Abstract
Background:
Research has suggested 2 potential mechanisms by which the periodontal inflammatory response may communicate to distant organs: 1) direct translocation of periodontal bacteria from the oral cavity to another organ system; and 2) inflammation as a result of metastatic periodontal inflammation. The purpose of this scoping review is to explore these mechanisms as potential mediators between periodontitis and Alzheimer’s disease.
Methods:
A reiterative literature search of peer-reviewed articles was performed in the PubMed and Scopus databases using keywords or combinations such as Alzheimer’s disease AND periodontitis OR periodontal disease AND inflammation.
Results:
A total of 777 articles were identified. After eliminating duplicates and reviewing titles and abstracts, 84 articles were selected for full-text review. Following full-text review, 19 articles met the eligibility criteria for the study.
Discussion:
The review of the literature highlights how periodontitis may contribute to neuroinflammation by the introduction of periodontal bacteria and/or proinflammatory cytokines locally produced at the periodontium.
Conclusion:
Inflammation is an important mechanism in the onset and progression of both periodontitis and Alzheimer’s disease. Nevertheless, further studies are necessary to better understand the multifactorial pathogenesis of Alzheimer’s disease.
Keywords: Aggregatibacter actinomycetemcomitans, Alzheimer’s disease, cytokines, Fusobacterium nucleatum, immune response, inflammation, interleukin, periodontitis, Porphyromonas gingivalis, Treponema denticola, tumor necrosis factor-alpha
Abstract
Contexte :
La recherche a suggéré 2 possibilités de mécanismes par lesquels la réponse inflammatoire parodontale peut communiquer avec des organes distants : 1) la translocation directe des bactéries parodontales de la cavité buccale vers un autre système organique; et 2) l’inflammation découlant d’une inflammation parodontale métastatique. Le but de cet examen de la portée est d’explorer ces mécanismes en tant que médiateurs potentiels entre la parodontite et la maladie d’Alzheimer.
Méthodologie :
Une recherche documentaire réitérative d’articles évalués par des pairs a été effectuée dans les bases de données PubMed et Scopus en utilisant les mots-clés ou des combinaisons de mots-clés tels que maladie d’Alzheimer ET parodontite OU maladie parodontale ET inflammation (en anglais).
Résultats :
Un total de 777 articles a été répertorié. Après avoir éliminé les doublons et examiné les titres et les résumés, 84 articles ont été sélectionnés pour être examinés dans leur intégralité. À la suite de l’examen du texte complet, 19 articles répondaient aux critères d’admissibilité de l’étude.
Discussion :
L’analyse documentaire souligne comment la parodontite peut contribuer à la neuroinflammation en introduisant des bactéries parodontales ou des cytokines pro-inflammatoires produites localement au niveau du parodonte.
Conclusion :
L’inflammation est un mécanisme important dans l’apparition et la progression à la fois de la parodontite et de la maladie d’Alzheimer. Néanmoins, d’autres études sont nécessaires pour mieux comprendre la pathogenèse multifactorielle de la maladie d’Alzheimer.
PRACTICAL IMPLICATIONS OF THIS RESEARCH.
Oral health professionals need to recognize and address the influence of chronic periodontal inflammation on systemic health.
Dental hygienists are preventive oral health care providers, who are positioned to reduce the burden and slow the onset of chronic conditions.
BACKGROUND
According to the World Health Organization, chronic diseases are multifactorial pathological conditions that result in approximately 35 million or 60% of annual deaths worldwide.1 Risk factors associated with chronic inflammatory disease include age, genetics, obesity, physical inactivity, unhealthy diet, and tobacco use.2 Intermittent increases in inflammation are expected during injury and/or infection. However, chronic disease results from an inability to achieve tissue homeostasis between the local irritant and the host inflammatory immune response (HIIR).3 Although the gravity of chronic disease depends on the affected organ system and initial inflammatory stimulus, there is a shared biological process: 1) the host recognizes detrimental irritants and/or infection; 2) inflammatory pathways are activated; 3) inflammatory markers are released; and 4) inflammatory cells and cytokines are recruited.3, 4 The release of proinflammatory cytokines will lead to the activation of immune cells as well as the production and further release of cytokines. The most prominent proinflammatory cytokines identified during chronic innate inflammation are interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α ).5, 6-7
Periodontal disease, which includes gingivitis, is one of the most prevalent chronic conditions in the world, affecting between 20% and 50% of the global population.8 In the advanced stage, also termed periodontitis (PD), chronic inflammation results in alveolar bone destruction and tooth loss.9 Periodontal inflammation becomes chronic when the HIIR is unable to eliminate bacteria from the sulcus of gingival tissues, resulting in the prolonged release of destructive inflammatory cells.9 The most putative bacteria found in the oral cavity during PD are Porphyromonas (P.) gingivalis, Aggregatibacter (A.) actinomycetemcomitans, Treponema (T.) denticola, and Fusobacterium (F.) nucleatum.10 These gram-negative organisms can also be found at clinical healthy sites, albeit at different rates than in periodontal pockets.11 Regardless, the presence of these microbes in individuals with no evidence of disease progression suggests PD is the net effect of the inflammatory processes—not the mere presence of bacteria. To rephrase, biological interactions between the disease-susceptible host and irritating bacteria determine the severity of disease.6,9,12
Alzheimer’s disease (AD) is a chronic neuroinflammatory disease that leads to atrophy of brain tissues, cognitive impairments, and death. 13 A hallmark symptom of AD is the progressive shrinkage of the hippocampus, which is responsible for storing long-term memories, spatial processing, and navigation. 14 More than 55 million people live with dementia worldwide; AD represents 60% to 70% of cases.13 In Canada, over 700,000 adults are living with AD or another form of dementia.15 The risk of AD significantly increases with age, reaching nearly one-third in persons aged 85 years.16 As the population ages and life span increases, the prevalence of dementia will increase even further and is expected to affect approximately 81.1 million people by 2040.17
There are several different stages of AD including preclinical, mild cognitive impairment, and severe dementia. 18 During the preclinical phase, patients do not show signs or symptoms of AD, but neuropathologic changes and inflammatory biomarkers are still present. Biological changes of AD can begin years, possibly even decades, before the patient experiences any clinical symptoms of the disease.18-19 Early detection of AD is necessary to prevent and/or delay onset and progression. Currently, however, a definitive diagnosis is made postmortem by brain tissue autopsy.13
A trademark finding of AD is the excessive buildup of amyloid plaques and tau tangles in the brain.20 The amyloid hypothesis proposes that cerebrum accumulation of amyloid is the main etiologic factor for brain tissue destruction and shrinkage.20 However, with advances in amyloid imaging, many individuals without AD have exhibited amyloid deposits.20-21 Additionally, AD patients with very few amyloid deposits have been identified.20- 21 These findings suggest amyloid is not toxic under normal physiological conditions and does not directly initiate tissue destruction. While amyloid deposits have been found in individuals who do not experience neurodegeneration, the same cannot be said for tauopathies. The tau hypothesis suggests tau tangle pathology precedes amyloid plaque formation and is the main cause of neurodegeneration in AD.20 This hypothesis is based on the finding of tau tangles in the brains of patients with mild dementia and no amyloid pathology.22 Unfortunately, tau research is still in the early stages since decades of research have primarily focused on the amyloid hypothesis.
Amyloid plaques and tau tangles are not the only features observed in the AD brain—inflammation is also present. The inflammatory hypothesis suggests the pathogenesis and progression of AD is a result of alterations in the brain via amyloid plaques and tau tangles, which cause a localized HIIR.23 The presence of amyloid plaques and tau tangles activates immune system cells in the brain called microglia, which results in upregulated proinflammatory cytokines, IL-1, IL-6, and TNF-α.23 -25 Elevated proinflammatory cytokines can then produce additional amyloid plaques, tau tangles, and proinflammatory mediators.25 Similar to PD, biological interactions between the disease-susceptible host and the irritants determine the severity of inflammation and subsequent destruction.
The focal infection theory states that the etiology for many chronic diseases is a localized infection.26 If the human body is viewed as a single entity, it is possible that a local disruption of homeostasis cannot be an isolated phenomenon. To clarify, irritating microbes and/or inflammatory mediators are unlikely to remain confined to the originating organ system. PD has been associated with several chronic diseases, such as cardiovascular disease, diabetes mellitus, adverse pregnancy outcomes, and osteoporosis.8 Studies have demonstrated that non-surgical periodontal therapy (NSPT) can reduce the number of pathogenic bacteria and inflammation, making therapeutic care by a dental hygienist critical for vulnerable populations.27, 28
One study suggested that PD increases an individual’s risk of developing AD and vice versa, alluding to a 2-way street. 29 This hypothesis was supported by a study completed by Qi et al.30 who found that with each lost tooth the risk of cognitive impairment increased by 1.4% and the risk of dementia increased by 1.1%. Although PD is the most common cause of tooth loss, accounting for 70% of missing teeth, 31 the actual association between tooth loss and cognitive decline is still unclear. Research has suggested 2 potential mechanisms by which PD may communicate to distant organs: 1) direct migration of periodontal bacteria from the oral cavity to another organ system, which initiates a secondary inflammatory reaction distant from the originating organ; and 2) inflammation as a result of metastatic periodontal inflammation.4 The purpose of this scoping review is to explore these mechanisms as potential mediators between PD and AD. These findings will enhance oral health professionals’ understanding of oral–systemic comorbidity as well as their role in the management of chronic conditions.
METHODOLOGY
This research project used a scoping review approach to summarize existing literature; to identify gaps in that literature; and to disseminate research, policy, and practice implications from the literature.32, 33 A scoping review adopts a broad search strategy while allowing reproducibility, transparency, and reliable analysis of the current literature.33 The literature on this topic was reviewed using the 5-step methodological framework for conducting a scoping review (Table 1).32
Identification of research question
The research question that was explored in this scoping review was, “What is the association between periodontitis and Alzheimer’s disease?” This question evolved following a preliminary search of the literature. To address the specific gap in the literature, the HIIR as the result of migrating periodontal bacteria and/or proinflammatory cytokines was the focus. Because the primary gram-negative bacteria found in individuals with PD are P. gingivalis, A. actinomycetemcomitans, T. denticola, and F. nucleatum, these microbes were used to gain a full representation of the possible association. Similarly, because the key proinflammatory cytokines crucial for an innate immune response are IL-1, IL-6, and TNF-α, these cytokines were used to gain a full representation of the HIIR in the 2 conditions.
Identification of relevant studies
A reiterative literature review on the impact of PD on the onset and progression of AD was performed (Table 2). Original peer-reviewed articles published in English-language journals from 2000 to 2022 were sought and selected from PubMed and Scopus databases. The following search parameter was entered into the search engine: (“Alzheimer’s Disease” OR Alzheimer’s) AND (periodontitis OR “periodontal disease”). The screening of records included the analysis of the title and the abstract to eliminate records not related to the topics of review.
Selection of relevant studies
After the screening phase, the duplicates were removed, and the complete texts of the articles were analyzed. Articles taken into consideration were literature reviews, meta-analyses, in vitro experiments, and randomized controlled trials. Results obtained from the literature search were manually screened according to inclusion and exclusion criteria. The articles considered to be potentially eligible were those reporting on the association between PD and AD. The potentially eligible articles were finally subjected to a full-text analysis to verify their use for qualitative analysis.
Charting the data
Microsoft Excel (Microsoft Corporation, United States of America) was used to chart the data and to summarize the articles that were included in the scoping review (Table 3). The Excel chart listed author(s), year of publication, journal source, methodology used, outcomes, and significant findings.
Summarizing and reporting results
A descriptive summary of the characteristics of the included studies was prepared by the primary investigator (AL). Study quality was determined by year of publication, investigated medical condition, clearly explained inclusion/exclusion criteria, and the appropriate discussion of valid results.
Table 1.
Six steps of the Arksey and O’Malley scoping review process
|
Arksey and O’Malley framework |
Steps undertaken in this scoping review |
|
Step 1: Identify the research question |
From the previous literature, what is known about the relationship between Alzheimer’s disease and periodontitis? |
|
Step 2: Identify relevant studies |
Described in the methods section. A reiterative systemic search of PubMed and Scopus was performed. |
|
Step 3: Study selection |
Described in the methods section. |
|
Step 4: Chart the data |
Described in the methods section. The same author from step 3 charted the data based on the research question and the inclusion and exclusion criteria. |
|
Step 5: Collect, summarize, and report the results |
Described in the discussion section. |
|
Step 6: Consultation (optional) |
Omitted. |
Table 2.
Search strategy
|
Electronic databases: PubMed, Scopus |
|
Search string: “periodontitis” or “periodontal disease”; and “Alzheimer’s disease” |
|
Filter: none |
|
Language: English |
|
Inclusion criteria: review articles, observational studies, preclinical studies explicit evaluation of the correlation between periodontal inflammation and Alzheimer’s disease, as evidenced by periodontal bacteria or markers of chronic inflammation |
|
Exclusion criteria: published prior to 2000 |
|
Additional sources: reference lists of all screened studies |
Figure 1.
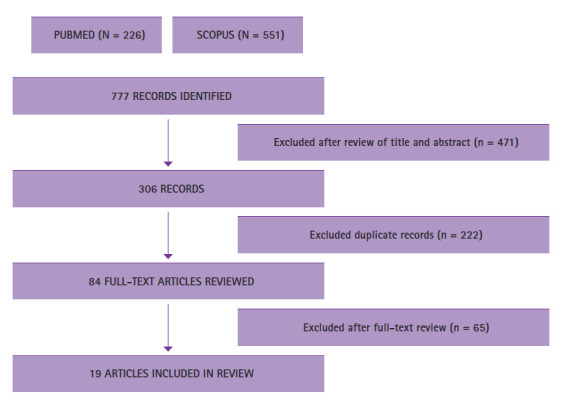
Flowchart of the different phases of review
RESULTS
Identification of potential studies
The combined PubMed and Scopus search resulted in 777 potentially eligible articles. These articles were screened by title and abstract for eligibility. The screening of articles and removal of duplicates resulted in 84 articles that qualified for full-text reading (Figure 1). After full-text reading, 19 articles fulfilled the inclusion criteria and were processed for data extraction. More specifically, 1 systematic review, 2 cohort studies, 7 case–control studies, 2 cross-sectional studies, 5 animal studies, 1 experimental study on brain tissue, and 1 randomized controlled trial were selected. Table 3 summarizes the relevant findings from each study. To avoid the risk of selection bias, general search terms were chosen to guarantee that articles reporting P. gingivalis, A. actinomycetemcomitans, T. denticola, F. nucleatum, IL-1, IL-6, and TNF-α as outcomes would also be included. Limitation of detailed design, inconsistency, indirectness, imprecision, and publication bias were evaluated during this workflow.
Translocation of periodontal bacteria
One of the goals of this scoping review was to explore the association between PD and AD as evidenced by the possible migration of periodontal bacteria. Specifically, translocation of P. gingivalis, A. actinomycetemcomitans, T. denticola, and F. nucleatum was investigated. Thirteen studies evaluated the role of periodontal bacteria in the onset and progression of AD: 2 cohort studies, 5 animal studies, 1 experimental study on post-mortem brain tissue, 4 case–control, and 1 controlled clinical trial. The results of these studies revealed that infection by P. gingivalis appears to be potentially related to the pathogenesis of AD by activating the HIIR, causing neuroinflammation and neurodegeneration. Further research is needed to better understand the possible correlation between AD and A. actinomycetemcomitans, T. denticola, and F. nucleatum.
Metastatic periodontal inflammation
This scoping review also explored the association between PD and AD as evidenced by the presence of proinflammatory cytokines. Six studies evaluated the simultaneous inflammation during PD and AD: 3 case–control, 2 cross-sectional, and 1 systematic review. The outcomes showed that increased levels of IL-1, IL-6, and TNF-α in the presence of PD and AD suggest overlapping mechanisms between these conditions.
Table 3.
Articles used for data extraction
|
Author(s) and Year |
Study design |
Topic of review |
Findings |
|
Dominy et al., 201935 |
Cohort study |
Porphyromonas gingivalis |
P. gingivalis in the brain plays a central role in the pathogenesis of AD |
|
Ishida et al., 201759 |
Experimental study on mice |
Porphyromonas gingivalis |
Periodontitis is a risk factor for Alzheimer’s disease |
|
Kamer et al., 202052 |
Systematic review |
Causal relationship between periodontal disease and Alzheimer’s disease |
Periodontal disease could induce systemic inflammation, blood-brain barrier disruption, neuroinflammation, brain amyloid, neurodegeneration, and cognitive impairment |
|
Cestari et al., 201657 |
Case–control |
Oral infections and cytokine levels in patients with Alzheimer’s disease |
Increased levels of cytokines suggest their implication in the overlapping mechanisms between oral infections and Alzheimer’s |
|
Farhad et al., 201353 |
Case–control |
Tumor necrosis factor-α |
TNF-α in patients with Alzheimer’s disease and periodontitis was approximately three-fold higher than in patients only with Alzheimer’s disease |
|
Kamer et al., 201560 |
Cross-sectional |
Periodontal inflammation and amyloid plaques |
Periodontal inflammation is associated with amyloid accumulation in brain in areas that are prone to amyloid accumulation in patients with Alzheimer’s disease |
|
Hategan et al., 202154 |
Cross-sectional |
Cognitive dysfunction and periodontal inflammatory cytokines |
Subjects with periodontitis had cognitive dysfunction; interleukin-1β may play a role in this process |
|
Poole et al., 201336 |
Experimental study on brain tissue |
Porphyromonas gingivalis |
Associative hypothesis between P. gingivalis and Alzheimer’s disease |
|
Ide et al., 201638 |
Cohort study |
Porphyromonas gingivalis |
Study suggests there is a direct relationship between P. gingivalis and cognitive decline |
|
Hayashi et al., 201940 |
Experimental study on mice |
Porphyromonas gingivalis |
P. gingivalis worsens the prognosis in Alzheimer’s disease |
|
Liu et al., 201741 |
Experimental study on mice |
Porphyromonas gingivalis |
Study supports the periodontal infection hypothesis of Alzheimer’s disease |
|
Nie et al., 201942 |
Experimental study on mice |
Porphyromonas gingivalis |
Study supports periodontitis related Alzheimer’s disease initiation and pathological progression |
|
Ding et al., 201855 |
Experimental study on mice |
Oral infection with Porphyromonas gingivalis |
Periodontal infection by P. gingivalis may cause cognitive impairments |
|
Sochocka et al., 201756 |
Case–control |
Pro- and anti-inflammatory cytokines |
Results confirm that the presence of cognitive decline and the additional source of proinflammatory mediators, such as periodontal health problems, aggravate systemic inflammation |
|
Leblhuber et al., 202039 |
Case–control |
Porphyromonas gingivalis |
Data support a possible association between specific periodontal pathogens and cognitive impairment |
|
Cortexyme Inc., 202245 |
Randomized, double-blind, placebo-controlled clinical trial |
Gingipains |
Reduction of P. gingivalis in the saliva is associated with cognitive improvement |
|
Laugisch et al., 201846 |
Case–control |
Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, and Treponema species |
Periodontal pathogens may enter the brain and stimulate a local immune response. However, [they] do not act as a trigger for developing AD |
|
Díaz-Zúñiga et al., 201947 |
Case–control |
Aggregatibacter actinomycetemcomitans |
Aggregatibacter actinomycetemcomitans causes specific inflammatory and immune responses in brain cells |
|
Taati Moghadam et al., 202248 |
Case–control |
Porphyromonas gingivalis, Fusobacterium nucleatum, Prevotella intermedia, Aggregatibacter actinomycetemcomitans, and Streptococcus mutans |
There was a significant relationship between increased number of pathogenic bacteria in oral microbiome and higher concentration of cytokines in patient’s blood |
DISCUSSION
Communication mechanisms between periodontitis and Alzheimer’s disease
Although PD and AD affect different tissues and have distinct etiologies, they share common risk factors, such as age, genetics, obesity, physical inactivity, unhealthy diet, and tobacco use.2 Additionally, both conditions have chronic destructive properties due to unresolved inflammation. Dental hygienists are trained to address several risk factors by providing tobacco cessation, nutritional counselling, and oral hygiene instruction to both clients and caregivers alike. Additionally, studies have shown reductions in the presence of pathogenic bacteria and systemic proinflammatory cytokines as a result of NSPT.27, 28 Since dental hygienists are the primary oral health providers who typically deliver NSPT to their clients, they can play an integral role in the care of persons with AD. To date, a direct connection between PD and AD cannot be confirmed. However, the results presented in Table 3 suggest an indirect relationship. The 2 proposed communication mechanisms between PD and AD are related to transient bacteremia and metastatic inflammation.
Translocation of periodontal bacteria
An important cause of neuroinflammation may be gram-negative bacteria, which reach the brain after systemic spread from their primary habitat, the periodontium.34 Periodontal bacteria can enter the vascular system through ulcerated epithelium shortly after common activities such as toothbrushing, mastication, and dental procedures.35 A study completed by Poole et al.36 investigated the presence of periodontal pathogenic bacteria in brain tissues of mice with AD pathology. In addition to finding P. gingivalis in mice brains, Poole and colleagues also demonstrated intracellular localization of gingipains.36 Gingipains are proteolytic enzymes produced by P. gingivalis that are capable of degrading host proteins while activating host systems and cells.37 In a study completed by Dominy et al.35 gingipains were identified in over 90% of postmortem AD brains. Although the gingipains were found throughout the brain, they were most prominent in regions associated with memory, such as the hippocampus.35
P. gingivalis is not the only organism that can enter the brain. However, it has the ability to disrupt homeostasis even at low concentrations.34 Ide et al.38 and Leblhuber et al.39 both found that P. gingivalis was associated with cognitive impairment in AD patients, and the cognitive impairment was significantly greater than in patients with AD who did not have PD. In other words, the presence of P. gingivalis may have advanced the cognitive impairment seen during AD. This finding was partly confirmed by Hayashi et al.40 and Liu et al.41 who both found worsening ability to learn and memorize after oral exposure to P. gingivalis. Furthermore, Nie et al.42 reported that P. gingivalis induced the production of amyloid plaques in mice, a prominent feature of AD. These studies suggest that chronic oral inflammation due to P. gingivalis could result in symptoms and neuropathology consistent with that of AD.
It is hypothesized that inhibition of gingipain activity could prevent or slow the progression of associated chronic diseases. 43 To date, several classes of gingipain inhibitors have been recognized, including gingipain N-terminal prodomains, synthetic compounds, inhibitors from natural sources, antibiotics, antiseptics, antibodies, and bacteria.43 Dominy et al. 35 demonstrated a reduction of P. gingivalis, amyloid plaque, and TNF-α in the brains of mice after oral administration of a synthesized small-molecule gingipain inhibitor. Human clinical trials are currently underway to test the efficacy, safety, and tolerability of gingipain inhibitors in older adults with mild to moderate AD.44 A year-long trial followed 643 adults with mild to moderate AD and found reductions of P. gingivalis in patients’ saliva at week 24.45 This finding also significantly correlated with improved outcomes in cognition at the end of the trial.45 The study also found that gingival pocket depths were reduced in 242 patients with P. gingivalis DNA detected in saliva.45 Future studies should investigate the use of gingipain inhibitors during NSPT provided by dental hygienists for the improvement of both PD and AD.
Unfortunately, the possible implications of A. actinomycetemcomitans, T. denticola, and F. nucleatum in the etiology and/or progression of AD have not been as widely investigated as P. gingivalis. The literature on these oral microbes and their association with AD is rather limited and contradictory. For this reason, it is difficult to provide an adequate and up-to-date assessment of the possible bilateral comorbidity of these complex chronic diseases. Nevertheless, 3 studies that discussed these particular oral microbes were included in this scoping review. A study conducted by Laugisch et al.46 concluded that periodontal pathogens, such as P. gingivalis, A. actinomycetemcomitans, and T. denticola have the capability to enter the brain and stimulate a local HIIR, although their association with AD was not found to be significant. The Laugisch study included participants who were younger than 70 years and with other forms of dementia as controls to AD patients rather than healthy patients, which could have skewed the results. These parameters varied from other comparable studies. In contrast, Díaz-Zúñiga et al.47 concluded that A. actinomycetemcomitans can cause a HIIR in brain cells and could potentially increase the risk of AD. Additionally, a case–control study by Taati Moghadam et al.48 showed significant associations between changes in P. gingivalis and F. nucleatum during AD. However, A. actinomycetemcomitans were relatively comparable with the control group.48
Metastatic periodontal inflammation
Inflammation is a prominent component of both PD and AD and presents a strong, plausible link between both conditions. During a typical HIIR, cytokines with a proinflammatory function are elevated in the infected tissue and can be useful in the detection of infections. Cytokines are recruited locally. However, cytokine receptors may become overwhelmed through chronic exposure, allowing for cytokines to spread into the systemic circulation.49-51 A systematic review by Kamer et al.52 concluded that periodontally derived cytokines could reach the brain by both systemic and neural pathways, which then amplify brain cytokine pools. Farhad et al.53 demonstrated this phenomenon with a significant increase of TNF-α in persons with AD and PD compared to those with AD and a healthy periodontium.
Continued cytokine production can have a deleterious effect on the host. A study published by Hategan et al.54 concluded that increased levels of IL-1, both locally and systemically, may be a facilitator of cognition. This finding was also demonstrated by Ding et al.57 and Liu et al.43, who reported cognitive impairment in association with increased levels of TNF-α, IL-6, and IL-1β in mice brain tissue as a result of an induced oral infection. A case-control study completed by Sochocka et al.56 also concluded that PD and cognitive abilities are correlated with the relative changes in systemic measures of proinflammatory cytokines as a reflection of systemic inflammation. Furthermore, an experimental study on mice completed by Ishida et al.59 and a cross-sectional study completed by Kamer et al.60 found that the overproduction of TNF-α and IL-1β, due to PD, increased amyloid deposits in the brain. A case–control study completed by Cestari et al.57 demonstrated an association between IL-6 in patients with AD and PD, suggesting their implication in the overlapping mechanisms between AD and PD. Cestari and colleagues also found high levels of TNF-α in patients with PD. However, an association could not be made with AD.57
It is evident that the current literature supports proinflammatory cytokines as a key component in the pathogenesis of AD. However, this literature is primarily at an animal and case–control study level. A logical hypothesis is that the reduction of proinflammatory cytokines might be a viable strategy for disrupting the disease process. Randomized controlled trials (RCTs) have demonstrated that NSPT, such as scaling and root planing, can reduce systemic inflammatory cytokines.27,28 Given that the presence of proinflammatory cytokines is a definite risk factor for AD, adequate treatment or prevention of PD may assist in the delay of chronic disease.52, 51 However, current studies have not yet demonstrated cognitive improvement in association with proinflammatory cytokine modulation.58 Thus, more rigorous and higher level studies, such as RCTs, will be required to further support these findings.
Limitations of this scoping review
Despite the consistent evidence supporting an association between PD and AD, higher-level studies were limited for review. Therefore, this study’s findings should be interpreted with caution. This scoping review excluded articles not published in English-language journals, possibly biasing study results. One of the major limitations of this scoping review was the quality of each eligible study. Further higher-level studies, such as RCTs, are necessary to authenticate findings. Another major limitation of the scoping review is the limited inclusion of medical literature. AD has been associated with other members of the human microflora—found outside the oral cavity. Given the narrow concentration on oral microbes, there is a possibility of selection bias.
Further research is necessary to establish the definite nature of the association between AD and PD. Future longitudinal human studies involving detailed evaluation of cognitive function in susceptible persons with a history of PD will be required to verify such correlations. Furthermore, evidence of periodontal bacteria crossing the blood-brain barrier and directly leading to brain tissue atrophy also requires additional research, most appropriately by animal or human cadaver studies. However, the results of animal experiments often do not translate into replications in human trials.
CONCLUSION
Like many hypothesized bidirectional relationships, it is difficult to determine which condition preceded the other or if they are definite catalysts to each other. Longitudinal monitoring of patients suggests an association between PD and AD. However, it remains to be determined as to whether this phenomenon is due to the direct invasion of periodontal bacteria or the indirect effect of systemic changes because of a localized inflammatory response. The current literature supports the plausibility of both mechanisms; that is, periodontal bacteria and the HIIR related to PD may intensify or initiate systemic inflammation, eventually favoring the onset of AD. However, this association may also be due to high prevalence of PD diagnosis among patients with dementia and inadequate oral care. Additional studies are necessary to better understand the multifactorial pathogenesis of this complex neuroinflammatory disease.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to disclose.
Footnotes
CDHA Research Agenda category: risk assessment and management
References
- World Health Organization. Preventing chronic diseases: A vitalinvestment. WHO Global Report. Geneva : : WHO ; ; 2005 . 182p. [Google Scholar]
- Kotas ME , Medzhitov R Homeostasis, inflammation, and disease susceptibility Cell 2015 ; 160 ( 5 ): 816 – 827 doi: 10.1016/j.cell.2015.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L , Deng H , Cui H , et al. Inflammatory responses and inflammation-associated diseases in organs Oncotarget 2017 ; 9 ( 6 ): 7204 – 7218 doi: 10.18632/oncotarget.23208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasturk H , Kantarci A Activation and resolution of periodontal inflammation and its systemic impact Periodontol 2000 2015 ; 69 ( 1 ): 255 – 273 doi: 10.1111/prd.12105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso EM , Reis C , Manzanares-Céspedes MC Chronic periodontitis, inflammatory cytokines, and interrelationship with other chronic diseases Postgraduate Medicine 2018 ; 130 ( 1 ): 98 – 104 doi: 10.1080/00325481.2018.1396876 [DOI] [PubMed] [Google Scholar]
- Pan W , Wang Q , Chen Q The cytokine network involved in the host immune response to periodontitis Int J Oral Sci 2019 ; 11 ( 30 ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleerup HS , Hasselbalch SG , Simonsen AH Biomarkers for Alzheimer’s disease in saliva: a systematic review Disease Markers 2019 ; 2019 : 1 – 11 doi: 10.1155/2019/4761054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazir MA Prevalence of periodontal disease, its association with systemic diseases and prevention Int J Health Sci (Qassim) 2017 ; 11 ( 2 ): 72 – 80 [PMC free article] [PubMed] [Google Scholar]
- Cekici A , Kantarci A , Hasturk H , Van Dyke TE Inflammatory and immune pathways in the pathogenesis of periodontal disease Periodontol 2000 2014 ; 64 ( 1 ): 57 – 80 doi: 10.1111/prd.12002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paster BJ , Olsen I , Aas JA , Dewhirst FE The breadth of bacterial diversity in the human periodontal pocket and other oral sites Periodontol 2000 2006 ; 42 ( 1 ): 80 – 87 doi: 10.1111/j.1600-0757.2006.00174.x [DOI] [PubMed] [Google Scholar]
- Ximénez-Fyvie LA , Haffajee AD , Socransky SS Comparison of the microbiota of supra- and subgingival plaque in health and periodontitis J Clin Periodontol 2000 ; 27 ( 9 ): 648 – 657 doi: 10.1034/j.1600-051x.2000.027009648.x [DOI] [PubMed] [Google Scholar]
- Yu N , Van Dyke TE Periodontitis: a host mediated disruption of microbial homeostasis Curr Oral Health Rep 2020 ; 7 ( 1 ): 3 – 11 doi: 10.1007/s40496-020-00256-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Alzheimer’s Disease and Related Dementias: What is Alzheimer’s Disease? [Internet]. Published 2021 April 7 [cited 2021 Sept 29]. Available from: www.cdc.gov/aging/aginginfo/alzheimers.htm
- Wilckens KA , Stillman CM , Waiwood AM , et al. Exercise interventions preserve hippocampal volume: a meta-analysis Hippocampus 2021 ; 31 ( 3 ): 335 – 347 doi: 10.1002/hipo.23292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Association. Alzheimer’s & Dementia in Canada [Internet]. N.d. [cited 2022 March 15]. Available from: www.alz.org/ca/dementia-alzheimers-canada.asp
- Alzheimer’s Association. What is Alzheimer’s Disease? [Internet]. N.d. [cited 2020 Oct 16]. Available from: https://alz.org/alzheimers-dementia/what-is-alzheimers
- Ferri CP , Prince M , Brayne C , et al. Global prevalence of dementia: a Delphi consensus study Lancet 2005 ; 366 ( 9503 ): 2112 – 2117 doi: 10.1016/S0140-6736(05)67889-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Association 2016 Alzheimer’s disease facts and figures Alzheimers Dement 2016 ; 12 ( 4 ): 459 – 509 doi: 10.1016/j.jalz.2016.03.001 [DOI] [PubMed] [Google Scholar]
- Villemagne VL , Burnham S , Bourgeat P , et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study Lancet Neurol 2013 ; 12 ( 4 ): 357 – 367 doi: 10.1016/S1474-4422(13)70044-9 [DOI] [PubMed] [Google Scholar]
- Kametani F , Hasegawa M Reconsideration of amyloid hypothesis and tau hypothesis in Alzheimer’s disease Front Neurosci 2018 ; 12 : 25 doi: 10.3389/fnins.2018.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edison P , Archer HA , Hinz R , et al. Amyloid, hypometabolism, and cognition in Alzheimer disease: An [11C]PIB and [18F]FDG PET study Neurology 2007 ; 68 ( 7 ): 501 – 508 doi: 10.1212/01.wnl.0000244749.20056.d4 [DOI] [PubMed] [Google Scholar]
- J De Paula V , Guimarães F , Diniz B , Forlenza O Neurobiological pathways to Alzheimer’s disease: Amyloid-beta, TAU protein or both? Dementia e Neuropsychologia 2009 ; 3 : 188 – 194 doi: 10.1590/S1980-57642009DN30300003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney JW , Bemiller SM , Murtishaw AS , Leisgang AM , Salazar AM , Lamb BT Inflammation as a central mechanism in Alzheimer’s disease Alzheimers Dement (NY) 2018 ; 4 : 575 – 90 doi: 10.1016/j.trci.2018.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL , McGeer EG The amyloid cascade-inflammatory hypothesis of Alzheimer disease: implications for therapy Acta Neuropathol 2013 ; 126 ( 4 ): 479 – 497 doi: 10.1007/s00401-013-1177-7 [DOI] [PubMed] [Google Scholar]
- Kamer AR , Craig RG , Dasanayake AP , Brys M , Glodzik-Sobanska L , de Leon MJ Inflammation and Alzheimer’s disease: Possible role of periodontal diseases Alzheimers Dement 2008 ; 4 ( 4 ): 242 – 250 doi: 10.1016/j.jalz.2007.08.004 [DOI] [PubMed] [Google Scholar]
- Rocca JP , Fornaini C , et al. Focal infection and periodontitis: a narrative report and new possible approaches Int J Microbiol 2020 ; 2020 doi: 10.1155/2020/8875612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar Arregocés FM , Del Hierro Rada M , Sáenz Martinez MJ , et al. Systemic inflammatory response to non-surgical treatment in hypertensive patients with periodontal infection Medicine 2021 ; 100 ( 13 ): e24951doi: 10.1097/MD.0000000000024951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY , Kim HN Changes in inflammatory cytokines in saliva after non-surgical periodontal therapy: a systematic review and meta-analysis Int J Environ Res Public Health 2020 ; 18 ( 1 ): E194 doi: 10.3390/ijerph18010194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju X , Kapellas K , Jamieson LM , Mueller N , Wang X The association between periodontal disease and dementia: a systematic review and meta-analysis Dental Oral Biology and Craniofacial Research 2019 ; 2019 ( 1 ): 1 – 11 doi: 10.31487/j.DOBCR.2019.01.005 [DOI] [Google Scholar]
- Qi X , Zhu Z , Plassman BL , Wu B Dose-response meta-analysis on tooth loss with the risk of cognitive impairment and dementia J Am Med Dir Assoc 2021 ; 22 ( 10 ): 2039 – 2045 doi: 10.1016/j.jamda.2021.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Children’s Wisconsin. Periodontal Disease [Internet]. N.d. [cited 2022 March 15]. Available from: https://childrenswi.org/medical-care/dental-care/dental-and-oral-health/periodontal-disease
- Arksey H , O’Malley L Scoping studies: towards a methodological framework Int J Soc Res Methodol 2005 ; 8 ( 1 ): 19 – 32 doi: 10.1080/1364557032000119616 [DOI] [Google Scholar]
- Levac D , Colquhoun H , O’Brien KK Scoping studies: advancing the methodology Implementation Sci 2010 ; 5 ( 1 ): 69 doi: 10.1186/1748-5908-5-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein PS , Steffen MJ , Smith C , et al. Serum antibodies to periodontal pathogens are a risk factor for Alzheimer’s disease Alzheimers Dement 2012 ; 8 ( 3 ): 196 – 203 doi: 10.1016/j.jalz.2011.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominy SS , Lynch C , Ermini F , et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors Sci Adv 2019 ; 5 ( 1 ): eaau3333 doi: 10.1126/sciadv.aau3333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole S , Singhrao SK , Chukkapalli S , et al. Active invasion of Porphyromonas gingivalis and infection-induced complement activation in ApoE-/- mice brains J Alzheimers Dis 2015 ; 43 ( 1 ): 67 – 80 doi: 10.3233/JAD-140315 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick R , Wijeyewickrema L , Pike R The gingipains: Scissors and glue of the periodontal pathogen, Porphyromonas gingivalis Future Microbiol 2009 ; 4 : 471 – 487 doi: 10.2217/fmb.09.18 [DOI] [PubMed] [Google Scholar]
- Ide M , Harris M , Stevens A , et al. Periodontitis and cognitive decline in Alzheimer’s disease PLoS One 2016 ; 11 ( 3 ): e0151081 doi: 10.1371/journal.pone.0151081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblhuber F , Huemer J , Steiner K , Gostner JM , Fuchs D Knock-on effect of periodontitis to the pathogenesis of Alzheimer’s disease? Wien Klin Wochenschr 2020 ; 132 ( 17-18 ): 493 – 498 doi: 10.1007/s00508-020-01638-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K , Hasegawa Y , Takemoto Y , et al. Continuous intracerebroventricular injection of Porphyromonas gingivalis lipopolysaccharide induces systemic organ dysfunction in a mouse model of Alzheimer’s disease Exp Gerontol 2019 ; 120 : 1 – 5 doi: 10.1016/j.exger.2019.02.007 [DOI] [PubMed] [Google Scholar]
- Liu Y , Wu Z , Nakanishi Y , et al. Infection of microglia with Porphyromonas gingivalis promotes cell migration and an inflammatory response through the gingipain-mediated activation of protease-activated receptor-2 in mice Sci Rep 2017 ; 7 ( 1 ): 11759 doi: 10.1038/s41598-017-12173-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie R , Wu Z , Ni J , et al. Porphyromonas gingivalis infection induces amyloid-β accumulation in monocytes/macrophages J Alzheimers Dis 2019 ; 72 ( 2 ): 479 – 494 doi: 10.3233/JAD-190298 [DOI] [PubMed] [Google Scholar]
- Olsen I , Potempa J Strategies for the inhibition of gingipains for the potential treatment of periodontitis and associated systemic diseases J Oral Microbiol 2014 ; 6 : 10 3402/jom v6 24800 doi: 10.3402/jom.v6.24800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raha D , Broce S , Haditsch U , et al. COR388, a novel gingipain inhibitor, decreases fragmentation of APOE in the central nervous system of Alzheimer’s disease patients Alzheimers Dement 2020 ; 16 ( S9 ): e040578 doi: 10.1002/alz.040578 [DOI] [Google Scholar]
- Cortexyme Inc. GAIN Trial: A randomized, double-blind, placebo-controlled study of COR388 in subjects with Alzheimer’s disease. clinicaltrials.gov; 2022 [cited 2022 March 10]. Available from:https://clinicaltrials.gov/ct2/show/NCT03823404
- Laugisch O , Johnen A , Maldonado A , et al. Periodontal pathogens and associated intrathecal antibodies in early stages of Alzheimer’s disease J Alzheimers Dis 2018 ; 66 ( 1 ): 105 – 114 doi: 10.3233/JAD-180620 [DOI] [PubMed] [Google Scholar]
- Díaz-Zúñiga J , Muñoz Y , Melgar-Rodríguez S , et al. Serotype b of Aggregatibacter actinomycetemcomitans triggers pro-inflammatory responses and amyloid beta secretion in hippocampal cells: a novel link between periodontitis and Alzheimer´s disease? J Oral Microbiol 2019 ; 11 ( 1 ): 1586423 doi: 10.1080/20002297.2019.1586423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taati Moghadam M , Amirmozafari N , Mojtahedi A , Bakhshayesh B , Shariati A , Masjedian Jazi F Association of perturbation of oral bacterial with incident of Alzheimer’s disease: a pilot study J Clin Lab Anal 2022 ; 36 ( 7 ): e24483 doi: 10.1002/jcla.24483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts A , Crimmins EM , Gatz M Inflammation as a potential mediator for the association between periodontal disease and Alzheimer’s disease Neuropsychiatr Dis Treat 2008 ; 4 ( 5 ): 865 – 876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RPH , Ho YS , Leung WK , Goto T , Chang RCC Systemic inflammation linking chronic periodontitis to cognitive decline Brain Behav Immun 2019 ; 81 : 63 – 73 doi: 10.1016/j.bbi.2019.07.002 [DOI] [PubMed] [Google Scholar]
- Abbayya K , Puthanakar N , Naduwinmani S , et al. Association between periodontitis and Alzheimer’s disease N Am J Med Sci 2015 ; 7 ( 6 ): 241 doi: 10.4103/1947-2714.159325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamer AR , Craig RG , Niederman R , Fortea J , Leon MJ de Periodontal disease as a possible cause for Alzheimer’s disease Periodontol 2000 2020 ; 83 ( 1 ): 242 – 271 doi: 10.1111/prd.12327 [DOI] [PubMed] [Google Scholar]
- Farhad SZ , Amini S , Khalilian A , et al. The effect of chronic periodontitis on serum levels of tumor necrosis factor-alpha in Alzheimer disease Dent Res J (Isfahan) 2014 ; 11 ( 5 ): 549 – 552 [PMC free article] [PubMed] [Google Scholar]
- Hategan SI , Kamer SA , Craig RG , et al. Cognitive dysfunction in young subjects with periodontal disease Neurol Sci 2021 ; 42 ( 11 ): 4511 – 4519 doi: 10.1007/s10072-021-05115-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y , Ren J , Yu H , Yu W , Zhou Y Porphyromonas gingivalis, a periodontitis causing bacterium, induces memory impairment and age-dependent neuroinflammation in mice Immun Ageing 2018 ; 15 : 6 doi: 10.1186/s12979-017-0110-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sochocka M , Sobczyński M , Sender-Janeczek A , et al. Association between periodontal health status and cognitive abilities. The role of cytokine profile and systemic inflammation Curr Alzheimer Res 2017 ; 14 ( 9 ): 978 – 990 doi: 10.2174/1567205014666170316163340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cestari JAF , Fabri GMC , Kalil J , et al. Oral infections and cytokine levels in patients with Alzheimer’s disease and mild cognitive impairment compared with controls J Alzheimers Dis 2016 ; 52 ( 4 ): 1479 – 1485 doi: 10.3233/JAD-160212 [DOI] [PubMed] [Google Scholar]
- Wang WY , Tan MS , Yu JT , Tan L Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease Ann Transl Med 2015 ; 3 ( 10 ): 136 doi: 10.3978/j.issn.2305-5839.2015.03.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida N , Ishihara Y , Ishida K , et al. Periodontitis induced by bacterial infection exacerbates features of Alzheimer’s disease in transgenic mice npj Aging Mech Dis 2017 ; 3 ( 1 ): 1 – 7 doi: 10.1038/s41514-017-0015-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamer AR , Pirraglia E , Tsui W , et al. Periodontal disease associates with higher brain amyloid load in normal elderly Neurobiol Aging 2015 ; 36 ( 2 ): 627 – 633 doi: 10.1016/j.neurobiolaging.2014.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]


