
Participant experience
All participants with ID, along with the two carer participants, participated in exit interviews. Qualitative feedback, including programme highlights and suggestions for improvement, were elicited, and thematically analysed. Core themes emerging from the exit interviews are summarised below.
Programme benefits
Participants highlighted several beneficial impacts from being involved in the Get Healthy! programme, including a sense of pride and achievement; improved knowledge of and commitment to healthy lifestyle change; increased opportunities for positive social interactions; and improved ability to set future healthy lifestyle goals. Table 10 provides contextualised data illustrating these positive impacts.
Table 10.
Programme highlights and benefits: qualitative participant feedback
| Programme highlight | Quotes from participants with intellectual disability | Quotes from carer participants | |
|---|---|---|---|
| Programme fostered a sense of pride and achievement |
“When I was riding the bikes [the instructor] would say, ‘come on, keep on going, keep on going’. I thought that was good because it made me feel like I was losing weight...pushing that bit more.” Participant F “I liked them [reward stickers] …That means I went on the bike with a high score… [so I was motivated] I did more”. Participant E |
“I just really really like the whole [program] environment. I mean, [the instructor] was just amazing with the guys. She was always extremely positive. All the guys wanted to go each there because of her. … she was so calm and confident, that she was able to transmit that energy to the guys and then when they were there they wanted to be part of the activity, they wanted to be part of the exercise.” Carer 2 ““[Their] confidence grew with the [bike] equipment. In the beginning they were really hesitant and just did two minutes, then towards the end they were competing against each other- you know, pushing buttons themselves, and setting things up and all that sort of stuff. So the equipment confidence grew, they jumped in.” Carer 2 |
|
| Programme Increased knowledge of and commitment to healthy lifestyles: | Healthy eating |
“I liked learning about how much sugar was in the coca cola… “[I eat] better now [post-program]- at home, eat salad” Participant D “[I liked] learning about different foods, and what you can have and can’t have. I thought it [the program] was very good because it helped me out a lot.” Participant F “Well I [still] love eating food and sometimes I love ice-cream. But only one. Not every day”. Participant A “I eat a small amount now…a small one [plate of pasta] and vegetables and that…I started on the program, drinking it [water]…[and now I] eat fruit. Its really healthy- eat bananas and that and eat grapes” Participant E |
“The guys used to come back and talk a lot about the healthy eating choices, and what they spoke about in the education sessions. And I know that they liked going out to different restaurants or shopping centres and participating in a healthy choice option. And there were a few of them that, you know, on days where they had brought their own morning tea or lunch in from home, you could see that they had made that healthy, conscious choice about buying the right things.” Carer 2 |
| Physical activity |
“[I exercise more now because] It’s healthy for your heart” Participant D Yeah, walk- park- everyday [now]” …Exercise is very good. Very good idea. Makes sense. I loved it so much”. Participant A “I think exercising does you good because if you’re not exercising then you’re watching TV and I think you’ve got to get out, get away from it and go for a walk” Participant F |
“A few of the guys [in the program], their doctors had actually reported improvements and were asking what was going on. One participant who was in the group, his respiratory doctor, said his lung capacity was, he couldn’t believe the change that had happened. Because you know that test where you blow into the thing and the balls go up- he said it was like a different person from when he did it the last time, to when he happened to do it just towards the end of the program.” Carer 2 | |
| The programme provided valued opportunities for social interaction |
“[I liked exercising] with my friends… I liked the people that taught me how to do it [exercise] Participant F” “[I liked spending time with] the staff’ Participant E |
“I think they enjoyed mixing with the university staff and the other different physiotherapists … they developed a bit of a friendship with a few people there. And also some of the other, some of the others participants- patients- some of the other older people coming in, they enjoyed having a chat with them every week as well” Carer 1 “They all had fun [doing the program together], they were always- because I used to drive them [to the program] on the Tuesdays- they were lined up at my car ready to jump in to go every week. I was never having to chase anyone or find out where they were.” Carer 2 |
|
| The programme helped participants to identify future healthy lifestyle goals |
“I do need help, because sometimes I’ve got problems with what I eat…I think I have to cut back on sweets. I eat too much from the top [of the food pyramid]…. [I’m going to] give up sugar, it’s not good. Too many Coca Colas not good” Participant A “I want to change, so I get back to the size that I was before, instead of all of this weight, a healthier weight, maybe get help staying away from fatty foods” Participant F |
“[one participant] you made such a positive change in her life that now when we are discussing her future goals, one of her goals is that she says she wants to do a cooking program. And the reason she wants to do a cooking program is because she wants to learn how to eat healthy…So I just want to point out that the program has really made a positive change in someone’s life.” Carer 1 | |
Programme problems and challenges
Participants with ID did not identify many areas for programme improvement, despite being explicitly asked. One participant stated finding that using the bike, “made me tired”, and another participant described struggling with motivation to get out of bed and attend the programme: “Maybe getting out of bed [to come, was hard]. I wanted to stay snuggly and warm and I didn’t want to get out of a warm bed” (participant F). Carer participants, however, identified several areas for programme improvement. These are summarised in Table 11.
Table 11.
Programme problems and challenges: qualitative participant feedback
| Programme challenge | Quotes from carer participants |
|---|---|
|
The programme was inadequately resourced: While the costs of the programme sessions were covered by the research team, transport to and from the sessions as well re-imbursement for carer time, was not covered. These out-of-pocket costs created financial stress for the participating organisation |
“There wasn’t any additional resources or, um, I don’t know, supports we had available to us for the program. Like it was, time that I had to put aside out of my week and the other carer had to do the same, and we had, you know, to stop other clients using the vehicle so that we could use the vehicle on a Thursday [to get to the program]. So it was a bit of a challenge because we had clients from all different parts of [the disability organization]…like on the days we couldn’t get a vehicle, the taxi to get us all there ended up being, like $120 bucks, just in a taxi to do a turn around there and back” Carer 2 |
| Communication between programme facilitators and formal and informal carers was inadequate | “I did get a copy of handouts [from the program] because I would request it, but in other cases the guys were getting a copy of the handouts but I’m not sure if the support worker or the homes they were living were getting a copy as well. So you don’t know if the guys are taking the paper then they don’t want it anymore and then they trash it… the group homes or the families where they are living need to told, even if its on an email so the group homes or families have access to the same information because some guys are very particular about their things being touched.” Carer 1 |
| Issues with malfunctioning physical activity equipment created stress for some participants | “Towards the end of the program the straps on the exercise bike on the seat broke, and, um, because they weren’t working for a few weeks, it just was a little bit difficult for some of our clients to say, use the exercise bikes without the straps because they’d got used to them.” Carer 2 |
| Healthy eating component of the programme was too theoretical | “For the nutrition [sessions]….I think it would have been more beneficial for the guys to learn in practical ways about food… I think that the talking was good, but I just know from experience that they need practice…. so perhaps teaching them to make a healthy lunch would have been a bit better than to just talk about it… Because I know that they learn by seeing, by doing, by touching” Carer 1 |
|
Lifestyle changes may not be sustained once programme is over: While carers highlighted a number of benefits resulting from programme participation, they expressed concern that changes may be unsustainable without further buy-in from family members and paid support staff |
“If they [participants with ID] don’t have a constant support, or a program in place with someone, or a group of people will be taking them every week to continue these [healthy lifestyle] approaches, …it just won’t happen. They won’t independently go and do it. Either they need the assistance to travel somewhere, or they need someone’s guidance to help them use the equipment in the gym, and …they need someone there to give them that push”. Carer 2 So I think that it’s got to be a real commitment, not just from the practitioners perspective but also from the families perspective, because without their support they can't really do it alone. The ones that did [make healthy lifestyle changes] had extra support, whether that was in a group home or it was at home. So, yeah, it’s got to be a group agreement, it’s not just the participant. Because if the participant wants to lose weight, they want to do exercise but they live in a group home unless the carer takes them out they wont be able to do exercise. Its compromising, it’s finding a comprise, within the organisations where they are living. And keeping them accountable as well, do you know what I mean?” Carer 1 |
Discussion
Results from the Get Healthy! feasibility pilot indicate that the programme was well received by a small group of adult participants with moderate ID and their carers. The programme has potential to positively impact several indicators of cardiometabolic health.
Reflections on programme feasibility
Screening: Only participants screened by GPs as safe to participate were included in the trial, however, GPs were not required to provide programme facilitators with details of each participant’s specific health conditions. Unfortunately, not all participants and/or carers in this feasibility trial were able to reliably self-report relevant medical conditions. For the planned efficacy trial we therefore recommend replacing the generic medical consent form, which only asks if any restrictions should be placed on the person’s participation, with a more detailed form prompting the GP’s to indicate whether or not the person has a known diagnosis of: high blood pressure, diabetes, asthma, allergies, cardiac complications, lipid abnormalities, musculoskeletal conditions, or psychiatric or behavioural issues that may impact on programme participation. GPs should also be requested to provide an up-to-date list of all medications the person is currently prescribed. Knowledge of these conditions can support programme facilitators to better manage risk and tailor the programme more effectively to each participant’s needs.
Increasing programme engagement
While overall programme attendance rates were acceptable and mean engagement scores were high, participants were notably less engaged in the nutrition component of the programme, compared to the physical activity sessions. Qualitative feedback from the exit interviews suggests that decreasing didactic teaching content and increasing practical activities related to food choice and preparation may increase engagement in nutrition sessions for the efficacy trial. An additional issue detracting from programme feasibility was limited carer involvement. Only two carers regularly attended the programme with participants, and no clear channels of communication were established between programme facilitators and carers who did not attend. Prior research has highlighted that carer buy-in can significantly improve the extent to which people with ID engage in and sustain healthy lifestyle behaviours [37–39]. Developing supplementary on-line or other written teaching content that carers can engage with remotely and developing a schedule of home-visits by programme facilitators, may help to build closer relationships with carers during the efficacy trial.
Improving data collection
Problems arose with the completeness and/or reliability of data from several of the outcome measures used in the feasibility pilot. A number of factors are likely to have contributed to this issue: Firstly, several of the measures (i.e. 24-h food recall, food photography, accelerometers, IPAQ-pr) required considerable carer support to complete. Retrospectively it is clear that the pilot protocol did not include a sufficiently robust carer training and follow-up schedule to ensure that full data sets were collected. The carer handouts and instructions sheets, for example, were not necessarily passed on from the participants with ID to their home carers and Get Healthy! programme facilitators did not have access to home carer contact information.
Since food photography [40–42], use of accelerometers [43, 44] and the IPAQ-proxy [26] have all been shown in previous studies to be reliable and viable to implement in adult populations with ID, we recommend keeping these measures in the protocol for the efficacy trial. However, the protocol should be modified to allow programme facilitators to liaise directly with carers to provide them with task training. A schedule of telephone prompts and face-to-face support should also be implemented during data collection periods.
Secondly, it is possible that several of the trial outcome measures, specifically, NAKS, PWI-ID and 10RM strength testing, were too complex and therefore inappropriate for our study participants, whom had a more ‘moderate’ spectrum of ID. The planned 10RM physical strength testing, for example, was unable to be implemented due to cognitive difficulties participants experienced using even a simplified rate of perceived exertion scale. Despite our AEP using clinical judgement to determine endpoint of 10RM testing (e.g. facial grimacing, perceived exertion, and technique safety), we believe that the values obtained do not represent individual’s true 10RM. To increase trial efficacy, we recommend replacing this measure with an objective assessment with simplified protocol measures (and reduced risk), such as a hand-grip strength test for upper body strength. Functional testing parameters, inclusive of normative data validated within this population remains limited, with future research looking to widen appropriate assessment selection.
Similarly, the NAKS measure may have been too complex for several participants in this study. While the NAKS has been validated in populations with mild ID [35], it requires participants to be able to meaningfully choose from four options. We recommend that a pre-testing protocol be implemented in the efficacy trial to assess whether participants are capable of meaningfully choosing from four options. Another issue of concern that arose with administrations of the NAKS was presence of carers, who in some cases attempted to ‘prompt’ participants with correct answers. For the planned efficacy trial, we recommend administering the NAKS without a carer present wherever possible. Should the participant wish to have a carer present in a support capacity, we recommend providing additional guidance to the carer to refrain from prompting the participant’s answers.
In the PWI-ID validation study [45], which included adults with mild and moderate ID, all participants were able to be administered at least the most basic (2-point scale) index. However, in our pilot, baseline pre-testing identified participants who were unable to be administered even this 2-point scale. This finding suggests several of our participants may have had a greater degree of intellectual impairment compared to the validation study cohort. The other issue of concern we experienced with the PWI-ID involved participants passing the pre-testing phase but then scoring at the top of the response range across all seven domains. Such a scoring pattern is most likely the result of acquiescent responding, a known issue among populations with ID [46]. The original validation study for this measure [45] also encountered this issue with data from 32% of respondents needing to be removed prior to analysis due to suspected acquiescent responding. We recommend excluding suspected acquiescent response data from analysis in the efficacy trial. Participants who fail the baseline pre-testing protocol should not have the measure re-administered at endpoint.
Reflections on potential programme efficacy
Efficacy data from the trial are exploratory in nature, given the small sample size, and multiple missing data-points. Preliminary findings, however, indicate that compared to baseline, most participants in the ‘Get Healthy!’ programme recorded clinically meaningful reductions in waist circumference and some improvement in measures of cardiovascular fitness. Some participants also displayed clinically meaningful improvements in physical strength at programme endpoint. BMI, quality of life, and objectively measured health literacy did not appear to improve from baseline to endpoint. Dietary intake patterns were mixed and analysis was limited due to incomplete data.
The decreases in waist circumference recorded for all but one participant is a promising finding, given that waist circumference provides a relatively simple and accurate reflection of central adiposity [47, 48]. Decreased central adiposity, in turn, is a strong predictor of lower risk for hypertension, diabetes mellitus, dyslipidemia, metabolic syndrome, and coronary heart disease [49, 50]. Reassuringly, given the lack of weight loss among study participants, this finding holds true irrespective of changes to BMI [50].
Study participants displayed some improvements in cardiovascular fitness from baseline to endpoint based on the YMCA sub-maximal testing protocol. Our participants significantly increased their clinical cardiovascular fitness throughout this intervention. Participants not only increased (178%) their duration of cycling (2.81 vs. 7.80 min) but also their workload (70.83 vs. 120 W) by 69% post-intervention, while maintaining a steady HR (70–65% APMHR). This indicates that participants were able to exercise longer at an increased workload, using the same amount of energy, indicating increased cardiovascular fitness. This is supported by the number of participants able to complete the YMCA sub-maximal testing protocol post-intervention (3 participants vs 0 participants pre-intervention). Based on post-intervention data, the average estimated VO2 was 2.14 L/min (31.51 ml/kg/min) indicating ‘poor’ cardiovascular fitness [51]. Poor cardiovascular ability to sustain prolonged physical work is a powerful predictor of morbidity and all-cause mortality as well as cardiovascular specific mortality [52, 53]. Improvements in measures of cardiovascular fitness, if confirmed in a sufficiently powered efficacy trial, would thus be another strong argument to implement the programme more widely among this at-risk population. Given that no participants were able to complete this incremental YMCA protocol pre-intervention, in addition to the poor cardiovascular fitness measured, we suggest fellow researchers consider the inclusion of a steady-state cardiovascular cycling protocol, such as the Astrand Rhyming Test, or modification to the YMCA step test to further increase data collection and efficacy.
Improvements in measures of physical strength were also noted for some participants from baseline to programme endpoint. Again, this result, if replicated in a sufficiently powered efficacy trial, would be promising in terms of cardiovascular risk reduction. Improved physical strength has been shown to have an attenuating effect on premature all-cause mortality [54], as well as lifestyle-related disease such as diabetes [55], stroke [56] and obesity [54]. Our physical strength data highlights poor upper and lower body strength for adults with ID. Of particular note, is lower limb endurance and falls risk, indicative through the 30-sec STS data. Despite our cohort having a mean age of 46, this 30-sec STS data indicates increased falls risk for adults aged 60–64 years. Despite our intervention showing clinically meaningful improvements (12.40 pre- vs. 15.33 post-intervention) in this outcome measure, post-intervention data continued to represent increased falls risk for an age bracket 14–18 years their senior, highlighting the need for continued exercise interventions and health supports in this population.
A point of further discussion includes the relatively large age range of the study participants (28–62 years of age). Despite concerted efforts of the research team to recruit people with ID 40 + years of age, due to the nature of the disability service providers who expressed interest in this study, we received a large age range of eligible participants. We must highlight the variances in physiological adaptations based on the ageing process, particularly on the ability to build muscular strength and improve cardiovascular fitness as a limitation of this study. This large age range could be a contributing factor in the diversity of change seen across our physical outcome measures. Further efficacy studies should look to either narrow the demographic age bracket of participants, or perhaps target the exercise intervention dependent on age.
Conclusion
The ‘Get Healthy!’ feasibility pilot was well attended and positively received by participants and carers. Outcome data, while exploratory in nature, suggests the programme has potential to improve several important indicators of cardiometabolic health including waist circumference, cardiovascular fitness and physical strength. Problems with missing data-points and potentially unreliable data were identified, however, and several of the study outcome measures will require modification or replacement prior to implementing a full-scale efficacy trial. Further attention should also be given to improving carer buy-in to maximise data collection and programme impact and sustainability.
Acknowledgements
We thank programme participants and their support networks for their participation and feedback. Ward PB, Curtis J, Tzarimas C, Watkins A, Rosenbaum S, and Samaras K also contributed to the overall project idea and funding application. Student researchers Guo, X; Bartter, J; Chen, A; Chan, J.; Wang, Q; Heinonen, T; Chub, C and Tripodi, E. helped to develop programme content. The UNSW Lifestyle Clinic kindly donated space and equipment for the physical activity component of the programme. We thank all these contributors for their support.
Abbreviations
- AEP
Accredited Exercise Physiologist
- BMI
Body mass index
- CV Fitness
Cardiovascular fitness
- ESSA
Exercise and Sports Science Australia
- HEIFA
Healthy Eating Index for Australian Adults
- ID
Intellectual disability
- IPAQ-pr
International Physical Activity Questionnaire-proxy respondent
- NAKS
Nutrition and Activity Knowledge Scale for Use with People with an Intellectual Disability
- PWI-ID
Personal wellbeing index-intellectual disability
- STS
Sit to stand
- WC
Waist circumference
- WFR
Weighted food record
Authors’ contributions
JB interpreted the physical activity data and was a major contributor to writing the manuscript. ST supported nutrition data interpretation and contributed to writing the manuscript. RR and HS interpreted the nutrition related data and contributed to writing the manuscript. EE provided insight into the quality-of-life measures and contributed to writing the manuscript. CS interpreted exit interview data and was a major contributor in writing the manuscript. JT reviewed all data analysis and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Funding
The pilot feasibility study was funded by the NSW Department of Family and Community Services under grant RG161748. The funding body has not been involved in designing the ‘Get Healthy!’ programme or the study protocol.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was conducted in accordance to the ethics approval granted by the UNSW HREC (Approval number: HC17471).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Carmela Salomon, Email: carmela.odonoghue@yahoo.com.au.
Jessica Bellamy, Email: j.bellamy@unsw.edu.au.
Elizabeth Evans, Email: liz.evans@cid.org.au.
Renae Reid, Email: renaereiddietitian@hotmail.com.
Michelle Hsu, Email: michelle.hsu@sydney.edu.au.
Scott Teasdale, Email: scott.teasdale@health.nsw.gov.au.
Julian Trollor, Email: j.trollor@unsw.edu.au.
References
- 1.Havercamp SM, Scott HM. National health surveillance of adults with disabilities, adults with intellectual and developmental disabilities, and adults with no disabilities. Disabil Health J. 2015;8(2):165–172. doi: 10.1016/j.dhjo.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organisation. International statistical classification of diseases and related health problems. 11th ed: World Health Organisation; 2019.
- 3.Trollor J, Srasuebkul P, Xu H, Howlett S. Cause of death and potentially avoidable deaths in Australian adults with intellectual disability using retrospective linked data. BMJ Open. 2017;7(2):e013489. doi: 10.1136/bmjopen-2016-013489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoey E, Staines A, Walsh D, Corby D, Bowers K, Belton S, et al. An examination of the nutritional intake and anthropometric status of individuals with intellectual disabilities: results from the SOPHIE study. J Intellect Disabil. 2017;21(4):346–365. doi: 10.1177/1744629516657946. [DOI] [PubMed] [Google Scholar]
- 5.Dairo YM, Collett J, Dawes H, Oskrochi GR. Physical activity levels in adults with intellectual disabilities: a systematic review. Prev Med Rep. 2016;4:209–219. doi: 10.1016/j.pmedr.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melville CA, Oppewal A, Elinder LS, Freiberger E, Guerra-Balic M, Hilgenkamp TI, et al. Definitions, measurement and prevalence of sedentary behaviour in adults with intellectual disabilities—a systematic review. Prev Med Rep. 2017;97:62–71. doi: 10.1016/j.ypmed.2016.12.052. [DOI] [PubMed] [Google Scholar]
- 7.Balogh RS, Lake JK, Lin E, Wilton A, Lunsky Y. Disparities in diabetes prevalence and preventable hospitalizations in people with intellectual and developmental disability: a population-based study. Diabet Med. 2015;32:235–42. doi: 10.1111/dme.12573. [DOI] [PubMed] [Google Scholar]
- 8.Rimmer JH, Yamaki K, Lowry BD, Wang E, Vogel LC. Obesity and obesity-related secondary conditions in adolescents with intellectual/developmental disabilities. J Intellect Disabil Res. 2010;54(9):787–794. doi: 10.1111/j.1365-2788.2010.01305.x. [DOI] [PubMed] [Google Scholar]
- 9.Salomon C, Britt H, Pollack A, Trollor J. Primary care for people with an intellectual disability—what is prescribed? An analysis of medication recommendations from the BEACH dataset. BJGP Open. 2018;2:bjgpopen18X101540031. doi: 10.3399/bjgpopen18X101541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li H, Fujiura G, Magaña S, Parish S. Health care expenditures of overweight and obese US adults with intellectual and developmental disabilities. Res Dev Disabil. 2018;75:1–10. doi: 10.1016/j.ridd.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rimmer JH, Yamaki K. Obesity and intellectual disability. Ment Retard Dev Disabil Res Rev. 2006;12(1):22–27. doi: 10.1002/mrdd.20091. [DOI] [PubMed] [Google Scholar]
- 12.Emerson E, Baines S, Allerton L, V. W. Health inequalities & people with learning disabilities in the UK. Improving Health and Lives: Learning Disability Observatory. 2011.
- 13.Kuijken NMJ, Naaldenberg J, van der Nijhuis-Sanden MW, de Valk Schrojenstein-Lantman HMJ. Healthy living according to adults with intellectual disabilities: towards tailoring health promotion initiatives. J Intellect Disabil Res. 2016;60:228–241. doi: 10.1111/jir.12243. [DOI] [PubMed] [Google Scholar]
- 14.Trollor JN, Eagleson C, Turner B, Salomon C, Cashin A, Iacono T, et al. Intellectual disability health content within nursing curriculum: an audit of what our future nurses are taught. Nurse Educ Today. 2016;45:72–79. doi: 10.1016/j.nedt.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Trollor JN, Ruffell B, Tracy J, Torr J, Durvasula S, Iacono T, et al. Intellectual disability health content within medical curriculum: an audit of what our future doctors are taught. BMC Med Educ. 2016;16(1):105. doi: 10.1186/s12909-016-0625-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dusseldorp E, Genugten LV, Buuren SV, Verheijden MW, Empelen PV. Combinations of techniques that effectively change health behavior: evidence from Meta CART analysis. Health Psychol. 2014;33:1530. doi: 10.1037/hea0000018. [DOI] [PubMed] [Google Scholar]
- 17.Heller T, McCubbin JA, Drum C, Peterson J. Physical activity and nutrition health promotion interventions: what is working for people with intellectual disabilities? Intellect Dev Disabil. 2011;49(1):26–36. doi: 10.1352/1934-9556-49.1.26. [DOI] [PubMed] [Google Scholar]
- 18.Willems M, Waninge A, Hilgenkamp TI, van Empelen P, Krijnen WP, van der Schans CP, et al. Effects of lifestyle change interventions for people with intellectual disabilities: systematic review and meta-analysis of randomized controlled trials. J Appl Res Intellect Disabil. 2018;31:949. doi: 10.1111/jar.12463. [DOI] [PubMed] [Google Scholar]
- 19.Salomon C, Bellamy J, Evans E, Reid R, Hsu M, Teasdale S, et al. ‘Get Healthy!’A physical activity and nutrition program for older adults with intellectual disability: pilot study protocol. Pilot Feasibil Stud. 2018;4(1):1–11. doi: 10.1186/s40814-018-0333-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salomon C, Whittle E, Bellamy J, Evans E, Teasdale S, Samaras K, et al. A qualitative exploration of barriers and enablers of healthy lifestyle engagement for older Australians with intellectual disabilities. Res Pract Intellect Dev Disabil. 2019;6(2):182–191. [Google Scholar]
- 21.Roy R, Hebden L, Rangan A, Allman-Farinelli M. The development, application, and validation of a healthy eating index for Australian adults (HEIFA – 2013) Nutrition. 2016;32(4):434–440. doi: 10.1016/j.nut.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Reiman MP, Manske RC. Functional testing in human performance. 139 tests for sport, fitness, and occupational setting. Australia: Human Kinetics; 2009.
- 23.Czernichow S, Kengne AP, Stamatakis E, Hamer M, Batty GD. Body mass index, waist circumference and waist-to-hip ratio: Which is the better discriminator of cardiovascular disease mortality risk? Evidence from an individual-participant meta-analysis of 82 864 participants from nine chohort studies. Int Asso Stud Obes. 2011;12:680–687. doi: 10.1111/j.1467-789X.2011.00879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heyward, V.H. Advanced fitness assessment and exercise prescription (6th edition). Australia: Human Kinetics. 2010.
- 25.Golding LA. YMCA Fitness Testing and Assessment Manual. Champaign: Human Kinetics; 2000.
- 26.Christian-Jones, C. Validation of a proxy respondent version of the international physical activity questionnaire for use with adults with intellectual disabilities: Bangor University; 2013.
- 27.Harris L, Melville C, Jones N, et al. A single-blind, pilot randomised trial of a weight management intervention for adults with intellectual disabilities and obesity: study protocol. Pilot Feasibil Stud. 2015;1:1–12. doi: 10.1186/2055-5784-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suni, J. Health-related fitness test battery for middle-aged adults – with emphasis on musculoskeletal and motor tests. University of Jyvaskyla. 2000.
- 29.Harris C, Wattles AP, DeBeliso M, Sevene-Adams PG, Berning JM, Adams KJ. The seated medicine ball throw as a test of upper body power in older adults. J Strength Cond Res. 2011;58(8):2344–2348. doi: 10.1519/JSC.0b013e3181ecd27b. [DOI] [PubMed] [Google Scholar]
- 30.American College of Sports Medicine (ACSM). ACSM’s guidelines for exercise testing and prescription (7th edition). IN, USA: Lippincott Williams & Wilkins. 2006.
- 31.Jones CJ, Rikli RE, Beam WC. A 30-sec chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport. 1999;70(2):113–119. doi: 10.1080/02701367.1999.10608028. [DOI] [PubMed] [Google Scholar]
- 32.Cummins, L. Personal wellbeing index- Intellectual Disability (English), 3rd ed: Deakin University. 2005.
- 33.Bergstrom H, Hagstromer M, Hagberg J, Elinder LS. A multi-component universal intervention to improve diet and physical activity among adults with intellectual disabilities in community residences: a cluster randomised controlled trial. Res Dev Disabil. 2013;34:3847–3857. doi: 10.1016/j.ridd.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 34.Humphries K, Traci MA, Seekins T. Nutrition and adults with intellectual or developmental disabilities: systematic literature review results. Intellect Dev Disabil. 2009;47(3):163–185. doi: 10.1352/1934-9556-47.3.163. [DOI] [PubMed] [Google Scholar]
- 35.Illingworth K, Moore KA, McGillivray J. The development of the nutrition and activity knowledge scale for use with people with an intellectual disability. J Appl Res Intellect Disabil. 2003;16:159–166. doi: 10.1046/j.1468-3148.2003.00158.x. [DOI] [Google Scholar]
- 36.Zanini A, Crisafulli E, D'Andria M, Gregorini C, Cherubino F, Zampogna E, et al. Minimum clinically important difference in 30-S sit-to-stand test after pulmonary rehabilitation in subjects with COPD. Respir Care. 2019;64(10):1261–1269. doi: 10.4187/respcare.06694. [DOI] [PubMed] [Google Scholar]
- 37.Heller T, Hsieh K, Rimmer J. Barriers and supports for exercise participation among adults with down syndrome. J Gerontol Soc Work. 2003;38(1–2):161–178. doi: 10.1300/J083v38n01_03. [DOI] [Google Scholar]
- 38.van Schijndel-Speet M, Evenhuis HM, van Wijck R, van Empelen P, Echteld MA. Facilitators and barriers to physical activity as perceived by older adults with intellectual disability. Intellect Dev Disabil. 2014;52(3):175–186. doi: 10.1352/1934-9556-52.3.175. [DOI] [PubMed] [Google Scholar]
- 39.Caton S, Chadwick D, Chapman M, Turnbull S, Mitchell D, Stansfield J. Healthy lifestyles for adults with intellectual disability: knowledge, barriers, and facilitators. J Intellect Dev Disabil. 2012;37(3):248–259. doi: 10.3109/13668250.2012.703645. [DOI] [PubMed] [Google Scholar]
- 40.Elinder LS, Brunosson A, Bergstrom H, Hagstromer M, Patterson E. Validation of personal digital photography to assess dietary quality among people with intellectual disability. J Intellect Disabil Res. 2012;56:221–226. doi: 10.1111/j.1365-2788.2011.01459.x. [DOI] [PubMed] [Google Scholar]
- 41.Elinder LS, Bergström H, Hagberg J, Wihlman U, Hagstromer M. Promoting a healthy diet and physical activity in adults with intellectual disabilities living in community residences: design and evaluation of a cluster-randomized intervention. BMC Public Health. 2010;10(1):761. doi: 10.1186/1471-2458-10-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ptomey LT, Erik WA, Goettz JR, Lee J, Sullivan D, Donnely JE. Digital Photography improves estimates of dietary intake in adolescents with intellectual and developmental disabilities. Disabil Health J. 2015;8:146–50. doi: 10.1016/j.dhjo.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 43.Matthews L, Hankey C, Penpraze V, Boyle S, Macmillan S, Miller S, et al. Agreement of accelerometer and a physical activity questionnaire in adults with intellectual disabilities. Prev Med Rep. 2011;52(5):361–364. doi: 10.1016/j.ypmed.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Harris L, Melville C, Jones N, et al. A single-blind, pilot randomised trial of a weight management intervention for adults with intellectual disabilities and obesity: study protocol. Pilot Feasibil Stud. 2015;1:1–12. doi: 10.1186/2055-5784-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGillivray JA, Lau ALD, Cummins RA, Davey G. The utility of the personal wellbeing index intellectual disability scale in an Australian sample. J Appl Res Intellect Disabil. 2009;22(3):276–286. doi: 10.1111/j.1468-3148.2008.00460.x. [DOI] [Google Scholar]
- 46.Sigelman CK, Budd EC, Spanhel CL, Schoenrock CJ. When in doubt, say yes: acquiescence in interviews with mentally retarded persons. Intellect Dev Disabil. 1981;19(2):53. [PubMed] [Google Scholar]
- 47.Kamel EG, McNeill G, Van Wijk MC. Change in intra-abdominal adipose tissue volume during weight loss in obese men and women: correlation between magnetic resonance imaging and anthropometric measurements. Int J Obes Relat Metab Disord. 2000;24(5):607–13. doi: 10.1038/sj.ijo.0801204. [DOI] [PubMed] [Google Scholar]
- 48.Ross R, Rissanen JRH. Sensitivity associated with the identification of visceral adipose tissue levels using waist circumference in men and women: effects of weight loss. Int J Obes Relat Metab Disord. 1996;20(6):533–538. [PubMed] [Google Scholar]
- 49.Berglund G, Ljungman S, Hartford M, Wilhelmsen L, Bjorntorp P. Type of obesity and blood pressure. Hypertension. 1982;4(5):692. doi: 10.1161/01.HYP.4.5.692. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Rimm EB, Stampfer MJ, Willett WC, Hu FB. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr. 2005;81(3):555–63. doi: 10.1093/ajcn/81.3.555. [DOI] [PubMed] [Google Scholar]
- 51.Glass S, Gregory B. ACSM’s Metabolic Calculations Handbook. Baltimore: Lippincott Wiliiams & Wilkins; 2007. [Google Scholar]
- 52.Després JP. Physical activity, sedentary behaviours, and cardiovascular health: when will cardiorespiratory fitness become a vital sign? Can J Cardiol. 2016;32(4):505–13. doi: 10.1016/j.cjca.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 53.Kokkinos P, Myers J, Franklin B, Narayan P, Lavie CJ, Faselis C. Cardiorespiratory fitness and health outcomes: a call to standardize fitness categories. Mayo Clin Proc. 2018;39(3):333–336. doi: 10.1016/j.mayocp.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 54.Dankel SJ, Loenneke JP, Loprinzi PD. Determining the importance of meeting muscle-strengthening activity guidelines: is the behavior or the outcome of the behavior (strength) a more important determinant of all-cause mortality? Mayo Clin Proc. 2016;91(2):166–174. doi: 10.1016/j.mayocp.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 55.Sigal RJ, Kenny GP, Boule NG, Wells GA, Prud'homme D, Fortier M. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147(6):357–69. doi: 10.7326/0003-4819-147-6-200709180-00005. [DOI] [PubMed] [Google Scholar]
- 56.Weiss A, Suzuki T, Bean J, Fielding RA. High intensity strength training improves strength and functional performance after stroke. Am J Phys Med Rehabil. 2000;79(4):369–76. doi: 10.1097/00002060-200007000-00009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.