Abstract
Background and Objectives
Nonvalvular atrial fibrillation (NVAF) is associated with an increased risk of dementia. Oral anticoagulants (OACs) are essential for stroke prevention in NVAF, and studies have shown a possible protective effect on dementia. However, findings have been inconsistent and hampered by methodological limitations. Thus, we assessed whether the use of OACs is associated with a decreased incidence of dementia in patients with NVAF. In addition, we explored the impact of the cumulative duration of OAC use on the incidence of dementia.
Methods
Using the UK Clinical Practice Research Datalink, we formed a cohort of all patients aged 50 years or older with an incident diagnosis of NVAF between 1988 and 2017 and no prior OAC use, with a follow-up until 2019. Patients were considered unexposed until 6 months after their first OAC prescription for latency considerations and exposed thereafter until the end of follow-up. We used time-dependent Cox regression models to estimate hazard ratios (HRs), adjusted for 54 covariates, with 95% CIs for dementia associated with OAC use, compared with nonuse. We also assessed whether the risk varied with the cumulative duration of OAC use, compared with nonuse, by comparing prespecified exposure categories defined in a time-varying manner and by modeling the HR using a restricted cubic spline.
Results
The cohort included 142,227 patients with NVAF, with 8,023 cases of dementia over 662,667 person-years of follow-up (incidence rate 12.1, 95% CI 11.9–12.4 per 1,000 person-years). OAC use was associated with a decreased risk of dementia (HR 0.88, 95% CI 0.84–0.92) compared with nonuse. A restricted cubic spline also indicated a decreased risk of dementia, reaching a low at approximately 1.5 years of cumulative OAC use and stabilizing thereafter. Moreover, OAC use decreased the risk in patients aged 75 years and older (HR 0.84, 95% CI 0.80–0.89), but not in younger patients (HR 0.99, 95% CI 0.90–1.10).
Discussion
In patients with incident NVAF, OACs were associated with a decreased risk of dementia, particularly in elderly individuals. This warrants consideration when weighing the risks and benefits of anticoagulation in this population.
Classification of Evidence
This study provides Class II evidence that in patients with NVAF, OAC use (vs nonuse) is associated with a decreased risk of dementia.
Oral anticoagulation is essential to prevent physical disability and mortality associated with stroke occurrence in patients with atrial fibrillation (AF) but may also play a role in preventing cognitive decline in this population. Indeed, these patients have an increased risk of dementia compared with the general population,1,2 including not only vascular dementia but also Alzheimer disease.3 This increase in risk may arise from different mechanisms such as ischemic stroke, repetitive microembolisms, silent strokes, and hypoperfusion.3 Patients with AF experiencing an ischemic stroke have a more than 2-fold increased risk of dementia.4 However, an increased risk of dementia has also been shown independently of stroke occurrence.2 Hence, not only are OACs expected to reduce the risk of dementia through the prevention of overt ischemic stroke, they may also protect against subclinical brain infarcts and be warranted even in patients with a lower risk of stroke.
In patients with nonvalvular AF (NVAF), the potential preventive role of OACs on the risk of cognitive decline has been assessed in several observational studies. Two early studies did not report associations between OAC use and cognitive decline in patients with NVAF.5,6 More recently, some studies identified protective effects of OACs on the risk of dementia; however, these associations were of differing strengths and several studies had notable limitations, including possible selection bias,7-9 exposure and/or outcome misclassification,7,8 no latency considerations,7,9,10 immortal time bias,9,10 inclusion of patients with prevalent AF and/or OAC use,9,10 and limited sample size.10 In addition, it was not clear whether these observed associations were occurring independent of overt ischemic stroke during follow-up.
The introduction of direct oral anticoagulants (DOACs), which have at least comparable efficacy and a lower risk of intracranial hemorrhage compared with warfarin,11 are easier to use with less drug and food interactions, and potentially improve adherence, has raised further discussion on whether DOACs may provide better prevention of dementia than warfarin.12-14
Consequently, recent guidelines,15 reviews,14,16 and reports12 have called for further studies to provide more conclusive evidence and highlighted specific knowledge gaps regarding the potential dose-response relationship between the duration of untreated AF16 and the risk of dementia and the prescription of OACs to patients at low risk of stroke. Hence, we conducted a population-based cohort study to assess whether the use of OACs was associated with the risk of dementia among patients with NVAF, compared with nonuse. Our primary research question was to investigate the association between OAC use and the risk of dementia in patients with NVAF.
Methods
Data Source
We used the UK Clinical Practice Research Datalink (CPRD), a primary care electronic medical records database for more than 15 million patients from more than 700 general practices, with data available since 1987.17 Covering approximately 7% of the UK population, these data are broadly representative of the entire population in the UK for age, sex, and ethnicity.17 The CPRD contains demographic and lifestyle information, prescriptions, diagnoses, and referrals to specialists and hospitals. Drug prescriptions written by the general practitioner are recorded automatically during issue using a coded drug dictionary based on the British National Formulary.17 In addition, the Read code classification system is used to record medical diagnoses during patient consultations.17,18 To ensure accuracy and completeness of data, quality control audits are regularly conducted, and numerous studies have shown the validity and high quality of the recorded data.17
Study Population
We assembled a cohort of all patients aged 50 years or older with an incident diagnosis of AF between January 1, 1988, and December 31, 2017. Cohort entry was defined as the date of first AF diagnosis within the study period. We excluded patients with less than 1 year of registration with a practice before cohort entry and those with a prior AF diagnosis, a history of valvular surgery, or rheumatic valvular disease at any time before cohort entry to identify a cohort of patients with incident NVAF. We also excluded patients with prior hyperthyroidism because AF rarely requires long-term oral anticoagulation in this context. To avoid biases related to the inclusion of prevalent users, we excluded patients prescribed OACs before cohort entry. Finally, we excluded patients with a diagnosis of dementia, cognitive impairment, and those prescribed a cholinesterase inhibitor (donepezil, galantamine, and rivastigmine) or memantine at any time before cohort entry. Because the diagnosis of dementia can be delayed by several months due to the progressive nature of its symptoms, follow-up started 6 months after cohort entry to exclude cases of dementia that occurred before AF diagnosis but were not yet diagnosed. Patients were followed up until a diagnosis of dementia, death from any cause, end of registration with the general practice, or end of the study period (December 31, 2019), whichever occurred first.
Exposure
Exposure to OACs (apixaban, dabigatran, rivaroxaban, and warfarin) was defined in a time-varying manner whereby patients were considered unexposed until 6 months after their first OAC prescription and exposed thereafter until the end of follow-up, regardless of switching or treatment discontinuation. The 6-month lag period accounts for a biologically plausible latency period because OACs initiated shortly before the diagnosis of dementia are unlikely to have influenced its onset. This approach also minimizes the potential for reverse causality and detection bias. Edoxaban was not considered among OACs because it was approved for use in the UK for patients with NVAF at the end of 2015.19 Thus, it is likely that very few patients were exposed to edoxaban and those initiating the drug may not have a sufficient follow-up to assess an outcome such as dementia.
Outcome
The primary outcome was the first diagnosis of dementia during follow-up, identified using relevant Read codes, irrespective of the type of dementia (list of Read codes available in eTable 1, links.lww.com/WNL/C578). Read codes reflect diagnoses made in routine clinical practice by general practitioners after referrals to specialists or further investigation, where necessary. Hence, their validity is good, with 83%–90% of diagnoses identified through Read codes confirmed as dementia or Alzheimer disease.20-22 We evaluated the risk of all types of dementia collectively in the primary analysis because it has previously been suggested that OACs may decrease the risk of both vascular and nonvascular dementia.3
Covariates
We adjusted all models for age (50–59, 60–69, 70–79, 80–89, and 90 years and older), sex, smoking, body mass index (BMI) (≤25, >25 to ≤30, >30.0 kg/m2), and calendar year at cohort entry, in addition to the following risk factors and comorbidities, measured at any time before cohort entry: alcohol abuse, substance abuse, diabetes, hypertension, hyperlipidemia, coronary heart disease, heart failure, stroke, TIA, systemic embolism, peripheral arterial disease, a history of bleeding, chronic obstructive pulmonary disease, chronic kidney disease, liver disease, Parkinson disease, cancer (other than nonmelanoma skin cancer), a history of falls, depression, and head trauma. Hypertension, diabetes, and hyperlipidemia were defined using diagnostic codes or related medications. All models were also adjusted for use of the following drugs measured in the year before cohort entry: antiplatelet drugs, beta-blockers, diuretics, nonsteroidal anti-inflammatory drugs, antidepressants, antipsychotics, anxiolytics, antiarrhythmic drugs, and hormone replacement therapy. We also adjusted for the total number of distinct drug classes prescribed in the year before cohort entry and the number of physician visits as a surrogate marker for overall health. Missing information for BMI and smoking was classified in a separate category. Scores that are used to estimate the risk of ischemic stroke in AF such as CHADS223 or CHA2DS2-VASc24 were not adjusted for because the individual components of these scores were included in all models.
Statistical Analysis
We used descriptive statistics to summarize the characteristics of the cohort. We estimated the crude incidence rates and 95% CIs of dementia for each exposure group based on the Poisson distribution. In the primary analysis, we fitted time-dependent Cox proportional hazards models to estimate the hazard ratio (HR) and 95% CIs of dementia associated with the use of OACs compared with nonuse, adjusted for the covariates listed earlier.
In secondary analyses, we first estimated whether the incidence of dementia varies with the cumulative duration of OAC use. Cumulative duration of use was calculated by summing the durations of all OAC prescriptions during follow-up, with a 30-day grace period between 2 nonoverlapping prescriptions. We estimated HRs using time-dependent Cox proportional hazards models according to 3 prespecified categories (≤2, >2 to ≤5, and >5 years) measured in a time-varying manner. Furthermore, cumulative duration of use was modeled using a restricted cubic spline with 5 interior knots to produce a smooth curve of the HR as a function of cumulative duration of OAC use.25 To assess effect measure modification, we performed stratified analyses by age (aged 75 years or younger vs older than 75 years), sex, a history of chronic kidney disease, a history of ischemic stroke (including TIAs), CHADS2, and CHA2DS2-VASc score measured at cohort entry. To further minimize potential confounding by indication, we repeated the primary analyses with users of antiplatelet drugs as the reference group. In addition, we repeated the primary analyses for each type of dementia separately (vascular dementia, Alzheimer disease, other, and not specified). As an exploratory analysis, we also assessed the risk of dementia associated with DOACs compared with vitamin K antagonists (VKAs). For this analysis, we identified all patients with a new DOAC or VKA prescription after NVAF diagnosis, with cohort entry defined as the date of this first prescription. Patients were followed up until treatment discontinuation or crossover to the other exposure group, a diagnosis of dementia, death, end of registration with CPRD, or end of the study period, whichever occurred first. We used standardized mortality ratio weighting, estimated from propensity scores (PS) including all covariates described earlier and time between NVAF diagnosis and OAC initiation, to balance baseline characteristics at cohort entry. We also used inverse probability of censoring weighting (IPCW) to account for potential informative censoring. Weighted Cox proportional hazards models were used to estimate the HR and 95% CI of dementia associated with DOAC use compared with VKA use. This analysis was restricted to the years 2011–2017 because DOACs were first marketed in 2011 in the United Kingdom for stroke prevention in NVAF. Finally, to assess whether OACs may prevent dementia beyond their protective effect on the risk of ischemic stroke, we accounted for the potential impact of ischemic stroke on the incidence of dementia by censoring patients who experienced an ischemic stroke or TIA during follow-up, using IPCW. We repeated this analysis censoring patients with an ischemic stroke (not TIA) during follow-up, and next, we restricted the analysis to patients without a previous stroke/TIA at anytime before cohort entry and censored patients who experienced an ischemic stroke or TIA during follow-up.
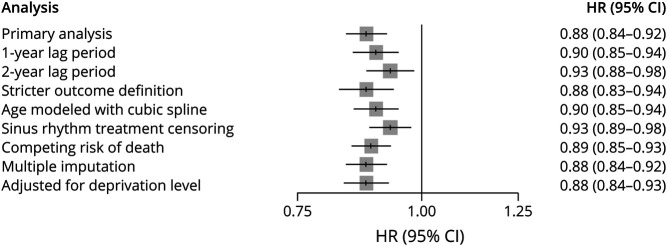
We performed 7 sensitivity analyses to examine the robustness of our results. First, given that the optimal latency time window is unknown, we varied the exposure lag time to 1 and 2 years. Second, to evaluate the impact of potential outcome misclassification, we used a stricter outcome definition requiring either 2 diagnostic codes for dementia or a diagnostic code in combination with a consultation with a neurologist or geriatrician, all within 1 year. For this analysis, the date of the second qualifying event was considered as the outcome date. Third, we repeated the primary analysis with age modeled flexibly using cubic splines. Fourth, we censored patients on prescription of treatments to restore sinus rhythm (e.g., pharmacologic or electrical cardioversion). Fifth, we also explored the potential impact of the competing risk of death during follow-up using IPCW.26 Sixth, we used multiple imputation to address missing values for BMI and smoking. In brief, we used the multiple imputation by chained equations method27 and combined results from 5 imputed datasets. Seventh, in a post hoc sensitivity analysis, we additionally adjusted for deprivation level using the UK Index of Multiple Deprivation, a measure of socioeconomic status that encompasses domains such as education, skills, and training.
Last, we conducted a supplementary time-conditional PS-matched analysis28 to further explore potential for residual confounding. In brief, among the base cohort of patients with NVAF and starting chronologically, each patient initiating an OAC was matched 1:1 without replacement on age (±2 years), sex, year of AF diagnosis, and time-conditional PS to a patient not exposed up to the same point in time. Thus, in exposure sets defined at the point of OAC prescription, potential comparators included all patients with the same AF duration as the corresponding exposed patient and a consultation within 60 days of the date of OAC prescription. Time-conditional PS included time-varying patient characteristics measured at the point of the time-based exposure sets and were used to identify the comparator patient most similar to the patient who initiated an OAC. Trimming was performed within each exposure set for the positivity assumption. Covariates with imbalances after matching were additionally adjusted for in the analysis. For the matched pairs formed, cohort entry was defined as the date of OAC initiation and the corresponding date of consultation for the matched comparator patient. The matched sets were followed up until an incident diagnosis of dementia, switching from no use to use of an OAC, death from any cause, end of registration with the practice, or end of the study period, whichever occurred first. We fitted a Cox proportional hazards model with robust standard errors to estimate the HR and 95% CI of dementia associated with OAC use—an estimate of the average treatment effect in the treated group (i.e., patients who initiated OACs). We also determined the 2- and 5-year risk differences and the corresponding number needed to treat (NNT). The HRs and 95% CIs were then computed for each type of dementia separately. All analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC).
Standard Protocol Approvals, Registrations, and Patient Consents
The study protocol was approved by the Independent Scientific Advisory Committee of the CPRD (No.19_277) and the research ethics board of the Jewish General Hospital in Montreal, Canada, who waived the requirement for informed consent.
Data Availability
This study is based partly on data from the CPRD obtained under license from the UK Medicines and Healthcare products Regulatory Agency. Data are provided by patients and collected by the UK National Health Service as part of their care and support. The interpretation and conclusions contained in this study are those of the author/s alone. Because electronic health records are classified as “sensitive data” by the UK Data Protection Act, information governance restrictions (to protect patient confidentiality) prevent data sharing through public deposition. Data are available with approval through the individual constituent entities controlling access to the data. Specifically, the primary care data can be requested through application to the CPRD (cprd.com).
Results
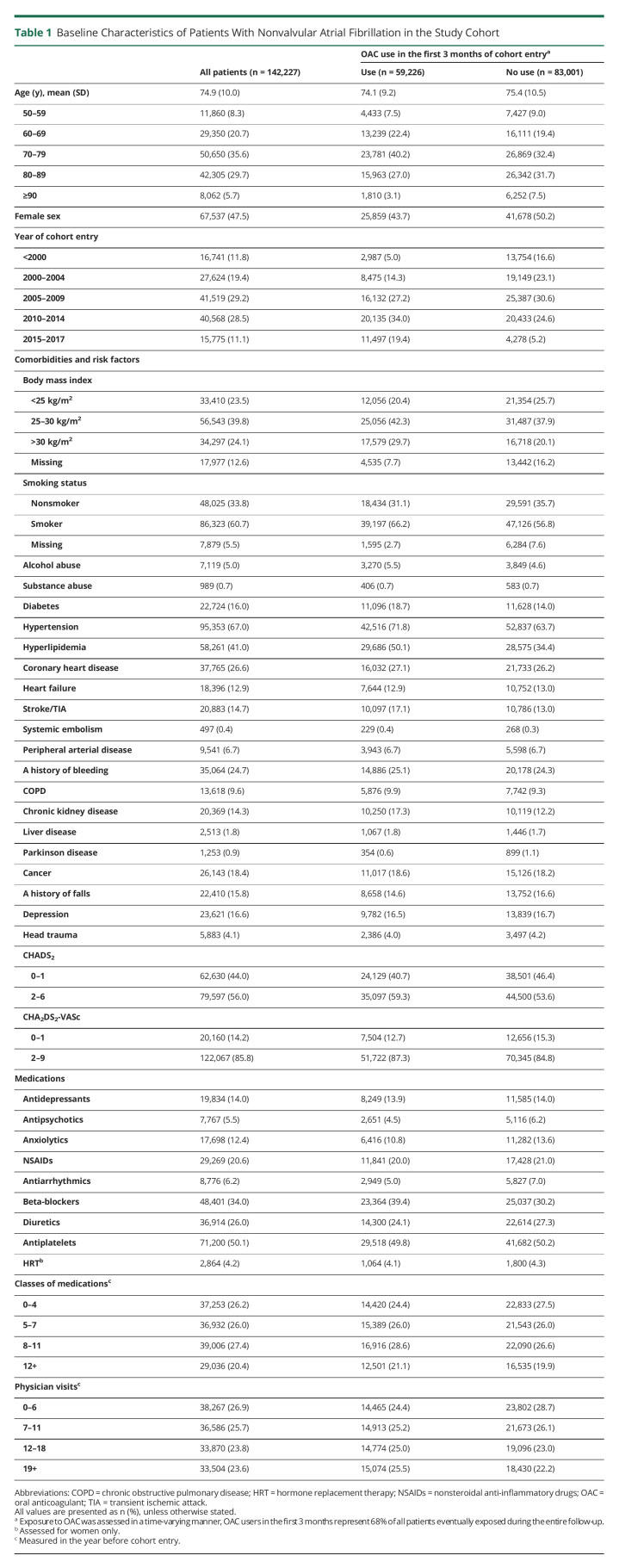
We initially identified 327,452 patients with AF during the study period. After applying exclusion criteria, the final study cohort consisted of 142,227 patients with NVAF (eFigure 1, links.lww.com/WNL/C579). The mean age was 74.9 years (SD 10.0 years), and 74,690 (52.5%) were men (Table 1).
Table 1.
Baseline Characteristics of Patients With Nonvalvular Atrial Fibrillation in the Study Cohort
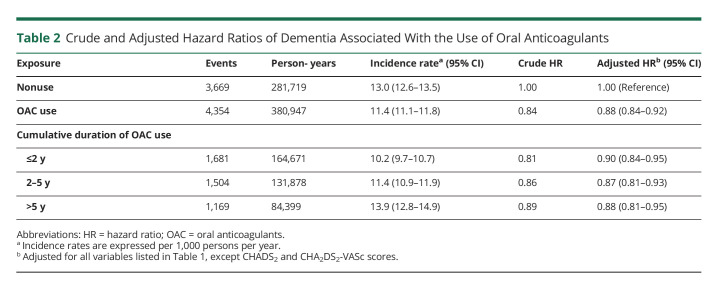
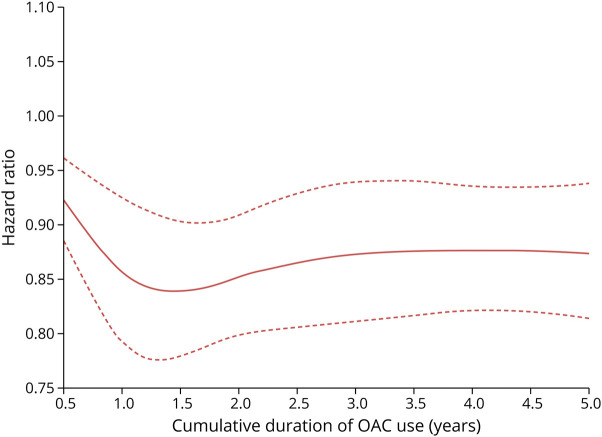
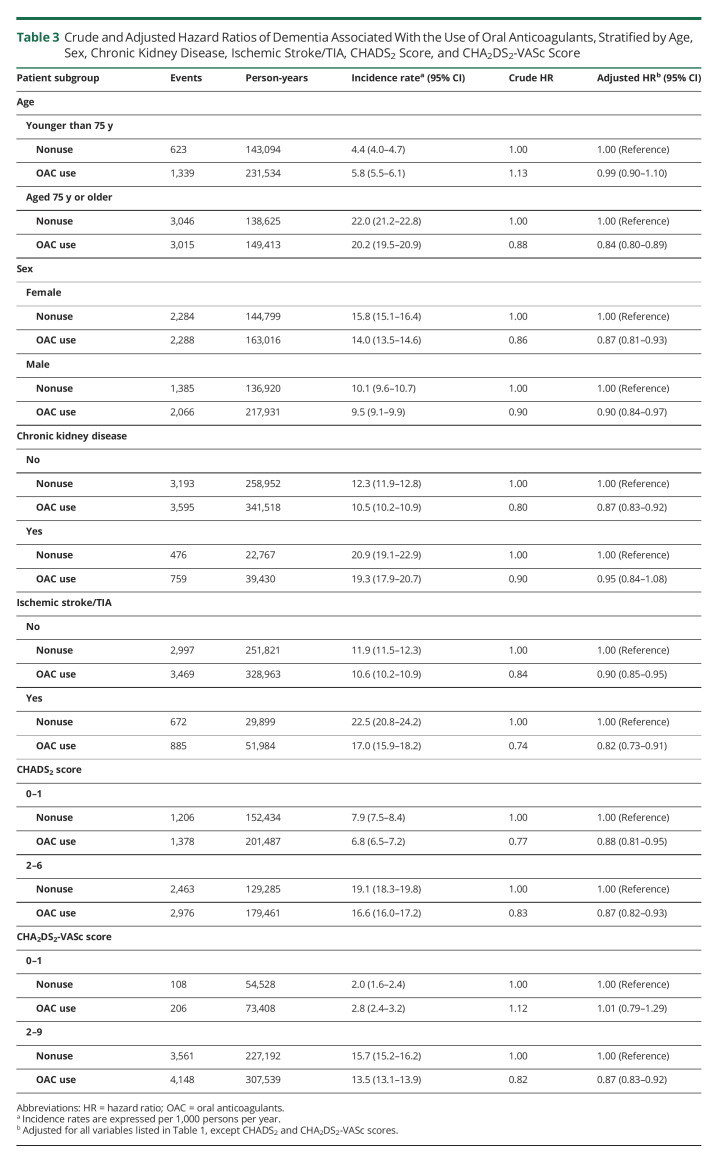
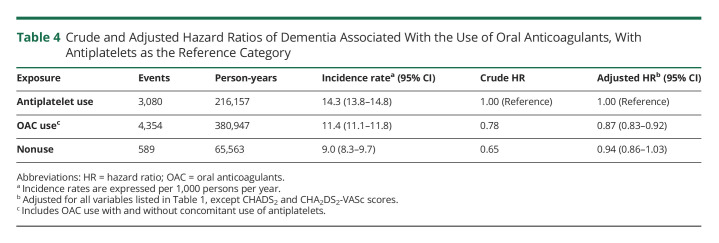
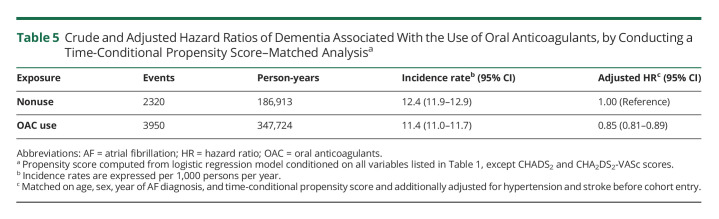
There were 8,023 incident cases of dementia over 662,667 person-years of follow-up, yielding an incidence rate of 12.1 (95% CI 11.9–12.4) per 1,000 person-years. OAC use was associated with a 12% reduction in the risk of dementia (HR 0.88, 95% CI 0.84–0.92), compared with nonuse, which did not vary with cumulative duration of use (Table 2). The spline, which modeled the HR as a function of cumulative duration of OAC use, also indicated a decreased risk of dementia, which reached a low at approximately 1.5 years and stabilized thereafter (Figure 1). OAC use was associated with a decreased risk of dementia in patients aged 75 years and older (HR 0.84, 95% CI 0.80–0.89), but not in younger patients (HR 0.99, 95% CI 0.90–1.10) (Table 3). The risk did not vary by sex, previous chronic kidney disease, previous ischemic stroke/TIA, or CHADS2 score. However, only patients with CHA2DS2-VASc score above 1 had a decreased risk of dementia associated with OAC use (HR 0.87, 95% CI 0.83–0.92). After considering antiplatelet use as an active comparator, OAC use was still associated with a decreased risk of dementia (HR 0.87, 95% CI 0.83–0.92) (Table 4). In addition, when assessing the risk of each dementia type separately, there was an association between OAC use and Alzheimer disease (HR 0.90, 95% CI 0.82–0.99) and unspecified dementia (HR 0.77, 95% CI 0.71–0.83), but not with other types of dementia (eTable 2, links.lww.com/WNL/C578). The risk of dementia was similar when comparing DOAC with VKA use (eTable 4). Finally, OAC use was associated with a reduced risk of dementia on censoring patients with stroke/TIA (HR 0.85, 95% CI 0.81–0.89), patients with stroke only (HR 0.86, 95% CI 0.82–0.90), or patients with stroke/TIA, excluding those without previous stroke/TIA at anytime before cohort entry (HR 0.86, 95% CI 0.81–0.91) (eTable 5). In the supplementary time-conditional PS-matched analysis, there was a similar association between OAC use and the risk of dementia (HR 0.85, 95% CI 0.81–0.89) as in the primary analysis (Table 5). The 2- and 5-year risk differences were 0.3% and 0.9%, respectively. Consequently, this generated corresponding NNT estimates of 298 and 114, respectively. In this supplementary analysis, OAC use was also associated with a decrease in the risk of unspecified dementia (eTable 3). Last, in all sensitivity analyses, results were consistent with those of the primary analysis (Figure 2 and eTables 6–12).
Table 2.
Crude and Adjusted Hazard Ratios of Dementia Associated With the Use of Oral Anticoagulants
Figure 1. Restricted Cubic Spline of Hazard Ratio for Risk of Dementia as a Function of Cumulative Duration of OAC Usea.
a. Cumulative duration of oral anticoagulant use begins at 0.5 years because patients were considered unexposed for the first 180 days of use. OACs = oral anticoagulants.
Table 3.
Crude and Adjusted Hazard Ratios of Dementia Associated With the Use of Oral Anticoagulants, Stratified by Age, Sex, Chronic Kidney Disease, Ischemic Stroke/TIA, CHADS2 Score, and CHA2DS2-VASc Score
Table 4.
Crude and Adjusted Hazard Ratios of Dementia Associated With the Use of Oral Anticoagulants, With Antiplatelets as the Reference Category
Table 5.
Crude and Adjusted Hazard Ratios of Dementia Associated With the Use of Oral Anticoagulants, by Conducting a Time-Conditional Propensity Score–Matched Analysisa
Figure 2. Forest Plot Summarizing the Results of Sensitivity Analyses for the Association Between the Use of OAC and Risk of Dementia.
OACs = oral anticoagulants.
Classification of Evidence
This study provides Class II evidence that in patients with NVAF, OAC use (vs nonuse) is associated with a decreased risk of dementia.
Discussion
In this large population-based cohort of patients with NVAF, OAC use was associated with a 12% decreased risk of dementia compared with non-use, which was evident within 1–2 years of use. This association was also present in patients aged 75 years and older and in those with a CHA2DS2-VASc score ≥2. The main findings were robust in numerous sensitivity analyses and remained consistent in the time-conditional PS-matched analysis.
The potential association between OAC use and the risk of dementia in patients with NVAF has been assessed in several observational studies, yet inconsistent results and methodological limitations have hampered definitive conclusions.7-10 Two studies reported 10% and 29% decreases, respectively, in the risk of dementia associated with OAC use.7,8 Both assessed exposure to OACs during a brief time window after NVAF diagnosis. However, patients may initiate OACs after this window, leading to considerable exposure misclassification, as acknowledged in one of these studies.7 Immortal time bias was present in 2 other studies reporting strong protective associations with 39% and 60% decreased risk of dementia, respectively.9,10 In addition, none of these studies considered an exposure latency time window, and most did not account for potential delays in dementia diagnosis. Our study comprised a well-defined cohort of patients with incident NVAF, without prior use of OACs. Some previous studies included patients with valvular AF7,10 or only those hospitalized with AF.7 Hence, our cohort may have a different baseline risk for dementia. Regarding the cumulative duration of exposure to OACs, our findings suggest that an association with a decreased risk of dementia is present relatively early after initiation of therapy.
Clinical guidelines for the management of NVAF recommend the use of OACs for stroke prevention, including in elderly and frail patients.13,15,29 However, elderly patients may have an increased risk of bleeding, given their multiple comorbidities or comedications.30 Thus, the decision to prescribe OACs is typically based on careful balancing of the perceived benefits and harms of treatment. Nevertheless, OACs remain underprescribed in the elderly individuals, even in those without any specific contraindications.31 Two previous studies have assessed the risk of dementia according to age, though with mixed results.7,8 Our findings suggest additional benefit of OACs in elderly patients with NVAF owing to their protective effect on dementia. In older adults, dementia is one of the leading causes of disability32 and projected to be a major public health crisis of the next decade,32 with 7.7 million new cases arising globally each year.3 NVAF is a notable risk factor for dementia,3 and the potential decreased risk associated with OACs in the elderly individuals warrants consideration during clinical decision-making. These findings may also explain the similar decreased risk of dementia associated with OAC use in patients with a CHA2DS2-VASc score ≥2 (i.e., high risk) but not with lower scores because all patients aged 75 years and older are classified as high risk by this score.
We also reported significantly lower risks of unspecified dementia and Alzheimer disease associated with OAC use. Although our analysis did not suggest an association with vascular dementia, the estimate may have been affected by residual confounding because patients with a high risk of stroke may be more likely to be prescribed OACs and to either develop vascular dementia or be labeled with vascular dementia rather than with unspecified dementia or Alzheimer disease. Alzheimer disease also has a strong vascular component,33 which may explain the observed protective association with OAC use. The decreased risk of unspecified dementia is also consistent with these findings, given that Alzheimer disease and vascular dementia are the most common types. OACs were also associated with a decreased risk of dementia after censoring patients who experienced a stroke or TIA during follow-up, which suggest that oral anticoagulation may be protective against dementia in patients with NVAF beyond overt ischemic stroke prevention. Finally, as an exploratory objective, we compared the risk of dementia between DOAC users and VKA users, and we did not observe any difference. However, due to their recent introduction into the market, the duration of follow-up among DOAC users was relatively short and prevented ideal comparison with VKA users. In a previous study of Korean patients with AF with a mean follow-up of less than 1.3 years, no association was found between the use of DOACs overall and the risk of dementia, compared with warfarin use.34 Hence, further studies with longer follow-up are still required to address this research question.
Our large population-based study has several notable strengths. First, the CPRD is one of the world's largest medical records databases of community-dwelling patients, enabling the selection of a cohort that is representative of the general population. Second, we selected a well-defined cohort of patients with incident NVAF without previous use of OACs to avoid potential biases associated with the inclusion of prevalent users. Third, we used exposure lags to account for a biologically plausible latency time window. Fourth, we used a time-varying exposure, which prevented time-related biases present in some prior studies. Fifth, we conducted a supplementary time-conditional PS-matched analysis that resulted in a similar protective association between OAC use and the risk of dementia, suggesting that the conclusions of this study remain robust to potential residual confounding. Some potential limitations must also be considered. Prescriptions recorded in the CPRD include those issued by general practitioners—misclassification may occur if patients do not follow the treatment regimen provided. In addition, prescriptions issued by specialists are not available in the CPRD. However, in the UK, NVAF is managed extensively by general practitioners,35 who are likely to issue most prescriptions, suggesting that the data sufficiently capture exposure to OACs. Outcome misclassification is also possible. In particular, dementia diagnoses may be under-recorded in the CPRD, resulting in low sensitivity. However, given that the specificity is high, there should be limited impact on our results.36 Furthermore, to address any potential limitations in specificity, we used a stricter outcome definition in sensitivity analyses, with results consistent with the primary analysis. However, the validity of dementia subtypes is less well-defined, and many recorded dementia cases are coded as dementia not otherwise specified. Finally, given the observational nature of the study, residual confounding is possible, with educational level and frailty being possible sources. In a post hoc analysis, we additionally adjusted for the UK Index of Multiple Deprivation, which can partially account for confounding due to education level, and obtained findings similar to those of our primary analysis. On the contrary, we were not able to specifically adjust for frailty. We attempted to indirectly account for it through adjustment for 54 variables in the primary analysis and an additional age, sex, and time-conditional PS-matched analysis that gave consistent results.
Overall, the use of OACs was associated with a decreased risk of dementia in patients with NVAF, specifically those aged 75 years and older. The proportion of older adults is projected to increase drastically in many high-income countries,32 leading to the recognition of dementia as an upcoming public health crisis.32 Regarding this, OAC therapy may be an effective strategy to reduce its burden in patients with NVAF. Thus, when balancing the risks and benefits of OAC use in these patients, the potential benefit of OACs in preventing dementia is an important consideration for the management of NVAF.
Acknowledgment
A. Rahman is supported by a Tomlinson Doctoral Fellowship from McGill University and a Canada Graduate Scholarship—Doctoral from the Canadian Institutes of Health Research. E.E.M. Moodie is a Canada Research Chair in Statistical Methods for Precision Medicine and holds a chercheur de merité career award from the Fonds de recherche Québec, Santé (FRQ-S). M. Durand is supported by a clinician-researcher salary award from the FRQ-S. J.R. Guertin is supported by a researcher salary award from FRQ-S.
Glossary
- AF
atrial fibrillation
- BMI
body mass index
- CPRD
Clinical Practice Research Datalink
- DOAC
direct oral anticoagulant
- HRs
hazard ratios
- IPCW
inverse probability of censoring weighting
- NNT
number needed to treat
- NVAF
nonvalvular atrial fibrillation
- OAC
oral anticoagulant
- PS
propensity score
- VKA
vitamin K antagonist
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
Study Funding
This work was supported by a grant-in-aid from the Heart and Stroke Foundation of Canada (G-19-0026399).
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Bunch TJ, Weiss JP, Crandall BG, et al. Atrial fibrillation is independently associated with senile, vascular, and Alzheimer's dementia. HeartRhythm. 2010;7(4):433-437. doi: 10.1016/j.hrthm.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Kalantarian S, Stern TA, Mansour M, Ruskin JN. Cognitive impairment associated with atrial fibrillation: a meta-analysis. Ann Intern Med. 2013;158(5_Part_1):338-346. doi: 10.7326/0003-4819-158-5-201303050-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alonso A, Arenas de Larriva AP. Atrial fibrillation, cognitive decline and dementia. Eur Cardiol Rev. 2016;11(1):49-53. doi: 10.15420/ecr.2016:13:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwok CS, Loke YK, Hale R, Potter JF, Myint PK. Atrial fibrillation and incidence of dementia: a systematic review and meta-analysis. Neurology. 2011;76(10):914-922. doi: 10.1212/wnl.0b013e31820f2e38. [DOI] [PubMed] [Google Scholar]
- 5.Park H, Hildreth A, Thomson R, O'Connell J. Non-valvular atrial fibrillation and cognitive decline: a longitudinal cohort study. Age Ageing. 2007;36(2):157-163. doi: 10.1093/ageing/afl164. [DOI] [PubMed] [Google Scholar]
- 6.Mavaddat N, Roalfe A, Fletcher K, et al. Warfarin versus aspirin for prevention of cognitive decline in atrial fibrillation: randomized controlled trial (Birmingham Atrial Fibrillation Treatment of the Aged Study). Stroke. 2014;45(5):1381-1386. doi: 10.1161/strokeaha.113.004009. [DOI] [PubMed] [Google Scholar]
- 7.Friberg L, Rosenqvist M. Less dementia with oral anticoagulation in atrial fibrillation. Eur Heart J. 2018;39(6):453-460. doi: 10.1093/eurheartj/ehx579. [DOI] [PubMed] [Google Scholar]
- 8.Mongkhon P, Fanning L, Lau WCY, et al. Oral anticoagulant and reduced risk of dementia in patients with atrial fibrillation: a population-based cohort study. HeartRhythm. 2020;17(5):706-713. doi: 10.1016/j.hrthm.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Kim D, Yang PS, Yu HT, et al. Risk of dementia in stroke-free patients diagnosed with atrial fibrillation: data from a population-based cohort. Eur Heart J. 2019;40(28):2313-2323. doi: 10.1093/eurheartj/ehz386. [DOI] [PubMed] [Google Scholar]
- 10.Ding M, Fratiglioni L, Johnell K, et al. Atrial fibrillation, antithrombotic treatment, and cognitive aging: a population-based study. Neurology. 2018;91(19):e1732-e1740. doi: 10.1212/wnl.0000000000006456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. doi: 10.1016/s0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 12.Rivard L, Friberg L, Conen D, et al. Atrial fibrillation and dementia: a report from the AF-SCREEN international collaboration. Circulation. 2022;145(5):392-409. doi: 10.1161/circulationaha.121.055018. [DOI] [PubMed] [Google Scholar]
- 13.Hindricks G, Potpara T, Dagres N, et al. ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 20202021;42:373-498. [DOI] [PubMed] [Google Scholar]
- 14.Ferri C, Del Pinto R. Is there a connection among atrial fibrillation, anticoagulant treatment, and dementia? Eur Heart J Suppl. 2020;22(Supplement_E):E79-E81. doi: 10.1093/eurheartj/suaa066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrade JG, Aguilar M, Atzema C, et al. The 2020 Canadian cardiovascular society/Canadian heart rhythm society comprehensive guidelines for the management of atrial fibrillation. Can J Cardiol. 2020;36(12):1847-1948. doi: 10.1016/j.cjca.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Mongkhon P, Naser AY, Fanning L, et al. Oral anticoagulants and risk of dementia: a systematic review and meta-analysis of observational studies and randomized controlled trials. Neurosci Biobehav Rev. 2019;96:1-9. doi: 10.1016/j.neubiorev.2018.10.025. [DOI] [PubMed] [Google Scholar]
- 17.Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: clinical practice research Datalink (CPRD). Int J Epidemiol. 2015;44(3):827-836. doi: 10.1093/ije/dyv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chisholm J. The Read clinical classification. BMJ. 1990;300(6732):1092. doi: 10.1136/bmj.300.6732.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Worms L. Uk's Nice Recommends Once-Dialy Lixiana (Edoxaban) for Preventing Stroke and Systemic Embolism in Patients with Non-valvular Atrial Fibrillation. Daiichi-Sankyo; 2015. [Google Scholar]
- 20.Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA. Statins and the risk of dementia. Lancet. 2000;356(9242):1627-1631. doi: 10.1016/s0140-6736(00)03155-x. [DOI] [PubMed] [Google Scholar]
- 21.Khan NF, Harrison SE, Rose PW. Validity of diagnostic coding within the general practice research database: a systematic review. Br J Gen Pract. 2010;60(572):e128-e136. doi: 10.3399/bjgp10x483562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunn N, Mullee M, Perry VH, Holmes C. Association between dementia and infectious disease. Alzheimer Dis Assoc Disord. 2005;19(2):91-94. doi: 10.1097/01.wad.0000165511.52746.1f. [DOI] [PubMed] [Google Scholar]
- 23.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicing stroke: results from the national registry of atrial fibrillation. JAMA. 2001;285(22):2864-2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 24.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263-272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 25.Harrell FE. Regression Modeling Strategies with Applications to Linear Models, Logistic Regression and Survival Analysis. Spinger-Verlag; 2001. [Google Scholar]
- 26.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550-560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20(1):40-49. doi: 10.1002/mpr.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suissa S, Moodie EEM, Dell'Aniello S. Prevalent new-user cohort designs for comparative drug effect studies by time-conditional propensity scores. Pharmacoepidemiol Drug Saf. 2017;26(4):459-468. doi: 10.1002/pds.4107. [DOI] [PubMed] [Google Scholar]
- 29.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the heart rhythm society in collaboration with the society of thoracic surgeons. Circulation. 2019;140(2):e125-e151. doi: 10.1161/CIR.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 30.Green L, Tan J, Morris JK, et al. A three-year prospective study of the presentation and clinical outcomes of major bleeding episodes associated with oral anticoagulant use in the UK (ORANGE study). Haematologica. 2018;103(4):738-745. doi: 10.3324/haematol.2017.182220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinberg BA, Greiner MA, Hammill BG, et al. Contraindications to anticoagulation therapy and eligibility for novel anticoagulants in older patients with atrial fibrillation. Cardiovasc Ther. 2015;33(4):177-183. doi: 10.1111/1755-5922.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin A, O'Connor S, Jackson C. A scoping review of gaps and priorities in dementia care in Europe. Dementia (London). 2020;19(7):2135-2151. doi: 10.1177/1471301218816250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toledo JB, Arnold SE, Raible K, et al. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer's Coordinating Centre. Brain. 2013;136(9):2697-2706. doi: 10.1093/brain/awt188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee SR, Choi EK, Park SH, et al. Comparing warfarin and 4 direct oral anticoagulants for the risk of dementia in patients with atrial fibrillation. Stroke. 2021;52(11):3459-3468. doi: 10.1161/strokeaha.120.033338. [DOI] [PubMed] [Google Scholar]
- 35.Adderley NJ, Ryan R, Nirantharakumar K, Marshall T. Prevalence and treatment of atrial fibrillation in UK general practice from 2000 to 2016. Heart. 2019;105(1):27-33. doi: 10.1136/heartjnl-2018-312977. [DOI] [PubMed] [Google Scholar]
- 36.Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;58(4):323-337. doi: 10.1016/j.jclinepi.2004.10.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study is based partly on data from the CPRD obtained under license from the UK Medicines and Healthcare products Regulatory Agency. Data are provided by patients and collected by the UK National Health Service as part of their care and support. The interpretation and conclusions contained in this study are those of the author/s alone. Because electronic health records are classified as “sensitive data” by the UK Data Protection Act, information governance restrictions (to protect patient confidentiality) prevent data sharing through public deposition. Data are available with approval through the individual constituent entities controlling access to the data. Specifically, the primary care data can be requested through application to the CPRD (cprd.com).