Abstract
Background Spontaneous intracranial hypotension (SIH) is a secondary cause of headache and an underdiagnosed disease. The clinical presentation can be highly variable. It typically presents with isolated classic orthostatic headache complaints, but patients can develop significant complications such as cerebral venous thrombosis (CVT).
Objective To report 3 cases of SIH diagnosis admitted and treated in a tertiary-level neurology ward.
Methods Review of the medical files of three patients and description of clinical and surgical outcomes.
Results Three female patients with SIH with a mean age of 25.6 ± 10.0 years old. The patients had orthostatic headache, and one of them presented with somnolence and diplopia because of a CVT. Brain magnetic resonance imaging (MRI) ranges from normal findings to classic findings of SIH as pachymeningeal enhancement and downward displacement of the cerebellar tonsils. Spine MRI showed abnormal epidural fluid collections in all cases, and computed tomography (CT) myelography could determine an identifiable cerebrospinal fluid (CSF) leak in only one patient. One patient received a conservative approach, and the other two were submitted to open surgery with laminoplasty. Both of them had uneventful recovery and remission in surgery follow-up.
Conclusion The diagnosis and management of SIH are still a challenge in neurology practice. We highlight in the present study severe cases of incapacitating SIH, complication with CVT, and good outcomes with neurosurgical treatment.
Keywords: Intracranial Hypotension, Hospitalization, Headache, Neurosurgical Procedures, Venous Thrombosis
RESUMO
Antecedentes Hipotensão intracraniana espontânea (HIE) é uma causa secundária de cefaleia e uma doença subdiagnosticada. A apresentação clínica pode ser muito variável. Tipicamente, se apresenta com queixas isoladas de cefaleia ortostática clássica, porém pode evoluir com complicações significativas como trombose venosa cerebral (TVC).
Objetivo Relatar 3 casos de diagnóstico de hipotensão intracraniana espontânea manejados em uma enfermaria de neurologia de nível terciário.
Métodos Revisão dos prontuários de três pacientes e descrição dos resultados clínicos e cirúrgicos.
Resultados Três pacientes do sexo feminino com média de idade de 25.6 ± 10.0 anos. As pacientes apresentavam cefaleia ortostática e uma delas apresentou sonolência e diplopia devido a TVC. A ressonância magnética (RM) do encéfalo varia de achados normais até achados clássicos de HIE como realce paquimeníngeo e deslocamento inferior das tonsilas cerebelares. A RM da coluna mostrou coleções anormais de líquido epidural em todos os casos e a mielografia por tomografia computadorizada (TC) foi capaz de determinar fístula liquórica identificável em apenas uma paciente. Uma paciente recebeu abordagem conservadora e as outras duas foram submetidas a cirurgia aberta com laminoplastia. Ambas tiveram recuperação e remissão sem intercorrências no seguimento cirúrgico.
Conclusão O diagnóstico e manejo da hipotensão intracraniana ainda são desafios na prática neurológica. Destacamos no presente estudo casos graves, complicação com TVC e bons resultados com tratamento neurocirúrgico.
Palavras-chave: Hipotensão Intracraniana, Hospitalização, Cefaleia, Procedimentos Neurocirúrgicos, Trombose Venosa
INTRODUCTION
Labeled as "hypoliquorrhea" in the first description, spontaneous intracranial hypotension (SIH) is a secondary cause of headache syndrome and an underdiagnosed disease with an estimated incidence of 5 per 100,000 people per year. Spontaneous intracranial hypotension is often an imprecise term because just about one-third of the cases have low cerebral spinal fluid (CSF) pressure. Most of the etiologies are associated with spinal liquoric fistulas or CSF leaks. 1
The main etiologies are: 1) dural tears; 2) dural weakness involving nerve root sleeves; and 3) CSF-venous fistula. Common causes of ventral dural tears are disk herniations and osteophytes. Cerebrospinal fluid extravasation from nerve root sleeves typically shows the occurrence of a meningeal diverticulum. Cerebrospinal fluid venous-fistula constitutes an abnormal connection of subarachnoid space to a spinal epidural vein and imposes a challenge to identifying CSF leakage in imaging studies. 2 Connective tissue matrix disorders, such as Marfan and Ehlers-Danlos syndrome, and trauma, are risk factors for SIH development. Lumbar puncture and trivial traumas or physical exercise are other possible causes. 3
The International Classification of Headache Disorders 3 rd edition (ICHD-3) defines diagnostic criteria for the condition: 1) headache must have a temporal relation to the low CSF pressure or leakage; 2) the presence of low CSF pressure (< 60 mm H 2 O) and evidence of CSF leak in imaging study (head magnetic resonance imaging [MRI], spinal MRI, myelography, myelographic computed tomography [CT], dynamic subtraction myelography); 3) no other better ICHD-3 diagnosis. 4
Complementary imaging studies are paramount for the management of SIH. Brain MRI typically shows signs of “sagging brain” as a result of dilatation of intracranial venous structures to compesate CSF spine loss. Subdural effusions, a decrease in the distance from the optic chiasma to the pituitary gland, contrast enhancement of dura mater, slit ventricular, and enlargement of the pituitary gland are typical findings. A spine MRI showing epidural fluid collections in T2-weighted images demonstrates the CSF spinal leakage, even though the exact point is not identified routinely. Finally, dynamic myelography and dynamic myelography CT are used to show the leakage and contrast extravasation to epidural space. 5
Spontaneous intracranial hypotension treatment encompasses general measures and specific procedures to cease CSF leakage. General measures comprise bed rest, increasing caffeine intake (200 mg tid – 300 mg qid) and oral overhydration. This conservative approach is a short course treatment. If the patient fails to improve, epidural blood patch (EBP) or operative surgical treatment are considered for definitive treatment. 1
Data related to SIH is limited worldwide. The present study aims to report three patients with SIH diagnosis treated at a general neurology ward.
METHODS
We accessed the medical records of three patients admitted to the neurology ward of Hospital São Paulo, Universidade Federal de São Paulo, São Paulo, Brazil, from September 2008 to January 2021, who had their final diagnosis as “liquoric fistula” CSF leak in the International Classification of Diseases – 10 (ICD-10) upon medical discharge.
The following variables were described: age, gender, presenting symptoms, duration of symptoms, neuroimaging, treatment, and clinical outcome. We followed-up all patients for 1 year for symptom recurrence. All patients were submitted to brain/spine MRI and dynamic myelography CT. Only one patient was submitted to head CT prior to brain/spine MRI for immediate differential diagnosis of secondary headache in the emergency department. Lumbar puncture was not performed in any of the patients because a normal CSF pressure does not exclude SIH.
RESULTS
The 3 patients ( Table 1 ) were women, with a mean age of 25.6 ± 10.0 years. Orthostatic headache was the main symptom, and one patient had an extensive cerebral venous thrombosis (CVT) ( Figure 1 ). Symptoms were present for 49.0 ± 61.73 days before seeking the neurology emergency room and hospitalization time was 39.9 ± 7.2 days. The main reason for a prolonged period of hospitalization was the unavailability of iohexol intrathecal radiographic contrast (Omnipaque, GE Healthcare, United States) in the hospital for CT myelography ( Figure 2 ) A limited-resource setting and the neurosurgical planning also contributed to this extended hospitalization. Patient 2 was the only patient submitted to a brain CT to rule out other possible critical diagnoses in the emergency department. Brain MRI had typical findings of SIH in two patients, and one had a normal exam. Spine MRI identified epidural CSF collection in all three patients, while CT spine myelography could determine the point of CSF leakage in only one. Treatment included general clinical measures (patient 1) and neurosurgical intervention (patients 2 and 3) with full recovery of the symptoms. These patients had no recurrence of the symptoms in 1 year of follow-up.
Table 1. Summary of characteristics of the patients admitted to the neurology ward.
| Patient | 1 | 2 | 3 |
|---|---|---|---|
| Age (years old) | 15 | 35 | 27 |
| Gender | Female | Female | Female |
| Symptoms | Orthostatic headache relieved by decubitus | Orthostatic headache relieved by decubitus Photophobia/phonophobia Nausea/vomit Dizziness Diplopia Excessive somnolence |
Orthostatic headache relieved by decubitus Photopohobia/phonophobia Nausea/vomit |
| Duration of symptoms | 4 months | 19 days | 8 days |
| Brain CT | − | Brain: cerebral venous thrombosis in superior sagittal sinus, straight sinus, bilateral transverse sinus, right sigmoid sinus, and right internal jugular vein. Bilateral tonsillar cerebellar downward displacement (0,7 cm). Decreased supratentorial and infratentorial ventricular space. | − |
| Brain/Spine MRI | Brain: no significant abnormalities Spine: posterior epidural CSF collection C7–T12 |
Brain: cerebral venous thrombosis in superior sagittal sinus, straight sinus, bilateral transverse sinus, right sigmoid sinus, and right internal jugular vein. Bilateral subdural effusions (thickness < 0.4 cm). Diffuse pachymeningeal enhancement on T1 weighted image after contrast. Bilateral tonsillar cerebellar downward displacement (0,5 cm). Decreased supratentorial and infratentorial ventricular space. ( Figure 1 ) Spine: anterolateral epidural CSF collection C2–T3 and T12–sacral segments. Posterior epidural CSF collection T8–T12 |
Brain: bilateral subdural collections (thickness < 0.9 cm). Diffuse pachymeningeal enhancement on T1 weighted image after contrast. Enlargement of the pituitary gland. Spine: anterior epidural CSF collection C6–T3. Posterior epidural CSF collection T6–T10. Epidural cervical contrast enhancement suggesting venous epidural plexus engorgement. |
| Myelography dynamic CT | Bilateral foraminal contrast extravasation C4. Posterior epidural contrast extravasation T4–L1. | Lateral and posterior epidural contrast extravasation C2–C7 ( Figure 2 ) |
Anteroposterior epidural contrast extravasation C5–T4. Linear contrast enhancement T3–T4. |
| Treatment | Conservative treatment | Neurosurgical treatment ( Figure 3 ) |
Neurosurgical treatment |
| Length of hospitalization | 31 days | 43 days | 44 days |
Abbreviations: CSF, cerebrospinal fluid; CT, computed tomography; MRI, magnetic resonance imaging.
Figure 1.
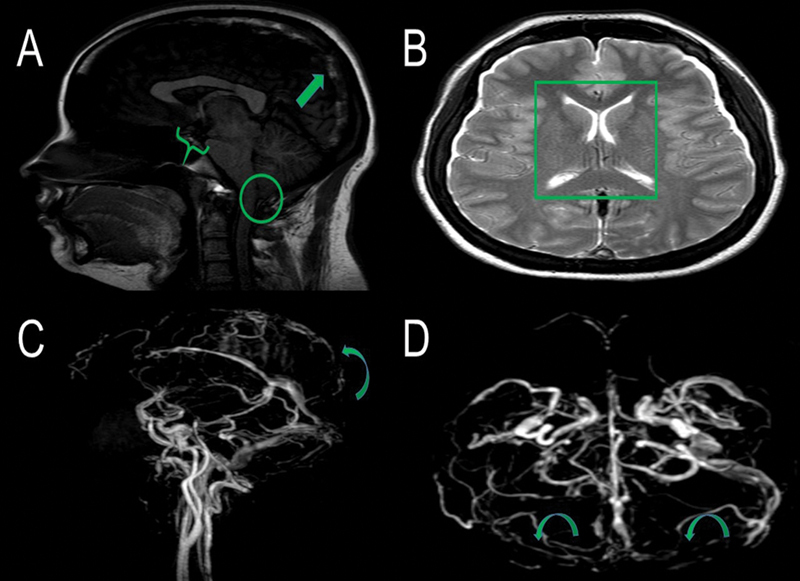
Brain magnetic resonance imaging (MRI) sagittal T1-weighted image (without contrast administration) (A) shows a hyperintense thrombus in the superior sagittal sinus (arrow), decreased mamillopontine distance (0,3 cm) (brace) and cerebelar tonsilar ectopia (0.5 cm) (CIRCLE). Brain MRI axial T2 weighted (B) image demonstrates collapsed lateral ventricles space (square). Brain MRI venography (C and D) shows a contrast filling defect in the topography of superior sagittal and bilateral transverse sinus suggesting CVT (curve arrows)
Figure 2.
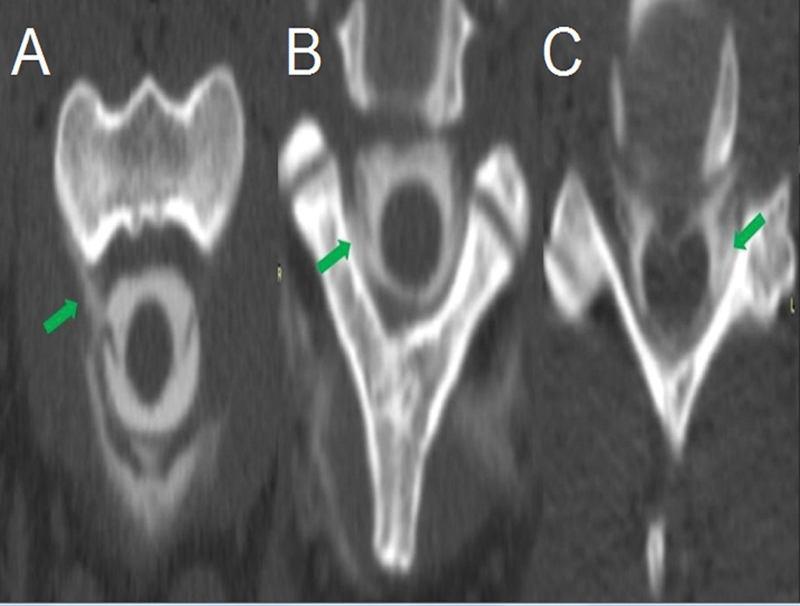
Computed tomography spine myelography shows contrast extravasation on right anterior C2 (A), right posterior C2-C3 (B) and left posterior C5-C6 (C) levels (arrows). From C2 to C7, there was contrast extravasation. These findings were consistent with CSF leak.
Patients diagnosed with spontaneous cerebrospinal hypotension refractory to conservative treatment were referred to surgical treatment. In anesthetic induction, intrathecal fluorescein was used at a concentration of 20%, diluted 1/10 with saline solution. A lumbar puncture injected 1ml of this solution after anesthetic induction. They underwent surgery in the prone position, with exposure of the cervical midline, with upper thoracic spine extension. A laminotomy was performed, guided by the points of fluorescein leakage in the interlaminar spaces, which had its detection extended using Glow800 filter (Leica Microsystems, Germany). Patient 2 had a fistulous orifice through the dura mater on its posterolateral aspect ( Figure 3 ). It could be explored and tightly closed with a simple suture strategy with 5.0 prolene and protected with fibrin glue. Patient 3 presented a large amount of fluid leakage without an identifiable orifice of CSF leak. During surgical exploration after extensive laminotomy, we could not identify the exact point of the dural tears. They were probably located in the anterior region of the dural sac, inaccessible to the posterior surgical approach. We performed an epidural packing, covering all the exposed epidural regions with a thin layer of Gelfoam and sealing with fibrin glue before replacing the lamina.
Figure 3.
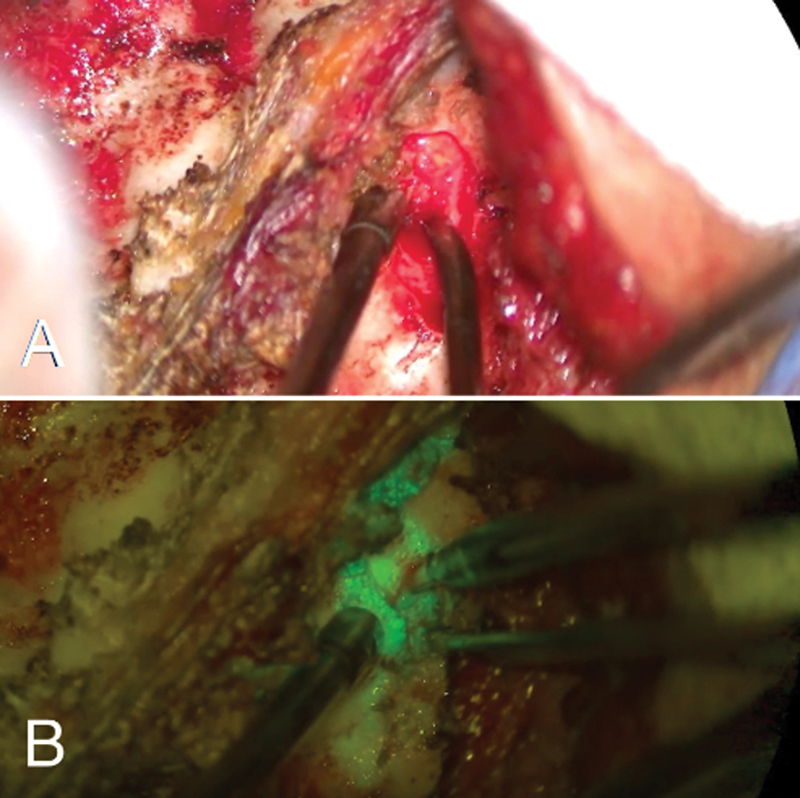
Conventional microscopy on posterior approach to cervical spine (A). The filter helps to accurately identify the fluorescein leak point, previously undetectable by conventional microscopy (B).
DISCUSSION
Spontaneous intracranial hypotension predominates in female and middle-aged patients. 6 Orthostatic headache is the most common symptom of SIH. A recent meta-analysis of 144 articles on SIH and 1,694 patients estimates a frequency of 92% of orthostatic headache in SIH. 7 Previous studies reveal a frequency of 75-80%. 1 The original description was of a headache that worsened within 15 minutes after standing up. However, the headache pattern can have a wide variety, like exertional or thunderclap. It can even be aggravated by decubitus, mimicking intracranial hypertension. 8 The tinnitus and auditory disturbances rates are estimated at 20 to 28%. Phonophobia (11%) and nausea/vomiting (54%) are common, and SIH is often misdiagnosed with migraine. 7 Diplopia and somnolence are infrequent symptoms. 1
Cerebral venous thrombosis is a rare complication of SIH. It encompasses 1 to 2% of patients with SIH. Slow flow of venous blood due to venous engorgement, increased viscosity of cerebral venous content by diminished CSF reabsorption in the dural sinus, and the mechanical distortion of the cerebral venous system are responsible for an increased risk of cerebral thrombosis. 9 Patient 2 initially received anticoagulation because of CVT, but it was interrupted upon the SIH diagnosis. Anticoagulation, in this scenario, may cause intracranial bleeding, increasing the size of previous subdural hematoma. Therefore, treatment should favor the CSF leakage closure. There is no final consensus regarding anticoagulation in these cases. 9
Pachymeningeal enhancement and "brain sagging" are the most typical signs in brain MRI. Other commonly reported findings are subdural effusions, venous, and pituitary gland engorgement. 10 11 12 In this series, one patient had a normal brain MRI, and ∼ 19% of SIH cases were present with a normal exam. 7 Head CT has low sensitivity and specificity for SIH-associated findings. A recent review of 40 cases with SIH showed that head CT was normal in 83.3% of the patients. 13 We performed head CT only in patient 2 to rule out other secondary causes of headache that would require immediate treatment due to the severity of her clinical symptoms.
In the spine MRI, epidural fluid collection is the most common finding. The frequency can vary from 67 to 100% in SIH. A Japanese study assessed the distribution of epidural fluid collections (ventral, dorsal, and circular). It detected a tendency of dorsal fluid accumulation at the thoracic level, ventral at the cervical and lumbar spine, and circular patterns at the junction levels. This distribution may be influenced by normal spine curvature that can collapse the dural sac inside the curvature direction. 14 We observed a similar pattern in two patients.
Computed tomography myelography is the method of choice in identifying the CSF leakage. Findings are determined by the extravasation flow: fast or short flow or no identifiable leakage. Slow-flow leakages are easier to determine and appear in the nerve root sleeve. It is paramount to observe that between 46 and 55% of CSF leaks will be undetermined. 15 The spinal fistula leaks are classified into type 1 (1a – ventral dural tear; 1b – dorsolateral dural tear), type 2 (meningeal diverticulum), type 3 (CSF-venous fistula), and type 4 (indeterminate/unknown). 16 In our case series, one patient had CSF bilateral cervical foraminal extravasation, which suggests a type 1b slow-flow leak. In the remaining patients, the leak could not be identified.
Complications of SIH include dural venous sinus thrombosis, subdural hematoma, and subarachnoid hemorrhage. Some of these complications are potentially life-threatening and should be recognized by imaging studies. 17
Treatment of SIH involves bed rest, oral hydration, and increasing caffeine intake. 1 Prednisone or fludrocortisone can be added. 18 19 However, a recent large meta-analysis found a response rate of 28% to these clinical measures. 7
EBP is a choice in patients who show no improvement with the conservative treatment. The procedure has the advantage of low adverse risks. The main complications are transient during or right after the procedure, including headache or back pains and transient paresthesia. 20 In a recent meta-analysis, nontargeted EBP and targeted EBP have similar success rates (69 versus 70%, respectively). EBP performed with a large quantity of autologous blood (> 20 ml) has a better response compared to the use of a small amount of blood (< 20 ml) (77 versus 66%, respectively). The meta-analysis did not include any randomized clinical trials, so its results must be interpreted with caution. 7
A neurosurgical approach is indicated for SIH refractory to EBP with a specific CSF leak site in imaging exams. The surgical exploration tries to localize extradural CSF and the leak site, performing direct dural repair. When it is impossible to determine the exact point of the leak, the main technique used is epidural packing, which consists in the USE of blood-soaked Gelfoam, fibrin glue, and muscle to generate a barrier where CSF leak can be reduced and the dural defect can close. 21 Our patients had possibly cervical leak, and performing EBP in the cervical region is a potentially high-risk procedure for neurological complications, such as direct spinal injury and spinal cord compression or infarct. Other potential complications are transient bradycardia, cranial nerve palsy, and seizures. Cervical EBP for cervical leaks compared with lumbar EBP tends to provide long-term relief 22 , and the efficacy of lumbar EBP is not fully established in this scenario. 23 The anesthesiology department of our institution does not execute cervical EBP because of the high risk of the procedure. Nontargeted EBP had lower efficacy than targeted EBP in several studies. 24 25 26 27 There is no final consensus on the potential difference of efficacy between targeted and nontargeted EBP. 7 It is important to notice that EBP has predictors of negative response, such as the presence of ≥ 4 brain MRI abnormalities (defined as pachymeningeal enhancement, brain sag, subdural fluids, pituitary enlargement, or venous engorgement). 24 Patient 2 had four poor predictors for EBP in brain MRI, and patient 3 had three poor predictors in brain MRI and had venous epidural plexus engorgement in spine MRI. We considered those patients unlikely to benefit from EBP.
Considering these specificities, we favored neurosurgical treatment for definitive treatment. Direct fistula repair using a microscopic filter and fluorescein was executed in patient 2 and epidural packing technique in patient 3. Both patients described symptom resolution in < 24 hours. They had no symptom recurrence or complications after surgery in a 1-year follow-up period. We believe that using the microscopic filter associated with intrathecal fluorescein provided less invasiveness to the surgical procedure, as the opening of the lamina could be restricted to the leak point.
Conflict of Interest The authors have no conflict of interests to declare.
Authors' Contributions
DGM: had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis; DGM, VLB, EMLO: contributed to the study conception and design, data acquisition, analysis and interpretation, and critical revision of the manuscript for important intellectual content; EPVF, HBF: contributed to the critical revision of the manuscript for important intellectual content; FJO: contributed to the study conception, design, and critical revision of the manuscript for important intellectual content; TLCC: contributed to the study conception, design, and acquisition of data. All authors and contributors have agreed to conditions noted on the Authorship Agreement Form.
References
- 1.Urbach H, Fung C, Dovi-Akue P, Lützen N, Beck J.Spontaneous Intracranial Hypotension Dtsch Arztebl Int 2020117(27-28):480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kranz P G, Malinzak M D, Amrhein T J, Gray L. Update on the Diagnosis and Treatment of Spontaneous Intracranial Hypotension. Curr Pain Headache Rep. 2017;21(08):37. doi: 10.1007/s11916-017-0639-3. [DOI] [PubMed] [Google Scholar]
- 3.Friedman D I.Headaches Due to Low and High Intracranial Pressure. Continuum (Minneap Minn) 2018. Aug;24(4, Headache):1066-1091. [DOI] [PubMed]
- 4. The International Classification of Headache Disorders 3 rd edition (ICHD-3) . Headache attributed to low cerebrospinal fluid (CSF) pressure 2021. [Acessed on 04/09/2021]. Available at:https://ichd-3.org/7-headache-attributed-to-non-vascular-intracranial-disorder/7-2-headache-attributed-to-low-cerebrospinal-fluid-pressure/
- 5.Kranz P G, Gray L, Malinzak M D, Amrhein T J. Spontaneous Intracranial Hypotension: Pathogenesis, Diagnosis, and Treatment. Neuroimaging Clin N Am. 2019;29(04):581–594. doi: 10.1016/j.nic.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Rahman M, Bidari S S, Quisling R G, Friedman W A.Spontaneous intracranial hypotension: dilemmas in diagnosis Neurosurgery 201169014–14., discussion 14 [DOI] [PubMed] [Google Scholar]
- 7.D'Antona L, Jaime Merchan M A, Vassiliou A. Clinical Presentation, Investigation Findings, and Treatment Outcomes of Spontaneous Intracranial Hypotension Syndrome: A Systematic Review and Meta-analysis. JAMA Neurol. 2021;78(03):329–337. doi: 10.1001/jamaneurol.2020.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schievink W I. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA. 2006;295(19):2286–2296. doi: 10.1001/jama.295.19.2286. [DOI] [PubMed] [Google Scholar]
- 9.Zhang D, Wang J, Zhang Q, He F, Hu X. Cerebral Venous Thrombosis in Spontaneous Intracranial Hypotension: A Report on 4 Cases and a Review of the Literature. Headache. 2018;58(08):1244–1255. doi: 10.1111/head.13413. [DOI] [PubMed] [Google Scholar]
- 10.Kranz P G, Tanpitukpongse T P, Choudhury K R, Amrhein T J, Gray L. Imaging Signs in Spontaneous Intracranial Hypotension: Prevalence and Relationship to CSF Pressure. AJNR Am J Neuroradiol. 2016;37(07):1374–1378. doi: 10.3174/ajnr.A4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mokri B.Spontaneous Intracranial Hypotension. Continuum (Minneap Minn) 2015. Aug;21(4 Headache):1086-108. [DOI] [PubMed]
- 12.Hoffmann J, Goadsby P J. Update on intracranial hypertension and hypotension. Curr Opin Neurol. 2013;26(03):240–247. doi: 10.1097/WCO.0b013e328360eccc. [DOI] [PubMed] [Google Scholar]
- 13.Li C, Raza H K, Chansysouphanthong T, Zu J, Cui G. A clinical analysis on 40 cases of spontaneous intracranial hypotension syndrome. Somatosens Mot Res. 2019;36(01):24–30. doi: 10.1080/08990220.2019.1566122. [DOI] [PubMed] [Google Scholar]
- 14.Yagi T, Horikoshi T, Senbokuya N, Murayama H, Kinouchi H. Distribution Patterns of Spinal Epidural Fluid in Patients with Spontaneous Intracranial Hypotension Syndrome. Neurol Med Chir (Tokyo) 2018;58(05):212–218. doi: 10.2176/nmc.oa.2017-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kranz P G, Luetmer P H, Diehn F E, Amrhein T J, Tanpitukpongse T P, Gray L. Myelographic Techniques for the Detection of Spinal CSF Leaks in Spontaneous Intracranial Hypotension. AJR Am J Roentgenol. 2016;206(01):8–19. doi: 10.2214/AJR.15.14884. [DOI] [PubMed] [Google Scholar]
- 16.Schievink W I, Maya M M, Jean-Pierre S, Nuño M, Prasad R S, Moser F G. A classification system of spontaneous spinal CSF leaks. Neurology. 2016;87(07):673–679. doi: 10.1212/WNL.0000000000002986. [DOI] [PubMed] [Google Scholar]
- 17.Girão M MV, Sousa R MP, Ribeiro M C, Cardoso T AMO, França Júnior M C, Reis F. Spontaneous intracranial hypotension and its complications. Arq Neuropsiquiatr. 2018;76(08):507–511. doi: 10.1590/0004-282X20180070. [DOI] [PubMed] [Google Scholar]
- 18.Russo C, Buono V, Fenza G, Zandolino A, Serino A, Manto A. Spontaneous intracranial hypotension: two steroid-responsive cases. Pol J Radiol. 2018;83:e229–e233. doi: 10.5114/pjr.2018.76380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rizk M, El Khatib M, Yamout B. Spontaneous intracranial hypotension syndrome treated with fludrocortisone. A A Case Rep. 2015;4(01):8–11. doi: 10.1213/XAA.0000000000000105. [DOI] [PubMed] [Google Scholar]
- 20.Davies M J, Davies M A, Sharpe R, Cordato D, Schwartz R. Epidural Blood Patch as a Diagnostic and Therapeutic Intervention in Spontaneous Intracranial Hypotension: A Novel Approach to Management. World Neurosurg. 2020;137:e242–e250. doi: 10.1016/j.wneu.2020.01.163. [DOI] [PubMed] [Google Scholar]
- 21.Cohen-Gadol A A, Mokri B, Piepgras D G, Meyer F B, Atkinson J L.Surgical anatomy of dural defects in spontaneous spinal cerebrospinal fluid leaksNeurosurgery 2006;58(4, Suppl 2):ONS-238–ONS-245, discussion ONS-245 [DOI] [PubMed]
- 22.Kapoor S G, Ahmed S. Cervical epidural blood patch–A literature review. Pain Med. 2015;16(10):1897–1904. doi: 10.1111/pme.12793. [DOI] [PubMed] [Google Scholar]
- 23.Wang E, Wang D. Successful treatment of spontaneous intracranial hypotension due to prominent cervical cerebrospinal fluid leak with cervical epidural blood patch. Pain Med. 2015;16(05):1013–1018. doi: 10.1111/pme.12418. [DOI] [PubMed] [Google Scholar]
- 24.Pagani-Estévez G L, Cutsforth-Gregory J K, Morris J M. Procedural predictors of epidural blood patch efficacy in spontaneous intracranial hypotension. Reg Anesth Pain Med. 2019;•••:rapm-2018-000021. doi: 10.1136/rapm-2018-000021. [DOI] [PubMed] [Google Scholar]
- 25.He F F, Li L, Liu M J, Zhong T D, Zhang Q W, Fang X M. Targeted Epidural Blood Patch Treatment for Refractory Spontaneous Intracranial Hypotension in China. J Neurol Surg B Skull Base. 2018;79(03):217–223. doi: 10.1055/s-0037-1606312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith K A. Spontaneous intracranial hypotension: Targeted or blind blood patch. J Clin Neurosci. 2016;25:10–12. doi: 10.1016/j.jocn.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Cho K I, Moon H S, Jeon H J, Park K, Kong D S. Spontaneous intracranial hypotension: efficacy of radiologic targeting vs blind blood patch. Neurology. 2011;76(13):1139–1144. doi: 10.1212/WNL.0b013e318212ab43. [DOI] [PubMed] [Google Scholar]


