Abstract
Advanced age is the most established risk factor for developing age-related macular degeneration (AMD), one of the leading causes of visual impairment in the elderly, in Western and developed countries. Similarly, after middle age, there is an exponential increase in pathological molecular and cellular events that can induce senescence, traditionally defined as an irreversible loss of the cells’ ability to divide and most recently reported to also occur in select post-mitotic and terminally differentiated cells, such as neurons. Together these facts raise the question as to whether or not cellular senescence, may play a role in the development of AMD. A number of studies have reported the effect of ocular-relevant inducers of senescence using primarily in vitro models of poorly polarized, actively dividing retinal pigment epithelial (RPE) cell lines. However, in interpretating the data, the fidelity of these culture models to the RPE in vivo, must be considered. Fewer studies have explored the presence and/or impact of senescent cells in in vivo models that present with phenotypic features of AMD, leaving this an open field for further investigation. The goal of this review is to discuss the current thoughts on the potential role of senescence in AMD development and progression, considering the model systems used and their relevance to human disease.
Keywords: Age-related macular degeneration, Senescence, Aging, Retinal pigment epithelium
1. Introduction
Amongst the bevy of retinal degenerations, age-related macular degeneration (AMD) remains a major contributor to vision loss in the elderly in many parts of the World (Zhou et al., 2021). Since the first descriptive reports of the aging macula, in the middle of the 19th century (de Jong, 2016), significant progress has been made in characterizing the pathobiology, and genetic, epidemiological, and environmental risk factors associated with the disease. And though drusen, extracellular material that accumulate outside the retinal pigment epithelium (RPE), characteristic of dry AMD, have also been identified in some young individuals (Pedersen et al., 2018), this is more often than not, an exception to the rule, as age is an established risk factor for the disease (Heesterbeek et al., 2020). That AMD is a neurodegenerative disease of the aging retina (Hadziahmetovic and Malek, 2020) raises the question as to whether or not senescence plays a defining role in its occurrence and progression. On first glance it would seem that senescence, a biological consequence of aging, would be central to AMD and therefore an established contributor to disease. However, how and the extent to which senescence may either impart a positive or negative affect in AMD remains an unanswered question and is currently the focus of investigation in many research groups. The goal of this review, written following the 2021 Stephen J. Ryan Initiative for Macular Research meeting by members of the sub-group focused on discussing “Cellular and Organelle Aging in AMD”, is to discuss the current state of the literature and begin to tease out the potential role of senescence in AMD. Throughout, we have embedded discussion points on impeding factors that have held up progress in this field, while focusing on identifying relevant future avenues of research to be pursued including targeting senescence as therapy for AMD, if justified.
1.1. Pathobiology of AMD
The pathobiology of AMD is complex and our knowledge of it has evolved with the emergence of new imaging modalities allowing evaluation of the retinal layers of patients in real time (Fleckenstein et al., 2021). AMD can largely be sub-classified into early dry AMD, intermediate AMD, geographic atrophy (GA), and non-exudative and exudative macular neovascularization (MNV) (Fleckenstein et al., 2021). The early stages of AMD, referred to as ‘early’ and ‘intermediate’ AMD, involve the accumulation of medium-or large-sized lipid- and protein-rich extracellular deposits to the RPE. Vascular changes at the level of the choriocapillaris also emerge at this stage and include choriocapillary dropout (Mullins et al., 2011). Capillary degeneration, along with photoreceptor and in particular RPE atrophy are hallmarks of GA, resulting in significant compromise to central vision. Finally, in some patients, the invasion of vessels originating from the choroid into the outer retina results in MNV, and extensive vision loss.
Our initial understanding of the pathology of AMD was primarily based on examination of ocular tissues from donors, often collected with variable post-mortem times and sample processing methods, which impacted the quality of the tissue. Improved techniques and stricter tissue processing protocols have not only confirmed early findings but also identified new distinct pathological changes in the macula, including but not limited to sub-retinal drusenoid deposits/reticular pseudodrusen and outer retinal tubulations (Chen et al., 2020; Rudolf et al., 2008). Of importance, the overall clinicopathological picture of AMD supports the involvement of an assortment of cells in the different stages of the disease including the photoreceptors, microglial cells, RPE, and choroidal endothelial cells, all potentially vulnerable to aging associated events such as cellular senescence (Fig. 1) (Malek and Lad, 2014; Tuttle et al., 2021).
Figure 1.
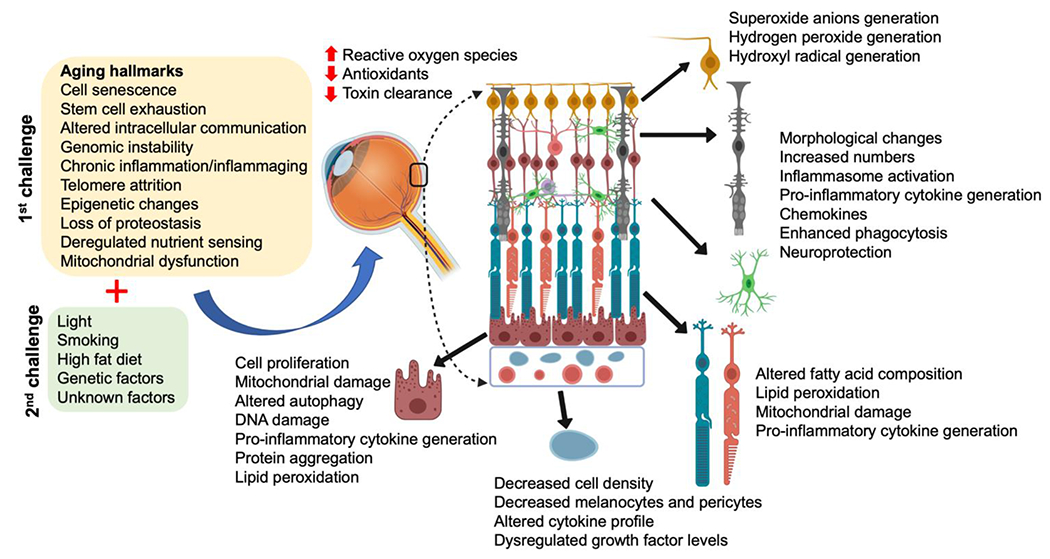
Overview of aging and AMD: The combination of aging (1st hit) and other stressors/modifiers (2nd hit) can impact the integrity and function of ocular cells in the posterior pole triggering AMD. Cells affected include the retinal ganglion cells (yellow), microglial (green) and Müller cells (dark grey), photoreceptors (aqua/orange), retinal pigment epithelial cells (burgundy) and choriocapillaris/endothelial cells (light blue). Select consequences have been listed next to each cell. Figure modified from (Hadziahmetovic and Malek, 2020).
1.2. Aging and cell senescence
The risk of developing AMD increases markedly with age. Likewise, pathological molecular and cellular conditions that can induce senescence also increase exponentially after middle age. Even though aging is difficult to precisely define, it corresponds to an overall decline in many physiological functions with age. In 2013, nine hallmarks of aging were proposed, including stem cell exhaustion, altered intercellular communication, genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, and increased cellular senescence (Lopez-Otin et al., 2013). These hallmarks often overlap, and of note, several are associated with cellular senescence potentially triggered by persistent DNA damage, mutations and/or oxidative stress (Campisi et al., 2019). In particular, oxidative stress, mitochondrial dysfunction, and impaired proteostasis are known to contribute to AMD development and progression (Ferrington et al., 2021; Fleckenstein et al., 2021; Paraoan et al., 2020) (Fig. 1).
Cellular senescence was originally described to entail an irreversible loss in the cells’ ability to divide concomitant with resistance to cell death-inducing stimuli (Hayflick and Moorhead, 1961). Consequently, these cells accumulate with age. More recently, cellular senescence phenotypes have been observed in post-mitotic neurons and brain tissue (Jurk et al., 2012; Musi et al., 2018; von Zglinicki et al., 2021). Senescent cells characteristically secrete many inflammatory factors, as part of the complex senescence-associated secretory phenotype (SASP) (Coppe et al., 2008), and can contribute to many pathologies, including pulmonary fibrosis (Chilosi et al., 2013; Wiley et al., 2019), atherosclerosis (Childs et al., 2016), osteoarthritis (Jeon et al., 2017), type 2 diabetes (Aguayo-Mazzucato et al., 2019; Sone and Kagawa, 2005) and eye diseases including diabetic retinopathy (Crespo-Garcia et al., 2021; Oubaha et al., 2016), and glaucoma (Blasiak et al., 2017; Skowronska-Krawczyk et al., 2015). Senescent cells drive these pathologies largely by secreting pro-inflammatory cytokines, danger-associated molecular patterns (DAMPS) and chemotactic chemokines (Basisty et al., 2020). The SASP is dynamic and in some cases beneficial, such as during wound healing (Demaria et al., 2014). Here, we summarize the characteristics of senescent cells (Fig. 2).
Figure 2.
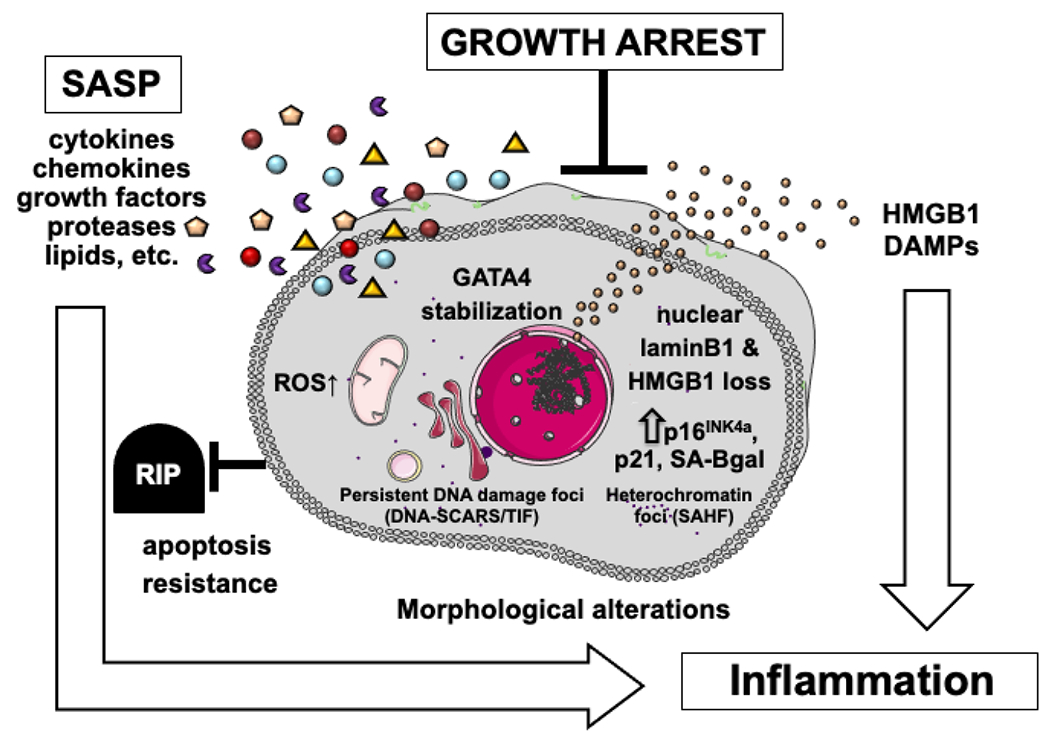
Definition of senescent cells: Cellular senescence is a cell fate in which both intrinsic and extrinsic signals can cause an irreversible cell cycle arrest, accompanied by many phenotypic changes. These phenotypic changes have also been reported in non-replicative senescence. Senescent cells acquire a complex, often pro-inflammatory, secretory phenotype termed the senescence-associated secretory phenotype (SASP), which can cause chronic inflammation. SA-βgal: senescence-associated beta-galactosidase; HMGB1: high mobility group box protein 1; DAMPs: damage-associated molecular patterns; ROS: reactive oxygen species; GATA4: GATA binding protein 4; SAHF: senescence-associated heterochromatin foci; DNA-SCARS: DNA segments with chromatin alterations reinforcing senescence; TIF: telomere dysfunction-induced foci.
Two major tumor suppressive pathways, governed by p16INK4a/Rb and p14ARF (P19Arf in mice)/p53/p21CIP1, initiate and maintain the senescent growth arrest. Many stressors enhance the expression of p16INK4a, which inhibits a cyclin-dependent kinase (CDK) and phosphorylation of the retinoblastoma (Rb) tumor suppressor protein, thus preventing activation of the pro-proliferative transcription factor E2F. On the other hand, p21CIP1 expression is regulated mainly by p53, which inhibits different CDK targets. The two pathways can act together synergistically to activate Rb proteins and arrest progression from G1 to the S phase of the cell cycle (Sherr, 1996; Wu et al., 2001). Increased expression of p16INK4a and/or p21CIP1 is commonly observed in senescent cells.
Genotoxic stress or oncogene activation can cause a senescence response, including the secretion of SASP factors; the SASP component includes pro-inflammatory cytokines such as interleukins (IL; e.g., IL-6, IL-8, IL-1α), chemokines (e.g., CXCL1 and CXCL10), and matrix metalloproteases (MMPs; e.g., MMP1, MMP3, MMP9) (Basisty et al., 2020; Coppe et al., 2008). These molecules are frequently associated with age-associated pathologies, including chronic inflammation. However, proteomic analyses of cultured human cells induced to senescence by different agents show that each SASP is unique, consisting of hundreds of largely distinct proteins (Basisty et al., 2020). Additionally, SASP components vary depending on species (e.g., human vs mouse) and cell type (e.g., epithelial vs stromal) (Coppe et al., 2008). On the other hand, certain core SASP factors, including growth differentiation factor-15 (GDF15), stanniocalcin-1 (STC1), Serpin Family E Member 1 (SERPINE-1), and MMP1, are expressed by many types of senescent cells (Basisty et al., 2020).
Oxidative stress, the overproduction of reactive oxygen species (ROS), can damage macromolecules, including nuclear and mitochondrial DNA. The primary sources of ROS are the mitochondrial electron transport chain and NADPH oxidases, enzymes in the plasma membrane and membranes of detoxifying organelles such as phagosomes. In all cases, ROS serve as either damaging or signaling molecules, and these divergent processes interact (Brennan et al., 2009; Dickinson and Chang, 2011; Finkel, 2003). For example, as ROS increases, it can sulfenylate cysteines on dynamin-related protein 1, the protein that initiates mitochondrial fission. This sulfenylation disrupts mitochondrial dynamics, resulting in cellular senescence in some cell types (Nishimura et al., 2018; Yu et al., 2020) potentially including RPE cells. Indeed, several studies show that mitochondrial dysfunction contributes to AMD pathology (Datta et al., 2017; Ferrington et al., 2016; La Cunza et al., 2021). Furthermore, a senescence response caused by mitochondrial dysfunction in the absence of genotoxic stress induces a distinct SASP lacking certain proinflammatory factors regulated by interleukin-1 receptor signaling. This mitochondrial dysfunction-associated senescence (MiDAS) (Wiley et al., 2016) may a good candidate to investigate further in AMD.
Finally, with regards to the retina and retinal diseases, early studies often used the terms aging and senescence interchangeably. The discovery of senescent characteristics has created a need to further review the literature in a more holistic way in an attempt to tease out the results of aging versus senescence with more clarity.
2. Requirements and challenges for assessing senescence in in vivo and ex vivo models
Modeling of AMD using both in vitro and in vivo platforms remains a vital tool in exploring the contributions of pathways to disease development and progression. Each present with challenges and limitations.
2.1. Limitations to animal models of AMD as platforms to investigate senescence
The multi-faceted nature of AMD has made recapitulating phenotypic features of the disease challenging, with an overall perception that animal models of human AMD remain to be developed. Yet, a number of in vivo models have been characterized incorporating either known genetic and environmental risk factors for the disease (Malek et al., 2005; Storti et al., 2019; Toomey et al., 2015) or through the discovery of pathways compromised simply during the aging process (Choudhary et al., 2020; Hu et al., 2013; Yao et al., 2022). These models present with different phenotypic features of AMD, most frequently basal laminar deposits below the RPE, and RPE phenotypic and degenerative changes. Importantly, these models were developed taking age into consideration and as such may serve as platforms to investigate potential senescence. The observation of choroidal changes similar to that observed in dry human AMD have not been reported in animal models.
There are a limited number of spontaneously developed animal models that have been used to investigate different aspects of senescence including the senescence-accelerated OXYS rats, though their ocular senescence markers remain to be investigated (Kozhevnikova et al., 2018), and the senescence-prone mouse strain 8 (SAMP8). Aged SAMP8 mice present with increased ocular autofluorescence, decreased scotopic retinal function, amyloid beta positive deposits below the RPE, RPE degenerative changes and increased p16Ink4a expression in the RPE, collectively supporting senescence mechanisms at play (Feng et al., 2016). Importantly, sub-retinal injection of an amyloid beta peptide in 5 month old C57BL/6 mice has been reported to impact retinal function, specifically triggering a decrease in the amplitudes of the a-, b- and c-waves on electroretinography, RPE pigmentary and degenerative changes, and increased expression of p16 Ink4a in RPE cells (Liu et al., 2015). However, as noted in Section 4, these studies need to be confirmed using multiple measures of senescence because p16 expression is not solely associated with senescence but can also be a marker inflammation and general aging (Liu et al., 2019b).
The ocular pathology of non-human primates has also been described in some detail and though with advanced age some species develop lipid-rich deposits, the time investment to use them as animal models (over 25 years versus 2 years for mice) limit their broad use for therapeutic testing. However, examination of the pathology of retinal cross-sections from one 16- and one 29-year-old Rhesus monkey has been informative, demonstrating Senescence-associated beta-galactosidase (SA-βgal) staining in the RPE adjacent to small, hard drusen (Mishima et al., 1999), indicating that senescence may be a feature of retinal aging in the monkey eye. One caveat for these studies is that SA-bgal also labels older lysosomes, and can be a measure of lysosome aging, rather than senescence per se. Evidence for senescence in the best model for AMD available, namely human donor tissue from AMD patients is fairly limited. Noteworthy has been immunolocalization of bone morphogenetic protein-4 (BMP4), capable of inducing expression p53 and p21CIP1 in RPE cells, within the RPE and Bruch’s membrane of patients with dry AMD (Zhu et al., 2009). In spite of this, there is a need to investigate senescence in current murine models that present with AMD phenotypes and more importantly human donor tissue, comprehensively, sub-classified based on clinical phenotype (Ferris et al., 2013) and ideally genotyped (at a minimum) (Fritsche et al., 2016; Pappas et al., 2021).
2.2. Limitations of existing in vitro models used to study senescence
The pros and cons of existing in vitro models to study important aspects of RPE cell biology have been recently discussed (Lakkaraju et al., 2020). Here, we will discuss the utility of these cell-based models to interrogate senescence in particular.
2.2.1. ARPE19, versus primary, versus stem cell derived RPE cells
Because the RPE is a terminally differentiated, postmitotic tissue, to accurately model non-replicative senescence in the RPE using in vitro models requires the use of non-dividing cell cultures. Many published studies on senescence in the RPE have used poorly differentiated cultures of the immortalized ARPE-19 cell line, which participates in the cell cycle and is susceptible to mitotic senescence. Therefore, conclusions regarding mechanisms or triggers that induce senescence in the RPE using immortalized RPE cell lines may have little relevance to RPE in vivo or to AMD. The minimum requirement to study senescence in RPE cell cultures is that the cells be well polarized, terminally differentiated, and express RPE differentiation markers (RPE 65, apical Na+,K+-ATPase, etc) to accurately reflect RPE in vivo. Well-characterized cell-based models include:
Primary RPE cultures established from freshly harvested retinas of mouse, porcine, or human donors recapitulate several features of RPE in situ. When grown on semi-permeable membrane supports (Transwell filters), these cultures form well-differentiated monolayers with tight junctions, trans-epithelial resistances (TER) greater than 300 ohm.cm2, and degrade photoreceptor outer segments with kinetics comparable to that found in vivo. For each of these models, there are established protocols that have been validated in multiple publications (Blenkinsop et al., 2013; Gibbs and Williams, 2003; Maminishkis et al., 2006; Samuel et al., 2017; Toops et al., 2014).
Primary RPE cultures have proven to be especially useful in understanding mechanisms of AMD pathogenesis. Long-term cultures of human fetal and porcine RPE have been shown to constitutively secrete apolipoprotein E (ApoE), a major component of drusen, and this could be further exacerbated by exposing the cultures to active complement components (Johnson et al., 2011). Adult human RPE cultures secrete osteopontin, an inflammatory mediator, in response to oxidative stress (Lekwuwa et al., 2021). These studies suggest that the RPE is sufficient to induce biogenesis of drusen constituents. But what could be the mechanism? Using porcine RPE cultures, recent studies demonstrated that complement activation and lipid dysregulation – two major pathways implicated in AMD – cause mitochondrial injury in the RPE. The resulting alteration of mitochondrial redox status drives the formation of ApoE biomolecular condensates as potential drusen precursors (La Cunza et al., 2021). Noteworthy, studies using RPE cultures established from adult human donors have identified defective autophagy, mitochondrial dysfunction, and decreased expression of nuclear hormone receptors in AMD donors compared with cells from unaffected controls (Ferrington et al., 2017; Ferrington et al., 2016; Hu et al., 2013; Zhang et al., 2020) (Fig. 3).
Figure 3.
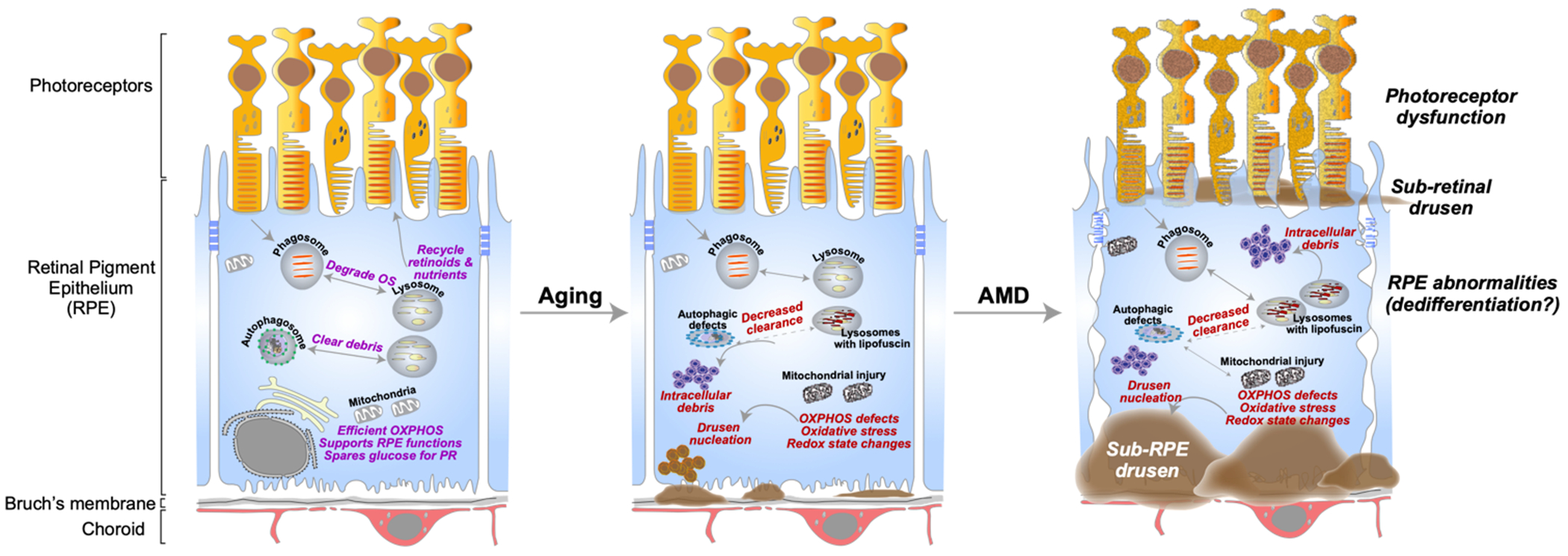
RPE dysfunction and dedifferentiation in aging and AMD: Left panel: The retinal pigment epithelium (RPE) performs numerous functions critical for photoreceptor health and vision. These include the daily phagocytosis and clearance of photoreceptor outer segments (OS) and recycling retinoids and nutrients to photoreceptors. The RPE relies on oxidative phosphorylation (OXPHOS) for its high energy needs and spares glucose for the photoreceptors. Middle panel: Age-related accumulation of vitamin A metabolites in the form of lipofuscin in RPE lysosomes interferes with critical functions such as autophagy and OXPHOS. Declining mitochondrial function can lead to redox state-mediated phase separation of proteins, resulting in the nucleation of drusen-like aggregates. Right panel: Genetic and environmental risk factors in AMD (complement activation, lipid dysregulation, etc.) can act as “tipping points” to exacerbate these deficits, increase drusen formation, and eventual cause RPE atrophy and photoreceptor dysfunction. Figure adapted from (La Cunza et al., 2021).
The limited availability of human donor tissue and number of passages that maintain expression of RPE cell markers, are the main drawbacks of primary cultures. While this is not a limitation for cultures from mice or pigs, one caveat for their use is the species-specific expression of genes associated with AMD: for instance, cholesterol ester transfer protein (CETP) is not expressed in mice, and humans are the only species that express three isoforms of APOE.
ES- and iPSC-RPE (Embryonic and induced pluripotent stem cell-derived RPE) cultures are now widely used not only to study various aspects of RPE cell biology (Hazim et al., 2017; Maruotti et al., 2015) but also in clinical trials launched by the National Eye Institute, for slowing or reversing vision loss associated with AMD. Cultures established from patient fibroblasts are especially useful to model disease conditions. Studies on iPSC-RPE generated from AMD donors with AMD-associated genetic risk alleles showed that these RPE cultures secreted high levels of complement proteins and drusen components including ApoE and identified nicotinamide as a potential modifier of these disease phenotypes (Saini et al., 2017).
Although iPSCs can constitute a freely available source to differentiate into RPE, there are a few significant caveats to keep in mind while studying senescence. First, iPSCs retain epigenetic memory that reflects the tissue of origin, developmental stage, and sex that can impact the efficiency of reprogramming (Efrat, 2020). This complex epigenetic regulation could in turn impact senescence response pathways in iPSC-RPE.
Immortalized RPE cell lines currently used include the human ARPE-19, human RPE-1, and rat RPE-J lines. RPE-1 (hTERT) is a telomerase immortalized line that exhibits virtually no features of RPE in situ. The cells are poorly differentiated, flat, and are more often used to study the development of the primary cilium, and not RPE biology (Lakkaraju et al., 2020). The rat RPE-J line was established by simian virus 40 (SV40)-mediated transformation, which resulted in hypodiploid RPE (Nabi et al., 1993). Moreover, these cells do not exhibit the correct apical localization of Na+,K+-ATPase and neural cell adhesion molecule (NCAM), which are required for RPE and photoreceptor function.
By far the most commonly used RPE cell line is ARPE-19, which was established by spontaneous immortalization of RPE from a 19-year-old donor (Dunn et al., 1996). Many studies on senescence have used poorly polarized or actively dividing ARPE-19 cells. These cells are fibroblastic in appearance, do not express key RPE proteins such as RPE65, and clear photoreceptor outer segments with very slow kinetics. Pertinent to studies on senescence, these poorly differentiated ARPE-19 cultures are very susceptible to oxidative stress (Glotin et al., 2008) or AMD-associated insults such as complement attack, in contrast to the highly robust primary RPE cultures (Radu et al., 2014; Tan et al., 2016). Therefore, using actively dividing ARPE-19 cultures to study senescence could identify pathways and mechanisms that would not be relevant for RPE in vivo.
Two recent protocols for culturing ARPE-19 cells have shown an improvement in cell phenotype and kinetics of outer segment clearance (Hazim et al., 2019; Samuel et al., 2017). These protocols involve growing the cells in high glucose with pyruvate for 3-4 months or in nicotinamide for 2 weeks. Using these protocols, ARPE-19 cells express RPE65 and other markers of differentiation. However, the TER is low, indicative of a leaky barrier, likely because ARPE-19 cells do not express claudin-19, which is a key component of the RPE tight junction in vivo. Because barrier function is directly linked to the ability of the RPE monolayer to withstand stressors such as complement and oxidative damage, this could potentially underlie the increased susceptibility of these cells to insults that induce senescence in dividing cells. Another caveat is that ARPE-19 cells also exhibit chromosomal abnormalities, which could be another confounder in their use for studies of RPE senescence. Researchers using ARPE-19 cells to study senescence should be mindful of these caveats and at the very least, culture the cells using protocols established by Hazim et al. It is important to establish that the cells express RPE markers and are not actively dividing before the start of the experiment. Because a feature of senescence in postmitotic cells is cell cycle re-entry, using mitotic cells could mask important mechanisms that could drive senescence in the RPE.
2.2.2. RF6A versus primary choroidal endothelial versus stem cell derived choroidal endothelial cells
Changes in the outer vasculature have been noted in all the sub-types of AMD, though the clinicopathological features are quite different. In early, intermediate AMD and GA, there is evidence of choriocapillary dropout, while in MNV the choroidal vasculature undergoes abnormal vessel growth. As such, studying the biology of the choroidal endothelial cells is paramount, yet understudied. This is in part because in vitro model systems for choroidal endothelial are limited. The most commonly used cell culture model system has been a spontaneously transformed cell line originally derived from a crude choroid-retina complex isolated from the fetus of the rhesus macaque monkey, called RF/6A, which originally was reported to expresses endothelial markers. Recent careful characterization of this cell line has identified low level expression of endothelial markers including von Willebrand factor, platelet endothelial cell adhesion molecule-1 (PECAM1), VE-cadherin and cadherin 5 in RF/6A cells (Makin et al., 2018). Additionally, functional assays using RF/6A cells, found these cells to be insensitive to VEGF-A stimulation as well as shear stress. Finally, when challenged with tumor necrosis factor alpha (TNFα), expression of the endothelial-specific protein E-selectin was prominently less than that in other endothelial cell lines. Collectively, these findings highlight limitations of this cell line (Makin et al., 2018). Alternative cell culture systems which may be used included primary cultures isolated from human donor eyes (Peavey and Malek, 2020; Peavey et al., 2022; Stewart et al., 2011), which are convenient should there be accessibility to non-fixed donor eyes with short post-mortem times. Of relevance to AMD has been the creation of a conditionally immortalized choroidal endothelial cell line with decreased binding affinity for the AMD-associated 402H variant compared to 402Y (Loeven et al., 2018). An immortalized human choroidal endothelial cell line generated using lentiviral vectors with endothelial-specific promoters to drive immortalization (Giacalone et al., 2019), and choroidal endothelial cells derived from mouse fibroblast iPSCs (Songstad et al., 2015), are also potential relevant cell culture models to incorporate in studies of AMD.
2.2.3. Senescence in spontaneously arising cell lines
Cells with a variety of phenotypes have been used to study age-related pathologies, including AMD. ARPE-19 cells, discussed above, is a spontaneously immortalized human RPE line that is not pre-malignant or malignant. Spontaneous immortalization is rare for primary human cells, but more common for mouse cells. In mouse cells many of the spontaneous immortalization events occur due to loss of p53 function (Harvey and Levine, 1991). This is significant because p53 can also regulate the SASP (Coppe et al., 2008; Davalos et al., 2013), Similarly human cells, particularly epithelial cells, are susceptible to losing p53 function upon spontaneous immortalization (Yaswen and Stampfer, 2002). Of interest, gene expression profiling of human embryonic stem cell derived (ESC)-RPE cells demonstrates greater similarity to primary RPE than to ARPE-19 cells (Klimanskaya et al., 2004). Finally, because gene expression profiles can differ substantially between primary and immortalized cells, caution and in vivo validation should be considered for interrogating aging, pathological and senescent phenotypes in intact tissues.
3. Aging-related changes in the outer retina
Given the impact of age-related vision loss on quality of life many laboratories have been investigating the morphological, physiological, and molecular changes in the aging eye using human ophthalmic data, human post-mortem specimens and animal models [reviewed in detail in (Campello et al., 2021)]. Common age-related ocular changes include cataract and low-light vision sensitivity, and chronic ocular diseases such as diabetic retinopathy, glaucoma and AMD, in which the neural retina, RPE and choroid are affected.
3.1. Retina
A key feature of the aging retina is a decline in visual function reflecting a decrease in photoreceptor activity. Studies in mice, rats, and humans (Ferdous et al., 2021; Gao and Hollyfield, 1992; Kovacs-Valasek et al., 2021) show an age-related reduction of scotopic a- and b-wave amplitudes and total number of photoreceptors. Interestingly, photoreceptor loss in aging is not uniform, rather pronounced in the peripheral retina as rods are more vulnerable to loss than cones (Curcio et al., 1993; Eliasieh et al., 2007). Surprisingly, these changes are not dependent on the diurnal/nocturnal lifestyle of the studied organism.
Another phenotype of aging is retinal thinning. Systematic studies (Samuel et al., 2011) have described age-related changes in cell number and density of the mouse retina and provided elegant quantification of dendritic arbors for all retinal neurons. Spectral domain-optical coherence tomography (SD-OCT) studies (Ferdous et al., 2021) have shown reduced thickness of the outer nuclear layer (ONL), which contains photoreceptor nuclei; confirmed by quantification of nuclei by histology. Interestingly, rod bipolar and horizontal cell dendrites extend into the ONL in aged animals suggesting changes in the extracellular matrix composition. Similar observations were made in examining the human aging retina (Eliasieh et al., 2007) potentially indicating high levels of metabolic activity in aged bipolar and horizontal cells.
A third aging phenotype of the retina is reactive gliosis (Mansour et al., 2008; Ramirez et al., 2001) often associated with aberrant activation of Müller glia cells and astrocytes, and elevated levels of glial fibrillary acidic protein (GFAP) immunoreactivity. Glial activation is characterized by increased expression of VEGF, cytokines, extracellular matrix modifying molecules and several interleukins, which maintain the retinal inflammatory environment, as well as an overall decline in microglial functionality [reviewed in (Ma and Wong, 2016)].
Finally, the vasculature is also affected in the aging retina. Multiple studies using optical coherence tomography angiography (OCTA) have shown a decrease in vessel density in the aging human retina. Additionally, detailed mouse and human studies have found a significant drop in vascular density in age-related eye conditions including glaucoma (Yip et al., 2019), AMD (Toto et al., 2017; Vaghefi et al., 2020) and diabetic retinopathy (Liu et al., 2019a; Tonade et al., 2017). Of note, in larger animal models including canines, an evaluation of the retina, uvea, and lens revealed that the presence of tumors rather than age was associated with an increase in the senescent markers gH2AX and p21Cip1 (Merz et al., 2019).
3.2. RPE
Like other postmitotic, metabolically active tissues, the RPE acquires specific functional deficits with age, which are exacerbated in AMD. One of the most important functions of the RPE is the daily phagocytosis and digestion of photoreceptor outer segment tips (Caceres and Rodriguez-Boulan, 2020). In the human eye, each RPE cell is in contact with ~40 photoreceptors, and the diurnal clearance of shed outer segments constitutes an enormous burden on the degradative machinery of the RPE. The importance of this process for photoreceptor health and vision is illustrated by the fact that mutations in specific phagocytic machinery are associated with inherited retinal degenerations: mutations in the MER tyrosine kinase (MERTK), which participates in outer segment ingestion, cause retinitis pigmentosa; whereas mutations in the actin motor protein myosin VIIa (MYO7A), which participates in phagosome transport in the RPE, result in Usher syndrome (Gal et al., 2000; Gibbs et al., 2003). However, there is to date limited evidence for defects in clearance of phagocytosed photoreceptor outer segments in the aging or AMD RPE. Studies on changes in RPE lysosomal hydrolase activities with age have yielded conflicting data, with one showing increased cathepsin D and acid phosphatase activities (Boulton et al., 1994) and another reporting a specific decrease in alpha-mannosidase activity but not in acid phosphatase (Wyszynski et al., 1989). Although decreased expression of the lysosomal membrane protein LAMP2 has been reported in AMD donor RPE compared to unaffected controls (Notomi et al., 2019), how this impacts photoreceptor outer segment degradation is yet to be investigated.
Decreased LAMP2 expression could however interfere with autophagy, an evolutionarily conserved mechanism to clear damaged proteins and organelles. Efficient autophagy is essential for postmitotic tissues like the RPE where debris cannot be dispersed among daughter cells after cell division. RPE from AMD donors exhibit defects in autophagosome biogenesis (decreased levels of lipidated LC3B) and autophagic flux (accumulation of long-lived proteins such as p62/SQSTM1) (Golestaneh et al., 2017; La Cunza et al., 2021). Declining autophagy and the resulting accumulation of undegraded debris can place additional stress on the aging RPE (Fig. 3).
Another feature of aging and AMD is mitochondrial dysfunction. RPE from AMD donors show increased mitochondrial fragmentation and mitochondrial DNA damage, and decreased oxidative phosphorylation (Ferrington et al., 2017; Golestaneh et al., 2017; La Cunza et al., 2021). Because the RPE is highly reliant on oxidative phosphorylation (OXPHOS) as it spares glucose for the photoreceptors, mitochondrial dysfunction with age could induce the RPE to switch to glycolysis as an energy source, and ultimately starve the photoreceptors (Kanow et al., 2017).
3.3. Choroid
The choroid is a complex tissue located between the sclera and neural retina and its underlying RPE. Playing a central role in providing oxygen and nutrients to the overlying retina, it is heavily vascularized and can be broadly divided into the Haller’s layer, composed of large blood vessels, Sattler’s layer, composed of medium diameter blood vessels, and the choriocapillaris, a network of fenestrated capillaries. It is home to a high concentration of melanin, which may in part protect the choroidal micro and macro-vessels from light toxicity. It is also rich in fibroblasts, resident immune cells, extracellular matrix molecules including collagen and elastic connective tissue. The integrity of the choroid is paramount to vision, as any damage has the potential to lead to degenerative changes in the retina due to lack of vascular support or abnormal neovascularization or edema. Indeed, OCT evaluation of choroidal thickness in non-AMD individuals aged 21 to 86 years, has revealed thinning in the fovea with age by approximately 3 μm/year (Wakatsuki et al., 2015). Studies on blood flow and choroidal vascularity, in which luminal and stromal components of the choroid have been measured have also found a decline with increase in age (Emeterio Nateras et al., 2014; Nivison-Smith et al., 2020). The reported changes in melanocytes with age are less clear and dependent on method of analysis. When measured with fluorescence, a decrease in melanocytes was observed with age (Weiter et al., 1986), while biochemical measurements showed no changes with age (Hayasaka, 1989). Finally, fewer adrenergic fibers with less varicosities, a decrease in nerve fibers to the submacular region, and a decrease in hyaluronic acid in the choroid, have been measured with age in studies with relatively small cohort sizes (Jablonski et al., 2007; Nuzzi et al., 1996; Tate et al., 1993). These studies remain to be corroborated in larger populations.
4. Senescence
Replicative senescence, is an adaptive mechanistic response to prolonged stress, associated with dividing cells and cells challenged with a cancerous signal (e.g. ras-induced senescence). However, an increasing number of studies show non-replicative senescence can occur in post-mitotic cells including neurons. Senescent cells are rare at young ages, but are generally only a minor population (a few percent) even at old ages (He and Sharpless, 2017). Although several markers of senescent cells have been reported, no single marker can confirm the presence and state of senescence in vivo. SA-βgal activity is a widely used marker and reflects the increase in lysosomal activities that generally accompany senescence-inducing stresses (Itahana et al., 2007). Therefore, SA-βgal staining is often used as a first step to identify senescent cells in culture and/or tissues. As a second step, expression of p16INK4a and/or p21CIP1 is also commonly used to identify senescent cells (Itahana et al., 2007; Lopez-Dominguez et al., 2021). However, upregulation of p16INK4a and p21CIP1 is not always caused by senescence. For example, a subpopulation of macrophages can express p16Ink4a and SA-βgal activity (Hall et al., 2016).
γ-H2AX and 53BP1 foci, markers of DNA double strand breaks, are also useful to identify senescent cells. Both often increase in primary cells from aged mice and humans, and skin of aged primates (Herbig et al., 2006). GATA Binding Protein 4 (GATA4) is a transcription factor that regulates SASP factors. It is usually degraded by autophagy, but, upon senescence, it is stabilized and activates nuclear factor kappa B (NF-κB), resulting in SASP secretion (Kang et al., 2015). High mobility group box 1 (HMGB1) typically resides in the nucleus but is expelled from the nucleus of senescent cells and secreted, where it functions as a damage-associated molecular pattern (DAMP) molecule (Davalos et al., 2013). Finally, bioactive lipids including prostaglandins, leukotrienes and monounsaturated fatty acids, were recently shown to increase in senescent cells (Cormenier et al., 2018; Wiley et al., 2021). Thus, multiple markers are best used to detect senescent cells (Gorgoulis et al., 2019). Collectively, these markers are appropriate endpoints and should be considered when evaluating senescence in the ocular space.
4.1. Evidence for senescence in the retina
Though there are no comprehensive reports on senescence in retinas of AMD donor tissues, several studies have investigated the presence of senescent markers in the retina of other ocular diseases including glaucoma and diabetic retinopathy, that may provide some insight relevant to AMD. In glaucoma, SA-βgal positive cells have been detected in the trabecular meshwork (Liton et al., 2005) and in retinal ganglion cells in the glaucomatous retina, concomitant with SASP expression (Skowronska-Krawczyk et al., 2015). In follow-up studies, the use of p16-3MR transgenic mice or administering a senolytic drug to remove senescent cells, induced by high intraocular pressure (IOP), has provided support for potential therapy (Rocha et al., 2020). Importantly, existing clinical data has shown that senolytic exposure as a treatment for other health complications, is not associated with decreased visual acuity, elevated intraocular pressure, or senolytic-related adverse ocular effects (El-Nimri et al., 2020). These data support the hypothesis that, when controlled for dosage and frequency, senolytic drugs might be applicable to treat ocular diseases including glaucoma.
In a mouse model of retinopathy of prematurity (ROP), cells devoid of oxygen become senescent and secrete SASP molecules, induce aberrant vasculogenesis (Oubaha et al., 2016), trigger the unfolded protein response (UPR) with consequent activation of classical senescence associated factors including p53 and p16Ink4a (Crespo-Garcia et al., 2021). Of note, use of metformin decreased the expression of these markers and could thus be a potential therapeutic for further investigation. That said, in the context of AMD, recent retrospective studies looking at the association between diabetic medication use and AMD risk, have not reached a consensus on the impact of metformin on AMD. A number of studies have found a decreased risk for AMD with metformin use (Blitzer et al., 2021; Brown et al., 2019) while others have reported no association and/or an increased hazard for AMD (Eton et al., 2022; Gokhale et al., 2022; Vergroesen et al., 2022). The study designs and cohorts used in these series of retrospectives varied, which may account for the different findings. Another therapeutic approach suggested to target senescence is intravitreal administration of small molecule inhibitors of senescence as well as removal of p16Ink4a-expressing cells, in retinal diseases associated with abnormal vascularization (Crespo-Garcia et al., 2021).
Certain markers associated with senescence have also been detected in aged retinas with microaneurysms (Lopez-Luppo et al., 2017). Systematic analysis of the retinal layers for the senescence phenotype found that neurons, but not glial cells, and blood vessels express senescence-associated markers. In an Alzheimer’s disease transgenic mouse characterized by amyloid beta immunoreactivity in the photoreceptor layer, rod degeneration concomitant with increased p16ink4a and p21 protein expression in the outer segments has been observed in 9-month-old mice, supporting a link between photoreceptor degeneration and senescence marker expression (Zhang et al., 2021). Interestingly, the Lopez-Luppo et al study found that cones rather than rods expressed the senescence marker p16Ink4a in retinas with microaneurysms. The finding that senescent cells are resistant to apoptosis, yet secrete SASP affecting surrounding cells, may explain why in several ocular diseases rod cell death precedes death of cones. These hypotheses and more detailed studies of senescence in the retina are critically needed, in order to move forward with a better understanding of the links between aging and senescence in retinal diseases including AMD.
4.2. Evidence for senescence in the RPE
Landmark studies in neurons conducted by Rita Levi-Montalcini in 1960 showed that as neurons mature, they undergo terminal differentiation and become resistant to apoptosis. In post-mitotic neurons, expression of cell cycle markers is accompanied by mitochondrial and endoplasmic reticulum stress, thought to be a prelude to cell death. However, because these post-mitotic cells are difficult to replace, it is likely that senescence may play a pro-survival role to preserve valuable cells. In postmitotic tissues like the RPE, the evidence for senescence is limited, and whether it plays a protective or detrimental role remains unclear (Sapieha and Mallette, 2018). Increased SA-βgal staining has been observed in aging human and non-human primate RPE. However, whether this is true senescence or increased immunoreactivity of long-lived lysosomes in postmitotic cells remains to be established. This distinction is especially important because SA-βgal activity has been detected in brain tissue independent of age or senescence.
As discussed earlier, the vast majority of published studies on senescence in the RPE have used the immortalized ARPE-19 cell line, which actively participates in the cell cycle and is susceptible to replicative senescence (Aryan et al., 2016). Therefore, conclusions regarding mechanisms or triggers that induce senescence in the RPE using poorly differentiated RPE cell lines might have little relevance to RPE cells in vivo or to AMD. Specifically, undifferentiated RPE cultures grown on plastic have been noted to show an age-related increase in the expression of p53, p16INK4a and p21CIP1. Similarly, sub-confluent human fetal RPE cells when exposed to oxidants begin to express senescent biomarkers (Sreekumar et al., 2022). To what extent these studies are representative of the aging RPE cells in vivo must be further evaluated using additional culture models and/or in vivo models. One example of in vitro – in vivo confirmation studies worth noting involved determining the effect of amyloid beta, a molecular component of drusen (Dentchev et al., 2003; Johnson et al., 2002) in RPE cells. Cultured undifferentiated RPE cells when exposed to amyloid-beta display characteristics of senescence including SA-βgal activity, increased p16INK4a expression, along with an increase in expression of pro-inflammatory molecules, such as IL-8, IL-33, MMP9 and VEGF (Cao et al., 2013; Liu et al., 2012; Yoshida et al., 2005). Amyloid-beta exposure also decreased tight junction-related proteins, such as ZO-1 and occlusion (Cao et al., 2013). Though this study involved the use of undifferentiated RPE cells, interestingly, in vivo studies involving subretinal injection of amyloid-beta also triggered an increase in the expression of p16Ink4a in the RPE, upregulated IL-6 and IL-8, compromised the integrity of basal infoldings and increased the formation of autophagic vacuoles in the RPE (Liu et al., 2015). Because the expression of p16, IL6, and IL-8 is also increased in inflammatory conditions, whether or not amyloid beta exposure in vivo stimulates additional markers of senescence and how this is relevant to AMD remains to be determined.
A few studies have used the more physiologically relevant RPE cultures, harvested from human donor eyes, to study senescence (Chaum et al., 2015; Sreekumar et al., 2016; Yamada et al., 2020). Polarized RPE grown on transwells treated with strong oxidants lead to mitochondrial damage and increased expression of p16INK4a. This is significant since in AMD, mitochondria display focal loss of cristae, decreased electron density of the matrix, and more advanced mitochondrial alterations, reflecting mitochondrial dysfunction (Feher et al., 2006; Ferrington et al., 2016; Ferrington et al., 2021). In general, the senescent MiDAS phenotype, that has been observed upon mitochondrial dysfunction, has a distinct SASP profile that differs from the classical SASP profile (Wiley and Campisi, 2016; Wiley et al., 2016). Future studies will need to determine whether or not RPE cells in AMD patients have a senescent MiDAS phenotype.
It is important to note, that in terminally differentiated neurons and cardiomyocytes, undigested lipids and proteins accumulate in the form of lipofuscin granules, which constitute a surrogate marker for aging. Lipofuscin also accumulates in RPE lysosomes with age; however, RPE lipofuscin is formed as a by-product of the visual cycle and almost entirely composed of vitamin A metabolites called bisretinoids (Sparrow, 2016). Whether RPE lipofuscin is a marker of aging or senescence, it is clear that it derails critical homeostatic functions and compromises RPE health. Bisretinoids in RPE lysosomes lead to a secondary accumulation of cholesterol, which activates acid sphingomyelinase. The resulting increase in ceramide interferes with microtubule based trafficking, leading to autophagic defects and makes the RPE susceptible to complement-mediated mitochondrial fragmentation (Kaur et al., 2018; Tan et al., 2016; Toops et al., 2015). Bisretinoids can also undergo photo-oxidation, leading to the production of free radicals and DNA damage (Ueda et al., 2016). These pathways could directly or indirectly drive RPE dysfunction and dedifferentiation (Fig. 3).
Finally, an important in vivo study recently demonstrated the relationship between Serpinf1, necessary for RPE PEDF production and cell senescence, revealing that the absence of Serpinf1 in mice, resulted in increased SA-βgal activity in RPE cells and induced the expression of senescence-associated genes (Rebustini et al., 2022). Additional in vivo studies investigating the distribution of putative senescent cells in in vivo models that present with AMD-like phenotypes as well as in human AMD donor tissues would complement studies such as that of Rebustini et al. in supporting an active role for senescence in the development and progression of AMD. Should additional data emerge supporting senescent RPE cells in AMD, an important question will be if these cells remain functional. Multinucleated RPE cells, post-mitotic cells, retain levels of phagocytic activity comparable to proliferative single nucleated cells (Chen et al., 2016). Further research is needed to determine how senescent RPE cells modify the microenvironment of the retina and lead to AMD.
4.3. Evidence for senescence in the choroid
As mentioned earlier accessibility to bona fide choroidal endothelial cells has impeded research, with researchers often using the RF/6A cell line as a launching point to examine cell senescence in vitro. In one such study, replicative senescence in vitro was attempted using RF/6A cells, in which cells were passaged frequently for greater than 20 passages (Cabrera et al., 2016). Beta galactosidase staining was found to increase along with the expression of p21Cip1, an inhibitor of cyclin-dependent kinase expression, which collectively was used to demonstrate the state of senescence in these high passage endothelial cells. When compared to low passage cell, high passage cells were found to be significantly less flexible and considerably stiffer, correlating with higher cytoskeletal Rho activity and greater susceptibility to complement injury, potentially supporting senescence-associated choroidal endothelial cell stiffening as a contributor to choriocapillary atrophy, observed in early dry AMD. On the other hand, there are a number of in vivo studies that provide evidence for senescent cells in the posterior pole using the experimental laser-induced choroidal neovascularization mouse model, which on flat mount and in cross-sections stain positively with SA-βgal (Chae et al., 2021) and point to the involvement of myeloid cells (Schlecht et al., 2021).
5. Anti-aging therapeutics and AMD
Anti-aging therapy can be thought of in two ways. The first is to prevent the aging process, the other is to reverse it. Many large cohort studies are looking at prevention, including the Age-Related Eye Disease Study (AREDS), in which the long term effect of antioxidants and zinc significantly reduce the risk of AMD progression and associated vision loss (Age-Related Eye Disease Study 2 Research et al., 2014; Age-Related Eye Disease Study Research, 2001; Chew et al., 2013; Seddon et al., 2016). Slowing the progression of AMD from intermediate to advanced stages could save eyesight, and even decrease mortality (Clemons et al., 2004). However, once a normal cell enters senescence, it appears unlikely to reverse. Recently, it was shown that many age-related pathologies can be improved by eliminating senescent cells, indicating that senescence-targeted therapy is a promising candidate for the treatment of specific age-related eye diseases associated with abnormal blood vessel growth (Crespo-Garcia et al., 2021; Rocha et al., 2020). Another consideration, for senescence targeted therapy is the density of senescent cells. Should a high density of senescent cells be observed, an important question to address prior to therapy is whether or not eliminating these cells would destabilized the existing tissue and potentially be more detrimental.
5.1. Pros and cons of senescence targeting drugs
Various molecules have been investigated to stop or at least slow aging. At least three major pathways regulate the SASP: the DNA damage response (DDR) pathway, the p38MAPK pathway, and the mTOR pathway. All these pathways converge on the NF-κB transcription factory, which drives the inflammatory phenotype. Persistent SASP expression produces a chronic pro-inflammatory microenvironment and can cause neighboring cells in tissues to function inappropriately. Some of these pathways could be inhibited by senomorphics: small molecules that can selectively inhibit certain aspects of the SASP and thus reduce some of the deleterious effects of senescent cells. Drug repurposing studies have shown that drugs such as rapamycin, an mTOR inhibitor, metformin, an AMPK activator, and ruxolitinib, a JAK inhibitor, block pathways that regulate the SASP and protect against age-related pathologies. However, when the treatment stops, the deleterious molecules are secreted again and pathologies triggered by senescent cells can recur (Crespo-Garcia et al., 2021). Additional consideration for therapy is potential off-target effects that may result in untoward effects. For example, one study has shown that cones are functionally dependent on the mTOR signaling pathway, and that stimulation of this pathway can delay cone death in a mouse model of retinitis pigmentosa (Punzo et al., 2009).
Eliminating senescent cells (senolysis) may be a more promising approach to diminish their adverse effects. Caveat being if the density of senescent cell is not such that eliminating them would compromise the integrity of the tissue. Transgenic mouse models, in which senescent cells can be eliminated throughout the body have uncovered a surprising number of age-related pathologies that are due, at least in part, to the presence of senescent cells (Gorgoulis et al., 2019). In addition, a number of small molecules can have senolytic effects, including tyrosine kinase inhibitors coupled with a flavonoid (dasatinib and quercetin) (Zhu et al., 2015), a Bcl inhibitor (ABT263), (Chang et al., 2016), and a glutaminase inhibitor (Johmura et al., 2021).
Currently, a Bcl-xL inhibitor UBX1325, is being tested in a phase 2a human clinical trial as a senolytic to treat refractory diabetic macular edema and neovascular age-related macular degeneration (NCT04537884). As discussed above, diabetic retinopathy is associated with senescence and eliminating senescent cells can improve the avascular area (Crespo-Garcia et al., 2021; Oubaha et al., 2016). One potential problem with senolytics is tissue atrophy due to the removal of specific, albeit small, populations of cells. Cells with low proliferative capacity, such as retinal cells, including photoreceptor cells and RGCs, are mostly post-mitotic and could be targeted by the drugs. Moreover, given this population of senescent cells is generally small (He and Sharpless, 2017), losing a small population in exchange for the possibility of maintaining tissue function may be acceptable for many age-related diseases. However, the continuous removal of p16-positive senescent cells in vivo can result in systemic perivascular fibrosis (Grosse et al., 2020). Further research is needed to determine the appropriate duration and interval of senolytic treatments to effectively clear senescent cells in the context of AMD.
5.2. Tools available to study senescence in vivo, ex vivo, and in animal models
Several mouse models have been developed to study senescence, either by inducing senescence conditionally, reprograming epigenetic changes with age, or eliminating senescent cells. One informative model created a cell type-selective mouse to generate senescent cells by crossing a floxed Ercc1 knockout (Ercc1-/fl), selectively deficient in transcription-coupled DNA repair, with Vav-iCre+/− (Yousefzadeh et al., 2021) to localize DNA damage to hematopoietic cells. This model demonstrated accelerated aging in immune cells, notably B cells, T lymphocytes, natural killer T cells, macrophages and monocytes, which all expressed significantly higher levels of p16Ink4a and p21Cip1, similar to that of wild-type aged mice. Aging effects in other tissues, such as kidney, pancreas and intervertebral discs, were also accelerated cell non-autonomously in this model. Another strategy deployed to study age-related epigenetic changes specifically in retinal ganglion cells (RGCs) expressed Yamanaka factors (excluding MYC) to alter the DNA methylation signature with age. This reprograming reversed vision loss following glaucomatous damage (Lu et al., 2020). While cellular senescence was not assessed in this model, elevated IOP, sufficient to cause glaucoma and progressive RGC degeneration, was associated with elevated p16INK4a expression (Skowronska-Krawczyk et al., 2015), and early elimination of senescent cells, either by p16Ink4a-dependent expression of a viral thymidine kinase gene and treatment with ganciclovir (Demaria et al., 2014) or by senolytic treatment (desatinib), which restores RGCs and evoked visual potentials (Rocha et al., 2020). Of relevance to AMD, rapid expression of SA-βgal in RPE cells has been reported in the laser induced experimental mouse model of CNV, the outer retinal degeneration model created by injecting sodium iodate to ablate the RPE, and in 9-week-old mice treated with doxorubicin to induce senescence (Chae et al., 2021; Sreekumar et al., 2022). Interestingly, retinal degeneration in mice subjected to doxorubicin is more evident than RPE degeneration and the atrophy of the photoreceptor outer nuclear layer is alleviated by treatment with nutlin-3a, a murine double minute 2 (MDM2, p53 target gene and E3 ubiquitin ligase) antagonist. By taking advantage of existing tools and by continuing to generate new conditional animal models, it should be possible to better understand whether or not specific cell types are particularly deleterious when senescent, and to develop therapeutics to more specifically target the most deleterious cell types.
5.3. Therapeutic targeting of ocular tissues in AMD
Which tissue compartment should be targeted in AMD is a critical point of discussion when considering therapies. The retina, RPE, and choroid are all involved in the pathogenesis of AMD and as such drugs that may improve their cellular health and function should be considered. So far, in AMD patients, the presence of senescent cells in the retina, a complex multicellular tissue, housing the light sensitive photoreceptors as well as microglial cells, both compromised in disease, has not been reported. Targeting the compromised RPE cells is attractive, as the RPE regulates homeostasis of ions and pH between photoreceptors and metabolic waste products from the retina, and transports nutrients to the retina. RPE cells also converts retinol to 11-cis-retinal, regulate photoreceptor outer segments, and secrete neurotrophic factors to maintain photoreceptor integrity. Furthermore, the RPE forms the blood-retina barrier. All these specialized functions depend on the expression and polar distribution of receptors, transporters, channels and enzymes that are markers of a differentiated RPE. Understanding the role of senescent RPE cells during the progression of degenerative diseases such as AMD are central prior to targeting them with senolytic treatments. Finally,
degenerative changes to the choroid play an important role in the different clinical sub-types of AMD. Loss of choroidal endothelial cells or choriocapillary dropout is a classic hallmark of early dry AMD. Morphometric analysis of the choriocapillary density and vascular lumen to stroma ratio in the outer choroid of donor tissue from patients with geographic atrophy reveal an even larger loss of the choriocapillaris (Sohn et al., 2019). This finding combined with the known vascular changes in wet AMD, support choroidal changes are an underlying pathological event in all clinical sub-types of AMD and therefore a valid tissue site for testing therapeutics. A common denominator in targeting any of these tissues is the need for further studies demonstrating the extent to which senescence is a factor in AMD and at which cellular levels does it occur.
5.4. Evidence needed to support senescence does in fact play a role in AMD and where should the field go from here
Currently, the data on senescence markers in the posterior pole in AMD patients or models are not definitive enough to conclude the extent to which senescence plays a role in the etiology of the disease. However, the potential that senescence may contribute to the pathogenesis of AMD, necessitates pursuing this line of research, in a more comprehensive way. The most convincing data to support a role for senescence would begin with detailed phenotypic profiling of a large cohort of donor tissue from AMD patients versus non-AMD, classified based on their clinical sub-type, sex, and genotype. This is the necessary minimum given the multi-factorial nature of AMD. The next stages would include molecular profiling including single cell transcriptomics and cell cyle profiling to help clarify which populations of AMD vulnerable cells express senescent markers, vital information should therapeutics need to be considered.
With regards to treatment there are a number of important questions to contemplate when considering the use of senolytic drugs: 1. Which cells should be targeted, the whole retina, microglial cells, photoreceptors, RPE cells, choroidal endothelial cells? 2. Are the senescent cells still active and functional?; 3. If senescent cells are removed, is it possible for the missing neurons to regenerate to avoid tissue atrophy?; 4. If senescent cells that are still functional, are removed, how will it affect vision?; 5. What if most of the cells of a given type are senescent, should we treat the tissue with senolytic drugs and risk losing other cell types? Finally, until studies on a molecular level are done, whether all cells assume the same type of senescence remains unknown; maybe some options could be specific to a given cell type.
6. Conclusions
The field of senescence is relatively young and new discoveries on its underlying mechanisms are published daily. Case in point, even though the fate of senescent cells involves resistance to death and secretion of SASPs, the phenotypic features, consequences, and triggers of senescence are cell and tissue specific, in part due to the diversity in the aging rate of organs throughout the body (Nie et al., 2022); adding to the complexity of this cellular process. It is therefore of great interest to understand the extent to which the retina and RPE undergo senescence in a manner similar to dividing cells, the degree to which the process differs, and/or if there are novel mechanisms underlying ocular senescence.
The discovery of senolytic factors has revolutionized the field of age-related conditions. Some of these compounds are safe for use in patients as they were previously FDA-approved drugs for other conditions. In addition, pre-clinical studies are promising (Boccardi and Mecocci, 2021). As an alternative, the use of senostatic/senomorphic drugs could be considered (Boccardi and Mecocci, 2021). These molecules quench the most deleterious segments of the SASP without removing the living cell. This type of approach most likely will require sustained treatment but might be the best option for now.
Since retinal neurons and RPE are largely non-dividing cells, removal will directly affect cell-cell connectivity with potential deleterious effects on activity. Although the technology is not there yet, one can imagine that replacement therapy or transdifferentiation approaches could be used to fill the space of missing cells. In particular, studies performed in lower vertebrates (Lahne et al., 2020) such as zebrafish are exciting, as it has been shown that Müller cells can undergo re-differentiation to the cell type that has been lost. Unfortunately, recent detailed studies have described an evolutionary change in the Muller glia’s ability to transdifferentiate in situ in response to damage (Hoang et al., 2020) and future work is needed to establish the feasibility of this approach in mammals (Eastlake et al., 2021) Current approaches including overexpression of cell-lineage specific transcription factors to transdifferentiate Muller cells into the specific neuron may prove to be more applicable (Todd et al., 2021) and further ideas are needed to fill this need in treating age-related eye conditions.
Finally, the degree to which senescence in the posterior pole may contribute to development and progression of AMD is of high interest. Hopefully, exploring the questions discussed along with studies in progress, including single cell transcriptomics and cell cycle profiling of donor retinal tissue in correlation with clinical stage of AMD, will provide much needed clarity and insight into the role of senescence in disease pathogenesis and provide support for senescent – associated ocular therapies, if indicated.
Highlights.
Advanced age is a major risk factor for developing age-related macular degeneration (AMD), a complex blinding disease.
Age-related changes are seen throughout the posterior pole, at the level of the retina, retinal pigment epithelium, and choroid, impacting vision and contributing to disease development.
The lack of therapies for AMD, necessitate further discovery of pathways that are affected in aging.
Recent observation of a senescence-like phenotype in post-mitotic, terminally differentiated cells in aged mice, has led to the hypothesis that senescence may play a role in AMD.
The extent to which senescent cells accumulate in the aged eye and the degree to which these cells may trigger AMD development needs to be investigated comprehensively in order to consider the use of senolytics and senomorphics for therapy.
Acknowledgements
Many thanks to the 2021 Stephen J. Ryan Initiative For Macular Research (RIMR) meeting for providing a unique forum for dialogue and scientific discussions on “Cellular and Organelle Aging in Age-Related Macular Degeneration”. Also, thanks to Pierre-Yves Desprez for scientific discussions. Figure 1 was created in part with BioRender.com.
Funding
This work was supported by generous funding from the National Eye Institute EY032751 (GM), EY028160 (GM), EY023299 (AL), EY030668 (AL) and EY027011 (DSK); The Japan Society for the Promotion of Science #201960725 (KK); Japan Eye Bank (KK); The Research to Prevent Blindness/American Macular Degeneration Foundation Catalyst Award for Innovative Research Approaches to AMD (AL); The BrightFocus Foundation Lorraine Maresca Award for Innovative Research in AMD (AL and DSK); The Edward N. and Della L. Thome Memorial Foundation Award Program in Age-Related Macular Degeneration Research (DSK); Research to Prevent Blindness (Duke Eye Center and the Gavin Herbert Eye Institute, UC, Irvine); and The Buck Institute flexible funds (JC).
Abbreviations
- AMD
age-related macular degeneration
- RPE
retinal pigment epithelium
- GA
geographic atrophy
- SASP
senescence-associated secretory phenotype
- DAMPS
danger-associated molecular patterns
- CDK
cyclin-dependent kinase
- Rb
retinoblastoma
- MMPs
matrix metalloproteases
- ROS
reactive oxygen species
- MiDAS
mitochondrial dysfunction-associated senescence
- SAMP8
senescence-prone mouse strain 8
- BMP4
bone morphogenetic protein-4
- iPSC-RPE
induced pluripotent stem cell derived RPE
- TER
transepithelial resistance
- ApoE
apolipoprotein E
- hTERT
human telomerase reverse transcriptase
- SD-OCT
Spectral domain-optical coherence tomography
- ONL
outer nuclear layer
- OCTA
optical coherence tomography angiography
- MERTK
MER tyrosine kinase
- MYO7A
actin motor protein myosin VIIa
- OXPHOS
oxidative phosphorylation
- SA-βgal
Senescence-associated beta-galactosidase
- GATA4
GATA Binding Protein 4
- HMGB1
High mobility group box 1
- IOP
intraocular pressure
- RGC
retinal ganglion cells
- TBK1
TANK-binding protein 1
- UPR
unfolded protein response
- NET
neutrophil extracellular traps
- IL
interleukin
- CETP
cholesterol ester transfer protein
- SV40
simian virus 40
- NCAM
neural cell adhesion molecule
- PECAM1
platelet endothelial cell adhesion molecule-1
- VEGF
vascular endothelial growth factor
- GDF15
growth differentiation factor-15
- STC1
stanniocalcin-1
- SERPINE-1
Serpin Family E Member 1
- TNFα
tumor necrosis factor alpha
- ESC
embryonic stem cell
- GFAP
glial fibrillary acidic protein
- LAMP
lysosomal membrane protein
- OXPHOS
oxidative phosphorylation
- NF-κB
nuclear factor kappa B
- DAMP
damage-associated molecular pattern
- DDR
DNA damage response
- MDM2
murine double minute 2
Footnotes
Ethics
Human ocular tissues were used with consent from the San Diego Eye bank under an IRB protocol approved by the University of California, San Diego Human Research Protection Program.
Declaration of competing interest
None.
References
- Age-Related Eye Disease Study 2 Research, G., Chew EY, Clemons TE, Sangiovanni JP, Danis RP, Ferris FL 3rd, Elman MJ, Antoszyk AN, Ruby AJ, Orth D, Bressler SB, Fish GE, Hubbard GB, Klein ML, Chandra SR, Blodi BA, Domalpally A, Friberg T, Wong WT, Rosenfeld PJ, Agron E, Toth CA, Bernstein PS, Sperduto RD, 2014. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthalmol 132, 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Age-Related Eye Disease Study Research, G., 2001. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol 119, 1417–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguayo-Mazzucato C, Andle J, Lee TB, Midha A, Talemal L, Chipashvili V, Hollister-Lock J, van Deursen J, Weir G, Bonner-Weir S, 2019. Acceleration of beta-cell aging determines diabetes and senolysis improves disease outcomes. Cell Metab 30, 129–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryan N, Betts-Obregon BS, Perry G, Tsin AT, 2016. Oxidative Stress Induces Senescence in Cultured RPE Cells. Open Neurol J 10, 83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basisty N, Kale A, Jeon OH, Kuehnemann C, Payne T, Rao C, Holtz A, Shah S, Sharma V, Ferrucci L, Campisi J, Schilling B, 2020. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol 18, e3000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasiak J, Piechota M, Pawlowska E, Szatkowska M, Sikora E, Kaarniranta K, 2017. Cellular senescence in age-related macular degeneration: Can autophagy and DNA damage response play a role? Oxid Med Cell Longev 2017, 5293258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenkinsop TA, Salero E, Stern JH, Temple S, 2013. The culture and maintenance of functional retinal pigment epithelial monolayers from adult human eye. Methods in molecular biology 945, 45–65. [DOI] [PubMed] [Google Scholar]
- Blitzer AL, Ham SA, Colby KA, Skondra D, 2021. Association of Metformin Use With Age-Related Macular Degeneration: A Case-Control Study. JAMA Ophthalmol 139, 302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccardi V, Mecocci P, 2021. Senotherapeutics: Targeting senescent cells for the main age-related diseases. Mech Ageing Dev 197, 111526. [DOI] [PubMed] [Google Scholar]
- Boulton M, Moriarty P, Jarvis-Evans J, Marcyniuk B, 1994. Regional variation and age-related changes of lysosomal enzymes in the human retinal pigment epithelium. Br J Ophthalmol 78, 125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan AM, Suh SW, Won SJ, Narasimhan P, Kauppinen TM, Lee H, Edling Y, Chan PH, Swanson RA, 2009. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat Neurosci 12, 857–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EE, Ball JD, Chen Z, Khurshid GS, Prosperi M, Ash JD, 2019. The Common Antidiabetic Drug Metformin Reduces Odds of Developing Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci 60, 1470–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera AP, Bhaskaran A, Xu J, Yang X, Scott HA, Mohideen U, Ghosh K, 2016. Senescence Increases Choroidal Endothelial Stiffness and Susceptibility to Complement Injury: Implications for Choriocapillaris Loss in AMD. Invest Ophthalmol Vis Sci 57, 5910–5918. [DOI] [PubMed] [Google Scholar]
- Caceres PS, Rodriguez-Boulan E, 2020. Retinal pigment epithelium polarity in health and blinding diseases. Curr Opin Cell Biol 62, 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campello L, Singh N, Advani J, Mondal AK, Corso-Diaz X, Swaroop A, 2021. Aging of the Retina: Molecular and Metabolic Turbulences and Potential Interventions. Annu Rev Vis Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, Kapahi P, Lithgow GJ, Melov S, Newman JC, Verdin E, 2019. From discoveries in ageing research to therapeutics for healthy ageing. Nature 571, 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Wang H, Wang F, Xu D, Liu F, Liu C, 2013. Abeta-induced senescent retinal pigment epithelial cells create a proinflammatory microenvironment in AMD. Invest Ophthalmol Vis Sci 54, 3738–3750. [DOI] [PubMed] [Google Scholar]
- Chae JB, Jang H, Son C, Park CW, Choi H, Jin S, Lee HY, Lee H, Ryu JH, Kim N, Kim C, Chung H, 2021. Targeting senescent retinal pigment epithelial cells facilitates retinal regeneration in mouse models of age-related macular degeneration. Geroscience 43, 2809–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, Janakiraman K, Sharpless NE, Ding S, Feng W, Luo Y, Wang X, Aykin-Burns N, Krager K, Ponnappan U, Hauer-Jensen M, Meng A, Zhou D, 2016. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med 22, 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaum E, Winborn CS, Bhattacharya S, 2015. Genomic regulation of senescence and innate immunity signaling in the retinal pigment epithelium. Mamm Genome 26, 210–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Messinger JD, Zhang Y, Spaide RF, Freund KB, Curcio CA, 2020. SUBRETINAL DRUSENOID DEPOSIT IN AGE-RELATED MACULAR DEGENERATION: Histologic Insights Into Initiation, Progression to Atrophy, and Imaging. Retina 40, 618–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Rajapakse D, Fraczek M, Luo C, Forrester JV, Xu H, 2016. Retinal pigment epithelial cell multinucleation in the aging eye-a mechanism to repair damage and maintain homoeostasis. Aging Cell 15, 436–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew EY, Clemons TE, Agron E, Sperduto RD, Sangiovanni JP, Kurinij N, Davis MD, Age-Related Eye Disease Study Research, G., 2013. Long-term effects of vitamins C and E, beta-carotene, and zinc on age-related macular degeneration: AREDS report no. 35. Ophthalmology 120, 1604–1611 e1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs BG, Baker DJ, Wijshake T, Conover CA, Campisi J, van Deursen JM, 2016. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 354, 472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilosi M, Carloni A, Rossi A, Poletti V, 2013. Premature lung aging and cellular senescence in the pathogenesis of idiopathic pulmonary fibrosis and COPD/emphysema. Transl Res 162, 156–173. [DOI] [PubMed] [Google Scholar]
- Choudhary M, Ismail EN, Yao PL, Tayyari F, Radu RA, Nusinowitz S, Boulton ME, Apte RS, Ruberti JW, Handa JT, Tontonoz P, Malek G, 2020. LXRs regulate features of age-related macular degeneration and may be a potential therapeutic target. JCI Insight 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemons TE, Kurinij N, Sperduto RD, Group AR, 2004. Associations of mortality with ocular disorders and an intervention of high-dose antioxidants and zinc in the Age-Related Eye Disease Study: AREDS Report No. 13. Arch Ophthalmol 122, 716–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J, 2008. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 6, 2853–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormenier J, Martin N, Desle J, Salazar-Cardozo C, Pourtier A, Abbadie C, Pluquet O, 2018. The ATF6alpha arm of the Unfolded Protein Response mediates replicative senescence in human fibroblasts through a COX2/prostaglandin E2 intracrine pathway. Mech Ageing Dev 170, 82–91. [DOI] [PubMed] [Google Scholar]
- Crespo-Garcia S, Tsuruda PR, Dejda A, Ryan RD, Fournier F, Chaney SY, Pilon F, Dogan T, Cagnone G, Patel P, Buscarlet M, Dasgupta S, Girouard G, Rao SR, Wilson AM, O’Brien R, Juneau R, Guber V, Dubrac A, Beausejour C, Armstrong S, Mallette FA, Yohn CB, Joyal JS, Marquess D, Beltran PJ, Sapieha P, 2021. Pathological angiogenesis in retinopathy engages cellular senescence and is amenable to therapeutic elimination via BCL-xL inhibition. Cell Metab 33, 818–832 e817. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Millican CL, Allen KA, Kalina RE, 1993. Aging of the human photoreceptor mosaic: evidence for selective vulnerability of rods in central retina. Invest Ophthalmol Vis Sci 34, 3278–3296. [PubMed] [Google Scholar]
- Datta S, Cano M, Ebrahimi K, Wang L, Handa JT, 2017. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog Retin Eye Res 60, 201–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos AR, Kawahara M, Malhotra GK, Schaum N, Huang J, Ved U, Beausejour CM, Coppe JP, Rodier F, Campisi J, 2013. p53-dependent release of Alarmin HMGB1 is a central mediator of senescent phenotypes. J Cell Biol 201, 613–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong PT, 2016. A Historical Analysis of the Quest for the Origins of Aging Macula Disorder, the Tissues Involved, and Its Terminology. Ophthalmol Eye Dis 8, 5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge RM, Vijg J, Van Steeg H, Dolle ME, Hoeijmakers JH, de Bruin A, Hara E, Campisi J, 2014. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell 31, 722–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentchev T, Milam AH, Lee VM, Trojanowski JQ, Dunaief JL, 2003. Amyloid-beta is found in drusen from some age-related macular degeneration retinas, but not in drusen from normal retinas. Mol Vis 9, 184–190. [PubMed] [Google Scholar]
- Dickinson BC, Chang CJ, 2011. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nature Chem Biol 7, 504–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM, 1996. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res 62, 155–169. [DOI] [PubMed] [Google Scholar]
- Eastlake K, Lamb WDB, Luis J, Khaw PT, Jayaram H, Limb GA, 2021. Prospects for the application of Muller glia and their derivatives in retinal regenerative therapies. Prog Retin Eye Res, 100970. [DOI] [PubMed] [Google Scholar]
- Efrat S, 2020. Epigenetic Memory: Lessons From iPS Cells Derived From Human beta Cells. Front Endocrinol (Lausanne) 11, 614234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Nimri NW, Moore SM, Zangwill LM, Proudfoot JA, Weinreb RN, Skowronska-Krawczyk D, Baxter SL, 2020. Evaluating the neuroprotective impact of senolytic drugs on human vision. Sci Rep 10, 21752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasieh K, Liets LC, Chalupa LM, 2007. Cellular reorganization in the human retina during normal aging. Invest Ophthalmol Vis Sci 48, 2824–2830. [DOI] [PubMed] [Google Scholar]
- Emeterio Nateras OS, Harrison JM, Muir ER, Zhang Y, Peng Q, Chalfin S, Gutierrez JE, Johnson DA, Kiel JW, Duong TQ, 2014. Choroidal blood flow decreases with age: an MRI study. Curr Eye Res 39, 1059–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eton EA, Wubben TJ, Besirli CG, Hua P, McGeehan B, VanderBeek BL, 2022. Association of metformin and development of dry age-related macular degeneration in a U.S. insurance claims database. Eur J Ophthalmol 32, 417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feher J, Kovacs I, Artico M, Cavallotti C, Papale A, Balacco Gabrieli C, 2006. Mitochondrial alterations of retinal pigment epithelium in age-related macular degeneration. Neurobiol Aging 27, 983–993. [DOI] [PubMed] [Google Scholar]
- Feng L, Cao L, Zhang Y, Wang F, 2016. Detecting Abeta deposition and RPE cell senescence in the retinas of SAMP8 mice. Discov Med 21, 149–158. [PubMed] [Google Scholar]
- Ferdous S, Liao KL, Gefke ID, Summers VR, Wu W, Donaldson KJ, Kim YK, Sellers JT, Dixon JA, Shelton DA, Markand S, Kim SM, Zhang N, Boatright JH, Nickerson JM, 2021. Age-Related Retinal Changes in Wild-Type C57BL/6J Mice Between 2 and 32 Months. Invest Ophthalmol Vis Sci 62, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrington DA, Ebeling MC, Kapphahn RJ, Terluk MR, Fisher CR, Polanco JR, Roehrich H, Leary MM, Geng Z, Dutton JR, Montezuma SR, 2017. Altered bioenergetics and enhanced resistance to oxidative stress in human retinal pigment epithelial cells from donors with age-related macular degeneration. Redox Biol 13, 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrington DA, Kapphahn RJ, Leary MM, Atilano SR, Terluk MR, Karunadharma P, Chen GK, Ratnapriya R, Swaroop A, Montezuma SR, Kenney MC, 2016. Increased retinal mtDNA damage in the CFH variant associated with age-related macular degeneration. Exp Eye Res 145, 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrington DA, Kenney MC, Atilano SR, Hurley JB, Brown EE, Ash JD, 2021. Mitochondria: The Retina’s Achilles’ Heel in AMD. Adv Exp Med Biol 1256, 237–264. [DOI] [PubMed] [Google Scholar]
- Ferris FL 3rd, Wilkinson CP, Bird A, Chakravarthy U, Chew E, Csaky K, Sadda SR, Beckman Initiative for Macular Research Classification, C., 2013. Clinical classification of age-related macular degeneration. Ophthalmology 120, 844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T, 2003. Oxidant signals and oxidative stress. Curr Opin Cell Biol 15, 247–254. [DOI] [PubMed] [Google Scholar]
- Fleckenstein M, Keenan TDL, Guymer RH, Chakravarthy U, Schmitz-Valckenberg S, Klaver CC, Wong WT, Chew EY, 2021. Age-related macular degeneration. Nat Rev Dis Primers 7, 31. [DOI] [PubMed] [Google Scholar]
- Fritsche LG, Igl W, Bailey JN, Grassmann F, Sengupta S, Bragg-Gresham JL, Burdon KP, Hebbring SJ, Wen C, Gorski M, Kim IK, Cho D, Zack D, Souied E, Scholl HP, Bala E, Lee KE, Hunter DJ, Sardell RJ, Mitchell P, Merriam JE, Cipriani V, Hoffman JD, Schick T, Lechanteur YT, Guymer RH, Johnson MP, Jiang Y, Stanton CM, Buitendijk GH, Zhan X, Kwong AM, Boleda A, Brooks M, Gieser L, Ratnapriya R, Branham KE, Foerster JR, Heckenlively JR, Othman MI, Vote BJ, Liang HH, Souzeau E, McAllister IL, Isaacs T, Hall J, Lake S, Mackey DA, Constable IJ, Craig JE, Kitchner TE, Yang Z, Su Z, Luo H, Chen D, Ouyang H, Flagg K, Lin D, Mao G, Ferreyra H, Stark K, von Strachwitz CN, Wolf A, Brandl C, Rudolph G, Olden M, Morrison MA, Morgan DJ, Schu M, Ahn J, Silvestri G, Tsironi EE, Park KH, Farrer LA, Orlin A, Brucker A, Li M, Curcio CA, Mohand-Said S, Sahel JA, Audo I, Benchaboune M, Cree AJ, Rennie CA, Goverdhan SV, Grunin M, Hagbi-Levi S, Campochiaro P, Katsanis N, Holz FG, Blond F, Blanche H, Deleuze JF, Igo RP Jr., Truitt B, Peachey NS, Meuer SM, Myers CE, Moore EL, Klein R, Hauser MA, Postel EA, Courtenay MD, Schwartz SG, Kovach JL, Scott WK, Liew G, Tan AG, Gopinath B, Merriam JC, Smith RT, Khan JC, Shahid H, Moore AT, McGrath JA, Laux R, Brantley MA Jr., Agarwal A, Ersoy L, Caramoy A, Langmann T, Saksens NT, de Jong EK, Hoyng CB, Cain MS, Richardson AJ, Martin TM, Blangero J, Weeks DE, Dhillon B, van Duijn CM, Doheny KF, Romm J, Klaver CC, Hayward C, Gorin MB, Klein ML, Baird PN, den Hollander AI, Fauser S, Yates JR, Allikmets R, Wang JJ, Schaumberg DA, Klein BE, Hagstrom SA, Chowers I, Lotery AJ, Leveillard T, Zhang K, Brilliant MH, Hewitt AW, Swaroop A, Chew EY, Pericak-Vance MA, DeAngelis M, Stambolian D, Haines JL, Iyengar SK, Weber BH, Abecasis GR, Heid IM, 2016. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet 48, 134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal A, Li Y, Thompson DA, Weir J, Orth U, Jacobson SG, Apfelstedt-Sylla E, Vollrath D, 2000. Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat Genet 26, 270–271. [DOI] [PubMed] [Google Scholar]
- Gao H, Hollyfield JG, 1992. Aging of the human retina. Differential loss of neurons and retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 33, 1–17. [PubMed] [Google Scholar]
- Giacalone JC, Miller MJ, Workalemahu G, Reutzel AJ, Ochoa D, Whitmore SS, Stone EM, Tucker BA, Mullins RF, 2019. Generation of an immortalized human choroid endothelial cell line (iChEC-1) using an endothelial cell specific promoter. Microvasc Res 123, 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs D, Kitamoto J, Williams DS, 2003. Abnormal phagocytosis by retinal pigmented epithelium that lacks myosin VIIa, the Usher syndrome 1B protein. Proc Natl Acad Sci U S A 100, 6481–6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs D, Williams DS, 2003. Isolation and culture of primary mouse retinal pigmented epithelial cells. Adv Exp Med Biol 533, 347–352. [DOI] [PubMed] [Google Scholar]
- Glotin AL, Debacq-Chainiaux F, Brossas JY, Faussat AM, Treton J, Zubielewicz A, Toussaint O, Mascarelli F, 2008. Prematurely senescent ARPE-19 cells display features of age-related macular degeneration. Free Radic Biol Med 44, 1348–1361. [DOI] [PubMed] [Google Scholar]
- Gokhale KM, Adderley NJ, Subramanian A, Lee WH, Han D, Coker J, Braithwaite T, Denniston AK, Keane PA, Nirantharakumar K, 2022. Metformin and risk of age-related macular degeneration in individuals with type 2 diabetes: a retrospective cohort study. Br J Ophthalmol. [DOI] [PubMed] [Google Scholar]
- Golestaneh N, Chu Y, Xiao YY, Stoleru GL, Theos AC, 2017. Dysfunctional autophagy in RPE, a contributing factor in age-related macular degeneration. Cell Death Dis 8, e2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgoulis V, Adams PD, Alimonti A, Bennett DC, Bischof O, Bishop C, Campisi J, Collado M, Evangelou K, Ferbeyre G, Gil J, Hara E, Krizhanovsky V, Jurk D, Maier AB, Narita M, Niedernhofer L, Passos JF, Robbins PD, Schmitt CA, Sedivy J, Vougas K, von Zglinicki T, Zhou D, Serrano M, Demaria M, 2019. Cellular senescence: Defining a path forward. Cell 179, 813–827. [DOI] [PubMed] [Google Scholar]
- Grosse L, Wagner N, Emelyanov A, Molina C, Lacas-Gervais S, Wagner K-D, Bulavin DV, 2020. Defined p16 high senescent cell types are indispensable for mouse healthspan. Cell Metabolism. [DOI] [PubMed] [Google Scholar]
- Hadziahmetovic M, Malek G, 2020. Age-Related Macular Degeneration Revisited: From Pathology and Cellular Stress to Potential Therapies. Front Cell Dev Biol 8, 612812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BM, Balan V, Gleiberman AS, Strom E, Krasnov P, Virtuoso LP, Rydkina E, Vujcic S, Balan K, Gitlin I, Leonova K, Polinsky A, Chernova OB, Gudkov AV, 2016. Aging of mice is associated with p16(Ink4a)-and beta-galactosidase-positive macrophage accumulation that can be induced in young mice by senescent cells. Aging (Albany NY) 8, 1294–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey DM, Levine AJ, 1991. p53 alteration is a common event in the spontaneous immortalization of primary BALB/c murine embryo fibroblasts. Genes & Dev 5, 2375–2385. [DOI] [PubMed] [Google Scholar]
- Hayasaka S, 1989. Aging changes in lipofuscin, lysosomes and melanin in the macular area of human retina and choroid. Jpn J Ophthalmol 33, 36–42. [PubMed] [Google Scholar]
- Hayflick L, Moorhead PS, 1961. The serial cultivation of human diploid cell strains. Exp Cell Res 25, 585–621. [DOI] [PubMed] [Google Scholar]
- Hazim RA, Karumbayaram S, Jiang M, Dimashkie A, Lopes VS, Li D, Burgess BL, Vijayaraj P, Alva-Ornelas JA, Zack JA, Kohn DB, Gomperts BN, Pyle AD, Lowry WE, Williams DS, 2017. Differentiation of RPE cells from integration-free iPS cells and their cell biological characterization. Stem Cell Res Ther 8, 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazim RA, Volland S, Yen A, Burgess BL, Williams DS, 2019. Rapid differentiation of the human RPE cell line, ARPE-19, induced by nicotinamide. Exp Eye Res 179, 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Sharpless NE, 2017. Senescence in Health and Disease. Cell 169, 1000–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesterbeek TJ, Lores-Motta L, Hoyng CB, Lechanteur YTE, den Hollander AI, 2020. Risk factors for progression of age-related macular degeneration. Ophthalmic Physiol Opt 40, 140–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbig U, Ferreira M, Condel L, Carey D, Sedivy JM, 2006. Cellular senescence in aging primates. Science 311, 1257. [DOI] [PubMed] [Google Scholar]
- Hoang T, Wang J, Boyd P, Wang F, Santiago C, Jiang L, Yoo S, Lahne M, Todd LJ, Jia M, Saez C, Keuthan C, Palazzo I, Squires N, Campbell WA, Rajaii F, Parayil T, Trinh V, Kim DW, Wang G, Campbell LJ, Ash J, Fischer AJ, Hyde DR, Qian J, Blackshaw S, 2020. Gene regulatory networks controlling vertebrate retinal regeneration. Science 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P, Herrmann R, Bednar A, Saloupis P, Dwyer MA, Yang P, Qi X, Thomas RS, Jaffe GJ, Boulton ME, McDonnell DP, Malek G, 2013. Aryl hydrocarbon receptor deficiency causes dysregulated cellular matrix metabolism and age-related macular degeneration-like pathology. Proc Natl Acad Sci U S A 110, E4069–4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itahana K, Campisi J, Dimri GP, 2007. Methods to detect biomarkers of cellular senescence: the senescence-associated beta-galactosidase assay. Methods in molecular biology 371, 21–31. [DOI] [PubMed] [Google Scholar]
- Jablonski MM, Iannaccone A, Reynolds DH, Gallaher P, Allen S, Wang X, Reiner A, 2007. Age-related decline in VIP-positive parasympathetic nerve fibers in the human submacular choroid. Invest Ophthalmol Vis Sci 48, 479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon OH, Kim C, Laberge RM, Demaria M, Rathod S, Vasserot AP, Chung JW, Kim DH, Poon Y, David N, Baker DJ, van Deursen JM, Campisi J, Elisseeff JH, 2017. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med 23, 775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johmura Y, Yamanaka T, Omori S, Wang TW, Sugiura Y, Matsumoto M, Suzuki N, Kumamoto S, Yamaguchi K, Hatakeyama S, Takami T, Yamaguchi R, Shimizu E, Ikeda K, Okahashi N, Mikawa R, Suematsu M, Arita M, Sugimoto M, Nakayama KI, Furukawa Y, Imoto S, Nakanishi M, 2021. Senolysis by glutaminolysis inhibition ameliorates various age-associated disorders. Science 371, 265–270. [DOI] [PubMed] [Google Scholar]
- Johnson LV, Forest DL, Banna CD, Radeke CM, Maloney MA, Hu J, Spencer CN, Walker AM, Tsie MS, Bok D, Radeke MJ, Anderson DH, 2011. Cell culture model that mimics drusen formation and triggers complement activation associated with age-related macular degeneration. Proc Natl Acad Sci U S A 108, 18277–18282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LV, Leitner WP, Rivest AJ, Staples MK, Radeke MJ, Anderson DH, 2002. The Alzheimer’s A beta -peptide is deposited at sites of complement activation in pathologic deposits associated with aging and age-related macular degeneration. Proc Natl Acad Sci U S A 99, 11830–11835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurk D, Wang C, Miwa S, Maddick M, Korolchuk V, Tsolou A, Gonos ES, Thrasivoulou C, Saffrey MJ, Cameron K, von Zglinicki T, 2012. Postmitotic neurons develop a p21-dependent senescence-like phenotype driven by a DNA damage response. Aging Cell 11, 996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C, Xu Q, Martin TD, Li MZ, Demaria M, Aron L, Lu T, Yankner BA, Campisi J, Elledge SJ, 2015. The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science 349, aaa5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanow MA, Giarmarco MM, Jankowski CS, Tsantilas K, Engel AL, Du J, Linton JD, Farnsworth CC, Sloat SR, Rountree A, Sweet IR, Lindsay KJ, Parker ED, Brockerhoff SE, Sadilek M, Chao JR, Hurley JB, 2017. Biochemical adaptations of the retina and retinal pigment epithelium support a metabolic ecosystem in the vertebrate eye. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur G, Tan LX, Rathnasamy G, La Cunza NR, Germer CJ, Toops KA, Fernandes M, Blenkinsop TA, Lakkaraju A, 2018. Aberrant early endosome biogenesis mediates complement activation in the retinal pigment epithelium in models of macular degeneration. Proc Natl Acad Sci U S A 115, 9014–9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimanskaya I, Hipp J, Rezai KA, West M, Atala A, Lanza R, 2004. Derivation and comparative assessment of retinal pigment epithelium from human embryonic stem cells using transcriptomics. Cloning Stem Cells 6, 217–245. [DOI] [PubMed] [Google Scholar]
- Kovacs-Valasek A, Postyeni E, Denes V, Mester A, Setalo G Jr., Gabriel R, 2021. Age-Related Alterations of Proteins in Albino Wistar Rat Retina. Cells Tissues Organs 210, 135–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhevnikova OS, Telegina DV, Devyatkin VA, Kolosova NG, 2018. Involvement of the autophagic pathway in the progression of AMD-like retinopathy in senescence-accelerated OXYS rats. Biogerontology 19, 223–235. [DOI] [PubMed] [Google Scholar]
- La Cunza N, Tan LX, Thamban T, Germer CJ, Rathnasamy G, Toops KA, Lakkaraju A, 2021. Mitochondria-dependent phase separation of disease-relevant proteins drives pathological features of age-related macular degeneration. JCI Insight 6, e142254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahne M, Nagashima M, Hyde DR, Hitchcock PF, 2020. Reprogramming Muller Glia to Regenerate Retinal Neurons. Annu Rev Vis Sci 6, 171–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakkaraju A, Umapathy A, Tan LX, Daniele L, Philp NJ, Boesze-Battaglia K, Williams DS, 2020. The cell biology of the retinal pigment epithelium. Prog Retin Eye Res, 100846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekwuwa M, Choudhary M, Lad EM, Malek G, 2021. Osteopontin accumulates in basal deposits of human eyes with age-related macular degeneration and may serve as a biomarker of aging. Mod Pathol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liton PB, Challa P, Stinnett S, Luna C, Epstein DL, Gonzalez P, 2005. Cellular senescence in the glaucomatous outflow pathway. Exp Gerontol 40, 745–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Cao L, Yang S, Xu L, Liu P, Wang F, Xu D, 2015. Subretinal injection of amyloid-beta peptide accelerates RPE cell senescence and retinal degeneration. Int J Mol Med 35, 169–176. [DOI] [PubMed] [Google Scholar]
- Liu H, Lessieur EM, Saadane A, Lindstrom SI, Taylor PR, Kern TS, 2019a. Neutrophil elastase contributes to the pathological vascular permeability characteristic of diabetic retinopathy. Diabetologia 62, 2365–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JY, Souroullas GP, Diekman BO, Krishnamurthy J, Hall BM, Sorrentino JA, Parker JS, Sessions GA, Gudkov AV, Sharpless NE, 2019b. Cells exhibiting strong p16 (INK4a) promoter activation in vivo display features of senescence. Proc Natl Acad Sci U S A 116, 2603–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XC, Liu XF, Jian CX, Li CJ, He SZ, 2012. IL-33 is induced by amyloid-beta stimulation and regulates inflammatory cytokine production in retinal pigment epithelium cells. Inflammation 35, 776–784. [DOI] [PubMed] [Google Scholar]
- Loeven MA, van Gemst JJ, Schophuizen CMS, Tilakaratna V, van den Heuvel LP, Day AJ, Klevering BJ, van der Vlag J, 2018. A Novel Choroidal Endothelial Cell Line Has a Decreased Affinity for the Age-Related Macular Degeneration-Associated Complement Factor H Variant 402H. Invest Ophthalmol Vis Sci 59, 722–730. [DOI] [PubMed] [Google Scholar]
- Lopez-Dominguez JA, Rodriguez-Lopez S, Ahumada-Castro U, Desprez PY, Konovalenko M, Laberge RM, Cardenas C, Villalba JM, Campisi J, 2021. Cdkn1a transcript variant 2 is a marker of aging and cellular senescence. Aging (Albany NY) 13, 13380–13392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Luppo M, Catita J, Ramos D, Navarro M, Carretero A, Mendes-Jorge L, Munoz-Canoves P, Rodriguez-Baeza A, Nacher V, Ruberte J, 2017. Cellular Senescence Is Associated With Human Retinal Microaneurysm Formation During Aging. Invest Ophthalmol Vis Sci 58, 2832–2842. [DOI] [PubMed] [Google Scholar]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G, 2013. The hallmarks of aging. Cell 153, 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Brommer B, Tian X, Krishnan A, Meer M, Wang C, Vera DL, Zeng Q, Yu D, Bonkowski MS, Yang JH, Zhou S, Hoffmann EM, Karg MM, Schultz MB, Kane AE, Davidsohn N, Korobkina E, Chwalek K, Rajman LA, Church GM, Hochedlinger K, Gladyshev VN, Horvath S, Levine ME, Gregory-Ksander MS, Ksander BR, He Z, Sinclair DA, 2020. Reprogramming to recover youthful epigenetic information and restore vision. Nature 588, 124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Wong WT, 2016. Aging Changes in Retinal Microglia and their Relevance to Age-related Retinal Disease. Adv Exp Med Biol 854, 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makin RD, Apicella I, Nagasaka Y, Kaneko H, Turner SD, Kerur N, Ambati J, Gelfand BD, 2018. RF/6A Chorioretinal Cells Do Not Display Key Endothelial Phenotypes. Invest Ophthalmol Vis Sci 59, 5795–5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek G, Johnson LV, Mace BE, Saloupis P, Schmechel DE, Rickman DW, Toth CA, Sullivan PM, Bowes Rickman C, 2005. Apolipoprotein E allele-dependent pathogenesis: a model for age-related retinal degeneration. Proc Natl Acad Sci U S A 102, 11900–11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek G, Lad EM, 2014. Emerging roles for nuclear receptors in the pathogenesis of age-related macular degeneration. Cell Mol Life Sci 71, 4617–4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maminishkis A, Chen S, Jalickee S, Banzon T, Shi G, Wang FE, Ehalt T, Hammer JA, Miller SS, 2006. Confluent monolayers of cultured human fetal retinal pigment epithelium exhibit morphology and physiology of native tissue. Invest Ophthalmol Vis Sci 47, 3612–3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour H, Chamberlain CG, Weible MW 2nd, Hughes S, Chu Y, Chan-Ling T, 2008. Aging-related changes in astrocytes in the rat retina: imbalance between cell proliferation and cell death reduces astrocyte availability. Aging Cell 7, 526–540. [DOI] [PubMed] [Google Scholar]
- Maruotti J, Sripathi SR, Bharti K, Fuller J, Wahlin KJ, Ranganathan V, Sluch VM, Berlinicke CA, Davis J, Kim C, Zhao L, Wan J, Qian J, Corneo B, Temple S, Dubey R, Olenyuk BZ, Bhutto I, Lutty GA, Zack DJ, 2015. Small-molecule-directed, efficient generation of retinal pigment epithelium from human pluripotent stem cells. Proc Natl Acad Sci U S A 112, 10950–10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz SE, Kershaw O, Petrick A, Gruber AD, Klopfleisch R, Breithaupt A, 2019. Tumour, but not Age-associated, Increase of Senescence Markers gammaH2AX and p21 in the Canine Eye. J Comp Pathol 173, 41–48. [DOI] [PubMed] [Google Scholar]
- Mishima K, Handa JT, Aotaki-Keen A, Lutty GA, Morse LS, Hjelmeland LM, 1999. Senescence-associated beta-galactosidase histochemistry for the primate eye. Invest Ophthalmol Vis Sci 40, 1590–1593. [PubMed] [Google Scholar]
- Mullins RF, Johnson MN, Faidley EA, Skeie JM, Huang J, 2011. Choriocapillaris vascular dropout related to density of drusen in human eyes with early age-related macular degeneration. Invest Ophthalmol Vis Sci 52, 1606–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musi N, Valentine JM, Sickora KR, Baeuerle E, Thompson CS, Shen Q, Orr ME, 2018. Tau protein aggregation is associated with cellular senescence in the brain. Aging Cell 17, e12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi IR, Mathews AP, Cohen-Gould L, Gundersen D, Rodriguez-Boulan E, 1993. Immortalization of polarized rat retinal pigment epithelium. J Cell Sci 104, 37–49. [DOI] [PubMed] [Google Scholar]
- Nie C, Li Y, Li R, Yan Y, Zhang D, Li T, Li Z, Sun Y, Zhen H, Ding J, Wan Z, Gong J, Shi Y, Huang Z, Wu Y, Cai K, Zong Y, Wang Z, Wang R, Jian M, Jin X, Wang J, Yang H, Han JJ, Zhang X, Franceschi C, Kennedy BK, Xu X, 2022. Distinct biological ages of organs and systems identified from a multi-omics study. Cell Rep 38, 110459. [DOI] [PubMed] [Google Scholar]
- Nishimura A, Shimauchi T, Tanaka T, Shimoda K, Toyama T, Kitajima N, Ishikawa T, Shindo N, Numaga-Tomita T, Yasuda S, Sato Y, Kuwahara K, Kumagai Y, Akaike T, Ide T, Ojida A, Mori Y, Nishida M, 2018. Hypoxia-induced interaction of filamin with Drp1 causes mitochondrial hyperfission-associated myocardial senescence. Sci Signal 11. [DOI] [PubMed] [Google Scholar]
- Nivison-Smith L, Khandelwal N, Tong J, Mahajan S, Kalloniatis M, Agrawal R, 2020. Normal aging changes in the choroidal angioarchitecture of the macula. Sci Rep 10, 10810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notomi S, Ishihara K, Efstathiou NE, Lee JJ, Hisatomi T, Tachibana T, Konstantinou EK, Ueta T, Murakami Y, Maidana DE, Ikeda Y, Kume S, Terasaki H, Sonoda S, Blanz J, Young L, Sakamoto T, Sonoda KH, Saftig P, Ishibashi T, Miller JW, Kroemer G, Vavvas DG, 2019. Genetic LAMP2 deficiency accelerates the age-associated formation of basal laminar deposits in the retina. Proc Natl Acad Sci U S A 116, 23724–23734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuzzi R, Finazzo C, Grignolo FM, 1996. [Changes in adrenergic innervation of the choroid during aging]. J Fr Ophtalmol 19, 89–96. [PubMed] [Google Scholar]
- Oubaha M, Miloudi K, Dejda A, Guber V, Mawambo G, Germain MA, Bourdel G, Popovic N, Rezende FA, Kaufman RJ, Mallette FA, Sapieha P, 2016. Senescence-associated secretory phenotype contributes to pathological angiogenesis in retinopathy. Sci Transl Med 8, 362ra144. [DOI] [PubMed] [Google Scholar]
- Pappas CM, Zouache MA, Matthews S, Faust CD, Hageman JL, Williams BL, Richards BT, Hageman GS, 2021. Protective chromosome 1q32 haplotypes mitigate risk for age-related macular degeneration associated with the CFH-CFHR5 and ARMS2/HTRA1 loci. Hum Genomics 15, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraoan L, Sharif U, Carlsson E, Supharattanasitthi W, Mahmud NM, Kamalden TA, Hiscott P, Jackson M, Grierson I, 2020. Secretory proteostasis of the retinal pigmented epithelium: Impairment links to age-related macular degeneration. Prog Retin Eye Res 79, 100859. [DOI] [PubMed] [Google Scholar]
- Peavey J, Malek G, 2020. Cell Line Authentication in Vision Research and Beyond: A Tale Retold. Invest Ophthalmol Vis Sci 61, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peavey J, Parmar VM, Malek G, 2022. Nuclear Receptor Atlases of Choroidal Tissues Reveal Candidate Receptors Associated with Age-Related Macular Degeneration. Cells 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen HR, Gilson SJ, Dubra A, Munch IC, Larsen M, Baraas RC, 2018. Multimodal imaging of small hard retinal drusen in young healthy adults. Br J Ophthalmol 102, 146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punzo C, Kornacker K, Cepko CL, 2009. Stimulation of the insulin/mTOR pathway delays cone death in a mouse model of retinitis pigmentosa. Nat Neurosci 12, 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radu RA, Hu J, Jiang Z, Bok D, 2014. Bisretinoid-mediated complement activation on retinal pigment epithelial cells is dependent on complement factor H haplotype. J Biol Chem 289, 9113–9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Ramirez AI, Salazar JJ, de Hoz R, Trivino A, 2001. Changes of astrocytes in retinal ageing and age-related macular degeneration. Exp Eye Res 73, 601–615. [DOI] [PubMed] [Google Scholar]
- Rebustini IT, Crawford SE, Becerra SP, 2022. PEDF Deletion Induces Senescence and Defects in Phagocytosis in the RPE. Int J Mol Sci 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha LR, Nguyen Huu VA, Palomino La Torre C, Xu Q, Jabari M, Krawczyk M, Weinreb RN, Skowronska-Krawczyk D, 2020. Early removal of senescent cells protects retinal ganglion cells loss in experimental ocular hypertension. Aging Cell 19, e13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolf M, Malek G, Messinger JD, Clark ME, Wang L, Curcio CA, 2008. Sub-retinal drusenoid deposits in human retina: organization and composition. Exp Eye Res 87, 402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini JS, Corneo B, Miller JD, Kiehl TR, Wang Q, Boles NC, Blenkinsop TA, Stern JH, Temple S, 2017. Nicotinamide Ameliorates Disease Phenotypes in a Human iPSC Model of Age-Related Macular Degeneration. Cell Stem Cell 20, 635–647 e637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel MA, Zhang Y, Meister M, Sanes JR, 2011. Age-related alterations in neurons of the mouse retina. J Neurosci 31, 16033–16044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel W, Jaworski C, Postnikova OA, Kutty RK, Duncan T, Tan LX, Poliakov E, Lakkaraju A, Redmond TM, 2017. Appropriately differentiated ARPE-19 cells regain phenotype and gene expression profiles similar to those of native RPE cells. Mol Vis 23, 60–89. [PMC free article] [PubMed] [Google Scholar]
- Sapieha P, Mallette FA, 2018. Cellular Senescence in Postmitotic Cells: Beyond Growth Arrest. Trends Cell Biol 28, 595–607. [DOI] [PubMed] [Google Scholar]
- Schlecht A, Thien A, Wolf J, Prinz G, Agostini H, Schlunck G, Wieghofer P, Boneva S, Lange C, 2021. Immunosenescence in Choroidal Neovascularization (CNV)-Transcriptional Profiling of Naive and CNV-Associated Retinal Myeloid Cells during Aging. Int J Mol Sci 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon JM, Silver RE, Rosner B, 2016. Response to AREDS supplements according to genetic factors: survival analysis approach using the eye as the unit of analysis. Br J Ophthalmol 100, 1731–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ, 1996. Cancer cell cycles. Science 274, 1672–1677. [DOI] [PubMed] [Google Scholar]
- Skowronska-Krawczyk D, Zhao L, Zhu J, Weinreb RN, Cao G, Luo J, Flagg K, Patel S, Wen C, Krupa M, Luo H, Ouyang H, Lin D, Wang W, Li G, Xu Y, Li O, Chung C, Yeh E, Jafari M, Ai M, Zhong Z, Shi W, Zheng L, Krawczyk M, Chen D, Shi C, Zin C, Zhu J, Mellon PL, Gao W, Abagyan R, Zhang L, Sun X, Zhong S, Zhuo Y, Rosenfeld MG, Liu Y, Zhang K, 2015. P16INK4a upregulation mediated by SIX6 defines retinal ganglion cell pathogenesis in glaucoma. Mol Cell 59, 931–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn EH, Flamme-Wiese MJ, Whitmore SS, Workalemahu G, Marneros AG, Boese EA, Kwon YH, Wang K, Abramoff MD, Tucker BA, Stone EM, Mullins RF, 2019. Choriocapillaris Degeneration in Geographic Atrophy. Am J Pathol 189, 1473–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sone H, Kagawa Y, 2005. Pancreatic beta cell senescence contributes to the pathogenesis of type 2 diabetes in high-fat diet-induced diabetic mice. Diabetologia 48, 58–67. [DOI] [PubMed] [Google Scholar]
- Songstad AE, Wiley LA, Duong K, Kaalberg E, Flamme-Wiese MJ, Cranston CM, Riker MJ, Levasseur D, Stone EM, Mullins RF, Tucker BA, 2015. Generating iPSC-Derived Choroidal Endothelial Cells to Study Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci 56, 8258–8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow JR, 2016. Vitamin A-aldehyde adducts: AMD risk and targeted therapeutics. Proc Natl Acad Sci U S A 113, 4564–4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreekumar PG, Ishikawa K, Spee C, Mehta HH, Wan J, Yen K, Cohen P, Kannan R, Hinton DR, 2016. The Mitochondrial-Derived Peptide Humanin Protects RPE Cells From Oxidative Stress, Senescence, and Mitochondrial Dysfunction. Invest Ophthalmol Vis Sci 57, 1238–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreekumar PG, Reddy ST, Hinton DR, Kannan R, 2022. Mechanisms of RPE senescence and potential role of alphaB crystallin peptide as a senolytic agent in experimental AMD. Exp Eye Res 215, 108918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart EA, Samaranayake GJ, Browning AC, Hopkinson A, Amoaku WM, 2011. Comparison of choroidal and retinal endothelial cells: characteristics and response to VEGF isoforms and anti-VEGF treatments. Exp Eye Res 93, 761–766. [DOI] [PubMed] [Google Scholar]
- Storti F, Klee K, Todorova V, Steiner R, Othman A, van der Velde-Visser S, Samardzija M, Meneau I, Barben M, Karademir D, Pauzuolyte V, Boye SL, Blaser F, Ullmer C, Dunaief JL, Hornemann T, Rohrer L, den Hollander A, von Eckardstein A, Fingerle J, Maugeais C, Grimm C, 2019. Impaired ABCA1/ABCG1-mediated lipid efflux in the mouse retinal pigment epithelium (RPE) leads to retinal degeneration. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LX, Toops KA, Lakkaraju A, 2016. Protective responses to sublytic complement in the retinal pigment epithelium. Proc Natl Acad Sci U S A 113, 8789–8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate DJ Jr., Oliver PD, Miceli MV, Stern R, Shuster S, Newsome DA, 1993. Age-dependent change in the hyaluronic acid content of the human chorioretinal complex. Arch Ophthalmol 111, 963–967. [DOI] [PubMed] [Google Scholar]
- Todd L, Hooper MJ, Haugan AK, Finkbeiner C, Jorstad N, Radulovich N, Wong CK, Donaldson PC, Jenkins W, Chen Q, Rieke F, Reh TA, 2021. Efficient stimulation of retinal regeneration from Muller glia in adult mice using combinations of proneural bHLH transcription factors. Cell Rep 37, 109857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonade D, Liu H, Palczewski K, Kern TS, 2017. Photoreceptor cells produce inflammatory products that contribute to retinal vascular permeability in a mouse model of diabetes. Diabetologia 60, 2111–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toomey CB, Kelly U, Saban DR, Bowes Rickman C, 2015. Regulation of age-related macular degeneration-like pathology by complement factor H. Proc Natl Acad Sci U S A 112, E3040–3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toops KA, Tan LX, Jiang Z, Radu R, Lakkaraju A, 2015. Cholesterol-mediated activation of acid sphingomyelinase disrupts autophagy in the retinal pigment epithelium. Mol Biol Cell 26:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toops KA, Tan LX, Lakkaraju A, 2014. A detailed three-step protocol for live imaging of intracellular traffic in polarized primary porcine RPE monolayers. Exp Eye Res 124C, 74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toto L, Borrelli E, Mastropasqua R, Di Antonio L, Doronzo E, Carpineto P, Mastropasqua L, 2017. Association between outer retinal alterations and microvascular changes in intermediate stage age-related macular degeneration: an optical coherence tomography angiography study. Br J Ophthalmol 101, 774–779. [DOI] [PubMed] [Google Scholar]
- Tuttle CSL, Luesken SWM, Waaijer MEC, Maier AB, 2021. Senescence in tissue samples of humans with age-related diseases: A systematic review. Ageing Res Rev 68, 101334. [DOI] [PubMed] [Google Scholar]
- Ueda K, Zhao J, Kim HJ, Sparrow JR, 2016. Photodegradation of retinal bisretinoids in mouse models and implications for macular degeneration. Proc Natl Acad Sci U S A 113, 6904–6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaghefi E, Hill S, Kersten HM, Squirrell D, 2020. Quantification of Optical Coherence Tomography Angiography in Age and Age-Related Macular Degeneration Using Vessel Density Analysis. Asia Pac J Ophthalmol (Phila) 9, 137–143. [DOI] [PubMed] [Google Scholar]
- Vergroesen JE, Thee EF, Ahmadizar F, van Duijn CM, Stricker BH, Kavousi M, Klaver CCW, Ramdas WD, 2022. Association of Diabetes Medication With Open-Angle Glaucoma, Age-Related Macular Degeneration, and Cataract in the Rotterdam Study. JAMA Ophthalmol 140, 674–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zglinicki T, Wan T, Miwa S, 2021. Senescence in Post-Mitotic Cells: A Driver of Aging? Antioxid Redox Signal 34, 308–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakatsuki Y, Shinojima A, Kawamura A, Yuzawa M, 2015. Correlation of Aging and Segmental Choroidal Thickness Measurement using Swept Source Optical Coherence Tomography in Healthy Eyes. PLoS One 10, e0144156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiter JJ, Delori FC, Wing GL, Fitch KA, 1986. Retinal pigment epithelial lipofuscin and melanin and choroidal melanin in human eyes. Invest Ophthalmol Vis Sci 27, 145–152. [PubMed] [Google Scholar]
- Wiley CD, Brumwell AN, Davis SS, Jackson JR, Valdovinos A, Calhoun C, Alimirah F, Castellanos CA, Ruan R, Wei Y, Chapman HA, Ramanathan A, Campisi J, Jourdan Le Saux C, 2019. Secretion of leukotrienes by senescent lung fibroblasts promotes pulmonary fibrosis. JCI Insight 4, e130056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley CD, Campisi J, 2016. From Ancient pathways to aging cells: Connecting metabolism and cellular senescence. Cell Metab 23, 1013–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley CD, Sharma R, Davis SS, Lopez-Dominguez JA, Mitchell KP, Wiley S, Alimirah F, Kim DE, Payne T, Rosko A, Aimontche E, Deshpande SM, Neri F, Kuehnemann C, Demaria M, Ramanathan A, Campisi J, 2021. Oxylipin biosynthesis reinforces cellular senescence and allows detection of senolysis. Cell Metabolism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley CD, Velarde MC, Lecot P, Liu S, Sarnoski EA, Freund A, Shirakawa K, Lim HW, Davis SS, Ramanathan A, Gerencser AA, Verdin E, Campisi J, 2016. Mitochondrial Dysfunction Induces Senescence with a Distinct Secretory Phenotype. Cell Metab 23, 303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Timmers C, Maiti B, Saavedra HI, Sang L, Chong GT, Nuckolls F, Giangrande P, Wright FA, Field SJ, Greenberg ME, Orkin S, Nevins JR, Robinson ML, Leone G, 2001. The E2F1–3 transcription factors are essential for cellular proliferation. Nature 414, 457–462. [DOI] [PubMed] [Google Scholar]
- Wyszynski RE, Bruner WE, Cano DB, Morgan KM, Davis CB, Sternberg P, 1989. A donor-age-dependent change in the activity of alpha-mannosidase in human cultured RPE cells. Invest Ophthalmol Vis Sci 30, 2341–2347. [PubMed] [Google Scholar]
- Yamada K, Kaneko H, Shimizu H, Suzumura A, Namba R, Takayama K, Ito S, Sugimoto M, Terasaki H, 2020. Lamivudine Inhibits Alu RNA-induced Retinal Pigment Epithelium Degeneration via Anti-inflammatory and Anti-senescence Activities. Transl Vis Sci Technol 9, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao PL, Parmar VM, Choudhary M, Malek G, 2022. NURR1 expression regulates retinal pigment epithelial-mesenchymal transition and age-related macular degeneration phenotypes. Proc Natl Acad Sci U S A 119, e2202256119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaswen P, Stampfer MR, 2002. Molecular changes accompanying senescence and immortalization of cultured human mammary epithelial cells. Int J Biochem Cell Biol 34, 1382–1394. [DOI] [PubMed] [Google Scholar]
- Yip VCH, Wong HT, Yong VKY, Lim BA, Hee OK, Cheng J, Fu H, Lim C, Tay ELT, Loo-Valdez RG, Teo HY, Lim Ph A, Yip LWL, 2019. Optical Coherence Tomography Angiography of Optic Disc and Macula Vessel Density in Glaucoma and Healthy Eyes. J Glaucoma 28, 80–87. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Ohno-Matsui K, Ichinose S, Sato T, Iwata N, Saido TC, Hisatomi T, Mochizuki M, Morita I, 2005. The potential role of amyloid beta in the pathogenesis of age-related macular degeneration. J Clin Invest 115, 2793–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefzadeh MJ, Flores RR, Zhu Y, Schmiechen ZC, Brooks RW, Trussoni CE, Cui Y, Angelini L, Lee KA, McGowan SJ, Burrack AL, Wang D, Dong Q, Lu A, Sano T, O’Kelly RD, McGuckian CA, Kato JI, Bank MP, Wade EA, Pillai SPS, Klug J, Ladiges WC, Burd CE, Lewis SE, LaRusso NF, Vo NV, Wang Y, Kelley EE, Huard J, Stromnes IM, Robbins PD, Niedernhofer LJ, 2021. An aged immune system drives senescence and ageing of solid organs. Nature 594, 100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Ma J, Li J, Wang D, Wang Z, Wang S, 2020. Mitochondrial phosphatase PGAM5 modulates cellular senescence by regulating mitochondrial dynamics. Nat Commun 11, 2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Gao F, Ma Y, Xue T, Shen Y, 2021. Identification of early-onset photoreceptor degeneration in transgenic mouse models of Alzheimer’s disease. iScience 24, 103327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Jiang N, Chu Y, Postnikova O, Varghese R, Horvath A, Cheema AK, Golestaneh N, 2020. Dysregulated metabolic pathways in age-related macular degeneration. Sci Rep 10, 2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Duan PC, Liang JH, Zhang XF, Pan CW, 2021. Geographic distributions of age-related macular degeneration incidence: a systematic review and meta-analysis. Br J Ophthalmol 105, 1427–1434. [DOI] [PubMed] [Google Scholar]
- Zhu D, Wu J, Spee C, Ryan SJ, Hinton DR, 2009. BMP4 mediates oxidative stress-induced retinal pigment epithelial cell senescence and is overexpressed in age-related macular degeneration. J Biol Chem 284, 9529–9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, O’Hara SP, LaRusso NF, Miller JD, Roos CM, Verzosa GC, LeBrasseur NK, Wren JD, Farr JN, Khosla S, Stout MB, McGowan SJ, Fuhrmann-Stroissnigg H, Gurkar AU, Zhao J, Colangelo D, Dorronsoro A, Ling YY, Barghouthy AS, Navarro DC, Sano T, Robbins PD, Niedernhofer LJ, Kirkland JL, 2015. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14, 644–658. [DOI] [PMC free article] [PubMed] [Google Scholar]


