Abstract
Increasing the proportion of locally produced plant protein in currently meat-rich diets could substantially reduce greenhouse gas emissions and loss of biodiversity1. However, plant protein production is hampered by the lack of a cool-season legume equivalent to soybean in agronomic value2. Faba bean (Vicia faba L.) has a high yield potential and is well suited for cultivation in temperate regions, but genomic resources are scarce. Here, we report a high-quality chromosome-scale assembly of the faba bean genome and show that it has expanded to a massive 13 Gb in size through an imbalance between the rates of amplification and elimination of retrotransposons and satellite repeats. Genes and recombination events are evenly dispersed across chromosomes and the gene space is remarkably compact considering the genome size, although with substantial copy number variation driven by tandem duplication. Demonstrating practical application of the genome sequence, we develop a targeted genotyping assay and use high-resolution genome-wide association analysis to dissect the genetic basis of seed size and hilum colour. The resources presented constitute a genomics-based breeding platform for faba bean, enabling breeders and geneticists to accelerate the improvement of sustainable protein production across the Mediterranean, subtropical and northern temperate agroecological zones.
Subject terms: Plant genetics, Natural variation in plants, Plant breeding, Plant evolution, Genome evolution
Using a high-quality chromosome-scale assembly of the faba bean genome, the genetic basis of seed size and hilum colour is explored.
Main
Faba bean (Vicia faba L., 2n = 12) was domesticated in the near East more than 10,000 years bp3,4 and its broad adaptability, value as a restorative crop in rotations and high nutritional density have propelled it to the status of a global crop grown on all continents except Antarctica5. Despite its global importance, no extant wild progenitor has been found. Nonetheless, the finding of Neolithic charred wild faba bean seeds points to pre-domestication use of this species by hunter–gatherers and possible domestication in the Levant3. The presence of several closely related species (Vicia narbonensis, Vicia palaestina and Vicia kalakhensis) in the same region6 gives hope that a wild progenitor may yet be found. Faba bean exhibits such extreme variation in seed size that some taxonomists defined the primitive, small-seeded ‘paucijuga’ forms7 or small-seeded ‘minor’ forms8 as separate subspecies from the medium–large ‘faba’ types. However, the absence of reproductive barriers between any of these forms means that ‘major’, ‘minor’, ‘equina’ and ‘paucijuga’ forms are now regarded as botanical types resulting from sustained human selection on growth habit and seed size over many thousands of years9. Faba bean continues to be relevant in the twenty-first century as humanity strives to lower agricultural greenhouse gas emissions by replacing meat or milk protein with plant-based alternatives10. It is the highest yielding of all grain legumes11 and has a favourable protein content (approximately 29%) compared with other cool-season pulses such as pea, lentil and chickpea, making it a suitable candidate to meet challenging projected future protein demands. Furthermore, the high biological nitrogen fixation rates of faba bean12 and the long duration of nectar-rich, pollinator-friendly flowers13 provide important ecosystem services, which means that cultivation of faba bean is increasingly seen as key for sustainable intensification strategies. Conversely, its partially allogamous mating system and estimated 13-Gb genome size, coupled with a low seed multiplication rate, have made it a challenging target for breeders14. Substantial progress has been made in faba bean genomics and pre-breeding research. The mining of the first faba bean transcriptomes and development of single-nucleotide polymorphism (SNP)-based genetic maps, which showed strong collinearity with model legumes, set the scene for the identification of the WD40 transcription factor underlying the Zero Tannin1 locus15, whereas a combination of high-resolution mapping, transcriptomic and metabolomic approaches led to the cloning of the VC1 gene, which controls seed content of the antinutrients vicine and convicine, paving the way for safer exploitation of the crop in the human food chain16. However, the lack of a reference genome sequence greatly complicated these studies, and improved faba bean genomic resources are urgently needed to accelerate crop improvement.
Sequence of the giant faba bean genome
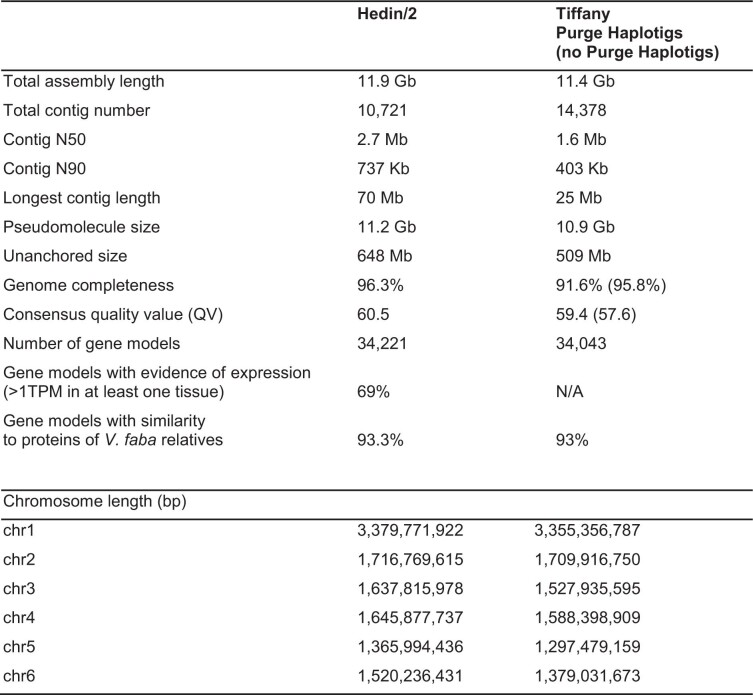
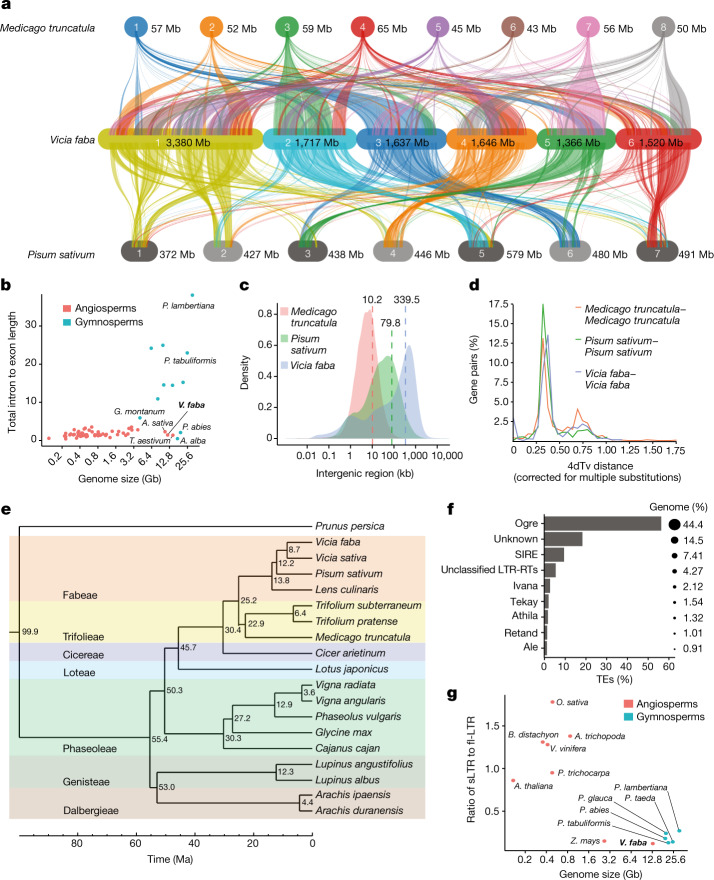
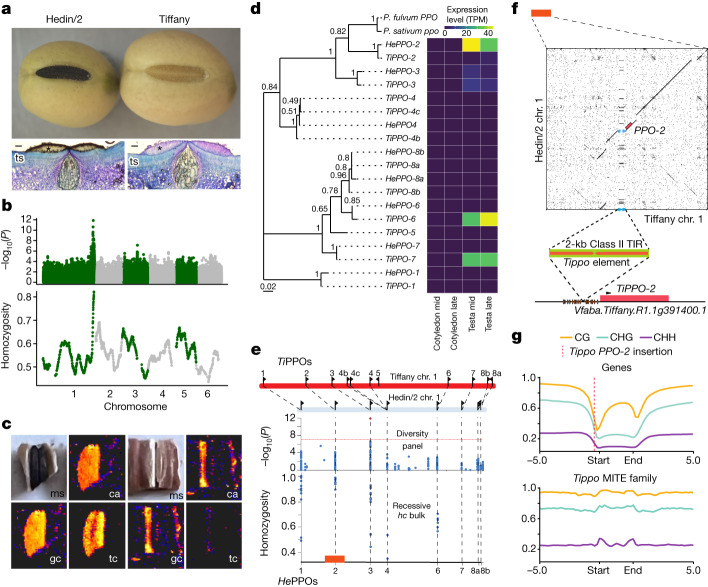
The 13-Gb faba bean genome (2n = 2x = 12) is one of the largest diploid field crops (Extended Data Fig. 1a,b) and its dominant repeat family members are longer17,18 (up to 25 kb) than those in similarly sized polyploid cereal genomes19. The biggest of its six chromosomes holds the equivalent of an entire human genome. Although aiding cytogenetics20, these properties made genome assembly very challenging before the emergence of long and accurate reads. We chose the inbred line ‘Hedin/2’ as a reference genotype owing to its high autofertility and productivity, combined with an early maturing spring habit and exceptional degree of homozygosity. We sequenced its genome with PacBio HiFi long reads to 20-fold coverage and assembled 11.9 Gb of sequence, more than half of which was represented by contigs longer than 2.7 Mb (Extended Data Table 1). Linkage information afforded by a genetic map (Supplementary Table 1) and chromosome conformation capture sequencing (Hi-C) data placed 11.2 Gb (94%) into chromosomal pseudomolecules (Fig. 1a and Supplementary Fig. 1a). Chromatin immunoprecipitation sequencing for centromeric histone H3 pinpointed the locations of the centromeres in the Hedin/2 assembly, and arm ratios were consistent with karyotypes (Supplementary Fig. 1b). The single metacentric chromosome 1 was the only one to adopt a Rabl configuration, evident from the presence of both a main and an anti-diagonal on that chromosome in Hi-C interaction plots (Fig. 1a). This supports the notion that chromosome arms need to be of approximately equal size to spatially juxtapose in interphase. Some regions of the Hi-C contact matrices were empty for lack of mapped short reads (Fig. 1a). These white regions coincided with the locations of enormous (up to 752 Mb) satellite arrays and aligned well with cytological maps of those repeats (Fig. 1b,c). Assembly evaluation with Merqury21 revealed the genome to be 96.3% complete, with a consensus quality value of 60.5, indicating a high accuracy of our Hedin/2 assembly (Extended Data Table 1 and Extended Data Fig. 1c). A good collinear agreement between the genetic and physical maps further validated the accurate assignment of contigs to chromosomes (Supplementary Fig. 1a). In addition, the long terminal repeat (LTR) assembly index score of 10.5 supports the contiguity of our assembly. We also collected HiFi data (tenfold coverage) for the German cultivar ‘Tiffany’ and assembled these into a set of contigs with an N50 of 1.6 Mb, spanning 11.4 Gb (Extended Data Table 1). Similar to Hedin/2, Merqury assessments supported the high quality of the Tiffany assembly (Extended Data Table 1 and Extended Data Fig. 1d). This level of completeness and contiguity was sufficient to arrange the contigs into pseudomolecules guided by the Hedin/2 reference (Extended Data Table 1 and Extended Data Fig. 2). In the future, the Hedin/2 assembly is expected to become the nucleus of a faba pan-genome.
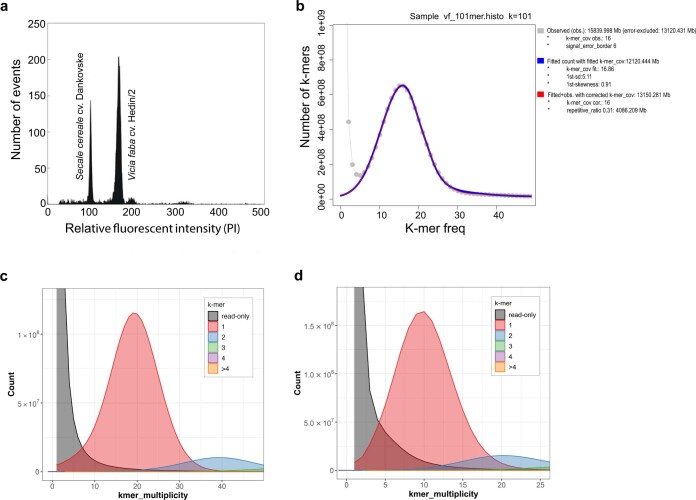
Extended Data Fig. 1. K-mer and flow-cytometry based genome size estimation.
a, Estimation of nuclear genome size of V. faba Hedin/2 using flow cytometry. The histogram of relative nuclear DNA content was obtained after flow cytometric analysis of propidium-iodide stained nuclei of V. faba ‘Hedin/2’ and Secale cereale cv. Dankovske (2C = 16.19 pg), which served as the internal reference standard. The low level threshold was set to channel 20 on a 512-channel scale histogram of relative fluorescence intensity; all remaining fluorescence events were recorded. 2C nuclear DNA content of V. faba Hedin/2 was estimated at 26.36 pg (± 0.26 s.d.). b, k-mer based estimation of genome size using Hedin/2 raw HiFi sequencing data. c, k-mer spectrum plot produced by Merqury for the Hedin/2 genome assembly. K-mer multiplicity refers to the number of times a k-mer is found in sequencing reads. Colour corresponds to the number of times a k-mer is found in the assembly. d, k-mer spectrum plot produced by Merqury for the Tiffany genome assembly.
Extended Data Table 1.
Genome assembly and annotation statistics
Summary of genome assembly and annotation statistics for V. faba Hedin/2 and Tiffany genomes.
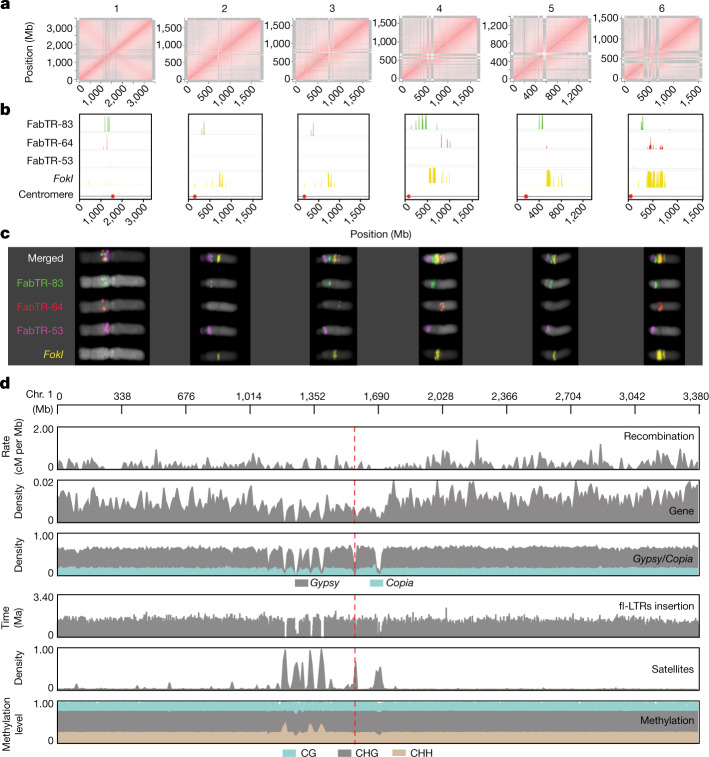
Fig. 1. Gigabase-size chromosome-scale assembly of faba bean.
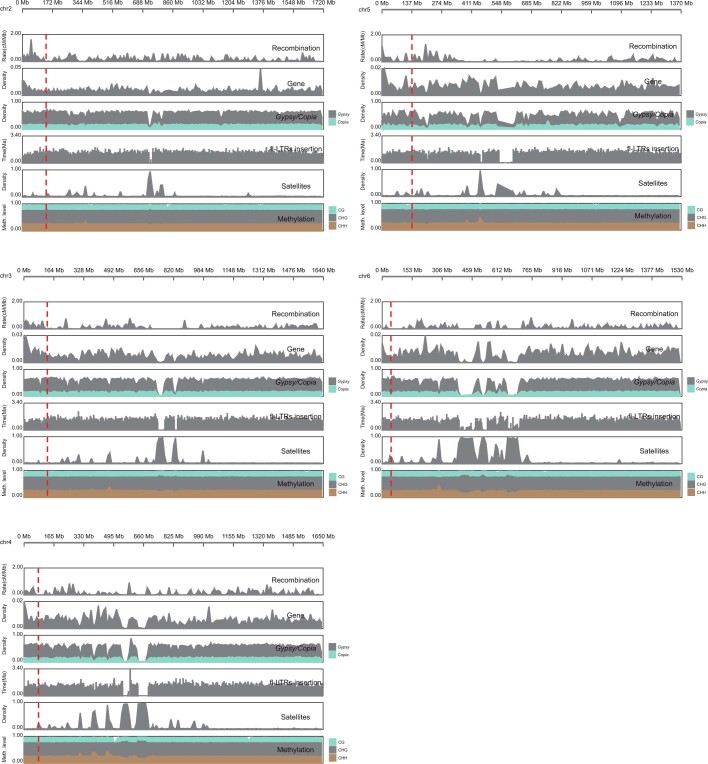
a, Intrachromosomal contact matrix of assembled chromosomes. The red colour intensity indicates the normalized Omni-C Hi-C links between 1-Mb windows on each chromosome. The antidiagonal pattern in chromosome 1 represents the Rabl configuration. b, Distribution of major families of satellite repeats (FabTR-83 in green, FabTR-64 in red, FabTR-53 in magenta and FokI in yellow). c, Distribution of major families of satellite repeats on metaphase chromosomes visualized by multicolour fluorescent in situ hybridization. d, Distribution of genomic components including recombination (cM per Mb), gene density, LTR retrotransposons of Gypsy and Copia, full-length LTR-retrotransposon (fl-LTR) insertions, satellite repeats and DNA methylation (CH, CHG and CHH context) on chromosome 1. The red dashed line represents the centromere position.
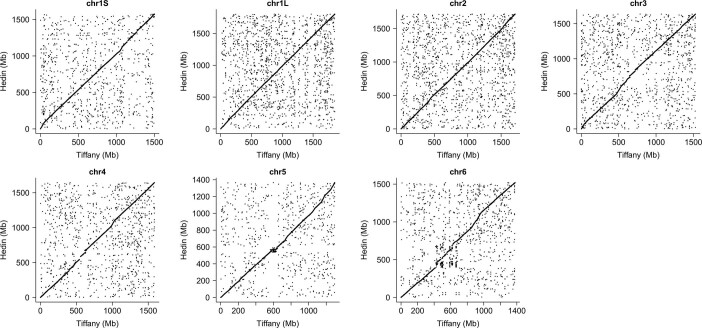
Extended Data Fig. 2. Hedin/2 and Tiffany chromosome alignments.
Dot plots showing alignments between Hedin/2 and Tiffany chromosomes.
Driving forces of genome size expansion
The genome sequence of Hedin/2 was annotated with RNA sequencing data from nine diverse tissues (Supplementary Table 2), resulting in a total of 34,221 protein-coding genes (Supplementary Table 3). A similar number of gene models (34,043) was also predicted in the Tiffany assembly. The predicted Hedin/2 gene models captured 96% of single-copy orthologues conserved in Embryophyta according to the BUSCO metric (Supplementary Table 4). Gene density was uniform along the chromosomes (except for the positions of satellite DNA arrays) without the proximal–distal gradient typically observed for grass chromosomes22. Meiotic recombination displayed a similar distribution with an average of 27 genes per centimorgan (Fig. 1d and Extended Data Fig. 3). Thus, despite its large genome, faba bean may be more amenable to genetic mapping than cereals, in which up to one-third of genes are locked in non-recombining pericentric regions22. Gene order was highly collinear and syntenic with other legumes (Fig. 2a). To further validate gene annotation, we aligned 262 Medicago truncatula genes related to symbiosis with rhizobia or arbuscular mycorrhizal fungi and found putative orthologues for them all. In addition, using RNA sequencing, we verified that a large subset of these genes was responsive to inoculation, as expected23–25 (Supplementary Table 5).
Extended Data Fig. 3. Genomic features of Hedin/2 chromosomes 2–6.
Distribution of genomic components including recombination (cM/Mb), gene density, retrotransposons of Gypsy and Copia superfamilies, full-length (FL) LTR insertions, satellite repeats and DNA methylation (CH, CHG and CHH context) on chromosomes 2 to 6.
Fig. 2. Evolution and synteny analysis in faba bean.
a, Syntenic relationship of faba bean (middle) with Medicago (top) and pea (bottom). b, Intron versus exon lengths across angiosperms and gymnosperms. A. alba, Abies Alba; A. sativa, Avena sativa; G. montanum, Geum montanum; P. abies, Picea abies; P. lambertiana, Pinus lambertiana; P. tabuliformis, Pinus tabuliformis; T. aestivum, Triticum aestivum. c, Lengths of legume intergenic regions. d, Distribution of the transversion rates at the fourfold degenerate sites (4dTv) of paralogous gene pairs. e, Phylogenetic relationships between faba bean and other crop legumes in the Papilionoideae clade. The numbers on the branches indicate the estimated divergence time (Ma). f, Summary of the faba bean retrotransposon composition by family. g, Ratio of sLTR to fl-LTR plotted against genome size in gymnosperms and angiosperms. The ratio for other species was retrieved from ref. 32. A. trichopoda, Amborella trichopoda; B. distachyon, Brachypodium distachyon; O. sativa, Oryza sativa; P. abies, Picea abies; P. glauca, Picea glauca; P. taeda, Pinus taeda; P. trichocarpa, Populus trichocarpa; V. vinifera, Vitis vinifera; Z. mays, Zea mays.
In contrast to gymnosperms, with similarly gigantic genomes26,27, introns in faba bean genes were not larger than in angiosperms with smaller genomes (Fig. 2b), but the intergenic space was more expanded (Fig. 2c). Moreover, the number of multicopy gene families in faba bean was similar to related diploid species (Supplementary Table 6 and Supplementary Fig. 2), in contrast to soybean, which is considered a partially diploidized tetraploid28. Likewise, nucleotide substitution rates between paralogous and orthologous gene pairs place the last whole-genome duplication (WGD) event in the faba bean lineage at 55 million years ago (Ma), well before the split from other Papilionoideae29 (Fig. 2e and Supplementary Fig. 3), a taxon that also includes pea and lentil (Lens culinaris), species from which faba bean diverged around 12.2 and 13.8 Ma, respectively. Although we did not find evidence for a recent WGD in faba bean, more genes were duplicated in tandem than in pea and lentil (Supplementary Fig. 4a). These duplications post-date the last WGD and occurred later than tandem duplications in Arabidopsis thaliana and M. truncatula (Supplementary Fig. 4b), two species whose genomes were also rich in such events and coincided with recent transposable element (TE) expansion. Overall, there were 1,108 syntenic clusters of tandemly duplicated genes in Hedin/2 and Tiffany, some of which differed in copy number. Of note, the agronomically relevant family of leghaemoglobins had expanded (Supplementary Table 7). Despite this, the absence of a lineage-specific WGD or widespread gene family expansion means that the proliferation of repeat elements largely explains why the faba genome is more than seven times larger than that of its close relative common vetch (Vicia sativa)30.
Approximately 79% of the Hedin/2 assembly was annotated as transposon-derived (Supplementary Table 8). By far, the largest group is the LTR retrotransposons (RLX), accounting for 63.7% of the genome sequence. Other groups of TEs represent only minor fractions of the genome (Supplementary Table 8). Among the RLX, those of the Gypsy (RLG) superfamily outnumber Copia (RLC) elements by more than 2:1 (Fig. 1d and Extended Data Fig. 3). The Ogre family of Gypsy elements alone make up almost half (44%) of the genome, confirming its status as a major determinant of genome size in the Fabaceae18 (Fig. 2f). The great length of individual elements (up to 35 kb for Ogre and 32 kb of SIRE, the longest and second-longest elements, respectively), together with their abundance, partially explains the large size of the faba bean genome (Supplementary Fig. 5). In addition, a large and diverse set of satellite repeat families that differ in their monomer sequences and genome abundance31 accounted for 9.4% of the total assembly length, with the most abundant satellite family FokI representing 4% (0.475 Gb). FokI, together with several other highly amplified satellites, forms prominent heterochromatic bands on faba bean chromosomes (Fig. 1c). The TE density was remarkably invariable along all six chromosomes, mirroring gene density and recombination rate, and inverse to the density of satellite arrays (Fig. 1d and Extended Data Fig. 3).
The persistence of retrotransposons as full-length copies can tell us about the balance between genome size expansion by retrotransposition and shrinkage by elimination through recombination. Modelling the solo-LTRs (sLTRs) as the product of the recombination between the LTRs of a single element, and assuming the canonical Ogre to comprise LTRs of 4,161 bp and an internal domain of 11,655 bp, the 395,657 sLTRs represent a loss of 6.26 Gb of DNA from the genome (55.6% of the current assembly size). This loss would be even greater if recombination between LTRs of different individual Ogre elements as well as DNA double-strand break repair-mediated internal truncations were considered. However, unlike plant species with smaller genomes, there were generally many fewer sLTRs in faba bean relative to the number of full-length LTRs, similar to large gymnosperm genomes (Fig. 2g), indicating slower removal than spreading of RLX32. The V. sativa genome of 1.65 Gb was earlier reported to comprise 22.5% of Ogre elements and to have 1.6 sLTRs for each full-length Ogre, considerably more than found for V. faba18.
Efficient genome-wide methylation
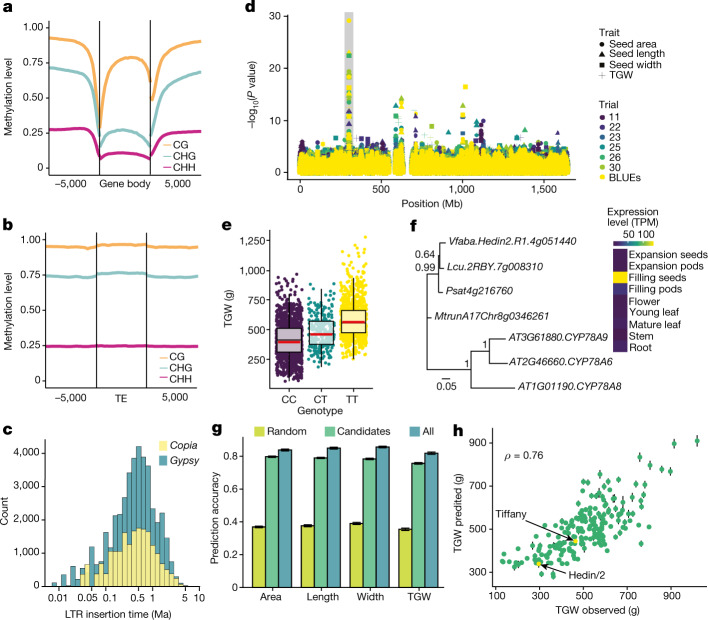
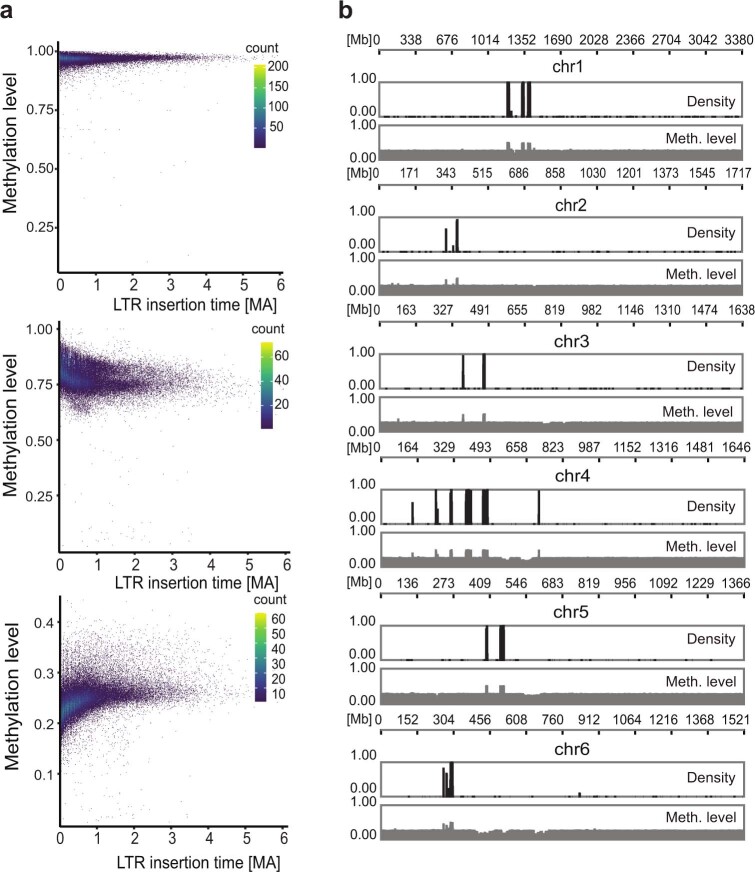
In addition to the relatively slow RLX elimination rate, it is also possible that lower levels of methylation could have accelerated TE proliferation through less efficient silencing. We found that most cytosines in the faba bean genomes were methylated: 95.8% in CG, 88.2% in CHG and 14% in CHH contexts (Fig. 1d and Extended Data Fig. 3), placing it among the most highly methylated plant genomes32. Gene body methylation followed the canonical pattern (Fig. 3a) observed in other plants33: CG methylation was enriched in internal exons and introns (Supplementary Fig. 6a), in contrast to low methylation in first exons, and may be related to transcriptional repression34. Genes with a high level of gene body methylation were more highly expressed in young leaf tissue (Supplementary Fig. 6b) and also tended to be longer (average 3.3 kb). The elements of the major superfamilies of RLX, Gypsy and Copia, occupied 48% and 11% of the genome, respectively. They were also heavily methylated, more so in their bodies than in their flanking regions (Fig. 3b). The most recent transposon burst occurred less than 1 Ma, but many structurally intact elements were between 3 and 5 million years old (Fig. 3c). Both young and old insertions were invariably methylated in all three sequence contexts (Extended Data Fig. 4a). In contrast to other plant taxa35, RLX insertion times and methylation levels were uncoupled. Conspicuous islands of elevated CHH methylation also coincided with the abundant satellite repeat FabTR-83 (Extended Data Fig. 4b), which accounts for 1.1% of the genome. Generally, the faba bean methylation machinery appeared fully functional, efficiently methylating all classes of repetitive elements, suggesting that methylation deficiency is unlikely to have a role in genome expansion. This is supported by investigation of genes involved in RNA-directed DNA methylation36, for which gene copy number in faba bean is similar to V. sativa, pea and lentil (Supplementary Table 9).
Fig. 3. TE methylation and seed size genetics.
a, Global distribution of DNA methylation levels at protein-coding genes including a 5-kb region upstream of the transcription start site and downstream of the transcription end site. b, DNA methylation patterns for TEs and their 5-kb flanking regions. c, Distribution of Copia (RLC) and Gypsy (RLG) retrotransposons based on insertion time. d, Combined Manhattan plot of GWAS analysis of seed area, seed width, seed length and thousand grain weight (TGW) for chromosome 4. BLUE, best linear unbiased estimator. e, Effect plot for TGW for the SNP marker within Vfaba.Hedin2.R1.4g051440 at position 299,823,118 on chromosome 4 (highlighted by a grey bar in part d). n = 2,499 data points distributed over six trials. In the box plots, the horizontal black central line represents the median, the red line indicates the mean, the box ranges from the first to third quartile, and the vertical black lines extend to the smallest or largest point within the 1.5× interquartile range. f, Phylogenetic tree showing the relationships between Vfaba.Hedin2.R1.4g051440 and its orthologues in pea, lentil, Medicago and Arabidopsis and its expression levels in nine diverse tissues of Hedin/2 in transcripts per million (TPM). The branch lengths are measured in the number of substitutions per site. Numbers next to branches indicate bootstrapping support. g, Genomic best linear unbiased prediction accuracies for seed traits using 15 randomly chosen SNPs (random), the 15 seed-size-associated markers (candidates) or all markers (all). h, Mean genomic best linear unbiased prediction of TGW using the 15 seed-size-associated SNPs plotted against the mean observed values. The Pearson correlation coefficient is indicated. Error bars indicate the standard deviation of a fivefold genomic best linear unbiased prediction cross-validation scheme with predicted values computed from ten replicate runs (g,h; see Methods).
Extended Data Fig. 4. Methylation landscape in faba bean.
a, Landscape of CG (top), CHG (middle) and CHH (bottom) methylation with different TE insertion ages. b, CHH methylation peaks on FabTR-83 satellite repeats.
Integration of QTL and variation data
The faba bean genome sequence provides a unified frame of reference for genetic mapping, gene expression profiling and comparative genomics. To assist the adoption of the new infrastructure among faba bean breeders and geneticists, we mapped markers from two commonly used genotyping platforms, the Illumina Infinium 1,536 SNP and the Illumina Oligo Pool Array assays. Moreover, we projected genetic maps of both different biparental crosses and derived consensus genetic maps onto the genome assembly. This provided physical coordinates to quantitative trait loci (QTLs) for disease resistance and phenology. Marker maps and QTL intervals can be browsed interactively at https://pulses.plantinformatics.io (Supplementary Fig. 7 and Supplementary Note 1).
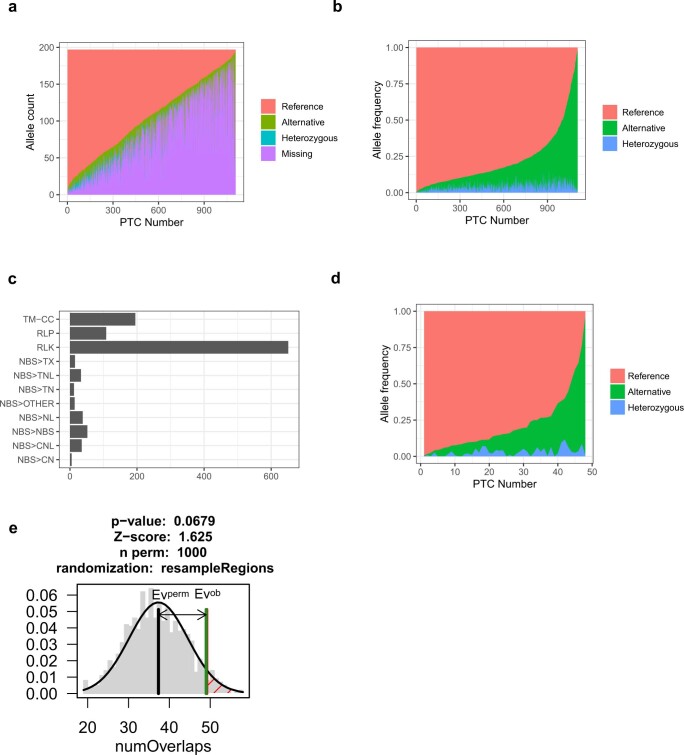
The genome sequence has also paved the way for sequence-based genotyping. We mined the Hedin/2 assembly for oligonucleotide probes for use in single primer enrichment technology (SPET)37, a reduced-representation genotyping method with high throughput and low per-sample costs. A panel of 197 cultivated accessions from a diversity collection designed for trait mapping was profiled with a 90,000 probe SPET assay with at least one probe in each predicted gene (Supplementary Table 10). Sequence reads were mapped to the Hedin/2 assembly and 1,081,031 segregating variants (SNPs) uniformly distributed along the genome were called. Analysing the functional impact of SNPs and short insertions and deletions (indels) found in genic regions, we identified 1,042 SNPs and 65 indels introducing a premature termination codon in at least one of 197 accessions. The premature termination codons interrupted transcripts of 933 genes, including 39 resistance gene analogues (Extended Data Fig. 5 and Supplementary Table 11). We provide a full atlas of genes and accessions carrying premature termination codons to facilitate functional studies (Supplementary Table 12).
Extended Data Fig. 5. Variants resulting in premature termination codons.
Variants (SNPs and short InDels) resulting in premature termination codons (PTCs) identified from SPET data. a, Distribution of allele counts including missing genotype calls. b, Distribution of allele frequencies excluding missing genotype calls. c, Summary of resistance gene analogues (RGAs) identified in the Hedin/2 genome annotation. d, Distribution of allele frequencies of variants resulting in PTC in RGAs. e, Permutation test for over-representation of PTCs in RGAs.
Genetics of seed size
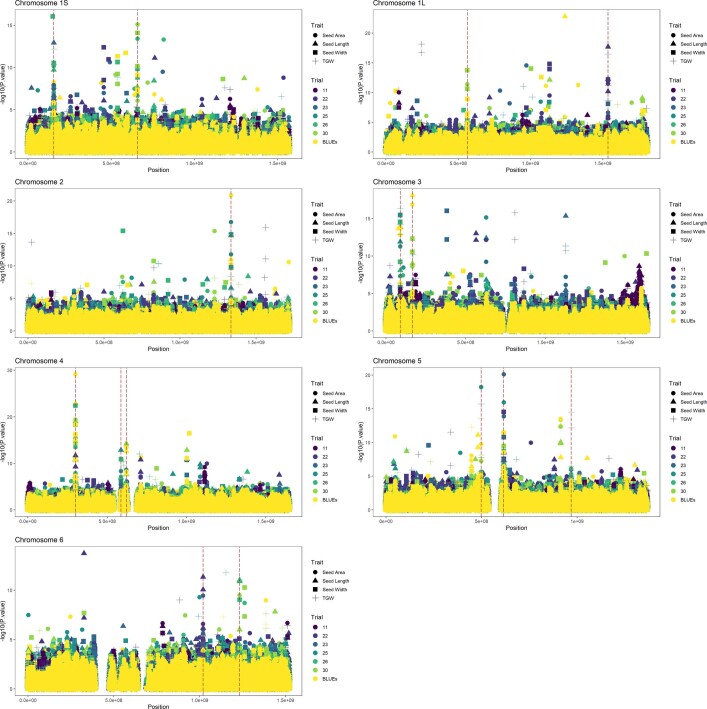
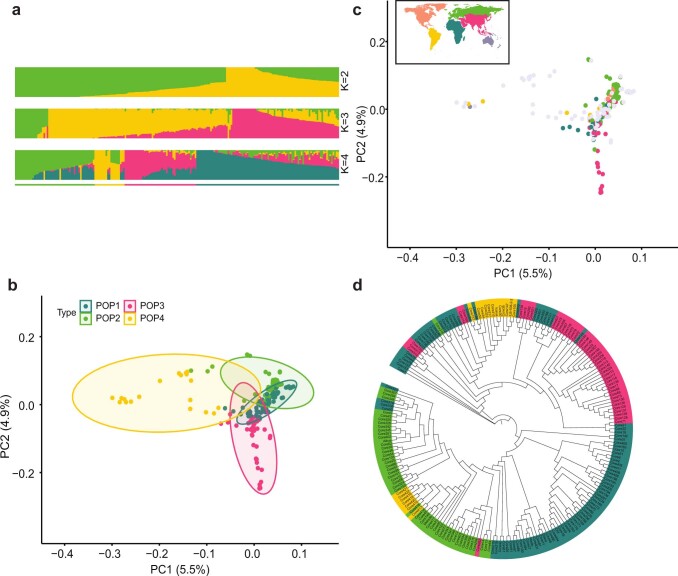
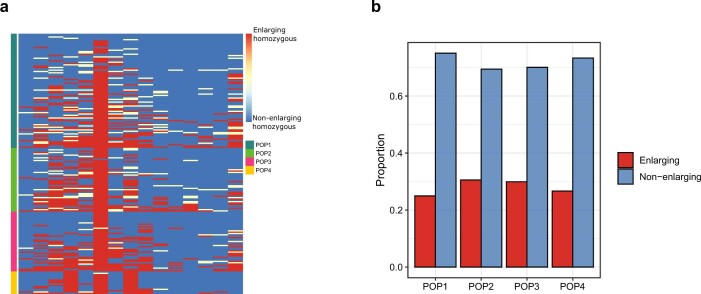
Despite impressive variation and critical agronomic importance, the genetics underlying faba bean seed size have remained obscure, with only a few large seed weight QTL regions detected38. We collected seed size data for the 197 accessions at two locations for 3 years and combined these with the SPET marker data to carry out a high-resolution genome-wide association study (GWAS). This identified 15 marker–trait associations, which were stable across trials and GWAS methods (Fig. 3d, Extended Data Fig. 6, Supplementary Figs. 8–11 and Supplementary Tables 13 and 14). The most prominent signal was located on chromosome 4 within the Vfaba.Hedin2.R1.4g051440 gene (Fig. 3d,e and Supplementary Fig. 12), which is highly expressed in faba bean seeds, resides within a previously identified seed weight QTL region38 (Supplementary Note 1) and is homologous to the Arabidopsis CYP78A genes known to regulate seed size39 (Fig. 3f). Vfaba.Hedin2.R1.4g051440 is thus likely to contribute to seed size variation in faba bean, but does not explain the majority of the variation for this complex trait (Fig. 3e). By contrast, using all 15 high-confidence markers, we were able to predict seed size with nearly as high accuracy as when using the full set of genomic markers (Fig. 3g,h), indicating that we have identified a large proportion of the key loci, and associated candidate genes, controlling faba bean seed size. To investigate whether seed size has been a driver of population differentiation, we carried out population structure analysis by model-based ancestry estimation, and principal component analysis divided the diversity panel into four groups, corresponding to their geographical origin (Extended Data Fig. 7). All populations had a similar proportion of seed-enlarging alleles and all seed-enlarging alleles were present in all populations, with the exception of population 4, which comprised relatively few accessions that all harboured the seed-enlarging allele of Vfaba.Hedin2.R1.4g051440 (Extended Data Fig. 8 and Supplementary Table 13). This allele distribution across populations suggests extensive historical sharing of germplasm by breeders across geographical regions.
Extended Data Fig. 6. Manhattan plots of seed size GWAS.
Seed traits are shown as shapes. Trials are shown as colours. BLUE - best linear unbiased estimator. Candidate SNPs are shown as dashed lines.
Extended Data Fig. 7. Genetic diversity and population structure.
a, Population structure grouped by ADMIXTURE. b, Principal component analysis (PCA) of 197 accessions grouped with regard to subpopulations. c, PCA of 197 accessions coloured based on geographic origin. An African cluster extends to South America, a European cluster spans North America, an Asian cluster and other clusters are consistent with the observed ADMIXTURE result. 113 accessions (57.36%) could be assigned to a specific group, and 84 were identified as admixed resulting from hybridization between two or more of the four groups found with the analysis based on the membership coefficient (>=0.6). d, Neighbour joining (NJ) tree showing relationships among the 197 accessions.
Extended Data Fig. 8. Seed enlarging alleles across populations.
a, distribution of seed enlarging alleles for 15 significantly associated SNPs (columns) across accessions and subpopulations (rows). b, Proportion of seed enlarging alleles in each subpopulation.
Hilum colour candidate-gene mapping
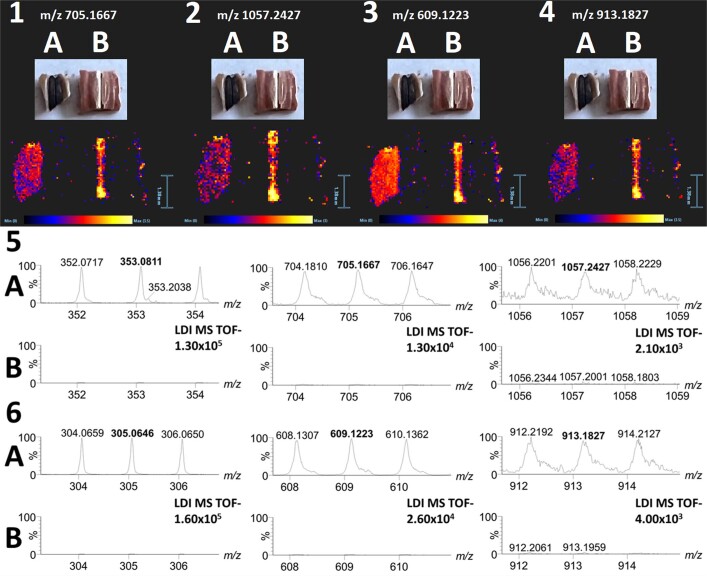
The two sequenced genotypes, Hedin/2 and Tiffany, differ not only in seed size but also in seed hilum colour (Figs. 3h and 4a). This is an important Mendelian quality trait, with pale hila being preferred by human consumers40. Similarly to seed size, no candidate genes have yet been identified. To reveal the molecular basis of the trait, we carried out a GWAS for hilum colour and identified a single prominent peak that was coincident both with the previously mapped Hilum Colour (HC) locus40 and peak homozygosity in a recessive pale hilum bulk of segregants from a cross between pale and dark hilum faba bean varieties (Fig. 4b). We found the most highly associated GWAS marker in a polyphenol oxidase (PPO) gene residing in a cluster of eight fully intact and highly conserved PPO genes in the Hedin/2 assembly. In pea, PPO variation controls hilum colour. At the syntenic PI locus, a frameshifted, non-functional form of the single PPO copy conferring a pale hilum is fixed in all modern pea varieties41. The pattern of pigmentation (Fig. 4a) and content of oligomeric phenolic compounds such as dimers and trimers of chlorogenic acid, gallocatechin and tetracosylcaffeate on the surface of pigmented and non-pigmented hila in faba bean (Fig. 4c and Extended Data Fig. 9) were very similar to those observed in pea40. Together with the genetic data, this indicates that differential PPO activity is responsible for hilum colour variation in both pea and faba bean, but it was unclear which faba bean PPO (or PPOs) may be causative.
Fig. 4. Rearrangements at the complex PPO locus give rise to changes in PPO gene expression and hilum colour.
a, Whole seeds of dark hilum Hedin/2 and pale hilum Tiffany are shown above light microscope images of a transverse section (ts) of the dark (left) and pale (right) hila. Asterisks indicate the counter palisade cells of the hilum where PPO activity is indicated by brown pigmentation. Scale bars, 20 µm. b, GWAS with hilum colour scored as a binary trait in the NORFAB diversity panel (top) and for homozygosity of pale hilum parent alleles in an 84-component recessive pseudo-F2 pale hilum bulk (bottom). c, Optical image of Hedin/2 (left) and Tiffany (right) hilum specimens subjected to laser desorption–ionization mass spectrometry imaging (ms), and the laser desorption–ionization mass spectrometry imaging signal distribution for chlorogenic acid (ca), epi-gallocatechin (gc) and tetracosylcaffeate (tc), showing absence of signal from these compounds from the hilum area of the pale hilum genotype. d, Phylogenetic tree showing the relationships between the causative pea gene and 8 and 11 PPO copies found in a tandem arrangement at the Hedin/2 and Tiffany HC locus, respectively. He and Ti prefixes denote Hedin/2- and Tiffany-specific versions of PPO paralogues. The branch lengths are measured in the number of substitutions per site. Numbers next to branches indicate bootstrapping support. e, From top to bottom: to-scale schematic of the chromosome 1 PPO cluster showing the order and orientation of PPOs in Tiffany and Hedin/2, with syntenic PPO copies joined by dashed lines; close-up of hilum colour associations in the NORFAB diversity panel and homozygosity in the pale hilum (hc) bulk are also shown. The red block shows the region expanded in the dot plot in panel f. f, Dot plot of 20 kb upstream and downstream of HePPO-2 (3,291,947,464) and TiPPO-2 (3,263,562,398) showing an approximately 2-kb MITE, named ‘Tippo’, inserted in Tiffany among predicted transcription factor-binding sites (brown ovals) in close proximity to the RNA polymerase II-binding site (TATA box is shown as a green oval) and transcription start site (arrowhead) of PPO-2. TIR, Terminal inverted repeat. g, Genome-wide methylation status of genes (top) compared with the Tippo MITE family (bottom).
Extended Data Fig. 9. Phenolic compounds in faba bean hila.
Laser desorption-ionisation mass spectrometry imaging (LDI-MSI) showing polymerization of phenolic compounds associated with dark hilum colour in faba bean. Distribution of dimer and trimer of chlorogenic acid (1 and 2) and gallocatechin (3 and 4) on the surface of pigmented (A, Hedin/2) and nonpigmented (B, Tiffany) hila. Zoomed spectra of monomer, dimer and trimer of chlorogenic acid (5; signals at m/z 353.0811; 705.1667 and 1057.2427) and gallocatechin (6; signals at m/z 305.0646, 609.1223, 913.1827) collected from pigmented (A, Hedin/2) and non-pigmented (B, Tiffany) hila (the spectra showing particular signals are zoomed on the same intensity for both genotypes, A and B, e.g. the intensity 1.30x105 is set for spectra 5A and 5B, etc.), spectra were collected from the compact surface without the hilar groove area and edges of the seed coat fragment. The identity of chlorogenic acid and gallocatechin dimers and trimers was further confirmed by characteristic fragments observed during related MS/MS experiment (i.e. anions of caffeic and chlorogenic acids cleaved from chlorogenic acid dimer and trimer at m/z 179.0341 and 353.0811, respectively, and products of retro-Diels Alder cleavage at m/z 179.0375 and 125.0232 from gallocatechin dimers and trimers, respectively).
To clarify, we compared the phylogeny and structure of the PPO clusters of the two fully sequenced genotypes Hedin/2 (dark) and Tiffany (pale). VfPPO-2 shared the highest level of identity with the causal pea gene Psat1g2063360 (Fig. 4d), whereas the most strongly associated GWAS marker was found in VfPPO-3, and the pale hilum bulk homozygosity peak was localized between VfPPO-2 and VfPPO-3, suggesting that the causal polymorphism resided at the proximal end of the cluster (Fig. 4e). Structurally, apart from large differences in intergenic distances between syntenic PPO genes caused mainly by Ogre insertions, the most striking features of the Hedin/2–Tiffany comparison were the triplication of VfPPO-4 in Tiffany and the absence of VfPPO-5 in Hedin/2 (Fig. 4e), prompting us to investigate whether these structural variations were associated with variation in PPO gene expression. We first established that transcription of the PPO gene cluster was almost exclusively confined to the maternal testa tissue (which encompasses the hilum), rather than the cotyledon in both genotypes (Fig. 4d, Supplementary Tables 15 and 16 and Supplementary Fig. 13). In Hedin/2 testa, VfPPO-2, and to a lesser extent VfPPO-3, accounted for nearly all PPO expression. By contrast, Tiffany testa PPO expression was dominated by VfPPO-6 and VfPPO-7 (Fig. 4d). A detailed annotation and comparative repeat analysis of the PPO cluster region (Supplementary Fig. 14) highlighted an approximately 2-kb AT-rich MITE insertion in the TiPPO-2 promoter region (Fig. 4f), which interrupts the sequence of the predicted VfPPO-2 promoter and belongs to a class of MITE associated with high levels of methylation (Fig. 4g). Together, our results suggest that regulation of expression of VfPPO-2 controls hilum colour variation in faba bean. Beyond suggesting a causative mechanism for pale hilum in faba bean, our analysis illustrates that increased copy number does not necessarily correlate with trait expression and emphasizes the utility of complete genome sequences from multiple genotypes.
Discussion
Faba bean is one of the earliest domesticated crops. It was part of the Neolithic package of crops that the early farmers took with them as they left the fertile crescent42. Concern about faba bean toxicity was voiced in classical antiquity43. In the twenty-first century, nutritional quality remains a central breeding goal: new faba bean varieties should be low in the alkaloid glycosides vicine and convicine as well as in tannins. Furthermore, essential amino acids should be balanced better to accommodate human dietary needs, whereas seed phytate and protease inhibitors should be reduced to improve nutrient bioavailability, all the while taking care not to alter seed size or compromise pest resistance and at the same time improving yield stability. Faba bean breeders can now face these complex challenges enabled by genomic resources and insights. Ubiquitous and frequent recombination will allow rapid introgression of new traits into elite material and permits powerful and broadly applicable mapping approaches exploiting the high SNP densities provided by SPET genotyping. Pinpointing causative variants can still be difficult in genomic regions with tandemly duplicated genes, but our investigation of hilum colour demonstrates that these challenges can be overcome using high-quality long-read assemblies coupled with transcriptomics. Repeats and their methylation influence genome evolution, but can also affect gene expression variation where the repeat elements insert within the regulatory regions of genes. Our rich genome-wide repeat annotation now sheds light on these effects, adding an important component to the genomics-based breeding platform. Faba bean appears to be an isolated species and does not hybridize with others in the genus Vicia38, effectively barring the use of wild relatives in faba bean breeding. However, stable Agrobacterium-mediated transformation of faba bean embryo axes has been reported44. Together with target gene identification, this opens up the possibility of gene editing. Expanding the platform further by cataloguing and exploiting as much of the segregating variation of domesticated faba bean as possible is especially important, as we do not know its wild progenitor. Population-scale resequencing of mutants, genebank collections and elite cultivars, along with pan-genome assemblies of representatives of major germplasm groups, can now proceed, supported by the resources and methods presented here.
Methods
Genome assembly and validation
PacBio HiFi reads were assembled using hifiasm v0.11-r302 (ref. 45) with default parameters. The dovetail Omni-C data were aligned to the resulting contigs using minimap2 v2.20 (ref. 46) to accurately order and orient the contigs. Similarly, the genetic markers from a consensus genetic map have been previously reported47, and the 25K SNP array markers mapped in the NV644 × NV153 recombinant inbred lines (F6) were aligned to the preliminary contigs using minimap2 to assign contigs to chromosomes. Subsequently, the pseudomolecule construction was done with the TRITEX pipeline48. The final order and orientation of contigs in each chromosome were inspected and corrected manually with complementary support of Omni-C and the NV644 × NV153-derived genetic map. The assembled contigs were taxonomically classified using Kraken2 v2.1.1 (ref. 31) with a database including sequences of plants, insects and bacteria, and with BlobTools v1.1 (ref. 49). The genome completeness and consensus accuracy were evaluated by Merqury v1.3 (ref. 50). The levels of homozygosity, heterozygosity and duplication were assessed by various tools such as Merqury, FindGSE v1.94 (ref. 21) and GenomeScope v1.0 (ref. 51). The centromere regions were identified in each chromosome using chromatin immunoprecipitation followed by sequencing (ChIP–seq) with the CENH3 (a centromere-specific histone H3 variant) antibody as previously reported52. In brief, the raw reads from ChIP–seq were trimmed by cutadapt v.1.15 (ref. 53) and mapped to the preliminary pseudomolecules using minimap2. The alignments were converted to BAM format using SAMtools v1.15.1 (ref. 54) and sorted by Novosort v3.06.05 (http://www.novocraft.com). The read depth was then calculated in 100-kb windows. Finally, the order of each chromosome was determined with regard to centromere positions (short-to-long arm), matching with the karyotype map of faba bean.
Estimation of genome size using flow cytometry
Nuclear genome size was estimated by flow cytometry as previously described55. In brief, intact leaf tissues of the V. faba accession Hedin/2 and Secale cereale cv. Dankovske (2C = 16.19 pg DNA)56, which served as the internal reference standard, were chopped together in a glass Petri dish containing 500 μl Otto I solution (0.1 M citric acid and 0.5% v/v Tween 20; Otto, 1990). The crude suspension was filtered through a 50-μm nylon mesh. Nuclei were then pelleted (300g for 2 min) and resuspended in 300 µl of Otto I solution. After 15 min of incubation on ice, 600 µl of Otto II solution supplemented with 50 µg ml−1 RNase and 50 µg ml−1 propidium iodide was added. Samples were analysed using a CyFlow Space flow cytometer (Sysmex Partec GmbH) equipped with a 532-nm green laser. The gain of the instrument was adjusted so that the peak representing G1 nuclei of the reference standard was positioned approximately on channel 100 on a histogram of relative propidium fluorescence intensity when using a 512-channel scale. The low-level threshold was set to channel 20 to eliminate particles with the lowest fluorescent intensity from the histogram; all remaining fluorescent events were recorded with no further gating used. Twelve Hedin/2 plants were sampled, and each sample was analysed three times, each time on a different day. A minimum of 5,000 nuclei per sample were analysed by the FloMax software (Sysmex Partec GmbH), and 2C DNA contents (in pg) were calculated from the means of the G1 peak positions by applying the formula: 2C nuclear DNA content = (sample G1 peak mean) × (standard 2C DNA content)/(standard G1 peak mean). The mean nuclear DNA content (2C) was then calculated for each species and DNA contents (in pg) were converted to the number of base pairs (in bp) using the conversion factor 1 pg DNA = 0.978 Gb (ref. 57).
Genome size estimation and quality assessment
The distribution of the k-mer (K = 101) frequency was estimated from PacBio HiFi reads using Jellyfish v2.2.10 (ref. 58). The output histograms were used to estimate the genome size and heterozygosity using findGSE v1.94 (ref. 52). The completeness of the assembly was assessed by two independent approaches: (1) self-alignment of HiFi reads to the assembly by minimap2 v2.20 followed by single variant (SV) calling using Sniffles v1.0.11 (ref. 59); and (2) BUSCO v3.0.2b60 analysis with Embryophyta database 9.
Enzymatic methylation sequencing
DNA for methylome sequencing was extracted using the Qiagen DNEasy Plant 96 kit in accordance with the manufacturer’s instructions, and checked for intactness on a 1% agarose gel and quantitated using the Thermo Fisher Quant-iT PicoGreen dsDNA Assay. Of Hedin/2 genomic DNA, 200 ng was combined with 0.001 ng of CpG-methylated pUC19 control DNA and 0.02 ng of unmethylated bacteriophage Lambda control DNA, then brought to a volume of 50 µl using EB buffer. The input DNA was sheared to 350–400 bp on the S220 Focused-Ultrasonicator Instrument (Covaris) using the following protocol: duty factor = 10; peak incident power = 175; cycles per burst = 200; time = 2 times 30 s. The sheared DNA was used to prepare a large insert NEBnext Enzymatic Methyl-seq library following the manufacturer’s instructions (https://www.neb.com/-/media/nebus/files/manuals/manuale7120.pdf). Four libraries were constructed with different sequencing indexes. Index PCR was performed with five PCR cycles to include indexes and amplify the libraries. The final libraries were quantified by quantitative PCR, pooled at equimolar concentrations and sequenced for 500 cycles (2 × 250 bp paired-end reads) on an SP-flow cell of the Novaseq6000 system (Illumina).
Tiffany genome assembly
The distribution of k-mers (K = 51) was estimated from PacBio HiFi reads using KAT v2.4.2 (ref. 61). The output histograms were used to estimate genome size and heterozygosity using findGSE v1.94. Assembly was performed using hifiasm v0.15.5-r350. The completeness of the assembly was assessed by aligning HiFi reads back to contigs and calling structural variants using Sniffles v2.0.7. Despite there being no obvious heterozygous peak on the k-mer plots, we observed a higher proportion of BUSCO duplicate genes in Tiffany than in Hedin/2 and a slight overestimation of genome size with findGSE. In addition, we also noted a number of short contigs with read coverage about half of the expected, suggesting the presence of regions of heterozygosity in the otherwise mostly homozygous genome. We therefore performed haplotig purging using purge_haplotigs v1.1.2 (ref. 62) (purge_haplotigs cov -l 3 -m 7 -h 25). The quality of the purged assembly was further evaluated using Merqury v1.3. Chromosome-level scaffolds were constructed with RagTag v2.0.1 (ref. 63) using the haplotig-purged assembly. To confirm the success of scaffolding, Hedin/2 and Tiffany chromosomes were aligned using GSAlign v1.0.22 (ref. 64). We compared two approaches for Tiffany annotation to choose the one most suitable for comparative analyses: (1) individual annotation of genomes; and (2) a ‘transfer and gap fill’ approach (Supplementary Fig. 15). We observed that when genomes were individually annotated using the same pipeline, a proportion of syntenic genes had a different exon structure. These differences were substantially reduced when the Hedin/2 annotation was transferred onto Tiffany, suggesting that they might not reflect true biological differences. Artefactual differences in annotation, even when using the same pipeline, which could confound comparative analyses, have previously been reported65,66. We therefore used a transfer and gap fill approach, in which the Hedin/2 annotation was transferred onto Tiffany using Liftoff v1.6.1 (ref. 67). To prevent the formation of chimeric gene models, caused, for example, by SVs, transferred models with in-frame stop codons were removed and replaced by Tiffany genes. Gene models unique to Tiffany were also added to the annotation. Overall, we observed that the transfer and gap fill approach resulted in more syntenic genes and more genes with the same coding sequence (CDS) length in both accessions.
Repetitive DNA annotation
De novo repeat finding was done on Hedin/2 pseudomolecules with RepeatModeler v2.0.1 (ref. 68) with sample sizes of 1,000,000 bp and with LTR_retriever v2.9.0 (ref. 69) and LTRharvest70. De novo elements were clustered with cd-hit-est v4.8.1 (ref. 71); element classification was aided by comparing to RepBase release 20181926, core-repeat domains from GyDB 2.0 (ref. 72) and REXdb Viridiplantae v3.0 (ref. 73). For the retrotransposons, sLTRs and full-length elements were specified as such by LTR_retriever and LTRharvest. Repeat masking was done with RepeatMasker v4.2.1 (http://www.repeatmasker.org) using de novo repeat libraries. Transposable element sequences encoding conserved protein domains were also identified based on their similarities to the REXdb v3.0 database using DANTE v0.1.1 (https://github.com/kavonrtep/dante). Satellite repeats were annotated using similarity searches to a custom database of satellite DNA families described in our previous studies18,31,74,75. The distribution of satellite repeats on metaphase chromosomes of V. faba was examined using fluorescence in situ hybridization (FISH). Chromosome preparation, probe labelling and FISH were performed as previously described31, with hybridization and washing temperatures adjusted to account for the probe AT/CG content to allow for 10–20% mismatches. Chromosomes were counterstained with 4′,6-diamidino-2-phenylindole (DAPI), mounted in Vectashield medium (Vector Laboratories) and examined using a Zeiss AxioImager.Z2 microscope with an Axiocam 506 mono camera. Images were captured and processed using ZEN 3.2 software (Carl Zeiss).
Gene model annotation
The repeat sequences were masked using RepeatMasker v4.1.1 (http://www.repeatmasker.org) with a custom repeat library generated by RepeatModeler v2.0.1 (using the Hedin/2 assembly). The gene annotation was conducted using BRAKER v2.1.6 (ref. 76) (etpmode, min_contig 10000). The RNA sequencing libraries (Supplementary Table 2) were aligned using STAR 2.7.8a77,78. The protein database Viridiplantae OrthoDB v10.1 (ref. 79) (https://onlinelibrary.wiley.com/doi/10.1111/tpj.14546merged), with the translated sequences of the previously published V. faba transcriptome assembly16, was used as input for BRAKER, together with alignments generated by mapping the faba transcriptome assembly using GMAP v2020-10-14 (ref. 80). In addition, M. truncatula genes (‘Mt4.0v2_Genes’) and P. sativum genes (‘pissa.Cameor.gnm1.ann1.7SZR’) were aligned using GMAP v2020-10-14. The generated alignments were used to polish the BRAKER gene models. To account for any gene models missed by BRAKER prediction but present in the Hedin/2 transcriptome assembly, the gene models from GMAP faba transcriptome alignments and BRAKER were compared using bedtools v2.30.0, retaining only the GMAP genes that did not have an intersection with the BRAKER gene models. For these genes, a further filtration was done to eliminate any short (less than 50 amino acids) translated proteins, in-frame stop codons or low (less than 200 reads) expression featureCounts, subread v2.0.1 (ref. 81).
Completeness of the annotation was assessed for Hedin/2 and Tiffany by aligning one Iso-Seq dataset82 and assembled transcriptomes produced for faba bean cultivars Hiverna, Dozah and Farah. Transcriptomes were mapped using GMAP v2020-10-14 and comparisons between those mappings and the annotations were made using bedtools83. Gene models that had been removed by polishing, but which intersected mapped transcripts, were rescued if the transcript was not a putative transposable element. R genes were detected on the unpolished and polished annotations using RGAugury v1.0 (ref. 84). R genes present in the unpolished annotation but not in the polished annotation were also rescued. The coding potential for each transcript was computed with CPC2 v2.0 (ref. 85). The mRNAs with low coding potential were reclassified as long non-coding RNAs. Genes of which at least 50% overlapped a transposable element domain were removed. Finally, any proteins that contained in-frame stop codons after phase correction were also removed. The completeness of the final gene set was assessed using BUSCOv5.2.2 with the embryophyta_odb10 and fabales_odb10 databases.
Symbiotic gene discovery
Total RNA sequencing was carried out for three biological replicates per condition. Eighteen libraries were prepared, and paired-end Illumina HiSeq mRNA sequencing (2 × 100 bp RNA sequencing) was performed by GeneWiz, which produced around 2 × 70 million reads per library on average. Adaptor sequences were removed using CLC Genomics Workbench 11 (CLC Bio Workbench, Qiagen). Only inserts of at least 30 nt were conserved for further analysis. Reads were mapped to the Hedin/2 genome using CLC Genomics Workbench 11 according to the manufacturer’s recommendations. The mapped reads for each transcript were normalized as total counts and used for calculating gene expression. Intact and broken pairs were counted as one. The total counts of each transcript under different conditions were compared using proportion-based test statistics86 implemented in the CLC Genomic Workbench suite. This β-binomial test compares the proportions of counts in a group of samples against those of another group of samples. Different weights were given to the samples, depending on their sizes (total counts). The weights were obtained by assuming a β-distribution on the proportions in a group, and estimating these, along with the proportion of a binomial distribution, by the method of moments. The result was a weighted t-type test statistic. We then calculated a false discovery rate correction for multiple-hypothesis tests87. Only genes with a minimum of ten reads for all compared conditions were considered in the analysis.
Orthologous gene family identification
Genes from 19 legume species (Supplementary Table 6) were clustered to determine the orthologues relationship. The protein sequences from these species were aligned to each other using BLASTP v2.2.26 (ref. 88) (-evalue 1 × 10−5). The results were then used to cluster the gene families by OrthoMCL v2.0.9 (ref. 89).
Phylogenetic analysis and divergence time estimation
The single-copy genes identified from 19 legume species (Supplementary Table 6) by OrthoMCL v2.0.9 were selected for the phylogenetic analysis. The fourfold degenerate synonymous site (4d locus) was extracted to build the evolutionary tree by PhyML v3.0 (ref. 90) and TreeBest v1.9.2 (https://github.com/Ensembl/treebest). Molecular clocks and divergence times were estimated using MCMCTREE v4.4 in the PAML v4.5 package91 using the phylogenetic tree and the divergence time of known species (from published literature or using Timetree (http://www.timetree.org/)).
Whole-genome duplication
The whole-genome duplication of V. faba, M. truncatula and P. sativum were estimated using the collinearity within each genome. First, synteny regions were identified using MCScanX v2.0 (ref. 92). Then, the gene pairs in the synteny regions were used for 4dtv (fourfold degenerate transversions) calculation. The transversion rate was corrected by the HKY93 model. The synonymous (Ks) and non-synonymous (Ka) substitutions were estimated by KaKs_Calculator v1.2 (ref. 94).
Tandem duplicate gene discovery
Tandem duplicated genes were also discovered using the CRBHits v0.0.4 package95 function tandemdups. To confirm the results, genes were also classified using DupGen_finder v25Apr2019 (ref. 96), with A. thaliana serving as the outgroup. V. sativa was excluded from TD analysis owing to suspected fragmentation of its structural annotation, which could result in inflation in the number of genes annotated as tandem duplications (TDs) (Supplementary Table 17). The age of duplications was estimated using T = Ks/2r, r = 1.5 × 10−8. Ks was calculated using CRBHits using method ‘Li’. Synteny between Hedin/2 and Tiffany genes was analysed using CRBHits v0.0.4 package function rbh2dagchainer (type = ‘idx’, gap_length = 1, max_dist_allowed = 20), which internally uses the DAGchainer algorithm97. Syntenic tandem duplicated gene (TDG) clusters were discovered by connecting TDG clusters in individual genomes using the syntenic gene pairs found between Hedin/2 and Tiffany. To minimize the effect of unplaced genes on copy number variation analysis, as unplaced genes can result in spurious copy number variation calls, we corroborated the synteny-based results with Orthofinder v2.5.4 (ref. 98) analysis. Only clusters that had the same or higher copy number in the same genotype, based on both synteny and Orthofinder results (for Orthofinder, only genes on the matching chromosomes and unplaced contigs were considered), were retained for further analysis. Syntenic clusters were functionally annotated with human readable descriptions using prot-scriber v0.1.0 (https://github.com/usadellab/prot-scriber).
SPET library preparation and sequencing
Quantified genomic DNA using the Qubit 2.0 Fluorometer (Invitrogen) was used for library preparation, applying the Allegro Targeted Genotyping protocol (NuGEN Technologies), which relies on a panel of probes. Of DNA in solution, 20 ng µl−1 was used as input following the manufacturer’s instructions. All libraries were quantified using the Qubit 2.0 Fluorometer and library size was verified using the High Sensitivity DNA assay from Bioanalyzer (Agilent Technologies) or the High Sensitivity DNA assay from Caliper LabChip GX (Caliper Life Sciences). Libraries were also quantified by quantitative PCR, using the CFX96 Touch Real-Time PCR Detection System (Bio-Rad Laboratories). Samples were sequenced at IGA Technology Services (IGATech). DNA sequencing was performed on the Illumina NovaSeq 6000 (Illumina) in a 2 × 150 PE configuration, generating an average of 7.73 million sequenced read pairs per accession.
Phenotyping and field trials
Seed traits were quantified using a MARViN seed analyzer (MARViTECH) on seeds harvested from trials at Sejet Plant Breeding, Sejet (55.82° N, 9.94° E) in 2019 (trial 23), 2020 (trial 26) and 2021 (trial 30), and at Nordic Seed, Dyngby (55.96° N, 10.25° E) in 2018 (trial 11), 2019 (trial 22) and 2020 (trial 25). Hilum colour was scored by visual inspection.
SNP calling and GWAS
The SPET raw reads were trimmed with cutadapt v1.15 and aligned to the Hedin/2 genome using minimap2 v2.20. The alignments were sorted using Novosort v3.06.05 (http://www.novocraft.com), and BCFtools v.1.8 was used to call SNPs and short indels. The missing data in the VCF file were imputed using Beagle v5. The population structure analysis was performed with ADMIXTURE v1.3.0 (ref. 99) with K values ranging from two to ten. A fivefold cross-validation error for each K was used to select the best K. The principle component analysis was performed using Plink v1.90b6.9 and the linkage disequilibrium (LD) blocks were identified using LDBlockshow v1.40. GWAS was performed with GEMMA v0.98.5 (ref. 100), BLINK101, FarmCPU102 and EMMAX+EMMA200 (ref. 103) using imputed SNP matrices. BLINK and FarmCPU were run using GAPIT3 v3.1 R package with three principal components. Only SNPs found by at least two methods were considered as candidates and it was further required that the signal was found in more than a single trial. The values used for the GWAS were the means of each genotype in each trial and the best linear unbiased estimator (BLUE). The BLUEs were computed with the lme4 package in R by first using the model:
Where yijk is the score of accession i in environment j in block k, µ is the overall mean of the trait, Gi is the effect of accession i, Ej is the effect of environment j, Gi×Ej is the interaction effect between accession i and environment j, Bjk is the effect of block k in environment j, and εijk is the residual. All effects except the mean were random effects. The significance of each random effect except G were then tested one at a time using lmertest package in R. Only effects with a P value larger than 0.05 were included in the final model. The final model had G and µ as a fixed effect and all others as random. The BLUEs were then extracted from G.
Prediction accuracies of seed-size-related traits were investigated by fivefold cross-validation using the genomic best linear unbiased prediction (gBLUP) method. The fitted model in matrix notation is of the form y = 1μ + Zu + e, where y is a vector of observed phenotypic records (BLUEs), μ is the intercept, 1 is a vector of ones, Z is a design matrix linking records to accessions, u is a vector of (genomic) breeding values of the accessions assumed u∼N(0, G), where is the additive genetic variance and G is the genomic relationship matrix (GRM). The GRM was constructed as G = ZZ′/2pi(1 − pi)104, where Z is the SNP matrix centred for the allele frequencies and pi is the allele frequency for the marker i. Finally, e is a vector of random residuals assumed e∼N(0, I), where I is an identity matrix and is the residual variance. Three prediction scenarios were investigated by varying the available markers for GMR calculation: (1) only candidate genome-wide association signals, (2) random samples of the same size as before repeated 100 times, and (3) all available SNP markers. Cross-validations were replicated ten times and averages ± standard deviations were reported. The ‘mixed.solve’ function from the rrBLUP v4.6.1 (ref. 105) R package was used for all calculations.
Premature termination codon and resistance gene analogue identification
SNPs and indels were filtered to retain biallellic variants only. Variants annotated as ‘stop_gained’ by SNPEff v4.3 (ref. 106) were extracted, and only polymorphic variants with at least one homozygous reference and one homozygous alternative genotype were retained. Resistance gene analogues were identified using the RGAugury v1.0 pipeline84. Enrichment of premature termination codons in resistance gene analogues was calculated using the permTest function of regionerR v1.18.1 (ref. 107) and 1,000 permutations (randomize.function=resampleRegions, evaluate.function=numOverlaps). All genes were provided as a universe for resampleRegions function.
Identification of candidate gene for seed size
Positions of the most highly significant and stable SNPs associated with seed size were compared with positions of Hedin/2 protein-coding genes. Orthologues of the gene overlapping variant were identified using Orthofinder v2.5.4 (-M msa -S diamond -A mafft -T fasttree). Multiple sequence alignment of selected protein sequences was performed using Clustal Omega v1.2.4. The evolutionary history was inferred using the maximum likelihood method and JTT matrix-based model as implemented in MEGA X v10.2.6 (ref. 108) with 100 bootstrap replicates. Publicly available expression data16 for nine diverse tissues of Hedin/2 were used to quantify gene expression using Kallisto v0.44.0 (ref. 109). LD patterns in the genomic interval surrounding the candidate gene were investigated using LDBlockShow v1.40 (ref. 110).
Hilum colour and histology
To examine hilum morphology, seed coat-containing hilum from inbred lines Hedin/2 (dark hilum) and Tiffany (pale hilum) were dissected from mature dry seed, saturated with 2% sucrose solution under vacuum for 1 h and embedded in cryo-gel media (Cryo-gel Leica). Samples were cut in cryotome (Leica CM1950) into 15-µm transversal sections and stained with Toluidine blue O (0.01%, w/v in water; Sigma Aldrich) as previously described41,111. Observation and photography were done on an Olympus BX 51 microscope (Olympus) in bright field, and figures were documented with an Apogee U4000 digital camera (Apogee Imaging Systems). For the investigation of metabolite content of surface layers of the hilum by laser desorption–ionization imaging mass spectrometry (LDI-MS), seeds were mechanically cracked and hila with a small part of surrounding tissue were separated from the rest of seed coats using microscissors (MicroSupport), fixed using a double-sided tape on MALDI plates with outer surfaces facing up and analysed as previously described41,112. LDI-MSI experiments were done using a high-resolution tandem mass spectrometer (HRTMS) Synapt G2-S (Waters). The vacuum MALDI ion source used was equipped with a 350-nm 1-kHz Nd:YAG solid-state laser. Parameters of the mass spectrometer were set as follows: extraction voltage at 10 V, collision energies: trap collision energy (TrapCE) at 4 eV and transfer collision energy (TransferCE) at 2 eV. TrapCE at 25 eV and low mass (LM) resolution at 10 were used for MS/MS experiments. Instrument calibration was done using red phosphorus (1 mg.mL−1, suspension in acetone). Mass imaging data collection was driven by HDImaging 1.5 software (Waters). The laser beam size was 60 μm. Spectra were collected in positive and negative ionization mode with laser energy at 300 arb. Laser repetition rate was set up at 1,000 Hz. Mass range was 50–1,200 Da. To fine-map the HC locus, a cross was made between inbred lines Disco (♀pale) and Hedin/2 (♂dark). F4 seeds from 337 F3 progeny of 21 F2 individuals shown by flanking marker analysis to be heterozygous across the HC interval were scored for hilum colour, resulting in a 253 dark to 84 pale hilum ratio (χ2 = 0.00098, P = 0.9749 for fit to expected 3:1 ratio). A pool composed of equimolar quantities of DNA from each of the 84 recessive pseudo-F2 individuals was created and subjected to SPET re-sequencing alongside DNA samples of the parent lines. To study expression of the PPO gene family in mid to late pod fill, individual plants of Hedin/2 and Tiffany were grown in the glasshouse until the most mature pods on lower nodes had almost reached maturity and the uppermost nodes were still in flower, giving a gradient of seed development. All pods were then harvested and dissected into pod wall, testa, cotyledon, funicle and embryo axis samples (Supplementary Fig. 16); fresh weights of each tissue were recorded. Because all pods on a given node are not fertilized synchronously and do not necessarily progress through development at the same rate, and on the basis of insights from our previous studies of faba bean seed development113, we categorized individual pods into mid and late pod-fill stages in terms of the ratio of cotyledon weight to the total seed weight (Supplementary Fig. 17).
PPO locus comparative sequence analysis
To identify PPO homologues in Hedin/2 and Tiffany proteomes, the protein sequence from the pea PPO1/Pl gene (Psat1g206360) was used as a BLAST v2.12.0 query. Multiple sequence alignment of PPO protein sequences was performed using Clustal Omega v1.2.4. The evolutionary history was inferred using the maximum likelihood method and JTT matrix-based model as implemented in MEGA X with 100 bootstrap replicates. The complete PPO regions (from the beginning of the first to the end of the last PPO gene and 10,000 bp flanking sequences on both sides) were extracted and aligned using minimap2 v2.24-r1122. Then, 20,000 bp downstream and upstream from the transcription start of PPO-2 were extracted and similarity between sequences was visualized using FlexiDot v1.06 (ref. 114).
Gene expression analysis
RNA was extracted from 100 mg of flash-frozen dissected tissue (testa and cotyledon) using a Sigma Spectrum Kit (STRN250) according to the manufacturer’s directions, except that incubation was made at room temperature after DNA digestion. Although extraction of RNA from cotyledons was performed exactly as per manufacturer’s specifications, testa tissue was disrupted in an extraction buffer consisting of CTAB, PVP, 2 M Tris pH 8, 0.5 M EDTA pH 8, 4 M NaCl, spermidine and β-mercaptoethanol, followed by precipitation with 8 M lithium chloride (instead of the kit’s lysis step). Total RNA was quantified using Qubit RNA IQ assay and normalized before preparation of directional mRNA sequencing libraries using standard methods. Between 4.1 and 5.6 million Illumina PE150 short reads per library (3× replicates, 2× tissues and 2× genotypes) were generated. Hedin/2 and Tiffany gene expression were quantified using Kallisto v0.44.0 by pseudo-aligning RNA sequencing reads to respective reference transcripts. Transcript-level abundance was converted to gene-level abundance using tximport.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41586-023-05791-5.
Supplementary information
This file contains Supplementary Figs. 1–17, Supplementary Tables 3, 4, 6, 13 and Supplementary Note (Pretzel instructions). The file supports assembly and structural annotation quality control, gene family and genome duplication analysis, transposable element and DNA methylation analysis, GWAS and gene expression profiling quality control, further information supporting hilum colour and seed size candidate loci. Supplementary Note includes instructions for accessing integrated faba bean datasets using Pretzel.
This file contains supplementary Tables 1, 2, 5, 7–12 and 14–17. The file supports genome assembly and annotation, expression profiling of roots and nodules, copy number variation and transposable element analysis, SPET data and GWAS analysis and expression profiling of Hedin/2 and Tiffany seeds.
Acknowledgements
This research was supported by grants from the Innovation Fund Denmark (‘NORFAB’, 5158-00004B) to J.S.; FACCE-JPI ERA-NET SusCrop Profaba to S.U.A., D.M.O., F.L.S. and A.H.S.; German Leibniz Association in the frame of the Leibniz Junior Research groups (J118/2021/REPLACE) to M.J.; the German Federal Ministry of Education and Research (de.NBI, 031A536B) to H.G.; Novo Nordisk Fund (#NNF20OC0065157) to A.H.S.; the Jane and Aatos Erkko Foundation grant (PanFaba) to A.H.S.; the Alexander von Humboldt Foundation in the framework of Sofja Kovalevskaja Award to A.A.G.; the Biotechnology and Biological Sciences Research Council award BB/P023509/1 to D.M.O.; the German Research Foundation (DFG) project number 497667402 to A.A.G. and B.U.; the Czech Science Foundation project number GACR 20-24252S to J.M.; the European Research Council under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 834221) to J.S.; the BMBF-funded de.NBI Cloud within the German Network for Bioinformatics Infrastructure (de.NBI) (031A532B, 031A533A, 031A533B, 031A534A, 031A535A, 031A537A, 031A537B, 031A537C, 031A537D and 031A538A); and Grains Research Development Corporation as well as Agriculture Victoria for PacBio sequencing, parts of the public dataset curation for pulses.plantinformatics.io (DAV 1905-003RTX) and RNA sequencing data (BioProject ID PRJNA395480). We acknowledge CSC-IT Center for Science, Finland and ELIXIR CZ Research Infrastructure (Czech Ministry of Education, Youth and Sports grant no. LM2018131) for generous computational resources; and A. Fiebig for help with data submission.
Extended data figures and tables
Author contributions
M.J. performed Hedin/2 genome assembly, methylation data analysis, hilum colour GWAS and wrote the first manuscript draft. A.A.G. performed Tiffany genome assembly, analysed seed coat and embryo expression data and compiled the premature termination codon atlas. A.A.G., J.K. and B.I. analysed synteny, tandem gene duplications and intron size variation. J.K., A.A.G. and L.I.F. contributed with gene annotation and data submission. S.U.A., D.M.O., L.I.F., D.A. and P.S. designed the SPET assay. D.A. and A.O.W. performed hilum colour bulk segregant analysis (BSA). P.B. carried out seed coat MS. E.B. and L.L.J. contributed with SPET data analysis and submission and prediction of seed size. P.-E.C. and R.B. generated and analysed rhizobial and mycorrhizal symbiosis RNA sequencing data. K.C. generated seed coat and embryo RNA sequencing libraries. J.D. and J.C. estimated genome size using flow cytometry. H.G., A.H.S., J.M., P.Neumann, P.Novák, J.T. and J.K. annotated and analysed repetitive elements. A.Hallab, P.T., B.U. and L.U.H. performed gene functional annotation. A.Himmelbach, S.P. and N.S. generated methylome sequencing data. S.K. and G.K.-G. contributed to PacBio sequencing, provided RNA sequencing data used for variant discovery, and integrated and visualized QTL and variation data online. A.K. and J.M. generated and analysed ChIP–seq data. L.K. and P.S. performed seed coat histochemistry. P.K. contributed seed coat analytical chemistry. T.W.M. carried out phenotype data validation and seed size GWAS. M.M., D.M.O., A.H.S., S.U.A., I.S., G.A., M.N. and H.K. edited the manuscript with input from all authors. J.O., L.K.N. and A.S. carried out SPET sample preparation and hilum colour phenotyping. P.Novák developed the faba bean genome browser. K.C. and T.R.-S.-H. prepared seed coat and embryo RNA sequencing libraries. L.A.R. contributed cytogenetic analysis. A.H.J.W. and R.J.S. generated HiFi data. H.Z. provided gene family, evolution, diversity and phylogenetic analysis. F.L.S. provided plant material. S.U.A., J.S., N.T. and A.M.T. provided resources. D.M.O. and D.A. genotyped pure Hedin/2 stock and generated the genetic map. S.U.A., A.H.S., D.M.O. and M.J. designed the study. S.U.A. coordinated the project.
Peer review
Peer review information
Nature thanks Aureliano Bombarely, Eric von Wettberg and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
Raw data are available under European Nucleotide Archive study ID PRJEB52541. Genome assemblies and annotations for Hedin/2 and Tiffany are available for download at www.fabagenome.dk and can be accessed via an interactive genome browser (http://w3lamc.umbr.cas.cz/lamc/resources.html).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Murukarthick Jayakodi, Agnieszka A. Golicz, Jonathan Kreplak
Contributor Information
Donal Martin O’Sullivan, Email: d.m.osullivan@reading.ac.uk.
Alan H. Schulman, Email: alan.schulman@helsinki.fi
Stig Uggerhøj Andersen, Email: sua@mbg.au.dk.
Extended data
is available for this paper at 10.1038/s41586-023-05791-5.
Supplementary information
The online version contains supplementary material available at 10.1038/s41586-023-05791-5.
References
- 1.Hyland JJ, Henchion M, McCarthy M, McCarthy SN. The role of meat in strategies to achieve a sustainable diet lower in greenhouse gas emissions: a review. Meat Sci. 2017;132:189–195. doi: 10.1016/j.meatsci.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Zander P, et al. Grain legume decline and potential recovery in European agriculture: a review. Agron. Sustain. Dev. 2016;36:26. doi: 10.1007/s13593-016-0365-y. [DOI] [Google Scholar]
- 3.Tanno K-i, Willcox G. The origins of cultivation of Cicer arietinum L. and Vicia faba L.: early finds from Tell el-Kerkh, north-west Syria, late 10th millennium b.p. Veget. Hist. Archaeobot. 2006;15:197–204. doi: 10.1007/s00334-005-0027-5. [DOI] [Google Scholar]
- 4.Caracuta V, et al. 14,000-year-old seeds indicate the Levantine origin of the lost progenitor of faba bean. Sci. Rep. 2016;6:37399. doi: 10.1038/srep37399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warsame AO, O’Sullivan DM, Tosi P. Seed storage proteins of faba bean (Vicia faba L): current status and prospects for genetic improvement. J. Agric. Food Chem. 2018;66:12617–12626. doi: 10.1021/acs.jafc.8b04992. [DOI] [PubMed] [Google Scholar]
- 6.Khattab A, Maxted N, Bisby FA. Close relatives of the fababean from Syria: a new species of Vicia and notes on V. hyaeniscyamus (Leguminosae) Kew Bull. 1988;43:535–540. doi: 10.2307/4118984. [DOI] [Google Scholar]
- 7.Muratova, V. Bulletin of Applied Botany of Genetics and Plant Breeding Supplement 50, 1–298 (1931).
- 8.Hanelt P, Schäfer H, Schultze-Motel J. Die Stellung von Vicia faba L. in der Gattung Vicia L. und Betrachtungen zu dieser Kulturart. Kulturpflanze. 1972;20:263–275. doi: 10.1007/BF02095463. [DOI] [Google Scholar]
- 9.Cubero JI, Suso MJ. Primitive and modern forms of Vicia faba. Kulturpflanze. 1981;29:137–145. doi: 10.1007/BF02014744. [DOI] [Google Scholar]
- 10.Vranken L, Avermaete T, Petalios D, Mathijs E. Curbing global meat consumption: emerging evidence of a second nutrition transition. Environ. Sci. Policy. 2014;39:95–106. doi: 10.1016/j.envsci.2014.02.009. [DOI] [Google Scholar]
- 11.Cernay C, Pelzer E, Makowski D. A global experimental dataset for assessing grain legume production. Sci. Data. 2016;3:160084. doi: 10.1038/sdata.2016.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herridge DF, Peoples MB, Boddey RM. Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil. 2008;311:1–18. doi: 10.1007/s11104-008-9668-3. [DOI] [Google Scholar]
- 13.Bailes EJ, Pattrick JG, Glover BJ. An analysis of the energetic reward offered by field bean (Vicia faba) flowers: nectar, pollen, and operative force. Ecol. Evol. 2018;8:3161–3171. doi: 10.1002/ece3.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adhikari KN, et al. Conventional and molecular breeding tools for accelerating genetic gain in faba bean (Vicia faba L.) Front. Plant Sci. 2021;12:744259. doi: 10.3389/fpls.2021.744259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Webb A, et al. A SNP-based consensus genetic map for synteny-based trait targeting in faba bean (Vicia faba L.) Plant Biotechnol. J. 2016;14:177–185. doi: 10.1111/pbi.12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Björnsdotter E, et al. VC1 catalyses a key step in the biosynthesis of vicine in faba bean. Nat. Plants. 2021;7:923–931. doi: 10.1038/s41477-021-00950-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macas J, Neumann P. Ogre elements—a distinct group of plant Ty3/gypsy-like retrotransposons. Gene. 2007;390:108–116. doi: 10.1016/j.gene.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Macas J, et al. In depth characterization of repetitive DNA in 23 plant genomes reveals sources of genome size variation in the legume tribe fabeae. PLoS ONE. 2015;10:e0143424. doi: 10.1371/journal.pone.0143424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang W, Jääskeläinen M, Li S-P, Schulman AH. BARE retrotransposons are translated and replicated via distinct RNA pools. PLoS ONE. 2013;8:e72270. doi: 10.1371/journal.pone.0072270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuchs J, Strehl S, Brandes A, Schweizer D, Schubert I. Molecular-cytogenetic characterization of the Vicia faba genome—heterochromatin differentiation, replication patterns and sequence localization. Chromosome Res. 1998;6:219–230. doi: 10.1023/A:1009215802737. [DOI] [PubMed] [Google Scholar]
- 21.Rhie A, Walenz BP, Koren S, Phillippy AM. Merqury: reference-free quality, completeness, and phasing assessment for genome assemblies. Genome Biol. 2020;21:245. doi: 10.1186/s13059-020-02134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mascher M, et al. A chromosome conformation capture ordered sequence of the barley genome. Nature. 2017;544:427–433. doi: 10.1038/nature22043. [DOI] [PubMed] [Google Scholar]
- 23.de Bruijn, F. J. in The Model Legume Medicago truncatula (ed. de Bruijn, F. J.) Ch. 8 (Wiley, 2019).
- 24.Courty PE, Smith P, Koegel S, Redecker D, Wipf D. Inorganic nitrogen uptake and transport in beneficial plant root–microbe interactions. Crit. Rev. Plant Sci. 2015;34:4–16. doi: 10.1080/07352689.2014.897897. [DOI] [Google Scholar]
- 25.Wipf D, Krajinski F, van Tuinen D, Recorbet G, Courty P-E. Trading on the arbuscular mycorrhiza market: from arbuscules to common mycorrhizal networks. New Phytol. 2019;223:1127–1142. doi: 10.1111/nph.15775. [DOI] [PubMed] [Google Scholar]
- 26.De La Torre AR, et al. Insights into conifer giga-genomes. Plant Physiol. 2014;166:1724–1732. doi: 10.1104/pp.114.248708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niu S, et al. The Chinese pine genome and methylome unveil key features of conifer evolution. Cell. 2022;185:204–217.e14. doi: 10.1016/j.cell.2021.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Schmutz J, et al. Genome sequence of the palaeopolyploid soybean. Nature. 2010;463:178–183. doi: 10.1038/nature08670. [DOI] [PubMed] [Google Scholar]
- 29.Cannon SB, et al. Multiple polyploidy events in the early radiation of nodulating and nonnodulating legumes. Mol. Biol. Evol. 2014;32:193–210. doi: 10.1093/molbev/msu296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xi, H., Nguyen, V., Ward, C., Lui, Z. & Searle, I. R. Chromosome-level assembly of the common vetch (Vicia sativa) reference genome. Gigabyte10.46471/gigabyte.38 (2022). [DOI] [PMC free article] [PubMed]
- 31.Ávila Robledillo L, et al. Satellite DNA in Vicia faba is characterized by remarkable diversity in its sequence composition, association with centromeres, and replication timing. Sci. Rep. 2018;8:5838. doi: 10.1038/s41598-018-24196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cossu RM, et al. LTR retrotransposons show low levels of unequal recombination and high rates of intraelement gene conversion in large plant genomes. Genome Biol. Evol. 2017;9:3449–3462. doi: 10.1093/gbe/evx260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bewick AJ, Schmitz RJ. Gene body DNA methylation in plants. Curr. Opin. Plant Biol. 2017;36:103–110. doi: 10.1016/j.pbi.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brenet F, et al. DNA methylation of the first exon is tightly linked to transcriptional silencing. PLoS ONE. 2011;6:e14524. doi: 10.1371/journal.pone.0014524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L, et al. DNA methylome analysis provides evidence that the expansion of the tea genome is linked to TE bursts. Plant Biotechnol. J. 2019;17:826–835. doi: 10.1111/pbi.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erdmann RM, Picard CL. RNA-directed DNA methylation. PLoS Genet. 2020;16:e1009034. doi: 10.1371/journal.pgen.1009034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barchi L, et al. Single primer enrichment technology (SPET) for high-throughput genotyping in tomato and eggplant germplasm. Front. Plant Sci. 2019;10:1005. doi: 10.3389/fpls.2019.01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khazaei H, O’Sullivan DM, Sillanpää MJ, Stoddard FL. Use of synteny to identify candidate genes underlying QTL controlling stomatal traits in faba bean (Vicia faba L.) Theor. Appl. Genet. 2014;127:2371–2385. doi: 10.1007/s00122-014-2383-y. [DOI] [PubMed] [Google Scholar]
- 39.Adamski NM, Anastasiou E, Eriksson S, O’Neill CM, Lenhard M. Local maternal control of seed size by KLUH/CYP78A5-dependent growth signaling. Proc. Natl Acad. Sci. USA. 2009;106:20115–20120. doi: 10.1073/pnas.0907024106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khazaei H, et al. Flanking SNP markers for vicine–convicine concentration in faba bean (Vicia faba L.) Mol. Breeding. 2015;35:38. doi: 10.1007/s11032-015-0214-8. [DOI] [Google Scholar]
- 41.Balarynová J, et al. The loss of polyphenol oxidase function is associated with hilum pigmentation and has been selected during pea domestication. New Phytol. 2022;235:1807–1821. doi: 10.1111/nph.18256. [DOI] [PubMed] [Google Scholar]
- 42.Gopher, A., Lev-Yadun, S. & Abbo, S. Breaking Ground: Plant Domestication in the Neolithic Levant: the “Core-area One-event” Model (Emery and Claire Yass Publications in Archaeology, The Institute of Archaeology, Tel Aviv University, 2021).
- 43.Scarborough J. Beans, Pythagoras, taboos, and ancient dietetics. Classic. World. 1982;75:355–358. doi: 10.2307/4349404. [DOI] [Google Scholar]
- 44.Hanafy M, Pickardt T, Kiesecker H, Jacobsen H-J. Agrobacterium-mediated transformation of faba bean (Vicia faba L.) using embryo axes. Euphytica. 2005;142:227–236. doi: 10.1007/s10681-005-1690-4. [DOI] [Google Scholar]
- 45.Cheng H, Concepcion GT, Feng X, Zhang H, Li H. Haplotype-resolved de novo assembly using phased assembly graphs with hifiasm. Nat. Methods. 2021;18:170–175. doi: 10.1038/s41592-020-01056-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li H. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics. 2018;34:3094–3100. doi: 10.1093/bioinformatics/bty191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carrillo-Perdomo E, et al. Development of new genetic resources for faba bean (Vicia faba L.) breeding through the discovery of gene-based SNP markers and the construction of a high-density consensus map. Sci. Rep. 2020;10:6790. doi: 10.1038/s41598-020-63664-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Monat C, et al. TRITEX: chromosome-scale sequence assembly of Triticeae genomes with open-source tools. Genome Biol. 2019;20:284. doi: 10.1186/s13059-019-1899-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wood DE, Salzberg SL. Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol. 2014;15:R46. doi: 10.1186/gb-2014-15-3-r46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laetsch D, Blaxter M. BlobTools: interrogation of genome assemblies [version 1; peer review: 2 approved with reservations] F1000Res. 2017;6:1287. doi: 10.12688/f1000research.12232.1. [DOI] [Google Scholar]
- 51.Sun H, Ding J, Piednoël M, Schneeberger K. findGSE: estimating genome size variation within human and Arabidopsis using k-mer frequencies. Bioinformatics. 2017;34:550–557. doi: 10.1093/bioinformatics/btx637. [DOI] [PubMed] [Google Scholar]
- 52.Vurture GW, et al. GenomeScope: fast reference-free genome profiling from short reads. Bioinformatics. 2017;33:2202–2204. doi: 10.1093/bioinformatics/btx153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011;17:10–12. doi: 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 54.Li H, et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doležel J, Greilhuber J, Suda J. Estimation of nuclear DNA content in plants using flow cytometry. Nat. Protoc. 2007;2:2233–2244. doi: 10.1038/nprot.2007.310. [DOI] [PubMed] [Google Scholar]
- 56.Doležel J, Sgorbati S, Lucretti S. Comparison of three DNA fluorochromes for flow cytometric estimation of nuclear DNA content in plants. Physiol. Plant. 1992;85:625–631. doi: 10.1111/j.1399-3054.1992.tb04764.x. [DOI] [Google Scholar]
- 57.Dolezel J. Nuclear DNA content and genome size of trout and human. Cytometry A. 2003;51:127–128. doi: 10.1002/cyto.a.10013. [DOI] [PubMed] [Google Scholar]
- 58.Marçais G, Kingsford C. A fast, lock-free approach for efficient parallel counting of occurrences of k-mers. Bioinformatics. 2011;27:764–770. doi: 10.1093/bioinformatics/btr011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sedlazeck FJ, et al. Accurate detection of complex structural variations using single-molecule sequencing. Nat. Methods. 2018;15:461–468. doi: 10.1038/s41592-018-0001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simão FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31:3210–3212. doi: 10.1093/bioinformatics/btv351. [DOI] [PubMed] [Google Scholar]
- 61.Mapleson D, Garcia Accinelli G, Kettleborough G, Wright J, Clavijo BJ. KAT: a k-mer analysis toolkit to quality control NGS datasets and genome assemblies. Bioinformatics. 2017;33:574–576. doi: 10.1093/bioinformatics/btw663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roach MJ, Schmidt SA, Borneman AR. Purge Haplotigs: allelic contig reassignment for third-gen diploid genome assemblies. BMC Bioinformatics. 2018;19:460. doi: 10.1186/s12859-018-2485-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alonge, M. et al. Automated assembly scaffolding using RagTag elevates a new tomato system for high-throughput genome editing. Genome Biol.23, 258 (2022). [DOI] [PMC free article] [PubMed]
- 64.Lin H-N, Hsu W-L. GSAlign: an efficient sequence alignment tool for intra-species genomes. BMC Genomics. 2020;21:182. doi: 10.1186/s12864-020-6569-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.König S, Romoth LW, Gerischer L, Stanke M. Simultaneous gene finding in multiple genomes. Bioinformatics. 2016;32:3388–3395. doi: 10.1093/bioinformatics/btw494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bayer PE, et al. Assembly and comparison of two closely related Brassica napus genomes. Plant Biotechnol. J. 2017;15:1602–1610. doi: 10.1111/pbi.12742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shumate A, Salzberg SL. Liftoff: accurate mapping of gene annotations. Bioinformatics. 2021;37:1639–1643. doi: 10.1093/bioinformatics/btaa1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Flynn JM, et al. RepeatModeler2 for automated genomic discovery of transposable element families. Proc. Natl Acad. Sci. USA. 2020;117:9451–9457. doi: 10.1073/pnas.1921046117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ou S, Jiang N. LTR_retriever: a highly accurate and sensitive program for identification of long terminal repeat retrotransposons. Plant Physiol. 2017;176:1410–1422. doi: 10.1104/pp.17.01310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ellinghaus D, Kurtz S, Willhoeft U. LTRharvest, an efficient and flexible software for de novo detection of LTR retrotransposons. BMC Bioinformatics. 2008;9:18. doi: 10.1186/1471-2105-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang Y, Niu B, Gao Y, Fu L, Li W. CD-HIT Suite: a web server for clustering and comparing biological sequences. Bioinformatics. 2010;26:680–682. doi: 10.1093/bioinformatics/btq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Llorens C, et al. The Gypsy Database (GyDB) of mobile genetic elements: release 2.0. Nucleic Acids Res. 2010;39:D70–D74. doi: 10.1093/nar/gkq1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Neumann P, Novák P, Hoštáková N, Macas J. Systematic survey of plant LTR-retrotransposons elucidates phylogenetic relationships of their polyprotein domains and provides a reference for element classification. Mob. DNA. 2019;10:1. doi: 10.1186/s13100-018-0144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ávila Robledillo L, et al. Extraordinary sequence diversity and promiscuity of centromeric satellites in the legume tribe Fabeae. Mol. Biol. Evol. 2020;37:2341–2356. doi: 10.1093/molbev/msaa090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vondrak T, et al. Characterization of repeat arrays in ultra‐long nanopore reads reveals frequent origin of satellite DNA from retrotransposon‐derived tandem repeats. Plant J. 2020;101:484–500. doi: 10.1111/tpj.14546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brůna T, Hoff KJ, Lomsadze A, Stanke M, Borodovsky M. BRAKER2: automatic eukaryotic genome annotation with GeneMark-EP+ and AUGUSTUS supported by a protein database. NAR Genom. Bioinform. 2021;3:lqaa108. doi: 10.1093/nargab/lqaa108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dobin A, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dobin A, Gingeras TR. Mapping RNA‐seq reads with STAR. Curr. Protoc. Bioinformatics. 2015;51:11.14.1–11.14.19. doi: 10.1002/0471250953.bi1114s51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kriventseva EV, et al. OrthoDB v10: sampling the diversity of animal, plant, fungal, protist, bacterial and viral genomes for evolutionary and functional annotations of orthologs. Nucleic Acids Res. 2019;47:D807–D811. doi: 10.1093/nar/gky1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu TD, Watanabe CK. GMAP: a genomic mapping and alignment program for mRNA and EST sequences. Bioinformatics. 2005;21:1859–1875. doi: 10.1093/bioinformatics/bti310. [DOI] [PubMed] [Google Scholar]
- 81.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 82.Lyu JI, et al. Unraveling the complexity of faba bean (Vicia faba L.) transcriptome to reveal cold-stress-responsive genes using long-read isoform sequencing technology. Sci. Rep. 2021;11:21094. doi: 10.1038/s41598-021-00506-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li P, et al. RGAugury: a pipeline for genome-wide prediction of resistance gene analogs (RGAs) in plants. BMC Genomics. 2016;17:852. doi: 10.1186/s12864-016-3197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kang Y-J, et al. CPC2: a fast and accurate coding potential calculator based on sequence intrinsic features. Nucleic Acids Res. 2017;45:W12–W16. doi: 10.1093/nar/gkx428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baggerly KA, Deng L, Morris JS, Aldaz CM. Differential expression in SAGE: accounting for normal between-library variation. Bioinformatics. 2003;19:1477–1483. doi: 10.1093/bioinformatics/btg173. [DOI] [PubMed] [Google Scholar]
- 87.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. 1995;57:289–300. [Google Scholar]
- 88.Altschul SF, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li L, Stoeckert CJ, Roos DS. OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res. 2003;13:2178–2189. doi: 10.1101/gr.1224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guindon S, Delsuc F, Dufayard J-F, Gascuel O. Estimating maximum likelihood phylogenies with PhyML. Methods Mol. Biol. 2009;537:113–137. doi: 10.1007/978-1-59745-251-9_6. [DOI] [PubMed] [Google Scholar]
- 91.Yang Z. PAML: a program package for phylogenetic analysis by maximum likelihood. Comput. Appl. Biosci. 1997;13:555–556. doi: 10.1093/bioinformatics/13.5.555. [DOI] [PubMed] [Google Scholar]
- 92.Wang Y, et al. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40:e49. doi: 10.1093/nar/gkr1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hasegawa M, Kishino H, Yano T-A. Dating of the human–ape splitting by a molecular clock of mitochondrial DNA. J. Mol. Evol. 1985;22:160–174. doi: 10.1007/BF02101694. [DOI] [PubMed] [Google Scholar]
- 94.Zhang Z, et al. KaKs_Calculator: calculating Ka and Ks through model selection and model averaging. Genomics Proteomics Bioinformatics. 2006;4:259–263. doi: 10.1016/S1672-0229(07)60007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ullrich KK. CRBHits: from conditional reciprocal best hits to codon alignments and Ka/Ks in R. J. Open Source Softw. 2020;5:2424. doi: 10.21105/joss.02424. [DOI] [Google Scholar]
- 96.Qiao X, et al. Gene duplication and evolution in recurring polyploidization–diploidization cycles in plants. Genome Biol. 2019;20:38. doi: 10.1186/s13059-019-1650-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Haas BJ, Delcher AL, Wortman JR, Salzberg SL. DAGchainer: a tool for mining segmental genome duplications and synteny. Bioinformatics. 2004;20:3643–3646. doi: 10.1093/bioinformatics/bth397. [DOI] [PubMed] [Google Scholar]
- 98.Emms DM, Kelly S. OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biol. 2019;20:238. doi: 10.1186/s13059-019-1832-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Alexander DH, Lange K. Enhancements to the ADMIXTURE algorithm for individual ancestry estimation. BMC Bioinformatics. 2011;12:246. doi: 10.1186/1471-2105-12-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhou X, Stephens M. Genome-wide efficient mixed-model analysis for association studies. Nat. Genet. 2012;44:821–824. doi: 10.1038/ng.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang M, Liu X, Zhou Y, Summers RM, Zhang Z. BLINK: a package for the next level of genome-wide association studies with both individuals and markers in the millions. GigaScience. 2018;8:2. doi: 10.1093/gigascience/giy154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu X, Huang M, Fan B, Buckler ES, Zhang Z. Iterative usage of fixed and random effect models for powerful and efficient genome-wide association studies. PLoS Genet. 2016;12:e1005767. doi: 10.1371/journal.pgen.1005767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kang HM, et al. Variance component model to account for sample structure in genome-wide association studies. Nat. Genet. 2010;42:348–354. doi: 10.1038/ng.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.VanRaden PM. Efficient methods to compute genomic predictions. J. Dairy Sci. 2008;91:4414–4423. doi: 10.3168/jds.2007-0980. [DOI] [PubMed] [Google Scholar]
- 105.Endelman JB. Ridge regression and other kernels for genomic selection with R package rrBLUP. Plant Genome. 2011;4:250–255. doi: 10.3835/plantgenome2011.08.0024. [DOI] [Google Scholar]
- 106.Cingolani P, et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin) 2012;6:80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gel B, et al. regioneR: an R/Bioconductor package for the association analysis of genomic regions based on permutation tests. Bioinformatics. 2015;32:289–291. doi: 10.1093/bioinformatics/btv562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bray NL, Pimentel H, Melsted P, Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016;34:525–527. doi: 10.1038/nbt.3519. [DOI] [PubMed] [Google Scholar]
- 110.Dong S-S, et al. LDBlockShow: a fast and convenient tool for visualizing linkage disequilibrium and haplotype blocks based on variant call format files. Brief. Bioinform. 2020;22:bbaa227. doi: 10.1093/bib/bbaa227. [DOI] [PubMed] [Google Scholar]
- 111.Zablatzká L, Balarynová J, Klčová B, Kopecký P, Smýkal P. Anatomy and histochemistry of seed coat development of wild (Pisum sativum subsp. elatius (M. Bieb.) Asch. et Graebn. and domesticated pea (Pisum sativum subsp. sativum L.) Int. J. Mol. Sci. 2021;22:4602. doi: 10.3390/ijms22094602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Krejčí P, et al. Combination of electronically driven micromanipulation with laser desorption ionization mass spectrometry—the unique tool for analysis of seed coat layers and revealing the mystery of seed dormancy. Talanta. 2022;242:123303. doi: 10.1016/j.talanta.2022.123303. [DOI] [PubMed] [Google Scholar]
- 113.Warsame AO, Michael N, O’Sullivan DM, Tosi P. Seed development and protein accumulation patterns in faba bean (Vicia faba, L.) J. Agric. Food Chem. 2022;70:9295–9304. doi: 10.1021/acs.jafc.2c02061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Seibt KM, Schmidt T, Heitkam T. FlexiDot: highly customizable, ambiguity-aware dotplots for visual sequence analyses. Bioinformatics. 2018;34:3575–3577. doi: 10.1093/bioinformatics/bty395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file contains Supplementary Figs. 1–17, Supplementary Tables 3, 4, 6, 13 and Supplementary Note (Pretzel instructions). The file supports assembly and structural annotation quality control, gene family and genome duplication analysis, transposable element and DNA methylation analysis, GWAS and gene expression profiling quality control, further information supporting hilum colour and seed size candidate loci. Supplementary Note includes instructions for accessing integrated faba bean datasets using Pretzel.
This file contains supplementary Tables 1, 2, 5, 7–12 and 14–17. The file supports genome assembly and annotation, expression profiling of roots and nodules, copy number variation and transposable element analysis, SPET data and GWAS analysis and expression profiling of Hedin/2 and Tiffany seeds.
Data Availability Statement
Raw data are available under European Nucleotide Archive study ID PRJEB52541. Genome assemblies and annotations for Hedin/2 and Tiffany are available for download at www.fabagenome.dk and can be accessed via an interactive genome browser (http://w3lamc.umbr.cas.cz/lamc/resources.html).