Abstract
Aims
Orthopaedic surgery requires grafts with sufficient mechanical strength. For this purpose, decellularized tissue is an available option that lacks the complications of autologous tissue. However, it is not widely used in orthopaedic surgeries. This study investigated clinical trials of the use of decellularized tissue grafts in orthopaedic surgery.
Methods
Using the ClinicalTrials.gov (CTG) and the International Clinical Trials Registry Platform (ICTRP) databases, we comprehensively surveyed clinical trials of decellularized tissue use in orthopaedic surgeries registered before 1 September 2022. We evaluated the clinical results, tissue processing methods, and commercial availability of the identified products using academic literature databases and manufacturers’ websites.
Results
We initially identified 4,402 clinical trials, 27 of which were eligible for inclusion and analysis, including nine shoulder surgery trials, eight knee surgery trials, two ankle surgery trials, two hand surgery trials, and six peripheral nerve graft trials. Nine of the trials were completed. We identified only one product that will be commercially available for use in knee surgery with significant mechanical load resistance. Peracetic acid and gamma irradiation were frequently used for sterilization.
Conclusion
Despite the demand for decellularized tissue, few decellularized tissue products are currently commercially available, particularly for the knee joint. To be viable in orthopaedic surgery, decellularized tissue must exhibit biocompatibility and mechanical strength, and these requirements are challenging for the clinical application of decellularized tissue. However, the variety of available decellularized products has recently increased. Therefore, decellularized grafts may become a promising option in orthopaedic surgery.
Cite this article: Bone Joint Res 2023;12(3):179–188.
Keywords: Decellularized tissue, Clinical trials, Orthopedic surgery, Autologous tissue harvesting, orthopaedic clinical trials, orthopaedic surgeries, clinical studies, strength, knee surgery, knee joint, peripheral nerve, tissue grafts, shoulder surgery, ankle surgery
Article focus
Although autografts are frequently used in orthopaedic surgery, decellularized allografts/xenografts have attracted attention because of the limited harvest volume and the risk of donor site morbidity for autografts.
The extent to which decellularized tissue grafts are used in orthopaedic surgery is unclear; therefore, this study comprehensively investigated their use in orthopaedic surgery clinical trials.
We hypothesized that few commercially available decellularized products would be identified in orthopaedic clinical trials.
Key messages
There have been 27 trials investigating the use of decellularized tissue in orthopaedic surgery, nine of which were completed, and 16 product names were identified.
We identified one decellularized product that will complete at least one clinical trial with favourable outcomes for knee joint grafts requiring mechanical load resistance that makes clinical use difficult.
The variety of decellularized products used in orthopaedic surgery requiring mechanical strength and biocompatibility has recently increased, suggesting that they could be a promising option.
Strengths and limitations
This is the first study to comprehensively investigate the clinical use of orthopaedic decellularized grafts.
We comprehensively surveyed the tissue processing methods, clinical use, and outcomes of decellularized products in trials found on the CTG and ICTRP databases.
This study may have omitted products and studies not registered on the CTG and ICTRP databases.
Introduction
The tissues treated in orthopaedic surgery are mainly those of the musculoskeletal system, including bone, cartilage, muscles, ligaments, and tendons. Autologous and allogenic orthopaedic tissue transplantations are effective treatments when the volume of these tissues is reduced by injury or degenerative disease.1 While autologous tissue is biocompatible, there is a limited harvest volume and a risk of donor site morbidity. Allografts avoid these problems but have their own disadvantages, including lower biocompatibility and potential disease transmission.2,3 To overcome the limitations of autografts and allografts, research has endeavoured, through in vitro tissue engineering, to create artificial grafts able to perform some of the biological functions of real tissue.4 This is achieved using artificial biological scaffolds. Such scaffolds require a 3D microenvironment able to maintain the growth of seed cells and cytokines. A well-constructed scaffold is a prerequisite for good biocompatibility when the graft is implanted.5 The biomaterials used to construct artificial biological scaffolds are generally composed of one or two extracellular matrix (ECM) components, such as collagen, fibronectin, and hyaluronic acid. These are selected and constructed to mimic the natural ECM. However, these scaffolds cannot reproduce the dynamic complexity of the natural microenvironment or perform the range of functions of natural ECMs.6 The ECM structure differs significantly in different types of tissue, even within the same individual. Conversely, the ECM structure of a given type of tissue is conserved across different species.7 Therefore, xenotransplantation using natural tissue from other species, in which the ECM shares the structure of equivalent human ECM, has attracted attention. Xenografts are more available than allografts because of their animal origin. However, there is a risk of rejection by xenogeneic antigens8 and disruption of the ECM structure by tissue processing methods.9 Therefore, the use of xenogeneic bone in orthopaedic surgery is not recommended because of poor biocompatibility and poor clinical results compared to those achieved using allogenic bone. Because of these disadvantages, xenogeneic bone tissue is seldom commercially available.10 A further issue with xenotransplantation is the difficulty of both removing cellular components and preserving the ECM, as one tends to preclude the other.11 However, if such limitations can be overcome, then xenotransplantation would certainly be a desirable option in orthopedics, a field in which reconstructive and reparative surgeries are common. In recent years, the US Food and Drug Administration (FDA) approved the use of decellularized and antigenically weakened ECM from allogenic or xenogeneic tissues or organs.12 Although a few reports have described the use of decellularized grafts in orthopaedic surgery,11 there have been no comprehensive investigations into the clinical use of orthopaedic decellularized grafts. Therefore, this study surveyed and analyzed the clinical applications and indications of decellularized tissues in orthopaedic surgery and the decellularization and sterilization methods used in each product. We hypothesized that not many decellularized products that had passed clinical trials would be commercially available in orthopaedic surgery.
Methods
Ethical approval
No ethical approval or consent was required for this study because data were obtained from internet databases and involved no humans or animals.
Clinical trial search methods
Using the two leading databases of the clinical trial, ClinicalTrials.gov (CTG), a registry of clinical trials maintained by the National Library of Medicine of the USA National Institutes of Health, and the International Clinical Trials Registry Platform (ICTRP), maintained by the World Health Organization, we thoroughly surveyed clinical trials in orthopaedics that used decellularized tissue. We searched for trials registered by 1 September 2022, using the search terms “decellularized OR decellularization OR acellular OR acellularization OR xenograft OR allograft”. The status of extracted clinical trials and details of decellularized products used in trials were reviewed by 10 October 2022.
Trial selection and data extraction
The study included clinical trials that assessed the use of allogenic or xenogeneic decellularized tissue for orthopaedic grafting purposes. Studies with a status of ‘not yet recruiting’, those unrelated to orthopaedics, those concerned with the treatment of skin ulcers using decellularized tissue-derived dressings, those in which decellularized tissue transfer was not performed, and those in which decellularized tissues such as injectable or wrapping materials that do not require mechanical strength were excluded. Two independent reviewers (MI and JI) assessed the trials returned in our search for eligibility. The senior author (KI) was asked to provide a final decision in cases of disagreement.
Scrutiny of the medical products used in the clinical trials
The diseases or parts of the body to which any decellularized tissue was applied, the status of the clinical trials, the name of the tissue product, the type of derived tissue, and the clinical results of the application of each product were carefully scrutinized and recorded using the PubMed (Medline), Web of Science, Cochrane, and EMBASE databases, as well as the websites of the companies responsible for the sale and manufacture of the relevant medical technology. If still unsure, we emailed the clinical trial or manufacturer’s representative if the product name was known, or the clinical trial representative if the product name was unsure. ‘Not available (N/A)’ was assigned (Tables I and IV) when the representatives refused information, or there was no response to our email from the representatives, and further information collection was not possible. Because this study was intended as an overview of the decellularized products used in trials extracted from the database, no quantitative evaluation was performed and no statistical analysis was required.
Table I.
Overview of nine clinical trials for the treatment of shoulder rotator cuff injuries using decellularized tissue.
| Trial no. | Condition | Clinical trial name | Length | Status | Product name | Source tissue | Country | Hospital/Company | Clinical reports | Decellularization method/Sterilization method |
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | Rotator cuff tear | Use of graft jacket for rotator cuff repair | 2007 to 2011 | Completed | GRAFTJACKET allograft | Human dermis | USA | Southern California Orthopaedic Institute/Wright Medical | Barber,13 Gupta14 | N/A; tissue underwent no general sterilization and freezing15 |
| S2 | Rotator cuff tear | Outcome evaluation of allograft scaffold augmentation for arthroscopic repair of full thickness of rotator cuff tear | 2013 to 2016 | Completed | CGDerm | Human dermis | South Korea | Hallym University Medical Center Kangnam/CGBio | Lee16 | N/A |
| S3 | Rotator cuff tear | Clinical outcomes and structural integrity of arthroscopic superior capsular reconstruction using cryopreserved acellular dermal matrix with increased elasticity and thickness in patients with irreparable rotator cuff tear | 2019 to 2020 | Completed | CGDerm | Human dermis | South Korea | Ewha Womans University Seoul Hospital/CGBio | Shin17 | N/A |
| S4 | Rotator cuff tear | Acellular dermis in rotator cuff repair | 2013 to 2021 | Completed | N/A | Human dermis | UK | The Royal Orthopaedic Hospital NHS Trust | N/A | N/A |
| S5 | Rotator cuff tear | Allograft reconstruction of massive rotator cuff tears vs partial repair alone | 2015 to 2021 | Completed | Allopatch HD | Human dermis | Canada | Nova Scotia Health Authority/MTF Sports Medicine | Agrawal18 | N/A; harvested by sterile techniques; tissue underwent no general sterilization19 |
| S6 | Rotator cuff tear | Arthroscopic superior capsular reconstruction – study of different types of grafts | 2018 to 2022 | Active, not recruiting | N/A | Pig dermis, bovine pericardium, allogenic/autologous femoral fascia, allogeneic Achilles tendon | Portugal | Hospital de Egas Moniz, Centro Hospitalar Lisboa Ocidental, General Hospital of Santo António, etc. | N/A | N/A |
| S7 | Rotator cuff tear | Massive rotator cuff tear reconstruction | 2018 to 2023 | Recruiting | GRAFTJACKET allograft | Human dermis | Canada | Nova Scotia Health Authority/Wright Medical | Barber,13 Gupta14 | N/A; tissue underwent no general sterilization and freezing15 |
| S8 | Rotator cuff tear | Comparison of partial rotator cuff repair vs superior capsular reconstruction for irreparable rotator cuff tears | 2020 to 2024 | Recruiting | N/A | Human dermis | USA | University Hospitals Cleveland Medical Centre, Lake Health, Midwest Orthopaedics at Rush | N/A | N/A |
| S9 | Rotator cuff tear | An outcomes study utilizing Allomend (R) HD for superior capsular reconstruction | 2021 to 2025 | Recruiting | AlloMend | Human dermis | USA | Western Orthopaedics Research and Education Foundation/AlloSource | N/A | Detergents or enzymes/electron irradiation not required20 |
N/A, not available.
Table IV.
Overview of five clinical trials for the treatment of peripheral nerve injury of the upper limbs using decellularized tissue.
| Trial no. | Condition | Clinical trial name | Length | Status | Product name | Source tissue | Country | Hospital/Company | Clinical reports | Decellularization method/sterilization method |
|---|---|---|---|---|---|---|---|---|---|---|
| N1 | Peripheral nerve injury of upper limb | A comparative post-marketing study of commercially available peripheral nerve gap repair options | 2009 to 2014 | Terminated (transitioning from 361 HCT/P Tissue to a Biological (BLA)) | Avance nerve graft | Human peripheral nerve | USA | Georgia Hand, Shoulder and Elbow, Indiana Hand Centre, University of Kentucky/Axogen Corporation | Safa34 | 50 mM phosphate + 100 nM Na, 0.14% Triton X-200, 0.6 mM sulfobetaine-16/gamma irradiation35 |
| N2 | Peripheral nerve injury of upper limb | Human acellular nerve graft for repair of peripheral nerve defects: a prospective, multicentre clinical study | 2009 to 2017 | Completed | N/A | Human peripheral nerve | China | The Sixth Affiliated Hospital of Shanghai Jiao Tong University /Guangzhou Zhongda Medical Devices Company | Zhu36 | 46 mM Triton X-100, 96 mM sodium deoxycholate;36 N/A |
| N3 | Peripheral nerve injury of upper limb | Human acellular nerve graft for repair of pure sensory nerve defects: a prospective, multicentre clinical study | 2009 to 2017 | Completed | N/A | Human peripheral nerve | China | The First Affiliated Hospital of Sun Yat-sen University/Guangzhou Zhongda Medical Devices Company | Zhu36 | 46 mM Triton X-100, 96 mM sodium deoxycholate;36 N/A |
| N4 | Peripheral nerve injury of upper limb | Comparison of processed nerve allograft and collagen nerve cuffs for peripheral nerve repair (RECON) | 2015 to 2021 | Completed | Avance nerve graft | Human peripheral nerve | USA | Virginia Commonwealth University Medical Centre, University of Pennsylvania, University of Florida/Axogen Corporation | Axogen37 | 50 mM phosphate + 100 nM Na, 0.14% Triton X-200, 0.6 mM sulfobetaine-16/gamma irradiation35 |
| N5 | Peripheral nerve injury of upper limb | BMAC nerve allograft study | 2017 to 2021 | Unknown status (Previously: active, not recruiting) | Avance nerve graft | Human peripheral nerve | USA | Brooke Army Medical Centre, Walter Reed National Military Medical Centre, Curtis National Hand Centre at MedStar Union Memorial Hospital/Axogen Corporation | Safa34 | 50 mM phosphate + 100 nM Na, 0.14% Triton X-200, 0.6 mM sulfobetaine-16/gamma irradiation35 |
| N6 | Peripheral nerve injury of upper limb | Use of a nerve regeneration conduit (NerVFIX) in the treatment of nerve section of the wrist | 2020 to 2023 | Recruiting | NerVFIX | Human artery or vein from umbilical cord | France | Clinique de la Main - Nantes Atlantique, Institut Chirurgical de la Main et du Membre Supérieur/TBF Genie Tissulaire | Barnouin38 | N/A; gamma irradiation (25 to 32 kGy), freezing39 |
BLA, biologic license application; BMAC, bone marrow aspirate concentrate; CM, carpometacarpal; HCT/P, human cell & tissue products; N/A, not available.
Results
Extracted clinical trials
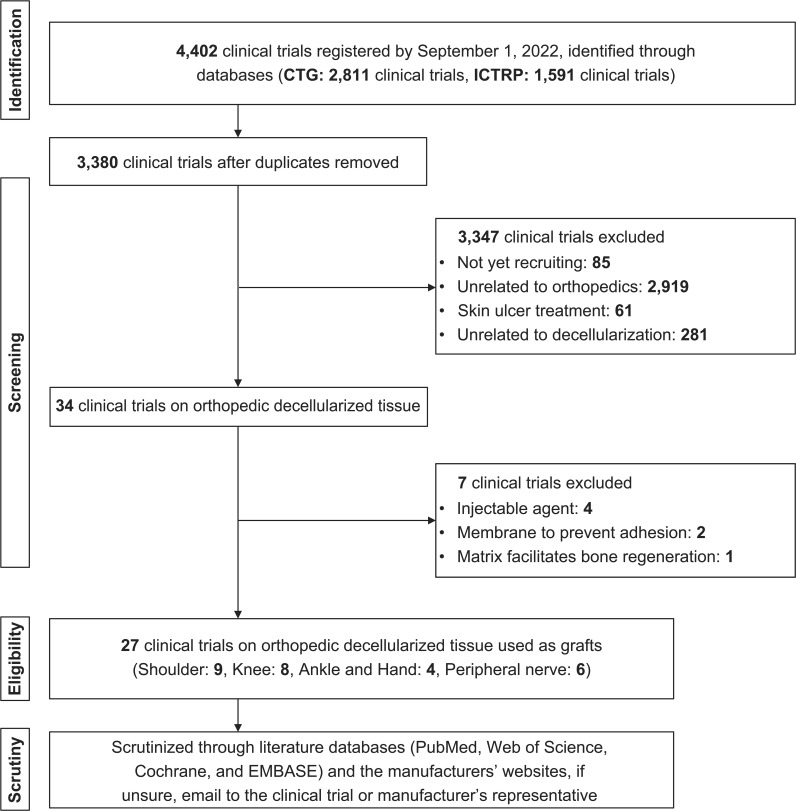
We identified 4,402 clinical studies (CTG: 2,811, ICTRP: 1,591). From these, 1,022 duplicates were removed, resulting in 3,380 trials being screened. After screening, 85 trials with a status of “not yet recruiting”, 2,919 trials unrelated to orthopaedics, 61 trials related to the treatment of skin ulcers using decellularized tissue-derived dressings, and 281 trials that did not use decellularized medical devices were excluded. Furthermore, from the remaining 34 clinical trials, seven trials were excluded: four using injectable agents, two using membranes to prevent adhesion, and one using a matrix that facilitates bone regeneration; thus, 27 clinical trials were finally eligible for inclusion in our study (Figure 1).
Fig. 1.
Flowchart of the clinical trial search process and the identification of decellularized products used in trials. We identified 4,402 trials initially and eventually reviewed a total of 27 trials: nine in the shoulder field, eight in the knee field, four in the ankle and hand field, and six in the peripheral nerve field. CTG, ClinicalTrials.gov; ICTRP, International Clinical Trials Registry Platform.
Target disease or body part and clinical trial status
Of the 27 trials included in our review, nine (S1 to 9) were related to shoulder injuries, including studies of rotator cuff patch grafting40 and superior capsule reconstruction (SCR)41 for rotator cuff injuries (Table I); eight (K1 to 8) were related to knee injuries, including four studies on grafts for osteochondral injury, three on grafts for anterior cruciate ligament (ACL) reconstruction, and one on grafts for meniscal transplantation (Table II); and four trials (AH1 to 4) were related to ankle or hand disorders, including one study on graft for osteochondral injury of the ankle, one on graft for lateral ligament reconstruction of the ankle, one on graft for void-filling after trapeziectomy, and one on graft for scaphotrapeziotrapezoid osteoarthritis (Table III). The remaining six trials (N1 to N6) were on nerve grafts for peripheral nerve injury of the upper limb (Table IV). Five trials (S1 to S5) in the shoulder field, one trial (AH3) in the hand field, and three trials (N2 to N4) in the nerve field were completed (Tables I and IV).
Table II.
Overview of eight clinical trials for the treatment of knee disorders using decellularized tissue.
| Trial no. | Condition | Clinical trial name | Length | Status | Product name | Source tissue | Country | Hospital/Company | Clinical reports | Decellularization method/sterilization method |
|---|---|---|---|---|---|---|---|---|---|---|
| K1 | Cartilage injury | Chondrofix osteochondral allograft prospective study | 2011 to 2014 | Terminated (inadequate enrolment and decreased need for clinical data to support product) | Chondrofix | Human osteochondral plug | USA | Plancher Orthopaedics and Sports Medicine, Cartilage Repair Centre, Brigham and Women’s Hospital, Orthopaedic Research Foundation, etc/Zimmer Biomet | Farr21 | N/A; gamma irradiation, freezing22 |
| K2 | Cartilage injury | Second line treatment of knee osteochondral lesion with treated osteochondral graft | 2016 to 2021 | Terminated (low accrual rate) | OD-PHOENIX | Human osteochondral plug | France | TBF Genie Tissulaire | N/A | N/A; gamma irradiation (25 to 32 kGy), freezing23 |
| K3 | Cartilage injury | Clinical study of decalcification bone scaffold for cartilage lesions of the knee | 2018 to 2023 | Recruiting | N/A | Decalcification bone | China | Peking University Third Hospital | N/A | N/A |
| K4 | Cartilage injury | Clinical trial to evaluate the efficacy and safety of MegaCarti in knee cartilage defects | 2020 to 2027 | Active, not recruiting | MegaCarti | Human cartilage | South Korea | Yonsei University Health System, Gangnam Severance Hospital/L&C Bio | N/A | N/A; gamma irradiation24 |
| K5 | ACL injury | Safety and efficacy study of the Z-Lig medical device compared to allograft | 2010 to ? | Unknown status | Z-Lig | Porcine bone-patellar tendon | Belgium, Italy, Netherlands, South Africa, Spain | Ghent University Hospital, Aarhus University Hospital, Istituti Ortopedici Rizzoli, etc/Aperion Biologics | Zaffagnini25 | Enzymes and α-galactosidase/electron irradiation26 |
| K6 | ACL injury | OrthoPure XT pilot clinical study | 2018 to 2020 | Withdrawn (unspecified business decision/strategic reason) | OrthoPure XT | Porcine tendon | USA | St. Joseph’s Outpatient Surgery Centre, OrthoIndy Hospital South, Jewish Hospital/TRX Orthopedics | Hunt27 | 0.1% sodium dodecyl sulfate and protease inhibitor28/antibiotics, peracetic acid29 |
| K7 | ACL injury | Clinical evaluation of dCELL ACL scaffold for reconstruction of the anterior cruciate ligament | 2015 to 2021 | Active, not recruiting | dCELL ACL scaffold | Porcine tendon | Poland, Spain, UK | Robert Jones and Agnes Hunt Hospital NHS Foundation Trust, Hospital Universitari de Bellvitage, Klinika Chirurgii Endoskopowej Sp. z o.o. etc/Tissue Regenix | Hunt27 | 0.1% sodium dodecyl sulfate and protease inhibitor28/antibiotics, peracetic acid29 |
| K8 | Meniscus injury | Clinical evaluation of dCELL meniscus for partial arthroplasty of the meniscus | 2014 to 2018 | Suspended | dCELL meniscus | Porcine meniscus | Poland, UK | "Ortotrauma" Spółka Z Ograniczoną, The Hillingdon Hospitals NHS Foundation Trust, the Robert Jones & Agnes Hunt Orthopaedic Hospital NHS Foundation Trust/Tissue Regenix | N/A | 0.1% sodium dodecyl sulfate and protease inhibitor;28,30 N/A |
ACL, anterior cruciate ligament; N/A, not available.
Table III.
Overview of four clinical trials for the treatment of ankle and hand disorder using decellularized tissue.
| Trial no. | Condition | Clinical trial name | Length | Status | Product name | Source tissue | Country | Hospital/Company | Clinical reports | Decellularization method/sterilization method |
|---|---|---|---|---|---|---|---|---|---|---|
| AH1 | Ankle: osteochondral lesions of the talus | OD-PHOENIX in talus osteochondral lesion | 2017 to 2021 | Terminated (low accrual rate) | OD-PHOENIX | Human osteochondral plug | Belgium, Israel | AZ Monica, Poriya Medical Centre/TBF Genie Tissulaire | N/A | N/A; gamma irradiation (25 to 32 kGy), freezing23 |
| AH2 | Ankle: lateral collateral ligament instability of the ankle | The comparison of clinical and radiological outcomes between modified Broström operation and lateral ankle ligament reconstruction using acellular dermal matrix augmentation in chronic lateral ankle instability: A 5 year randomized controlled trial | 2020 to 2025 | Recruiting | SureDerm | Human dermis | South Korea | Wonkwang University Hospital/Hans Biomed Corporation | N/A | Enzymes, EDTA/freeze-dried31 |
| AH3 | Hand: CM arthritis of the thumb | Use of FlexHD as Post Trapeziectomy Spacer | 2013 to 2017 | Completed | FlexHD | Human dermis | USA | Cedars-Sinai Department of Hand Surgery/Ethicon | Yao32 | Non-ionic surfactant (Triton X-100)/alcohol, peracetic acid33 |
| AH4 | Hand: STT arthritis | Use of a treated, devitalized and sterile meniscus segment (MENISC-T) in the treatment of STT osteoarthritis | 2021 to 2023 | Recruiting | MENISC-T | Human meniscus | France | Institut Chirurgical de la Main et du Membre Supérieur/TBF Genie Tissulaire | N/A | N/A |
CM, carpometacarpal; EDTA, ethylenediaminetetraacetic acid; N/A, not available; STT, scaphotrapeziotrapezoid.
Decellularized tissue products used and the type of derived tissue
We identified 16 decellularized tissue product names used in 27 clinical trials: GRAFTJACKET (Wright Medical, USA), CGDerm (CGBio, South Korea),16 Allopatch HD (MTF Biologics, USA), AlloMend (AlloSource, USA),20 Chondrofix (Zimmer Biomet, USA),21 OD-PHOENIX (TBF Genie Tissulaire, France),23 Z-lig (APEIRON Biologics, Austria),25 MegaCarti (L&C Bio, South Korea),24 dCELL ACL Scaffold (Tissue Regenix, UK),27,28,30 OrthoPure XT (Tissue Regenix),27,28,30 dCELL Meniscus (Tissue Regenix),28,30 SureDerm (Hans Biomed Corporation, South Korea),31 FlexHD (Ethicon, USA),32 MENISC-T (TBF Genie Tissulair), Avance Nerve Graft (Axogen, USA),34 and NerVFIX (TBF Genie Tissulair).38 GRAFTJACKET, CGDerm, and OD-PHOENIX were used in two trials (S1 and S7, S2 to S3, and K2 and AH1, respectively), and Avance in three trials (N1, N4, and N5). The product names used in six trials (S4, S6, S8, K3, and N2 to N3) were not provided. Of the 16 products, Chondrofix, OD-PHOENIX, and Z-lig contained bone tissues, and the rest were sourced from the soft-tissue or cartilage tissue alone.
Clinical results, decellularization methods, and sterilization methods
Clinical results were presented for 16 trials: S1 to S3, S5, and S7;13,16-18 K1, K5 to K7;21,25,27 AH3,32 and N1 to N6.34,36-38 The decellularization methods were provided in nine products in 12 trials (S9, K5 to K8, AH2 and AH3, and N1 to N5). AlloMend was treated without detergents or enzymes.20 Z-lig was treated with enzymes for decellularization and with α-galactosidase for α-galactose removal.26 The remaining seven products were decellularized with sodium dodecyl sulphate, protease inhibitors, enzymes, ethylenediaminetetraacetic acid, Triton X-200, phosphate, sulfobetaine-16, and Triton X-100.28,30,31,33,35,36 The sterilization methods were provided in 13 products in 17 trials (S1, S5, S7, S9, K1, K2, K4 to K7, AH1 to AH3, and N1, N4 to N6). Allopatch HD and GRAFTJACKET were only decellularized after the tissue had been harvested in a sterile manner.15,19 The remaining 11 products were sterilized using gamma irradiation and freezing,21-23,39 electron beam irradiation,20,26 antibiotics and peracetic acid (animal study),29 gamma radiation,24,35 freezing,31 and alcohol and peracetic acid33 (Tables I and IV).
Discussion
The most important findings of this study were, firstly, that there have been 27 orthopaedic clinical trials using decellularized tissue and only nine of these were completed. Secondly, decellularized tissue has been used for only a few sites and conditions in orthopaedics. The first of these findings verified our hypothesis.
Rotator cuff injuries were the most common condition treated in the trials surveyed. In this context, decellularized tissue was used as a patch, as an augmentation for rotator cuff repair, and as a graft for SCR.41 In general, repairs for small rotator cuff tears have good outcomes; however, massive tears, which account for 40% of rotator cuff tears, display poorer outcomes with primary repair alone.42 Following debridement and primary repair, the treatment options for massive rotator cuff tears include: reverse shoulder arthroplasty; transfer of the latissimus dorsi, pectoralis major, and trapezius muscles; and SCR.41,42 However, these methods do not provide anatomical repair or reconstruction. No improvement was reported in patient symptoms after the anatomical repair of a two-tendon rotator cuff tear using decellularized porcine small intestine mucosa tissue as a patch.43 The poor results of this procedure may be explained by the fact that the small intestine mucosa is an inappropriate graft because its mechanical strength is inferior to that of the rotator cuff.44 Lee et al16 reported retear rates of 9.1% in the group with CGDerm as a patch augmentation for repair of large rotator cuff tears and 38.1% in the repair-alone group (p = 0.034). Shin et al17 reported a visual analogue scale for pain improvement from 4.0 to 0.7 (p < 0.001) after SCR with CGDerm. Agrawal18 used Allopatch HD for patch repair of massive rotator cuff tears. On MRI, performed a median of 16.8 months after the procedure, they found that 85.7% (12/14 patients) of the repaired rotator cuffs were intact, indicating good clinical outcomes. Barber et al13 conducted a prospective randomized study on 22 patients treated with GRAFTJACKET augmentation for massive rotator cuff tears and 22 without augmentation. MRI performed a mean of 14.5 months after surgery found that the repaired rotator cuff was intact in 85% of patients in the GRAFTJACKET group versus 40% of those in the non-augmentation group (p < 0.01). In a prospective observational study with a mean follow-up of three years, all 24 patients with massive rotator cuff tears treated with GRAFTJACKET displayed significant improvements in pain, range of motion, and muscle strength.14 FDA has approved GRAFTJACKET for use as reinforcement for rotator cuffs, ligaments, and joint capsules,45 but not for use as a graft bridge for rotator cuff tears with gaps larger than 1 cm.14 This prohibition of the use of GRAFTJACKET as a bridge is likely because the strength of the dermis comprising the product is inferior to that of the rotator cuff. However, an increasing number of completed clinical trials in recent years and good clinical outcomes suggest that decellularized tissues will be a promising device for rotator cuff treatment.
Chondrofix, a decellularized human osteochondral plug currently unavailable, produced poor clinical results of a high failure rate of 72% (23/32 knees) at a two-year follow-up.21 OD-PHOENIX is derived from the human osteochondral plug; however, the clinical trial has been terminated because of the low accrual rate. We could identify incompleted trials in the osteochondral injury field. Z-lig is a decellularized porcine bone-patellar tendon graft. It has been used with just three patients but no failure was observed at two-year follow-up evaluations.25 Z-lig is the world’s first artificial biological device to receive the CE marking, which was awarded in 2014, as a graft for ACL revision or multiple ligament reconstruction. However, this product is no longer on the market, suggesting that problems may have been identified. OrthoPure XT and dCELL ACL scaffold are identical in terms of indications, tissue derivation, and manufacturer and appear to be identical products. The K6 trial (withdrawn due to business decision) appears to have been USA-based, presumably to obtain FDA approval and USA market access. The K7 trial appears to have been Europe-based, presumably to obtain CE mark approval and European market access. OrthoPure XT was reported to have received CE marking on 1 June 2020, for its use in ACL revision grafts or multiple ligament reconstruction surgeries. Neil Hunt,27 the principal investigator of the K7 trial, reported favourable clinical outcomes after 48 months of primary ACL reconstruction using OrthoPure XT at the European Society of Sports Traumatology, Knee Surgery & Arthroscopy in 2022. The status of the dCELL meniscus trial on partial meniscus reconstruction using decellularized porcine meniscus-derived was “suspended”. We could identify incomplete trials in the knee field, including the dCELL ACL Scaffold trial. However, it would be changed to “completed”. Studies using bovine bone-patellar tendon grafts for ACL reconstruction were conducted in the 1980s using a technique whereby glutaraldehyde cross-linked porcine heart valves were transplanted into humans. However, the large amounts of glutaraldehyde used for cross-linking resulted in poor biocompatibility of the tissue, inappropriate biomechanical properties, and a lack of integration with the host tissue. Hence, the clinical results of bovine tendon tissue use in humans were poor.46 Therefore, the fact that OrthoPure XT is about to enter the market with distribution agreements in the UK and Italy is a significant step forward.47,48 However, the practical application of decellularized tissues for meniscus disorders is not yet available. As mentioned, there is a trade-off between ECM maintenance and adequate decellularization when developing these products.11 The challenge of maintaining both graft strength and biocompatibility is likely to be greater in tissue for the knee, due to the heavier load on the graft than occurs elsewhere on the body.49
In terms of the ankle joint, the AH1 trial using OD-PHOENIX for osteochondral lesions of the talus bone was terminated for the same reason as the K2 trial; however, the AH2 trial using SureDerm to augment the lateral ligament is ongoing, and the product is expected to be put into practical use. Yao et al32 evaluated the clinical outcomes of FlexHD following its use as a void-filling spacer after trapeziectomy for carpometacarpal (CM) arthropathy. At six-month follow-ups, no complications were observed, and patients reported improvements in pain and grip strength compared to preoperative levels (p < 0.01). The AH3 trial is also in progress, which studies the placement of a decellularized meniscus segment at the joint interposition for scaphotrapeziotrapezoid arthropathy.
Safa et al34 conducted a trial of Avance Nerve Graft and found that, in 624 nerve repairs with various gaps between transects, the mean meaningful recovery rate was 82% (91% for gaps smaller than 15 mm and 69% for gaps of 50 to 70 mm), which was comparable to results previously reported for autologous nerve grafts. It was also recently reported that the group using Avance Nerve Graft showed significantly better recovery than those using conduits for digital nerve injury (N5).37 Other decellularized allogenic nerves and umbilical cord vessels (NerVFIX) have been used as scaffolds for peripheral nerve repair, yielding favourable results.36,38 One of the reasons for the good clinical results in the hand and peripheral nerve field may be partially explained by the fact that these grafts need less mechanical strength than those applied to the knee.
An issue with the decellularized products identified in the current study was the use of dermal tissue patches for rotator cuff repair. Because the dermis is weaker than the rotator cuff,44 these patches may not be suitable for rotator cuff repair, as the rotator cuff is subject to relatively high loads. However, the technology to decellularize thicker tissue more suitable for rotator cuff repair, while maintaining both the tissue strength and the ECM, has not yet been developed.50 Stronger and thicker grafts more suitable for the rotator cuff may provide a more “anatomical” repair than SCR for large tears. We also identified some issues with the processing of tissues in the studies evaluated. Glutaraldehyde is used as a chemical cross-linking agent to stabilize tissue strength. However, its use compromises the integrity of collagen fibres and inhibits the infiltration of host cells into the graft.51 Gamma irradiation sterilization destroys collagen fibres and impairs tissue regeneration.52 Sterilization using peracetic acid, which is highly acidic and a strong oxidant, can affect the physical and chemical properties of the tissue.53
As previously mentioned, decellularized tissue used in orthopaedic surgeries requires both the biocompatibility necessary in other medical fields and the endurance of high mechanical loads. This latter requirement is specific to orthopaedics, making its clinical application in this field particularly difficult. Overcoming this issue will lead to the widespread use of decellularized tissue, which is highly demanded in orthopaedics. Therefore, development of decellularized tissues that are biocompatible and endure high mechanical loads is in high demand.
This study had several limitations. First, because the survey was conducted using the CTG and ICTRP databases, it may not have included clinical trials or products that were not registered on these databases. However, these databases reflect registries of 18 countries and appear to be highly comprehensive. Second, other tissue products may have unpublished tissue processing methods. However, this is unavoidable considering the risk of product features and specifications being leaked to the public. Third, there was little information available on the approval of the 16 products by relevant medical authorities; only Z-lig and OrthoPure XT have achieved CE marking.
In conclusion, of the 27 clinical trials of decellularized tissue in orthopaedics identified by a comprehensive survey, nine were completed. Despite the demand for decellularized tissue, not many decellularized tissue products are commercially available now, particularly for the knee joint. However, the variety of available decellularized products, including OrthoPure XT for knee surgery, has recently increased. Therefore, decellularized grafts may become a promising option in orthopaedic surgery.
Author contributions
M. Itoh: Methodology, Formal analysis, Investigation, Writing – original draft.
J. Itou: Investigation.
S. Imai: Investigation.
K. Okazaki: Supervision.
K. Iwasaki: Conceptualization, Methodology, Supervision, Writing – review & editing.
Funding statement
The authors disclose receipt of the following financial or material support for the research, authorship, and/or publication of this article: this work was supported by AMED (Japan Agency for Medical Research and Development), grant number 20pc0101059h0001.
ICMJE COI statement
The authors declare no competing interests.
Acknowledgements
The authors would like to thank Mitsuru Yuba and Yusuke Tsuboko for their assistance in searching for clinical trials. We would also like to thank Enago (www.enago.jp) for the English language review.
Ethical review statement
No ethical approval or consent was required for this study because data were obtained from internet databases and involved no humans or animals.
Open access funding
Open access funding was supported by AMED (Japan Agency for Medical Research and Development), grant number 20pc0101059h0001.
© 2023 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/
Contributor Information
Masafumi Itoh, Email: itoumasafumi@gmail.com.
Junya Itou, Email: jun718@gmail.com.
Shinya Imai, Email: imashin@toki.waseda.jp.
Ken Okazaki, Email: okazaki.ken@twmu.ac.jp.
Kiyotaka Iwasaki, Email: iwasaki@waseda.jp.
References
- 1. Osawa Y, Seki T, Okura T, Takegami Y, Ishiguro N, Hasegawa Y. Long-term outcomes of curved intertrochanteric varus osteotomy combined with bone impaction grafting for non-traumatic osteonecrosis of the femoral head. Bone Joint J. 2021;103-B(4):665–671. doi: 10.1302/0301-620X.103B4.BJJ-2020-1107.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gazdag AR, Lane JM, Glaser D, Forster RA. Alternatives to autogenous bone graft: efficacy and indications. J Am Acad Orthop Surg. 1995;3(1):1–8. doi: 10.5435/00124635-199501000-00001. [DOI] [PubMed] [Google Scholar]
- 3. Wellings EP, Houdek MT, Owen AR, et al. Comparison of free vascularized fibular flaps and allograft fibular strut grafts to supplement spinopelvic reconstruction for sacral malignancies. Bone Joint J. 2021;103-B(8):1414–1420. doi: 10.1302/0301-620X.103B8.BJJ-2020-2302.R1. [DOI] [PubMed] [Google Scholar]
- 4. Hu WS. Engineering perspectives in tissue engineering. Cytotechnology. 1992;10(3):187–188. doi: 10.1007/BF00146669. [DOI] [PubMed] [Google Scholar]
- 5. Zhang W, Zhu Y, Li J, et al. Cell-derived extracellular matrix: basic characteristics and current applications in orthopedic tissue engineering. Tissue Eng Part B Rev. 2016;22(3):193–207. doi: 10.1089/ten.TEB.2015.0290. [DOI] [PubMed] [Google Scholar]
- 6. Ravindran S, Gao Q, Kotecha M, et al. Biomimetic extracellular matrix-incorporated scaffold induces osteogenic gene expression in human marrow stromal cells. Tissue Eng Part A. 2012;18(3–4):295–309. doi: 10.1089/ten.tea.2011.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Swindle MM, Makin A, Herron AJ, Clubb FJ, Frazier KS. Swine as models in biomedical research and toxicology testing. Vet Pathol. 2012;49(2):344–356. doi: 10.1177/0300985811402846. [DOI] [PubMed] [Google Scholar]
- 8. Robson SC, Schulte am Esch J, Bach FH. Factors in xenograft rejection. Ann N Y Acad Sci. 1999;875:261–276. doi: 10.1111/j.1749-6632.1999.tb08509.x. [DOI] [PubMed] [Google Scholar]
- 9. Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: structure and function. Acta Biomater. 2009;5(1):1–13. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 10. Bracey DN, Cignetti NE, Jinnah AH, et al. Bone xenotransplantation: a review of the history, orthopedic clinical literature, and a single-center case series. Xenotransplantation. 2020;27(5):e12600. doi: 10.1111/xen.12600. [DOI] [PubMed] [Google Scholar]
- 11. Nie X, Wang DA. Decellularized orthopaedic tissue-engineered grafts: biomaterial scaffolds synthesised by therapeutic cells. Biomater Sci. 2018;6(11):2798–2811. doi: 10.1039/c8bm00772a. [DOI] [PubMed] [Google Scholar]
- 12. Kehoe S, Zhang XF, Boyd D. FDA approved guidance conduits and wraps for peripheral nerve injury: a review of materials and efficacy. Injury. 2012;43(5):553–572. doi: 10.1016/j.injury.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 13. Barber FA, Burns JP, Deutsch A, Labbé MR, Litchfield RB. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy. 2012;28(1):8–15. doi: 10.1016/j.arthro.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 14. Gupta AK, Hug K, Berkoff DJ, et al. Dermal tissue allograft for the repair of massive irreparable rotator cuff tears. Am J Sports Med. 2012;40(1):141–147. doi: 10.1177/0363546511422795. [DOI] [PubMed] [Google Scholar]
- 15. Wagner CT, Owens RT, Harper JR, McQuillan DJ. 10 - Human-derived acellular matrices for dermal replacement In Orgill D, Blanco C. Biomaterials for Treating Skin Cambridge, UK: Woodhead Publishing; 2009. 142 173 [Google Scholar]
- 16. Lee GW, Kim JY, Lee HW, Yoon JH, Noh K-C. Clinical and Anatomical Outcomes of Arthroscopic Repair of Large Rotator Cuff Tears with Allograft Patch Augmentation: A Prospective, Single-Blinded, Randomized Controlled Trial with a Long-term Follow-up. Clin Orthop Surg. 2022;14(2):263–271. doi: 10.4055/cios21135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shin SJ, Lee S, Hwang JY, Lee W, Koh KH. Superior capsular reconstruction using acellular dermal allograft combined with remaining rotator cuff augmentation improved shoulder pain and function at 1 year after the surgery. Arthroscopy. 2022;38(4):1089–1098. doi: 10.1016/j.arthro.2021.10.027. [DOI] [PubMed] [Google Scholar]
- 18. Agrawal V. Healing rates for challenging rotator cuff tears utilizing an acellular human dermal reinforcement graft. Int J Shoulder Surg. 2012;6(2):36–44. doi: 10.4103/0973-6042.96992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.No authors listed AlloPatch HD® acellular dermisAcellular Dermis. MTF Biologics. [12 January 2023]. https://www.mtfbiologics.org/docs/default-source/packageinserts/2020/pi-42-rev-11.pdf date last. accessed.
- 20.Stilwell R, Delaney R.The Biomechanics of Allomend® acellular dermal matrix: biocompatibility study. [12 January 2023]. https://allosource.org/wp-content/uploads/2020/11/AlloMend-ADM-Biocompatibility-Study.pdf date last. accessed.
- 21. Farr J, Gracitelli GC, Shah N, Chang EY, Gomoll AH. High failure rate of a decellularized osteochondral allograft for the treatment of cartilage lesions. Am J Sports Med. 2016;44(8):2015–2022. doi: 10.1177/0363546516645086. [DOI] [PubMed] [Google Scholar]
- 22. Reynolds KL, Bishai SK. In situ evaluation of chondrofix® osteochondral allograft 25 months following implantation: a case report. Osteoarthritis and Cartilage. 2014;22:S155–S156. [Google Scholar]
- 23.No authors listed Brochure - PHOENIX bone allograftsBone Allografts. [12 January 2023]. https://www.tbf-lab.com/tbf-ortho/index.php/en/downloads/brochures-flyers/phoenix-product-range/viewdocument/13 date last. accessed.
- 24. Kwoen M-J, Kim S-Y, Choi Y-H, et al. The use of acellular allogenic dermal matrix in soft tissue management around the implants. Journal of Dental Implant Research. 2019;38(1):6–12. [Google Scholar]
- 25. Zaffagnini S, Grassi A, Marcheggiani Muccioli GM, et al. Anterior cruciate ligament reconstruction with a novel porcine xenograft: the initial Italian experience. Joints. 2015;3(2):85–90. doi: 10.11138/jts/2015.3.2.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stone KR, Abdel-Motal UM, Walgenbach AW, Turek TJ, Galili U. Replacement of human anterior cruciate ligaments with pig ligaments: a model for anti-non-gal antibody response in long-term xenotransplantation. Transplantation. 2007;83(2):211–219. doi: 10.1097/01.tp.0000250598.29377.13. [DOI] [PubMed] [Google Scholar]
- 27.Neil H. Paris, France: 20th ESSKA Congress: First in human clinical data from a novel xenograft biologic scaffold used to treat primary acl reconstruction. 24 & 48 Month Follow-up. 29 April 2022. [Google Scholar]
- 28. Jones G, Herbert A, Berry H, Edwards JH, Fisher J, Ingham E. Decellularization and characterization of porcine superflexor tendon: a potential anterior cruciate ligament replacement. Tissue Eng Part A. 2017;23(3–4):124–134. doi: 10.1089/ten.tea.2016.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Herbert A, Jones GL, Ingham E, Fisher J. A biomechanical characterisation of acellular porcine super flexor tendons for use in anterior cruciate ligament replacement: investigation into the effects of fat reduction and bioburden reduction bioprocesses. J Biomech. 2015;48(1):22–29. doi: 10.1016/j.jbiomech.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stapleton TW, Ingram J, Katta J, et al. Development and characterization of an acellular porcine medial meniscus for use in tissue engineering. Tissue Eng Part A. 2008;14(4):505–518. doi: 10.1089/tea.2007.0233. [DOI] [PubMed] [Google Scholar]
- 31. Lee KC, Lee NH, Ban JH, Jin SM. Surgical treatment using an allograft dermal matrix for nasal septal perforation. Yonsei Med J. 2008;49(2):244–248. doi: 10.3349/ymj.2008.49.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yao CA, Ellis CV, Cohen MJ, Kulber DA. Preserving the posttrapeziectomy space with a human acellular dermal matrix spacer: a pilot case series of patients with thumb carpometacarpal joint arthritis. Plast Reconstr Surg Glob Open. 2013;1(7):e65. doi: 10.1097/GOX.0b013e3182aa8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eberli D, Rodriguez S, Atala A, Yoo JJ. In vivo evaluation of acellular human dermis for abdominal wall repair. J Biomed Mater Res A. 2010;93(4):1527–1538. doi: 10.1002/jbm.a.32636. [DOI] [PubMed] [Google Scholar]
- 34. Safa B, Jain S, Desai MJ, et al. Peripheral nerve repair throughout the body with processed nerve allografts: results from a large multicenter study. Microsurgery. 2020;40(5):527–537. doi: 10.1002/micr.30574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hudson TW, Zawko S, Deister C, et al. Optimized acellular nerve graft is immunologically tolerated and supports regeneration. Tissue Eng. 2004;10(11–12):1641–1651. doi: 10.1089/ten.2004.10.1641. [DOI] [PubMed] [Google Scholar]
- 36. Zhu S, Liu J, Zheng C, et al. Analysis of human acellular nerve allograft reconstruction of 64 injured nerves in the hand and upper extremity: a 3 year follow-up study. J Tissue Eng Regen Med. 2017;11(8):2314–2322. doi: 10.1002/term.2130. [DOI] [PubMed] [Google Scholar]
- 37.No authors listed Axogen, Inc. Announces Positive Topline Results from Phase 3 RECON(SM) Study for Avance® Nerve Graft. Globe News Wire. [26 January 2023]. https://www.globenewswire.com/news-release/2022/05/04/2436081/0/en/Axogen-Inc-Announces-Positive-Topline-Results-from-Phase-3-RECON-SM-Study-for-Avance-Nerve-Graft.html date last. accessed.
- 38.Vanmierlo B, Ardouin L, Verstreken F.Results of a nerve generation guide from human umbilical cord vessels in the treatment of nerve sections in the hand. [30 January 2023]. https://www.tbf-lab.com/nervfix/images/abstract-2019-nervfix-a0477-nerve-regeneration-from-human-umbilical-cord-vessels.pdf date last. accessed.
- 39.No authors listed NerVFIX conduit allogénique de régénération nerveuse. Group Lépine. [12 January 2023]. https://www.tbf-lab.com/nervfix/images/documentation-nervfix_2021.pdf date last. accessed.
- 40. Chalmers PN, Tashjian RZ. Patch augmentation in rotator cuff repair. Curr Rev Musculoskelet Med. 2020;13(5):561–571. doi: 10.1007/s12178-020-09658-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459–470. doi: 10.1016/j.arthro.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 42. Bedi A, Dines J, Warren RF, Dines DM. Massive tears of the rotator cuff. J Bone Joint Surg Am. 2010;92-A(9):1894–1908. doi: 10.2106/JBJS.I.01531. [DOI] [PubMed] [Google Scholar]
- 43. Iannotti JP, Codsi MJ, Kwon YW, Derwin K, Ciccone J, Brems JJ. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88-A(6):1238–1244. doi: 10.2106/JBJS.E.00524. [DOI] [PubMed] [Google Scholar]
- 44. Barber FA, Herbert MA, Coons DA. Tendon augmentation grafts: biomechanical failure loads and failure patterns. Arthroscopy. 2006;22(5):534–538. doi: 10.1016/j.arthro.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 45. Longo UG, Lamberti A, Maffulli N, Denaro V. Tendon augmentation grafts: a systematic review. Br Med Bull. 2010;94:165–188. doi: 10.1093/bmb/ldp051. [DOI] [PubMed] [Google Scholar]
- 46.Teitge R. Bovine xenograft reconstruction of the ACL. The crucial ligaments. New York, USA: Churchill Livingstone; 1988. pp. 529–534. [Google Scholar]
- 47.No authors listed Tissue Regenix Group plc - UK distribution partner for OrthoPure® XT. IP Group. Aug 24, 2020. [12 January 2023]. https://www.ipgroupplc.com/media/portfolio-news/2020/2020-08-24a date last. accessed.
- 48.No authors listed Tissue Regenix Group plc - Distribution agreement for OrthoPure® XT. IP Group. Mar 9, 2022. [17 January 2023]. https://www.ipgroupplc.com/media/portfolio-news/2022/2022-03-09 date last. accessed.
- 49. Morrison JB. Function of the knee joint in various activities. Biomed Eng. 1969;4(12):573–580. [PubMed] [Google Scholar]
- 50. O’Connor Mooney R, Davis NF, Hoey D, Hogan L, McGloughlin TM, Walsh MT. On the automatic decellularisation of porcine aortae: a repeatability study using a non-enzymatic approach. Cells Tissues Organs. 2016;201(4):299–318. doi: 10.1159/000445107. [DOI] [PubMed] [Google Scholar]
- 51. Botes L, Laker L, Dohmen PM, et al. Advantages of decellularized bovine pericardial scaffolds compared to glutaraldehyde fixed bovine pericardial patches demonstrated in a 180-day implant ovine study. Cell Tissue Bank. 2022;23(4):791–805. doi: 10.1007/s10561-021-09988-8. [DOI] [PubMed] [Google Scholar]
- 52. Yang X, Feng J, Wang F, Hu Y. Irradiation sterilization used for allogenetic tendon: a literature review of current concept. Cell Tissue Bank. 2019;20(2):129–139. doi: 10.1007/s10561-019-09756-9. [DOI] [PubMed] [Google Scholar]
- 53. Tao M, Ao T, Mao X, et al. Sterilization and disinfection methods for decellularized matrix materials: review, consideration and proposal. Bioact Mater. 2021;6(9):2927–2945. doi: 10.1016/j.bioactmat.2021.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]