Abstract
The pathophysiology of chronic kidney disease–mineral and bone disorder accounts for an inverse relationship between bone mineralization and vascular calcification in progressive nephropathy. Inverse associations between bone mineral density (BMD) and calcified atherosclerotic plaque are also observed in individuals of European and African ancestry without nephropathy, suggesting a mechanistic link between these processes that is independent of kidney disease. Despite lower dietary calcium intake and serum 25-hydroxyvitamin D (25(OH)D) concentrations, African Americans have higher BMD and develop osteoporosis less frequently than do European Americans. Moreover, despite having more risk factors for cardiovascular disease, African Americans have a lower incidence and severity of calcified atherosclerotic plaque formation than do European Americans. Strikingly, evidence is now revealing that serum 25(OH)D and/ or 1,25 dihydroxyvitamin D levels associate positively with atherosclerosis but negatively with BMD in African Americans; by contrast, vitamin D levels associate negatively with atherosclerosis and positively with BMD in individuals of European ancestry. Biologic phenomena, therefore, seem to contribute to population-specific differences in vitamin D metabolism, bone and vascular health. Genetic and mechanistic approaches used to explore these differences should further our understanding of bone–blood vessel relationships and explain how African ancestry protects from osteoporosis and calcified atherosclerotic plaque, provided that access of African Americans to health care is equivalent to individuals of European ethnic origin. Ultimately, in our opinion, a new mechanistic understanding of the relationships between bone mineralization and vascular calcification will produce novel approaches for disease prevention in aging populations.
Introduction
The association between metabolic bone disease and vascular calcification is widely reported in patients with advanced nephropathy. Emerging evidence supports relationships between susceptibility to osteoporosis and calcified atherosclerotic plaque in the absence of kidney disease. This Review summarizes the evidence indicating that maintenance of bone and vascular health is biologically linked and discusses the marked racial differences in susceptibility to low bone density and athero sclerosis. African Americans, despite lower dietary calcium ingestion and serum 25 hydroxyvitamin D (25(OH)D) levels, are less likely to develop osteo porosis than are European Americans. Similarly, African Americans develop lower levels of calcified atherosclerotic plaque than do European Americans, despite greater exposure to conventional cardiovascular disease risk factors (for example, hypertension and diabetes mellitus). Potential biologic mechanisms and genetic susceptibility to development of bone and vascular disease are also discussed here. Moreover, we describe the processes of calcium and vitamin D metabolism as well as novel risk factors that could mediate crosstalk between blood vessels and bone health in individuals with preserved kidney function.
CKD, bone and vascular calcium
Chronic kidney disease–mineral and bone disorder (CKD–MBD) provides a mechanistic explanation of the events that link progressive nephropathy with the development of low bone mineralization and vascular calcification.1 A central component of CKD–MBD is phosphate retention, which leads to an elevation of fibroblast growth factor 23 (FGF-23) levels, as early as chronic kidney disease (CKD) stage 2. Hyperphosphatemia may worsen if hypocalcemia leads to parathyroid hormone (PTH)-induced increases in bone resorption, which releases calcium from bone, but also liberates additional phosphate from the bone calcium–phosphate reservoir. This model, however, remains under debate.2 Although bone mineral density (BMD) and vascular calcification are tightly linked in patients with CKD, this process is best viewed as the response of bone and systemic vasculature to an overarching environmental insult—progressive kidney failure. Individual patients with CKD have their own inherent genetic susceptibility to osteoporosis and atherosclerosis; however, the environmental effects of CKD could be a more powerful risk factor than innate genetic risk. Ultimately, in CKD, hyperphosphatemia seems to drive osteoporosis and arterial calcification.
Atherosclerosis and bone health
Inverse relationships between skeletal and vascular calcification are clearly present in individuals with preserved kidney function. In 1964, Anderson et al.3 reported that atherosclerotic calcification of the aorta accompanied osteoporosis in individuals who were routinely assessed in bone clinics. This observation supported the hypothesis proposed by Elkeles in 1957 that a relationship existed between osteoporosis and vascular calcification, particularly in postmenopausal women.4 These sentinel reports were verified nearly 30 years later in European, European American and African American populations.5–13 A major issue involved determining whether these phenomena were related or simply reflected the simultaneous effects of aging on bone and blood vessels. Two longitudinal studies strongly indicated that bone metabolism and calcified atherosclerotic plaque formation are mechanistically related.6,11 Further support was provided by cross-sectional studies, as the observed inverse associations between BMD and calcified atherosclerotic plaque persisted after adjustment for age and conventional cardiovascular disease (CVD) risk factors such as BMI and LDL cholesterol.12,13
Animal models of osteoporosis also provided evidence that genetic mechanisms underlie the relationships between BMD and calcified atherosclerotic plaque. Indeed, mice in which genes that encode inhibitors of mineralization are not expressed have important vascular phenotypes.14 For example, mice that do not express matrix Gla protein (encoded by Mgp), osteo pontin (Spp1), fetuin A (also known as α-2-HS glyco protein; encoded by Ahsg), klotho (Kl), and osteoprotegerin (also known as TNFRSF11B; encoded by TnfrsfF11b) demonstrate not only bone, cartilage, and skeletal abnormalities, but also arterial calcification.15–19 These mice generally have normal kidney function, with the exception of the Ahsg deficient mouse, in which accelerated vascular calcification precedes development of CKD.17 Interestingly, circulating osteoprotegerin concentrations are elevated in humans with high levels of coronary and aortic calcified atherosclerotic plaque.20 This observation seems to contradict studies using the TnfrsfF11b knockout mouse, in which the mice had elevated vascular calcification. This relationship is suggestive of a U-shaped response curve, whereby an optimal level of local TnfrsfF11b expression has an inhibitory effect on the development of arterial calcification, but where calcification can occur below or above this level. Moreover, we predict that high levels of circulating osteoprotegerin could occur as a response to ectopic mineralization or be related to a bone metabolic problem. Nonetheless, these results strongly indicate the presence of biologic relationships between bone and blood vessel health in the absence of CKD (Figure 1).
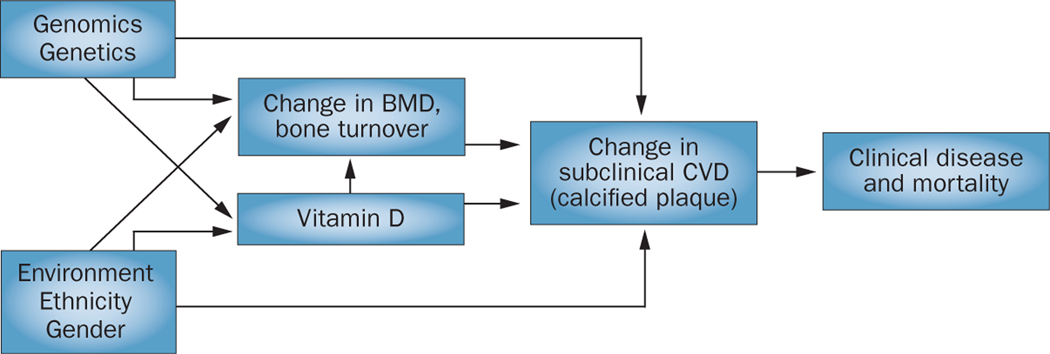
Figure 1 |.
Relationships between bone and vascular calcium and phosphate deposition and dependence upon the environment, ethnicity, sex, and genetic factors. Abbreviations: BMD, bone mineral density; CVD, cardiovascular disease.
Proposed mechanistic links
Although calcified atherosclerotic plaques have a morpho logic appearance similar to those observed in bone formation,21 the events that contribute to the initial calcium phosphate (CaPi) precipitation and hydroxyapatite formation in the artery are poorly understood. A number of models, from passive to active, have been proposed to explain these initial events, and are described below (Figure 2).22–25 The microenvironment of advanced atherosclerotic lesions resembles hyper trophic cartilage in the endochondral growth plate prior to mineralization during long bone development. Cell death, alkaline phosphatase, acidic CaPi complexes, and anoxia are all present in areas that will soon mineralize and form immature CaPi mineral complexes.26–31 Thus, atherosclerotic plaque calcification might be initiated locally in the arterial wall, perhaps independently from bone metabolism.
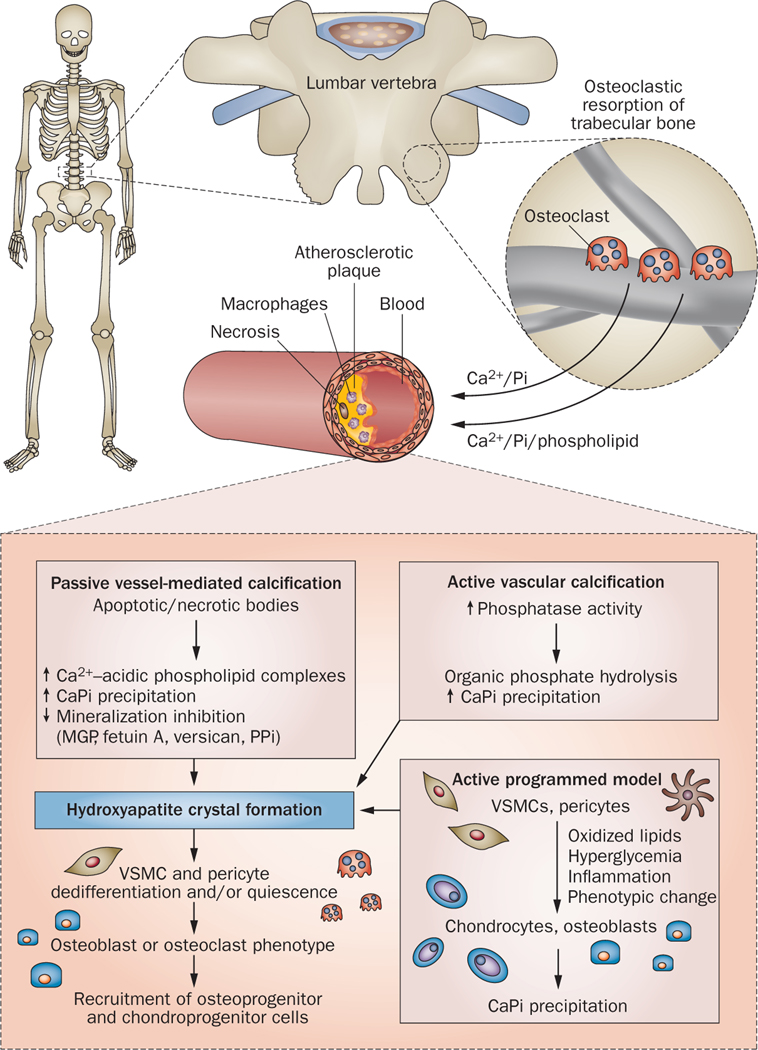
Figure 2 |.
Proposed mechanistic links between metabolic bone disease, atherosclerosis, and vascular calcification. Passive, vessel-mediated calcification in an advanced atherosclerotic lesion may involve apoptosis and/or necrosis of cells in core regions of plaques, which results in the development of an area rich in acidic phospholipids complexed with Ca2+, and/or an elevated local CaPi ion product. CaPi precipitation could then lead to the formation and growth of amorphous CaPi and eventually to hydroxyapatite crystals in the vessel. The loss of local inhibitors of mineralization could remove the block to local crystal formation and growth and allow mineralization to progress. The presence of plaque mineral crystals could then facilitate a process whereby local cells dedifferentiate and/or become quiescent and assume a phenotype consistent with the bone remodeling process (that is, osteoblasts and/or osteoclasts), or lead to recruitment of pluripotent circulating cells (osteoprogenitor or chondroprogenitor cells) to the site. More active models of vascular calcification involve increased activity of local enzymes (phosphatases), which catalyze amorphous CaPi precipitation and crystal formation through increases in free inorganic phosphate generated through hydrolysis of organic phosphates. Active programmed models in which resident vascular cells independently assume a phenotype consistent with mineralizing cells (chondrocytes or osteoblasts) and initiate the initial precipitation of CaPi have also been suggested. This phenotypic change might occur, in part, in response to oxidized lipids, hyperglycemia, or inflammation in the vessel wall. Postulated mechanisms linking bone loss directly to mineralization in the artery include the release of Ca2+ and Pi from bone, perhaps in the form of CaPi complexes, which then localize to atherosclerosis-prone vascular sites where they form a nidus for future mineralization or lead to locally elevated Ca2+ and/or Pi levels. These increased levels would then promote spontaneous CaPi precipitation or growth of arterial CaPi precipitates or hydroxyapatite. Abbreviations: CaPi, calcium phosphate; MGP, matrix Gla protein; PPi, pyrophosphate; VSMC, vascular smooth muscle cell.
Passive models
Simple passive models of vessel-mediated calcification involve apoptosis and/or necrosis of cells in core regions of plaques, resulting in the development of an area rich in acidic phospholipids complexed with Ca2+, and/or simply an elevated local Ca*Pi ion product, perhaps from intracellular sarcoplasmic reticulum and mitochondrial Ca2+ stores in dead or dying cells. Nucleated or spontaneous CaPi precipitation could then lead to the formation and growth of amorphous CaPi and, eventually, hydroxyapatite crystals in the blood vessel. 22–25
Alternatively, or perhaps in conjunction with the above scenario, the loss of local inhibitors of mineralization, including γ-carboxyglutamic-acid-containing proteins (for example, matrix Gla protein), fetuin A, acidic proteoglycan aggregates (such as versican core protein, which act as calcium sequestrants) and pyrophosphate or other polyphosphates, which can act as crystal poisons, could remove the block to local crystal formation and growth and enable mineralization to progress. The presence of plaque mineral crystals could then cause local cells to dedifferentiate and/or become quiescent,31 or lead to the recruitment of pluripotent circulating cells (that is, osteoprogenitor or chondroprogenitor cells) to the site of calcification, resulting in a tissue phenotype with similarities to bone by the presence of cells with osteoblastic and/or osteoclastic appearances.
Active models
Active models of vascular calcification involve increased activity of local phosphatases which catalyze CaPi precipitation and crystal formation through increases in free inorganic phosphate generated through hydrolysis of organic phosphates such as nucleoside diphosphates and pyrophosphates, leading to a local elevation in the Ca*Pi ion product. Active programmed models in which resident vascular cells (including pericytes and vascular smooth muscle cells [VSMCs]) independently (in absence of pre-existing mineral) acquire a phenotype similar to that of mineralizing cells (that is, chondrocytes or osteoblasts) and initiate the initial precipitation of CaPi have also been suggested.32 This phenotypic change has been postulated to occur, in part, in response to oxidized lipids, hyperglycemia, or inflammation in the vessel wall—conditions that have been shown to promote mineral formation in vitro in cultured VSMCs.21,24,25,32
Postulated mechanisms that directly link bone loss with calcification in the artery include the release of Ca2+ and phosphate from bone, perhaps in the form of CaPi complexes, via increased osteoclast-mediated resorptive processes.32 The released mineral ions and/or complexes may then localize to vascular sites that are susceptible to atherosclerosis where they form a nidus for future mineralization or lead to locally elevated Ca2+ and/or phosphate levels, promoting spontaneous CaPi precipitation or growth of arterial CaPi precipitates. In the absence of inhibitors of vascular calcification (such as fetuin or matrix Gla protein), the growth of these precipitates is likely to occur regardless of the mechanism underlying the initial CaPi precipitate. Substantial evidence suggests that the elevation of phosphate levels is important in the initiation and propagation of plaque mineral.33 Clearly, the inverse relationships between bone mineralization and vascular calcification indicate that a net transfer of mineral ions from bone to mineralization-prone sites occurs in arteries. Sex hormones, calciotropic hormones, calcification inhibitors, cell differentiation machinery, and inflammatory factors have been suggested as being important in these models and may underlie, in part, the observed ethnic and sex differences in bone mineralization and vascular calcification.
Ethnicity and serum vitamin D
Consistent inverse relationships are seen between bone mineralization and vascular health in diverse population groups of both African and European ethnic origin. Despite these common relationships, interesting and unique population-specific characteristics are also present, which require detailed investigation. Serum 25(OH)D concentrations are markedly lower in individuals of African ancestry than in those of European ancestry,34–36 which might be attributable to the association of darker skin pigmentation with a low efficiency of vitamin D activation.37 However, this hypothesis fails to account for a multitude of potentially related biologic observations. If individuals with African ancestry truly had higher frequencies of 25(OH)D deficiency, this effect should, theoretically, lead to a lower BMD and put them at higher risk of osteoporosis than individuals of European ancestry; data from epidemiologic and clinical studies conducted in the USA have, however, revealed the opposite trends.38,39 Indeed, despite lower 25(OH)D levels and generally lower dietary calcium ingestion, African Americans have greater bone mass, higher bone density and lower rates of osteoporosis than do European Americans.38,39 Therefore, the lower plasma 25(OH)D levels observed in African Americans might be physiologically normal. In our opinion, the normal range of 25(OH)D levels in individuals with African ancestry is likely to be lower than in individuals of European origin35,36 and, as such, the appropriate ‘normal’ range for 25(OH)D levels needs to be determined in different population groups.40–42 In addition, young adult African Americans have higher levels of active (intact PTH and 1,25 dihydroxyvitamin D (1,25(OH)2D) and increased renal tubular calcium reabsorption than do European Americans.43–45 These factors are also likely to contribute to racial differences in susceptibility to bone disease.
In agreement with the recognized racial differences in the risk of developing calcified athero sclerotic plaque and osteoporosis, the association of serum vitamin D (1,25(OH)2D) and vitamin D precursor or metabolite levels with these disease processes differ in different populations (Box 1). BMD and calcified athero sclerotic plaque have negative relationships in both African Americans and European Americans; however, vitamin D and/or vitamin D precursor levels are positively associated with BMD in European Americans and negatively associated in African Americans.42,46 In addition, 25(OH)D levels were negatively associated with calcified atherosclerotic plaque in European Americans and positively associated in African Americans.42,46 These findings strongly indicate that biologic differences exist across population groups. We believe that osteoporosis favors the development of atheromatous intimal calcification, which is associated with an increased risk of CVD events and death in the presence of increasing coronary artery calcified atherosclerotic plaque.47,48 Medial calcification is commonly present in patients with CKD and/or diabetes; however, whether low BMD favors development of medial calcification remains unknown.
Box 1 |. Relationship between vitamin D, BMD and atherosclerosis by ethnicity.
BMD and calcified atherosclerotic plaque are inversely related in patients without nephropathy
Serum vitamin D levels are positively associated with calcified atherosclerotic plaque in African Americans and negatively associated in European Americans
Serum vitamin D levels are negatively associated with BMD in African Americans and positively associated in European Americans
Abbreviation: BMD, bone mineral density.
Current 25(OH)D target ranges are based largely on reference ranges set in populations of European origin. As mentioned above, this ‘normal’ range is likely to differ in African Americans. The African American–Diabetes Heart Study reported that higher ambient 25(OH)D serum levels were associated with higher levels of vascular calcified atherosclerotic plaque in a cross-sectional analysis; the opposite trend is observed in populations of European ancestry.46 This observation may cause concern regarding the safety of providing vitamin D supplements for presumed low vitamin D levels observed in African Americans. Aloia et al.49 supplemented 25(OH)D in calcium-replete postmenopausal African American women with osteopenia and found no changes in BMD even after 2 years, suggesting that replacing 25(OH)D to the desired levels in European American women was ineffective in African Americans. Additional evidence for population specific ranges for optimal circulating vitamin D levels is provided by our paradoxical observation that higher vitamin D levels were associated with lower BMD in African Americans.46 This initially surprising finding is supported by observations of higher fracture rates with higher 25(OH)D levels in African American participants in the Women’s Health Initiative (WHI), an observation directly opposing that seen in European American WHI participants.50 In concert with the racially variable relationships between 25(OH)D, bone and vascular health, the 2011 Institute of Medicine Report recommends vitamin D supplements only for maintaining bone health; benefits on cardiovascular health and cancer risk remain unproven.42 We feel that the optimal 25(OH)D levels in African Americans are likely to be lower than those in European Americans.35 At present, supplemental 25(OH)D in African Americans with levels <30 ng/ml does not seem to be indicated in those without osteopenia or osteoporosis.42
Vitamin D has been associated with both inhibition and progression of vascular calcification.51 Levels of 1,25(OH)2D in African Americans are typically equal or higher than those in European Americans. These increased levels could be a protective factor against calcified atherosclerotic plaque and osteoporosis in African-derived populations, although no evidence for such a relationship was found in our studies. Some evidence exists to suggest that higher administered doses of active vitamin D may underlie improved survival rates in African Americans with end-stage renal disease (ESRD), relative to European Americans.52,53 Clearly, the mechanisms underlying racial differences in vitamin D handling and tissue-specific responses require additional study.
Ethnicity and calcium metabolism
The existing literature provides evidence that systemic calcium, vascular, and bone metabolism are differentially regulated between populations of African and European origin (Table 1). Observations of racial differences in susceptibility to osteoporosis and vascular calcification date back to 1968.54 In addition to markedly lower 25(OH)D levels, African Americans also ingest less dietary calcium than European Americans,55 perhaps in part, owing to lactase deficiency resulting in reduced ingestion of dairy products.56 Lactase deficiency in African Americans often relates to variation in genes regulating lactose metabolism.57 Despite this reduced calcium intake, however, African Americans have lower rates of osteoporosis than do European Americans and show skeletal resistance to the effects of PTH, potentially contributing to lower rates of bone resorption.58 Lower rates of calcium-containing kidney stone formation are also seen in African Americans when compared with European Americans.59 In a small study of healthy individuals and patients with CKD, postprandial phosphorus and calcium excretion were lower in African Americans than in European Americans with and without CKD, whereas FGF-23 and PTH levels were similar in both populations.60 Polymorphisms in VDR, the gene that encodes the vitamin D3 receptor, affect PTH levels in patients with nephropathy;61 genetic variation in VDR could therefore be responsible for the observed differences between African Americans and European Americans.45 As discussed above, ethnic differences are present in the circulating levels of factors and/or hormones regulating bone and blood vessel mineralization. Moreover, differences in the tissue response to such factors and hormones might also exist. Indeed, Shao et al.62 revealed that the bone and vascular tissue in mice with diabetes and deficient in the LDL receptor responded differently to exogenous PTH administration—PTH reduced vascular calcification but enhanced bone mineralization in these knockout mice. Thus, bone tissue and blood vessels could also respond differently to regulatory factors in African Americans and European Americans; relationships between PTH and BMD or calcified atherosclerotic plaque, however, were not observed in our prior report.13
Table 1 |.
Factors that potentially affect racial variation in bone and vascular disease
| Parameter | African Americans | European Americans |
|---|---|---|
| Calcified atherosclerotic plaque | ↓ | ↑ |
| Osteoporosis | ↓ | ↑ |
| Serum 25 hydroxyvitamin D | ↓ | ↑ |
| Serum 1,25 dihydroxyvitamin D | ↑ | ↓ |
| Serum intact parathyroid hormone | ↑ | ↓ |
| Dietary calcium ingestion | ↓ | ↑ |
| Renal tubular calcium reabsorption | ↑ | ↓ |
| Calcium containing kidney stones | ↓ | ↑ |
Abbreviations: ↑, increased risk of the specified parameter; ↓, decreased risk of the specified parameter.
Numerous unique metabolic characteristics related to calcium and phosphate handling distinguish African Americans from European Americans; these characteristics may have a genetic basis. A genome-wide association study performed in Icelandic and Dutch Europeans revealed that variants in CLDN14, which encodes Claudin-14, were associated with susceptibility to calcium nephrolithiasis and increased urinary calcium excretion (with lower serum bicarbonate concentrations), as well as lower BMD in the hip and spine. These observations thereby link genes that encode proteins involved in renal calcium metabolism and those involved in bone health.63
The variable risk of developing calcified atherosclerotic plaque between African Americans and European Americans is considered to be one of the most striking biologic effects related to differences in calcium metabolism between populations.64–68 It is widely reported that African Americans in the general population are at higher risk of myocardial infarction and CVD events than are European Americans.69 This increased risk likely reflects higher rates of conventional CVD risk factors in African Americans, including higher rates of hypertension and diabetes (with associated poorer regulation of blood pressure and diabetes), higher rates of albuminuria and subclinical nephropathy, and generally more restricted access to health care and lower socioeconomic status.
A different clinical pattern emerged when access of African Americans and European Americans to health care was equalized: African Americans with diabetes had approximately 50% lower rates of myocardial infarction compared with European Americans with diabetes in the Veteran’s Administration and Kaiser Permanente Healthcare Systems.70,71 These landmark studies recruited nearly 500,000 patients with diabetes and the results were consistent between reports. Although significantly higher rates of subclinical nephropathy were detected in African Americans in both of these healthcare system reports, CVD rates were significantly lower in African Americans. These results parallel those in patients receiving Medicare-supported renal replacement therapy.72 African Americans undergoing dialysis (who tend to receive equivalent access to medical care as European Americans) have markedly lower rates of myocardial infarction and longer survival rates than do European Americans.72–74 However, one report found poorer survival of young African American men (aged 18–30 years) than of European men at this age.75 Racial differences in susceptibility to CVD are likely to contribute to the lower mortality rates observed in African Americans with ESRD.76
Shared risk of CVD and low BMD
Equivalent access to health care might enable detection of biologic differences favoring lower rates of athero sclerosis (improved vascular health) in African Americans. Nearly all published reports reveal that African Americans have substantially lower rates of calcified atherosclerotic plaque than do European Americans. This phenomenon is not unique to patients with diabetes; populations with and without diabetes mellitus clearly reveal this effect.64–68 Even the large population-based Dallas Heart Study, one of the few reports that failed to observe lower levels of calcified atherosclerotic plaque in African Americans relative to European Americans, reported similar levels of calcified atherosclerotic plaque in African and European Americans despite excessive frequencies of conventional CVD risk factors in African Americans.77 Conventional CVD risk factors including hypertension, diabetes mellitus, smoking, and LDL-cholesterol levels fail to explain racially variable patterns of calcified atherosclerotic plaque.78 Novel CVD risk factors, including genetic polymorphisms, albuminuria, and pericardial adipose tissue, however, do seem to have a role.79–81 A landmark report by the Multi-Ethnic Study of Atherosclerosis (MESA) investigators demonstrated that African Americans with higher levels of calcified atherosclerotic plaque had significantly higher degrees of European ancestry than those with lower levels of calcified atherosclerotic plaque.79 These findings reverse the long-held belief that African Americans face a higher biologic risk of calcified atherosclerosis than do European Americans; these individuals are actually at lower biologic (inherited) risk, although the effect may be overcome by adverse environmental exposures. Similar results have been observed in the African American-Diabetes Heart Study (B. I. Freedman, unpublished work).
Two studies have provided proof-of-concept that circulating 25(OH)D, as well as susceptibility to calcified atherosclerotic plaque, may have a genetic basis. A genome-wide association study of serum 25(OH)D levels identified polymorphisms in the enzyme 1,25-dihydroxy vitamin D3 24-hydroxylase (encoded by the mitochondrial gene CYP24A1 on chromosome 20) as being significantly associated with 25(OH)D deficiency.82 CYP24A1 is involved in the degradation of 1,25-dihydroxyvitamin D3. In the same year, variation in CYP24A1 was associated with levels of coronary artery calcified atherosclerotic plaque.83 Hence, genes regulating vitamin D metabolism are associated with calcified atherosclerotic plaque. We investigated the potential role of three genes involved in bone formation in BMD and calcified atherosclerotic plaque. Several polymorphisms in the bone morphogenetic protein 7 gene (BMP7), but not in BMP2 or BMP4, were associated with an inverse relationship between BMD and calcified atherosclerotic plaque in European Americans with type 2 diabetes.84 Together, these studies suggest that an inverse association between BMD and calcified atherosclerotic plaque is biologically mediated and is not solely attributable to the effects of aging or shared environmental exposures.
Admixture mapping
African Americans are an admixed population group— their genomes are composed of approximately 80% African and 20% European ancestry. When continental isolation ended with forced migration during the slave trade, mixing of the African genome with European, Caribbean and other genomes ensued. This phenomenon led to the currently observed admixture in African Americans. The novel genetic mapping methodology admixture mapping (also called Mapping by Admixture Linkage Disequilibrium, MALD) can be useful in the setting of an admixed population group with differential disease risk in parental populations.85,86 MALD employs carefully selected genetic markers with markedly different frequencies between ancestral populations. The frequencies of these ancestry informative markers are compared across the genome in admixed cases with disease versus admixed controls without disease. Case-only studies can also be performed. Frequency differences in markers are compared between cases and controls to detect regions with excess (or reduced) frequencies of ancestral markers in given chromosomal regions. Associated disease genes likely reside under MALD peaks.
The most impressive discovery of a disease-associated gene using MALD was identification of the association between the genes that encode myosin-9 (MYH9) and apolipoprotein L1 (APOL1) with nondiabetic forms of nephropathy in African Americans.87–89 African Americans have higher rates of nondiabetic nephropathy including focal segmental glomerulosclerosis (FSGS), HIV-associated collapsing glomerulopathy, and hypertension-attributed nephropathy than do European Americans.90 Socioeconomic factors, conventional risk factors for renal disease (including prevalence and severity of hypertension and HIV infection) failed to account for this higher frequency of nephropathy in African Americans. Marked familial aggregation of nondiabetic ESRD is observed in African Americans.91,92 As such, MALD identified a region on chromosome 22q with a significant 10% excess frequency of African ancestry among African American cases with FSGS compared with African American non-nephropathy controls. Fine mapping under this peak subsequently identified the major APOL1 association with nondiabetic ESRD in African Americans, with odds ratios of 7.3, 17, and 29, for hypertension-attributed ESRD, idiopathic FSGS, and HIV-associated nephropathy; these genetic associations are among the most powerful in complex human disease.89,93
These findings provide a strong rationale for using this genetic methodology to detect genes simultaneously regulating bone and vascular health in African Americans. Candidate gene approaches are limited by the need to test several hundred genes that are potentially involved in disease progression, as well as by our limited understanding of the pathophysiology of these syndromes.94 Not only could genes such as MGP, SPP1, AHSG, KL and TNFRSF11B have a role in human disease as they do in animal models, but their receptors and signaling pathways could also be involved. It is far more appropriate to perform unbiased genome-wide mapping for these disorders. We believe that applying MALD mapping to the complex and related phenotypes of BMD and calcified atherosclerotic plaque in the admixed African American population has great potential in this regard.
As African Americans have biologically lower rates of calcified atherosclerotic plaque and osteoporosis than do European Americans, both of these related disorders seem to originate from European ancestry.79 As such, we expect MALD to detect higher frequencies of European ancestry markers in chromosomal regions harboring disease-associated genes in African Americans who have low BMD or high levels of calcified atherosclerotic plaque. Indeed, MESA identified an excess frequency of European ancestry in African Americans with high levels of calcified atherosclerotic plaque.79 Based on the results of animal and human studies, it seems likely that polymorphisms in genes that regulate bone mineralization, calcium, phosphate, and vitamin D metabolism are involved in susceptibility to both calcified atherosclerotic plaque and osteoporosis in individuals without advanced nephropathy.
Conclusions
Recognition of population-specific differences in susceptibility to osteoporosis and calcified atherosclerotic plaque among individuals without advanced nephropathy is an important first step towards designing studies that will enable identification of mechanisms linking bone mineralization and vascular calcification, particularly in African Americans. This research provides insights into potential pathways, which may be targeted to improve health outcomes in many ethnic groups. This work is particularly important with respect to our increasingly aging populations who are at high risk of developing these debilitating conditions.
Key points.
Inverse relationships exist between bone mineral density (BMD) and calcified atherosclerotic plaque in individuals of European and African ancestry without nephropathy
African Americans have higher BMD and develop osteoporosis less often than do European Americans, despite lower serum 25-hydroxyvitamin D levels and dietary calcium intake
European Americans have higher incidence rates and severity of calcified atherosclerotic plaque relative to African Americans despite fewer cardiovascular disease risk factors
Inherited phenomena are likely to contribute to population-specific differences in vitamin D metabolism, vascular calcification, and bone mineralization
Molecular genetic approaches will enhance our understanding of bone and vascular health
Acknowledgments
The authors wish to thank D. W. Bowden, C. D. Langefeld, J. Divers, J. J. Carr, and N. Allred for their assistance with this work. This project was funded in part by NIH grant DK071891.
Footnotes
Competing interests
The authors declare no competing interests.
Contributor Information
Barry I. Freedman, Department of Internal Medicine, Section on Nephrology, Wake Forest School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157-1053, USA.
Thomas C. Register, Department of Pathology, Wake Forest School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157-1053, USA.
References
- 1.Hruska KA et al. Cardiovascular risk in chronic kidney disease (CKD): the CKD-mineral bone disorder (CKD-MBD). Pediatr. Nephrol 25, 769–778 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Isakova T. et al. Postprandial mineral metabolism and secondary hyperparathyroidism in early CKD. J. Am. Soc. Nephrol 19, 615–623 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson J, Barnett E. & Nordin BE The relation between osteoporosis and aortic calcification. Br. J. Radiol 37, 910–912 (1964). [DOI] [PubMed] [Google Scholar]
- 4.Elkeles A. A comparative radiological study of calcified atheroma in males and females over 50 years of age. Lancet 273, 714–715 (1957). [DOI] [PubMed] [Google Scholar]
- 5.Browner WS et al. Association between low bone density and stroke in elderly women. The study of osteoporotic fractures. Stroke 24, 940–946 (1993). [DOI] [PubMed] [Google Scholar]
- 6.Kiel DP et al. Bone loss and the progression of abdominal aortic calcification over a 25 year period: the Framingham Heart Study. Calcif. Tissue Int 68, 271–276 (2001). [DOI] [PubMed] [Google Scholar]
- 7.Marcovitz PA et al. Usefulness of bone mineral density to predict significant coronary artery disease. Am. J. Cardiol 96, 1059–1063 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Ness J. & Aronow WS Comparison of prevalence of atherosclerotic vascular disease in postmenopausal women with osteoporosis or osteopenia versus without osteoporosis or osteopenia. Am. J. Cardiol 97, 1427–1428 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Silverman SL et al. Comparison of fracture, cardiovascular event, and breast cancer rates at 3 years in postmenopausal women with osteoporosis. J. Am. Geriatr. Soc 52, 1543–1548 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Tanko LB et al. Relationship between osteoporosis and cardiovascular disease in postmenopausal women. J. Bone Miner. Res 20, 1912–1920 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Schulz E. et al. Aortic calcification and the risk of osteoporosis and fractures. J. Clin. Endocrinol. Metab 89, 4246–4253 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Carr JJ et al. Calcified atherosclerotic plaque and bone mineral density in type 2 diabetes: the diabetes heart study. Bone 42, 43–52 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Divers J. et al. Relationships between calcified atherosclerotic plaque and bone mineral density in African Americans with type 2 diabetes. J. Bone Miner. Res 26, 1554–1560 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofbauer LC et al. Vascular calcification and osteoporosis—from clinical observation towards molecular understanding. Osteoporos. Int 18, 251–259 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Luo G. et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 386, 78–81 (1997). [DOI] [PubMed] [Google Scholar]
- 16.Speer MY et al. Inactivation of the osteopontin gene enhances vascular calcification of matrix Gla protein-deficient mice: evidence for osteopontin as an inducible inhibitor of vascular calcification in vivo. J. Exp. Med 196, 1047–1055 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schafer C. et al. The serum protein α−2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J. Clin. Invest 112, 357–366 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuro-o M. et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390, 45–51 (1997). [DOI] [PubMed] [Google Scholar]
- 19.Bucay N. et al. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 12, 1260–1268 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abedin M. et al. Relation of osteoprotegerin to coronary calcium and aortic plaque (from the Dallas Heart Study). Am. J. Cardiol 99, 513–518 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Doherty TM et al. Genetic determinants of arterial calcification associated with atherosclerosis. Mayo Clin. Proc 79, 197–210 (2004). [DOI] [PubMed] [Google Scholar]
- 22.Abedin M, Tintut Y. & Demer LL Vascular calcification: mechanisms and clinical ramifications. Arterioscler. Thromb. Vasc. Biol 24, 1161–1170 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Demer LL & Tintut Y. Mineral exploration: search for the mechanism of vascular calcification and beyond: the 2003 Jeffrey M. Hoeg Award lecture. Arterioscler. Thromb. Vasc. Biol 23, 1739–1743 (2003). [DOI] [PubMed] [Google Scholar]
- 24.Giachelli CM et al. Regulation of vascular calcification: roles of phosphate and osteopontin. Circ. Res 96, 717–722 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Demer LL Vascular calcification and osteoporosis: inflammatory responses to oxidized lipids. Int. J. Epidemiol 31, 737–741 (2002). [DOI] [PubMed] [Google Scholar]
- 26.Irving JT & Wuthier RE, Histochemistry and biochemistry of calcification with special reference to the role of lipids. Clin. Orthop. Relat. Res 56, 237–260 (1968). [PubMed] [Google Scholar]
- 27.Cotmore JM, Nichols G Jr & Wuthier RE Phospholipid-calcium phosphate complex: enhanced calcium migration in the presence of phosphate. Science 172, 1339–1341 (1971). [DOI] [PubMed] [Google Scholar]
- 28.Ennever J, Vogel JJ & Riggan LJ Calcification by proteolipid from atherosclerotic aorta. Atherosclerosis 35, 209–213 (1980). [DOI] [PubMed] [Google Scholar]
- 29.Register TC, McLean FM, Low MG & Wuthier RE Roles of alkaline phosphatase and labile internal mineral in matrix vesicle-mediated calcification. Effect of selective release of membrane-bound alkaline phosphatase and treatment with isosmotic pH 6 buffer. Biol. Chem 261, 9354–9360 (1986). [PubMed] [Google Scholar]
- 30.Shanahan CM et al. Expression of mineralisation-regulating proteins in association with human vascular calcification. Z. Kardiol 89 (Suppl. 2), 63–68 (2000). [DOI] [PubMed] [Google Scholar]
- 31.Burton DG, Matsubara H. & Ikeda K: Pathophysiology of vascular calcification: pivotal role of cellular senescence in vascular smooth muscle cells. Exp. Gerontol 45, 819–824 (2010). [DOI] [PubMed] [Google Scholar]
- 32.Giachelli CM Vascular calcification mechanisms. J. Am. Soc. Nephrol 15, 2959–2964 (2004). [DOI] [PubMed] [Google Scholar]
- 33.Ellam TJ & Chico TJ Phosphate: the new cholesterol? The role of the phosphate axis in non-uremic vascular disease. Atherosclerosis 220, 310–318 (2012). [DOI] [PubMed] [Google Scholar]
- 34.Aloia JF et al. Optimal vitamin D status and serum parathyroid hormone concentrations in African American women. Am. J. Clin. Nutr 84, 602–609 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Acheson LS Bone density and the risk of fractures: should treatment thresholds vary by race? JAMA 293, 2151–2154 (2005). [DOI] [PubMed] [Google Scholar]
- 36.Aloia JF African Americans, 25-hydroxyvitamin D, and osteoporosis: a paradox. Am. J. Clin. Nutr 88, 545S–550S (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rostand SG Vitamin D, blood pressure, and African Americans: toward a unifying hypothesis. Clin. J. Am. Soc. Nephrol 5, 1697–1703 (2010). [DOI] [PubMed] [Google Scholar]
- 38.George A. et al. Racial differences in bone mineral density in older men. J. Bone Miner. Res 18, 2238–2244 (2003). [DOI] [PubMed] [Google Scholar]
- 39.Looker AC, Melton LJ III, Borrud LG & Shepherd JA Lumbar spine bone mineral density in US adults: demographic patterns and relationship with femur neck skeletal status. Osteoporos. Int 23, 1351–1360 (2012). [DOI] [PubMed] [Google Scholar]
- 40.Ross AC et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J. Clin. Endocrinol. Metab 96, 53–58 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shapses SA & Manson JE Vitamin D and prevention of cardiovascular disease and diabetes: why the evidence falls short. JAMA 305, 2565–2566 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ross AC, Taylor CL & Yaktine AL (Eds) Dietary reference intakes for calcium and vitamin D (The National Academies Press, Washington DC, 2011). [PubMed] [Google Scholar]
- 43.Bell NH et al. Evidence for alteration of the vitamin D-endocrine system in blacks. J. Clin. Invest 76, 470–473 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bell NH et al. Demonstration of a difference in urinary calcium, not calcium absorption, in black and white adolescents. J. Bone Miner. Res 8, 1111–1115 (1993). [DOI] [PubMed] [Google Scholar]
- 45.Hustmyer FG, DeLuca HF & Peacock M. ApaI, BsmI, EcoRV and TaqI polymorphisms at the human vitamin D receptor gene locus in Caucasians, blacks and Asians. Hum. Mol. Genet 2, 487 (1993). [DOI] [PubMed] [Google Scholar]
- 46.Freedman BI et al. Vitamin D, adiposity, and calcified atherosclerotic plaque in African Americans. J. Clin. Endocrinol. Metab 95, 1076–1083 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Detrano R. et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N. Engl. J. Med 358, 1336–1345 (2008). [DOI] [PubMed] [Google Scholar]
- 48.Agarwal S. et al. Coronary calcium score and prediction of all-cause mortality in diabetes: the diabetes heart study. Diabetes Care 34, 1219–1224 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aloia JF, Talwar SA, Pollack S. & Yeh J. A randomized controlled trial of vitamin D3 supplementation in African American women. Arch. Intern. Med 165, 1618–1623 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cauley JA et al. Serum 25 hydroxyvitamin D and clinical fracture risk in a multiethnic cohort of women: the Women’s health initiative (WHI). J. Bone Miner. Res 26, 2378–2388 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davies MR & Hruska KA Pathophysiological mechanisms of vascular calcification in end-stage renal disease. Kidney Int. 60, 472–479 (2001). [DOI] [PubMed] [Google Scholar]
- 52.Wolf M. et al. Impact of activated vitamin D and race on survival among hemodialysis patients. J. Am. Soc. Nephrol 19, 1379–1388 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalantar-Zadeh K. et al. Impact of race on hyperparathyroidism, mineral disarrays, administered vitamin D mimetic, and survival in hemodialysis patients. J. Bone Miner. Res 25, 2724–2734 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dent CE, Engelbrecht HE & Godfrey RC Osteoporosis of lumbar vertebrae and calcification of abdominal aorta in women living in Durban. Br. Med. J 4, 76–79 (1968). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Newby PK et al. Race and region are associated with nutrient intakes among black and white men in the United States. J. Nutr 141, 296–303 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bersaglieri T. et al. Genetic signatures of strong recent positive selection at the lactase gene. Am. J. Hum. Genet 74, 1111–1120 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Swallow DM Genetics of lactase persistence and lactose intolerance. Annu. Rev. Genet 37, 197–219 (2003). [DOI] [PubMed] [Google Scholar]
- 58.Cosman F. et al. Resistance to bone resorbing effects of PTH in black women. J. Bone Miner. Res 12, 958–966 (1997). [DOI] [PubMed] [Google Scholar]
- 59.Stamatelou KK et al. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int. 63, 1817–1823 (2003). [DOI] [PubMed] [Google Scholar]
- 60.Gutierrez OM et al. Racial differences in postprandial mineral ion handling in health and in chronic kidney disease. Nephrol. Dial. Transplant 25, 3970–3977 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yokoyama K. et al. Apa I polymorphism in the vitamin D receptor gene may affect the parathyroid response in Japanese with end-stage renal disease. Kidney Int. 53, 454–458 (1998). [DOI] [PubMed] [Google Scholar]
- 62.Shao JS et al. Teriparatide (human parathyroid hormone (1–34)) inhibits osteogenic vascular calcification in diabetic low density lipoprotein receptor-deficient mice. J. Biol. Chem 278, 50195–50202 (2003). [DOI] [PubMed] [Google Scholar]
- 63.Thorleifsson G. et al. Sequence variants in the CLDN14 gene associate with kidney stones and bone mineral density. Nat. Genet 41, 926–930 (2009). [DOI] [PubMed] [Google Scholar]
- 64.Newman AB et al. Racial differences in coronary artery calcification in older adults. Arterioscler. Thromb. Vasc. Biol 22, 424–430 (2002). [DOI] [PubMed] [Google Scholar]
- 65.Freedman BI et al. The impact of ethnicity and sex on subclinical cardiovascular disease: the Diabetes Heart Study. Diabetologia 48, 2511–2518 (2005). [DOI] [PubMed] [Google Scholar]
- 66.Bild DE et al. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 111, 1313–1320 (2005). [DOI] [PubMed] [Google Scholar]
- 67.Carnethon MR et al. Racial/ethnic differences in subclinical atherosclerosis among adults with diabetes: the Multiethnic Study of Atherosclerosis. Diabetes Care 28, 2768–2770 (2005). [DOI] [PubMed] [Google Scholar]
- 68.Budoff MJ et al. Ethnic differences of the presence and severity of coronary atherosclerosis. Atherosclerosis 187, 343–350 (2006). [DOI] [PubMed] [Google Scholar]
- 69.American Diabetes Association. http://www.diabetes.org
- 70.Young BA, Maynard C. & Boyko EJ Racial differences in diabetic nephropathy, cardiovascular disease, and mortality in a national population of veterans. Diabetes Care 26, 2392–2399 (2003). [DOI] [PubMed] [Google Scholar]
- 71.Karter AJ et al. Ethnic disparities in diabetic complications in an insured population. JAMA 287, 2519–2527 (2002). [DOI] [PubMed] [Google Scholar]
- 72.Young BA et al. Racial and ethnic differences in incident myocardial infarction in end-stage renal disease patients: The USRDS. Kidney Int. 69, 1691–1698 (2006). [DOI] [PubMed] [Google Scholar]
- 73.Buckalew VM Jr & Freedman BI Effects of race on albuminuria and risk of cardiovascular and kidney disease. Expert Rev. Cardiovasc. Ther 9, 245–249 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Collins AJ et al. United States Renal Data System. USRDS 2011 Annual Data Report: Atlas of chronic kidney disease and end-stage renal disease in the United States. Am. J. Kidney Dis 59 (Suppl. 1), e1–e420 (2012). [DOI] [PubMed] [Google Scholar]
- 75.Kucirka LM et al. Association of race and age with survival among patients undergoing dialysis. JAMA 306, 620–626 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Buckalew VM & Freedman BI Reappraisal of the impact of race on survival in patients on dialysis. Am. J. Kidney Dis 55, 1102–1110 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jain T. et al. African Americans and Caucasians have a similar prevalence of coronary calcium in the Dallas Heart Study. J. Am. Coll. Cardiol 44, 1011–1017 (2004). [DOI] [PubMed] [Google Scholar]
- 78.Wagenknecht LE et al. Correlates of coronary artery calcified plaque in blacks and whites with type 2 diabetes. Ann. Epidemiol 21, 34–41 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wassel CL et al. Genetic ancestry is associated with subclinical cardiovascular disease in African-Americans and Hispanics from the multi-ethnic study of atherosclerosis. Circ. Cardiovasc. Genet 2, 629–636 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Divers J. et al. Ethnic differences in the relationship between pericardial adipose tissue and coronary artery calcified plaque: African-American-diabetes heart study. J. Clin. Endocrinol. Metab 95, 5382–5389 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Divers J. et al. Ethnic differences in the relationship between albuminuria and calcified atherosclerotic plaque: the African American-diabetes heart study. Diabetes Care 33, 131–138 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang TJ et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet 376, 180–188 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shen H. et al. Association of the vitamin D metabolism gene CYP24A1 with coronary artery calcification. Arterioscler. Thromb. Vasc. Biol 30, 2648–2654 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Freedman BI et al. Bone morphogenetic protein 7 (BMP7) gene polymorphisms are associated with inverse relationships between vascular calcification and BMD: the Diabetes Heart Study. J. Bone Miner. Res 24, 1719–1727 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith MW et al. A high-density admixture map for disease gene discovery in african americans. Am. J. Hum. Genet 74, 1001–1013 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Divers J, Moossavi S, Langefeld CD & Freedman BI Genetic admixture: a tool to identify diabetic nephropathy genes in African Americans. Ethn. Dis 18, 384–388 (2008). [PubMed] [Google Scholar]
- 87.Kopp JB et al. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat. Genet 40, 1175–1184 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kao WH et al. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat. Genet 40, 1185–1192 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Genovese G. et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329, 841–845 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Freedman BI et al. The Apolipoprotein L1 (APOL1) gene and nondiabetic nephropathy in African Americans. J. Am. Soc. Nephrol 21, 1422–1426 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ferguson R, Grim CE & Opgenorth TJ A familial risk of chronic renal failure among blacks on dialysis? J. Clin. Epidemiol 41, 1189–1196 (1988). [DOI] [PubMed] [Google Scholar]
- 92.Freedman BI, Spray BJ, Tuttle AB & Buckalew VM Jr. The familial risk of end-stage renal disease in African Americans. Am. J. Kidney Dis 21, 387–393 (1993). [DOI] [PubMed] [Google Scholar]
- 93.Kopp JB et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J. Am. Soc. Nephrol 22, 2129–2137 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Razzaque MS The dualistic role of vitamin D in vascular calcifications. Kidney Int. 79, 708–714 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]