Abstract
Background and objectives
Since the outbreak of COVID-19, more and more studies have proved that it has an impact on the male reproductive system. The purpose of this article is to investigate the effects of COVID-19 on male semen parameters, further analyze the incidence and risk factors of diseases related to semen parameters, and put forward to corresponding preventive measures.
Methods
Retrieved from PubMed, Embase, The Cochrane Library, Web of Science, Clinicaltrails, CNKI, CBM, Wanfang Database and VIP to collect research on the effects of COVID-19 on the male reproductive system. The literature search was conducted until January 2022. In strict accordance with the inclusion and exclusion criteria, two researchers independently screened the literature and comprehensively analyzed five cohort studies on the impact of COVID-19 on male reproductive system. The Newcastle-Ottawa Scale (NOS) was used to evaluate the quality of the included cohort studies, and Revman 5.4.1 was applied for statistical analysis.
Results
Semen volume (RR = −0.10; 95% CI: 0.45, 0.26; P = 0.60), there was no significant difference between the test group and the control group; Sperm count (RR = −45.28; 95% CI: 66.38, 24.19; P = 0.0001), the sperm count of the COVID-19 test group was lower than that of the control group, and the difference was statistically significant; Sperm concentration (RR = −15.65 × 106; 95%CI: 31.52 × 106, 0.21 × 106; P = 0.05), there was no significant difference between the test group and the control group; progressive sperm motility (RR = 4.31; 95% CI: 4.62, 13.24; P = 0.34), there was no effect on progressive sperm motility in the COVID-19 test group compared with the control group.
Conclusions
COVID-19 can reduce semen quality and affect male fertility. However, due to the limitations of this study, this conclusion needs to be further verified by large-sample, high-quality prospective cohort studies on the long-term effects of COVID-19 on male reproductive function.
Keywords: COVID-19, Semen volume, Sperm count, Sperm concentration, Male fertility
1. Introduction
In December 2019, The Coronavirus Disease 2019 (COVID-19) broke out in Wuhan, Hubei Province, and then spread rapidly around the world in a brief period. As of March 7, 2022, COVID-19 has confirmed more than 440 million cases worldwide, with a cumulative death rate of more than 6 million. At present, there is still controversy at home and abroad about whether COVID-19 has an impact on the male reproductive system. Sacha [1] et al. concluded that COVID-19 had essentially no effect on semen parameters in patients; the trial by Scroppo [2], Best [3] et al. showed that semen samples from patients diagnosed with COVID-19 were tested and ultimately showed a reduction in sperm concentration, sperm count, and progressive sperm motility. With the opening of the third-child policy, whether it will have an impact on male reproduction is also an urgent concern to people. Given the potential impact of COVID-19 on the male reproductive system and unknown outcomes, it is necessary to determine reproductive outcomes in male patients with clinical symptoms or confirmed COVID-19. In this study, we collected published literature through an evidence-based medicine approach to objectively evaluate the effects of COVID-19 on semen parameters and develop appropriate defensive measures to provide a high-quality basis for their primary preventive care services.
2. Methods
2.1. Scheme and registration
Registered with PROSPERO as CRD42020181812.
2.2. Search strategy
PubMed, Embase, The Cochrane Library, Web of Science, Clinicaltrails, CNKI, CBM, Wanfang Database and VIP database were searched by computer. All database documents are from inception to May 2021. Using the combination of free words and subject words, the search language is not limited. Search words include: SARS-COV-2, COVID-19, male reproductive system, semen, spermatozoa, etc. The search strategy in The Cochrane Library as shown in the box1.
2.3. Inclusion and exclusion criteria
Inclusion criteria: (1) Published literature or ongoing clinical trials data; (2) Cohort studies or case-control studies with COVID-19 and control groups, whether or not double-blind; (3) Patients in the experimental group were at the stage of COVID-19 infection or recovery. The control group was non-COVID-19 normal healthy people; (4) The variable detection methods in literature were consistent.
Exclusion criteria: (1) No control group or poor balance between groups, no comparability; (2) The research contents are inconsistent or do not meet the inclusion criteria; (3) Cross-sectional studies, reviews, experience summaries, theoretical discussions, experimental animal studies, case reports, and repeated published studies; (4) The experimental groups or control groups with diseases or treatments related to the reproductive system, such as vasectomy history, infertility history, etc.; medications that affect semen production, such as testosterone or hormone therapy; received chemotherapy, such as immunosuppressant drugs. (5) The full text cannot be obtained or the outcome indicators are inconsistent; (6) No valid data were extracted or there were obvious errors in the data.
2.4. Literature quality assessment
The Newcastle-Ottawa Scale (NOS) was used to evaluate the quality of the literature of prospective cohort studies, which mainly consisted of the following three aspects: (1) The selection of the study population (4 points): ①whether the exposure group is representative: 1 point from a random sample of the general population, no points from special groups, such as nurses, volunteers, etc.; ②selection of non-exposed group: 1 point for being from the same population as the exposed group, otherwise no points; ③confirmation of exposure: 1 point for patients diagnosed with COVID-19 by definite nasopharyngeal swabs or oral specimens receive; otherwise no points; ④1 point for absence of urinary tract disease and COVID-19 symptoms prior to follow-up, otherwise no points. (2) Comparability between groups (2 points): whether the relevant confounding factors were adjusted or not: 2 points for adjusting age and important confounding factors related to the disease, 1 point for adjusting age or important confounding factors related to the disease, and no points for not adjusting either. (3) Result evaluation (3 points): ①evaluation of outcome: 1 point for clear recording of semen parameter data, otherwise no score; ②follow-up years: 1 point for more than 60 days of follow-up, otherwise no points; ③cohort population lost visit rate: less than 25% scored 1 point, otherwise no score. The results of the literature scoring are shown in Table 2. NOS scores out of 9, with 0 to 3, 4 to 6, and 7 to 9 being low, medium, and high quality studies in that order.
Table 2.
NOS scores for the included studies.
2.5. Literature screening and data extraction
The data will be collected and screened independently by two evaluators. if there are differences, they will be resolved through discussion and judged by the third researcher if necessary. The extraction contents included publication year, first author, study site, total number of patients in control group and experimental group, mean age, clinical symptoms, BMI (body mass index; normal range: 18.5–23.9), outcome indicators, NOS score and so on. After extracting the data, the third researcher will check the extracted results, and if there is any difference in the data, they can be handled through group discussions or consultation with professional statisticians.
2.6. Statistical methods
Five articles were grouped and analyzed according to the type of outcome index. Calculation of the combined RR value and 95% CI,two-side P-value <0.05 was considered statistically significant. Q and I2 were used to assess heterogeneity between studies. I2 ≤ 50% or P ≥ 0.1 demonstrated no significant heterogeneity, and a fixed-effects model was used. I2>50% or P<0.1 indicated a significant heterogeneity, and a random-effects model was applied. For data that cannot be analyzed using meta-analysis, descriptive statistics are used directly. Funnel plots were used to assess publication bias. Review Manager (RevMan)5.4.1 software was used for all statistical analyses.
3. Results
3.1. Screening process and eligible studies
A total of 1119 studies were included, and 398 duplicate articles were excluded. After title and abstract screening, 476 studies were excluded. After full-text screening, an additional 240 studies were excluded due to the following criteria: review articles (n = 137), reporting incomplete data (n = 23), review articles (n = 35), and full-text articles (n = 45). Finally, five prospective cohort studies were included in this meta-analysis [[3], [4], [5], [6], [7]], and all languages were in English. The flow chart of the study selection is summarized in Fig. 1.
Fig. 1.
Summary of the literature identification and selection process. ∗ signifies PubMed = 244, Cochrane library = 28, Embase = 534, Web of science = 173, Clinicaltrails = 0, CBM = 16, CNKI = 21, Wanfang = 89,VIP = 14.
3.2. Study characteristics
A total of five studies were included in this meta-analysis, including two from China, one from the US, one from Germany, and one from Turkey. A summary of the included studies is presented in Table 1.
Table 1.
Basic characteristics of the included literature.
| Study | Study design | Study location | Number (experimental/control) | Age (experimental/control) | BMI (experimental/control) | Clinical characterristics (percentage) | Duration of Symptoms, d |
|---|---|---|---|---|---|---|---|
| Best 2021 [3] | Prospective cohort study | the US | 30/30 | 40/42 | 26.25 NA |
abcde | 12 ± 8.97 |
| Holtmann 2020 [4] | Prospective cohort study | Germany | 14/14 | 42.2 ± 9.9 33.4 ± 13.1 |
25.6 ± 2.9 24.6 ± 2.6 |
abcde | 8.6 ± 9.1 |
| Temiz 2021 [5] | Prospective cohort study | Turkey | 10/10 | 38.00 ± 8.28 36.64 ± 9.63 |
25.5 ± 2.08 26.5 ± 2.71 |
NA | NA |
| Guo 2021 [6] | Prospective cohort study | China | 41/50 | 26.0/265 | NA | acd | NA |
| Li 2020 [7] | Prospective cohort study | China | 23/22 | 40.8 ± 8.5 40.5 ± 5.9 |
25.1 ± 4.51 NA |
a | NA |
|
Study |
Comorbidity (number) | Time between positive oropharyngeal swab and semen collection, d | Time between end of symptoms and semen collection, d | Outcome index |
|---|---|---|---|---|
| Best 2021 [3] | NA | 37 | NA | ①②③ |
| Holtmann 2020 [4] | none | 43.5 ± 6,2 | 34.9 ± 11.7 | ①②③④ |
| Temiz 2021 [5] | diabetes (1) | NA | NA | ①②③④ |
| Guo 2021 [6] |
diabetes (1) | 73 ± 10 | 56 ± 17.03 | ①②③④ |
| Li 2020 [7] |
NA | 25.8 | NA | ③ |
BMI: body mass index.
a: fever; b: testicular pain; c: cough; d: dyspean; e: chills.
NA: (not applicable).
①: semen volume; ②: sperm count; ③: sperm concentration; ④: progressive sperm motility.
3.3. Quality evaluation of the included studies
The included articles were all prospective cohort studies, and their risk of bias was evaluated according to the NOS scale. Five of the included literatures had the study score of ≥6 stars, and the quality of the included studies was high. The specific scores are shown in Table 2.
4. Meta-analysis results
4.1. A meta-analysis of the relationship between COVID-19 and semen volume
In this study, there were four literatures about the effect of COVID-19 on male semen volume (normal range: 2–6 mL) [[3], [4], [5], [6]], and the results suggested that there was no heterogeneity among the studies (I2 = 0%, P = 0.74), which was analyzed by fixed effect model. RR = −0.10 [−0.45, 0.26], Z = 0.52, P = 0.60>0.05. It was suggested that the semen volume of men in the COVID-19 test group was lower than that in the control group, but the difference was not statistically significant. The results of Meta analysis are shown in Fig. 2.
Fig. 2.
Forest plot between COVID-19 and semen volume.
4.2. A meta-analysis of the relationship between COVID-19 and sperm count
Sperm count is an important indicator to evaluate male fertility. In this study, four literatures were compared on the influence of male sperm (normal range: 33-46 × 106)count [[3], [4], [5], [6]], with small heterogeneity among studies (I2 = 12%, P = 0.33). Meta-analysis was conducted using fixed effect model, and the results showed that: RR = −45.28 [−66.38, −24.19], Z = 4.21, P = 0.0001 < 0.05, suggesting that the sperm count of the COVID-19 test group decreased compared with the control group, and the difference was statistically significant. The meta-analysis results are shown in Fig. 3.
Fig. 3.
Forest plot between COVID-19 and sperm count.
4.3. A meta-analysis of the relationship between COVID-19 and sperm concentration
A total of 5 articles reported the effect of COVID-19 on sperm concentration (normal range:12-16 × 106/mL) [[3], [4], [5], [6], [7]]. The heterogeneity between the results of the study is large (I2 = 72%, P = 0.006), which is analyzed by random effect model. RR = − 15.65 × 106 [- 31.52 × 106, 0.21 × 106], Z = 1.93, P = 0.05, suggesting that the sperm concentration of the COVID-19 test group was lower than that of the control group, but the difference was not statistically significant. The results of Meta analysis are shown in Fig. 4.
Fig. 4.
Forest plot between COVID-19 and sperm concentration.
4.4 A meta-analysis of the relationship between COVID-19 and progressive sperm motility. Progressive sperm motility (normal range: >32%) is one of the important indicators to ensure normal sperm survival and fertilization ability. Three studies compared the effect of forward movement between the experimental group and the control group of COVID-19 [[4], [5], [6]]. There was heterogeneity among studies (I2 = 66%, P = 0.05). The random effects model was used for meta-analysis, and the results showed that RR = −1.57 [−12.09, 8.96], Z = 0.29, P = 0.77 > 0.05, suggesting that the progressive sperm motility in experimental group decreased compared with control group, but the difference was not statistically significant. The meta-analysis results are shown in Fig. 5.
Fig. 5.
Forest plot between COVID-19 and progressive sperm motility.
4.5. Sensitivity analysis
In the meta-analysis of the effects of COVID-19 on male semen parameters, 5 studies compared the sperm concentration of the experimental group and the control group [[3], [4], [5], [6], [7]], and heterogeneity test suggested that there was a large heterogeneity between the included studies (I2 = 72%, P = 0.006). The random-effect model was used for meta-analysis, and the results showed that: RR = −15.65 × 106 [−31.52 × 106, 0.21 × 106], Z = 1.93, P = 0.05, indicating that there was no significant difference between the experimental group and the control group. After excluding the included literature one by one, none of the study results showed significant changes, suggesting that the study results are stable and COVID-19 may not have an effect on sperm concentration.
Three studies compared progressive motility between the experimental group and the control group [[4], [5], [6]], and heterogeneity test suggested that there was a large heterogeneity among the included studies (I2 = 66%, P = 0.05). Meta-analysis was conducted using the random effect model, and the results showed that: RR = −1.57 [−12.09, 8.96], Z = 0.29, P = 0.77, indicating that there was no statistically significant difference between the experimental group and the control group. After eliminating the included literatures one by one, it was found that the results of Guo [6]et al. had a great impact on heterogeneity, and the heterogeneity was reduced after eliminating the study (I2 = 0%, P = 0.96), and the fixed-effect model was used for meta-analysis. The results showed that RR = 4.31 [−4.62, 13.24], Z = 0.95 P = 0.34, suggesting that COVID-19 may not affect progressive sperm motility, which is a robust and reliable conclusion. The meta-analysis results are shown in Fig. 6.
Fig. 6.
Forest map after sensitivity analysis of COVID-19 and progressive sperm motility.
4.6. Test of publication bias
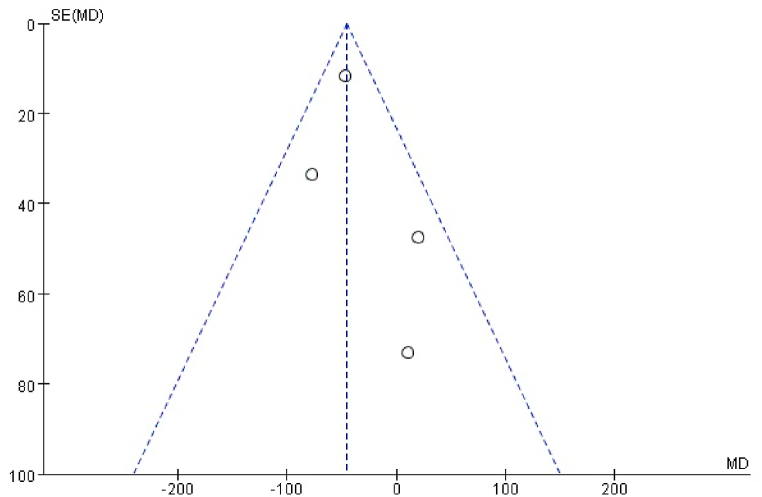
Funnel plot was used to analyze publication bias. A small number of meta-analysis studies were included in this study, and the research indicators were different, resulting in a further decrease in the number of included studies. The influence of COVID-19 on sperm count was selected to draw a funnel diagram, the distribution in the figure is uneven, as shown in Fig. 7. Considering the small number of studies in the included articles, the small sample size and regional differences, so we think the risk of publication bias is high.
Fig. 7.
Funnel plot of the influence of the included literature on sperm count.
5. Discussion
Studies have shown that severe Acute Respiratory syndrome Coronavirus 2 (SARS-COV-2) has a strong binding affinity with angiotensin-converting enzyme 2 (ACE2) [8]. Transmembrane protease serine 2 (TMPRSS2) cleaves ACE2 protein and promotes cell invasion [9], leading to SARS-COV-2 endocytosis. ACE2 was highly expressed in renal tubular epithelial cells, testicular seminiferous tubules, interstitial cells, adrenal gland and prostate [10], while TMPRSS2 was enriched in kidney, prostate, seminal vesicle and epididymis [11]. It suggests that the testis is a highly susceptible organ to SARS-CoV-2 infection, and SARS-CoV-2 can cause substantial damage to testicular tissue, leading to destruction of testicular cells and impaired spermatogenesis, impairing male fertility and eventually possibly causing male infertility. Semen parameter detection is the primary method to evaluate male fertility. In this study, the effects of COVID-19 on male semen parameters were systematically summarized by systematic evaluation method, and semen volume, sperm count, sperm concentration and progressive sperm motility were selected for meta analysis. The final results showed that the sperm count in the COVID-19 test group decreased, and the difference was statistically significant. The semen volume and sperm concentration of experimental group reduced, but the difference was not statistically significant. Compared with the control group, the progressive sperm motility basically had no effect, it suggests that COVID-19 can affect semen quality and reduce fertility in men.
Patients with COVID-19 often have clinical symptoms such as high fever, testicular and epididymal pain, scrotal discomfort and so on [12,13]. It has been shown that high fever or testicular pain may reduce the quality of semen parameters and damage sperm DNA integrity [14], affecting fertility [4,14,15]. In the literature of Best [3], Guo [6], Li [7]et al., some patients in the experimental group had clinical symptoms such as fever, but the semen parameters of symptomatic and asymptomatic patients were not compared, and it was not possible to verify whether semen parameters of patients with clinical symptoms differed from those of patients without clinical symptoms by subgroup analysis. In addition, the proportion of febrile patients was uneven across the test groups, and the reactions such as scrotal heat stress can make COVID-19 febrile patients likely to have lower levels of semen parameters than non-febrile patients. Therefore, when selecting study subjects from different overall, confounding factors such as clinical characteristics of patients should be noted to increase feasibility and scientific validity. In the sensitivity analysis of progressive sperm motility, the heterogeneity was greatly reduced after excluding the experimental data of Guo [6] et al. The experimental group Guo [6] included were patients in the recovered stage. And compared with the data of the two groups in the other acute active stage, semen parameters of patients in the convalescent stage increased the heterogeneity between studies. At the time of study inclusion, the time frame could be determined based on the duration of infection in COVID-19 patients as well as the sperm production cycle.
In Hajizadeh [16] et al. ‘s trial, the disruption of COVID-19 to the male reproductive system remained undiminished at 60 days of follow-up. In the experiment of Guo [6] et al., in which semen from 22 patients with COVID-19 were tested 84 days after recovery from hospital discharge, the total sperm count and sperm concentration of the patients were higher than in the first sampling, indicating that the sperm count may recover, but whether it is reversible and the duration of recovery is unknown. The spermatogenesis cycle is about 70 days. For families with fertility requirements, COVID-19 male patients should undergo regular semen parameter examinations after at least 70 days of asymptomatic recovery, with a follow-up of more than six months. It was suggested that sarS-COV-2 induced inflammation or oxidative stress in male reproductive system may be related to immune mechanism. Testicular inflammation or genital tract inflammation, etc., may lead to the production of anti-sperm antibodies. Therefore, for male patients with COVID-19, semen DFI detection is also a necessary choice, and it may also become a decisive factor in the examination of male infertility in the later stage [17].
This study has the following deficiencies: 1. The sample size of some experimental data is insufficient, and the mean ± standard( ‾x ± s)deviation of some research data is not directly provided. The data obtained after conversion may have certain errors. 2. There are limitations in this study. The different study indicators in different literature make it necessary to perform different meta-analyses when extracting data, resulting in a smaller number of papers included in each analysis and greater heterogeneity among studies, which reduces the credibility of the study results. 3. A small number of meta-analysis studies were included in this study, so only a funnel plot of sperm number was drawn to evaluate publication bias. Considering the small sample size and regional differences of the included studies, the risk of publication bias may be high. 4. Due to the limited number of clinical cases retrieved and the high heterogeneity of many research indicators in the article, there is some bias in the results. 5. No late follow-up data were available for all studies, it was impossible to know whether SARS-COV-2 would have long-term effects on male semen.
In summary, COVID-19 has a direct effect on male semen parameters and may threaten male fertility, but the long-term effects on patients and whether they are reversible are not yet known. Therefore, it is necessary for patients to undergo regular long-term semen routine and DFI tests after recovery. And further studies with higher quality and larger samples need to be included at a later stage to further reveal the effects of COVID-19 on the male reproductive system.
Ethics statement
None.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding
This research was supported by the National Natural Science Foundation of China (Grant no. 81973647); Sichuan Science and Technology Department Seedling project (Grant no. 2021JDRC0168); and Xinglin Scholar Research Premotion Project of Chengdu University of TCM (Grant no. QJJJ2021007).
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest’s statement
The authors declare no competing interests.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e14776.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Sacha C.R., Vagios S., Hammer K.C., Fitz V.W., Souter I., Bormann C.L. When and where during COVID-19: the effect of at-home semen collection on sperm parameters, fertilization rate, and blastocyst rate. Fertil. Steril. 2020;114(3):e555. doi: 10.1016/j.fertnstert.2020.09.110. [DOI] [Google Scholar]
- 2.Scroppo F.I., Costantini E., Zucchi A., Illiano E., Trama F., Brancorsini S., et al. COVID-19 disease in clinical setting: impact on gonadal function, transmission risk, and sperm quality in young males. J. Basic Clin. Physiol. Pharmacol. 2021 doi: 10.1515/jbcpp-2021-0227. [DOI] [PubMed] [Google Scholar]
- 3.Best J.C., Kuchakulla M., Khodamoradi K., Lima T.F.N., Frech F.S., Achua J., et al. Evaluation of SARS-CoV-2 in human semen and effect on total sperm number: a prospective observational study. World J. Men's Heal. 2021;39 doi: 10.5534/WJMH.200192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holtmann N., Edimiris P., Andree M., Doehmen C., Baston-Buest D., Adams O., et al. Assessment of SARS-CoV-2 in human semen-a cohort study. Fertil. Steril. 2020;114(2):233–238. doi: 10.1016/j.fertnstert.2020.05.028. Epub 2020/07/12. PubMed PMID: 32650948; PubMed Central PMCID: PMCPMC7256599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Temiz M.Z., Dincer M.M., Hacibey I., Yazar R.O., Celik C., Kucuk S.H., et al. Investigation of SARS-CoV-2 in semen samples and the effects of COVID-19 on male sexual health by using semen analysis and serum male hormone profile: a cross-sectional, pilot study. Andrologia. 2021;53(2) doi: 10.1111/and.13912. Epub 2020/11/28. PubMed PMID: 33244788; PubMed Central PMCID: PMCPMC7744846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo T.H., Sang M.Y., Bai S., Ma H., Wan Y.Y., Jiang X.H., et al. Semen parameters in men recovered from COVID-19. Asian J. Androl. 2021 doi: 10.4103/aja.aja_31_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li H.G., Xiao X.Y., Zhang J., Zafar M.I., Wu C.L., Long Y.T., et al. Impaired spermatogenesis in COVID-19 patients. EclinicalMed. 2020;28 doi: 10.1016/j.eclinm.2020.100604. PubMed PMID: WOS:000645903100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheikhzadeh Hesari F., Hosseinzadeh S.S., Asl Monadi Sardroud M.A. Review of COVID-19 and male genital tract. Andrologia. 2021;53(1) doi: 10.1111/and.13914. Epub 2020/11/26. PubMed PMID: 33236375; PubMed Central PMCID: PMCPMC7744899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agolli A., Yukselen Z., Agolli O., Patel M.H., Bhatt K.P., Concepcion L., et al. SARS-CoV-2 effect on male infertility and its possible pathophysiological mechanisms. Discoveries. 2021;9(2):e131. doi: 10.15190/d.2021.10. Epub 2021/11/25. PubMed PMID: 34816001; PubMed Central PMCID: PMCPMC8605861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu J., Zhou B., Zhang L., Balaji K.S., Wei C., Liu X., et al. Expressions and significances of the angiotensin-converting enzyme 2 gene, the receptor of SARS-CoV-2 for COVID-19. Mol. Biol. Rep. 2020;47(6):4383–4392. doi: 10.1007/s11033-020-05478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moshrefi M., Ghasemi-Esmailabad S., Ali J., Findikli N., Mangoli E., Khalili M.A. The probable destructive mechanisms behind COVID-19 on male reproduction system and fertility. J. Assist. Reprod. Genet. 2021;38(7):1691–1708. doi: 10.1007/s10815-021-02097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdel-Moneim A. COVID-19 pandemic and male fertility: clinical manifestations and pathogenic mechanisms. Biochemistry (Mosc.) 2021;86(4):389–396. doi: 10.1134/s0006297921040015. Epub 2021/05/05. PubMed PMID: 33941061; PubMed Central PMCID: PMCPMC7978437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J., Thomsen T., Sell N., Goldsmith A.J. Abdominal and testicular pain: an atypical presentation of COVID-19. Am. J. Emerg. Med. 2020;38(7):1542.e1. doi: 10.1016/j.ajem.2020.03.052. e3. Epub 2020/04/11. PubMed PMID: 32273141; PubMed Central PMCID: PMCPMC7138186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banihani S.A. Human semen quality as affected by SARS-CoV-2 infection: an up-to-date review. Andrologia. 2022;54(2) doi: 10.1111/and.14295. PubMed PMID: WOS:000712794800001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navarra A., Albani E., Castellano S., Arruzzolo L., Levi-Setti P.E. Coronavirus disease-19 infection: implications on male fertility and reproduction. Front. Physiol. 2020;11 doi: 10.3389/fphys.2020.574761. Epub 2020/12/15. PubMed PMID: 33312128; PubMed Central PMCID: PMCPMC7704452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hajizadeh Maleki B., Tartibian B. COVID-19 and male reproductive function: a prospective, longitudinal cohort study. Reproduction. 2021;161(3):319–331. doi: 10.1530/rep-20-0382. Epub 2021/02/02. PubMed PMID: 33522983. [DOI] [PubMed] [Google Scholar]
- 17.Haghpanah A., Masjedi F., Alborzi S., Hosseinpour A., Dehghani A., Malekmakan L., et al. Potential mechanisms of SARS-CoV-2 action on male gonadal function and fertility: current status and future prospects. Andrologia. 2021;53(1) doi: 10.1111/and.13883. Epub 2020/10/28. PubMed PMID: 33108833; PubMed Central PMCID: PMCPMC7645932. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.