Abstract
Background
Evidence from rodents indicated that after recent stress, reduced expression of tight junction protein claudin-5 may weaken the blood-brain barrier and allow interleukin-6 to induce depressive symptoms. Our aims were to prove this pathomechanism in humans.
Methods
We used a large population genetic database (UK Biobank, n = 277 501) to test whether variation in the CLDN5 gene could modulate effects of the IL6 gene variant in stress-induced depression. Three-way interaction of functional polymorphisms, rs885985 of CLDN5, and rs1800795 of IL6 with recent stressful life events were tested on current depressive symptoms. Analyses were performed in male and female populations as well.
Results
The 3-way interaction including recent stress yielded highly significant results on current depressive symptoms in the UK Biobank sample, which was more pronounced in men and could be replicated on trend level in an independent cohort (NewMood, n = 1638). None of any other associations or interactions, including, for example, childhood stressors and lifetime depression as an outcome, yielded significance.
Conclusions
These findings provide genetic evidence in humans for the interaction among interleukin-6, claudin-5, and recent stress, suggesting that inflammation is involved in the development of depression and that stress-connected brain entry of inflammatory molecules is a key factor in this pathomechanism. These genetic polymorphisms may help to identify people at higher risk for recent stress-induced depression.
Keywords: major depressive disorder, CLDN5, IL6, blood-brain barrier, biomarker
Significance Statement.
Evidence suggests involvement of tight-junction protein claudin-5 of the blood-brain barrier in the development of depression after experiencing stress. Animal data suggested that stress-induced decrease in claudin-5 expression may allow for interleukin-6 penetration of the brain and, consequently, depression. Because the theory remained untested in humans, our study aimed to translate these findings using large population genetic cohorts. We could show a highly significant interaction of functional single nucleotide polymorphisms of CLDN5 and IL6 genes with recent stress for depressive symptoms. These findings are important, because (1) they provide the first evidence, to our knowledge, for the mechanism in humans; (2) provide biomarkers (i.e., stress and the polymorphisms) and drug targets in the form of claudin-5 and interleukin-6 proteins for current depression that can be investigated in animals and have relevance in humans; and (3) indicate a potential role for stress-induced alteration of the blood-brain barrier behind depression.
INTRODUCTION
Treating major depressive disorder (MDD) is highly challenging and, at the same time, in exceptional demand. Although this disorder causes one of the largest global burdens on healthcare systems (Otte et al., 2016), its underlying pathomechanism remains only partially discovered. Because currently available medications are not sufficiently effective in a large portion of the cases, there is an urgent need for identifying novel biomarkers and therapeutic targets in depression research, focusing on distinct patterns of various subtypes of the disorder (Gonda et al., 2019). For the development of biomarkers and novel therapeutic targets, a better understanding of the underlying biology of this disorder is of major importance.
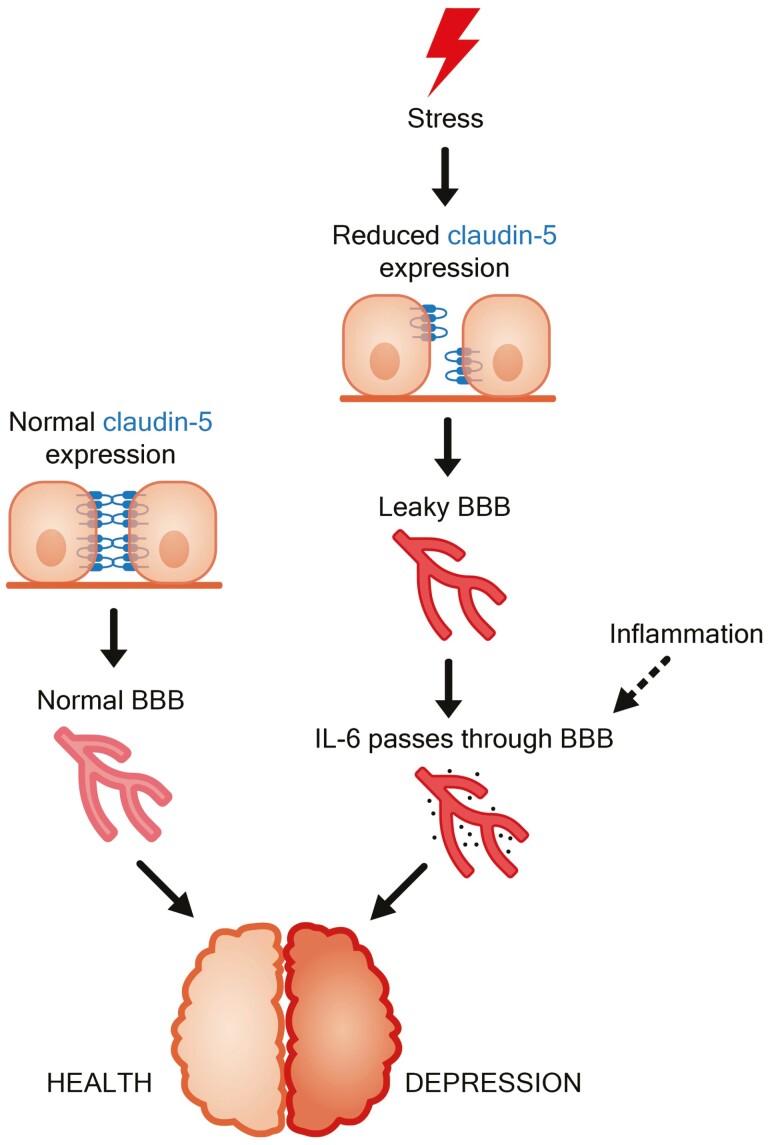
One potential target of interest in biomarker research of depression is the blood-brain barrier (BBB), which surrounds the central nervous system by tight junction molecules, possessing the largest surface area among the biological barriers in the human body (Abbott et al., 2010). Previous results from animal experiments and post-mortem human samples revealed that important changes occurred in the integrity of the BBB during depression (Menard et al., 2017; Dion-Albert et al., 2022b). Chronically stressed mice showed depressive-like behavior and downregulation of mRNA expression of claudin-5, the most abundant tight junction protein of the BBB (Greene et al., 2019), with increased BBB permeability (Lee et al., 2018). A reduced level of cyclic adenosine monophosphate, a molecule that increases the expression of CLDN5 (Ishizaki et al., 2003), could also be observed in mice after stress (Zhang et al., 2020). Menard et al. demonstrated a stress-susceptible depressive phenotype consisting of downregulation of cldn5 mRNA, abnormal blood vessel morphology, and infiltration of interleukin-6 (IL-6) cytokine in the nucleus accumbens after chronic social defeat stress in male mice; these changes were reversed by administration of the antidepressant imipramine (Menard et al., 2017). A follow-up study indicated similar alterations in the prefrontal cortex of female animals (Dion-Albert et al., 2022b). A reduced level of CLDN5 expression was also shown in post-mortem, mainly suicidal samples of depressed human individuals (Menard et al., 2017; Dion-Albert et al., 2022b). Although the stress-dependent effects remain untested in humans, the regulation of the CLDN5 gene strongly indicates that claudin-5 reduction may be a consequence of recent stressors (Dudek et al., 2020). Therefore, it is likely that in susceptible individuals, stress-induced downregulation of CLDN5 may increase permeability of the BBB for peripheral molecules such as interleukins.
In depression, the importance of peripheral cytokines, notably IL-6, has already been demonstrated (Sluzewska et al., 1996; Lanquillon et al., 2000; Howren et al., 2009; Dowlati et al., 2010; Nobis et al., 2020). A meta-analysis revealed that in sera of clinical- and non-clinical MDD samples, IL-6 cytokine levels were significantly elevated (Howren et al., 2009). Treatment-resistant clinical cases of MDD showed similar results (Hodes et al., 2014). Yet IL-6 effects may also depend on previous stress exposures. In rodents, stress resilience was promoted by depletion of IL-6 (Hodes et al., 2014), and correlations were revealed between hippocampal IL-6 expression and stress-induced anhedonia (Taler et al., 2021).
Based on the above, attenuation of BBB function by reduced CLDN5 expression may promote effects of peripheral cytokines, including IL-6, during stress to induce depression (Figure 1). The elements of this hypothesis originate mainly from animal experiments. It is widely accepted that the translatability of preclinical studies has been limited and raised controversies about the use of animal models in depression research. Therefore, we tested whether the above hypothesis could be proven in humans. To test the hypothetic joint contribution of claudin-5 and IL-6 in stress-induced depression in humans, a genetic association study was conducted using functional genetic polymorphisms that influence gene and possibly protein functions. In humans, rs885985, a functional polymorphism of CLDN5, may contribute to alteration of isoform expression because in vitro experiments showed that the encoded longer isoform of the corresponding protein is unable to be built into the cell membrane (Cornely et al., 2017). In the case of IL6, minor allele carriers of functional polymorphism rs1800795 show elevated plasma levels of peripheral IL-6 (Brull et al., 2001; Jones et al., 2001). A human genetic study even indicated that the effects of rs1800795 in the IL6 gene are only relevant in depression in interaction with recent stress without main effects (Kovacs et al., 2016).
Figure 1.
Supposed consecutive steps leading to stress-induced depression. Stress directly affects brain function, disrupting claudin-5 expression to cause a more permeable blood-brain barrier (BBB), and thus peripheral inflammatory cytokines, like interleukin (IL)-6, may enter the brain to cause an increased risk for depression.
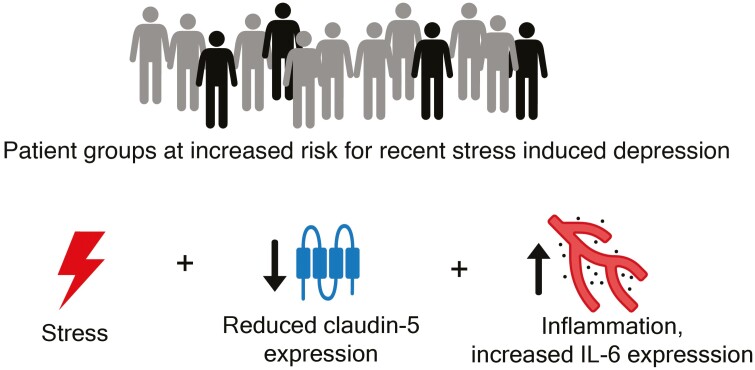
In this study, we hypothesized and tested whether the presence of minor alleles of rs885985 (CLDN5) and rs1800795 (IL6) in humans contribute to the development of depressive symptoms after experiencing recent stressful stimuli and thus could be risk factors for the disorder (Figure 2). Accordingly, our aim was to test the effects of 3-way (gene–gene–environment) interaction among rs885985 of CLDN5, rs1800795 of IL6, and recent stress on current depressive symptoms in UK Biobank (UKB) and replicate significant findings in the independent NewMood (NM) cohort. Significant 3-way interactions could prove our hypothesis. To reveal potential differences between recent and distal stressors, we also tested early stress effects, namely, childhood adversities. The more relevant outcome variable, lifetime depression, was used for this parameter.
Figure 2.
Inheritably and environmentally induced factors leading to increased risk for recent stress-induced depression.
METHODS
Population Genetic Samples
UK Biobank
The UKB data collection procedure was previously published (Bycroft et al., 2017 “preprint”, Bycroft et al., 2018) and was approved by North West Centre for Research Ethics Committee (11/NW/0382). Genetic data were extracted from blood samples of participants who visited the UKB assessment center at the baseline of the study, and a custom genotype calling pipeline was applied by Affymetrix Research Services Laboratory to optimize the process for biobank-scale genotyping (Bycroft et al., 2018). UKB Axiom Array was used in the majority of participants (approximately 440 000), and Affymetrix UK BiLEVE Axiom Array was applied for approximately 50 000 participants (Welsh et al., 2017). A subset of 277 501 individuals with white British ancestry, who were genotyped for rs885985 (CLDN5) and rs1800795 (IL6) and aged between 39 and 72 years, were included from the UKB database (UKB Resource under application no. 1602).
NewMood
The NM Study [New Molecules in Mood Disorders, LHSM-CT-2004-503474, Sixth Framework Program of the European Union (Deakin et al., 2011)] was carried out in accordance with the Declaration of Helsinki and was approved by the Scientific and Research Ethics Committee of the Medical Research Council (Budapest, Hungary, [ad.225/KO/2005; ad.323-60/2005-1018EKU and ad.226/KO/2005; ad.323-61/2005-1018 EKU]) and the North Manchester Local Research Ethics Committee (Manchester, United Kingdom [REC reference no.: 05/Q1406/26]). All participants provided written informed consent prior to participation. Genetic data by a saliva sample kit was collected from participants and Illumina’s CoreExom PsychChip for rs885985 of CLDN5, and Sequenom MassARRAY technology (Sequenom Inc., San Diego, CA, USA) under the ISO 9001:2000 quality management requirements for rs1800795 of IL6 were used to determine single nucleotide polymorphisms (SNPs). A total of 1638 participants (aged between 18 and 60 years) of self-reported European ethnicity, genotyped for rs885985 of CLDN5 and rs1800795 of IL6, were included in the current study.
Quality Control Steps for Genetic Data
UK Biobank
UKB genetic data quality control (QC) steps were previously published (Nora Eszlari et al., 2021). We performed genetic quality control steps a priori and to conduct principal component (PC) analyses on the whole genome of participants included this study (n = 277 501). The quality control steps included filtering and excluding participants of high relatedness, extreme heterozygosity or mismatching sex, minor allele frequency (MAF) filtering (MAF > 0.01), Hardy-Weinberg equilibrium tests (HWE > 0.00001), calculating the missingness per marker and participant (missingness rate >0.01 was excluded), and calculation of linkage disequilibriums.
NewMood
QC steps for the genetic data in NM were also previously published (Eszlari et al., 2019). Essentially, we performed the same QC steps as in UKB. To obtain PCs, we filtered and excluded relatives, extreme heterozygosity, and individuals with mismatched sex. SNPs were filtered according to MAF (> 0.01), HWE (> 0.00001), missingness (missingness rate >0.01 was excluded), and linkage disequilibriums.
Phenotypes
UK Biobank
Sociodemographic information; age (Field ID 21003) and sex (Field ID 31) were collected in the UKB. Current depressive symptoms were determined by the sum of 4 item scores [“Frequency of depressed mood in last 2 weeks” (Field ID 2050), “Frequency of unenthusiasm/disinterest in last 2 weeks” (Field ID 2060), “Frequency of tenseness/restlessness in last 2 weeks” (Field ID 2070) and “Frequency of tiredness/lethargy in last 2 weeks” (Field ID 2080)], each measured on a 4-point Likert scale at the baseline time point (Hullam et al., 2019), while lifetime depression was derived using ICD10 codes of F32 or F33 (Field IDs 130894 and 130896). Proximal stress factors were assessed based on UKB Field ID 6145 (“Illness, injury, bereavement, stress in last 2 years”), and Field ID 20487 (“Felt hated by family member as a child”), 20488 (“Physically abused by family as a child”), 20489 (“Felt loved as a child”), 20490 (“Sexually molested as a child”), and 20491 (“Someone to take to doctor when needed as a child”) were utilized to register early stress. In case of current depressive symptoms and childhood stress factors, mean values were used and calculated by dividing the sum of the item scores by the number of answered questions.
NewMood
Sociodemographic information (age, sex) were recorded in background questionnaires. Current depressive symptoms were measured based on the Brief Symptom Inventory (“thoughts of ending your life,” “feeling lonely,” “feeling blue,” “feeling no interest in things,” “feeling hopeless about the future,” “feelings of worthlessness”) (Derogatis, 1993) plus 4 items (“poor appetite,” “trouble falling asleep,” “thoughts of death or dying,” “feelings of guilt”) and were self-reported along with lifetime depression. The List of Threatening Experiences questionnaire (Brugha et al., 1985) was used to record recent negative life events in the previous 1 year. Reduced form of Childhood Trauma Questionnaire (Bernstein et al., 1994) plus 2 items from the background questions asking about early parental loss (“When I was growing up I lost my mother,” “When I was growing up I lost my father”) were used to determine early stress factors. For current depressive symptoms and childhood stressors, item scores divided by answered questions were used in the analyses as previously reported (Kristof et al., 2021).
Statistical Analyses
Descriptive statistics and regression analyses were calculated with Plink 2.0 (Chang et al., 2015; Chang SPaC, 2015) and R 4.1.1 (R Core Team, 2021) for the above-mentioned cohorts.
To test our hypotheses, we used the following linear and logistic regression models—for continuous and binary outcomes, respectively—in both cohorts with both sexes together or men and women separately: (1) 3-way interaction with the 2 SNPs and recent negative life events on current depressive symptoms; (2) 3-way interaction with the 2 SNPs and childhood adversities on lifetime depression; (3) interaction analyses with recent negative life events in case of current depressive symptoms; (4) interaction analyses with childhood adversities in case of lifetime depression; (5) epistasis (rs885985 of CLDN5 and rs1800795 of IL6) analyses on current depressive symptoms; (6) epistasis (rs885985 of CLDN5 and rs1800795 of IL6) analyses on lifetime depression; (7) main effect analyses on current depressive symptoms; and (8) main effect analyses on lifetime depression.
In all analyses, except the sex-specific ones—age, sex, type of the genotyping array, and the first 10 PCs—calculated for the whole genome were included as covariates.
For main effect and 2-way epistasis interaction analyses Plink 2.0 (Chang et al., 2015; Chang SPaC, 2015) was used. For 3-way interaction, R 4.1.1 version (R Core Team, 2021) with “tidyverse” (Wickham et al., 2019), “jtools” (Long, 2020), and “interaction” (Long, 2019) packages were utilized to calculate and visualize results.
To correct for multiple testing, we applied the Bonferroni method, where P = 0.0007 was used as the threshold of significance. The threshold was calculated based on the 12 tests multiplied by 3 types of division (whole cohort, male and female participants only), multiplied by the 2 cohorts (0.05/ [12 * 3 * 2] = 0.0007).
RESULTS
Descriptive Statistics
The genotype distribution of the 2 cohorts could be seen in supplementary Table 1. The MAF of rs885985 (CLDN5) and of rs1800795 (IL6) corresponded with 1000Genomes reference population (European subpopulation) (Auton et al., 2015) (supplementary Table 2). The HWE values did not significantly deviate from the expected values (supplementary Table 2). Supplementary Table 3 contains the descriptive statistics of continuous variables and supplementary Table 4 contains lifetime depression status of the participants in the 2 cohorts.
Main hypothesis:
Gene-gene-environment 3-way interaction analysis among rs885985 (CLDN5), rs1800795 (IL6) and recent negative live events on current depressive symptoms
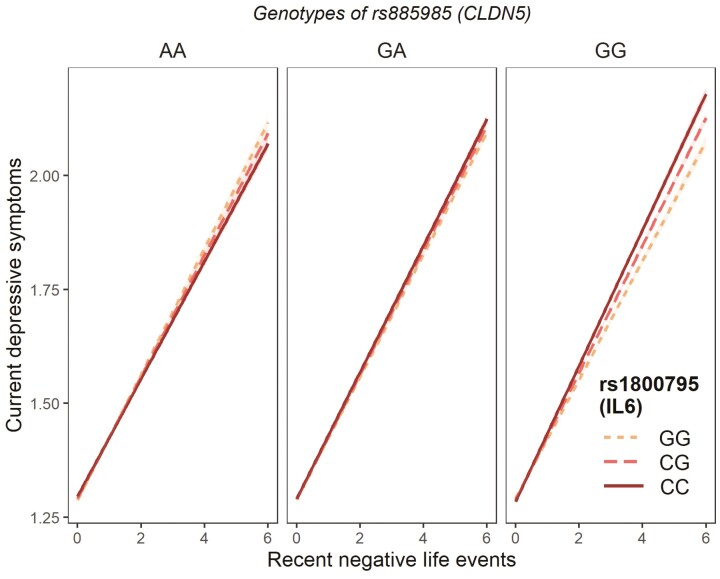
The 3-way interaction analysis (Table 1) provided highly significant associations between rs885985 of CLDN5 and rs1800795 of IL6 in interaction with recent stress on current depressive symptoms (β = 0.0093; P = 0.0003). The effect size and significance level were similar when only men were included (β = 0.0141; P = 0.0002). The results survived correction for multiple testing, where the Bonferroni threshold was P = 0.0007. Significant association was not detected when only women were included into the analysis (β = 0.0055; P = 0.1208). Based on the results, minor allele carrier status, in additive manner at both SNPs (“G” of rs885985 (CLDN5) and “C” of rs1800795 (IL6)) in interaction with recent stress, represented a risk for current depressive symptoms, especially in men (Figure 3).
Table 1.
Summary table of 3-way interaction effects of rs885985 (CLDN5) and rs1800795 (IL6) with recent negative life events on current depressive symptoms in UK Biobank
| Additive model | |||||
|---|---|---|---|---|---|
| Tested population | Participants | β | SE | t | P |
| Whole cohort | 277 501 | 0.0093 | 0.0026 | 3.5922 | 0.0003* |
| Males | 128 752 | 0.0141 | 0.0038 | 3.6905 | 0.0002* |
| Females | 148 749 | 0.0055 | 0.0035 | 1.5514 | 0.1208 |
Abbreviations: β, regression coefficient; P, asymptotic P-value; SE, standard error of β; t, T-statistics. Nominally significant (P < 0.05) results are marked in bold; results surviving correction for multiple testing (P < 0.0007) is marked with an asterisk.
Figure 3.
Results of the 3-way interaction analysis on current depressive symptoms among rs885985 of CLDN5 and rs1800795 of IL6 and recent negative life events in male subjects in the UK Biobank cohort. Based on our results, homozygous genotype of minor alleles of the examined functional polymorphisms (rs885985 of CLDN5 – “G”, rs1800795 of IL6 – “C”) represented the highest risk in terms of current depressive symptoms after experiencing recent stress in male subjects. Current depressive symptoms score increases along with the number of recent negative life events in association with the presence of minor alleles of the 2 functional polymorphisms. The risk effect of the minor alleles increases additively; with more minor allele, the association became more pronounced. (Because of the small effect size [β = 0.0141], the current depressive symptoms score only shows a subset of the whole scale.)
In the NM cohort, results could be replicated only on trend level in the whole cohort (β = 0.0553; P = 0.0972) (Table 2). The direction of the effect was the same as in the UKB cohort.
Table 2.
Summary table of 3-way interaction effects of rs885985 (CLDN5) and rs1800795 (IL6) with recent negative life events on current depressive symptoms in NewMood
| Additive model | |||||
|---|---|---|---|---|---|
| Tested population | Participants | β | SE | t | P |
| Whole cohort | 1638 | 0.0553 | 0.0333 | 1.6593 | 0.0972 |
Abbreviations: β, regression coefficient; P, asymptotic P value; SE, standard error of β; t, T-statistics. Due to the large reduction in sample size and statistical power, analysis was conducted in only the whole cohort.
Additional Analyses for Further Characterization
Further analyses did not yield significance on either current depressive symptoms or lifetime depression after correction for multiple testing (Table 3). Only rs1800795 of IL6 showed association at nominal significance level with current depressive symptoms (β = 0.0033; P = 0.0185) and with lifetime depression (OR = 1.0285; P = 0.0014).
Table 3.
Analyses on current depressive symptoms
| Analysis description | Whole cohort | ||
|---|---|---|---|
| β | P | Supplementary table no. | |
| Gene–gene interaction analysis of rs885985 (CLDN5) and rs1800795 (IL6) on current depressive symptoms | 0.0010 | 0.6188 | S6 |
| Gene–environment interaction analysis of rs885985 (CLDN5) with recent negative life events on current depressive symptoms | 0.0010 | 0.4735 | S8 |
| Gene–environment interaction analysis of rs1800795 (IL6) with recent negative life events on current depressive symptoms | −0.0008 | 0.5715 | S9 |
| Direct association analyses of rs885985 (CLDN5) with current depressive symptoms | −0.0013 | 0.3559 | S12 |
| Direct association analyses of rs1800795 (IL6) with current depressive symptoms | 0.0033 | 0.0185 | S13 |
| Direct association analyses of rs1800795 (IL6) on lifetime depression | 1.0285 | 0.0014 | S15 |
| Analyses on lifetime depression | |||
| Analysis description | Whole cohort | ||
| OR | P | Supplementary table no. | |
| 3-Way interaction analysis among rs885985 (CLDN5), rs1800795 (IL6), and childhood adversities on lifetime depression | 1.0083 | 0.8299 | S5 |
| Gene–gene interaction analysis of rs885985 (CLDN5) and rs1800795 (IL6) on lifetime depression | 0.9721 | 0.0247 | S7 |
| Gene–environment interaction analyses of rs885985 (CLDN5) with childhood adversities on lifetime depression | 0.9965 | 0.7831 | S10 |
| Gene–environment interaction analyses of rs1800795 (IL6) with childhood adversities on lifetime depression | 1.0027 | 0.8314 | S11 |
| Direct association analyses of rs885985 (CLDN5) on lifetime depression | 0.9923 | 0.3807 | S14 |
| Direct association analyses of rs1800795 (IL6) on lifetime depression | 1.0285 | 0.0014 | S15 |
Abbreviations: β, regression coefficient; OR, odds ratio; P, asymptotic P-value (without multiple hypothesis correction).
DISCUSSION
Significant gene–gene–environment 3-way interaction analysis showed that the rs885985 functional polymorphism of CLDN5 of the BBB plays a role in mediating the effects of recent stress on current depressive symptoms in interaction with rs1800795 of IL6 in humans. Neither rs885985 of CLDN5 without stress, in interaction with early-life stress, nor the IL-6 polymorphism alone or in interaction with early-life stress showed consistent, significant risk for current depressive symptoms or lifetime depression in our analyses.
These findings confirm our original hypothesis (Figure 1) and suggest that a potential decrease in CLDN5 after recent stress and elevated IL-6 levels (that are proxied by these functional polymorphisms) may promote risk for depression in humans. In addition, our findings also point out that this effect of CLDN5 is specific to recent stress–induced depression and is, perhaps, more pronounced in men, although further studies are needed to validate the revealed potential sex differences.
By proving our main hypothesis, we could translate previous findings from animal experiments, suggesting that inflammation is involved in the development of depression and stress-connected brain entry of inflammatory molecules is a key factor that regulates inflammation-related depression in humans.
Our findings support that following recent stress, decrease in claudin-5 and increase in IL-6 concentrations are risk factors for the development of depressive symptoms in humans. For the selection of this genetic variant, based on in vitro experiments (Cornely et al., 2017), we supposed that in minor allele(s) at rs885985 of CLDN5, claudin-5 turnover is decreased or disrupted. This assumption is supported by the following: the G, minor allele of rs885985 (CLDN5) encodes for glutamine, where the A, major allele encodes for a STOP codon. This alteration could result in translation of 2 isoforms of claudin-5 protein; the length is 218 amino acids in the G allele and 308 amino acids in the A allele. Despite the different genotypes of rs885985 (CLDN5), only the shorter form of claudin-5 protein was detected in human tissues so far (Cornely et al., 2017). Forced expression of the longer isoform of claudin-5 was possible only in vitro; however, the long, 308-amino acid protein remained in the intracellular compartment and could not be built into the cell membrane (Cornely et al., 2017). Our results suggest that the appearance of depressive symptoms could be the consequence of the translation of an elevated amount of the longer isoform, which is unable to be incorporated into the BBB, or the protein expression is merely attenuated by other mechanisms also caused by the “stop-gain” nature of this variant.
In IL6, the presence of a minor allele of rs1800795 (IL6) is associated with elevated IL-6 concentrations in the blood (Brull et al., 2001; Jones et al., 2001). The elevated level of IL-6 passing through a more disrupted BBB (by minor allele of rs885985 of CLDN5) would be more likely to contribute to depression. Our study, though indirectly and non-causally, supports that one significant mechanism for recent stress-induced depression may be through reduced expression of CLDN5 in the BBB and the consequent elevated penetration of IL-6 into the brain.
Sex Differences Behind Stress-Induced Depression
Previous findings from animal and post-mortem human samples revealed the same biological mechanisms in male and female subjects, although in different brain areas (Menard et al., 2017; Dion-Albert et al., 2022b). These dissimilarities could explain the different characteristics of depression symptoms in male and female individuals (Dion-Albert et al., 2022a) and may also explain the potential sex differences in our analyses. In our study, significant 3-way interaction results were obtained only in the whole sample and in men; however, we could only replicate it with trend-level significance in the whole NM cohort. In contrast, association of IL-6 polymorphism with lifetime depression showed a strong trend in the whole sample and in women. Substantially higher incidence of lifetime depression in women (Labaka et al., 2018) and the higher fluctuation of mood parameters caused by the hormonal cycles even in women without premenstrual dysphoric disorder (Gonda et al., 2008) could give an explanation for this phenomenon. We have to emphasize that none of the sex-specific analyses could reliably be replicated in the NM population, which necessitates further replication studies.
Toward the Identification of New Therapeutic Targets for Stress and Neuroinflammation-Associated Subtypes of Depression
Our findings may promote efforts trying to develop claudin-5- and IL-6–based therapies. Numerous existing molecules, like steroids, valproic acid, and GSK3β inhibitors, already indirectly increase or hinder decrease of claudin-5 expression and can be considered as CLDN5 enhancers (Hashimoto et al., 2021). In effect, GSK3β inhibitors extend the cellular half-life of tight junction proteins (including claudin-5), which might also counteract the effects of minor allele of rs885985 (CLDN5) in humans. This may even further enhance the value of the findings with rs885985 (CLDN5) in combination with stress and rs1800795 (IL6) because its presence may guide efforts in selecting those patients who might benefit most from a CLDN5-enhancing (e.g., GSK3β inhibitors) therapy. Furthermore, more specific drugs for such patients can be within reach, because specific molecular targeting is possible for claudins (Suzuki et al., 2012; Wöll et al., 2014). Our study suggests that such therapy may be beneficial in patients exposed to recent stress and exhibiting elevated IL-6 levels. Therefore, findings highlight claudin-5 as a potential drug target in this specific subgroup of patients and may guide patient selection for future clinical studies.
CONCLUSION
In the present study, we showed that CLDN5 polymorphism rs885985 interacts with recent stress and rs1800795 of IL6 in promoting current depressive symptoms. We also evidenced at a nominal significance level that rs1800795 of IL6 is associated with lifetime depression. Analyses conducted in men and women suggested potential sex differences. These findings translate previous results of animal and human post-mortem data onto the genetic level and characterize them in a large human population. Our results suggest that recent stress may cause BBB disruption and IL-6 penetration into the brain, which increases the risk of depression in humans. In addition, we also point out that rs885985 (CLDN5) and rs1800795 (IL6) may have predictive value as biomarker in recent stress-induced depression and that CLDN5 and IL6 may serve as potential drug targets in specified vulnerable groups.
Limitations
The current study employed a population genetics approach, and, as such, it is non-causal in nature because studies relying on population genetic samples cannot directly investigate temporal patterns and causal relationships (Lynch and Ho, 2020). The exact impact of the investigated polymorphisms on protein levels in vivo is partially unsolved for rs885985 (CLDN5) and for rs1800795 (IL6), which should be addressed by follow-up experiments. In most studies, minor allele carrier status of rs1800795 (IL6) was associated with an elevated level of IL-6; however, there are contradicting results (Fishman et al., 1998).
The result could be replicated in the independent NM cohort on a trend level; however, the direction of this 3-way interaction effect was the same in the 2 cohorts. On one hand, the weak replication may be caused by the difference in the sizes of the cohorts, which also caused differences in the statistical power. On the other hand, it may be derived from the differences in age distribution of the 2 independent cohorts, knowing that, among other factors, the integrity of the BBB could also change with age (Erdő et al., 2016). Whereas in NM the majority of participants were younger (mean age = 30.88 years), in the UKB cohort only individuals older than 39 years participated (mean age = 56.88 years) (supplementary Table 3). Moreover, the differences in cohort sizes did not allow us to perform the replication in male and female subpopulations in the NM population; therefore, we could not draw a clear conclusion on sex differences.
Supplementary Material
Acknowledgments
This work was supported by the Hungarian Academy of Sciences (MTA-SE Neuropsychopharmacology and Neurochemistry Research Group); the Hungarian Brain Research Program (2017-1.2.1-NKP-2017-00002, KTIA_NAP_13-1-2013- 0001, KTIA_NAP_13-2- 2015-0001, Hungarian Brain Research Program 3.0: NAP2022-I-4/2022); by the Thematic Excellence Program (2020-4.1.1.-TKP2020) of the Ministry for Innovation and Technology in Hungary, within the framework of the Neurology and Translational Biotechnology thematic programs of the Semmelweis University; by TKP2021-EGA-25, which has been implemented with the support provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021-EGA funding scheme; and by the National Research, Development and Innovation Office, Hungary, with grants K 143391, and 2019-2.1.7-ERA-NET-2020-00005 under the frame of ERA PerMed (ERAPERMED2019-108). This action has received funding from the ERA-NET COFUND Program with co-funding from the European Union Horizon 2020 research and innovation program. PP is an international research fellow of Japan Society for the Promotion of Science (Postdoctoral Fellowships for Research in Japan, standard program, P20809). NE has been supported by the New National Excellence Program of the Ministry for Innovation and Technology (ÚNKP-20-4-II-SE-9, ÚNKP-21-4-II-SE-1), and by the ÚNKP-22-4-II-SE-1 New National Excellence Program of the Ministry for Culture and Innovation, all from the source of the National Research, Development and Innovation Fund.
Contributor Information
Zsofia Gal, Department of Pharmacodynamics, Faculty of Pharmacy, Semmelweis University, Budapest, Hungary; NAP3.0-SE Neuropsychopharmacology Research Group, Hungarian Brain Research Program, Semmelweis University, Budapest, Hungary.
Dora Torok, Department of Pharmacodynamics, Faculty of Pharmacy, Semmelweis University, Budapest, Hungary; NAP3.0-SE Neuropsychopharmacology Research Group, Hungarian Brain Research Program, Semmelweis University, Budapest, Hungary.
Xenia Gonda, NAP3.0-SE Neuropsychopharmacology Research Group, Hungarian Brain Research Program, Semmelweis University, Budapest, Hungary; Department of Psychiatry and Psychotherapy, Semmelweis University, Budapest, Hungary.
Nora Eszlari, Department of Pharmacodynamics, Faculty of Pharmacy, Semmelweis University, Budapest, Hungary; NAP3.0-SE Neuropsychopharmacology Research Group, Hungarian Brain Research Program, Semmelweis University, Budapest, Hungary.
Ian Muir Anderson, Division of Neuroscience and Experimental Psychology, School of Biological Sciences, Faculty of Biological, Medical and Human Sciences, The University of Manchester and Manchester Academic Health Sciences Centre, Manchester, United Kingdom.
Bill Deakin, Division of Neuroscience and Experimental Psychology, School of Biological Sciences, Faculty of Biological, Medical and Human Sciences, The University of Manchester and Manchester Academic Health Sciences Centre, Manchester, United Kingdom.
Gabriella Juhasz, Department of Pharmacodynamics, Faculty of Pharmacy, Semmelweis University, Budapest, Hungary; NAP3.0-SE Neuropsychopharmacology Research Group, Hungarian Brain Research Program, Semmelweis University, Budapest, Hungary.
Gyorgy Bagdy, Department of Pharmacodynamics, Faculty of Pharmacy, Semmelweis University, Budapest, Hungary; NAP3.0-SE Neuropsychopharmacology Research Group, Hungarian Brain Research Program, Semmelweis University, Budapest, Hungary.
Peter Petschner, NAP3.0-SE Neuropsychopharmacology Research Group, Hungarian Brain Research Program, Semmelweis University, Budapest, Hungary; Bioinformatics Center, Institute of Chemical Research, Kyoto University, Uji, Kyoto, Japan; Research Unit for Realization of Sustainable Society, Kyoto University, Gokasho, Uji, Kyoto, Japan.
Interest Statement
G.B. was a member of the board of directors at Gedeon Richter. J.F.W.D. has share options in P1vital and has performed research, consultancy, and speaking engagements for AstraZeneca, Autifony, Bristol-Myers Squibb, Eli Lilly, Janssen-Cilag, P1vital, Schering Plough, and Servier, for which all fees have been paid to the University of Manchester to reimburse them for the time taken. The funders had no role in the data collection, analyses, interpretation of data, in the design of the study, in writing of the manuscript, or in the decision to publish the results. Other authors declare no conflict of interest.
Data Availability
N.M. data presented in this study are available on reasonable request from the corresponding author. The data are not publicly available due to ethical considerations.
UKB data can be accessed under standard terms and conditions of UK Biobank Limited. This study used UK Biobank data under application number 1602.
REFERENCES
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ (2010) Structure and function of the blood-brain barrier. Neurobiol Dis 37:13–25. [DOI] [PubMed] [Google Scholar]
- Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini JL, McCarthy S, McVean GA, Abecasis GR; 1000 Genomes Project Consortium (2015) A global reference for human genetic variation. Nature 526:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J (1994) Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry 151:1132–1136. [DOI] [PubMed] [Google Scholar]
- Brugha T, Bebbington P, Tennant C, Hurry J (1985) The list of threatening experiences: a subset of 12 life event categories with considerable long-term contextual threat. Psychol Med 15:189–194. [DOI] [PubMed] [Google Scholar]
- Brull DJ, Montgomery HE, Sanders J, Dhamrait S, Luong L, Rumley A, Lowe GD, Humphries SE (2001) Interleukin-6 gene -174g>c and -572g>c promoter polymorphisms are strong predictors of plasma interleukin-6 levels after coronary artery bypass surgery. Arterioscler Thromb Vasc Biol 21:1458–1463. [DOI] [PubMed] [Google Scholar]
- Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, Motyer A, Vukcevic D, Delaneau O, O’Connell J, Cortes A, Welsh S, Young A, Effingham M, McVean G, Leslie S, Allen N, Donnelly P, Marchini J (2018) The UK Biobank resource with deep phenotyping and genomic data. Nature 562:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ (2015) Second-generation PLINK: rising to the challenge of larger and richer datasets. GigaScience 4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SPaC (2015) PLINK 2.0. In, PLINK 2.0 Edition. Accessed August 5, 2022. https://www.cog-genomics.org/plink/2.0/
- Cornely RM, Schlingmann B, Shepherd WS, Chandler JD, Neujahr DC, Koval M (2017) Two common human CLDN5 alleles encode different open reading frames but produce one protein isoform. Ann N Y Acad Sci 1397:119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deakin JF, Harro J, Anderson IM (2011) NewMood: a productive European model of collaboration for translational research in depression. Eur Neuropsychopharmacol 21:1–2. [DOI] [PubMed] [Google Scholar]
- Derogatis LR (1993) BSI brief symptom inventory. Administration, scoring, and procedures manual (4th ed.). Minneapolis, MN: National Computer Systems. [Google Scholar]
- Dion-Albert L, Bandeira Binder L, Daigle B, Hong-Minh A, Lebel M, Menard C (2022a) Sex differences in the blood-brain barrier: implications for mental health. Front Neuroendocrinol 65:100989. [DOI] [PubMed] [Google Scholar]
- Dion-Albert L, Cadoret A, Doney E, Kaufmann FN, Dudek KA, Daigle B, Parise LF, Cathomas F, Samba N, Hudson N, Lebel M, Campbell M, Turecki G, Mechawar N, Menard C; Signature Consortium (2022b) Vascular and blood-brain barrier-related changes underlie stress responses and resilience in female mice and depression in human tissue. Nat Commun 13:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL (2010) A meta-analysis of cytokines in major depression. Biol Psychiatry 67:446–457. [DOI] [PubMed] [Google Scholar]
- Dudek KA, Dion-Albert L, Lebel M, LeClair K, Labrecque S, Tuck E, Ferrer Perez C, Golden SA, Tamminga C, Turecki G, Mechawar N, Russo SJ, Menard C (2020) Molecular adaptations of the blood-brain barrier promote stress resilience vs. depression. Proc Natl Acad Sci USA 117:3326–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdő F, Denes L, de Lange E (2016) Age-associated physiological and pathological changes at the blood–brain barrier: A review. J Cereb Blood Flow Metab 37:4–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eszlari N, Millinghoffer A, Petschner P, Gonda X, Baksa D, Pulay AJ, Réthelyi JM, Breen G, Deakin JFW, Antal P, Bagdy G, Juhasz G (2019) Genome-wide association analysis reveals KCTD12 and miR-383-binding genes in the background of rumination. Transl Psychiatry 9:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eszlari N, Bruncsics B, Millinghoffer A, Hullam G, Petschner P, Gonda X, Breen G, Antal P, Bagdy G, Deakin JFW, Juhasz G (2021) Biology of perseverative negative thinking: the role of timing and folate intake. Nutrients 13:4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman D, Faulds G, Jeffery R, Mohamed-Ali V, Yudkin JS, Humphries S, Woo P (1998) The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest 102:1369–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda X, Telek T, Juhász G, Lazary J, Vargha A, Bagdy G (2008) Patterns of mood changes throughout the reproductive cycle in healthy women without premenstrual dysphoric disorders. Prog Neuropsychopharmacol Biol Psychiatry 32:1782–1788. [DOI] [PubMed] [Google Scholar]
- Gonda X, Petschner P, Eszlari N, Baksa D, Edes A, Antal P, Juhasz G, Bagdy G (2019) Genetic variants in major depressive disorder: from pathophysiology to therapy. Pharmacol Ther 194:22–43. [DOI] [PubMed] [Google Scholar]
- Greene C, Hanley N, Campbell M (2019) Claudin-5: gatekeeper of neurological function. Fluids Barriers CNS 16:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y, Campbell M, Tachibana K, Okada Y, Kondoh M (2021) Claudin-5: a pharmacological target to modify the permeability of the blood-brain barrier. Biol Pharm Bull 44:1380–1390. [DOI] [PubMed] [Google Scholar]
- Hodes GE, et al. (2014) Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci USA 111:16136–16141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J (2009) Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 71:171–186. [DOI] [PubMed] [Google Scholar]
- Hullam G, Antal P, Petschner P, Gonda X, Bagdy G, Deakin B, Juhasz G (2019) The UKB environment of depression: from interactions to synergistic effects. Sci Rep 9:9723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki T, Chiba H, Kojima T, Fujibe M, Soma T, Miyajima H, Nagasawa K, Wada I, Sawada N (2003) Cyclic AMP induces phosphorylation of claudin-5 immunoprecipitates and expression of claudin-5 gene in blood-brain-barrier endothelial cells via protein kinase A-dependent and -independent pathways. Exp Cell Res 290:275–288. [DOI] [PubMed] [Google Scholar]
- Jones KG, Brull DJ, Brown LC, Sian M, Greenhalgh RM, Humphries SE, Powell JT (2001) Interleukin-6 (IL-6) and the prognosis of abdominal aortic aneurysms. Circulation 103:2260–2265. [DOI] [PubMed] [Google Scholar]
- Kovacs D, Eszlari N, Petschner P, Pap D, Vas S, Kovacs P, Gonda X, Bagdy G, Juhasz G (2016) Interleukin-6 promoter polymorphism interacts with pain and life stress influencing depression phenotypes. J Neural Transm (Vienna) 123:541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristof Z, Eszlari N, Sutori S, Gal Z, Torok D, Baksa D, Petschner P, Sperlagh B, Anderson IM, Deakin JFW, Juhasz G, Bagdy G, Gonda X (2021) P2RX7 gene variation mediates the effect of childhood adversity and recent stress on the severity of depressive symptoms. PLoS One 16:e0252766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labaka A, Goñi-Balentziaga O, Lebeña A, Pérez-Tejada J (2018) Biological sex differences in depression: a systematic review. Biol Res Nurs 20:383–392. [DOI] [PubMed] [Google Scholar]
- Lanquillon S, Krieg JC, Bening-Abu-Shach U, Vedder H (2000) Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology 22:370–379. [DOI] [PubMed] [Google Scholar]
- Lee S, Kang BM, Kim JH, Min J, Kim HS, Ryu H, Park H, Bae S, Oh D, Choi M, Suh M (2018) Real-time in vivo two-photon imaging study reveals decreased cerebro-vascular volume and increased blood-brain barrier permeability in chronically stressed mice. Sci Rep 8:13064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JA (2019) Interactions: comprehensive, user-friendly toolkit for probing interactions. in, R package version 1.1.0 Edition. Accessed August 5, 2022. https://interactions.jacob-long.com/ [Google Scholar]
- Long JA (2020) jtools: Analysis and presentation of social scientific data. in, R package version 2.1.0 edition. Accessed August 5, 2022. https://jtools.jacob-long.com/ [Google Scholar]
- Lynch M, Ho W-C (2020) The Limits to estimating population-genetic parameters with temporal data. Genome Biol Evol 12:443–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard C, et al. (2017) Social stress induces neurovascular pathology promoting depression. Nat Neurosci 20:1752–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobis A, Zalewski D, Waszkiewicz N (2020) Peripheral markers of depression. J Clin Med 9:3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, Mohr DC, Schatzberg AF (2016) Major depressive disorder. Nat Rev Dis Primers 2:16065. [DOI] [PubMed] [Google Scholar]
- R Core Team (2021) R: a language and environment for statistical computing. 4.1.1 edition. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Sluzewska A, Rybakowski J, Bosmans E, Sobieska M, Berghmans R, Maes M, Wiktorowicz K (1996) Indicators of immune activation in major depression. Psychiatry Res 64:161–167. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Kondoh M, Takahashi A, Yagi K (2012) Proof of concept for claudin-targeted drug development. Ann N Y Acad Sci 1258:65–70. [DOI] [PubMed] [Google Scholar]
- Taler M, Aronovich R, Henry Hornfeld S, Dar S, Sasson E, Weizman A, Hochman E (2021) Regulatory effect of lithium on hippocampal blood-brain barrier integrity in a rat model of depressive-like behavior. Bipolar Disord 23:55–65. [DOI] [PubMed] [Google Scholar]
- Welsh S, Peakman T, Sheard S, Almond R (2017) Comparison of DNA quantification methodology used in the DNA extraction protocol for the UK Biobank cohort. BMC Genomics 18:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H, et al. (2019) Welcome to the Tidyverse. J Open Source Softw 4:1686. [Google Scholar]
- Wöll S, Schlitter AM, Dhaene K, Roller M, Esposito I, Sahin U, Türeci O (2014) Claudin 18.2 is a target for IMAB362 antibody in pancreatic neoplasms. Int J Cancer 134:731–739. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lu W, Wang Z, Zhang R, Xie Y, Guo S, Jiao L, Hong Y, Di Z, Wang G, Aa J (2020) Reduced neuronal cAMP in the nucleus accumbens damages blood-brain barrier integrity and promotes stress vulnerability. Biol Psychiatry 87:526–537. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
N.M. data presented in this study are available on reasonable request from the corresponding author. The data are not publicly available due to ethical considerations.
UKB data can be accessed under standard terms and conditions of UK Biobank Limited. This study used UK Biobank data under application number 1602.