Abstract
The treatment landscape for hemophilia has been rapidly changing with introduction of novel therapies. Gene therapy for hemophilia is a promising therapeutic option for sustained endogenous factor production to mitigate the need for prophylactic treatment to prevent spontaneous and traumatic bleeding. Etranacogene dezaparvovec is an investigational factor IX (FIX) gene transfer product that utilizes the adeno-associated virus (AAV) 5 vector with a liver-specific promoter and a hyperactive FIX transgene. Here, the development of etranacogene dezaparvovec and available efficacy and safety data from clinical trials are reviewed. Overall, etranacogene dezaparvovec provides sustained FIX expression for more than 2 years and allows for a bleed and infusion-free life in the majority of patients. Safety, efficacy, and quality-of-life data will inform shared decision-making for patients who are considering gene therapy. Long-term follow-up regarding duration of expression and safety are crucial.
Keywords: gene therapy, hemophilia, hemophilia B
Plain Language Summary
Factor IX Padua gene therapy to boost clotting factor and prevent bleeding for people living with hemophilia B
People living with hemophilia have low or missing clotting factor, which can lead to bleeding that is unexpected or caused by a traumatic event (such as a sports injury or surgery). There are two main types of hemophilia: clotting factor (F)VIII deficiency (known as hemophilia A) and FIX deficiency (known as hemophilia B). People living with the severe or moderately severe forms of hemophilia (clotting factor levels below 3% of normal) need regular treatment, typically by infusions into the vein, to stop or prevent bleeding and damage to their joints. Gene therapy is currently being investigated as a new treatment option that introduces a working copy of the clotting factor gene to the liver. Following treatment, clotting factor is produced by the liver. Etranacogene dezaparvovec [Et-ra-na-co-gene dez-a-par-vo-vec] is a form of gene therapy for people living with hemophilia B. This form of gene therapy includes a modified form of FIX (FIX Padua) which produces high levels of FIX activity compared with normal FIX. It is being tested to see whether individuals will have low rates of bleeding and not need to treat themselves with clotting factor. In the clinical trials, participants with FIX levels below 2% (of normal) receive a single gene therapy infusion. The results of the trials have so far shown that patients given etranacogene dezaparvovec have continuous production of FIX, whereby they have reported much less bleeding and factor treatment. Questions relating to the safety of the gene therapy and how long it works will hopefully be answered through long-term follow-up of the patients once the trials are completed.
Introduction
Hemophilia B [factor IX (FIX) deficiency] is an X-linked inherited bleeding disorder that affects 25,000 people globally. Hemophilia severity is classified as severe <1% factor activity, moderate 1–5% factor activity, and mild 6–40% factor activity. Bleeding complications are most significant in patients with moderate-to-severe hemophilia B (FIX ⩽ 2%) and include traumatic and spontaneous bleeding into joints and muscle (Table 1).1,2 Life-threatening internal bleeding may also occur. Recurrent bleeding into the joints results in painful and debilitating arthropathy which negatively impacts quality of life and is associated with high levels of health care utilization and financial investment. 3
Table 1.
| Hemophilia severity | Clotting factor level | Spontaneous bleeding in the absence of prophylaxis | Prophylaxis to prevent bleeding | Eligible for gene therapy clinical trials |
|---|---|---|---|---|
| Severe | <1 IU/dL | Frequent | Yes | Yes |
| Moderate | 1–5 IU/dL | Occasional | Yes, depending on bleeding phenotype | Yes, if clotting factor level 1–2 IU/dL |
| Mild | 5–40 IU/dL | Rare | Yes, depending on bleeding phenotype | No |
| Normal | >40 IU/dL | No | No | No |
IU/dL, international units per deciliter.
The current standard of care for hemophilia B is prophylactic replacement therapy with standard half-life (SHL) clotting factor infused 2–3 times weekly or extended half-life (EHL) clotting factor infused every 7–14 days. 1 Prophylaxis effectively reduces bleeding and ameliorates the bleeding phenotype, but it is associated with substantial treatment burden as well as cost over time. 4 Subcutaneous FIX replacement 5 and rebalancing therapies6–8 are under investigation. Regardless of prophylaxis type, patients still need factor replacement for breakthrough bleeds, trauma, and surgery.
The goal of gene therapy for hemophilia is to provide durable endogenous FIX production with FIX levels high enough such that prophylactic replacement may be discontinued, and factor replacement is not needed for high-intensity activities, surgery, trauma, or bleeding. Gene therapy has been estimated to be cost-effective compared with on-demand and prophylactic treatment in severe hemophilia B. 9
This review describes the development of gene transfer product etranacogene dezaparvovec and evaluates the clinical trial data currently available.
Overview of gene therapy for hemophilia B
Gene transfer for hemophilia B includes an adeno-associated virus 5 (AAV5) vector construct containing a transgene. The first AAV gene therapy was studied in humans for cystic fibrosis in 1998 10 and for hemophilia in 2000. 11 Vectors act as a highly ordered set of proteins that access receptors on cell surface and deliver DNA to nucleus. Advantages of AAV as a viral vector include low rate of host genome integration (⩽0.01%), ability to target a wide range of cells, transgene expression in non-dividing cells, and transgene expression over multiple years. 12
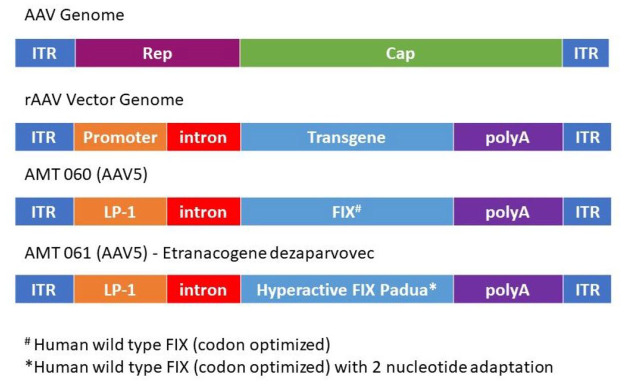
Wild-type AAVs (wtAAVs) include two inverted terminal repeats (ITRs) and rep (replication) and cap (capsid) genes (Figure 1). 12 Recombinant AAV (rAAV) are bioengineered for optimal gene transfer including self-complementary (sc) sequences to promote efficient transduction.13–15 rAAV vector genome includes the ITRs, and the rep and cap genes are replaced with a promoter, intron, transgene of interest, and polyadenylation (polyA) signal sequences (Figure 1). 12 AAV vectors are non-replicating. After transduction, the expression cassette forms a stable episomal DNA, which supports stable long-term gene expression.
Figure 1.
Schematic representation of AAV gene therapy. The wild-type AAV genome contains inverted terminal repeats (ITRs) and rep (replication) and cap (capsid) genes. To create a rAAV vector genome, rep and cap are replaced by a promoter, intron, and polyA genes, and the transgene of interest. In the case of the AAV5 vector for AMT-060, the transgene is human wild-type FIX (codon optimized) and the promoter is liver-specific promotor 1 (LP1) and SV40 intron. AMT-061 was created by replacing the hFIX transgene with a hyperactive FIX Padua.
Source: Adapted from Doshi et al. 12
A potential limitation of AAV vectors are anti-AAV neutralizing antibodies (NAbs). Natural exposure to wtAAV triggers a humoral immune response and antibody production. wtAAV NAbs cross-react with the rAAV capsid. NAb-coated vector cannot bind to the target cell receptor and vector transduction is blocked, limiting the efficacy of vector-based gene therapy.16–18 In addition to the humoral immune response, AAV capsid proteins displayed on transduced hepatocytes may trigger a vector dose-dependent cytotoxic T-cell response which results in clearance (apoptosis) of transduced hepatocytes and loss of transgene expression.17–19 In response to gene therapy, a humoral antibody response is expected in all recipients to the AAV vector serotype which limits repeat dosing. 20
The rate of preexisting NAbs varies by AAV serotype.21–23 The prevalence of NAbs for AAV5 is lower than for AAV2 and AAV8 which have been utilized in other gene therapy studies.24,25 Cross-reactivity may be less than with other serotypes since AAV5 has less capsid homology compared with other serotypes. In addition, the capsid-specific T-cell response with AAV5 appears to be lower than with other AAV serotypes.20,26
Development of etranacogene dezaparvovec (AMT-061)
AMT-060 (AAV5-wt-hFIX) is the predecessor to etranacogene dezaparvovec. It includes an AAV5 vector, and the F9 transgene includes a codon-optimized wt-hFIX under the control of a liver-specific promoter (LP1; Figure 1). The AAV5 vector was selected due to the immunologic advantages described above. This product was evaluated in a phase 1/2 study as described below.
To further optimize FIX expression and decrease the vector dose-dependent immune response, etranacogene dezaparvovec (AAV5-Padua hFIX) was developed via site-directed mutagenesis to include FIX Padua. FIX Padua differs from wt-hFIX by a substitution at position 338 (R338 L-Padua; Figure 1). FIX Padua is associated with elevated FIX levels and a faster rate of factor X activation. A thrombophilic phenotype is seen when FIX levels are supraphysiologic (>700% of normal).27,28 The efficacy of this hyperactive F9 transgene was initially demonstrated in mouse and dog models and then in early-stage clinical trials.29–31 Use of a higher potency FIX allows for lower vector dose to decrease the risk of vector dose-dependent immune response. Compared with other gene transfer products that use mammalian cells lines, etranacogene dezaparvovec utilizes an insect cell line and a baculovirus production system. 32 The baculovirus production system, first developed in the mid-1980s, is now an established system for manufacturing gene therapy vectors and the basis for several commercially available products. 33 The use of recombinant technology, large viral genome, inherits capacity of baculovirus to infect insect cells, and other features confer advantages compared with other platforms: faster manufacturing speed, flexible product design, safety features, and large-scale production. 33 Etranacogene dezaparvovec is manufactured per Good Manufacturing Practices.
Active clinical trials of AMT-060 and etranacogene dezaparvovec (AMT-061)
Clinical trial data are collected from peer-reviewed publications and scientific meeting abstracts. For meeting abstracts, see individual references plus meeting slides. 34 Publications report FIX activity in both percentage and international unit (IU)/dL. For the purposes of this article, all data are presented in IU/dL with 1% = 1 IU/dL.
In general, participants are eligible for the clinical trials if they are previously treated males aged 18 years and older and have FIX activity ⩽2 IU/dL of normal and severe bleeding phenotype. Participants are excluded if they have a history of inhibitor, active hepatitis B or C, uncontrolled human immunodeficiency virus (HIV) infection, advanced liver disease, or uncontrolled comorbidity.26,32,35
Efficacy endpoints include FIX activity levels measured by a central one-stage activated partial thromboplastin time (aPTT) assay, bleeding rates, factor usage, discontinuation of prophylaxis, and quality of life. Particular attention is paid to transaminitis requiring immunosuppression and/or resulting in decreased FIX expression. Safety endpoints include treatment-related adverse events (TRAEs), NAbs to AAV5, immunologic/inflammatory response, FIX inhibitor development, and vector shedding with detection of vector DNA in nasal secretions, saliva, feces, urine, semen, and whole blood.
NAbs to AAV5 are measured by a green-fluorescent protein (GFP)–based assay, 26 AAV5-luciferase transgene assay, and/or an enzyme-linked immunosorbent assay (ELISA) which detects both immunoglobulin (Ig)M and IgG antibodies. 16 T-cell response to AAV5 antigen is measured using an Enzyme-Linked ImmunoSpot (ELISPOT) assay of interferon-gamma production.
Trial participants are followed for up to 5 years post-treatment.
Evaluation of AMT-060
AMT-060 has been evaluated in a phase 1/2 dose-escalation multinational clinical trial (NCT-AMT-060-01; ClinicalTrials.gov identifier NCT02396342). 26 Participants included adult men with moderate (n = 1) or severe (n = 9) hemophilia B with severe bleeding phenotype. Participants were excluded for preexisting NAbs to AAV5 based on the GFP-based assay.
Per study protocol, prophylaxis was tapered and discontinued by 12 weeks if FIX was ⩾2 IU/dL. Tapering doses of prednisone was prescribed if participants had alanine aminotransferase (ALT) >1.5–2 times baseline. Five participants were treated with a vector dose 5 × 1012 genome copies (gc)/kg (low dose) in cohort 1. After dosing for cohort 1, five participants were treated with 2 × 1013 gc/kg (high dose) in cohort 2. Participants in cohort 1 were notably older. In addition, the participants in cohort 1 had more baseline arthropathy which is likely related to differences in prior treatment history (i.e. later initiation of prophylaxis in the older cohort). Participant characteristics plus 1-year and 5-year efficacy and safety data are presented in Table 2.26,36 Overall, FIX activity levels remained stable over the course of follow-up with change in disease severity from severe to mild in five participants, severe to moderate in four participants, and moderate to mild in one participant. Of nine participants previously on prophylaxis, eight discontinued prophylaxis. No participant resumed prophylaxis during the follow-up phase. Factor usage decreased by 2.1 million units over 1 year across all 10 participants. 26
Table 2.
Participant characteristics and efficacy and safety data for the AMT-060 phase 1/2 clinical trial.26,34,36.
| Cohort 1
(n = 5) Vector dose = 5 × 1012 (low) |
Cohort 2
(n = 5) Vector dose = 2 × 1013 (high) |
|||
|---|---|---|---|---|
| Age, years; median (range) | 69 (35–72) | 35 (33–46) | ||
| Baseline disease severity, n | ||||
| Severe | 4 | 5 | ||
| Moderate | 1 (FIX activity 1.5 IU/dL) | 0 | ||
| Prior treatment, n | ||||
| Prophylaxis | 5 | 4 | ||
| On-demand | 0 | 1 | ||
| Hemophilia Joint Health Scores, median (range) | 27 (2–49) | 6 (0–17) | ||
| CRM status, a n | ||||
| + | 2 | 1 | ||
| – | 3 | 3 | ||
| ± | 0 | 1 | ||
| Preexisting NAb | ||||
| GFP detected at baseline, b n | 0 | 0 | ||
| Preexisting NAb | ||||
| ELISA, n | ||||
| IgG | 2 | 0 | ||
| IgM | 1 | 4 | ||
| Infection history | ||||
| HIV positive | 1 | 0 | ||
| History of hepatitis C | 4 | 2 | ||
| Duration of follow-up | 1 year | 5 years | 1 year | 4.5 years |
| Efficacy | ||||
| FIX activity, c IU/dL mean (95% CI) | 4.4 (1.5–7.3) | 5.2 (2.0–8.4) | 6.9 (2.6–11.3) | 7.4 (4.2–10.6) |
| Hemophilia severity post-treatment, n | ||||
| Severe | 0 | 0 | 0 | 0 |
| Moderate | 3 | 3 | 1 | 1 |
| Mild | 2 | 2 | 4 | 4 |
| Reduction in ABR, % | ||||
| Total | 48% | 55% | 49% | 100% |
| Spontaneous | 53% | NA | 70% | Not reported |
| Reduction in factor consumption | 81% | 84% | 73% | 100% |
| Prophylaxis discontinued and not resumed (n = 9) | 4/5 | 4/5 | 4/4 | 4/4 |
| Safety | ||||
| Any TRAE | 4 events in 3 participants | 1 new event in 1 participant | 10 events in 3 participants | No new events |
| ALT elevation | 1 event in 1 participant; ALT returned to normal 2 weeks after starting treatment | No new events | 3 events d in 2 participants; ALT returned to normal at 6 weeks after starting treatment in 1 participant and after 10 weeks in the other participant | No new events |
| Treatment with tapering doses of prednisolone for ALT elevation, n | 1 | 2 | ||
| Loss of FIX expression, n | 0 | 0 | ||
| T-cell-mediated anti-AAV5 capsid response, n | 1 (not sustained and deemed not clinically relevant) | 0 | ||
AAV5, adeno-associated virus 5; ABR, annualized bleed rate; ALT, alanine aminotransferase; CI, confidence interval; CRM, cross-reacting material; ELISA, enzyme-linked immunosorbent assay; FIX, factor IX; GFP, green-fluorescent protein; HIV, human immunodeficiency virus; IgG, immunoglobulin G; IgM, immunoglobulin M; IU/dL, international units per deciliter; NA, not available; NAb, neutralizing antibody; TRAE, treatment-related adverse event.
Assays for CRM were not completed at baseline. Most individuals showed post-AMT-060 activity/antigen ratios ~1:1. Ratios lower than 1:1 suggest CRM positivity.
Three participants in cohort 1 retrospectively tested positive using the Luciferase assay.
Central one-stage activated partial thromboplastin time assay. Mean FIX protein concentrations varied between 1.3% and 12.3% in 8 of the 10 participants; 2 participants in cohorts 1 and 2 had mean FIX protein concentrations higher than 50% during the 1-year and 26-week follow-up periods. This discrepancy was attributed to genetic mutations that resulted in full production of a non-functional protein (Miesbach et al., 26 Supplemental Table 4).
1 event that was not treated was attributed to antibiotics.
Evaluation of etranacogene dezaparvovec (AMT-061)
Etranacogene dezaparvovec was evaluated in a phase 2b dose confirmation study (ClinicalTrials.gov identifier NCT03489291). 32 Three adult males with severe to moderately severe hemophilia B were enrolled. None of the participants had target joints at the time of enrollment. All had preexisting AAV5 NAbs as measured by the Luciferase assay (48, 44, and 25). All three participants received a vector dose of 2 × 1013 gc/kg (high dose) administered as a 500 mL intravenous infusion over 1 h.
A single dose of SHL FIX was administered prior to treatment to all participants and if needed at day 3. Indications for resuming prophylaxis were two or more consecutive FIX levels <2 IU/dL or based on clinical judgment and participant preference if FIX level was 2–5 IU/dL. Taper of prednisone was prescribed if participants had ALT ⩾2 times baseline.
The primary efficacy endpoint was FIX activity ⩾5 IU/dL measured by the central one-stage aPTT assay 6 weeks after dosing.
The initial 26-week follow-up data of a planned interim analysis were published by Von Drygalski et al., 32 and 2-year follow-up data were presented at the 2020 American Society of Hematology (ASH) Annual Meeting 37 (Table 3). All participants met the primary efficacy endpoint and had sustained FIX production and bleed protection.
Table 3.
| Phase 2b (n = 3) | HOPE-B phase 3 (n = 54) | |||
|---|---|---|---|---|
| Participant characteristics | ||||
| Age, years | 43, 47, 50 | Mean (SD, minimum, maximum) 41.5 (15.8, 19–75) |
||
| Baseline disease severity, n | ||||
| Severe | 2 | 44 | ||
| Moderate | 1 (FIX activity 1 IU/dL) | 10 (FIX activity ⩾1 and ⩽2 IU/dL) | ||
| ABR (treated and untreated) | 3, 1, 5 | Mean 3.98 |
||
| Prior treatment, n | ||||
| Prophylaxis | 3 | 54 | ||
| On-demand | 0 | 0 | ||
| Preexisting NAb | 3 (48, 44, 25) | % (maximum titer) | ||
| Luciferase assay, n | 23 (42.6, 3212.3) Median (1st and 3rd quartiles) 56.9 (23.8, 282.5) |
|||
| Preexisting NAb | ||||
| ELISA, n | ||||
| IgG | 0 | Not available | ||
| IgM | 1 | |||
| Infection history, n | ||||
| HIV positive | 2 | 3 | ||
| History of hepatitis B | 0 | 3 | ||
| History of hepatitis C | 3 | 31 | ||
| Duration of follow-up | 26 weeks | 2 years | 26 weeks | 1 year |
| Efficacy | ||||
| FIX activity, a IU/dL | 51.0, 33.2, 57.0 | 44.7, 36.3, 51.6 | Mean (SD, minimum, maximum) 37.2 (±19.6) |
Mean (SD, minimum, maximum) 41.5 (±21.7; 5.9, 113.0) |
| Reduction in ABR Total Spontaneous |
No bleeds | All participants had reduction in bleeding, only 1 participant had bleeding | 83% 83% |
66.6% 84.5% |
| Factor consumption | None after day 3 post-infusion | 1 participant with 1 infusion for suspected bleed and 1 infusion for confirmed bleed | Use of FIX decreased from a mean of 270,769 IU per year in lead in phase to 12,537 in weeks 0–26 | 96% reduction in number of infusions per year; use of FIX decreased from a mean of 257,070 IU per year in lead in phase to 8401 IU per year in months 7–12 |
| Prophylaxis discontinued and not resumed, n (%) | 3 (100) | 3 (100) | 52 (96) | 52 (96) |
| Zero bleeds, n (%) | 3 (100) | 2 (67) | 39 (72) | 40 (74) |
| Safety | ||||
| TRAE | 2 events in 1 participant | No new events | 88 events b in 37 participants | 3 new events for total of 91 events^ in 39 participants |
| ALT elevation | 1 event in 1 participant | No new events | 9 participants | No new events |
| Treatment with tapering doses of prednisolone for ALT elevation, n | 0 | 0 | 9 participants; all discontinued by week 26 | No new events |
| Loss of FIX expression, n | 0 | 0 | 0 | 0 |
| T-cell-mediated anti-AAV5 capsid response | 0 | 0 | Not available | Not available |
AAV5, adeno-associated virus 5; ABR, annualized bleed rate; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ELISA, enzyme-linked immunosorbent assay; FIX, factor IX; HIV, human immunodeficiency virus; HOPE-B, Health Outcomes with Padua gene: Evaluation in Hemophilia B; IgG, immunoglobulin G; IgM, immunoglobulin M; IU/dL, international units per deciliter; NAb, neutralizing antibody; SD, standard deviation; TRAE, treatment-related adverse event.
Central one-stage activated thromboplastin time assay.
TRAEs which occurred in ⩾5% of the safety population in the post-treatment follow-up included influenza like illness, headache, ALT increased, AST increased, fatigue, creatine phosphokinase increased in blood, nausea, infusion-related reactions, dizziness, and arthralgia. No FIX inhibitors were reported.
One participant had two bleeds, both treated with a single dose of FIX. He had six spontaneous bleeds in the year prior to treatment. The other two participants did not use FIX. Regarding safety, there were two TRAE in one participant that resolved without intervention: mild headache on the day of dosing and slightly elevated C-reactive protein on day 14. One participant had worsening of preexisting avascular necrosis (AVN) and underwent two hip surgeries. The worsening of AVN was not attributed to gene therapy. There were no clinically significant elevations in transaminases and no participants were treated with corticosteroids or other immunosuppression during the initial 26 weeks. 32 One participant had elevated of aspartate aminotransferase (AST) to 62 U/L (upper limit of normal 34 U/L) at 18 months. This elevation resolved and there was no change in FIX activity. 37
Etranacogene dezaparvovec is now being evaluated in the phase 3 clinical trial Health Outcomes with Padua gene: Evaluation in Hemophilia B (HOPE-B, ClinicalTrials.gov identifier NCT3569891). 35 This trial of adult men with FIX ⩽ 2 IU/dL of normal and treatment with continuous prophylaxis for ⩾2 months included a 26-week lead-in phase, dosing visit, post-treatment follow-up through week 52, and then long-term follow-up every 6 months until year 5. Preexisting AAV5 NAbs were not used as an exclusion criterion.
The primary endpoints are FIX activity at 26 weeks after dosing (central one-stage aPTT assay), FIX activity 52 weeks after dosing, and 52-week annualized bleed rate (ABR) compared with lead-in. Secondary endpoints included rates of total, spontaneous, traumatic, and treated/untreated bleeds; FIX consumption; and correlation of FIX activity levels and safety with preexisting anti-AAV5 antibody titers over 26 weeks (6 months) follow-up. Post hoc analysis evaluated FIX activity (central one-stage aPTT assay) and safety at 26 weeks after dosing in participants with and without preexisting NAbs to AAV5.
Seventy-five patients were screened for enrollment into the study. Eight did not meet eligibility during screening. Thirteen stopped study participation prior to dosing related to clinical factors, COVID-19 pandemic, and withdrawal of consent. Fifty-four participants were dosed. 35
Participant characteristics plus 26- and 52-week efficacy and safety are shown in Table 3.35,38 Fifty-participants (96%) discontinued prophylaxis. Of the two participants who continued prophylaxis, one participant only completed 10% of the gene therapy infusion due to anaphylaxis and one had an NAb titer of 3212.35,39 No participant that stopped prophylaxis resumed prophylaxis by week 52.
Impact of preexisting AAV5 NAbs on safety and efficacy
In the clinical trial of AMT-060, participants were excluded for preexisting NAbs to AAV5 based on the GFP-based assay performed for study eligibility. As part of the study procedures, the participants were also tested for NAb by ELISA-based assays. Two participants had positive titers of anti-AAV5 IgG and five had positive titers of anti-AAV5 IgM at baseline. The subject with the highest preexisting NAb of 340 achieved highest post-infusion FIX activity.16,26 Based on these results, participants in the etranacogene dezaparvovec trials were not excluded based on preexisting AAV5 NAbs. In the phase 2b trial, all three participants had preexisting AAV5 NAb (by Luciferase assay) including two participants who were enrolled even though they were denied entry into another gene therapy trial. 40 In HOPE-B, participants with and without preexisting AAV5 NAbs were compared for efficacy, as measured by bleeding outcomes, and safety (Table 4). 40 – 42 The highest titer that resulted in FIX activity of ⩾2 IU/dL was 678.2. 40 The mean FIX activity was 32.7 (<2–90.4) in those with preexisting NAb compared with 41.3 IU/dL (range = 9.4–97.1) in those without preexisting NAb. Statistical analysis showed a trend to lower FIX activity in the preexisting NAb group (R2 = 0.078). Percentage of total bleeds was higher in those with preexisting NAbs. Long-term follow-up is indicated to determine whether this difference in FIX activity results in higher bleeding rates over time.
Table 4.
| Anti-AAV5 NAb
positive (n = 23) |
Anti-AAV5 NAb
negative (n = 31) |
|
|---|---|---|
| Maximum titer | 3212.3 | – |
| Median titer (1st quartile and 3rd quartile) | 56.9 (23.8, 282.5) | – |
| FIX activity, a IU/dL, mean (range) | 32.7 (<2–90.4) | 41.3 (8.4–97.1) |
| Bleeds, n (%) | ||
| Total | 13 (35) | 8 (23) |
| Spontaneous, untreated | 2 | 1 |
| Spontaneous, treated | 2 | 2 |
| Traumatic, untreated | 1 | 4 |
| Traumatic, treated | 5 | 1 |
| Unknown cause, untreated | 1 | 0 |
| Unknown cause, treated | 2 | 0 |
| TRAE, n (%) | ||
| Transient transaminitis, requiring corticosteroids | 2 (8.7) | 7 (22.6) |
| Infusion-related reactions | 5 (21.7) | 2 (6.5) |
| Headache | 2 (8.7) | 5 (16.1) |
| Influenza-like illness | 4 (17.4) | 3 (9.7) |
| Correlation of preexisting NAbs with FIX activity up to 628 b (n = 52) | ||
| Pearson’s product–moment correlation (95% CI) | –0.28 (0.51, –0.00) | |
| Spearman’s correlation coefficient (95% CI) | –0.27 (–0.50, 0.01) | |
| R 2 | 0.078 | |
AAV5, adeno-associated virus 5; CI, confidence interval; FIX, factor IX; HOPE-B, Health Outcomes with Padua gene: Evaluation in Hemophilia B; IU/dL, international units per deciliter; NAb, neutralizing antibody; TRAE, treatment-related adverse event.
Central one-stage activated thromboplastin time assay.
The correlation analysis was completed for 52 participants. Two participants excluded from the full analysis set including one participant with titer of 3212.3 and FIX activity <2 IU/dL.
Infusion-related reactions in HOPE-B
Seven participants (13%) had gene-therapy-infusion-related reactions on the day of dosing. 39 Symptoms included itchy eyes, hives, headache, dizziness, chest tightness, flushing, fever, and epigastric pain. In the first participant, to have a suspected hypersensitivity reaction of moderate intensity, the infusion was stopped after ~10% of the dose, and the reaction was managed with a combination of diphenhydramine, methylprednisolone, famotidine, epinephrine, meperidine, and intravenous fluids. In the subsequent six participants, the infusions were completed. One participant with mild reaction had his infusion temporarily interrupted and was treated with diphenhydramine and hydrocortisone. One participant with mild reaction had his infusion temporarily interrupted and treatment with diphenhydramine. One participant with mild reaction had his infusion temporarily interrupted and resumed at a slower rate, and treatment with chlorphenamine and hydrocortisone. The other three participants had no intervention.
Oncogenesis concerns: evaluation of a case of hepatocellular carcinoma in HOPE-B
Of significance, HOPE-B was placed on clinical hold in December 2021 to investigate the diagnosis of hepatocellular carcinoma (HCC) in a 69-year-old White, non-Hispanic male with moderately severe hemophilia B. At week 52, a hepatic lesion was identified on per study protocol screening ultrasound and confirmed on abdominal computed tomography (CT). Biopsy confirmed the diagnosis of HCC. HCC was treated with surgical resection and transarterial chemoembolization. Results of an extensive evaluation including integration site analysis and whole genome sequencing on the tumor suggested that the cancer was related to preexisting risk factors, including history of hepatitis B and C, rather than the gene therapy.43,44 On integration site analysis, integrations were infrequent and there was no dominant integration site. On whole genome sequencing, common HCC oncogene mutations were identified and there were no AAV integration sites near oncogenes. The trial was resumed in April 2021 when U.S. Food and Drug Administration (FDA) lifted the clinical hold. The study protocol was updated to increase ultrasound screening from once to twice per year. The case highlights the importance of short- and long-term monitoring after gene therapy especially in participants with preexisting risk factors for HCC.
Comparison with other hemophilia B gene therapy in clinical development
Several other gene therapy products as well as other novel treatments are in clinical development for hemophilia B. 45 Comparison of results is beyond the scope of this article. However, as data from each trial are made available, clinicians, patients, and other stakeholders may consider the following: eligibility criteria used for the trial, as regulatory approval may be limited to those patients who meet the trial inclusion/exclusion criteria; vector dose to achieve therapeutic effect, since higher doses may be associated with more toxicity; FIX levels achieved as well as predictability and durability of response; bleed outcomes; impact on quality of life; adverse events; and use of prophylactic or treatment corticosteroids or other immunosuppressant agents. In evaluating the FIX response, it will be important to make assessments in the context of the FIX assay utilized. Robinson et al. 46 recently described discrepancies in FIX activity measured between different one-stage assays and between one-stage assays and chromogenic assays for FIX Padua gene therapy products. Further work is needed in this area.
Limitations of therapy and unknowns
Etranacogene dezaparvovec (AMT 061) safety and efficacy are limited to men who meet the eligibility criteria of the trials. This notably excludes females, children, and patients with a history of inhibitors.
Safety and efficacy were seen with preexisting AAV5 NAbs up to 678.2. The analysis of the impact of NAb on outcomes in HOPE-B excluded the participant with the highest titer of ~3200. Ongoing evaluation is needed to identify the maximum titer at which safety and efficacy are not compromised and to inform whether patients with preexisting NAbs may be included in all trials.
Trial data have shown a wide range of FIX response. The etiology of this variable response is known and is likely multifactorial and related to unknown effects related to the immune system and liver.
Optimal evaluation and management of transaminitis must be defined. First, alternative causes of transaminitis should be considered such as autoimmune, obesity (non-alcoholic steatohepatitis), tumors, medications, drugs, alcohol, exercise, and hereditary hemochromatosis. Further research is indicated to determine which causes of transaminitis negatively impact on transgene expression and clinical response. Second, optimal use of immunosuppressive agents to protect transgene expression deserves further study.
As noted above, after gene therapy, all patients are expected to develop high-titer AAV5 antibodies which limit the potential of redosing using the same or other AAV5 construct. Novel strategies are required for the management of patients who do not respond to initial treatment or lose transgene expression over time.
Future concepts
The final 1-year analysis of HOPE-B will be used for marketing authorization applications. 35 The FDA granted AMT-061 breakthrough designation, and the European Medicines Agency (EMA) granted it PRIority MEdicines Scheme (PRIME) eligibility. Both these designations support the rapid development of AMT-061.
Even after approval, gene therapy recipients will need both short- and long-term monitoring for safety and efficacy. Recipients may be enrolled in the World Federation of Hemophilia Gene Therapy Registry and the American Thrombosis and Hemostasis Network (ATHN) 14 study: Hemophilia Gene Therapy Outcomes Study. 47 Hemophilia Treatment Centers must prepare to implement gene therapy outside of clinical trials or work with referral centers such that all patients have access to treatment. 48 In a recent single institution study of men with severe hemophilia A or B, 41% would accept gene therapy. 49 As gene therapy products are approved for commercial use, clear, standardized terminology, and shared decision-making tools which incorporate safety and efficiency data may help with implementation.50–52
Conclusion
Etranacogene dezaparvovec with hyperactive FIX Padua in an AAV5 construct is a promising therapy for individuals with moderately severe and severe hemophilia, including those with preexisting AAV5 NAbs. Results from phase 2B and phase 3 trials show sustained FIX expression and associated decrease in bleeds and factor usage. Most significant TRAEs are infusion-related reactions and transaminitis which can be overcome with close monitoring and medical management. Final week 52 data from HOPE-B are anxiously awaited along with long-term follow-up.
Footnotes
Author contributions: The author wrote and edited the manuscript.
The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval/patient consent: Ethical approval and informed consent were not required for this review.
Funding: The author received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Courtney D. Thornburg  https://orcid.org/0000-0002-5665-8958
https://orcid.org/0000-0002-5665-8958
References
- 1.Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia 2020; 26(Suppl. 6): 1–158. [DOI] [PubMed] [Google Scholar]
- 2.White GC, 2nd, Rosendaal F, Aledort LM, et al. Definitions in hemophilia. Recommendation of the scientific subcommittee on factor VIII and factor IX of the scientific and standardization committee of the International Society on Thrombosis and Haemostasis. Thromb Haemost 2001; 85: 560. [PubMed] [Google Scholar]
- 3.Buckner TW, Bocharova I, Hagan K, et al. Health care resource utilization and cost burden of hemophilia B in the United States. Blood Adv 2021; 5: 1954–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li N, Sawyer EK, Maruszczyk K, et al. Adult lifetime cost of hemophilia B management in the US: payer and societal perspectives from a decision analytic model. J Med Econ 2021; 24: 363–372. [DOI] [PubMed] [Google Scholar]
- 5.Mahlangu J, Levy H, Lee M, et al. Efficacy and safety of subcutaneous prophylaxis with dalcinonacog alfa in adults with haemophilia B. Haemophilia 2021; 27: 574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pasi KJ, Rangarajan S, Georgiev P, et al. Targeting of antithrombin in hemophilia A or B with RNAi therapy. N Engl J Med 2017; 377: 819–828. [DOI] [PubMed] [Google Scholar]
- 7.Peterson JA, Maroney SA, Mast AE.Targeting TFPI for hemophilia treatment. Thromb Res 2016; 141(Suppl. 2): S28–S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shapiro AD.Concizumab: a novel anti-TFPI therapeutic for hemophilia. Blood Adv 2021; 5: 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolous NS, Chen Y, Wang H, et al. The cost-effectiveness of gene therapy for severe hemophilia B: microsimulation study from the United States perspective. Blood. Epub ahead of print 25April2021. DOI: 10.1182/blood.2021010864. [DOI] [PubMed] [Google Scholar]
- 10.Wagner JA, Reynolds T, Moran ML, et al. Efficient and persistent gene transfer of AAV-CFTR in maxillary sinus. Lancet 1998; 351: 1702–1703. [DOI] [PubMed] [Google Scholar]
- 11.Kay MA, Manno CS, Ragni MV, et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet 2000; 24: 257–261. [DOI] [PubMed] [Google Scholar]
- 12.Doshi BS, Arruda VR. Gene therapy for hemophilia: what does the future hold. Ther Adv Hematol 2018; 9: 273–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCarty DM, Monahan PE, Samulski RJ. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther 2001; 8: 1248–1254. [DOI] [PubMed] [Google Scholar]
- 14.Raj D, Davidoff AM, Nathwani AC. Self-complementary adeno-associated viral vectors for gene therapy of hemophilia B: progress and challenges. Expert Rev Hematol 2011; 4: 539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown HC, Zakas PM, George SN, et al. Target-cell-directed bioengineering approaches for gene therapy of hemophilia A. Mol Ther Methods Clin Dev 2018; 9: 57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majowicz A, Nijmeijer B, Lampen MH, et al. Therapeutic hFIX activity achieved after single AAV5-hFIX treatment in hemophilia B Patients and NHPs with pre-existing Anti-AAV5 NABs. Mol Ther Methods Clin Dev 2019; 14: 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baruteau J, Waddington SN, Alexander IE, et al. Gene therapy for monogenic liver diseases: clinical successes, current challenges and future prospects. J Inherit Metab Dis 2017; 40: 497–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colella P, Ronzitti G, Mingozzi F. Emerging issues in AAV-mediated in vivo gene therapy. Mol Ther Methods Clin Dev 2018; 8: 87–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mingozzi F, High KA. Immune responses to AAV in clinical trials. Curr Gene Ther 2007; 7: 316–324. [DOI] [PubMed] [Google Scholar]
- 20.Pipe S, Leebeek FWG, Ferreira V, et al. Clinical considerations for capsid choice in the development of liver-targeted AAV-based gene transfer. Mol Ther Methods Clin Dev 2019; 15: 170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stanford S, Pink R, Creagh D, et al. Adenovirus-associated antibodies in UK cohort of hemophilia patients: a seroprevalence study of the presence of adenovirus-associated virus vector-serotypes AAV5 and AAV8 neutralizing activity and antibodies in patients with hemophilia A. Res Pract Thromb Haemost 2019; 3: 261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayes G, Andreeva T, Gregg K, et al. Global seroprevalence of pre-existing immunity again various AAV serotypes in the hemophilia A population. Res Pract Thromb Haemost 2019; 3: OC 31.5. [Google Scholar]
- 23.Boutin S, Monteilhet V, Veron P, et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther 2010; 21: 704–712. [DOI] [PubMed] [Google Scholar]
- 24.Mingozzi F, Chen Y, Edmonson SC, et al. Prevalence and pharmacological modulation of humoral immunity to AAV vectors in gene transfer to synovial tissue. Gene Ther 2013; 20: 417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Q, Huang W, Zhang H, et al. Neutralizing antibodies against AAV2, AAV5 and AAV8 in healthy and HIV-1-infected subjects in China: implications for gene therapy using AAV vectors. Gene Ther 2014; 21: 732–738. [DOI] [PubMed] [Google Scholar]
- 26.Miesbach W, Meijer K, Coppens M, et al. Gene therapy with adeno-associated virus vector 5-human factor IX in adults with hemophilia B. Blood 2018; 131: 1022–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simioni P, Tormene D, Tognin G, et al. X-linked thrombophilia with a mutant factor IX (factor IX Padua). N Engl J Med 2009; 361: 1671–1675. [DOI] [PubMed] [Google Scholar]
- 28.Samelson-Jones BJ, Finn JD, George LA, et al. Hyperactivity of factor IX Padua (R338L) depends on factor VIIIa cofactor activity. JCI Insight 2019; 4: e128683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nair N, Rincon MY, Evens H, et al. Computationally designed liver-specific transcriptional modules and hyperactive factor IX improve hepatic gene therapy. Blood 2014; 123: 3195–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crudele JM, Finn JD, Siner JI, et al. AAV liver expression of FIX-Padua prevents and eradicates FIX inhibitor without increasing thrombogenicity in hemophilia B dogs and mice. Blood 2015; 125: 1553–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.George LA, Sullivan SK, Giermasz A, et al. Hemophilia B gene therapy with a high-specific-activity factor IX variant. N Engl J Med 2017; 377: 2215–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Von Drygalski A, Giermasz A, Castaman G, et al. Etranacogene dezaparvovec (AMT-061 phase 2b): normal/near normal FIX activity and bleed cessation in hemophilia B. Blood Adv 2019; 3: 3241–3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Felberbaum RS. The baculovirus expression vector system: a commercial manufacturing platform for viral vaccines and gene therapy vectors. Biotechnol J 2015; 10: 702–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.uniQure. Events & presentations, http://uniqure.com/investors-newsroom/events-presentations.php (accessed 21 July 2021).
- 35.Pipe S, Recht M, Key N, et al. First data from the phase 3 HOPE-B gene therapy trial: efficacy and safety of etranacogene dezaparvovec (AAV5-Padua hFIX variant; AMT-061) in adults with severe or moderate-severe hemophilia B treated irrespective of pre-existing anti-capsid neutralizing antibodies. Blood 2020; 136: LBA-6. [Google Scholar]
- 36.Leebeek FW, Meijer K, Coppens M, et al. AMT-060 gene therapy in adults with severe or moderate-severe hemophilia B confirm stable FIX expression and durable reductions in bleeding and factor IX consumption for up to 5 years. Blood 2020; 136: 26. [Google Scholar]
- 37.von Drygalski A, Giermasz A, Castaman G, et al. Etranacogene dezaparvovec (AAV5-Padua hFIX variant), an enhanced vector for gene transfer in adults with severe or moderate-severe hemophilia B: two year data from a Phase 2b trial. Blood 2020; 136: 13. [Google Scholar]
- 38.Pipe S, Leebeek FW, Recht M, et al. 52 week efficacy and safety of etranacogene dezaparvovec in adults with severe or moderate-severe hemophilia B: data from the phase 3 HOPE-B gene therapy trial. Res Pract Thromb Haemost 2021; 5, https://abstracts.isth.org/abstract/52-week-efficacy-and-safety-of-etranacogene-dezaparvovec-in-adults-with-severe-or-moderate-severe-hemophilia-b-data-from-the-phase-3-hope-b-gene-therapy-trial/ [Google Scholar]
- 39.Recht M, Leebeek FW, Miesbach W, et al. Management of infusion reactions: lessons from the Phase 3 HOPE-B gene therapy trial of etranacogene dezaparvovec in adults with hemophilia B. Res Pract Thromb Haemost 2021; 5, https://abstracts.isth.org/abstract/management-of-infusion-reactions-lessons-from-the-phase-3-hope-b-gene-therapy-trial-of-etranacogene-dezaparvovec-in-adults-with-hemophilia-b [Google Scholar]
- 40.Leebeek FW, Miesbach W, Recht M, et al. Clinical outcomes in adults with hemophilia B with and without pre-existing neutralizing antibodies to AAV5: 6 month data from the phase 3 etranacogene dezaparvovec HOPE-B gene therapy trial. Res Pract Thromb Haemost 2021; 5, https://abstracts.isth.org/abstract/clinical-outcomes-in-adults-with-hemophilia-b-with-and-without-pre-existing-neutralizing-antibodies-to-aav5-6-month-data-from-the-phase-3-etranacogene-dezaparvovec-hope-b-gene-therapy-trial/ [Google Scholar]
- 41.Recht M, Leebeek FW, Miesbach W, et al. Clinical outcomes in patients with and without pre-existing neutralizing antibodies to the vector: 6 month data from the phase 3 HOPE-B gene therapy trial of etranacogene dezaparvovec. Mol Ther 2021; 29: 45, http://uniqure.com/ASGCT%202021-HOPE%20B-Oral_11May_clean.pdf [Google Scholar]
- 42.Leebeek FW, Miesbach W, Recht M, et al. Clinical outcomes in adults with hemophilia B with and without pre-existing neutralizing antibodies to AAV5: 6 month data from the phase 3 etranacogene dezaparvovec HOPE-B gene therapy trial, http://uniqure.com/investors-newsroom/ISTH_Oral%20Presentation_HOPE%20B%20NAbs_Draft%203_June%2018th%20final.pdf (accessed 21 July 2021).
- 43.uniQure. uniQure announces findings from reported case of hepatocellular carcinoma (HCC) in hemophilia B gene therapy program, https://www.globenewswire.com/news-release/2021/03/29/2200653/0/en/uniQure-Announces-Findings-from-Reported-Case-of-Hepatocellular-Carcinoma-HCC-in-Hemophilia-B-Gene-Therapy-Program.html (accessed 21 July 2021).
- 44.Schmidt ML, Foster G, Coppens M, et al. Liver safety case report from the phase 3 HOPE-B gene therapy trial in adults with hemophilia B. Res Pract Thromb Haemost 2021; 5, https://abstracts.isth.org/abstract/liver-safety-case-report-from-the-phase-3-hope-b-gene-therapy-trial-in-adults-with-hemophilia-b [Google Scholar]
- 45.Butterfield JSS, Hege KM, Herzog RW, et al. A molecular revolution in the treatment of hemophilia. Mol Ther 2020; 28: 997–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robinson MM, George LA, Carr ME, et al. Factor IX assay discrepancies in the setting of liver gene therapy using a hyperfunctional variant factor IX-Padua. J Thromb Haemost 2021; 19: 1212–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Konkle BA, Recht M, Hilger A, et al. The critical need for postmarketing surveillance in gene therapy for haemophilia. Haemophilia 2021; 27(Suppl. 3): 126–131. [DOI] [PubMed] [Google Scholar]
- 48.Miesbach W, Pasi KJ, Pipe SW, et al. Evolution of haemophilia integrated care in the era of gene therapy: treatment centre’s readiness in United States and EU. Haemophilia 2021; 27: 511–514. [DOI] [PubMed] [Google Scholar]
- 49.Krumb E, Lambert C, Hermans C. Patient selection for hemophilia gene therapy: real-life data from a single center. Res Pract Thromb Haemost 2021; 5: 390–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hart DP, Branchford BR, Hendry S, et al. Optimizing language for effective communication of gene therapy concepts with hemophilia patients: a qualitative study. Orphanet J Rare Dis 2021; 16: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sidonio RF, Jr, Pipe SW, Callaghan MU, et al. Discussing investigational AAV gene therapy with hemophilia patients: a guide. Blood Rev 2021; 47: 100759. [DOI] [PubMed] [Google Scholar]
- 52.Nossair F, Thornburg CD. The role of patient and healthcare professionals in the era of new hemophilia treatments in developed and developing countries. Ther Adv Hematol 2018; 9: 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]