Abstract
Background:
Large electricity-generating wind turbines emit both audible sound and inaudible infrasound at very low frequencies that are outside of the normal human range of hearing. Sufferers of wind turbine syndrome (WTS) have attributed their ill-health and particularly their sleep disturbance to the signature pattern of infrasound. Critics have argued that these symptoms are psychological in origin and are attributable to nocebo effects.
Objectives:
We aimed to test the effects of 72 h of infrasound (1.6–20 Hz at a sound level of dB pk re , simulating a wind turbine infrasound signature) exposure on human physiology, particularly sleep.
Methods:
We conducted a randomized double-blind triple-arm crossover laboratory-based study of 72 h exposure with a washout conducted in a noise-insulated sleep laboratory in the style of a studio apartment. The exposures were infrasound ( dB pk), sham infrasound (same speakers not generating infrasound), and traffic noise exposure [active control; at a sound pressure level of 40–50 dB and 70 dB transient maxima, night (2200 to 0700 hours)]. The following physiological and psychological measures and systems were tested for their sensitivity to infrasound: wake after sleep onset (WASO; primary outcome) and other measures of sleep physiology, wake electroencephalography, WTS symptoms, cardiovascular physiology, and neurobehavioral performance.
Results:
We randomized 37 noise-sensitive but otherwise healthy adults (18–72 years of age; 51% female) into the study before a COVID19-related public health order forced the study to close. WASO was not affected by infrasound compared with sham infrasound ( min; 95% CI: , 3.88, ) but was worsened by the active control traffic exposure compared with sham by 6.07 min (95% CI: 0.75, 11.39, ). Infrasound did not worsen any subjective or objective measures used.
Discussion:
Our findings did not support the idea that infrasound causes WTS. High level, but inaudible, infrasound did not appear to perturb any physiological or psychological measure tested in these study participants. https://doi.org/10.1289/EHP10757
Introduction
Large electricity-generating wind turbines emit both audible sound and inaudible infrasound at very low frequencies that are outside of the normal human range of hearing. There have been several studies published recently on the effects, or lack thereof, of audible wind turbine sound on sleep1–4 including a meta-analysis incorporating several of these studies with wake after sleep onset (WASO) as core outcome.5 Despite these previous studies generally finding no effect of audible wind turbine noise (WTN) on WASO, other observers6–8 have proposed that people who live in the vicinity of wind turbines suffer from wind turbine syndrome (WTS described in this case series9) with dizziness, sleep disturbance, and other symptoms. The causes of this syndrome have been the subject of substantial international controversy.10–13 Proponents have contended that the symptoms that compose this syndrome are caused by low frequency subaudible infrasound generated by wind turbines.14 Critics have argued that these symptoms are psychological in origin and are attributable to nocebo effects.9,15–18 The Australian National Health and Medical Research Council (NHMRC) Wind Farms and Human Health Reference Group concluded that the available evidence was not sufficient to establish which, if either, of these explanations is correct.10 One of the reasons for this is because there had been to our knowledge no robustly designed, double-blind, controlled, and adequately powered studies of the health effects of exposure to sustained infrasound of the type emitted by a wind turbine. This absence of evidence has the potential to complicate initiatives to decarbonize electricity generation due to community concerns about the possibility for this measurable, but inaudible, infrasound to affect their health.
Our principal hypothesis was that exposure to infrasound in healthy individuals, at a level of dB pk re compared with the sham infrasound, increases WASO—a measure of sleep disturbance—and worsens other measures of sleep quality, mood, WTS symptoms, and other electrophysiological measures. In addition, as a positive control, we also tested whether audible traffic noise, a mixture of road (motorbike, truck, car) and aircraft noise (at a sound level of 40–50 dB and 70 dB transient maxima) had an adverse impact on these same outcomes, when compared with sham infrasound.
Methods
Study Design, Setting, and Ethics
This was a randomized, double-blind, three-way crossover study (equal ratios of all possible sequences) in noise-sensitive participants exposed during three noncontiguous 72-h periods to a) wind turbine simulated infrasound at dB peak, with reference to in air (test exposure); b) no added sound (sham, negative control, speakers not generating infrasound); and c) traffic noise [positive control mixture of road and air traffic with background levels of 40–50 dB , at night (2200–0700 hours) and 70 dB transient maxima]. The metrics and are common measures used to indicate the noise level of traffic noise but are not applicable to infrasound, which has a frequency range of Hz. Although any number of metrics could be used to measure the amplitude of infrasound, in this study the use of dB pk is adopted, corresponding to the peak amplitude of the infrasound waveform, to enable comparison with the dB pk level measured at a working wind farm, as described in more detail in the section “Experimental Exposures (Infrasound, Sham, and Traffic).”
The study was conducted exclusively at the Woolcock Institute of Medical Research, Glebe, New South Wales, Australia, in our sleep laboratory which is shielded from external sound. The ambient underlying noise level in the sleep laboratory in the nighttime period (i.e., without the generated traffic noise) was dB with the air-conditioning operating in the room. Air-conditioning was not turned off during the experiment to prevent ambient elevated temperature disturbing sleep. The ambient underlying level of infrasound was 80–85 dB pk predominantly in the frequencies of Hz (i.e., below the frequency range of the simulated infrasound).
During each test period starting around noon, the participants were subjected to one of the three noise conditions continuously for 72 h (including 3 normal nocturnal sleep periods) without leaving the testing setting (bedroom with ensuite approximating a studio apartment). The testing protocol within each 72-h period is described in Figure S1. Each noise condition was separated by a washout period during which participants lived normally outside of the laboratory environment.
This study was registered in the Australian and New Zealand Clinical Trials Registry (ACTRN12617000001392) before the first participant was randomized. A full copy of the final study protocol followed throughout the study without amendment and was lodged in the registry before the first participant was randomized after it was approved by the Sydney Local Health District Ethics Committee at The Royal Prince Alfred Hospital (protocol nos. X16-0073 and HREC/16/RPAH/91) and was performed in accordance with the Declaration of Helsinki, the 2006 Australian Clinical Trials Handbook Version 1.0, and the guidelines of the NHMRC for human research.19 Participants also gave written informed consent. Participants were offered an AUD1,000 payment upon completion ( in the laboratory) and the study paid for all transportation and meal costs.
Randomization Sequence, Allocation Concealment, and Blinding
The randomization sequence was a simple 1:1:1 ratio with no blocking that was computer generated by an investigator (C.T.C.) who played no role in participant selection or data collection and never met any participants or undertook statistical analysis. The sequence was secured in our password-protected research database and was only accessible by that investigator and the acoustic and data engineers who played no role in the screening for eligibility or the decision to randomize participants. The principal investigator (PI) and lead study coordinator assessed eligibility and consent before the PI irrevocably recorded the decision to randomize against the participants’ screening number and this was recorded by the study database.
Participants and study staff were blinded to the infrasound and sham infrasound exposures (given that the infrasound is inaudible). Study investigators and outcome assessors/processors were blinded to study allocation by using engineering staff who were solely responsible for delivering the exposures based on the randomization schedule and who never disclosed to any other study staff what exposure had been used, would be used, or was occurring. Infrasound or sham generation were controlled from a locked box kept outside the participants’ room only accessible by the engineers. Engineering staff did not meet the participants. The audible positive control (loud traffic noise) by its nature cannot be subject to either participant or investigator blinding and was an open-label exposure.
We asked all of the participants and staff during our informal conversations with them whether they could sense that infrasound was playing. None of the participants said they could sense infrasound was playing. None of the staff who processed the electrophysiology measurements [including polysomnography (PSG)] or were in the presence of the participants reported being able to tell whether infrasound or sham infrasound was being delivered. Statistical analyses were undertaken by investigators (N.S.M. and G.C.) who were also unable to tell which condition was infrasound. Exposures were labeled numerically (1 vs. 2 vs. 3) in the database rather than descriptively (traffic vs. infrasound vs. sham) until after the principal analyses were completed and presented to the chief investigators.
Participants
We undertook a two-stage screening process (online and clinical screening). Online screening selected for adults who were fluent in English, y old, and who were noise sensitive (21-Question Weinstein Noise Sensitivity Scale, with a score above the reported mean for the measurement of ),20 did not have severe insomnia (7-question Insomnia Severity Index of ),21 or any other detectable medical or psychiatric morbidity (including caffeine, alcohol, tobacco, or hypnotic dependence) that would preclude residing in a controlled environment for 72 h. We also excluded women who reported being pregnant or breastfeeding owing to the unknown risks to unborn children and babies. To benchmark the sample for mood and sleepiness levels, we also measured the Depression Anxiety and Stress Scale (DASS-21) and the Epworth Sleepiness Scale (ESS).22,23 We measured participants’ attitudes to windfarms (“How concerned are you about the health effects of infrasound generated from wind farms?”) with a Likert scale (0–6, with 0 being completely unconcerned to 6, extremely concerned); we planned to test this in the context of a main effect of infrasound as a potential explanatory variable (Figure S2).
Online-screened eligible participants were invited to proceed to the clinical screening stage. The clinical screening involved sending participants an Actiwatch 2 (Philips Respironics) using light and movement measurements to determine at-home sleep/wake patterns which were then clinically interpreted by a sleep psychologist (D.J.B.). Instructions were provided on how to use the watch and to wear it for a minimum period of 7 nights. Participants were excluded if that actigraphy recording showed abnormal sleep patterns, such as unusually short/late/early and shift work and/or transmeridian travel within 2 wk preceding randomization. Eligible participants were then invited to attend an in-person screening at the Woolcock Institute of Medical Research for audiological testing, psychological review and, if relevant, sign informed consent documents. Psychological screening was conducted by a psychologist (D.J.B.) who interviewed participants and reviewed their psychological history and questionnaire data [including assessments of claustrophobia (the Claustrophobia Questionnaire or CLQ)]24 and determined whether they were likely to tolerate 72 h in a studio apartment style accommodation.
Audiological screening was conducted to exclude any participants who had an existing hearing loss. Only those with normal audiometry in the opinion of the audiologist and neurotologist (M.S.W.) were included. As per a standard audiological examination, otoscopy, tympanometry, and pure tone audiometry (PTA) were conducted.
Otoscopy was conducted to check for the presence of excessive wax or anything that could obstruct probe measurements for tympanometry. Tympanometry provides quantitative information on the function of structures and the presence of fluid in the middle ear and was done to exclude those with underlying middle ear pathology that may impact upon sound transmission to the inner ear. Tympanograms were recorded using a Madsen OTOflex 100 (Natus Medical) system with a 226-Hz probe tone.25 Static compliance (in millimho), middle ear pressure (in decapascals) and ear canal volume (in cubic centimeters) were recorded. Only those with clinically normal middle ear function as indicated by equipment norms (static compliance of mmho and middle ear pressure of between and 100 daPa) were included in the study.
PTA was conducted using a personal computer-based audiometer (Oscilla USB-350B; Inmedico A/F), and thresholds were obtained following standard clinical practice using the Hughson-Westlake procedure.26 Thresholds were recorded at 250, 500, 1,000, 2,000, 4,000, and 8,000 Hz for air conduction (AC) and at 500, 1,000, 2,000, and 4,000 Hz for bone conduction (BC); 125 Hz AC thresholds were not tested owing to the risk of vibrotactile stimulation, which could affect the reliability of the results. Only participants with AC and BC thresholds at dB Hearing Level (HL) (i.e., clinically normal hearing) were included in the study.
Experimental Exposures (Infrasound, Sham, and Traffic)
The infrasound attributable to wind turbines was simulated digitally using a trapezoidal-shaped waveform with 16 harmonics in the frequency range of 0.8–20 Hz at a sound level of dB pk re (measurable but inaudible to all participants).16 This infrasound level is higher than what has been recorded both inside and outside a dwelling where people have previously reported WTS from exposure experienced at 1,100 ft (335 meters) from a wind turbine located at the Shirley Wind Farm, Wisconsin, USA.14,27 The Shirley wind project has eight Nordex100 wind turbines. The simulated wind turbine infrasound was generated by a Teensy microprocessor fitted with an SGTL5000 audio processor and the signal fed to a purpose-built Direct Current (DC)-coupled class D amplifier and four 18-in JBL subwoofer loudspeakers in four fully sealed timber enclosures faced with heavy protective mesh so that the participants could not observe the speakers in operation. The simulated wind turbine infrasound comprised sinusoidal harmonics in the frequency range specified with monotonically decreasing amplitude and selected phase shift, resulting in a trapezoidal waveform as observed in field measurements (Figure S3).5 The sham infrasound exposure involved use of the same equipment but with the loudspeakers wired in antiphase so that the cones moved by an equivalent amount but did not generate infrasound.
Because of the limitation in the physical size and type of loudspeakers, the 0.8-Hz fundamental frequency could not be generated at the required sound level and so the frequencies generated commenced at the second harmonic at 1.6 Hz. The peak sound level was nonetheless maintained as specified.
A mixture of road (motorcycles, trucks, cars) and aircraft noise (referred to herein as traffic noise) was generated via a Bose audio sound system and audio digital player at a sound level of 40–50 dB and 70 dB (loud enough to disrupt normal conversation). The traffic noise was timed specifically to disrupt the last 3 h of sleep as a positive control condition to increase WASO. The six sound files that constituted this exposure are available as supplemental audio files 1–6 upon request to the authors. The counts of sound events labeled airplane, motorbike, truck, or car can be found in Figure S4 for the full 72 h. Traffic noise events occurred approximately three to four times an hour during daylight and early sleep but, between and 0700 hours, the traffic noise events occurred closer to 15 events an hour (for a person with a normal wake time about 0700 hours). The events in the last 3 h of sleep were clustered noise events occurring one after the other. A typical noise event outside of the time 0400–0700 hours would be experienced as a truck going past. But between the 0400–0700 hours, the experience would be six trucks going past one after the other.
The infrasound sound levels were measured continuously above the pillow of each participant using a GRAS 46AZ one-half inch low frequency microphone and preamplifier set connected to the microprocessor. Software was used to enable the overall dB pk sound level and narrow band frequency analysis to be measured continuously and stored at 1-s intervals for later processing. The sound levels of the traffic noise were measured using an NTi XL2 sound level meter (having a frequency bandwidth of 10 Hz–20 kHz) with its microphone placed adjacent to the infrasound microphone. There was no attempt to address noise from the subjects such as snoring, talking in their sleep, or turning over in bed because it was considered that this extraneous noise would not contribute to the , which is measured over the whole nighttime period. A review of the sound levels was made after each session to identify any unusual extraneous noise. The equipment was calibrated before and after each test period with a Bruel & Kjaer 4231 sound level calibrator to confirm there was no drift in calibration. After the experiment, an acoustic engineer (O.J.) compared the exposure received to the exposure allocated by the randomized sequence to check for correct allocation and also for any equipment malfunction or performance issues.
Neurophysiological Measurements of Sleep and Wake
The primary and secondary outcomes were neurophysiological measurements of sleep and wake. Participants were measured using PSG at three time points per visit (1 sleep study per night). PSG was measured using Alice 6 LDX and Sleepware G3 (Philips Respironics) at electroencephalographic (EEG) derivations (F3-A2, Fz-A2, F4-A1, C3-A2, Cz-A2, C4-A1, Pz-A2, O1-A2, Oz-A2, O2-A1), left and right electrooculogram (EOG), chin electromyogram (EMG), and lead II electrocardiogram (ECG). Additional PSG sensors, such as nasal cannula and thermistor (to measure airflow), thoracic and abdomen respiratory bands, and leg EMG were measured during the first night of each visit. For nights 2 and 3, only EEG, EOG, ECG, and Chin EMG were measured. The PSG was manually scored by a sleep technician blinded to noise conditions (except traffic) according to the American Academy of Sleep Medicine guideline that was operational at the time when the study started (version 2.2).28
To reduce laboratory-induced variability in sleep behavior, individualized habitual sleep and wake times were calculated based on the average sleep and wake times from the 7 d of actigraphy measured during screening. Participants’ sleep opportunity period in the laboratory protocol was calculated using their habitual sleep and wake times with an additional 30-min windows on each end. Participants could then elect a set time inside those 30-min windows to be their go-to-sleep and wake-up times that they would then follow for the duration of the protocol.
Our primary outcome, WASO, was measured in minutes and was calculated from the first epoch of any sleep detected on the polysomnogram until the last epoch of any sleep detected on the PSG or the participant’s individually calculated habitual wake-up time, whichever happened last. Sleep technicians and staff were instructed to keep in-room disruption (e.g., fixing signals or physiological sensors) to a minimum to reduce artificially induced wake impacting the WASO calculation. All other PSG outcomes were scored according to the guideline and analyzed in the order in which they were listed in the preregistration.
EEG microstructure (power spectral density and spindle density) was analyzed by importing the PSG as a European data format (EDF) and sleep stage file into an in-house–built program.29–31 Preprocessing consisted of manual artifact detection and visual signal quality checks prior to exporting the EDF file. If judged that of the primary EEG channel (Cz-A2) was artifact, we substituted EEG channel (C3-A2). Where both EEG channels were deemed nonvalid, PSG recordings were excluded from analysis. The program calculates power for five frequency bands [beta ; sigma ; alpha ; theta ; delta ] in both rapid eye movement stage (REM) and non-REM sleep. The output of this program provides the “power” describing the density of each predefined EEG frequency bands during the PSG recording. This program also automatically detects the number of sleep spindles per minute during non-REM sleep (11–16 Hz) and fast and slow frequency spindles (fast 14–16 Hz; slow 11–13 Hz). Sleep spindles are a distinctive feature of non-REM sleep that are thought to play a role in memory and sleep stability.
Additional Secondary and Tertiary Outcome Measures
Neurocognitive battery and affective scales.
The battery of tests was repeated 12 times per visit and therefore a maximum of 36 times per participant. The exact sequence and timing of the battery are provided in Figure S1 and Table S2.
VAS.
Participants were asked to plot on a scale/line to indicate the momentary severity of symptoms reported in WTS (see Figure S5 for an example). The distance from the left end of the line to the participants’ response was recorded in millimeters. The 19 symptoms measured were: Headaches, Ringing in the ear, Itchy Skin, Blurred Vision, Dizziness, Racing Heart, Nausea, Tiredness, Feeling Faint, Sleepiness, Difficulty Concentrating, Difficulty Remembering, Fatigue, Irritability, Muscle Spasms, Disruption while falling asleep, Awakening from Sleep, and Anxiety. We also measured an additional visual analog scale (VAS) asking how annoying the noise is right now (Figure S5). The statistical processing of the 20 VAS is described in the “Statistical Analyses” section.
Karolinska Drowsiness Test.
Participants were asked to look at a dot directly in front of them at eye level ( away) for 2.5 min followed by 2.5 min with eyes closed and, last, another 2.5 min with their eyes opened. EEG was simultaneously measured (Alice Respironics G3 Sleepware). The alpha density in the eyes-open and the eyes-closed states, as measured in the EEG evaluates the level of wakefulness in participants during the course of the test. EEG data was exported into an EDF that was processed using an in-house analysis program.29,30
N-back.
The N-back test is a computerized test which involved the participant monitoring a series of stimuli (letters) and requires them to respond whenever a stimulus was presented in the same location as the one presented two trials previously. The task requires monitoring, updating, and manipulation of memorized information and is, therefore, assumed to place great demand on a number of key processes within working memory. The total number of correct responses out of 48 trials is the outcome. Before unblinding, we excluded all scores correct as evidence that a participant had not followed instructions.
Tower of London.
This is a neuropsychological test of planning, executive/spatial function, and working memory.32,33 The participant is instructed to move three colored balls on three pegs from an initial state to a goal state in the minimum number of moves necessary. Each trial contains multiple problems of increasing difficulty, requiring the participant to set more subgoals to reach the illustrated goal and requires preplanning. Each trial has a prespecified number of moves that the participant must aim to complete the trial within. The variables analyzed in this task are the mean of time and the grand mean number of errors across all puzzles attempted.
Psychomotor vigilance task.
This test is a measurement of simple reaction time (RT) over a 10-min period using an independent hand-held box with an light emitting diode (LED) display and two buttons that can be depressed using your thumbs. Participants are instructed to respond to a red LED display counting up in milliseconds that is stopped by pressing the right button as fast as possible. Stimulus delivery is randomly spaced between 2 and 10 s after the last one. An overall mean RT over the 10 min of the test period is generated (mean RT) and reciprocated to normalize the distribution.34,35
End-of-visit questionnaire.
Participants were asked four computerized questionnaires before exiting the laboratory environment at the end of each visit. These questionnaire, which are described in Table S1 are the Insomnia Severity Index (modified to a 3-day version), Kessler-10, Warwick—Edinburgh Mental Wellbeing Scale, Depression Anxiety Scale, and Stress Scale DASS-21).
Cardiovascular and Blood Measurements
Twenty-four–hour blood pressure.
Participants had ambulatory blood pressure measured over a 24-h period each visit using the Oscar2 device (SunTech Medical). Blood pressure measurements were taken at the brachial location, using a blood pressure cuff sized according to each participant’s arm. This procedure occurred from the morning of the second day to the third day of each visit. Blood pressure readings occurred at half-hourly intervals while participants were awake and hourly when participants were asleep (50 measurements per visit). Participants were instructed to keep their arm relaxed during readings and were notified there would be two inflations of the cuff (arterial and central blood pressure). Data was recorded and downloaded using provided programs by SunTech Medical and exported into comma-separated files for SAS import and statistical analysis.
Endothelial function test.
This procedure was undertaken once per visit at approximately noon after the last night and after the completion of noise exposure. In this 15-min test, participants were required to rest in a supine position on a bed while vascular tone was measured at the index fingers of each hand using the EndoPAT device (Itamar Medical). Vascular tone was measured at a baseline resting state at both fingers for 5 min. Following the reading at rest, a blood pressure cuff was inflated for 5 min on one arm to a pressure of above their resting blood systolic pressure or , whichever occurred first. A baseline blood pressure measurement was taken prior to the test to determine the pressure of cuff inflation. Following the occlusion period, the blood pressure cuff was fully deflated to allow measurement of endothelial mediated vasodilation (endothelial function). The cuff then remained deflated for another 5 min of rest.
Blood-based cardiovascular markers.
A fasted blood sample was collected on the final morning of each visit. Due to infection and safety procedures surrounding venipuncture, participants were briefly taken away from the noise exposure to perform blood draws in a separate room. Blood samples were commercially processed using standard assays by a local pathology company (Laverty Pathology, Sydney, Australia). Four serum variables were prespecified as of interest and were entered from the pathology reports: cortisol [a potential measure of stress; limit of detection (LOD) was ], highly sensitive C-reactive protein (HsCRP; a measure of inflammation; LOD of ), glucose and insulin (measures of metabolic homeostasis that might be perturbed by sleep disruption; the glucose LOD was and the insulin LOD was ). When a value was reported as being below the level of detection for any of these variables, we entered that value as being at that level (except HsCRP). Only HsCRP was ever reported with a lower than detectible level result (see the “Results” section).
Pulse wave velocity.
This procedure was undertaken once per visit at hours the morning after the second night and while in the presence of the speakers. This 10-min test required participants to be supine on the bed with a baseline rest period of 5 min prior to measurements using the SphygmaCor-XCEL (AtCor medical). A blood pressure cuff was attached around the participants’ thigh, and their carotid pulse was located using a high-fidelity pressure tonometer at the neck on the same side of the body. The carotid and femoral pulse waves were then simultaneously acquired from the thigh cuff and the tonometer. Pulse wave velocity was calculated based upon the distance between the middle of the thigh cuff and the site of the carotid pulse using established techniques.36 Recordings were deemed technically acceptable if pulse waves could be simultaneously acquired for at least 10 s.
Twenty-four–hour urinary catecholamines.
On the morning of the third day, participants were provided with a 24-h urine collection container and asked to void their bladder immediately prior to urine collection commencement. All urinary output was then collected in the container until the following morning. Urinary catecholamines were commercially processed using standard assays used by a local pathology company (Laverty Pathology, Sydney, Australia). The pathology service assayed these urine samples for creatinine, noradrenaline, adrenaline, and dopamine. Noradrenaline, adrenaline, and dopamine levels were divided by the amount of creatinine for statistical analyses. The minimum levels of detectable noradrenaline and adrenaline values are , creatinine is detectable at , and dopamine is detectable above levels of . Values at the LOD indicated in the pathology report were entered in as being at that point (e.g., of adrenaline was data entered as 38).
Statistical Analyses
In our prestudy planning, we calculated that in a crossover study design, a sample size of 38 participants would provide power to detect a difference in the primary end point, WASO, of 15 min between infrasound and sham infrasound (Cohen’s ). Our analysis code is available in the University of Sydney’s data repository and can be accessed on request of the authors. Repeated linear mixed models were performed for the primary and secondary end points in SAS (version 9.4; SAS Institute, Inc.), including all randomized participants in the groups they were randomized to and using the least squares means procedure to address missing data. For the primary and secondary outcomes, Participant Identification Numbers were coded as random effects, and we used the exposure received, the order it was received, the night of exposure and interactions between the exposure by night, and night by order as fixed effects. We regarded as statistically significant, and exposure effects were tested using the least squares means option. Tertiary outcomes were analyzed using the same model without the night effect or any of its interactions but, instead, included a time-point term indicating how far through the sequence the test was made. Blood pressure had 50 repeated measurements per visit and the repeated daytime test battery data, including symptom VAS scores, had 12 repeated measurements per visit.
Because a large proportion of VAS scores were 0/100 and to reduce the number of end points, a post hoc decision was made to use principal components factor analysis (proc factor). We used squared multiple correlations of each variable with the 19 other variables (including the noise annoyance scale) as the prior communality estimates. We selected five factors to be extracted based on an examination of the scree plot and evaluation of the interpretability of the resultant rotated factors. Varimax orthogonal rotation was implemented after extraction. The five factors were labeled according to the variables that loaded on them, as follows: Fatigue, Irritability, Nausea and Dizziness, Sleep Disturbance, and Tinnitus. Scores for each factor for each subject on each of 36 occasions of measurement were calculated. These factor scores had 3 added to them and then were log-transformed to approximate a normal distribution. The log-transformed factor scores were used as dependent variables in five mixed effects regression models.
Results
Between 19 April 2017 and 23 March 2020, we randomized 37 participants (see Table 1 for demographic information), 2 of whom withdrew themselves from the study after one exposure (see the “Adverse Events” section) and 1 of whom completed infrasound and sham infrasound exposures, but not the positive control exposure because our facility was closed by a COVID-related public health directive (5 missed exposure visits in total). Within conditions data collection was interrupted for 2 participants on night 1 (power cut) and night 2 (a staff scheduling error) that meant we had to send those participants home for 1 night.
Table 1.
Participant demographics in the laboratory-based three-arm crossover study of 72 h of exposure to simulated wind turbine infrasound, sham infrasound, and traffic noise.
| Variable | Descriptive statistics | Range |
|---|---|---|
| Sex, | ||
| Females | 19 | |
| Males | 18 | |
| Age [y ()] | 18–72 | |
| Weinstein Noise Sensitivity Score (0–126 points) () | 64–108 | |
| Insomnia Severity Scale (0–28 points) () | 0–16 | |
| DASS-2123—Depression (0–21 points) () | 0–16 | |
| DASS-2123—Anxiety (0–21 points) () | 0–12 | |
| Epworth Sleepiness Scale (0–24 points) () | 0–13 | |
| Attitude toward windfarm (0–6) [median] | 3 | 0–6, including 7 participants with scores |
Note: There were no data missing for any of these variables; baseline data was complete in all participants. Weinstein scores had to be to be eligible (approximately the median value in community-dwelling people, rather than a cutoff value indicative of a person being noise sensitive) and an Insomnia Severity Index (ISI) of . ISI scores indicate no insomnia; , subthreshold insomnia; and , moderate severity insomnia. Participants had to be at least 18 years of age. No other metrics reported were subject to inclusion/exclusion for a participant to be eligible. Scores on the DASS Depression scale indicate no depression, scores between 10 and 13 indicate mild depression, and scores between 14 and 20 indicate moderate depression. Scores on the DASS Anxiety scale indicate no anxiety, scores between 8 and 9 mild anxiety, and scores between 10 and 14 moderate anxiety. Scores on the Epworth Sleepiness Scale indicate clinically significant daytime sleepiness, and scores severe daytime sleepiness (see the “Participants” section in the “Methods” section for more detail). Attitude toward windfarm was scored from 0 (completely unconcerned) to 6 (extremely concerned). DASS, Depression Anxiety and Stress Scale; SD, standard deviation.
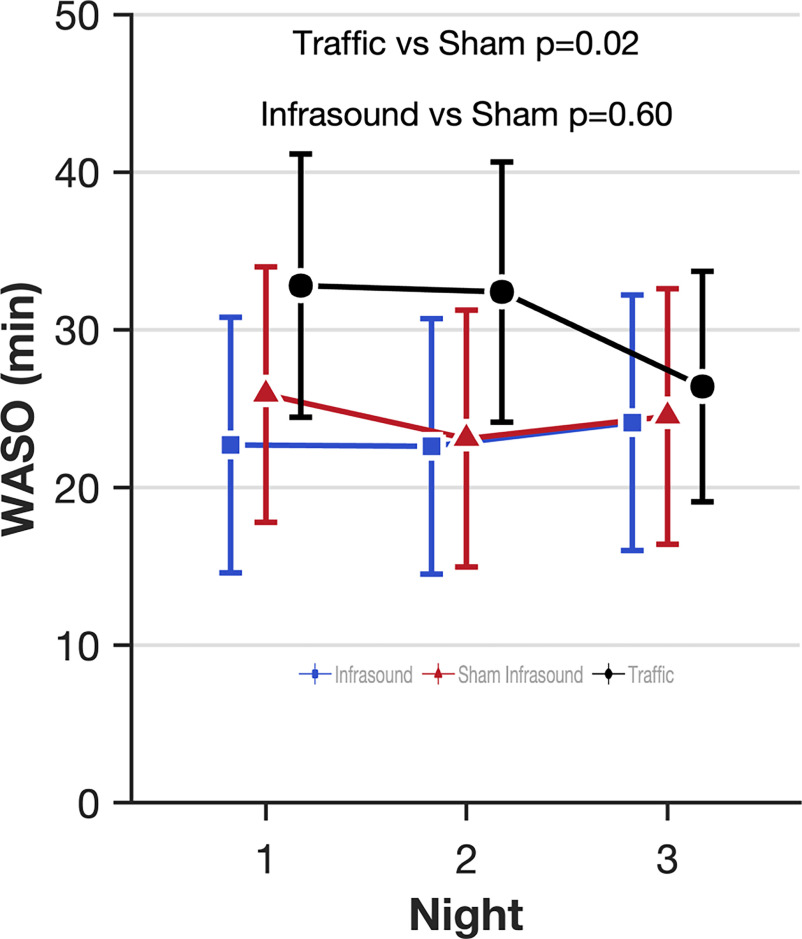
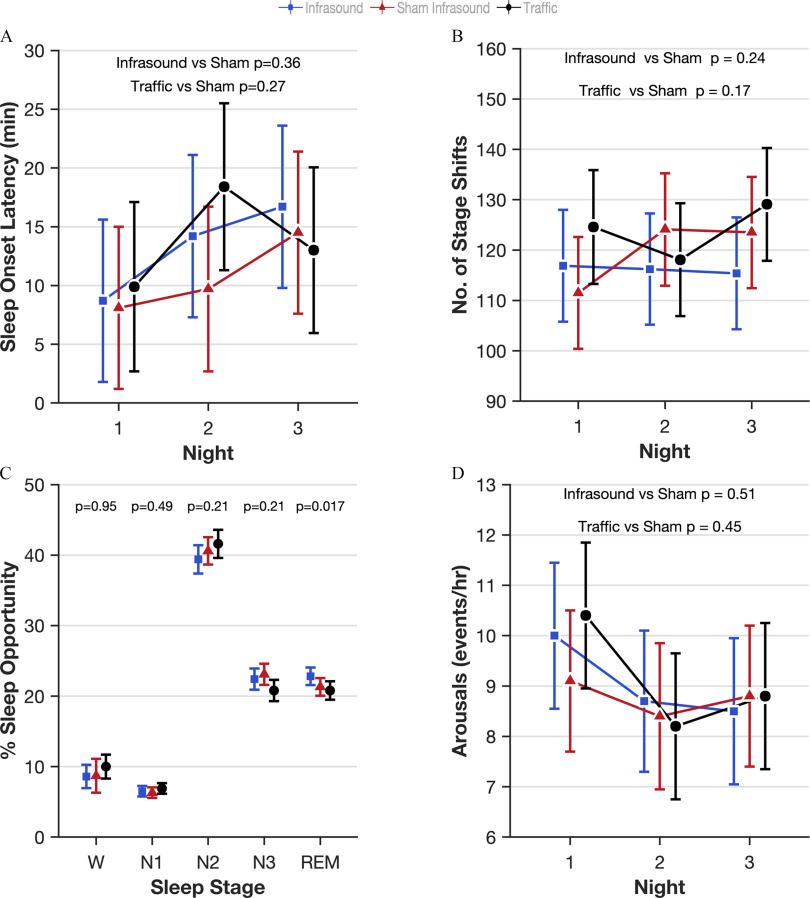
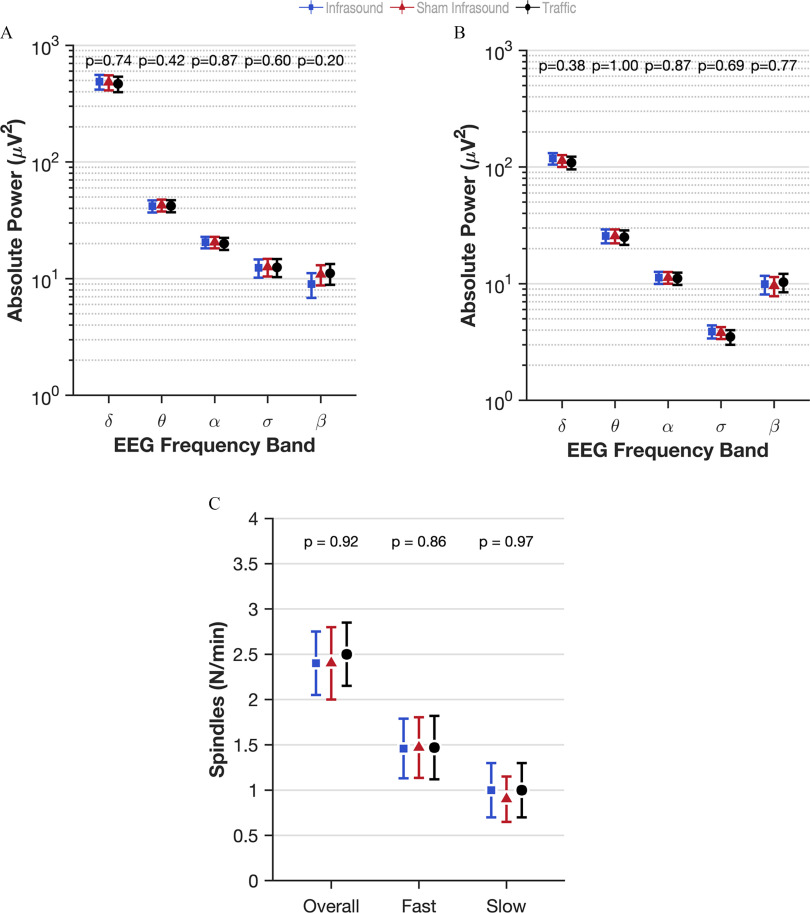
The lead authors (N.S.M. and G.C.) asked all of the staff and all of the participants whether they were able to differentiate in any way between infrasound and sham infrasound. None of them were able to. The sound engineer checked whether participants were exposed to the condition that they were scheduled to be exposed to in the randomization sequence. For one participant, the first and third visit, traffic and infrasound respectively were inadvertently switched compared with the randomization schedule due to human error. All randomized participants were analyzed for primary and secondary outcomes. Analysis was by intention-to-treat, that is, according to the randomly assigned exposure condition. The model estimated effects of infrasound and traffic noise compared with sham infrasound for all outcomes are shown in Table 2. Because there was no main effect of infrasound on sleep, we did not test for an interaction with prestudy attitudes to the health effects of windfarms. Five of 62 outcomes listed in Table 2 were found to be significantly different in the infrasound exposure (8.1% compared with the false positive rate of 5%): blood pressure [; 95% confidence interval (CI): , ]; insulin (; 95% CI: , ); percentage REM (% REM) sleep (1.5%; 95% CI: 0.3, 2.7); Warwick–Edinburgh Mental Wellbeing Scale (1.9 points; 95% CI: 0.2, 3.5); and change in power in the alpha frequency in the eyes-closed condition (; 95% CI: , ). Figure 1 shows the estimated effect of infrasound, sham infrasound, and traffic noise on the primary outcome (WASO) after 3 nights of each exposure, indicating the lack of effect of infrasound on WASO compared with sham and the effect of traffic noise on the first 2 nights. There was no evidence of a first-night effect whereby participants’ WASO were worse on the first night they spent in the laboratory (). Figure 2 shows the estimated effects on other standard electrophysiological measures of human sleep quality, including sleep onset latency (Figure 2A), sleep stage shifts (Figure 2B), the arousal index (Figure 2C), and the distribution of the five sleep stages (Wake, N1, N2, N3, and REM; Figure 2D) for each of the three exposures. Figure 3 shows the quantitative analysis of the EEG at Cz (top middle of the scalp) in the five frequency bands during sleep (delta, theta, alpha, sigma, beta) for each exposure in non-REM sleep (Figure 3A), REM sleep (Figure 3B), and the sleep spindle analysis (Figure 3C), again showing the lack of perturbed human sleep quality/continuity that could be attributable to infrasound.
Table 2.
Effects of infrasound and traffic noise on outcome measures in 37 participants in the in the laboratory-based three-arm crossover study of 72 h of exposure to simulated wind turbine infrasound, sham infrasound, and traffic noise ().
| Variable | Units of measurement | Sham ; actual/potential obs | Infrasound ; actual/potential obs | Traffic ; actual/potential obs | Difference (95% CI), -value infrasound vs. sham | Difference (95% CI), -value traffic vs. sham |
|---|---|---|---|---|---|---|
| Wake after sleep onset | Min | ; 107/111 | ; 108/111 | ; 101/111 | (, 3.88), 0.601 | 6.07 (0.75, 11.39), 0.02 |
| Electrophysiological measurements of sleep (secondary outcomes) | ||||||
| Sleep onset latency | Min | ; 107/111 | ; 108/111 | ; 101/111 | 2.48 (, 7.82), 0.36 | 3.0 (, 8.4), 0.27 |
| Arousal index | Events/h | ; 107/111 | ; 108/111 | ; 101/111 | 0.29 (, 1.16), 0.51 | 0.34 (, 1.21), 0.45 |
| Sleep stage % Wake | % of TST | ; 107/111 | ; 108/111 | ; 101/111 | (, 1.6), 0.95 | 1.3 (, 3.0), 0.11 |
| Sleep stage % N1 | % of TST | ; 107/111 | ; 108/111 | ; 101/111 | 0.2 (, 0.7), 0.49 | 0.6 (0.1, 1.1), 0.015 |
| Sleep stage % N2 | % of TST | ; 107/111 | ; 108/111 | ; 101/111 | (, 0.6), 0.21 | 1.0 (, 2.9), 0.28 |
| Sleep stage % N3 | % of TST | ; 107/111 | ; 108/111 | ; 101/111 | (, 0.4), 0.21 | (, ), 0.0001 |
| Sleep stage % REM | % of TST | ; 107/111 | ; 108/111 | ; 101/111 | 1.5 (0.3, 2.7), 0.017 | (, 0.7), 0.42 |
| Sleep stage shifts | Count | ; 107/111 | ; 108/111 | ; 101/111 | (, 2.4), 0.24 | 4.2 (, 10.2), 0.17 |
| Delta power—NREM | ; 106/111 | ; 107/111 | ; 100/111 | 5.61 (, 38.25), 0.74 | (, 18.52), 0.39 | |
| Theta power—NREM | ; 105/111 | ; 107/111 | ; 100/111 | (, 0.99), 0.42 | (, 1.08), 0.47 | |
| Alpha power—NREM | ; 105/111 | ; 107/111 | ; 100/111 | 0.07 (, 0.96), 0.87 | (, 0.47), 0.35 | |
| Sigma power—NREM | ; 105/111 | ; 107/111 | ; 100/111 | (, 0.44), 0.60 | (, 0.59), 0.97 | |
| Beta power—NREM | ; 105/111 | ; 107/111 | ; 100/111 | (, 1.01), 0.20 | 0.11 (, 3.12), 0.94 | |
| Delta power—REM | ; 105/111 | ; 107/111 | ; 99/111 | 4.92 (, 16.02), 0.38 | (, 7.14), 0.47 | |
| Theta power—REM | ; 105/111 | ; 107/111 | ; 99/111 | 0.00 (, 1.15), 1.00 | (, 0.57), 0.31 | |
| Alpha power—REM | ; 105/111 | ; 107/111 | ; 99/111 | (, 0.46), 0.87 | (, 0.26), 0.34 | |
| Sigma power—REM | ; 105/111 | ; 107/111 | ; 99/111 | 0.08 (, 0.47), 0.69 | (, 0.08), 0.12 | |
| Beta power—REM | ; 105/111 | ; 107/111 | ; 99/111 | 0.34 (, 2.57), 0.77 | 0.72 (, 3.00), 0.54 | |
| Spindles in NREM | Count | ; 106/111 | ; 107/111 | ; 101/111 | (, 31.0), 0.60 | 2.4 (, 45.3), 0.91 |
| Average Spindle density in NREM | Count/min | ; 106/111 | ; 107/111 | ; 101/111 | 0.01 (, 0.14), 0.91 | 0.03 (, 0.16), 0.69 |
| Average fast spindle density in NREM | Count/min | ; 106/111 | ; 107/111 | ; 101/111 | 0.02 (, 0.12), 0.73 | 0.03 (, 0.13), 0.59 |
| Average slow spindle density in NREM | Count/min | ; 106/111 | ; 107/111 | ; 101/111 | (, 0.08), 0.81 | (, 0.09), 0.96 |
| Cardiovascular measurements | ||||||
| Pulse transit time | ms | ; 33/37 | ; 36/37 | ; 30/37 | (, 2.5), 0.41 | (, 4.5), 0.99 |
| Pulse wave velocity | m/s | ; 33/37 | ; 36/37 | ; 30/37 | 0.50 (, 1.25), 0.20 | (, 0.66), 0.73 |
| Reactive hyperemia (endothelial function test) | Index | ; 35/37 | ; 32/37 | ; 33/37 | 0.00 (, 0.24), 0.97 | (, 0.16), 0.51 |
| Blood pressure—systolic (24-h) | mmHg | ; 1,212/1,850 | ; 1,246/1,850 | ; 1,196/1,850 | (, ), | ( , ), 0.002 |
| Blood pressure—diastolic (24-h) | mmHg | ; 1,212/1,850 | ; 1,246/1,850 | ; 1,196/1,850 | (, 0.1), 0.07 | (, 0.3), 0.19 |
| Heart rate | bpm | ; 1,212/1,850 | ; 1,246/1,850 | ; 1,196/1,850 | 0.1 (, 0.7), 0.80 | (, 0.1), 0.07 |
| Blood and urine measurements | ||||||
| Serum cortisol | nmol/L | ; 28/37 | ; 23/37 | ; 28/37 | (, 28), 0.38 | (, 17), 0.20 |
| Glucose | mmol/L | ; 28/37 | ; 24/37 | ; 29/37 | 0.0 (, 0.1), 0.88 | 0.0 (, 0.1), 0.88 |
| Insulin | mU/L | ; 28/37 | ; 24/37 | ; 29/37 | (, ), 0.025 | 0.6 (, 2.0), 0.44 |
| HsCRP | mg/L | ; 27/37 | ; 20/37 | ; 25/37 | (, 1.4), 0.87 | 0.1 (, 1.5). 0.93 |
| Urinary creatinine | mmol/24 h | ; 31/37 | ; 32/37 | ; 32/37 | 0.3 (, 1.4), 0.54 | 0.0 (, 1.1), 0.95 |
| Urinary noradrenaline (adjusted)a | nmol/24 h/creatinine | ; 31/37 | ; 32/37 | ; 32/37 | 0.1 (, 4.4), 0.98 | 4.1 (, 8.4), 0.06 |
| Urinary adrenaline (adjusted)a | nmol/24 h/creatinine | ; 31/37 | ; 32/37 | ; 32/37 | (, 0.6), 0.81 | 0.2 (, 0.9), 0.49 |
| Urinary dopamine (adjusted)a | nmol/24 h/creatinine | ; 31/37 | ; 32/37 | ; 32/37 | 8.8 (, 25.0), 0.28 | 8.7 (, 24.7), 0.28 |
| Electrophysiological measurements of wake (derived from the Karolinska Drowsiness Test) | ||||||
| Eyes open—delta | ; 392/444 | ; 403/444 | ; 379/444 | 4.98 (, 13.58), 0.26 | 10.81 (2.06, 19.55), 0.016 | |
| Eyes open—theta | ; 392/444 | ; 403/444 | ; 379/444 | 0.89 (, 1.94), 0.095 | 1.07 (0.01, 2.13), 0.048 | |
| Eyes open—alpha | ; 392/444 | ; 403/444 | ; 379/444 | (, 0.63), 0.44 | (, 0.55), 0.35 | |
| Eyes open—sigma | ; 392/444 | ; 403/444 | ; 379/444 | (, 0.17), 0.69 | (, 0.03), 0.09 | |
| Eyes open—beta | ; 392/444 | ; 403/444 | ; 379/444 | (, 1.29), 0.97 | (, ), 0.014 | |
| Eyes closed—delta | ; 391/444 | ; 403/444 | ; 378/444 | 10.24 (, 22.95), 0.11 | 1.93 (, 14.86), 0.77 | |
| Eyes closed—theta | ; 391/444 | ; 403/444 | ; 378/444 | 1.39 (, 3.39), 0.17 | 0.56 (, 2.59), 0.59 | |
| Eyes closed—alpha | ; 391/444 | ; 403/444 | ; 378/444 | (, ), 0.045 | (, 0.96), 0.31 | |
| Eyes closed—sigma | ; 391/444 | ; 403/444 | ; 378/444 | 0.07 (, 0.35), 0.65 | (, 0.15), 0.33 | |
| Eyes closed—beta | ; 391/444 | ; 403/444 | ; 378/444 | 0.33 (, 1.78), 0.66 | (, 0.37), 0.14 | |
| Affective symptoms of WTS (based on a 5-factor PCA of the original 20 visual analog scales) | ||||||
| Factor 1: Fatigue | Log-standardized | ; 404/444 | ; 403/444 | ; 375/444 | 0.01 (, 0.05), 0.41 | 0.03 (, 0.07), 0.09 |
| Factor 2: Irritability | Log-standardized | ; 404/444 | ; 403/444 | ; 375/444 | 0.01 (, 0.04), 0.60 | 0.02 (, 0.05), 0.16 |
| Factor 3: Nausea and Dizziness | Log-standardized | ; 404/444 | ; 403/444 | ; 375/444 | 0.00 (, 0.03), 0.87 | (, 0.01), 0.19 |
| Factor 4: Sleep disturbance | Log-standardized | ; 404/444 | ; 403/444 | ; 375/444 | 0.00 (, 0.02), 0.96 | 0.02 (0.01, 0.04), 0.01 |
| Factor 5: Tinnitus | Log-standardized | ; 404/444 | ; 403/444 | ; 375/444 | (, 0.02), 0.79 | 0.03 (, 0.05), 0.09 |
| Behavioral performance measures | ||||||
| PVT reciprocal mean reaction time | 1/s | ; 407/444 | ; 412/444 | ; 390/444 | 0.00 (, 0.05), 0.99 | 0.01 (, 0.06), 0.83 |
| Tower of London grand mean execution test | s | ; 409/444 | ; 414/444 | ; 384/444 | 0.1 (, 0.7), 0.64 | (, 0.4), 0.62 |
| Tower of London grand number of errors | % | ; 409/444 | ; 414/444 | ; 384/444 | 0.2 (, 1.2), 0.75 | 0.9 (, 2.0), 0.08 |
| N-back—total number correct | Count | ; 403/444 | ; 411/444 | ; 379/444 | 0.5 (, 1.2), 0.20 | 1.1 (0.3, 1.8), 0.005 |
| End-of-visit questionnaire | ||||||
| Insomnia Severity Index (3-d modified) | Points | ; 106/111 | ; 106/111 | ; 106/111 | (, 0.9), 0.68 | 2.1 (1.0, 3.3), |
| Warwick–Edinburgh Mental Wellbeing Scale | Points | ; 106/111 | ; 106/111 | ; 106/111 | 1.8 (0.2, 3.5), 0.03 | (, 1.7), 0.99 |
| Kessler-10 | Points | ; 106/111 | ; 106/111 | ; 106/111 | 0.0 (, 0.8), 1.00 | 0.1 (, 0.9), 0.89 |
| Depression Anxiety Stress Scale–Depression | Points | ; 106/111 | ; 106/111 | ; 106/111 | (, 1.3), 0.88 | 0.8 (, 2.2), 0.24 |
| Depression Anxiety Stress Scale—Anxiety | Points | ; 106/111 | ; 106/111 | ; 106/111 | 0.1 (, 1.0), 0.83 | 0.6 (, 1.5) 0.22 |
| Depression Anxiety Stress Scale—Stress | Points | ; 106/111 | ; 106/111 | ; 106/111 | (, 0.5), 0.23 | 0.2 (, 1.5), 0.76 |
Note: Actual/potential obs gives the amount of observed and recorded data being used by the mixed model compared with the potential number of observations (i.e., complete data) so readers can gauge how much missing data for each of the conditions the model is having to deal with. %, Percentage; alpha frequency band, 8–12 Hz; beta frequency band, 15–32 Hz; CI, confidence interval; delta frequency band, 0.5–4.5 Hz; HsCRP, highly sensitive C-reactive protein; N1, non-REM sleep stage 1; N2, non-REM sleep stage 2; N3, non-REM sleep stage 3; NREM, non-REM sleep; obs, observation; PCA, principal component analysis; PVT, psychomotor vigilance task (simple reaction time task); REM, rapid eye movement sleep; SEM, standard error of the mean; sigma frequency band, 12–15 Hz; theta frequency band, 4.5–8 Hz; TST, total sleep time; WTS, wind turbine syndrome.
Unit of measurement is the variable measured divided (adjusted) by the simultaneously measured creatinine ().
Figure 1.
Effect estimates of infrasound and traffic on wake after sleep onset (WASO) over 3 nights in the laboratory-based study of the three-arm crossover study of 72 h of exposure to simulated wind turbine infrasound, sham infrasound, and traffic noise. The mixed model estimates of the effect of infrasound and traffic noise on electrophysiologically measured human WASO. The primary outcome of this study was WASO as a measure of the effects of noise on sleep perturbation. WASO is the amount of time spent awake between sleep onset and final wake-up time. We measured sleep for 3 nights under each of the three exposures [infrasound in blue squares, sham infrasound in red triangles, and traffic noise in black circles; min difference between infrasound and sham infrasound (95% CI: , , ); Table 2]. Error bars indicate the 95% CIs. Effects estimates are derived from mixed models of repeated measures where the participants were classed as random effects and exposure (3 levels), the order the exposure was received (1, 2, 3), the night of exposure (1, 2, 3), and interactions between the exposure by night and night by order as fixed effects. The least squares means procedure was used to address missing data. The exact numerical values for the estimated means and 95% CIs can be found in Table S3. Note: CI, confidence interval.
Figure 2.
Effect estimates of infrasound and traffic on measures of sleep quality over 3 nights in the laboratory-based study of the three-arm crossover study of 72 h of exposure to simulated wind turbine infrasound, sham infrasound, and traffic noise. Infrasound is represented in blue squares, sham infrasound in red triangles, and traffic noise in black circles. Error bars are 95% CI. Effects estimates are derived from mixed models of repeated measures where the participants were classed as random effects and exposure (3 levels), the order the exposure was received (1, 2, 3), the night of exposure (1, 2, 3), and interactions between the exposure by night and night by order as fixed effects. The least squares means procedure was used to address missing data. The exact numerical values for the estimated means and 95% CIs can be found in Table S3. (A) Sleep onset latency is the amount of time taken to fall asleep [2.48 min difference between infrasound and sham (95% CI: , , ); Table 2]. (B) Number of sleep stage shifts is a measure of sleep stability [ shifts difference between infrasound and sham (95% CI: , , )]. (C) Proportions of the sleep period scored as each of the traditional sleep stages plotted together to test whether infrasound causes perturbation to sleep depth. Numerical values above each plot are the -values for the difference between infrasound and sham infrasound. -Values above the stacked columns are comparing infrasound to sham infrasound. (D) Arousal index is the number of cortical arousals detected during each hour of sleep as a measure of sleep quality [0.29 events/h difference between infrasound and sham (95% CI: , 1.16, )]. Note: %, percentage; 1, non-REM sleep stage 1; 2, non-REM sleep stage 2; 3, non-REM sleep stage 3; CI, confidence interval; REM, rapid eye movement stage; SEM, standard error of the mean; W, wake.
Figure 3.
Effect estimates of infrasound and traffic on quantitative measures of electroencephalography during sleep in the laboratory-based study of the three-arm crossover study of 72 h of exposure to simulated wind turbine infrasound, sham infrasound, and traffic noise. Infrasound is represented in blue squares, sham infrasound in red triangles, and traffic noise in black circles. Absolute power derived from overnight electrophysiology transformed into five frequency bands as a measure of cortical activity in (A) NREM and (B) REM (delta , theta , alpha sigma , beta ). Sleep spindle density in NREM overall and density of fast and slow spindles in NREM. (C) Fast and slow . Numerical values above each plot are the -values for the difference between infrasound and sham infrasound. Effects estimates are derived from mixed models of repeated measures where the participants were classed as random effects and exposure (3 levels), the order the exposure was received (1, 2, 3), the night of exposure (1, 2, 3), and interactions between the exposure by night and night by order as fixed effects. The least squares means procedure was used to address missing data. The exact numerical values for the estimated means and 95% CIs can be found in Table S3. Point estimates are indicated graphically by the shapes and 95% CIs indicated by the bars. Note: CI, confidence interval; NREM, non-REM sleep; REM, rapid eye movement sleep.
Adverse Events
There were no serious adverse events (unplanned hospitalizations or deaths). There were four adverse events in participants, who we describe in this section as participants A, B, C, and D. Participant A completed only one visit while being exposed to infrasound. They complained that the tips of their hair were being made brittle by the EEG paste and declined further contact and participation. We found no pattern of complaints in their data that would match WTS after reviewing all their objective and subjective data after unblinding. Participant B completed only one visit while being exposed to sham infrasound. They complained of social isolation and did not enjoy being in a windowless room and then declined further contact and participation. Participant C completed all visits and during the first visit was exposed to traffic noise and had a mild asthma attack possibly triggered by a bushfire event in Sydney that weekend. This participant was immediately seen by a respiratory physician in our clinic to update their asthma management before they left the laboratory. Participant D completed all visits but emailed the study coordinator to report feeling on edge in the immediate days after returning home from the second visit (infrasound exposure). We then reviewed their data where they did not report having, or later retrospectively report having, that experience in the laboratory environment.
Data Quality
Our quality assurance for our blood pressure/heart rate data indicated that other than the missing data at a participant level (where 2 visits were missed because of equipment availability and a staff scheduling accident plus the 5 missed visits from people dropping out and the final COVID shutdown plus 1 collection missed because of missing visits missing values) that the missing data were randomly distributed among different times of the day and night but that the data loss proportion was greater than the ideal proportions used clinically (i.e., having complete data).37 Our missing data rate (1,523 missing values) after allowance for the people in whom it was not collected (i.e., 400 missing measurements) was 71%, which is not as good as the 85% target. These missing data were due to equipment slippage and the failure of the blood pressure cuff to inflate properly.
Urinary analyses were part of the protocol that people could opt out of and some participants declined to consent. Table 2 reports we missed 16 of the potential urine samples we could theoretically have collected, and this is accounted for by five missed visits (5), plus two people opting out of urine in all visits (6), plus four people opting out of urine on one visit (4; one of whom was actually the final COVID-affected participant and we could not collect their sample because of the start of the Public Health Order that shut down research in the state and our study’s data collection phase), plus one missed sample (1) in conjunction with the staffing scheduling error (). Two values for noradrenaline and 48 values for adrenaline were at the lower detectable limit.
HsCRP data were missing for 39/111 potential measurements. These are accounted for by five missed visits (5), the one visit cut short at the end by the public health order (1), plus one person opting out of all blood collection (3), plus one person opting out of blood on one visit (1), plus two people opting out of blood collection on two visits (4), 8 samples returned a value at or below the LODs and were entered as being missing data (; ), and 17 samples were missing owing to insufficient blood volume collection to send for testing. There were no samples missing because of pathology service errors or a failed sample in transit ().
Cortisol data were missing for 32/111 potential measurements. These are accounted for by five missed visits (5), the one visit cut short at the end by the public health order (1), plus one person opting out of all blood collection (3), plus one person opting out of blood on one visit (1), plus two people opting out of blood collection on two visits (4), and 18 samples were missing owing to insufficient blood volume collection to send for testing. There were no samples missing because of pathology service errors or a failed sample in transit or being below the LODs ().
Glucose and insulin data were missing for 30/111 potential measurements. These are accounted for by five missed visits (5), the one visit cut short at the end by the public health order (1), plus one person opting out of all blood collection (3), plus one person opting out of blood on one visit (1), plus two people opting out of blood collection on two visits (4), and 16 samples were missing owing to insufficient blood volume collection to send for testing. There were no samples missing because of pathology service errors or a failed sample in transit or being below the LODs ().
Discussion
This study found that 72 h of the simulated wind turbine infrasound ( dB pk re ) in controlled laboratory conditions did not worsen any measure of sleep quality compared with the same speakers being present but not generating infrasound (sham infrasound). The positive control condition, audible traffic noise (sound level of 40–50 dB and 70 transient maxima) worsened the primary measure of sleep quality, WASO, by min compared with the sham infrasound. This effect was evident on the first 2 nights where the magnitude of the effect is similar to a previous report.38 The lack of an effect on the third night of traffic noise might have been caused by the build-up of homeostatic sleep pressure caused by the sleep disturbance on the first 2 nights.38,39 Furthermore, none of the staff or participants involved in the study reported being able to distinguish the infrasound condition from the sham infrasound and none of the participants displayed objective or subjective features consistent with WTS. From our list of 22 secondary outcome measures of sleep electrophysiology only one (% REM sleep) was significantly different in the infrasound exposure compared with sham. Furthermore, the effect estimate was in the opposite direction to that which was hypothesized and was not offset by changes in other sleep stages. As far as we can tell, this is the first study to investigate the effects on human sleep of simulated wind turbine infrasound in double-blind conditions.
We also measured the effects of infrasound on a wide range of nonsleep-related physiological and psychological measures. Four of the 39 tertiary outcomes we analyzed demonstrated an estimated effect of infrasound. These very small differences were not systematically in an adverse direction. Hence, we believe these effects have been detected by chance. We note that the design of the study, with many repeated measurements for each participant, meant there was a very high level of statistical power. Three of the measures (systolic blood pressure, insulin, and the Warwick–Edinburgh Mental Wellbeing Scale) improved by a small amount in association with exposure to infrasound compared with sham. In addition, the amount of alpha power during the eyes-closed condition of the Karolinska Drowsiness Test changed by a very small amount (, Cohens ) in association with infrasound, and it is not clear whether this direction of effect is helpful or harmful. We conclude that these findings suggest the absence of detectable health effects of infrasound on humans in our study.
People who suffer from WTS report that their symptoms begin quickly when they are exposed to infrasound from wind turbines and are then sustained.9,40 Our scientifically robust study provides evidence to address this claim. The Australian NHMRC report10 that gave rise to our study made note of this “absence of evidence” rather than concluding an “evidence of absence” owing to the lack of any laboratory-controlled double-blind experiments of sufficient duration and intensity to hypothetically induce WTS in a human. Our study attempted to address this absence of evidence by rigorously simulating wind turbine infrasound in the frequency range of 1.6–20 Hz at a sound level of pk re (measurable but inaudible) and comparing it in a double-blind randomized exposure study to a sham infrasound exposure using the same equipment but with the speakers wired in antiphase so that they did not generate infrasound. Furthermore, all previous studies we are aware of testing wind turbine infrasound (not audible WTN) in double-blind conditions have exposed people to 7.5 or 23 min of infrasound and not 72 h as we have here.15,16,18,41
Our original sample size goal was 40 people, with an assumption that 2 people would drop out during the study. We actually randomized and analyzed data from 37 people and had 2 people drop out after completing only one of the planned three exposure periods before COVID forced us to close the study. The last participant, who was still in the study on the day it was closed, had completed both infrasound and sham infrasound conditions; therefore, we have not considered this person as having dropped out. The power of the study remains strong owing to the triple-arm crossover design coupled with multiple repeated measurements of numerous physiological and psychological outcomes. For instance, the primary and secondary outcomes were measured nine times per person, which allowed us to estimate the difference between exposures for the primary outcome (WASO) within a span of min for the full width of the 95% confidence limit. The greatest number of repeated measures was for blood pressure where we collected blood pressure measurements across the three exposure per person. The width of the 95% confidence limits for the difference between exposures in 24-h systolic blood pressure measurement was only . This helps improve our confidence that there are no meaningful adverse health effects of this specific formulation of infrasound in these humans. In addition, we found no evidence for a first-night effect in our primary outcome where humans often have a poor sleep in their first night in an unfamiliar laboratory environment compared with their subsequent stays.
The study has some limitations. It is possible that, despite the application of a noise-sensitivity eligibility criterion and also having seven participants who were somewhat concerned or concerned about the health effects of infrasound generated by wind turbines, we inadvertently recruited a group of people who were not sensitive to the effects of infrasound. It remains possible that some humans are sensitive to infrasound. This hypothesis has been tested in a short term study in double-blind conditions in people who said that wind turbines make them feel ill. Those participants were unable to reliably detect infrasound and did not physiologically react to it.40 All of our quantitative electrophysiology measurements of the brain were taken from a single channel, so it remains possible that infrasound could have effects on other brain regions. It is also possible that our outcome measures lacked sensitivity for detecting adverse health effects of noise and the lack of effect of traffic noise on a number of secondary and tertiary outcome measures could be evidence for that lack of sensitivity. The background noise level in the sleeping environment created by the air-conditioning system was measured at about 39 dB , which is above the recommended levels of the World Health Organization night noise guidelines of 35 dB through the night and is louder than background recordings in sleep laboratories internationally that have undertaken noise exposure studies.42 This might have caused some sleep perturbation in some people that might have biased our results toward a null effect. However, we do not think that this would have been a powerful biasing effect. Our reasoning is that the sleep recordings in the people in this study when they were in the sham condition were in a very healthy range compared with reference values.43 In addition, the sound levels in the two rooms used are experienced as a white noise background that is sometimes used by people as a sleep aid at considerably louder levels, albeit with a poor evidence base to be able to tell whether it is helpful or harmful.44 Nevertheless, a background level of 39 dB is somewhat louder than optimal for a control condition in a study of noise effects on sleep. However, on balance, we felt it was important to have a comfortable environment for the participants given many individuals in our country would have home air-conditioning or fans. Although the sham and traffic conditions might appear to be similar using averaged overnight sound pressure levels (39 dB in the sham condition vs. 40–50 dB in the traffic noise condition) they are actually quite different because the traffic noise includes infrequent, abrupt 70-dB noise peaks designed to awaken humans (Figure S4). Presumably, if we had employed these noise maxima peaks more often we would have disrupted sleep to a greater degree. Our data completeness for our primary and secondary outcomes was high and most data losses were at random and of small numbers, which can be dealt with by the statistical techniques we used. Our analyses of some tertiary outcome variables, including the blood-derived variables and the urinary catecholamines and also the heart rate and blood pressure data, should be interpreted with some caution given that they did suffer from higher than expected missing data proportions.37
Conclusion
Our study found no evidence that 72 h of exposure to a sound level of dB pk re of simulated wind turbine infrasound in double-blind conditions perturbed any physiological or psychological variable. None of the 36 people exposed to infrasound developed what could be described as WTS. Our study is unique because it measured the effects of infrasound alone on sleep. This study suggests that the infrasound component of WTN is unlikely to be a cause of ill-health or sleep disruption, although this observation should be independently replicated.
Supplementary Material
Acknowledgments
The contributions of the authors were as follows: experiment design and application for funding (N.S.M., B.G.T., R.T., D.J.B., C.T.C., N.G., M.S.W., C.L.P., G.B.M., and R.R.G.), principal investigators (N.S.M. and R.R.G.), protocol development (N.S.M., G.C., and R.K.), lead study coordinator (G.C.), medical oversight (G.B.M. and R.R.G.), psychological screening (D.J.B.), audiological and neurological screening (C.R.W. and M.S.W.), staff coordination (G.C., B.G.T., and C.A.E.), grant budget control (B.G.T.), noise simulation speaker construction and acoustic engineering quality assurance and quality control (R.T. and O.J.), electroencephalography data quality and processing oversight (G.C. and A.L.D.), statistical analyses (N.S.M. and G.C.), manuscript drafting (N.S.M. and G.C.), and manuscript planning committee (N.S.M., B.G.T., R.T., G.B.M., and R.R.G.).
We acknowledge the people who collected or processed data: C. Berry, N. Hurst, E. Argaet, L. Fratturo, S. Theocharous, S. Haffar, B. Zhang, S. Shulka, M. Nguyen, I. Wood, J. Nguyen, H. Smith, N. Charlwood, C. Low, K. Kremerskothen, V. Fuchsova, B. Tiik, A. Bertolin, S. Yuhedran, A. Radowiecka, J. Ponahajba, E. Olszewska, M. Bronisz, C. Lorilla, M. Allado, N. Mutombe, H. Linn, M. Balenzuela, I. Basic, C. Geha, B. Pan, D. Jia, R. Wallis, K. O’Doherty, and I. Juria. We also acknowledge the members of the data safety monitoring board (DSMB): S. Loughran, G. Hamilton, S. O’Leary, and F. Garden (DSMB statistician), as well as the data engineers, G. Unger and Z. Zhou, and graphic design assistance, R. Wassing.
The funder played no role in the conduct of the study or the decision to publish.
Trial Registration: ANZCTR (ACTRN12617000001392).
Funding for this study was granted by the National Health and Medical Research Council of Australia (NHMRC) through the Targeted Call for Research into Wind Farms and Human health, APP1113615.
References
- 1.Ageborg Morsing J, Smith M, Ögren M, Thorsson P, Pedersen E, Forssén J, et al. . 2018. Wind turbine noise and sleep: pilot studies on the influence of noise characteristics. Int J Environ Res Public Health 15(11):2573, PMID: , 10.3390/ijerph15112573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lechat B, Scott H, Decup F, Hansen KL, Micic G, Dunbar C, et al. . 2022. Environmental noise-induced cardiovascular responses during sleep. Sleep 45(3):zsab302, PMID: , 10.1093/sleep/zsab302. [DOI] [PubMed] [Google Scholar]
- 3.Michaud DS, Keith SE, Guay M, Voicescu S, Denning A, McNamee JP. 2021. Sleep actigraphy time-synchronized with wind turbine output. Sleep 44(9):zsab070, PMID: , 10.1093/sleep/zsab070. [DOI] [PubMed] [Google Scholar]
- 4.Smith MG, Ögren M, Thorsson P, Hussain-Alkhateeb L, Pedersen E, Forssén J, et al. . 2020. A laboratory study on the effects of wind turbine noise on sleep: results of the polysomnographic WiTNES study. Sleep 43(9):zsaa046, PMID: , 10.1093/sleep/zsaa046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liebich T, Lack L, Hansen K, Zajamšek B, Lovato N, Catcheside P, et al. . 2021. A systematic review and meta‐analysis of wind turbine noise effects on sleep using validated objective and subjective sleep assessments. J Sleep Res 30(4):e13228, PMID: , 10.1111/jsr.13228. [DOI] [PubMed] [Google Scholar]
- 6.Nissenbaum MA, Aramini JJ, Hanning CD. 2012. Effects of industrial wind turbine noise on sleep and health. Noise Health 14(60):237–243, PMID: , 10.4103/1463-1741.102961. [DOI] [PubMed] [Google Scholar]
- 7.Salt AN, Kaltenbach JA. 2011. Infrasound from wind turbines could affect humans. Bull Sci Technol Soc 31(4):296–302, 10.1177/0270467611412555. [DOI] [Google Scholar]
- 8.Schomer PD, Erdreich J, Pamidighantam PK, Boyle JH. 2015. A theory to explain some physiological effects of the infrasonic emissions at some wind farm sites. J Acoust Soc Am 137(3):1356–1365, PMID: , 10.1121/1.4913775. [DOI] [PubMed] [Google Scholar]
- 9.Pierpont N. 2009. Wind Turbine Syndrome: a Report on a Natural Experiment. https://www.windturbinesyndrome.com/wind-turbine-syndrome/ [accessed 14 March 2022].
- 10.NHMRC (National Health and Medical Research Council). 2015. Information Paper: Evidence on Wind Farms and Human Health. https://www.nhmrc.gov.au/about-us/publications/nhmrc-information-paper-evidence-wind-farms-and-human-health [accessed 14 March 2022].
- 11.Council of Canadian Academies. 2015. Understanding the Evidence: Wind Turbine Noise. The Expert Panel on Wind Turbine Noise and Human Health. https://cca-reports.ca/reports/understanding-the-evidence-wind-turbine-noise/ [accessed 14 March 2022].
- 12.Knopper LD, Ollson CA. 2011. Health effects and wind turbines: a review of the literature. Environ Health 10:78, PMID: , 10.1186/1476-069X-10-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCunney RJ, Mundt KA, Colby WD, Dobie R, Kaliski K, Blais M. 2014. Wind turbines and health: a critical review of the scientific literature. J Occup Environ Med 56(11):e108–e130, PMID: , 10.1097/JOM.0000000000000313. [DOI] [PubMed] [Google Scholar]
- 14.Tonin R. 2018. A review of wind turbine-generated infrasound: source, measurement and effect on health. Acoust Aust 46(1):69–86, 10.1007/s40857-017-0098-3. [DOI] [Google Scholar]
- 15.Chapman S, Crichton F. 2017. Wind Turbine Syndrome: a Communicated Disease. https://open.sydneyuniversitypress.com.au/9781743324967.html [accessed 14 March 2022].
- 16.Tonin R, Brett J, Colagiuri B. 2016. The effect of infrasound and negative expectations to adverse pathological symptoms from wind farms. J Low Freq Noise V A 35(1):77–90, 10.1177/0263092316628257. [DOI] [Google Scholar]
- 17.Rubin GF, Burns M, Wessely S. 2014. Possible psychological mechanisms for “wind turbine syndrome”. On the windmills of your mind. Noise Health 16(69):116–122, PMID: , 10.4103/1463-1741.132099. [DOI] [PubMed] [Google Scholar]
- 18.Crichton F, Dodd G, Schmid G, Gamble G, Petrie KJ. 2014. Can expectations produce symptoms from infrasound associated with wind turbines? Health Psychol 33(4):360–364, PMID: , 10.1037/a0031760. [DOI] [PubMed] [Google Scholar]
- 19.NHMRC. 2007. National Statement on Ethical Conduct in Human Research (Updated 2018). http://www.nhmrc.gov.au/guidelines/publications/e72 [accessed March 14 2022].
- 20.Ekehammar B, Dornic S. 1990. Weinstein’s Noise Sensitivity Scale: reliability and construct validity. Percept Mot Skills 70(1):129–130, PMID: , 10.2466/pms.1990.70.1.129. [DOI] [PubMed] [Google Scholar]
- 21.Morin CM, Belleville G, Belanger L, Ivers H. 2011. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 34(5):601–608, PMID: , 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johns MW. 1991. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep 14(6):540–545, PMID: , 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 23.Lovibond SH, Lovidbond PF. 1995. Manual for the Depression Anxiety Stress Scales. 2nd ed. Sydney, New South Wales, Australia: Psychology Foundation. [Google Scholar]
- 24.Radomsky AS, Rachman S, Thordarson DS, McIsaac HK, Teachman BA. 2001. The Claustrophobia Questionnaire. J Anxiety Disord 15(4):287–297, PMID: , 10.1016/s0887-6185(01)00064-0. [DOI] [PubMed] [Google Scholar]
- 25.Onusko EM. 2004. Tympanometry. Am Fam Physician 70(9):1713–1720, PMID: . [PubMed] [Google Scholar]
- 26.Carhart R, Jerger JF. 1959. Preferred method for clinical determination of pure-tone thresholds. J Speech Hear Disord 24(4):330–345, 10.1044/jshd.2404.330. [DOI] [Google Scholar]
- 27.Walker B, Hessler GF, Hessler DM, Rand R, Schomer P. 2012. A Cooperative Measurement Survey and Analysis of Low Frequency and Infrasound at the Shirley Wind Farm in Brown County, Wisconsin. Report No. 122412-1. https://apps.psc.wi.gov/ERF/ERFview/viewdoc.aspx?docid=178200 [accessed 14 March 2022].
- 28.Berry RB, Brooks R, Gamaldo CE, Harding SM, Lloyd RM, Marcus CL, et al. . 2015. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.2. Darien, IL: American Academy of Sleep Medicine. [Google Scholar]
- 29.D’Rozario AL, Cross NE, Vakulin A, Bartlett DJ, Wong KKH, Wang D, et al. . 2017. Quantitative electroencephalogram measures in adult obstructive sleep apnea—potential biomarkers of neurobehavioural functioning. Sleep Med Rev 36:29–42, PMID: , 10.1016/j.smrv.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Wang D, Bai XX, Williams SC, Hua SC, Kim JW, Marshall NS, et al. . 2015. Modafinil increases awake EEG activation and improves performance in obstructive sleep apnea during continuous positive airway pressure withdrawal. Sleep 38(8):1297–1303, PMID: , 10.5665/sleep.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vakulin A, D’Rozario A, Kim JW, Watson B, Cross N, Wang D, et al. . 2016. Quantitative sleep EEG and polysomnographic predictors of driving simulator performance in obstructive sleep apnea. Clin Neurophysiol 127(2):1428–1435, PMID: , 10.1016/j.clinph.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Phillips L, Gilhooly K, Logie R, Della Sala S, Wynn V. 2003. Age, working memory, and the Tower of London task. Eur J Cogn Psychol 15(2):291–312, 10.1080/09541440244000148. [DOI] [Google Scholar]
- 33.Shallice T. 1982. Specific impairments of planning. Philos Trans R Soc Lond B Biol Sci 298(1089):199–209, PMID: , 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- 34.Dorrian J, Rogers NL, Dinges DF. 2004. Psychomotor vigilance performance: neurocognitive assay sensitive to sleep loss. In: Sleep Deprivation. Clinical Issues, Pharmacology, and Sleep Loss Effects. Kushida CA, ed. 1st ed. Boca Raton, FL: CRC Press, 39–70. [Google Scholar]
- 35.Dinges DF, Powell JW. 1985. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Methods Instrum Comput 17(6):652–655, 10.3758/BF03200977. [DOI] [Google Scholar]
- 36.Butlin M, Qasem A. 2017. Large artery stiffness assessment using SphygmoCor technology. Pulse (Basel) 4(4):180–192, PMID: , 10.1159/000452448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGrath BP. 2002. Ambulatory blood pressure monitoring. Med J Aust 176(12):558–592, PMID: , 10.5694/j.1326-5377.2002.tb04590.x. [DOI] [PubMed] [Google Scholar]
- 38.Borbély AA. 1982. A two process model of sleep regulation. Hum Neurobiol 1(3):195–204, PMID: . [PubMed] [Google Scholar]
- 39.Basner M, Müller U, Elmenhorst EM. 2011. Single and combined effects of air, road, and rail traffic noise on sleep and recuperation. Sleep 34(1):11–23, PMID: , 10.1093/sleep/34.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chapman S, St George A, Waller K, Cakic V. 2013. The pattern of complaints about Australian wind farms does not match the establishment and distribution of turbines: support for the psychogenic, ‘communicated disease’ hypothesis. PLoS One 8(10):e76584, PMID: , 10.1371/journal.pone.0076584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maijala PP, Kurki I, Vainio L, Pakarinen S, Kuuramo C, Lukander K, et al. . 2021. Annoyance, perception, and physiological effects of wind turbine infrasound. J Acoust Soc Am 149(4):2238, PMID: , 10.1121/10.0003509. [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization. 2009. Night Noise Guidelines for Europe. https://apps.who.int/iris/handle/10665/326486 [accessed 3 November 2022].
- 43.Mitterling T, Högl B, Schönwald SV, Hackner H, Gabelia D, Biermayr M, et al. . 2015. Sleep and respiration in 100 healthy Caucasian sleepers—a polysomnographic study according to American Academy of Sleep Medicine standards. SLEEP 38(6):867–875, PMID: , 10.5665/sleep.4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riedy SM, Smith MG, Rocha S, Basner M. 2021. Noise as a sleep aid: a systematic review. Sleep Med Rev 55:101385, PMID: , 10.1016/j.smrv.2020.101385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.