Abstract
The current study was conducted to evaluate the effects of different administration routes of bacterial lipopolysaccharide (LPS) on intestinal mucosal morphological, immunological, and microbial barrier functions in goslings. First, we compared intestinal villi morphology of goslings under intraperitoneal or oral LPS treatment through hematoxylin and eosin staining. Then, we determined the signatures of the microbiome in the ileum mucosa of goslings subjected to oral LPS treatment at 0, 2, 4, and 8 mg/kg BW by 16S sequencing, and analyzed the changes in intestinal barrier functions and permeability, levels of LPS in the ileum mucosa, plasma, and liver tissue, and the induced inflammatory response of Toll-like receptor 4 (TLR4). As a result, intraperitoneal LPS injection resulted in a thicker intestinal wall in the ileum within a short time, whereas villus height was less affected; in contrast, oral LPS treatment exerted a stronger influence on villus height but not on intestinal wall thickness. We also found that oral LPS treatment affected the structure of the intestinal microbiome, reflected by changes in the clustering of intestinal microbiota. The average abundance of Muribaculaceae showed an increasing trend with increasing LPS levels, and that of the genus Bacteroides decreased, compared with the control group. In addition, oral LPS treatment with 8 mg/kg BW affected the intestinal epithelial morphology, damage the mucosal immune barrier, downregulated the expression of tight junction proteins, increased circulating D-lactate levels, and stimulated the secretion of various inflammatory mediators and activation of the TLR4/MyD88/NFκB pathway. This study presented the injuries of intestinal mucosal barrier function induced by LPS challenges in goslings and provided a scientific model for searching the novel strategies to attenuate the immunological stress and gut injury caused by LPS.
Key words: gosling, lipopolysaccharide challenge, intestinal mucosal barrier function
INTRODUCTION
Geese are economically important because they supply nutritious meat and high-quality liver fat for cooking as well as feathers for the clothing industry (Lu et al., 2015; Gao et al., 2016). China has the largest geese farming industry in the world, with recently approximately 60,000 gees slaughtered per year (data from the China Agriculture Research System of MOF and MARA, 2020). Intensive poultry farms are typically prone to outbreaks of infections with pathogenic bacteria and environmental pollution. Lipopolysaccharide (LPS) is the major component of the outer surface membrane of almost all gram-negative bacteria, including Escherichia coli and Salmonella spp., and excessive LPS can severely affect the survival rate and growth performance of geese (Yang et al., 2012; Xi et al., 2022). In a previous study, we found that gut-derived LPS translocation caused by gut microbiota dysbiosis in goslings with gout increased the risk of systemic inflammation (Xi et al., 2019). LPS can elicit inflammatory responses in various eukaryotes by activating signaling pathways and promoting gene expression (Lu et al., 2008). In geese, LPS injection involves a gradually enhanced inflammatory response and reduces endocrine functioning in adult Yangzhou geese (Ying et al. 2017,2020). A splenic oxidative stress model established through intraperitoneal LPS injection showed ferroptosis-related indicators and cytokine expression in Magang goslings (Li et al., 2022). More importantly, geese exhibit different LPS tolerance thresholds, compared with ducks, adult chickens, and other poultry (Yang et al., 2012), however, few respective studies are available. Thus, it is necessary to study the harmful consequences of bacterial LPS challenge on geese production to maintain acceptable survival rates and health of goslings.
LPS challenge may deteriorate intestinal epithelial integrity, jeopardize the barrier functions of the gastrointestinal tract (Flaviana et al., 2019), and inhibit growth in poultry. Many studies on chickens have shown that immunological stress induced through intraperitoneal or intravenous administration of LPS can change intestinal morphology, downregulate mRNA expression of tight junction (TJ) proteins, and increase circulating D-lactate levels and diamine oxidase (DAO) activity, which are 2 sensitive biomarkers reflecting gut permeability (Li et al., 2015; Hu et al., 2021). LPS also plays an important role in activating the nuclear factor kappa B (NF-κB) pathway and inducing intestinal inflammatory responses in chickens (Cheng et al., 2017). Some studies also found that Pekin ducks showed similar response patterns to LPS challenge as chickens (Xie et al., 2021). However, few studies have evaluated the effects of LPS on intestinal barrier function in geese. In addition, most previous studies on the effects of LPS treatment on intestinal function in poultry used intraperitoneal injection rather than oral administration (Li et al., 2015; Horvatić et al., 2019; Hu et al., 2021; Xie et al., 2021). However, LPS in the diet, water, or feces in poultry production mainly enters the body through the gastrointestinal tract after oral intake (Yang et al., 2012; Cheled-Shoval et al., 2014). This should be considered because the route of LPS administration has also been shown to play an important role with regard to metabolic and immune responses to LPS challenge (Zebeli et al., 2013). Therefore, we analyzed the effect of LPS treatment on intestinal morphology, barrier functions, and inflammatory responses in goslings, and explored differences in the damage mechanisms of the intestinal barrier between different LPS administrations.
MATERIALS AND METHODS
Experimental Design
This study was conducted at the Experimental Animal Center of the Jiangsu Academy of Agricultural Sciences (Nanjing, China) in 2021. All goslings were purchased from Anhui Tianzhijiao Goose Co. Ltd. (Chuzhou, China). In Experiment 1, 56 one-day-old male Taizhou goslings (Anser anser domesticus) were randomly assigned to 7 groups of 8 goslings, each, and were prefed for 7 d. At 8 d of age, the goslings were intraperitoneally injected with 0, 0.25, 0.5, 1, 2, 4, and 8 mg/kg BW LPS (from Escherichia coli, serotype O55:B5, 2880, Sigma-Aldrich, St. Louis, MO; diluted with PBS) at a volume of 1 mL. Blood samples were collected 12 h after LPS injection to determine the appropriate LPS concentration for further experiments. In Experiment 2, 80 one-day-old male Taizhou goslings were randomly assigned to 2 groups of 40 goslings, each. After prefeeding for 7 d and at 8 d of age, the goslings in the LPS group (LPS) were injected intraperitoneally with 4 mg/kg BW LPS in 1 mL (based on the results of Experiment 1). The control group was injected with 1 mL PBS at the same time. Tissue samples of 16 goslings (8 individuals per group) were collected 4, 12, 24, and 48 h after LPS injection to analyze time-dependent effects of intraperitoneal LPS treatment. Considering that bacterial LPS in geese mainly enters the body through the gastrointestinal tract, Experiment 3 was conducted by exposing goslings to LPS by oral perfusion at various dosages for 3 d: 48 one-day-old male Taizhou goslings were randomly assigned to 4 groups of 12 individuals, each, and were prefed for 7 d. Then, from 8 to 10 d of age, the goslings were treated by oral perfusion with 0 (HN0, control), 2 (HN2), 4 (HN4), and 8 (HN8) mg/kg BW LPS in 1 mL at 7:00 each day. Tissues of all goslings were collected 24 h after the last perfusion with LPS. All goslings were kept in a thermostatic house and in stainless steel cages of identical size (1.20 × 1.00 × 0.50 m which were mounted 0.50 m above the ground, with 8 goslings per cage in Experiments 1 and 2, and 12 goslings per cage in Experiment 3. The goslings were fed according to standard management conditions, with feed and water provided ad libitum. The ambient temperature was maintained at 30°C from d 0 to 3, 28°C from d 4 to 6, and 26°C from d 7 to 10. Relative humidity was approximately 60% throughout the experimental period.
Plasma Biochemistry, Endotoxin, and Secretory Immunoglobulin A Determination
Goslings were slaughtered by decapitation as per the respective experimental design, and blood samples were collected immediately (5 mL/individual) in tubes without anticoagulant. Blood samples were incubated at 37°C for 2 h and were centrifuged at 1,500 × g for 15 min. The obtained sera were stored in 0.6-mL Eppendorf tubes at −80°C until analyses. Concentrations of glutamic oxalacetic transaminase (GOT), glutamic pyruvic transaminase (GPT), creatinine (Cr), and urea nitrogen (UN) were determined via enzymatic colorimetry using a microplate spectrophotometer (Promega Corporation, Madison, WI). The respective kits were supplied by the Jiancheng Bioengineering Institute (Nanjing, China); the codes were C010 (GOT), C009 (GPT), C011 (Cr), and C013 (UN). Plasma D-lactate concentration was quantified using a D-lactate colorimetric assay kit (catalog no. K667-100; BioVision Inc., San Francisco, CA), according to the manufacturer's protocol, and its determination range was 0.01 to 10 nmol/mL. Endotoxin accumulation was detected using a conventional limulus amebocyte lysate assay. The details on tissue collection and homogenization of this protocol refer to Ceasrine et al. (2022), and these steps are necessary to ensure sample opacity will not interfere with the assay. The amount of water for homogenizing the samples in should be optimized for each sample type, and we found 1 mL worked well for 100 mg of liver or mucosa tissues. Then, the following steps of run the assay and data analysis are based on the manufacturer's instructions (Xiamen Limulus Assay Biotechnology Company Ltd., Xiamen, China). Briefly, standards are generated by serial dilution for a standard curve, and samples are diluted prior to being loaded onto the plate. Samples and standards are assayed for endotoxin levels [EU/mL, equivalent to EU/100 mg for tissues]. The detection ranges of LPS in the Limulus assay ranged from 0.015 to 10 EU/mL. We also used a chicken secretory immunoglobulin A (sIgA) ELISA kit to process samples for sIgA measurement, according to the manufacturer's instructions (SenBeiJia, Nanjing, China). All materials used for blood collection and endotoxin measurement were pyrogen-free. All assays were performed according to the respective manufacturer's instructions. The samples were tested in triplicates. Intra- and interassay coefficients of variation were <10 and <15%, respectively.
Intestinal Morphology
Goslings were necropsied to examine the small intestine which was exposed and separated from the mesentery after blood sample collection. The ileum samples (about 4 cm segments in the mid-ileum) were collected from Meckel's diverticulum to the cecal junction for histological analyses. Samples were washed using ice-cold PBS (pH 7.4), fixed in 4% paraformaldehyde, embedded in paraffin, and were sectioned to 3 μm thickness (4 slices per gosling). Villus height and crypt depth of 15 well-oriented villi per segment were measured using a Nikon ECLIPSE 80i light microscope equipped with a computer-assisted morphometric system (Nikon Corporation, Tokyo, Japan).
Goblet Cell Staining
Samples for goblet cell staining were prepared according to established procedures for intestinal morphology analysis (Li et al., 2015). The combined Alcian blue/periodic acid Schiff stain technique was employed to measure intestinal goblet cell density. Deparaffinized and rehydrated sections were stained with 1.0% Alcian blue solution (Alcian blue in 3% acetic acid solution), were washed gently using double-distilled H2O for 10 min, oxidized in 1.0% periodic acid solution for 15 min, rinsed again in double-distilled H2O for 10 min, and were then placed in periodic acid Schiff solution for 30 min. Goblet cells were counted in 15 well-oriented villi per section using a Nikon ECLIPSE 80i light microscope (Nikon Corporation). Goblet cell density was calculated (goblet cell count divided by corresponding villus length), averaged, and was expressed as the goblet cell numbers per 100 μm villus length.
Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick-End Labeling Assay
A terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) kit (Vazyme Biotech Co., Ltd., Nanjing, China) was used to detect DNA damage-based apoptosis in the mucosal epithelium. Briefly, ileum tissues were fixed with 4% paraformaldehyde. After washing the tissues with PBS, the TUNEL assay was performed using a fluorescein-conjugated probe according to the manufacturer's protocol. The nuclei were stained with DAPI. The slides were washed and imaged with a fluorescent microscope. Apoptosis was analyzed using an image analyzer to calculate the positive cell rate.
RT-PCR
Cecal tonsil tissues of goslings were collected after mesenteric separation and were stored in 2-mL RNase/DNase-free Eppendorf tubes at −80°C until RT-PCR. Total RNA was extracted from the tissues using TRIzol reagent (Life Technologies, Grand Island, NY) and was reverse-transcribed using a Reverse Transcription Levels kit (TaKaRa, Dalian, China), according to the manufacturer's instructions. β-Actin was used as an invariant control. Primers were designed using Primer Premier 5.0 (Premier, Canada), and their sequences are listed in Table 1. RT-PCR was performed using SYBR Premix Ex TaqTM (Roche, Switzerland). All RT-PCRs were performed in triplicates. The relative expression levels of the target genes were determined using the 2− ΔΔCt method.
Table 1.
Primer sequences for RT-PCR.
| Gene name | Forward primer (5′–3′) | Reversed primer (5′–3′) | Product length (bp) |
|---|---|---|---|
| β-Actin | TGACGCAGATCATGTTTGAGA | GCAGAGCGTAGCCCTCATAG | 159 |
| OCLN | CGCCACGTTCTTCACCCACTC | CTCATCTGCTTCTTCGCCCACA | 129 |
| CLDN5 | GTCCCGCTCTGCTGGTTC | CCCTATCTCCCGCTTCTGG | 84 |
| ZO1 | CTTCAGGTGTTTCTCTTCCTCCTC | CTGTGGTTTCATGGCTGGATC | 131 |
| TLR4 | GGTGCCACATCCATACAAT | TAGGTCAGTCAGAGAGGATA | 173 |
| MyD88 | CCCTGGGGAAAGACTAAGAGC | AAGAAGGTGTCGGAGGATGGT | 100 |
| NFκB | GCCCAATGCCTCCAACTTAAA | ATATCATCTTTCTGAACCTTGTCAC | 108 |
| IL1β | ACTGGGCATCAAGGGCTA | GGTAGAAGATGAAGCGGGTC | 122 |
| IL8 | CCAAGCACACCTCTCTTCCA | GCAAGGTAGGACGCTGGTAA | 164 |
OCLN: occludin; CLDN5: claudin-5; ZO1: zona occludens-1; TLR4: Toll-like receptor 4; MyD88: myeloid differentiation primary response gene 88; NFκB: nuclear factor-κB; IL1β: interleukin-1β; IL8: interleukin-8.
16S rRNA Sequencing of the Ileum Mucosa
The ileum mucosa was scratched carefully using a sterile glass microscope slide, and the samples were placed in sterile internally threaded cryogenic 2-mL vials, snap-frozen in liquid N2, and stored at −80°C until 16S rDNA analysis. DNA from ileal mucosa samples was extracted using a MicroElute Genomic DNA Kit (D3096-01, Omega, Inc., Norcross, GA) according to the manufacturer's instructions. Sample blanks consisting of unused swabs were processed through DNA extraction to control for contamination. Total DNA was eluted in 50 µL elution buffer using a modified procedure described by the manufacturer (QIAGEN, Hilden, Germany) and was stored at −80°C.
Using total DNA as a template and 16S rDNA primers (343F: 5′-TACGGRAGGCAGCAG-3′; 798R: 5′-AGGGTATCTAATCCT-3′), we amplified the V3 to V4 region of bacterial 16S rRNA. All reactions were carried out in 25 µL total volume containing 25 ng genomic DNA, 12.5 µL PCR Premix, 2.5 µL of each primer, and PCR-grade water to adjust the volume. PCR products were normalized using an AxyPrep Mag PCR Normalizer (Axygen Biosciences, Union City, CA), which allowed omitting quantification, regardless of the PCR reaction volume submitted for sequencing. Amplicon pools were prepared for sequencing using AMPure XT beads (Beckman Coulter Genomics, Danvers, MA; OE Biotech Co., Ltd., Shanghai, China). Size and quantity of the amplicon library were assessed using a LabChip GX (Perkin Elmer, Waltham, MA) and a Kapa Library Quantification Kit for Illumina (Kapa Biosciences, Woburn, MA), respectively. The PhiX Control library (v3) (Illumina, San Diego, CA) was added to the amplicon library (at expected 30%). The library was clustered at a density of approximately 570 K/mm2. The libraries were sequenced either on 300PE MiSeq runs, where one library was sequenced with both protocols using standard Illumina sequencing primers, thereby eliminating the need for a third (or fourth) index read. The reads were filtered using QIIME software (http://qiime.org/tutorials/processing_illumina_data.html). The CD-HIT pipeline was used to select operational taxonomic units (OTU) by producing an OTU table. Sequences were assigned at 97% similarity to the OTUs. Representative sequences were chosen for each OTU, and taxonomic data were assigned to each representative sequence using the RDP classifier (Wang et al., 2007). The OTU nucleotide sequences were made available on GenBank under the accession SAMN31842828. To estimate alpha diversity, the OTU table was rarified, and the following 4 metrics were calculated: the Chao1 metric to estimate richness, observed species metric as a count of unique OTUs found in the sample, the Shannon index, and the Simpson index. Principal coordinate analysis (PCoA) based on Bray-Curtis was used to estimate the dissimilarity in the community structure (β-diversity). Kyoto Encyclopedia of Genes and Genomes (KEGG) predictions of bacterial community functions were made using STAMP software v2.1.3 (Pasks et al., 2014).
Data Analyses
One-way ANOVA with post hoc Tukey's multiple comparison test was performed for assessing the effect of LPS dosage, while 2-way ANOVA with post hoc Tukey's multiple comparison test was performed to examine the effects of LPS dosage and action time. Significance was declared if P < 0.05 and a trend was reported if 0.05 < P < 0.10. All analyses were performed using GraphPad Prism software v7.0 (GraphPad Prism Software, San Diego, CA).
RESULTS
Effects of Intraperitoneal LPS Treatment on Plasma LPS Concentrations and Intestinal Morphology
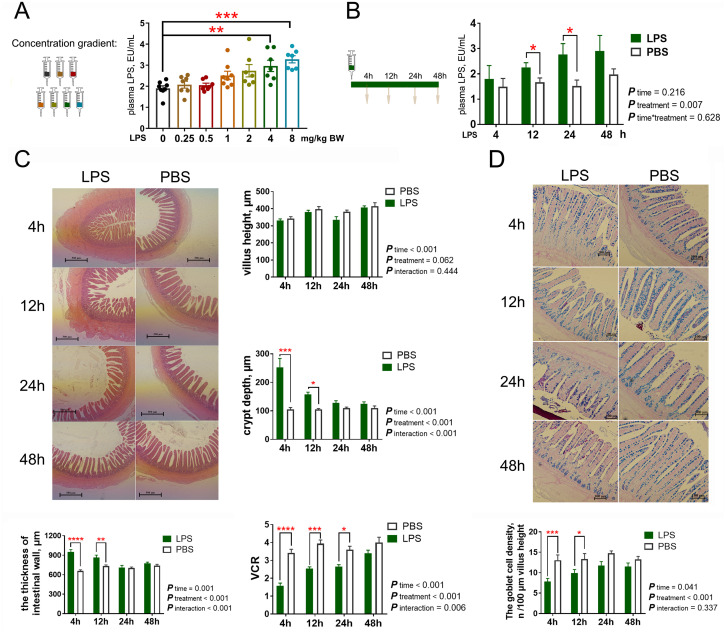
The effects of intraperitoneal LPS treatment on plasma LPS concentrations and intestinal morphology are visualized in Figure 1. Compared with the control (0 mg/kg), LPS injection at 4 and 8 mg/kg increased plasma LPS concentrations (P < 0.05), and the difference between the control and the 8 mg/kg LPS treatment was highly significant (P < 0.01). Based on this result, we chose 4 mg/kg as the optimal concentration in the subsequent experiments. Intraperitoneal LPS treatment consistently affected plasma LPS concentrations within 48 h after injection (P < 0.05), however, this irritation reaction first increased and then decreased over time. The significant differences of plasma LPS concentrations appeared at 12 and 24 h after LPS injection, compared with the PBS treatments. Unlike plasma LPS concentrations, the intestinal irritation reaction caused by intraperitoneal LPS treatment would be strong at first and then decrease with time. LPS treatment resulted in higher crypt depth and thicker intestinal wall, lower ratio between villus height and crypt depth, and lower goblet density in the ileum at 4 and 12 h (P < 0.05, each); however, no significant difference in crypt depth, intestinal wall thickness, and goblet density between the LPS and PBS treatments occurred at 24 and 48 h (P > 0.05), and the significant difference in villus:crypt depth ratio (VCR) between the 2 groups was not observed at 48 h (P > 0.05). In addition, villus heights in the ileum did not differ between the LPS and PBS treatments at any time point (P > 0.05).
Figure 1.
Intraperitoneal LPS challenge impacts on plasma LPS concentration and intestinal morphology in goslings. (A) The levels of plasma LPS among goslings with different LPS injection levels. (B) The levels of plasma LPS with different sampling times when goslings under a 4 mg/kg BW (body weight) LPS injection. (C) Morphological observation of the epithelial tissue in ileum with HE staining, and the comparison of intestinal wall thickness, villus height, crypt depth, and VCR among groups. (D) Morphological observation of the epithelial tissue in ileum with AB-PAS stain, and density of intestinal goblet cells. n = 8 goslings. Abbreviations: AB-PAS stain: the combined Alcian blue/periodic acid Schiff stain; HE staining, hematoxylin and eosin staining; LPS, lipopolysaccharide; VCR, villus height/crypt depth ratio.
Oral LPS Administration Affects Plasma LPS Concentrations and Intestinal Morphology
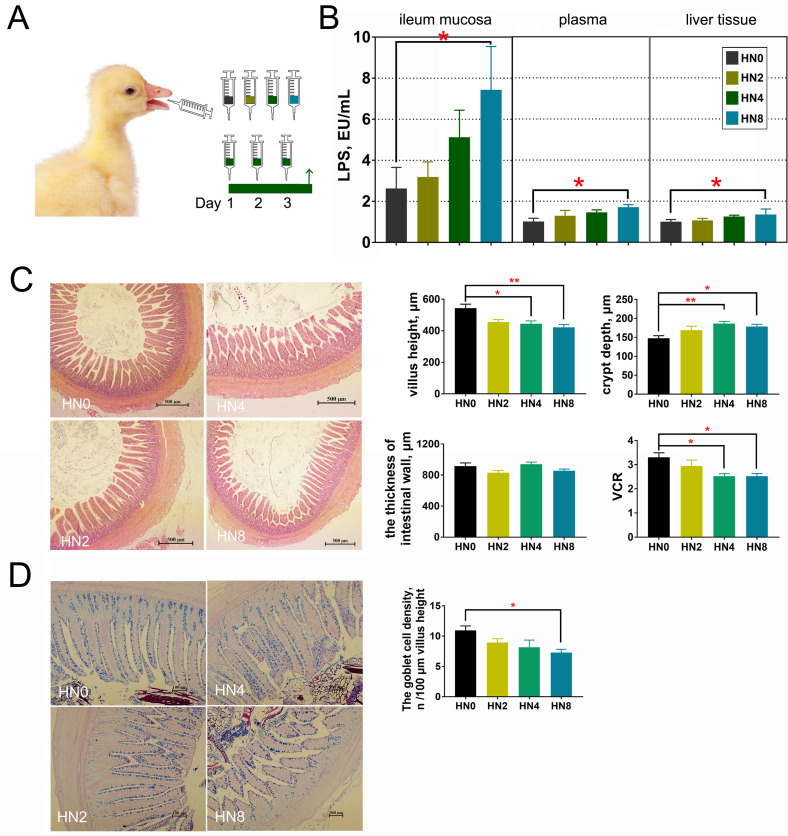
The effects of oral LPS administration on intestinal morphology and renal and liver functioning are shown in Figure 2. Goslings in the HN8 group had higher LPS concentrations in the ileum mucosa, plasma, and liver than those in the HN0 group (P < 0.05), and the LPS content in the ileum mucosa was markedly higher than that in other tissues. Meanwhile, when LPS was administered to goslings at 4 mg/kg, villus height and VCR in the ileum decreased significantly compared to those of goslings without LPS (P < 0.05), while crypt depth in the HN4 group increased significantly (P < 0.01). Similarly, goslings in the HN8 group also had lower villus height (P < 0.01) and VCR (P < 0.05) and larger crypt depth (P < 0.05) than those in the HN0 group. Goblet density in the ileum also decreased when goslings were administered LPS at 8 mg/kg (P < 0.05). The intestinal wall thickness in the ileum did not differ between the groups (P > 0.05).
Figure 2.
Oral LPS challenge affected plasma LPS concentration and intestinal morphology in goslings. (A) The experiment design. (B) The LPS levels in ileum mucosa, plasma, and liver tissues of goslings with different LPS levels when oral administration [unit: EU/mL, equivalent to EU/100 mg for mucosa and liver tissues]. (C) Morphological observation of the epithelial tissue in ileum with HE staining, and the comparison of intestinal wall thickness, villus height, crypt depth, and VCR among groups. (D) Morphological observation of the epithelial tissue in ileum with AB-PAS stain, and density of intestinal goblet cells. n = 12 goslings. Abbreviations: AB-PAS stain, the combined Alcian blue/periodic acid Schiff stain; HE staining, hematoxylin and eosin staining; LPS, lipopolysaccharide; VCR, villus height/crypt depth ratio.
Oral LPS Administration Affects Intestinal Barrier Functions
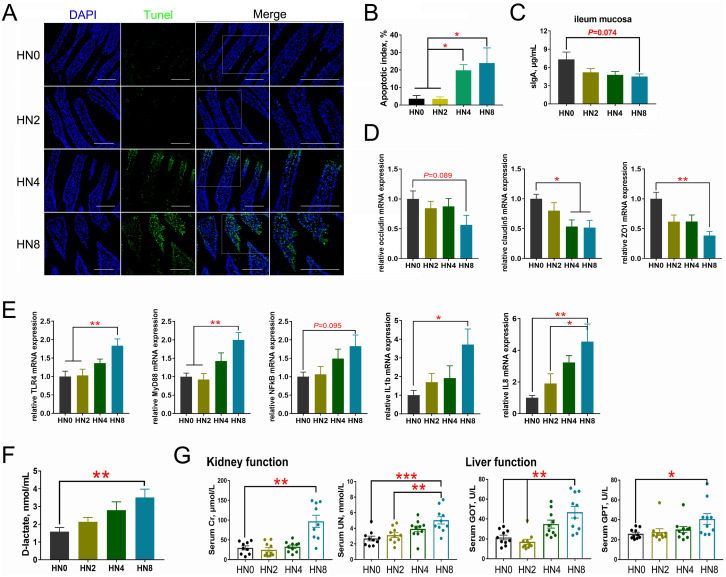
The effects of oral LPS administration on intestinal epithelium apoptosis, sIgA concentrations, TJ proteins, inflammatory responses, and intestinal permeability are shown in Figure 3. The TUNEL assay showed that LPS treatment induced apoptosis of the intestinal epithelium, and the apoptosis indices in the HN4 and HN8 groups increased compared with the other 2 groups (P < 0.05). Cell apoptosis only occurred at the tip of the small intestinal villi in the 4 mg/kg LPS treatment, whereas a larger range of apoptosis was observed at 8 mg/kg LPS. Further, the sIgA concentration of ileum mucosa in the HN8 group showed a decreasing trend, compared to that in the control group (P = 0.074). The mRNA expression of several TJ proteins in ileum mucosa, including occludin (OCLN; P = 0.089), claudin5 (CLDN5; P < 0.05), and zonulin1 (ZO1; P < 0.01), was downregulated in the HN8 group, compared to that in the controls, and the expression of CLDN5 (P < 0.05) mRNA was also downregulated in the HN4 group. In addition, expression of the proinflammatory factors IL1β (P < 0.05) and IL8 (P < 0.01) in the cecal tonsils was significantly upregulated in the HN8 group compared with that in the HN0 group. Compared with the HN0 and HN2 groups, LPS treatment in the HN8 group also increased the mRNA levels of TLR4 (P < 0.01) and MyD88 (P < 0.01) in the cecal tonsils. Compared with the HN0 group, the mRNA expression level of NFκB in the HN8 group showed an increasing trend (P = 0.095). Moreover, oral administration of LPS at 8 mg/kg increased circulating D-lactate concentrations, compared with the controls (P < 0.01). Regarding renal and liver functions, administration of 8 mg/kg LPS markedly increased plasma Cr (P < 0.01), UN (P < 0.01), GOT (P < 0.01), and GPT (P < 0.05) levels, while plasma UN (P < 0.01) and GOT (P < 0.01) levels were higher in the HN4 groups than in the controls.
Figure 3.
Oral LPS challenge impacts on the intestinal epithelium apoptosis, sIgA concentrations, TJ proteins and inflammation responses as well as intestinal permeability in goslings. (A) TUNEL assay of ileum mucosa with the positive cells showing up as green, scale bar = 50 μm. (B) The apoptotic index. (C) The sIgA levels in ileum mucosa. (D) The mRNA expressions of tight junction (TJ) proteins in ileum mucosa. (E) The mRNA expression levels of proinflammatory cytokines and the TLRs/NFκB inflammatory signaling pathway in cecal tonsil among groups. (F) The comparisons of the activity of D-lactic acid. (G) The liver (GPT and GOT) and kidney (UN and Cr) functions of goslings with oral LPS challenge. n = 12 goslings. Abbreviations: CLDN5, claudin-5; Cr, creatinine; GOT, glutamic-oxalacetic transaminase; GPT, glutamic-pyruvic transaminase; IL1β, interleukin-1β; IL8, interleukin-8; LPS, lipopolysaccharide; MyD88, myeloid differentiation primary response gene 88; NFκB, nuclear factor-κB; OCLN, occludin; TLR4, Toll-like receptor 4; UN, urea nitrogen; ZO1, zona occludens-1.
Oral LPS Treatment Affects Intestinal Microbial Communities
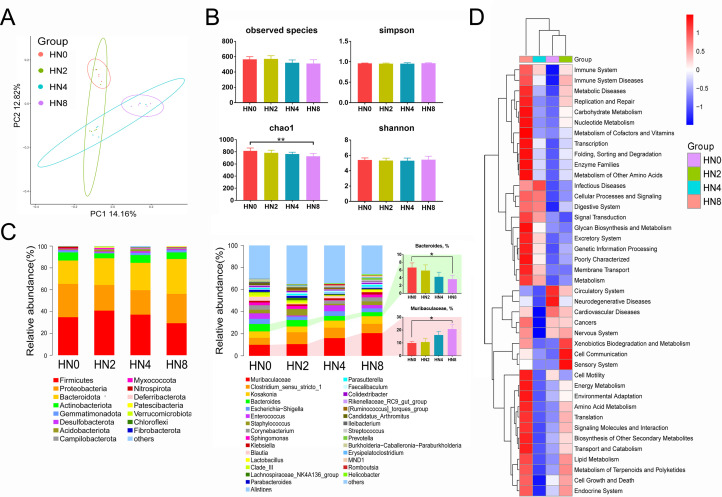
In the analysis of microbiota in the ileum mucosa of goslings treated with LPS orally by 16Sr RNA (V3−V4) gene sequencing (Figure 4), after quality control and chimera removal, 1,708,194 valid tags were retained, with an average of 71,174 tags per sample, and they were identified to be of bacterial origin. These sequences were assigned to 1,566 OTUs of bacterial species based on a 97% similarity cut-off. A comparison of bacterial 16S rRNA datasets based on PCoA demonstrated that the microbiota showed clear differences between the HN0 and HN8 groups, and such differences in bacterial community structure increased with increasing LPS concentrations. The key components of biodiversity, including species richness, evenness, and rarity, were measured using the Shannon, Simpson, and Chao1 indices. The Chao1 indices in the HN8 group were higher (P < 0.01) than those in the HN0 group, whereas other biodiversity indices did not differ between the groups. The overall composition of the microbiota as well as the average relative abundance at the phylum and genus levels are shown in Figure 4C. The predominant phyla were Firmicutes, Proteobacteria, and Bacteroidetes, and the predominant taxa on genus level were Muribaculaceae, Clostridium_sensu_stricto_1, Kosakonia, Bacteroides, Escherichia-Shigella, and Enterococcus. The average abundance of Muribaculaceae increased with increasing LPS levels, whereas that of Bacteroides decreased. Additionally, according to the KEGG prediction, we found that oral treatment with LPS may affect physiological processes, including the immune system, immune system diseases, metabolic diseases, replication and repair, and infectious diseases.
Figure 4.
Oral LPS challenge impacts on intestinal microbial communities of goslings. (A) The PCoA analysis. (B) The Alpha diversity indices (the observed species, Chao1, and Shannon indices). (C) The overall composition of microbiota and average relative abundance in the experimental groups at phylum and genus levels. (D) The average abundance of Bacteroidetes and Proteobacteria among groups; n = 6 goslings. (E) The KEGG prediction of the bacterial community functions. KEGG, Kyoto Encyclopedia of Genes and Genomes; PCoA, principal coordinate analysis.
DISCUSSION
Numerous studies have shown that LPS challenge leads to compromised intestinal barrier functioning in poultry owing to gut dysbacteriosis, oxidative stress damage, inflammatory responses, destruction of TJ fractures, and cell ultrastructure (Wu et al., 2013; Lucke et al., 2018; Tulkens et al., 2020). The typical phlogogenic concentrations of LPS when injected intraperitoneally in broiler chickens range from 0.25 to 1 mg/kg BW (Chen et al., 2018; Konieczka et al., 2019; Sun et al., 2020), however, we found that at least 4 mg/kg BW LPS were required to significantly alter the serum LPS levels in goslings. Moreover, to achieve such alterations, the oral concentrations of LPS must be at least 8 mg/kg BW, which markedly affected LPS levels in the ileum mucosa, serum, and liver. Our results thus differed remarkably from those of previous studies on other poultry, which may indicate stronger intestinal endotoxin tolerance in geese.
Alterations in intestinal barrier functioning frequently occur with modifications of intestinal morphology (Wen et al., 2019; Xiong et al., 2020). In the present study, we found that LPS injection resulted in intestinal wall thickening in the ileum of goslings within a short time, whereas villus height was less affected. In contrast, oral LPS treatment markedly affected villus height but not intestinal wall thickness. Although the thickness of the intestinal wall can be significantly affected by the degree of bowel distension (Wiesner et al., 2002), abnormal wall thickening can result from immune-mediated, infectious, or vascular causes, toxic effects, and other factors (Marín-Díez and Crespo Del Pozo, 2021). LPS administered through intraperitoneal injection is believed cause immune responses of the bowel wall or surrounding lymph nodes. Meanwhile, in our study, the stimulating effects of LPS injection did not seem to affect villus height for a short time, whereas oral LPS administration did. Most previous studies demonstrated that LPS injection can affect nutrient digestibility and growth performance by adversely affecting intestinal barrier functioning, as evidenced by decreased villus height and increased crypt depth (Thongsong at al., 2018; Gu et al., 2022); however, we propose a direct correlation between the degree of exposure to LPS and the degree of villi damage. Oral administration facilitated the development of direct injuries to the intestinal villi compared with other administration routes. Crypt depth and VCR significantly changed though either form of LPS administration, which is consistent with the results of other studies (Sun et al., 2020; Gu et al., 2022). Goblet cells synthesize and secrete bioactive molecules that are components of mucus, such as mucins and trefoil factors (Dharmani et al., 2009). The mucus layer coating the intestinal epithelium forms a physical barrier that protects against endogenous and exogenous irritants and microbial attachment and invasion (Einerhand et al., 2002). Compared to the controls, intraperitoneal injection with 4 mg/kg BW LPS or oral administration of 8 mg/kg BW LPS reduced goblet cell density in the present study, which may have weakened the intestinal mucous barrier functions and increased intestinal permeability. However, lower goblet density may be reconstituted slowly after acute LPS intraperitoneal injection, and goblet density did not differ between goslings with LPS and PBS injection at 24 and 48 h, suggesting a certain resilience of the intestinal barrier. In summary, although LPS administration through both routes affected intestinal epithelium morphology and decreased goblet cell density, different treatment methods may result in different main damage processes in the gosling gut.
The mucosal immunological barrier, mainly comprising goblet cells and IgA+ plasma cells, is an important component of the innate defense barrier against intestinal pathogens (Li et al., 2018). LPS can reduce mucin secreted by goblet cells, thereby affecting the mucosal immunological barrier in broiler chickens (Gadde et al., 2017). In the current study, oral LPS treatment at 8 mg/kg markedly reduced goblet cell and sIgA concentrations in the ileum of goslings, indicating that oral LPS treatment leads to injury of the intestinal mucosal immunological barrier functions in goslings. Intestinal sIgA serves as the first-line defense in protecting the intestinal barrier from harmful toxins, antigens, and microorganisms (Mantis et al., 2011), and oral LPS treatment at a high level may weaken this protection. The physical mucosal barrier, which is mainly composed of epithelial cells, is a further important component that prevents the translocation of luminal macromolecular content into inner tissues. The present study showed that the proportion of TUNEL-positive cells was increased by oral LPS treatment at a high dosage, indicating an increase in epithelial cell apoptosis. Interestingly, we found that at 4 mg/kg LPS, cell apoptosis only occurred at the tip of the intestinal villi, whereas at 8 mg/kg, a larger range of apoptosis was observed, which means that damage to intestinal villi due to oral LPS treatment progresses starting from the tip. We predicted that such alterations would be associated with decreased protein expression of mucin and increased permeability of the mucous layer following oral LPS treatment (Gadde et al., 2017). Cellular apoptosis may be the mechanism underlying gut mucosal barrier impairment during severe infection. Additionally, TJ proteins play a critical role in maintaining intestinal physical mucosal barrier functions because they mechanically seal the gaps between intestinal epithelial cells (Tulkens et al., 2020). TJs are multiprotein complexes composed of transmembrane proteins, peripheral membrane (scaffolding) proteins, and regulatory molecules including kinases; CLDN family proteins are important transmembrane proteins, OCLN protein is the junctional adhesion molecule, and ZO family proteins are peripheral membrane proteins that are crucial for TJ assembly (Zeisel et al., 2019). Our experiments showed that the mRNA expression of TJ proteins, including OCLN, CLDN5, and ZO1, was also downregulated when LSP was administered orally at a high dosage, likely because of possible TJ loss. Consistent with our results, LPS-induced alterations in the intestinal mRNA abundance of genes related to TJ function have been observed in broiler chickens (Li et al., 2015; Chen et al., 2018), Cherry Valley ducklings (Xia et al., 2021) and Pekin ducks (Xie et al., 2021). Moreover, plasma D-LA, a product of bacterial fermentation of carbohydrates, is a sensitive marker to assess intestinal injury and to monitor intestinal permeability (Sun et al., 2001), and in the current study, it was also increased under oral LPS treatment at a high level. As further evidence for increased intestinal permeability, renal and liver functions were also affected by oral LPS treatment at a high concentration, represented by the increased LPS levels in the ileum mucosa, plasma, and liver tissue and the deterioration of liver (plasma GOT and GPT increased) and kidney (plasma Cr and UN increased) function of goslings in our study. The loss of the intestinal barrier due to gut dysbiosis may cause translocation of gut-derived LPS, or even bacteria, and thereby interfere with liver and kidney functions; such mechanisms may be considered external manifestation of the “gut–liver–kidney axis” theory (Xi et al., 2019). Taken together, in the current study, oral LPS treatment impaired intestinal barrier functions, represented by increased intestinal permeability, impaired intestinal morphology, promoted epithelial cell apoptosis, and disrupted intestinal TJ and the immunological barrier.
The gut microbiome is an important part of the intestinal mucosal barrier and is crucial for maintaining host health (Ma et al., 2018). The emergence of intestinal barrier damage is typically accompanied by dysbiosis of the intestinal microbiota (Sun et al., 2022). Previous studies have shown that LPS treatment can lead to an inflammatory response and microflora disorders in rats (Yang et al., 2018). In the present study, the microbiota of goslings subjected to various LPS treatments showed few differences in alpha diversity, but they clustered separately from those of the controls, based on PCoA analysis, indicating that repeated oral LPS challenge may affect the classification of intestinal microbiota subtypes (Lucke et al., 2018). Meanwhile, increasing differences in LPS concentrations between groups resulted in more pronounced separation of clusters. Moreover, oral LPS treatment increased the abundance of Bacteroidetes at the phylum level. At the genus level, compared to the control group, the average abundance of Muribaculaceae displayed an increasing trend with increasing LPS levels, whereas that of Bacteroides decreased. Muribaculaceae is a further predominant family of gut Bacteroidales, except Bacteroidaceae, which is referred to as family S24-7 and has a relative abundance of 20 to 30% in the mouse gut as detected by fluorescence in situ hybridization (Salzman et al., 2002). Muribaculaceae are functionally distinct from neighboring families on the genome level, and they are versatile with respect to complex carbohydrate degradation (Lagkouvardos et al., 2019). However, to date, it has not yet been found that LPS challenge leads to the proliferation of Muribaculaceae in the gut of poultry or other animals. Additional in-depth genetic analysis and functional studies of Muribaculaceae would be required to understand their precise roles in the specific host-derived ecosystems they colonize. Bacteroides is a prominent Bacteroidetes representative in the gastrointestinal tract of healthy individuals, whose members may be commensals, mutualists, or pathogens (Davenport et al., 2017). It is noteworthy that LPS exposure in the gut of healthy individuals arose primarily from Bacteroides rather than from Escherichia coli. Bacteroides LPS is typically used as a potent innate immune activator, and it is structurally distinct from E. coli LPS and inhibits innate immune signaling and endotoxin tolerance (Vatanen et al., 2016). Hence, Bacteroides may be involved in maintaining mucosal immune homeostasis in the healthy intestinal microenvironment of goslings. With regard to the decrease in Bacteroides in the present study, we suspected that when exogenous immunostimulation (such as through E. coli LPS) increases, the hosts’ microecosystems may attempt to inhibit proliferation of Bacteroides for self-protection and to reduce immune stimulation and inflammatory reactions (Vatanen et al., 2016; Di Lorenzo et al., 2020; Kordahi et al., 2021). There should be a dynamic balance that is both collaborative and constrained between intestinal microorganisms and the host, as well as between microbes. KEGG prediction also supported this assumption, and genera with significant abundance variation were more strongly enriched in pathways such as immune system and immune system diseases.
In a previous study on a model of LPS-induced intestinal injury, many inflammatory mediators, such as proinflammatory cytokines TNF-α, IL-1β, IL-6, and chemokine IL-8, were found to be induced and released in the intestinal tissue (Liu et al., 2019). In the current study, oral LPS treatment at high concentrations significantly increased IL-1β and IL-8 levels in the cecal tonsils of goslings. Similar studies have shown that LPS injection enhances the levels of these proinflammatory cytokines in chickens (Wu et al., 2013; French et al., 2020). Bacterial LPS induces acute inflammatory responses in the host by activating the NF-κB signaling pathway (Liu et al., 2019). Upon phosphorylation and degradation of IκB-α, the downstream NF-κB signaling pathway can be activated through p65 translocation into the nucleus to alter the expression of related inflammatory genes induced by LPS (Cheng et al., 2017). In the present study, oral treatment with 8 mg/kg LPS markedly increased mRNA abundance of TLR4, MyD88, and NF-κB in the cecal tonsils, compared with the control group, indicating that oral LPS treatment caused intestinal inflammation in goslings by activating the TLR4/MyD88/NFκB pathway.
In conclusion, the results of the present study suggest that oral LPS challenge leads to injury of intestinal mucosal barrier function in goslings. These negative effects are represented by changes in intestinal epithelial morphology and increased permeability, presumably through damage to the mucosal immune barrier, modulation of TJ protein expression, imbalance of intestinal microbiota, and stimulation of the secretion of various inflammatory mediators. Meanwhile, intestinal damage processes differ between LPS administration routes in goslings, and further studies are required to determine the differences in molecular mechanisms that lead to the damage of intestinal mucosal barrier function by different LPS challenge procedures. This study provided a scientific model for searching the novel strategies to attenuate the immunological stress and gut injury caused by LPS together with further investigating the underlying mechanisms of the novel strategies mitigating stress and injury induced by LPS.
ACKNOWLEDGMENTS
This study was supported by the National Science Foundation of China (grant no. 31902190), China Agriculture Research System of MOF and MARA (grant no. CARS-42-20), Jiangsu Agriculture Science and Technology Innovation Fund (JASTIF) [CX (21) 2016] and National Key R&D Program of China (2022YFD1300400).
DISCLOSURES
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled, “Bacterial lipopolysaccharide with different administration routes affects intestinal mucosal morphological, immunological, and microbial barrier functions in goslings.”
REFERENCES
- Ceasrine A.M., Green L.A., Bilbo S.D. Protocol to measure endotoxin from opaque tissues in mice using an optimized kinetic limulus amebocyte lysate assay. STAR Protoc. 2022;3 doi: 10.1016/j.xpro.2022.101669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheled-Shoval S.L., Gamage N.S., Amit-Romach E., Forder R., Marshal J., Van Kessel A., Uni Z. Differences in intestinal mucin dynamics between germ-free and conventionally reared chickens after mannan-oligosaccharide supplementation. Poult. Sci. 2014;93:36–44. doi: 10.3382/ps.2013-03362. [DOI] [PubMed] [Google Scholar]
- Chen Y., Zhang H., Cheng Y., Li Y., Wen C., Zhou Y. Dietary l-threonine supplementation attenuates lipopolysaccharide-induced inflammatory responses and intestinal barrier damage of broiler chickens at an early age. Br. J. Nutr. 2018;19:1254–1262. doi: 10.1017/S0007114518000740. [DOI] [PubMed] [Google Scholar]
- Cheng P., Wang T., Li W., Muhammad I., Wang H., Sun X., Yang Y., Li J., Xiao T., Zhang X. Baicalin alleviates lipopolysaccharide-induced liver inflammation in chicken by suppressing TLR4-mediated NFκB pathway. Front. Pharmacol. 2017;8:547. doi: 10.3389/fphar.2017.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport E.R., Sanders J.G., Song S.J., Amato K.R., Clark A.G., Knight R. The human microbiome in evolution. BMC Biol. 2017;15:127. doi: 10.1186/s12915-017-0454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmani P., Srivastava V., Kissoon-Singh V., Chadee K. Role of intestinal mucins in innate host defense mechanisms against pathogens. J. Innate Immun. 2009;1:123–135. doi: 10.1159/000163037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo F., Pither M.D., Martufi M., Scarinci I., Guzmán-Caldentey J., Łakomiec E., Jachymek W., Bruijns S.C.M., Santamaría S.M., Frick J.S., van Kooyk Y., Chiodo F., Silipo A., Bernardini M.L., Molinaro A. Pairing bacteroides vulgatus LPS Structure with Its Immunomodulatory Effects on Human Cellular Models. ACS Cent. Sci. 2020;6:1602–1616. doi: 10.1021/acscentsci.0c00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einerhand A.W., Renes I.B., Makkink M.K., van der Sluis M., Buller H.A., Dekker J. Role of mucins in inflammatory bowel disease: important lessons from experimental models. Eur. J. Gastroent. Hepatol. 2002;14:757–765. doi: 10.1097/00042737-200207000-00008. [DOI] [PubMed] [Google Scholar]
- Flaviana D.L., Cristina D.C., Alba S., Antonio M. Lipopolysaccharide structures of Gram-negative populations in the gut microbiota and effects on host interactions. FEMS Microbiol. Rev. 2019;43:257–272. doi: 10.1093/femsre/fuz002. [DOI] [PubMed] [Google Scholar]
- French C.E., Sales M.A., Rochell S.J., Rodriguez A., Erf G.F. Local and systemic inflammatory responses to lipopolysaccharide in broilers: new insights using a two-window approach. Poult. Sci. 2020;99:6593–6605. doi: 10.1016/j.psj.2020.09.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadde U.D., Oh S., Lee Y., Davis E., Zimmerman N., Rehberger T., Lillehoj H.S. Dietary Bacillus subtilis based direct-fed microbials alleviate LPS-induced intestinal immunological stress and improve intestinal barrier gene expression in commercial broiler chickens. Res. Vet. Sci. 2017;114:236–243. doi: 10.1016/j.rvsc.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Gao G., Zhao X., Li Q., He C., Zhao W., Liu S., Ding J. Genome and metagenome analyses reveal adaptive evolution of the host and interaction with the gut microbiota in the goose. Sci. Rep. 2016;6:32961. doi: 10.1038/srep32961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y.F., Chen Y.P., Jin R., Wang C., Wen C., Zhou Y.M. Dietary chitooligosaccharide supplementation alleviates intestinal barrier damage, and oxidative and immunological stress in lipopolysaccharide-challenged laying hens. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvatić A., Guillemin N., Kaab H., McKeegan D., O'Reilly E., Bain M., Kuleš J., Eckersall P.D. Quantitative proteomics using tandem mass tags in relation to the acute phase protein response in chicken challenged with Escherichia coli lipopolysaccharide endotoxin. J. Proteomics. 2019;192:64–77. doi: 10.1016/j.jprot.2018.08.009. [DOI] [PubMed] [Google Scholar]
- Hu R., Lin H., Wang M., Zhao Y., Liu H., Min Y., Yang X., Gao Y., Yang M. Lactobacillus reuteri-derived extracellular vesicles maintain intestinal immune homeostasis against lipopolysaccharide-induced inflammatory responses in broilers. J. Anim. Sci. Biotechnol. 2021;12:25. doi: 10.1186/s40104-020-00532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konieczka P., Barszcz M., Kowalczyk P., Szlis M., Jankowski J. The potential of acetylsalicylic acid and vitamin E in modulating inflammatory cascades in chickens under lipopolysaccharide-induced inflammation. Vet. Res. 2019;50:65. doi: 10.1186/s13567-019-0685-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordahi M.C., Stanaway I.B., Avril M., Chac D., Blanc M.P., Ross B., Diener C., Jain S., McCleary P., Parker A., Friedman V., Huang J., Burke W., Gibbons S.M., Willis A.D., Darveau R.P., Grady W.M., Ko C.W., DePaolo R.W. Genomic and functional characterization of a mucosal symbiont involved in early-stage colorectal cancer. Cell Host Microbe. 2021;29:1589–1598. doi: 10.1016/j.chom.2021.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagkouvardos I., Lesker T.R., Hitch T., Gálvez E., Smit N., Neuhaus K., Wang J., Baines J.F., Abt B., Stecher B., Overmann J., Strowig T., Clavel T. Sequence and cultivation study of Muribaculaceae reveals novel species, host preference, and functional potential of this yet undescribed family. Microbiome. 2019;7:28. doi: 10.1186/s40168-019-0637-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R.X., Li J., Zhang S.Y., Mi Y.L., Zhang C.Q. Attenuating effect of melatonin on lipopolysaccharide-induced chicken small intestine inflammation. Poult. Sci. 2018;97:2295–2302. doi: 10.3382/ps/pey084. [DOI] [PubMed] [Google Scholar]
- Li Y., Zhang H., Chen Y.P., Yang M.X., Zhang L.L., Lu Z.X., Zhou Y.M., Wang T. Bacillus amyloliquefaciens supplementation alleviates immunological stress in lipopolysaccharide-challenged broilers at early age. Poult. Sci. 2015;94:1504–1511. doi: 10.3382/ps/pev124. [DOI] [PubMed] [Google Scholar]
- Li W., Zhou X., Xu S., Cao N., Li B., Chen W., Yang B., Yuan M., Xu D. Lipopolysaccharide-induced splenic ferroptosis in goslings was alleviated by polysaccharide of atractylodes macrocephala koidz associated with proinflammatory factors. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Song M., Yun W., Lee J., Kim H., Cho J. Effect of carvacrol essential oils on immune response and inflammation-related genes expression in broilers challenged by lipopolysaccharide. Poult. Sci. 2019;98:2026–2033. doi: 10.3382/ps/pey575. [DOI] [PubMed] [Google Scholar]
- Lu L., Chen Y., Wang Z., Li X., Chen W., Tao Z., Shen J., Tian Y., Wang D., Li G., Chen L., Chen F., Fang D., Yu L., Sun Y., Ma Y., Li J., Wang J. The goose genome sequence leads to insights into the evolution of waterfowl and susceptibility to fatty liver. Genome Biol. 2015;16:89. doi: 10.1186/s13059-015-0652-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y.C., Yeh W.C., Ohashi P.S. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Lucke A., Böhm J., Zebeli Q., Metzler-Zebeli B.U. Dietary deoxynivalenol and oral lipopolysaccharide challenge differently affect intestinal innate immune response and barrier function in broiler chickens. J. Anim. Sci. 2018;96:5134–5143. doi: 10.1093/jas/sky379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N., Guo P., Zhang J., He T., Kim S.W., Zhang G., Xi M. Nutrients mediate intestinal bacteria-mucosal immune crosstalk. Front. Immunol. 2018;9:5. doi: 10.3389/fimmu.2018.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantis N.J., Rol N., Corthesy B. Secretory IgA's complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011;4:603–611. doi: 10.1038/mi.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín-Díez E., Crespo Del Pozo J. Diagnostic approach to small-bowel wall thickening: beyond Crohn's disease and cancer. Radiol. (Engl. Ed.) 2021;63:519–530. doi: 10.1016/j.rxeng.2020.11.008. [DOI] [PubMed] [Google Scholar]
- Parks D.H., Tyson G.W., Hugenholtz P., Beiko R.G. STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics. 2014;30:3123–3124. doi: 10.1093/bioinformatics/btu494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman N.H., de Jong H., Paterson Y., Harmsen H.J., Welling G.W., Bos N.A. Analysis of 16S libraries of mouse gastrointestinal microflora reveals a large new group of mouse intestinal bacteria. Microbiology. 2002;148:3651–3660. doi: 10.1099/00221287-148-11-3651. [DOI] [PubMed] [Google Scholar]
- Sun X.Q., Fu X.B., Rong Z., Lu Y., Deng Q., Jiang X.G., Sheng Z.Y. Relationship between plasma D(-)-lactate and intestinal damage after severe injuries in rats. World J. Gastroenterol. 2001;7:555–558. doi: 10.3748/wjg.v7.i4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Guo L., Xu G., Li Z., Appiah M.O., Yang L., Lu W. Quercetin reduces inflammation and protects gut microbiota in broilers. Molecules. 2022;27:3269. doi: 10.3390/molecules27103269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Xu G., Dong Y., Li M., Yang L., Lu W. Quercetin protects against lipopolysaccharide-induced intestinal oxidative stress in broiler chickens through activation of Nrf2 pathway. Molecules. 2020;25:1053. doi: 10.3390/molecules25051053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongsong B., Suthongsa S., Pichyangkura R., Kalandakanond-Thongsong S. Effects of chito-oligosaccharide supplementation with low or medium molecular weight and high degree of deacetylation on growth performance, nutrient digestibility and small intestinal morphology in weaned pigs. Livest. Sci. 2018;209:60–66. [Google Scholar]
- Tulkens J., Vergauwen G., Van Deun J., Geeurickx E., Dhondt B., Lippens L., De Scheerder M.A., Miinalainen I., Rappu P., De Geest B.G., Vandecasteele K., Laukens D., Vandekerckhove L., Denys H., Vandesompele J., De Wever O., Hendrix A. Increased levels of systemic LPS-positive bacterial extracellular vesicles in patients with intestinal barrier dysfunction. Gut. 2020;69:191–193. doi: 10.1136/gutjnl-2018-317726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatanen T., Kostic A.D., d'Hennezel E., Siljander H., Franzosa E.A., Yassour M., Kolde R., Vlamakis H., Arthur T.D., Hämäläinen A.M., Peet A., Tillmann V., Uibo R., Mokurov S., Dorshakova N., Ilonen J., Virtanen S.M., Szabo S.J., Porter J.A., Lähdesmäki H., Huttenhower C., Gevers D., Cullen T.W., Knip M., DIABIMMUNE Study Group. Xavier R.J. Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell. 2016;165:842–853. doi: 10.1016/j.cell.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Garrity G.M., Tiedje J.M., Cole J.R. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z.Y., Liu W.H., Li X., Chen W.G., Liu Z.C., Wen J.B., Liu Z.P. A protective role of the NRF2-Keap1 pathway in maintaining intestinal barrier function. Oxid. Med. Cell Longev. 2019;2019 doi: 10.1155/2019/1759149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner W., Mortelé K.J., Ji H., Ros P.R. Normal colonic wall thickness at CT and its relation to colonic distension. J. Comput. Assist. Tomogr. 2002;26:102–106. doi: 10.1097/00004728-200201000-00015. [DOI] [PubMed] [Google Scholar]
- Wu Q.J., Zhou Y.M., Wu Y.N., Zhang L.L., Wang T. The effects of natural and modified clinoptilolite on intestinal barrier function and immune response to LPS in broiler chickens. Vet. Immunol. Immunopathol. 2013;153:70–76. doi: 10.1016/j.vetimm.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Xi Y., Huang Y., Li Y., Yan J., Shi Z. The effects of dietary protein and fiber levels on growth performance, gout occurrence, intestinal microbial communities, and immunoregulation in the gut-kidney axis of goslings. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Y., Yan J., Li M., Ying S., Shi Z. Gut microbiota dysbiosis increases the risk of visceral gout in goslings through translocation of gut-derived lipopolysaccharide. Poult. Sci. 2019;98:5361–5373. doi: 10.3382/ps/pez357. [DOI] [PubMed] [Google Scholar]
- Xia D., Yang L., Li Y., Chen J., Zhang X., Wang H., Zhai S., Jiang X., Meca G., Wang S., Huang L., Zhu S., Fu Y., Ma W., Zhu Y., Ye H., Wang W. Melatonin alleviates ochratoxin A-induced liver inflammation involved intestinal microbiota homeostasis and microbiota-independent manner. J. Hazard. Mater. 2021;413 doi: 10.1016/j.jhazmat.2021.125239. [DOI] [PubMed] [Google Scholar]
- Xie Y., Wen M., Zhao H., Liu G., Chen X., Tian G., Cai J., Jia G. Effect of zinc supplementation on growth performance, intestinal development, and intestinal barrier function in Pekin ducks with lipopolysaccharide challenge. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W., Huang J., Li X.Y., Zhang Z., Jin M.L., Wang J., Xu Y.W., Wang Z.L. Icariin and its phosphorylated derivatives alleviate intestinal epithelial barrier disruption caused by enterotoxigenic Escherichia coli through modulate p38 MAPK in vivo and in vitro. FASEB J. 2020;34:1783–1801. doi: 10.1096/fj.201902265R. [DOI] [PubMed] [Google Scholar]
- Yang X., Liu L., Jiang D., Wang C., Sun A., Shi Z. Improving geese production performance in ``goose-fish’’ production by competitive reduction of pathogenic bacteria in pond water. J. Integr. Agric. 2012;11:993–1001. [Google Scholar]
- Yang Y., Qu C., Liang S., Wang G., Han H., Chen N., Wang X., Luo Z., Zhong C., Chen Y., Li L., Wu W. Estrogen inhibits the overgrowth of Escherichia coli in the rat intestine under simulated microgravity. Mol. Med. Rep. 2018;17:2313–2320. doi: 10.3892/mmr.2017.8109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying S., Guo J., Dai Z., Zhu H., Yu J., Ma W., Li J., Akhtar M.F., Shi Z. Time course effect of lipopolysaccharide on Toll-like receptors expression and steroidogenesis in the Chinese goose ovary. Reproduction. 2017;153:509–518. doi: 10.1530/REP-17-0011. [DOI] [PubMed] [Google Scholar]
- Ying S., Qin J., Dai Z., An H., Zhu H., Chen R., Yang X., Wu W., Shi Z. Effects of LPS on the secretion of gonadotrophin hormones and expression of genes in the hypothalamus-pituitary-ovary (HPG) axis in laying Yangzhou geese. Animals. 2020;10:2259. doi: 10.3390/ani10122259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebeli Q., Mansmann D., Sivaraman S., Dunn S.M., Ametaj B.N. Oral challenge with increasing doses of LPS modulated the patterns of plasma metabolites and minerals in periparturient dairy cows. Innate Immunity. 2013;19:298–314. doi: 10.1177/1753425912461287. [DOI] [PubMed] [Google Scholar]
- Zeisel M.B., Dhawan P., Baumert T.F. Tight junction proteins in gastrointestinal and liver disease. Gut. 2019;68:547–561. doi: 10.1136/gutjnl-2018-316906. [DOI] [PMC free article] [PubMed] [Google Scholar]