Abstract
The outbreak of novel coronavirus disease 2019 (COVID-19), caused by the novel coronavirus (SARS-CoV-2), has had a significant impact on human health and the economic development. SARS-CoV-2 3CL protease (3CLpro) is highly conserved and plays a key role in mediating the transcription of virus replication. It is an ideal target for the design and screening of anti-coronavirus drugs. In this work, seven β-nitrostyrene derivatives were synthesized by Henry reaction and β-dehydration reaction, and their inhibitory effects on SARS-CoV-2 3CL protease were identified by enzyme activity inhibition assay in vitro. Among them, 4-nitro-β-nitrostyrene (compound a) showed the lowest IC50 values of 0.7297 µM. To investigate the key groups that determine the activity of β-nitrostyrene derivatives and their interaction mode with the receptor, the molecular docking using the CDOCKER protocol in Discovery Studio 2016 was performed. The results showed that the hydrogen bonds between β-NO2 and receptor GLY-143 and the π-π stacking between the aryl ring of the ligand and the imidazole ring of receptor HIS-41 significantly contributed to the ligand activity. Furthermore, the ligand-receptor absolute binding Gibbs free energies were calculated using the Binding Affinity Tool (BAT.py) to verify its correlation with the activity of β-nitrostyrene 3CLpro inhibitors as a scoring function. The higher correlation(r2=0.6) indicates that the absolute binding Gibbs free energy based on molecular dynamics can be used to predict the activity of new β-nitrostyrene 3CLpro inhibitors. These results provide valuable insights for the functional group-based design, structure optimization and the discovery of high accuracy activity prediction means of anti-COVID-19 lead compounds.
Keywords: SARS-CoV-2, 3CLpro inhibitor, β-nitrostyrene derivatives, Molecular docking, Receptor-ligand interaction, Binding Gibbs free energies
Graphical abstract
1. Introduction
The COVID-19 outbreak caused by SARS-CoV-2 has aroused an unprecedented medical and economic crisis worldwide and posed a huge challenge to the global health system [1,2]. It has been included in the list of priority diseases of the World Health Organization [3]. Since the outbreak of COVID-19, many efforts have been made to develop vaccines, drug treatments, in addition to physical isolation measures such as wearing masks to stop the spread of the disease. WHO reported that as of 21 October 2022, 172 COVID-19 vaccines were in clinical development and 199 were in preclinical development [4]. The SARS-CoV-2 surface spike (S) protein targeted vaccine is one of the most widely approved vaccines for COVID-19 prevention [5]. However, because the S protein takes account of a large number of mutations of COVID-19 (https://covdb.stanford.edu/page/mutation-viewer/#omicron), there are researchers worry about the mutation will essentially lead to vaccine-elicited immune escape [6,7], making the ability of the virus spreading stronger and reducing the vaccine efficacy [8]. Although vaccines of different generations have been developed and used, they have not prevented the spread of the newly appeared SARS-CoV-2 mutations [9].
Therefore, according to the common characteristics of SARS-CoV-2 mutations, some conservative targets that play key role in their replication cycle are selected to specifically inhibit their replication process, so as to develop broad-spectrum small-molecule anti-SARS-CoV-2 drugs that are effective against various mutations of SARS-CoV-2 to combat the rapid mutation of virus strains. Similar to other coronavirus, SARS-CoV-2 contains an important 3CL protease (3CLpro or Mpro), which processes its polyprotein and is conserved in various variants of SARS-CoV-2, making it a potential target for inhibitors of various variants of SARS-CoV-2 [10,11]. Besides, there is no protease similar to 3CLpro binding site in the human body, which ensures the high selectivity of inhibitor molecules. Therefore, the 3CL protease of SARS-CoV-2 can be used as an ideal target for the design and screening of anti-SARS-CoV-2 drugs. Currently, some 3CLpro inhibitors as anti-SARS-CoV-2 drugs have been approved for clinical treatment, such as Nirmatrelvir (PF-07321332) [12] and Ensitrelvir [13]. While both drugs have high 3CLpro inhibitory activity and good efficacy against COVID-19, Nirmatrelvir has metabolic stability issues [14] and needs to be used with ritonavir, and Ensitrelvir has reported that it might have teratogenic side effects [15].
Different functional groups have important effects on drug-receptor interaction [16]. In this regard, Andrews et al. [17] elaborated the contribution of different functional groups to drug-receptor interaction. He et al. predicted drug-target interaction networks based on functional groups and biological features [18]. In terms of the discovery of new 3CLpro inhibitors, Zhai et al. [19] found through calculation and experiment that pyrimidinetrione and quinoxaline were newly found functional groups in 3CLpro inhibitors, thus they are of high interest for lead optimization. Konno et al. [20] discovered that peptidomimetic compounds with a unique benzothiazolyl ketone as a warhead group, which display potent activity against 3CLpro of SARS-CoV-2. Bai et al. [21] reported that compounds of peptidomimetic nitrile warheads showed the potential to develop new generations of SARS-CoV-2 3CL protease inhibitors.
For newly discovered ligand structures that exhibit preliminary inhibitory activity against a target, lead compound optimization is often required to find derivatives with higher activity, which requires understanding of the ligand binding pose and interaction between ligand and target binding site [22]. In this regard, the crystal structure of the complex of target protein and small molecule inhibitor can provide the most intuitive view for the analysis of their interaction. Presently, there have been some studies on the crystal structure of the complex of 3CLpro and small molecule inhibitors of SARS-CoV-2 [11,[23], [24], [25], [26], [27]], which provides convenience for subsequent researchers to investigate the interaction between 3CLpro and the ligands for the optimization of the lead compounds. For many compounds synthesized by chemists, molecular docking can be used to obtain their predicted binding poses with the target protein quickly and cheaply, which facilitates the analysis of their interaction. There have been many reports on the molecular docking of 3CLpro of SARS-CoV-2 with small molecule inhibitors [28], [29], [30], [31]. However, although molecular docking is a quick and convenient means to study receptor-ligand interaction, the correlations between the ligand activity and the scores of scoring functions from different docking software are not ideal at present [32], [33], [34], which seriously affects the efficiency of lead compound optimization. In order to obtain more accurate predictive activity of ligands, people attempt to compute the binding Gibbs free energy of the receptor and ligand using molecular dynamics [35,36] through absolute free energy and relative free energy computing methods [37], [38], [39]. In general, the file preprocessing and system preparation of free energy calculation are very complicated, which limit the application of this method. The appearance of some operating procedure software, such as BFEE [40], PyAutoFEP [41] and BAT.py [42], simplifies the operation of free energy calculation. The application of free energy as scoring function in the prediction of ligand activity is expected to improve the prediction accuracy and accelerate the optimization process of lead compounds [43].
β-nitrostyrene derivatives are a class of compounds containing nitro group on the β-C of the double bond of styrene which are usually in trans configuration. Though they have been studied for their potential medical applications [44], [45], [46]. At present, there are no reports on the research of these compounds as 3CLpro inhibitors. In this work, a series of β-nitrostyrene derivatives were synthesized and their structures were characterized with 1H and 13C nuclear magnetic resonance(NMR), Fourier transform infrared spectroscopy (FTIR) and mass spectrum(MS). Their inhibitory effect on 3CL protease of SARS-CoV-2 was also examined in vitro. The molecular docking of β-nitrostyrene derivatives into 3CL protease binding site were studied. This study is of significance for the discovery of new functional groups with 3CLpro inhibition property and the subsequent optimization of lead compounds to obtain new SARS-CoV-2 drugs with high activity and low side effects.
2. Results and discussion
2.1. Chemistry
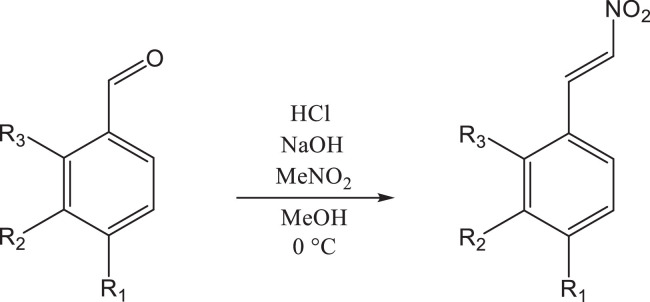
Since different substituents have important effects on the interaction between the compound and the target, a series of β-nitrostyrene derivatives have been synthesized. The substituents on the aryl ring contain the electron drawing substituents such as -NO2, -CN, -Br, -F and electron donating substituents such as –OCH3. As shown in Table 1 , the target compounds (a-g) with -CN, -NO2, -F, -Br and –OCH3 on the different position of the aryl ring, were synthesized by the Henry reaction and the subsequent β-dehydration reaction of nitroalcohols compounds in one pot [47] (Scheme 1) Because substituents changed the electron density distribution of β-nitrostyrene, the interaction of these compounds with 3CL protease were affected and thus their inhibitory activity to 3CL protease may also be changed. All synthesized compounds were purified by column chromatography and the structures were characterized and confirmed by 1H NMR, 13C NMR, FTIR, MS respectively.
Table 1.
Different substituents at positions R1-R3 in Scheme 1 for the synthesized β-nitrostyrene derivatives.
| Compound | Substituted Groups |
||
|---|---|---|---|
| R1 | R2 | R3 | |
| a | NO2 | H | H |
| b | H | H | Br |
| c | H | H | H |
| d | CN | H | H |
| e | OCH3 | H | H |
| f | OCH3 | OCH3 | H |
| g | F | H | H |
Scheme 1.
Synthesis of β-Nitrostyrene derivatives.
2.2. Characterization of the compound a-g
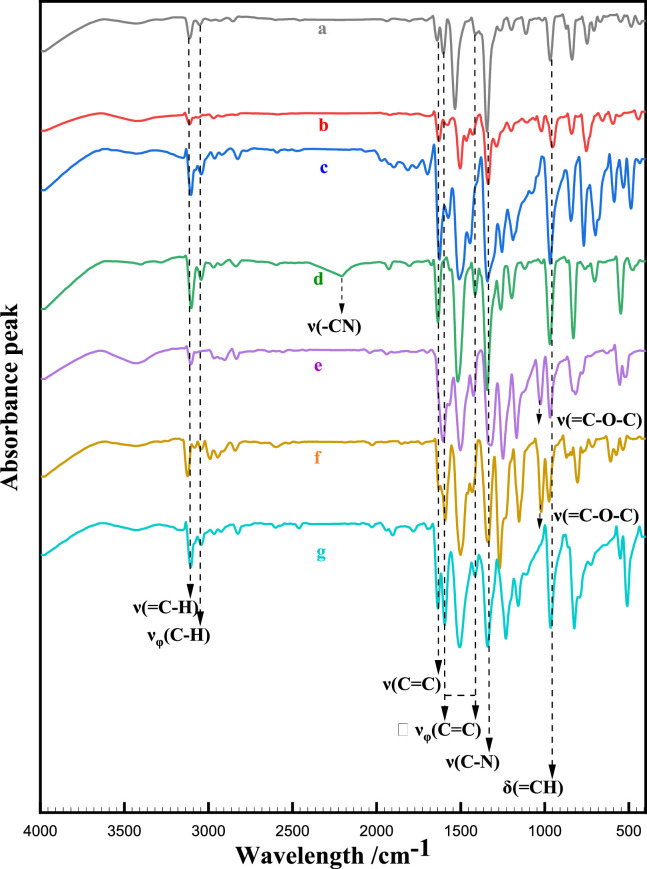
The structures of compounds a-g were confirmed through 1H NMR, 13C NMR, FTIR, and MS analyses. The results of FTIR spectroscopy are presented in Fig. 1 and Table 2 , while other structural characterization data can be found in the supporting information. It can be seen from Fig. 1 that all the compounds appear the narrow and sharp peaks in the range of 3100–3125 cm−1 comes from the stretching vibration of the =C—H bond υ(=C—H) on the alkenyl group connecting the aryl ring and the nitro group in compounds a-g [48,49]. The absorption peaks in the range of 960–975 cm−1 shows the out-of-plane bending of the =C—H of alkenyl bond δ(=C—H), and the absorption peak in the range of 1610–1645 cm−1 shows the stretching vibration of the alkenyl group υ(C=C) connected to the trans-substituted nitro group [50,51].
Fig. 1.
FTIR spectroscopy of compound a-g.
Table 2.
The FTIR results of β-nitrostyrene derivatives(cm−1).
| Compound | υ(=C—H) | δ(=C—H) | υ(C=C) | υφ(C=C) | υφ(C—H) | υ(C—N) | υ(-C N) | υ(=C—O-C) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| a | 3116 | 968 | 1645 | 1605 | 1595 | 1537 | 1414 | 3051 | 1344 | — | — |
| b | 3112 | 954 | 1633 | 1631 | 1582 | 1500 | 1463 | 3057 | 1344 | — | — |
| c | 3105 | 968 | 1627 | 1626 | 1577 | 1508 | 1450 | 3043 | 1344 | — | — |
| d | 3103 | 970 | 1635 | 1636 | 1562 | 1516 | 1419 | 3045 | 1342 | 2214 | — |
| e | 3105 | 968 | 1630 | 1608 | 1567 | 1506 | 1422 | 3043 | 1326 | — | 1029 |
| f | 3124 | 974 | 1629 | 1628 | 1595 | 1503 | 1432 | 3045 | 1342 | — | 1029 |
| g | 3107 | 966 | 1637 | 1593 | 1631 | 1501 | 1432 | 3041 | 1340 | — | — |
The weak absorption peak in the range of 3040–3057 cm−1, υφ(C—H) (φ represents the aryl ring structure), comes from the stretching vibration of C—H in the aryl ring of β-nitrostyrene compounds. The absorption peaks at 1450–1600 cm−1, υφ(C=C), come from the stretching vibration of the C = C bond in the aryl ring skeleton [52]. The strong absorption peak in the range of 1340–1350 cm−1, υ(C—N), comes from the stretching vibration of the C—N bond connecting the nitro group [53]. The absorption peak at 2214 cm−1, υ(-C N), in compound d comes from the stretching vibration of -C N. The absorption peak at 1029 cm−1 in compounds e and f, υ(=C—O-C), comes from the stretching vibration of the C—O-C bond in the methoxy ether group [53,54].
2.3. In vitro inhibitory activity analysis
The potential of β-nitrostyrene in therapeutic application has long been studied. Carter et al. [55,56] investigated the effects of β-nitrostyrene on cell proliferation and macrophage immune responses. The results showed that β-nitrostyrene derivatives could inhibit the proliferation of cancer cells and induce programmed cell death. Another study revealed the mechanistic activity of β-nitrostyrene derivative Cyt-Rx20 in preventing the growth of breast cancer cells at G2/M phase [57]. In addition, β-nitrostyrene derivatives have antibacterial and antifungal activities [58], and Milhazes et al. demonstrated the mechanistic pathway of antibacterial activity by studying the correlation between REDOX potential and antimicrobial activity of a series of β-nitrostyrene derivatives [59]. Another study conducted by Park and Pei showed that β-nitrostyrene derivatives could inhibit protein tyrosine phosphatases (PTPs) to interrupt cell signaling in microorganisms [58,60]. Alfarisi et al. screened β-nitrostyrene derivatives against Staphylococcus aureus, Pseudomonas aeruginosa, Candida albicans, and Aspergillus Niger [61]. However, there are no reports about their effects on SARA-CoV-2 virus.
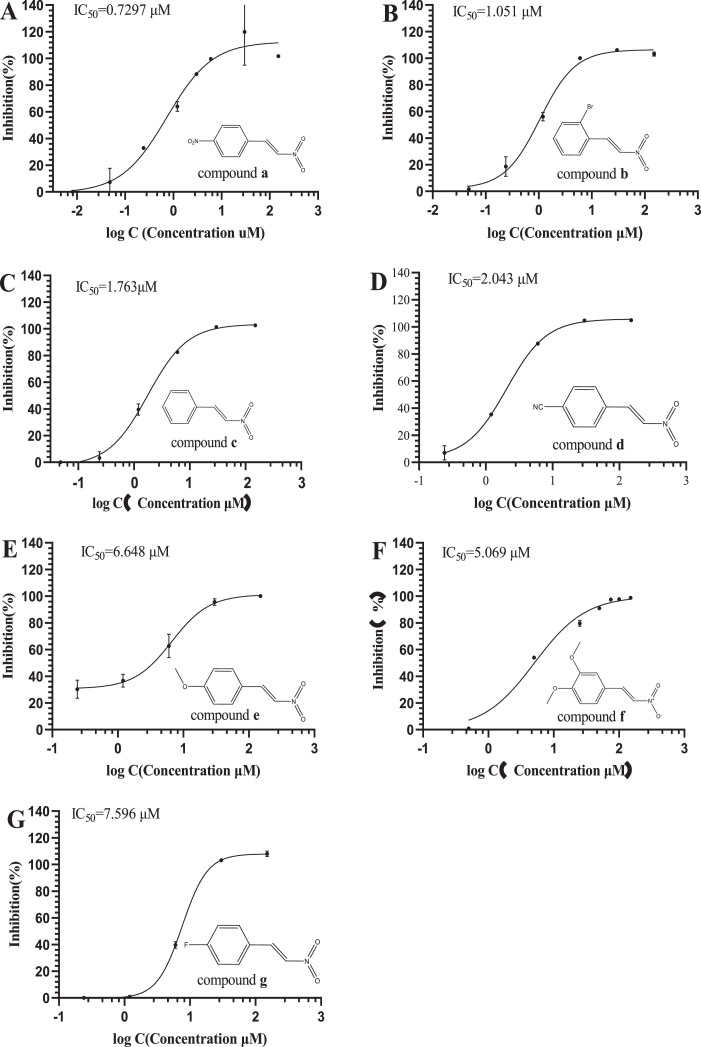
In this study, the inhibitory effect of β-nitrostyrene derivatives on 3CL protease activity of SARS-CoV-2 was evaluated in vitro, and the results are summarized in Fig. 2 150 µM was chosen for the initial concentration of all the synthesized compounds to evaluate their inhibitory activity to 3CL protease of SARS-CoV-2. Subsequently, half-maximal inhibitory concentrations (IC50) were assayed by serial dilution method using 2019-nCoV Mpro/3CLpro Inhibitor Screening Kit (#P0312S, Beyotime, China) with Ebselen as a positive control (IC50= 0.8000 µΜ). The results showed that 4-nitro-β-nitrostyrene (compound a) and 2‑bromo-β-nitrostyrene (compound b) have the higher inhibitory activity than β-nitrostyrene (compound c) without any substituents, however, other compounds such as 4-cyano-β-nitrostyrene (compound d), 4-dimethoxyl-β-nitrostyrene (compound e), 3,4-dimethoxyl-β-nitrostyrene (compound f) and 4-fluoro-β-nitrostyrene (compound g) have lower inhibitory activity than β-nitrostyrene (compound c). Among all seven compounds, 4-nitro-β-nitrostyrene (compound a) with strong electron-withdrawing substituent has the highest inhibitory activity(IC50= 0.7297 µΜ), while 4-fluoro-β-nitrostyrene with a similar electron-withdrawing substituent -F has the lowest activity. (IC50= 7.5960 µΜ) The reason needs further exploring, thus, the molecular docking of the seven compounds to the binding site of 3CL protease was carried out subsequently.
Fig. 2.
Dose-response curves for IC50 determination of compounds a-g were generated by nonlinear regression and are shown in pictures A-G, respectively.
2.4. Molecular docking analysis
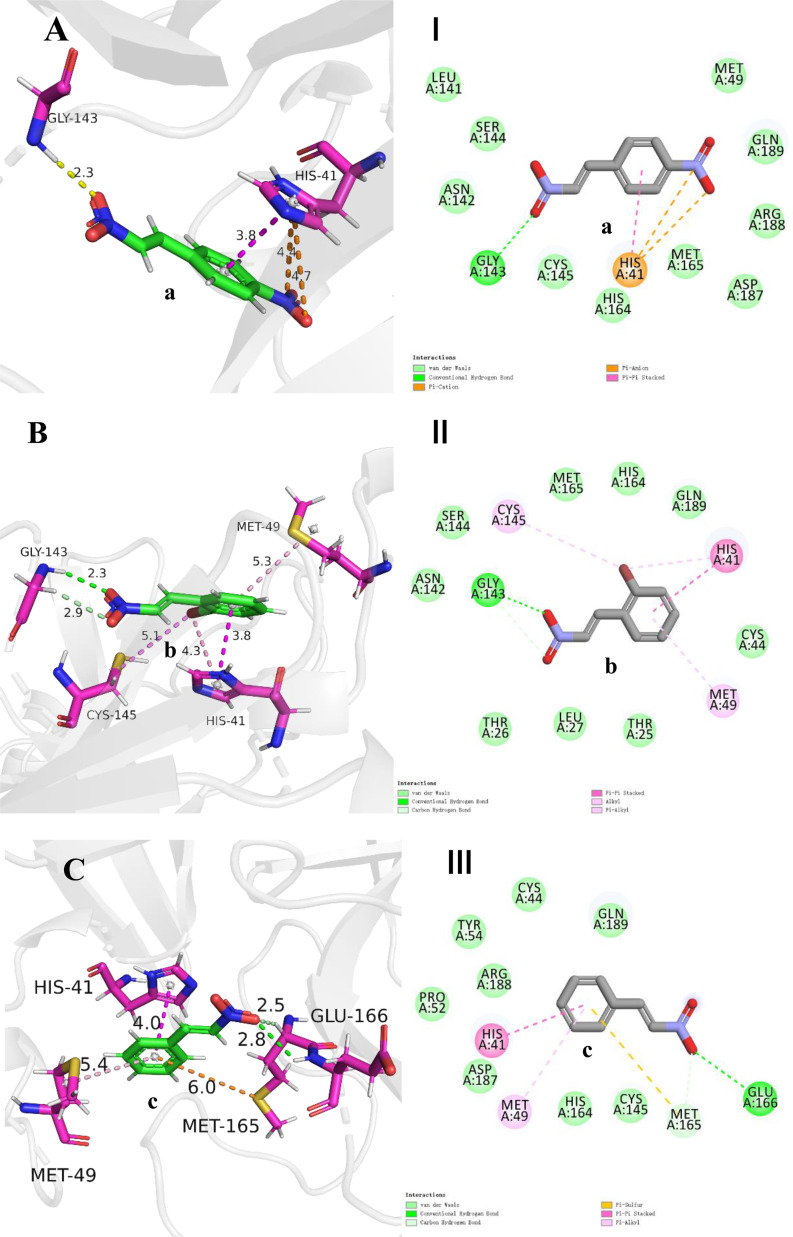
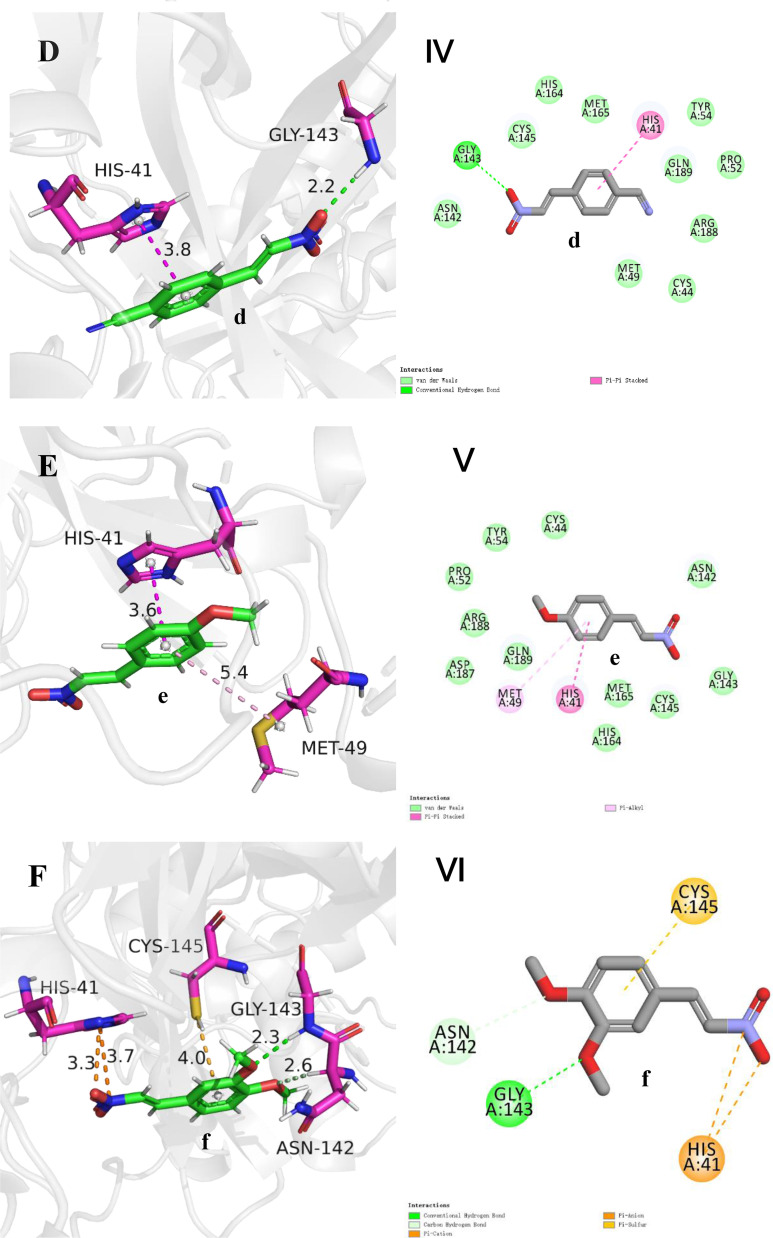
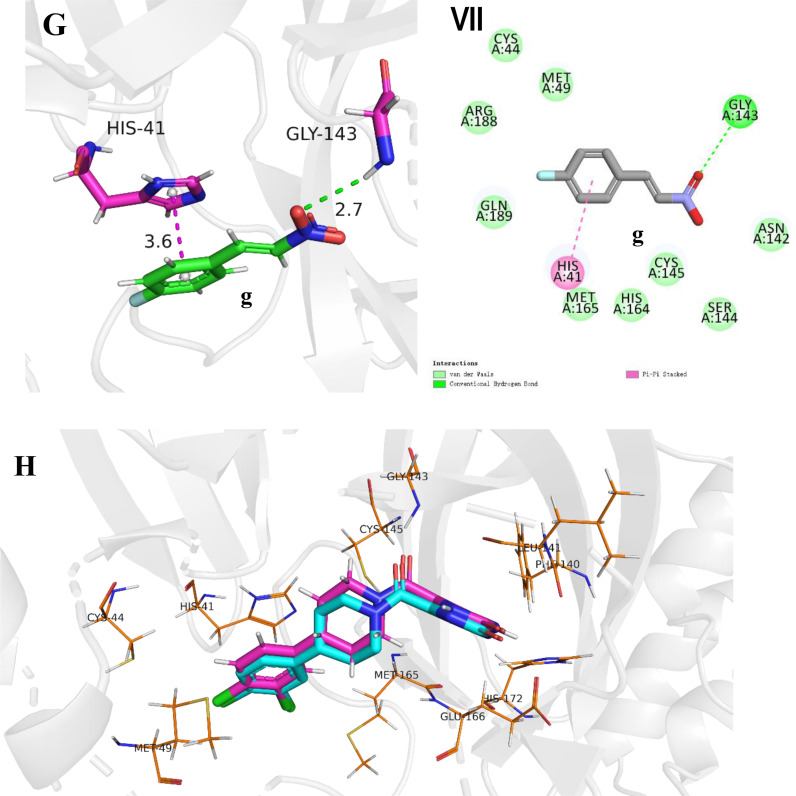
In order to explore the binding mode of different substrates to 3CL protease and to further understand the factors affecting the activity of the ligand. The molecular docking of compounds a-g to the 3CL protease was carried out using the CDOCKER module in Discovery Studio 2016 based on the scoring function of –CDOKER_ENERGY [62] with default docking calculation parameters. The top 10 docking poses sorted by the –CDOKER_ENERGY (kcal/mol) for each compound were output as the docking pose results. The pose with the highest –CDOKER_ENERGY was selected as the accepted best pose for each compound. The structure of 3CL protease (PDB ID: 7RN4) [23,62] was obtained from the protein data bank(https://www.rcsb.org/), and the ligands were docked into the receptor pockets occupied by inhibitor H69 in complex with 3CL protease in 7RN4 respectively. The top values of the docking function of –CDOKER_ENERGY (kcal/mol) of compounds a-g were listed in Table 3 and the corresponding docking poses were shown in Fig. 3 . In general, retrospective studies attest that docking can often recapitulate experimental ligand poses. CDOCKER is a molecular docking method based on CHARMm force field. For pose prediction, it usually gives a relatively ideal predictive pose. The reliability of the CDOCKER predictive pose has been evaluated by the root-mean-square deviation (RMSD) between the ligand poses from docking and those in ligand-receptor complex crystal structures by other people [63,64]. In this study, the retrospective studies attest was done by docking the ligand H69 to the original position in the crystal structure of the complex (PDB ID: 7RN4) and the result is shown in Fig. 3H. The RMSD is 0.5792 Å, which shows that the protocol and scoring function employed in this study can predict the pose of ligand in protein binding sites with high confidence, further, the geometries obtained from molecular docking can be used for the analysis of receptor-ligand interaction patterns. The binding forces of ligand and receptor generally include cation-anion interaction, hydrogen bond, van der Waals force, π-π interaction, π-alkyl interaction, cation-anion interaction, π-ion interaction and so on, among which cation-anion interaction, hydrogen bond and π-π interaction contribute more to the receptor-ligand non-covalent interaction [65,66]. The catalytic center of 3CL protease is a unit containing CYS145-HIS41 dimer [67]. It can be seen from Fig. 3 that the π-π interaction between aryl ring of β-nitrostyrene derivatives and imidazole ring of HIS-41 of the receptor is prevalent (π-S interaction not π-π interaction between aryl ring and -SH of CYS-145 for compound f). As an important hydrogen bond donor, nitro group has hydrogen bond interaction with N—H bond of LY-143 in most ligands, especially in ligands with higher activity, for examples, a strong hydrogen bond interaction exists in ligands a and b (in Fig. 3A and 3B, there is a shorter hydrogen bond length of 2.3 Å). However, there is a weaker hydrogen bond (bond length 2.7 Å) in compound g with low activity. For compound f, no hydrogen bond formed between -NO2 of the ligand and GLY-143, which may be the key reason for its low activity though other hydrogen bonds, such as those between –OCH3 and GLY-143, compensate parts for the absence of the former. The highest activity of compound a may also be due to the extra π-charge interaction between the -NO2 group on aryl ring and the imidazole of HIS-41 (Fig. 3A).
Table 3.
Scoring values of CDOCKER_ENERGY of 7 compounds using CDOCKER docking.
| Compounds | –CDOCKER_ENERGY (kcal/mol) | IC50 (µΜ) |
|---|---|---|
| a | 13.7900 | 0.7297 |
| b | 13.2580 | 1.0510 |
| c | 13.7804 | 1.7630 |
| d | 13.3979 | 2.0430 |
| e | 14.6718 | 6.6480 |
| f | 13.0223 | 5.0690 |
| g | 12.4077 | 7.5960 |
Fig. 3.
Binding modes between ligands and 3CL protease. Picture A-G show interactions between β-nitrostyrene derivatives and protein amino acids in 3D mode and distance between two atoms in unit Å. Picture I-VII show interactions between β-nitrostyrene derivatives and protein amino acids in 2D mode by DS 2016. Picture H show the results of docking the ligand H69 to the original structure of the complex (PDB ID:7RN4) for retrospective studies. The result of molecular docking is indicated in cyans, and the pose of H69 in the original crystal structure is indicated by magenta.
By analyzing the ligand molecular docking pose, we can qualitatively evaluate some interaction modes that affect the activity of ligand. If the activity of the ligand can be quantitatively predicted from molecular modeling, the accuracy and efficiency of virtual screening through molecular docking can be improved more effectively. However, there is often a poor correlation between the scores given by scoring functions and experimental binding affinities [64,68] Table 3 gives the scoring values of molecular docking of 7 compounds using CDOCKER docking. It can be seen that for the molecular docking of β-nitrostyrene derivatives to 3CLpro, the score (–CDOCKER_ENERGY) has poor correlation with the experimental activity (IC50), and the coefficient r2 is only 0.03. Therefore, like most other molecular docking scoring functions, it is not suitable to be used as scoring function to predict the inhibitory activity of β-nitrostyrene derivatives to 3CLpro directly. It is urgent to find ways to improve the reliability of prediction methods for searching new β-nitrostyrene derivatives with high 3CLpro inhibitor activity.
2.5. Binding gibbs free energy
Since the ligand-receptor binding Gibbs free energy obtained by molecular dynamics calculation has a better agreement with the experimental activity of ligand, in recent years, more and more people are carrying out virtual screening of drugs through the calculation of ligand-receptor binding Gibbs free energy [69,70]. Due to the complexity and hardworking of the configuration and preparation of the calculation of the binding Gibbs free energy, we adopted the process-based software BAT.py to simplify the process of the binding Gibbs free energy calculation. The Binding Affinity Tool (BAT.py) is designed by Heinzelmann and Gilson [71] using Python language for the fully automate absolute binding free energy(ABFE) calculations starting from a cocrystal structure or a docked complex of the ligand-protein complex. In our experiment, the structures of compounds a-g synthesized were docked into the binding site of 3CL protease (PDB ID: 7RN4) oriented by the original inhibitor of H69 with DS2016 CDOCKER protocol. BAT.py uses the pmemd.cuda module in AMBER18, which runs on Graphics Processing Units (GPUs) with a very high performance and a reduced cost (https://ambermd.org/GPUPerformance.php). It allows for free-energy-based high-throughput screenings of ligands to a given receptor, and can also be applied for parameter testing and optimization. The calculation of binding Gibbs free energy in the BAT.py is defined as follows:
| (1) |
In Eq. (1), the index refers to the attachment of restraints in the bound state, and represents the release of restraints with the ligand in bulk. The and refer to the ligand and the protein (receptor), respectively. denotes to the conformational restraints and is abbreviated for translational/rotational restraints. The term is the Gibbs free energy of transferring the ligand from the receptor binding site to bulk with all restraints applied. In our study, the double decoupling method (DD) [72] of the simultaneous decoupling was used.
Eq. (2) shows the double decoupling procedure:
| (2) |
The transfer free energy is equal to the sum of four terms in the equation. The index refers to decoupling, to electrostatic interactions and to Lennard-Jones interactions. These calculations were performed for the ligand in the binding site (bound) and in bulk (unbound) separately (the bulk calculations being performed with the ligand in a separate box).
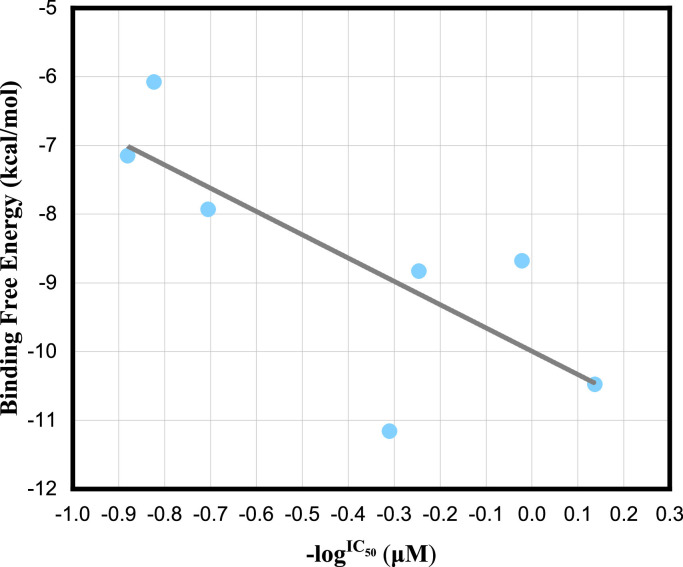
To ascertain the level of agreement between the binding Gibbs free energy and experiment inhibitory results, a linear regression between binding Gibbs free energy and -logIC50 of seven β-nitrostyrene derivatives is shown in Fig. 4 , which shows that higher binding Gibbs free energy correlates with stronger 3CL protease inhibition (coefficient r2=0.60). The binding Gibbs free energies ( ranged from 6.78 to 11.16 kcal/mol. Compound a with the lowest IC50 value has a good binding Gibbs free energy of 10.48 ± 1.47 kcal/mol, while compound g with the highest IC50 value has the minimal binding Gibbs free energy. It can be seen from the good correlation between the calculated binding Gibbs free energy and the experimental activity that it is a promising scoring function for the screening of β-nitrostyrene derivatives as 3CLpro inhibitors.
Fig. 4.
Correlation between the IC50 of seven inhibitors and binding Gibbs free energy. A linear regression shows that lower binding Gibbs free energy correlates with stronger 3CL protease inhibition.
3. Methods and materials
3.1. Reagents and instruments
4-nitro-benzaldehyde, 2‑bromo-benzaldehyde, benzaldehyde, 4-cyano-benzaldehyde, 4‑methoxy-benzaldehyde, 3, 4-benzal-dehyde, 4-fluoro-Benzaldehyde, sodium hydroxide, are all reagent pure. Anhydrous methanol, anhydrous ethanol, nitromethane, Chloroform-d and 36% HCl (g/g) are all analytical pure reagents. All the above reagents were purchased from HEOWNS (China). The water is distilled water. Deuterated chloroform and deuterated DMSO were purchased from CIL (America).
ZF-7 three-purpose ultraviolet analyzer was made in Shanghai Jiapeng Technology Co., LTD., China. RE-3000 rotary evaporation instrument was from Shanghai Yarong Biochemical Instrument Factory. AV400 nuclear magnetic resonance spectrometer (Brock Spectral Instruments, America) was employed for NMR analysis; The FTIR spectra were recorded in a FTIR spectrometer (BRUKER TENSOR 27) with the wavenumber of 4000 - 400 cm−1. Gas chromatography - mass spectrometry (GCMS-QP2010 Ultra, SHIMADZU, Japan) was used with a chromatographic column of 30 m length, 0.25 mm inner diameter, and 0.25 µm film thickness (Agilent DB5-MS). SpectraMax M2 multifunction microplate Reader, (Molecular Devices, USA.) was used to determine the 3CL protease inhibitory activity of the compounds.
3.2. Synthesis of β-nitrostyrene derivatives
All of the compounds listed in Table 1 were prepared according to the Scheme 1 and the detailed operations were described subsequently. All the structures of compound a-g were confirmed by 1H NMR, 13C NMR on Bruker 400 MHz spectrometers with TMS (δ=0.00) as internal standard.
After 4-nitro-benzaldehyde (2 g, 10.3 mmol), 2‑bromo-benzaldehyde (1 g, 4.3 mmol), benzaldehyde (3 g, 20.1 mmol), 4-cyano-benzaldehyde (2 g, 11.48 mmol), 4‑methoxy-benzaldehyde (1 g, 5.58 mmol), 3, 4-benzaldehyde (1 g, 4.78 mmol) and 4-fluoro-Benzaldehyde (1 g, 4.3 mmol) were solvated in 2 mL absolute methanol in 50 mL round-bottom flasks respectively, nitromethane (1.89 mL, 0.493 mL, 0.888 mL, 1.024 mL, 0.817 mL, 0.541 mL, 0.363 mL correspondingly) was added and the mixture was stirred for 0.1 h at room temperature. Subsequently, when the mixed system was fully cooled in the ice salt bath, the pre-cooled NaOH (10.5 mol/L, 0.65 mL) aqueous solution was slowly added and some white sediment formed. 0.5 mL of absolute methanol was supplemented to dilute the mixture under stirring. Thereafter, the reaction was performed in the ice salt bath for 5–10 min. 5 mL ice water was added to the reaction system, so that the sediment is dissolved. Subsequently, 10 mL pre-cooled HCl solution (:=2:5) was added as quickly as possible, so that a large amount of precipitation was produced. The precipitation was filtered and washed with absolute ethanol. Then it was filtered and dried overnight. Finally, it was purified by silica gel column chromatography (PE/EA) and recrystallized using absolute ethanol to give the object compounds.
3.3. FTIR
An appropriate amount of compounds a-g and KBr powder (with a mass ratio of about 1:150) were thoroughly ground in an agate mortar. Then, a suitable amount was uniformly spread into a mold, compressed into pellets, and placed on the sample holder for spectrum scanning in the range of 4000 - 400 cm−1.
3.4. GCMS
The GC was programmed to heat from 100 °C to 300 °C at a rate of 10 °C/min and hold for 5 min. The inlet temperature was set to 240 °C and the carrier gas (He) was controlled by constant pressure. The MS was operated in EI mode. The ion source temperature was set to 220 °C and the interface temperature was set to 240 °C. The solvent cut-off time was 3 min. The results of mass spectrometry (MS) analyses on compounds a-g are in Fig. S8. - Fig. S14. within the supporting information.
3.5. Molecular docking
3.5.1. Receptor preparation
Prior to docking analysis, crystal structure of SARS-CoV-2 3CL protease protein (PDB ID: 7RN4), downloaded from the website of protein data bank (https://www.rcsb.org/, accessed June 20, 2022.), was used for the docking study. The water molecules in the crystal were removed. The H atoms were added with Discovery Studio 2016 [73].
3.5.2. Ligand preparation
ChemBioDraw 14.0 was used to draw the two-dimensional structure of β-nitrostyrene derivatives, which was imported into ChemBioDraw 3D 14.0 software to construct the initial geometric configurations of seven molecules. The energy minimization calculation with the semi-empirical AM1 method in GAMESS [74] was performed on the structures of the seven β-nitrostyrene derivatives respectively, and the stable geometric configurations of the β-nitrostyrene derivatives were established. The optimization results were saved as sdf format and imported into Discovery Studio 2016 software for subsequent calculations.
3.5.3. Docking
The Discovery Studio 2016 software with CDOCKER protocol was used for docking, and seven β-nitrostyrene derivatives were docked into 3CL protease (PDB ID: 7RN4) respectively. In the pretreated receptor protein, the position of the original inhibitor in the crystal H69(6- [4-(3,4-dichlorophenyl) piperidine-1-carbonyl] pyrimidine-2,4(1H,3H)‑dione) was defined as the binding site and the coordinate (11.733625, −1.108042, 18.162292) as the docking center. A radius of 10.00 Å sphere was used to delimit the docking region of the ligand, in which the ligand takes the binding interaction with the relevant amino acid residues around the active pocket. Finally, CDOCKER produces 10 suitable docking positions with high docking scores for each ligand. Discovery Studio 2016 Client was employed to make the graphical images to show the interactions of potential residues with ligands. It also produces 2D images representing the forces stabilizing ligand molecules within the receptor binding pocket as Ⅰ-Ⅶ shown in Fig. 3 [75]. The best docking pose with the highest – CDOCKER_ENERGY was selected for binding Gibbs free energy calculations.
3.6. Binding Gibbs free energy calculation
The operating procedure software Binding Affinity Tool (BAT.py) designed by Heinzelmann and Gilson [71] using Python language was employed for computing the fully automate absolute binding free energy(ABFE) of β-nitrostyrene derivatives with 3CL protease of SARS-CoV-2. It uses AMBER molecular dynamics computing software as the background support, and uses pmemd.cuda module [76] running on GPU to complete the molecular dynamics calculation. The 3CLpro receptor structure was obtained from the H69–3CLpro co-crystal structure (PDBID: 7RN4) by removing the conformer, water and H69 ligand. The receptor was protonated with H++ website (http://newbiophysics.cs.vt.edu/H++/) at pH=7.2. The ligand poses of the β-nitrostyrene derivatives were from the molecular docking result of CDOCKER previously. The original protein sequence numbering was used here, using AMBER masks to define atom. The three anchor atoms of the receptor, P1, P2 and P3, were determined as :44@CA:21@CA and :87@CA respectively. Protein backbone dihedrals conformational restraints was employed by setting rec_bb = yes. The ligand anchor search parameters l1_x, l1_y, l1_z, l1_range, min_adis and max_adis are 5.38, 6.75, −5.13, 22.50, 2.00 and 9.00 respectively. The restrained range of the residues is from 176 to 200. The receptor force field(receptor_ff) is protein.ff14SB, The ligand force field(ligand_ff) is gaff2. The ligand pH(ligand_pH) is 7.2.
These parameters for the Binding Gibbs free energy are all included in an input file. Some main parameters are listed in Table 4 . Other parameters used are the default values in the example input file accompanied with the software distribution(input-dd.in).
Table 4.
Some main parameters listed for computing the Binding Gibbs free energies.
| Parameter | Value | Implication |
|---|---|---|
| calc_type | dock | Docked or crystal structure |
| ligand_name | UNK | Ligand name in the docked poses or crystal structure |
| P1 | :44@CA | Protein anchors, using original protein sequence |
| P2 | :21@CA | Protein anchors, using original protein sequence |
| P3 | :87@CA | Protein anchors, using original protein sequence |
| fe_type | dd-rest | Type of free energy calculations (rest, dd, sdr, dd-rest, sdr-rest, custom) |
| water_model | TIP3P | Water model (SPCE, TIP4PEW or TIP3P) |
| num_waters | 23,000 | Number of waters in the system |
| neutralize_only | no | Neutralize only or also ionize (yes or no) |
| cation | Na+ | Cation for neutralization/ionization |
| anion | Cl− | Anion for neutralization/ionization |
| ion_conc | 0.15 | Salt concentration for all boxes |
| Temperature | 310.15(K) | Temperature of simulation and analysis |
| rec_bb | yes | Use protein backbone dihedrals conformational restraints |
| bb_start | 176 | First residue of the restrained range (original protein numbering) |
| bb_end | 200 | Last residue of the restrained range |
| l1_x | 5.38(Å) | X distance between P1 and center of L1 search range |
| l1_y | 6.75(Å) | Y distance between P1 and center of L1 search range |
| l1_z | −5.13(Å) | Z distance between P1 and center of L1 search range |
| l1_range | 22.50(Å) | Search radius for the first ligand anchor L1 |
| min_adis | 2.00(Å) | Minimum distance between anchors |
| max_adis | 9.00(Å) | Maximum distance between anchors |
| receptor_ff | protein.ff14SB | Force field for receptor atoms |
| ligand_ff | gaff2 | Force field for ligand atoms (gaff or gaff2 available) |
| ligand_ph | 7.0 | Choose pH for ligand protonation |
3.7. Activity assay of SARS-CoV-2 3CLpro
Fluorescence resonance energy transfer (FRET) assay method was used to determine the inhibitory activity of β-nitrostyrene compounds against SARS-CoV-2 3CL protease with SARS-CoV-2 Mpro/3CLpro Inhibitor Screening Kit #P0312S (Beyotime, China). Based on the manufacturer's instructions for activity testing, a 96-well black microplate was used in this experiment. The total volume of each well was 100 µL, and two parallel wells were set for each sample. Each well was included with evenly mixed 0.5 µL SARS-CoV-2 3CL protease, 92.5 µL assay buffer and 5 µL DMSO solubilized β-nitrostyrene derivatives with different concentrations (prepared by 5 times dilution, such as: 150 µM, 30 µM, 6 µM, 1.2 µM, 0.24 µM, and 0 µM (negative control)) respectively. Then the microplate was kept for 10 min incubation at room temperature. Subsequently, 2 µL fluorescent substrate (Dabcyl-KTSAVLQSGFRKME-Edans) was added to each well and quickly placed into a SpectraMax M2 microplate reader for the detection of fluorescence change with excitation wavelength of 340 nm and emission wavelength of 490 nm within 20 min at intervals 30 s. The enzyme activity was calculated by the increase with time in fluorescence upon continuous monitoring of the reactions of each sample. Reactions were run at 37 ℃. The initial velocities of the fluorescence changes were plotted against the different inhibitor concentrations to obtain the IC50 values by fitting with GraphPad Prism version 9.0 software.
4. Conclusion
The discovery of a new active functional group for a target is of great significance for the development of new drugs to make up for the deficiencies of existing drugs. In this study, seven β-nitrostyrene derivatives with different substituents were synthesized by Henry reaction and β-dehydration reaction, and their inhibitory activities against 3CLpro of SARS-CoV-2 were assayed. All synthesized compounds were characterized and confirmed by 1H NMR, 13C NMR, FTIR, MS respectively. It was found that β-nitrostyrene was a new scaffold with 3CLpro inhibitory activity. Among the seven derivatives, the 4-nitro-β-nitrostyrene (compound a) has the highest on with 0.7297 µM. In order to found the key groups that determine the activity of β-nitrostyrene derivatives and the interaction mode with the receptor, molecular docking was carried out for seven β-nitrostyrene derivatives using CDOCKER protocol. It was found that the hydrogen bonds between β-NO2 and receptor GLY-143 and the π-π stacking between the aryl ring of the ligand and imidazole ring of receptor HIS-41 contribute significantly to the ligand activity, and other groups in the aryl ring of the ligand also have important effects on the ligand-receptor interaction. The π-charge interaction between the aryl nitro group of the compound a and the imidazole group of HIS-41 of the receptor contributes to the activity enhancement. These results provide important guidance for the lead optimization and the design of new and more active derivatives.
A scoring function having good correlation with experimental activity is very important to improve the reliability of the virtual screening results. In this paper, the molecular dynamics for the calculation of binding Gibbs free energies between compound a-g and 3CLpro was carried with BAT.py and AMBER18, and the correlation coefficient r2 between the binding Gibbs free energy ( and the experimental activity (-log IC50) is 0.60. It can be seen that binding Gibbs free energy calculation provides a reliable scoring function for screening new high-activity β-nitrostyrene derivatives by the calculation of binding Gibbs free energy of 3CL protease with the ligand.
CRediT authorship contribution statement
Ze-jun Jia: Investigation, Writing – original draft, Software, Visualization. Xiao-wei Lan: Data curation, Supervision, Methodology. Kui Lu: Validation, Data curation. Xuan Meng: Visualization, Formal analysis. Wen-jie Jing: Methodology, Conceptualization. Shi-ru Jia: Resources, Formal analysis. Kai Zhao: Resources, Funding acquisition, Conceptualization. Yu-jie Dai: Resources, Writing – review & editing, Funding acquisition, Conceptualization.
Declaration of Competing Interest
All co-authors declare that there are no conflicts of interest.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (22105145), the COVID-19 Prevention and Control Program of Tianjin University of Science and Technology (grant No.2020STCv0016) and the Natural Science Foundation of Tianjin (grant No.19JCZDJC34800).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.molstruc.2023.135409.
Appendix. Supplementary materials
Data Availability
Data will be made available on request.
References
- 1.Toit A.Du. Outbreak of a novel coronavirus. Nat. Rev. Microbiol. 2020;18(3):123. doi: 10.1038/s41579-020-0332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassetti M., Vena A., Giacobbe D.R. The novel Chinese coronavirus (2019-nCoV) infections: challenges for fighting the storm. Eur. J. Clin. Invest. 2020;50(3):e13209. doi: 10.1111/eci.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hui D.S.C., Zumla A. Severe acute respiratory syndrome: historical, epidemiologic, and clinical features. Infect. Dis. Clin. North Am. 2019;33(4):869–889. doi: 10.1016/j.idc.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Health World. Organization COVID-19 vaccine tracker. and landscape. 2022 [Google Scholar]
- 5.Hashimoto M., Nagata N., Homma T., Maeda H., Dohi K., Seki N.M., Yoshihara K., Iwata-Yoshikawa N., Shiwa-Sudo N., Sakai Y., Shirakura M., Kishida N., Arita T., Suzuki Y., Watanabe S., Asanuma H., Sonoyama T., Suzuki T., Omoto S., Hasegawa H. Immunogenicity and protective efficacy of SARS-CoV-2 recombinant S-protein vaccine S-268019-b in cynomolgus monkeys. Vaccine. 2022;40(31):4231–4241. doi: 10.1016/j.vaccine.2022.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harvey W.T., Carabelli A.M., Jackson B., Gupta R.K., Thomson E.C., Harrison E.M., Ludden C., Reeve R., Rambaut A., Peacock S.J., Robertson D.L. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021;19(7):409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tao K., Tzou P.L., Nouhin J., Gupta R.K., de Oliveira T., Kosakovsky Pond S.L., Fera D., Shafer R.W. The biological and clinical significance of emerging SARS-CoV-2 variants. Nat. Rev. Genet. 2021;22(12):757–773. doi: 10.1038/s41576-021-00408-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cele S., Jackson L., Khoury D.S., Khan K., Moyo-Gwete T., Tegally H., San J.E., Cromer D., Scheepers C., Amoako D.G., Karim F., Bernstein M., Lustig G., Archary D., Smith M., Ganga Y., Jule Z., Reedoy K., Hwa S.H., Giandhari J., Blackburn J.M., Gosnell B.I., Abdool Karim S.S., Hanekom W., von Gottberg A., Bhiman J.N., Lessells R.J., Moosa M.S., Davenport M.P., de Oliveira T., Moore P.L., Sigal A. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature. 2022;602(7898):654–656. doi: 10.1038/s41586-021-04387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong W., Parkkila S., Wu X., Aspatwar A. SARS-CoV-2 variants and COVID-19 vaccines: current challenges and future strategies. International Reviews of Immunology. 2022:1–22. doi: 10.1080/08830185.2022.2079642. [DOI] [PubMed] [Google Scholar]
- 10.Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y., Zhang B., Li X., Zhang L., Peng C., Duan Y., Yu J., Wang L., Yang K., Liu F., Jiang R., Yang X., You T., Liu X., Yang X., Bai F., Liu H., Liu X., Guddat L.W., Xu W., Xiao G., Qin C., Shi Z., Jiang H., Rao Z., Yang H. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582(7811):289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L., Lin D., Sun X., Curth U., Drosten C., Sauerhering L., Becker S., Rox K., Hilgenfeld R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. 2020;368(6489):409–412. doi: 10.1126/science.abb3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanne J.H. British Medical Journal Publishing Group; 2022. Covid-19: FDA Authorises Pharmacists to Prescribe Paxlovid. [DOI] [PubMed] [Google Scholar]
- 13.Lim S.P. Targeting SARS-CoV-2 and host cell receptor interactions. Antiviral Res. 2023;210 doi: 10.1016/j.antiviral.2022.105514. 105514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joyce R.P., Hu V.W., Wang J. The history, mechanism, and perspectives of nirmatrelvir (PF-07321332): an orally bioavailable main protease inhibitor used in combination with ritonavir to reduce COVID-19-related hospitalizations. Med Chem Res. 2022;31(10):1637–1646. doi: 10.1007/s00044-022-02951-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.L. Shionogi & Co. 2022. Filled for Domestic Approval of COVID-19 vaccine S-268019 and Post-Marketing Safety Surveillance of Xocova® Tablets 125mg.https://www.shionogi.com/content/dam/shionogi/global/investors/ir-library/presentation/2022/Web_COVID_20221124_E_v2.pdf [Google Scholar]
- 16.Schaeffer L. In: The Practice of Medicinal Chemistry. third ed. Wermuth C.G., editor. Academic Press; New York: 2008. Chapter 21 - The role of functional groups in drug–receptor interactions; pp. 464–480. [Google Scholar]
- 17.Andrews P.R., Craik D.J., Martin J.L. Functional group contributions to drug-receptor interactions, Journal of Medicinal Chemistry. 1984;27(12):1648–1657. doi: 10.1021/jm00378a021. [DOI] [PubMed] [Google Scholar]
- 18.He Z., Zhang J., Shi X.H., Hu L.L., Kong X., Cai Y.D., Chou K.C. Predicting drug-target interaction networks based on functional groups and biological features. PloS. 2010;5(3):e9603. doi: 10.1371/journal.pone.0009603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhai T., Zhang F., Haider S., Kraut D., Huang Z. An integrated computational and experimental approach to identifying inhibitors for SARS-CoV-2 3CL protease. Frontiers in Molecular Biosciences. 2021;8:661424. doi: 10.3389/fmolb.2021.661424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konno S., Kobayashi K., Senda M., Funai Y., Seki Y., Tamai I., Schäkel L., Sakata K., Pillaiyar T., Taguchi A., Taniguchi A., Gütschow M., Müller C.E., Takeuchi K., Hirohama M., Kawaguchi A., Kojima M., Senda T., Shirasaka Y., Kamitani W., Hayashi Y. 3CL protease inhibitors with an electrophilic arylketone moiety as anti-SARS-CoV-2 agents, Journal of Medicinal Chemistry. 2021;65(4):2926–2939. doi: 10.1021/acs.jmedchem.1c00665. [DOI] [PubMed] [Google Scholar]
- 21.Bai B., Arutyunova E., Khan M.B., Lu J., Joyce M.A., Saffran H.A., Shields J.A., Kandadai A.S., Belovodskiy A., Hena M., Vuong W., Lamer T., Young H.S., Vederas J.C., Tyrrell D.L., Lemieux M.J., Nieman J.A. Peptidomimetic nitrile warheads as SARS-CoV-2 3CL protease inhibitors. RSC Medicinal Chemistry. 2021;12(10):1722–1730. doi: 10.1039/d1md00247c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du X., Li Y., Xia Y.L., Ai S.M., Liang J., Sang P., Ji X.L., Liu S.Q. Insights into protein–ligand interactions: mechanisms models methodsm. Int J Mol Sci. 2016;17(2):144. doi: 10.3390/ijms17020144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y., Zhang B., Li X., Zhang L., Peng C., Duan Y., Yu J., Wang L., Yang K., Liu F., Jiang R., Yang X., You T., Liu X., Yang X., Bai F., Liu H., Liu X., Guddat L.W., Xu W., Xiao G., Qin C., Shi Z., Jiang H., Rao Z., Yang H. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582(7811):289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 24.Dai W., Zhang B., Jiang X.-.M., Su H., Li J., Zhao Y., Xie X., Jin Z., Peng J., Liu F.J.S. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science. 2020;368(6497):1331–1335. doi: 10.1126/science.abb4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Günther S., Reinke P.Y., Fernández-García Y., Lieske J., Lane T.J., Ginn H.M., Koua F.H., Ehrt C., Ewert W., Oberthuer D.J.S. X-ray screening identifies active site and allosteric inhibitors of SARS-CoV-2 main protease. Science. 2021;372(6542):642–646. doi: 10.1126/science.abf7945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oerlemans R., Ruiz-Moreno A.J., Cong Y., Kumar N.D., Velasco-Velazquez M.A., Neochoritis K., Smith J., Reggiori F., Groves M.R., Dömling A. Repurposing the HCV NS3-4A Protease Drug Boceprevir as COVID-19. RSC Med Chem. 2021;12(3):370–379. doi: 10.1039/d0md00367k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kneller D.W., Li H., Galanie S., Phillips G., Labbé A., Weiss K.L., Zhang Q., Arnould M.A., Clyde A., Ma H., Ramanathan A., Jonsson C.B., Head M.S., Coates L., Louis J.M., Bonnesen P.V., Kovalevsky A. Structural, electronic, and electrostatic determinants for inhibitor binding to subsites S1 and S2 in SARS-CoV-2 main protease, J Med Chem. 2021;64(23):17366–17383. doi: 10.1021/acs.jmedchem.1c01475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerçek Z., Ceyhan D., Erçağ E. Synthesis and molecular docking study of novel COVID-19 inhibitors. TUrk. J. Chem. 2021;45(3):704–718. doi: 10.3906/kim-2012-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohamed N.M., Eltelbany R.F.J.C. Synthetic coumarin derivatives as SARS-CoV-2 major protease inhibitors: design, synthesis, bioevaluation and molecular docking. ChemistrySelect. 2021;6(47):13616–13626. [Google Scholar]
- 30.Alotaibi S.H., Amer H.H., Touil N., Abdel-Moneim A.S., Soliman M.M., Zaki Y.H. Synthesis, Characterization and Molecular Docking of New Nucleosides and Schiff Bases Derived from Ampyrone as Antiviral Agents to Contain the COVID-19 Virus. Polycycl. Aromat. Compd. 2022 [Google Scholar]
- 31.Venkateshan M., Muthu M., Suresh J., Kumar R.Ranjith. Azafluorene derivatives as inhibitors of SARS CoV-2 RdRp: synthesis, physicochemical, quantum chemical, modeling and molecular docking analysis. J. Mol. Struct. 2020;1220 doi: 10.1016/j.molstruc.2020.128741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng T., Li X., Li Y., Liu Z., Wang R. Comparative assessment of scoring functions on a diverse test set. J Chem Inf Model. 2009;49(4):1079–1093. doi: 10.1021/ci9000053. [DOI] [PubMed] [Google Scholar]
- 33.Xu W., Lucke A.J., Fairlie D.P. Comparing sixteen scoring functions for predicting biological activities of ligands for protein targets. J. Mol. Graph. Modell. 2015;57:76–88. doi: 10.1016/j.jmgm.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 34.Fujimoto K.J., Minami S., Yanai T. Machine-learning- and knowledge-based scoring functions incorporating ligand and protein fingerprints. ACS Omega. 2022;7(22):19030–19039. doi: 10.1021/acsomega.2c02822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malmstrom R.D., Watowich S.J. Using free energy of binding calculations to improve the accuracy of virtual screening predictions. J. Chem. Inf. Model. 2011;51(7):1648–1655. doi: 10.1021/ci200126v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee H.S., Jo S., Lim H.S., Im W. Application of binding free energy calculations to prediction of binding modes and affinities of MDM2 and MDMX inhibitors. J Chem Inf Model. 2012;52(7):1821–1832. doi: 10.1021/ci3000997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gapsys V., Yildirim A., Aldeghi M., Khalak Y., Van der Spoel D., de Groot B.L.J.C.C. Accurate absolute free energies for ligand–protein binding based on non-equilibrium approaches. Communications Chemistry. 2021;4(1):61. doi: 10.1038/s42004-021-00498-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woo H.J., Roux B. Calculation of absolute protein–ligand binding free energy from computer simulations. Proc Natl Acad Sci U S A. 2005;102(19):6825–6830. doi: 10.1073/pnas.0409005102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cournia Z., Allen B., Sherman W. Relative binding free energy calculations in drug discovery: recent advances and practical considerations. J Chem Inf Model. 2017;57(12):2911–2937. doi: 10.1021/acs.jcim.7b00564. [DOI] [PubMed] [Google Scholar]
- 40.Fu H., Gumbart J.C., Chen H., Shao X., Cai W., Chipot C. BFEE: a user-friendly graphical interface facilitating absolute binding free-energy calculations, J Chem Inf Model. 2018;58(3):556–560. doi: 10.1021/acs.jcim.7b00695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.L.Carvalho Martins, Cino E.A., Ferreira R.S. PyAutoFEP: an automated free energy perturbation workflow for GROMACS integrating enhanced sampling. methods, J Chem Theory Comput. 2021;17(7):4262–4273. doi: 10.1021/acs.jctc.1c00194. [DOI] [PubMed] [Google Scholar]
- 42.Heinzelmann G., Gilson M.K. Automated docking refinement and virtual compound screening with absolute binding free energy calculations, bioRxiv. 2020;2020(04):15.043240. [Google Scholar]
- 43.Homeyer N., Stoll F., Hillisch A., Gohlke H. Binding free energy calculations for lead optimization: assessment of their accuracy in an industrial drug design context. J Chem Theory Comput. 2014;10(8):3331–3344. doi: 10.1021/ct5000296. [DOI] [PubMed] [Google Scholar]
- 44.Alfarisi S., Santoso M., Kristanti A.N., Siswanto I., Puspaningsih N.N.T. Synthesis, antimicrobial study, and molecular docking simulation of 3, 4-dimethoxy-β-nitrostyrene derivatives as candidate PTP1B inhibitor. Sci Pharm. 2020;88(3) 37. [Google Scholar]
- 45.Milhazes N., Calheiros R., Marques M.P.M., Garrido J., Cordeiro M.N.D., Rodrigues C., Quinteira S., Novais C., Peixe L., Borges F.J.B. β-Nitrostyrene derivatives as potential antibacterial agents: a structure–property–activity relationship study. Bioorg Med Chem. 2006;14(12):4078–4088. doi: 10.1016/j.bmc.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 46.Ramzan A., Padder S.A., Masoodi K.Z., Shafi S., Tahir I., Rehman R.U., Prasad R., Shah A.H. β-Nitrostyrene derivatives as broad range potential antifungal agents targeting fungal cellwall. Eur J Med Chem. 2022;240:114609. doi: 10.1016/j.ejmech.2022.114609. [DOI] [PubMed] [Google Scholar]
- 47.Current Patent Assignee: ShangHai HuaJin Biotechnology - CN108658778, 2018.
- 48.Tools for infrared and Raman spectroscopy, Infrared and Raman Spectroscopy1995, pp. 63–188.
- 49.Slovetskii V.I. IR spectra of nitro compounds. Bull. Acad. Sci. USSR Div. Chem. Sci. 1970;19(10):2086–2091. [Google Scholar]
- 50.Clavijo R.E., Araya-Maturana R., Cassels B.K., Weiss-López B. Infrared spectra of nitrostyrene derivatives. Spectrochim. Acta Part A. 1994;50(12):2105–2115. [Google Scholar]
- 51.Philip D., John A., Panicker C.Y., Varghese H.T., FT-Raman FT-IR and surface enhanced Raman scattering spectra of sodium salicylate. Spectrochim. Acta Part A. 2001;57(8):1561–1566. doi: 10.1016/s1386-1425(01)00395-x. [DOI] [PubMed] [Google Scholar]
- 52.Gunasekaran S., Sailatha E., Srinivasan S., Kumaresan S. FTIR, FT Raman spectra and molecular structural confirmation of isoniazid. Indian J. Pure Appl. Phys. 2009;47 [Google Scholar]
- 53.Alfarisi S., Santoso M., Kristanti A.N., Siswanto I., Puspaningsih N.N.T. Synthesis, Antimicrobial Study, and Molecular Docking Simulation of 3,4-Dimethoxy-β-Nitrostyrene Derivatives as Candidate PTP1B Inhibitor. Sci Pharm. 2020;88(3) 37. [Google Scholar]
- 54.Calheiros R., Milhazes N., Borges F., Marques M.P.M. β-Nitrostyrene derivatives—a conformational study by combined Raman spectroscopy and ab initio MO calculations. J. Mol. Struct. 2004;692(1):91–106. doi: 10.1039/b405290k. [DOI] [PubMed] [Google Scholar]
- 55.Carter K.C., Finnon Y.S., Daeid N.N., Robson D.C., Waddell R. The effect of nitrostyrene on cell proliferation and macrophage immune responses. Immunopharmacol. Immunotoxicol. 2002;24(2):187–197. doi: 10.1081/iph-120003749. [DOI] [PubMed] [Google Scholar]
- 56.Rahmani-Nezhad S., Safavi M., Pordeli M., Ardestani S.K., Khosravani L., Pourshojaei Y., Mahdavi M., Emami S., Foroumadi A., Shafiee A. Synthesis, in vitro cytotoxicity and apoptosis inducing study of 2-aryl-3-nitro-2H-chromene derivatives as potent anti-breast cancer agents. Eur. J. Med. Chem. 2014;86:562–569. doi: 10.1016/j.ejmech.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 57.Hung A.C., Tsai C.H., Hou M.F., Chang W.L., Wang C.H., Lee Y.C., Ko A., Hu S.C., Chang F.R., Hsieh P.W., Yuan S.S. The synthetic β-nitrostyrene derivative CYT-Rx20 induces breast cancer cell death and autophagy via ROS-mediated MEK/ERK pathway. Cancer Lett. 2016;371(2):251–261. doi: 10.1016/j.canlet.2015.11.035. [DOI] [PubMed] [Google Scholar]
- 58.Nicoletti G., Cornell H., Hugel H., White K., Nguyen T., Zalizniak L., Nugegoda D. Synthesis and antimicrobial activity of nitroalkenyl arenes. Anti-Infect. Agents. 2013;11:179–191. [Google Scholar]
- 59.Milhazes N., Calheiros R., Marques M.P., Garrido J., Cordeiro M.N., Rodrigues C., Quinteira S., Novais C., Peixe L., Borges F. Beta-nitrostyrene derivatives as potential antibacterial agents: a structure-property-activity relationship study. Bioorg. Med. Chem. 2006;14(12):4078–4088. doi: 10.1016/j.bmc.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 60.Park J., Pei D. trans-β-nitrostyrene derivatives as slow-binding inhibitors of protein tyrosine phosphatases. Biochemistry. 2004;43(47):15014–15021. doi: 10.1021/bi0486233. [DOI] [PubMed] [Google Scholar]
- 61.Alfarisi S., Santoso M., Kristanti A.N., Siswanto I., Puspaningsih N.N.T. Synthesis, Antimicrobial Study, and Molecular Docking Simulation of 3,4-Dimethoxy-β-Nitrostyrene Derivatives as Candidate PTP1B Inhibitor. Scientia Pharmaceutica. 2020;88(3) 37. [Google Scholar]
- 62.Wu G., Robertson D.H., Brooks C.L., 3rd, Vieth M. Detailed analysis of grid-based molecular docking: a case study of CDOCKER-A CHARMm-based MD docking algorithm. J. Comput. Chem. 2003;24(13):1549–1562. doi: 10.1002/jcc.10306. [DOI] [PubMed] [Google Scholar]
- 63.X. Ding, R.L. Hayes, J.Z. Vilseck, M.K. Charles, C.L. Brooks, 3rd, CDOCKER and λ-dynamics for prospective prediction in D₃R Grand Challenge 2, Journal of computer-aided molecular design 32(1) (2018) 89-102. [DOI] [PMC free article] [PubMed]
- 64.Wang S., Jiang J.-.H., Li R.-.Y., Deng P.J.B.c. Docking-based virtual screening of TβR1 inhibitors: evaluation of pose prediction and scoring functions. BMC Chemistry. 2020;14(1):1–8. doi: 10.1186/s13065-020-00704-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schug K.A., Lindner W. Noncovalent binding between guanidinium and anionic groups: focus on biological- and synthetic-based arginine/guanidinium interactions with phosph [on]ate and sulf [on]ate residues. Chem. Rev. 2005;105(1):67–114. doi: 10.1021/cr040603j. [DOI] [PubMed] [Google Scholar]
- 66.Toth G., Bowers S.G., Truong A.P., Probst G.J.C.p.d. The role and significance of unconventional hydrogen bonds in small molecule recognition by biological receptors of pharmaceutical relevance. Curr Pharm Des. 2007;13(34):3476–3493. doi: 10.2174/138161207782794284. [DOI] [PubMed] [Google Scholar]
- 67.Domínguez-Villa F.X., Durán-Iturbide N.A., Ávila-Zárraga J.G. Synthesis, molecular docking, and in silico ADME/Tox profiling studies of new 1-aryl-5-(3-azidopropyl)indol-4-ones: potential inhibitors of SARS CoV-2 main protease. Bioorg. Chem. 2021;106 doi: 10.1016/j.bioorg.2020.104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Plewczynski D., Łaźniewski M., Augustyniak R., Ginalski K. Can we trust docking results? Evaluation of seven commonly used programs on PDBbind database. J Comput Chem. 2011;32(4):742–755. doi: 10.1002/jcc.21643. [DOI] [PubMed] [Google Scholar]
- 69.Boz E., Stein M. Accurate receptor-ligand binding free energies from fast QM conformational chemical space sampling. Int J Mol Sci. 2021;22(6):3078. doi: 10.3390/ijms22063078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu W., Liu Z., Liu H., Westerhoff L.M., Zheng Z. Modeling, Free Energy Calculations Using the Movable Type Method with Molecular Dynamics Driven Protein–Ligand Sampling. Journal of Chemical Information and Modeling. 2022;62(22):5645–5665. doi: 10.1021/acs.jcim.2c00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heinzelmann G., Gilson M., py B.A.T. A fully automated python tool for high-performance absolute binding free energy calculations. Sci Rep. 2020 [Google Scholar]
- 72.Bruce Macdonald H.E., Cave-Ayland C., Ross G.A., Essex J.W. Ligand Binding Free Energies with Adaptive Water Networks: two-Dimensional Grand Canonical Alchemical Perturbations. J. Chem. Theory Comput. 2018;14(12):6586–6597. doi: 10.1021/acs.jctc.8b00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.SYSTèMES D. BIOVIA Discovery Studio, Dassault Syst mes BIOVIA, discovery studio modeling environment, Release 2017. Dassault Syst. 2016 [Google Scholar]
- 74.Alexeev Y., Mazanetz M.P., Ichihara O., Fedorov D.G. GAMESS as a free quantum-mechanical platform for drug research. Current topics in medicinal chemistry. 2012;12(18):2013–2033. doi: 10.2174/156802612804910269. [DOI] [PubMed] [Google Scholar]
- 75.Khan W., Ashfaq U., Aslam S., Saif S., Aslam T., Tusleem K., Maryam A., Tahir Ul Qamar M. Anticancer screening of medicinal plant phytochemicals against Cyclin-Dependent Kinase-2 (CDK2): an in-silico approach. Adv. Life Sci. 2017;4:113–119. [Google Scholar]
- 76.Götz A.W., Williamson M.J., Xu D., Poole D., Grand S.Le, Walker R.C. Routine Microsecond Molecular Dynamics Simulations with AMBER on GPUs. 1. Generalized Born. J. Chem. Theory Comput. 2012;8(5):1542–1555. doi: 10.1021/ct200909j. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.