Abstract
We describe two new species of Fulvifomes based on morphological observations and phylogenetic investigations. Both species were identified as Phellinus rimosus by former mycologists, but both are morphologically distinct from authenticated specimen of P. rimosus. Fulvifomes boninensis is characterized by perennial basidiomata, a sulcate pileus surface becoming rimose, lack of a distinct crust on the pileus surface, subdimitic hyphal system in the context, and ellipsoid basidiospores. This species is endemic to the Bonin Islands, Japan, and is specific to the host Morus boninensis, a red-listed tree species. Fulvifomes imazekii is characterized by perennial basidiomata, sulcate and velutinous pileus surface, lack of a crust on the pileus surface, dimitic hyphal system in the context, and broadly ellipsoid basidiospores. This species is specific to Berchemiella berchemiaefolia, and is known only from Mt. Yokogura-yama, in Kochi Prefecture, Japan. Fulvifomes boninensis and F. imazekii are considered threatened, because of their high host specificity each with a threatened tree species as well as the limited distribution of the former and the extremely small number of “mature individuals” of the latter. The following new combinations were also proposed: Fulvifomes aulaxinus, F. pappianus, and F. tepperi.
Keywords: host specificity, Hymenochaetales, Phellinus, polypore, red list
1. Introduction
Polyporus rimosus Berk. was erected by Berkeley (1845) based on a specimen collected by J. Drummond at Swan River, West Australia, and lectotypification of P. rimosus was required when the West Australian holotype was lost. Based on our observation of specimens at K, we concur with Larsen's (1990) choice of a lectotype probably collected from Mauritius - from among discordant elements of Berkeley's specimens of P. igniarius var. scaber Berk. (1839), a name included by Berkeley (1845) as a synonym.
Polyporus rimosus was considered a member of Phellinus Quél. by most mycologists during the late 20th century, but Fiasson and Niemelä (1984) divided Phellinus into several genera, and accommodated this fungus into Fulvifomes Murrill as F. rimosus (Berk.) Fiasson & Niemelä. Wagner and Fischer (2002) accepted Fulvifomes as a distinct genus from Phellinus based on its nuclear-encoded large subunit rRNA gene (LSU) sequences, and accepted this fungus as a member of Fulvifomes.
Ito and Imai (1940) reported “F. rimosus” from Japan as Fomes rimosus Berk. (instead of “(Berk.) Cooke”) based on specimens collected at the Bonin Islands. Imazeki (1940) also applied this name to a specimen collected in Kochi Prefecture, Shikoku Island, Japan. Specimens indicated host specificity, with those collected at the Bonin Islands found only on the island endemic tree Morus boninensis Koidz. (Moraceae), whereas those from Kochi prefecture were only on Berchemiella berchemiaefolia (Makino) Nakai (Rhamnaceae) according to the herbarium records. This fungus is now red-listed in Japan as a “critically endangered + endangered (CR+EN)” species, because of its limited distribution in Japan and serious reduction in number of host trees (Ministry of the Environment, 2015a).
Meanwhile, the name F. rimosus (= Phellinus rimosus) has been applied to a wide range of fungi occurring in tropical to temperate areas, but some of them do not correspond with the lectotype of Polyporus rimosus (Hattori et al., 2014). Basidiospores of the Japanese specimens, for example, were described as “subglobose, yellow brown, and 5-6 × 4-5 µm” (Imazeki, 1940), while those of the lectotype are “broadly ellipsoid to subglobose, light brown to brown, and 5.5-7.5 × 4-6 µm” (Hattori et al., 2014). Moreover, the two Japanese populations show a disjunct distribution in Bonin Islands and a limited area of Shikoku Island and are associated with different host tree species, suggesting that they possibly represent two distinct species.
In this article, we performed molecular phylogenetic analyses based on partial sequences of LSU and the internal transcribed spacer (ITS) region of the ribosomal RNA gene, along with morphological observations of the Japanese specimens. Morphological characteristics were also examined for some authenticated specimens of Fulvifomes that are possibly conspecific with the Japanese populations. Then, scientific names were provided for the Japanese populations with detailed descriptions.
2. Materials and methods
2.1. Morphological studies
Morphological characteristics were examined for the Japanese specimens as well as authenticated specimens of some potentially synonymous species, with F. rimosus described from paleotropic areas and Australia. Microscopic observations were of dried specimens mounted in 5% (w/v) KOH solution or Melzer's reagent. Side view basidiospore measurements were taken from materials mounted in Melzer's reagent. The following abbreviations were used for basidiospore characterizations and measurements: IKI− = both non-amyloid and non-dextrinoid; r = the ratio of length/width of a basidiospore; R = arithmetic mean of r; n = n1/n2 indicates the n1 measurement of basidiospores from n2 specimens. Herbaria with specimens were abbreviated according to Index Herbariorum (http://sweetgum.nybg.org/science/ih/). All specimens without herbarium code indications are stored in the Mycological Herbarium of Forestry and Forest Products Research Institute (TFM).
2.2. Molecular phylogeny
DNA was extracted from cultured mycelia using a Maxwell® 16 Tissue DNA Purification Kit (Promega, Fitchburg), or following Hosaka and Castellano (2008). The LSU and ITS regions were amplified and sequenced with the following primers: LR0R/LR5 for LSU (Vilgalys & Hester, 1990) and ITS4/ITS5 (White, Bruns, Lee, & Taylor, 1990) for ITS. PCR reactions for LSU and ITS were performed as described by Sotome et al. (2014). PCR products were purified using ExoSAP-IT (GE Healthcare, Tokyo) or a MonoFas DNA Purification Kit (GL Sciences, Tokyo). Some amplified fragments were cloned in the pGEM-T Easy cloning vector (Promega, Fitchburg) and transformed into competent cells (Escherichia coli Migula, JM109). The DNA of up to four transformed clones from each sample was amplified as above. DNA sequences were determined using a Big Dye Terminator 3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City) with an ABI 3130 DNA sequencer (Applied Biosystems).
The LSU and ITS sequences were initially aligned by the MAFFT v. 7 online program (Katoh & Standley, 2013) using the E-INS-I strategy (June/2021). Trailing ends of the alignment were trimmed and alignments were manually edited when necessary in BioEdit v. 7 (Hall, 1999). Dataset congruence was tested with the incongruence length difference (ILD) test (Farris et al. 1994), as implemented in PAUP* 4.0a167 (“partition-homogeneity test”) (Swofford, 2002). A P-value of < 0.05 was considered statistically significant. After testing for congruence, the individual gene datasets were combined, and phylogenetic analyses were conducted. For the LSU dataset and the combined LSU and ITS dataset, Fomitiporia punctata (P. Karst.) Murrill and Fo. torreyae Y.C. Dai & B.K. Cui were selected as outgroups based on the results of Wagner and Fischer (2002) and Ota et al. (2014). Maximum likelihood (ML) analyses were generated by RaxML 8.2.10 (Stamatakis, 2014) using the GTR+G model on the partitioned dataset. The topology was evaluated with 1,000 bootstrap (BS) replicates (Felsenstein, 1985). Bayesian inference (BI) analyses were performed using MrBayes 3.2.7 (Ronquist et al., 2012). The best-fit substitution model for BI analysis was estimated using MrModeltest 2.4 (Nylander, 2004) under the Akaike information criterion. BI analysis was run with four chains of Metropolis-coupled Markov chain Monte Carlo iterations for 5,000,000 generations, and trees were sampled every 100 generations. The first 12,500 (25%) trees were discarded as burn-in and the remaining trees were kept and combined into one 50% majority-rule consensus tree. Bayesian Posterior Probabilities (BPP) were obtained from the 50% majority consensus of the remaining trees. The aligned data sets and resulting trees were deposited in TreeBASE under the accession number S28398.
3. Results
3.1. Phylogenetic analyses
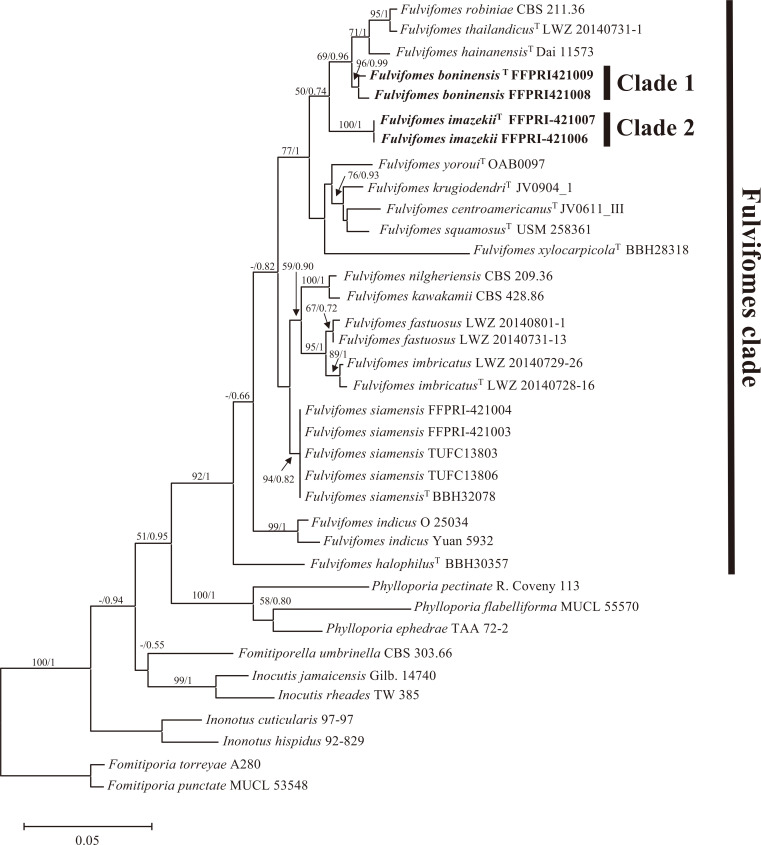
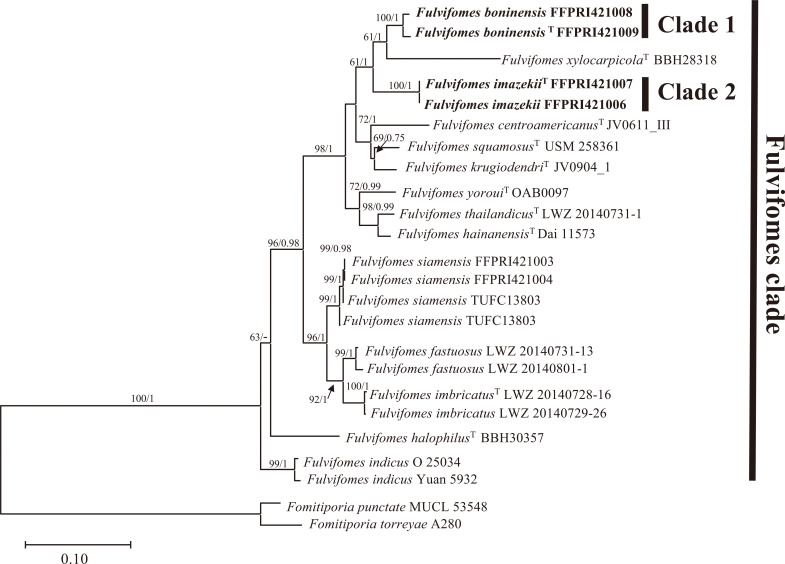
In total, 16 new sequences of the LSU and ITS regions were generated and submitted to the DDBJ/EMBL/GenBank database, as shown in Table 1 (accession nos. LC315775-LC315791). Additional 28 LSU and 17 ITS sequences were retrieved from available databases and used in constructing the alignment datasets (Table 2). The LSU data set was 790 characters, of which 530 were constant, 147 variable, and parsimony informative. The combined LSU and ITS data set was 1,520 characters (790 for LSU and 730 for ITS) of which 1,050 were constant, 359 variable, and parsimony informative. An ILD test showed no significant incongruence between the LSU and ITS datasets (P = 0.111). The BI analyses were performed with the LSU and combined LSU and ITS dataset. The best-fit evolutionary model for each dataset was GTR+G+I. The ML, BI trees based on LSU dataset and the combined LSU and ITS dataset showed the same topologies respectively. The resulting phylogenetic analyses of the LSU dataset and the combined LSU and ITS dataset are summarized in Figs 1 and 2. All species of Fulvifomes used in these phylogenetic analyses are included in a clade labeled as “Fulvifomes clade” in Figs. 1 and 2. In all analyses of this study, Japanese “Fulv. rimosus” were divided into two distinct clades (Clade 1 and 2) with strong support (BS values above 96% and BPP above 99%) and were separated from other Fulvifomes species.
Table 1. List of species and their GenBank accession numbers newly deposited in this study.
| Species and strain nos. | Locality | Culture Bank | GenBank accession nos. | |
| LSU | ITS | |||
| Fulvifomes boninensis | ||||
| FFPRI421008 | Chichijima Island, Japan | FFPRI | LC315776 | LC315785 |
| FFPRI421009 T | Hahajima Island, Japan | FFPRI | LC315777 | LC315786 |
| Fulvifomes imazekii | ||||
| FFPRI421006 | Kochi, Japan | FFPRI | LC315778 | LC315787 |
| FFPRI421007 T | Kochi, Japan | FFPRI/ | LC315779 | LC315788 |
| Fulvifomes siamensis | ||||
| FFPRI421003 | Hahajima Island, Japan | FFPRI | LC315780 | LC315789 |
| FFPRI421004 | Hahajima Island, Japan | FFPRI | LC315781 | LC315790 |
| TUFC13803 | Chichijima Island, Japan | FMRC | LC315782 | LC315791 |
| TUFC13806 | Chichijima island, Japan | FMRC | LC315783 | - |
T Type materials. FFPRI: Microbial Genetic Resources of FFPRI Genebank.
FMRC: Fungus/Mushroom Resource and Research Center
Table 2. List of species, strains or specimens and GenBank accession numbers of sequences used in this study.
| Species | Strain or specimen nos. | GenBank accession nos. | |
| LSU | ITS | ||
| Fomitiporella umbrinella | CBS 303.66 | AY059036 | - |
| Fomitiporia punctata | MUCL 53548 | JX093834 | JX093790 |
| Fomitiporia torreyae | A280 | LC033549 | LC033540 |
| Fulvifomes centroamericanus T | JV0611_III | KX960764 | KX960763 |
| Fulvifomes fastuosus | LWZ 20140731-13 | KR905668 | KR905674 |
| Fulvifomes fastuosus | LWZ 20140801-1 | KR905669 | KR905675 |
| Fulvifomes hainanensis T | Dai 11573 | JX866779 | KC879263 |
| Fulvifomes halophilus T | BBH30357 | JX104734 | JX104687 |
| Fulvifomes imbricatus T | LWZ 20140728-16 | KR905670 | KR905677 |
| Fulvifomes imbricatus | LWZ 20140729-26 | KR905671 | KR905679 |
| Fulvifomes indicus | Yuan 5932 | JX866777 | AB735976 |
| Fulvifomes indicus | O 25034 | KC879259 | KC879262 |
| Fulvifomes kawakamii | CBS 428.86 | AY059028 | - |
| Fulvifomes krugiodendri T | JV0904_1 | KX960765 | KX960762 |
| Fulvifomes nilgheriensis | CBS 209.36 | AY059023 | - |
| Fulvifomes robiniae | CBS 211.36 | AY059038 | - |
| Fulvifomes siamensis T | BBH32078 | JX104753 | JX104706 |
| Fulvifomes squamosus T | USM 258361 | MF479266 | MF479267 |
| Fulvifomes thailandicus T | LWZ 20140731-1 | KR905665 | KR905672 |
| Fulvifomes xylocarpicola T | BBH28318 | JX104718 | JX104671 |
| Fulvifomes yoroui T | OAB0097 | MN017120 | MN017126 |
| Inocutis jamaicensis | Gilb. 14740 | AY059048 | - |
| Inocutis rheades | TW 385 | AF311019 | - |
| Inonotus cuticularis | 97-97 | AF311010 | |
| Inonotus hispidus | 92-829 | AF311014 | - |
| Phylloporia ephedrae | TAA 72-2 | AF411826 | - |
| Phylloporia flabelliforma | MUCL 55570 | KU198350 | KU198358 |
| Phylloporia pectinata | R. Coveny 113 | AF411823 | - |
T Type materials.
Fig. 1 - Maximum likelihood tree based on the LSU data set. BS values above 50% and BPP above 0.7 are shown. T indicates sequence derived from type materials.
Fig. 2 - Maximum likelihood tree based on the combined LSU and ITS data set. BS values above 50% and BPP above 0.7 are shown. T indicates sequence derived from type materials.
3.2. Descriptions of the new species
Fulvifomes boninensis T. Hatt., Y. Ota & Sotome, sp. nov.
MycoBank no.: MB 841421.
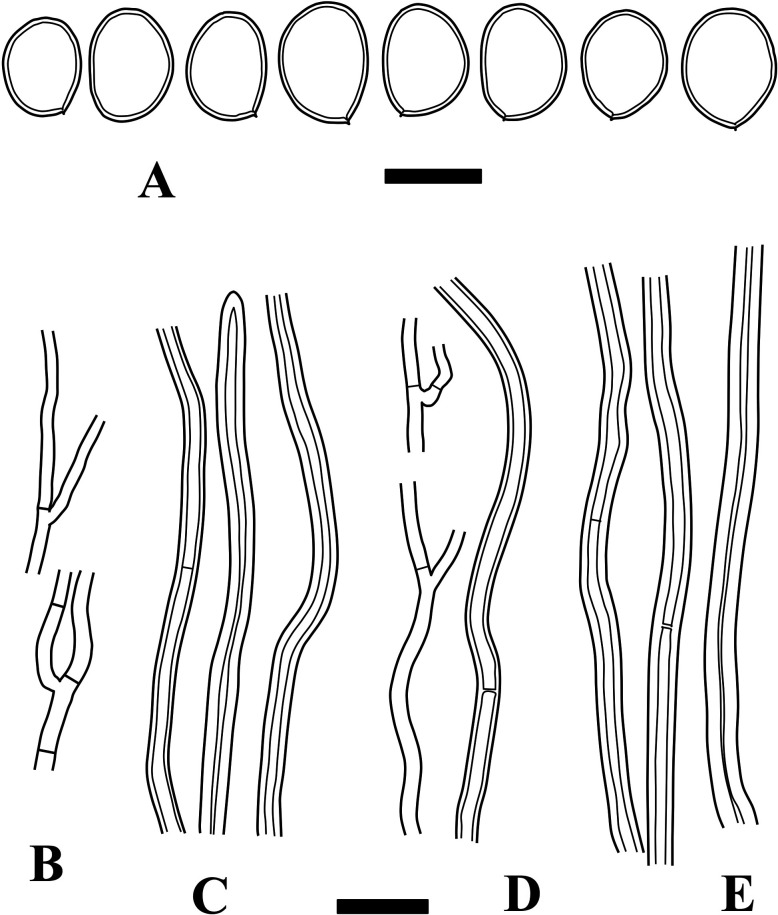
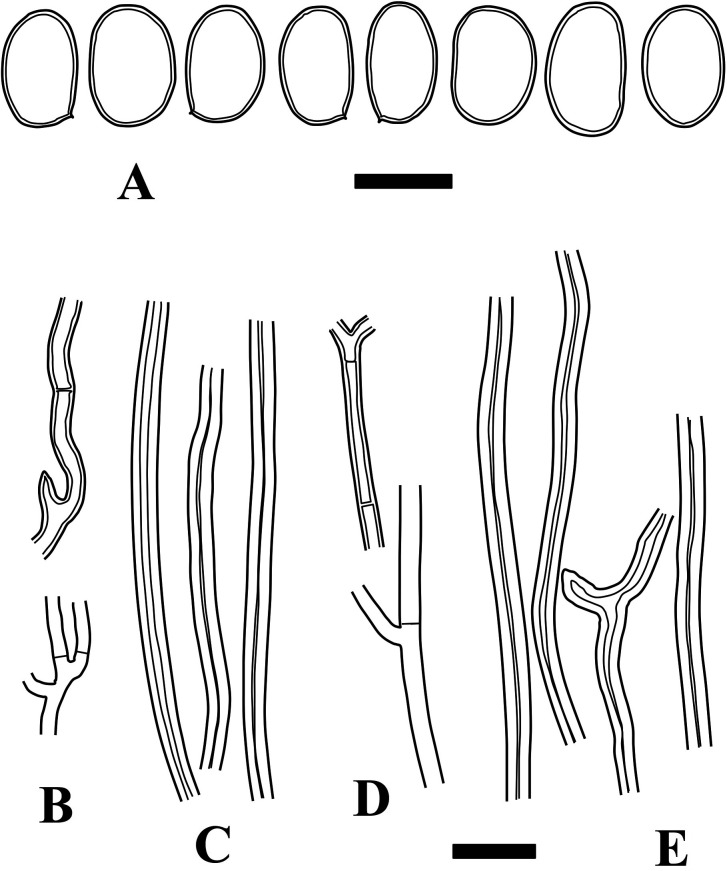
Fig. 3 - Microscopic structures of Fulvifomes boninensis (holotype). A: Basidiospores. B: Generative hyphae from trama. C: Skeletoid hyphae from trama. D: Generative hyphae from context. E: Skeletoid hyphae from context. Bars: A 5 µm; B-E 10 µm.
Basidiomata perennial, sessile. Pileus semicircular, convex to ungulate, pileus surface sulcate when young, becoming rimose. Context woody, without a distinct crust. Pores 4-6/mm. Hyphal system subdimitic. Basidiospores ellipsoid, light brown to rusty brown, 5-6.5 × 3.5-5 µm.
Type: JAPAN, Bonin Islands, Hahajima I, Sekimon, on dead trunk of Morus boninensis, 13 Nov 2013, leg. T. Hattori et al. (TFM: F-28831; ex-type culture, FFPRI421009).
Gene sequences ex-holotype: LC315777 (LSU), LC315786 (ITS).
Etymology: boninensis, from the type locality.
Basidiomata perennial, sessile, broadly attached, solitary. Pileus semicircular to irregular, triquetrous to convex when young, later becoming ungulate, up to 15 × 10 cm, 9 cm thick; pileus surface at first densely sulcate at least near the margin, velutinous to glabrous, yellowish brown to brown, becoming glabrous, rough with irregular projections, later deeply rimose with irregular cracks, dark brown to almost black; pileus margin entire, dull and obtuse. Context woody, homogeneous, yellowish brown, up to 1 cm thick in holotype, without a crust in young specimens, later becoming covered with crust-like layer on pileus surface, crust-like layer continuous and not well-differentiated from context. Tubes indistinctly stratified, woody, concolorous with context, each layer up to 3 mm deep; pores angular to round, 4-6/mm, dissepiments thick, entire, pore surface glancing, yellowish brown; with sterile marginal zone up to 5 mm wide.
Hyphal system subdimitic; tramal hyphae semi-parallel to partly interwoven, not agglutinated; tramal generative hyphae thin- to slightly thick-walled, occasionally branched, septate without clamp-connections, hyaline to light brown, 1.5-2.5 µm wide; tramal skeletoid hyphae thick-walled to almost solid, aseptate to occasionally septate, light brown to rusty brown, 2-4 µm wide, not well-differentiated from generative hyphae; contextual generative hyphae thin- to slightly thick-walled, occasionally branched, septate without clamp-connections, hyaline to light brown, 1.5-3 µm wide; contextual skeletoid hyphae thick-walled, unbranched or rarely branched, aseptate to occasionally septate, brown, 2-4.5 µm wide, not well-differentiated from generative hyphae. Setae or setal hyphae lacking. Basidia not seen. Basidiospores ellipsoid to broadly ellipsoid, slightly thick-walled, light brown to rusty brown, IKI−, 5-6.5 × 3.5-5 µm, 5.6 × 4.3 µm on average, R = 1.29, r: 1.19-1.58, n = 54/3 (Holotype only: 5-6.5 × 3.5-4.5 µm, 5.6 × 4.2 µm on average, R = 1.33, r: 1.22-1.39, n = 28).
Habitat and distribution: on cut stumps and dead standing trees of Morus boninensis. Chichijima I. and Hahajima I., Bonin Islands, Japan.
Additional specimens examined: JAPAN, Hahajima I., Sekimon, on M. boninensis, leg. Y. Hayashi, 1 Jan 1977 (F-28878); the same place, the same date (F-28879; F-28880); the same place, 3 Jan 1977 (F-28881); Chichijima I., Renju-dani, on M. boninensis, leg. T. Hirano, 21 Jan 1976 (F-11583); the same place, leg. H. Neda, 12 Mar 1990 (F-16016); Chichijima I., Nagatani, leg. H. Neda, 12 Mar 1990 (F-16015); Chichijima I., Mikazuki-yama, on M. boninensis, leg. T. Hattori et al., 21 Jun 2015 (F-28882; voucher of culture FFPRI421008).
Remarks: This species can be distinguished from other Fulvifomes species by a combination of lack of a distinct crust on the pileus surface, subdimitic hyphal system in the context, and ellipsoid basidiospores measuring 5-6.5 µm long in this genus. This is keyed out at lead number 24 (Hattori et al., 2014), and has larger basidiospores than F. swieteniae Murrill and F. xylocarpicola T. Hatt., Sakayaroj & E.G.B. Jones, also keyed out at lead 24. Fulvifomes rimosus and F. robiniae both have a rimose pileus surface without distinct crusts, but the former can be differentiated from F. boninensis by its smaller pores (6-7/mm), larger basidiospores (5.5-7.5 µm long), and skeletoid hyphae with a distinct lumen (Hattori et al., 2014), and the latter by its smaller pores (7-8/mm) and subglobose basidiospores (Gilbertson & Ryvarden, 1987). Fulvifomes hainanensis and F. thailandicus are phylogenetically related to this fungus, but the former has basidiomata drying lighter in weight, with an orange brown pileus surface, and larger pores (3-4/mm; Zhou, 2014), while the latter has basidiomata lighter in weight, an applanate pileus, and smaller pores (6-7/mm; Zhou, 2015).
Fulvifomes imazekii T. Hatt., Y. Ota & Sotome, sp. nov. Figs. 4, 8B, C, 9B.
MycoBank no.: MB 841422.
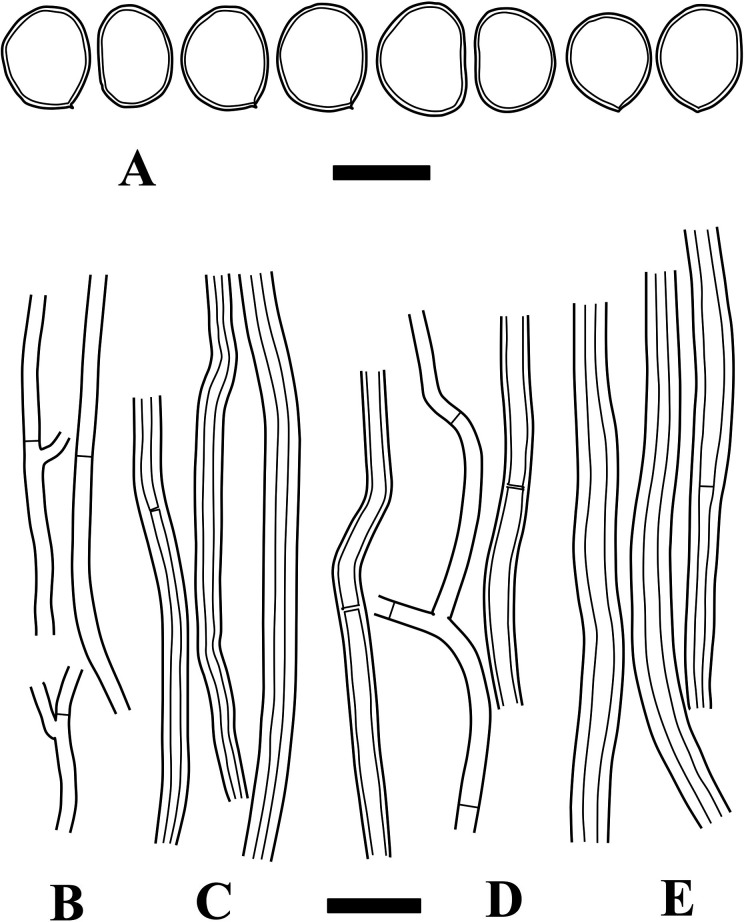
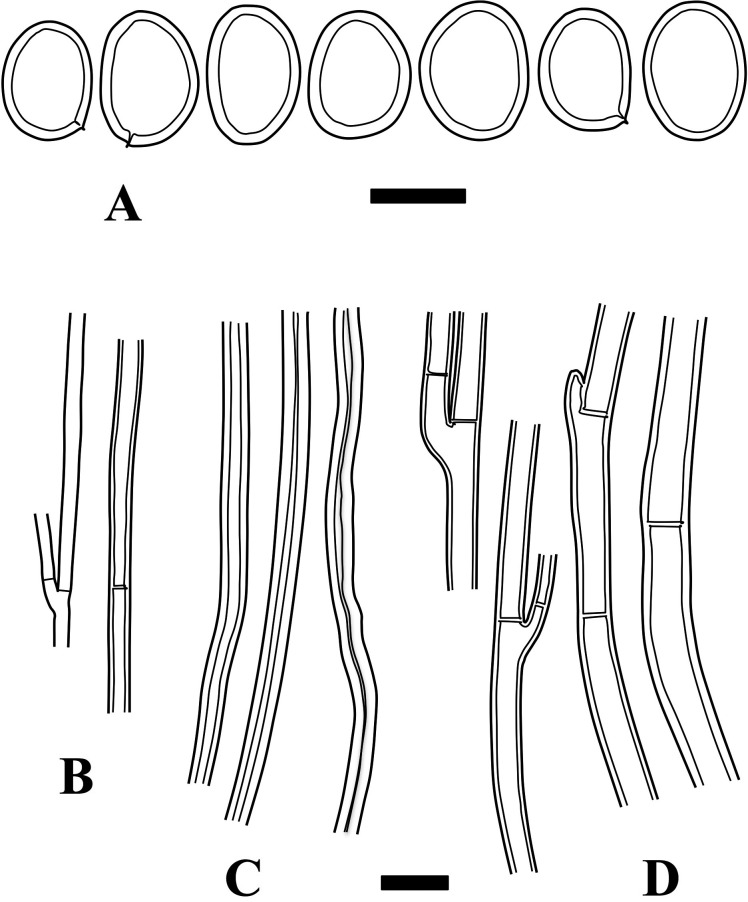
Fig. 4 - Microscopic structures of Fulvifomes imazekii (holotype). A: Basidiospores. B: Generative hyphae from trama. C: Skeletal hyphae from trama. D: Generative hyphae from context. E: Skeletal hyphae from context. Bars: A 5 µm; B-E 10 µm.
Basidiomata perennial, sessile. Pileus semicircular, applanate to convex, pileus surface sulcate and shortly tomentose. Context fibrous-corky, usually without crust, but crustose lines present in context. Pores 6-7/mm. Hyphal system dimitic in context. Basidiospores broadly ellipsoid to leniform, light brown to rusty brown, 4.5-6.5 × 3.5-5 µm.
Type: JAPAN, Kochi Pref., Ochi-cho, Mt. Yokogura-yama, on Berchemiella berchemiaefolia, 15 Jun 2013, leg. K. Ikeuchi (TFM: F-28883; ex-type culture, FFPRI 421007).
Gene sequences ex-holotype: LC315779 (LSU), LC315788 (ITS).
Etymology: imazekii, from name of Japanese mycologist Rokuya Imazeki (1904-1991), who reported this fungus for the first time.
Basidiomata perennial, sessile, broadly attached, solitary. Pileus semicircular to irregular, applanate to convex, occasionally triquetrous to ungulate, up to 18 × 12 cm, 7 cm thick; pileus surface at first sulcate, shortly tomentose to velutinous, yellowish brown, brown to dark brown near the base; becoming fibrous-rough, occasionally with cracks, dark brown to almost black in old specimens; pileus margin entire, acute to obtuse. Context fibrous-corky, woody-corky near the tubes; with crustose layer(s) inside of the context as thin black line or discontinuous lines; usually without distinct crust on pileus surface, but occasionally with partial crust as thin black line below tomentum, otherwise, pileus surface sometimes becoming crust-like (but continuous from context) near the base in old specimens. Tubes not distinctly stratified, fibrous corky, yellowish brown; pores angular to round, 6-7/mm, dissepiments entire, pore surface glancing, yellowish brown.
Hyphal system dimitic; tramal hyphae semi-parallel to partly interwoven; tramal generative hyphae thin- to slightly thick-walled, occasionally branched, septate without clamp-connections, hyaline to light brown, 1.5-3.5 µm wide; tramal skeletal hyphae thick-walled to almost solid, aseptate, rarely with ambiguous septa, light brown to rusty brown, 3-5.5 µm wide; contextual generative hyphae thin- to slightly thick-walled, occasionally branched, septate without clamp-connections, hyaline to light brown, 2-4 µm wide; contextual skeletal hyphae thick-walled with wide lumen, unbranched or rarely branched, aseptate, rarely with ambiguous septa, rusty brown, 3-6 µm wide. Setae or setal hyphae lacking. Basidia not seen. Basidiospores broadly ellipsoid to leniform, slightly thick-walled, light brown to rusty brown, IKI−, 4.5-6.5 × 3.5-5 µm, 5.3 × 4.4 µm on average, R = 1.23, r: 1.12-1.39, n = 61/2 (Holotype only: 4.5-6 × 3.5-4.5 µm, 5.1 × 4.1 µm on average, R = 1.24, r: 1.12-1.39, n = 35).
Habitat and distribution: On trunk of Berchemiella berchemiaefolia. Mt. Yokogura-yama, Kochi pref., Japan.
Additional specimens examined: JAPAN, Kochi Pref., Yokogura-yama, on B. berchemiaefolia, Aug. 1938, leg. R. Imazeki (TNS-F-206909); the same place, on B. berchemiaefolia, 8 Nov 1965, leg. T. Kobayashi (F-10700); the same place, 12 Mar 1980, leg. K. Aoshima (F-23248); the same place, on B. berchemiaefolia, 1 May 1998, leg. T. Suda (F-13103); the same place, 21 Jul 1996, leg. Y. Abe (F-18705; voucher of culture FFPRI421006); the same place, on B. berchemiaefolia, 18 Aug 2016, leg. K. Ikeuchi (F-28884).
Remarks: Fulvifomes imazekii can be differentiated from F. boninensis by its more distinctly velutinous pileus surface, fibrous-corky context, and smaller pores. As with F. boninensis, this species is also keyed out by Hattori et al. (2014) at lead 24 (in that case without a crust) or lead 10-11 (with a crust). Fulvifomes imazekii can be distiguished from F. swieteniae and F. xylocarpicola by its distinctly sulcate and velutinous pileus surface and broadly ellipsoid basidiospores; from F. fastuosus (Lév.) Bondartseva & S. Herrera, by its single and smaller pileus, sulcate and tomentose pileus surface, and lack of a crust covering all parts of the pileus, from F. halophilus T. Hatt., Sakayaroj & E.B.G. Jones by its broadly ellipsoid basidiospores, and from F. nilgheriensis (Mont.) Bondartseva & S. Herrera by its lack of a distinct and persistent back line (crust) under the tomentum.
3.3. Other authenticated specimens examined
Fulvifomes aulaxinus (Bres.) T. Hatt., comb. nov. Figs. 5, 8D, 9C.
MycoBank no.: MB 841423.
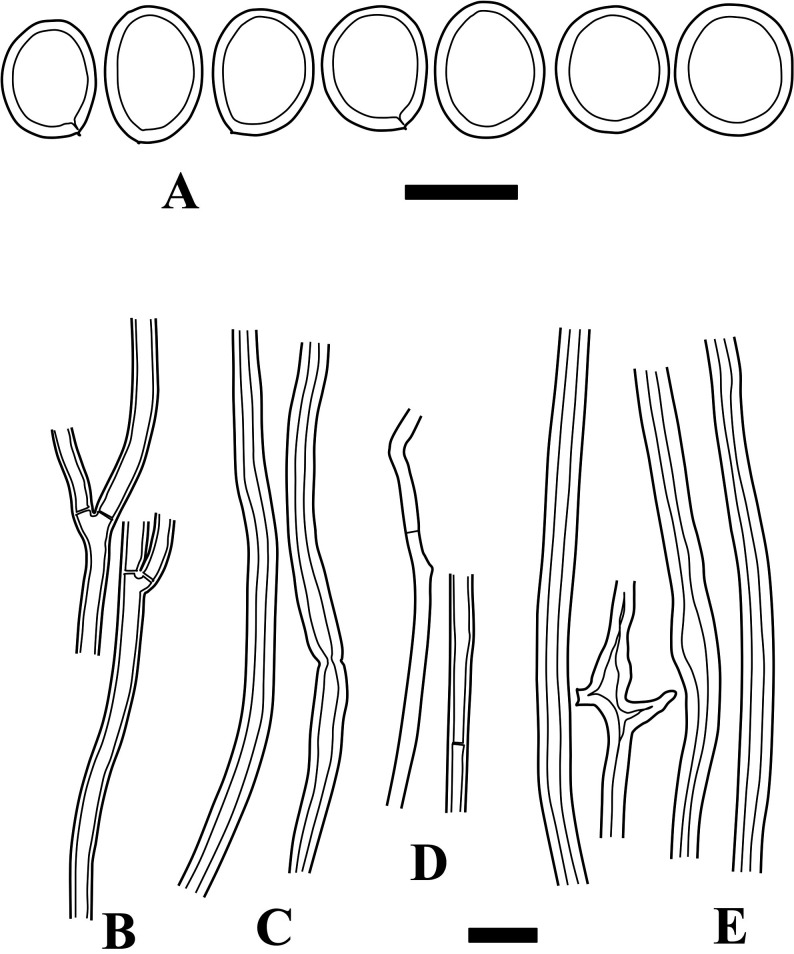
Fig. 5 - Microscopic structures of Fulvifomes aulaxinus (holotype). A: Basidiospores. B: Generative hyphae from trama. C: Skeletal hyphae from trama. D: Generative hyphae from context. E: Skeletal hyphae from context. Bars: A 5 µm; B-E 10 µm.
Basionym: Fomes aulaxinus Bres., Annls mycol. 10: 497 (1912).
Basidiomata perennial, sessile, broadly attached, solitary. Pileus semicircular, convex, triquetrous or ungulate; pileus surface densely sulcate near the margin, slightly velutinous near the margin, almost glabrous near the base, not cracked, brown to dark brown; pileus margin entire, obtuse. Context woody, yellowish brown, whitish mycelial strands abundantly present within context, up to 1 cm thick, crust-like layer present on pileus surface, but not well-differentiated from context, almost black, up to 2 mm thick. Tubes indistinctly stratified, woody, up to 2 cm deep in holotype; pores angular, 4-5/mm, dissepiments entire, moderately thick, grayish brown; with sterile marginal zone.
Hyphal system dimitic; tramal hyphae semiparallel, partly agglutinated; tramal generative hyphae thin-walled, septate without clamp-connections, hyaline to yellow, 1.5-2.5 µm wide; tramal skeletal hyphae thick-walled, aseptate, unbranched, light brown to rusty brown, 2-3.5 µm wide; contextual hyphae as in trama; hyphae of crust-like layer interwoven and highly agglutinated, brown. Basidia not seen. Setae absent. Basidiospores (ellipsoid to) oblong ellipsoid, slightly thick-walled, light brown, IKI−, (5.5-) 6-7 × 3.5-4.5 µm, 6.4 × 4.2 µm on average, R = 1.55, r: 1.39-1.73, n = 31.
Specimen examined: Fjibodas, Java, 1908 (holotype, FH).
Remarks: This species can be characterized by its densely sulcate pileus surface, thick crust-like layer on the pileus surface, and oblong ellipsoid basidiospores up to 7 µm long. The holotype resembles young specimens of F. boninensis, but has more oblong and larger basidiospores. Ryvarden (1988) concluded that this was a synonym of F. rimosus, but it is distinct from the latter in its distinctly sulcate pileus surface, dimitic hyphal system in context, and more oblong basidiospores. Lowe (1957) considered that this is a prior name for Fomes mcgregori Bres. These two species share a similar sulcate pileus surface, hyphal characteristics, and oblong basidiospores, but the holotype of F. mcgregori has widely effused basidiomata, thin pilei, a distinct crust as a thin black line, and smaller basidiospores (5-6 × 3-4 µm) compared with those of F. aulaxinus. Therefore, we kept them as two distinct species. For characteristics of F. mcgregori, see Ryvarden (1988) as Phellinus mcgregori (Bres.) Ryvarden.
Fomes dialeri Bres. & Torrend, Brotéria, sér. Bot. 4: 218 (1905)
Specimen examined: Merurú, Mozanbique, leg. L. G. Dialer (isotype, FH).
Remarks: It was treated as a synonym of F. rimosus by Ryvarden (1988), but it represents Fulvifomes pappianus. For a detailed description, see below.
Fulvifomes pappianus (Bres.) T. Hatt., comb. nov. Figs. 6, 8E, 9D.
MycoBank no.: MB 841424.
Fig. 6 - Microscopic structures of Fulvifomes pappianus (isotype). A: Basidiospores. B: Generative hyphae from trama. C: Skeletal hyphae from trama. D: Generative hyphae from context. Bars: A 5 µm; B-D 10 µm.
Basionym: Fomes pappianus Bres., Annuar. R. Ist. Bot. Roma 6: 178 (1896).
= Fomes dialeri Bres. & Torrend.
Basidiomata perennial, sessile, broadly attached, solitary. Pileus probably semicircular, convex to triquetrous; pileus surface glabrous, smooth, partly warty, yellowish brown near the margin, dark grayish brown near the base. Context fibrous-corky to felty, brown, crust present as a thin black line near the base [“with distinct crust” according to Lowe (1957)]. Tubes indistinctly stratified, corky, each layer up to 8 mm deep; pores angular, partly elongated, 2-4/mm, dissepiments entire, brown.
Hyphal system dimitic in trama, monomitic in context; tramal hyphae almost parallel, non-agglutinated; tramal generative hyphae thin- to slightly thick-walled, septate without clamp-connections, hyaline to light brown, 1.5-2.5 µm wide; tramal skeletal hyphae thick-walled, aseptate, unbranched, light brown to brown, 2-4.5 µm wide; contextual generative hyphae thin to slightly thick-walled, light brown to brown, 2-7 µm wide. Basidia not seen. Setae absent. Basidiospores ellipsoid, thick-walled, light brown to brown, IKI−, 6-7.5 × 4.5-5.5 µm, 7.0 × 5.0 µm on average, R = 1.4, r: 1.27-1.5, n = 30.
Specimen examined: Colonia Africa, leg. Pappi, Apr 1844 (isotype, FH).
Remarks: This fungus can be characterized by its almost glabrous and smooth pileus surface, fibrous-corky context, monomitic hyphal system in context, and ellipsoid basidiospores. It is unclear if it always has a thin crust on the pileus surface or not. Reid (1975) considered this and F. dialeri as synonyms of Phellinus badius (Cooke) G. Cunn., but Larsen (1990) rejected these synonymies. Ryvarden (1988) concluded that this is a synonym of F. rimosus, but F. pappianus is distinct from F. rimosus owing to the former having fibrous corky context, larger pores, and a monomitic hyphal system in context. Fulvifomes mangrovicus (Imazeki) T. Hatt. is similar to F. pappianus with regard to its hyphal and basidiospore characteristics, but the former has a rough pileus surface with appressed fibrils or irregular protuberances, smaller pores (5-6/mm), and wider contextual hyphae up to 10 µm wide (Hattori et al., 2014).
Fulvifomes tepperi (Lloyd) T. Hatt., comb. nov. Figs. 7, 8F, 9E.
MycoBank no.: MB 841425.
Fig. 7 - Microscopic structures of Fulvifomes tepperi (holotype). A: Basidiospores. B: Generative hyphae from trama. C: Skeletal hyphae from trama. D: Generative hyphae from context. E: Skeletal hyphae from context. Bars: A 5 µm; B-E 10 µm.
Basionym: Fomes tepperi Lloyd, Mycol. Writ. 4 (Syn. gen. Fomes): 256 (1915).
Basidiomata sessile. Pileus semicircular, ungulate; pileus surface sulcate, glabrous, rimose with deep cracks, dark brown, partly grayish brown; pileus margin partly eroded, obtuse. Context woody, rusty brown, up to 1 mm thick, without crust or with indistinct crust-like layer on pileus surface. Tubes not stratified, woody, concolorous with context, up to 1.5 cm deep; pores irregular, angular to elongated, partly sinuous, 1-3/mm, up to 3 mm long in elongated pores, dissepiments entire, moderately thick, rusty brown.
Hyphal system dimitic; tramal hyphae semiparallel to partly interwoven, partly agglutinated; tramal generative hyphae thin- to slightly thick-walled, septate without clamp-connections, hyaline to light brown, 2-3.5 µm wide; tramal skeletal hyphae thick-walled to solid, aseptate, unbranched, occasionally branched, light brown to brown, 2.5-4 µm wide; contextual generative hyphae thin to slightly thick-walled, hyaline to light brown, 2-3 µm wide: contextual skeletal hyphae thick-walled, aseptate, but occasionally with secondary septa, light brown to brown, 3-7 µm wide. Basidia not seen. Setae absent. Basidiospores ellipsoid in side view, subglobose in face view, thick-walled, light brown, IKI−, 6-8 × 5-6.5 µm, 7.1 × 5.9 µm on average, R = 1.21, r: 1.13-1.29 in side view, n = 28.
Specimen examined: Norwood, S. Australia, leg. J. G. O Tepper (holotype, BPI: US0310673).
Remarks: This species can be characterized by its large and irregular pores, and flattened basidiospores that are ellipsoid in the side view and subglobose in the face view. This is keyed out at lead 24 in Hattori et al. (2014), but is easily discriminated from others by the above characteristics.
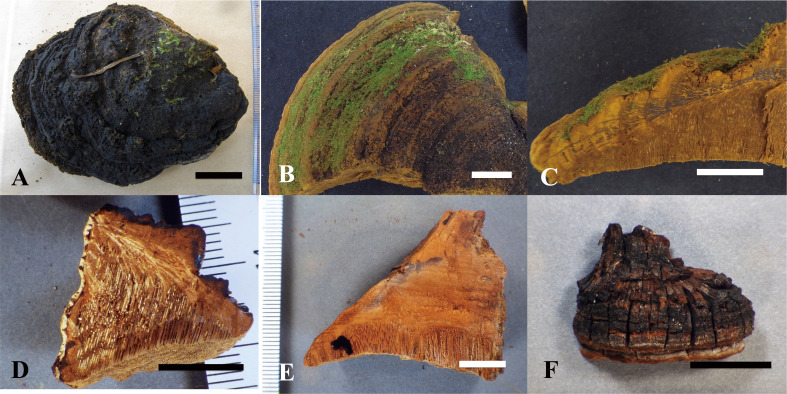
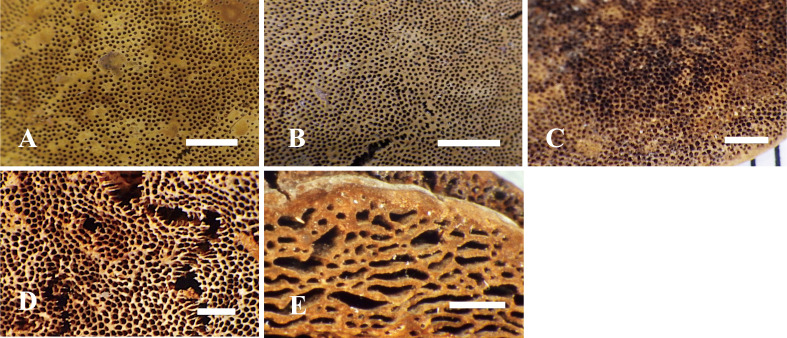
Fig. 8 - Basidiomata of the type specimens examined. A: Pileus surface of Fulvifomes boninensis (holotype). B: Pileus surface of F. imazekii (holotype). C: Vertically cut section of F. imazekii (holotype). D: Vertically cut section of F. aulaxinus (holotype). E: Vertically cut section of F. pappianus (isotype, FH). F: Vertical pileus view of F. tepperi (holotype). Bars: A-C 3 cm; D-F 1 cm.
Fig. 9 - Pore surface of the type specimens examined. A: Fulvifomes boninensis (holotype). B: F. imazekii (holotype). C: F. aulaxinus (holotype). D: F. pappianus (isotype, FH). E: F. tepperi (holotype). Bars: 2 mm.
4. Discussion
“Fulvifomes rimosus” has been considered a widely distributed species in Southern Europe, Africa, Asia, and Australia (Ryvarden & Johansen, 1980; Dai, 2010; Ryvarden & Melo, 2017). Japanese specimens determined as “F. rimosus”, however, actually represent two undescribed species of Fulvifomes. Some names synonymized with “F. rimosus” were also revealed to represent morphologically distinct species in the present study. Consequently, specimens determined as “F. rimosus” are likely in need of reexamination to determine their correct identities. We suggest that this name is better treated as an ambiguous name, because 1) several cryptic species have been included under the traditional usages of “F. rimosus,” and 2) the lectotype is from Mauritius, a tropical island east of Madagascar, while locality of the missing holotype is in temperate West Australia. It is doubtful the missing holotype and the designed lectotype really represent the same taxon. In this article, we did not include sequences of F. rimosus sensu stricto, because lectotypification of F. rimosus does not readily allow identification of modern authenticated specimens from which DNA can be extracted.
Recently, a number of Fulvifomes species have been described from subtropical and tropical areas of Asia on the basis of molecular phylogeny and morphological observations (Hattori et al., 2014; Zhou, 2014; 2015). More than 30 species of Fulvifomes are already known, but most of them are morphologically similar with distinctly pileate basidiomata, lack of hymenial setae and setal hyphae, and subglobose to ellipsoid and colored basidiospores (Hattori et al., 2014). Molecular phylogenetic studies and detailed morphological observations may lead to the description of more Fulvifomes species from tropical and subtropical areas (Ji et al., 2017; Olou et al., 2019; Liu et al., 2020).
Fulvifomes boninensis is known only on Morus boninensis at present. Morus boninensis is a tree species that is endemic to the Bonin Islands, and was intensively logged during the last quarter of the 19th century and the beginning of the 20th century owing to its high-quality wood (Tani et al., 2003). This species is now seriously declining and threatened because of replacement by exotic trees, particularly Bischofia javanica Blume, and gene contamination with allied species in addition to overharvesting. It is now classified as a Critically Endangered species (CR) in the Japanese red data book (Ministry of the Environment, 2015b).
Morus acidosa Griff. is another species of Morus L. seen in the Bonin Islands. It is an exotic species introduced from Okinawa, but is now widely distributed in the Bonin Islands (Toyoda, 1981). Despite its common occurrence in these islands, however, F. boninensis has never been recorded on M. acidosa. Most of the recent collections or records of F. boninensis were made on huge cut stumps with a heart rot cavity, and one specimen was made on a recently dead standing tree of M. boninensis. Perhaps, this fungus is associated with a heart rot of M. boninensis.
Fulvifomes boninensis is known only from the Chichijima and Hahajima Islands, which cover areas of 23.45 km2 and 19.88 km2, respectively. Its host tree M. boninensis has a severely fragmented distribution, and is in continuous decline due to the threats indicated above. Therefore, 1) the extent of occurrence of F. boninensis is less than 100 km2; 2) its distribution is severely fragmented; and 3) it shows continuing decline in the area and in its quality of habitat. This fungus is considered to be Critically Endangered (CR) under criterion B1(a,b(iii)) of the IUCN Red List criteria (IUCN Species Survival Commission, 2012).
Fulvifomes imazekii also shows host specificity, and is known only on B. berchemiaefolia. Recent collections were made on living or recently dead trees (K. Ikeuchi, personal communications), suggesting that this is possibly a heart rot fungus of living B. berchemiaefolia. Berchemiella berchemiaefolia is a rare but widely distributed species in warm temperate areas in Japan as well as southern Korea and some parts of China (Kitamura & Murata, 1976; Ota, 2000), but this fungus only known to occur on a few trees of B. berchemiaefolia at Mt. Yokogura-yama, which is situated in Kochi Prefecture, Japan. This tree is now red-listed as a near threatened species (NT) in this prefecture (Kochi Prefecture, 2010), although detailed investigations are ongoing for a revision of the prefectural red list (N. Kuroiwa, personal communications).
According to herbarium records, F. imazekii is hitherto known only from a single site that covers an area of less than 10 km2, and its perennial basidiocarps are known on fewer than 10 trees in the site at present (K. Ikeuchi, personal communications). For evaluation of IUCN threat status of lignicolous macrofungi, Dahlberg and Mueller (2011) recommended an estimate of two “mature individuals” for each host log or trunk; therefore the number of “mature individuals” of this fungus can be estimated as less than 50. Thus, this fungus can be assessed as Critically Endangered (CR) under criterion D (IUCN Species Survival Commission, 2012), if its distribution is entirely restricted to this site. This fungus has been repeatedly collected from the type locality since its first record by Imazeki (1940), but it has never been recorded from other areas of Japan even after intensive collection by Japanese mycologists for nearly 80 years. We consider the distribution of this fungus to be restricted to an extremely limited area.
It is widely believed that most wood-inhabiting polypores do not show distinct host specificity in tropical areas, but some in Malaysia actually show preferences for certain tree families or genera (Hattori et al., 2012; Hattori, 2017). Some Fulvifomes spp. distributed in tropical and subtropical areas also show preferences for certain trees. Fulvifomes fastuosus (Lév.) Bondartseva & S. Herrera is always associated with Dipterocarpaceae trees in Pasoh Forest Reserve, a lowland forest of Malaysia (Hattori et al., 2012). Fulvifomes halophilus and F. xylocarpicola, described from Thailand, are known only on mangrove tree Xylocarpus granatum J. König (Meriaceae) at present (Sakayaroj et al., 2012; Hattori et al., 2014). Fulvifomes robiniae (Murrill) Murrill usually occurs on Robinia L. (Fabaceae) in America (Gilbertson & Ryvarden, 1987), whereas F. squamosus Salvador-Montoya & Drechsler-Santos is mainly found on living trees of Acacia macracantha Humb. & Bonpl. ex Willd. in Peru (Salvador-Montaya et al., 2018). We suggest that several Fulvifomes species may have highly specific host preferences, at least at the local population level, even in tropical areas, and host range could be an important characteristic of Fulvifomes spp.
Regarding its macro-morphological characteristics, F. boninensis lacks a distinct crust on the pileus surface as a thin black line, while a partial crust inside the context is almost consistently observed in F. imazekii. As shown in Hattori et al. (2014), the presence of a pileus crust is considered one of the distinctive characteristics for Fulvifomes species. The hyphal system is another important characteristic that varies according to the species in this genus (Hattori et al., 2014). Fulvifomes boninensis consistently has a subdimitic hyphal system with thick-walled and aseptate to ambiguously septate skeletoid hyphae that are not well-differentiated from generative hyphae, while F. imazekii has a dimitic hyphal system with skeletal hyphae more distinctly differentiated from generative hyphae.
Disclosure
The authors declare no conflicts of interest. All the experiments undertaken in this study comply with the current laws of Japan.
Acknowledgments
This study was financially supported, in part, by a Grant from the Institute for Fermentation, Osaka (no. G-24-1-36) and by a Grant-in-Aid for Scientific Research (no. 25292096) from the Japan Society for the Promotion of Science. We thank Mr. Tomio Okino (Ehime pref.) and Ms. Keiko Ikeuchi (Ehime pref.) for the donation of specimens of F. imazekii and information about biology of this fungus; Mr. Nobuhito Kuroiwa (Kochi Prefectural Forest Technology Center) for information about Berchemiella berchemiaefolia; and Ms. Ritsuko Shimada (Tokyo pref.) and Mr. Akitsugu Mukai (Tokyo pref.) for their guidance in the Bonin Islands. We also thank the curators of the following herbaria for loan of specimens: Farlow Reference Library and Herbarium of Cryptogamic Botany, Harvard University (FH), U.S. National Fungus Collections, Systematic Botany and Mycology Laboratory (BPI), Royal Botanic Gardens, Kew (K), and National Museum of Nature and Science (TNS). This study was undertaken with permissions from Forestry Agency, Ministry of Agriculture and Fisheries, Ministry of the Environment, and Tokyo Metropolitan.
References
- Berkeley, M. J. (1839) .Contribution toward a flora of Van Diemen's Land. Annals and Magazine of Natural History Ser. 1. 3, 322–327. [Google Scholar]
- Berkeley, M. J. (1845) .Decades of fungi. Decades III-VII. Australian fungi. London Journal of Botany, 4, 42–73. [Google Scholar]
- Dahlberg, A., & Mueller, G. M. (2011) .Applying IUCN red-listing criteria for assessing and reporting on the conservation status of fungal species. Fungal Ecology, 4, 147–162. https://doi.org/10.1016/j.funeco.2010.11.001 [Google Scholar]
- Dai, Y. C. (2010) .Hymenochaetaceae (Basidiomycota) in China. Fungal Diversity, 45, 131–343. http://doi.org/10.1007/s13225-010-0066-9 [Google Scholar]
- Farris, J. S., Källersjö, M., Kluge, A. G., & Bult, C. (1994) .Testing significance of incongruence. Cladistics. 10, 315, 319. https://doi.org/10.1111/j.1096-0031.1994.tb00181.x [Google Scholar]
- Felsenstein, J. (1985) .Confidence limits on phylogenies: an approach using the bootstrap. Evolution, 39, 783–791. [DOI] [PubMed] [Google Scholar]
- Fiasson, J. L., & Niemelä, T. (1984) .The Hymenochaetales: a revision of the European poroid taxa. Karstenia, 24, 14–28. [Google Scholar]
- Gilbertson, R. L., & Ryvarden, L. (1987) .North American polypores, vol 2. Oslo: Fungiflora. [Google Scholar]
- Hall, T. A. (1999) .BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41, 95–98. [Google Scholar]
- Hattori, T. (2017) .Biogeography of polypores in the Malesian region, Southeast Asia. Mycoscience, 58, 1–13. https://doi.org/10.1016/j.myc.2016.09.004 [Google Scholar]
- Hattori, T., Sakayaroj, J., Jones, E. B. G., Suetrong, S., Preedanon, S., & Klaysuban, A. (2014) .Three species of Fulvifomes (Basidiomycota, Hymenochaetales) associated with rots on mangrove tree Xylocarpus granatum in Thailand. Mycoscience, 55, 344–354. http://dx.doi.org/10.1016/j.myc.2014.01.001 [Google Scholar]
- Hattori, T., Yamashita, S., & Lee, S. S. (2012) .Diversity and conservation of wood-inhabiting polypores and other aphyllophoraceous fungi in Malaysia. Biodiversity and Conservation, 21, 2375–2396. http://dx.doi.org/10.1007/s10531-012-0238-x [Google Scholar]
- Hosaka, K., & Castellano, M. A. (2008) .Molecular phylogenetics of Geastrales with special emphasis on the position of Sclerogaster. Bulletin of the National Museum of Nature and Science. Series B, Botany, 34, 161–173. [Google Scholar]
- Imazeki, R. (1940) .Observations on Japanese fungi V. On several Fomes with brown context (in Japanese with English descriptions). The Journal of Japanese Botany, 16, 583–588. [Google Scholar]
- Ito, S., & Imai, S. (1940) .Fungi of the Bonin Islands V. Transactions of the Sapporo Natural History Society, 16, 120–138. [Google Scholar]
- IUCN Species Survival Commission (2012) .IUCN red list categories and criteria, Version 3.1. 2nd ed. http://cmsdocs.s3.amazonaws.com/keydocuments/Categories_and_Criteria_en_web%2Bcover%2Bbckcover.pdf. Accessed 28 Jul 2017. [Google Scholar]
- Ji, X. H., Wu, F., Dai, Y. C., & Vlasák, J. (2017) .Two new species of Fulvifomes (Hymenochaetales, Basidiomycota) from America. MycoKeys, 22, 1–13. https://doi.org/10.3897/mycokeys.22.12380 [Google Scholar]
- Katoh, K., & Standley, D. M. (2013) .MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution, 30, 772–780. https://doi.org/10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura, S., & Murata, G. (1976) .Coloured illustrations of woody plants of Japan vol. 1. Osaka: Hoikusha. [Google Scholar]
- Kochi prefecture (2010) .Redlist of higher plants in Kochi prefecture(in Japanese). http://www.pref.kochi.lg.jp/soshiki/030701/files/2011012600100/2011012600100_www_pref_kochi_lg_jp_uploaded_attachment_42619.pdf Accessed 29 Nov 2019. [Google Scholar]
- Larsen, M. J. (1990) .Reexamination of the nomenclatural types of Polyporus rimosusBerk. and P. badius Berk. Mycotaxon, 37, 353–361. [Google Scholar]
- Liu, G. H., Ji, X. H., Wang, Y. P., & Chen, J. J. (2020) .A new species ofFulvifomes(Basidiomycota) from China. Phytotaxa, 470, 194–202. https://doi.org/10.11646/phytotaxa.470.2.8 [Google Scholar]
- Lowe, J. L. (1957) .Polyporaceae of North America: the genusFomes. Technical Publications. New York State University College of Forestry, 80, 1-97. [Google Scholar]
- Ministry of the Environment (2015. a) .Red data book 2014. Threatened wildlife of Japan. Vol. 9, bryophytes, algae, lichens, fungi. Tokyo: Gyosei Co. [Google Scholar]
- Ministry of the Environment (2015. b) .Red data book 2014. Threatened wildlife of Japan. Vol. 8, higher plants. Tokyo: Gyosei Co. [Google Scholar]
- Nylander, J. A. A. (2004) .MrModeltest v2. Program distributed by the author. Uppsala: Evolutionary Biology Centre, Uppsala University. [Google Scholar]
- Olou, B.A., Ordynets, A., & Langer, E. (2019) .First new species of Fulvifomes(Hymenochaetales, Basidiomycota) from tropical Africa. Mycological Progress, 18, 1383–1393. https://doi.org/10.1007/s11557-019-01536-9 [Google Scholar]
- Ota, K. (2000) .Rhamnaceae. In: Takahashi, H., & Katsuyama T. (Eds.), Woody plants of Japan, Choripetalae 2 (in Japanese, pp. 500–517). Tokyo: Yamakei. [Google Scholar]
- Ota, Y., Hattori, T., Nakamura, H., Terashima, Y., Lee, S. S., Miyuki, Y., & Sotome, K. (2014) .Taxonomy and phyloenetic position of Fomitiporia torreyae, a causal agent of trunk rot on Sanbu-sugi, a cultivar of Japanese cedar (Cryptomeria japonica) in Japan. Mycologia, 106, 66–76. http://dx.doi.org/10.3852/13-045 [DOI] [PubMed] [Google Scholar]
- Reid, D. A. (1975) .Type studies of the larger Basidiomycetes described from Southern Africa. Contributions from the Bolus Herbarium, 7, 1–255. [Google Scholar]
- Ronquist, F., Teslenko, M., Van Der Mark, P., Ayres, D. L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M. A., & Huelsenbeck, J. P. (2012) .MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61, 539–542. https://doi.org/10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryvarden, L. (1988) .Type studies in the Polyporaceae 20. Species described by G. Bresadola. Mycotaxon, 33, 303–327. [Google Scholar]
- Ryvarden, L., & Johansen, I. (1980) .A preliminary polypore flora of East Africa. Oslo: Fungiflora. [Google Scholar]
- Ryvarden, L., & Melo, I. (2017) .Poroid fungi of Europe, 2nd ed. Synopsis Fungorum, 37, 1–431. [Google Scholar]
- Sakayaroj, J., Preedanon, S., Suetrong, S., Klaysuban, A., Jones, E. B. G., & Hattori, T. (2012) .Molecular characterization of basidiomycetes associated with the decayed mangrove tree Xylocarpus granatum in Thailand. Fungal Diversity, 56, 145–156. http://dx.doi.org/10.1007/s13225-012-0195-4 [Google Scholar]
- Salvador-Montoya, C. A., Popoff, O. F., Reck, M., Drechsler‑Santos, E. R. (2018) .Taxonomic delimitation of Fulvifomes robiniae (Hymenochaetales, Basidiomycota) and related species in America: F. squamosus sp. nov. Plant Systematics and Evolution, 304, 445–459. https://doi.org/10.1007/s00606-017-1487-7 [Google Scholar]
- Sotome, K., Maekawa, N., Nakagiri, A., Lee, S. S., & Hattori, T. (2014) .Taxonomic study of Asian species of poroid Auriculariales. Mycological Progress, 13, 987–997. http://dx.doi.org/10.1007/s11557-014-0984-0 [Google Scholar]
- Stamatakis, A. (2014) .RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics, 30, 1312–1313. https://doi.org/10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swofford, D. L. (2002) .PAUP*: phylogenetic analysis using parsimony (*and other methods). Version 4. Sunderland: Sinauer Associates. [Google Scholar]
- Tani, N., Kawahara, T., Yoshimaru, H., & Hoshi, Y. (2003) .Developent of SCAR markers distinguishing pure seedlings of the endangered species Morus boninenis from M. boninenis × M. acidosa hybrids for conservation in Bonin (Ogasawara) Islands. Conservation Genetics, 4, 605–612. http://dx.doi.org/10.1023/A:1025655331429 [Google Scholar]
- Toyoda, T. (1981) .Flora of Bonin Is. (in Japanese). Kamakura: Aboc-Sha. [Google Scholar]
- Vilgalys, R., & Hester, M. (1990) .Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology, 172, 4239–4246. http://dx.doi.org/10.1128/jb.172.8.4238-4246.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, T., & Fischer, M. (2002) .Proceedings towards a natural classification of the worldwide taxa Phellinus s.l. and Inonotuss.l., and phylogenetic relationships of allied genera. Mycologia, 94, 998–1016. https://doi.org/10.1080/15572536.2003.11833156 [PubMed] [Google Scholar]
- White, T. J., Bruns, T., Lee, S., & Taylor, J. (1990) .Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis, M. A., Gelfand, D. H., Sninsky, J. J., & White, T. J. (Eds.), PCR protocols: a guide to methods and applications (pp. 315–322). NY: Academic Press. https://doi.org/10.1016/b978-0-12-372180-8.50042-1 [Google Scholar]
- Zhou, L. W. (2014) .Fulvifomes hainanensis sp. nov. and F. indicus comb. nov. (Hymenochaetales, Basidiomycota) evidenced by a combination of morphology and phylogeny. Mycoscience, 55, 70–77. http://dx.doi.org/10.1016/j.myc.2013.05.006 [Google Scholar]
- Zhou, L. W. (2015) .Fulvifomes imbricatus and F. thailandicus (Hymenochaetales, Basidiomycota): two new species from Thailand based on morphological and molecular evidence. Mycological Progress, 14, 89. http://dx.doi.org/10.1007/s11557-015-1116-1 [Google Scholar]