Abstract
The respiratory system attempts to maintain normal levels of oxygen and carbon dioxide. However, airflow limitation, parenchymal abnormalities and dysfunction of the respiratory pump may be compromised in individuals with advanced COPD, eventually leading to respiratory failure, with reduced arterial oxygen tension (hypoxaemia) and/or increased arterial carbon dioxide tension (PaCO2; hypercapnia). Hypoxaemia may persist in individuals with severe COPD despite smoking cessation and optimisation of pharmacotherapy. Long-term oxygen therapy (LTOT) can improve survival in those with severe daytime hypoxaemia, whereas those with less severe hypoxaemia may only have improved exercise capacity and dyspnoea. Changes in respiratory physiology that occur during sleep further predispose to hypoxaemia, particularly in individuals with COPD. However, the major cause of hypoxaemia is hypoventilation. Noninvasive ventilation (NIV) may reduce mortality and need for intubation in individuals with COPD and acute hypercapnic respiratory failure. However, NIV may also improve survival and quality of life in individuals with stable, chronic hypercapnia and is now suggested for those with prolonged hypercapnia (e.g. PaCO2 >55 mmHg 2–6 weeks after hospital discharge) when clinically stable and after optimisation of medical therapy including LTOT if indicated. Many questions remain about the optimal mode, settings and goal of NIV therapy.
Short abstract
In COPD, long-term oxygen therapy improves survival in severe daytime hypoxaemia and noninvasive ventilation reduces the need for intubation after acute hypercapnic respiratory failure and improves survival in stable in chronic hypercapnia. https://bit.ly/3yaLYTb
COPD and hypoxaemic respiratory failure
The progressive deterioration of pulmonary function seen in individuals with COPD may lead to an increased risk of persistent hypoxaemia. Thus, although the UPLIFT study showed that only 2% of the 5993 participants (mean forced expiratory volume in 1 s (FEV1): 48% predicted) were on supplemental oxygen [1], 80% of the end-stage COPD individuals participating in the National Emphysema Treatment Trial used oxygen therapy [2]. In COPD, ventilation/perfusion disturbances resulting from progressive airflow limitation and emphysematous destruction of the pulmonary capillary bed are the most important contributors to hypoxaemia. Obesity and disturbed ventilatory responses to hypoxia may also play a role [3, 4]. Persistent hypoxaemia should not be confused with the hypoxaemia related to an acute exacerbation of COPD (AECOPD), which may often persist up to several weeks after the event but resolves in 40–50% of cases [5, 6].
Hypoxaemia during exercise occurs in approximately 40% of individuals with moderate to severe COPD who have normoxaemia at rest [7–9]. Nocturnal oxygen desaturation occurs in almost half of all individuals with COPD [10–12] and is attributed to a combination of hypoventilation and gas exchange abnormalities resulting from alteration in ventilatory control and thoracic wall configuration, especially during rapid eye movement (REM) sleep [13–15], as discussed in greater detail below.
Detrimental effects of chronic hypoxaemia in COPD
In COPD, chronic hypoxaemia appears to contribute to mortality and has been associated with complications such as systemic inflammation and pulmonary hypertension [16]. Cohort studies have indicated that the need for long-term oxygen therapy (LTOT) is one of the most powerful determinants of mortality in COPD [17, 18].
Pulmonary hypertension is relatively common in advanced disease, with prevalence varying between 50 and 90% in individuals with COPD and emphysema listed for lung transplant or lung volume reduction surgery [19]. Mean pulmonary arterial pressure rarely exceeds 35 mmHg in hypoxaemic individuals [20]. The pathogenesis of pulmonary hypertension in COPD is complex and not solely related to alveolar hypoxia, but due to 1) an interaction between hyperinflation and right and left ventricular dysfunction, 2) an increased pulmonary vascular resistance due to pulmonary vascular remodelling in conjunction with loss of small pulmonary vessels and prolonged hypoxic pulmonary vasoconstriction, and 3) a tobacco-induced remodelling of pulmonary arteries [21]. Whether isolated night-time hypoxaemia is eventually responsible for ever-increasing pulmonary artery pressures and subsequent cor pulmonale remains unclear [22, 23].
Polycythaemia, frequently reported in hypoxaemic individuals with COPD in the past, is less frequently encountered, possibly due to the widespread prescription of LTOT [24]. Skeletal muscle and neurocognitive dysfunction have also been reported in chronically hypoxaemic COPD (with neurocognitive dysfunction rare in the absence of chronic hypoxaemia) [25].
Effects of LTOT in individuals with COPD and severe daytime hypoxaemia
The 2015 British Thoracic Society (BTS) [26] and the 2020 American Thoracic Society (ATS) [27] guidelines recommend that stable individuals with COPD and severe resting hypoxaemia (arterial oxygen tension (PaO2) <55 mmHg (7.3 kPa)), or with resting hypoxaemia PaO2 <60 mmHg (8.0 kPa) combined with peripheral oedema, a haematocrit >55% or pulmonary hypertension, should be considered for LTOT. They recommend LTOT to be used for 15–24 h·day−1 [27]. The Thoracic Society of Australia and New Zealand recommends titrating oxygen to maintain a PaO2 >60 mmHg (8.0 kPa) or an oxygen saturation measured by pulse oximetry (SpO2) >90% awake at rest [28]. These recommendations are based on the inclusion criteria of two randomised controlled trials (RCTs), the Medical Research Council (MRC) trial [29] and the Nocturnal Oxygen Treatment Trial (NOTT) [30], dating from 1980 and 1981, respectively. In the MRC trial, participants were randomised to receive oxygen or no oxygen for at least 15 h·day−1 at a flow of 2 L·min−1 [29]. In the NOTT, participants were either treated with nocturnal or continuous oxygen at a flow of 1–4 L·min−1 [30]. Inclusion criteria of these two trials are summarised in table 1, with an emphasis on the changes in available therapy since the trials were performed more than 40 years ago. Participants enrolled in the MRC study [29] or NOTT [30] were not blinded to their treatment and the primary end-point was mortality. Interestingly, participant compliance was assessed by weighing cylinders after use or recording the time of use (MRC) and with timers that recorded gas flow duration (NOTT) [29, 30]. In the latter study, those prescribed 24 h·day−1 oxygen used it 17 h·day−1 (less than prescribed) and those prescribed nocturnal oxygen only used it 12 h·day−1 (more than prescribed) [30].
TABLE 1.
Inclusion and exclusion criteria of the Medical Research Council (MRC) study and Nocturnal Oxygen Treatment Trial (NOTT) [29, 30]
| Trial | Inclusion criteria | Exclusion criteria |
| MRC [ 29 ] | Stable individuals with chronic bronchitis or emphysema with FEV1 <1.2 L, aged <70 years PaO2 40–60 mmHg (5.3–8.0 kPa) when breathing room air, repeated after 3 weeks One or more episodes of ankle oedema |
Restrictive disorders, pulmonary embolism, systemic hypertension, coronary artery disease |
| NOTT [ 30 ] | Stable participants PaO2 <55 mmHg (7.3 kPa) (at least two occasions) PaO2 <59 mmHg (7.9 kPa) (at least two occasions) plus one of the following: oedema, haematocrit >55%, or P pulmonale on ECG Lung function: FEV1/FVC <70% after bronchodilator TLC >80% pred Age >35 years |
Previous LTOT Other diseases that may be expected to influence mortality |
FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; LTOT: long-term oxygen therapy; PaO2: arterial oxygen tension; TLC: total lung capacity.
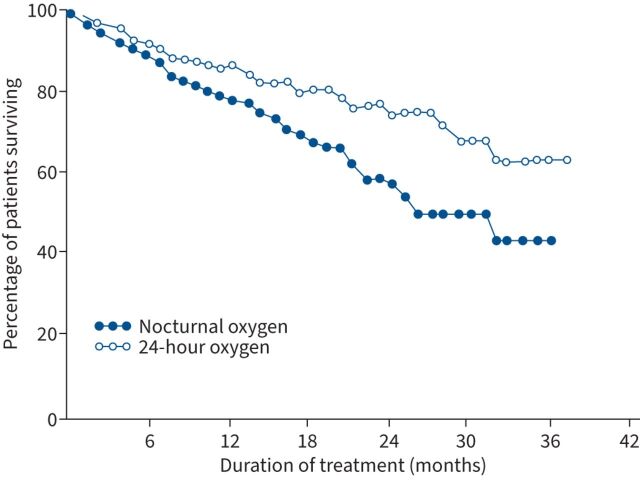
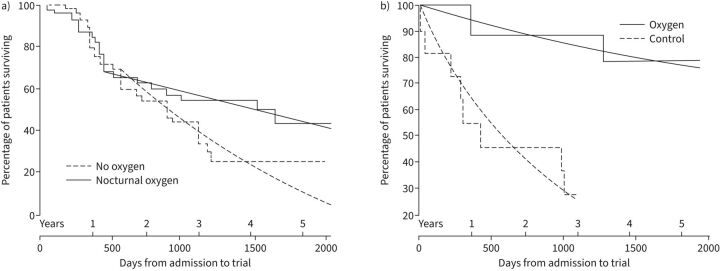
The NOTT [30], which had 203 participants, indicated a 2-year mortality risk reduction of 55% (relative risk 0.45, 95% CI 0.25–0.81, in those prescribed LTOT (24 h·day−1) compared with subjects prescribed only nocturnal oxygen (figure 1). Similarly, the MRC study [29], with 87 participants, indicated a 5-year mortality risk reduction of 59% in those with LTOT versus no oxygen (relative risk 0.41, 95% CI 0.17–0.98) (figure 2). For the NOTT [30], the number of individuals needed to be treated (NNT) to keep one additional individual alive over a given time period was 11.5 for the first year and 5.4 for the second [30]. For the MRC trial, the NNT was 4.8 [29].
FIGURE 1.
Overall mortality in the Nocturnal Oxygen Treatment Trial study [30]. Ordinate is fraction of individuals surviving; abscissa is time from randomisation or duration of treatment. Open circles represent the continuous oxygen therapy group, closed circles represent the nocturnal oxygen therapy group. Of the total group, 80 nocturnal and 87 continuous O2 therapy individuals were followed for 12 months, and 29 nocturnal and 37 continuous O2 therapy individuals were followed for 24 months. Reproduced and modified from [30] with permission.
FIGURE 2.
Mortality in a) male and b) female individuals in the Medical Research Council study [29]. Smooth curves indicate expected proportions surviving from 500 days, at constant risk of 11.9% per annum for those on oxygen and 29% per annum for controls (males) and of 5.7% per annum for those on oxygen and 36.5% per annum for controls (females). Reproduced and modified from [29] with permission.
Although methodologically debatable, the late Dr. Tom Petty merged the two trials’ findings such that the composite results suggested a dose–response relationship between hours per day of oxygen used and survival [31]. His proposed concept of “the more hours oxygen, the better” is, however, not currently supported by the Swedevox registry, which covers virtually all patients on LTOT in Sweden [32], and which has not shown any survival benefit for those prescribed oxygen 24 versus 15 h·day−1. These data should be interpreted with caution, as the relevance of their findings is limited by lack of any data concerning the actual number of hours per day patients used their oxygen, compared to what was prescribed by the physician. It may be that there is a threshold effect (though the optimal number of hours per day is unclear) or that prescription of supplemental oxygen might limit mobility/exercise in certain cases and enhance it in others, which is also important. Of note, of the individuals meeting criteria for LTOT, half had died after a median of 1.1 years of follow-up, emphasising the severity of illness for such patients [32].
In the NOTT [30], subgroup analysis suggested that continuous LTOT improved survival compared with nocturnal oxygen alone in individuals with more obvious signs of respiratory failure and, at the same time, less pronounced markers of pulmonary vascular disease (table 2). In the same trial, continuous oxygen therapy was associated with a reduction in pulmonary vascular resistance levels compared with nocturnal oxygen, although a relationship between greater decreases in pulmonary vascular resistance and reduced mortality was not seen, possibly due to small numbers of participants in the subgroups.
TABLE 2.
Mortality (%) according to baseline characteristics, with identification of subgroups with a statistically significant improved survival with continuous long-term oxygen therapy (LTOT) compared with nocturnal oxygen only (Nocturnal Oxygen Treatment Trial [30])
| Variable of interest | Continuous LTOT (%) | Nocturnal oxygen (%) |
| PaCO2 ≥43 mmHg (5.7 kPa) | 19.6 | 46.0 |
| pH <7.40 | 16.0 | 42.2 |
| FVC <1.89 L | 20.8 | 43.5 |
| Mean room air SaO2 during sleep <85% | 24.4 | 50.0 |
| Haematocrit <47.4% | 21.7 | 41.5 |
| Mean PAP <27 mmHg | 17.5 | 37.0 |
| Pulmonary vascular resistance <279 dynes·s·cm−5 | 12.8 | 33.3 |
FVC: forced vital capacity; PaCO2: arterial carbon dioxide tension; PAP: pulmonary artery pressure; SaO2: arterial oxygen saturation.
Overall, it remains unclear whether LTOT reduces healthcare use. One retrospective study [33] found fewer hospitalisations over 3 years in the LTOT group compared with conventional therapy (mean difference: −1.17, 95% CI −1.73–−0.59), whereas an observational study could not confirm that finding [34].
Stationary home oxygen equipment is often the only source of supplemental oxygen. This may restrict physical activity. More recently, lightweight oxygen therapy equipment has been developed to complement the stationary equipment with ambulatory oxygen therapy. However, these more portable devices have not necessarily come with observed clinical benefits. Some studies have reported that portable oxygen sources increased daily exposure to oxygen by no more than 30 min·day−1, whereas other studies revealed that individuals used their ambulatory oxygen at home [35–37]. Moreover, people with COPD still often consider currently available portable oxygen sources strenuous and burdensome [38].
Effects of LTOT in COPD with mild daytime hypoxaemia
The effects of supplemental oxygen in those with less severe daytime hypoxaemia are less clear [39]. That question was recently addressed in the Long-Term Oxygen Treatment Trial (LOTT) [40], an RCT which compared the effects of LTOT versus no oxygen in 783 individuals with either moderate hypoxaemia at rest (defined as room air SpO2 of 89–93%) or with no hypoxaemia at rest and only moderate exertional desaturation. The latter was defined as an SpO2<90% for ≥10 s and an SpO2≥80% for ≥5 min during a 6-min walk test). Those with isolated exertional hypoxaemia were randomly assigned to oxygen during both exertion and sleep. The intervention was not blinded. The primary end-point was a composite end-point of time to death or first hospitalisation over a 6-year period.
There was no difference between treatments in time to death, confirming the results of an older and smaller RCT of 135 participants [41]. Statistically and/or clinically meaningful differences in health-related quality of life (HRQoL) between treatments were not found after 12 months. Based in part on the LOTT trial [40], the 2020 ATS guideline [27] states not to prescribe LTOT to individuals with COPD and chronic moderate hypoxaemia while the 2020 Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommendations [39] say not to prescribe it routinely.
Effects of LTOT in COPD individuals with isolated night-time hypoxaemia
A French study [42] and the recently published INOX trial [43] addressed the impact of night-time oxygen therapy on mortality in individuals with only nocturnal oxygen desaturation. In these studies, desaturation was defined as an SpO2<90% for >30% of the time during nocturnal oximetry. Taken together, these studies do not show any improvement in mortality with up to 3 years of nocturnal oxygen therapy [43]. Moreover, night-time oxygen therapy did not affect [43] daytime HRQoL nor did it delay progression to LTOT. Its impact on sleep quality was mixed [44].
Regarding pulmonary artery pressure, studies have yielded variable results, showing either no difference or a small decrement in pulmonary arterial pressure of −3.7 mmHg compared with room air [42, 45, 46]. Currently, screening for isolated (i.e. daytime normoxia, and in the absence of signs and symptoms of obstructive sleep apnoea (OSA)) nocturnal oxygen desaturation is not recommended.
Ambulatory oxygen in individuals with COPD and isolated exercise-induced desaturation
The 2015 BTS [26] and the 2020 ATS [27] oxygen guidelines state that ambulatory oxygen should not be routinely offered to individuals with isolated exertional hypoxaemia. Indeed, its effects are mostly limited to acute improvement in exercise capacity, whereas its effects on HRQoL and dyspnoea are inconsistent. A few cross-over or parallel-group RCTs of ambulatory oxygen [47–49] have been published, of which only one included blinding to the intervention [48]. Ambulatory oxygen resulted in inconsistent improvements in HRQoL: the St. George Respiratory Questionnaire score remained unaffected, whereas a small statistically significant improvement in Chronic Respiratory Disease Questionnaire score was noted, which did not reach the minimal clinically important difference. However, statistically and clinically significant improvements in different domains of the Short Form Health Survey score, among which physical role, general health, social functioning and emotional role favouring ambulatory oxygen were noted [49]. Acute improvements in exercise capacity were also seen with ambulatory oxygen, with increases in the 5-min walk test of 28.9 m (95% CI 16.1–41.9 m) and endurance time by 5.8 min (95% CI 2.23–9.37 min) [47]. A reduction in Borg dyspnoea score by 1.1 unit (95% CI 0.53–1.69 units) at the end of exercise in favour of ambulatory oxygen was also reported [26]. Therefore, ambulatory oxygen should primarily be limited to patients who exhibit and perceive a clinically relevant improvement in exercise tolerance or dyspnoea. According to two recent studies, the beneficial effects of portable oxygen on exercise capacity and dyspnoea may be further enhanced using automatically titrating oxygen systems, which regulate oxygen flow to maintain a predefined SpO2 target during walking, when compared with the usually proposed oxygen supplementation via constant flow oxygen systems [50, 51].
Whether ambulatory oxygen affects survival in individuals with COPD and isolated exercise-induced desaturation remains to be investigated, as the subgroup of individuals with exertion-only desaturation enrolled in the LOTT study were required to use supplemental oxygen during sleep as well as during exercise [40]. Despite decades of use, many important questions about the use of LTOT remain.
Research questions about supplemental oxygen in COPD
1) Do COPD patients with early pulmonary haemodynamic impairment benefit from a lower threshold for starting LTOT?
2) Which individuals really benefit from ambulatory oxygen therapy? Is it possible to reduce the burden of ambulatory oxygen to individuals and their care givers?
3) Is there benefit of LTOT in individuals with COPD and resting moderate hypoxaemia in the absence of clinically important comorbid conditions?
4) What is the benefit of LTOT in individuals with pulmonary hypertension and night-time hypoxaemia (as these were excluded from the INOX trial [43])?
5) By what mechanism(s) does LTOT increase survival in COPD?
6) What is the optimal daily duration of exposure to oxygen to improve survival in COPD individuals on LTOT?
7) In which patients can LTOT be discontinued?
Changes in respiration with sleep
Sleep is a complex biological process that is evolutionarily conserved across a wide range of living organisms. The purpose(s) of sleep are not well understood, but sleep is important for memory/learning and immune function, among other factors. Relevant to the current discussion, sleep state is associated with a number of physiological changes in all people, which impact oxygenation and ventilation.
The greatest changes are state-dependent changes in ventilatory control during sleep. The respiratory system is a negative feedback control system designed to maintain a given arterial carbon dioxide tension (PaCO2). When transitioning from wake to sleep, and then from non-REM to REM sleep, the PaCO2 set-point increases (to 45–50 mmHg), leading to a reduction in minute ventilation of approximately 15% in REM compared to wake, primarily through a reduction in tidal volume [52]. A number of other changes occur, particularly during REM sleep, when breathing can be irregular and shallow. There is reduced muscle activation compared to wakefulness and, during REM sleep, skeletal muscle atonia with exception of the diaphragm. The fall in upper airway muscle activity can lead to upper airway narrowing or even collapse with some snoring and/or OSA. End-expiratory lung volumes fall both with change from sitting to supine position [53] and with sleep onset, typically by about 0.3 L [54, 55]. In those without chronic lung disease, there will be a small decrease in PaO2 and in SaO2 as a result of all the changes above.
Changes in respiration with sleep and impact on COPD
Given all of the changes mentioned earlier, it is perhaps not surprising that sleep and adequate ventilation is a struggle for those with COPD. When cataloguing symptoms of people with COPD, sleep difficulties are very prevalent (often only behind dyspnoea and fatigue) [56, 57]. Objective sleep measured using polysomnography is also poor [58]. Perhaps as a result, sedative hypnotic use can be common, even though there are concerns about the safety of these drugs in those with COPD [59].
Even a change to the supine/recumbent position can increase dyspnoea in individuals with severe COPD [60]. This may be due to less efficiency of the respiratory muscles to generate inspiratory and expiratory airflow [61], increased airways resistance at lower lung volumes when supine leading to increased expiratory flow limitation [62] or other changes in gas exchange that necessitate increased ventilation to maintain PaCO2. When measured during wakefulness, the change in body position from sitting to supine is associated with a rapid increase in neural drive to the diaphragm (using diaphragm electromyography) and the change in neural drive is correlated with the subjective change in dyspnoea. These authors also noted a decrease in lung compliance with the change in position in both controls and those with COPD that will additionally tax already challenged muscles of respiration. Thus, many individuals with COPD may report orthopnoea and try to sleep upright as a result.
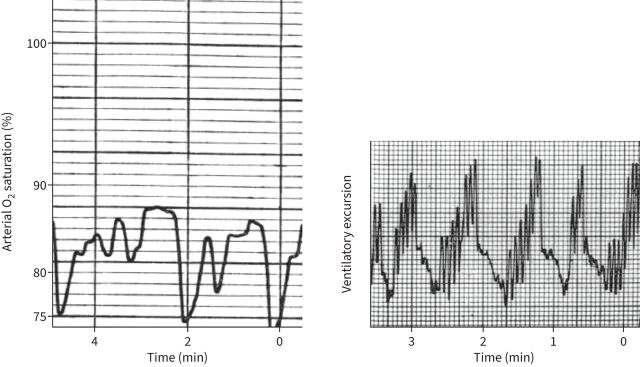
A long-described feature of sleep and COPD has been nocturnal oxygen desaturation (NOD), in which oxygen saturation is often lower at night (sometimes dramatically so) compared to daytime levels (figure 3) [63]. In particular, the drop in oxygen can be more severe than that seen during exercise during the day and may be particularly pronounced for those already with mild to moderate hypoxaemia during the day (and who rest on the steep part of the oxyhaemoglobin desaturation curve) [11, 64]. Some studies have suggested that NOD is associated with worse sleep either subjectively or objectively [65, 66] and it has been associated with increased mortality [67]. The major mechanism of NOD is hypoventilation, with contributions from both a change in PaCO2 set-point, particularly during REM sleep (i.e. “won't breathe”) [68–70] when ventilation can drop nearly 40% from waking values, and perhaps an increased upper airway resistance even in those without OSA (i.e. “can't breathe”) [71]. For example, Luo et al. [69] measured the change in neural drive to the diaphragm in those with severe COPD and showed state-related reductions in neural drive that were worse in REM sleep. O’Donoghue et al. [68] cleverly used a helium–oxygen mixture with lower density than air that should reduce any upper airway contribution to reduce ventilation, but did not see any increase in ventilation when switching from air to the helium mixture in those with COPD. Changes in lung volume both with the supine position and sleep, and consequent changes in ventilation/perfusion matching, may also contribute a small amount to the drop in oxygen saturation [55]. These desaturation events have consequences, with increases in both systemic and pulmonary blood pressure [72]. It is also worth noting the observation that people with COPD are reported to die more frequently at night than expected [73].
FIGURE 3.
Example of nocturnal oxygen desaturation, with evidence of hypoventilation as the cause. Reproduced from [63] with permission.
At present, it is poorly understood how the sleep PaCO2 set-point changes over time in those with COPD, how this change during sleep impacts wake PaCO2 levels and what other factors are important. For example, do changes in PaCO2 during sleep precede changes in PaCO2 levels during wakefulness? Kitamjima et al. [74] documented episodic nocturnal hypercapnia (eNH) in those without daytime hypercapnia and, interestingly, those with eNH were more likely to have had more frequent exacerbations of their COPD. Some individuals during AECOPD have acute elevations in PaCO2 that resolve with therapy and time, suggesting that COPD therapies can influence the set-point on the order of days to weeks [75]. Finally, coexisting diseases such as obesity and/or OSA can contribute to chronic hypoventilation (see below).
Although ventilation decreases during sleep, tidal expiratory flow limitation can still be observed due to severe disease, adoption of the supine position and/or lung volume changes [62, 76]. Tidal expiratory flow limitation suggests dynamic inflation or intrinsic positive end expiratory pressure (PEEPi), which will have implications for work of breathing and might be matched/offset by application of an external expiratory positive airway pressure (EPAP) [77].
OSA
OSA is defined by repetitive collapse of the upper airway, which leads to repetitive episodes of hypoxaemia and arousals from sleep [78]. Severity is traditionally assessed by the number of apnoeas (absence of airflow) and hypopneas (reduction in airflow associated with drop in oxygen saturation and/or arousal from sleep) per hour of sleep. This fragmentation of sleep is associated with neurocognitive (daytime sleepiness and reduced HRQoL), cardiovascular (particularly hypertension, but also pulmonary hypertension and myocardial infarction) and metabolic consequences (diabetes mellitus). Traditional risk factors include male sex, obesity and age. OSA is a global problem, with nearly a billion people likely affected, the vast majority undiagnosed and untreated [79]. Continuous positive airway pressure (CPAP) therapy is the gold standard for treatment, yet adherence to therapy is not optimal.
By chance alone, the high prevalence of both COPD and OSA would suggest a great number of people with both disorders. Prevalence estimates have varied substantially depending on the population studied (likely due to differences in age, gender, rates of obesity and smoking), with about 0.5% of people in the general population, but more than two-thirds when looking at Veterans Administration or pulmonary rehabilitation populations [80]. Whether these diseases simply exist together in the same person or interact in some way uniquely detrimental—as thought by Flenley [81]—is not known, but the term “overlap syndrome” is frequently used [81]. Observational data by Marin et al. [82] suggested worse outcomes with overlap syndrome compared to either disorder alone and small case studies suggested that individuals with overlap syndrome had more pulmonary hypertension and right heart failure (cor pulmonale) [83, 84]. Additionally, data suggest that those with OSA develop hypercapnia even with relatively preserved lung function [85].
Most interesting are data suggesting that treatment of OSA might be beneficial for those with COPD. For example, Konikkara et al. [86] found that those with COPD diagnosed with OSA and adherent to therapy had fewer emergency room visits and hospital admissions in the 6 months after starting treatment compared to the 6 months before CPAP therapy. More recently, CPAP usage by individuals with overlap syndrome was associated with reduced all-cause hospitalisations and emergency room visits, severe AECOPD and healthcare costs using US claims data [87].
Oxygenation versus ventilation
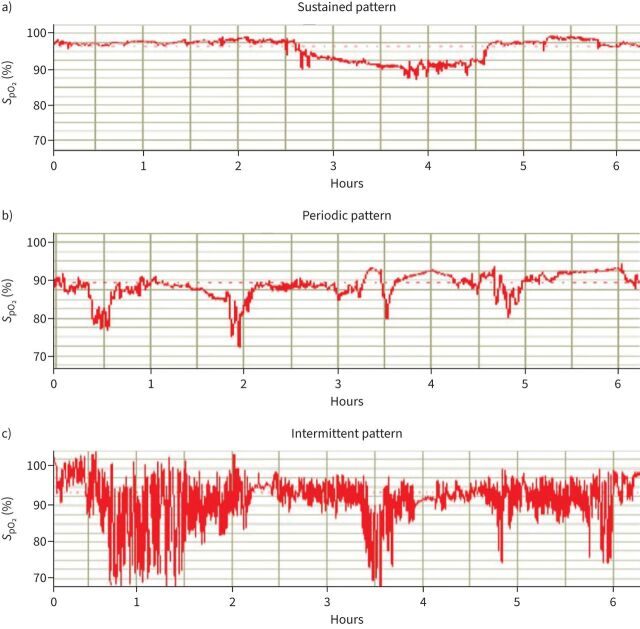
From the data above, it is clear that the major abnormality in COPD during sleep is hypoventilation leading to a decrease in oxygen saturation. However, measurements of overnight oxygen saturation (nocturnal oximetry) are more available then respiratory polygraphy, polysomnography or surrogate measures of PaCO2 such as transcutaneous CO2. Thus, the clinician may be forced to rely on home oximetry to try and determine whether OSA is present. While different patterns of oxygen desaturation have been described (figure 4) with an “intermittent” pattern reported to be suggestive for OSA [88], studies overall have found relatively modest diagnostic value of home oximetry for detecting OSA [89, 90]. At the same time, there may be harm for individuals with overlap syndrome who are prescribed nocturnal oxygen therapy and who will also not benefit from CPAP therapy. For example, when Alford et al. [91] administered supplemental oxygen to those with overlap syndrome, obstructive events lasted longer with a higher PaCO2 and lower pH at the end of those events. Thus, a high index of suspicion is needed when considering comorbid OSA. Similarly, patients with COPD and comorbid OSA might have hypercapnia due to combination of COPD and severe OSA/obesity hypoventilation syndrome. The optimal therapy of patients with COPD and OSA might depend on the relative severity of the two disorders. That is, patients with severe upper airway collapsibility/OSA in the setting of obesity who have only relatively modest lower airways disease might have improvement in hypercapnia with CPAP alone. Alternatively, those with mild upper airway obstruction but severe lower airways disease as the main contributor of hypercapnia should be treated with NIV [92].
FIGURE 4.
Overnight oximetry patterns: a) sustained, b) periodic and c) intermittent. The intermittent pattern has been suggested to be specific for obstructive sleep apnoea, while the other patterns are thought to be more consistent with COPD. However, the data are more mixed (see text). Reproduced and modified from [85] with permission. SpO2: oxygen saturation measured by pulse oximetry.
Points for clinical practice and future research questions about sleep and COPD
1) Nocturnal oxygen desaturation is common in individuals with severe COPD and is caused largely by hypoventilation.
2) OSA is common in those with COPD and treatment of OSA with CPAP therapy may improve outcomes.
3) How does night-time hypercapnia lead to daytime hypercapnia?
4) Can CPAP therapy be used to match PEEPi to decrease work of breathing or does titration of EPAP to PEEPi improve synchrony with NIV?
5) Does treatment of OSA with COPD (or weight loss) in those with hypercapnic COPD lead to improvements in gas exchange?
NIV in stable individuals with chronic hypercapnic respiratory failure due to COPD
Up to 25% of individuals with COPD at GOLD stages 3 and 4 may suffer from chronic hypercapnia (PaCO2>45 mmHg). Additional LTOT treatment of chronic hypercapnic respiratory failure due to COPD may include long-term NIV. Physiological and clinical studies, systematic reviews and meta-analyses have assessed the usefulness of NIV in these individuals through measurements of stabilisation of PaCO2 levels, reduction in hospitalisation rate and improvement in HRQoL [93–99].
NIV should be applied to effectively reduce hypercapnia by means of appropriate levels of inspiratory positive airway pressure (IPAP). With “high” IPAP levels, NIV resulted in improvements in HRQoL and reduction in mortality [100, 101]. In one study [101], participants were recruited from 36 respiratory units in Germany and Austria over a 7-year recruitment period. NIV was targeted to reduce baseline PaCO2 by at least 20% or to achieve PaCO2 values lower than 48.1 mmHg. One-year mortality was 12% in the intervention group and 33% in the control group; hazard ratio 0.24 (95% CI 0.11–0.49, p=0.0004). It is interesting that despite the relatively high reported prevalence of chronic hypercapnia in individuals with most severe stages of COPD [93], the recent randomised controlled trials [75, 101, 102] have faced great challenges with recruitment necessitating long inclusion periods.
Furthermore, use of long-term NIV might result in a reduction in hospitalisations and be cost effective when initiated and continued in individuals successfully treated for acute-on-chronic hypercapnic respiratory failure due to an exacerbation of their disease [102, 103]. However, the best results are observed when NIV is applied after an appropriate time span after AECOPD (e.g. 1 month) in order to avoid to include individuals with spontaneously reversible hypercapnia who would not benefit from long-term NIV [102]. A trial on continuation of NIV after AECOPD with acute type 2 respiratory failure is ongoing, so this still needs to be addressed prospectively [104].
Physiological mechanisms of improved outcomes with NIV in COPD
Four potential mechanisms may explain the effectiveness of NIV in these individuals:
Respiratory muscle unloading
The pressure support (defined as IPAP minus EPAP) unloads the inspiratory muscles. In stable patients with COPD, the dynamic PEEPi is higher in hypercapnic patients. As a result, the application of an external PEEP (or EPAP) may counteract the dynamic PEEPi associated with lung hyperinflation and reduce work of breathing [105–107].
Sleep quality and correction of hypoventilation
Physiological studies have shown that NIV may improve alveolar ventilation by increasing the tidal volume and reducing the respiratory rate. This is particularly important as sleep hypoventilation and related night-to-morning change in PaCO2 (potentially at least in part due to reduced CO2 responsiveness) might be an additional factor in the development of pulmonary hypertension. In addition, chronic hypercapnia may further impair diaphragmatic function and have a deleterious effect on the central respiratory drive [105, 108, 109].
Resetting of the respiratory centres
Compared with LTOT alone, addition of night NIV results in significant improvements in daytime PaO2 and PaCO2, total sleep time, sleep efficiency and overnight PaCO2. The degree of improvement in daytime PaCO2 is significantly correlated with the improvement in mean overnight PaCO2 [110].
Cardiovascular effects
It has been reported that nocturnal NIV applied over 3 months improved heart rate variability, reduced circulating natriuretic peptide levels and enhanced the functional performance of individuals with advanced COPD, suggesting that NIV may reduce the impact of cardiac comorbidities. These beneficial effects must be balanced by the observation that acute application of high IPAP in individuals with severe stable COPD and hypercapnia may transiently reduce oxygen delivery despite adequate levels of arterial oxygen saturation through reduced cardiac output [111, 112].
Guidelines for NIV in COPD
There is no global recommendation on when to start long-term NIV in individuals with COPD. In the frame of internationally accepted standards, criteria for referral, initiation and performance of home NIV should be stated by national guidelines according to the health services available in each country, developing also national learning management systems for professional and nonprofessional caregivers.
The European Respiratory Society guidelines suggest with various levels of evidence that long-term NIV should be used [113]:
in individuals with chronic stable hypercapnic COPD,
in individuals with COPD following a life-threatening episode of acute hypercapnic, respiratory failure requiring acute NIV, if hypercapnia persists following the episode,
titrating NIV to normalise or reduce PaCO2 levels,
using fixed pressure support mode as first-choice ventilator mode.
The German Respiratory Society guidelines recommend starting long-term NIV [114, 115]:
in individuals with symptoms of chronic respiratory failure and PaCO2>50 mmHg during daytime spontaneous breathing or >55 mmHg during night-time spontaneous breathing,
after acute hypercapnic exacerbations needing mechanical ventilation if there is persistency of hypercapnia (defined as PaCO2 >53 mmHg at least 14 days following acute mechanical ventilation).
The ATS guidelines recommend with various grades of certainty [116, 117]:
the use of nocturnal NIV in addition to usual care for stable hypercapnic individuals,
a screening for obstructive sleep apnoea before starting,
not initiating long-term NIV during an admission for acute-on-chronic hypercapnic respiratory failure, but after reassessment at 2–4 weeks after resolution,
not using an in-laboratory overnight polysomnogram to titrate NIV in individuals initiating NIV,
NIV should be aimed at normalisation of PaCO2.
Other national recommendations or experiences have been reported [118–121].
Practical recommendations
Practical recommendations on long-term NIV can be summarised as follows [122]:
The physiological target of NIV (to correct hypoventilation and reduce or normalise daytime PaCO2 during NIV and spontaneous breathing) must be defined and checked. In the study by Köhnlein et al. [101], the mean±sd ventilator setting at hospital discharge in the home oxygen therapy plus home NIV group were an IPAP of 21.6±4.7 cmH2O and an EPAP of 4.8±1.6 cmH2O. In the study by Murphy et al. [102] the median applied IPAP was 24 cmH2O (interquartile range (IQR) 22–26 cmH2O), with EPAP of 4 cmH2O (IQR, 4–5 cmH2O).
In individuals with COPD and chronic hypercapnia, NIV is effective in improving arterial blood gases and in unloading inspiratory muscles independent on whether it is set on the basis of comfort or physiologically targeted. However, physiological setting of EPAP to overcome upper airway collapse (e.g. OSA) and/or dynamic PEEPi may improve patient–ventilator synchrony [123].
Oronasal masks are the most used interface for the delivery of home NIV in individuals with obstructive hypoventilation syndrome and COPD. However, there is no difference in the efficacy or tolerance of oronasal or nasal masks [124].
As a key component in the long-term management of underweight individuals under NIV, nutritional status and dietary intake-related problems should be addressed [125].
There is the need to monitor pressure changes in the use of external humidification devices in some home NIV ventilators [126, 127].
Short, valid and easy to use tools such as questionnaires are needed to monitor NIV in clinical practice and for organisation of home mechanical ventilation services [128].
Use of telemonitoring for NIV
Telemonitoring programmes may be useful for safety and effectiveness of long-term NIV. Transferring this chronic management to the home environment is a big challenge: monitoring has to be noninvasive, reliable and easy to use, data security needs to be ensured, signals need to be integrated and preferably automatically processed and algorithms need to be developed based on clinically relevant outcomes. Many remote health monitoring systems are available, ensuring safety, feasibility, effectiveness, sustainability and flexibility to face individual needs. The legal problems associated with telemonitoring are still controversial, but most ventilator devices can report hours of use (compliance), tidal volumes and respiratory rate among other parameters that might be useful for clinicians. National and European Union governments should develop guidelines and ethical, legal, regulatory, technical and administrative standards for remote medicine. The economic advantages, if any, of this new approach must be compared to a “gold standard” of home care that is very variable among different countries and within each country [129, 130].
Other uses of NIV in those with COPD
NIV can improve exercise tolerance in different clinical conditions. Long-term home NIV administered also during walking resulted in improved oxygenation, decreased dyspnoea and increased walking distance. The use of a portable NIV device during walking can improve dyspnoea and walking distance in individuals with moderate to severe COPD. Patients who do not already receive long-term NIV therapy are more likely to benefit compared to those undergoing long-term NIV. CPAP and different modalities of assisted ventilation have been delivered through different interfaces during exercise training programs. Patients on long-term NIV may benefit from exercising with the same ventilators, interfaces and settings as used at home [131, 132].
To conclude, recent studies add new comprehension of the role of home NIV; in the light of these, long-term NIV should be tailored to the individual after appropriate evaluation of each case, whereas more generalised use of domiciliary NIV in patients with COPD cannot be justified by the available evidence.
Future research questions regarding COPD and NIV
Studies are needed to better define which individuals will have survival benefit from long-term NIV compared with the usual LTOT. Future trials should include clear therapeutic end-points (e.g. PaCO2 levels) and should consider technical aspects, such as ventilator mode, settings and duration of NIV, and account for the presence of comorbidities (e.g. obesity and OSA) and COPD phenotypes.
Further studies are needed to evaluate selection criteria, the right time to start home NIV, the optimal ventilatory settings and the follow-up programme. Better understanding of the resolution of hypercapnia after an AECOPD is needed to address timing of evaluation for home NIV after an exacerbation.
There is still a lack of information about the optimal ventilatory settings for home use. High IPAP levels and backup respiratory rates have both positive (respiratory muscle unloading and improvement in gas exchange) and potentially negative (increased hyperinflation, increased patient–ventilator asynchronies, barotrauma and cardiovascular) effects that need to be better evaluated.
The cost-effectiveness of home NIV is still unclear. Further evidence is required to identify patients most likely to benefit and to establish optimum time points for starting NIV and equipment settings [103].
The mortality rate following initiation of home NIV is still to be clearly defined, but it is reported to be high. Initiation of home NIV following an acute admission and low levels of NIV adherence are poor prognostic features. Whether mode, settings or hospital versus at-home NIV setup impacts adherence are unknown [133–139].
Footnotes
Provenance: Commissioned article, peer reviewed.
Previous articles in this series: No. 1: Montes de Oca M, Laucho-Contreras ME. Smoking cessation and vaccination. Eur Respir Rev 2023; 32: 220187. No. 2: Volpato E, Farver-Vestergaard I, Brighton LJ, et al. Nonpharmacological management of psychological distress in people with COPD. Eur Respir Rev 2023; 32: 220170.
This article has an editorial commentary: https://doi.org/10.1183/16000617.0028-2023
Number 3 in the Series “Nonpharmacological interventions in COPD: state of the art and future directions” Edited by Geert M. Verleden and Wim Janssens
Conflict of interest: R.L. Owens has received grant NIH/NHLBI R01HL142114 paid to his institution, outside the submitted work. The remaining authors have nothing to disclose.
References
- 1.Tashkin DP, Celli B, Senn S, et al. . A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008; 359: 1543–1554. doi: 10.1056/NEJMoa0805800 [DOI] [PubMed] [Google Scholar]
- 2.Martinez FJ, Foster G, Curtis JL, et al. . Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med 2006; 173: 1326–1334. doi: 10.1164/rccm.200510-1677OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franssen FM, O'Donnell DE, Goossens GH, et al. . Obesity and the lung: 5. Obesity and COPD. Thorax 2008; 63: 1110–1117. doi: 10.1136/thx.2007.086827 [DOI] [PubMed] [Google Scholar]
- 4.Bradley CA, Fleetham JA, Anthonisen NR. Ventilatory control in patients with hypoxemia due to obstructive lung disease. Am Rev Respir Dis 1979; 120: 21–30. doi: 10.1164/arrd.1979.120.1.21 [DOI] [PubMed] [Google Scholar]
- 5.Spece LJ, Epler EM, Duan K, et al. . Reassessment of home oxygen prescription after hospitalization for chronic obstructive pulmonary disease. A potential target for deimplementation. Ann Am Thorac Soc 2021; 18: 426–432. doi: 10.1513/AnnalsATS.202004-364OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levi-Valensi P, Weitzenblum E, Pedinielli JL, et al. . Three-month follow-up of arterial blood gas determinations in candidates for long-term oxygen therapy. A multicentric study. Am Rev Respir Dis 1986; 133: 547–551. doi: 10.1164/arrd.1986.133.4.547 [DOI] [PubMed] [Google Scholar]
- 7.Dantzker DR, D'Alonzo GE. The effect of exercise on pulmonary gas exchange in patients with severe chronic obstructive pulmonary disease. Am Rev Respir Dis 1986; 134: 1135–1139. doi: 10.1164/arrd.1986.134.6.1135 [DOI] [PubMed] [Google Scholar]
- 8.O'Donnell DE, D'Arsigny C, Fitzpatrick M, et al. . Exercise hypercapnia in advanced chronic obstructive pulmonary disease: The role of lung hyperinflation. Am J Respir Crit Care Med 2002; 166: 663–668. doi: 10.1164/rccm.2201003 [DOI] [PubMed] [Google Scholar]
- 9.Andrianopoulos V, Franssen FM, Peeters JP, et al. . Exercise-induced oxygen desaturation in COPD patients without resting hypoxemia. Respir Physiol Neurobiol 2014; 190: 40–46. doi: 10.1016/j.resp.2013.10.002 [DOI] [PubMed] [Google Scholar]
- 10.McNicholas WT. Impact of sleep in COPD. Chest 2000; 117: 48S–53S. doi: 10.1378/chest.117.2_suppl.48S [DOI] [PubMed] [Google Scholar]
- 11.Lewis CA, Fergusson W, Eaton T, et al. . Isolated nocturnal desaturation in COPD: prevalence and impact on quality of life and sleep. Thorax 2009; 64: 133–138. doi: 10.1136/thx.2007.088930 [DOI] [PubMed] [Google Scholar]
- 12.Lacasse Y, Sériès F, Vujovic-Zotovic N, et al. . Evaluating nocturnal oxygen desaturation in COPD–revised. Respir Med 2011; 105: 1331–1337. doi: 10.1016/j.rmed.2011.04.003 [DOI] [PubMed] [Google Scholar]
- 13.Fletcher EC, Gray BA, Levin DC. Nonapneic mechanisms of arterial oxygen desaturation during rapid-eye-movement sleep. J Appl Physiol Respir Environ Exerc Physiol 1983; 54: 632–639. doi: 10.1152/jappl.1983.54.3.632 [DOI] [PubMed] [Google Scholar]
- 14.Catterall JR, Douglas NJ, Calverley PM, et al. . Transient hypoxemia during sleep in chronic obstructive pulmonary disease is not a sleep apnea syndrome. Am Rev Respir Dis 1983; 128: 24–29. doi: 10.1164/arrd.1983.128.1.24 [DOI] [PubMed] [Google Scholar]
- 15.Hudgel DW, Martin RJ, Capehart M, et al. . Contribution of hypoventilation to sleep oxygen desaturation in chronic obstructive pulmonary disease. J Appl Physiol Respir Environ Exerc Physiol 1983; 55: 669–677. doi: 10.1152/jappl.1983.55.3.669 [DOI] [PubMed] [Google Scholar]
- 16.Kent BD, Mitchell PD, McNicholas WT. Hypoxemia in patients with COPD: cause, effects, and disease progression. Int J Chron Obstruct Pulmon Dis 2011; 6: 199–208. doi: 10.2147/COPD.S10611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Owusuaa C, Dijkland SA, Nieboer D, et al. . Predictors of mortality in chronic obstructive pulmonary disease: a systematic review and meta-analysis. BMC Pulm Med 2022; 22: 125. doi: 10.1186/s12890-022-01911-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singanayagam A, Schembri S, Chalmers JD. Predictors of mortality in hospitalized adults with acute exacerbation of chronic obstructive pulmonary disease. Ann Am Thorac Soc 2013; 10: 81–89. doi: 10.1513/AnnalsATS.201208-043OC [DOI] [PubMed] [Google Scholar]
- 19.Thabut G, Dauriat G, Stern JB, et al. . Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest 2005; 127: 1531–1536. doi: 10.1378/chest.127.5.1531 [DOI] [PubMed] [Google Scholar]
- 20.Scharf SM, Iqbal M, Keller C, et al. . Hemodynamic characterization of patients with severe emphysema. Am J Respir Crit Care Med 2002; 166: 314–322. doi: 10.1164/rccm.2107027 [DOI] [PubMed] [Google Scholar]
- 21.Bunel V, Guyard A, Dauriat G, et al. . Pulmonary arterial histologic lesions in patients with COPD with severe pulmonary hypertension. Chest 2019; 156: 33–44. doi: 10.1016/j.chest.2019.02.333 [DOI] [PubMed] [Google Scholar]
- 22.Fletcher EC, Luckett RA, Miller T, et al. . Pulmonary vascular hemodynamics in chronic lung disease patients with and without oxyhemoglobin desaturation during sleep. Chest 1989; 95: 757–764. doi: 10.1378/chest.95.4.757 [DOI] [PubMed] [Google Scholar]
- 23.Chaouat A, Weitzenblum E, Kessler R, et al. . Outcome of COPD patients with mild daytime hypoxaemia with or without sleep-related oxygen desaturation. Eur Respir J 2001; 17: 848–855. doi: 10.1183/09031936.01.17508480 [DOI] [PubMed] [Google Scholar]
- 24.Fremault A, Janssens W, Beaucage F, et al. . Modification of COPD presentation during the last 25 years. COPD 2010; 7: 345–351. doi: 10.3109/15412555.2010.510546 [DOI] [PubMed] [Google Scholar]
- 25.Dodd JW, Getov SV, Jones PW. Cognitive function in COPD. Eur Respir J 2010; 35: 913–922. doi: 10.1183/09031936.00125109 [DOI] [PubMed] [Google Scholar]
- 26.Hardinge M, Annandale J, Bourne S, et al. . British Thoracic Society guidelines for home oxygen use in adults. Thorax 2015: Suppl. 1, 70: i1–i43. doi: 10.1136/thoraxjnl-2015-206865 [DOI] [PubMed] [Google Scholar]
- 27.Jacobs SS, Krishnan JA, Lederer DJ, et al. . Home oxygen therapy for adults with chronic lung disease. An Official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med 2020; 202: e121–e141. doi: 10.1164/rccm.202009-3608ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDonald CF, Whyte K, Jenkins S, et al. . Clinical practice guideline on adult domiciliary oxygen therapy: executive summary from the Thoracic Society of Australia and New Zealand. Respirology 2016; 21: 76–78. doi: 10.1111/resp.12678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stuart-Harris C. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema: report of the Medical Research Council Working Party. Lancet 1981; 1: 681–686. [PubMed] [Google Scholar]
- 30.Nocturnal Oxygen Therapy Trial Group . Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Nocturnal Oxygen Therapy Trial Group. Ann Intern Med 1980; 93: 391–398. doi: 10.7326/0003-4819-93-3-391 [DOI] [PubMed] [Google Scholar]
- 31.Petty TL. Home oxygen–a revolution in the care of advanced COPD. Med Clin North Am 1990; 74: 715–729. doi: 10.1016/S0025-7125(16)30547-8 [DOI] [PubMed] [Google Scholar]
- 32.Ahmadi Z, Sundh J, Bornefalk-Hermansson A, et al. . Long-term oxygen therapy 24 vs 15 h/day and mortality in chronic obstructive pulmonary disease. PLOS One 2016; 11: e0163293. doi: 10.1371/journal.pone.0163293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bao H, Wang J, Zhou D, et al. . Community physician-guided long-term domiciliary oxygen therapy combined with conventional therapy in stage IV COPD patients. Rehabil Nurs 2017; 42: 268–273. doi: 10.1002/rnj.233 [DOI] [PubMed] [Google Scholar]
- 34.Crockett AJ, Moss JR, Cranston JM, et al. . The effects of home oxygen therapy on hospital admission rates in chronic obstructive airways disease. Monaldi Arch Chest Dis 1993; 48: 445–446. [PubMed] [Google Scholar]
- 35.Vergeret J, Brambilla C, Mounier L. Portable oxygen therapy: use and benefit in hypoxaemic COPD patients on long-term oxygen therapy. Eur Respir J 1989; 2: 20–25. [PubMed] [Google Scholar]
- 36.Lacasse Y, Lecours R, Pelletier C, et al. . Randomised trial of ambulatory oxygen in oxygen-dependent COPD. Eur Respir J 2005; 25: 1032–1038. doi: 10.1183/09031936.05.00113504 [DOI] [PubMed] [Google Scholar]
- 37.Casaburi R, Porszasz J, Hecht A, et al. . Influence of lightweight ambulatory oxygen on oxygen use and activity patterns of COPD patients receiving long-term oxygen therapy. COPD 2012; 9: 3–11. doi: 10.3109/15412555.2011.630048 [DOI] [PubMed] [Google Scholar]
- 38.Dakkak J, Tang W, Smith JT, et al. . Burden and unmet needs with portable oxygen in patients on long-term oxygen therapy. Ann Am Thorac Soc 2021; 18: 1498–1505. doi: 10.1513/AnnalsATS.202005-487OC [DOI] [PubMed] [Google Scholar]
- 39.Global Initiative for Chronic Obstructive Lung Disease (GOLD) . Global Strategy for the Diagnosis, management, and prevention of Chronic Obstructive Pulmonary Disease. 2020. Available from: https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf
- 40.Albert RK, Au DH, Blackford AL, et al. . A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med 2016; 375: 1617–1627. doi: 10.1056/NEJMoa1604344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Górecka D, Gorzelak K, Sliwiński P, et al. . Effect of long-term oxygen therapy on survival in patients with chronic obstructive pulmonary disease with moderate hypoxaemia. Thorax 1997; 52: 674–679. doi: 10.1136/thx.52.8.674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaouat A, Weitzenblum E, Kessler R, et al. . A randomized trial of nocturnal oxygen therapy in chronic obstructive pulmonary disease patients. Eur Respir J 1999; 14: 1002–1008. doi: 10.1183/09031936.99.14510029 [DOI] [PubMed] [Google Scholar]
- 43.Lacasse Y, Sériès F, Corbeil F, et al. . Randomized trial of nocturnal oxygen in chronic obstructive pulmonary disease. N Engl J Med 2020; 383: 1129–1138. doi: 10.1056/NEJMoa2013219 [DOI] [PubMed] [Google Scholar]
- 44.Calverley PM, Brezinova V, Douglas NJ, et al. . The effect of oxygenation on sleep quality in chronic bronchitis and emphysema. Am Rev Respir Dis 1982; 126: 206–210. doi: 10.1164/arrd.1982.126.2.206 [DOI] [PubMed] [Google Scholar]
- 45.Fletcher EC, Luckett RA, Goodnight-White S, et al. . A double-blind trial of nocturnal supplemental oxygen for sleep desaturation in patients with chronic obstructive pulmonary disease and a daytime PaO2 above 60 mmHg. Am Rev Respir Dis 1992; 145: 1070–1076. doi: 10.1164/ajrccm/145.5.1070 [DOI] [PubMed] [Google Scholar]
- 46.Fletcher EC, Donner CF, Midgren B, et al. . Survival in COPD patients with a daytime PaO2 greater than 60 mmHg with and without nocturnal oxyhemoglobin desaturation. Chest 1992; 101: 649–655. doi: 10.1378/chest.101.3.649 [DOI] [PubMed] [Google Scholar]
- 47.Nonoyama ML, Brooks D, Guyatt GH, et al. . Effect of oxygen on health quality of life in patients with chronic obstructive pulmonary disease with transient exertional hypoxemia. Am J Respir Crit Care Med 2007; 176: 343–349. doi: 10.1164/rccm.200702-308OC [DOI] [PubMed] [Google Scholar]
- 48.Moore RP, Berlowitz DJ, Denehy L, et al. . A randomised trial of domiciliary, ambulatory oxygen in patients with COPD and dyspnoea but without resting hypoxaemia. Thorax 2011; 66: 32–37. doi: 10.1136/thx.2009.132522 [DOI] [PubMed] [Google Scholar]
- 49.Eaton T, Garrett JE, Young P, et al. . Ambulatory oxygen improves quality of life of COPD patients: a randomised controlled study. Eur Respir J 2002; 20: 306–312. doi: 10.1183/09031936.02.00301002 [DOI] [PubMed] [Google Scholar]
- 50.Schneeberger T, Jarosch I, Leitl D, et al. . Automatic oxygen titration versus constant oxygen flow rates during walking in COPD: a randomised controlled, double-blind, crossover trial. Thorax 2021; in press [ 10.1136/thoraxjnl-2020-216509] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kofod LM, Westerdahl E, Kristensen MT, et al. . Effect of automated oxygen titration during walking on dyspnea and endurance in chronic hypoxemic patients with COPD: a randomized crossover trial. J Clin Med 2021; 10: 4820. doi: 10.3390/jcm10214820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Douglas NJ, White DP, Pickett CK, et al. . Respiration during sleep in normal man. Thorax 1982; 37: 840–844. doi: 10.1136/thx.37.11.840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yap JC, Watson RA, Gilbey S, et al. . Effects of posture on respiratory mechanics in obesity. J Appl Physiol 1995; 79: 1199–1205. doi: 10.1152/jappl.1995.79.4.1199 [DOI] [PubMed] [Google Scholar]
- 54.Ballard RD, Irvin CG, Martin RJ, et al. . Influence of sleep on lung volume in asthmatic patients and normal subjects. J Appl Physiol 1990; 68: 2034–2041. doi: 10.1152/jappl.1990.68.5.2034 [DOI] [PubMed] [Google Scholar]
- 55.Hudgel DW, Devadatta P. Decrease in functional residual capacity during sleep in normal humans. J Appl Physiol Respir Environ Exerc Physiol 1984; 57: 1319–1322. doi: 10.1152/jappl.1984.57.5.1319 [DOI] [PubMed] [Google Scholar]
- 56.Agusti A, Hedner J, Marin JM, et al. . Night-time symptoms: a forgotten dimension of COPD. Eur Respir Rev 2011; 20: 183–194. doi: 10.1183/09059180.00004311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kinsman RA, Yaroush RA, Fernandez E, et al. . Symptoms and experiences in chronic bronchitis and emphysema. Chest 1983; 83: 755–761. doi: 10.1378/chest.83.5.755 [DOI] [PubMed] [Google Scholar]
- 58.McSharry DG, Ryan S, Calverley P, et al. . Sleep quality in chronic obstructive pulmonary disease. Respirology 2012; 17: 1119–1124. doi: 10.1111/j.1440-1843.2012.02217.x [DOI] [PubMed] [Google Scholar]
- 59.Chen SJ, Yeh CM, Chao TF, et al. . The use of benzodiazepine receptor agonists and risk of respiratory failure in patients with chronic obstructive pulmonary disease: a nationwide population-based case-control study. Sleep 2015; 38: 1045–1050. doi: 10.5665/sleep.4808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elbehairy AF, Faisal A, McIsaac H, et al. . Mechanisms of orthopnoea in patients with advanced COPD. Eur Respir J 2021; 57: 2000754. doi: 10.1183/13993003.00754-2020 [DOI] [PubMed] [Google Scholar]
- 61.Heijdra YF, Dekhuijzen PN, van Herwaarden CL, et al. . Effects of body position, hyperinflation, and blood gas tensions on maximal respiratory pressures in patients with chronic obstructive pulmonary disease. Thorax 1994; 49: 453–458. doi: 10.1136/thx.49.5.453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eltayara L, Ghezzo H, Milic-Emili J. Orthopnea and tidal expiratory flow limitation in patients with stable COPD. Chest 2001; 119: 99–104. doi: 10.1378/chest.119.1.99 [DOI] [PubMed] [Google Scholar]
- 63.Trask CH, Cree EM. Oximeter studies on patients with chronic obstructive emphysema, awake and during sleep. N Engl J Med 1962; 266: 639–642. doi: 10.1056/NEJM196203292661303 [DOI] [PubMed] [Google Scholar]
- 64.Mulloy E, McNicholas WT. Ventilation and gas exchange during sleep and exercise in severe COPD. Chest 1996; 109: 387–394. doi: 10.1378/chest.109.2.387 [DOI] [PubMed] [Google Scholar]
- 65.Cormick W, Olson LG, Hensley MJ, et al. . Nocturnal hypoxaemia and quality of sleep in patients with chronic obstructive lung disease. Thorax 1986; 41: 846–854. doi: 10.1136/thx.41.11.846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krachman SL, Chatila W, Martin UJ, et al. . Physiologic correlates of sleep quality in severe emphysema. COPD 2011; 8: 182–188. doi: 10.3109/15412555.2011.560583 [DOI] [PubMed] [Google Scholar]
- 67.Fletcher EC, Miller J, Divine GW, et al. . Nocturnal oxyhemoglobin desaturation in COPD patients with arterial oxygen tensions above 60 mm Hg. Chest 1987; 92: 604–608. doi: 10.1378/chest.92.4.604 [DOI] [PubMed] [Google Scholar]
- 68.O'Donoghue FJ, Catcheside PG, Eckert DJ, et al. . Changes in respiration in NREM sleep in hypercapnic chronic obstructive pulmonary disease. J Physiol 2004; 559: 663–673. doi: 10.1113/jphysiol.2004.066084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Luo YM, He BT, Wu YX, et al. . Neural respiratory drive and ventilation in patients with chronic obstructive pulmonary disease during sleep. Am J Respir Crit Care Med 2014; 190: 227–229. doi: 10.1164/rccm.201402-0302LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Becker HF, Piper AJ, Flynn WE, et al. . Breathing during sleep in patients with nocturnal desaturation. Am J Respir Crit Care Med 1999; 159: 112–118. doi: 10.1164/ajrccm.159.1.9803037 [DOI] [PubMed] [Google Scholar]
- 71.Ballard RD, Clover CW, Suh BY. Influence of sleep on respiratory function in emphysema. Am J Respir Crit Care Med 1995; 151: 945–951. doi: 10.1164/ajrccm.151.4.7697271 [DOI] [PubMed] [Google Scholar]
- 72.Fletcher EC, Levin DC. Cardiopulmonary hemodynamics during sleep in subjects with chronic obstructive pulmonary disease. The effect of short- and long-term oxygen. Chest 1984; 85: 6–14. doi: 10.1378/chest.85.1.6 [DOI] [PubMed] [Google Scholar]
- 73.McNicholas WT, Fitzgerald MX. Nocturnal deaths among patients with chronic bronchitis and emphysema. Br Med J 1984; 289: 878. doi: 10.1136/bmj.289.6449.878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kitajima T, Marumo S, Amimoto H, et al. . Relationship between episodic nocturnal hypercapnia and history of exacerbations in patients with advanced chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2022; 17: 1553–1563. doi: 10.2147/COPD.S361914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Struik FM, Sprooten RT, Kerstjens HA, et al. . Nocturnal non-invasive ventilation in COPD patients with prolonged hypercapnia after ventilatory support for acute respiratory failure: a randomised, controlled, parallel-group study. Thorax 2014; 69: 826–834. doi: 10.1136/thoraxjnl-2014-205126 [DOI] [PubMed] [Google Scholar]
- 76.Calverley PM, Koulouris NG. Flow limitation and dynamic hyperinflation: key concepts in modern respiratory physiology. Eur Respir J 2005; 25: 186–199. doi: 10.1183/09031936.04.00113204 [DOI] [PubMed] [Google Scholar]
- 77.McKenzie J, Nisha P, Cannon-Bailey S, et al. . Overnight variation in tidal expiratory flow limitation in COPD patients and its correction: an observational study. Respir Res 2021; 22: 319. doi: 10.1186/s12931-021-01913-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet 2014; 383: 736–747. doi: 10.1016/S0140-6736(13)60734-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Benjafield AV, Ayas NT, Eastwood PR, et al. . Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med 2019; 7: 687–698. doi: 10.1016/S2213-2600(19)30198-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shawon MS, Perret JL, Senaratna CV, et al. . Current evidence on prevalence and clinical outcomes of co-morbid obstructive sleep apnea and chronic obstructive pulmonary disease: a systematic review. Sleep Med Rev 2017; 32: 58–68. doi: 10.1016/j.smrv.2016.02.007 [DOI] [PubMed] [Google Scholar]
- 81.Flenley DC. Sleep in chronic obstructive lung disease. Clin Chest Med 1985; 6: 651–661. doi: 10.1016/S0272-5231(21)00402-0 [DOI] [PubMed] [Google Scholar]
- 82.Marin JM, Soriano JB, Carrizo SJ, et al. . Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med 2010; 182: 325–331. doi: 10.1164/rccm.200912-1869OC [DOI] [PubMed] [Google Scholar]
- 83.Bradley TD, Rutherford R, Grossman RF, et al. . Role of daytime hypoxemia in the pathogenesis of right heart failure in the obstructive sleep apnea syndrome. Am Rev Respir Dis 1985; 131: 835–839. doi: 10.1164/arrd.1985.131.6.835 [DOI] [PubMed] [Google Scholar]
- 84.Hawrylkiewicz I, Sliwinski P, Gorecka D, et al. . Pulmonary haemodynamics in patients with OSAS or an overlap syndrome. Monaldi Arch Chest Dis 2004; 61: 148–152. doi: 10.4081/monaldi.2004.693 [DOI] [PubMed] [Google Scholar]
- 85.Resta O, Foschino Barbaro MP, Brindicci C, et al. . Hypercapnia in overlap syndrome: possible determinant factors. Sleep Breath 2002; 6: 11–18. doi: 10.1055/s-2002-23151 [DOI] [PubMed] [Google Scholar]
- 86.Konikkara J, Tavella R, Willes L, et al. . Early recognition of obstructive sleep apnea in patients hospitalized with COPD exacerbation is associated with reduced readmission. Hosp Pract 2016; 44: 41–47. doi: 10.1080/21548331.2016.1134268 [DOI] [PubMed] [Google Scholar]
- 87.Sterling KL, Pépin JL, Linde-Zwirble W, et al. . Impact of positive airway pressure therapy adherence on outcomes in patients with obstructive sleep apnea and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2022; 206: 197–205. doi: 10.1164/rccm.202109-2035OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yoshizaki A, Nagano T, Izumi S, et al. . Characteristics of the nocturnal desaturation waveform pattern of SpO2 in COPD patients: an observational study. Respir Res 2021; 22: 276. doi: 10.1186/s12931-021-01868-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lajoie AC, Series F, Bernard S, et al. . Reliability of home nocturnal oximetry in the diagnosis of overlap syndrome in COPD. Respiration 2020; 99: 132–139. doi: 10.1159/000505299 [DOI] [PubMed] [Google Scholar]
- 90.Scott AS, Baltzan MA, Wolkove N. Examination of pulse oximetry tracings to detect obstructive sleep apnea in patients with advanced chronic obstructive pulmonary disease. Can Respir J 2014; 21: 171–175. doi: 10.1155/2014/948717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alford NJ, Fletcher EC, Nickeson D. Acute oxygen in patients with sleep apnea and COPD. Chest 1986; 89: 30–38. doi: 10.1378/chest.89.1.30 [DOI] [PubMed] [Google Scholar]
- 92.Verbraecken J, McNicholas WT. Respiratory mechanics and ventilatory control in overlap syndrome and obesity hypoventilation. Respir Res 2013; 14: 132. doi: 10.1186/1465-9921-14-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dreher M, Neuzeret PC, Windisch W, et al. . Prevalence of chronic hypercapnia in severe chronic obstructive pulmonary disease: data from the HOmeVent registry. Int J Chron Obstruct Pulmon Dis 2019; 14: 2377–2384. doi: 10.2147/COPD.S222803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Raveling T, Vonk J, Struik FM, et al. . Chronic non-invasive ventilation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2021; 8: CD002878. doi: 10.1002/14651858.CD002878.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Clini E, Vitacca M, Foglio K, et al. . Long-term home care programmes may reduce hospital admissions in COPD with chronic hypercapnia. Eur Respir J 1996; 9: 1605–1610. doi: 10.1183/09031936.96.09081605 [DOI] [PubMed] [Google Scholar]
- 96.Clini E, Sturani C, Porta R, et al. . Outcome of COPD patients performing nocturnal non-invasive mechanical ventilation. Respir Med 1998; 92: 1215–1222. doi: 10.1016/S0954-6111(98)90424-3 [DOI] [PubMed] [Google Scholar]
- 97.Clini E, Sturani C, Rossi A, et al. . The Italian multicentre study on noninvasive ventilation in chronic obstructive pulmonary disease patients. Eur Respir J 2002; 20: 529–538. doi: 10.1183/09031936.02.02162001 [DOI] [PubMed] [Google Scholar]
- 98.McEvoy RD, Pierce RJ, Hillman D, et al. . Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomised controlled trial. Thorax 2009; 64: 561–566. doi: 10.1136/thx.2008.108274 [DOI] [PubMed] [Google Scholar]
- 99.Owens RL. Long-term domiciliary noninvasive ventilation for COPD. Respir Care 2021; 66: 1120–1127. doi: 10.4187/respcare.09052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dreher M, Ekkernkamp E, Walterspacher S, et al. . Noninvasive ventilation in COPD: impact of inspiratory pressure levels on sleep quality. Chest 2011; 140: 939–945. doi: 10.1378/chest.11-0253 [DOI] [PubMed] [Google Scholar]
- 101.Köhnlein T, Windisch W, Köhler D, et al. . Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med 2014; 2: 698–705. doi: 10.1016/S2213-2600(14)70153-5 [DOI] [PubMed] [Google Scholar]
- 102.Murphy PB, Rehal S, Arbane G, et al. . Effect of home noninvasive ventilation with oxygen therapy vs oxygen therapy alone on hospital readmission or death after an acute COPD exacerbation: a randomized clinical trial. JAMA 2017; 317: 2177–2186. doi: 10.1001/jama.2017.4451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hall J, Turner AM, Dretzke J, et al. . Cost-effectiveness of domiciliary non-invasive ventilation in patients with chronic obstructive pulmonary disease. Thorax 2022; 77: 976–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ankjærgaard KL, Tønnesen P, Laursen LC, et al. . Home non invasive ventilation (NIV) treatment for COPD patients with a history of NIV-treated exacerbation; a randomized, controlled, multi-center study. BMC Pulm Med 2016; 16: 32. doi: 10.1186/s12890-016-0184-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ambrosino N, Nava S, Bertone P, et al. . Physiologic evaluation of pressure support ventilation by nasal mask in patients with stable COPD. Chest 1992; 101: 385–391. doi: 10.1378/chest.101.2.385 [DOI] [PubMed] [Google Scholar]
- 106.Vitacca M, Ceriana P, Prediletto I, et al. . Intrinsic dynamic positive end-expiratory pressure in stable patients with chronic obstructive pulmonary disease. Respiration 2020; 99: 1129–1135. doi: 10.1159/000511266 [DOI] [PubMed] [Google Scholar]
- 107.Nava S, Ambrosino N, Rubini F, et al. . Effect of nasal pressure support ventilation and external PEEP on diaphragmatic activity in patients with severe stable COPD. Chest 1993; 103: 143–150. doi: 10.1378/chest.103.1.143 [DOI] [PubMed] [Google Scholar]
- 108.Elliott MW, Mulvey DA, Moxham J, et al. . Domiciliary nocturnal nasal intermittent positive pressure ventilation in COPD: mechanisms underlying changes in arterial blood gas tensions. Eur Respir J 1991; 4: 1044–1052. [PubMed] [Google Scholar]
- 109.Juan G, Calverley P, Talamo C, et al. . Effect of carbon dioxide on diaphragmatic function in human beings. N Engl J Med 1984; 310: 874–879. doi: 10.1056/NEJM198404053101402 [DOI] [PubMed] [Google Scholar]
- 110.Meecham Jones DJ, Paul EA, Jones PW, et al. . Nasal pressure support ventilation plus oxygen compared with oxygen therapy alone in hypercapnic COPD. Am J Respir Crit Care Med 1995; 152: 538–544. doi: 10.1164/ajrccm.152.2.7633704 [DOI] [PubMed] [Google Scholar]
- 111.Sin DD, Wong E, Mayers I, et al. . Effects of nocturnal noninvasive mechanical ventilation on heart rate variability of patients with advanced COPD. Chest 2007; 131: 156–163. doi: 10.1378/chest.06-1423 [DOI] [PubMed] [Google Scholar]
- 112.Ambrosino N, Nava S, Torbicki A, et al. . Haemodynamic effects of pressure support and PEEP ventilation by nasal route in patients with stable chronic obstructive pulmonary disease. Thorax 1993; 48: 523–528. doi: 10.1136/thx.48.5.523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ergan B, Oczkowski S, Rochwerg B, et al. . European Respiratory Society guidelines on long-term home non-invasive ventilation for management of COPD. Eur Respir J 2019; 54: 1901003. doi: 10.1183/13993003.01003-2019 [DOI] [PubMed] [Google Scholar]
- 114.Windisch W, Geiseler J, Simon K, et al. . German national guideline for treating chronic respiratory failure with invasive and non-invasive ventilation: Revised Edition 2017 - Part 1. Respiration 2018; 96: 66–97. doi: 10.1159/000488001 [DOI] [PubMed] [Google Scholar]
- 115.Windisch W, Geiseler J, Simon K, et al. . German national guideline for treating chronic respiratory failure with invasive and non-invasive ventilation – revised edition 2017: part 2. Respiration 2018; 96: 171–203. doi: 10.1159/000488667 [DOI] [PubMed] [Google Scholar]
- 116.Macrea M, Oczkowski S, Rochwerg B, et al. . Long-term noninvasive ventilation in chronic stable hypercapnic chronic obstructive pulmonary disease. An Official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med 2020; 202: e74–e87. doi: 10.1164/rccm.202006-2382ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Orr JE, Coleman JM 3rd, McSparron JI, et al. . Summary for clinicians: clinical practice guideline for long-term noninvasive ventilation in chronic stable hypercapnic chronic obstructive pulmonary disease. Ann Am Thorac Soc 2021; 18: 395–398. doi: 10.1513/AnnalsATS.202009-1171AG [DOI] [PubMed] [Google Scholar]
- 118.Janssens JP, Michel F, Schwarz EI, et al. . Long-term mechanical ventilation: recommendations of the Swiss Society of Pulmonology. Respiration 2020; 99: 867–902. doi: 10.1159/000510086 [DOI] [PubMed] [Google Scholar]
- 119.van den Biggelaar RJM, Hazenberg A, Cobben NAM, et al. . Home mechanical ventilation: the Dutch approach. Pulmonology 2022; 28: 99–104. doi: 10.1016/j.pulmoe.2021.11.001 [DOI] [PubMed] [Google Scholar]
- 120.Mineiro MA, Guimarães MJ, Winck JC. Organization of home mechanical ventilation in Portugal: characterization of current centers and a pathway to uniformization. Pulmonology 2020; 26: 84–89. doi: 10.1016/j.pulmoe.2019.05.006 [DOI] [PubMed] [Google Scholar]
- 121.Cosío BG, Hernández C, Chiner E, et al. . Spanish COPD Guidelines (GesEPOC 2021): non-pharmacological treatment update. Arch Bronconeumol 2022; 58: 345–351. doi: 10.1016/j.arbres.2021.08.010 [DOI] [PubMed] [Google Scholar]
- 122.Ambrosino N. The history of home ventilation: what have we learned? The Buyer's Guide 2014–2015: 31–34. [Google Scholar]
- 123.Vitacca M, Barbano L, D'Anna S, et al. . Comparison of five bilevel pressure ventilators in patients with chronic ventilatory failure: a physiologic study. Chest 2002; 122: 2105–2114. doi: 10.1378/chest.122.6.2105 [DOI] [PubMed] [Google Scholar]
- 124.Lebret M, Léotard A, Pépin JL, et al. . Nasal versus oronasal masks for home non-invasive ventilation in patients with chronic hypercapnia: a systematic review and individual participant data meta-analysis. Thorax 2021; 76: 1108–1116. doi: 10.1136/thoraxjnl-2020-215613 [DOI] [PubMed] [Google Scholar]
- 125.Ambrosino N, Clini E. Long-term mechanical ventilation and nutrition. Respir Med 2004; 98: 413–420. doi: 10.1016/j.rmed.2003.11.008 [DOI] [PubMed] [Google Scholar]
- 126.Duiverman ML. “Tricks and tips for home mechanical ventilation” Home mechanical ventilation: set-up and monitoring protocols. Pulmonology 2021; 27: 144–150. doi: 10.1016/j.pulmoe.2020.08.002 [DOI] [PubMed] [Google Scholar]
- 127.Collada-Carrasco J, Lamolda-Puyol C, Luján M, et al. . The addition of a humidifier device to a circuit and its impact on home ventilator performance: a bench study. Pulmonology 2020; 26: 363–369. doi: 10.1016/j.pulmoe.2019.11.004 [DOI] [PubMed] [Google Scholar]
- 128.Ribeiro C, Conde S, Oliveira P, et al. . Portuguese adaptation of the S3-non-invasive ventilation (S3-NIV) questionnaire for home mechanically ventilated patients. Pulmonology 2022; 28: 262–267. doi: 10.1016/j.pulmoe.2020.11.006 [DOI] [PubMed] [Google Scholar]
- 129.Ambrosino N, Vitacca M, Dreher M, et al. . Tele-monitoring of ventilator-dependent patients: a European Respiratory Society statement. Eur Respir J 2016; 48: 648–663. doi: 10.1183/13993003.01721-2015 [DOI] [PubMed] [Google Scholar]
- 130.Angelucci A, Aliverti A. Telemonitoring systems for respiratory patients: technological aspects. Pulmonology 2020; 26: 221–232. doi: 10.1016/j.pulmoe.2019.11.006 [DOI] [PubMed] [Google Scholar]
- 131.Vitacca M, Ambrosino N. Non-invasive ventilation as an adjunct to exercise training in chronic ventilatory failure: a narrative review. Respiration 2019; 97: 3–11. doi: 10.1159/000493691 [DOI] [PubMed] [Google Scholar]
- 132.Majorski DS, Magnet FS, Thilemann S, et al. . Portable NIV for patients with moderate to severe COPD: two randomized crossover trials. Respir Res 2021; 22: 123. doi: 10.1186/s12931-021-01710-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Borel JC, Pepin JL, Pison C, et al. . Long-term adherence with non-invasive ventilation improves prognosis in obese COPD patients. Respirology 2014; 19: 857–865. doi: 10.1111/resp.12327 [DOI] [PubMed] [Google Scholar]
- 134.Vanfleteren LE, Spruit MA, Groenen M, et al. . Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013; 187: 728–735. doi: 10.1164/rccm.201209-1665OC [DOI] [PubMed] [Google Scholar]
- 135.Duiverman ML, Windisch W, Storre JH, et al. . The role of NIV in chronic hypercapnic COPD following an acute exacerbation: the importance of patient selection? Ther Adv Respir Dis 2016; 10: 149–157. doi: 10.1177/1753465815624645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tuggey JM, Plant PK, Elliott MW. Domiciliary non-invasive ventilation for recurrent acidotic exacerbations of COPD: an economic analysis. Thorax 2003; 58: 867–871. doi: 10.1136/thorax.58.10.867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Clini EM, Magni G, Crisafulli E, et al. . Home non-invasive mechanical ventilation and long-term oxygen therapy in stable hypercapnic chronic obstructive pulmonary disease patients: comparison of costs. Respiration 2009; 77: 44–50. doi: 10.1159/000127410 [DOI] [PubMed] [Google Scholar]
- 138.Dretzke J, Blissett D, Dave C, et al. . The cost-effectiveness of domiciliary non-invasive ventilation in patients with end-stage chronic obstructive pulmonary disease: a systematic review and economic evaluation. Health Technol Assess 2015; 19: 1–246. doi: 10.3310/hta19810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Patout M, Lhuillier E, Kaltsakas G, et al. . Long-term survival following initiation of home non-invasive ventilation: a European study. Thorax 2020; 75: 965–973. doi: 10.1136/thoraxjnl-2019-214204 [DOI] [PubMed] [Google Scholar]