Abstract
Following the World Trade Center (WTC) collapse on September 11, 2001, more than 40,000 people were exposed to a complex mixture of inhalable nanoparticles and toxic chemicals. While many developed chronic respiratory symptoms, to what degree olfaction was compromised is unclear. A previous WTC Medical Monitoring and Treatment Program study found that olfactory and nasal trigeminal thresholds were altered by the toxic exposure, but not scores on a 20-odor smell identification test.
Objectives
To employ a well-validated 40-item smell identification test to definitively establish whether the ability to identify odors is compromised in a cohort of WTC-exposed individuals and, if so, whether the degree of compromise is associated with self-reported severity of rhinitic symptoms.
Methods
The University of Pennsylvania Smell Identification Test (UPSIT) was administered to 99 WTC-exposed persons and 99 matched normal controls. The Sino-Nasal Outcomes Test (SNOT-20) was administered to the 99 WTC-exposed persons and compared to the UPSIT scores.
Results
The mean (SD) UPSIT scores were lower in the WTC-exposed group than in age-, sex-, and smoking history-matched controls [respective scores: 30.05 (5.08) vs 35.94 (3.76); p = 0.003], an effect present in a subgroup of 19 subjects additionally matched on occupation (p < 0.001). Fifteen percent of the exposed subjects had severe microsmia, but only 3% anosmia. SNOT-20 scores were unrelated to UPSIT scores (r = 0.20; p = 0.11).
Conclusion
Exposure to WTC air pollution was associated with a decrement in the ability to identify odors, implying that such exposure had a greater influence on smell function than previously realized.
Keywords: Anosmia, Hyposmia, Rhinosinusitis, World Trade Center, SNOT-20, UPSIT, Smell, Rhinology, Occupational medicine
Introduction
Air pollution, which is comprised of complex mixtures of particulate matter, gases, and organic compounds, can damage the nasal passages, including the olfactory receptor cells within the olfactory neuroepithelium, the outermost element of the olfactory system (Holt 1996; Trevino 1996; Doty and Hastings 2001). In residents living in highly polluted cities, such as Mexico City, both the upper and lower respiratory tracts frequently become chronically inflamed (Moss et al. 2001; Calderon-Garciduenas et al. 2001, 2003b). Under these conditions, particulates often accumulate within the nasal respiratory epithelium and the nasal respiratory epithelial barrier can break down (Calderon-Garciduenas et al. 2002, 2003a, 2004, 2008b).
Decreased ability to smell, as measured by quantitative tests, is common in highly polluted urban areas (Hudson et al. 2006; Calderon-Garciduenas et al. 2010; Guarneros et al. 2009), as well as in occupational settings where workers are chronically exposed to airborne particulates, metals, and other pollutants (Adams and Crabtree 1961; Ahlstrom et al. 1986; Schwartz et al. 1989; Lucchini et al. 1999; Dalton et al. 2003; Antunes et al. 2007). While such exposure to airborne toxins is usually reflected in olfactory epithelial damage, damage to other elements of the olfactory pathway can also occur. Thus, ultrafine particulate matter (UFPM < 100 nm) is present at autopsy in the olfactory bulbs of dogs, children, and young adults who have been chronically exposed to extreme air pollution, reflecting transit of materials from the nasal cavity into the brain via the olfactory fila (Calderon-Garciduenas et al. 2008a, b, 2010). Disruption of nasal and olfactory barriers, increased apurinic and apyrimidinic DNA sites in olfactory bulb and hippocampal tissues, and white matter hyperintense prefrontal lesions by MRI have been noted in those who have been chronically exposed to high levels of urban air pollution (Block and Calderon-Garciduenas 2009).
Although loss of smell function is well documented in long-term residents of highly polluted cities, shorter-term influences of extreme air pollutants on the ability to smell are less well documented. Following the aftermath of the World Trade Center (WTC) collapse on September 11, 2001, thousands of New York residents, as well as fire fighters and others involved in the demolition and cleanup of Ground Zero, were exposed to extremely high levels of air pollutants for time periods lasting up to 6 months. Chronic respiratory symptoms subsequently appeared in thousands of individuals exposed to the resulting pollution, a number of which have subsequently increased in frequency since the time of exposure (Brackbill et al. 2006; Reibman et al. 2009).
This study is the second of two studies on the topic of smell loss in persons evaluated at Mount Sinai Medical Center as part of the World Trade Center Medical Monitoring and Treatment Program. In the first of these studies, 102 persons who worked at the World Trade Center (WTC) site were administered a 20-odorant smell identification test, an odor detection threshold test for the rose-like odorant phenyl ethyl alcohol, and a nasal trigeminal nerve irritation threshold test employing n-butanol (Dalton et al. 2010). Relative to matched normal controls, no significant influences on smell identification ability were found, although the olfactory and trigeminal thresholds were elevated in the exposed WTC subjects. No associations were present between the olfactory test measures and prior health history, nasal patency (as measured by acoustic rhinometry), mucus transit time (as measured by the saccharine test), duration of WTC exposure, job title, or history of respirator use.
The finding of no significant effect on the measure of smell identification was unexpected, since smell identification test scores are usually correlated with detection thresholds (Doty et al. 1994). Thus, it is possible that the previously employed smell identification test lacked adequate power to detect an exposure-related decrement, given that the reliability and sensitivity of smell identification tests are related to the number of odorants they contain (Doty et al. 1995). Since decrements in the ability to identify smells are more noticeable to patients than simple alterations in general sensitivity, smell identification deficits likely reflect greater functional disability. Thus, one goal of the present study was to employ the well-validated 40-item University of Pennsylvania Smell Identification Test (UPSIT) (Doty et al. 1984b) to determine whether a more extensive smell identification test might detect a difference between exposed and non-exposed cohorts. If such a difference is observed, this would suggest that the extreme exposures incurred in the aftermath of 9/11 may have had greater functional consequences than previously appreciated. A second goal of this study was to ascertain whether chemosensory disturbance, if found, is associated with self-reported severity of upper respiratory symptoms (Piccirillo et al. 2002). In the earlier cohort, exposure was associated with nasal inflammation, as measured by a number of inflammatory markers (Dalton et al. 2010).
Methods
Subjects
Subject recruitment consisted of sequentially approaching all individuals over the age of 18 years who came in for their regularly scheduled visits in the Monitoring and Treatment Program at Mount Sinai Hospital from March to July of 2008. To be eligible to receive clinical services in this unique program, the participant had to have been a rescue, recovery, debris cleanup, or related support services worker in either lower Manhattan (south of Canal Street), the Staten Island Landfill, or the barge loading piers. Moreover, he or she must have been on-site for either 4 h on September 11–14, 2001, 24 h or more during the month of September, or for at least 80 h during the months of September, October, November, and December combined (Herbert et al. 2006). Controls were obtained from a database of normal subjects tested in earlier studies outside of the New York metropolitan area and matched, on a one-to-one basis, to the exposed cohort on age, sex, and smoking behavior (current, previous, past) (Doty et al. 1984b). Approval involving human subjects was granted by the Institutional Review Board at Mount Sinai Hospital as well as the Population Protection Committee of the World Trade Center Monitoring and Treatment Program. Written informed consent was given to all patients who voluntarily participated in the study. No compensation was provided.
A total of 100 exposed subjects were recruited, of which 99 completed both UPSIT and the SNOT-20 questionnaire. One woman failed to complete the UPSIT and she was excluded from the study. The final study group, whose demographics are presented in Table 1, was comprised of 72 exposed men and 27 exposed women. The controls were individually matched to this group on the basis of age, sex, and smoking history (current, past, never). The mean (SD) age of the study group was 48.0 (0.7). Four subjects (4%) were active smokers, 28 were former smokers (28.2%), and 7 subjects (7%) had a history of prior sinus surgery. Exact matches on occupation between the cases and controls were available from the control database for only 19 individuals; thus, this subset of 19 pairs was analyzed separately.
Table 1.
Patient demographics
| N (%) | |
|---|---|
| Male gender | 72 (72.8%) |
| Age (years ± SD) | 48.0 ± 8.8 |
| Ethnicity | |
| Caucasian | 44 (44.4%) |
| Black | 12 (12.1%) |
| Hispanic | 40 (40.4%) |
| American Indian | 0 (0%) |
| Asian | 3 (3.0%) |
| Other | 0 (0%) |
| Occupation | |
| Law enforcement | 24 (24.2%) |
| Firefighter | 4 (4.0%) |
| Constructiona | 11 (11.1%) |
| Laborerb | 37 (37.4%) |
| Health workerc | 5 (5.1%) |
| Otherd | 18 (18.2%) |
| Current smoker | 4 (4.0%) |
| History of smoking | 28 (28.2%) |
| History of sinus surgery | 7 (7.0%) |
Includes ironworker, electrician, carpenter, plumber
Includes asbestos handler, building cleaner
Includes nurses and EMS
Includes media, photographers, volunteers, transportation authorities
Olfactory testing
In light of the earlier extensive threshold testing of a WTC-exposed cohort (Dalton et al. 2010), no threshold testing was performed in this study. All subjects were administered the UPSIT (Doty et al. 1984b). This well-validated and reliable 40-odorant test (test–retest r > 0.90) employs microencapsulated odorants in a forced-choice format; i.e., a subject is required to provide an answer even if no smell is perceived. The test is comprised of four 10-page booklets, with each page containing a different microencapsulated scented strip and an associated multiple choice question. Stimuli were released using a pencil tip. After informed consent, the UPSIT was self-administered in a private room. Following testing, which generally took 10–15 min, the test was scored by one of the investigators (SCD), and the patient was informed of the results relative to a normative database of ~4,000 normal individuals (Doty 1995).
Nasal symptom questionnaire
Given our expectation that WTC-exposed persons would have increased levels of chronic rhinosinusitis, we administered the Sino-Nasal Outcomes Test (SNOT-20), a standardized and clinically validated questionnaire, for evaluating the symptoms of rhinosinusitis (Piccirillo et al. 2002). The average score for the 20 items was calculated (range of possible scores: 0–100). The SNOT-20 survey took patients approximately 5–10 min to complete and was given after the administration of the UPSIT.
Statistical analysis
The UPSIT scores were skewed to the left, so they were transformed to achieve normality before parametric analysis using the following equation: log (41-UPSIT score). The UPSIT scores of the exposed subjects were compared to their age-, sex-, and smoking history-matched controls. The data were analyzed using a general linear model with the within-subject factor of group (exposed, controls) and the between-subject factors of sex (male, female), age, and the interaction of sex and age. Data from the subgroup of 19 subjects for whom exact occupational matches were additionally analyzed using a one-way repeated measures analysis of variance (ANOVA). Non-parametric analyses using the Wilcoxin matched-pairs signed-ranks test were additionally employed to confirm significant group effects.
Results
Mean UPSIT (SD) scores were 30.05 (5.08) for the WTC-exposed group and 35.94 (3.76) for the control group. This difference was statistically significant [group F(1, 95) = 9.38, p = 0.003]. The age covariate was also significant [F(1, 95) = 8.91, p = 0.004], reflecting the well-established age-related decrement in smell function. No other sources of variance were significant, including the interaction between age and exposure group (p > 0.20). The Wilcoxin test confirmed the significant group effect (Z = 6.89, p < 0.001). Pearson r values computed between age and UPSIT scores for the control and exposed groups were significant at the 0.05 α level for the control group (r = −0.23, p = 0.022), but not for the WTC-exposed group (r = −0.19, p = 0.065). The one-way ANOVA performed on the 19 age-, sex-, and occupation-matched pairs also revealed a significant group main effect [respective UPSIT (SD) scores = 29.68 (6.03) & 35.37 (3.76); F(1,18) = 14.24, p = 0.001], which was confirmed by the Wilcoxon test (Z = 3.03, p = 0.002).
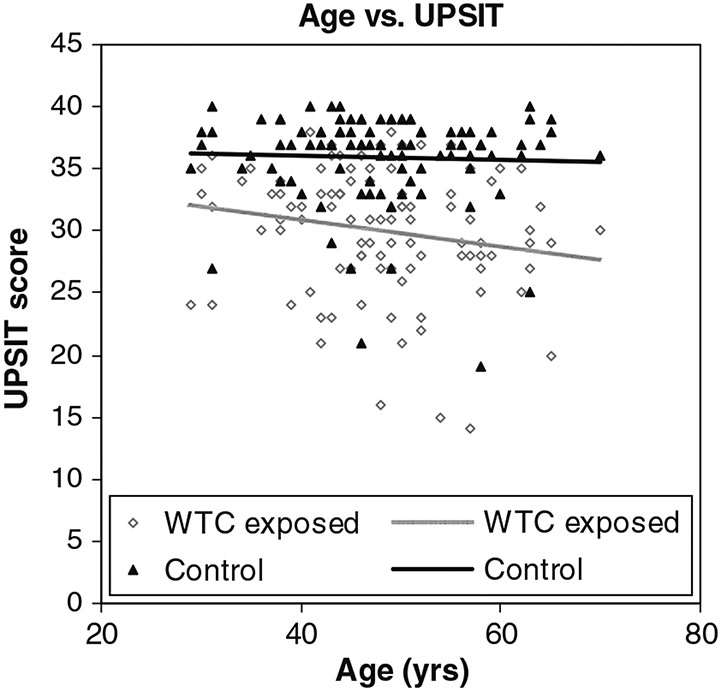
A scatter plot of the raw data for the cases (hollow diamonds) and controls (solid triangles) is shown in Fig. 1 as a function of age with imposed linear regression lines. Despite significant differences from controls, severe smell loss was relatively rare in the study population, as indexed by UPSIT norms (Doty 1995). Thus, only 3% of the exposed subjects exhibited UPSIT scores indicative of anosmia and only 15% had severe microsmia. In contrast, 25% of the cases had scores that fell within the normal range, and 31% had scores that fell within the mild microsmia range. Moderate microsmia was present in 25% of the exposed group.
Fig. 1.
Age versus UPSIT in WTC-exposed and control populations
The mean (SD) SNOT-20 score for the 99 WTC-exposed subjects was 41.16 (23.79). Relative to available norms, this score is indicative of marked rhinitic symptomatology (Piccirillo et al. 2002). No meaningful association existed between the UPSIT and SNOT-20 scores (Pearson r = 0.16; p = 0.11).
Discussion
The present research suggests that, even several years post-exposure, the ability to accurately identify odors is compromised in many individuals who worked at or near the WTC in the aftermath of the 9/11 attack. This implies that the extent of olfactory loss goes beyond the previously demonstrated adverse influences on olfactory and nasal trigeminal thresholds (Dalton et al. 2010). However, total anosmia or severe microsmia was present in less than a fifth of our subjects, and normal or near-normal function was present in about half of the study cohort. These prevalence figures are similar to those reported for chronic upper and lower respiratory disease in this cohort (Herbert et al. 2006).
Despite the fact that previous studies have reported decreased smell function in populations chronically exposed to air pollutants, the influences of such exposures on measures of smell identification ability have not been large. While Calderon-Garciduenas et al. (2010) found significantly lower UPSIT scores in 62 young residents of polluted sections of Mexico City than in 25 young residents of the rural town of Polotitlan, only a one and a half point difference in mean UPSIT scores was present (34.24 vs. 35.76), even though abnormal scores occurred three times more often in the Mexico City cohort (35.5% MC vs. 12%). Using a 16-item smell identification test, Guarneros et al. (2009) found no difference in odor identification test scores of 30 Mexico City residents from those of 30 Tlaxcala residents but did find both odor discrimination and detection threshold sensitivity deficits in the Mexico City residents.
As noted earlier, Dalton et al. (2010), who employed a 20-odor smell test in which each odorant was presented twice, also failed to find a smell identification deficit in WTC pollution–exposed subjects, although 8% of the exposed individuals who were classified as anosmic by their threshold scores also exhibited poor smell identification test scores, a frequency higher than that in their control group (1%). Additionally, these investigators found that 17–20% of their sample performed poorly on their smell identification test—a figure not different from the 18% that we found to have anosmia or severe microsmia. The reason why a significant decrement in odor identification ability was detected in the present study, but not in their study, is not clear. It may reflect subject sampling issues, the use of a more reliable test with a larger number of odorants, the nature of the response alternatives, the statistical design that was employed, the cut-off values used to define dysfunction, or other factors.
The physiological basis of the olfactory loss observed in the present study is unknown. One possibility is that the smell dysfunction is secondary to chronic rhinosinusitis induced by airborne toxins. If this is the case, the effect is likely due to inflammation within the olfactory epithelium, as has been documented by Kern (2000), rather than by blockage of airflow to the olfactory epithelium. Numerous studies have noted a direct correlation between histopathological changes in the olfactory mucosa and measures of olfactory function in patients with chronic rhinosinusitis (Downey et al. 1996; Moll et al. 1998; Apter et al. 1992; Simola and Malmberg 1998; Doty and Mishra 2001). Strong relationships are not found between airway patency and olfactory dysfunction except in cases of severe blockage, as from extreme polyposis (Doty and Mishra 2001). This lack of association is supported by the earlier WTC finding of no relationship between olfactory test scores and acoustic rhinometric measures of nasal patency (Dalton et al. 2010), as well as our current finding of a lack of association between UPSIT and SNOT-20 scores.
It is of interest that prior studies have reported olfactory dysfunction from a number of the same chemicals and toxins that were found in the dust from Ground Zero, including polychlorinated biphenyls (PCBs), volatile organic chemicals (VOCs), and various metals (Gobba 2006; Herbert et al. 2006). Dust samples from the WTC have been shown to contain, among other things, phthalate esters, various hydrocarbons, concrete, gypsum, glass fiber particles, cellulose, and asbestos (Lioy et al. 2002). PM2.5 particles (particulate matter with a mass median aerodynamic diameter < 2.5 μM), which are the most volatile and most problematic from a health perspective, collected 1–2 days after the WTC contained mainly calcium-based compounds, such as sulfate (gypsium) and calcium carbonate (calcite), presumably from crushed building materials such as cement, concrete aggregate, ceiling tiles, and wall board (McGee et al. 2003). Many of the aforementioned chemical and particulate agents are capable of damaging or desquamating the olfactory mucosa, although if Bowman glands and the basal stem cells are intact, regeneration usually occurs after such damage (Mackay-Sim 2003). Cigarette smoke, which contains particulate matter, activates caspase-3—a key effector enzyme in neuronal apoptosis that mediates proteolytic cell death within the olfactory epithelium (Vent et al. 2004). Some nanoparticles, if inhaled in sufficient quantity for long time periods, traverse the olfactory receptor cells to enter into the olfactory bulb (Calderon-Garciduenas et al. 2008a, b, 2010). Although Bowman glands secrete multiple detoxification enzymes that act on a wide range of toxic substrates, such enzymes can be rapidly overwhelmed and, for some ligands, can produce metabolites injurious to the olfactory membrane (Ding and Dahl 2003).
The present study found a statistically significant association between the UPSIT scores and age, an association that is well documented in dozens of other olfactory studies (Doty 1994). While the magnitude of the association was relatively small (rs = 0.19 and 0.23) and was significant at the 0.05 α level only for the controls, the difference in the magnitude of these coefficients is negligible. This relatively weak association likely reflects the fact that most subjects in this study were less than 65 years of age—an age when the major decline in smell function begins (Doty et al. 1984a).
There are several limitations to the present research. First, in common with the earlier WTC study (Dalton et al. 2010), our control group was based on a database of volunteers from a geographic region outside of New York City, namely Philadelphia. Whether this meaningfully influenced our findings is unknown, although unlikely. As pointed out in the earlier WTC study, air quality data are similar in Philadelphia and New York; if anything, Philadelphia has slightly higher levels of VOCs (Kleinman et al. 2002). In accord with this earlier study, we carefully matched our general sample on age, gender, and smoking habits and, in addition, matched a subgroup on occupation as well. Nonetheless, access to a larger group of occupation-matched controls would have been preferred. Second, the subjects in both the exposed and the control groups were volunteers, conceivably resulting in sampling biases, such as oversampling persons with concerns about their ability to smell. If such a bias was present, however, it is difficult to see how it would differentially influence the two groups. Third, the sample size of this study, like that of the earlier study (Dalton et al. 2010), is relatively small compared to most epidemiological studies seeking to establish associations between multiple subject variables. A larger cohort in which multiple variables could be assessed would have been desirable.
Although extremely unfortunate, the WTC disaster has provided a unique opportunity to understand how relatively short-term exposure to very high levels of air pollutants, including nanoparticles, alters the sense of smell. Smell loss is of critical importance to those who depend on olfaction to detect leaking natural gas, smoke, or a range of toxic chemicals, making our findings of potential value to investigators monitoring the health of first responders to disasters. It is well established that smell loss affects safety, the flavor of foods and beverages, and overall quality of life.
Conclusion
Workers and others exposed to the toxic chemicals and airborne particulates associated with the aftermath of the WTC 9/11 attack exhibited a statistically significant decrement in the ability to identify odors. These findings imply that such exposure had a greater influence on smell function than previously realized. While SNOT-20 scores documented continuing sinonasal complaints, there was no meaningful statistical association between odor identification test and SNOT-20 test scores. The degree to which improvement in smell function will occur over time is unknown. Smell loss may have particularly significant consequences for the first-responder group of police and fire fighters who often rely on their sense of smell to identify public safety hazards.
Acknowledgments
Supported in part by the Alpha Omega Alpha Carolyn L. Kuckein Student Research Fellowship, the World Trade Center Monitoring and Treatment Program at Mount Sinai Hospital, and grant NIEHS P30 ES013508 to the University of Pennsylvania. We thank Dr. Jay F. Piccirillo for allowing us to use the SNOT-20 in this study.
Footnotes
Conflict of interest statement RLD is President of and major shareholder in Sensonics, Inc, the manufacturer of the smell test used in this study. No other conflicts are noted.
Contributor Information
Kenneth W. Altman, Department of Otolaryngology-Head and Neck Surgery, Mount Sinai School of Medicine, One Gustave L. Levy Place, Box 1189, New York, NY 10029, USA
Shaun C. Desai, Department of Otolaryngology-Head and Neck Surgery, Mount Sinai School of Medicine, One Gustave L. Levy Place, Box 1189, New York, NY 10029, USA
Jacqueline Moline, Department of Community and Preventive Medicine, Mount Sinai School of Medicine, New York, NY 10029, USA.
Rafael E. de la Hoz, Department of Community and Preventive Medicine, Mount Sinai School of Medicine, New York, NY 10029, USA; Department of Medicine, Mount Sinai School of Medicine, New York, NY 10029, USA
Robin Herbert, Department of Community and Preventive Medicine, Mount Sinai School of Medicine, New York, NY 10029, USA.
Patrick J. Gannon, Touro University College of Medicine, Hackensack, NJ 07601, USA
Richard L. Doty, Smell and Taste Center and Department of Otorhinolaryngology: Head and Neck Surgery, School of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA
References
- Adams RG, Crabtree N (1961) Anosmia in alkaline battery workers. Br J Ind Med 18:216–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlstrom R, Berglund B, Berglund U, Lindvall T, Wennberg A (1986) Impaired odor perception in tank cleaners. Scand J Work Environ Health 12:574–581 [DOI] [PubMed] [Google Scholar]
- Antunes MB, Bowler R, Doty RL (2007) San Francisco/Oakland Bay Bridge Welder Study: olfactory function. Neurology 69:1278–1284 [DOI] [PubMed] [Google Scholar]
- Apter AJ, Mott AE, Cain WS, Spiro JD, Barwick MC (1992) Olfactory loss and allergic rhinitis [clinical conference]. J Allergy Clin Immunol 90:670–680 [DOI] [PubMed] [Google Scholar]
- Block ML, Calderon-Garciduenas L (2009) Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci 32:506–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackbill RM, Thorpe LE, DiGrande L, Perrin M, Sapp JH, Wu D, Campolucci S et al. (2006) Surveillance for World Trade Center disaster health effects among survivors of collapsed and damaged buildings. MMWR Surveill Summ 55:1–18 [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Rodriguez-Alcaraz A, Valencia-Salazar G, Mora-Tascareno A, Garcia R, Osnaya N, Villarreal-Calderon A et al. (2001) Nasal biopsies of children exposed to air pollutants. Toxicol Path 29:558–564 [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Azzarelli B, Acuna H, Garcia R, Gambling TM, Osnaya N, Monroy S et al. (2002) Air pollution and brain damage. Toxicol Path 30:373–389 [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Maronpot RR, Torres-Jardon R, Henriquez-Roldan C, Schoonhoven R, Acuna-Ayala H, Villarreal-Calderon A et al. (2003a) DNA damage in nasal and brain tissues of canines exposed to air pollutants is associated with evidence of chronic brain inflammation and neurodegeneration. Toxicol Path 31:524–538 [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Mora-Tiscareno A, Fordham LA, Valencia-Salazar G, Chung CJ, Rodriguez-Alcaraz A, Paredes R et al. (2003b) Respiratory damage in children exposed to urban pollution. Pediatr Pulmonol 36:148–161 [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Reed W, Maronpot RR, Henriquez-Roldan C, Gado-Chavez R, Calderon-Garciduenas A, Dragustinovis I et al. (2004) Brain inflammation and Alzheimer’s-like pathology in individuals exposed to severe air pollution. Toxicol Path 32:650–658 [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Mora-Tiscareno A, Ontiveros E, Gomez-Garza G, Barragan-Mejia G, Broadway J, Chapman S et al. (2008a) Air pollution, cognitive deficits and brain abnormalities: a pilot study with children and dogs. Brain Cogn 68:117–127 [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Solt AC, Henriquez-Roldan C, Torres-Jardon R, Nuse B, Herritt L, Villarreal-Calderon R et al. (2008b) Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol Pathol 36:289–310 [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Franco-Lira M, Henriquez-Roldan C, Osnaya N, Gonzalez-Maciel A, Reynoso-Robles R, Villarreal-Calderon R et al. (2010) Urban air pollution: influences on olfactory function and pathology in exposed children and young adults. Exp Toxicol Pathol 62:91–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton P, Cowart B, Dilks D, Gould M, Lees PS, Stefaniak A, Emmett E (2003) Olfactory function in workers exposed to styrene in the reinforced-plastics industry. Am J Ind Med 44:1–11 [DOI] [PubMed] [Google Scholar]
- Dalton PH, Opiekun RE, Gould M, McDermott R, Wilson T, Maute C, Ozdener MH et al. (2010) Chemosensory loss: functional consequences of the World Trade Center Disaster. Environ Health Perspect. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Dahl AR (2003) Olfactory mucosa: composition, enzymatic localization, and metabolism. In: Doty RL (ed) Handbook of olfaction and gustation, 2nd edn. Marcel Dekker, New York, pp 51–73 [Google Scholar]
- Doty RL (1994) Smell and taste in the elderly. In: Albert ML, Knoefel JE (eds) Clinical neurology of aging, 2nd edn. Oxford University Press, New York, pp 465–479 [Google Scholar]
- Doty RL (1995) The Smell Identification Test™ Administration manual, 3rd edn. Sensonics, Inc., Haddon Hts [Google Scholar]
- Doty RL, Hastings L (2001) Neurotoxic exposure and olfactory impairment. Clin Occupat Environ Med 1:547–575 [Google Scholar]
- Doty RL, Mishra A (2001) Olfaction and its alteration by nasal obstruction, rhinitis, and rhinosinusitis. Laryngoscope 111:409–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L (1984a) Smell identification ability: changes with age. Science 226:1441–1443 [DOI] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Dann M (1984b) Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav 32:489–502 [DOI] [PubMed] [Google Scholar]
- Doty RL, Smith R, McKeown DA, Raj J (1994) Tests of human olfactory function: principal components analysis suggests that most measure a common source of variance. Percept Psychophys 56:701–707 [DOI] [PubMed] [Google Scholar]
- Doty RL, McKeown D, Lee WW, Shaman P (1995) Test-retest reliability of 10 olfactory tests. Chem Senses 20:645–656 [DOI] [PubMed] [Google Scholar]
- Downey LL, Jacobs JB, Lebowitz RA (1996) Anosmia and chronic sinus disease. Otolaryngol Head Neck Surg 115:24–28 [DOI] [PubMed] [Google Scholar]
- Gobba F (2006) Olfactory toxicity: long-term effects of occupational exposures. Int Arch Occup Environ Health 79:322–331 [DOI] [PubMed] [Google Scholar]
- Guarneros M, Hummel T, Martinez-Gomez M, Hudson R (2009) Mexico City air pollution adversely affects olfactory function and intranasal trigeminal sensitivity. Chem Senses 34:819–826 [DOI] [PubMed] [Google Scholar]
- Herbert R, Moline J, Skloot G, Metzger K, Baron S, Luft B, Markowitz S, Udasin I, Harrison D, Stein D, Todd A, Enright P, Stellman JM, Landrigan PJ, Levin SM (2006) The World Trade Center disaster and the health of workers: five-year assessment of a unique medical screening program. Environ Health Perspect 114:1853–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt GR (1996) Effects of air pollution on the upper aerodigestive tract. Otolaryngol Head Neck Surg 114:201–204 [DOI] [PubMed] [Google Scholar]
- Hudson R, Arriola A, Martinez-Gomez M, Distel H (2006) Effect of air pollution on olfactory function in residents of Mexico City. Chem Senses 31:79–85 [DOI] [PubMed] [Google Scholar]
- Kern RC (2000) Chronic sinusitis and anosmia: pathologic changes in the olfactory mucosa. Laryngoscope 110:1071–1077 [DOI] [PubMed] [Google Scholar]
- Kleinman LI, Daum PH, Imre D, Nunnermacker LJ, Springston SR, Weinstein-Lloyd J (2002) Ozone production rate and hydrocarbon reactivity in 5 urban areas: a cause of high ozone concentration in Houton. Geophys Res Lett 29:1467–1471 [Google Scholar]
- Lioy PJ, Weisel CP, Millette JR, Eisenreich S, Vallero D, Offenberg J, Buckley B et al. (2002) Characterization of the dust/smoke aerosol that settled east of the World Trade Center (WTC) in lower Manhattan after the collapse of the WTC 11 September 2001. Environ Health Perspect 110:703–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini R, Apostoli P, Perrone C, Placidi D, Albini E, Migliorati P, Mergler D et al. (1999) Long-term exposure to “low levels” of manganese oxides and neurofunctional changes in ferroalloy workers. Neurotoxicology 20:287–297 [PubMed] [Google Scholar]
- Mackay-Sim A (2003) Neurogenesis in the adult olfactory neuroepithelium. In: Doty RL (ed) Handbook of olfaction and gustation, 2nd edn. Marcel Dekker, New York, pp 93–113 [Google Scholar]
- McGee JK, Chen LC, Cohen MD, Chee GR, Prophete CM, Haykal-Coates N, Wasson SJ et al. (2003) Chemical analysis of World Trade Center fine particulate matter for use in toxicologic assessment. Environ Health Perspect 111:972–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll B, Klimek L, Eggers G, Mann W (1998) Comparison of olfactory function in patients with seasonal and perennial allergic rhinitis. Allergy 53:297–301 [DOI] [PubMed] [Google Scholar]
- Moss OR, Gross EA, James RA, Janszen DB, Ross PW, Roberts KC, Howard AM et al. (2001) Respiratory tract toxicity in rats exposed to Mexico City air. Res Rep Health Eff Inst 100:1–24 [PubMed] [Google Scholar]
- Piccirillo JF, Merritt MG Jr, Richards ML (2002) Psychometric and clinimetric validity of the 20-Item Sino-Nasal Outcome Test (SNOT-20). Otolaryngol Head Neck Surg 126:41–47 [DOI] [PubMed] [Google Scholar]
- Reibman J, Liu M, Cheng Q, Liautaud S, Rogers L, Lau S, Berger KI et al. (2009) Characteristics of a residential and working community with diverse exposure to World Trade Center dust, gas, and fumes. J Occup Environ Med 51:534–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz BS, Doty RL, Monroe C, Frye R, Barker S (1989) Olfactory function in chemical workers exposed to acrylate and methacrylate vapors. Am J Pub Health 79:613–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simola M, Malmberg H (1998) Sense of smell in allergic and nonallergic rhinitis. Allergy Allergy 53:190–194 [DOI] [PubMed] [Google Scholar]
- Trevino RJ (1996) Air pollution and its effect on the upper respiratory tract and on allergic rhinosinusitis. Otolaryngol Head Neck Surg 114:239–241 [DOI] [PubMed] [Google Scholar]
- Vent J, Robinson AM, Gentry-Nielsen MJ, Conley DB, Hallworth R, Leopold DA, Kern RC (2004) Pathology of the olfactory epithelium: smoking and ethanol exposure. Laryngoscope 114(8):1383–1388 [DOI] [PubMed] [Google Scholar]