Abstract
U.S. smoking-related disparities persist, but data evaluating how smoking patterns across diverse populations have changed by birth cohort are lacking. Worldwide, smoking continues to exact harm, especially to low- and middle-income nations with less historical data for smoking analyses. The Cancer Intervention and Surveillance Modeling Network (CISNET) Lung Working Group previously generated smoking histories for the whole U.S. population using an age, period, and birth cohort (APC) methodological framework. These inputs have been used in numerous models to simulate future patterns of smoking and to evaluate the potential impact of policies. However, the absence of detailed model-ready inputs on smoking behaviors for diverse U.S. populations has been a barrier to research evaluating future trends in smoking-related disparities or the projected impacts of policies across sociodemographic groups. This supplement issue provides new estimates of smoking behaviors with detailed historical data by race/ethnicity, educational attainment, family income, and for each of the 50 U.S. states and Washington, DC. All-cause mortality relative risks associated with smoking by race/ethnicity and educational attainment are also available for the first time. Finally, the supplement issue presents comprehensive smoking histories for Brazil, demonstrating the application of this methodology to resource-limited settings. Collectively, these data aim to offer insight into future U.S. and global smoking disparities and accelerate research on tobacco control policies that advance health equity. This effort will allow tobacco simulation models to account comprehensively for population diversity, thereby enabling researchers to develop more sophisticated analyses of tobacco use and control interventions.
A BRIEF HISTORY
Since publication of the first Surgeon General’s Report on Smoking and Health in 1964,1 the U.S. has benefited from major improvements to health and life expectancy, partly due to decades of progress in tobacco prevention and control that drastically reduced smoking and consequently smoking-related morbidity and mortality in the population.2 Social norms around smoking changed dramatically over this time period; as Americans grew to understand the harms of smoking and secondhand smoke exposure, many quit, and quit in droves, and many fewer young people started to smoke.1
As public health progress unfolded, differences in how communities experienced that progress came to light and in 1998 Surgeon General’s Report on smoking disparities was published, “Tobacco Use among U.S. Racial/Ethnic Minority Groups,” focused on 4 groups: African Americans, American Indians and Alaska Natives, Asian Americans and Pacific Islanders, and Hispanics.3 Since then, research examining smoking disparities has proliferated, expanding beyond race/ethnicity to encompass disparities by SES, geographic region, and other sociodemographic characteristics or comorbid health conditions.4–6 From 2000 to 2022, 1,306 articles related to smoking disparities have been indexed through PubMed (Query: (Smoking[MeSH Major Topic]) AND (Disparities)).
Research related to smoking disparities was synthesized in a 2017 National Cancer Institute Tobacco Control Monograph detailing how smoking disparities have been shaped by multiple factors at the individual, interpersonal, community, and societal levels across the life course.4 At the societal level, tobacco control interventions such as tobacco taxation, smoke-free air laws, and access to smoking cessation treatment improved public health, but low SES and racial/ethnic minority groups were less likely to benefit.4 Meanwhile, sophisticated tobacco industry marketing campaigns contributed directly to disparities by targeting women, racial/ethnic minorities, homeless people, and other vulnerable groups.7–13 Beyond the U.S., global context also matters: both the WHO Framework Convention on Tobacco Control and international trade agreements have shaped tobacco policies across different countries.14–18
In the U.S., public health authorities have continued to emphasize the importance of understanding smoking disparities and achieving health equity goals for racial/ethnic minorities. Native Americans have the highest smoking rates of any racial/ethnic group, individuals of low SES continue to bear the brunt of tobacco-related disease and death,19 and differences by state have been magnified by the slow adoption of tobacco control policies in the South and Midwest.20 Although targeted tobacco control interventions have been developed to address smoking disparities,21 including ‘Tips from Former Smokers’ campaigns tailored for racial/ethnic minorities and other priority populations,22,23 their long-term impact on smoking disparities will need to be evaluated. How sociodemographic differences in cigarette smoking will unfold over the coming decades will depend on accurate information about smoking within and across populations, and whether that information is used to inform policy and regulation.
A COHORT LENS
Cohort analyses are particularly crucial to understanding smoking trajectories in U.S. populations. As each birth cohort comes of age, they face their own unique socio-cultural and policy environments during key life stages relevant to smoking uptake (adolescence, young adulthood) and cessation (middle and older ages). For instance, an individual born in 1975 was surrounded by a different tobacco product and policy landscape and a different set of social norms surrounding smoking as a teenager during the 1990s, compared to someone born in 1960 who would have been at peak smoking initiation ages during the 1970s and 1980s. Major events, policies, and social changes have defined the lives, and influenced the behaviors, of entire birth cohorts during the most significant ages of smoking initiation and cessation. Examples are disruptions caused by the 20th century’s 2 world wars, during which cigarettes were provided to American soldiers as rations, inducing higher smoking initiation in certain birth cohorts24; college education subsidies provided by the G.I. Bill to military veterans improved social mobility and decreased smoking among veterans who used them25; and the women’s liberation movement whose feminist slogans were co-opted by Philip Morris to market Virginia Slim cigarettes to young women.12 In more recent history, the National Truth campaign, launched in 2000 as a smoking prevention educational campaign, reduced smoking initiation among adolescents born in the 1980s.26 Without a cohort lens, researchers may attribute changes in smoking patterns to contemporaneous issues of a given period, rather than acknowledging the impact of shifts in the lived experiences of different generations.
To understand how public health interventions (or lack thereof) are impacting the smoking behaviors of newer generations, it is necessary to examine and compare changes by birth cohort or generation (e.g., people born in the 2000s versus people born in the 1980s). Researchers have studied smoking trajectories by birth cohort for the U.S. population (Figure 1),27,28 but the cohort perspective has been relatively absent from the smoking disparities literature. This literature has mostly documented trends in cross-sectional prevalence by period (calendar year), but such analyses combine information from multiple birth cohorts into a single metric, usually age-adjusted or crude prevalence, thereby masking trends as they vary across generations within specific subpopulations. Examining changes in prevalence and potential disparities by birth cohort, within advantaged and disadvantaged groups, offers a more accurate assessment. In addition, trend analyses of a specific subpopulation (e.g., people of low SES) by period implicitly assume homogeneity across birth cohorts. Therefore, disaggregating the population by birth cohort provides a more comprehensive view—one that better reflects societal changes.
Figure 1.
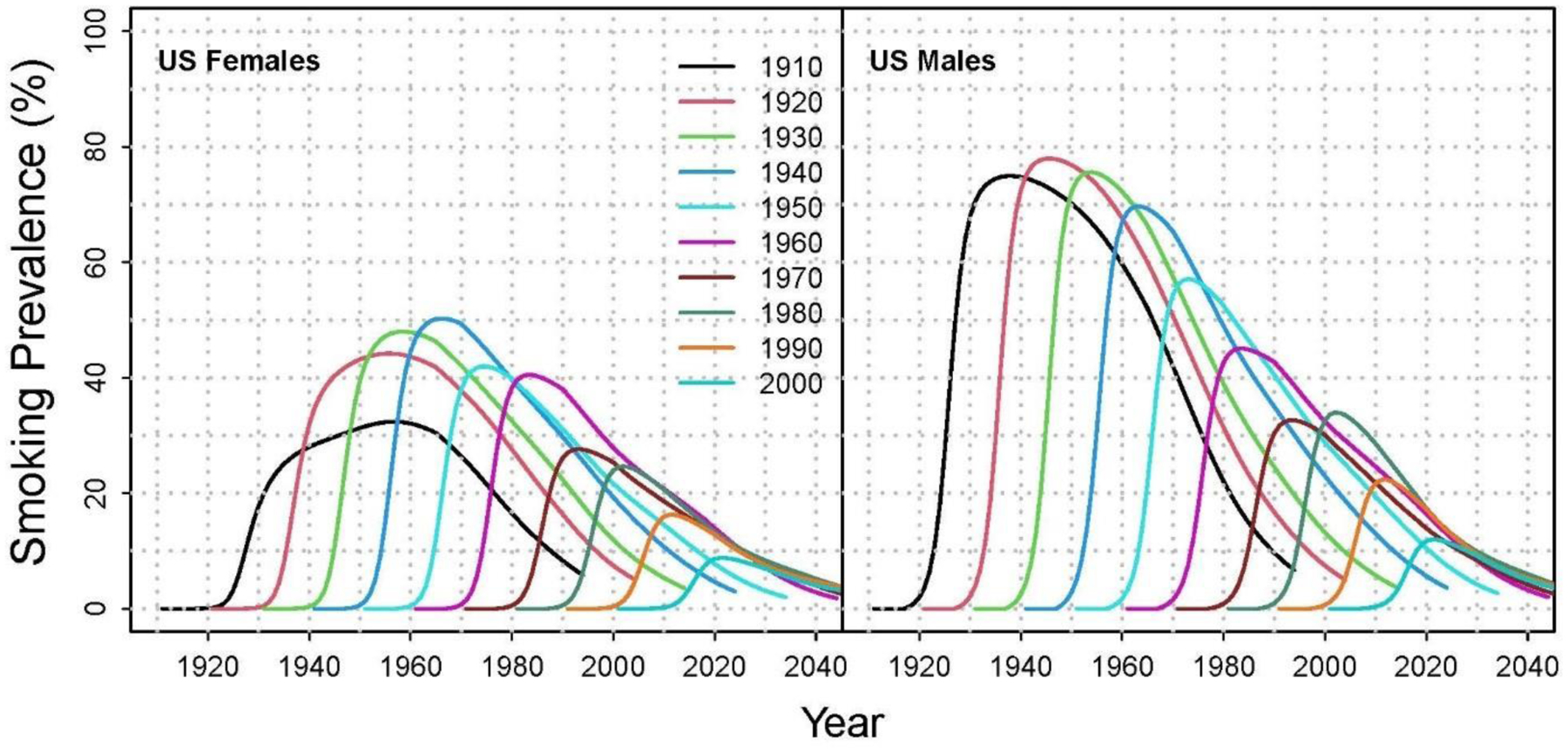
Smoking prevalence by birth cohort for the U.S. population.
Notes: Lines represent individual birth cohorts from 1910 (black line, farthest to left) to 2000 (teal line, farthest to right). Data reflect increasing smoking prevalence among females from 1910‒1940 birth cohorts, and declines from 1950‒2000 birth cohorts; among males, smoking prevalence increased from the 1910 to 1920 birth cohorts, with declines in most subsequent cohorts. Estimates of current smoking prevalence by cohort and gender were generated by the Cancer Intervention and Surveillance Modeling Network (CISNET) Lung Working Group using the age, period, and birth cohort (APC) methodological framework.27,28
For instance, much larger proportions of recent generations have completed college degrees compared to those born earlier, reflecting the integration of women and racial/ethnic minorities in higher education institutions, as well as changing expectations for future employment.29 Thus, the 1950 birth cohort of people with a college education has a substantially different demographic profile than that of the 1990 birth cohort with college degrees. Because college education is often completed earlier in life, this characteristic is then carried forward, affecting the educational mix in future years.
Other broader demographic developments over time, such as patterns of immigration,30 combined with differences in birth rates,31 mean that among U.S. births, larger shares have come from Hispanic women, reflecting the rising proportion of Hispanics in the overall population. This translates into recent birth cohorts being more comprised of Hispanics compared to older birth cohorts.32 The cohort perspective, therefore, facilitates trend analysis of smoking by sociodemographic groups that may be changing in size and composition.
MOVING BEYOND SMOKING PREVALENCE
Most reports analyze data and trends in smoking prevalence—the main metric used to evaluate smoking progress in the population, and the primary point of comparison between advantaged and disadvantaged groups. However, overall smoking prevalence is a function of multiple underlying drivers at the individual level. To reconstruct individual-level smoking histories across the population, information is needed regarding who started smoking (ever use) and when (initiation), who is smoking now (current use), how long people who smoke continue to do so (duration), the intensity with which they smoke (cigarettes per day), and who quit (former use) and when (cessation). These behavioral components represent different points for intervention and metrics of progress, and collectively shape disparities in smoking prevalence across populations, and across time and age. For example, similar overall smoking prevalence estimates for White and Black Americans may mask key differences: Black Americans start smoking at older ages compared to Whites, but have less success with quitting.33 As a result, Black Americans tend to have longer smoking duration, and thus a higher risk of lung cancer.33–36 Black Americans are more susceptible to lung cancer than other racial/ethnic groups, even though they smoke fewer cigarettes per day;37 as a result, Black Americans are less eligible for lung cancer screening (based on calculated pack-years) compared to White Americans.34,38–42 These findings contributed to the recent 2021 recommendation by the U.S. Preventive Services Task Force to extend lung cancer screening eligibility to smokers with fewer pack years.43 A comprehensive analysis of historical smoking patterns and behaviors within each key U.S. subpopulation can better inform future health equity efforts to help target policy or treatment interventions for specific groups by age, gender, and sociodemographic factors (i.e., race/ethnicity, SES).
APPLYING THE AGE-PERIOD-COHORT (APC) FRAMEWORK
The CISNET Lung Working Group has previously constructed APC models for the whole U.S. population’s smoking history using data from the 1965‒2018 National Health Interview Surveys (NHIS).27,28 These analyses generated estimates of the distribution of individual-level parameters for smoking initiation, cigarettes smoked per day, and smoking cessation by age, gender, and birth cohort, starting with the generation born in 1890. Collectively these parameters are validated by reproducing observed trends in smoking prevalence in the U.S. population. These estimates are internally consistent, forming a comprehensive picture of the smoking experience for specific groups. For example, Figure 1 presents smoking prevalence by birth cohort for the entire U.S. population generated by the CISNET Lung Working Group.
The APC approach can be applied across different sociodemographic groups, thereby facilitating comparisons of smoking patterns across groups accounting for age, period, and cohort differences. This process allows historical comparisons of the specific smoking metrics by race/ethnicity, SES, and geographic location, thereby providing a more complete picture the components affecting past and present smoking disparities. Research in this arena could help further develop strategies that aim to achieve tobacco and health equity but have yet to achieve their full potential.
LEVERAGING HISTORICAL DATA TO MODEL THE FUTURE
The CISNET smoking history parameters are age- and cohort-specific, and the statistical distribution of individual-level inputs that have been leveraged to make projections about future smoking trends for the population as a whole (Figure 2). They are widely used as inputs for simulation models of tobacco use, tobacco control policy, and lung cancer screening for the U.S. population.27,44–49 These models have been used to evaluate the potential impact of tobacco policies on smoking and lung cancer,27,46,48,50–52 the benefits and harms of different lung cancer screening strategies,38,53–55 their effectiveness and cost-effectiveness,45,56–58 as well as the effect of smoking cessation in lung cancer screening settings.59,60 Tobacco simulation models are useful tools for surveillance and policy evaluation; recent analyses have begun to apply such models for analyzing and predicting changes in smoking disparities by race, income, and mental health status.4,49,61,62 However, models are only as reliable as the smoking inputs applied, and the lack of detailed empirical data inputs for specific subpopulations, developed in a systematic and consistent way, has limited the development of modeling and evaluation tools to monitor progress towards health equity aims. Moreover these tools require mortality inputs that reflect differences by smoking status for specific populations; although relative risk estimates for mortality by smoking status are available for the general U.S. population,63–65 the lack of such estimates for different sociodemographic groups is also an impediment to health equity research progress.
Figure 2.
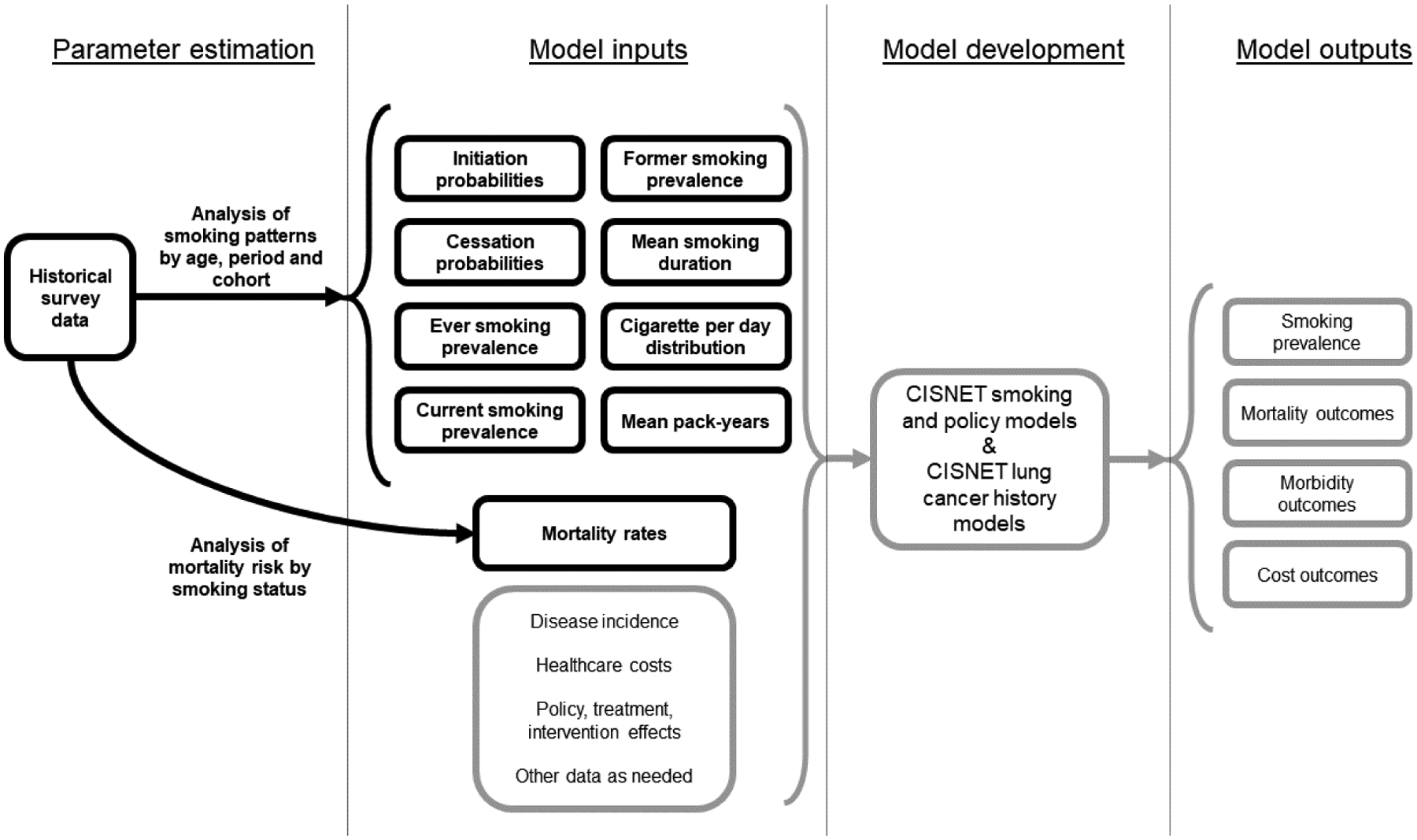
The CISNET Lung Working Group approach to modeling smoking, smoking-related mortality, and lung cancer.
Notes: Black lines with text in bold typeface represent research and data published in this supplement. Age, period, and cohort analyses were conducted for the U.S. population by race/ethnicity, education, income, and state, and for the Brazil population. Mortality analyses were conducted for the U.S. population by race/ethnicity and education only.
This supplement presents the most detailed and comprehensive historical analysis of U.S. smoking patterns and disparities to date. Using decades of data from the NHIS and a rigorously validated APC methodology, the CISNET Lung Working Group examines smoking histories for specific U.S. subpopulations as they vary by race/ethnicity, family income, level of educational attainment, and state of residence. As the data become more sparse, the methodologic issues become more challenging. Figure 3 presents applications of the APC methodology in previously published studies, and their extensions to new sociodemographic groups analyzed within this supplement issue. Five of the papers in this supplement issue cover U.S. populations: 4 examining smoking histories for specific subpopulations (Figure 3), and a fifth paper examining mortality associated with smoking by age, gender, race/ethnicity, and education.
Figure 3.
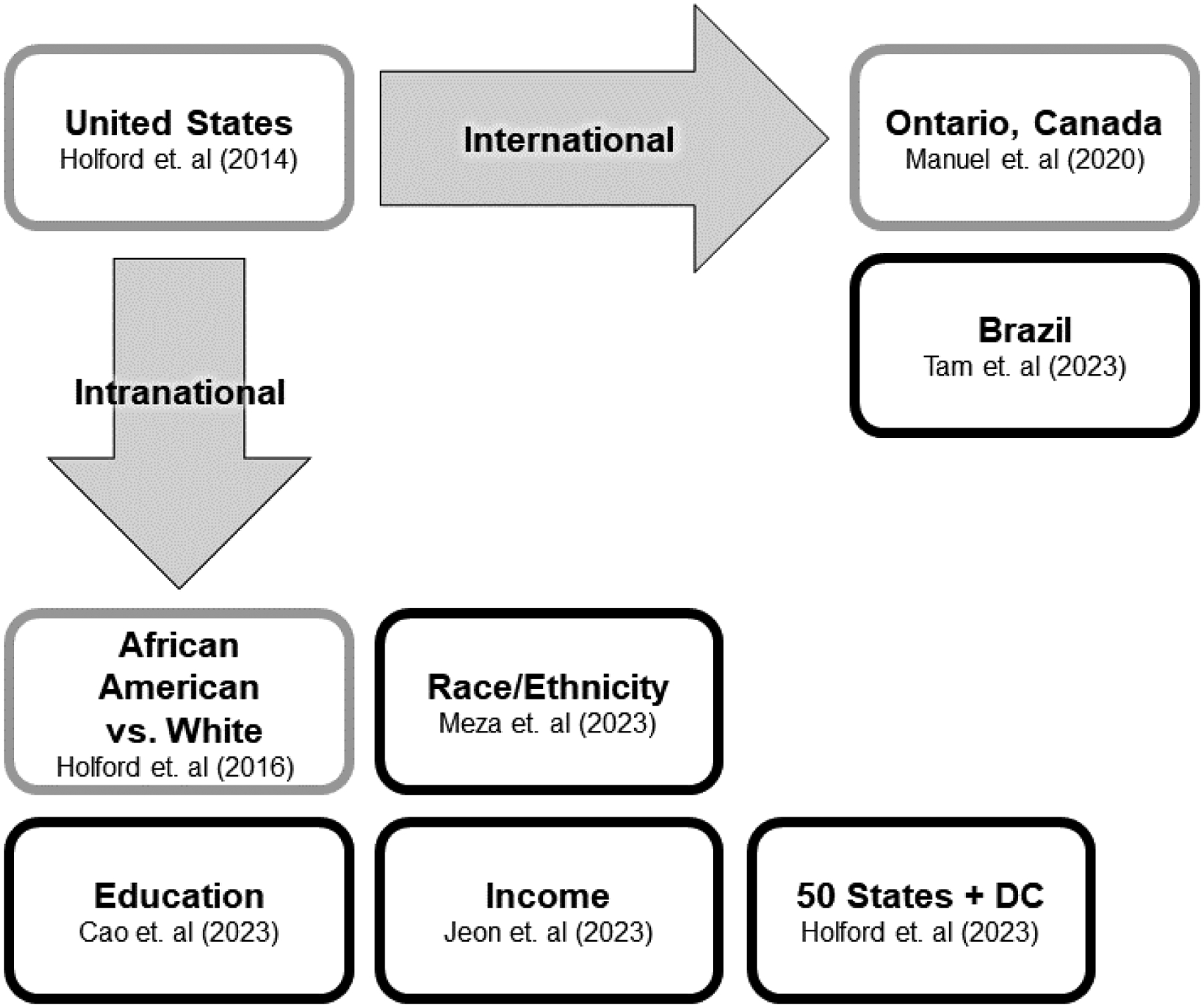
Extensions of the age-period-cohort framework for population smoking histories.
Notes: Gray outlines represent previously published applications of the Age-Period-Cohort (APC) framework for smoking histories. Black outlines represent APC extensions to new populations published in this supplement.
Throughout these papers, the authors refer to harms caused by smoking commercial cigarettes, and not sacred tobacco used for traditional or ceremonial Indigenous practices.66 The term “gender” is used to refer to males (men/boys) and females (women/girls), consistent with recommendations that “sex” be used to refer to biological factors, and “gender” for social factors such as smoking.67 Other genders are not considered because survey measures have historically treated gender as binary.
Race/Ethnicity
Meza et al.’s analysis68 of smoking patterns by race/ethnicity presents a major advance: historical smoking histories by age, gender, and birth cohort for American Indian and Alaska Natives (AIANs)—the group that bears the largest burden of health harms caused by cigarette smoking - at the national level. For the first time, smoking parameters are also available for the Hispanic, Non-Hispanic White, Non-Hispanic Black, and the Asian and Pacific Islander populations.
Education
Cao et al.’s analyses69 of smoking-related disparities by SES generally affirm an inverse relationship between level of education—a major component of SES—and smoking outcomes, where less educated individuals have higher smoking prevalence, while more educated individuals have lower prevalence. However, their analyses of changing birth-cohort smoking patterns by level of educational attainment reveal novel findings among those with the lowest levels of education in the U.S.
Income
Trends in smoking disparities by level of family or household income—another important determinant of individual SES—are challenging to analyze due to data missingness, the variety of income sources within a household, and poverty thresholds being contingent on family structures.70 Jeon et al.’s analysis71 was therefore quite the methodological undertaking. Authors addressed well-known income data challenges to impute missing income data. The result is an extensive historical analysis of smoking behaviors across U.S. birth cohorts as they vary by poverty status going back to 1983.
States
Local and state researchers and policymakers will now benefit from detailed state-specific smoking parameters published in this supplement issue by Holford et al.72 The authors generated comprehensive smoking histories for each of the 50 U.S. states and the District of Columbia. Now rich, detailed historical information reflects each state’s unique smoking trajectories as shaped by the societal and policy norms of their populations.
Mortality Risk
Parameters for smoking behaviors alone are not sufficient to conduct modeling analyses for disparate groups; mortality risk estimates are also necessary (Figure 2). The Jeon et al. mortality analysis73 begins to fill this gap: the paper presents detailed relative risk of mortality estimates by race/ethnicity (Hispanic, non-Hispanic Black, and non-Hispanic White) and education according to smoking status. The Jeon et al.73 mortality analysis, together with the smoking parameters provided by Meza et al.68 and Cao et al.,69 respectively, now offer a full suite of data to inform modeling, evaluation, and surveillance tools for specific race/ethnicity and education groups. Future analyses of smoking-specific mortality risk estimates by state and family income could provide similar opportunities.
MOVING THE APC FRAMEWORK BEYOND U.S. BORDERS
The APC methodology has been successfully extended to numerous U.S. subpopulations, accounting for wide-ranging social, demographic, and geographical diversity across the country and its implications for smoking. Manuel et al. have successfully adapted the APC framework to produce smoking histories for the population of Ontario, Canada using biennial cross-sectional data from 2003‒2013.74 Although far fewer data points are available for this modeling, it would be remiss not to extend this methodology to other countries, especially low- and middle-income nations that are disproportionately impacted by the global burden of smoking. In Latin America, ~70 million people smoke, which translates into substantial harm to public health. Brazil has made progress towards reducing smoking, but as the most populous nation of Latin America, its lower overall prevalence still reflects over 20 million75 people who smoke in this country. This supplement issue provides the first extensions of the APC methodology to Brazil (Figure 3).
Brazil
The CISNET Lung Working Group and experts at the Brazilian National Cancer Institute (Instituto Nacional de Câncer José Alencar Gomes da Silva [INCA]) estimated detailed smoking histories of the Brazil population for birth cohorts from 1950 to 2000. With fewer historical data points available from the National Household Sample Survey (PNAD) and National Health Surveys (PNS), Tam et al. adapted the APC approach for Brazil.76 The work demonstrates the feasibility of adapting this rigorous validated methodology to a resource-limited setting, indicating that similar studies could be developed in other countries with limited data.
Looking Ahead
The U.S. studies consider race/ethnicity, education, income, and state independently, but future smoking parameters could be estimated to evaluate intersectional populations, such as racial/ethnic groups by SES. As well, the smoking parameters generated for the Brazil population could be further extended to consider differences within the country by region, race/ethnicity, or SES. Moreover, similar analyses are currently being conducted to derive estimates for 2 more Latin American countries: Mexico and Argentina. The present set of studies should not be viewed as comprehensive; they merely offer a beginning.
Progress towards health equity aims has been inadequate—hampered in part by the lack of critical information needed to understand smoking behaviors within specific populations. This supplement issue aims to accelerate tobacco control progress and scale up research on tobacco-related health disparities and their resulting impacts on marginalized populations. The data generated in this supplement issue are publicly available for users to examine and download through an interactive website at apps.cisnetsmokingparameters.org/. The data can be used to make projections about future smoking within specific subpopulations under different assumptions for baseline or ‘status quo’ conditions, or under policies that impact only smoking initiation (e.g., Tobacco 21 laws52), only cessation,60 or both.77 Researchers can also use the data to evaluate changes in smoking disparities between groups over time, and to assess the relative effects of different policy interventions on population health.
Promising research evaluating policies as they impact tobacco use disparities for diverse populations is already being published.77–89 There are now opportunities for researchers to combine the smoking parameters produced in this supplement issue with emerging research on the effects of tobacco policies or regulations for specific vulnerable subgroups. Future surveillance and policy modeling to explicitly study historically marginalized populations—largely excluded from such scholarship—are sorely needed. Lastly, the application of APC approaches to other countries and sub-populations should facilitate analyses of historical smoking patterns and the development of context-specific models. These, in turn, can inform the development of local policies aimed at reducing not only smoking-related morbidity and mortality, but also reducing smoking-related disparities globally.
ACKNOWLEDGMENTS
This paper was supported through National Cancer Institute (NCI) grants U01CA199284 and U01CA253858. JT, DL, JJ, TH and RM also acknowledge support from NCI grant U54CA229974. JT is also supported by the National Institute on Drug Abuse (K01DA056424). EJF is an employee of the NCI. The ideas presented in this paper are that of the authors and do not reflect the official policy of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No financial disclosures were reported by the authors of this paper.
REFERENCES
- 1.U.S. Department of Health Education and Welfare. Smoking and health: report of the Advisory Committee to the Surgeon General of the public health service. U.S. Department of Health, Education, and Welfare, Public Health Service; 1964. [Google Scholar]
- 2.Holford TR, Meza R, Warner KE, et al. Tobacco control and the reduction in smoking-related premature deaths in the United States, 1964–2012. JAMA. 2014;311(2):164–171. 10.1001/jama.2013.285112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Department of Health and Human Services. Tobacco use among US racial/ethnic minority groups: A report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 1998. [Google Scholar]
- 4.U.S. National Cancer Institute. A Socioecological Approach to Addressing Tobacco-Related Health Disparities. Bethesda, MD: U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute; 2017. [Google Scholar]
- 5.Doogan NJ, Roberts ME, Wewers ME, et al. A growing geographic disparity: Rural and urban cigarette smoking trends in the United States. Prev Med. 2017;104:79–85. 10.1016/j.ypmed.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams JM, Steinberg ML, Griffiths KG, Cooperman N. Smokers With Behavioral Health Comorbidity Should Be Designated a Tobacco Use Disparity Group. Am J Public Health. 2013;103(9):1549–1555. 10.2105/AJPH.2013.301232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iglesias-Rios L, Parascandola M. A historical review of R.J. Reynolds’ strategies for marketing tobacco to Hispanics in the United States. Am J Public Health. 2013;103(5):e15–e27. 10.2105/AJPH.2013.301256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Silva J, O’Gara E, Villaluz NT. Tobacco industry misappropriation of American Indian culture and traditional tobacco. Tob Control. 2018;27(e1):e57–e64. 10.1136/tobaccocontrol-2017-053950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown-Johnson CG, England LJ, Glantz SA, Ling PM. Tobacco industry marketing to low socioeconomic status women in the U.S.A. Tob Control. 2014;23(e2):e139–e146. 10.1136/tobaccocontrol-2013-051224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith EA, Thomson K, Offen N, Malone RE. “If you know you exist, it’s just marketing poison”: meanings of tobacco industry targeting in the lesbian, gay, bisexual, and transgender community. Am J Public Health. 2008;98(6):996–1003. 10.2105/AJPH.2007.118174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Apollonio DE, Malone RE. Marketing to the marginalised: tobacco industry targeting of the homeless and mentally ill. Tob Control. 2005;14(6):409–415. 10.1136/tc.2005.011890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toll BA, Ling PM. The Virginia Slims identity crisis: an inside look at tobacco industry marketing to women. Tob Control. 2005;14(3):172–180. 10.1136/tc.2004.008953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cruz TB, Rose SW, Lienemann BA, et al. Pro-tobacco marketing and anti-tobacco campaigns aimed at vulnerable populations: A review of the literature. Tob Induc Dis. 2019;17:68. 10.18332/tid/111397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jarman H Normalizing Tobacco? The Politics of Trade, Investment, and Tobacco Control. Milbank Q. 2019;97(2):449–479. 10.1111/1468-0009.12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Appau A, Drope J, Labonté R, Stoklosa M, Lencucha R. Disentangling regional trade agreements, trade flows and tobacco affordability in sub-Saharan Africa. Global Health. 2017;13(1):81. 10.1186/s12992-017-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung-Hall J, Craig L, Gravely S, Sansone N, Fong GT. Impact of the WHO FCTC over the first decade: a global evidence review prepared for the Impact Assessment Expert Group. Tob Control. 2019;28(Suppl 2):s119–s128. 10.1136/tobaccocontrol-2018-054389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Craig L, Fong GT, Chung-Hall J, Puska P. Impact of the WHO FCTC on tobacco control: perspectives from stakeholders in 12 countries. Tob Control. 2019;28(Suppl 2):s129–s135. 10.1136/tobaccocontrol-2019-054940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drope J, Lencucha R. Tobacco control and trade policy: proactive strategies for integrating policy norms. J Public Health Policy. 2013;34(1):153–164. 10.1057/jphp.2012.36. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Health Disparities Related to Commercial Tobacco and Advancing Health Equity. Smoking & Tobacco Use. https://www.cdc.gov/tobacco/health-equity/index.htm. Published 2022. Updated June 27, 2022. Accessed September 2, 2022.
- 20.American Lung Association. State of Tobacco Control 2022: 20 Years Proven Policies to Prevent and Reduce Tobacco Use. Chicago, IL: American Lung Association; January 26 2022. [Google Scholar]
- 21.Drope J, Liber AC, Cahn Z, et al. Who’s still smoking? Disparities in adult cigarette smoking prevalence in the United States. CA Cancer J Clin. 2018;68(2):106–115. 10.3322/caac.21444. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Tips from Former Smokers. Real Stories. https://www.cdc.gov/tobacco/campaign/tips/stories/index.html. Published 2022. Updated September 5. Accessed November 18, 2022.
- 23.Kong G, Singh N, Krishnan-Sarin S. A Review of Culturally Targeted/Tailored Tobacco Prevention and Cessation Interventions for Minority Adolescents. Nicotine Tob Res. 2012;14(12):1394–1406. 10.1093/ntr/nts118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bedard K, Desch, xea, nes O. The Long-Term Impact of Military Service on Health: Evidence from World War II and Korean War Veterans. Am Econ Rev. 2006;96(1):176–194. 10.1257/000282806776157731. [DOI] [PubMed] [Google Scholar]
- 25.Rumery ZR, Patel N, Richard P. The Association Between the Use of the Education Benefits from the G.I. Bill and Veterans’ Health. Mil Med. 2018;183(5–6):e241–e248. 10.1093/milmed/usx102. [DOI] [PubMed] [Google Scholar]
- 26.Farrelly MC, Nonnemaker J, Davis KC, Hussin A. The Influence of the National truth® Campaign on Smoking Initiation. Am J Prev Med. 2009;36(5):379–384. 10.1016/j.amepre.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 27.Jeon J, Holford TR, Levy DT, et al. Smoking and Lung Cancer Mortality in the United States From 2015 to 2065: A Comparative Modeling Approach. Ann Intern Med. 2018;169(10):684–693. 10.7326/M18-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holford TR, Levy DT, McKay LA, et al. Patterns of birth cohort-specific smoking histories, 1965–2009. Am J Prev Med. 2014;46(2):e31–e37. 10.1016/j.amepre.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.U.S. Census Bureau. CPS Historical Time Series Tables. https://www.census.gov/data/tables/time-series/demo/educational-attainment/cps-historical-time-series.html. Published 2022. Updated February 24. Accessed November 16, 2022.
- 30.Migration Policy Institute. U.S. Immigration Trends https://www.migrationpolicy.org/programs/data-hub/us-immigration-trends. Published 2022. Accessed October 3, 2022.
- 31.Centers for Disease Control and Prevention. Health, United States, 2020–2021: Births. https://www.cdc.gov/nchs/hus/topics/births.htm. Published 2022. Updated August 12. Accessed October 3, 2022.
- 32.Pew Research Center. Key facts about U.S. Latinos for National Hispanic Heritage Month https://www.pewresearch.org/fact-tank/2022/09/23/key-facts-about-u-s-latinos-for-national-hispanic-heritage-month/. Published 2022. Updated September 23. Accessed October 3, 2022.
- 33.Holford TR, Levy DT, Meza R. Comparison of Smoking History Patterns Among African American and White Cohorts in the United States Born 1890 to 1990. Nicotine Tob Res. 2016;18(Suppl 1):S16–S29. 10.1093/ntr/ntv274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinsky PF. Racial and ethnic differences in lung cancer incidence: how much is explained by differences in smoking patterns? (United States). Cancer Causes Control. 2006;17(8):1017–1024. 10.1007/s10552-006-0038-2. [DOI] [PubMed] [Google Scholar]
- 35.Tammemagi CM, Pinsky PF, Caporaso NE, et al. Lung Cancer Risk Prediction: Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial Models and Validation. J Natl Cancer Inst. 2011;103(13):1058–1068. 10.1093/jnci/djr173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stram DO, Park SL, Haiman CA, et al. Racial/Ethnic Differences in Lung Cancer Incidence in the Multiethnic Cohort Study: An Update. J Natl Cancer Inst. 2019;111(8):811–819. 10.1093/jnci/djy206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haiman CA, Stram DO, Wilkens LR, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med. 2006;354(4):333–342. 10.1056/NEJMoa033250. [DOI] [PubMed] [Google Scholar]
- 38.Meza R, Jeon J, Toumazis I, et al. Evaluation of the Benefits and Harms of Lung Cancer Screening With Low-Dose Computed Tomography: Modeling Study for the US Preventive Services Task Force. JAMA. 2021;325(10):988–997. 10.1001/jama.2021.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams RM, Li T, Luta G, et al. Lung cancer screening use and implications of varying eligibility criteria by race and ethnicity: 2019 Behavioral Risk Factor Surveillance System data. Cancer. 2022;128(9):1812–1819. 10.1002/cncr.34098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryan BM. Lung cancer health disparities. Carcinogenesis. 2018;39(6):741–751. 10.1093/carcin/bgy047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aldrich MC, Mercaldo SF, Sandler KL, Blot WJ, Grogan EL, Blume JD. Evaluation of USPSTF Lung Cancer Screening Guidelines Among African American Adult Smokers. JAMA Oncol. 2019;5(9):1318–1324. 10.1001/jamaoncol.2019.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tammemägi MC, Katki HA, Hocking WG, et al. Selection criteria for lung-cancer screening. N Engl J Med. 2013;368(8):728–736. 10.1056/NEJMoa1211776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.U.S. Preventive Services Task Force. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(10):962–970. 10.1001/jama.2021.1117. [DOI] [PubMed] [Google Scholar]
- 44.Cadham CJ, Cao P, Jayasekera J, et al. Cost-Effectiveness of Smoking Cessation Interventions in the Lung Cancer Screening Setting: A Simulation Study. J Natl Cancer Inst. 2021. 10.1093/jnci/djab002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Criss SD, Cao P, Bastani M, et al. Cost-Effectiveness Analysis of Lung Cancer Screening in the United States: A Comparative Modeling Study. Ann Intern Med. 2019;171(11):796–804. 10.7326/M19-0322. [DOI] [PubMed] [Google Scholar]
- 46.Tam J, Levy DT, Jeon J, et al. Projecting the effects of tobacco control policies in the USA through microsimulation: a study protocol. BMJ Open. 2018;8(3). 10.1136/bmjopen-2017-019169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levy DT, Borland R, Lindblom EN, et al. Potential deaths averted in USA by replacing cigarettes with e-cigarettes. Tob Control. 2018;27(1):18–25. 10.1136/tobaccocontrol-2017-053759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Apelberg BJ, Feirman SP, Salazar E, et al. Potential Public Health Effects of Reducing Nicotine Levels in Cigarettes in the United States. N Engl J Med. 2018;378(18):1725–1733. 10.1056/NEJMsr1714617. [DOI] [PubMed] [Google Scholar]
- 49.Mendez D, Le TTT. Consequences of a match made in hell: the harm caused by menthol smoking to the African American population over 1980–2018. Tob Control. 2022;31(4):569–571. 10.1136/tobaccocontrol-2021-056748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moolgavkar SH, Holford TR, Levy DT, et al. Impact of reduced tobacco smoking on lung cancer mortality in the United States during 1975–2000. J Natl Cancer Inst. 2012;104(7):541–548. 10.1093/jnci/djs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levy DT, Tam J, Sanchez-Romero LM, et al. Public health implications of vaping in the USA: the smoking and vaping simulation model. Popul Health Metr. 2021;19(1):19. 10.1186/s12963-021-00250-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.IOM (Institute of Medicine). Health Implications of Raising the Minimum Age for Purchasing Tobacco Products. Washington, DC: The National Academies Press; 2015. [PubMed] [Google Scholar]
- 53.de Koning HJ, Meza R, Plevritis SK, et al. Benefits and Harms of Computed Tomography Lung Cancer Screening Strategies: A Comparative Modeling Study for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160(5):311‒320. 10.7326/m13-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meza R, ten Haaf K, Kong CY, et al. Comparative analysis of 5 lung cancer natural history and screening models that reproduce outcomes of the NLST and PLCO trials. Cancer. 2014;120(11):1713–1724. 10.1002/cncr.28623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McMahon PM, Meza R, Plevritis SK, et al. Comparing benefits from many possible computed tomography lung cancer screening programs: extrapolating from the National Lung Screening Trial using comparative modeling. PLoS One. 2014;9(6):e99978. 10.1371/journal.pone.0099978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cao P, Jeon J, Meza R. Evaluation of benefits and harms of adaptive screening schedules for lung cancer: A microsimulation study. J Med Screen. 2022:9691413221118194. 10.1177/09691413221118194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toumazis I, de Nijs K, Cao P, et al. Cost-effectiveness Evaluation of the 2021 US Preventive Services Task Force Recommendation for Lung Cancer Screening. JAMA Oncol. 2021;7(12):1833–1842. 10.1001/jamaoncol.2021.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han SS, Ten Haaf K, Hazelton WD, et al. The impact of overdiagnosis on the selection of efficient lung cancer screening strategies. Int J Cancer. 2017;140(11):2436–2443. 10.1002/ijc.30602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meza R, Cao P, Jeon J, et al. Impact of Joint Lung Cancer Screening and Cessation Interventions Under the New Recommendations of the U.S. Preventive Services Task Force. J Thorac Oncol. 2022;17(1):160–166. 10.1016/j.jtho.2021.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cao P, Smith L, Mandelblatt JS, et al. Cost-Effectiveness of a Telephone-Based Smoking Cessation Randomized Trial in the Lung Cancer Screening Setting. JNCI Cancer Spectr. 2022;6(4). 10.1093/jncics/pkac048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tam J, Warner KE, Zivin K, Taylor GMJ, Meza R. The Potential Impact of Widespread Cessation Treatment for Smokers With Depression. Am J Prev Med. 2021. 10.1016/j.amepre.2021.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tam J, Taylor GMJ, Zivin K, Warner KE, Meza R. Modeling smoking-attributable mortality among adults with major depression in the United States. Prev Med. 2020:106241. 10.1016/j.ypmed.2020.106241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thun MJ, Carter BD, Feskanich D, et al. 50-year trends in smoking-related mortality in the United States. N Engl J Med. 2013;368(4):351–364. 10.1056/NEJMsa1211127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Inoue-Choi M, Christensen CH, Rostron BL, et al. Dose-Response Association of Low-Intensity and Nondaily Smoking With Mortality in the United States. JAMA Netw Open. 2020;3(6):e206436. 10.1001/jamanetworkopen.2020.6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.U.S. Department of Health and Human Services. The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. In. Atlanta, GA. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014. [Google Scholar]
- 66.Boudreau G, Hernandez C, Hoffer D, et al. Why the World Will Never Be Tobacco-Free: Reframing “Tobacco Control” Into a Traditional Tobacco Movement. Am J Public Health. 2016;106(7):1188–1195. 10.2105/AJPH.2016.303125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Clayton JA, Tannenbaum C. Reporting Sex, Gender, or Both in Clinical Research? JAMA. 2016;316(18):1863–1864. 10.1001/jama.2016.16405. [DOI] [PubMed] [Google Scholar]
- 68.Meza R, Cao P, Jeon J, et al. Patterns of Birth Cohort-Specific Smoking Histories by Race and Ethnicity in the US. Am J Prev Med. 2023;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cao P, Jeon J, Tam J, et al. Smoking Disparities by Level of Educational Attainment and Birth Cohort in the US. Am J Prev Med. 2023;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.U.S. Census Bureau. How the Census Bureau Measures Poverty. https://www.census.gov/topics/income-poverty/poverty/guidance/poverty-measures.html. Published 2022. Updated September 22. Accessed December 1, 2022.
- 71.Jeon J, Cao P, Fleischer NL, et al. Birth Cohort-Specific Smoking Patterns by Family Income in the US. Am J Prev Med. 2023;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Holford TR, McKay L, Jeon J, et al. Smoking Histories by State in the US. Am J Prev Med. 2023;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jeon J, Inoue-Choi M, Mok Y, et al. Relative Risk of All-Cause Mortality by Smoking Status, Race/Ethnicity, and Education in the United States. Am J Prev Med. 2023;Under Review for Supplement Inclusion. [Google Scholar]
- 74.Manuel DG, Wilton AS, Bennett C, Rohit Dass A, Laporte A, Holford TR. Smoking patterns based on birth-cohort-specific histories from 1965 to 2013, with projections to 2041. Health Rep. 2020;31(11):16–31. 10.25318/82-003-x202001100002-eng. [DOI] [PubMed] [Google Scholar]
- 75.Malta DC, Gomes CS, Andrade FMDd, et al. Tobacco use, cessation, secondhand smoke and exposure to media about tobacco in Brazil: results of the National Health Survey 2013 and 2019. Rev Bras Epidemiol. 2021;24. 10.1590/1980-549720210006.supl.2. [DOI] [PubMed] [Google Scholar]
- 76.Tam J, Jaffri M, Mok Y, et al. Patterns of Birth Cohort-Specific Smoking Histories in Brazil. Am J Prev Med. 2023;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tam J, Jeon J, Thrasher JF, et al. Estimated Prevalence of Smoking and Smoking-Attributable Mortality Associated with Graphic Health Warnings on Cigarette Packages in the US From 2022 to 2100. JAMA Health Forum. 2021;2(9):e212852. 10.1001/jamahealthforum.2021.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smith CE, Hill SE, Amos A. Impact of population tobacco control interventions on socioeconomic inequalities in smoking: a systematic review and appraisal of future research directions. Tob Control. 2021;30(e2):e87–e95. 10.1136/tobaccocontrol-2020-055874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Colston DC, Xie Y, Patrick ME, et al. Tobacco 21 laws may reduce smoking and tobacco-related health disparities among youth in the U.S. Prev Med Rep. 2022;27:101762. 10.1016/j.pmedr.2022.101762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Colston DC, Xie Y, Thrasher JF, et al. Examining Truth and State-Sponsored Media Campaigns as a Means of Decreasing Youth Smoking and Related Disparities in the United States. Nicotine Tob Res. 2021;24(4):469–477. 10.1093/ntr/ntab226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Titus AR, Xie Y, Thrasher JF, et al. A longitudinal analysis of smoke-free laws and smoking initiation disparities among young adults in the United States. Addiction. 2022;117(3):730–738. 10.1111/add.15658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Titus AR, Xie Y, Colston DC, et al. Smoke-Free Laws and Disparities in Youth Smoking in the U.S., 2001–2018. Am J Prev Med. 2021;61(6):841–851. 10.1016/j.amepre.2021.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kalousova L, Levy D, Titus AR, et al. Cigarette taxes, prices, and disparities in current smoking in the United States. SSM Popul Health. 2020;12:100686. 10.1016/j.ssmph.2020.100686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Titus AR, Kalousova L, Meza R, et al. Smoke-Free Policies and Smoking Cessation in the United States, 2003–2015. Int J Environ Res Public Health. 2019;16(17):3200. 10.3390/ijerph16173200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Donahoe JT, Norton EC, Elliott MR, Titus AR, Kalousová L, Fleischer NL. The Affordable Care Act Medicaid Expansion and Smoking Cessation Among Low-Income Smokers. Am J Prev Med. 2019;57(6):e203–e210. 10.1016/j.amepre.2019.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brown T, Platt S, Amos A. Equity impact of interventions and policies to reduce smoking in youth: systematic review. Tob Control. 2014;23(e2):e98–e105. 10.1136/tobaccocontrol-2013-051451. [DOI] [PubMed] [Google Scholar]
- 87.Brown T, Platt S, Amos A. Equity impact of population-level interventions and policies to reduce smoking in adults: A systematic review. Drug Alcohol Depend. 2014;138:7–16. 10.1016/j.drugalcdep.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 88.Parks MJ, Patrick ME, Levy DT, Thrasher JF, Elliott MR, Fleischer NL. Tobacco Taxation and Its Prospective Impact on Disparities in Smoking Initiation and Progression Among Young Adults. J Adolesc Health. 2021;68(4):765–772. 10.1016/j.jadohealth.2020.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nanninga S, Lehne G, Ratz T, Bolte G. Impact of Public Smoking Bans on Social Inequalities in Children’s Exposure to Tobacco Smoke at Home: An Equity-Focused Systematic Review. Nicotine Tob Res. 2019;21(11):1462–1472. 10.1093/ntr/nty139. [DOI] [PubMed] [Google Scholar]


