Abstract
Background
The strength of association between comorbidities and asthma has never been ranked in relation to the prevalence of the comorbidity in the nonasthma population. We investigated the strength of association between comorbidities and asthma.
Methods
A comprehensive literature search was performed for observational studies reporting data on comorbidities in asthma and nonasthma populations. A pairwise meta-analysis was performed and the strength of association calculated by anchoring odds ratios and 95% confidence intervals with the rate of comorbidities in nonasthma populations via Cohen's d method. Cohen's d=0.2, 0.5 and 0.8 were cut-off values for small, medium and large effect sizes, respectively; very large effect size resulted for Cohen's d >0.8. The review was registered in the PROSPERO database; identifier number CRD42022295657.
Results
Data from 5 493 776 subjects were analysed. Allergic rhinitis (OR 4.24, 95% CI 3.82–4.71), allergic conjunctivitis (OR 2.63, 95% CI 2.22–3.11), bronchiectasis (OR 4.89, 95% CI 4.48–5.34), hypertensive cardiomyopathy (OR 4.24, 95% CI 2.06–8.90) and nasal congestion (OR 3.30, 95% CI 2.96–3.67) were strongly associated with asthma (Cohen's d >0.5 and ≤0.8); COPD (OR 6.23, 95% CI 4.43–8.77) and other chronic respiratory diseases (OR 12.85, 95% CI 10.14–16.29) were very strongly associated with asthma (Cohen's d >0.8). Stronger associations were detected between comorbidities and severe asthma. No bias resulted according to funnel plots and Egger's test.
Conclusion
This meta-analysis supports the relevance of individualised strategies for disease management that look beyond asthma. A multidimensional approach should be used to assess whether poor symptom control is related to uncontrolled asthma or to uncontrolled underlying comorbidities.
Short abstract
Bronchiectasis, allergic rhinitis, nasal congestion, allergic conjunctivitis and hypertensive cardiomyopathy were strongly associated with asthma; COPD and other chronic respiratory diseases were very strongly associated with asthma. https://bit.ly/3XJxOTe
Introduction
Patients with asthma are more likely to suffer from a greater number of comorbidities than nonasthmatic subjects [1]. Rhinitis and rhinosinusitis, obesity, sleep disorders, gastro-oesophageal reflux disease (GORD), COPD and mental health disorders are among the most common conditions that can occur as asthma comorbidities [2].
The presence of comorbidities is related to poor asthma outcomes. Asthma exacerbations, worsening of asthma control and impairment of quality of life are more frequent in asthmatic subjects with other chronic conditions [3]. Furthermore, there is a higher economic burden in asthmatic patients affected by concomitant diseases, and in particular, psychological disorders have the greatest economic impact [4]. Several comorbidities are also associated with an increased risk of all-cause mortality in asthma [5]. Therefore, assessment and treatment of comorbidities is mandatory in asthma management [6], in accordance with an individualised management approach, which has been termed “treatable traits” [7–9]. Through this approach, specific characteristics of patients including phenotypes of airways disease, overlapping disorders, comorbidities, environmental and lifestyle factors, that potentially contribute to respiratory health, and are potentially amenable to specific treatments, could be systemically investigated and managed. In this regard, targeting treatable traits in severe asthma may lead to improvements in health-related quality of life, asthma control and reduced primary care acute visits [10].
In order to investigate the real burden of comorbidities in the asthma population, observational real-world studies provide data that may be generalisable to routine clinical practice [11]. For this purpose, calculation of odds ratios has been extensively used in epidemiological studies [12], and while statistically significant odds ratios indicate the presence of relationships between comorbidities and asthma, they do not provide sufficient information on the effect size [12]. A more informative approach is to anchor odds ratios and their 95% confidence intervals with the rate of comorbidities in nonasthma populations [12, 13].
In a post hoc analysis [14] it was shown that it is possible to rank the strength of association between specific chronic respiratory disorders (i.e. asthma, COPD, interstitial lung disease) and related comorbidities using the validated method of Chen et al. [12]. This statistical approach [12] provides information about the strength of relationship between a risk factor and a disease by using an effective method to clinically interpret odds ratios looking beyond the simple statistical significance that, conversely, could be misinterpreted as the effect size. Interestingly, results obtained from the theorem of Chen et al. [12] can be graphically represented to make the interpretation of the strength of association easier for clinicians [14].
Therefore, the aim of this systematic review and meta-analysis was to use this methodology to quantify the strength of association between comorbidities and asthma in observational real-world studies, in relation to the prevalence of the comorbidity in the nonasthma population, thereby looking beyond the simple statistical significance of the odds ratios.
Materials and methods
Search strategy and study eligibility
This quantitative synthesis was registered in the international prospective register of systematic reviews (PROSPERO identifier number CRD42022295657), and performed in agreement with the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) and Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guidelines [15, 16]. The flow diagram is shown in figure 1. This study satisfied all the recommended items reported by the MOOSE checklist (supplementary table S1) [16].
FIGURE 1.
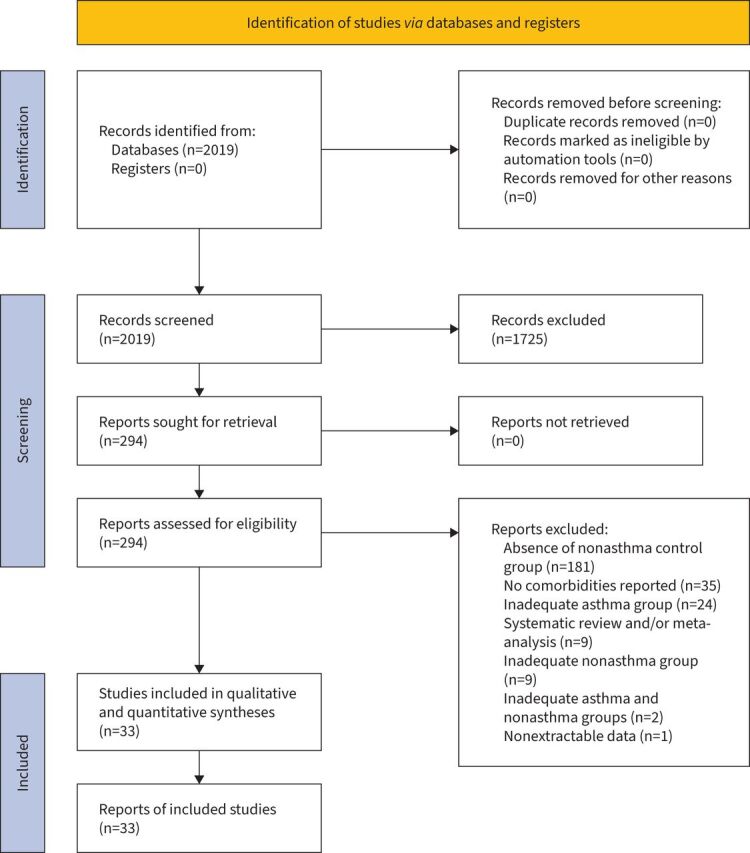
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) 2020 flow diagram for the identification of the studies included in the pairwise meta-analysis.
A comprehensive literature search was performed for observational studies written in English and reporting data on comorbidities in asthma populations compared to nonasthma populations.
In this regard, the Population, Exposure, Control and Outcome(s) (PICO) framework was applied to develop the literature search strategy, as reported previously [17]. The “population” included asthmatic and nonasthmatic subjects; the “exposure” regarded asthma; the “comparison” was performed with respect to a nonasthmatic group; and the assessed “outcome” was the strength of association of various comorbidities with asthma.
The search was performed in ClinicalTrials.gov, the Cochrane Central Register of Controlled Trials, Embase, European Union Clinical Trials Register, MEDLINE, Scopus and Web of Science, in order to provide for relevant studies published with no time limit up to 6 October 2021. The research string was as follows: asthma AND comorbidity AND (real OR observational OR cross-sectional OR retrospective OR prospective OR cohort) NOT (children OR pediatric) NOT (COVID-19 OR SARS-CoV2). As an example, supplementary table S2 reports the literature search terms used for Ovid MEDLINE.
Literature search results were uploaded into EPPI-Reviewer 4 (EPPI-Centre Software, London, UK), a web-based software programme for managing and analysing data in literature reviews that facilitates collaboration among reviewers during the study selection process.
Study selection
Observational studies (cohort, case–control, cross-sectional) comparing asthma versus nonasthma populations and reporting data on comorbidities were selected. When the design was not clearly reported in the study, it was assessed using previously published criteria [18].
Two reviewers independently examined the relevant studies identified from the literature search. The studies were selected in agreement with aforementioned criteria and any difference in opinion concerning the eligibility was resolved by discussion leading to consensus [19].
Data extraction
Data were extracted in agreement with Data Extraction for Complex Meta-Analysis (DECiMAL) recommendations [20] from published papers and/or supplementary data files, checked for study characteristics and duration of observation, number and characteristics of analysed subjects, number of asthmatic subjects, asthma diagnosis, age, gender, smoking habit and study quality assessment via the Newcastle–Ottawa Scale (NOS) score and Joanna Briggs Institute (JBI) critical appraisal checklist tool.
The inter- and intra-rater reliability for data abstraction was assessed via Cohen's κ score, as described previously [21]. Cohen's κ ≥0.80 indicated excellent agreement, coefficients between 0.61 and 0.80 represented substantial agreement, coefficients between 0.41 and 0.61 moderate agreement and <0.41 fair to poor agreement [21].
End-points
The end-point of this quantitative analysis was to assess the strength of association between comorbidities and asthma.
Data synthesis and analysis
A pairwise meta-analysis was performed and associations were expressed as OR (95% CI). Since data were selected from a series of studies performed by researchers operating independently and a common effect size cannot be assumed, a binary random-effects model was used in order to balance the study weights and adequately estimate the 95% confidence interval of the mean distribution of the odds ratios for the investigated variables [22–25].
Results were further analysed using the method validated by Chen et al. [12] to provide the strength of association between comorbidities and asthma via graphical representation, as described previously [14]. Briefly, the strength of association between comorbidities and asthma was investigated by calculating the OR (95% CI) between asthmatic and nonasthma populations according to the Cohen's d values, representing the standardised mean difference between two group means. Cohen's d=0.2, 0.5 and 0.8 are defined cut-off values for small, medium and large effect sizes, respectively [12, 26]. In this study, the calculated odds ratios equivalent to Cohen's d values were plotted according to the specific percentage of outcomes (prevalence of each comorbidity) in the nonasthma population [12]. Detailed equations delimiting the effect sizes were: weak association Y= −0.398×X/(1.929+X)−0.002×X+1.818; moderate association Y= −1.869×X/(1.236+X)−0.17×X+4.322; and strong association Y= −5.473×X/(1.081+X)−0.030×X+9.370; very strong association resulted in OR (95% CI) values greater than those of strong association [14].
Subgroup analyses were performed according to asthma severity.
Detailed information on data synthesis and analysis are reported in the supplementary data file.
Quality of the studies, risk of bias and evidence profile
The NOS was used to assess the quality of cohort and case–control studies [27] and the methodological quality of cross-sectional studies was evaluated by using the JBI critical appraisal checklist tool [28].
According to the NOS, a study can be awarded with a maximum of one star for each item within the selection and outcome (for cohort studies) or exposure (for case–control studies) categories, and a maximum of two stars can be given for comparability [27]. In the present quantitative analysis, the NOS quality assessment score was established to be in the range between zero and a maximum of eight stars, since the categories outcome for cohort studies and exposure for case–control studies were modified to fit the intrinsic characteristics of observational studies reporting the rates of various comorbidities retrospectively, which lack the assessment of nonresponse and follow-up rates. Studies reporting a NOS score ≥7 were considered of high quality, whereas those reporting a NOS score ≤6 were considered of low quality.
The checklist of the JBI critical appraisal checklist tool [28] consisted of eight question items assessing the inclusion criteria for the definition and detailed description of the sample; use of valid and reliable way to measure the exposure; use of objective and standard criteria to measure the condition; identification of and strategies to deal with confounding factors; use of a valid and reliable way to measure outcomes; and suitability of statistical analysis. In the present quantitative analysis, each item of the JBI checklist was rated as “yes” (1 point) and “no”, “unclear” or “not applicable” (0 points). The quality assessment score was calculated on the proportion of “yes” responses for the possible maximum score and judged at high risk, moderate risk or low risk of bias in agreement with the percentage of the achieved score: ≤49%, 50–69% or ≥70%, respectively.
The test for heterogeneity (I2) was performed to quantify the between-study dissimilarity [29] and sensitivity analyses were carried out according to study design to identify the studies that introduced substantial levels of heterogeneity (I2>50%) [30].
The risk of publication bias was assessed by applying the funnel plot and Egger's test as previously described [31–33] if ≥10 studies were included in the meta-analysis [34]. The equation of Egger's test was as follows: SND=a+b×precision, where SND represents the standard normal deviation (log of the odds ratio divided by its standard error), and precision represents the reciprocal of the standard error. Evidence of asymmetry from Egger's test was considered to be significant at p<0.1, and the graphical representation of 90% confidence bands is presented [31–33].
Two reviewers independently assessed the quality of studies, risk bias and evidence profile, and any difference in opinion was resolved by consensus.
Software and statistical significance
OpenMeta-Analyst was used to perform the pairwise meta-analysis [29] and GraphPad Prism (version 5.0, www.graphpad.com; La Jolla, CA, USA) software to graph the data. The statistical significance was assessed for p<0.05.
Results
Study characteristics
Out of the 2019 potentially relevant records screened by title and abstract, 33 studies were deemed eligible for qualitative and quantitative syntheses. Quantitative synthesis included data obtained from 15 cross-sectional studies [6, 35–48], 14 cohort studies [49–62] and four case–control studies [63–66]. Results were obtained from 5 493 776 subjects: 878 224 asthmatics, of who 1791 were affected by severe asthma, and 4 615 552 nonasthmatic subjects.
Two cohort studies [49, 57] and two case–control studies [63, 65] achieved a NOS score ≥7 and were considered of high quality, while 12 cohort studies [50–56, 58–62] and two case–control studies [64, 66] obtained a NOS score ≤6 and were regarded as of low quality. Quality assessment of cross-sectional studies indicated that five [6, 39, 43, 46, 48] were at low risk of bias, eight [35–38, 41, 44, 45, 47] were at medium risk of bias and two [40, 42] were at high risk of bias.
The inter-rater reliability for data abstraction was excellent before and after the learning process (Cohen's κ 0.96 and 1.00, respectively). The intra-rater reliability produced a Cohen's κ of 1.00 after the learning process.
Detailed patient demographics and study characteristics have been summarised in table 1.
TABLE 1.
Main characteristics of the observational studies included in the pairwise meta-analysis. When necessary, age, sex and smoking habit were reported as weighted arithmetic mean between asthma and nonasthma populations
| First author, year [ref.] | Country | Study characteristics | Duration of observation (years) | Subjects analysed | Asthmatic subjects | Groups of comparison | Subjects’ characteristics | Age (years) | Male (%) | Diagnosis of asthma | Current smokers (%) | NOS quality assessment | JBI checklist tool+ | |||
| Selection | Comparability | Outcome#/exposure¶ | Total | |||||||||||||
| Chalitsios, 2021 [49] | UK | Population-based, retrospective, longitudinal, cohort study | 13 | 658 749 | 138 123 (21.0) | Asthma versus nonasthma control | Subjects selected from the UK Clinical Practice Research Database | 51.8 | 41.0 | Read codes for asthma | 20.4 | **** | ** | ** | 8 | |
| Landré, 2020 [50] | France | Retrospective, cohort study | 26 | 12 345 | 372 (3.0) | Asthma versus nonasthma control | Subjects selected from the French GAZEL cohort of community-dwelling adults | 69.8 | 74.0 | Diagnosis defined by questionnaire | 7.0 | *** § | ƒ | 3 | ||
| Carter, 2019 [54] | UK | Retrospective, cohort study | ≈12 | 362 544 | 60 424 (16.7) | Asthma versus nonasthma control | Subjects admitted to NHS hospitals in the UK | 48.6 | 26.5 | Diagnostic ICD-10 and OPCS-4 disease codes | NA | *** § | ** | * ƒ | 6 | |
| Kim, 2019 [55] | Korea | Population-based, retrospective, longitudinal, cohort study | ≈11 | 226 118 | 113 059 (50.0) | Asthma versus nonasthma control | Subjects randomly selected from the Korean National Health Insurance Service Database | ≥20.0 | 37.3 | Asthma or status asthmaticus diagnostic ICD-10 codes: J45 or J46 of a physician diagnosis | NA | *** § | ** | * ƒ | 6 | |
| Kim, 2019 [40] | US | Population-based, retrospective, cross-sectional survey | ≈4 | 643 885 | 44 420 (6.9) | Asthma versus nonasthma control | Representative sample of civilian, non-institutionalised subjects of USA selected from the National Health Interview Survey | ≥18.0 | 48.0 | Diagnosis defined by questionnaire | 16.0 | High bias | ||||
| Bourdin, 2019 [63] | France | Population-based, retrospective, case–control study | 3 | 2760 | 690 (25.0) | Severe asthma versus nonasthma control | Subjects randomly selected from a French representative claims database | 61.0 | 34.3 | Diagnosis based on GINA recommendations (severe asthma patients received ≥1 dispensing for OMA and/or ≥10 dispensings of a medium or high dose of ICS+LABA) | NA | *** | ** | ** | 7 | |
| Toppila-Salmi, 2019 [64] | Finland | Population-based, retrospective, case–control study | 1 | 2890 | 1118 (38.7) | Asthma (includes severe asthma) versus nonasthma control | Subjects randomly selected from the Finnish Drug Reimbursement Register | 53.0 | 37.0 | Drug reimbursement decision of diagnosed asthma granted by prior physician's certificate, which includes background information, clinical examination results, lung function test results and findings and conclusions after asthma treatment test for 6 months | NA | *** | ** | * | 6 | |
| Varsano, 2017 [39] | Israel | Population-based, retrospective, cross-sectional study | 1 | 39 991 | 19 991 (50.0) | Nonsevere and severe asthma versus nonasthma control | Subjects selected from an Israeli population present in a national electronic healthcare insurance provider database | 42.2 | 23.8 | Asthma diagnostic ICD-9 CM code of a physician's diagnosis of bronchial asthma | 20.0 | Low bias | ||||
| Weatherburn, 2017 [38] | UK | Population-based, retrospective, cross-sectional study | NA | 1 424 378 | 84 505 (5.9) | Asthma versus nonasthma control | Representative sample of the Scottish population selected from the UK NHS database of primary care practice | ≥18.0 | 49.1 | Primary-care physician's diagnosis | 24.5 | Moderate bias | ||||
| Bozek, 2016 [37] | Poland | Population-based, retrospective, cross-sectional study | 1 | 2099 | 1023 (48.7) | Asthma versus nonasthma control | Representative population of all regions of Poland randomly selected from patient databases | 67.9 | 46.4 | Diagnosis based on clinical criteria according to GINA recommendations and a positive reversibility test after salbutamol according to the ATS/ERS criteria | 6.9 | Moderate bias | ||||
| Peng, 2015 [56] | Taiwan | Nationwide, retrospective, population-based, cohort study | ≈3 | 63 855 | 12 771 (20.0) | Asthma versus nonasthma control | Subjects randomly selected from the National Health Insurance Research Database of Taiwan | 53.7 | 45.8 | Asthma diagnostic ICD-9 CM code: 493 | NA | *** § | ** | * ƒ | 6 | |
| Van den Bemt, 2016 [57] | The Netherlands | Dynamic historical, longitudinal, cohort study | ≈20 | 2385 | 795 (33.3) | Asthma versus nonasthma control | Subjects selected from the Continuous Morbidity Registration Nijmegen database | 33.3 | 41.1 | Physician's diagnosis | NA | **** | ** | ** | 8 | |
| Yao, 2016 [58] | China | Population-based, retrospective, longitudinal, cohort study | 6 | 84 474 | 28 158 (33.3) | Asthma versus nonasthma control | Subjects randomly selected from the National Health Insurance Research Database of Taiwan | 54.5 | 46.3 | Asthma diagnostic ICD-9 CM code: 493; subjects who had ≥1 hospitalisation or ≥3 visits for outpatient medical services for asthma | NA | *** § | ** | * ƒ | 6 | |
| Cheng, 2015 [59] | Taiwan | Nationwide, retrospective, longitudinal, cohort study | 11 | 52 275 | 10 455 (20.0) | Asthma versus nonasthma control | Subjects randomly selected from the National Health Insurance Research Database of Taiwan | 59.8 | 41.3 | Asthma diagnostic ICD-9 CM code: 493; diagnosis by pulmonologist or rheumatologist on clinical judgement or pulmonary function test | NA | *** § | ** | * ƒ | 6 | |
| Alcázar Navarrete, 2015 [36] | Spain | Cross-sectional study | NA | 57 | 40 (70.2) | Asthma versus nonasthma control | Outpatients in an ambulatory setting | 60.8 | 31.6 | Previous physician diagnosis of bronchial asthma | 7.0 | Moderate bias | ||||
| Chung, 2014 [60] | Taiwan | Nationwide, retrospective, population-based, cohort study | 6 | 156 513 | 31 356 (20.0) | Asthma versus nonasthma control | Subjects randomly selected from the National Health Insurance Research Database of Taiwan | 38.9 | 49.0 | Asthma diagnostic ICD-9 code: 493 from ambulatory case visits or admission records | NA | *** § | ** | * ƒ | 6 | |
| Chung, 2014 [61] | Taiwan | Nationwide, retrospective, population-based, cohort study | 11 | 72 587 | 14 518 (20.0) | Asthma versus nonasthma control | Subjects randomly selected from the National Health Insurance Research Database of Taiwan | 52.1 | 45.7 | Asthma diagnostic ICD-9 CM code: 493 | NA | *** § | ** | * ƒ | 6 | |
| Steppuhn, 2014 [48] | Germany | Population-based, retrospective, cross-sectional survey | 2 | 43 189 | 2242 (5.2) | Asthma versus nonasthma control | Adults randomly selected for the national telephone health interview survey in Germany | 49.0 | 48.6 | Self-reported physician's diagnosis | 29.8 | Low bias | ||||
| Huang, 2014 [65] | Taiwan | Nationwide, prospective, population–based, case–control study (comorbidities were assessed retrospectively) | 3 | 140 344 | 35 086 (25.0) | Asthma versus nonasthma control | Subjects randomly selected from the National Health Insurance Research Database of Taiwan | 47.7 | 44.1 | Diagnosis by board-certified internist, clinical immunologist, pulmonologist or other medical experts | NA | **** | ** | ** | 8 | |
| Chen, 2014 [62] | Taiwan | Nationwide, retrospective, longitudinal, population-based, cohort study | 11 | 55 150 | 11 030 (20.0) | Asthma versus nonasthma control | Subjects randomly selected from the National Health Insurance Research Database of Taiwan | 60.9 | 41.7 | Asthma diagnostic ICD-9 CM code: 493 | NA | *** § | ** | * ƒ | 6 | |
| Sundbom, 2013 [47] | Sweden | Population-based, retrospective, cross-sectional survey | 1 | 25 610 | 1830 (7.1) | Asthma versus nonasthma control | Subjects randomly selected for the 2008 GA2LEN survey | 43.7 | 49.0 | Diagnosis defined by questionnaire | 13.8 | Moderate bias | ||||
| Marcon, 2013 [66] | Italy | Population-based, retrospective, multi-case–control study | ≈3 | 662 | 360 (54.4) | Mild asthma versus nonasthma control | Subjects randomly selected from the general population belonging to the Italian Study on Asthma in Young Adults cohort and to the Italian branch of the European Community Respiratory Health Survey cohort | 43.8 | 49.0 | Diagnosis defined by questionnaire and lung function tests | 22.4 | **** | * | 5 | ||
| Lu, 2013 [46] | Singapore | Population-based, retrospective, cross-sectional survey | 1 | 2809 | 106 (3.8) | Asthma versus nonasthma control | Adults randomly selected from the Singapore National Mental Health Survey | 20.0–59.0 | 38.2 | Self-report of a doctor's diagnosis | NA | Low bias | ||||
| Traister, 2013 [51] | USA | Retrospective, cohort study | ≈6 | 160 | 59 (36.9) | Asthma versus nonasthma control | Outpatients selected by random computer-generated sequence | 44.6 | 33.7 | Asthma diagnostic ICD-9 CM code: 493 and spirometry tests | 35.1 | *** § | ƒ | 3 | ||
| Patel, 2013 [45] | USA | Population-based, retrospective, cross-sectional survey | 8 | 22 172 | 2873 (13.0) | Asthma versus nonasthma control | Representative sample of civilian, non-institutionalised subjects of USA selected from the National Health and Nutrition Examination Survey | 46.7 | 48.1 | Self-report of a physician's diagnosis | NA | Moderate bias | ||||
| Iribarren, 2012 [52] | USA | Prospective, cohort study (comorbidities were assessed retrospectively) | 13 | 407 190 | 203 595 (50.0) | Asthma versus nonasthma control | Adults selected from the Kaiser Permanente Northern California healthcare plan | 44.6 | 34.0 | Medical records of hospitalisation with primary discharge code ICD-9 CM 493.00−493.99 or ≥1 secondary code for asthma with a principal ICD-9 code for acute asthma-related respiratory conditions, or outpatient or ED visits for asthma | 17.4 | *** § | ** | * ƒ | 6 | |
| Luyster, 2012 [44] | USA and UK | Retrospective, cross-sectional study | NA | 282 | 222 (78.7) | Nonsevere asthma and severe asthma versus nonasthma control | Participants selected from the retrospective multicentre Severe Asthma Research Program cohort study | 31.5 | 47.4 | Evaluation and classification according to the ATS definition of refractory asthma; diagnosis of severe asthma required continuous oral corticosteroid use or high-dose ICS use and ≥2 of the 7 minor criteria [67] | 0.0 | Moderate bias | ||||
| Cazzola, 2011 [6] | Italy | Population-based retrospective, cross-sectional study | 1 | 909 638 | 55 500 (6.1) | Asthma versus nonasthma control | Subjects selected from the Health Search Database of the Italian College of General Practitioners | >14.0 | 47.3 | Asthma diagnostic ICD-9 CM code: 493 | NA | Low bias | ||||
| Hakola, 2011 [53] | Finland | Prospective, cohort study (comorbidities were assessed retrospectively) | 1–4 | 64 951 | 2196 (3.4) | Persistent asthma versus nonasthma control | Finnish public sector employees selected from national registers | 44.1 | 20.0 | Physician's diagnosis confirmation by the Social Insurance Institution of Finland | 18.3 | *** § | * ƒ | 5 | ||
| Ng, 2007 [43] | Singapore | Population-based, retrospective, cross-sectional survey | 1 | 1092 | 61 (5.6) | Asthma versus nonasthma control | Older adults selected from the National Mental Health Survey of Elderly of Singapore | ≥60.0 | NA | Self-report of a doctor's diagnosis | NA | Low bias | ||||
| Adams, 2006 [42] | Australia | Population-based, retrospective, cross-sectional household telephone interview survey | 1 | 7443 | 834 (11.2) | Asthma versus nonasthma control | Adults selected from the Collaborative Health and Well-being Survey | ≥18.0 | 50.9 | Self-report of a doctor's diagnosis | NA | High bias | ||||
| Goodwin, 2003 [41] | USA | Retrospective, cross-sectional study | ≈2 | 998 | 176 (17.6) | Asthma versus nonasthma control | Primary care patients | 18.0–70.0 | 25.1 | Asthma diagnostic ICD-9 CM code: 493 of a primary-care physician's diagnosis | NA | Moderate bias | ||||
| Goodwin, 2003 [35] | Germany | Population-based, retrospective, cross-sectional, core survey | 1 | 4181 | 236 (5.6) | Nonsevere and severe asthma versus nonasthma control | Representative community sample of adults | 41.1 | 41.0 | Questionnaire and physician's diagnosis | NA | Moderate bias | ||||
Data are presented as n or n (%), unless otherwise stated. NOS: Newcastle–Ottawa Scale; JBI: Joanna Briggs Institute; NHS: National Health Service; ICD: International Statistical Classification of Diseases and Related Health Problems; OPCS: Office of Population Censuses and Surveys Classification of Interventions and Procedures; NA: not available; GINA: Global Initiative for Asthma; OMA: omalizumab; ICS: inhaled corticosteroids; LABA: long-acting β2-adrenoceptor agonists; CM: Clinical Modification; ATS: American Thoracic Society; ERS: European Respiratory Society; ED: emergency department. #: cohort studies could not be assigned a star for the outcome item “adequacy of follow-up of cohorts”, as outcomes of interest were all assessed retrospectively and there was no mention of losses; ¶: case–control studies could not be assigned a star for the exposure item “non-response rate”, as outcomes of interest were all assessed retrospectively; +: each of the eight items of the JBI tool was rated as “yes” (1 point) and “no” or “not applicable” (0 points). The score for each cross-sectional study was calculated on the proportion of “yes” responses for the possible maximum score and rated as high, moderate or low risk of bias according to the achieved score expressed as percentage (high bias: ≤49.0%; moderate bias: 50.0–69.0%; low bias ≥70.0%); §: no star could be assigned for the selection item “demonstration that outcome of interest was not present at start of study”, as outcomes of interest were already present at baseline; ƒ: no star could be assigned for the outcome item “was follow-up long enough for outcomes to occur”, as outcomes of interest were already present at baseline.
Strength of association between comorbidities and asthma
Psychiatric and neurological comorbidities
Alcohol and drug use disorders, anorexia or bulimia, blindness, deafness, epilepsy and learning disability were weakly associated with asthma. The upper 95% CI of alcohol use disorder reached moderate association level. Affective disorder, Alzheimer's disease, anxiety, depression, migraine, panic attack, phobia, psychiatric disorder, somatoform disorder and suicidal ideation were moderately associated with asthma. The upper 95% CI of panic attack (OR 2.09, 95% CI 1.59–2.75), phobia (OR 2.24, 95% CI 1.52–3.25), psychiatric disorder (OR 1.78, 95% CI 1.38–2.20) and suicidal ideation (OR 2.25, 95% CI 1.30–3.81) reached strong association levels. Detailed results are shown in figure 2a, supplementary figure S1 and supplementary table S3.
FIGURE 2.
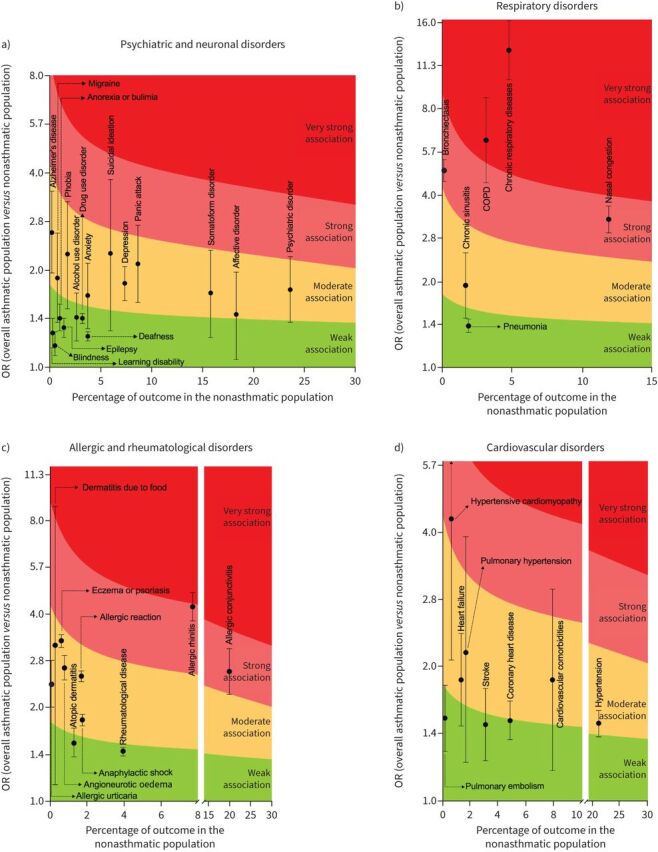
Analysis of the strength of association of specific a) psychiatric and neuronal disorders, b) respiratory disorders, c) allergic disorders and d) cardiovascular disorders with asthma.
Respiratory comorbidities
A weak association with asthma was observed for pneumonia, and a moderate association observed for chronic sinusitis. Bronchiectasis (OR 4.89, 95% CI 4.48–5.34) and nasal congestion (OR 3.30, 95% CI 2.96–3.67) were strongly associated with asthma. COPD (OR 6.23, 95% CI 4.43–8.77) and other chronic respiratory diseases (OR 12.85, 95% CI 10.14–16.29) were very strongly associated with asthma. The term “chronic respiratory diseases” was extracted exactly as it appeared from the primary papers. Since the overall classification of chronic respiratory diseases was reported in only one study [62] (International Classification of Diseases (ninth revision) codes 491, 492, 494, 496, 416.8, 416.9), the term “chronic respiratory diseases” should be considered unspecific in this meta-analysis. Detailed results are shown in figure 2b, supplementary figure S2 and supplementary table S3.
Allergic and rheumatological comorbidities
Dermatitis and rheumatological disease were weakly associated with asthma, although the upper 95% CI of atopic dermatitis achieved a moderate degree of association. Allergic reaction, allergic urticaria, anaphylactic shock, angioneurotic oedema, dermatitis due to food, and eczema or psoriasis were moderately associated with asthma. The upper 95% CI of dermatitis due to food achieved a strong to very strong association level (OR 3.19, 95% CI 1.14–8.89). Allergic conjunctivitis (OR 2.63, 95% CI 2.22–3.11) and allergic rhinitis (OR 4.24, 95% CI 3.82–4.71) were strongly associated with asthma, with the upper 95% CI of allergic rhinitis reaching the level of very strong association. Detailed results are shown in figure 2c, supplementary figure S3 and supplementary table S3.
Cardiovascular comorbidities
Pulmonary embolism, coronary heart disease and stroke were weakly associated with asthma, with their upper 95% CI reaching a moderate level of association; heart failure and hypertension were moderately associated with asthma. Cardiovascular comorbidities and pulmonary hypertension were moderately associated, and their upper 95% CI reached strong association (OR 1.86, 95% CI 1.17–2.96 and OR 2.14, 95% CI 1.22–3.88, respectively). Hypertensive cardiomyopathy was strongly associated with asthma and the upper 95% CI reached a very strong association (OR 4.24, 95% CI 2.06–8.90). Detailed results are shown in figure 2d, supplementary figure S4 and supplementary table S3.
Metabolic comorbidities
Diabetes, dyslipidaemia and thyroid disorders were weakly associated with asthma and, among these, the upper 95% CI of thyroid disorders reached moderate association; obesity was moderately associated with asthma. Detailed results are shown in figure 3a, supplementary figure S5 and supplementary table S3.
FIGURE 3.
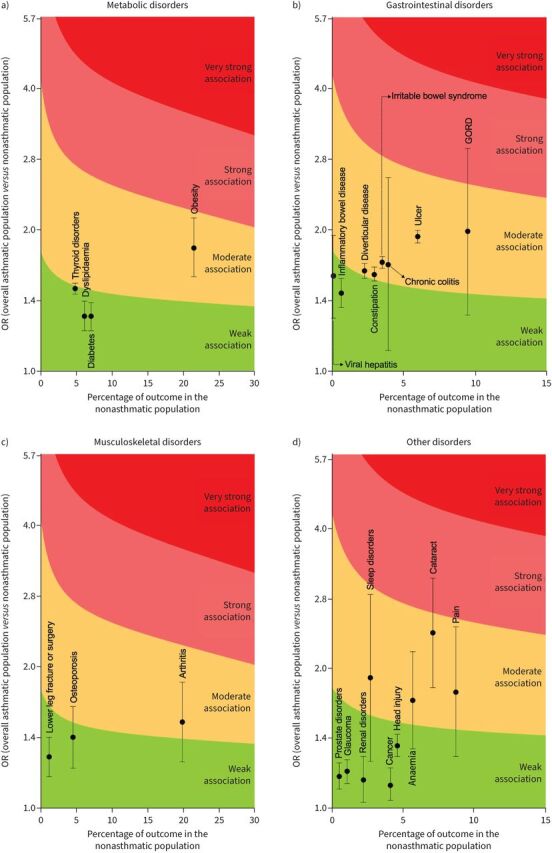
Analysis of the strength of association of specific a) metabolic disorders, b) gastrointestinal disorders, c) musculoskeletal disorders and d) other disorders with asthma. GORD: gastro-oesophageal reflux disease.
Gastrointestinal comorbidities
Inflammatory bowel disease and viral hepatitis were weakly associated with asthma, and the upper 95% CI of viral hepatitis reached a moderate association. Chronic colitis, constipation, diverticular disease, GORD, irritable bowel syndrome and ulcer were moderately associated with asthma, with the upper 95% CI of GORD reaching a strong association (OR 1.99, 95% CI 1.32–2.99). Detailed results are shown in figure 3b, supplementary figure S6 and supplementary table S3.
Musculoskeletal comorbidities
Osteoporosis and lower leg fracture or surgery were weakly associated with asthma, with the upper 95% CI of osteoporosis reaching a moderate association; arthritis was moderately associated with asthma. Detailed results are shown in figure 3c, supplementary figure S6 and supplementary table S3.
Other comorbidities
Cancer, head injury, glaucoma, prostate disorders and renal disorders were weakly associated with asthma. Anaemia, cataract, pain and sleep disorders were moderately associated with asthma, with the upper 95% CI of cataract reaching a strong association (OR 2.37, 95% CI 1.82–3.10). Detailed results are shown in figure 3d, supplementary figure S7 and supplementary table S3.
Strength of association between comorbidities and severe asthma
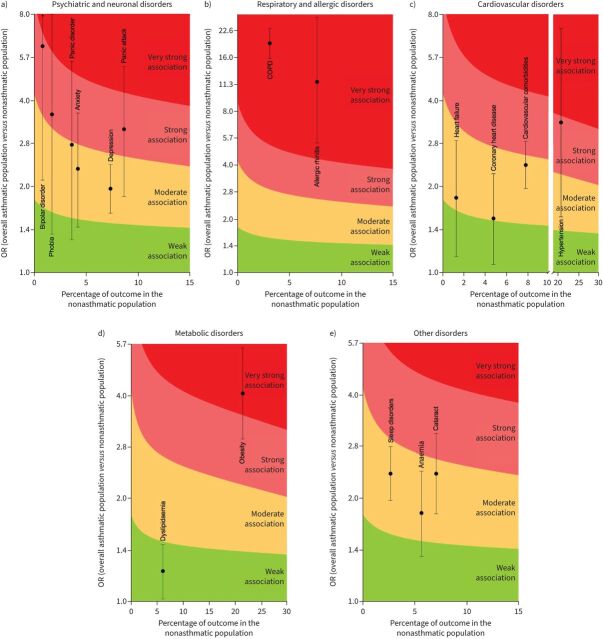
Moderate association was observed for anxiety, depression and panic disorder and a strong association was observed for panic attack, phobia and bipolar disorder. The upper 95% CI of panic attack (OR 3.16, 95% CI 1.84–5.24), panic disorders (OR 2.78, 95% CI 1.30–5.46), phobia (OR 3.56, 95% CI 1.37–7.96) and bipolar disorder (OR 6.16, 95% CI 2.10–15.2) reached a very strong association (figure 4a).
FIGURE 4.
Analysis of the strength of association of specific a) psychiatric and neurological disorders, b) respiratory and allergic disorders, c) cardiovascular disorders, d) metabolic disorders and e) other disorders with severe asthma.
Allergic rhinitis (OR 11.71, 95% CI 5.33–26.98) and COPD (OR 19.27, 95% CI 15.87–23.41) were very strongly associated with severe asthma (figure 4b).
Cardiovascular comorbidities, coronary heart disease and heart failure were moderately associated with severe asthma, with the upper 95% CI of cardiovascular comorbidities reaching a strong association (OR 2.38, 95% CI 1.97–2.88). Hypertension had a strong association with severe asthma and the upper 95% CI reached a very strong association (OR 3.35, 95% CI 1.57–7.14) (figure 4d).
Dyslipidaemia was weakly associated with severe asthma, whereas obesity (OR 4.06, 95% CI 2.99–5.51) showed a very strong association (figure 4e).
Anaemia, cataract and sleep disorders had a moderate association with severe asthma, and the upper 95% CI of cataract reached a strong association (OR 2.37, 95% CI 1.82–3.10) (figure 4c).
Detailed results concerning the strength of association between comorbidities and severe asthma are shown in supplementary figure S8 and supplementary table S4.
Quality of evidence and risk of bias
The NOS and JBI critical appraisal checklist tool scores are shown in table 1.
Generally, a substantial level of heterogeneity was detected for the association between the reported comorbidities and asthma (supplementary figures S1–S7). Sensitivity analyses performed according to study design generally did not resolve the substantial level of heterogeneity (data not shown). Regarding severe asthma, no heterogeneity was detected for the association with the reported comorbidities (supplementary figure S8).
The analysis of funnel plots confirmed heterogeneity, although the visual inspection evidenced neither dispersion nor asymmetry, with most outcome points clustering symmetrically around the top of the plots. Egger's tests confirmed the lack of significant publication bias. Detailed analysis of bias is reported in supplementary figures S9 and S10.
Discussion
Although several previous meta-analyses [1, 68–75] have investigated the association between comorbidities and asthma, this is the first study to have ranked via the Cohen's d approach the strength of association between multiple comorbidities and asthma or severe asthma, incorporating the prevalence of the comorbidity in the nonasthma population in this analysis. Thus, the strength of the association was determined by both the odds ratio and the prevalence of the comorbidity in the nonasthma population. More specifically, for a given OR (95% CI), the strength of the association between comorbidities and asthma was higher for those comorbidities which were more prevalent in the nonasthma population.
This study gives real-world evidence that allergic conjunctivitis, allergic rhinitis, bronchiectasis, hypertensive cardiomyopathy and nasal congestion are strongly associated with asthma, with COPD and other chronic respiratory diseases being very strongly associated. Concerning severe asthma, a strong association was found for panic attack, phobia, bipolar disorders and hypertension, and a very strong association resulted for allergic rhinitis, COPD and obesity. The clinical assessment of those comorbidities more strongly associated with asthma is crucial to achieve better asthma control and promote a change towards a patient-centred asthma management.
It is well recognised that disorders co-occurring with asthma enhance the complexity and heterogeneity of the disease [76]. The 2022 Global Initiative for Asthma (GINA) document [77] recommends the active management of comorbid conditions, as they may lead to the impairment of symptoms control, poor quality of life, interactions between medications and greater healthcare use, especially in severe asthma. When not recognised and properly treated, comorbidities may result in acute asthma flare-ups [78] or even cause symptoms that mimic asthma, potentially leading to misdiagnosis and inappropriate treatment [76]. However, paradoxically, the current GINA recommendations [77] report only a few comorbidities to be managed in asthmatic patients, namely obesity, GORD, anxiety and depression, food allergy and anaphylaxis, rhinitis, sinusitis and nasal polyps. Evidently, several comorbidities strongly to very strongly associated with asthma and severe asthma were missed in the section “Managing asthma with multimorbidity” of the GINA document [77], such as relevant comorbidities in the domains of respiratory disorders (bronchiectasis), cardiovascular disorders (i.e. hypertension, hypertensive cardiomyopathy) and psychiatric and neuronal disorders (i.e. bipolar disorder, phobia, panic attack). Perhaps future recommendations for asthma management should include these disorders as comorbidities to be managed in asthma.
Considering respiratory comorbidities, conflicting evidence still exists in literature concerning the real frequency estimates of bronchiectasis in asthma, ranging between 0.8% and 67.0% [38, 79–81]. This could be due to the use of different methodologies for diagnosis, high heterogeneity across the studies and small sample sizes [82]. Two recent meta-analyses [70, 75] demonstrated that coexistence of bronchiectasis correlated to greater asthma severity and increased risk of acute exacerbation.
The association between allergic rhinitis and asthma has been described by the united airway theory, the concept supported by epidemiological and pathophysiological evidence that explains the frequent interaction between the upper and lower airways [83]. Approximately 30.0–80.0% of asthmatic subjects are affected by allergic rhinitis [84], and moderate-to-severe allergic rhinitis could impair asthma control [85]. Thus, the “Allergic Rhinitis and its Impact on Asthma” document recommends assessing the presence of allergic rhinitis in asthmatic patients in order to optimise symptom management [86]. In observational studies, treatment of allergic rhinitis resulted in the improvement of upper and lower airway outcomes and reduced the risk for asthma-related hospitalisation and emergency department visits [87, 88].
The very strong level of association between COPD and asthma or severe asthma could be related to potential misclassification between the diseases due to the overlap of symptoms and clinical features, particularly in elderly patients and smokers who experience poor symptom control, frequent exacerbations, progressive lung function deterioration and impaired quality of life [77].
The association between asthma and cardiovascular diseases has been described in previous studies [89–91]. The strong association observed between hypertension and severe asthma could be in part related to systemic inflammation [92, 93], although hypertension may be a steroid-induced complication related to the use of inhaled corticosteroids in asthma [13]. Interestingly, the strong association between hypertensive cardiomyopathy and asthma could be also indirectly related to systemic inflammation, representing the natural progression of hypertensive heart disease associated to the chronic use of corticosteroids in asthmatic patients [94]. Unfortunately, the design of the primary studies included in this quantitative synthesis and the meta-analytical approach itself do not allow an assessment of the cause-and-effect relationship between cardiovascular disease and asthma [95].
Concerning obesity, it is proven that obese subjects are at increased risk of developing asthma, and obesity is associated with poor asthma outcomes [96, 97].
Of note, the present meta-analysis confirms data obtained from previous studies regarding the burden of psychiatric disorder in asthmatic subjects [1, 6, 68]. Phobia, panic attack and bipolar disorders were found to be strongly associated with severe asthma. Factors implicated in this relationship could be identified in expectation and fear of further asthma attacks, hyperventilation as a trigger for asthma symptoms and poor adherence to asthma treatment in patients with psychiatric diagnoses [6]. All these factors could impair asthma outcomes. Alternatively, phobia and panic attacks might lead to vocal cord dysfunction, which may present as severe asthma attacks.
Fortunately, we found that although chronic sinusitis, GORD and sleep disorders were significantly associated with asthma, the level of association was not strong.
The present study has limitations that warrant consideration. Our systematic review and meta-analysis was rigorously conducted according to MOOSE guidelines [16]; nevertheless, the effect estimates were characterised by a substantial level of heterogeneity observed across studies that was neither resolved by sensitivity analyses nor explained by subgroup analyses. The observational studies included in this quantitative synthesis may be intrinsically susceptible to heterogeneity based on data or design, such as differences in investigated populations or outcomes, survey recruitment, measurement instruments, timing of outcome measurements and data reporting [98].
In this regard, meta-analyses assessing broadly framed questions may assemble highly heterogeneous studies, especially when addressing the prevalence of phenomena in heterogeneous diseases such as asthma [77, 98]. Furthermore, the assessment of comorbid disorders greatly varies across the studies depending on the analysed population, diagnostic criteria and measurement tools [99]. Besides, observational studies are not always specifically designed to assess comorbidities as primary outcomes; therefore, we must acknowledge that the estimated odds ratios might be partly affected by lack of precision and potentially by diagnostic confusion, as a result of miscoding in claims-based patient treatment records. In addition, considering that observational studies are intrinsically susceptible to biases due to the extreme diversity of the included population, design and assessed outcomes, the interpretation of summary estimates resulting from this meta-analysis may be potentially problematic [16]. Certainly, randomised controlled trials (RCTs) are considered to be more reliable than observational studies, at least on the assessment of treatment effectiveness. However, it has been reported that results of well-performed observational studies do not systematically differ from those of RCTs on the same topic and that RCTs are often inadequate in reporting adverse events [100–102]. Moreover, generalisability can be limited for results from RCTs, because patients with multiple comorbidities are often excluded from these studies [103].
In recent well-performed meta-analyses of observational studies [98, 104], heterogeneity was confirmed by both funnel plot and Egger's test. Conversely, despite the substantial level of heterogeneity detected in our analysis, both funnel plot and Egger's test indicated that the association between comorbidities and asthma were not affected by significant bias. The clinical interpretation of this finding is that the direction of our effect estimates is correct and that perhaps results of future well-designed studies may report smaller 95% CI values.
Unexpectedly, we observed fewer comorbidities associated with severe asthma than with the general asthma population. This may be explained by considering that fewer studies specifically enrolled severe asthmatic subjects. However, when detected, the strength of association between severe comorbidities and asthma was generally higher compared to the analysis performed on the general asthma population.
Finally, considering that we analysed multiple outcomes, we cannot exclude that Type I error may have affected some of the reported result. However, the sample size of the investigated population suggests that our research was more than sufficiently powered to detect the comorbidities at the frequencies reported in the control group [105].
In conclusion, several comorbidities are strongly and very strongly associated with asthma and severe asthma. It is important to implement individualised strategies for asthma management that look beyond asthma [7–9]. According to the Brussels Declaration regarding the need for the change in asthma management, well-performed pragmatic and observational studies are needed to corroborate the findings of the present meta-analysis and assess the real impact of comorbidities that are more strongly associated with asthma and in particular with severe asthma [106, 107]. Indeed, this quantitative synthesis is a first step to help clinicians to better place each asthmatic patient in the context of their own comorbidities according to disease severity. This is of interest because we have demonstrated that even nonsevere patients may have a galaxy of concomitant disorders to be managed along with asthma. Correct diagnosis of these comorbidities is pivotal to optimise asthma management by a multidimensional approach and to assess whether poor symptom control is related to uncontrolled asthma or to uncontrolled underlying comorbidities. In turn, multidimensional assessment enables the detection of treatable traits, representing an effective approach for addressing the complexity of asthma [10]. However, although here we have quantified “how big is a big odds ratio” [12] for association between comorbidities and asthma by providing useful clinical implications, the question remains whether the level of severity of asthma is primary related to the severity of asthma itself or, especially in severely asthmatic patients, if asthma severity is due to untreated or undetected treatable traits related to pulmonary and/or extrapulmonary comorbidities [108].
Points for clinical practice
A multidimensional approach should be focused upon assessing whether poor symptom control is related to uncontrolled asthma or to uncontrolled/undiagnosed underlying comorbidities.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0202-2022.SUPPLEMENT (1.5MB, pdf)
Acknowledgements
We thank Beatrice Ludovica Ritondo (University of Rome “Tor Vergata”, Rome, Italy) for her support on the PRISMA 2020 flow diagram, PROSPERO and graphical representation of results.
Provenance: Submitted article, peer reviewed.
Data sharing: Specific data calculations are available from P. Rogliani and L. Calzetta upon request; P. Rogliani and L. Calzetta are the guarantors of this systematic review and meta-analysis.
Author contributions: P. Rogliani, R. Laitano, J. Ora, R. Beasley and L. Calzetta had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. P. Rogliani and L. Calzetta designed the statistical analyses. P. Rogliani and L. Calzetta wrote the first draft of the article, in consultation with R. Laitano, J. Ora and R. Beasley for data interpretations. All authors revised the article critically for important intellectual content, gave final approval of the version to be published, and agreed to be accountable for all aspects of the article in ensuring that questions related to the accuracy or integrity of any part of the article were appropriately investigated and resolved.
Conflict of interest: P. Rogliani participated as a lecturer and advisor in scientific meetings and courses under the sponsorship of Almirall, AstraZeneca, Biofutura, Boehringer Ingelheim, Chiesi Farmaceutici, GlaxoSmithKline, Menarini Group, Mundipharma, and Novartis, and her department was funded by Almirall, Boehringer Ingelheim, Chiesi Farmaceutici Novartis, and Zambon, outside the submitted work.
Conflict of interest: R. Laitano has nothing to disclose.
Conflict of interest: J. Ora has nothing to disclose.
Conflict of interest: R. Beasley reports grants and personal fees from AstraZeneca, grants from GlaxoSmithKline and Genentech, personal fees from Avillion and Theravance, outside the submitted work.
Conflict of interest: L. Calzetta has participated as advisor in scientific meetings under the sponsorship of Boehringer Ingelheim and Novartis; received nonfinancial support from AstraZeneca; a research grant partially funded by Chiesi Farmaceutici, Boehringer Ingelheim, Novartis, and Almirall; is or has been a consultant to ABC Farmaceutici, Edmond Pharma, Zambon, Verona Pharma, and Ockham Biotech; and his department was funded by Almirall, Boehringer Ingelheim, Chiesi Farmaceutici, Novartis, and Zambon, outside the submitted work.
References
- 1.Su X, Ren Y, Li M, et al. Prevalence of comorbidities in asthma and nonasthma patients: a meta-analysis. Medicine 2016; 95: e3459. doi: 10.1097/MD.0000000000003459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO) . Asthma. www.who.int/news-room/fact-sheets/detail/asthma. Date last accessed: 22 February 2022.
- 3.Tay TR, Radhakrishna N, Hore-Lacy F, et al. Comorbidities in difficult asthma are independent risk factors for frequent exacerbations, poor control and diminished quality of life. Respirology 2016; 21: 1384–1390. doi: 10.1111/resp.12838 [DOI] [PubMed] [Google Scholar]
- 4.Chen W, Lynd LD, Fitzgerald JM, et al. Excess medical costs in patients with asthma and the role of comorbidity. Eur Respir J 2016; 48: 1584–1592. doi: 10.1183/13993003.01141-2016 [DOI] [PubMed] [Google Scholar]
- 5.Sumino K, O'Brian K, Bartle B, et al. Coexisting chronic conditions associated with mortality and morbidity in adult patients with asthma. J Asthma 2014; 51: 306–314. doi: 10.3109/02770903.2013.879881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cazzola M, Calzetta L, Bettoncelli G, et al. Asthma and comorbid medical illness. Eur Respir J 2011; 38: 42–49. doi: 10.1183/09031936.00140310 [DOI] [PubMed] [Google Scholar]
- 7.Agusti A, Bel E, Thomas M, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J 2016; 47: 410–419. doi: 10.1183/13993003.01359-2015 [DOI] [PubMed] [Google Scholar]
- 8.Pavord ID, Beasley R, Agusti A, et al. After asthma: redefining airways diseases. Lancet 2018; 391: 350–400. doi: 10.1016/S0140-6736(17)30879-6 [DOI] [PubMed] [Google Scholar]
- 9.Agustí A, Bafadhel M, Beasley R, et al. Precision medicine in airway diseases: moving to clinical practice. Eur Respir J 2017; 50: 1701655. doi: 10.1183/13993003.01655-2017 [DOI] [PubMed] [Google Scholar]
- 10.McDonald VM, Clark VL, Cordova-Rivera L, et al. Targeting treatable traits in severe asthma: a randomised controlled trial. Eur Respir J 2020; 55: 1901509. doi: 10.1183/13993003.01509-2019 [DOI] [PubMed] [Google Scholar]
- 11.Price D, Brusselle G, Roche N, et al. Real-world research and its importance in respiratory medicine. Breathe 2015; 11: 27–38. doi: 10.1183/20734735.015414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen H, Cohen P, Chen S. How big is a big odds ratio? Interpreting the magnitudes of odds ratios in epidemiological studies. Commun Stat Simul Comput 2010; 39: 860–864. doi:101080/03610911003650383 [Google Scholar]
- 13.Szumilas M. Explaining odds ratios. J Can Acad Child Adolesc Psychiatry 2010; 19: 227–229. doi: 10.1007/s00787-010-0087-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogliani P, Cazzola M, Calzetta L. Cardiovascular disease in chronic respiratory disorders and beyond. J Am Coll Cardiol 2019; 73: 2178–2180. doi: 10.1016/j.jacc.2018.11.068 [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015; 4: 1. doi: 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283: 2008–2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 17.Morgan RL, Whaley P, Thayer KA, et al. Identifying the PECO: a framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ Int 2018; 121: 1027–1031. doi: 10.1016/j.envint.2018.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Understanding observational studies. Drug Ther Bull 2016; 54: 105–108. doi: 10.1136/dtb.2016.9.0426 [DOI] [PubMed] [Google Scholar]
- 19.Li T, Higgins JP, Deeks JJ. Reliability and reaching consensus. In: Higgins J, Thomas J, Chandler J, et al., eds. Cochrane Handbook for Systematic Reviews of Interventions version 62. 2021. https://training.cochrane.org/handbook/current. Date last updated: February 2021. [Google Scholar]
- 20.Pedder H, Sarri G, Keeney E, et al. Data extraction for complex meta-analysis (DECiMAL) guide. Syst Rev 2016; 5: 212. doi: 10.1186/s13643-016-0368-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gianinazzi ME, Rueegg CS, Zimmerman K, et al. Intra-rater and inter-rater reliability of a medical record abstraction study on transition of care after childhood cancer. PLoS One 2015; 10: e0124290. doi: 10.1371/journal.pone.0124290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borenstein M, Hedges LV, Higgins JPT, et al. Introduction to Meta-Analysis. Chichester, John Wiley & Sons, 2009. [Google Scholar]
- 23.DeCoster J. Meta-Analysis Notes. 2004. Available from: www.stat-help.com/notes.html.
- 24.Turner JR, Durham TA. Meta-methodology: conducting and reporting meta-analyses. J Clin Hypertens 2014; 16: 91–93. doi: 10.1111/jch.12215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cazzola M, Calzetta L, Page C, et al. Influence of N-acetylcysteine on chronic bronchitis or COPD exacerbations: a meta-analysis. Eur Respir Rev 2015; 24: 451–461. doi: 10.1183/16000617.00002215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd Edn. New York, Routledge, 1988; pp. 413–414. [Google Scholar]
- 27.Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analysis. 2022. Available from https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 28.Moola S, Munn Z, Tufanaru C, et al. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E, Munn Z, eds. JBI Manual for Evidence Synthesis. 2020. Available from https://synthesismanual.jbi.global. [Google Scholar]
- 29.Wallace BC, Dahabreh IJ, Trikalinos TA, et al. Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Softw 2012; 49: 1–15. doi: 10.18637/jss.v049.i05 [DOI] [Google Scholar]
- 30.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sterne JAC, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol 2000; 53: 1119–1129. doi: 10.1016/S0895-4356(00)00242-0 [DOI] [PubMed] [Google Scholar]
- 32.Sterne JAC, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol 2001; 54: 1046–1055. doi: 10.1016/S0895-4356(01)00377-8 [DOI] [PubMed] [Google Scholar]
- 33.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins J, Thomas J, Chandler J, et al., eds. Cochrane Handbook for Systematic Reviews of Interventions. 2022. https://training.cochrane.org/handbook/current.
- 35.Goodwin RD, Jacobi F, Thefeld W. Mental disorders and asthma in the community. Arch Gen Psychiatry 2003; 60: 1125–1130. doi: 10.1001/archpsyc.60.11.1125 [DOI] [PubMed] [Google Scholar]
- 36.Alcázar Navarrete B, Gómez-Moreno G, Aguilar-Salvatierra A, et al. Xerostomia relates to the degree of asthma control. J Oral Pathol Med 2015; 44: 273–277. doi: 10.1111/jop.12228 [DOI] [PubMed] [Google Scholar]
- 37.Bozek A, Rogala B, Bednarski P. Asthma, COPD and comorbidities in elderly people. J Asthma 2016; 53: 943–947. doi: 10.3109/02770903.2016.1170139 [DOI] [PubMed] [Google Scholar]
- 38.Weatherburn CJ, Guthrie B, Mercer SW, et al. Comorbidities in adults with asthma: population-based cross-sectional analysis of 1.4 million adults in Scotland. Clin Exp Allergy 2017; 47: 1246–1252. doi: 10.1111/cea.12971 [DOI] [PubMed] [Google Scholar]
- 39.Varsano S, Segev D, Shitrit D. Severe and non-severe asthma in the community: a large electronic database analysis. Respir Med 2017; 123: 131–139. doi: 10.1016/j.rmed.2016.12.017 [DOI] [PubMed] [Google Scholar]
- 40.Kim M, Tillis W, Patel P, et al. Association between asthma/chronic obstructive pulmonary disease overlap syndrome and healthcare utilization among the US adult population. Curr Med Res Opin 2019; 35: 1191–1196. doi: 10.1080/03007995.2019.1565531 [DOI] [PubMed] [Google Scholar]
- 41.Goodwin RD, Olfson M, Shea S, et al. Asthma and mental disorders in primary care. Gen Hosp Psychiatry 2003; 25: 479–483. doi: 10.1016/S0163-8343(03)00071-9 [DOI] [PubMed] [Google Scholar]
- 42.Adams RJ, Wilson DH, Taylor AW, et al. Coexistent chronic conditions and asthma quality of life: a population-based study. Chest 2006; 129: 285–291. doi: 10.1378/chest.129.2.285 [DOI] [PubMed] [Google Scholar]
- 43.Ng TP, Chiam PC, Kua EH. Mental disorders and asthma in the elderly: a population-based study. Int J Geriatr Psychiatry 2007; 22: 668–674. doi: 10.1002/gps.1728 [DOI] [PubMed] [Google Scholar]
- 44.Luyster FS, Teodorescu M, Bleecker E, et al. Sleep quality and asthma control and quality of life in non-severe and severe asthma. Sleep Breath 2012; 16: 1129–1137. doi: 10.1007/s11325-011-0616-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel MR, Janevic MR, Heeringa SG, et al. An examination of adverse asthma outcomes in U.S. adults with multiple morbidities. Ann Am Thorac Soc 2013; 10: 426–431. doi: 10.1513/AnnalsATS.201302-032OC [DOI] [PubMed] [Google Scholar]
- 46.Lu Y, Feng L, Lim L, et al. Asthma, life events and psychiatric disorders: a population-based study. Soc Psychiatry Psychiatr Epidemiol 2013; 48: 1273–1282. doi: 10.1007/s00127-013-0655-5 [DOI] [PubMed] [Google Scholar]
- 47.Sundbom F, Lindberg E, Bjerg A, et al. Asthma symptoms and nasal congestion as independent risk factors for insomnia in a general population: results from the GA2LEN survey. Allergy 2013; 68: 213–219. doi: 10.1111/all.12079 [DOI] [PubMed] [Google Scholar]
- 48.Steppuhn H, Langen U, Keil T, et al. Chronic disease co-morbidity of asthma and unscheduled asthma care among adults: results of the national telephone health interview survey German Health Update (GEDA) 2009 and 2010. Prim Care Respir J 2014; 23: 22–29. doi: 10.4104/pcrj.2013.00107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chalitsios CV, McKeever TM, Shaw DE. Incidence of osteoporosis and fragility fractures in asthma: a UK population-based matched cohort study. Eur Respir J 2021; 57: 2001251. doi: 10.1183/13993003.01251-2020 [DOI] [PubMed] [Google Scholar]
- 50.Landré B, Nadif R, Goldberg M, et al. Asthma is associated with frailty among community-dwelling adults: the GAZEL cohort. BMJ Open Respir Res 2020; 7: e000526. doi: 10.1136/bmjresp-2019-000526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Traister RS, Fajt ML, Whitman-Purves E, et al. A retrospective analysis comparing subjects with isolated and coexistent vocal cord dysfunction and asthma. Allergy Asthma Proc 2013; 34: 349–355. doi: 10.2500/aap.2013.34.3673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iribarren C, Tolstykh IV, Miller MK, et al. Adult asthma and risk of coronary heart disease, cerebrovascular disease, and heart failure: a prospective study of 2 matched cohorts. Am J Epidemiol 2012; 176: 1014–1024. doi: 10.1093/aje/kws181 [DOI] [PubMed] [Google Scholar]
- 53.Hakola R, Kauppi P, Leino T, et al. Persistent asthma, comorbid conditions and the risk of work disability: a prospective cohort study. Allergy 2011; 66: 1598–1603. doi: 10.1111/j.1398-9995.2011.02729.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carter P, Lagan J, Fortune C, et al. Association of cardiovascular disease with respiratory disease. J Am Coll Cardiol 2019; 73: 2166–2177. doi: 10.1016/j.jacc.2018.11.063 [DOI] [PubMed] [Google Scholar]
- 55.Kim SY, Min C, Oh DJ, et al. Bidirectional association between asthma and migraines in adults: two longitudinal follow-up studies. Sci Rep 2019; 9: 18343. doi: 10.1038/s41598-019-54972-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peng YH, Wu BR, Su CH, et al. Adult asthma increases dementia risk: a nationwide cohort study. J Epidemiol Community Health 2015; 69: 123–128. doi: 10.1136/jech-2014-204445 [DOI] [PubMed] [Google Scholar]
- 57.van den Bemt L, Luijks H, Bor H, et al. Are asthma patients at increased risk of clinical depression? A longitudinal cohort study. J Asthma 2016; 53: 43–49. doi: 10.3109/02770903.2015.1059852 [DOI] [PubMed] [Google Scholar]
- 58.Yao C-W, Shen T-C, Lu C-R, et al. Asthma is associated with a subsequent risk of peripheral artery disease: a longitudinal population-based study. Medicine 2016; 95: e2546. doi: 10.1097/MD.0000000000002546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheng CM, Wu YH, Tsai SJ, et al. Risk of developing Parkinson's disease among patients with asthma: a nationwide longitudinal study. Allergy 2015; 70: 1605–1612. doi: 10.1111/all.12758 [DOI] [PubMed] [Google Scholar]
- 60.Chung WS, Lin CL, Ho FM, et al. Asthma increases pulmonary thromboembolism risk: a nationwide population cohort study. Eur Respir J 2014; 43: 801–807. doi: 10.1183/09031936.00043313 [DOI] [PubMed] [Google Scholar]
- 61.Chung WS, Lin CL, Chen YF, et al. Increased stroke risk among adult asthmatic patients. Eur J Clin Invest 2014; 44: 1025–1033. doi: 10.1111/eci.12336 [DOI] [PubMed] [Google Scholar]
- 62.Chen MH, Li CT, Tsai CF, et al. Risk of dementia among patients with asthma: a nationwide longitudinal study. J Am Med Dir Assoc 2014; 15: 763–767. doi: 10.1016/j.jamda.2014.06.003 [DOI] [PubMed] [Google Scholar]
- 63.Bourdin A, Fabry-Vendrand C, Ostinelli J, et al. The burden of severe asthma in France: a case-control study using a medical claims database. J Allergy Clin Immunol Pract 2019; 7: 1477–1487. doi: 10.1016/j.jaip.2018.12.029 [DOI] [PubMed] [Google Scholar]
- 64.Toppila-Salmi S, Chanoine S, Karjalainen J, et al. Risk of adult-onset asthma increases with the number of allergic multimorbidities and decreases with age. Allergy 2019; 74: 2406–2416. doi: 10.1111/all.13971 [DOI] [PubMed] [Google Scholar]
- 65.Huang HL, Ho SY, Li CH, et al. Bronchial asthma is associated with increased risk of chronic kidney disease. BMC Pulm Med 2014; 14: 80. doi: 10.1186/1471-2466-14-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marcon A, Girardi P, Ferrari M, et al. Mild asthma and chronic bronchitis seem to influence functional exercise capacity: a multi-case control study. Int Arch Allergy Immunol 2013; 161: 181–188. doi: 10.1159/000345137 [DOI] [PubMed] [Google Scholar]
- 67.Wenzel SE, Fahy JV, Irvin C, et al. Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. Am J Respir Crit Care Med 2000; 162: 2341–2351. doi: 10.1164/ajrccm.162.6.ats9-00 [DOI] [PubMed] [Google Scholar]
- 68.Wu MK, Wang HY, Chen YW, et al. Significantly higher prevalence rate of asthma and bipolar disorder co-morbidity: a meta-analysis and review under PRISMA guidelines. Medicine 2016; 95: e3217. doi: 10.1097/MD.0000000000003217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee JH, An J, Won HK, et al. Prevalence and impact of comorbid laryngeal dysfunction in asthma: a systematic review and meta-analysis. J Allergy Clin Immunol 2020; 145: 1165–1173. doi: 10.1016/j.jaci.2019.12.906 [DOI] [PubMed] [Google Scholar]
- 70.Zhang SQ, Xiong XF, Wu ZH, et al. Clinical features of asthma with comorbid bronchiectasis: a systematic review and meta-analysis. Medicine 2021; 100: e23858. doi: 10.1097/MD.0000000000023858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gao YH, Zhao HS, Zhang FR, et al. The relationship between depression and asthma: a meta-analysis of prospective studies. PLoS One 2015; 10: e0132424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang L, Deng ZR, Zu MD, et al. The comorbid relationship between migraine and asthma: a systematic review and meta-analysis of population-based studies. Front Med 2021; 7: 609528. doi: 10.3389/fmed.2020.609528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang L, Zhang X, Zheng J, et al. Co-morbid psychological dysfunction is associated with a higher risk of asthma exacerbations: a systematic review and meta-analysis. J Thorac Dis 2016; 8: 1257–1268. doi: 10.21037/jtd.2016.04.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ye G, Baldwin DS, Hou R. Anxiety in asthma: a systematic review and meta-analysis. Psychol Med 2021; 51: 11–20. doi: 10.1017/S0033291720005097 [DOI] [PubMed] [Google Scholar]
- 75.Lan G, Huang C, Liu Y, et al. How does comorbid bronchiectasis affect asthmatic patients? A meta-analysis. J Asthma 2021; 58: 1314–1328. doi: 10.1080/02770903.2019.1656230 [DOI] [PubMed] [Google Scholar]
- 76.Gibson PG, McDonald VM, Granchelli A, et al. Asthma and comorbid conditions – pulmonary comorbidity. J Allergy Clin Immunol Pract 2021; 9: 3868–3875. doi: 10.1016/j.jaip.2021.08.028 [DOI] [PubMed] [Google Scholar]
- 77.Global Initiative for Asthma (GINA) . Global Strategy for Asthma Management and Prevention. 2022. https://ginasthma.org/wp-content/uploads/2022/07/GINA-Main-Report-2022-FINAL-22-07-01-WMS.pdf. Date last accessed: 13 September 2022.
- 78.Boulet LP, Boulay MÈ. Asthma-related comorbidities. Expert Rev Respir Med 2011; 5: 377–393. doi: 10.1586/ers.11.34 [DOI] [PubMed] [Google Scholar]
- 79.Coman I, Pola-Bibián B, Barranco P, et al. Bronchiectasis in severe asthma: clinical features and outcomes. Ann Allergy Asthma Immunol 2018; 120: 409–413. doi: 10.1016/j.anai.2018.02.016 [DOI] [PubMed] [Google Scholar]
- 80.Dimakou K, Gousiou A, Toumbis M, et al. Investigation of bronchiectasis in severe uncontrolled asthma. Clin Respir J 2018; 12: 1212–1218. doi: 10.1111/crj.12653 [DOI] [PubMed] [Google Scholar]
- 81.Padilla-Galo A, Olveira C, Fernández de Rota-Garcia L, et al. Factors associated with bronchiectasis in patients with uncontrolled asthma; the NOPES score: a study in 398 patients. Respir Res 2018; 19: 43. doi: 10.1186/s12931-018-0746-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Crimi C, Ferri S, Crimi N. Bronchiectasis and asthma: a dangerous liaison? Curr Opin Allergy Clin Immunol 2019; 19: 46–52. doi: 10.1097/ACI.0000000000000492 [DOI] [PubMed] [Google Scholar]
- 83.Khan DA. Allergic rhinitis and asthma: epidemiology and common pathophysiology. Allergy Asthma Proc 2014; 35: 357–361. doi: 10.2500/aap.2014.35.3794 [DOI] [PubMed] [Google Scholar]
- 84.Compalati E, Ridolo E, Passalacqua G, et al. The link between allergic rhinitis and asthma: the united airways disease. Expert Rev Clin Immunol 2010; 6: 413–423. doi: 10.1586/eci.10.15 [DOI] [PubMed] [Google Scholar]
- 85.Brożek JL, Bousquet J, Agache I, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines – 2016 revision. J Allergy Clin Immunol 2017; 140: 950–958. doi: 10.1016/j.jaci.2017.03.050 [DOI] [PubMed] [Google Scholar]
- 86.Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA2LEN and AllerGen). Allergy 2008; 63: Suppl. 86, 8–160. doi: 10.1111/j.1398-9995.2007.01620.x [DOI] [PubMed] [Google Scholar]
- 87.Price D, Kemp L, Sims E, et al. Observational study comparing intranasal mometasone furoate with oral antihistamines for rhinitis and asthma. Prim Care Respir J 2010; 19: 266–273. doi: 10.4104/pcrj.2010.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Crystal-Peters J, Neslusan C, Crown WH, et al. Treating allergic rhinitis in patients with comorbid asthma: the risk of asthma-related hospitalizations and emergency department visits. J Allergy Clin Immunol 2002; 109: 57–62. doi: 10.1067/mai.2002.120554 [DOI] [PubMed] [Google Scholar]
- 89.Tattersall MC, Guo M, Korcarz CE, et al. Asthma predicts cardiovascular disease events: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol 2015; 35: 1520–1525. doi: 10.1161/ATVBAHA.115.305452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Veenendaal M, Westerik JAM, van den Bemt L, et al. Age- and sex-specific prevalence of chronic comorbidity in adult patients with asthma: a real-life study. NPJ Prim Care Respir Med 2019; 29: 14. doi: 10.1038/s41533-019-0127-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tomisa G, Horváth A, Sánta B, et al. Epidemiology of comorbidities and their association with asthma control. Allergy Asthma Clin Immunol 2021; 17: 95. doi: 10.1186/s13223-020-00502-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barnig C, Levy BD. Innate immunity is a key factor for the resolution of inflammation in asthma. Eur Respir Rev 2015; 24: 141–153. doi: 10.1183/09059180.00012514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Couillard S, Jackson DJ, Wechsler ME, et al. Workup of severe asthma. Chest 2021; 160: 2019–2029. doi: 10.1016/j.chest.2021.07.008 [DOI] [PubMed] [Google Scholar]
- 94.Drazner MH. The progression of hypertensive heart disease. Circulation 2011; 123: 327–334. doi: 10.1161/CIRCULATIONAHA.108.845792 [DOI] [PubMed] [Google Scholar]
- 95.Ejima K, Li P, Smith DL, et al. Observational research rigour alone does not justify causal inference. Eur J Clin Invest 2016; 46: 985–993. doi: 10.1111/eci.12681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Camargo CA, Weiss ST, Zhang S, et al. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch Intern Med 1999; 159: 2582–2588. doi: 10.1001/archinte.159.21.2582 [DOI] [PubMed] [Google Scholar]
- 97.Mosen DM, Schatz M, Magid DJ, et al. The relationship between obesity and asthma severity and control in adults. J Allergy Clin Immunol 2008; 122: 507–511. doi: 10.1016/j.jaci.2008.06.024 [DOI] [PubMed] [Google Scholar]
- 98.Imrey PB. Limitations of meta-analyses of studies with high heterogeneity. JAMA Netw Open 2020; 3: e1919325. doi: 10.1001/jamanetworkopen.2019.19325 [DOI] [PubMed] [Google Scholar]
- 99.Griffo R, Spanevello A, Temporelli PL, et al. Italian survey on prevalence and disease management of chronic heart failure and chronic obstructive pulmonary disease comorbidity in ambulatory patients. SUSPIRIUM study rationale and design. Monaldi Arch Chest Dis 2014; 82: 29–34. doi: 10.4081/monaldi.2014.40 [DOI] [PubMed] [Google Scholar]
- 100.Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med 2000; 342: 1887–1892. doi: 10.1056/NEJM200006223422507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med 2000; 342: 1878–1886. doi: 10.1056/NEJM200006223422506 [DOI] [PubMed] [Google Scholar]
- 102.Ioannidis JPA, Lau J. Completeness of safety reporting in randomized trials: an evaluation of 7 medical areas. JAMA 2001; 285: 437–443. doi: 10.1001/jama.285.4.437 [DOI] [PubMed] [Google Scholar]
- 103.Boyd CM, Vollenweider D, Puhan MA. Informing evidence-based decision-making for patients with comorbidity: availability of necessary information in clinical trials for chronic diseases. PLoS One 2012; 7: e41601. doi: 10.1371/journal.pone.0041601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sabitova A, McGranahan R, Altamore F, et al. Indicators associated with job morale among physicians and dentists in low-income and middle-income countries: a systematic review and meta-analysis. JAMA Netw Open 2020; 3: e1913202. doi: 10.1001/jamanetworkopen.2019.13202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mascha EJ. Alpha, beta, meta: guidelines for assessing power and Type I error in meta-analyses. Anesth Analg 2015; 121: 1430–1433. doi: 10.1213/ANE.0000000000000993 [DOI] [PubMed] [Google Scholar]
- 106.Holgate S, Bisgaard H, Bjermer L, et al. The Brussels Declaration: the need for change in asthma management. Eur Respir J 2008; 32: 1433–1442. doi: 10.1183/09031936.00053108 [DOI] [PubMed] [Google Scholar]
- 107.Barnish M, Turner S. The value of pragmatic and observational studies in health care and public health. Pragmat Obs Res 2017; 8: 49–55. doi: 10.2147/POR.S137701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rogliani P, Sforza M, Calzetta L. The impact of comorbidities on severe asthma. Curr Opin Pulm Med 2020; 26: 47–55. doi: 10.1097/MCP.0000000000000640 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0202-2022.SUPPLEMENT (1.5MB, pdf)