Abstract
Tissues and organs are composed of many diverse cell types, making cell-specific gene expression profiling a major challenge. Herein we report that endogenous enzymes, unique to a cell of interest, can be utilized to enable cell-specific metabolic labeling of RNA. We demonstrate that appropriately designed “caged” nucleosides can be rendered active by serving as a substrate for cancer-cell specific enzymes to enable RNA metabolic labeling, only in cancer cells. We envision that the ease and high stringency of our approach will enable expression analysis of tumor cells in complex environments.
Isolating and profiling RNA molecules from specified cells is a long-standing challenge in molecular biology. Metabolic labeling using nucleosides endowed with several types of chemical functional groups such as alkyne, azide, and more recently vinyl groups enable tracking and analysis by fluorescence or enrichment (Figure 1a).1–5
Figure 1.
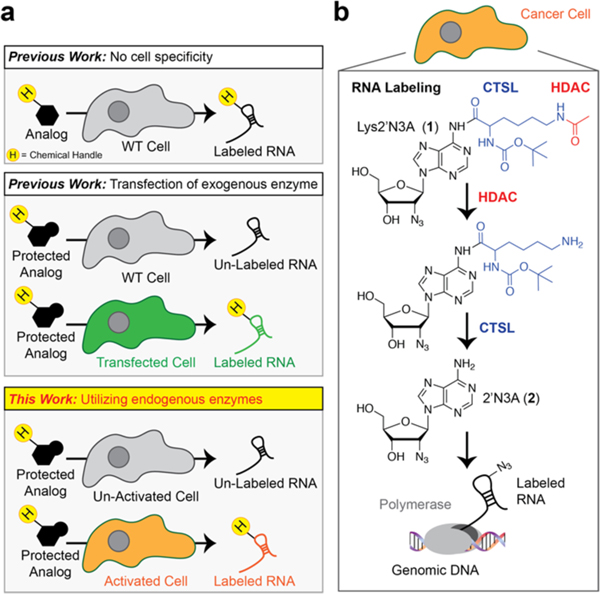
Outline of cell-type specific metabolic labeling of RNA. (a) In metabolic labeling experiments, a chemically modified nucleoside analog is added to cells. The chemical modifications on the nucleobase/nucleoside analogs render them “inert” to endogenous metabolic pathways while the expression of specific metabolic enzymes can control their incorporation. Most studies to date rely on the overexpression of exogenous enzymes to control metabolic incorporation. (b) Our approach uses endogenous enzymes to “uncage” inert intermediates for eventual incorporation into RNA to apply cell-specific metabolic labeling of RNA in cancer cells. H = handle for conjugation such as alkyne or azide. HDAC = histone deacetylase. CTSL = cathepsin L protease.
A major limitation with chemical approaches to track RNA expression is the inability to tag RNAs from cell types of interest. Expression of exogenous enzymes (Figure 1a) that, when paired with modified nucleosides/nucleobases, can enable cell-specific metabolic labeling of RNA.6–11 However, each of these methods relies on expression of an exogenous enzyme, which requires laborious procedures to produce expression constructs; further, the utilization in living animals takes extensive time to implement. Overall, these limitations suggest additional methods need to be explored for cell-specific metabolic labeling of RNA, but may also present an opportunity for more sophisticated chemical approaches toward this problem.
An alternative approach, which has yet to be demonstrated, is utilizing endogenous enzymes to control metabolic labeling (Figure 1b). Unique cell types often use the expression of cell-specific enzymes to regulate signaling pathways that control cell functions such as cell motility, and metabolic activity to achieve maximal fitness.12 The ability to develop novel chemical approaches for cell-specific metabolic labeling that do not require exogenous enzyme expression or cellular engineering not only would be incredibly useful for understanding cell-specific RNA expression but also could be seamlessly integrated with ongoing efforts in the field to utilize metabolic labeling to understand RNA–protein interactions,13 RNA localization,14–16 and even transcriptional dynamics.17
Herein, we present the synthesis and characterization of a novel metabolic reporter which is only metabolically incorporated into RNA in cancer cells. We envision this report will serve as a benchmark toward the development of enzyme-responsive metabolic reporters for measuring RNA expression within unique cell types and provide novel inroads toward utilizing chemical approaches to characterize gene expression within specific cancer cells.
Cancer cells are notorious for having unique enzyme expression and activity profiles to control growth and metastasis.18,19 For example, histone deacetylases (HDACs) are known to be highly overexpressed and hyperactive in cancer cells to control gene expression.20 Cathepsin proteases are another class of enzymes that are notoriously overexpressed in cancer cells, particularly metastatic cancers, to control degradation of the extracellular matrix and enable epithelial-to-mesenchymal transition (EMT) for subsequent metastases.21 These unique enzymatic signatures of cancer cells have been utilized for pro-drug approaches,22 but have not been employed for metabolic labeling, and as such may provide inroads to distinguish cancer cell from normal cell RNA in complex tumor environments.
Our initial hypothesis was that we could achieve cancer-cell specific metabolic RNA labeling through the design of Lys-2′N3A (compd 1; Figure 1b). Lys-2′N3A is designed to be an AND-gate (an AND-gate relies on two inputs to get a desired output) responsive nucleoside, needing both HDAC and Cathepsin L protease (CTSL) enzymes to “uncage” the N-6 protecting group to liberate 2′N3A (compd 2; Figure 1b). Therefore, our design should enable highly stringent cell-specific RNA metabolic labeling. A terminal acetylated amine would be a substrate for HDAC enzyme, liberating a lysine amine, which would serve as a substrate for CTSL cleavage. We have demonstrated previously the liberated product, 2′N3A, is nontoxic in cells and yields robust RNA labeling.7
Lys2′N3A and 2′N3A were synthesized in five steps starting with commercially available Vidarabine (Synthesis and Characterization in Supporting Information). Using high-performance liquid chromatography (HPLC), we demonstrated that Lys-2′N3A is a substrate and can be deacetylated by HDAC1, a broad spectrum HDAC enzyme,22 and subsequently the N6-amide bond cleaved by CTSL. Deacylation of Lys-2′N3A is necessary for CTSL cleavage of the N6-amide (Figure S1; Supporting Information). These results suggest that Lys-2′N3A should be a substrate for dual activity of HDAC and CTSL enzymes in cells.
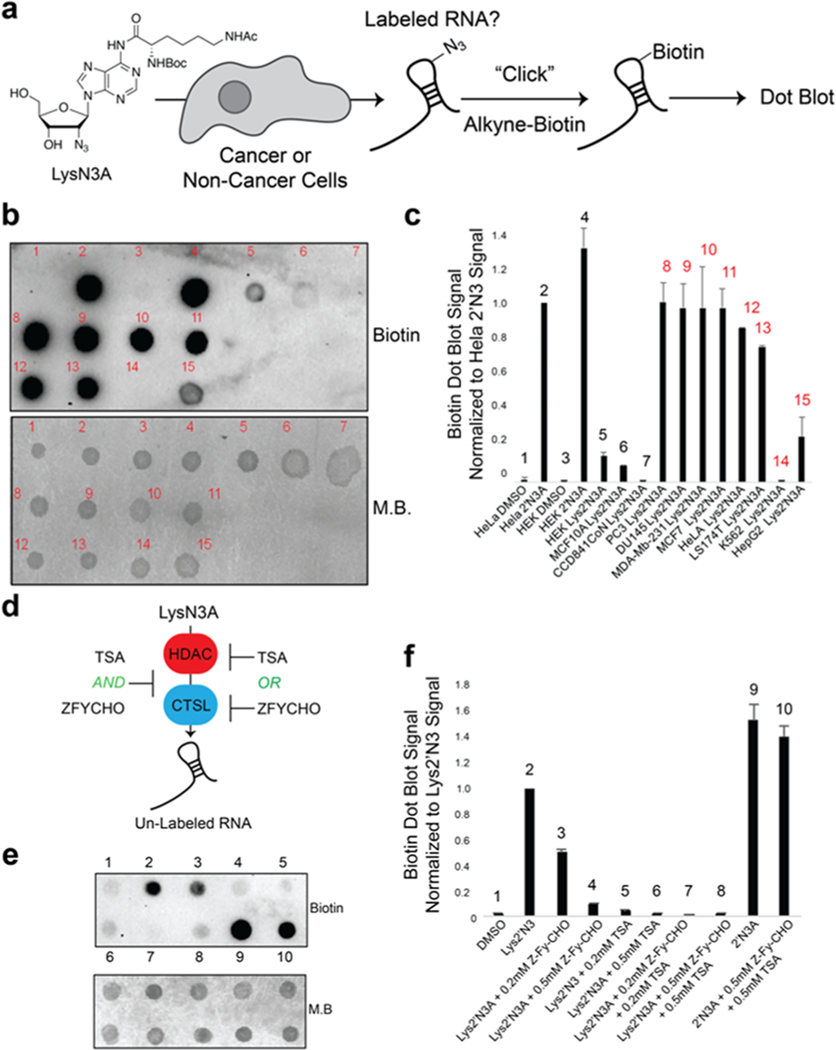
We next utilized an in-cell screening assay (Figure 2a) to determine if Lys-2′N3A would be preferentially incorporated into cancer cell RNA. Briefly, Lys-2′N3A was incubated in the media of either cancer or noncancer cells at 1 mM concentration for 24 h. Total RNA was harvested and biotinylated using CuAAC and alkyne biotin. Biotinylation was assessed using dot blot. We screened both cancerous and noncancerous cell lines. As shown in Figure 2b and c, we did not observe RNA incorporation above background in noncancer cell lines (Table S1). In contrast, we observed varying degrees of incorporation in cancer cell lines, with highly invasive cancers MDA-MB-231 and MCF7 (metastatic breast cancers), PC3 (pancreatic), or lung (LS174T) having very high incorporation into RNA. Interestingly, K562 cells (chronic myelogenous leukemia) did not have measurable incorporation of liberated 2′N3A. This may be due to the nonmetastatic nature of such cancers, which do not undergo EMT and therefore would not have the need for expression of CTSL, undifferentiated state of the cells, or specificity of the patient line from which the line is derived. We also compared both 2′N3A or Lys-2′N3A incubation for toxicity and using an MTT assay. We did not detect toxicity, consistent with our previous work using 2′N3A for RNA metabolic labeling (Figure S2).7 Concentration and time titration experiments revealed similar incorporation characteristics between 2′N3A and Lys-2′N3A in the metastatic cancer line MDA-MB-231 (Figure S3). Control experiments demonstrate that we could not detect 2′N3A in cellular DNA by LC-MS/MS (Figure S4).
Figure 2.
Demonstration of cancer cell-specific metabolic labeling of RNA in cells. (a) Schematic of cell-based screen used for determining cell-specific metabolic labeling using Lys2′N3A. Cells were treated with 1 mM concentration of Lys2′N3A or 2′N3A for 24 h. Total RNA was harvested and biotinylated using copper(I)-catalyzed azide−alkyne cycloaddition (CuAAC) with alkyne biotin. Biotinylation was assessed using dot blot. (b) Representative dot-blot analysis demonstrating that Lys2′N3A is only “uncaged” in cancer cells to enable 2′N3A incorporation into RNA. M.B. = methylene blue dye to demonstrate loading onto the dot blot membrane. Cell types are listed in Table S1. (c) Bar chart representing integrated signal from biological duplicate dot blots as in panel b. Red numbering above bar chart represents cancer types. Data were normalized to the HeLa-2′N3A dot blot sample. (d) Schematic of utilizing HDAC (histone deacetylase) and CTSL (cathepsin L protease) enzyme inhibitors to demonstrate their activity and 2′N3A incorporation into RNA. Cells were incubated with noted concentrations of inhibitors then treated with 0.2 mM final concentration and incubated for 5 h. RNA was then isolated and biotinylated for dot blot. (e) Representative dot blot analysis demonstrating that HDAC (histone deacetylase) and CTSL (cathepsin L protease) enzyme inhibitors suppress Lys2′N3A uncaging in HeLa cells. (f) Bar chart representing integrated signal from biological duplicate dot blots as in panel e. Data were normalized to the HeLa-2′N3A dot blot sample.
To further characterize the expression of HDAC and CTSL enzymes, we performed Western blot analysis for HDAC enzymes 1, 3, 4, 6, and 8, which represent a large portion of the complete HDAC class I and class II family of enzymes, as well as CTSL on cancer and noncancerous lines (Figure S5). We observed more variability among cell lines (cancer and noncancer) for HDAC enzymes. Much higher signal was observed by Western for CTSL in MDA-MB-231 cells, with some observed in HeLa (Figure S5). We did not observe evidence of expression in HEK293 or K562 cells. Overall, these data suggest that “de-caging” of the Lys2′N3A is gated primarily by CTSL, but that HDAC expression is important for the liberation of the lysine substrate for CTSL cleavage.
We utilized known inhibitors of HDAC and CTSL enzymes to test if their incubation in cell media would decrease subsequent 2′N3A incorporation into cellular RNA (Figure 2d). Trichostatin A is a broad inhibitor of HDAC activity. ZFY-CHO is a well-established specific inhibitor of cathepsin L activity. As demonstrated in Figure 2e and f and Figure S6, incubation of inhibitors at 0.2 mM and 0.5 mM with Lys2′N3A resulted in a marked decrease of incorporation of 2′N3A into cellular RNA (see also Figure S6). These experiments support the notion that HDAC and CTSL decrease subsequent 2′N3A incorporation into cellular RNA.
The utility of bio-orthogonal reporters can be demonstrated in cellular imaging. We also employed imaging to better understand the cell specificity of our metabolic labeling approach. We used cancerous (MDA-MB-231) and noncancerous (HEK293T) cells. As demonstrated in Figure S6, we observed that Lys-2′N3A is not incorporated into RNA noncancer cells even in coculture situations. These results further demonstrate the cell selectivity of cancer cell labeling for Lys-2′N3A.
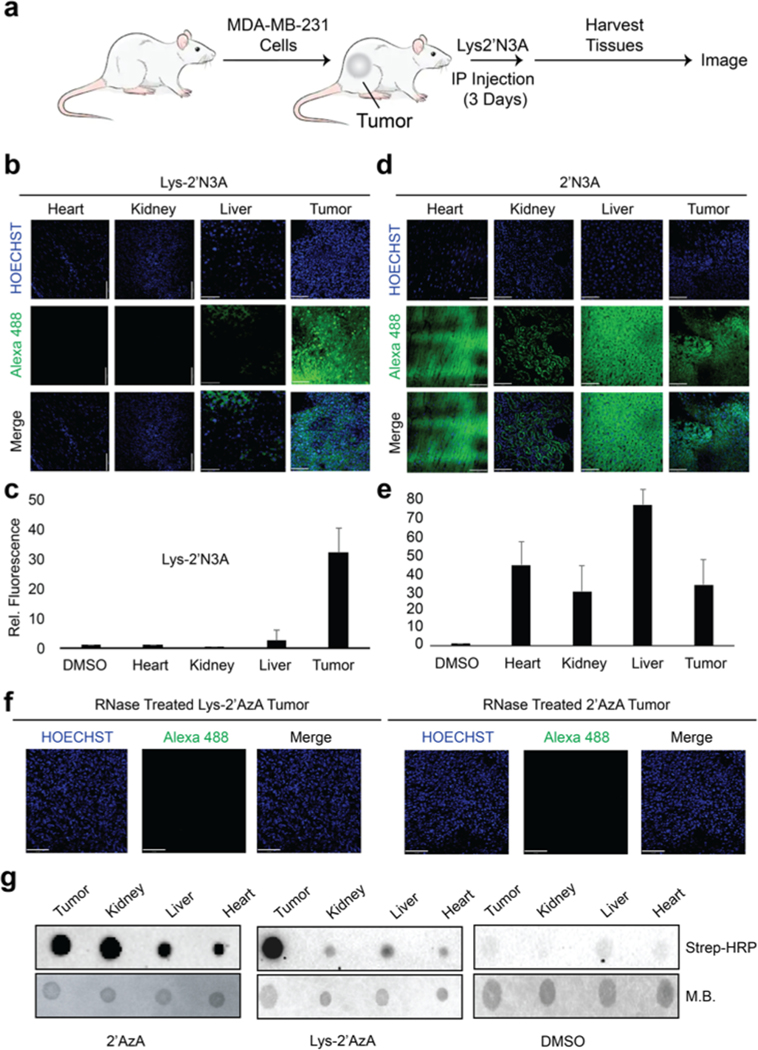
We further aimed to test our approach in vivo. We established an in vivo xenograft tumor model of MDA-MB-231 cells (Figure 3a; Methods in Supporting Information). After 1 month, mice were subjected to an intraperitoneal injection of 2′N3A or Lys-2′N3A. Organs were harvested and imaged using CuAAC. We observed bright fluorescence in the tumors of Lys-2′N3A injected animals, with a slight signal within the livers of the same animals (Figure 3b and c; Figure S7). In stark contrast, we observed fluorescence in the tumor and many organs with 2′N3A injected animals (Figure 3d and e). Lastly, we treated tumor slices from both animals with RNase (Figure 3f) and observed a dramatic loss in signal, suggesting the signal was originating from RNA labeling. We isolated total RNA from organs and subjected isolated total RNA to biotinylation followed by analysis by dot blot. As shown in Figure 3g, RNA biotinylation profiles were similar to those observed in imaging experiments. Overall, these results suggest our strategy can selectively mark tumors using cell-selective metabolic labeling of RNA in vivo.
Figure 3.
Imaging cell-specific RNA labeling in vivo. (a) Outline of experiments used for RNA labeling in vivo. 100 μL of either DMSO, 2′N3A, or Lys2′N3A were IP injected at a concentration of 2 mg/mL once per 24 h over 3 days. After 3 days, the mice were sacrificed, and the tumors and organs were collected and sectioned for imaging. (b) Imaging of isolated organs and tumor tissue from experiments exposing mice to Lys2′N3A. (c) Integration of fluorescence signal from slices represented in panel b. (d) Imaging of isolated organs and tumor tissue from experiments exposing mice to 2′N3A. (e) Integration of fluorescence signal from slices represented in panel d. (f) Imaging experiments demonstrating loss of fluorescent signal after RNase treatment. Sections were then incubated in a 0.2 mg/mL solution of RNase A in 0.5% Triton in 1× PBS pH 7.4 for 1 h, followed by 3× washes with DPBS. Slices were then imaged. (g) Dot blot on isolated RNA from various tissues following IP injection. IP injections were followed as described in panel a. Isolated RNA was then subjected to CuAAC with biotin alkyne. Dot blot was performed as in Figure 2. M.B. = methylene blue dye to demonstrate loading onto the dot blot membrane.
Herein, we have presented our efforts to develop novel chemical approaches to metabolically label RNA in complex environments. We have demonstrated that endogenous enzymes can be utilized to control metabolic labeling and achieve exquisite cell selectivity. We have further demonstrated that the specificity is achieved by two highly reactive enzymes in cancer cells, HDAC and CTSL, for precursor processing and eventual RNA labeling. Specificity was demonstrated not only in vitro through coculture systems but also in vivo, to mark tumor cells with high selectivity. We envision such an approach could be extended to other enzymes in the context of different diseases or specific organs in living animals. Further, probes such as the ones described here have potential to be employed for transcriptome-wide analysis, as the azide functionality has been utilized for RNA biotinylation and enrichment in RNA sequencing workflows. Such directions are currently underway in our lab and will be reported in due course.
Supplementary Material
ACKNOWLEDGMENTS
We thank members of the Spitale lab for their careful reading and critique of the manuscript. This work is supported by the Ono Pharma Breakthrough Science Initiative Award to R.C.S. R.C.S. is a Pew Biomedical Scholar. S.B. is supported by an NIH training grant 5T32CA009054-40.
Footnotes
Complete contact information is available at: https://pubs.acs.org/10.1021/jacs.2c02404
The authors declare no competing financial interest.
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.2c02404.
Experimental methods, synthetic schemes, and spectra for all compounds (PDF)
Contributor Information
Samantha Beasley, Department of Pharmaceutical Sciences, University of California—Irvine, Irvine, California 92697, United States.
Abigail Vandewalle, Department of Pharmaceutical Sciences, University of California—Irvine, Irvine, California 92697, United States.
Monika Singha, Department of Pharmaceutical Sciences, University of California—Irvine, Irvine, California 92697, United States.
Kim Nguyen, Department of Pharmaceutical Sciences, University of California—Irvine, Irvine, California 92697, United States.
Whitney England, Department of Pharmaceutical Sciences, University of California—Irvine, Irvine, California 92697, United States.
Eric Tarapore, Department of Developmental & Cellular Biology, University of California—Irvine, Irvine, California 92697, United States.
Nan Dai, New England Biolabs, Ipswich, Massachusetts 01938, United States.
Ivan R. Corrêa, Jr., New England Biolabs, Ipswich Massachusetts 01938, United States
Scott X. Atwood, Department of Developmental & Cellular Biology, University of California—Irvine, Irvine, California 92697, United States
Robert C. Spitale, Department of Pharmaceutical Sciences and Department of Chemistry, University of California—Irvine, Irvine, California 92697, United States
REFERENCES
- (1).Gupta M; Singha M; Rasale DB; Zhou Z; Bhandari S; Beasley S; Sakr J; Parker SM; Spitale RC Mutually Orthogonal Bioconjugation of Vinyl Nucleosides for RNA Metabolic Labeling. Org. Lett 2021, 23 (18), 7183–7187. [DOI] [PubMed] [Google Scholar]
- (2).Jao CY; Salic A. Exploring RNA transcription and turnover in vivo by using click chemistry. Proc. Natl. Acad. Sci. U. S. A 2008, 105 (41), 15779–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Kubota M; Nainar S; Parker SM; England W; Furche F; Spitale RC Expanding the Scope of RNA Metabolic Labeling with Vinyl Nucleosides and Inverse Electron-Demand Diels-Alder Chemistry. ACS Chem. Biol 2019, 14 (8), 1698–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Nainar S; Beasley S; Fazio M; Kubota M; Dai N; Correa IR Jr.; Spitale RC Metabolic Incorporation of Azide Functionality into Cellular RNA. Chembiochem 2016, 17 (22), 2149–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Zheng Y; Beal PA Synthesis and evaluation of an alkyne-modified ATP analog for enzymatic incorporation into RNA. Bioorg. Med. Chem. Lett 2016, 26 (7), 1799–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Hida N; Aboukilila MY; Burow DA; Paul R; Greenberg MM; Fazio M; Beasley S; Spitale RC; Cleary MD EC-tagging allows cell type-specific RNA analysis. Nucleic Acids Res. 2017, 45 (15), e138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Miller MR; Robinson KJ; Cleary MD; Doe CQ TU-tagging: cell type-specific RNA isolation from intact complex tissues. Nat. Methods 2009, 6 (6), 439−41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Nainar S; Cuthbert BJ; Lim NM; England WE; Ke K; Sophal K; Quechol R; Mobley DL; Goulding CW; Spitale RC An optimized chemical-genetic method for cell-specific metabolic labeling of RNA. Nat. Methods 2020, 17 (3), 311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Nguyen K; Fazio M; Kubota M; Nainar S; Feng C; Li X; Atwood SX; Bredy TW; Spitale RC Cell-Selective Bioorthogonal Metabolic Labeling of RNA. J. Am. Chem. Soc 2017, 139 (6), 2148–2151. [DOI] [PubMed] [Google Scholar]
- (10).Nguyen K; Kubota M; Arco JD; Feng C; Singha M; Beasley S; Sakr J; Gandhi SP; Blurton-Jones M; Fernandez Lucas J; Spitale RC A Bump-Hole Strategy for Increased Stringency of Cell-Specific Metabolic Labeling of RNA. ACS Chem. Biol 2020, 15 (12), 3099–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Wang D; Zhang Y; Kleiner RE Cell- and Polymerase-Selective Metabolic Labeling of Cellular RNA with 2′-Azidocytidine. J. Am. Chem. Soc 2020, 142 (34), 14417–14421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Sonawane AR; Platig J; Fagny M; Chen CY; Paulson JN; Lopes-Ramos CM; DeMeo DL; Quackenbush J; Glass K; Kuijjer ML Understanding Tissue-Specific Gene Regulation. Cell Rep 2017, 21 (4), 1077–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Danan C; Manickavel S; Hafner M. PAR-CLIP: A Method for Transcriptome-Wide Identification of RNA Binding Protein Interaction Sites. Methods Mol. Biol 2022, 2404, 167–188. [DOI] [PubMed] [Google Scholar]
- (14).Li Y; Aggarwal MB; Nguyen K; Ke K; Spitale RC Assaying RNA Localization in Situ with Spatially Restricted Nucleobase Oxidation. ACS Chem. Biol 2017, 12 (11), 2709–2714. [DOI] [PubMed] [Google Scholar]
- (15).Engel KL; Lo HG; Goering R; Li Y; Spitale RC; Taliaferro JM Analysis of subcellular transcriptomes by RNA proximity labeling with Halo-seq. Nucleic Acids Res. 2022, 50 (4), e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Li R; Zou Z; Wang W; Zou P, Metabolic incorporation of electron-rich ribonucleosides enhances APEX-seq for profiling spatially restricted nascent transcriptome. Cell Chem. Biol 2022. DOI: 10.1016/j.chembiol.2022.02.005. [DOI] [PubMed] [Google Scholar]
- (17).Muhar M; Ebert A; Neumann T; Umkehrer C; Jude J; Wieshofer C; Rescheneder P; Lipp JJ; Herzog VA; Reichholf B; Cisneros DA; Hoffmann T; Schlapansky MF; Bhat P; von Haeseler A; Kocher T; Obenauf AC; Popow J; Ameres SL; Zuber J. SLAM-seq defines direct gene-regulatory functions of the BRD4-MYC axis. Science 2018, 360 (6390), 800–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Hanahan D; Weinberg RA Hallmarks of cancer: the next generation. Cell 2011, 144 (5), 646–74. [DOI] [PubMed] [Google Scholar]
- (19).Hainaut P; Plymoth A. Targeting the hallmarks of cancer: towards a rational approach to next-generation cancer therapy. Curr. Opin Oncol 2013, 25 (1), 50–1. [DOI] [PubMed] [Google Scholar]
- (20).Li Y; Seto E. HDACs and HDAC Inhibitors in Cancer Development and Therapy. Cold Spring Harb Perspect Med. 2016, 6 (10), a026831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Olson OC; Joyce JA Cysteine cathepsin proteases: regulators of cancer progression and therapeutic response. Nat. Rev. Cancer 2015, 15 (12), 712–29. [DOI] [PubMed] [Google Scholar]
- (22).Ueki N; Lee S; Sampson NS; Hayman MJ Selective cancer targeting with prodrugs activated by histone deacetylases and a tumour-associated protease. Nat. Commun 2013, 4, 2735. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.