Abstract
Background:
The cannabinoid cannabidiol (CBD) is currently under investigation as a pharmacotherapy for alcohol use disorder. The aim of the present study was to examine whether acute and chronic treatment with pure CBD would decrease alcohol seeking and consumption behaviors or alter drinking patterns in male baboons with extensive histories of daily alcohol intake (1g/kg/day).
Methods:
Seven male baboons self-administered oral alcohol (4% w/v) in a validated chained schedule of reinforcement (CSR) procedure that modeled periods of anticipation, seeking, and consumption. In Experiment 1, CBD (5–40 mg/kg) or vehicle (peanut oil, USP) was administered orally 15- or 90-minutes prior to the start of the session. In Experiment 2, oral doses of CBD (10–40 mg/kg) or vehicle were administered for 5 consecutive days during ongoing alcohol access under the CSR. In addition, behavioral observations were conducted to assess potential drug side effects (e.g., sedation, motor incoordination) following chronic CBD treatment immediately after the session and 24-hours after drug administration.
Results:
Across both experiments, baboons self-administered an average of 1 g/kg/day of alcohol under baseline conditions. Administration of acute or chronic CBD at doses (150–1200 mg total CBD dose/day) that encompassed purported therapeutic range did not significantly reduce alcohol seeking, self-administration or intake (g/kg), and drinking patterns (i.e., number of drinks/bouts, bout duration, nor interdrink interval) also were not altered. There were no observable behavioral disruptions following CBD treatment.
Conclusions:
In sum, the current data do not support use of pure CBD as an effective pharmacotherapy to reduce ongoing excessive drinking.
Keywords: Alcohol, Cannabidiol, reinforcement, self-administration, alcohol-related cues
1. Introduction
Alcohol use disorder (AUD) is a highly prevalent, highly comorbid, chronic condition that affects over 100 million people globally (Burnette et al. 2022). Within the United States alone, AUD contributes to approximately 88,000 deaths annually (Mason and Heyser, 2021), and produces an economic burden of $250 billion dollars across the USA (Ray et al. 2019). The hallmark characteristics of AUD include an impaired ability to stop or control alcohol intake despite adverse consequences (Mason and Heyser, 2021). AUD is associated with comorbid mood disorders (anxiety, depression), as well as alcohol-associated conditions such as acute and chronic inflammation, and neuropathy (Castillo-Carniglia et al. 2019, Rehm 2011, Julian et al. 2019). Despite the high prevalence of AUD, the limited available treatments are only modestly effective and are severely under-utilized (Burnette et al. 2022), indicating a need for novel strategies and pharmacotherapies.
Cannabidiol (CBD) is one of the main constituents of Cannabis sativa L.; certain strains of Cannabis sativa L. are cultivated to contain high amounts of CBD (e.g., CBD-dominant) and CBD is also extracted from hemp and sold in a variety of formulations (e.g., edibles, tinctures, oils). An oral formulation of CBD has demonstrated efficacy as an anticonvulsant (FDA, 2018) and has advanced to clinical trials to evaluate therapeutic potential to alleviate pain, anxiety, inflammation, and depression (Devinsky et al. 2014, Kogan et al. 2007 Blessing et al. 2015). The exact mechanism of action of CBD is unclear, but it has been proposed to act in part via the endogenous cannabinoid system as a partial agonist at and a neuroimmune modulator of the cannabinoid type-2 receptor (CB2) and possibly via a negative allosteric mechanism at cannabinoid type-1 receptors (CB1) (LaPrairie et al. 2015). CBD also has pharmacodynamic effects on nicotinic acetylcholine receptors (nAChR), serotoninergic 1A receptors (5-HT1A), and mu-opioid receptors (Gonzalez-Cuevas et al. 2018). CBD has been considered a pharmacotherapeutic candidate for AUD treatment due to its anxiolytic, anti-convulsant, anti-inflammatory and neuroprotective effects (Campos et al. 2016). Furthermore, CBD has no detectable abuse liability (Haney et al. 2015; Babalonis et al. 2017) and an oral CBD formulation is already FDA-approved for human use to treat epileptic seizures.
To date, several preclinical studies conducted in rodents have seen promising results for CBD effects on alcohol intake and alcohol-associated behaviors. In mice, chronic administration of CBD (30–120 mg/kg, ascending dose order) decreased voluntary alcohol intake and preference in a two-bottle choice paradigm (Viudez-Martinez et al. 2018). Further, chronic CBD (30 mg/kg/day) reduced the reinforcing and motivational properties of alcohol in an operant paradigm (Viudez-Martinez et al. 2018). In this same study, CBD given during a test of reinstatement to alcohol-seeking behavior reduced responding after treatment with 60 mg/kg then 120 mg/kg CBD (Viudez-Martinez et al. 2018). A separate study with rats also demonstrated effectiveness of CBD to reduce stress-induced and cue-induced reinstatement (Gonzalez-Cuevas et al. 2018). Rodent studies of CBD have also shown promise in affecting behaviors comorbid with AUD in humans. In rats, CBD reduced anxiety-like behaviors in alcohol naive and alcohol experienced animals (Gonzalez-Cuevas et al. 2018). Further, several studies in rodents have indicated neuroprotective effects of CBD on alcohol-induced neurodegeneration (Liput et al. 2013; Hamelink et al. 2005), as well as reduced hepatic inflammation, metabolic dysregulation and liver steatosis induced by chronic and binge alcohol feeding (Liput et al. 2013; Hamelink et al. 2005; Wang et al. 2005; Yang et al. 2014). To date, the effects of CBD on alcohol seeking and self-administration have not yet been tested in nonhuman primate models.
Our laboratory employs a baboon model where alcohol is self-administered under a chained-schedule of reinforcement (CSR) that was designed to evaluate seeking and consumption within the same session (Weerts et al. 2006). The CSR procedure reliably produces alcohol self-administration at high levels (∼1.0 g/kg per day). Blood alcohol levels (BALs) in excess of 0.08% after comparable alcohol intake has been demonstrated in multiple prior studies in using this procedure in our baboons (Kaminski et al. 2008; Kaminski et al. 2014; Holtyn et al. 2014; Holtyn et al. 2017a). Further, alcohol metabolism and pharmacokinetic parameters in baboons are more similar to humans than rodents (Fridman and Popova, 1988; Jolivette and Ward, 2005), which may yield information on potential drug interactions with alcohol that may not be apparent in rodent models. Other AUD pharmacotherapies we have assessed include naltrexone, varenicline, baclofen, mifepristone, and novel benzodiazepine-GABA receptor modulators (Duke et al. 2012; Holtyn et al. 2017a; Holtyn et al. 2017b; Holtyn et al. 2019; Kaminski et al. 2012a; Kaminski et al. 2012b; Kaminski et al. 2014). Thus, the CSR procedure in baboons provides insightful information and can improve prediction of efficacy of potential therapeutics in humans.
Therefore, the aim of the current study was to determine if acute and chronic administration of CBD could reduce alcohol seeking and self-administration in baboons with an extensive history of daily alcohol self-administration. Additionally, daily behavioral observations were assessed to verify any potential disruptions to species-typical behaviors associated with administration of CBD following alcohol administration.
2. Methods
2.1. Subjects
Seven adult male baboons (Papio anubis; Southwest Foundation for Biomedical Research, San Antonio, TX) were housed singly in cages that also served as the experimental chambers. All baboons had extensive histories of chronic alcohol self-administration under the CSR paradigm (Mean ± SEM: 10.4 ± 2.2 years). Baboons were not food deprived and received primate chow (50 to 73 kcal/kg), fresh fruit or vegetables, and a children’s chewable multivitamin daily. Water was available ad libitum, except during experimental sessions. The facilities were maintained in accordance with USDA and AAALAC standards. The protocol was approved by the JHU Animal Care and Use Committee and followed the Guide for the Care and Use of Laboratory Animals (2011).
2.2. Apparatus
Sessions were conducted in modified cages as described previously (Weerts et al., 2006). Each cage contained a panel with 2 vertically operated levers, 2 different colored jewel lights mounted above each lever, and a drinkometer (connected to a calibrated 1,000-ml bottle) with 2 white and 2 green lights that surrounded a protruding drink spout. Contact with the spout operated a solenoid valve that delivered fluid for up to 5 seconds or until contact stopped. A separate panel contained 3 colored cue lights. A speaker was mounted above the cages for the presentation of auditory stimuli. Experimental events were controlled remotely using Med Associates (Fairfax, VT) software and hardware interfaced with a computer.
2.3. Drugs
Ethyl alcohol (190 Proof; Pharmco-AAPER, Brookville, CT) was diluted with reverse osmosis water to concentrations of 4% w/v alcohol. Cannabidiol (synthetic) was provided by the National Institute on Drug Abuse (NIDA) Drug Supply Program. CBD was mixed with 1mL of 100% peanut oil using sonication and vortex for an oral suspension. Peanut oil was used as it dissolves lipid-soluble cannabinoids for increased bioavailability (Zgair et al. 2016). CBD (5, 10, 20 and 40 mg/mL) in the peanut oil vehicle was then mixed with peanut butter and spread between two crackers prior to oral administration (see details below). Total doses of CBD in the current study (150–1200 mg total CBD dose/day) were chosen to encompass the purported therapeutic range of CBD in humans (e.g., 100–900 mg/day; White 2019) and represent human equivalent doses of ~250–2000 mg total CBD dose/day; Mordenti and Chapell, 1989).
2.4. Chained Schedule of Reinforcement (CSR) Procedure
Each baboon self-administered alcohol under the CSR (Figure 1A). The CSR includes 3 components, each associated with distinct stimuli (cues) and behavioral contingencies (schedule requirements), which modeled periods of anticipation (Component 1), seeking (Component 2), and consumption (Component 3). This procedure allows for examination of drug effects on responding in the presence of alcohol-related cues that is maintained by conditioned reinforcement (i.e., responding that produces access to alcohol or “seeking”), as well as alcohol self-administration and consumption within the same session. Our laboratory has previously validated this procedure in baboons, demonstrating its sensitivity to pharmacological treatments (Kaminski et al., 2008; Weerts et al., 2006; Holtyn et al., 2017a). Daily sessions began at the same time (0830h) and were signaled by a 3-second tone. During Component 1, all instrumental responses were recorded but had no programmed consequence. The red cue light was illuminated, and a fixed-time (FT) 20-minute schedule was in effect. After 20 minutes elapsed, the red cue light was turned off, and the yellow cue light was illuminated, signaling Component 2.
Figure 1.
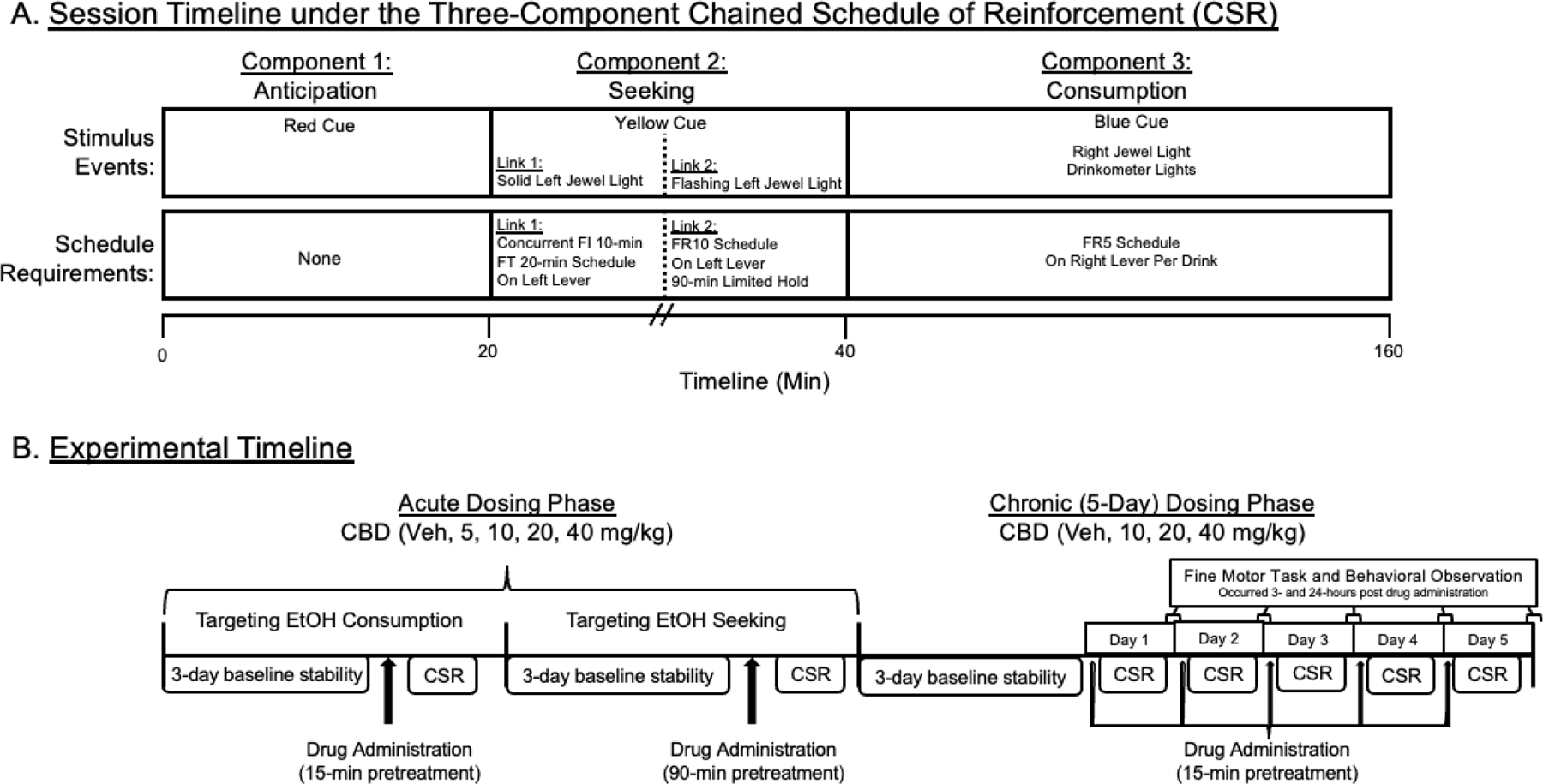
(A) The session timeline for the three-component chain-schedule of reinforcement (CSR). CBD = cannabidiol. Veh = Vehicle. FR = fixed ratio. FI = fixed interval. FT = fixed time. (B) The experimental timeline is shown for the acute and chronic (5-day) dosing of cannabidiol for self-administration, fine motor task, and behavioral observations.
Component 2 consisted of 2 links. During the first link, the jewel light over the left lever was illuminated, and an alternate fixed-interval (FI) 10-minute, FT 20-minute schedule was in effect on the left lever. The first link ended either (i) with the first response on the left lever after 10 minutes elapsed or (ii) automatically after 20 minutes, whichever occurred first. During the second link, the jewel light over the left lever flashed and a fixed-ratio (FR) 10 schedule was in effect on the left lever. Completion of the FR 10 ended Component 2; the yellow cue light and the jewel light were turned off, and Component 3 was initiated. Failure to complete the FR 10 in Component 2 within 90 minutes terminated the session (i.e., no access to alcohol).
During Component 3, the blue cue light and the jewel light over the right lever were illuminated, and the opportunity to orally self-administer alcohol was available according to an FR 10 schedule on the right lever. Completion of each FR turned the jewel light off and turned on the white lights on the drinkometer faceplate, indicating drink availability. Contact with the drinkometer spout turned off the white lights, turned on the green lights on the drinkometer faceplate, and initiated the delivery of fluid for the duration of spout contact or for a programmed maximum duration (5 seconds), whichever came first. That is, each baboon controls the volume of each ‘drink’ depending on mouth contact with the spout up to 5-sec; each ‘drink’ consumed is about 35 ml. Following each drink, all drinkometer lights were turned off, and the jewel light over the right lever was again illuminated. Component 3 ended after 120 minutes, and all programmed stimuli were turned off. Drug administration procedures (described below) were initiated after the following criterion was met: alcohol self-administration intake was ≥ 0.8 g/kg with no increasing or decreasing trends (i.e., ± 20%) for three consecutive days.
2.4.1. Experiment 1: Acute CBD Treatment prior to CSR sessions
Once baseline criterion was met, test conditions occurred with treatment of CBD (5–40 mg/kg) at either 15- or 90-minutes prior to the start of CSR sessions (Figure 1B). The 15-minute pretreatment time was selected so that the blood concentrations of CBD, which occurs approximately 2 hours following oral administration and are maintained for approximately 4 hours (Bergeria et al. 2022), would occur during Component 3 (i.e., alcohol consumption). A total of 5 baboons (GB, HA, LN, RS, WI) completed all CBD doses with a 15-minute pretreatment time. The 90-minute pretreatment time was selected so that the peak blood plasma concentrations of CBD would occur during Component 2 (i.e., alcohol seeking). A total of 6 baboons (DK, HA, HS, LN, RS, WI) completed self-administration under this time condition. A vehicle condition followed each CBD treatment once the baseline CSR criterion was re-established. CBD doses were counterbalanced across subjects. Finally, all acute CBD treatments were separated by at least one week to allow for adequate clearance of CBD and its metabolites (Bergeria et al. 2022). The average number of days between acute CBD administration and its matched vehicle condition were 11.6 (range: 4–32) and 15.3 (range: 4–41) days for 15- and 90-minute pretreatment conditions, respectively.
2.4.2. Experiment 2: Chronic CBD Treatment during CSR sessions
Following Experiment 1, the stability criterion was again met, and CBD treatments (10–40 mg/kg) were administered for 5 consecutive days during ongoing daily alcohol CSR sessions (Figure 1B). CBD and vehicle treatments were administered 15 minutes prior to the start of each alcohol session. CBD treatments were administered in ascending dose order (>1 week between treatments) with the vehicle condition randomized within the dose order. An ascending dose order design was used to examine the potential side effects associated with repeated administration of CBD, which was coupled with behavioral observations (see below) to quantify any adverse reactions. All seven baboons (DK, GB, HS, LN, RAF, RS, WI) completed chronic CBD dosing in Experiment 2.
2.5. Fine Motor Task and Behavioral Observation Procedure.
A fine motor task and behavioral observations were conducted by trained technicians immediately after the CSR session (i.e., after the 2-hours of access to alcohol for self-administration) and 24 hours after the administration of CBD or vehicle. Baboons performed a 2-minute fine motor task, where a custom board containing 6 individual depressed cups, ~1” apart on a Plexiglas board is used. The cups are spaced to fit between the cage bars and fit small, highly preferred food items (e.g., single raisins, peanuts, or M&Ms). The current study used M&Ms. The technician placed the M&Ms, offered the board to the baboon, and started the stopwatch to time the task. Baboons were given up to 2 minutes to retrieve all six M&Ms from the cups, to measure fine motor coordination, motor speed and performance. During the task, technicians recorded the number of M&Ms taken, dropped, and total time (s) to retrieve all 6 M&Ms or 120s maximum time if not all were taken. During the task technicians also recorded occurrence of any limb or body tremors, ataxia, or signs of sedation (lip droop, slow movements) as defined previously (Weerts et al., 1998). In addition, daily intake of food (g) and water (ml) was recorded at the same time each day to allow for detection of changes in intake.
2.6. Data Analysis
For the self-administration procedures, the primary dependent variables were: 1) alcohol seeking in Component 2, defined as the FI responses on the left lever and the latency (s) to complete the FI response requirement, and 2) total alcohol consumption, defined as the number of responses on the active self-administration lever and the total volume of 4% alcohol consumed (in mLs). Alcohol intake (in g/kg) was calculated based on individual body weights and the total volume of alcohol consumed. To analyze any effects of CBD on drinking patterns, a secondary analysis of CBD on drinking bouts was conducted. As defined previously, drinking bouts were defined as 2 or more drinks with less than 5 minutes between each drink, beginning from the first drink (Kaminski and Weerts 2014; Holtyn et al. 2017a). For the bout analysis, outcomes analyzed were 1) time to first drink, 2) total # drinks, 3) # drinking bouts, 4) inter-bout-interval. As the majority of drinks occur in the first bout, we also analyzed 1) # drinks in bout 1, 2) inter-drink-interval for bout 1 drinks, and 3) bout 1 duration.
Outcomes for the acute studies were analyzed separately using a two-way, repeated-measures analysis of variance (ANOVA) with the within-subject factors of Treatment (Vehicle, CBD) and Dose (5, 10, 20, 40 mg/kg). Differences between active doses and matched vehicles were assessed with paired comparisons using Sidak’s post-hoc test. Outcomes for the chronic CBD dosing sessions were analyzed separately using two-way, repeated-measures ANOVA with within-subject factors of Day and Treatment. Data that were determined to be largely non-normally distributed (via Shapiro-Wilk test; results shown in Supplemental Table 1) were analyzed with the Friedman’s test, followed by Wilcoxon signed-rank tests with a Bonferroni correction applied. For all statistical analyses, a p-value of 0.05 or less was considered significant. Statistics were performed using SPSS Statistics 28 and GraphPad Prism 9.5.
2. Results
3.1. Experiments 1: Alcohol Seeking and Self-Administration Following Acute CBD Treatment
During baseline sessions, alcohol intake was stable prior to acute CBD treatments with a grand mean of 1.06 g/kg (SEM = 0.07 g/kg). There were no differences between matched vehicle administrations on any outcome tested (p’s>0.05), therefore the grand mean is shown in all figures for legibility. Data from matched vehicle administrations are shown in Supplemental Figures 1 and 2.
Figure 2 illustrates the alcohol seeking (Figure 2A–B) and self-administration (Figure 2C–E) following vehicle and CBD (5–40 mg/kg) given 15-minutes prior to session start (i.e., 35 minutes prior to component 2 (seeking) and 45–55 minutes prior to component 3 (self-administration)). Across all primary dependent outcomes, there was no effect of CBD treatment observed on alcohol seeking or alcohol self-administration behavior relative to vehicle responding (p’s > 0.05). Figure 3 illustrates the alcohol seeking (Figure 3A–B) and self-administration (Figure 3C–E) following vehicle and CBD (5–40 mg/kg) given 90-minutes prior to session start (i.e., 110 minutes prior to component 2 (seeking) and 120–130 minutes prior to component 3 (self-administration)). Similarly, there were no alterations in alcohol seeking or alcohol self-administration behaviors across any of the primary outcomes across CBD treatment (p’s > 0.05).
Figure 2.
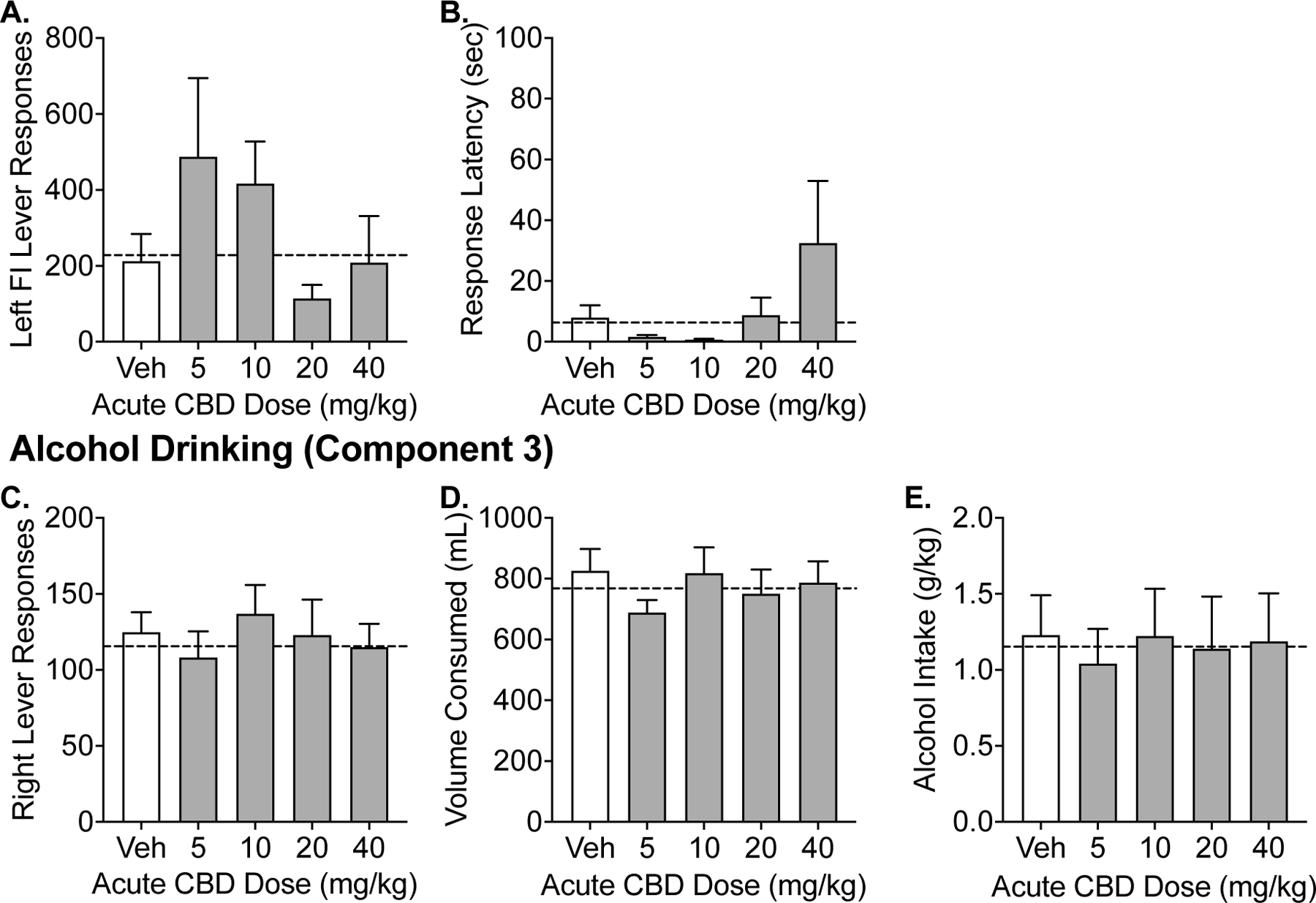
Experiment 1. Effects of acute administration (15 minutes prior to session start) of CBD on alcohol seeking and drinking in the CSR procedure. Data shown are the group means (+ SEM) of the fixed-interval responses and latencies during Component 2 and self-administration responses, volume consumed, g/kg alcohol intake during Component 3. Baseline responding is indicated by the horizontal, dashed lines.
Figure 3.
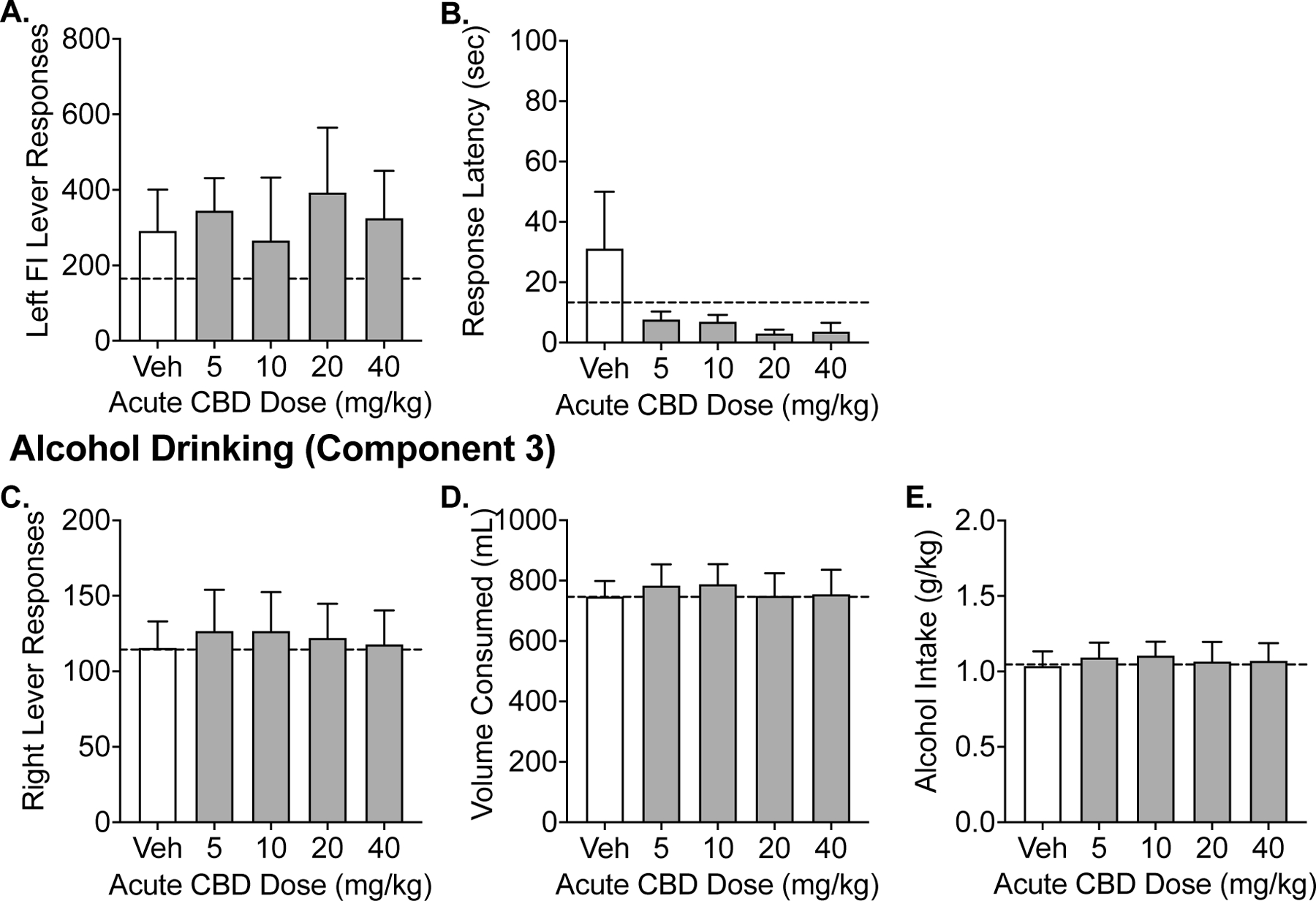
Experiment 1. Effects of acute administration (90 minutes prior to session start) of CBD on alcohol seeking and drinking in the CSR procedure. Data shown are the group means (+ SEM) of the fixed-interval responses and latencies during Component 2 and self-administration responses, volume consumed, g/kg alcohol intake during Component 3. Baseline responding is indicated by the horizontal, dashed lines.
3.2. Experiments 2: Alcohol Seeking and Self-Administration Following Chronic CBD Treatment
During baseline sessions prior to chronic CBD treatment, the grand mean alcohol intake was 0.99 g/kg (SEM = 0.08 g/kg). Across the 5-days of CBD treatment, there were no effects of day on any primary outcomes (seeking (left lever responses, response latency) or self-administration (right lever responses, mL consumed, g/kg intake); p’s > 0.05). Figure 4 illustrates the alcohol seeking (Figure 4A–B) and self-administration (Figure 4C–E) vehicle and CBD (10–40 mg/kg). Across all primary dependent outcomes, chronic CBD did not alter alcohol seeking or alcohol self-administration behavior relative to vehicle responding (p’s > 0.05).
Figure 4.
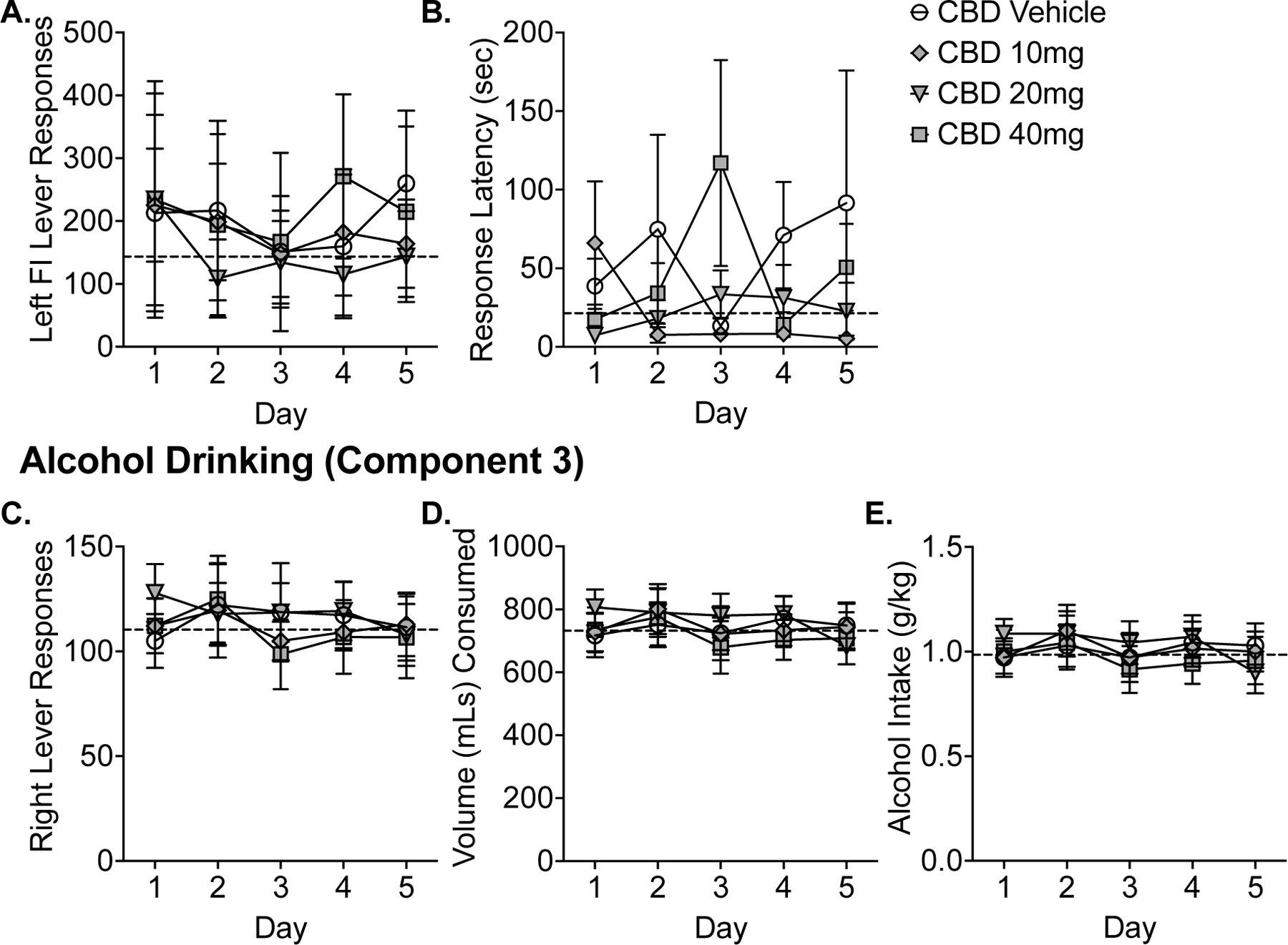
Experiment 2. Effects of chronic administration of CBD on alcohol seeking and drinking in the CSR procedure. Data shown are the group means (+ SEM) by day for the 5-day period for the fixed-interval responses and latencies during Component 2 and self-administration responses, volume consumed, g/kg alcohol intake during Component 3. A grand mean of baseline responding is indicated by the horizontal, dashed lines.
3.3. Bout analysis
To determine if CBD had effects on drinking patterns of baboons during component 3, we analyzed drinking bouts (≥ 2 drinks less than 5 minutes apart). Under baseline conditions, 53.3% (±7.7%; range: 32–88%) of drinks occurred in the first bout, with an average of 3.2 bouts per session (±0.5 bouts; range: 1.6–5.1 bouts) and a median inter-bout-interval of 24.0 minutes ((±3.6 minutes; range: 11.4–40.2 minutes). Outcomes from the bout analysis following acute and chronic CBD are presented in Table 1. Across all outcomes, neither acute nor chronic CBD altered alcohol drinking patterns relative to vehicle responding (p’s > 0.05).
Table 1.
Changes in drinking patterns (Mean (SD)) following acute and chronic CBD administration.
| Outcomes | Baseline (grand mean) | Vehicle (grand mean) | 5 mg/kg CBD | 10 mg/kg CBD | 20 mg/kg CBD | 40 mg/kg CBD | CBD effect: ANOVA F (or Friedman’s test χ2)a | p-value |
|---|---|---|---|---|---|---|---|---|
| Acute (15-min) CBD Pretreatment (N=5) | ||||||||
|
| ||||||||
| Time to first drink (s) | 4.6 (0.5) | 5.1 (2.0) | 3.8 (0.7) | 4.2 (0.8) | 4.0 (0.9) | 4.4 (0.8) | χ2 = 5.49 | p = 0.60 |
| Total # drinks | 22.9 (4.3) | 25.0 (5.9) | 21.6 (7.6) | 27.4 (8.4) | 24.4 (10.3) | 23.0 (6.9) | F (1,12) = 3.27 | p = 0.72 |
| Total # Bouts | 3.0 (1.8) | 3.0 (1.5) | 2.0 (1.0) | 3.4 (2.1) | 3.6 (2.8) | 3.6 (1.8) | F (1,12) = 0.55 | p = 0.50 |
| Median inter-bout-interval (s) | 1354.0 (677.1) | 1121.0 (406.9) | 1381.0 (336.2) | 1197.0 (187.2) | 953.5 (656.2) | 639.3 (310.2) | F (1,6) = 0.47 | p = 0.57 |
| # drinks in Bout 1 | 14.2 (4.1) | 14.7 (4.8) | 17.0 (8.9) | 17.8 (6.2) | 13.8 (8.4) | 11.6 (3.1) | F (1,12) = 1.47 | p = 0.27 |
| Bout 1 duration | 416.3 (171.4) | 510.7 (370.3) | 428.1 (230.7) | 494.1 (225.1) | 325.9 (316.5) | 455.6 (271.1) | F (1,12) = 0.28 | p = 0.62 |
| Inter-drink-interval in Bout 1 (s) | 10.2 (7.4) | 11.4 (11.2) | 11.1 (12.5) | 5.3 (1.8) | 5.8 (1.5) | 8.1 (5.4) | χ2= 6.78 | p = 0.45 |
|
| ||||||||
| Acute (90-min) CBD Pretreatment (N=6) | ||||||||
|
| ||||||||
| Time to first drink (s) | 11.6 (14.7) | 8.5 (8.8) | 4.6 (1.1) | 4.3 (0.4) | 4.7 (0.7) | 5.4 (1.5) | χ2 = 8.40 | p = 0.30 |
| Total # drinks | 22.7 (8.1) | 23.0 (8.6) | 25.3 (13.4) | 25.3 (12.6) | 24.3 (10.9) | 23.3 (11.2) | F (1,12) = 1.31 | p = 0.30 |
| Total # Bouts | 3.2 (1.3) | 2.5 (0.9) | 2.8 (1.6) | 2.5 (1.2) | 3.2 (3.1) | 2.5 (1.6) | F (1,12) = 0.31 | p = 0.60 |
| Median inter-bout-interval (s) | 1135.0 (388.2) | 1116.0 (462.1) | 1297.0 (1394.0) | 1678.0 (1995.0) | 553.9 (356.5) | 719.5 (504.8) | F (1,3) = 28.74 | p = 0.12 |
| # drinks in Bout 1 | 13.0 (4.0) | 15.6 (6.7) | 17.2 (10.9) | 17.0 (14.8) | 14.5 (5.7) | 13.0 (8.3) | F (1,12) = 0.03 | p = 0.88 |
| Bout 1 duration | 566.9 (411.6) | 752.1 (775.4) | 888.2 (953.3) | 935.2 (1464.0) | 595.1 (497.0) | 606.1 (535.3) | F (1,12) = 0.002 | p = 0.97 |
| Inter-drink-interval in Bout 1 (s) | 18.3 (13.3) | 20.7 (18.8) | 19.2 (16.5) | 44.3 (53.6) | 13.4 (9.1) | 18.4 (13.7) | χ2= 0.84 | p = 0.99 |
|
| ||||||||
| 5-Day Chronic (15-min) CBD Pretreatment (N=7) | ||||||||
|
| ||||||||
| Time to first drink (s) | 35.9 (72.6) | 75.4 (167.9) | -- | 39.1 (87.2) | 18.6 (34.3) | 148.1 (339.7) | χ2 = 3.86 | p = 0.28 |
| Total # drinks | 21.8 (6.1) | 22.7 (8.7) | -- | 22.5 (5.6) | 23.4 (7.0) | 21.7 (8.6) | F (1.98, 11.88) = 0.39 | p = 0.69 |
| Total # Bouts | 3.4 (1.2) | 3.6 (1.3) | -- | 3.9 (1.2) | 3.9 (1.2) | 3.8 (1.8) | F (2.27, 13.61) = 0.27 | p = 0.80 |
| Median inter-bout-interval (s) | 1556.0 (1044.0) | 1219.0 (846.5) | -- | 1286.0 (797.7) | 1224.0 (658.7) | 1254.0 (627.8) | F (1.27, 7.64) = 0.06 | p = 0.87 |
| # drinks in Bout 1 | 10.4 (5.1) | 10.7 (9.2) | -- | 9.8 (7.0) | 10.9 (8.1) | 8.9 (6.0) | F (1.33, 7.97) = 0.52 | p = 0.54 |
| Bout 1 duration | 444.8 (438.6) | 516.1 (783.5) | -- | 365.4 (462.7) | 495.9 (724.9) | 408.2 (595.9) | F (1.21, 7.28) = 1.32 | p = 0.30 |
| Inter-drink-interval in Bout 1 (s) | 21.7 (20.4) | 42.7 (70.0) | -- | 14.3 (13.8) | 21.3 (24.0) | 24.3 (32.2) | χ2= 3.00 | p = 0.39 |
Notes: SD = standard deviation, s = seconds, CBD = Cannabidiol, # = number.
Friedman’s test was used in data that were largely non-normal.
3.4. Behavioral Observations
Behavioral observations of baboons following chronic administration of CBD are summarized in Table 2. Following CBD administration and the CSR session, as well as 24h after CBD treatment, baboons showed little to no signs of adverse events, including nausea, deficits in locomotor behaviors and sedation. CBD did not affect performance on the fine motor task. Additionally, there were no observed changes in food or water intake following any CBD treatments.
Table 2.
Performance on the fine motor task (M&Ms retrieved, dropped, and time to complete task; Mean (SD)) and observations of adverse behavioral effects during chronic CBD treatment following alcohol self-administration session completion (Post-Session) and 24 hours after CBD administration. Data are number of baboons (n=7) with symptoms present on any of the five days.
| Characteristics | Vehicle | 10 mg/kg CBD | 20 mg/kg CBD | 40 mg/kg CBD |
|---|---|---|---|---|
| Fine Motor Task Post-Session | ||||
| M&Ms Taken (%) | 100 | 100 | 100 | 100 |
| M&Ms Dropped (%) | 4.3 | 2.9 | 1.4 | 3.3 |
| Time (sec) to complete task (SD) | 7.4 (2.6) | 7.4 (2.4) | 7.0 (1.3) | 7.8 (2.5) |
|
| ||||
| Behavioral Observations Post-Session | ||||
|
| ||||
| Ataxia | 0/7 | 0/7 | 0/7 | 0/7 |
| Slow in Moving | 5/7 | 3/7 | 3/7 | 3/7 |
| Lip Droop | 0/7 | 0/7 | 0/7 | 0/7 |
| Limb Tremor | 0/7 | 0/7 | 0/7 | 0/7 |
| Body Tremor | 0/7 | 0/7 | 0/7 | 0/7 |
|
| ||||
| Fine Motor Task 24-hours After CBD Administration (Pre-Session) | ||||
|
| ||||
| M&Ms Taken (%) | 100 | 100 | 100 | 100 |
| M&Ms Dropped (%) | 1.4 | 2.4 | 1.4 | 1.0 |
| Time (sec) to complete task (SD) | 7.6 (2.7) | 7.8 (2.1) | 8.8 (8.4) | 7.6 (1.7) |
|
| ||||
| Behavioral Observations 24-hours After CBD Administration (Pre-Session) | ||||
|
| ||||
| Ataxia | 0/7 | 0/7 | 0/7 | 0/7 |
| Slow in Moving | 1/7 | 2/7 | 1/7 | 2/7 |
| Lip Droop | 0/7 | 0/7 | 0/7 | 0/7 |
| Limb Tremor | 1/7 | 3/7 | 1/7 | 0/7 |
| Body Tremor | 0/7 | 0/7 | 0/7 | 0/7 |
Notes: SD = standard deviation, % = percent of total trials, CBD = Cannabidiol
4. Discussion
The present study examined the behavioral effect of acute and chronic administration of CBD on alcohol-seeking and alcohol self-administration during conditions of ongoing alcohol access in male baboons with long-term alcohol drinking experience. Using similar therapeutic dosing parameters as those in human clinical trials (Devinsky et al. 2016; Thiele et al. 2022), baboons were administered 5, 10, 20, and 40 mg/kg pure CBD acutely and for 5 consecutive days. Overall, CBD did not significantly reduce alcohol seeking, self-administration, or drinking patterns following either acute or chronic treatment. Further, there were no observable behavioral effects (e.g., sedation) following chronic CBD administration. In sum, therapeutically relevant doses of pure CBD initiated during active alcohol administration were not effective at reducing alcohol-drinking behaviors. These data do not support the use of CBD as an effective pharmacotherapy to reduce alcohol intake under conditions of active drinking and ongoing alcohol access.
The current study adds to the growing literature exploring the potential use of CBD and other cannabinoids for the treatment of AUD. CBD has multi-target pharmacology, including modulation of CB1 and CB2 receptors (Britch et al. 2021). Acute and chronic alcohol exposure modify the endocannabinoid system (Gonzalez-Cuevas et al. 2018; Maccioni et al. 2022), producing neurobiological alterations that modulate reward circuitry and the reinforcing effects of alcohol and other substances of abuse/misuse (Maldonado et al. 2006; Wolfe et al. 2022). Other groups have demonstrated that blockade of the CB1R by an inverse agonist reduced alcohol consumption, prevented acquisition of alcohol drinking, and deprivation-induced increases in alcohol consumption in rodents (Arnone et al. 1997; Freedland et al. 2001; Colombo et al. 1998; Serra et al. 2001; Serra et al. 2002). Further, activation of the CB2 receptor with the agonist JWH133 reduced alcohol conditioned place preference in mice (Martin-Sanchez et al. 2019). In the present study, CBD did not alter alcohol seeking or self-administration, nor did it disrupt patterns of alcohol drinking in these long-term alcohol drinking baboons. However, CBD functions as a negative allosteric modulator of the CB1R and a partial agonist of the CB2R (Laprairie et al. 2015). Therefore, it is possible that future studies investigating the effects of CB1 inverse agonists and CB2 agonists with greater efficacy at the CB2 receptor may serve as improved AUD pharmacotherapy targets.
Previous preclinical rodent studies have shown promising effects of CBD on alcohol reinforcement, motivation, and reinstatement (Gonzalez-Cuevas et al. 2018; Viudez-Martinez et al. 2018; Viudez-Martinez et al. 2020). In one study, transdermal CBD (2.5g CBD/100 g gel) reduced ethanol drug-seeking behavior following acute administration, and repeated treatment did not result in tolerance to these behavioral effects (Gonzalez-Cuevas et al. 2018). Another study found that acute intraperitoneal administration of 90 mg/kg CBD reduced alcohol consumption in male and female C57BL/6J mice relative to the vehicle control (Viudez-Martinez et al. 2020). Further, chronic administration of CBD (30–120 mg/kg; intraperitoneal, IP) reduced ethanol intake and preference in a two-bottle choice paradigm, and decreased ethanol self-administration (Viudez-Martinez et al 2018). A study using Sardinian alcohol preferring (sP) rats demonstrated reduced alcohol self-administration when treated with 12.5–100 mg/kg CBD (Maccioni et al. 2022).
In contrast to these findings, this study demonstrated that acute oral administration of CBD (5–40 mg/kg) did not alter alcohol seeking nor drinking behaviors, including patterns of intake, in any of the baboons. Additionally, chronic administration of CBD (10–40 mg/kg) did not have an effect on any outcomes. The discrepancies between our findings and previous reports in rodents may be related to species differences, the route of administration, and self-administration procedures used within each study. First, the rodent studies primarily used IP administration versus the oral route of administration used within the present study. While studies using IP administration demonstrate effective proof-of-concept designs, these designs often fail to translate clinically as this route of administration increases drug bioavailability, which may not be clinically meaningful (Ay Shoyaib et al. 2019). Studies utilizing clinically-relevant routes of administration (e.g., oral ingestion) are better suited for evaluating therapeutic effects that may be altered by drug formulations, bioavailability, and pharmacokinetics that are meaningful for clinical translation. Moreover, FDA-approved medications containing CBD (e.g., Epidiolex) or other cannabinoids (e.g., synthetic THC such as dronabinol and nabilone) are oral formulations, and those using CBD for medical purposes often use oral forms (e.g., edibles, tinctures and oils). Second, nonhuman primates are closer in phylogenetic origin than other laboratory species, and are thus genetically, anatomically, physiologically, and behaviorally more similar to humans than rodents. Drug metabolism and pharmacokinetic parameters in nonhuman primates are closely similar to humans. For example, rodents have faster rates of alcohol elimination than nonhuman primates and humans. Baboons (and humans) with larger body mass have lower metabolic rates than rodents and require smaller drug doses on a per weight basis. There are standard formulas for interspecies dose scaling that adjusts for such differences to estimate equivalent doses from between different animals and to humans. For example, a dose of 120 mg/kg CBD in a mouse is equivalent to a 15 mg/kg dose in a baboon. The total oral doses used within the present study (150–1200 mg/day) in baboons were chosen to encompass the purported therapeutic dose range of oral CBD in humans (i.e., 100–900 mg total CBD dose/day; White 2019). The human equivalent dosing of baboon doses of 10 mg/kg and 40 mg/kg CBD (300 and 1200 mg/day total dose, respectively) would be 7.76 mg/kg and 31.02 mg/kg (541 and 2171.5 mg/day total dose, respectively) based on interspecies dose scaling (Mordenti and Chapell, 1989). High doses of CBD (e.g., 25 mg/kg/day in humans; Devinsky et al. 2019) are associated with greater adverse effects (e.g., hepatic abnormalities, diarrhea, somnolence) which would limit the clinical efficacy of CBD in the treatment of AUD (for review, see Huestis et al. 2019).
The use of the CSR procedure in nonhuman primates has key differences over typical self-administration models in rodents that could be a factor in discrepancies in findings in rodents and baboons. For example, rodent alcohol self-administration procedures have relied on short sessions (~1 hour), short drinking histories (~40 days), and self-administration in the absence of drug-associated cues (e.g., Viudez-Martinez et al. 2018). The CSR procedure models the “too much, too fast, too often” drinking patterns associated with “at risk” problem drinking as defined by NIAAA (2007). Briefly, binge drinking (drinking too much, too fast) is defined by NIAAA as alcohol consumption sufficient to achieve a blood alcohol level of 0.08% or more within a 2–3 hour period which corresponds to 0.8–1.0 g/kg or four to five standard drinks (a standard drink in the US is 14g of ethyl alcohol). Excessive “at risk” drinking also includes drinking an average of more than 14 drinks per week for men and more than 7 drinks per week for women (drinking too much, drinking too often). Such patterns of alcohol drinking are associated with increased risk for the development of alcohol-related problems and AUD. Our baboons consume alcohol at binge levels (∼1.0 g/kg per day within 2 hours), with BALs exceeding 0.08 % and maintain this level of consumption 7 days per week for prolonged periods (Kaminski et al. 2008; Kaminski et al. 2014; Holtyn et al. 2014). Further, our baboons have an extensive history (Mean ± SEM: 10.4 ± 2.2 years) of long-term daily alcohol self-administration at binge levels in the presence of alcohol-related cues in the drinking environment. Therefore, the current study uniquely models aspects of human problem drinking under carefully controlled laboratory conditions and without confounds common to human research. Baboon models of alcohol drinking are a critical bridge between preclinical rodent studies and humans. NHP models allow for long term behavioral investigations, an important consideration for interpretating results relating to alcohol reinforcement.
There are several considerations to the present study. Importantly, the present experiment assessed CBD effects on alcohol seeking and self-administration during ongoing access to alcohol. It remains to be seen if CBD may be useful for reducing the reinstatement of alcohol seeking after a period of abstinence or attenuate withdrawal symptoms in those physically dependent on alcohol. Further, CBD has been shown to have other effects that could be beneficial to populations with AUD, such as neuroprotective effects and beneficial effects on hepatotoxicity as has been shown in some rodent studies (Liput et al. 2013; Hamelink et al. 2005; Wang et al. 2005; Yang et al. 2014). While this study was not designed to explicitly measure hepatic effects of CBD, our baboons are routinely screened for liver function (i.e. levels of liver enzymes present in blood samples), and we saw no beneficial or detrimental effects of CBD across the year where baboons were receiving acute and chronic treatments. This study did not include a positive control (e.g., naltrexone). However, we have demonstrated previously that acute and chronic naltrexone reduces alcohol drinking behaviors in these baboons under the CSR procedure (Holtyn et al. 2017) and facilitates extinction of alcohol-directed responding (Kaminski et al. 2012). Given the lack of effectiveness of CBD in the current report, these results in the context of our past findings with naltrexone (Kaminski et al. 2012; Holtyn et al 2017) suggest that CBD would not function as an effective pharmacotherapy in reducing alcohol drinking behaviors. The potential use of CBD for the treatment of alcohol-withdrawal related outcomes has yet to be determined. Finally, this study was conducted in male baboons only, precluding any conclusions related to CBD effectiveness in females or potential sex differences in this model. However, while sex differences have been observed in a previous report examining acute and chronic CBD administration on alcohol behaviors in mice, CBD was only effective at reducing drinking in male mice and not in female mice (Viudez-Martinez et al. 2020). This study provides a thorough examination of CBD effects on alcohol seeking and self-administration behaviors across a range of doses given acutely and chronically. In sum, the current data do not support use of pure CBD as an effective pharmacotherapy to reduce ongoing alcohol drinking.
Supplementary Material
Highlights.
Cannabidiol (CBD) has been proposed as a treatment to reduce alcohol drinking
In nonhuman primates, we tested effects of CBD on alcohol seeking and drinking
Neither acute nor chronic (5 days) administration of CBD affected alcohol behaviors
No adverse events of CBD were observed throughout treatment
These data do not support the use of pure CBD for reducing ongoing alcohol drinking
Acknowledgments
The authors wish to thank the NIDA Drug Supply Program for providing CBD. The authors would like to thank Samuel Womack and Susan James for excellent technical assistance.
Role of the funding source
This work was supported by R01AA15971 (PI: Elise Weerts) from NIH/NIAAA. NIAAA had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
EW and CM have received funds from MyMD pharmaceuticals, Inc. and MIRA-1 Pharmaceuticals, Inc for contract preclinical research. EW received support for clinical research projects funded by Cultivate Biologics LLC, and Canopy Growth Corp. The research reported in the manuscript is not related to the above contracted research. CAZ has no conflicts to report.
CRediT authorship contribution statement
CM: Investigation, Data Curation, Formal analysis, Writing - Review & Editing; CAZ: Formal analysis, Writing - Original Draft; EW : Conceptualization, Methodology, Validation, Investigation, Project administration, Writing - Review & Editing. All authors reviewed and approved the final manuscript before submission, and agree to be accountable for all aspects of this work.
References
- Al Shoyaib A, Archie SR, Karamyan VT, 2019. Intraperitoneal Route of Drug Administration: Should it Be Used in Experimental Animal Studies? Pharm Res 37(1): 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone M, Maruani J, Chaperon F, Thiebot MH, Poncelet M, Soubrie P, Le Fur G, 1997. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 132(1), 104–106. [DOI] [PubMed] [Google Scholar]
- Babalonis S, Haney M, Malcolm RJ, Lofwall MR, Votaw VR, Sparenborg S, Walsh SL, 2017. Oral cannabidiol does not produce a signal for abuse liability in frequent marijuana smokers. Drug Alcohol Depend 172, 9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeria CL, Spindle TR, Cone EJ, Sholler D, Goffi E, Mitchell JM, Winecker RE, Bigelow GE, Flegel R, Vandrey R, 2022. Pharmacokinetic Profile of ∆9-Tetrahydrocannabinol, Cannabidiol and Metabolites in Blood following Vaporization and Oral Ingestion of Cannabidiol Products. J Anal Toxicol 46(6), 583–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessing EM, Steenkamp MM, Manzanares J, Marmar CR, 2015. Cannabidiol as a Potential Treatment for Anxiety Disorders. Neurotherapeutics 12(4), 825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britch SC, Babalonis S, Walsh SL, 2021. Cannabidiol: pharmacology and therapeutic targets. Psychopharmacology (Berl) 238(1), 9–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette EM, Nieto SJ, Grodin EN, Meredith LR, Hurley B, Miotto K, Gillis AJ, Ray LA, 2022. Novel Agents for the Pharmacological Treatment of Alcohol Use Disorder. Drugs 82(3), 251–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos AC, Fogaca MV, Sonego AB, Guimaraes FS, 2016. Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol Res 112, 119–127. [DOI] [PubMed] [Google Scholar]
- Castillo-Carniglia A, Keyes KM, Hasin DS, Cerda M, 2019. Psychiatric comorbidities in alcohol use disorder. Lancet Psychiatry 6(12), 1068–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Fa M, Guano L, Lobina C, Loche A, Reali R, Gessa GL, 1998. Reduction of voluntary ethanol intake in ethanol-preferring sP rats by the cannabinoid antagonist SR-141716. Alcohol Alcohol 33(2), 126–130. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Cilio MR, Cross H, Fernandez-Ruiz J, French J, Hill C, Katz R, Di Marzo V, Jutras-Aswad D, Notcutt WG, Martinez-Orgado J, Robson PJ, Rohrback BG, Thiele E, Whalley B, Friedman D, 2014. Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia 55(6), 791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Nabbout R, Miller I, Laux L, Zolnowska M, Wright S, Roberts C, 2019. Long-term cannabidiol treatment in patients with Dravet syndrome: An open-label extension trial. Epilepsia 60(2), 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke AN, Kaminski BJ, Weerts EM, 2014. Baclofen effects on alcohol seeking, self-administration and extinction of seeking responses in a within-session design in baboons. Addict Biol 19(1), 16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration. FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy 2018.
- Freedland CS, Sharpe AL, Samson HH, Porrino LJ, 2001. Effects of SR141716A on ethanol and sucrose self-administration. Alcohol Clin Exp Res 25(2), 277–282. [PubMed] [Google Scholar]
- Fridman EP, Popova VN, 1988. Species of the genus Papio (Cercopithecidae) as subjects of biomedical research: I. Biological basis of experiments on baboons. J Med Primatol 17(6), 291–307. [PubMed] [Google Scholar]
- Gonzalez-Cuevas G, Martin-Fardon R, Kerr TM, Stouffer DG, Parsons LH, Hammell DC, Banks SL, Stinchcomb AL, Weiss F, 2018. Unique treatment potential of cannabidiol for the prevention of relapse to drug use: preclinical proof of principle. Neuropsychopharmacology 43(10), 2036–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelink C, Hampson A, Wink DA, Eiden LE, Eskay RL, 2005. Comparison of cannabidiol, antioxidants, and diuretics in reversing binge ethanol-induced neurotoxicity. J Pharmacol Exp Ther 314(2), 780–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Malcolm RJ, Babalonis S, Nuzzo PA, Cooper ZD, Bedi G, Gray KM, McRae-Clark A, Lofwall MR, Sparenborg S, Walsh SL, 2016. Oral Cannabidiol does not Alter the Subjective, Reinforcing or Cardiovascular Effects of Smoked Cannabis. Neuropsychopharmacology 41(8), 1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtyn AF, Kaminski BJ, Wand GS, Weerts EM, 2014. Differences in extinction of cue-maintained conditioned responses associated with self-administration: alcohol versus a nonalcoholic reinforcer. Alcohol Clin Exp Res 38(10), 2639–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtyn AF, Kaminski BJ, Weerts EM, 2017a. Baclofen and naltrexone effects on alcohol self-administration: Comparison of treatment initiated during abstinence or ongoing alcohol access in baboons. Drug Alcohol Depend 179, 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtyn AF, Tiruveedhula VV, Stephen MR, Cook JM, Weerts EM, 2017b. Effects of the benzodiazepine GABA(A) alpha1-preferring antagonist 3-isopropoxy-beta-carboline hydrochloride (3-ISOPBC) on alcohol seeking and self-administration in baboons. Drug Alcohol Depend 170, 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtyn AF, Weerts EM, 2019. Evaluation of mifepristone effects on alcohol-seeking and self-administration in baboons. Exp Clin Psychopharmacol 27(3), 227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis MA, Solimini R, Pichini S, Pacifici R, Carlier J, Busardo FP, 2019. Cannabidiol Adverse Effects and Toxicity. Curr Neuroppharmacolol 17(10), 974–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolivette LJ, Ward KW, 2005. Extrapolation of human pharmacokinetic parameters from rat, dog, and monkey data: Molecular properties associated with extrapolative success or failure. J Pharm Sci 94(7), 1467–1483. [DOI] [PubMed] [Google Scholar]
- Julian T, Glascow N, Syeed R, Zis P, 2019. Alcohol-related peripheral neuropathy: a systematic review and meta-analysis. J Neurol 266(12), 2907–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski BJ, Duke AN, Weerts EM, 2012. Effects of naltrexone on alcohol drinking patterns and extinction of alcohol seeking in baboons. Psychopharmacology (Berl) 223(1), 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski BJ, Goodwin AK, Wand G, Weerts EM, 2008. Dissociation of alcohol-seeking and consumption under a chained schedule of oral alcohol reinforcement in baboons. Alcohol Clin Exp Res 32(6), 1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski BJ, Van Linn ML, Cook JM, Yin W, Weerts EM, 2013. Effects of the benzodiazepine GABAA alpha1-preferring ligand, 3-propoxy-beta-carboline hydrochloride (3-PBC), on alcohol seeking and self-administration in baboons. Psychopharmacology (Berl) 227(1), 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski BJ, Weerts EM, 2014. The effects of varenicline on alcohol seeking and self-administration in baboons. Alcohol Clin Exp Res 38(2), 376–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan NM, Mechoulam R, 2007. Cannabinoids in health and disease. Dialogues Clin Neurosci 9(4), 413–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprairie RB, Bagher AM, Kelly ME, Denovan-Wright EM, 2015. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol 172(20), 4790–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liput DJ, Hammell DC, Stinchcomb AL, Nixon K, 2013. Transdermal delivery of cannabidiol attenuates binge alcohol-induced neurodegeneration in a rodent model of an alcohol use disorder. Pharmacol Biochem Behav 111, 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccioni P, Bratzu J, Carai MAM, Colombo G, Gessa GL, 2022. Reducing Effect of Cannabidiol on Alcohol Self-Administration in Sardinian Alcohol-Preferring Rats. Cannabis Cannabinoid Res 7(2), 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado R, Valverde O, Berrendero F, 2006. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci 29(4), 225–232. [DOI] [PubMed] [Google Scholar]
- Martin-Sanchez A, Warnault V, Montagud-Romero S, Pastor A, Mondragon N, De La Torre R, Valverde O, 2019. Alcohol-induced conditioned place preference is modulated by CB2 cannabinoid receptors and modifies levels of endocannabinoids in the mesocorticolimbic system. Pharmacol Biochem Behav 183, 22–31. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Heyser CJ, 2021. Alcohol Use Disorder: The Role of Medication in Recovery. Alcohol Res 41(1), 07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordenti J, and Chappell W 1989. Toxicokinetics in new drug development.” eds. Yacobi A, Skelly J, and Batra V, Pergamon Press, New York, pp. 42–96. [Google Scholar]
- National Research Council, 2011. Guide for the Care and Use of Laboratory Animals, eighth edition The National Academies Press, Washington, DC. [Google Scholar]
- NIAAA, 2007. Helping Patients Who Drink Too Much, A Clinician’s Guide, updated edition 2005, NIH Publication No. 07–3769
- Ray LAP, Bujarski SP, Grodin EP, Hartwell EP, Green RM, Venegas AB, Lim AM, Gillis AM, Miotto KM, 2019. State-of-the-art behavioral and pharmacological treatments for alcohol use disorder. Am J Drug Alcohol Abuse 45(2), 124–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, 2011. The risks associated with alcohol use and alcoholism. Alcohol Res Health 34(2), 135–143. [PMC free article] [PubMed] [Google Scholar]
- Serra S, Brunetti G, Pani M, Vacca G, Carai MA, Gessa GL, Colombo G, 2002. Blockade by the cannabinoid CB(1) receptor antagonist, SR 141716, of alcohol deprivation effect in alcohol-preferring rats. Eur J Pharmacol 443(1–3), 95–97. [DOI] [PubMed] [Google Scholar]
- Serra S, Carai MA, Brunetti G, Gomez R, Melis S, Vacca G, Colombo G, Gessa GL, 2001. The cannabinoid receptor antagonist SR 141716 prevents acquisition of drinking behavior in alcohol-preferring rats. Eur J Pharmacol 430(2–3), 369–371. [DOI] [PubMed] [Google Scholar]
- Thiele EA, Bebin EM, Filloux F, Kwan P, Loftus R, Sahebkar F, Sparagana S, Wheless J, 2022. Long-term cannabidiol treatment for seizures in patients with tuberous sclerosis complex: An open-label extension trial. Epilepsia 63(2), 426–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viudez-Martinez A, Garcia-Gutierrez MS, Manzanares J, 2020. Gender differences in the effects of cannabidiol on ethanol binge drinking in mice. Addict Biol 25(3), e12765. [DOI] [PubMed] [Google Scholar]
- Viudez-Martinez A, Garcia-Gutierrez MS, Navarron CM, Morales-Calero MI, Navarrete F, Torres-Suarez AI, Manzanares J, 2018. Cannabidiol reduces ethanol consumption, motivation and relapse in mice. Addict Biol 23(1), 154–164. [DOI] [PubMed] [Google Scholar]
- Wang Y, Mukhopadhyay P, Cao Z, Wang H, Feng D, Hasko G, Mechoulam R, Gao B, Pacher P, 2017. Cannabidiol attenuates alcohol-induced liver steatosis, metabolic dysregulation, inflammation and neutrophil-mediated injury. Sci Rep 7(1), 12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerts EM, Ator NA, Grech DM, Griffiths RR, 1998. Zolpidem physical dependence assessed across increasing doses under a once-daily dosing regimen in baboons. J Pharmacol Exp Ther 285(1), 41–53. [PubMed] [Google Scholar]
- Weerts EM, Goodwin AK, Kaminski BJ, Hienz RD, 2006. Environmental cues, alcohol seeking, and consumption in baboons: effects of response requirement and duration of alcohol abstinence. Alcohol Clin Exp Res 30(12), 2026–2036. [DOI] [PubMed] [Google Scholar]
- White MW, 2019. A Review of Human Studies Assessing Cannabidiol’s (CBD) Therapeutic Actions and Potentials. J Clin Pharmacol 59(7), 923–934. [DOI] [PubMed] [Google Scholar]
- Wolfe SA, Vozella V, Roberto M, 2022. The Synaptic Interactions of Alcohol and the Endogenous Cannabinoid System. Alcohol Res 42(1), 03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Rozenfeld R, Wu D, Devi LA, Zhang Z, Cederbaum A, 2014. Cannabidiol protects liver from binge alcohol-induced steatosis by mechanisms including inhibition of oxidative stress and increase in autophagy. Free Radic Biol Med 68, 260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgair A, Wong JC, Lee JB, Mistry J, Sivak O, Wasan KM, Hennig IM, Barrett DA, Constantinescu CS, Fischer PM, Gershkovich P, 2016. Dietary fats and pharmaceutical lipid excipients increase systemic exposure to orally administered cannabis and cannabis-based medicines. Am J Transl Res 8(8), 3448–3459. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


