Abstract
Introduction
COVID-19 particularly impacted patients with co-morbid conditions, including cancer. Patients with melanoma have not been specifically studied in large numbers. Here, we sought to identify factors that associated with COVID-19 severity among patients with melanoma, particularly assessing outcomes of patients on active targeted or immune therapy.
Methods
Using the COVID-19 and Cancer Consortium (CCC19) registry, we identified 307 patients with melanoma diagnosed with COVID-19. We used multivariable models to assess demographic, cancer-related, and treatment-related factors associated with COVID-19 severity on a 6-level ordinal severity scale. We assessed whether treatment was associated with increased cardiac or pulmonary dysfunction among hospitalized patients and assessed mortality among patients with a history of melanoma compared with other cancer survivors.
Results
Of 307 patients, 52 received immunotherapy (17%), and 32 targeted therapy (10%) in the previous 3 months. Using multivariable analyses, these treatments were not associated with COVID-19 severity (immunotherapy OR 0.51, 95% CI 0.19 – 1.39; targeted therapy OR 1.89, 95% CI 0.64 – 5.55). Among hospitalized patients, no signals of increased cardiac or pulmonary organ dysfunction, as measured by troponin, brain natriuretic peptide, and oxygenation were noted. Patients with a history of melanoma had similar 90-day mortality compared with other cancer survivors (OR 1.21, 95% CI 0.62 – 2.35).
Conclusions
Melanoma therapies did not appear to be associated with increased severity of COVID-19 or worsening organ dysfunction. Patients with history of melanoma had similar 90-day survival following COVID-19 compared with other cancer survivors.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-023-10708-6.
Keywords: COVID-19, Melanoma, Immune therapy, Targeted therapy, Cancer
Introduction
SARS-CoV-2, the virus responsible for the COVID-19 pandemic, has resulted in particularly severe morbidity and mortality for patients at increased age, and with health co-morbidities, such as obesity, diabetes, cardiovascular disease including cardiomyopathy, and cancer [1]. A growing number of large studies have assessed the impact of COVID-19 among patients with various types of cancer, and among patients with various modalities of anti-cancer therapy [2, 3]. Given the diverse types of malignancy and treatment scenarios, many questions remain, particularly in less common tumor types.
Melanoma is the most lethal type of skin cancer and is an archetypal malignancy for the development of both targeted therapy (with BRAF and MEK inhibitors) and especially immunotherapy. Melanoma was the first malignancy where many checkpoint inhibitors were first approved and most aggressively combined (including agents targeting programmed death-1 [PD-1], cytotoxic T lymphocyte antigen-4 [CTLA-4], and lymphocyte antigen gene-3 [LAG-3]). In addition, melanoma have unique risk factors that differ from those for other malignancies (e.g., exposure to ultraviolet light rather than cigarette smoke, alcohol, obesity, or chronic inflammation), thus theoretically conferring a better prognosis.
Leveraging detailed patient reports to the COVID-19 and Cancer Consortium (CCC-19) registry—consisting of 12,661 patients with cancer and COVID-19 reported from 93 different centers, including 307 patients with melanoma—we sought to assess the factors, including recent targeted or immunotherapy, that were associated with COVID-19 severity and survival. We also assessed whether these therapies synergized with COVID-19 to produce exacerbated organ dysfunction. Finally, we compared survival following COVID-19 among patients with melanoma to those with all other cancer types in the database.
Methods
Study design
This was a registry-based retrospective cohort study that used data from the CCC19 registry, a centralized multi-institutional registry of patients who had COVID-19 and a diagnosis of cancer (either past or current). The registry is maintained as an electronic database using REDCap Software at Vanderbilt University Medical Center, and its schema and format have been previously described [4]. Records for the present study were accrued from March 17, 2020, to December 31, 2021, and included patients with a diagnosis of SARS-CoV-2 infection confirmed by serology or polymerase chain reaction tests. Patients with non-invasive cancers including non-melanoma skin cancer, in situ carcinoma, or precursor hematologic neoplasms were excluded. The analysis also excluded patients with inadequate data quality (quality score ≥ 5 according to our previously defined metric [4]) and those with incomplete follow-up to assess the outcomes of interest. Patients with cancer types other than melanoma were excluded when assessing the associations between modality of systemic anti-cancer therapy with COVID-19 severity among patients with melanoma. Patients with active cancer status or active systemic anti-cancer therapy were excluded when assessing the association between presence of non-active melanoma verses non-active other cancers with 30-day and 90-day mortality.
This study was approved by Institutional Review Board of Vanderbilt University Medical Center and participating sites. Informed consent was waived since data were anonymized and posed minimal risk to study participants.
Data elements
The primary outcome was a 6-level ordinal scale of COVID-19 severity based on a patient’s most severe reported disease status: none of the complications listed here, hospital admission without supplemental oxygen, hospital admission with supplemental oxygen, intensive care unit admission, mechanical ventilation use, and death from any cause. Secondary outcomes were death from any cause within 30 days and 90 days after COVID-19 diagnosis. Class of recent (i.e., within 3 months prior to COVID-19 diagnosis) systemic anti-cancer therapy was defined as cytotoxic chemotherapy, targeted therapy, immunotherapy, or none [3].
Statistical analysis
All statistical analysis methods and data elements were pre-specified in a statistical analysis plan. Standard descriptive statistics were used to summarize baseline characteristics and outcomes among patients with melanoma: categorical and continuous variables were summarized as counts (%) and median (interquartile range [IQR]), respectively. Laboratory measurements (BNP, CRP, troponin) and receipt of supplemental oxygen were summarized among patients with melanoma hospitalized at baseline. Ordinal logistic regression models with an offset for (log) follow-up time were used to determine whether class of recent anti-cancer therapy was associated with COVID-19 severity [5]. The model was adjusted for melanoma as the primary cancer type, age (quadratic term to accommodate a non-linear association), sex, race and ethnicity, geographical region of patient residence (defined as Sunbelt region or not), smoking status, obesity, comorbidities (cardiovascular, pulmonary. renal disease, diabetes, immunosuppression), Eastern Cooperative Oncology Group (ECOG) performance status, cancer status (remission or no evidence of disease, active and stable/responding, active and progressing, or unknown), and time period of COVID-19 diagnosis. Binary logistic regression models were used to determine whether type of non-active cancer (melanoma or other) was associated with 30-day and 90-day mortality. In addition to the covariates listed above but not including cancer status, the model was adjusted for corticosteroids as a COVID-19 treatment ever. Variables were assessed for collinearity before inclusion in multivariable models. Multiple imputation (10 imputations, missingness rates < 6%) using additive regression, bootstrapping, and predictive mean matching was used to impute missing and unknown data, except unknown ECOG status and unknown cancer status, which were included as “unknown” categories. Results were combined using Rubin’s rules and are reported as odds ratios (ORs) with 95% confidence intervals (CIs). All tests were two-sided and a 95% CI that did not cross 1.0 was considered significantly significant. All analyses were conducted using R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria), including the Hmisc and rms extension packages.
Results
We identified 307 patients with melanoma in the CCC19 registry. Among these, median age was 64 years (IQR 55–75 years), 167 (54%) were male, 14% were races other than non-Hispanic White, and 81 (26%) resided in the US Sunbelt region, Spain, or Mexico whereas 225 (73%) were from US states outside the sunbelt or Canada (Table 1). Regarding comorbid conditions, 105 (34%) had obesity, 69 (22%) had cardiovascular conditions, 56 (18%) had pulmonary comorbidities, and 56 (18%) had diabetes. In terms of melanoma disease status, 168 (55%) were in remission or had no evidence of disease, 70 (23%) had active melanoma that was stable or responding, and 37 (12%) had active melanoma that was progressing. Regarding melanoma treatment, 86 (28%) had systemic therapy in the 3 months preceding COVID-19 diagnosis, including immunotherapy (n = 52, 17%), targeted therapy (n = 32, 10%), or cytotoxic chemotherapy (n = 11, 4%).
Table 1.
Demographics for all melanoma patients
| Characteristics | All patients |
|---|---|
| N = 307 | |
| Age [Median (IQR)] | 64 (55–75) |
| Sex | |
| Female | 140 (46%) |
| Male | 167 (54%) |
| Race | |
| Non-Hispanic White | 259 (84%) |
| Non-Hispanic Black | 8 (3%) |
| Hispanic | 14 (5%) |
| Other | 22 (7%) |
| Missing/Unknown | 4 (1%) |
| Geographic location | |
| US Sunbelt region, Spain, or Mexico | 81 (26%) |
| US states outside of Sunbelt region or Canada | 225 (73%) |
| Missing/Unknown | 1 (0%) |
| Smoking status | |
| Never | 179 (58%) |
| Current or Former | 118 (38%) |
| Missing/Unknown | 10 (3%) |
| Obesity | |
| Not obese | 200 (65%) |
| Obese | 105 (34%) |
| Missing/Unknown | 2 (1%) |
| Comorbidities | |
| Diabetes mellitus | 56 (18%) |
| Pulmonary comorbidities | 56 (18%) |
| Cardiovascular comorbidities | 69 (22%) |
| Renal comorbidities | 38 (12%) |
| Immunosuppressed | 44 (14%) |
| Missing/Unknown | 2 (1%) |
| ECOG Status | |
| 0 | 129 (42%) |
| 1 | 62 (20%) |
| 2 + | 28 (9%) |
| Unknown | 87 (28%) |
| Missing | 1 (0%) |
| Cancer Status | |
| Remission/NED | 168 (55%) |
| Active, stable/responding | 70 (23%) |
| Active, progressing | 37 (12%) |
| Unknown | 32 (10%) |
| Recent systemic cancer therapy | |
| Systemic therapy in the last 3 months | 86 (28%) |
| Cytotoxic Therapy | 11 (4%) |
| Targeted Therapy | 32 (10%) |
| Immunotherapy | 52 (17%) |
| No systemic therapy in the last 3 months | 215 (70%) |
| Missing/Unknown | 6 (2%) |
| COVID-19 treatments | |
| Low-dose steroids ever | |
| Yes | 41 (13%) |
| No | 256 (83%) |
| Missing/Unknown | 10 (3%) |
| High-dose steroids ever | |
| Yes | 37 (12%) |
| No | 254 (83%) |
| Missing/Unknown | 16 (5%) |
| Monoclonal antibodies | |
| Yes | 10 (3%) |
| No | 290 (94%) |
| Missing/Unknown | 7 (2%) |
| Timing of COVID diagnosis | |
| Jan—Apr 2020 | 63 (21%) |
| May—Aug 2020 | 102 (33%) |
| Sep—Dec 2020 | 86 (28%) |
| Jan—Apr 2021 | 32 (10%) |
| May—Aug 2021 | 15 (5%) |
| Sep—Dec 2021 | 9 (3%) |
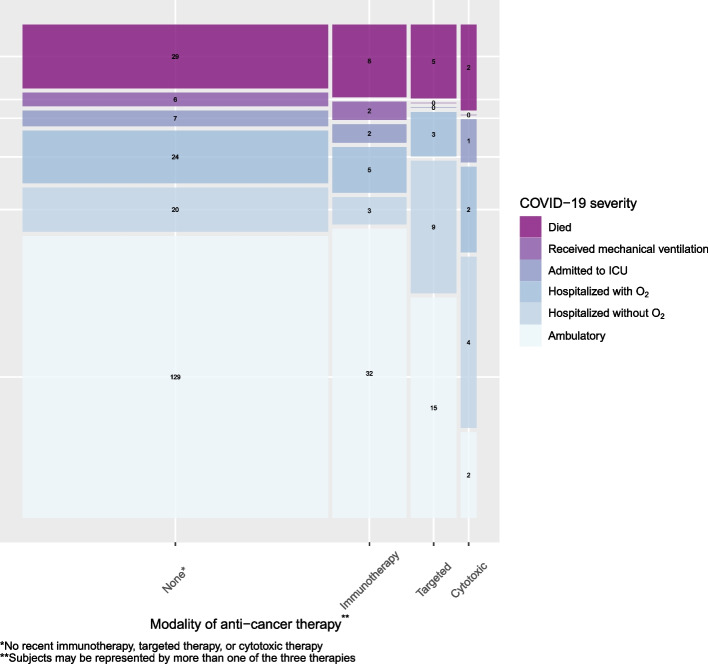
Among the 307 patients with melanoma, over a median follow up of 90 days from COVID-19 diagnosis, 127 (41%) were hospitalized at any time, including 85 (28%) that required supplemental oxygen, 40 (13%) that required ICU admission, and 44 (14%) that died due to any cause (Table S1). Among hospitalized patients, attribution was noted as definitely related to COVID-19 (n = 91, 72%), possibly related to COVID-19 (n = 26, 20%), and unrelated to COVID-19 (n = 10, 8%). COVID-19 severity is summarized by class of recent anti-cancer therapy in Fig. 1. On multivariable analyses, no class of therapy was associated with COVID-19 severity, including for immunotherapy (OR 0.51, 95% CI 0.19 – 1.39), targeted therapy (OR 1.89, 95% CI 0.64 – 5.55), or chemotherapy (OR 0.63, 95% CI 0.18 – 2.19) (Table 2). Age exhibited a significant non-linear (i.e., linear-quadratic) association with COVID-19 severity, such that older patients had higher COVID-19 severity. Pulmonary comorbidities (OR 2.27, 95% CI 1.12 – 4.62), diabetes (OR 2.12, 95% CI 1.01 – 4.47), ECOG 2 + (vs. ECOG 0; OR 12.5, 95% CI 4.22 – 37.2), active and progressing cancer (vs. remission, OR 3.22, 95% CI 1.21 – 8.58) were all associated with higher COVID-19 severity, whereas location in the sunbelt region (OR 0.35, 95% 0.17 – 0.75) and later diagnosis (after May 2020) was associated with lower COVID-19 severity (OR 0.10 – 0.18 compared with Jan – April 2020).
Fig. 1.
Distribution of the ordinal COVID-19 severity outcome, stratified by modality of anti-cancer therapy received within 3 months prior to COVID-19 diagnosis. Note: The ordinal COVID-19 severity outcome was based on a patient’s most severe reported disease status during follow-up (for example, a patient who was hospitalized and later died is included in “Died”). The width of the boxes is proportional to the number of patients who received each anti-cancer therapy; the height of the boxes is proportional to the number of patients in each outcome level. Patient numbers are listed for each outcome level
Table 2.
Variables associated with COVID-19 Severity
| COVID-19 Severity | ||
|---|---|---|
| Predictors | Odds Ratios | CI |
| Recent cytotoxic therapy | 0.63 | 0.18 – 2.19 |
| Recent targeted therapy | 1.89 | 0.64 – 5.55 |
| Recent immunotherapy | 0.51 | 0.19 – 1.39 |
| No systemic therapy, ever | 0.91 | 0.29 – 2.87 |
| Melanoma as the primary cancer (Yes) | 0.83 | 0.40 – 1.71 |
| Age, per decade | ||
| Linear | 0.74 | 0.46 – 1.20 |
| Quadratic | 1.24 | 1.12 – 1.37 |
| Sex (Male vs Female) | 1.80 | 0.96 – 3.36 |
| Race (Non-Hispanic White vs Other) | 1.14 | 0.50 – 2.60 |
| Sunbelt region (yes vs no) | 0.35 | 0.17 – 0.75 |
| Smoking status (Ever vs Never) | 1.42 | 0.78 – 2.60 |
| Obesity (yes vs no) | 0.50 | 0.27 – 0.93 |
| Cardiovascular comorbidities (yes vs no) | 0.96 | 0.46 – 2.00 |
| Renal disease (yes vs no) | 1.81 | 0.72 – 4.56 |
| Pulmonary comorbidities (yes vs no) | 2.27 | 1.12 – 4.62 |
| Diabetes (yes vs no) | 2.12 | 1.01 – 4.47 |
| Immunosuppressed (yes vs no) | 1.55 | 0.66 – 3.64 |
| ECOG status (1 vs 0) | 0.93 | 0.42 – 2.08 |
| ECOG status (2 + vs 0) | 12.53 | 4.22 – 37.19 |
| ECOG status (Unknown vs 0) | 1.49 | 0.72 – 3.07 |
| Cancer status (Active, stable/responding vs remission) | 1.37 | 0.56 – 3.38 |
| Cancer status (Active, progressing vs remission) | 3.22 | 1.21 – 8.58 |
| Cancer status (Unknown vs remission) | 1.05 | 0.38 – 2.91 |
| COVID Diagnosis (May—Aug 2020 vs Jan—Apr 2020) | 0.18 | 0.08 – 0.40 |
| COVID Diagnosis (Sep—Dec 2020 vs Jan—Apr 2020) | 0.17 | 0.08 – 0.38 |
| COVID Diagnosis (Jan—Apr 2021 vs Jan—Apr 2020) | 0.10 | 0.03 – 0.31 |
| COVID Diagnosis (May—Aug 2021 vs Jan—Apr 2020) | 0.18 | 0.05 – 0.63 |
| COVID Diagnosis (Sep—Dec 2021 vs Jan—Apr 2020) | 0.14 | 0.02 – 0.83 |
Because melanoma therapies can cause end-organ toxicities (e.g. immune-related adverse events impacting many different organs, and BRAF/MEK inhibitors causing cardiomyopathy), we assessed organ function in relation to systemic therapy. Among hospitalized patients, there was no obvious difference in brain natriuretic peptide, troponin, supplemental oxygen requirement, or C-reactive protein (Supplementary Table 2). This was true regardless of therapy class, although numbers were small and limited definitive conclusions.
Finally, we assessed 30- and 90-day mortality among patients with a history of melanoma (but no active cancer) and compared these with cancer survivors with other cancers, hypothesizing that patients with a history of melanoma may have fewer comorbid conditions and perhaps have other less quantifiable advantages (e.g. excess sun exposure correlating with a more active lifestyle). We observed approximately similar mortality at 30 and 90 days (15% vs. 12%; 17% vs. 16%) among patients with a history of melanoma compared with other cancers (Supplemental Table 3). On multivariable analyses, patients with melanoma had similar odds of 30-day mortality (OR 1.38, 95% CI 0.69 – 2.76) and 90-day mortality (OR 1.21, 95% CI 0.62 – 2.35). Factors associated with inferior survival are listed in Table 3, but broadly overlapped with factors associated with higher COVID-19 severity in the full melanoma cohort.
Table 3.
Factors associated with 30 and 90 day mortality in patients without cancer or cancer treatment in the previous 3 months
| 30 day mortality | 90 day mortality | |||
|---|---|---|---|---|
| OR | CI | OR | CI | |
| Cancer type (Melanoma vs Other) | 1.38 | 0.69—2.76 | 1.21 | 0.62—2.35 |
| Steroids as a COVID-19 treatment, ever | 2.03 | 1.5—2.73 | 2.38 | 1.81—3.14 |
| Age, per decade | ||||
| Linear | 1.42 | 0.88—2.3 | 1.33 | 0.9—1.97 |
| Quadratic | 1.06 | 0.98—1.15 | 1.06 | 0.99—1.13 |
| Sex (Male vs Female) | 1.47 | 1.11—1.94 | 1.58 | 1.22—2.04 |
| Race (Non-Hispanic White vs Other) | 0.91 | 0.68—1.22 | 0.98 | 0.75—1.28 |
| Sunbelt region (yes vs no) | 0.70 | 0.47—1.06 | 0.78 | 0.54—1.12 |
| Smoking status (Ever vs Never) | 1.21 | 0.9—1.62 | 1.07 | 0.81—1.4 |
| Obesity (yes vs no) | 1.09 | 0.81—1.46 | 0.98 | 0.75—1.28 |
| Cardiovascular comorbidities (yes vs no) | 0.97 | 0.72—1.3 | 1.11 | 0.85—1.46 |
| Renal disease (yes vs no) | 1.35 | 1—1.82 | 1.41 | 1.06—1.86 |
| Pulmonary comorbidities (yes vs no) | 1.35 | 0.99—1.83 | 1.37 | 1.03—1.83 |
| Diabetes (yes vs no) | 1.30 | 0.98—1.73 | 1.16 | 0.89—1.51 |
| Immunosuppressed (yes vs no) | 1.37 | 0.84—2.25 | 1.70 | 1.1—2.63 |
| ECOG status (1 vs 0) | 1.86 | 1.18—2.95 | 1.94 | 1.29—2.93 |
| ECOG status (2 + vs 0) | 3.44 | 2.13—5.55 | 4.00 | 2.58—6.18 |
| ECOG status (Unknown vs 0) | 1.66 | 1.11—2.47 | 1.52 | 1.06—2.17 |
| COVID Diagnosis (2021 vs 2020) | 0.43 | 0.29—0.65 | 0.42 | 0.29—0.61 |
Discussion
In this study, we found that recent anti-cancer therapy, irrespective of modality, was not strongly associated with COVID-19 severity among patients with melanoma. Further, recent therapy did not obviously predispose patients to compromised organ function, a finding of clinical relevance given the varied and frequent organ involvement with immune-related adverse events. To our knowledge, this is the first systematic effort to study outcomes following BRAF and MEK inhibitors and COVID-19. Finally, melanoma was associated with modestly inferior COVID-19 survival in univariate but not multivariable analyses in a cohort of cancer survivors, contrasting with our hypothesis that it might be associated with improved outcomes.
Several studies have demonstrated the impact of various cancer treatments on COVID-19 outcomes in patients with cancer. Certain treatments have been associated with inferior outcomes, including several multiagent chemotherapy regimens (rituximab + cyclophosphamide, doxorobucin, vincristine, and predisione [R-CHOP], platinum + etoposide) and B cell malignancy directed therapies [3, 6]. Initially, clinicians had a high degree of concern that immune checkpoint inhibitors might synergize with SARS-CoV2 to produce enhanced lung or other organ inflammation, based on theoretical concern and early reports [7, 8]. Subsequent studies however have largely suggested that these agents are not associated with inferior COVID-19 outcomes [3, 9]. Although subclinical effects are difficult to rule out, our study provides additional evidence that this type of relationship is not present, at least when assessing COVID-19 severity and measures of organ function in hospitalized patients. In addition, our study is the largest to specifically study melanoma, where high rates of combination ipilimumab and nivolumab are used. This combination is associated with pneumonitis, myocarditis, and fatal toxicities more often than single agent anti-PD-1 regimens [10–12].
Similarly, we assessed targeted therapy in melanoma, specifically BRAF and MEK inhibitors. These agents are associated with modest rates of myocardial dysfunction, pyrexia, and elevated liver transaminase levels, all of which may confound or delay a diagnosis of COVID-19 infection, or could worsen organ function in the setting of concomitant COVID-19 infection. Although there was a potential trend towards increasing COVID-19 severity in this cohort, it was far from statistically significant, and there was no evidence of increasing organ dysfunction. Thus, BRAF and MEK inhibitors appear to join other non-chemotherapy agents that have no obvious impact on COVID-19 outcomes [3, 13].
Patients with a history of melanoma (defined as those without active melanoma or melanoma therapy in the prior 3 months) had a marginally inferior survival on univariate analyses compared with survivors of other cancers, although this difference was not present after adjustment for other prognostic variables. We originally hypothesized that melanoma may be associated with fewer co-morbidities than many other cancers since it is not associated with smoking, and thus more active lifestyle and better COVID-19 outcomes [14]. It is possible though that patients with melanoma do not have less co-morbidities, or that adjustments for comorbidities masked this difference. Regardless, despite the large number of patients, there were only 89 melanoma survivors, limiting the generalizability of these conclusions.
This study has limitations: even though it came from the largest registry of patients with cancer and COVID-19 (CCC19), the number of patients receiving different therapies remained modest. Further, teasing out other potentially relevant interactions (e.g. whether a patient was early on therapy, or whether only patients of a certain age on various therapies were impacted) was particularly difficult. However, we do provide evidence for overall safety of these regimens in patients with advanced melanoma.
In conclusion, patients with melanoma on targeted or immune therapy did not experience more severe COVID-19 or obviously enhanced organ damage in this study. Patients with a history of melanoma paradoxically appeared to have worse clinical outcomes compared with unselected cancer survivors, although this difference was not significant on multivariable modeling. This study adds to the growing body of evidence for the safety of most immune and targeted therapies in the context of COVID-19, and suggests additional studies into risk factors for adverse outcomes in cancer patients and survivors.
Supplementary Information
Additional file 1: Supplemental Table 1. Outcomes summarized over full patient cohort. Supplementary Table 2. Organ function in hospitalized patients based on therapy type. Supplemental Table 3. Survival in patients without active cancer or treatment in the previous 3 months.
Acknowledgements
We thank all members of the CCC19 steering committee for their invaluable guidance of the CCC19 consortium.
COVID-19 and Cancer Consortium (CCC-19)
List of participants in CCC-19 by institution
Sonya A. Reid1, MD, MPH; Alicia Beeghly1, MPH, PhD; Alaina J. Brown1, MD, MPH; Alex Cheng1, PhD; Sarah Croessmann1, PhD; Elizabeth J. Davis1, MD; Kyle T. Enriquez, MSc BS; Erin A. Gillaspie1, MD, MPH; Daniel Hausrath1, MD; Douglas B. Johnson1, MD, MSCI; Xuanyi Li1, MD; Sanjay Mishra1, MS, PhD; David A. Slosky1, MD; Carmen C. Solorzano1, MD, FACS; Matthew D. Tucker1, MD; Karen Vega-Luna1, MA; Lucy L. Wang1, BA, Trisha M. Wise-Draper3, MD, PhD; Syed A. Ahmad3, MD, FACS; Punita Grover3, MD; Shuchi Gulati3, MD; Jordan Kharofa3, MD; Tahir Latif3, MBBS, MBA; Michelle Marcum3, MS; Davendra P. S. Sohal3, MD, MPH; Olga Zamulko3, MD, Toni K. Choueiri, MD4; Ziad Bakouny4, MD, MSc; Jean M. Connors4, MD; George D. Demetri4, MD, FASCO; Narjust Duma4, MD; Dory A. Freeman4, BS; Antonio Giordano4, MD, PhD; Chris Labaki4, MD; Alicia K. Morgans4, MD, MPH; Anju Nohria4, MD; Renee-Maria Saliby4, MD, MSc; Andrew L. Schmidt4, MD; Eliezer M. Van Allen4, MD; Wenxin Xu4, MD; Rebecca L. Zon4, MD, Clara Hwang5, MD; Shirish M. Gadgeel5, MD; Sheela Tejwani5, MD, Christopher R. Friese6, PhD, RN, AOCN, FAAN; Leslie A. Fecher6, MD; Anne Boldt6, MD; James J. Yoon6, MD, Brandon Hayes-Lattin7, MD, FACP; Aaron M. Cohen7, MD, MS; Shannon McWeeney7, PhD; Eneida R. Nemecek7, MD, MS, MBA; Staci P. Williamson7, BS, Mehmet A. Bilen8, MD; Cecilia A. Castellano8; Deepak Ravindranathan8, MD, MS, Gary H. Lyman9, MD, MPH, FASCO, FRCP; Jerome J. Graber9, MD, MPH; Petros Grivas9, MD, PhD; Jessica E. Hawley9, MD; Elizabeth T. Loggers9, MD, PhD; Ryan C. Lynch9, MD; Elizabeth S. Nakasone9, MD, PhD; Michael T. Schweizer9, MD; Lisa Tachiki9, MD; Shaveta Vinayak9, MD, MS; Michael J. Wagner9, MD; Albert Yeh9, MD, Sumit A. Shah10, MD, MPH; Elwyn C. Cabebe10, MD; Michael J. Glover10, MD; Alokkumar Jha10, PhD; Ali Raza Khaki10, MD; Lidia Schapira10, MD, FASCO; Julie Tsu-Yu Wu10, MD, PhD, Daniel B. Flora11, MD, PharmD; Goetz Kloecker11, MD; Barbara B. Logan11, MS; Chaitanya Mandapakala11, MD, Elizabeth Wulff-Burchfield12, MD; Anup Kasi12, MD, MPH; Crosby D. Rock12, MD, Dimitrios Farmakiotis13, MD, FACP, FIDSA; Panos Arvanitis13, MS; Pamela C. Egan13, MD; Hina Khan13, MD; Elizabeth J. Klein13, BA; Adam J. Olszewski13, MD; Kendra Vieira13, BS; Jeremy L. Warner13, MD, MS, FAMIA, FASCO, Lisa B. Weissmann14, MD; Padmanabh S. Bhatt14, MD; Chinmay Jani14, MD; Melissa G. Mariano14, DO; Carey C. Thomson14, MD, FCCP, MPH, Matthew Puc15, MD; Theresa M. Carducci15, MSN, RN, CCRP; Karen J. Goldsmith15, BSN, RN; Susan Van Loon15, RN, CTR, CCRP, Daniel Y. Reuben16, MD, MS; Mariam Alexander16, MD, PhD; Sara Matar16, MD; Sarah Mushtaq16, MD, Keith E. Stockerl-Goldstein17, MD; Omar Butt17, MD, PhD; Mark A. Fiala17, MSW; Jeffrey P. Henderson17, MD, PhD; Ryan S. Monahan17, MBA; Alice Y. Zhou17, MD, PhD, Philip E. Lammers18, MD, MSCI, Sanjay G. Revankar19, MD, FIDSA, Salvatore A. Del Prete20, MD; Michael H. Bar20, MD, FACP; Anthony P. Gulati20, MD; K. M. Steve Lo20, MD; Suzanne J. Rose20, MS, PhD, CCRC, FACRP; Jamie Stratton20, MD; Paul L. Weinstein20, MD, Shilpa Gupta21, MD; Nathan A. Pennell21, MD, PhD, FASCO; Manmeet S. Ahluwalia21, MD, FACP; Scott J. Dawsey21, MD; Christopher A. Lemmon21, MD; Amanda Nizam21, MD; Nima Sharifi21, MD, Claire Hoppenot22, MD; Ang Li, MD, MS , Susan Halabi23, PhD, FASCO, FSCT, FASA; Hannah Dzimitrowicz23, MD; Tian Zhang23, MD, MHS, Sharad Goyal24, MD; Minh-Phuong Huynh-Le24, MD, MAS, Peter Paul Yu25, MD, FACP, FASCO; Jessica M. Clement25, MD; Ahmad Daher25, MD; Mark E. Dailey25, MD; Rawad Elias25, MD; Asha Jayaraj25, MD; Emily Hsu25, MD; Alvaro G. Menendez25, MD; Oscar K. Serrano25, MD, MBA, FACS, Melissa K. Accordino26, MD, MS; Divaya Bhutani26, MD; Dawn Hershman26, MD, MS, FASCO; Matthew A. Ingham26, MD; Gary K. Schwartz26, MD, Eric H. Bernicker27, MD, John F. Deeken28, MD; Danielle Shafer28, DO, Erika Ruíz-García29, MD, MCs; Ana Ramirez29, MD; Diana Vilar-Compte29, MD, MsC, Mark A. Lewis30, MD; Terence D. Rhodes30, MD, PhD; David M. Gill30, MD; Clarke A. Low30, MD, Sandeep H. Mashru31, MD; Abdul-Hai Mansoor31, MD, Grant C. Lewis32, MD; Stephanie J. Smith32, RN, MSN, OCN; Howard A. Zaren32, MD, FACS, Gayathri Nagaraj33, MD; Mojtaba Akhtari33, MD; Dan R. Castillo33, MD; Eric Lau33, DO; Mark E. Reeves33, MD, PhD, Stephanie Berg34, DO; Natalie Knox34, BS; Timothy E. O'Connor34, MD, Eric B. Durbin35, DrPH, MS, Amit A. Kulkarni36, MD; Heather H. Nelson36, PhD, MPH; Zohar Sachs36, MD, PhD, Rachel P. Rosovsky37, MD, MPH; Kerry L. Reynolds37, MD; Aditya Bardia37, MD; Genevieve Boland37, MD, PhD, FACS; Justin F. Gainor37, MD; Leyre Zubiri37, MD, PhD, Thorvardur R. Halfdanarson38, MD; Tanios S. Bekaii-Saab38, MD, FACP; Aakash Desai38, MD, MPH; Irbaz B. Riaz38, MD, MS; Surbhi Shah38, MD; Katherine E. Smith38, MD; Colt Williams38, MD, Nathaniel Bouganim39, MD, FRCP(C); Arielle Elkrief39, MD, FRCP(C); Justin Panasci39; Donald C. Vinh39, MD, FRCP(C), Gregory J. Riely40, MD, PhD; Rimma Belenkaya40, MA, MS; John Philip40, MS, Bryan Faller41, MD, Rana R. McKay42, MD; Archana Ajmera42, MSN, ANP-BC, AOCNP; Sharon S. Brouha42, MD, MPH; Sharon Choi42, MD, PhD; Albert Hsiao42, MD, PhD; Seth Kligerman42, MD; Taylor K. Nonato42; Erin G. Reid42, MD, Sibel Blau43, MD, Sachin R. Jhawar44, MD; Daniel Addison44, MD; James L. Chen44, MD; Margaret E. Gatti-Mays44, MD; Vidhya Karivedu44, MBBS; Joshua D. Palmer44, MD; Daniel G. Stover44, MD; Sarah Wall44, MD; Nicole O. Williams44, MD, Monika Joshi45, MD, MRCP; Hyma V. Polimera45, MD; Lauren D. Pomerantz45; Marc A. Rovito45, MD, FACP, Elizabeth A. Griffiths46, MD; Pragati G. Advani46, MD, MPH; Igor Puzanov46, MD, MSCI, FACP, Salma K. Jabbour47, MD; Christian F. Misdary47, MD; Mansi R. Shah47, MD, Gerald Batist48, MD, FACP, FRCP; Erin Cook48, MSN; Miriam Santos Dutra48, PhD; Cristiano Ferrario48, MD; Wilson H. Miller Jr.48, MD, PhD, Babar Bashir49, MD, MS; Christopher McNair49, PhD; Sana Z. Mahmood49, BA, BS; Vasil Mico49, BS; Andrea Verghese Rivera49, MD, Natasha C. Edwin50 MD; Melissa Smits50, APC, Deborah B. Doroshow51, MD, PhD; Matthew D. Galsky51, MD; Michael Wotman51, MD, Alyson Fazio52, APRN-BC; Julie C. Fu52, MD; Kathryn E. Huber52, MD; Mark H. Sueyoshi52, MD, Vadim S. Koshkin53, MD; Hala T. Borno53, MD; Daniel H. Kwon53, MD; Eric J. Small53, MD; Sylvia Zhang53, MS, Samuel M. Rubinstein54, MD; William A. Wood54, MD, MPH; Tessa M. Andermann54, MD; Christopher Jensen54, MD, Daniel W. Bowles55, MD; Christoper L. Geiger55, MD, Lawrence E. Feldman56, MD; Kent F. Hoskins56, MD; Gerald Gantt Jr.56, MD; Li C. Liu56, PhD; Mahir Khan56, MD; Ryan H. Nguyen56, DO; Mary Pasquinelli56, APN, DNP; Candice Schwartz56, MD; Neeta K. Venepalli56, MD, MBA, Blanche H. Mavromatis57, MD; Ragneel R. Bijjula57, MD; Qamar U. Zaman57, MD, David M. Aboulafia58, MD; Brett A. Schroeder58, MD, Umit Topaloglu59, PhD, FAMIA; Saif I. Alimohamed59, MD, Joan K. Moore60, MSN, RN, OCN, CCRP, Prakash Peddi61, MD; Lane R. Rosen61, MD; Briana Barrow McCollough61, BSc, CCRC, Navid Hafez62, MD, MPH; Roy Herbst62, MD, PhD; Patricia LoRusso62, DO, PhD; Maryam B. Lustberg62, MD, MPH; Tyler Masters62, MS; Catherine Stratton62, BA.
Affiliations: 1 Vanderbilt University Medical Center, Nashville, TN, USA; 2Georgetown Lombardi Comprehensive Cancer Center at Georgetown University, Washington DC, USA; 3University of Cincinnati Cancer Center, Cincinnati, OH, USA; 4Dana-Farber Cancer Institute, Boston, MA, USA; 5Henry Ford Cancer Institute, Henry Ford Hospital, Detroit, MI, USA 6University of Michigan Rogel Cancer Center, Ann Arbor MI, USA 7Knight Cancer Institute at Oregon Health and Science University, Portland, OR, USA, 8Winship Cancer Institute of Emory University, Atlanta GA, USA, 9Fred Hutchinson Cancer Center, Seattle WA, USA, 10Stanford Cancer Institute at Stanford University, Palo Alto CA, USA 11St. Elizabeth Healthcare, Edgewood, KY, USA, 12The University of Kansas Cancer Center, Kansas City, Kansas, USA, 13Brown University and Lifespan Cancer Institute, Providence RI, USA, 14Mount Auburn Hospital, Cambridge, MA, USA, 15Virtua Health, Marleton, NJ, USA, 16Hollings Cancer Center at the Medical University of South Carolina; Charleston, SC, USA, 17Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital, St. Louis, MO, USA; 18Baptist Cancer Center, Memphis, TN, USA; 19The Barbara Ann Karmanos Cancer Institute at Wayne State University School of Medicine, Detroit, MI, USA; 20Carl & Dorothy Bennett Cancer Center at Stamford Hospital, Stamford, CT, USA; 21Cleveland Clinic, Cleveland, OH, USA; 22Dan L Duncan Comprehensive Cancer Center at Baylor College of Medicine, Houston, TX, USA; 23Duke Cancer Institute at Duke University Medical Center, Durham, NC, USA; 24George Washington University, Washington, DC, USA; 25Hartford HealthCare Cancer Institute, Hartford, CT, USA; 26Herbert Irving Comprehensive Cancer Center at Columbia University, New York, NY, USA; 27Houston Methodist Cancer Center, Houston, TX, USA; 28Inova Schar Cancer Institute, Fairfax, VA, USA; 29Instituto Nacional de Cancerologia, Mexico City, Mexico; 30Intermountain Health Care, Salt Lake City, UT, USA; 31Kaiser Permanente Northwest, OR/WA, USA; 32Lewis Cancer and Research Pavilion @ St. Joseph’s/Candler, Savannah, GA, USA; 33Loma Linda University Cancer Center, Loma Linda, CA, USA; 34Loyola University Medical Center, Maywood, IL, USA; 35Markey Cancer Center at the University of Kentucky, Lexington, KY, USA; 36Masonic Cancer Center at the University of Minnesota, Minneapolis, MN, USA; 37Massachusetts General Hospital Cancer Center, Boston, MA, USA; 38Mayo Clinic, AZ/FL/MN, USA; 39McGill University Health Centre, Montreal, QC, Canada; 40Memorial Sloan Kettering Cancer Center, New York, NY, USA; 41Missouri Baptist Medical Center, St. Louis, MO, USA; 42Moores Comprehensive Cancer Center at the University of California, San Diego, La Jolla, CA, USA; 43Northwest Medical Specialties, Tacoma, WA, USA; 44The Ohio State University Comprehensive Cancer Center, Columbus, OH, USA; 45Penn State Health/Penn State Cancer Institute/St. Joseph Cancer Center, PA, USA; 46Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA; 47Rutgers Cancer Institute of New Jersey at Rutgers Biomedical and Health Sciences, New Brunswick, NJ, USA; 48Segal Cancer Centre, Jewish General Hospital, McGill University, Montreal, QC, Canada; 49Sidney Kimmel Cancer Center at Thomas Jefferson University, Philadelphia, PA, USA; 50ThedaCare Cancer Care, Appleton, WI, USA; 51Tisch Cancer Institute at the Icahn School of Medicine at Mount Sinai, New York, NY, USA; 52Tufts Medical Center Cancer Center, Boston and Stoneham, MA, USA; 53UCSF Helen Diller Family Comprehensive Cancer Center at the University of California at San Francisco, CA, USA; 54UNC Lineberger Comprehensive Cancer Center, Chapel Hill, NC, USA; 55University of Colorado Cancer Center, Aurora, CO, USA; 56University of Illinois Hospital & Health Sciences System, Chicago, IL, USA; 57UPMC Western Maryland, Cumberland, MD, USA; 58Virginia Mason Cancer Institute, Seattle, WA, USA; 59Wake Forest Baptist Comprehensive Cancer Center, Winston-Salem, NC, USA; 60WellSpan Health, York, PA, USA; 61Willis-Knighton Cancer Center, Shreveport, LA, USA; 62Yale Cancer Center at Yale University School of Medicine, New Haven, CT, USA.
Authors’ contributions
DBJ, BF, JW wrote the manuscript. BF, CH performed statistical analysis and prepared the figure. All authors (DBJ, MBA CH, TWD, HH, JA, ZB, CL, RMS, CH, SRKS, NB, CRF, LAF, JJY, BHL, MAB, CAC, GHL, LT, SAS, MJG, DBF, EWB, AK, SHA, DF, KV, EJK, LBW, CJ, MP, CCF, DYR, SM, ABF, BF, JLW) reviewed the manuscript. The author(s) read and approved the final manuscript.
Funding
REDCap is developed and supported by Vanderbilt Institute for Clinical and Translational Research grant support (UL1 TR000445 from NCATS / NIH). This study was partly supported by grants from the National Cancer Institute [grant number P30 CA068485 to Vanderbilt University Medical Center]. The study was partly supported by grants from the Melanoma Research Foundation (to DBJ). CRF is supported by P30 CA046592 and T32 CA236621. CCF is supported by T32 CA217834-05. LT received grant funding from the NIH 5T32CA009515-37.
Availability of data and materials
The data that support the findings of this study are available from COVID-19 and Cancer Consortium (CCC-19) but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. The dataset analyzed for the primary and secondary hypotheses will be made available upon request; requests should be sent to contact@ccc19.org. Individual deidentified patient data with site identifiers removed and geographic region of patient residence masked to a level no smaller than U.S. Census Divisions will be made available to researchers who provide a methodologically sound proposal, and whose proposed use of the data has been approved by an independent review committee identified for this purpose. External proposals can be submitted beginning 6 months and up to 72 months after publication of this article; the CCC19 is open to additional collaborators as well. All proposals should be directed to contact@ccc19.org; to gain access, data requestors will need to sign a data access agreement.
Declarations
Ethics approval and consent to participate
All methods were carried out in accordance with the Declaration of Helsinki. All protocols were approved at each institutional IRB (with the lead IRB Vanderbilt University Medical Center Human Research Protections Program). All data were anonymized and each institutional IRB approved a waiver of informed consent.
Consent for publication
Not applicable.
Competing interests
DBJ has served on advisory boards or as a consultant for BMS, Catalyst Biopharma, Iovance, Jansen, Mallinckrodt, Merck, Mosaic ImmunoEngineering, Novartis, Oncosec, Pfizer, Targovax, and Teiko, and has received research funding from BMS and Incyte. CRF has received institutional research support from the Merck Foundation and the National Comprehensive Cancer Network (via Pfizer). DF has received research support from Astellas, Viracor, and Merck, and consultant fee from Viracor. LAF has received clinical trial funding from BMS, Kartos, Array-Pfizer, EMD Serono, and as a consultant for Elsevier. GHL reports research grant support from Amgen to the Fred Hutchinson Cancer Center and consulting fees from Beyond Spring, G1 Therapeutics, Partner Therapeutics, Squibb, Samsung Bioepis, Merck, Jazz, TEVA, Seattle Genetics and Frensenius Kabi. Other authors declare no conflicts of interest relevant to the work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Douglas B. Johnson, Email: douglas.b.johnson@vumc.org
COVID-19 and Cancer Consortium:
Sonya A. Reid, Alaina J. Brown, Alex Cheng, Sarah Croessmann, Elizabeth J. Davis, Kyle T. Enriquez, Erin A. Gillaspie, Daniel Hausrath, Xuanyi Li, David A. Slosky, Carmen C. Solorzano, Matthew D. Tucker, Karen Vega-Luna, Lucy L. Wang, Trisha M. Wise-Draper, Syed A. Ahmad, Punita Grover, Shuchi Gulati, Jordan Kharofa, Tahir Latif, Michelle Marcum, Davendra P. S. Sohal, Olga Zamulko, Toni K. Choueiri, Jean M. Connors, George D. Demetri, Narjust Duma, Dory A. Freeman, Antonio Giordano, Alicia K. Morgans, Anju Nohria, Renee-Maria Saliby, Andrew L. Schmidt, Eliezer M. Van Allen, Wenxin Xu, Rebecca L. Zon, Shirish M. Gadgeel, Sheela Tejwani, Anne Boldt, Aaron M. Cohen, Shannon McWeeney, Eneida R. Nemecek, Staci P. Williamson, Deepak Ravindranathan, Jerome J. Graber, Petros Grivas, Jessica E. Hawley, Elizabeth T. Loggers, Ryan C. Lynch, Elizabeth S. Nakasone, Michael T. Schweizer, Shaveta Vinayak, Michael J. Wagner, Albert Yeh, Elwyn C. Cabebe, Michael J. Glover, Alokkumar Jha, Ali Raza Khaki, Lidia Schapira, Julie Tsu-Yu Wu, Goetz Kloecker, Barbara B. Logan, Chaitanya Mandapakala, Crosby D. Rock, Panos Arvanitis, Pamela C. Egan, Hina Khan, Adam J. Olszewski, Kendra Vieira, Lisa B. Weissmann, Padmanabh S. Bhatt, Melissa G. Mariano, Carey C. Thomson, Theresa M. Carducci, Karen J. Goldsmith, Susan Van Loon, Mariam Alexander, Sara Matar, Sarah Mushtaq, Keith E. Stockerl-Goldstein, Omar Butt, Mark A. Fiala, Jeffrey P. Henderson, Ryan S. Monahan, Alice Y. Zhou, Philip E. Lammers, Sanjay G. Revankar, Salvatore A. Del Prete, Michael H. Bar, Anthony P. Gulati, K. M. Steve Lo, Suzanne J. Rose, Jamie Stratton, Paul L. Weinstein, Shilpa Gupta, Nathan A. Pennell, Manmeet S. Ahluwalia, Scott J. Dawsey, Christopher A. Lemmon, Amanda Nizam, Nima Sharifi, Claire Hoppenot, Ang Li, Susan Halabi, Hannah Dzimitrowicz, Tian Zhang, Sharad Goyal, Minh-Phuong Huynh-Le, Peter Paul Yu, Jessica M. Clement, Ahmad Daher, Mark E. Dailey, Rawad Elias, Asha Jayaraj, Emily Hsu, Alvaro G. Menendez, Oscar K. Serrano, Melissa K. Accordino, Divaya Bhutani, Dawn Hershman, Matthew A. Ingham, Gary K. Schwartz, Eric H. Bernicker, John F. Deeken, Danielle Shafer, Erika Ruíz-García, Ana Ramirez, Diana Vilar-Compte, Mark A. Lewis, Terence D. Rhodes, David M. Gill, Clarke A. Low, Sandeep H. Mashru, Abdul-Hai Mansoor, Grant C. Lewis, Stephanie J. Smith, Howard A. Zaren, Gayathri Nagaraj, Mojtaba Akhtari, Dan R. Castillo, Eric Lau, Mark E. Reeves, Stephanie Berg, Natalie Knox, Timothy E. O’Connor, Eric B. Durbin, Amit A. Kulkarni, Heather H. Nelson, Zohar Sachs, Rachel P. Rosovsky, Kerry L. Reynolds, Aditya Bardia, Genevieve Boland, Justin F. Gainor, Leyre Zubiri, Thorvardur R. Halfdanarson, Tanios S. Bekaii-Saab, Aakash Desai, Irbaz B. Riaz, Surbhi Shah, Katherine E. Smith, Colt Williams, Nathaniel Bouganim, Arielle Elkrief, Justin Panasci, Donald C. Vinh, Gregory J. Riely, Rimma Belenkaya, John Philip, Bryan Faller, Rana R. McKay, Archana Ajmera, Sharon S. Brouha, Sharon Choi, Albert Hsiao, Seth Kligerman, Taylor K. Nonato, Erin G. Reid Sibel Blau, Sachin R. Jhawar, Daniel Addison, James L. Chen, Margaret E. Gatti-Mays, Vidhya Karivedu, Vidhya Karivedu, Joshua D. Palmer, Daniel G. Stover, Sarah Wall, Nicole O. Williams, Monika Joshi, Hyma V. Polimera, Lauren D. Pomerantz, Marc A. Rovito, Elizabeth A. Griffiths, Pragati G. Advani, Igor Puzanov, Salma K. Jabbour, Christian F. Misdary, Mansi R. Shah, Gerald Batist, Erin Cook, Miriam Santos Dutra, Cristiano Ferrario, Wilson H. Miller, Jr., Babar Bashir, Christopher McNair, Sana Z. Mahmood, Vasil Mico, Andrea Verghese Rivera, Natasha C. Edwin, Melissa Smits, Deborah B. Doroshow, Matthew D. Galsky, Michael Wotman, Alyson Fazio, Julie C. Fu, Kathryn E. Huber, Mark H. Sueyoshi, Vadim S. Koshkin, Hala T. Borno, Daniel H. Kwon, Eric J. Small, Sylvia Zhang, Samuel M. Rubinstein, William A. Wood, Tessa M. Andermann, Christopher Jensen, Daniel W. Bowles, Christoper L. Geiger, Lawrence E. Feldman, Kent F. Hoskins, Gerald Gantt, Jr., Li C. Liu, Mahir Khan, Ryan H. Nguyen, Mary Pasquinelli, Candice Schwartz, Neeta K. Venepalli, Blanche H. Mavromatis, Ragneel R. Bijjula, Qamar U. Zaman, David M. Aboulafiam, Brett A. Schroeder, Umit Topaloglu, Saif I. Alimohamed, Joan K. Moore, Prakash Peddi, Lane R. Rosen, Briana Barrow McCollough, Navid Hafez, Roy Herbst, Patricia LoRusso, Maryam B. Lustberg, Tyler Masters, and Catherine Stratton
References
- 1.Elkrief A, Wu JT, Jani C, et al. Learning through a Pandemic: The Current State of Knowledge on COVID-19 and Cancer. Cancer Discov. 2022;12(2):303–330. doi: 10.1158/2159-8290.CD-21-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grivas P, Khaki AR, Wise-Draper TM, et al. Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: a report from the COVID-19 and Cancer Consortium. Ann Oncol. 2021;32(6):787–800. doi: 10.1016/j.annonc.2021.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Covid, Cancer Consortium Electronic address jwvo, Covid, Cancer C. A Systematic Framework to Rapidly Obtain Data on Patients with Cancer and COVID-19: CCC19 Governance, Protocol, and Quality Assurance. Cancer cell. 2020;38(6):761–766. doi: 10.1016/j.ccell.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.French B, Shotwell MS. Regression Models for Ordinal Outcomes. JAMA. 2022;328(8):772–773. doi: 10.1001/jama.2022.12104. [DOI] [PubMed] [Google Scholar]
- 6.Rubinstein SM, Bhutani D, Lynch RC, et al. Patients Recently Treated for B-lymphoid Malignancies Show Increased Risk of Severe COVID-19. Blood Cancer Discov. 2022;3(3):181–193. doi: 10.1158/2643-3230.BCD-22-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo J, Rizvi H, Egger JV, Preeshagul IR, Wolchok JD, Hellmann MD. Impact of PD-1 Blockade on Severity of COVID-19 in Patients with Lung Cancers. Cancer Discov. 2020;10(8):1121–1128. doi: 10.1158/2159-8290.CD-20-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sullivan RJ, Johnson DB, Rini BI, et al. COVID-19 and immune checkpoint inhibitors: initial considerations. J Immunother Cancer. 2020;8(1):e000933. doi: 10.1136/jitc-2020-000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogiers A, Pires da Silva I, Tentori C, et al. Clinical impact of COVID-19 on patients with cancer treated with immune checkpoint inhibition. J Immunother Cancer. 2021;9(1):e001931. doi: 10.1136/jitc-2020-001931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naidoo J, Wang X, Woo KM, et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J Clin Oncol. 2017;35(7):709–717. doi: 10.1200/JCO.2016.68.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salem JE, Manouchehri A, Moey M, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19(12):1579–1589. doi: 10.1016/S1470-2045(18)30608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang DY, Salem JE, Cohen JV, et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018;4(12):1721–1728. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt AL, Tucker MD, Bakouny Z, et al. Association Between Androgen Deprivation Therapy and Mortality Among Patients With Prostate Cancer and COVID-19. JAMA Netw Open. 2021;4(11):e2134330. doi: 10.1001/jamanetworkopen.2021.34330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steenkamp L, Saggers RT, Bandini R, et al. Small steps, strong shield: directly measured, moderate physical activity in 65 361 adults is associated with significant protective effects from severe COVID-19 outcomes. Br J Sports Med. 2022;56(10):568–576. doi: 10.1136/bjsports-2021-105159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplemental Table 1. Outcomes summarized over full patient cohort. Supplementary Table 2. Organ function in hospitalized patients based on therapy type. Supplemental Table 3. Survival in patients without active cancer or treatment in the previous 3 months.
Data Availability Statement
The data that support the findings of this study are available from COVID-19 and Cancer Consortium (CCC-19) but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. The dataset analyzed for the primary and secondary hypotheses will be made available upon request; requests should be sent to contact@ccc19.org. Individual deidentified patient data with site identifiers removed and geographic region of patient residence masked to a level no smaller than U.S. Census Divisions will be made available to researchers who provide a methodologically sound proposal, and whose proposed use of the data has been approved by an independent review committee identified for this purpose. External proposals can be submitted beginning 6 months and up to 72 months after publication of this article; the CCC19 is open to additional collaborators as well. All proposals should be directed to contact@ccc19.org; to gain access, data requestors will need to sign a data access agreement.