Abstract
Chemotherapy—induced gastrointestinal dysfunction is a common occurrence associated with many different classes of chemotherapeutic agents. Gastrointestinal toxicity includes mucositis, diarrhea, and constipation, and can often be a dose-limiting complication, induce cessation of treatment and could be life threatening. The gastrointestinal epithelium is rich in rapidly dividing cells and hence is a prime target for chemotherapeutic drugs. The incidence of gastrointestinal toxicity, including diarrhea and mucositis, is extremely high for a wide array of chemotherapeutic and radiation regimens. In fact, 60%–100% of patients on high-dose chemotherapy suffer from gastrointestinal side effects. Unfortunately, treatment options are limited, and therapy is often restricted to palliative care. Therefore, there is a great unmet therapeutic need for preventing and treating chemotherapy-induced gastrointestinal toxicities in the clinic. In this review, we discuss our current understanding of the mechanisms underlying chemotherapy-induced diarrhea and mucositis, and emerging mechanisms involving the enteric nervous system, smooth muscle cells and enteric immune cells. Recent evidence has also implicated gut dysbiosis in the pathogenesis of not only chemotherapy-induced mucositis and diarrhea, but also chemotherapy-induced peripheral neuropathy. Oxidative stress induced by chemotherapeutic agents results in post-translational modification of ion channels altering neuronal excitability. Thus, investigating how chemotherapy-induced changes in the gut- microbiome axis may lead to gut-related toxicities will be critical in the discovery of new drug targets for mitigating adverse gastrointestinal effects associated with chemotherapy treatment.
1. Introduction
Current chemotherapeutic regimens do not differentiate between cancer cells and normal cells such as those lining the epithelium of the gastrointestinal tract. This non-specific targeting of rapidly dividing cells results in many gastrointestinal related side effects. Chemotherapy-induced gastrointestinal (GI) toxicities are prevalent among a wide array of chemotherapeutic and radiation regimens. It is estimated that 40% of patients receiving standard dose chemotherapy will develop GI-related toxicities, while significantly greater incidence rates have been reported in patients receiving higher drug doses. In fact, it is estimated that approximately 60%–100% of patients on high dose chemotherapy will experience GI toxicities (Cinausero et al., 2017; Dahlgren et al., 2021; Sonis, 2004). Clinical symptoms typically manifest as nausea, constipation, vomiting, diarrhea, abdominal pain, weight loss and ulcerations within the mucosa (Cinausero et al., 2017; Kwon, 2016; Sonis, 2004). The prevalence and severity of these GI toxicities is dependent on the type of chemotherapy and the dose regimen. Treatment of patients is often limited to symptom management and palliative care as currently no preventative treatments exist. Clinicians are frequently left with the difficult choice of interrupting or altering the chemotherapy regimen or even prematurely discontinuing treatment, ultimately reducing desirable outcomes, increasing hospital stays, impairing patients’ quality of life and increasing their economic burden (Cinausero et al., 2017; Elting et al., 2003). Given the pervasiveness and challenges in treating chemotherapy-induced gastrointestinal toxicities, it is of great interest to review the mechanisms contributing to development of these morbidities. A large number of chemotherapeutic agents used for the treatment of different cancers affect the epithelial barrier integrity. The gut epithelium contains rapidly dividing cells and therefore presents a significant target for the chemotherapeutic agents acting either directly or indirectly to initiate the disruption of the epithelial barrier. The current understanding of underlying mechanisms that contribute to these toxicities involve enterocytes, smooth muscle, enteric neurons, and immune cells.
2. Chemotherapy-induced diarrhea
Diarrhea is a common side effect of chemotherapy, especially in patients suffering from advanced cancers. It is estimated that about 50%–80% of cancer patients suffer from chemotherapy-induced diarrhea (CID) (Stein et al., 2010). CID is associated with a failure to retain fluid and electrolytes resulting in severe dehydration and electrolyte imbalances, malnutrition, or renal and cardiac dysfunction, all of which can lead to hospitalization and in severe cases, death. Diarrhea is characterized by increased stool frequency, liquidity, and decreased consistency of stools (Table 1). Diarrhea can be classified as secretory, osmotic, or infectious. Secretory diarrhea occurs due to increased chloride secretion from the epithelium to the lumen. The increase in intraluminal fluid can stimulate gastrointestinal motility and can be initiated by drugs acting on intestinal epithelial cells (Said, 2018). Osmotic diarrhea occurs as a result of increased water being drawn into the lumen. This can be a product of both malabsorption and maldigestion pathways. This diarrhea is often associated with high stool osmolarity gap (Said, 2018). Infectious diarrhea due to viruses such as Rotavirus or by bacteria including Escherichia coli and Salmonella, increase chloride secretion by crypt cells to produce watery diarrhea.
Table 1.
Diarrhea scoring criteria adapted from Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 (National Cancer Institute, 2017).
| Grade | Description |
|---|---|
| 1 | Increase of <4 stools per day over baseline |
| 2 | 4–6 stools per day |
| 3 | <7 stools per day, incontinence, hospitalization |
| 4 | Life threatening, urgent intervention |
| 5 | Death |
The pathophysiology of CID is complex and involves multiple factors. It has been suggested that acute damage to the intestinal epithelium, including to the architecture of the crypts and villi, plays a significant role in disrupting the secretory and absorptive functions of the intestinal wall. Preclinical studies have shown that chemotherapeutics, such as irinotecan, induce significant apoptosis in intestinal crypt cells within the colon and jejunum. The need to compensate for the enhanced rate of apoptosis can result in metaplasia of goblet cells and increased production of immature secretory cells, thus contributing to the exacerbated mucus production associated with CID (Gibson et al., 2003; Ikuno et al., 1995; McQuade, Stojanovska, et al., 2016). Impaired absorption across the intestinal epithelium due to atrophy of the villi can result in greater retention of contents within the lumen. This can produce an osmotic shift which drives water into the lumen, further contributing to the onset of diarrhea (Gibson & Keefe, 2006; McQuade, Stojanovska, et al., 2016; Stringer et al., 2007). CID is largely believed to be a form, or by-product, of intestinal mucositis characterized by mucosal injury presenting as inflammation and ulceration, resulting in alterations of intestinal microflora and GI secretion. Additionally, emerging evidence also implicates the enteric nervous system, which is known to regulate the motor and secretomotor functions of the GI tract. The mechanisms underlying the development of CID are discussed in detail in the subsequent sections. Table 2 provides a list of drugs that are known to cause CID and specific drug types commonly associated with CID have been described in more detail below.
Table 2.
Drugs associated with high incidence of chemotherapy-induced diarrhea.
| Drug class | Drug examples | Severity (diarrhea grade 1–4) |
|---|---|---|
| Topoisomerase inhibitors | Irinotecan | 16%−22% grade 3–4 (Stein et al., 2010) |
| Topotecan | ||
| Etoposide | ||
| Teniposide | ||
| Alkylating agents | Cyclophosphamide | All grades 20% (Boussios et al., 2012; Fraiser et al., 1991; McQuade, Stojanovska, et al., 2016; Sang et al., 1997) |
| Ifosfamide | ||
| Melphalan | ||
| Busulfan | ||
| Carmustine and lomustine | ||
| Dacarbazine | ||
| Chlorambucil | ||
| Melphalan | ||
| Mechlorethamine | ||
| Small molecules | Epidermal growth factor inhibitors | All grades 34%−96% (Secombe et al., 2020; Ustaris et al., 2015) |
| Human epidermal growth factor receptor 2 inhibitors (neratinib, Lapatinib) | ||
| Tyrosine kinase inhibitors | ||
| Mitogen-activated protein kinase kinase inhibitors | ||
| BCR-ABL Kinase inhibitors | ||
| Antimetabolites | 5-Fluorouracil | 80% all grades (Maroun et al., 2007) |
| Capecitabine | ||
| Methotrexate | ||
| Mitotic inhibitors | Paclitaxel | 50% grade 1–2 (Boussios et al., 2012) |
| Docetaxel | ||
| Vinblastine | ||
| vincristine | ||
| Platinum based agents | Cisplatin | 70%−80% grade 1–2 (McQuade, Stojanovska, et al., 2016) |
| Carboplatin | ||
| Oxaliplatin | ||
| m-TOR inhibitors | Everolimus | 34% grade 1–2 (Kamp et al., 2013); 1%−4% grade 3 (Stein et al., 2010) |
| Temsirolimus |
3. Chemotherapy-induced mucositis (CIM)
Damage to the gastrointestinal mucosa is a common consequence of both radiation therapy and chemotherapy. Chemotherapy-induced mucositis (CIM) is a major dose limiting side effect that can affect many parts of the gastrointestinal tract, although predominantly in the small intestine, oral and oropharyngeal mucosal linings (Cinausero et al., 2017; Dahlgren et al., 2021). As such CIM has been considered a great unmet clinical challenge to improving patient’s cancer outcomes. To date a majority of the research has been focused on oral mucositis rather than gastrointestinal mucositis. This is largely due to accessibility of the tissue area. In this section we first describe the structure of the intestinal epithelium and the function of constituent cell types. We then cover the specific pathobiological mechanisms as well as current and emerging treatment targets, with a focus on the gastrointestinal changes brought on by chemotherapy. For a more thorough review of chemotherapy induced oral mucositis and radiation therapy induced mucositis, readers are encouraged to consult the following reviews (Cinausero et al., 2017; Dahlgren et al., 2021; Kwon, 2016; Sonis, 2004).
3.1. Intestinal epithelium
The intestinal epithelium is a continuous monolayer of cells that forms a physical and biochemical barrier for host defense against luminal contents through a multitude of neuro-immune-epithelial interactions with the gut microbiota. These interactions are important for maintaining a symbiotic relationship with commensal gut bacteria. Perturbation of this steady state can result in microbial dysbiosis and invasion by pathogenic organisms, and lead to mucositis, dysregulation of intestinal activity and systemic inflammation.
The structure of the epithelium, and prevalence and function of the cell types constituting the epithelium vary between the small and the large intestines. In the small intestine, the epithelium extends into the lumen to form structures called villi, which greatly enhance the surface area for absorption of nutrients. Villi are not observed in the colon. In between the villi are invaginations called the crypts of Lieberkühn. Intestinal epithelial stem cells are present at the base of the crypts and give rise to transit-amplifying cells, which differentiate and mature into various cell types as they migrate towards the tip of the villi. These terminally differentiated cell-types, with the exception of Paneth cells, are subsequently shed into the lumen and as a result under homeostatic conditions the entire intestinal epithelium is renewed every 4–5 days (Allaire et al., 2018; van der Flier & Clevers, 2009). An unregulated increase in the intestinal epithelial cell death affects tissue restitution underlying many of the gastrointestinal toxicities.
The formation and maintenance of the crypt-villus organization of the intestinal epithelium rely on interactions between the epithelium and the underlying mesenchyme. Hedgehog signals from intestinal epithelial cells in the crypts induce bone morphogenetic protein (BMP) in the underlying mesenchyme, which in turn promotes the formation of villi, while inhibiting ectopic crypt formation. Studies have shown that inhibition of hedgehog signaling, or attenuation of BMP signal transduction prevents villi formation and overactivation of crypt-like domains (Haramis et al., 2004; He et al., 2004; Madison et al., 2005). Alternately, endogenous inhibitors of BMP such as noggin and gremlin produced in the inter-villus domains protect the crypts from BMP-mediated inhibition and sustains crypt proliferation. Furthermore, WNT-β-catenin and Notch signaling in intestinal epithelial cells in the crypts co-operate to maintain cell proliferation and determine cell fate i.e., differentiation into the various secretory and absorptive cell types (Crosnier et al., 2006; van der Flier et al., 2009).
The different species of epithelial cells include enterocytes (also referred to as colonocytes in the large intestine), which mediate absorption of nutrients and water, but can also secrete antimicrobial peptides; goblet cells, which secrete mucins and antimicrobial peptides, and play an important role in antigen sensing and priming of the immune system; enteroendocrine cells; and tuft cells that provide protection against helminths. Other cell types such as Paneth cells and microfold (M) cells are exclusively found in the small intestine. Paneth cells are intercalated with stem cells at the base of the crypts and secrete granules containing antimicrobial peptides, such as defensins, C-type lectin regenerating islet-derived III proteins, lysozyme C, cathelicidins, and phospholipases (Bevins & Salzman, 2011; Gallo & Hooper, 2012), to protect nearby stem cells, whereas M cells are specialized antigen presenting epithelial cells localized to Peyer’s patches. In the Peyer’s patches M cells present luminal antigens and live bacteria to the underlying mucosal immune system in order to prime the immune cells to the luminal microbial environment. The lymphoid follicle-associated epithelium of the Peyer’s patches can produce low levels of antimicrobial peptides, mucus, and IgA. Additionally, the intestinal epithelium is surveilled by subepithelial dendritic cells and resident macrophages, which sample the luminal microbial environment through transepithelial dendritic processes (Allaire et al., 2018; Peterson & Artis, 2014).
3.2. Pathobiological mechanisms of CIM
The pathological mechanisms that drive the specific changes in CIM are generally tissue (oral vs intestinal) and insult (chemotherapy vs radiation therapy) independent (Sonis, 2004). Chemotherapy-induced gastrointestinal mucositis is histologically characterized by crypt loss, villus atrophy, loss of renewal capacity, and impairment of the gut absorptive and barrier function (Bajic et al., 2018; Dahlgren et al., 2021). Originally thought to be an insult purely to the epithelium of the GI tract, we now understand CIM to be the result of a complex biological pathway that begins before patients often experience symptoms (Cinausero et al., 2017; Sonis, 2004). A well-established five-step model was initially proposed for the development of mucositis by a panelist established by The Multinational Association of Supportive Care in Cancer and the International Society for oral oncology. The initial clinical practice guidelines have been periodically modified over time (Bowen et al., 2019).
The first stage of mucositis begins with an initiation phase and the formation of reactive oxygen species (ROS) with direct damage to the DNA of cells throughout the tissue (submucosa, basal layers, endothelium) initiating both the innate immune response and the subsequent secondary messenger cascade likely within short order after DNA strand damage (Cinausero et al., 2017; Dahlgren et al., 2021). These initial events are followed by an upregulation and secondary messenger generation phase. ROS and the local immune cells further disrupt the tissue by stimulating macrophages and activating a host of secondary messengers such as Wnt/β-catenin, p53, caspase-1/3, Bcl-2 (Cinausero et al., 2017; Dahlgren et al., 2021; Sonis, 2004). NF-κB is likewise activated and is one of the most studied secondary messengers in the pathogenesis of CIM mainly because of its role in activating pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), IL-6, IL-1, IL-18, and IL-33 (Dahlgren et al., 2021; Guabiraba et al., 2014). This is then followed by a signal amplification phase, leading to increasing inflammation by the collective effects of many of the pathways that are activated by the aforementioned cytokines and secondary messengers. For example, the activation of TNF-α by NF-kB in turn activates yet more NF-kB which then activates MAPK and the JNK pathway and a breakdown of fibronectin and further macrophage activation. Ultimately the downstream effect of the NF-kB dependent and independent pathways is an increase in mucosal inflammation and increased apoptosis, resulting in an ulceration phase characterized by damage of the epithelial barrier promoting bacterial translocation and wound colonization. In this phase, loss of stem cells from the crypts due to apoptosis leads to a diminished renewal capacity and the inability to replenish the intestinal epithelial cells (IEC), and results in an ulcer formation allowing for increased translocation of luminal contents to the submucosa including bacterial colonization of the ulcer, ultimately promoting even more inflammatory signal cascades and activating pro apoptotic genes (Cinausero et al., 2017; Dahlgren et al., 2021; Sonis, 2004). Clinically, symptoms become evident at this stage, approximately 1–2 days after treatment in the case of CIM of the small intestines (enteritis) which is 10 times more likely to experience spontaneous apoptosis than the large intestines (Bowen et al., 2006; Dahlgren et al., 2021). Finally, a post ulcerative healing phase with cell proliferation and barrier recovery occurs once chemotherapy has ceased. This phase is characterized by the restoration of physiologically normal proliferation, maturation and differentiation of both progenitor stem cells within the crypts and subsequent epithelial layers (Cinausero et al., 2017; Dahlgren et al., 2021; Sonis, 2004).
Beyond the canonical DNA damage/ROS initiated pathway established by Bowen et al (Bowen et al., 2019), activation of toll-like receptors (TLRs), which are a group of pattern recognition receptors that can recognize pathogen associated molecular patterns such as lipoteichoic acid (LTA) and lipopolysaccharide (LPS) from various gram positive and gram-negative microbes is another important mechanism by which CIM may occur. TLRs are expressed throughout the gastrointestinal tract by epithelial and immune cells, and TLRs have been a focus of research due to their role in mediating the balance between the gut microbial flora and activated immunity. With specific regards to TLR-2, pre-clinical evidence has implicated TLR-2 in a mucosal protective role by downregulating the excessive activation of inflammatory cells and promoting the expression of the anti-inflammatory cytokine IL-10. However, some reports have also implicated TLRs in the induction of intestinal inflammation via production of NF-kB through MyD88-dependent and -independent pathways (Lupi et al., 2020; Wu et al., 2020). Studies examining the role different chemotherapeutics might play on the expression of TLRs have been recently examined by Wei et al. (Wei, Wen, & Xian, 2021), where the authors discussed the body of literature around TLRs with particular interest in TLR-2, TLR-4 and TLR-9. Interestingly, the authors found that while generally 5-FU and methotrexate (MTX) showed an increase in TLR-2, 4 and 9, conflicting evidence exists between animal models and species. Overall, findings from the clinical studies and animal model experiments suggested that the effects of chemotherapeutic agents on the expression of TLRs vary significantly with the types of drugs, dosages, species and routes of administration across models (Wei et al., 2021).
Drug metabolizing enzymes (DMEs) are an understudied but potentially important component of chemotherapy induced GI toxicity. In their recent review Tao et al. (2020) reported that altered activity of DMEs play an important role in chemotherapy-induced GI toxicity. The predominant DME in the gut are suggested to be the cytochrome P450 (CYP) and UDP-glucuronosyltransferase (UGT) families. These enzymes (particularly the CYP super family) are responsible for reactive metabolism with alkylating agents, antimetabolites, topoisomerase inhibitors and tyrosine kinase inhibitors. A noted example are the metabolizing effects on irinotecan (detailed below). Lapatinib has been demonstrated to inhibit CYP3A4, while paclitaxel, erlotinib, tamoxifen, ifosfamide, flutamide and docetaxel all activated the pregnane X receptor (PXR) and altered CYP3A4 expression (Tao et al., 2020).
3.3. Chemotherapeutics implicated in CIM and CID
Severity of CIM can often depend on the specific chemotherapeutic used. For example, the antimetabolites, 5-FU (Abdel-Rahman et al., 2016; Schwab et al., 2008) methotrexate, and irinotecan (Mayo et al., 2017; Stein et al., 2010), the alkylating agents such as cyclophosphamide, and cisplatin (Villa & Sonis, 2015), anthracyclines and lastly taxanes (Kwon, 2016) all tend to be more consistently associated with mucosal toxicities than bleomycin, hydroxyurea, or etoposide (Cinausero et al., 2017).
One of the most studied chemotherapeutics related to CIM/CID is irinotecan. Irinotecan is a prodrug that is metabolized by hepatic and systemic carboxylesterases to its active form, SN-38, which is 100–1000 times more potent than its parent compound (Stein et al., 2010). SN-38 inhibits topoisomerase I leading to single stranded DNA breaks. Activation of death signals induces pro-apoptotic proteins (caspase-9 and-3 and p53) via up-regulation of MAPK signaling and downregulates the anti-apoptotic PI3K/Akt and BcL-xL mediated pathways (Bowen et al., 2007; Koizumi et al., 2005; Takeba et al., 2007). SN-38 is inactivated through glucuronidation by hepatic uridine diphosphate glucuronosyltransferase to the inactive metabolite SN-38G and excreted into the gastrointestinal tract through the bile duct (Sun et al., 2020). In the GI tract β-glucuronidases produced by the gut bacteria cleave the glucuronide moiety, thus transforming the inactive SN-38G back to the active SN-38 (Bhatt et al., 2020). Consequently, accumulation of active SN-38 within the intestinal lumen leads to enhanced local toxicity and as a result causes significant damage to the intestinal wall (Stringer et al., 2008). As such, in animal models, irinotecan produces a dose and time dependent induction of villous atrophy and crypt ablation in the small intestine and severe colonic mucosal damage with crypt hypoplasia (Stringer, Gibson, Bowen, et al., 2009; Stringer, Gibson, Logan, et al., 2009). The damage to the mucosa contributes ultimately to the severe diarrhea, a hallmark of this chemotherapeutic. Diarrhea occurs within minutes of administration and can last up to 24h. The diarrhea produced immediately post drug administration is believed to be mediated through inhibition of acetylcholinesterase, which leads to increased cholinergic transmission (Stein et al., 2010). Acetylcholine is the major excitatory neurotransmitter in the gut and enhanced cholinergic signaling can lead to increased muscle contractions and fluid secretion, the basis for enhanced motility and secretion in diarrhea (Furness, 2007). Thus, atropine, the muscarinic receptor antagonist, is prescribed as a first-line therapy for mitigating the initial diarrhea (Schiller et al., 2017). However, late-onset diarrhea is chronic, atropine-insensitive, and is associated with significant intestinal mucositis (Bowen et al., 2007; Stein et al., 2010). Interestingly, CIM occurs more frequently with a combination of irinotecan and fluoropyrimidines such as capecitabine. For example, patients treated with the capecitabine, and irinotecan (XELIRI) regimen reported higher gastrointestinal toxicity than with fluorouracil and irinotecan (FOLFIRI). For combination regimens with fluorouracil and oxaliplatin (FOLFOX) or FOLFIRI, CIM has been estimated at 50% and 89%, respectively (Basile et al., 2019).
5-Fluorouracil (5-FU) is used in the treatment of variety of malignancies. 5-FU is converted to fluorodeoxyuridine monophosphate (FdUMP), which complexes with thymidylate synthase and prevents DNA replication and repair. 5-FU can also serve as a pyrimidine analog incorporating in place of uracil or thymine. This inhibition of DNA repair machinery results in cell death. 5-FU induced apoptosis of enterocytes results in a reduction of crypt and villi length, fusion of villi, enterocyte hyperplasia and increased apoptosis of crypt cells (Cinausero et al., 2017; Dahlgren et al., 2021; Kwon, 2016; Lee, 2014; Soares et al., 2008). The most common adverse effect reported with the systemic administration of 5-FU is diarrhea due to mucositis. As described above, mucositis involves epithelial damage due to ROS which can have significant effects on secretory processes resulting in diarrhea. Additionally, use of leucovorin in combination with fluoropyrimidines results in increased prevalence of this 5-FU induced diarrhea. The specific mechanisms whereby 5-FU induces toxicity are varied across the pre-clinical literature. In rats, Logan et al. (Logan, Gibson, Sonis, & Keefe, 2007) found that 5-FU elevated levels of TNF-α and IL-1β but not of NF-κB and IL-6 while other studies have noted increases in transcription of NF-kB (Lee, 2014). In general, a specific role for pro-inflammatory cytokines and proteins involved in apoptosis regulation has been suggested by many studies involving 5-FU (Basile et al., 2019; Lee, 2014; Ribeiro et al., 2016).
Platinum-based chemotherapeutic drugs, including cisplatin, form interstrand and intra-strand crosslinks with nucleic acid bases creating DNA adducts which inhibit DNA synthesis and transcription (Boussios et al., 2012; Stojanovska et al., 2015). The oxidative stress resulting from mitochondrial damage induces pro-apoptotic proteins (Bak, Bax, p38 MAPK, and p53), the activation of NF-kB and TNF-α signaling pathways as well as downstream activation of inflammatory cytokines and chemokines in the intestinal epithelium to induce gastrointestinal toxicity (Qi et al., 2019). Platinum agents are also known to elicit immunogenic cell death through activation of damage associated molecular patterns (DAMPs), such as high mobility group protein B1(HMGB1) and calreticulin (Stojanovska et al., 2015, 2016). When administered as single drug therapies, oxaliplatin and carboplatin exhibit lower GI toxicity compared to cisplatin (Hartmann & Lipp, 2003). This could be because preclinical studies have reported that the ileal mucosa is more sensitive to cisplatin (Cinausero et al., 2017; Yamamoto et al., 2013). The GI toxicity of platinum salts, oxaliplatin and cisplatin are significantly increased when administered in combination with 5-FU (Lee, 2014).
3.4. CIM and the microbiome
The role of the gut microbiome in mucosal inflammation has been well recognized, including in chemotherapy—induced effects (Bajic et al., 2018). Bacteria of the GI tract are essential to the normal physiological regulation of intestinal barrier functions, maintenance of selective intestinal permeability, inflammation and innate immune response, repair mechanisms, cell apoptosis, and oxidative stress (Dahlgren et al., 2021; Prisciandaro et al., 2011). Both the direct and indirect toxic effects of chemotherapeutics on the gut microbiome impact the clinical manifestations of CIM, where they ultimately may lead to the development of bacteremia and diarrhea or sepsis. Hong-Li et al reported that 5-FU resulted in significantly changed profiles of inflammatory cytokines/chemokines in serum and colon (Li et al., 2017). 5-FU diminished bacterial diversity, leading to the relative lower abundance of Firmicutes and decreased Firmicutes/Bacteroidetes (F/B) ratio in feces and cecum contents. These investigators found that fecal transplant from healthy mice prevented body weight loss and colon shortening of 5-FU treated mice, although restoring the dysbiotic microbiota from 5-FU-treated animals reversed these effects. Thus, these findings suggested that 5-FU induced intestinal mucositis could be alleviated by altering the gut microbiome (Li et al., 2017). Similarly, Chun-Yan et al found that diarrhea developed in the 5-FU groups but was attenuated after oral probiotic suspension of Lactobacillus casei variety rhamnosus (Lcr35) or Lactobacillus acidophilus and Bifidobacterium bifidum (LaBi). Additionally, they found that pro-inflammatory cytokines (TNF-α, IL-1β and Il-6) were upregulated after 5-FU treatment but were attenuated by the probiotic mixture (Yeung et al., 2015). Despite the fact that many studies find a strong link between the microbiome and the development of mucositis (Chang et al., 2018, 2020; da Silva Ferreira et al., 2020; Stringer & Logan, 2015; Wu et al., 2019; Wu et al., 2021) therapies designed to ameliorate gut dysbiosis have so far proven unsuccessful in treating CIM in the clinical setting (Dahlgren et al., 2021; Touchefeu et al., 2014). This could be due to challenges associated with the use of antibiotics or delivery of pharmacologically relevant doses of compatible strains of microbiota. Nonetheless, modulating the gut microbiome to alleviate chemotherapy-induced gastrointestinal toxicities by combining antibiotics with patient-specific probiotics/fecal microbiota transplants to target pathogenic bacteria and replenish the commensal microbiome remains an exciting prospect.
4. Emerging mechanisms underlying chemotherapy-induced gastrointestinal toxicity
It has been reported that 13%–50% of patients exhibit diarrhea for up to 10 years after cessation of cancer treatment (Denlinger & Barsevick, 2009). However, the pathophysiology underlying chronic diarrhea is not well understood. The development of diarrhea often involves aberrations in the transport of sodium, chloride, and water across the epithelium, and/or enhanced contractility of gastrointestinal smooth muscle function regulated by the enteric nervous system (ENS). The ENS is a semi-autonomous nervous system intrinsic to the gastrointestinal tract (Kulkarni et al., 2018). It is organized into two interconnected ganglionated plexuses—myenteric plexus (Auerbach’s) and submucosal (Meissner’s) plexus. The myenteric plexus lies between the outer longitudinal and circular muscle layers and is responsible for coordinating motor patterns of the intestinal wall. The submucosal plexus is located in the submucosal layer between the circular muscle and muscularis mucosa and regulates secretomotor activity (fluid and electrolyte homeostasis), vascular tone and epithelial cell functions. Nerve fibers from both the myenteric and submucosal plexuses project into the mucosa (Brehmer, 2006; Furness, 2007). For a comprehensive review of the enteric nervous system and the pathways underlying the motor and secretomotor reflexes that mediate gastrointestinal peristalsis and regulate secretory flux across the intestinal epithelium, respectively, readers are encouraged to refer to ‘The Enteric Nervous System’ by Furness (Furness, 2007).
Several chemotherapeutic agents, including platinum compounds and taxanes, are known to induce neuropathy in peripheral afferents innervating the limbs and the mechanisms underlying neurotoxicity have been extensively investigated (Colvin, 2019). It has been reported that enteric neurons are replaced every 2 weeks in the myenteric plexus of adult mice (Kulkarni et al., 2017), thus rendering neurogenesis as a potential target for disruption by chemotherapy drugs. However, it is not entirely clear whether the regeneration occurs under insult and injury or is a homeostatic mechanism in health conditions (Pawolski & Schmidt, 2020). Interestingly, enteric neurogenesis is reported to be modulated by the gut microbiome (Vicentini et al., 2021). However, the toxic effects of chemotherapy on the ENS are not well characterized in the clinic. Moreover, the role of enteric neurotoxicity in the induction and prolongation of symptoms associated with chemotherapy-induced diarrhea is unclear. Emerging evidence from preclinical studies indicates that several chemotherapeutic agents, such as oxaliplatin, cisplatin, 5-FU, irinotecan and vincristine induce enteric neuron toxicity and/or alter the neurochemical composition of enteric ganglia within the myenteric and/or submucosal plexuses (McQuade et al., 2020).
Oxidative stress has been reported as a key mechanism underlying mitochondrial damage and apoptosis in intestinal epithelial cells and enteric neurons following chemotherapy treatment (Cinausero et al., 2017; McQuade, Carbone, et al., 2016). Reactive oxygen/nitrogen species are known to alter ion channel function by inducing post-translational modifications directly to its structure or indirectly by modifying proteins regulating channel activity or gene expression. Several ion channels have been extensively examined in neurons under neuropathic or inflammatory conditions and provide a strong body of evidence for shared mechanisms and pathways. For example, voltage-gated sodium channels are important drivers of he action potential upstroke in neurons (Akbarali, 2014). Reactive nitrogen species (RNS) have been shown to increase the activity of inactivation-resistant voltage-gated sodium channels in hippocampal neurons (Annunziato et al., 2002), whereas in baroreceptor neurons RNS decreased the activity of tetrodotoxin-resistant and tetrodotoxin-sensitive voltage-gated sodium channels (Li et al., 1998). ROS/RNS can also increase the activity of several members of the TRP channel superfamily, such as TRPM2, TRPM7, TRPC3/4 and TRPV1, many of which are expressed in the gut and thus seem a likely target for dysregulation by the inflammation induced by chemotherapy (Akbarali, 2014; Drokhlyansky et al., 2020; Holzer, 2011). Oxidative stress can evoke prolonged sensitization of TRPV1 channels (Schilling & Eder, 2009). Oxidation of TRPV1 channels can also render them resistant to capsaicin-induced desensitization and activity of the desensitized receptor can be rescued following significant oxidative stress (Chuang & Lin, 2009). TRPV1 channels are non-selective cation channels known to respond to noxious stimuli, such as acidic pH (<5.9), noxious heat (>43°C) and capsaicin (Akbarali, 2014; Buckinx et al., 2013). They are highly expressed in nociceptive neurons, including those innervating the gut (Holzer, 2011), and have been implicated in chemotherapy-induced peripheral hypersensitivity (Aromolaran & Goldstein, 2017). Under physiological conditions, TRPV1-expressing gut-innervating neurons play an important role in maintaining homeostasis by responding to various changes in the gut (Chiu et al., 2013; Lai et al., 2020). Indeed, Lai et al. (2020) recently demonstrated that TRPV1-expressing primary afferent neurons protect against pathogenic bacteria invasion by regulating M cell density in ileum Peyer’s patches via release of CGRP. Although, during pathophysiological conditions, such as colitis, TRPV1 activation in sensory neurons exacerbated inflammation (Engel et al., 2012). It is important to note that the presence of TRPV1 channels in the ENS is contested with some studies indicating expression exclusively in extrinsic afferents innervating the gut (including primary afferent dorsal root ganglia neurons), while others have reported expression in enteric neurons (Buckinx et al., 2013; Holzer, 2011). This discrepancy could be due to the presence of different isoforms that display differing immunoreactivity (Allais et al., 2017; Buckinx et al., 2013). TRPM2, TRPM7 and TRPC3/4 are expressed in enteric neurons (Drokhlyansky et al., 2020). The P2X2 receptor is another ion channel target for oxidative stress in the intestine. The P2X2 receptor can mediate synaptic transmission between neurons and the neuromuscular junction (Akbarali, 2014). Oxidative stress has been shown to modulate P2X2 receptor activity. For example, Coddou et al. reported increased P2X2 receptor activity due to oxidative stress (Coddou et al., 2009). In contrast, Mason et al. (Mason, Bourke, & Kemp, 2004) reported that mitochondrial ROS production decreased ATP-activated P2X2 currents.
Interspersed between neurons are enteric glia. In the myenteric and submucosal plexuses, enteric glia have been observed surrounding neuronal cell bodies and like neurons, they extend projections into the musculature and the mucosal epithelium (Seguella & Gulbransen, 2021). Within the gastrointestinal tract, enteric glia have been reported to play an integral role in supporting epithelial barrier function, peristalsis and inflammation (Bhave et al., 2017; Rao et al., 2017; Seguella & Gulbransen, 2021) through interactions with neurons, immune cells, enterocytes and enteroendocrine cells (Bohórquez et al., 2014; Ibiza et al., 2016; Liu et al., 2013; Neunlist et al., 2007). Recent studies have reported up-regulation of molecular markers associated with reactive gliosis (S100β and GFAP) in enteric glia after treatment with chemotherapeutic agents, such as oxaliplatin, 5-FU and irinotecan (Costa et al., 2019; Gibson et al., 2016; Grundmann et al., 2019; Nogueira et al., 2017; Robinson et al., 2016). Furthermore, S-100β inhibition mitigated chemotherapy-induced oxidative stress, enteric neuropathy, and insult to the crypt-villi architecture (Costa et al., 2019). In enteric glia S-100β is known to stimulate inducible nitric oxide synthase (iNOS) in models of ulcerative colitis and celiac disease (Sharkey, 2015). Increased nitric oxide (NO) due to iNOS overstimulation via S100β-mediated activation of the RAGE/NF-kB pathway has been shown to disrupt epithelial barrier function (Grundmann et al., 2019) and promote neuron cell death (Costa et al., 2019; Villarreal et al., 2011). NO produced by iNOS can potentiate connexin 43 (CX43) hemichannel opening in enteric glia, stimulate CX43-mediated ATP release and drive neuron cell death during intestinal inflammation (Brown et al., 2016). Similarly, colonic inflammation due to chronic morphine treatment was associated with enhanced ATP currents in enteric glia. CX43 inhibition downregulated pro-inflammatory cytokines in the colon, reduced ATP currents and reversed chronic morphine-induced decrease in neuron density in the myenteric ganglia (Bhave et al., 2017). Consequently, upstream mechanisms underlying gliosis or downstream mechanisms modulating ATP release, for example, CX43 hemichannels, may be important targets for alleviating chemotherapy-induced gastrointestinal toxicity.
While several chemotherapeutic agents are reported to produce toxicity in cardiac muscle, skeletal muscle, vascular smooth muscle and the detrusor smooth muscle, not much is known about the direct effects on intestinal smooth muscle (Hayward et al., 2013; Iguchi et al., 2019; Min et al., 2015). Anthracycline compounds such as doxorubicin are known to induce chronic constipation and ileus in patients. Millington, Pascasio, Halligan, and De Chadarévian (2012) recently reported moderate-to-severe fibrosis, loss of myofilaments and/or hyalinization in the muscularis externa and the muscularis mucosae in the gastrointestinal tract of doxorubicin-treated patients. Gastrointestinal motility is a highly integrated phenomena and requires coordination between myenteric plexus neurons, interstitial cells (Interstitial Cells of Cajal and PDGFRα-positive cells), and smooth muscle (Sanders et al., 2012). Therefore, myopathy in intestinal smooth muscle cells can also impair intestinal motility. However, the mechanisms underlying intestinal smooth muscle toxicity are unclear. Anthracycline compounds such as doxorubicin are known to induce cardiotoxicity and cardiomyopathy via mitochondrial production of ROS (Min et al., 2015). Thus, oxidative stress could be a potential mechanism underlying doxorubicin effects on intestinal smooth muscle. During intestinal inflammation, oxidative stress altered the gating kinetics of the L-type calcium channel, CaV1.2b, in intestinal smooth muscle cells by mutating residues that are necessary for interactions with c-src kinase. Reduced channel phosphorylation resulted in decreased calcium influx, which impaired excitation-contraction coupling (Akbarali, 2014; Kang et al., 2004, 2007; Ross et al., 2007; 2010). Reduced calcium influx can also alter gene transcription in smooth muscles. For example, during colonic inflammation, tyrosine nitration of L-type calcium channels prevented depolarization-mediated CREB phosphorylation (Kang et al., 2010). Interestingly, doxorubicin has also been reported to inhibit smooth muscle activity without inducing changes in oxidative stress markers (Iguchi et al., 2019). In this study by Iguchi et al. (2019), doxorubicin impaired detrusor smooth muscle contractility by downregulating the large-conductance Ca2+-activated K+ channel and altering the expression of myosin-light chain kinase isoforms. Thus, inflammation-mediated dysregulation of excitation-contraction and/or excitation/transcription coupling in intestinal smooth muscle cells might underlie anthracycline-induced chronic constipation.
Macrophages are critical mediators of intestinal inflammation during the initial phase of chemotherapy-induced gastrointestinal mucositis. Increased bacterial translocation as a consequence of the breakdown of the gut epithelial barrier results in the activation of macrophages and thus helps sustain or even exacerbate intestinal inflammation (Sougiannis et al., 2021). Recently, Luo et al. (2018) demonstrated that macrophages, specifically those localized in intestinal smooth muscles, can also directly promote chemotherapy-induced diarrhea. In their studies Luo et al. demonstrated that genetic ablation of TRPV4 receptors expressed on muscularis macrophages inhibited irinotecan-induced diarrhea by preventing the release of PGE2 from macrophages and downstream activation of E-prostanoid receptor 1 and 3 on intestinal smooth muscles (Luo et al., 2018). This pathway was independent of enteric neurons. Thus, targeting muscularis macrophage activation might be a viable option for preventing not only chemotherapy-induced diarrhea but also a host of inflammation-driven gastrointestinal motility disorders.
5. Treatment of CID
Despite our growing understanding of the mechanisms for chemotherapy-induced GI toxicities, pharmacological treatments are limited. Currently, treatment of chemotherapy-induced diarrhea is based on the severity of the diarrhea.
The first line defense in treatment of CID is loperamide. Loperamide is a peripherally restricted mu opioid receptor agonist that decreases secretion and reduces intestinal motility by inhibition of enteric neurons. Patients receive escalating doses of loperamide to control symptoms. High dose loperamide remains the primary treatment for the acute management of CID (grades 1–2) with the clinical guidance of a maximum daily dosing of up to 24mg in man. However, in randomized clinical trial data, irinotecan-based therapy was found to progress to grades 3–4 diarrhea with a frequency ranging from 6% to 47% despite high doses of loperamide (Andreyev et al., 2014). Irinotecan-induced diarrhea therefore becomes refractory to loperamide. This may be due to the tolerance to opioids that develops in the myenteric neurons of the ileum (Muchhala et al., 2020).
The second line treatment in CID is often octreotide, a synthetic somatostatin analogue which decreases secretion and promotes absorption. Octreotide is generally recommended for patients refractory to loperamide (Zidan et al., 2001). The mechanism of action involves inhibition of serotonin, vasoactive intestinal peptide and gastrin and enhances intestinal motility (Brunton et al., 2017; Deng et al., 2017). However, prolonged use of octreotide may result in deleterious cardiovascular, CNS and endocrine effects.
Although other pharmacological interventions including corticosteroids may be used for inflammation, treatment for severe diarrhea are limited and can often result in cessation of therapy (Boussios et al., 2012).
5.1. Current and emerging pre-clinical strategies for treating CIM
Currently approved strategies for alleviating chemotherapy-induced GI toxicity are aimed towards managing symptoms associated with diarrhea and abdominal pain (Table 3). However, there is a major unmet clinical need to investigate and develop treatments for CIM, which is the basis of chemotherapy induced diarrhea (Cinausero et al., 2017; Dahlgren et al., 2021; Sonis, 2004). Novel strategies have aimed at targeting the pathway of interconnected pathobiological mechanisms including scavenging ROS with antioxidants, inhibiting pro-inflammatory cytokine (anti-inflammatory), and preventing runaway cell death particularly in the cryptic stem cells with apoptosis inhibitors (Dahlgren et al., 2021; Kwon, 2016).
Table 3.
Drugs used in the treatment of CID.
| Mechanism | Side effects | |
|---|---|---|
| Loperamide | Loperamide is a peripherally restricted mu opioid receptor agonist that decreases secretion and intestinal motility by decreasing excitability of enteric neurons (Brunton et al., 2017; Wadler et al., 1998) | Constipation, nausea, vomiting, cardiovascular effects |
| Deodorized tincture of opium | Centrally active mu-opioid receptor agonist inhibits gastrointestinal motility (Gershon et al., 1994) | CNS effects, tolerance |
| Octreotide | Somatostatin analogue that decreases hormone secretion (e.g., vasoactive intestinal polypeptide), reduces motility and pancreatic secretions and promotes absorption (Brunton et al., 2017; Deng et al., 2017; Zidan et al., 2001) | Nausea, bloating, hypo or hyperglycemia, gallstone formation, endocrine, cardiovascular and CNS side effects |
| Budesonide | Topically active corticosteroid that might restore mucosal function and fluid absorption; a 90% first-pass effect in the liver results in low systemic availability (Brunton et al., 2017; de Man et al., 2018; Ribeiro et al., 2016) | Endocrine effects |
| Atropine | Inhibition of acetylcholine at muscarinic receptors located at neuromuscular junction to inhibit motility (Brunton et al., 2017; Stein et al., 2010) | Cognitive impairment, xerostomia, constipation, blurred vision, dyspepsia |
| Antibiotics | Broad spectrum antibiotic that targets small intestinal bacterial overgrowth (Boussios et al., 2012; Brunton et al., 2017) | Diarrhea, increased risk of C. difficile infection |
| Bile acid sequestrants | Prevent water secretion into the colon induced by non-sequestered bile acids (Brunton et al., 2017) | |
| Beta-glucuronidase inhibitors | Inhibits removal of glucuronic acid moiety by gut commensal bacteria (Bhatt et al., 2020) |
For example, Amifostine, an aminothiol prodrug, which undergoes biotransformation to the active metabolite WR-1065, has been reported to reduce doxorubicin-induced CIM in rats and methotrexate-induced CIM in mice (Chen et al., 2013; Jaćević et al., 2018). The therapeutic effect of WR-1065 has been attributed to scavenging free radicals produced by oxidative stress and mitochondrial damage (Bensadoun, Schubert, Lalla, & Keefe, 2006). Currently Amifostine is FDA-approved for prophylactic treatment of oral mucositis in post-operative head-neck-cancer patients treated with radiotherapy (Antonadou et al., 2002) However, significant side-effects such as nausea and hypotension have limited its broader clinical use.
The profound role that inflammatory cytokines, such as interleukin-1β (IL-1β), NF-kB, and COX-2, play in inducing and potentiating the development of mucositis has made them a prime potential therapeutic target. IL-1β, for example, expressed and released by intestinal epithelial cells due to DNA damage perturbs the epithelial barrier by disrupting tight junction proteins (Kanarek et al., 2014; Kwon, 2016). IL-1 receptor antagonists, such as the endogenously produced interleukin-1 receptor antagonist (IL-1RA)(Daig, 2000), has been shown to inhibit irinotecan-induced mucosal damage, inflammatory cell infiltration, ulceration, and decrease 5-FU-induced intestinal mucosal damage while also expediting post-ulcerative healing (Kanarek et al., 2014; Wu et al., 2010, 2011). NF-kB has been established as a key component in the development of mucositis, and thus directly inhibiting NF-kB or its downstream messengers has been an extensively investigated strategy for reducing mucositis in preclinical studies (Chen et al., 2011; Cinausero et al., 2017; Kwon, 2016). Cyclooxygenase 2 (COX-2), which is involved in the synthesis of prostanoids, plays an important role in the amplification of CIM (Dahlgren et al., 2021; Sonis, 2004). Interestingly, in preclinical studies, COX-2 inhibition was reported to reduce the histological changes induced by 5-FU (de Miranda et al., 2020) and irinotecan (Javle et al., 2007). However, the clinical efficacy of COX-2 inhibition in the treatment of intestinal mucositis caused by radiation or chemotherapy was limited (Dahlgren et al., 2021; Javle et al., 2007; Lalla et al., 2014).
The gastrointestinal tract mucosa and submucosa rely on rapidly dividing stem cells within the crypts for renewal and normal physiological function, and therefore preventing apoptosis induced by chemotherapeutics is another well-established pre-clinical strategy to manage the development of mucositis. For example, CXCL9 mitigated 5-FU-induced intestinal mucositis and mucosal damage by protecting stem/progenitor cells against unregulated cell death induced by chemotherapy. CXCL9 treatment not only decreased chemotherapy-induced apoptosis in intestinal crypts but also increased the proliferation rate of intestinal crypts after treatment. Furthermore, administration of CXCL9 raised the therapeutic window of 5-FU in treated animals (Han et al., 2011). Likewise, GLP-1 and 2 and their metabolic enzyme (DPP-IV) have shown evidence for supporting mucosal regeneration after CIM, in part by reducing apoptosis (Boushey et al., 2001; Dahlgren et al., 2021).
Other potentially beneficial, but highly specific and cell restricted treatment targets outside of the traditional ROS—inflammatory cytokine— runaway apoptosis pathway have emerged in recent literature, such as the G-coupled estrogen receptor (GPER). GPER, which is expressed in intestinal crypts, has been reported to exhibit protective effects on intestinal architecture during colitis and ischemia by inhibiting inflammation-driven apoptosis of crypt cells, particularly during colitis and ischemia (Chai et al., 2019; Wang et al., 2021). Similarly, during treatment with 5-FU, Chen et al reported that the selective GPER agonist, G-1, significantly inhibited weight loss and inflammation-induced mucosal damage. Additionally, mucosal barrier function improved as G-1 treatment resulted in increased goblet cell density and reduced mucosal permeability due to up-regulation of ZO-1 Moreover, G-1 treatment did not alter the antitumor effect of 5-fluorouracil (Chen et al., 2021).
In a recent study, it was noted that levels of glutathione (GSH), a potent non-protein thiol antioxidant, and GSH/GSSG (glutathione disulfide) ratio (a potential indicator of cellular oxidative stress) in the small intestine mucosal tissue decreased compared to controls after 5-FU treatment. Therefore, modulating GSH synthesis can be explored as an intriguing strategy to reduce oxidative stress. Cystine and theanine, which is metabolized to glutamic acid in the liver, are essential precursors of GSH. In a recent study, Yoneda, Nishikawa, and Kurihara (2021) found that cystine and theanine (CT) administration decreased 5-FU-induced oxidative stress in the basal region of small intestinal crypts by increasing GSH levels and improving the GSH/GSSG ratio. CT-treated mice also exhibited reduced diarrhea and weight loss. Importantly CT did not affect the antitumor activity of 5-FU (Yoneda et al., 2021).
Ultimately, as has been discussed by many publications within the field of CIM, we feel that while all of these are individually exciting, progress has been slow and disappointing for a single solution to manage CIM effectively without ceasing or interrupting the chemotherapy regimen. A combinational therapeutic strategy which targets multiple or all of the individual components along the pathological pathway (ROS—Cytokines— Apoptosis) will likely yield the most promising clinical benefit (Cinausero et al., 2017; Dahlgren et al., 2021; Kwon, 2016; Sonis, 2004).
6. The gut and chemotherapy-induced peripheral neuropathy
Chemotherapy-induced peripheral neuropathy (CIPN) is a frequent and often dose-limiting complication of cancer therapy utilizing taxanes (docetaxel or paclitaxel), platinum compounds (cisplatin, carboplatin, and oxaliplatin) as well as other drugs such as vincristine, and bortezomib. Clinical manifestation of symptoms can vary greatly between patients, but often includes all or some of the following: thermal and mechanical hyperalgesia, tactile and thermal allodynia, spontaneous pain, paresthesia, dysesthesia as well as a variety of cognitive or motor impairments (Sałat, 2020). Currently no treatments exist that can reverse CIPN once it has developed, and pharmacological management of these neuropathies has been notoriously challenging with approximately 50% of patients being resistant to current pain management (Przewlocki & Przewlocka, 2005; Sałat, 2020). Over the last several years, rodent models of CIPN have been developed that demonstrate many of the neuropathological changes observed in patients undergoing cancer therapy as well as typical electrophysiological abnormalities associated with CIPN (Authier et al., 2009). Pre-clinical studies have shown long-lasting, bilateral neuropathy associated with a decrease in the density of peripheral intraepithelial nerve fibers (IENF), a hallmark of CIPN, as well as mechanical and cold allodynia utilizing a paclitaxel rodent model (Toma et al., 2017). While several explanations have been proposed for the development of CIPN, the mechanism(s) of paclitaxel-induced neuropathy and chronic pain are not well understood. However, there is growing evidence from animal models that paclitaxel and other cancer chemotherapeutic drugs can trigger an inflammatory response characterized by the production and release of pro-inflammatory cytokines, such as IL-1β and TNF-α (Shen et al., 2017; Vyas et al., 2014). Recent animal studies suggest that these cytokines that are released directly and indirectly by activated spinal glial cells within the dorsal root ganglia (DRG) are critical for the development of CIPN (Shen et al., 2017). Primary sensory afferents of the DRG innervate the intestinal mucosa and studies have recently demonstrated that intestinal inflammation and microbial dysbiosis can alter the excitability of DRG neurons (Kang et al., 2017; Mischel et al., 2018; Moore et al., 2002; Sessenwein et al., 2017). Therefore, inflammation emanating from the gut due to chemotherapy treatment might play an important role in the etiology of chemotherapy-induced peripheral hypersensitivity. Interestingly, several chemotherapeutic agents that are known to cause CIPN also induce gastrointestinal mucositis and perturb the intestinal epithelial barrier. Disruption of the epithelial barrier is known to promote dysbiosis of the gut bacteria and bacterial translocation across the intestinal epithelium can induce inflammatory responses through activation of pattern recognition receptors. For example, pre-clinical studies have shown that changes in the gut microbiome can result in loss of epithelial integrity and promote an inflammatory response within the colonic wall through activation of Toll-like receptors (TLR) on enteric glia cells (Bhave et al., 2017).
A growing body of preclinical evidence has implicated the gut microbiome in chemotherapy-induced neuropathic pain (Ramakrishna et al., 2019; Shen et al., 2017). Ramakrishna et al. (Ramakrishna et al., 2019) recently reported that fecal microbial transplant from healthy donor mice attenuated chemotherapeutic-induced hypersensitivity in mice treated with paclitaxel. Additionally, oxaliplatin-induced mechanical hyperalgesia was reduced in germ-free and in antibiotic treated mice and restored by replenishing the dysbiotic microbiota (Shen et al., 2017). However, it is unclear how the alterations to microbiota, or epithelial barrier result in chemotherapy-induced hyperalgesia.
An important constituent of the commensal microbiome are bacterial species that ferment fibers through complex enzymatic pathways and produce short chain fatty acids, acetate, propionate, and butyrate (70–140mM) (Venegas et al., 2019). Bonomo et al. (2020) recently reported that fecal microbial transplant from lean to obese, insulin-resistant mice increased serum butyrate levels. This was associated with reversal of peripheral neuropathy, mechanical allodynia, DRG hyperactivity, and transformation of macrophages in the DRG to an anti-inflammatory phenotype. Furthermore, butyrate administration decreased leukocyte population in the DRG and enhanced desensitization of TRPV1 channels (Bonomo et al., 2020). The gut epithelium plays a central role in preventing bacterial translocation and systemic inflammation. Butyrate is known to improve the integrity of the epithelium and to enhance some of the antimicrobial peptides preventing colonization by pathogenic bacteria (Schulthess et al., 2019; Zhao et al., 2018). Thus, loss of butyrate-producing commensal bacteria by way of direct effect from the chemotherapeutics or by indirectly altering the microenvironment may be one possible pathway through which the gut epithelial barrier is compromised and excitability of primary afferent neurons is altered to produce peripheral hypersensitivity.
7. Conclusion
Chemotherapy-induced gastrointestinal toxicities, including diarrhea and mucositis, are major hurdles in cancer treatment. Despite major advances in chemotherapy, treatment of gastrointestinal toxicity remains inadequate often resulting in hospitalization, cessation of treatment and decrease in quality of life. In this review, we highlighted our current understanding of the pathophysiology (Fig. 1) and potential pharmacological approaches to treat CID and CIM. An exciting prospect is the potential to approach future treatments based on the role the gut microbiome and metabolites may play on epithelial barrier function and enteric neurons. Future studies to dissect individual cell type specific responses in the gastrointestinal tract to chemotherapeutic agents will provide significant insights for treatment of gastrointestinal toxicities.
Fig. 1.
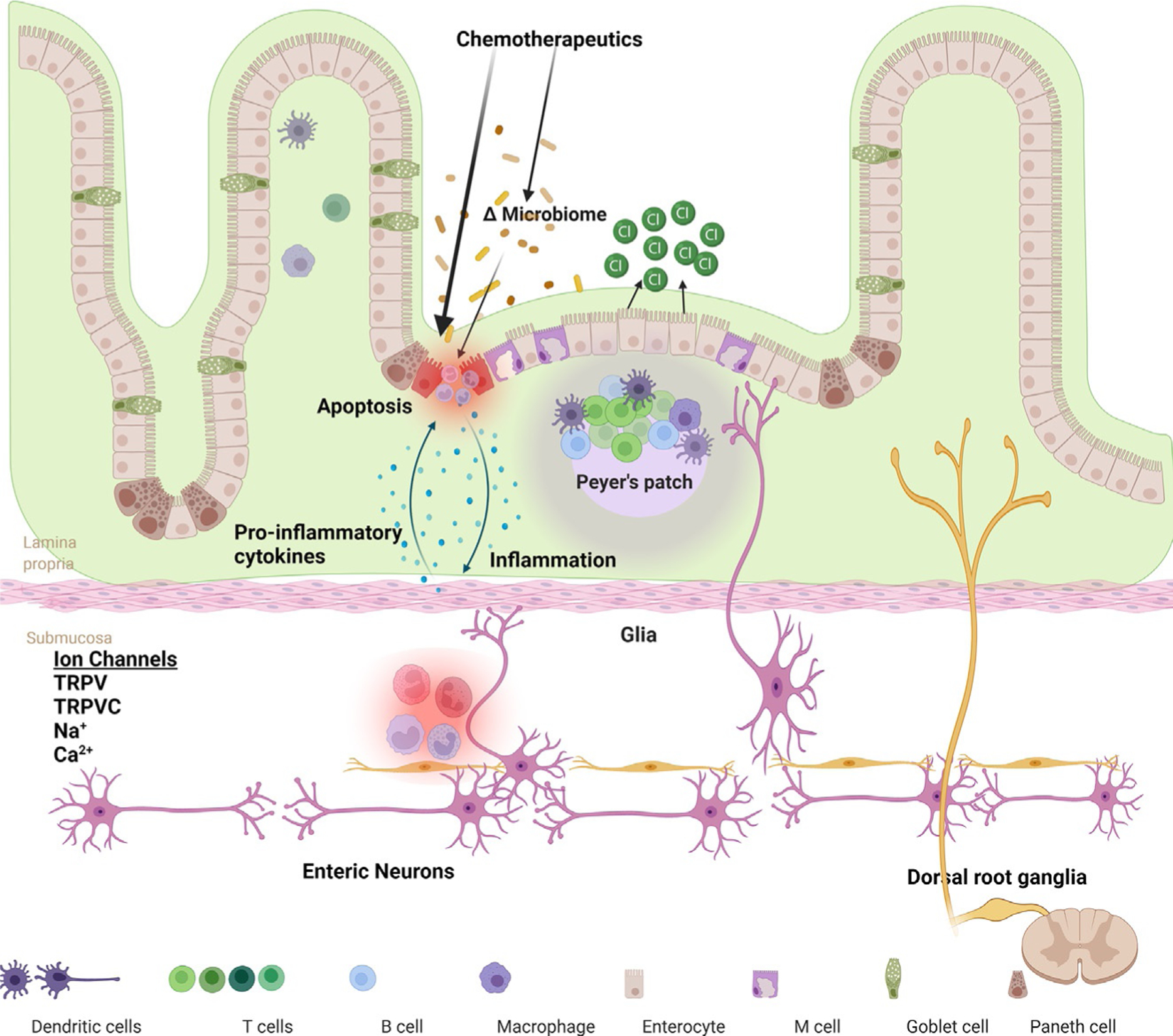
A schematic presentation of mechanisms that underlie the chemotherapy-induced gastrointestinal toxicity. Chemotherapeutics can alter gut microbiome directly or through effects on the epithelial cells. Disruption of the gut barrier results in bacterial translocation and an inflammatory response within the lamina propria. This can affect ion channels in enteric and extrinsic sensory neurons projecting from the dorsal root ganglia. Inflammation also induces increase in epithelial secretions resulting in diarrhea. Injury to DNA of intestinal epithelial cells induce apoptosis.
References
- Abdel-Rahman O, ElHalawani H, & Essam-Eldin S (2016). S-1-based regimens and the risk of oral and gastrointestinal mucosal injury: A meta-analysis with comparison to other fluoropyrimidines. Expert Opinion on Drug Safety, 15(1), 5–20. 10.1517/14740338.2016.1105959. [DOI] [PubMed] [Google Scholar]
- Akbarali HI (2014). Oxidative stress and ion channels. In Systems biology of free radicals and antioxidants (pp. 355–373). Berlin Heidelberg: Springer. 10.1007/978-3-642-30018-9_12. [DOI] [Google Scholar]
- Allaire JM, Crowley SM, Law HT, Chang SY, Ko HJ, & Vallance BA (2018). The intestinal epithelium: Central coordinator of mucosal immunity. Trends in Immunology, 39(9), 677–696. 10.1016/j.it.2018.04.002. [DOI] [PubMed] [Google Scholar]
- Allais L, De Smet R, Verschuere S, Talavera K, Cuvelier CA, & Maes T (2017). Transient receptor potential channels in intestinal inflammation: What is the impact of cigarette smoking? Pathobiology, 84(1), 1–15. 10.1159/000446568. [DOI] [PubMed] [Google Scholar]
- Andreyev J, Ross P, Donnellan C, Lennan E, Leonard P, Waters C, et al. (2014). Guidance on the management of diarrhoea during cancer chemotherapy. The Lancet Oncology, 15(10), e447–e460. 10.1016/S1470-2045(14)70006-3. [DOI] [PubMed] [Google Scholar]
- Annunziato L, Pannaccione A, Cataldi M, Secondo A, Castaldo P, Di Renzo G, et al. (2002). Modulation of ion channels by reactive oxygen and nitrogen species: A pathophysiological role in brain aging? Neurobiology of Aging, 23(5), 819–834. 10.1016/s0197-4580(02)00069-6. [DOI] [PubMed] [Google Scholar]
- Antonadou D, Pepelassi M, Synodinou M, Puglisi M, & Throuvalas N (2002). Prophylactic use of amifostine to prevent radiochemotherapy-induced mucositis and xerostomia in head-and-neck cancer. International Journal of Radiation Oncology, Biology, Physics, 52(3), 739–747. 10.1016/S0360-3016(01)02683-9. [DOI] [PubMed] [Google Scholar]
- Aromolaran KA, & Goldstein PA (2017). Ion channels and neuronal hyperexcitability in chemotherapy-induced peripheral neuropathy. Molecular Pain, 13. 10.1177/1744806917714693.174480691771469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Authier N, Balayssac D, Marchand F, Ling B, Zangarelli A, Descoeur J, et al. (2009). Animal models of chemotherapy-evoked painful peripheral neuropathies. Neurotherapeutics, 6(4), 620–629. 10.1016/j.nurt.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajic JE, Johnston IN, Howarth GS, & Hutchinson MR (2018). From the bottom-up: Chemotherapy and gut-brain axis dysregulation. Frontiers in Behavioral Neuroscience, 12. 10.3389/fnbeh.2018.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile D, Di Nardo P, Corvaja C, Garattini SK, Pelizzari G, Lisanti C, et al. (2019). Mucosal injury during anti-cancer treatment: From pathobiology to bedside. Cancers, 11(6), 857. 10.3390/cancers11060857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensadoun RJ, Schubert MM, Lalla RV, & Keefe D (2006). Amifostine in the management of radiation-induced and chemo-induced mucositis. Supportive Care in Cancer, 14(6), 566–572. 10.1007/s00520-006-0047-4. [DOI] [PubMed] [Google Scholar]
- Bevins CL, & Salzman NH (2011). Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nature Reviews Microbiology, 9(5), 356–368. 10.1038/nrmicro2546. [DOI] [PubMed] [Google Scholar]
- Bhatt AP, Pellock SJ, Biernat KA, Walton WG, Wallace BD, Creekmore BC, et al. (2020). Targeted inhibition of gut bacterial β-glucuronidase activity enhances anticancer drug efficacy. Proceedings of the National Academy of Sciences of the United States of America, 117(13), 7374–7381. 10.1073/pnas.1918095117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhave S, Gade A, Kang M, Hauser KF, Dewey WL, & Akbarali HI (2017). Connexin-purinergic signaling in enteric glia mediates the prolonged effect of morphine on constipation. FASEB Journal, 31(6), 2649–2660. 10.1096/fj.201601068R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohórquez DV, Samsa LA, Roholt A, Medicetty S, Chandra R, & Liddle RA (2014). An enteroendocrine cell—Enteric glia connection revealed by 3D electron microscopy. PLoS One, 9(2). 10.1371/journal.pone.0089881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonomo RR, Cook TM, Gavini CK, White CR, Jones JR, Bovo E, et al. (2020). Fecal transplantation and butyrate improve neuropathic pain, modify immune cell profile, and gene expression in the PNS of obese mice. Proceedings of the National Academy of Sciences of the United States of America, 117(42). 10.1073/pnas.2006065117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boushey RP, Yusta B, & Drucker DJ (2001). Glucagon-like peptide (GLP)-2 reduces chemotherapy-associated mortality and enhances cell survival in cells expressing a transfected GLP-2 receptor. Cancer Research, 61(2), 687–693. [PubMed] [Google Scholar]
- Boussios S, Pentheroudakis G, Katsanos K, & Pavlidis N (2012). Systemic treatment-induced gastrointestinal toxicity: Incidence, clinical presentation and management. Annals of Gastroenterology, 25(2), 106–118. [PMC free article] [PubMed] [Google Scholar]
- Bowen J, Al-Dasooqi N, Bossi P, Wardill H, Van Sebille Y, Al-Azri A, et al. (2019). The pathogenesis of mucositis: Updated perspectives and emerging targets. Supportive Care in Cancer, 27(10), 4023–4033. 10.1007/s00520-019-04893-z. [DOI] [PubMed] [Google Scholar]
- Bowen JM, Gibson RJ, Cummins AG, & Keefe DMK (2006). Intestinal mucositis: the role of the Bcl-2 family, p53 and caspases in chemotherapy-induced damage. Supportive Care in Cancer, 14(7), 713–731. 10.1007/s00520-005-0004-7. [DOI] [PubMed] [Google Scholar]
- Bowen JM, Gibson RJ, Cummins AG, Tsykin A, & Keefe DMK (2007). Irinotecan changes gene expression in the small intestine of the rat with breast cancer. Cancer Chemotherapy and Pharmacology, 59(3), 337–348. 10.1007/s00280-006-0275-9. [DOI] [PubMed] [Google Scholar]
- Brehmer A (2006). Structure of enteric neurons. In Beck FF, Clascá F, Haines DE, Korf H-W, Marani E, Putz R, … Zilles K (Eds.), Vol. 186. Advances in anatomy, embryology, and cell biology. Berlin Heidelberg: Springer. [PubMed] [Google Scholar]
- Brown IAM, McClain JL, Watson RE, Patel BA, & Gulbransen BD (2016). Enteric glia mediate neuron death in colitis through purinergic pathways that require connexin-43 and nitric oxide. Cellular and Molecular Gasteroenterology and Hepatology, 2(1). 10.1016/j.jcmgh.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton LL, Hilal-Dandan R, & Knollmann BC (2017). In Shanahan JF, & Lebowitz H (Eds.), Goodman & Gilman’s: The pharmacological basis of therapeutics (13 ed.). McGraw Hill. [Google Scholar]
- Buckinx R, Van Nassauw L, Avula LR, Alpaerts K, Adriaensen D, & Timmermans JP (2013). Transient receptor potential vanilloid type 1 channel (TRPV1) immunolocalization in the murine enteric nervous system is affected by the targeted C-terminal epitope of the applied antibody. Journal of Histochemistry & Cytochemistry, 61(6), 421–432. 10.1369/0022155413484764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai S, Liu K, Feng W, Liu T, Wang Q, Zhou R, et al. (2019). Activation of G protein–coupled estrogen receptor protects intestine from ischemia/reperfusion injury in mice by protecting the crypt cell proliferation. Clinical Science, 133(3), 449–464. 10.1042/CS20180919. [DOI] [PubMed] [Google Scholar]
- Chang CW, Lee HC, Li LH, Chiang Chiau JS, Wang TE, Chuang WH, et al. (2020). Fecal microbiota transplantation prevents intestinal injury, upregulation of toll-like receptors, and 5-fluorouracil/oxaliplatin-induced toxicity in colorectal cancer. International Journal of Molecular Sciences, 21(2), 386. 10.3390/ijms21020386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CW, Liu CY, Lee HC, Huang YH, Li LH, Chiau JSC, et al. (2018). Lactobacillus casei variety rhamnosus probiotic preventively attenuates 5-fluorouracil/oxaliplatin-induced intestinal injury in a syngeneic colorectal cancer model. Frontiers in Microbiology, 9(May). 10.3389/fmicb.2018.00983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Tian L, Zhang M, Sun Q, Zhang X, Li X, et al. (2013). Protective effect of amifostine on high-dose methotrexate-induced small intestinal mucositis in mice. Digestive Diseases and Sciences, 58(11), 3134–3143. 10.1007/s10620-013-2826-3. [DOI] [PubMed] [Google Scholar]
- Chen G, Zeng H, Li X, Liu J, Li Z, Xu R, et al. (2021). Activation of G protein coupled estrogen receptor prevents chemotherapy-induced intestinal mucositis by inhibiting the DNA damage in crypt cell in an extracellular signal-regulated kinase 1- and 2-dependent manner. Cell Death & Disease, 12(11), 1034. 10.1038/s41419-021-04325-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YK, Hou HA, Chow JM, Chen YC, Hsueh PR, & Tien HF (2011). The impact of oral herpes simplex virus infection and candidiasis on chemotherapy-induced oral mucositis among patients with hematological malignancies. European Journal of Clinical Microbiology & Infectious Diseases, 30(6), 753–759. 10.1007/s10096-010-1148-z. [DOI] [PubMed] [Google Scholar]
- Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J, et al. (2013). Bacteria activate sensory neurons that modulate pain and inflammation. Nature, 501(7465). 10.1038/nature12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang HH, & Lin S (2009). Oxidative challenges sensitize the capsaicin receptor by covalent cysteine modification. Proceedings of the National Academy of Sciences of the United States of America, 106(47), 20097–20102. 10.1073/pnas.0902675106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinausero M, Aprile G, Ermacora P, Basile D, Vitale MG, Fanotto V, et al. (2017). New frontiers in the pathobiology and treatment of cancer regimen-related mucosal injury. Frontiers in Pharmacology, 8(Jun). 10.3389/fphar.2017.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coddou C, Codocedo JF, Li S, Lillo JG, Acuna-Castillo C, Bull P, et al. (2009). Reactive oxygen species potentiate the P2X2 receptor activity through intracellular Cys430. Journal of Neuroscience, 29(39), 12284–12291. 10.1523/JNEUROSCI.2096-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin LA (2019). Chemotherapy-induced peripheral neuropathy: Where are we now? Pain, 160(1), S1–S10. 10.1097/j.pain.0000000000001540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa DVS, Bon-Frauches AC, Silva AMHP, Lima-Júnior RCP, Martins CS, Leitão RFC, et al. (2019). 5-Fluorouracil induces enteric neuron death and glial activation during intestinal mucositis via a S100B-RAGE-NFκB-dependent pathway. Scientific Reports, 9(1). 10.1038/s41598-018-36878-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosnier C, Stamataki D, & Lewis J (2006). Organizing cell renewal in the intestine: Stem cells, signals and combinatorial control. Nature Reviews. Genetics, 7(5), 349–359. 10.1038/nrg1840. [DOI] [PubMed] [Google Scholar]
- da Silva Ferreira AR, Wardill HR, Tissing WJE, & Harmsen HJM (2020). Pitfalls and novel experimental approaches to optimize microbial interventions for chemotherapy-induced gastrointestinal mucositis. Current Opinion in Supportive & Palliative Care, 14(2), 127–134. 10.1097/SPC.0000000000000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren D, Sjöblom M, Hellström PM, & Lennernäs H (2021). Chemotherapeutics-induced intestinal mucositis: Pathophysiology and potential treatment strategies. Frontiers in Pharmacology, 12. 10.3389/fphar.2021.681417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daig R (2000). Human intestinal epithelial cells secrete interleukin-1 receptor antagonist and interleukin-8 but not interleukin-1 or interleukin-6. Gut, 46(3), 350–358. 10.1136/gut.46.3.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Man FM, Goey AKL, van Schaik RHN, Mathijssen RHJ, & Bins S (2018). Individualization of irinotecan treatment: A review of pharmacokinetics, pharmacodynamics, and pharmacogenetics. Clinical Pharmacokinetics, 57(10), 1229–1254. 10.1007/S40262-018-0644-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Miranda JAL, Martins C.d. S., Fideles L.d. S., Barbosa MLL, Barreto JEF, Pimenta HB, et al. (2020). Troxerutin prevents 5-fluorouracil induced morphological changes in the intestinal mucosa: Role of cyclooxygenase-2 pathway. Pharmaceuticals, 13(1), 10. 10.3390/ph13010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C, Deng B, Jia L, & Tan H (2017). Efficacy of long-acting release octreotide for preventing chemotherapy-induced diarrhoea: Protocol for a systematic review. BMJ Open, 7(6), e014916. 10.1136/bmjopen-2016-014916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denlinger CS, & Barsevick AM (2009). The challenges of colorectal cancer survivorship. Journal of the National Comprehensive Cancer Network, 7(8), 883–894. 10.6004/jnccn.2009.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drokhlyansky E, Smillie CS, Van Wittenberghe N, Ericsson M, Griffin GK, Eraslan G, et al. (2020). The human and mouse enteric nervous system at single-cell resolution. Cell, 182(6), 1606–1622.e23. 10.1016/j.cell.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elting LS, Cooksley C, Chambers M, Cantor SB, Manzullo E, & Rubenstein EB (2003). The burdens of cancer therapy. Cancer, 98(7), 1531–1539. 10.1002/cncr.11671. [DOI] [PubMed] [Google Scholar]
- Engel MA, Khalil M, Mueller-Tribbensee SM, Becker C, Neuhuber WL, Neurath MF, et al. (2012). The proximodistal aggravation of colitis depends on substance P released from TRPV1-expressing sensory neurons. Journal of Gastroenterology, 47(3), 256–265. 10.1007/s00535-011-0495-6. [DOI] [PubMed] [Google Scholar]
- Fraiser LH, Kanekal S, & Kehrer JP (1991). Cyclophosphamide toxicity: Characterizing and avoiding the problem. Drugs, 42(5), 781–795. 10.2165/00003495-199142050-00005. [DOI] [PubMed] [Google Scholar]
- Furness JB (2007). The enteric nervous System. In Furness JB (Ed.), The enteric nervous system Blackwell Publishing. 10.1002/9780470988756. [DOI] [Google Scholar]
- Gallo RL, & Hooper LV (2012). Epithelial antimicrobial defence of the skin and intestine. Nature Reviews. Immunology, 12(7), 503–516. 10.1038/nri3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon MD, Kirchgessner AL, & Wade PR (1994). Functional anatomy of the enteric nervous system. In Johnson L, Alpers D, Jacobson E, & Walsh J (Eds.), Vol. 1. Physiology of the gastrointestinal tract (3rd ed., pp. 381–422). New York: Raven Press. [Google Scholar]
- Gibson RJ, Bowen JM, Inglis MRB, Cummins AG, & Keefe DMK (2003). Irinotecan causes severe small intestinal damage, as well as colonic damage, in the rat with implanted breast cancer. Journal of Gastroenterology and Hepatology, 18(9), 1095–1100. 10.1046/j.1440-1746.2003.03136.x. [DOI] [PubMed] [Google Scholar]
- Gibson RJ, Coller JK, Wardill HR, Hutchinson MR, Smid S, & Bowen JM (2016). Chemotherapy-induced gut toxicity and pain: Involvement of TLRs. Supportive Care in Cancer, 24(5). 10.1007/s00520-015-3020-2. [DOI] [PubMed] [Google Scholar]
- Gibson RJ, & Keefe DMK (2006). Cancer chemotherapy-induced diarrhoea and constipation: mechanisms of damage and prevention strategies. Support Care Cancer, 14, 890–900. 10.1007/s00520-006-0040-y. [DOI] [PubMed] [Google Scholar]
- Grundmann D, Loris E, Maas-Omlor S, Huang W, Scheller A, Kirchhoff F, et al. (2019). Enteric glia: S100, GFAP, and beyond. Anatomical Record, 302(8). 10.1002/ar.24128. [DOI] [PubMed] [Google Scholar]
- Guabiraba R, Besnard AG, Menezes GB, Secher T, Jabir MS, Amaral SS, et al. (2014). IL-33 targeting attenuates intestinal mucositis and enhances effective tumor chemotherapy in mice. Mucosal Immunology, 7(5), 1079–1093. 10.1038/mi.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Wu Z, Di J, Pan Y, Zhang H, Du Y, et al. (2011). CXCL9 attenuated chemotherapy-induced intestinal mucositis by inhibiting proliferation and reducing apoptosis. Biomedicine & Pharmacotherapy, 65(8), 547–554. 10.1016/j.bio-pha.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Haramis APG, Begthel H, van den Born M, van Es J, Jonkheer S, Offerhaus GJA, et al. (2004). De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science, 303(5664), 1684–1686. 10.1126/science.1093587. [DOI] [PubMed] [Google Scholar]
- Hartmann JT, & Lipp HP (2003). Toxicity of platinum compounds. Expert Opinion on Pharmacotherapy, 4(6), 889–901. 10.1517/14656566.4.6.889. [DOI] [PubMed] [Google Scholar]
- Hayward R, Hydock D, Gibson N, Greufe S, Bredahl E, & Parry T (2013). Tissue retention of doxorubicin and its effects on cardiac, smooth, and skeletal muscle function. Journal of Physiology and Biochemistry, 69(2). 10.1007/s13105-012-0200-0. [DOI] [PubMed] [Google Scholar]
- He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, et al. (2004). BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt–β-catenin signaling. Nature Genetics, 36(10), 1117–1121. 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- Holzer P (2011). Transient receptor potential (TRP) channels as drug targets for diseases of the digestive system. Pharmacology & Therapeutics, 131(1), 142–170. 10.1016/j.pharmthera.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibiza S, García-Cassani B, Ribeiro H, Carvalho T, Almeida L, Marques R, et al. (2016). Glial-cell-derived neuroregulators control type 3 innate lymphoid cells and gut defence. Nature, 535(7612), 440–443. 10.1038/nature18644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iguchi N, Dönmez Mİ, Carrasco A, Wilcox DT, Pineda RH, Malykhina AP, et al. (2019). Doxorubicin induces detrusor smooth muscle impairments through myosin dysregulation, leading to a risk of lower urinary tract dysfunction. American Journal of Physiology - Renal Physiology, 317(1). 10.1152/ajprenal.00090.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuno N, Soda H, Watanabe M, & Oka M (1995). Irinotecan (CPT-11) and characteristic mucosal changes in the mouse ileum and cecum. Journal of the National Cancer Institute, 87(24), 1876–1883. 10.1093/jnci/87.24.1876. [DOI] [PubMed] [Google Scholar]
- Jaćević V, Dragojević-Simić V, Tatomirović Ž, Dobrić S, Bokonjić D, Kovaćević A, et al. (2018). The efficacy of amifostine against multiple-dose doxorubicin-induced toxicity in rats. International Journal of Molecular Sciences, 19(8), 2370. 10.3390/ijms19082370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javle MM, Cao S, Durrani FA, Pendyala L, Lawrence DD, Smith PF, et al. (2007). Celecoxib and mucosal protection: Translation from an animal model to a phase I clinical trial of celecoxib, irinotecan, and 5-fluorouracil. Clinical Cancer Research, 13(3), 965–971. 10.1158/1078-0432.CCR-06-0551. [DOI] [PubMed] [Google Scholar]
- Kamp K, Gumz B, Feelders RA, Kwekkeboom DJ, Kaltsas G, Costa FP, et al. (2013). Safety and efficacy of everolimus in gastrointestinal and pancreatic neuroendocrine tumors after 177Lu-octreotate. Endocrine-Related Cancer, 20(6), 825–831. 10.1530/ERC-13-0254. [DOI] [PubMed] [Google Scholar]
- Kanarek N, Grivennikov SI, Leshets M, Lasry A, Alkalay I, Horwitz E, et al. (2014). Critical role for IL-1β in DNA damage-induced mucositis. Proceedings of the National Academy of Sciences of the United States of America, 111(6), E702–E711. 10.1073/pnas.1322691111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M, Mischel RA, Bhave S, Komla E, Cho A, Huang C, et al. (2017). The effect of gut microbiome on tolerance to morphine mediated antinociception in mice. Scientific Reports, 7(1), 42658. 10.1038/srep42658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M, Morsy N, Jin X, Lupu F, & Akbarali HI (2004). Protein and gene expression of Ca2+ channel isoforms in murine colon: Effect of inflammation. Pflugers Archiv European Journal of Physiology, 449(3). 10.1007/s00424-004-1339-5. [DOI] [PubMed] [Google Scholar]
- Kang M, Ross GR, & Akbarali HI (2007). COOH-terminal association of human smooth muscle calcium channel Ca v1.2b with Src kinase protein binding domains: Effect of nitrotyrosylation. American Journal of Physiology - Cell Physiology, 293(6). 10.1152/ajpcell.00308.2007. [DOI] [PubMed] [Google Scholar]
- Kang M, Ross GR, & Akbarali HI (2010). The effect of tyrosine nitration of L-type Ca2+ channels on excitation-transcription coupling in colonic inflammation. British Journal of Pharmacology, 159(6). 10.1111/j.1476-5381.2009.00599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi N, Hatano E, Nitta T, Tada M, Harada N, Taura K, et al. (2005). Blocking of PI3K/Akt pathway enhances apoptosis induced by SN-38, an active form of CPT-11, in human hepatoma cells. International Journal of Oncology, 26(5), 1301–1306. 10.3892/ijo.26.5.1301. [DOI] [PubMed] [Google Scholar]
- Kulkarni S, Ganz J, Bayrer J, Becker L, Bogunovic M, & Rao M (2018). Advances in enteric neurobiology: The “Brain” in the gut in health and disease. The Journal of Neuroscience, 38(44), 9346–9354. 10.1523/JNEUROSCI.1663-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni S, Micci MA, Leser J, Shin C, Tang SC, Fu YY, et al. (2017). Adult enteric nervous system in health is maintained by a dynamic balance between neuronal apoptosis and neurogenesis. Proceedings of the National Academy of Sciences of the United States of America, 114(18), E3709–E3718. 10.1073/pnas.1619406114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y (2016). Mechanism-based management for mucositis: Option for treating side effects without compromising the efficacy of cancer therapy. OncoTargets and Therapy, 9, 2007. 10.2147/OTT.S96899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai NY, Musser MA, Pinho-Ribeiro FA, Baral P, Jacobson A, Ma P, et al. (2020). Gut-innervating nociceptor neurons regulate peyer’s patch microfold cells and SFB levels to mediate Salmonella host defense. Cell, 180(1). 10.1016/j.cell.2019.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalla RV, Bowen J, Barasch A, Elting L, Epstein J, Keefe DM, et al. (2014). MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer, 120(10), 1453–1461. 10.1002/cncr.28592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CS (2014). Gastro-intestinal toxicity of chemotherapeutics in colorectal cancer: The role of inflammation. World Journal of Gastroenterology, 20(14), 3751. 10.3748/wjg.v20.i14.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HL, Lu L, Wang XS, Qin LY, Wang P, Qiu SP, et al. (2017). Alteration of gut microbiota and inflammatory cytokine/chemokine profiles in 5-fluorouracil induced intestinal mucositis. Frontiers in Cellular and Infection Microbiology, 7(Oct). 10.3389/fcimb.2017.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Chapleau MW, Bates JN, Bielefeldt K, Lee H-C, & Abboud FM (1998). Nitric oxide as an autocrine regulator of sodium currents in baroreceptor neurons. Neuron, 20(5), 1039–1049. 10.1016/S0896-6273(00)80484-5. [DOI] [PubMed] [Google Scholar]
- Liu YA, Chung YC, Pan ST, Shen MY, Hou YC, Peng SJ, et al. (2013). 3-D imaging, illustration, and quantitation of enteric glial network in transparent human colon mucosa. Neurogastroenterology and Motility, 25(5). 10.1111/nmo.12115. [DOI] [PubMed] [Google Scholar]
- Logan RM, Gibson RJ, Sonis ST, & Keefe DMK (2007). Nuclear factor-κB (NF-κB) and cyclooxygenase-2 (COX-2) expression in the oral mucosa following cancer chemotherapy. Oral Oncology, 43(4), 395–401. 10.1016/j.oraloncology.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Luo J, Qian A, Oetjen LK, Yu W, Yang P, Feng J, et al. (2018). TRPV4 channel signaling in macrophages promotes gastrointestinal motility via direct effects on smooth muscle cells. Immunity, 49(1). 10.1016/j.immuni.2018.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupi LA, Cucielo MS, Silveira HS, Gaiotte LB, Cesário RC, Seiva FRF, et al. (2020). The role of Toll-like receptor 4 signaling pathway in ovarian, cervical, and endometrial cancers. Life Sciences, 247. 10.1016/j.lfs.2020.117435, 117435. [DOI] [PubMed] [Google Scholar]
- Madison BB, Braunstein K, Kuizon E, Portman K, Qiao XT, & Gumucio DL (2005). Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development, 132(2), 279–289. 10.1242/dev.01576. [DOI] [PubMed] [Google Scholar]
- Maroun JA, Anthony LB, Blais N, Burkes R, Dowden SD, Dranitsaris G, et al. (2007). Prevention and management of chemotherapy-induced diarrhea in patients with colorectal cancer: A consensus statement by the Canadian Working Group on Chemotherapy-Induced Diarrhea. Current Oncology, 14(1), 13–20. 10.3747/co.2007.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason HS, Bourke S, & Kemp PJ (2004). Selective modulation of ligand-gated P2X purinoceptor channels by acute hypoxia is mediated by reactive oxygen species. Molecular Pharmacology, 66(6), 1525–1535. 10.1124/mol.104.000851. [DOI] [PubMed] [Google Scholar]
- Mayo BJ, Stringer AM, Bowen JM, Bateman EH, & Keefe DM (2017). Irinotecan-induced mucositis: The interactions and potential role of GLP-2 analogues. Cancer Chemotherapy and Pharmacology, 79(2), 233–249. 10.1007/s00280-016-3165-9. [DOI] [PubMed] [Google Scholar]
- McQuade RM, Al Thaalibi M, & Nurgali K (2020). Impact of chemotherapy-induced enteric nervous system toxicity on gastrointestinal mucositis. Current Opinion in Supportive & Palliative Care, 14(3), 293–300. 10.1097/SPC.0000000000000515. [DOI] [PubMed] [Google Scholar]
- McQuade RM, Carbone SE, Stojanovska V, Rahman A, Gwynne RM, Robinson AM, et al. (2016). Role of oxidative stress in oxaliplatin-induced enteric neuropathy and colonic dysmotility in mice. British Journal of Pharmacology, 173(24), 3502–3521. 10.1111/bph.13646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuade RM, Stojanovska V, Abalo R, Bornstein JC, & Nurgali K (2016). Chemotherapy-induced constipation and diarrhea: Pathophysiology, current and emerging treatments. Frontiers in Pharmacology, 7(Nov), 414. Frontiers Media S.A. 10.3389/fphar.2016.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millington KA, Pascasio JM, Halligan GE, & De Chadarévian JP (2012). Chemotherapy-related leiomyopathy: A suggested morphological explanation for the intestinal dysmotility affecting patients treated with anthracyclines. Modern Pathology, 25(2). 10.1038/modpathol.2011.115. [DOI] [PubMed] [Google Scholar]
- Min K, Kwon OS, Smuder AJ, Wiggs MP, Sollanek KJ, Christou DD, et al. (2015). Increased mitochondrial emission of reactive oxygen species and calpain activation are required for doxorubicin-induced cardiac and skeletal muscle myopathy. Journal of Physiology, 593(8). 10.1113/jphysiol.2014.286518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischel RA, Dewey WL, & Akbarali HI (2018). Tolerance to morphine-induced inhibition of TTX-R sodium channels in dorsal root ganglia neurons is modulated by gut-derived mediators. IScience, 2, 193–209. 10.1016/j.isci.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BA, Stewart TMR, Hill C, & Vanner SJ (2002). TNBS ileitis evokes hyperexcitability and changes in ionic membrane properties of nociceptive DRG neurons. American Journal of Physiology-Gastrointestinal and Liver Physiology, 282(6), G1045–G1051. 10.1152/ajpgi.00406.2001. [DOI] [PubMed] [Google Scholar]
- Muchhala KH, Jacob JC, Alam I, Hasan S, Khan A, Kang M, et al. (2020). Rapid tolerance to morphine in the myenteric neurons of the small intestine is independent of β-arrestin-2 and mediated by PKC. BioRxiv, 2020(07), 17.209437. 10.1101/2020.07.17.209437. [DOI] [Google Scholar]
- National Cancer Institute. (2017). Common terminology criteria for adverse events (CTCAE) common terminology criteria for adverse events (CTCAE) v5.0. https://ctep.cancer.gov.
- Neunlist M, Aubert P, Bonnaud S, Van Landeghem L, Coron E, Wedel T, et al. (2007). Enteric glia inhibit intestinal epithelial cell proliferation partly through a TGF-β1-dependent pathway. American Journal of Physiology - Gastrointestinal and Liver Physiology, 292(1). 10.1152/ajpgi.00276.2005. [DOI] [PubMed] [Google Scholar]
- Nogueira LT, Costa DVS, Gomes AS, Martins CS, Silva AMHP, Coelho-Aguiar JM, et al. (2017). The involvement of mast cells in the irinotecan-induced enteric neurons loss and reactive gliosis. Journal of Neuroinflammation, 14(1). 10.1186/s12974-017-0854-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawolski V, & Schmidt MHH (2020). Neuron–glia interaction in the developing and adult enteric nervous system. Cells, 10(1), 47. 10.3390/cells10010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson LW, & Artis D (2014). Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nature Reviews. Immunology, 14(3), 141–153. 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- Prisciandaro LD, Geier MS, Butler RN, Cummins AG, & Howarth GS (2011). Probiotic factors partially improve parameters of 5-fluorouracil-induced intestinal mucositis in rats. Cancer Biology & Therapy, 11(7), 671–677. 10.4161/cbt.11.7.14896. [DOI] [PubMed] [Google Scholar]
- Przewlocki R, & Przewlocka B (2005). Opioids in neuropathic pain. Current Pharmaceutical Design, 11(23), 3013–3025. 10.2174/1381612054865055. [DOI] [PubMed] [Google Scholar]
- Qi L, Luo Q, Zhang Y, Jia F, Zhao Y, & Wang F (2019). Advances in toxicological research of the anticancer drug cisplatin. Chemical Research in Toxicology, 32(8), 1469–1486. 10.1021/acs.chemrestox.9b00204. [DOI] [PubMed] [Google Scholar]
- Ramakrishna C, Corleto J, Ruegger PM, Logan GD, Peacock BB, Mendonca S, et al. (2019). Dominant role of the gut microbiota in chemotherapy induced neuropathic pain. Scientific Reports, 9(1), 20324. 10.1038/s41598-019-56832-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M, Rastelli D, Dong L, Chiu S, Setlik W, Gershon MD, et al. (2017). Enteric glia regulate gastrointestinal motility but are not required for maintenance of the epithelium in mice. Gastroenterology, 153(4), 1068–1081.e7. 10.1053/j.gastro.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro RA, Wanderley CWS, Wong DVT, Mota JMSC, Leite CAVG, Souza MHLP, et al. (2016). Irinotecan- and 5-fluorouracil-induced intestinal mucositis: Insights into pathogenesis and therapeutic perspectives. Cancer Chemotherapy and Pharmacology, 78(5), 881–893. 10.1007/s00280-016-3139-y. [DOI] [PubMed] [Google Scholar]
- Robinson AM, Stojanovska V, Rahman AA, McQuade RM, Senior PV, & Nurgali K (2016). Effects of oxaliplatin treatment on the enteric glial cells and neurons in the mouse ileum. Journal of Histochemistry and Cytochemistry, 64(9). 10.1369/0022155416656842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross GR, Kang M, & Akbarali HI (2010). Colonic inflammation alters Src kinase-dependent gating properties of single Ca2+ channels via tyrosine nitration. American Journal of Physiology - Gastrointestinal and Liver Physiology, 298(6). 10.1152/ajpgi.00056.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross GR, Kang M, Shirwany N, Malykhina AP, Drozd M, & Akbarali HI (2007). Nitrotyrosylation of Ca2+ channels prevents c-Src kinase regulation of colonic smooth muscle contractility in experimental colitis. Journal of Pharmacology and Experimental Therapeutics, 322(3). 10.1124/jpet.107.123075. [DOI] [PubMed] [Google Scholar]
- Said HM (2018). Physiology of the gastrointestinal tract (6th ed., pp. 1–2). Elsevier. [Google Scholar]
- Sałat K (2020). Chemotherapy-induced peripheral neuropathy: Part 1—current state of knowledge and perspectives for pharmacotherapy. Pharmacological Reports, 72(3), 486–507. 10.1007/s43440-020-00109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM, Koh SD, Ro S, & Ward SM (2012). Regulation of gastrointestinal motility-insights from smooth muscle biology. Nature Reviews Gastroenterology and Hepatology, 9(11). 10.1038/nrgastro.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang Q, Williamson S, & Young HM (1997). Projections of chemically identified myenteric neurons of the small and large intestine of the mouse. Journal of Anatomy, 190(2), 209–222. 10.1046/j.1469-7580.1997.19020209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller LR, Pardi DS, & Sellin JH (2017). Chronic diarrhea: Diagnosis and management. Clinical Gastroenterology and Hepatology, 15(2), 182–193.e3. 10.1016/j.cgh.2016.07.028. [DOI] [PubMed] [Google Scholar]
- Schilling T, & Eder C (2009). Importance of the non-selective cation channel TRPV1 for microglial reactive oxygen species generation. Journal of Neuroimmunology, 216(1–2), 118–121. 10.1016/j.jneuroim.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Schulthess J, Pandey S, Capitani M, Rue-Albrecht KC, Arnold I, Franchini F, et al. (2019). The short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity, 50(2). 10.1016/j.immuni.2018.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab M, Zanger UM, Marx C, Schaeffeler E, Klein K, Dippon J, et al. (2008). Role of genetic and nongenetic factors for fluorouracil treatment-related severe toxicity: A prospective clinical trial by the German 5-FU toxicity study group. Journal of Clinical Oncology, 26(13), 2131–2138. 10.1200/JCO.2006.10.4182. [DOI] [PubMed] [Google Scholar]
- Secombe KR, Van Sebille YZA, Mayo BJ, Coller JK, Gibson RJ, & Bowen JM (2020). Diarrhea induced by small molecule tyrosine kinase inhibitors compared with chemotherapy: Potential role of the microbiome. Integrative Cancer Therapies, 19. 10.1177/1534735420928493. 153473542092849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguella L, & Gulbransen BD (2021). Enteric glial biology, intercellular signalling and roles in gastrointestinal disease. Nature Reviews. Gastroenterology & Hepatology, 18(8), 571–587. 10.1038/s41575-021-00423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessenwein JL, Baker CC, Pradhananga S, Maitland ME, Petrof EO, Allen-Vercoe E, et al. (2017). Protease-mediated suppression of DRG neuron excitability by commensal bacteria. Journal of Neuroscience, 37(48). 10.1523/JNEUROSCI.1672-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey KA (2015). Emerging roles for enteric glia in gastrointestinal disorders. Journal of Clinical Investigation, 125(3). 10.1172/JCI76303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Lim G, You Z, Ding W, Huang P, Ran C, et al. (2017). Gut microbiota is critical for the induction of chemotherapy-induced pain. Nature Neuroscience, 20(9), 1213–1216. 10.1038/nn.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares PMG, Mota JMSC, Gomes AS, Oliveira RB, Assreuy AMS, Brito GAC, et al. (2008). Gastrointestinal dysmotility in 5-fluorouracil-induced intestinal mucositis outlasts inflammatory process resolution. Cancer Chemotherapy and Pharmacology, 63(1), 91–98. 10.1007/s00280-008-0715-9. [DOI] [PubMed] [Google Scholar]
- Sonis ST (2004). The pathobiology of mucositis. Nature Reviews Cancer, 4(4), 277–284. 10.1038/nrc1318. [DOI] [PubMed] [Google Scholar]
- Sougiannis AT, VanderVeen BN, Davis JM, Fan D, & Murphy EA (2021). Understanding chemotherapy-induced intestinal mucositis and strategies to improve gut resilience. American Journal of Physiology - Gastrointestinal and Liver Physiology, 320(5). 10.1152/AJPGI.00380.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein A, Voigt W, & Jordan K (2010). Chemotherapy-induced diarrhea: pathophysiology, frequency and guideline-based management. Therapeutic Advances in Medical Oncology, 2(1), 51–63. 10.1177/1758834009355164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojanovska V, Sakkal S, & Nurgali K (2015). Platinum-based chemotherapy: Gastrointestinal immunomodulation and enteric nervous system toxicity. American Journal of Physiology. Gastrointestinal and Liver Physiology, 308, 223–232. 10.1152/ajpgi.00212.2014.-The. [DOI] [PubMed] [Google Scholar]
- Stojanovska V, Stewart M, McQuade R, Timpani C, Sorensen J, Orbell J, et al. (2016). Platinum accumulation and changes in mitochondrial function of the longitudinal muscle and myenteric plexus following oxaliplatin administration. Neurogastroenterology & Motility, 28, 55. [Google Scholar]
- Stringer AM, Gibson RJ, Bowen JM, Logan RM, Ashton K, Yeoh ASJ, et al. (2009). Irinotecan-induced mucositis manifesting as diarrhoea corresponds with an amended intestinal flora and mucin profile. International Journal of Experimental Pathology, 90(5), 489–499. 10.1111/j.1365-2613.2009.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer AM, Gibson RJ, Bowen JM, Logan RM, Yeoh ASJ, & Keefe DMK (2007). Chemotherapy-induced mucositis: The role of gastrointestinal microflora and mucins in the luminal environment. The Journal of Supportive Oncology, 5(6), 259–267. [PubMed] [Google Scholar]
- Stringer AM, Gibson RJ, Logan RM, Bowen JM, Yeoh ASJ, & Keefe DMK (2008). Faecal microflora and β-glucuronidase expression are altered in an irinotecan-induced diarrhea model in rats. Cancer Biology & Therapy, 7(12), 1919–1925. 10.4161/cbt.7.12.6940. [DOI] [PubMed] [Google Scholar]
- Stringer AM, Gibson RJ, Logan RM, Bowen JM, Yeoh ASJ, Laurence J, et al. (2009). Irinotecan-induced mucositis is associated with changes in intestinal mucins. Cancer Chemotherapy and Pharmacology, 64(1), 123–132. 10.1007/s00280-008-0855-y. [DOI] [PubMed] [Google Scholar]
- Stringer AM, & Logan RM (2015). The role of oral flora in the development of chemotherapy-induced oral mucositis. Journal of Oral Pathology and Medicine, 44(2), 81–87. Blackwell Publishing Ltd. 10.1111/jop.12152. [DOI] [PubMed] [Google Scholar]
- Sun R, Zhu L, Li L, Song W, Gong X, Qi X, et al. (2020). Irinotecan-mediated diarrhea is mainly correlated with intestinal exposure to SN-38: Critical role of gut Ugt. Toxicology and Applied Pharmacology, 398, 115032. 10.1016/j.taap.2020.115032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeba Y, Kumai T, Matsumoto N, Nakaya S, Tsuzuki Y, Yanagida Y, et al. (2007). Irinotecan activates p53 with its active metabolite, resulting in human hepatocellular carcinoma apoptosis. Journal of Pharmacological Sciences, 104(3), 232–242. 10.1254/jphs.FP0070442. [DOI] [PubMed] [Google Scholar]
- Tao G, Huang J, Moorthy B, Wang C, Hu M, Gao S, et al. (2020). Potential role of drug metabolizing enzymes in chemotherapy-induced gastrointestinal toxicity and hepatotoxicity. Expert Opinion on Drug Metabolism & Toxicology, 16(11), 1109–1124. 10.1080/17425255.2020.1815705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma W, Kyte SL, Bagdas D, Alkhlaif Y, Alsharari SD, Lichtman AH, et al. (2017). Effects of paclitaxel on the development of neuropathy and affective behaviors in the mouse. Neuropharmacology, 117, 305–315. 10.1016/j.neuropharm.2017.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touchefeu Y, Montassier E, Nieman K, Gastinne T, Potel G, Bruley Des Varannes S, et al. (2014). Systematic review: The role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis—Current evidence and potential clinical applications. Alimentary Pharmacology & Therapeutics, 40(5). 10.1111/apt.12878. [DOI] [PubMed] [Google Scholar]
- Ustaris F, Saura C, Palma JD, Bryce R, Moran S, Neuman L, et al. (2015). Effective management and prevention of neratinib-induced diarrhea. American Journal of Hematology/Oncology, 11(11). [Google Scholar]
- van der Flier LG, & Clevers H (2009). Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annual Review of Physiology, 71(1), 241–260. 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- van der Flier LG, van Gijn ME, Hatzis P, Kujala P, Haegebarth A, Stange DE, et al. (2009). Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell, 136(5), 903–912. 10.1016/j.cell.2009.01.031. [DOI] [PubMed] [Google Scholar]
- Venegas DP, De La Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, et al. (2019). Short chain fatty acids (SCFAs)mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Frontiers in Immunology, 10(Mar). 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicentini FA, Keenan CM, Wallace LE, Woods C, Cavin J-B, Flockton AR, et al. (2021). Intestinal microbiota shapes gut physiology and regulates enteric neurons and glia. Microbiome, 9(1), 210. 10.1186/s40168-021-01165-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa A, & Sonis ST (2015). Mucositis. Current Opinion in Oncology, 27(3), 159–164. 10.1097/CCO.0000000000000180. [DOI] [PubMed] [Google Scholar]
- Villarreal A, Aviles Reyes RX, Angelo MF, Reinesf AG, & Ramos AJ (2011). S100B alters neuronal survival and dendrite extension via RAGE-mediated NF-κB signaling. Journal of Neurochemistry, 117(2). 10.1111/j.1471-4159.2011.07207.x. [DOI] [PubMed] [Google Scholar]
- Vyas D, Laput G, & Vyas AK (2014). Chemotherapy-enhanced inflammation may lead to the failure of therapy and metastasis. OncoTargets and Therapy, 7. 10.2147/OTT.S60114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadler S, Benson AB, Engelking C, Catalano R, Field M, Kornblau SM, et al. (1998). Recommended guidelines for the treatment of chemotherapy-induced diarrhea. Journal of Clinical Oncology, 16(9), 3169–3178. 10.1200/JCO.1998.16.9.3169. [DOI] [PubMed] [Google Scholar]
- Wang Q, Li Z, Liu K, Liu J, Chai S, Chen G, et al. (2021). Activation of the G protein–coupled estrogen receptor prevented the development of acute colitis by protecting the crypt cell. Journal of Pharmacology and Experimental Therapeutics, 376(2), 281–293. 10.1124/jpet.120.000216. [DOI] [PubMed] [Google Scholar]
- Wei L, Wen XS, & Xian CJ (2021). Chemotherapy-induced intestinal microbiota dysbiosis impairs mucosal homeostasis by modulating toll-like receptor signaling pathways. International Journal of Molecular Sciences, 22(17), 9474. 10.3390/ijms22179474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CH, Ko JL, Liao JM, Huang SS, Lin MY, Lee LH, et al. (2019). D-methionine alleviates cisplatin-induced mucositis by restoring the gut microbiota structure and improving intestinal inflammation. Therapeutic Advances in Medical Oncology, 11. 10.1177/1758835918821021. 175883591882102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Gan Y, Li M, Chen L, Liang J, Zhuo J, et al. (2020). Patchouli alcohol attenuates 5-fluorouracil-induced intestinal mucositis via TLR2/MyD88/NF-kB pathway and regulation of microbiota. Biomedicine & Pharmacotherapy, 124, 109883. 10.1016/j.biopha.2020.109883. [DOI] [PubMed] [Google Scholar]
- Wu Y, Wu J, Lin Z, Wang Q, Li Y, Wang A, et al. (2021). Administration of a probiotic mixture ameliorates cisplatin-induced mucositis and pica by regulating 5-HT in rats. Journal of Immunology Research, 2021, 1–16. 10.1155/2021/9321196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Han X, Qin S, Zheng Q, Wang Z, Xiang D, et al. (2010). Interleukin 1 receptor antagonist reduces lethality and intestinal toxicity of 5-fluorouracil in a mouse mucositis model. Biomedicine & Pharmacotherapy, 64(9), 589–593. 10.1016/j.biopha.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Wu Z, Han X, Qin S, Zheng Q, Wang Z, Xiang D, et al. (2011). Interleukin 1 receptor antagonist reduces lethality and intestinal toxicity of 5-Fluorouracil in a mouse mucositis model. Biomedicine & Pharmacotherapy, 65(5), 339–344. 10.1016/j.biopha.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Ishihara K, Takeda Y, Koizumi W, & Ichikawa T (2013). Changes in the mucus barrier during cisplatin-induced intestinal mucositis in rats. BioMed Research International, 2013, 1–8. 10.1155/2013/276186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung CY, Chan WT, Jiang CB, Cheng ML, Liu CY, Chang SW, et al. (2015). Amelioration of chemotherapy-induced intestinal mucositis by orally administered probiotics in a mouse model. PLoS One, 10(9), e0138746. 10.1371/journal.pone.0138746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda J, Nishikawa S, & Kurihara S (2021). Oral administration of cystine and theanine attenuates 5-fluorouracil-induced intestinal mucositis and diarrhea by suppressing both glutathione level decrease and ROS production in the small intestine of mucositis mouse model. BMC Cancer, 21(1), 1343. 10.1186/s12885-021-09057-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Chen F, Wu W, Sun M, Bilotta AJ, Yao S, et al. (2018). GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal Immunology, 11(3), 752–762. 10.1038/mi.2017.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidan J, Haim N, Beny A, Stein M, Gez E, & Kuten A (2001). Octreotide in the treatment of severe chemotherapy-induced diarrhea. Annals of Oncology, 12(2), 227–229. 10.1023/A:1008372228462. [DOI] [PubMed] [Google Scholar]


