Abstract
Active partitioning of low-copy number plasmids requires two proteins belonging to the ParA and ParB families and a cis-acting site which ParB acts upon. Active separation of clusters of plasmid molecules to the defined locations in the cell before cell division ensures stable inheritance of the plasmids. The central control operon of IncP-1 plasmids codes for regulatory proteins involved in the global transcriptional control of operons for vegetative replication, stable maintenance and conjugative transfer. Two of these proteins, IncC and KorB, also play a role in active partitioning, as the ParA and ParB homologues, respectively. Here we describe mapping the regions in KorB responsible for four of its different functions: dimerisation, DNA binding, repression of transcription and interaction with IncC. For DNA binding, amino acids E151 to T218 are essential, while repression depends not only on DNA binding but, additionally, on the adjacent region amino acids T218 to R255. The C-terminus of KorB is the main dimerisation domain but a secondary oligomerisation region is located centrally in the region from amino acid I174 to T218. Using three different methods (potentiation of transcriptional repression, potentiation of DNA binding and activation in the yeast two-hybrid system) we identify this region as also responsible for interactions with IncC. This IncC–KorB contact differs in location from the ParA–ParB/SopA–SopB interactions in P1/F but is similar to these systems in lying close to a masked oligomerisation determinant.
INTRODUCTION
A fundamental part of the bacterial cell cycle is the partitioning of chromosomes and plasmids to either side of the cell division plane. For low-copy number plasmids it is well established that this is a better-than-random process driven by an active partitioning apparatus (1). More recent data with Escherichia coli (2–4), Bacillus subtilis (5–8), Caulobacter crescentus (9), Streptomyces coelicolor (10) and Pseudomonas putida (11) have indicated that chromosomal segregation also appears to depend on active processes. Remarkably, one of the gene systems that drives active partitioning of many plasmids is also found on most bacterial chromosomes, except for those of the Enterobacteriaceae (12–14). The key features of the plasmid and chromosomal systems are two trans-acting genes, generically termed parA and parB, and a cis-acting site termed the centromere-like sequence by analogy to eukaryotic chromosomes (1). The ParB protein is a DNA-binding protein, which binds to the centromere-like sequence (15,16). The ParA protein is an ATPase whose activity is essential for partitioning (17–20). These Par functions, possibly together with as yet unidentified chromosomal functions, appear to be responsible for localisation of plasmid or chromosomal oriC region to the regions where DNA replication takes place and for the movement between these zones (2,21,22). The interaction between ParA and ParB appears to be a vital part of this process (21,23–26). Recent studies have roughly mapped the zones of interactions between the F and P1 ParA/ParB proteins (27,28) but it is uncertain whether the conclusions from these studies can be extrapolated to other systems.
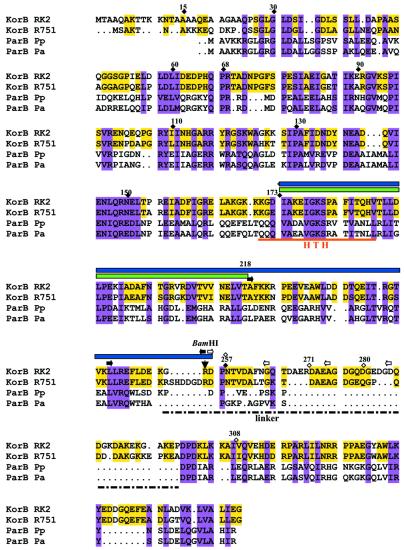
The IncP-1 plasmid Par system (19,29–31) has many interesting features not least because it also serves as a central control region which coordinates the expression of basic plasmid genes involved in plasmid replication, transfer and stable inheritance (30,32,33). Also, in the phylogenetic trees, the IncP-1 ParA and ParB homologues cluster with the chromosomally encoded members of the two families and away from other plasmid-encoded homologues (12). For simplicity, an alignment of KorB from IncP-1α and IncP-1β plasmids only with chromosomal ParB from P.putida and Pseudomonas aeruginosa are shown in Figure 1 to illustrate the sequence conservation and the structural features of these proteins. The Pseudomonas proteins are chosen because this genus appears most likely to be the natural host of the IncP-1 plasmids (34).
Figure 1.
Alignment of KorB from two subgroups of IncP-1 plasmids and ParB proteins of P.putida and P.aeruginosa. The regions of homology between four proteins are shown in pink. The similarities between KorBRK2 (IncP1-α) and KorBR751 (IncP-1β) are shown in yellow. Putative helix–turn–helix motif and linker region are underlined. The change in the codon for R254 introducing BamHI site is marked with an arrow. The symbols above the amino acids indicate the extent of deletions: black diamonds, deletions from N-terminus; open diamonds, deletions from C-terminus; black and white arrows, internal deletions from upstream and downstream of BamHI site, respectively. The green rectangle above the sequence shows region mapped in this work as ‘IncC interaction domain’ whereas blue rectangle corresponds to the fragment with the secondary dimerisation domain.
The IncP-1 ParB homologue KorB binds to 12 sites (OB1–OB12) in the RK2 plasmid genome (35–37). Class I sites (OB1, OB10 and OB12) lie immediately upstream of the –35 region of the promoters they are associated with (korAp, trfAp, klaAp), while Class II sites (OB2, OB9, OB10 and OB11) lie up to 189 bp upstream/downstream of a transcription start point (kfrAp, trbBp, trbAp, kleAp; note OB10 is Class I relative to trfAp and Class II relative to trbAp). KorB, bound at all these sites, can repress transcription from the adjacent promoter. The role of Class III (intergenic and intragenic) KorB-binding sites (OB3–OB8) is still unclear although the role of OB3 in the active partitioning has been suggested (38).
The ParA homologue IncC can potentiate transcriptional repression by KorB at Class I and Class II sites (39). incC codes for two forms of IncC, IncC1 and IncC2, differing at the N-terminus by 105 amino acids (40). IncC1 (364 amino acids) stabilises KorB–DNA complexes formed in vitro at all OBs except OB3 (37,39). With respect to partitioning, we know that IncC2 (254 amino acids) and KorB are needed for partitioning but that at least during growth in rich medium additional genes encoded in the kle, kla and klc regions are required for full stability (21,31). Nevertheless, IncC is essential to ensure that the foci that KorB forms on plasmid DNA are distributed regularly in the bacterial cell as expected for active partitioning (21). Whereas other plasmid-encoded partitioning proteins are known to have autoregulatory functions (1), such complex regulatory involvement of partitioning proteins has only been reported so far for other IncP-1 plasmids (31,41) and the chromosomal homologues of IncC and KorB such as Soj and SpoOJ of B.subtilis (25,26,42). There is no evidence that IncC has the ability to bind DNA. The co-regulatory effect it exerts on the activity of many plasmid promoters is absolutely dependent on the KorB protein, implying a direct interaction between these two proteins or modification of DNA structure by KorB to facilitate IncC binding (39). Direct interaction between KorB and IncC has recently been reported (43) and is likely to be the basis for IncC-related displacement of IncP-1 as well as heterologous plasmids encoding a KorB-binding site so long as KorB is also produced (39,44).
To provide further understanding of the ParB family and the subfamily that includes the chromosomal homologues, we have used deletion analysis to define the subregions of KorB. We describe mapping of the DNA-binding segment, definition of two KorB–KorB interaction zones and the region required for interaction with IncC. The localisation of the latter is different from the ParA/SopA recognition patch on ParB of P1 or SopB of F (27,28).
MATERIALS AND METHODS
Bacterial strains and growth
Escherichia coli K12 strains used were C600K (thr-1 leu-6 thi-1 lacY1 supE44 ton21 galK) and BL21 F– ompT hsdSB(rB–mB–) gal dcm (phage DE3) (Novagen, Inc.). Bacteria were generally grown in L broth (45) at 37°C or on L agar (L broth with 1.5% w/v agar) supplemented with antibiotics as appropriate: benzyl penicillin, sodium salt (150 µg ml–1 in liquid media and 300 µg ml–1 in agar plates) for penicillin resistance, kanamycin sulphate (50 µg ml–1) for kanamycin resistance and streptomycin sulphate (30 µg ml–1) for streptomycin resistance.
Yeast strains and growth conditions
Saccharomyces cerevisiae strain Y187 MATα ura3-52, his3-200, ade2-101, trp1-901, leu2-3112, met–, gal4Δ, gal80Δ, URA3::GAL1UAS-GAL1TATA-lacZ was used for β-galactosidase tests, and Y190 MATa ura3-52, his3-200, ade2-101, lys2-801, trp1-901, leu2-3112, gal4Δ, gal80Δ, URA3::GAL1UAS-GAL1TATA-lacZ, cyhr2, LYS2::GAL1UAS-HIS3TATA-His3 was used for complementation of HIS auxotrophy in the presence of 3-amino-1,2,4-triazole (3-AT). 3-AT was used to repress the basal activity of the His3 reporter gene, which otherwise results in non-specific background growth in the absence of exogenous histidine.
Yeast cells were grown in YPD medium (1% yeast extract, 2% bacto peptone, 2% glucose). Plasmid-containing yeast strains were grown in YNB medium (0.67% yeast nitrogen base, 3% glucose) supplemented with the mixture of appropriate nutrients lacking tryptophan, leucine or both, as required. Agar was added to a concentration of 2% for plates. To grow transformants for β-galactosidase filter-lift assay, YNB agar was supplemented with 0.5% glucose instead of 3% glucose. For β-galactosidase assay in the liquid cultures, the yeast cells were grown to logarithmic phase in YNB medium with 0.5% glucose and then transferred to YNB-ethanol (3%) medium for several hours. For testing the expression of the His reporter gene in Y190, YNB plates also contained 75 mM 3-AT. Plates were incubated at 30°C for 3–4 days.
Plasmids
The plasmids used are described below except for standard vectors (46).
Plasmids for regulatory studies were as follows. The transcriptional fusions to monitor the activity of RK2 promoters were constructed by insertion of PCR-amplified promoter fragments into the BamHI site of pGBT43 (10.4 kb KmR plasmid based on the pSC101 replicon) upstream of promoterless xylE cassette (30,47). pGBT30 (48) is an expression PnR vector based on the pMB1 replicon with lacIQ and tacp separated from λtR by the multiple cloning site from pUC18. To make use of the associated tacp SD sequence, the ATG codon of the inserted open reading frame (ORF) must directly follow the EcoRI site. ORFs were amplified by PCR with flanking EcoRI and SalI sites. pGBT36 was created by inserting the PCR product of the incC1 ORF as an EcoRI–SalI fragment into pGBT30. The upstream primer eliminated the ATG codon for KorA without changing the amino acid sequence of IncC (39). pGBT301 has the korB under the control of tacp (33).
Expression vector pGBT400 is an 11.6 kb IncQ SmR derivative of pDM1.1 (30) with the EcoRI site replaced by a SalI linker (BRL). pOLE1 is a pGBT400 derivative constructed by replacement of the BamHI–SalI fragment (lacIQ tacp) with lacIQ tacp-incC from pGBT36 (39).
C-terminal deletions of korB (pGBT303Δ) were obtained by Bal31 treatment of linearised pGBT303 with SalI as described previously (33). pGBT303 is a derivative of pGBT301 with an extra 1 kb of CAT (chloramphenicol acetyl-transferase) cassette inserted at the HindIII site downstream of the korB ORF to protect important plasmid DNA sequences from Bal31 treatment. N-terminal deletions were either obtained by amplification by PCR, with designed primers placing an ATG start codon in-frame with the remaining part of the protein, or by use of the unique restriction sites in the N-terminal part of korB such as NotI (43 nt from the start of korB), SacII (205 nt) and EcoRV (519 nt). Linearised plasmid was treated with DNA PolI Klenow fragment and ligated with an oligonucleotide carrying the EcoRI recognition site followed by ATG codon in-frame with the rest of KorB. To combine the N-terminal (of 173 amino acids) and C-terminal deletions (of 78 and 101 amino acids), pET28 (Novagen, Inc.) derivatives with korB gene truncated from the C-terminus were treated with EcoRV and then an appropriate oligomer was inserted as described above. All constructions were checked by sequencing of the new junction point. EcoRI–SalI fragments carrying different korB alleles were recloned into pGBT30 under tacp control.
Plasmids for protein purification were as follows. pET28a KmR (49; Novagen, Inc.) was modified with the help of the synthetic oligomer so that DNA sequences for His6-tag and thrombin cleavage sites (underlined in the presented sequence) precede almost directly the ATG codon of the ORF cloned as EcoRI–SalI fragments. N-terminal modification of over-produced proteins is represented by the following amino acid sequence: MGSSHHHHHHSSGLVPRGSHSEFM. All korB alleles were recloned into this modified pET28 and plasmids used to over-produce and purify the products.
Escherichia coli–Saccharomyces cerevisiae shuttle vectors used in the two-hybrid system were as follows. Two standard shuttle vectors, pGBT9 and pGAD424, were used (Clontech-Matchmaker) to fuse the polypeptides encoded by EcoRI–SalI fragments to the C-terminus of GAL4 DNA-binding domain (DBD) and GAL4 activation domain (AD), respectively. The system provides the ApR selection in E.coli and TRP+ or LEU+ selection in the yeast cells. Selected korB alleles were transferred to the shuttle vectors.
Plasmid DNA isolation, analysis, cloning and manipulation of DNA
Plasmid DNA was isolated by standard procedures (46). Large-scale plasmid purification was carried out by means of standard alkaline SDS extraction followed by CsCl ethidium bromide density gradient centrifugation. Digestion of plasmid DNA with restriction enzymes was carried out under conditions recommended by suppliers and run on agarose gels of concentration 0.8–2.0% (w/v). DNA sequencing was performed by internal sequencing facility (IBB, Warsaw) and AltaBioscience (Birmingham) using the Dye-terminator method in conjunction with an ABI 377 automated DNA sequencer. Standard PCR reactions (50) were performed as described previously (51). Fragments were amplified on a pCT690 (52) template for RK2. All PCR-derived clones were analysed by DNA sequencing to check their fidelity. DNA fragments (PCR amplified) for gel retardation studies were 3′-end-labelled with terminal transferase (Boehringer Manheim) and [α-32P]ddATP (Amersham).
Yeast transformation
Yeast transformation was performed using the standard PEG/LiAc method recommended by Clontech. Either single or double transformations were conducted and transformants selected and stored on minimal YNB agar.
Determination of β-galactosidase activity in yeasts
β-Galactosidase activity was monitored by the filter-lift assay with X-gal and quantitative liquid culture assay using o-nitrophenyl-β-d-galactopyranoside (ONPG) as a substrate (Clontech manual).
Determination of catechol 2,3-oxygenase activity
Catechol 2,3-oxygenase (the product of xylE) activity was assayed in logarithmically growing bacteria (OD600nm = 0.6) as described previously (53). Protein concentration was assayed by the Biuret method (54). Plasmid DNA was isolated from assayed cultures and XylE activities were normalised to the same plasmid level as the control.
Purification of His6-tailed polypeptides
Exponentially growing BL21 strains with pET28 derivatives (49,55) were induced with 0.5 mM IPTG at a cell density of ∼2 × 108 c.f.u./ml, grown for an additional 2 h with shaking at 37°C. The bacteria were harvested by centrifugation and sonicated. Over-produced His-tagged proteins were purified as recommended for soluble native proteins by Qiagen on Ni-agarose columns with an imidazole gradient in phosphate buffer at pH 6.0. Purification was monitored by SDS–PAGE using a Pharmacia PHAST gel system.
Analysis of protein–DNA interactions by electrophoretic mobility shift assay (EMSA)
A synthetic OB palindrome (37) 5′-gaattcTTTAGCGGCTAAAaagctt-3′ was cloned between EcoRI–HindIII into pUC18 (pKK113). Using PCR and primers, 5′-CGAAAGGGGGATGTGCTGC-3′ and 5′-GCTTCCGGCTCGTATGTTG-3′, annealing at positions 313 and 531 nt, respectively, on the pUC18 map, a fragment of 187 bp was amplified and labelled with terminal transferase. This labelled PCR product was either used directly for EMSA in the presence of 2 µg of salmon sperm DNA or cleaved with HaeIII to produce 78 and 109 bp fragments, the latter with OB, and used for EMSA with C-terminally truncated KorB polypeptides without non-specific DNA. Radioactive fragments were incubated with purified His6KorB (wild-type or truncated derivatives) and wild-type His6IncC and then separated by PAGE on 5% gels under conditions described previously (52).
Cross-linking with glutaraldehyde
His-tagged polypeptides purified on Ni-agarose columns were cross-linked with the use of glutaraldehyde (52) and separated on SDS–PAGE gels by Phast system (Pharmacia).
RESULTS
Construction of korB deletion mutants
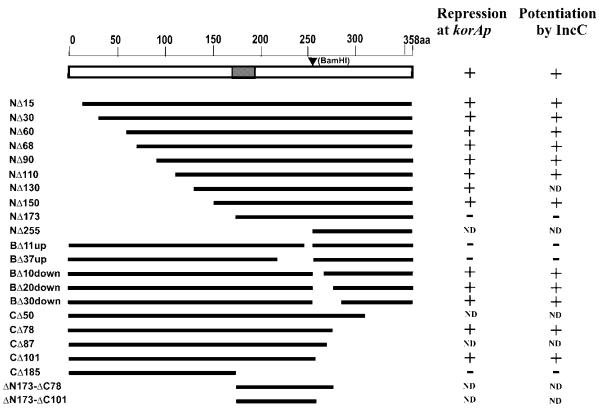
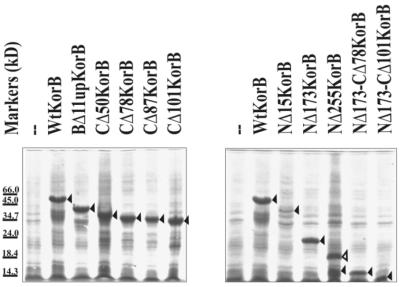
Deletion mutants were constructed to define the functional domains of KorB protein (Fig. 2). Using PCR and suitable restriction sites in the korB sequence, N-terminal deletions ranging from 15 to 173 amino acids were obtained. BAL31 digestion (33) was applied to get the C-terminal deletions removing up to 101 amino acids. A BamHI site was conveniently incorporated into the variable region between the two conserved domains of KorBRK2 and KorBR751 without changing the amino acid sequence (see Fig. 1). This site corresponds to R255 (change of codon CGC into CGG) of the polypeptide sequence. By linking the EcoRI–BamHI or BamHI–SalI fragments with PCR products, short internal deletions from 10 to 37 amino acids in length were created going from this site towards the N-terminus (upstream) or the C-terminus (downstream) of KorB. Finally, we combined the N-terminal deletion of 173 amino acids with two C-terminal deletions to produce short internal KorB fragments of 84 and 107 amino acids. The korB alleles were then incorporated into pGBT30 for over-production and regulatory studies in vivo, into the pET28 derivative for purification of His-tagged products and into the shuttle vectors pGAD424 and pGBT9 for the yeast two-hybrid system to create fusions with the GAL4 AD (ADGAL4) and DBD (DBDGAL4), respectively. We tried to determine whether korB deletion derivatives produced stable products. The extracts from IPTG-induced cultures of transformants of BL21(DE3) with pET28-korB alleles were separated by SDS–PAGE and the proteins of the right size were visualised. Representative extracts are shown in Figure 3. Using this criterion all the derivatives used, with the exception of the N-terminal 15 amino acid deletion, produced an apparently stable product of the expected size. Truncated product of NΔ15 is also visible on the gel but in smaller quantities.
Figure 2.
Deletion derivatives of KorB used in this study and summary of their transcriptional repressor activities. Deletions from the N-terminus are designated as NΔxx, from the C-terminus CΔxx and from the internal BamHI site introduced by site-directed mutagenesis as BΔxx, where xx corresponds to the number of amino acids deleted. For the internal deletions ‘up’ refers to a deletion towards the N-terminus while ‘down’ refers to a deletion towards the C-terminus. The putative helix–turn–helix motif is marked by the grey box. Repression of korAp–xylE transcriptional fusion in pDM3.1 is summarised to the right and indicates which KorB derivatives can still repress through OB1 (Class I) and whether this repression is potentiated by IncC. ND, not determined.
Figure 3.
Visualisation of korB truncation products. Escherichia coli BL21(DE3) with pET28 derivatives encoding korB alleles were grown for 3 h in the presence of 0.5 mM IPTG. Cell lysates were analysed by SDS–PAGE on 12.5% PHAST homogenous gels (Pharmacia) and stained with Coomassie blue. Dalton Markers VI were used as the molecular markers (Sigma). The major bands indicated by black arrows correspond to over-produced proteins. White arrow indicates very stable dimeric form of NΔ255KorB.
Repressor activity of truncated KorB products and its enhancement by IncC
To establish whether truncated forms of KorB were still able to repress transcription through binding to Class I operators, we placed pGBT30 derivatives with different korB alleles in trans to a pSC101 replicon with a korAp–xylE fusion (pDM3.1), along with a third plasmid based on the IncQ replicon initially carrying no IncP-1 gene (pDM1.1) but later to carry incC (pOLE1) (see below). The effectiveness as a repressor is given by the repression index (RIA)—the ratio between the activity of the korAp in the IPTG-induced cultures without korB derivatives (the empty expression vectors pGBT30 and pDM1.1) and with korB derivatives (the empty pDM1.1). All deletion derivatives tested affected the ability to repress, either by reducing or enhancing this activity (Table 1). The key points of the results are as follows. First, it was possible to make successive deletions from the N-terminus as far as amino acid 150 without abolishing repression. The shortest deletions, NΔ15 and NΔ30, reduced repressor activity but deletions of 60, 110 and 150 amino acids gave enhanced repressor activity. Deletion of 173 amino acids abolished repression. Secondly, from the C-terminus at least 101 amino acids could be removed without loss of repressor activity. Thirdly, with internal deletions, removal of amino acids 255–284 did not abolish repression but removal of amino acids 219–255 did abolish activity. These results are summarised in Figure 2.
Table 1. Potentiation of in vivo repressor activity of KorB by IncC protein monitored with korAp–xylE transcriptional fusion reporter plasmid pDM3.1 (30).
| korB derivative in transa | Repression index (RI) | Potentiationd | |
|---|---|---|---|
| RIAb without IncC (pDM1.1)a | RIBc with IncC(pPOLE1)a | RIB/RIA | |
| None | 1.1 | 1.0 | 1.0 |
| Wild-type KorB | 3.7 | 12.4 | 3.4 |
| NΔ15 | 1.8 | 4.2 | 2.3 |
| NΔ30 | 2.7 | 6.0 | 2.2 |
| NΔ60 | 10.1 | 17.2 | 1.7 |
| NΔ68 | 3.3 | 8.3 | 2.5 |
| NΔ90 | 3.6 | 8.3 | 2.3 |
| NΔ110 | 7.3 | 25.9 | 3.5 |
| NΔ150 | 11.1 | 23.1 | 2.1 |
| NΔ173 | 1.0 | 1.0 | 1.0 |
| BΔ11up | 0.9 | 1.1 | 1.2 |
| BΔ37up | 0.8 | 0.9 | 1.1 |
| BΔ10down | 2.2 | 5.5 | 2.5 |
| BΔ20down | 3.1 | 5.8 | 1.9 |
| BΔ30down | 3.2 | 10.2 | 3.2 |
| CΔ78 | 2.1 | 4.7 | 2.2 |
| CΔ101 | 3.0 | 7.3 | 2.4 |
akorB deletions were cloned into pGBT30 (44) where they were expressed from tacp. Strain C600K with pDM3.1 plasmid, based on the pSC101 replicon, and carrying korAp–xylE transcriptional fusion (30) was set up with relevant korB derivatives and either with pDM1.1 (an IncQ based expression vector) or pOLE1 (pDM1.1 derivative with incC under control of tacp; 39). Strain with pDM3.1, pGBT30 and pDM1.1 was used as a control. Cells were grown in the presence of 1 mM IPTG for 3 h to induce the expression of regulatory proteins.
bRepression index (RIA) was calculated as the ratio between the XylE activities in extracts of C600K (pDM3.1/pDM1.1/pGBT30) and C600K(pDM3.1/pDM1.1/pGBT30-korB alleles). This index gives the value of KorB (and KorB deletion derivatives) repressor activity in the three-plasmid system.
cRepressor index (RIB) was calculated as the ratio between the XylE activities in extracts of C600K (pDM3.1/pPOLE1/pGBT30) and C600K(pDM3.1/pPOLE1/pGBT30-korB alleles). This index gives the value of KorB (and KorB deletion derivatives) repressor activity in the presence of wild-type IncC in the three-plasmid system.
dPotentiation is defined as the ratio between RIB and RIA and indicates the factor by which IncC protein enhances the in vivo repressor activity of either wild-type or mutant KorB proteins.
To determine whether IncC can enhance repression by the korB deletion derivatives we included pOLE1 in the three-plasmid system because this plasmid carries incC under tacp control. The results in Table 1 express the repression in the presence of IncC as RIB—the ratio of XylE activity when only IncC is over-produced compared with XylE activity when the korB derivative and incC are expressed together under IPTG induction. Expression of IncC without korB present had no effect on korAp activity. The results showed that despite the differences in repression by the N-terminal deletion derivatives, their effect in all cases was potentiated 2–3-fold by the presence of IncC (RIB/RIA in Table 1). Similarly, repression by the longest C-terminal deletion derivative was still potentiated 2–3-fold by the presence of IncC. Finally, the same effect was observed for the internal deletion of amino acids 255–284. Those deletions that did not show repression (NΔ173 and BΔ37up) were also tested, but the presence of incC did not elicit any repressor activity. This indicated that we can delete residues M1 to E151 or from P257 to G358 and still have KorB acting as a repressor at korAp and that this activity can be additionally potentiated by the presence of IncC.
In vitro IncC potentiation of DNA-binding activity of truncated KorB
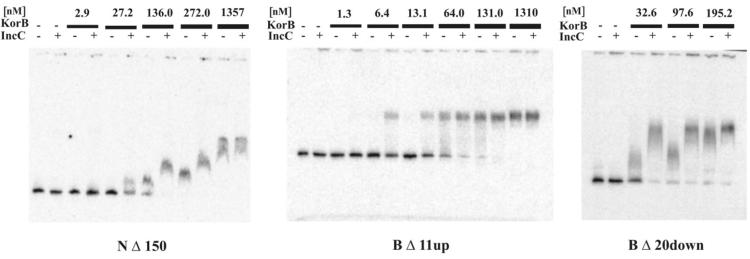
The wild-type KorB and some of the products of deletion derivatives were purified as N-terminally His-tagged proteins from BL21/pET28 extracts. The pUC18 fragment of 187 bp with a centrally located oligonucleotide corresponding to the consensus OB was radioactively labelled and used as the target in the mobility shift assays (Fig. 4).
Figure 4.
EMSA with representative N-terminal and internal deletion derivatives on DNA fragments containing a consensus OB site. The first two tracks on each gel demonstrate the conditions of DNA fragment incubation with no protein or IncC on its own. The numbers above the black lines refer to the concentration (nM) of His-tagged KorB truncation products used in the presence and absence of IncC. For NΔ150KorB, where indicated, IncC was added at 258 nM. For BΔ11upKorB and BΔ20downKorB, where indicated, IncC was added at 581 nM. Carrier DNA was added to the incubation mixtures at final concentration of 0.1 mg/ml.
Kapp was established experimentally for a selection of truncated KorB products alone and in the presence of IncC. The data are summarised in Table 2.
Table 2. IncC enhancement of KorB DNA-binding activity in vitro.
| Purified His-KorB and its deletion derivatives used | Kappa of KorB (nM) | Kappb of KorB (nM) in the presence of IncC | Kappa/Kappb |
|---|---|---|---|
| Wild-type KorB | 1125 | 385 | 2.9 |
| NΔ30 | 313 | 60 | 5.2 |
| NΔ60 | 167 | 34 | 4.9 |
| NΔ90 | 650 | 160 | 4.1 |
| NΔ150 | 135 | 27 | 5.0 |
| NΔ173 | No binding | No binding | ND |
| BΔ11up | 61 | 13 | 4.7 |
| BΔ37up | 520 | 346 | 1.5 |
| BΔ10down | 64 | 20 | 3.2 |
| BΔ20down | 29 | 16 | 1.8 |
aKapp was estimated experimentally in EMSA as the concentration of purified His-tagged KorB derivatives needed to shift 50% of the radioactive fragment containing OB. Kapp for NΔ173 could not be experimentally established, no binding to DNA was observed at 10 µM.
bKapp was estimated experimentally as the concentration of products of korB alleles needed to shift 50% of the labelled fragment in the presence of 581 nM IncC.
The His-tagged products of N-terminal deletions showed higher affinity towards the DNA fragment with OB than His-tagged wild-type KorB (NΔ150, Fig. 4) and the apparent affinity varied widely between deletion derivatives. Removal of 173 amino acids from the N-terminus gave a product that did not bind to DNA even at concentration of 10 µM. Therefore, its Kapp is at least 10 times greater than that of the wild-type and considerably more than this relative to the deletion derivatives closest in size. All the KorB derivatives with internal deletions still bound to DNA (deletions of up to 37 amino acids upstream and 20 amino acids downstream of R255). Note in particular that BΔ37up still bound to DNA despite having no repressor activity.
C-terminal deletion products showed weaker but still specific DNA-binding activity. As described previously (33), it was necessary with these proteins to demonstrate specificity by using two fragments, one with and the other without OB, but in the absence of unlabelled non-specific competitor DNA (Materials and Methods). This is consistent with the loss of dimerisation domain causing a major reduction in affinity for DNA. However, this means that any Kapp calculated on this basis is not comparable with the other proteins and is not therefore included in Table 2.
The presence of IncC in the reaction mixture increased the affinity of KorB for DNA 2–6-fold in the cases where KorB could bind to DNA (Table 2). The internal deletion BΔ37up retained the ability to interact with IncC. This limited the region of KorB necessary for interaction with IncC to amino acids E151 to T218 (Fig. 1).
Application of the yeast two-hybrid system to define the domain in KorB responsible for interaction with IncC
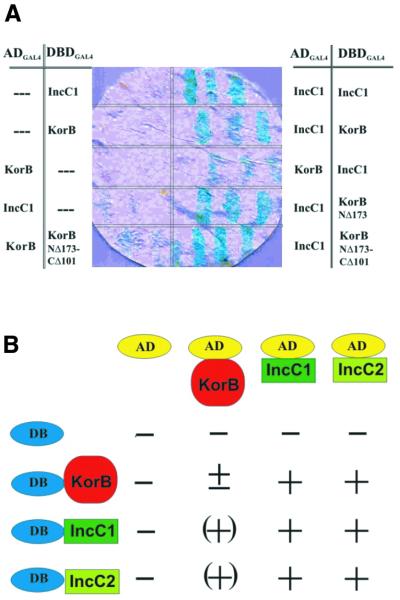
ORFs for KorB and IncC were cloned into vectors for the yeast two-hybrid system to create fusion proteins with GAL4 DBD (DBDGAL4) and AD (ADGAL4). Both IncC and KorB proteins were linked via their N-termini to GAL4 domains. IncC may code for two forms of IncC so we fused both products to GAL4 separately through N-terminal parts (IncC1 and IncC2). The interactions between the proteins were demonstrated by the colony-lift test for β-galactosidase activity followed by β-galactosidase assay on the liquid cultures of Y187 derivatives and confirmed by histidine prototrophy of Y190 double transformants in the presence of 3-AT. The interactions detected using this approach were quite weak but significant. Representative interactions are shown in Figure 5A and the summary of the results is presented in Figure 5B. From the IncC–KorB interaction pattern we concluded that when KorB was linked to the ADGAL4 it lost its ability to fold properly. This may explain the very weak KorB–KorB interactions observed, although strong KorB dimerisation has been well documented (33,35,36). Only when KorB was linked to DBDGAL4 (pGBT9-korB) and IncC to ADGAL4 (pGAD424-incC1) were the interactions between IncC and KorB easily detectable. The shorter form of IncC may interact with KorB. Two IncC molecules may interact with each other and this interaction seems to be stronger than that detected between IncC and KorB.
Figure 5.
Detection of KorB–KorB, IncC–IncC and KorB–IncC interactions by filter assays using the yeast two-hybrid system (as described in Materials and Methods). (A) Filter test for β-galactosidase activity corresponding to interactions detected between different fusion proteins and the relevant controls. (B) ‘+’ Corresponds to protein interactions and the dark blue colony colour (∼10 U of β-galactosidase), ‘(+)’ indicates weaker interactions (3–5 U), ‘±’ indicates pale blue colour (very weak interactions, 1–2 U), ‘–’ corresponds to no interactions and the white colour of the colony (<0.1 U). IncC1 corresponds to large (364 amino acids) and IncC2 to small (259 amino acids) forms of IncC.
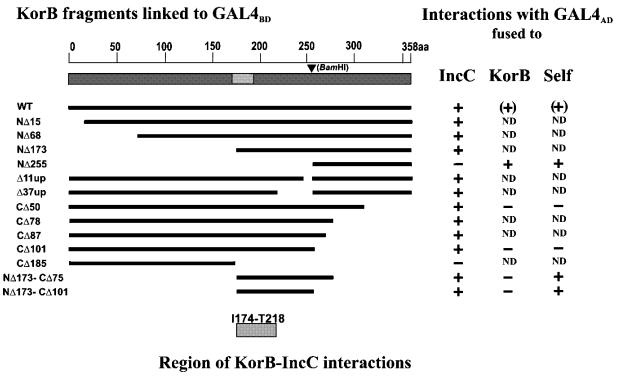
We selected representative korB mutants from the collection, cloned them into pGBT9 and introduced them into the S.cerevisiae strain Y187 with pGAD424-incC1 plasmid. In agreement with the regulatory studies, in vivo C-terminally truncated KorB proteins (deletions up to 101 amino acids from the C-terminus) were still able to interact with IncC to the same extent as the wild-type protein (Fig. 6). The two-hybrid system demonstrated that even a deletion of 173 amino acids from the N-terminus does not interfere with the ability of KorB to bind IncC. To confirm the presence of the ‘IncC-binding domain’ in the centre of KorB, we combined NΔ173 with two C-terminal deletions of CΔ78 and CΔ101, respectively obtaining clones producing polypeptides of 84 amino acids (from I174 to P257) and 107 amino acids (from I174 to D280). These two short polypeptides fused to DBDGAL4 were still able to interact with ADGAL4–IncC. To map the interaction domain even further, we used korB internal deletion derivatives in the two-hybrid system. We chose two deletion derivatives, BΔ11up and BΔ37up, having removed amino acids from between K244 and D256, and T218 and D256, respectively. Both deletion derivatives produced hybrid proteins DBDGAL4–KorB capable of interactions with ADGAL4–IncC. The fragments of KorB from M1 to D173 and from D256 to G358 showed no interactions with IncC. These results allowed us to limit the patch on KorB capable of interaction with IncC to the 45 amino acid segment from I174 to T218 in the KorB sequence (Fig. 1).
Figure 6.
Yeast two-hybrid system mapping of KorB region necessary for self-interactions and for interaction with IncC. korB alleles were fused to the DBD of GAL4 (DBDGAL4) in pGBT9 plasmid while either incC or korB (and its derivatives) were fused to the AD of GAL4 (ADGAL4) in pGAD424. Interactions between proteins were demonstrated by β-galactosidase filter assay and confirmed by β-galactosidase activity assay in the liquid culture. Symbols used in the diagram: +, interactions detected; –, no interactions detected; ND, not determined.
Dimerisation domains of KorB analysed by the yeast two-hybrid system
KorB molecules fused to both GAL4 domains gave only a weak positive signal in contrast to their established ability to dimerise (33,35,36). However, these interactions were significant enough to confirm the presence of a dimerisation domain in the C-terminus of KorB. Deletion of 101 amino acids from the C-terminus (DBDGAL4–KorBCΔ101) removed the ability to interact with wild-type KorB fused to ADGAL4, whereas the C-terminal 103 amino acids (DBDGAL4–KorBNΔ255) retained this ability (Fig. 6). The ability of other KorB fragments to interact with themselves and wild-type KorB was also tested. Both internal fragments of 107 amino acids (from I174 to D280) and 84 amino acids (from I174 to P257) showed strong self-interactions and cross-interactions between them. We could detect hardly any interactions of internal fragments with wild-type KorB (Fig. 5A) or with segments truncated either at the N-terminus (NΔ173) or C-terminus (CΔ101).
Glutaraldehyde cross-linking of the products of mutant korB alleles
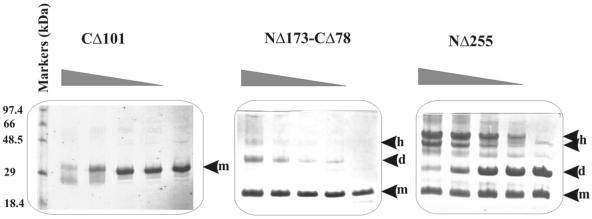
The oligomerisation ability of purified His-tagged proteins corresponding to some of the deletion derivatives described in this paper was tested by glutaraldehyde cross-linking. Previously, we have shown (33) that CΔ17 results in a monomeric form of truncated KorB. C-terminal deletions of up to 101 amino acids showed the same effect whereas N-terminal deletions up to 255 amino acids gave products that could be cross-linked (Fig. 7). The C-terminal 103 amino acids of KorB form very stable dimers and higher order complexes. The complexes are so stable that they can be detected even without glutaraldehyde under SDS–PAGE denaturing conditions. We also His-tagged and purified the internal parts of KorB (products of the deletions NΔ173–CΔ78 and NΔ173–CΔ101). Both fragments showed the ability to be cross-linked by glutaraldehyde confirming the presence of the potential secondary dimerisation domain in KorB, although this effect appears significantly less strong than that observed with the C-terminal 103 amino acids (only NΔ173–CΔ78 is shown in Fig. 7).
Figure 7.
Glutaraldehyde cross-linking of key korB deletion derivatives. His-tagged fragments of KorB polypeptide at a concentration of ∼0.3 mg/ml were incubated for 15 min with increasing concentrations of glutaraldehyde (0.001, 0.002, 0.005 and 0.01%) and then the samples were separated by SDS–PAGE on 20% Phast homogenous gels. Monomeric, dimeric and higher order complexes are indicated by m, d and h, respectively.
DISCUSSION
In this paper we have attempted to define key functional regions of KorB using a combination of genetic and in vitro methods. As described in the Introduction, KorB, the global regulatory protein and partitioning protein of IncP-1 plasmids (30,41), is known to bind DNA and function as a repressor in a way that depends on its oligomeric state (32). Its role in repression is modulated by IncC (33) and its role in partitioning is dependent on IncC (21,38), with which it is known to interact directly (33,39,43). The results we have presented above have helped us to localise regions either sufficient for or involved in each of these properties.
With respect to DNA-binding ability we have localised, by deletion analysis, the key region as lying between amino acids E151 and T218. Deletion of amino acids 1–173 destroyed DNA binding consistent with the position of the previously identified putative HTH motif (from K171 to T190) (56), which is present in all ParB family members (14). C-terminal deletions leaving amino acids 1–257 still bound DNA and the internal deletions covering T218 to D285 did the same. The estimated affinity for DNA (Kapp) of the various deletion derivatives varies over a wide range, suggesting that confirmation of the resulting proteins may differ and thus influence the stability of the protein–DNA complexes. Confirmation may be especially critical for KorB–DNA interactions as KorB is predicted to have a net negative charge so the juxtaposition of the acid residues relative to the DNA phosphate backbone should determine the degree of electrostatic repulsion.
For N-terminal deletion derivatives that still bind DNA there is a rough correlation between affinity for DNA and the ability to repress transcription at Class I OB. This contrasts with the ability to repress through a Class II OB, which is lost in all but the shortest N-terminal deletions (our unpublished results). We still do not understand exactly how KorB represses transcription at a distance so it would be premature to speculate what role the N-terminal domain plays. However, these results, along with previous ones (33), do point to much of the whole native protein structure of KorB being more essential for its other tasks—long range repression and possibly active partitioning—than for perhaps the simpler task of transcriptional repression through binding to proximal operators. The most striking of these results is that deletion of the region T218 to R255 gave a protein still able to bind DNA but no longer able to repress transcription at any of the promoters tested. This indicates that DNA binding alone is not sufficient for transcriptional repression even at Class I OBs, implying that direct interaction between KorB and RNAP may be necessary. This would be consistent with the observation that KorB does not prevent RNAP from binding but does stop isomerisation from closed to open complex (36).
With respect to oligomerisation, we have shown previously that the C-terminus is important for repression at a distance and for a multimeric state of KorB in vitro (33). Deletion of 255 amino acids from the N-terminus generated a 103 amino acid segment that could strongly dimerise as judged by cross-linking with glutaraldehyde in vitro (Fig. 7) and interactions in the yeast two-hybrid system in vivo (Fig. 6). Sequence alignments show that this C-terminal segment is highly conserved and is separated from the DNA-binding segment by a much more variable region (Fig. 1). The idea that all or part of this region forms a dimerisation domain is attractive.
Interestingly, the studies with the yeast two-hybrid system suggested that there is another segment that has potential to promote oligomerisation when both the N- and C-terminal regions are removed. These results have been confirmed also by glutaraldehyde cross-linking in vitro (Fig. 7). Deletion of 101 amino acid residues from the C-terminus renders KorB unable to form dimers in cross-linking reactions, but deletion of a further 173 amino acids from the N-terminus allows formation of dimers (oligomers) although with much lower efficiency than observed for the C-terminus under the same conditions. This may indicate that the secondary oligomerisation domain can function after a conformational change in KorB structure due, for example, to interaction with DNA, IncC or another macromolecule in the cell. A similar inhibitory function of one dimerisation domain over another was also observed for ParB of P1 (28).
Finally, we defined the patch in KorB sufficient for interactions with IncC. Combined in vitro and in vivo assays allowed us to localise this region to the central 45 amino acids in which the HTH motif has also been localised. So far, the domains of interactions with the ParA component of the partitioning apparatus have been defined in the N-terminal parts of ParB of P1 (the first 29 amino acids) (28) and SopB of F (N-terminal 180 amino acids) (27). So whereas the localisation of the primary dimerisation domain in ParB members of the family is highly conserved (27,33,56,57) there is no universal localisation of the domain of interaction with ParA. This may seem surprising in light of the apparent co-evolution of these two proteins as part of a ubiquitous stable inheritance mechanism. However, it seems likely that there has been selection for diversity in the specificity of the partitioning complexes. Given the differences in the centromere-like sequences, we might expect a corresponding diversity in the protein–DNA architecture of the partitioning complexes. In addition, the particular location in KorB of this interaction domain makes sense of the fact that IncC potentiates KorB DNA binding apparently by modulating the interaction of KorB with flanking sequences on either side of the core operator (37). IncC may distort KorB and make it more capable of productive contacts with these flanking regions. This coarse mapping of functional regions in KorB will now allow more detailed dissection of the different functions performed by KorB and will provide a fine analysis of the relationship between structure and function.
Acknowledgments
ACKNOWLEDGEMENTS
M.L. was supported in part by a Scholarship from the Darwin Trust of Edinburgh. K.K. was supported by project grant 048040 from The Wellcome Trust. The collaboration between G.J.B. and C.M.T. during this work was funded by a Research Initiative Grant from The Wellcome Trust (056022) which also provided partial support for A.A.B. G.D.C. was supported by a BBSRC Mres Studentship. This work was carried out in the context of EU Concerted Action MECBAD (Mobile Elements Contribution to Bacterial Adaptability and Diversity).
REFERENCES
- 1.Williams D.R. and Thomas,C.M. (1992) Active partitioning of bacterial plasmids. J. Gen. Microbiol., 138, 1–16. [DOI] [PubMed] [Google Scholar]
- 2.Gordon G.S., Sitnikov,D., Webb,C.D., Teleman,A., Straight,A., Losick,R., Murray,A.W. and Wright,A. (1997) Chromosome and low copy plasmid segregation in E.coli: visual evidence for distinct mechanisms. Cell, 90, 1113–1121. [DOI] [PubMed] [Google Scholar]
- 3.Niki H. and Hiraga,S. (1998) Polar localization of the replication origin and terminus in Escherichia coli nucleoids during chromosome partitioning. Genes Dev., 12, 1036–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niki H., Yamaichi,Y. and Hiraga,S. (2000) Dynamic organization of chromosomal DNA in Escherichia coli. Genes Dev., 14, 212–223. [PMC free article] [PubMed] [Google Scholar]
- 5.Webb C.D., Teleman,A., Gordon,S., Straight,A., Belmont,A., Lin,D.C.-H., Grossman,A.D., Wright,A. and Losick,R. (1997) Bipolar localization of the replication origin regions of chromosomes in vegetative and sporulating cells of B. subtilis.Cell, 88, 667–674. [DOI] [PubMed] [Google Scholar]
- 6.Webb C.D., Graumann,P.L., Kahana,J.A., Teleman,A., Silver,P.A. and Losick,R. (1998) Use of time-lapse microscopy to visualize rapid movement of the replication origin region of the chromosome during the cell cycle in Bacillus subtilis. Mol. Microbiol., 28, 883–892. [DOI] [PubMed] [Google Scholar]
- 7.Lewis P.J. and Errington,J. (1997) Direct evidence for active segregation of oriC regions of the Bacillus subtilis chromosome and co-localization with the SpoOJ partitioning protein. Mol. Microbiol., 25, 945–954. [DOI] [PubMed] [Google Scholar]
- 8.Glaser P., Sharpe,M., Raether,B., Perego,M., Ohlsen,K. and Errington,J. (1997) Dynamic, mitotic-like behavior of a bacterial protein required for accurate chromosome partitioning. Genes Dev., 11, 1160–1168. [DOI] [PubMed] [Google Scholar]
- 9.Mohl D.A. and Gober,J.W. (1997) Cell cycle-dependent polar localization of chromosome partitioning proteins in Caulobacter crescentus. Cell, 88, 675–684. [DOI] [PubMed] [Google Scholar]
- 10.Kim H.J., Calcutt,M.J., Schmidt,F.J. and Chater,K.F. (2000) Partitioning of the linear chromosome during sporulation of Streptomyces coelicolor A3(2) involves an oriC-linked parAB locus. J. Bacteriol., 182, 1313–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis R.A., Bignell,C.R., Zeng,W., Jones,A.C. and Thomas,C.M. (2002) Chromosome-loss from par mutants of Pseudomonas putida depends on growth medium and phase of growth. Microbiology, 148, 537–548. [DOI] [PubMed] [Google Scholar]
- 12.Hayes F. (2000) The partition system of multidrug resistance plasmid TP228 includes a novel protein that epitomizes an evolutionarily distinct subgroup of the ParA superfamily. Mol. Microbiol., 37, 528–541. [DOI] [PubMed] [Google Scholar]
- 13.Gerdes K., Moller-Jensen,J. and Bugge Jensen,R. (2000) Plasmid and chromosome partitioning: surprises from phylogeny. Mol. Microbiol., 37, 455–466. [DOI] [PubMed] [Google Scholar]
- 14.Yamaichi Y. and Niki,H. (2000) Active segregation by the Bacillus subtilis partitioning system in Escherichia coli. Proc. Natl Acad. Sci. USA, 97, 14656–14661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mori H., Mori,Y., Ichinose,C., Niki,H., Ogura,T., Kato,A. and Hiraga,S. (1989) Purification and characterization of SopA and SopB proteins essential for F plasmid partitioning. J. Biol. Chem., 264, 15535–15541. [PubMed] [Google Scholar]
- 16.Abeles A., Austin,S.J. and Reaves,L.D. (1989) Protein–DNA interactions in regulation of P1 plasmid replication. J. Bacteriol., 171, 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis M.A., Martin,K. and Austin,S. (1992) Biochemical activities of the ParA partition protein of the P1 plasmid. Mol. Microbiol., 6, 1141–1147. [DOI] [PubMed] [Google Scholar]
- 18.Davis M.A., Radnedge,L., Martin,K.A., Hayes,F. and Austin,S.J. (1996) The P1 ParA protein and its ATPase activity play a direct role in the segregation of plasmid copies to daughter cells. Mol. Microbiol., 21, 1029–1036. [DOI] [PubMed] [Google Scholar]
- 19.Motallebi-Veshareh M., Rouch,D.A. and Thomas,C.M. (1990) A family of ATPases involved in active partitioning of diverse bacterial plasmids. Mol. Microbiol., 4, 1455–1463. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe E., Wachi,M., Yamasaki,M. and Nagai,K. (1992) ATPase activity of SopA, a protein essential for active partitioning of F plasmid. Mol. Gen. Genet., 234, 249–352. [DOI] [PubMed] [Google Scholar]
- 21.Bignell C.R., Haines,A.S., Khare,D. and Thomas,C.M. (1999) Effect of growth rate and incC mutation on symmetric plasmid distribution by the IncP-1 partitioning apparatus. Mol. Microbiol., 34, 205–216. [DOI] [PubMed] [Google Scholar]
- 22.Niki H. and Hiraga,S. (1997) Subcellular distribution of actively partitioning F plasmid during the cell division cycle in E.coli. Cell, 90, 951–957. [DOI] [PubMed] [Google Scholar]
- 23.Hirano M., Mori,H., Onogi,T., Yamazoe,M., Niki,H., Ogura,T. and Hiraga,S. (1998) Autoregulation of the partition genes of the mini-F plasmid and the intracellular localization of their products in Escherichia coli. Mol. Gen. Genet., 257, 392–403. [DOI] [PubMed] [Google Scholar]
- 24.Erdmann N., Petroff,T. and Funnell,B.E. (1999) Intracellular localization of P1 ParB protein depends on ParA and parS. Proc. Natl Acad. Sci. USA, 96, 14905–14910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marston A.L. and Errington,J. (1999) Dynamic movement of the ParA-like Soj protein of B. subtilis and its dual role in nucleoid organization and developmental regulation. Mol. Cell, 4, 673–682. [DOI] [PubMed] [Google Scholar]
- 26.Quisel J.D., Lin,D.C. and Grossman,A.D. (1999) Control of development by altered localization of a transcription factor in B. subtilis. Mol. Cell, 4, 665–672. [DOI] [PubMed] [Google Scholar]
- 27.Kim S.-K. and Shim,J. (1999) Interaction between F plasmid partition proteins SopA and SopB. Biochem. Biophys. Res. Commun., 263, 113–117. [DOI] [PubMed] [Google Scholar]
- 28.Surtees J.A. and Funnell,B.E. (1999) P1 ParB domain structure includes two independent multimerization domains. J. Bacteriol., 181, 5898–5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidhauser T.J., Bechhofer,D.H., Figurski,D.H. and Helinski,D.R. (1989) Host-specific effects of the korA-korB operon and oriT region on the maintenance of miniplasmid derivatives of broad host-range plasmid RK2. Plasmid, 2, 99–112. [DOI] [PubMed] [Google Scholar]
- 30.Macartney D.P., Williams,D.R., Stafford,T. and Thomas,C.M. (1997) Divergence and conservation of the partitioning and global regulation functions in the central control region of the IncP plasmids RK2 and R751. Microbiology, 143, 2167–2177. [DOI] [PubMed] [Google Scholar]
- 31.Thorsted P.B., Macartney,D.P., Akhtar,P., Haines,A.S., Ali,N., Davidson,P., Stafford,T., Pocklington,M.J., Pansegrau,W., Wilkins,B.M., Lanka,E. and Thomas,C.M. (1998) Complete sequence of the IncPbeta plasmid R751: implications for evolution and organisation of the IncP backbone. J. Mol. Biol., 282, 969–990. [DOI] [PubMed] [Google Scholar]
- 32.Motallebi-Veshareh M., Balzer,D., Lanka,E., Jagura-Burdzy,G. and Thomas,C.M. (1992) Conjugative transfer functions of broad-host-range plasmid RK2 are co-regulated with vegetative replication. Mol. Microbiol., 6, 907–920. [DOI] [PubMed] [Google Scholar]
- 33.Jagura-Burdzy G., Macartney,D.P., Zatyka,M., Cunliffe,L., Cooke,G.D., Huggins,C., Khanim,F. and Thomas,C.M. (1999) Repression at a distance by the global regulator KorB of promiscuous IncP plasmids. Mol. Microbiol., 32, 519–532. [DOI] [PubMed] [Google Scholar]
- 34.Wilkins B.M., Chilley,P.M., Thomas,A.T. and Pocklington,M.J. (1996) Distribution of restriction enzyme recognition sequences on broad host range plasmid RP4: molecular and evolutionary implications. J. Mol. Biol., 258, 447–456. [DOI] [PubMed] [Google Scholar]
- 35.Balzer D., Ziegelin,G., Pansegrau,W., Kruft,V. and Lanka,E. (1992) KorB protein of promiscuous plasmid RP4 recognizes inverted sequence repetitions in regions essential for conjugative plasmid transfer. Nucleic Acids Res., 20, 1851–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams D.R., Motallebi-Vashareh,M. and Thomas,C.M. (1993) Multifunctional repressor KorB can block transcription by preventing isomerization of RNA polymerase-promoter complexes. Nucleic Acids Res., 21, 1141–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kostelidou K. and Thomas,C.M. (2000) The hierarchy of KorB binding at its 12 binding sites on the broad-host-range plasmid RK2 and modulation of this binding by IncC1 protein. J. Mol. Biol., 295, 411–422. [DOI] [PubMed] [Google Scholar]
- 38.Williams D.R., Macartney,D.P. and Thomas,C.M. (1998) The partitioning activity of the RK2 central control region requires only incC, korB and KorB-binding site O(B)3 but other KorB-binding sites form destabilizing complexes in the absence of O(B)3. Microbiology, 144, 3369–3378. [DOI] [PubMed] [Google Scholar]
- 39.Jagura-Burdzy G., Kostelidou,K., Pole,J., Khare,D., Jones,A., Williams,D.R. and Thomas,C.M. (1999) IncC of broad-host-range plasmid RK2 modulates KorB transcriptional repressor activity in vivo and operator binding in vitro. J. Bacteriol., 181, 2807–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas C.M. and Smith,C.A. (1986) The trfB region of broad host range plasmid RK2: the nucleotide sequence reveals incC and key regulatory gene trfB/korA/korD as overlapping genes. Nucleic Acids Res., 14, 4453–4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pansegrau W., Lanka,E., Barth,P.T., Figurski,D.H., Guiney,D.G., Haas,D., Helinski,D.R., Schwab,H., Stanisich,V.A. and Thomas,C.M. (1994) Complete nucleotide sequence of Birmingham IncP alpha plasmids. Compilation and comparative analysis. J. Mol. Biol., 239, 623–663. [DOI] [PubMed] [Google Scholar]
- 42.Cervin M.A., Spiegelman,G.B., Raether,B., Ohlsen,K., Perego,M. and Hoch,J.A. (1998) A negative regulator linking chromosome segregation to developmental transcription in Bacillus subtilis. Mol. Microbiol., 29, 85–95. [DOI] [PubMed] [Google Scholar]
- 43.Rosche T.M., Siddique,A., Larsen,M.H. and Figurski,D.H. (2000) Incompatibility protein IncC and global regulator KorB interact in active partition of promiscuous plasmid RK2. J. Bacteriol., 182, 6014–6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas C.M. and Hussain,A.A.K. (1984) The korB gene of broad host range plasmid RK2 is a major copy number control element which may act together with trfB by limiting trfA expression. EMBO J., 3, 1513–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kahn M.R., Kolter,R., Thomas,C.M., Figurski,D., Meyer,R., Remault,E. and Helinski,D.R. (1979) Plasmid cloning vehicles derived from plasmids ColE1, F, R6K and RK2. Methods Enzymol., 68, 268–280. [DOI] [PubMed] [Google Scholar]
- 46.Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual, 2nd Edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 47.Jagura-Burdzy G. and Thomas,C.M. (1992) Crosstalk between plasmid vegetative replication and conjugative transfer: repression of the trfA operon by trbA of broad host range plasmid RK2. Nucleic Acids Res., 20, 3939–3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jagura-Burdzy G., Ibbotson,J.P. and Thomas,C.M. (1991) The korF region of broad-host-range plasmid RK2 encodes two polypeptides with transcriptional repressor activity. J. Bacteriol., 173, 826–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Studier F.W. and Moffatt,B.A. (1981) Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol., 189, 113–130. [DOI] [PubMed] [Google Scholar]
- 50.Mullis K., Faloona,F., Scharf,S., Saiki,R., Horn,G. and Erlich,H. (1986) Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harb. Symp. Quant. Biol., 51, 263–273. [DOI] [PubMed] [Google Scholar]
- 51.Jagura-Burdzy G. and Thomas,C.M. (1994) KorA protein of promiscuous plasmid RK2 controls a transcriptional switch between divergent operons for plasmid replication and conjugative transfer. Proc. Natl Acad. Sci. USA, 91, 10571–10575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jagura-Burdzy G. and Thomas,C.M. (1995) Purification of KorA protein from broad host range plasmid RK2: definition of a hierarchy of KorA operators. J. Mol. Biol., 253, 39–50. [DOI] [PubMed] [Google Scholar]
- 53.Zukowski M.M., Gaffney,D.F., Speck,D., Kauffman,M., Findeli,A., Wisecup,A. and Lecoq,J.P. (1983) Chromogenic identification of genetic regulatory signals in Bacillus subtilis based on expression of a cloned Pseudomonas gene. Proc. Natl Acad. Sci. USA, 80, 1101–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gornall A.G., Bardwell,C.J. and David,M.M. (1949) Determination of serum proteins by means of the biuret reaction. J. Biol. Chem., 177, 751–766. [PubMed] [Google Scholar]
- 55.Arnold F.H. (1991) Metal-affinity separations: a new dimension in protein processing. Biotechnology, 9, 151–156. [DOI] [PubMed] [Google Scholar]
- 56.Lobocka M. and Yarmolinsky,M. (1996) P1 plasmid partition: a mutational analysis of ParB. J. Mol. Biol., 259, 366–382. [DOI] [PubMed] [Google Scholar]
- 57.Funnell B.E. (1991) The P1 plasmid partition complex at parS. The influence of Escherichia coli integration host factor and of substrate topology. J. Biol. Chem., 266, 14328–14337. [PubMed] [Google Scholar]