Abstract
Background:
Practice patterns and outcomes associated with use of oral anticoagulation (OAC) for arterial thromboembolism prevention following a hospitalization with new-onset atrial fibrillation (AF) during sepsis are unclear.
Methods:
Retrospective, observational cohort study of patients ≥40 years discharged alive following hospitalization with new-onset AF during sepsis across 21 hospitals in the Kaiser Permanente Northern California healthcare delivery system, years 2011–2018. Primary outcomes were ischemic stroke/transient ischemic attack (TIA), with a safety outcome of major bleeding events, both within 1-year of discharge alive from sepsis hospitalization. Adjusted risk differences for outcomes between patients who did and did not receive OAC within 30-days of discharge were estimated using marginal structural models fitted by inverse probability weighting using Super Learning within a target trial emulation framework.
Results:
Among 82,748 patients hospitalized with sepsis, 3992 (4.8%) had new-onset AF and survived to hospital discharge; mean age was 78±11 years, 53% were men, and 70% were White. Patients with new-onset AF during sepsis averaged 45±33% of telemetry-monitoring entries with AF, and 27% had AF present on the day of hospital discharge. Within 1-year of hospital discharge 89 (2.2%) patients experiencing stroke/TIA, 225 (5.6%) had major bleeding, and 1011 (25%) died. Within 30 days of discharge, 807 (20%) patients filled OAC prescriptions, which were associated with higher one-year adjusted risks of ischemic stroke/TIA (5.69% vs 2.32%; risk difference 3.37%; 95% CI 0.36, 6.38) and no significant difference in one-year adjusted risks of major bleeding (6.51% vs. 7.10%, risk difference −0.59%, 95% CI −3.09, 1.91). Sensitivity analysis of ischemic stroke-only outcomes showed risk difference of 0.15%, 95% CI −1.72, 2.03.
Conclusions:
After hospitalization with new-onset AF during sepsis, OAC use was uncommon and associated with potentially higher stroke/TIA risk. Further research to inform mechanisms of stroke and TIA and management of new-onset AF after sepsis is needed.
Keywords: sepsis, arrhythmia
Atrial fibrillation (AF) is common during sepsis.1 Approximately 5% of patients with sepsis develop new-onset AF, which is associated with increased short- and long-term risks of stroke and mortality.2 The coagulopathic state of sepsis3,4 and recovery from sepsis-induced organ dysfunction may predispose to thromboembolic complications of AF, whereas platelet dysfunction, renal failure, and other coagulopathy may also predispose to increased bleeding complications after sepsis. Thus, the relative risks and benefits of oral anticoagulation (OAC) for patients following a hospitalization with new-onset AF during sepsis may differ from those for ambulatory patients with primary AF included in prior randomized trials of OAC for arterial thromboembolic risk reduction after new-onset AF.5,6 However, little is known about current practice patterns and outcomes for OAC use following a hospitalization with new-onset AF during sepsis. We therefore sought to characterize OAC practices following new-onset AF during sepsis, and to evaluate the risks and benefits of anticoagulation in the year following the sepsis hospitalization.
Methods
This study was approved by the Kaiser Permanente Northern California (KPNC) institutional review board. A waiver of informed consent was obtained due to the nature of the study. Because of the sensitive nature of the data collected for this study, requests to access the dataset from qualified researchers trained in human subject confidentiality protocols may be sent to the corresponding author. Reporting of the study was aligned with STROBE and RECORD Guidelines7.
Study Design and Cohort Assembly
This was a retrospective observational study designed to describe practice patterns for use of OAC among patients with new-onset AF complicating a sepsis hospitalization, and to emulate a randomized trial8,9 of OAC prescribed after discharge from a hospitalization with new-onset AF during sepsis. We included adults aged ≥40 years who survived to hospital discharge following an index hospitalization for sepsis with new-onset AF across 21 KPNC hospitals between January 1, 2011 and September 30, 2017 and followed up for 1-year or until death. Sepsis was defined using the Sepsis-3 international consensus definitions by establishing ‘suspected infection’ based on a timed dyad of antibiotics and cultures, with organ dysfunction as defined by a Sequential Organ Failure Assessment (SOFA) score ≥2.10 Patients were included with sepsis present near admission, defined by cultures performed and antibiotics provided within 48 hours of admission and SOFA criteria met within 72 hours. AF was defined by the presence of atrial fibrillation in nurse annotation of cardiac rhythm flowsheets.11 In order to identify a cohort with new-onset AF, patients with diagnosis codes for AF 5 years before the index sepsis hospitalization were excluded. Patients were also excluded if they had used anticoagulation for any reason pre-admission or had non-AF indications for anticoagulation prior to or during sepsis (i.e., mechanical heart valves, venous thromboembolic disease), if they had a prior history of stroke, and if they did not have continual membership in KPNC during the 1-year follow up period (6% of patients with sepsis).
Exposure
OACs were defined as direct oral anticoagulants and vitamin K antagonists dispensed by KPNC network pharmacies after hospital discharge. Prior studies demonstrated that <6% of warfarin prescriptions were filled at out-of-network pharmacies12. The primary analysis defined OAC exposure as prescriptions dispensed within 30 days of hospital discharge, in order to identify OACs dispensed either at hospital discharge or at early post-hospitalization follow up appointments. Prescription durations were available from pharmacy records. Sensitivity analyses evaluated OAC exposure within 1 day after hospital discharge. Because most patients received warfarin, which necessitates frequent dosing adjustments, OAC discontinuation was defined for per protocol analyses as a gap between dispensed prescriptions of >30 days.12 No specific protocols were in place regarding anticoagulants for patients with AF during sepsis during the study period.
Outcomes
The primary outcome of interest was the composite outcome of ischemic stroke and transient ischemic attack, defined using a previously validated algorithm using International Classification of Diseases codes (Table S1).13 As a safety outcome to evaluate the potential risks and benefits of OACs initiated at hospital discharge after sepsis, we also evaluated the outcome of major bleeding events. Major bleeding was defined using previously validated codes including a hospitalization with any diagnosis of intracranial hemorrhage or a principal diagnosis of gastrointestinal hemorrhage.14–18 Outcomes were followed up from the day of discharge after sepsis hospitalization through one year after discharge, or death.
Covariates
We adjusted for variables deemed by clinician co-authors (AJW, LCM, VXL, ASG) to potentially confound estimates of the effect of OAC on outcomes of stroke, major bleeding, or censoring events such as death. Variables were considered potential confounders if they were potential determinants of OAC use, outcomes, or competing risks. Baseline covariates included pre-sepsis factors potentially associated with cardiovascular risk and sepsis severity, such as age at sepsis admission, body mass index, gender, race/ethnicity, Charlson comorbidity index19, CHA2DS2VASC stroke risk score20, antihypertensive, antiplatelet and statin use prior to sepsis, and smoking history. We also adjusted for intra-sepsis factors including source of sepsis, acute severity of illness (based on Laboratory and Acute Physiology score, version 2; [LAPS2]21 and SOFA score22), most extreme laboratory value measure (e.g. lowest or highest value, as appropriate) of complete blood count (e.g. white blood cell count, platelets, bands), serum chemistry parameters (e.g. bicarbonate, creatinine), anticoagulants, coagulation measures and lactate; use of intensive care or life-sustaining therapies (e.g. mechanical ventilation, hemodialysis), vasopressor type, fluid administration (total net intake and output; blood products transfused), the proportion of nursing rhythm documentation indicating AF in cardiac flowsheets, and the presence of AF on cardiac flowsheets on the day of hospital discharge. In per-protocol analyses, we also adjusted for time-varying, post-hospital discharge covariates that could influence censoring events of competing risk of death, anticoagulant use, and outcomes, including minor bleeding events; venous thromboembolism; diagnoses of AF, myocardial infarction, peripheral artery disease, falls, dementia, heart failure, and/or malignancy; head CT scan; procedures including percutaneous coronary intervention, coronary artery bypass graft; blood product transfusions; laboratory values including platelet count, serum creatinine, blood urea nitrogen, hemoglobin A1c, hemoglobin; systolic blood pressure; and new prescriptions of anti-platelet agents.
Statistical Analysis
The primary analysis aimed to emulate a hypothetical trial in which patients in the target population (survived hospitalization with new-onset AF during sepsis) would have been randomized within 30-days of hospital discharge to one of two treatment arms: OAC therapy or no OAC therapy. The one-year risk differences in outcomes of interest between these two treatment arms defined the primary effect measures. Formally, this approach was implemented by fitting saturated logistic marginal structural models (MSM) for the counterfactual discrete-time hazards defined by stochastic (in primary analysis) treatment within 30-days of hospital discharge. In order to estimate the risks and benefits of use of OACs after new-onset AF sepsis, the primary analysis was conducted within a “per-protocol” framework that censored patients at the time of study arm crossover (i.e., starting anticoagulants in the control arm or stopping in the treatment arm). Details of the approach for contrasting the stochastic intervention approach were described in prior methodologically-related work (see Appendix of 23).
Briefly, in order to avoid time-dependent biases such as immortal person-time,8 in the stochastic primary model patients could be assigned probabilistically to either one of the exposure arms in the 30-day period of eligibility for potential anticoagulation assignment after sepsis hospitalization, and then assigned based on whether they received OACs during the 30-day period thereafter. Inverse probability weighting (IPW) was implemented to address confounding and potential attribution bias due to death.24 Propensity scores were calculated for OAC initiation, anticoagulation continuation, and right-censoring due to the competing risk of death using an ensemble learning methodology known as Super Learning.25 In brief, Super Learning combines predicted values from various candidate learners through a weighted average. The selection of the optimal combination of learners is based on cross-validation to protect against over-fitting such that the resulting “super learner” performs asymptotically as well or better than any of the candidate learners considered. Each propensity score was estimated using the following 3 learner types: main-term-only logistic models, random forests (rng)26, and extreme gradient boosting regressions (xgb)27,28. The learner based on logistic regression was combined with 5 screeners that automatically select the first 5, 10, 20, and 30 covariates most associated with the independent variable of the propensity score (exposure or censoring event). The fifth screener selects all covariates considered in the analyses. The rng and xgb learners were combined with 2 screeners that automatically select the first 5 and 10 covariates most associated with the independent variable of the propensity score.
These machine learning approaches were used for both adapting the covariate adjustment set that best predicted anticoagulant dispensation within 30-days of hospital discharge (“covariate selection”) and to allow for complex, non-linear relationships between covariates and exposure/right-censoring. Following current guidelines and practice to address bias concerns over violation of the positivity assumption, inverse probability weights were truncated using the 99th percentile of the weights distribution in each analysis.29,30 Standard error estimates were obtained using the influence curve of the inverse probability estimator for the coefficients of the logistic marginal structural model and the delta method.31,32 The missingness indicator approach was used to address missing baseline and time-dependent covariate values.30,33,34 Longitudinal data were updated daily for 365 days after hospital discharge and structured to respect temporal ordering between covariates, exposure, and outcome using the LtAtStructuR R package35.
Multiple sensitivity analyses were conducted to assess the impact of specific assumptions on the results. In order to assess robustness to different specifications of the propensity score, we conducted sensitivity analyses using standard logistic regression-based propensity scores (rather than Super Learner) to estimate inverse probability weights. We also conduced secondary analyses defining anticoagulant treatment initiation eligibility as the day after hospital discharge, rather than 30-days after discharge (i.e., “static treatment assignment” models). Additionally, we conducted analyses within an intention to treat framework, with OAC assignment groups maintained throughout the follow up period if dispensed within the eligibility period – either within 30-days of sepsis hospitalization discharge in the stochastic treatment assignment analysis, or within one day of hospital discharge in the static treatment assignment analyses. We also conducted a sensitivity analysis using untruncated inverse probability weights. Lastly, we conducted a sensitivity analysis in which the outcome of interest was ischemic stroke (not including TIA). Statistical analyses were conducted using R version 3.6.2 (2019–12-12) based on the stremr R package.36 Study authors KKT, LCM and VXL had access to study data.
Results
Cohort characteristics
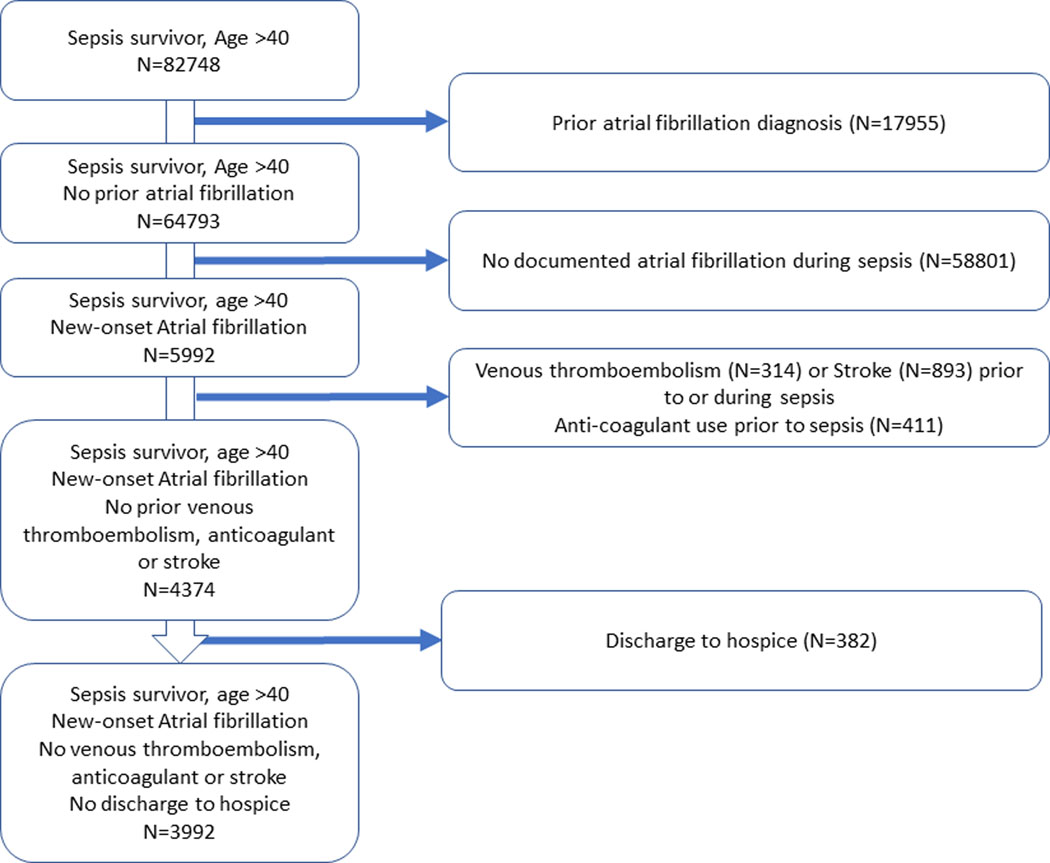
Among 82,748 patients hospitalized with sepsis, 3992 (4.8%) had new-onset AF and survived to hospital discharge (Figure 1). Characteristics of sepsis survivors with new-onset AF are shown in Table 1, and include mean age 78 ± 11 years old, 53% men, and 70% White race. Patients spent an average of 45 ± 33% of cardiac rhythm-documented time in an AF rhythm, 27% had AF present on telemetry on the day of hospital discharge, and 27% received anticoagulants during the sepsis hospitalization. The median CHA2DS2VASC stroke risk score was 4 (IQR 3–5). Cumulative incidence of ischemic stroke and TIA for the cohort 1 year after sepsis hospitalization is shown in Figure S1, with 89 (2.2%) patients experiencing stroke or TIA and 49 (1.2%) experiencing stroke.
Figure 1.
Flowchart describing study entry and exclusion to create the final study cohort.
Table 1.
Characteristics of patients who received and did not receive anticoagulant prescriptions within 30-days of hospital discharge.
| Characteristics | No anticoagulant within 30 days N=3185 | Anticoagulant within 30 days of hospital discharge N= 807 |
|---|---|---|
| N (%) unless otherwise specified | ||
| Pre-sepsis hospitalization | ||
| Age, median [IQR] | 80.0[71.0, 87.0] | 75.0 [67.0, 82.0] |
| Race and Ethnicity | ||
| Asian | 398 (12.5) | 104 (12.9) |
| Black | 203 (6.4) | 52 (6.4) |
| Hispanic | 295 (9.3) | 67 (8.3) |
| Other | 35 (1.1) | 7 (0.9) |
| White | 2254 (70.8) | 577 (71.5) |
| Sex, male | 1741 (54.7) | 469 (58.1) |
| Body mass index, median [IQR] |
26.5 [22.9, 31.2] | 29.2 [25.1, 34.9] |
| Smoking history | 1620 (50.9) | 429 (53.2) |
| Atherosclerotic cardiovascular disease score | 0.239 [0.126, 0.416] | 0.223 [0.115, 0.391] |
| Medications prior to sepsis hospitalization | ||
| Anti-platelet | 183 (5.7) | 26 (3.2) |
| Statin | 1570 (49.3) | 480 (59.5) |
| Anti-hypertensive | 2471 (77.6) | 682 (84.5) |
| Charlson comorbidity Index | 3.0 [2.0, 5.0] | 3.0 [1.0, 5.0] |
| Chronic pulmonary disease | 1212 (38.1) | 327 (40.5) |
| Hemiplegia or paraplegia | 46 (1.4) | 7 (0.9) |
| Acquired Immunodeficiency Syndrome | <5 | <5 |
| Chronic kidney disease | 1417 (44.5) | 362 (44.9) |
| Dementia | 219 (6.9) | 8 (1.0) |
| Diabetes | 1180 (37.0) | 370 (45.8) |
| Diabetes with chronic complications | 909 (28.5) | 287 (35.6) |
| Malignancy, including leukemia and lymphoma | 646 (20.3) | 108 (13.4) |
| Mild liver disease | 87 (2.7) | 13 (1.6) |
| Metastatic solid tumor | 184 (5.8) | 16 (2.0) |
| Peptic ulcer disease | 132 (4.1) | 20 (2.5) |
| Peripheral vascular disorder | 948 (29.8) | 228 (28.3) |
| Renal Disease | 1475 (46.3) | 374 (46.3) |
| Rheumatologic disease | 152 (4.8) | 34 (4.2) |
| Moderate or severe liver disease | 38 (1.2) | 6 (0.7) |
| Myocardial Infarction | 515 (16.2) | 122 (15.1) |
| CHA2DS2-VASc Score for Atrial Fibrillation Stroke Risk | 4.0 [3.0, 4.0] | 3.0 [2.0, 4.0] |
| During sepsis hospitalization | ||
| Percentage of cardiac flowsheet with atrial fibrillation, median [IQR] | 27.3 [8.64, 76.0] | 61.5 [24.6, 96.9] |
| Atrial fibrillation diagnosis code (all patients had atrial fibrillation on cardiac flowsheet) | 1996 (62.7) | 715 (88.6) |
| Atrial fibrillation on cardiac flowsheet on day of discharge | 687 (21.6) | 375 (46.5) |
| Maximum Sequential Organ Failure Assessment score | 4.0 [3.0, 6.0] | 3.000 [2.000, 5.000] |
| Laboratory Acute Physiology Score 2 | 111.0 [86.0, 138.0] | 104.0 [80.0, 128.0] |
| Medications | ||
| Anticoagulants | 448 (14.1) | 634 (78.6) |
| Aspirin | 1869 (58.7) | 556 (68.9) |
| Other Antiplatelet | 252 (7.9) | 55 (6.8) |
| Statin | 1549 (48.6) | 515 (63.8) |
| Inotrope (Dobutamine or Milrinone) | 108 (3.4) | 47 (5.8) |
| Vasopressor | 521 (16.4) | 136 (16.9) |
| Other inpatient procedures | ||
| First ejection fraction measure (%), median [IQR] | 60 [55, 65] | 60 [45, 64] |
| Intensive care unit admission | 1349 (42.4) | 366 (45.4) |
| Days of invasive mechanical ventilation, median [IQR] | 0.0 [0.0, 0.0] | 0.0 [0.0, 0.0] |
| Any invasive mechanical ventilation, N (%) | 397 (12.5) | 120 (14.9) |
| Days of non-invasive mechanical ventilation, median [IQR] | 0.0 [0.0, 0.0] | 0.0 [0.0, 0.0] |
| Any non-Invasive mechanical ventilation, N (%) | 515 (16.2) | 181 (22.4) |
| Hemodialysis | 94 (3.0) | 21 (2.6) |
| Head Computed tomography scan | 351 (11.0) | 57 (7.1) |
| Coronary artery bypass graft procedure | 16 (0.5) | 10 (1.2) |
| Percutaneous intervention procedure | 35 (1.1) | 11 (1.4) |
| Red blood cell transfusion | 445 (14.0) | 51 (6.3) |
| Cryoprecipitate transfusion | 62 (1.9) | 12 (1.5) |
| Platelet transfusion | 78 (2.4) | 9 (1.1) |
| Total blood product volume | 0.0 [0.0, 0.0] | 0.0 [0.0, 0.0] |
| Any blood product, N(%) | 503 (15.8) | 57 (7.1) |
| Other diagnoses during hospitalization | ||
| Peripheral arterial disease | 14 (0.4) | 5 (0.6) |
| Bleeding | 458 (14.4) | 73 (9.0) |
| Fall | 412 (12.9) | 41 (5.1) |
| Malignancy | 343 (10.8) | 36 (4.5) |
| Dementia | 355 (11.1) | 16 (2.0) |
| Heart failure | 1000 (31.4) | 366 (45.4) |
| Myocardial infarction | 237 (7.4) | 74 (9.2) |
| Hospital baseline vital signs and laboratory values (median [IQR]) | ||
| Systolic blood pressure mmHg | 139 [126, 152] | 139 [126, 152] |
| Heart rate, beats per minute | 86 [75, 96] | 89 [77, 100] |
| Hemoglobin | 10.0 [8.6, 11.3] | 10.7 [9.4, 12.0] |
| Hemoglobin A1c % | 6.5 [5.9, 7.9] | 6.5 [6.0, 7.8] |
| Blood urea nitrogen | 21.0 [15.0, 33.0] | 22.0 [16.0, 33.8] |
| Platelets, x 103 | 199 [144, 273] | 208 [158, 281] |
| Creatinine | 0.97 [0.74, 1.35] | 1.01 [0.78, 1.38] |
| Hospital most extreme selected laboratory values (median [IQR]) | ||
| Troponin I | 0.07 [0.03, 0.36] | 0.08 [0.03, 0.36] |
| Brain Natriuretic Peptide | 507 [249, 958] | 539 [299, 942] |
| Creatinine | 1.45 [1.05, 2.22] | 1.44 [1.04, 2.04] |
| Lactic Acid | 2.0 [1.4, 3.3] | 1.9 [1.3, 2.9] |
| Prothrombin time | 15.1 [14.0, 16.5] | 16.3 [14.8, 20.3] |
| Lowest platelet count x 103 | 150 [109, 203] | 151 [115, 203] |
| Hospital length of stay, days | 5.4 [3.7, 8.0] | 5.3 [3.8, 8.0] |
Patterns of anticoagulant prescriptions after sepsis with new-onset atrial fibrillation
The cumulative incidence of OAC prescriptions dispensed after hospital discharge among eligible patients with new-onset AF during a sepsis hospitalization are shown in Figure 2. Among 1057 (26%) patients dispensed OAC in the year after hospital discharge, 593 (56% of those receiving OAC) were dispensed within 1 day of discharge, and 807 (76% of those receiving OAC, including those within 1 day) were dispensed within 30 days of discharge. Use of OAC after sepsis increased over time: in 2011 18% of patients with new-onset AF during sepsis were filled OAC prescriptions within 30 days of discharge as compared with 24% in 2017, ptrend <0.001. (Table S2). Warfarin was the most commonly used OAC (82%), followed by dabigatran (13%). Among the 807 patients who started OAC within 30-days after hospital discharge, 118 received anti-platelet or aspirin concomitant with OAC and 512/807 (63%) met criteria for discontinuation (a gap between prescriptions of more than 30 days) of OAC, with median time from initial prescription to discontinuation of 112 (IQR 61–168) days. OACs were re-initiated among 312 (61% of those that discontinued) at a median of 24 (8–50) days after the first 30-day prescription gap, with 200 patients (25%) not resuming anticoagulants.
Figure 2.
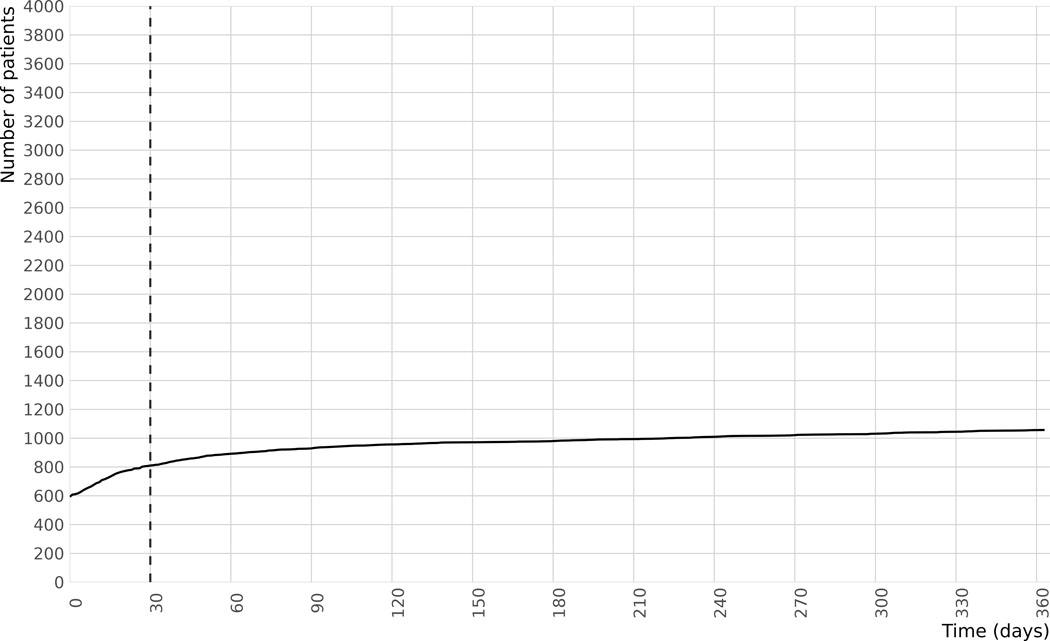
Cumulative incidence of new anticoagulant prescriptions in the year after discharge from a hospitalization with new-onset atrial fibrillation during sepsis. Day 0 is day of hospital discharge, total N of cohort is 3992, total N receiving anticoagulants is 1052.
OACs were more likely dispensed after sepsis to patients who were younger, with more frequent documentation of AF during sepsis (including on the day of hospital discharge), and who received anticoagulants during sepsis. In contrast, patients with higher sepsis severity, bleeding, falls, dementia or malignancy documented during sepsis were less likely to receive anticoagulants. The full list of factors associated with OAC dispensation within 30-days of discharge from a sepsis hospitalization are shown in Table S3.
Outcomes of patients prescribed anticoagulants after hospital discharge as compared to no anticoagulants
Inverse probability weights were a median of 1.3 (IQR 1.1–2.7; Table S4) among patients dispensed OACs and median 1.7 (IQR 1.2–2.5; Table S5) among those not dispensed anticoagulants. Stabilized inverse probability weights for the Super Learner (Table S6) show lower large weights as compared with the logistic propensity score models (Table S7) and Super Learner models demonstrated the lowest cross-validated risk as compared to other methods. In the primary stroke/TIA outcome analysis, 538 patients included initially in the OAC untreated arm crossed over to OAC treated and were censored in the per protocol approach, and 385 included initially in the OAC treated arm crossed over to untreated. Among patients with new-onset AF prescribed OAC within 30 days of discharge from a sepsis hospitalization, 26 (3.2%) had ischemic stroke or TIA, 52 (6.4%) had a major bleeding event, and 84 (10.4%) died within 1-year of hospital discharge. Patients with new-onset AF during sepsis who did not receive OAC within 30 days of hospital discharge had rates of ischemic stroke or TIA of 63 (2.0%), major bleeding of 173 (5.4%) and death of 927 (29.1%) within 1 year of sepsis. Primary MSM analysis comparing patients dispensed versus not dispensed OAC within 30-days of hospital discharge showed higher rates of stroke/TIA (risk difference 3.37%, 95% CI 0.36, 6.38) and no significant differences in major bleeding events (risk difference −0.59%, 95% CI −3.09, 1.91) (Figure 3A and 3B, Figure S2). Similar results were seen in sensitivity analyses (Table 2) for stroke/TIA outcomes using logistic regression to construct propensity scores (Figure S3), intention-to-treat (ITT) analysis of anticoagulants prescribed within 30-days (Figure S4A, Figure S4B), and in per protocol (Figure S5A, Figure S5B) and ITT analyses (Figure S6A, Figure S6B) evaluating OAC use within 24 hours of hospital discharge, and similarly for bleeding outcomes (Figures S7A-D). Sensitivity analyses using untruncated inverse probability weights (Table S8) showed similar findings to the analyses truncating weights at the 99%ile. Sensitivity analysis evaluating ischemic stroke as the outcome (not including TIA) showed a risk difference of 0.15% (95% CI −1.72, 2.03, p=0.87).
Figure 3.
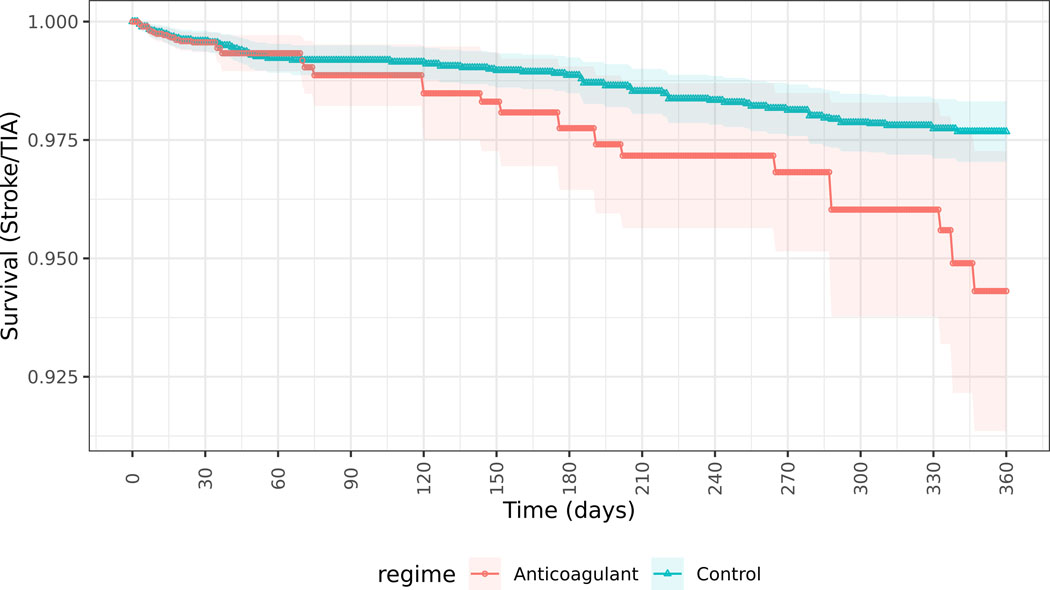
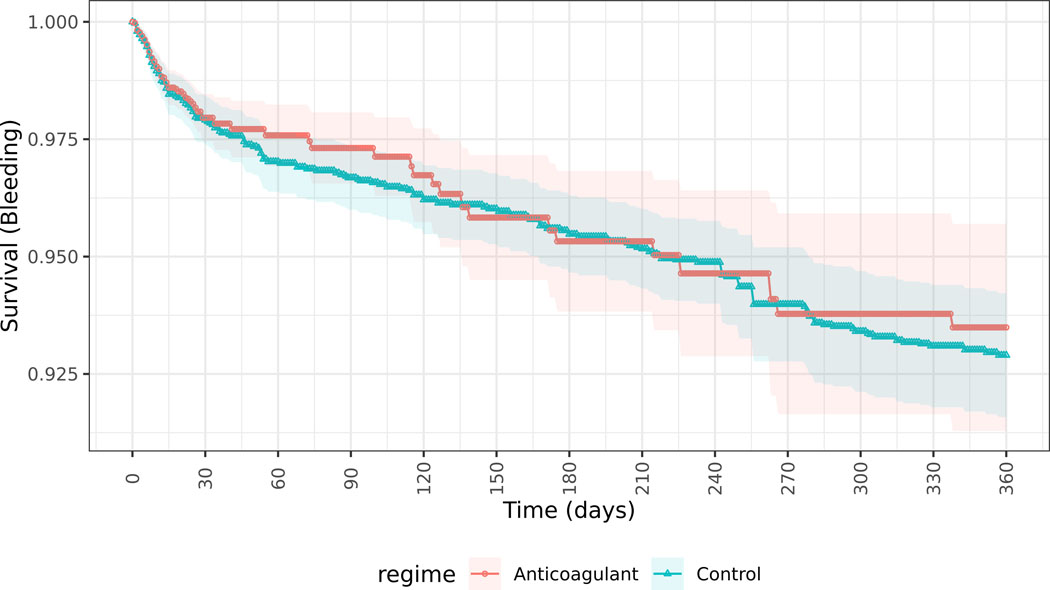
Survival curves of patients receiving anticoagulants (Blue) or not receiving anticoagulants (Red) within 30 days of discharge from hospitalization with sepsis and new-onset atrial fibrillation.
Figure 3a. Incidence of stroke and transient ischemic attack for patients receiving anticoagulants or not receiving anticoagulants within 30 days of discharge from hospitalization with sepsis and new-onset atrial fibrillation, estimated from marginal structural models with Super Learner-generated propensity scores and per protocol analysis.
Figure 3b. Incidence of major bleeding for patients receiving anticoagulants or not receiving anticoagulants within 30 days of discharge from hospitalization with sepsis and new-onset atrial fibrillation, estimated from marginal structural models with Super Learner-generated propensity scores and per protocol analysis.
Table 2.
Results of marginal structural models evaluating causal associations between anticoagulation and risks of stroke/transient ischemic attack (TIA) and bleeding within 1-year after hospitalization with sepsis and new-onset atrial fibrillation.
| Model | Cumulative one-year incidence of outcome in Anticoagulation-exposed per 100 participants | Cumulative one-year incidence of outcome in Anticoagulation-Unexposed per 100 participants | Risk difference (95% CI) Exposed-Unexposed per 100 participants |
|---|---|---|---|
| Outcome: Stroke/TIA | |||
|
Primary adjusted model: Anticoagulation within 30-days of discharge, per-protocol, Super Learner propensity score |
5.69 | 2.32 | 3.37 (0.36, 6.38) |
| Sensitivity Analyses | |||
| Anticoagulation within 30-days of discharge, per-protocol, logistic regression propensity score | 3.13 | 2.26 | 0.87 (−2.37, 4.12) |
| Anticoagulation within 30-days of discharge, intention to treat, Super Learner | 3.92 | 2.32 | 1.60 (0.0, 3.2) |
| Anticoagulation within 24 hours of hospital discharge, per protocol, Super Learner | 5.83 | 2.32 | 3.51 (−0.08, 7.09) |
| Anticoagulation within 24 hours of hospital discharge, intention to treat, Super Learner | 4.77 | 2.39 | 2.38 (−0.11, 4.85) |
| Outcome: Major Bleeding | |||
|
Primary adjusted model: Anticoagulation within 30-days of discharge, per-protocol, Super Learner propensity score |
6.51 | 7.1 | −0.59 (−3.09, 1.91) |
| Sensitivity Analyses | |||
| Anticoagulation within 30-days of discharge, per-protocol, logistic regression propensity score | 6.35 | 8.31 | −1.96 (−5.03, 1.12) |
| Anticoagulation within 30-days of discharge, intention to treat, Super Learner | 7.93 | 7.10 | 0.83 (−1.37, 3.03) |
| Anticoagulation within 24 hours of hospital discharge, per protocol, Super Learner | 7.47 | 7.10 | 0.37 (−3.59, 4.32) |
| Anticoagulation within 24 hours of hospital discharge, intention to treat, Super Learner | 7.67 | 7.11 | 0.56 (−2.78, 3.91) |
Discussion
We present multiple novel findings with regards to practice patterns and outcomes of anticoagulant use after a hospitalization with new-onset AF during sepsis. Across 21 hospitals, one in five patients with new-onset AF during sepsis were dispensed an anticoagulant within 30-days of discharge from a hospitalization with new-onset AF during sepsis, with a modest increase in OAC use from 2011 to 2017. Patients starting anticoagulants after a hospitalization with new-onset AF during sepsis differed across many characteristics from patients who did not receive anticoagulants, and frequently had interruptions, discontinuation, re-initiation and crossover of anticoagulant use in the 1-year period after sepsis. Overall, rates of stroke and TIA (~2%) were lower than rates of major bleeding (~5%) in the year after sepsis, with primary models showing increased stroke/TIA (and sensitivity analyses showing similar rates of stroke or stroke and TIA) without increased bleeding among patients dispensed anticoagulants within 30-days of discharge from a hospitalization with new-AF during sepsis, as compared to not dispensed anticoagulants after sepsis discharge.
Few prior studies have explored treatment patterns after hospital discharge for patients with new-onset AF during sepsis. A study using Quebec administrative claims data investigated outcomes after hospitalization among 102 patients with new-onset AF during sepsis and found that 27% received anticoagulants within 30 days after discharge, with a stroke rate of 1.67 per 100 person-years and no significant association between use of anticoagulants and stroke or bleeding risk.37 Similarly, a single center study of 32 patients with new-onset AF during sepsis found that 29% received anticoagulants after hospital discharge, but did not estimate stroke or bleeding risks. Thus, compared with our findings in a much larger, multicenter sample, prior studies also showed similarly low rates of anticoagulant use and similar stroke risks37,38 in the year following new-onset AF during sepsis. Further studies are needed to explore factors driving anticoagulant prescribing after new-onset AF in sepsis.
The finding that anticoagulants were associated with increased ischemic stroke risks was contrary to our expectations based on prior evidence from settings outside of sepsis.6 Based on observed prescribing patterns, we speculate that anticoagulants may have been targeted to patients with the highest perceived risks of AF recurrence or persistence, and the lowest perceived competing risks of death after sepsis. This was evidenced by the substantial difference in 1-year post-sepsis mortality in patients receiving versus not receiving anticoagulation near discharge. Thus, if a perceived (but unmeasured) indication for anticoagulants was associated with higher stroke or TIA risk, or if decisions to de-escalate medical care (and avoid anticoagulants) after sepsis were associated with lower risks of stroke (potentially through a higher competing risk of death), then residual confounding may contribute to our findings of increased stroke risk associated with anticoagulants despite our use of causal inference approaches to mitigate bias. However, the lack of predictive validity of CHA2DS2-VASc in the context of short-term sepsis-associated stroke risk39 decreases the likelihood that clinicians could accurately target anticoagulant prescribing to stroke risk following sepsis using tools developed for outpatient settings. Additionally, sensitivity analyses evaluating only stroke as an outcome (though limited by lower outcome event rates), or using alternative methods for confounding adjustment, did not suggest significantly higher rates of stroke with OAC. Despite these limitations, our findings suggest it is unlikely that anticoagulants started for new-onset AF during sepsis would achieve the 50–60%6 stroke risk reduction observed among ambulatory patients without secondary AF.
The absolute 1-year risks of ischemic stroke or TIA (~2%) after new-onset AF during sepsis were lower than previously reported 1-year stroke risks among community dwelling patients with AF without a secondary precipitant such as sepsis, but with similar median CHA2DS2-VASc scores (e.g., prior studies estimate a ~4% 1-year stroke risk for CHA2DS2-VASc of 4).5 As compared with AF diagnosed in the ambulatory care setting, we speculate that the lower rates of AF recurrence after onset during sepsis than in ‘non-secondary AF’ settings,38,40 high competing risks of death after sepsis, and potentially non-cardioembolic mechanisms of stroke after sepsis41 may have contributed to lower effectiveness of anticoagulation for stroke prevention after sepsis. Additional research using long-term rhythm monitoring42 is likely needed to determine characteristics of patients at highest risk for AF recurrence and stroke risk who may potentially benefit from anticoagulation following new-onset AF during sepsis.
Our study has additional strengths and limitations. Strengths include use of data from nearly 4000 patients across 21 hospitals, with detailed, electronic health record-based clinical information up to 5 years prior to sepsis onset, during the sepsis hospitalization, and for 1-year following sepsis to inform rigorous approaches to observational causal inference. Outcomes were defined by validated algorithms and results were robust to multiple sensitivity analyses. Limitations include the potential for residual confounding – as evidenced by higher crude mortality rates and different patient characteristics for patients who did and did not receive anticoagulants, potentially resulting in less competing risks of death, higher baseline stroke rates and lower bleeding risks among patients selected for anticoagulation that were incompletely accounted for with measured confounders. The absence of data regarding new decisions to limit treatments may that impact decisions to pursue anticoagulation after sepsis and censoring events, is an additional limitation. Super Learner resulted in lower proportions of observations with extreme weights than logistic regression, and lower cross-validated risks, potentially explaining differences in point estimates and standard errors between the two methods. Additional limitations include the use of warfarin - rather than direct oral anticoagulants - for most of the study cohort with inability to track adherence; the potential for prevalent user bias from initiation of anticoagulants during the sepsis hospitalization; use of electronic health record data not created for research purposes with unclear generalizability to other health systems, and the exclusion of patients who did not have continual membership in KPNC in the year after hospitalization.
In conclusion, a limited proportion of the patients with new-onset AF during sepsis initiated OAC following their sepsis hospitalization. Target trial emulation analyses did not suggest effectiveness of OAC initiated or continued after hospital discharge for reduction of 1-year stroke risks after new-onset atrial fibrillation during sepsis. Thus, additional strategies are needed to evaluate and reduce the risk of AF-associated stroke following sepsis.
Supplementary Material
What is Known.
New-onset atrial fibrillation (AF) during sepsis is common, associated with recurrence of AF, and ischemic stroke after hospital discharge; however, patterns of oral anticoagulant use and associated stroke and bleeding outcomes after sepsis are unclear.
What the Study Adds.
In a large, multicenter cohort of patients surviving a hospitalization with sepsis and new-onset AF, use of oral anticoagulants after hospital discharge was uncommon.
Patients with sepsis and new-onset AF who used oral anticoagulants at hospital discharge did not have lower ischemic stroke or higher bleeding risks than patients who were not prescribed anticoagulants.
Sources of Funding:
Funding for this study was supplied by National Instituted of Health National Heart Lung and BIood Institute R01 HL139751
Non-standard Abbreviations and Acronyms
- AF
Atrial Fibrillation
- CHA2DS2VASC
Congestive Heart Failure, Hypertension, Age ≥75 (Doubled), Diabetes, Stroke (Doubled), Vascular Disease, Age 65 to 74 and Sex Category (Female)
- CT
Computerized Tomography
- IPW
Inverse Probability Weighting
- IQR
Interquartile Range
- ITT
Intention-to-treat
- KPNC
Kaiser Permanente Northern California
- LAPS2
Laboratory and Acute Physiology Score, Version 2
- ML
Machine Learning
- MSM
Marginal Structural Models
- OAC
Oral Anticoagulant
- RECORD
REporting of studies Conducted using Observational Routinely collected Data
- RNG
Random Forests
- SOFA
Sequential Organ Failure Assessment
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
- TIA
Transient Ischemic Attack
- XGB
Extreme Gradient Boosting Regressions
Footnotes
Disclosures:
None
AJW drafted the manuscript. AJW, VXL, MD, LCM, ASG, and RD conceptualized and designed the work and interpreted results. KKT, PK, YWL, HC, and YD obtained the data and/or conducted analyses. All authors revised the manuscript for intellectual content and approved the final manuscript.
References
- 1.Walkey AJ, Wiener RS, Ghobrial JM, Curtis LH, Benjamin EJ. Incident Stroke and Mortality Associated with New-onset Atrial Fibrillation in Patients Hospitalized with Severe Sepsis. JAMA. 2011;306(20):2248–2254. doi: 10.1001/jama.2011.1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosch NA, Cimini J, Walkey AJ. Atrial Fibrillation in the ICU. Chest. 2018;154(6):1424–1434. doi: 10.1016/j.chest.2018.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Semeraro N, Ammollo CT, Semeraro F, Colucci M. Coagulopathy of Acute Sepsis. Semin Thromb Hemost. 2015;41(6):650–658. doi: 10.1055/s-0035-1556730 [doi] [DOI] [PubMed] [Google Scholar]
- 4.Ostrowski SR, Berg RM, Windeløv NA, Meyer MA, Plovsing RR, Møller K, Johansson PI. Coagulopathy, catecholamines, and biomarkers of endothelial damage in experimental human endotoxemia and in patients with severe sepsis: a prospective study. J Crit Care. 2013;28(5):586–596. doi: 10.1016/j.jcrc.2013.04.010 [DOI] [PubMed] [Google Scholar]
- 5.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, et al. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: Executive Summary. Circulation. 2014;130(23):2071–2104. doi: 10.1161/CIR.0000000000000040 [DOI] [PubMed] [Google Scholar]
- 6.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857–867. doi: 10.7326/0003-4819-146-12-200706190-00007 [DOI] [PubMed] [Google Scholar]
- 7.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Ann Intern Med. 2007;147(8):573. doi: 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 8.Hernan MA, Sauer BC, Hernandez-Diaz S, Platt R, Shrier I. Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. J Clin Epidemiol. 2016;79:70–75. doi:S0895–4356(16)30136–6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernan MA, Robins JM. Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available. Am J Epidemiol. 2016;183(8):758–764. doi: 10.1093/aje/kwv254 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding E, Albuquerque D, Winter M, Binici S, Piche J, Bashar SK, Chon S, Walkey AJ, McManus DD. Novel Method of Atrial Fibrillation Case Identification and Burden Estimation Using the MIMIC III Electronic Health Data Set. J Intensive Care Med. 2019;34(10):851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Go AS, Hylek EM, Chang Y, Phillips KA, Henault LE, Capra AM, Jensvold NG, Selby JV, Singer DE. Anticoagulation Therapy for Stroke Prevention in Atrial Fibrillation. JAMA. 2003;290(20):2685. doi: 10.1001/jama.290.20.2685 [DOI] [PubMed] [Google Scholar]
- 13.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031 [DOI] [PubMed] [Google Scholar]
- 14.Quinn GR, Singer DE, Chang Y, Go AS, Borowsky LH, Fang MC. How Well Do Stroke Risk Scores Predict Hemorrhage in Patients With Atrial Fibrillation? Am J Cardiol. 2016;118(5):697–699. doi: 10.1016/j.amjcard.2016.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kazi DS, Leong TK, Chang TI, Solomon MD, Hlatky MA, Go AS. Association of spontaneous bleeding and myocardial infarction with long-term mortality after percutaneous coronary intervention. J Am Coll Cardiol. 2015;65(14):1411–1420. doi: 10.1016/j.jacc.2015.01.047 [DOI] [PubMed] [Google Scholar]
- 16.Fang MC, Go AS, Chang Y, Borowsky LH, Pomernacki NK, Udaltsova N, Singer DE. A new risk scheme to predict warfarin-associated hemorrhage: The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol. 2011;58(4):395–401. doi: 10.1016/j.jacc.2011.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singer DE, Chang Y, Fang MC, Borowsky LH, Pomernacki NK, Udaltsova N, Go AS. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med. 2009;151(5):297–305. doi:151/5/297 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang MC, Chang Y, Hylek EM, Rosand J, Greenberg SM, Go AS, Singer DE. Advanced age, anticoagulation intensity, and risk for intracranial hemorrhage among patients taking warfarin for atrial fibrillation. Ann Intern Med. 2004;141(10):745–752. doi:141/10/745 [pii] [DOI] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 20.Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–272. doi: 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- 21.Escobar GJ, Gardner MN, Greene JD, Draper D, Kipnis P. Risk-adjusting hospital mortality using a comprehensive electronic record in an integrated health care delivery system. Med Care. 2013;51(5):446–453. doi: 10.1097/MLR.0b013e3182881c8e [DOI] [PubMed] [Google Scholar]
- 22.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 23.Schroeder EB, Neugebauer R, Reynolds K, Schmittdiel JA, Loes L, Dyer W, Pimentel N, Desai JR, Vazquez-Benitez G, Ho PM, et al. Association of Cardiovascular Outcomes and Mortality With Sustained Long-Acting Insulin Only vs Long-Acting Plus Short-Acting Insulin Treatment. JAMA Netw Open. 2021;4(9):e2126605. doi: 10.1001/jamanetworkopen.2021.26605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15(5):615–625. doi: 10.1097/01.ede.0000135174.63482.43 [DOI] [PubMed] [Google Scholar]
- 25.van der Laan MJ, Polley EC, Hubbard AE. Super learner. Stat Appl Genet Mol Biol. 2007;6:Article25. doi: 10.2202/1544-6115.1309 [doi] [DOI] [PubMed] [Google Scholar]
- 26.Breiman L.Random Forests. Mach Learn. 2001;45(1):5–32. [Google Scholar]
- 27.Chen T, Guestrin C, Benesty M, Khotilovich V, Tang Y. xgboost: Extreme Gradient Boosting R package https://cran.r-project.org/web/packages/xgboost/index.htm and https://github.com/dmlc/xgboost. [Google Scholar]
- 28.Chen T, Guestrin C. XGBoost. Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining. doi: 10.1145/2939672.2939785 [DOI] [Google Scholar]
- 29.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656–664. doi: 10.1093/aje/kwn164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blake HA, Leyrat C, Mansfield KE, Tomlinson LA, Carpenter J, Williamson EJ. Estimating treatment effects with partially observed covariates using outcome regression with missing indicators. Biom J. 2020;62(2):428–443. doi: 10.1002/bimj.201900041 [DOI] [PubMed] [Google Scholar]
- 31.van der Vaart A.Asymptotic Statistics. Cambridge: University Press; 1998. [Google Scholar]
- 32.Neugebauer R, Schmittdiel JA, van der Laan MJ. A Case Study of the Impact of Data-Adaptive Versus Model-Based Estimation of the Propensity Scores on Causal Inferences from Three Inverse Probability Weighting Estimators. Int J Biostat. 2016;12(1):131–155. doi: 10.1515/ijb-2015-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stuart EA. Matching methods for causal inference: A review and a look forward. Stat Sci. 2010;25(1):1–21. doi: 10.1214/09-STS313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hernán MA, McAdams M, McGrath N, Lanoy E, Costagliola D. Observation plans in longitudinal studies with time-varying treatments. Stat Methods Med Res. 2009;18(1):27–52. doi: 10.1177/0962280208092345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neugebauer R, Pimentel N, Hejazi N.LtAtStructuR R package. https://github.com/romainkp/LtAtStructuR. [Google Scholar]
- 36.Sofrygin O, van der Laan MJ, Neugebauer R. stremr R package. https://github.com/osofr/stremr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quon MJ, Behlouli H, Pilote L. Anticoagulant Use and Risk of Ischemic Stroke and Bleeding in Patients With Secondary Atrial Fibrillation Associated With Acute Coronary Syndromes, Acute Pulmonary Disease, or Sepsis. JACC Clin Electrophysiol. 2018;4(3):386–393. doi: 10.1016/j.jacep.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 38.Walkey AJ, Hammill BG, Curtis LH, Benjamin EJ. Long-term outcomes following development of new-onset atrial fibrillation during sepsis. Chest. 2014;146(5):1187–1195. doi: 10.1378/chest.14-0003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walkey AJ, Quinn EK, Winter MR, McManus DD, Benjamin EJ. Practice Patterns and Outcomes Associated With Use of Anticoagulation Among Patients With Atrial Fibrillation During Sepsis. JAMA Cardiol. 2016. Sep 1;1(6):682–90. doi: 10.1001/jamacardio.2016.2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lubitz SA, Yin X, Rienstra M, Schnabel RB, Walkey AJ, Magnani JW, Rahman F, McManus DD, Tadros TM, Levy D, et al. Long-term outcomes of secondary atrial fibrillation in the community: the Framingham Heart Study. Circulation. 2015;131(19):1648–1655. doi: 10.1161/CIRCULATIONAHA.114.014058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merdji H, Schini-Kerth V, Meziani F, Toti F. Long-term cardiovascular complications following sepsis: is senescence the missing link? Ann Intensive Care. 2021;11(1):166. doi: 10.1186/s13613-021-00937-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ko D, Dai Q, Flynn DB, Bosch NA, Helm RH, Monahan KM, Andersson C, Anderson CD, Walkey AJ. Meta-Analysis of Randomized Clinical Trials Comparing the Impact of Implantable Loop Recorder Versus Usual Care After Ischemic Stroke for Detection of Atrial Fibrillation and Stroke Risk. Am J Cardiol. 2022;162:100–104. doi: 10.1016/j.amjcard.2021.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.