Abstract
Background:
Perivascular spaces on brain magnetic resonance imaging (MRI) may indicate poor fluid drainage in the brain and have been associated with numerous neurological conditions. Cerebrovascular reactivity (CVR) is a marker of cerebrovascular function and represents the ability of cerebral blood vessels to regulate cerebral blood flow in response to vasodilatory or vasoconstrictive stimuli. We aimed to examine whether pathological widening of the perivascular space in older adults may be associated with deficits in CVR.
Methods:
Independently living older adults free of dementia or clinical stroke were recruited from the community and underwent brain MRI. Pseudo-continuous arterial spin labeling MRI quantified whole brain cerebral perfusion at rest and during CVR to hypercapnia and hypocapnia induced by visually guided breathing exercises. Perivascular spaces were visually scored using existing scales.
Results:
Thirty-seven independently living older adults (mean age = 66.3 years; SD = 6.8; age range 55–84 years; 29.7% male) were included in the current analysis. Multiple linear regression analysis revealed a significant negative association between burden of perivascular spaces and global CVR to hypercapnia (B = −2.0, 95% CI (−3.6, −0.4), p = 0.015), adjusting for age and sex. Perivascular spaces were not related to CVR to hypocapnia.
Discussion:
Perivascular spaces are associated with deficits in cerebrovascular vasodilatory response, but not vasoconstrictive response. Enlargement of perivascular spaces could contribute to, or be influenced by, deficits in CVR. Additional longitudinal studies are warranted to improve our understanding of the relationship between cerebrovascular function and perivascular space enlargement.
Keywords: Perivascular Spaces, Cerebrovascular reactivity, Small Vessel Disease
Perivascular spaces are fluid-filled spaces surrounding arterioles, capillaries and venules in the brain, which facilitate flow of substances and enable clearance of waste (Bakker et al., 2016; Wardlaw et al., 2020). Magnetic resonance imaging (MRI) of the brain has allowed in vivo visualization of perivascular spaces (Francis et al., 2019; Kwee and Kwee, 2007; Smith et al., 2019), which have been linked to numerous cerebrovascular pathologies, inflammation, neurodegenerative conditions, and cognitive decline (Debette et al., 2019; Gouveia-Freitas and Bastos-Leite, 2021; Paradise et al., 2021; Wuerfel et al., 2008). Widening of perivascular spaces may indicate poor fluid drainage due to accumulation of perivascular cell debris, proteins and waste along blood vessels (Hablitz and Nedergaard, 2021; Wardlaw et al., 2020). Although the precise mechanism of fluid transport and waste clearance in the brain is still under intensive investigation, fluid transport through perivascular spaces is thought to participate in the brain’s putative glymphatic clearance system. Specifically, it has been proposed that cerebral waste clearance may be facilitated in part by cerebrovascular vasomotion and pulsatility (Hablitz and Nedergaard, 2021; Jessen et al., 2015; Rasmussen et al., 2022; Ren et al., 2021), and changes in arterial pulsatility have been hypothesized to contribute to reduced waste clearance and accumulation of toxic solutes (Iliff et al., 2013; Mestre et al., 2018). While changes in cerebrovascular pulsatility and low cerebral blood flow (CBF) have both previously been linked to perivascular spaces in the basal ganglia (Blair et al., 2020; Liu et al., 2021; Mestre et al., 2018), few prior studies have examined associations between perivascular spaces and dynamic measures of cerebrovascular function.
Cerebrovascular reactivity (CVR) is a dynamic marker of cerebrovascular function and represents the ability of cerebral blood vessels to regulate CBF in response to vasoactive stimuli (Liu et al., 2019; Urback et al., 2017). Enlarged perivascular spaces may impede or diminish CVR through reduced CSF flow, endothelial damage, rigidity of arteriolar walls, or breakdown of the microvasculature (Wardlaw et al., 2020). Alternatively, diminished CVR may facilitate the development of enlarged perivascular spaces by preventing efficient fluid transport (Hablitz and Nedergaard, 2021). Prior studies have demonstrated a relationship between reduced CVR and white matter hyperintensities (Blair et al., 2020; Liem et al., 2009), lacunar infarction (Molina et al., 1999) and cerebral amyloid angiopathy (Beaudin et al., 2022). However, few studies have examined whether perivascular spaces specifically may be associated with diminished CVR (Blair et al., 2016, 2020; Hund-Georgiadis et al., 2001). To the best of our knowledge, only two studies have examined perivascular spaces as part of a total small vessel disease score in association with CVR using BOLD functional MRI (Blair et al., 2020). Only Blair et al (2020) reported the independent association between perivascular spaces and CVR, showing that greater basal ganglia perivascular spaces are associated with lower white matter CVR. Additionally, no studies to date have investigated CVR to vasodilation versus vasoconstriction in older adults with and without perivascular spaces.
In the present study, we aimed to determine whether older adults with perivascular spaces may exhibit reduced vasodilatory and/or vasoconstrictive CVR in response to hypercapnia and hypocapnia, respectively. We hypothesized that higher burden of perivascular spaces would be associated with an attenuated CVR response.
Methods
Participants
Participants were recruited from the community and all study procedures were conducted as part of the Vascular Senescence and Cognition (VaSC) Study at the University of Southern California (USC) and University of California, Irvine. Older adults aged 55 years or older who were living independently were included. Exclusion criteria included a history of clinical stroke, dementia, myocardial infarction, major neurological or psychiatric disorder impacting cognition, MRI contraindication, current organ failure, and other systemic or neurological illness that may impact central nervous system function. History of vascular risk factors, including hypertension, body mass index, dyslipidemia, diabetes, as well as history of other medical illnesses, was determined by clinical interview. Data will be made available via reasonable request to the authors and based on the conditions outlined the Data and Code Availability Statement. This study was approved by the local Institutional Review Board; all participants gave informed consent and underwent detailed clinical assessment and brain MRI.
Neuroimaging
All participants underwent a comprehensive neuroimaging protocol (Nation et al., 2018) and all MRI scans were conducted on a 3T scanner (Siemens MAGNETOM Prisma System). The following sequences were examined for the current analysis: 3D T1-weighted anatomical scan for qualitative assessment of brain structures and abnormalities (Scan parameters: TR = 2300 ms; TE = 2.98 ms; TI = 900 ms; flip angle = 9 deg; FOV = 256 mm; resolution = 1.0 × 1.0 × 1.2 mm3; Scan time = 9 minutes), T2-weighted scan for identification of perivascular spaces (Scan parameters: TR = 10000 ms; TE = 88.0 ms; flip angle = 120 deg; FOV = 210 mm; resolution = 0.8 × 0.8 × 3.5 mm3; Echo spacing = 9.8 ms; Echo trains per slice = 11; Scan time = 2 minutes), and 3D gradient and spin-echo (GRASE) pseudo-continuous arterial spin labeling (pCASL) for CBF. The scan parameters were as follows for pCASL: TR = 5000 ms; TE at University of Southern California = 36.3 ms; TE at University of California, Irvine = 37.46 ms; FOV = 240 mm; resolution = 2.5 × 2.5 × 3.4 mm3; slice thickness = 3.42 mm; number of slices = 24; labeling duration = 1517 ms; post-labeling delay = 2000 ms. There was a total of 32 acquisitions (1 M0 image + 1 dummy image + 30 alternating tag and control images), with a total scan time of 5 minutes 25 seconds, yielding 15 tag-control pair images.
Cerebral Blood Flow
Following the protocol as described in Yew et al. (2022), the pCASL scans were pre-processed using the ASLtbx pipeline, implemented in SPM12 within MATLAB (Wang, 2012; Wang et al., 2008). Pre-processing steps for pCASL scans included motion correction, co-registration to individual subject’s structural T1-weighted image, spatial smoothing with a 6 mm full-width at half-maximum Gaussian kernel, and tag-control subtraction resulting in 15 tag-control pairs for each subject with values for absolute CBF (mL/100g tissue/min). All CBF images were thresholded below 10 or above 150mL/100g/min to exclude CBF outside the expected physiological range of gray matter (Clark et al., 2021; Nation et al., 2013). Tag-control pairs were warped to MNI space and averaged to create mean CBF maps for each subject. Resulting mean CBF maps were visually inspected for quality and gross abnormalities (i.e., large signal dropout). Partial volume correction was performed by applying subject-specific gray matter masks derived from the gray matter tissue class segmentation of T1-weighted structural images (Petr et al., 2018). Segmented gray matter maps were thresholded at 0.3, binarized, and multiplied by the mean CBF maps to ensure CBF was limited to gray matter.
Perivascular Spaces
Perivascular spaces were identified in accordance with established neuroimaging standards for SVD (Smith et al., 2019) and scored using the perivascular spaces visual rating scale and user guide (Potter et al., 2015). Perivascular spaces were scored in the basal ganglia, centrum semiovale, and midbrain. Overall perivascular spaces score was the highest score of all anatomical regions. Scores ranged from 0 (no perivascular spaces) to 4 (>40 perivascular spaces, severe).
CO2 Manipulation
Hypercapnia and hypocapnia were induced using a breathing paradigm, described in detail elsewhere (Yew, Jang, Dutt et al., 2022). Briefly, visual stimuli were utilized to guide patient breathing. Breath hold and paced breathing exercises were performed during separate pCASL scans. Breath hold for 15 seconds was employed to induce hypercapnia. Paced breathing at 0.1 Hz using an exhale-inhale cycle was employed to induce hypocapnia. Details of the visual stimuli and illustrations of the temporal synchronization of breathing paradigms and pCASL acquisition is shown elsewhere. Participants were excluded from analyses if they could not adhere to breathing instructions.
Capnography
End tidal CO2 (etCO2) was indexed using capnography during MRI acquisition using an M3015A sidestream carbon dioxide extension module (Philips Medical Systems) connected to a nasal cannula into which participants breathed. To correct for sampling tubing latency, etCO2 time series were shifted by a pre-calibrated duration of time (i.e., 10 seconds in our set-up). Additional details of the protocol and extraction of etCO2 has previously been described in detail (Yew, Jang, Dutt et al., 2022). For breath hold (hypercapnia), maximum etCO2 was extracted for each tag-control pair (i.e., maximum positive peak across the acquisition interval for each tag-control pair) to capture increases in etCO2induced by breath hold. For paced breathing (hypocapnia) etCO2 was extracted at every expiration; etCO2 gradually decreased during the paced breathing task.
Cerebrovascular Reactivity
Cerebrovascular reactivity was defined as percent change in CBF per unit change in etCO2 (% ΔCBF/ΔmmHg etCO2) and determined as follows in every voxel:
Values were averaged across voxels and tag-control pairs to determine whole brain CBF in each breathing condition. CVR maps were generated as described in Yew, Jang, Dutt et al. (2022). Regional mean CVR values were extracted for our regions of interest (ROI), including frontal gyrus, orbitofrontal cortex, anterior cingulate cortex, amygdala, hippocampus, medial temporal lobe, parietal cortex, posterior cingulate cortex, precuneus, caudate, and thalamus.
Statistical Analyses
All analyses were performed using IBM SPSS Statistics 27 and R Version 3.6.1. Demographic variables were computed to characterize the sample. We examined the relationship between perivascular spaces (independent predictor) and CVR to hypercapnia and hypocapnia (dependent outcomes) using multiple linear regression, adjusting for age and sex. Multicollinearity was assessed, with a variance inflation factor above 4 indicating significant multicollinearity. We utilized multiple imputation for missing CVR to hypercapnia (N = 4) and hypocapnia (N = 3) values for our primary outcomes (CVR to hypercapnia and hypocapnia). Missing value pattern analysis was conducted to ensure data appeared missing at random. Multiple imputation was performed with 10 imputations, using linear regression and fully conditional specification (Markov chain Monte Carlo (MCMC) method), which was selected automatically by SPSS based on missing data patterns. No constraints were applied to imputed values. Pooled data from 10 imputations was used for all analyses. Sensitivity analysis was conducted for all analyses to compare results from pooled data (multiple imputations) versus original data (listwise deletion). Significance threshold was set at p < .05.
Results
A total of 37 participants were included in the current analysis. Age of study participants ranged from 55 to 84 years and years of education ranged from 9 to 20. Participant characteristics are reported in Table I. Scores on the perivascular spaces scale ranged from 1 (N = 10, 27%) to 3 (N = 6, 16.2%, Figure I). Range of scores were similar in the basal ganglia and centrum semiovale. Mean perivascular spaces score was 1.89 and mean perivascular spaces score by region is reported in Table I.
Table I.
Participant Characteristics, Demographics and Vascular Risk Factors
| N = 37 | |
|---|---|
| Age, M (SD) | 66.3 (6.8) |
| Sex Male, n (%) | 11 (29.7) |
| Education, M (SD) | 15.7 (2.7) |
| Vascular Risk Factors, n (%) | |
| Hypertension | 11 (29.7) |
| Dyslipidemia | 12 (32.4) |
| Diabetes | 1 (2.7) |
| Smoking History | 11 (29.7) |
| TIA | 2 (2.7) |
| Cardiovascular Disease | 4 (10.8) |
| Atrial Fibrillation | 3 (8.1) |
| Race, n (%) | |
| White | 27 (73.0) |
| Black | 3 (8.1) |
| Asian | 4 (10.8) |
| Other | 3 (8.1) |
| CVR to Hypocapnia, M (SD) | 11.3 (7.2) |
| CVR to Hypercapnia, M (SD) | 10.1 (3.2) |
| Perivascular Spaces, Basal Ganglia, M (SD) | 1.3 (0.6) |
| Perivascular Spaces, Centrum Semiovale, M (SD) | 1.8 (0.6) |
| Perivascular Spaces, Midbrain, M (SD) | 1.0 (0.2) |
Figure I.
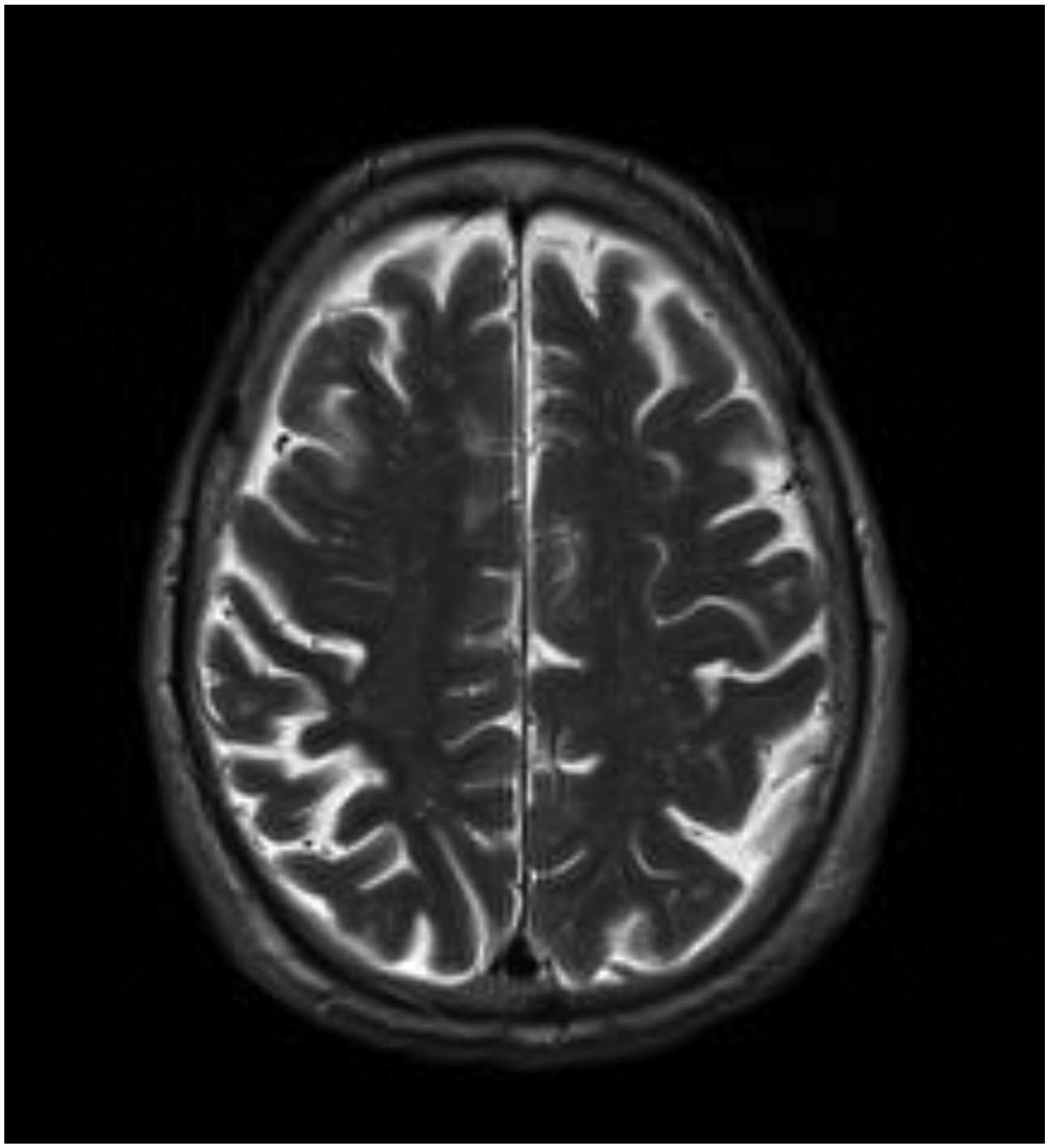
Enlarged perivascular spaces in the centrum semiovale on T2-weighted MRI
Perivascular Spaces and CVR to Hypercapnia
Mean CVR to hypocapnia in gray matter was 10.1% ΔCBF/ Δ mmHg etCO2. A significant association was observed between perivascular spaces and CVR to hypercapnia (B = −2.0, 95% CI (−3.6, −0.4), p = 0.015), adjusting for age and sex (Table II, Figure II–III). This model explained 28% of the variance in CVR to hypercapnia. Sensitivity analysis revealed the same results when listwise deletion was employed and when accounting for number of vascular risk factors.
Table II.
Association Between Perivascular Spaces and CVR to Hypercapnia
| Variable | Unstandardized Coefficients | t | Sig. | 95% Confidence Interval for B | ||
|---|---|---|---|---|---|---|
| B | Std. Error | Lower Bound | Upper Bound | |||
| Age (Years) | −0.11 | 0.08 | −1.43 | 0.153 | −0.26 | 0.04 |
| Sex (Male) | −0.13 | 1.05 | −0.12 | 0.904 | −2.19 | 1.93 |
| Perivascular Spaces Score | −2.02 | 0.82 | −2.46 | 0.015 | −3.64 | −0.40 |
Dependent Variable: CVR to Hypercapnia
Figure II.
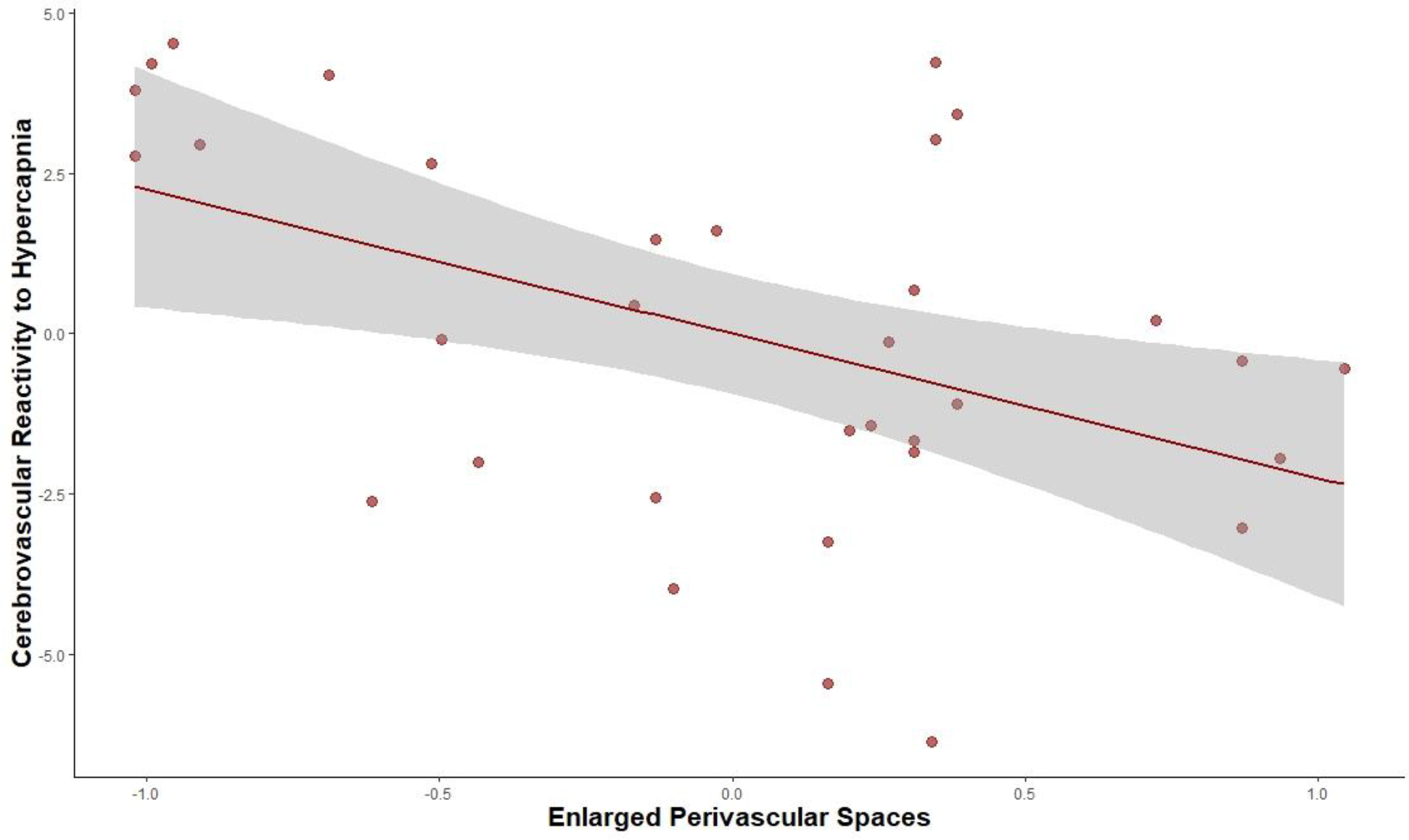
Partial Regression Plot of Association between Perivascular Spaces and CVR to Hypercapnia
Note: CVR to hypercapnia was measured as % ΔCBF/ΔmmHg etCO2 and enlarged perivascular was measured using the perivascular spaces visual rating scale. This partial regression plot accounts for age and sex, as adjusted in the reported regression models.
Figure III.
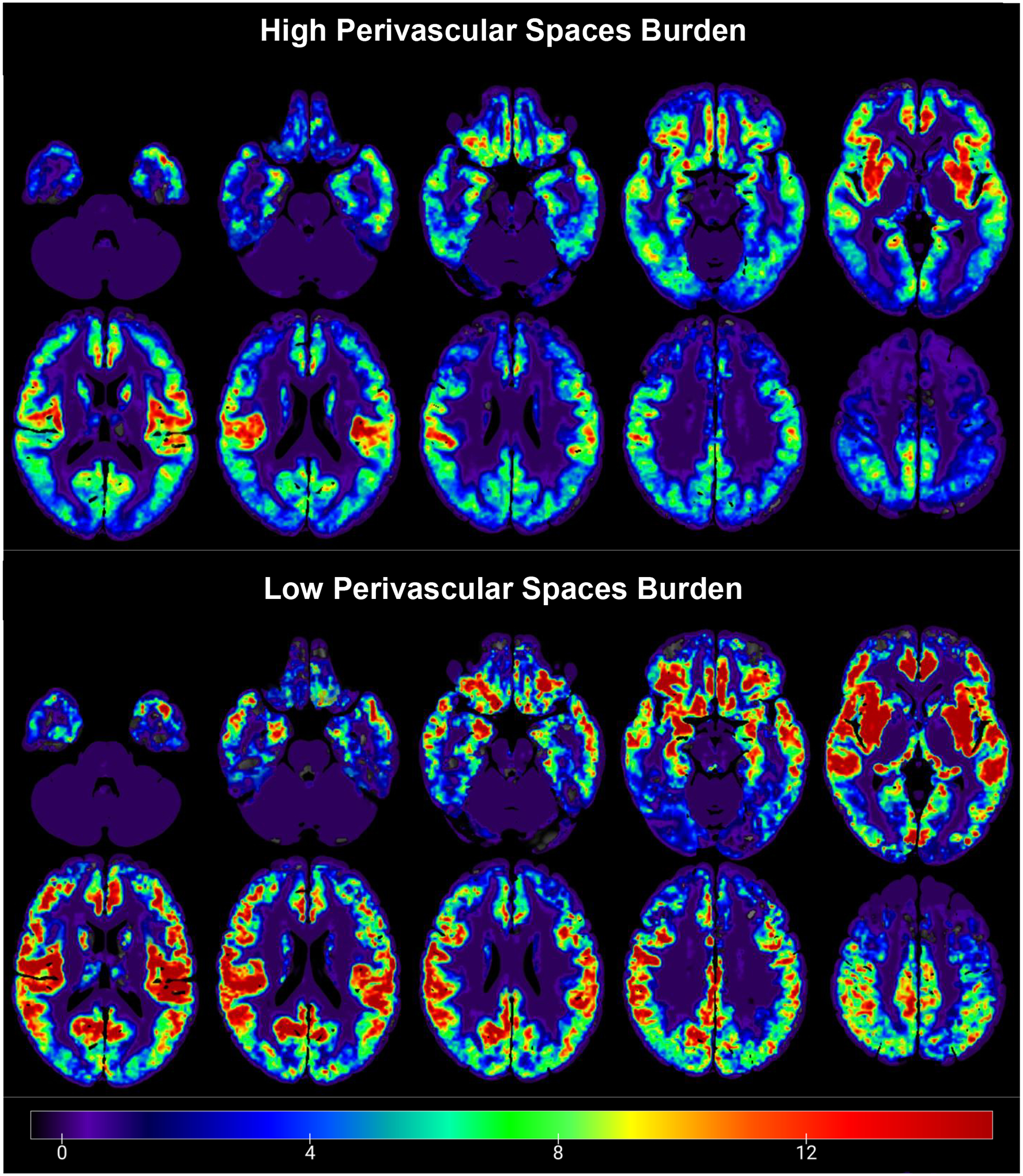
Average whole-brain gray matter cerebrovascular reactivity (CVR) maps contrasting high (scores of 2–4; top) versus low (scores of 0–1; bottom) perivascular spaces burden during breath hold (hypercapnia) condition. Warmer colors indicate higher CVR values (greater percent change in cerebral blood flow per mmHg change in etCO2), while colder colors show lower CVR values.
CVR to hypercapnia was associated with perivascular spaces specifically in the basal ganglia (B = −2.7, CI (−4.7, −0.8), p = .009) and marginally with centrum semiovale (B = −1.8, CI (−3.8, 0.1), p = .068). After adjusting for sex and age, significant associations were observed between perivascular spaces in the basal ganglia and CVR to hypercapnia in the right caudate (B = −7.3, 95% CI (−13.3, −1.3), p = .019), right posterior cingulate cortex (B = −6.4, 95% CI (−11.0, −1.8), p = .008) and right precuneus (B = −4.2, 95% CI (−7.8, −0.6), p = .025). Perivascular spaces in the centrum semiovale were significantly associated with CVR to hypercapnia in the right anterior cingulate cortex (B = −5.6, 95% CI (−10.5, −0.6), p = .030), right caudate (B = −6.6, 95% CI (−12.2, −1.0), p = .023), left lateral orbitofrontal cortex (B = −8.3, 95% CI (−15.7, −0.9), p = .030), left inferior frontal gyrus (B = −4.6, 95% CI (−9.0, −0.3), p = .039) and both left (B = −6.6, 95% CI (−11.3, −1.8), p = .008) and right (B = −4.9, 95% CI (−9.7, −0.1), p = .047) hippocampus.
Perivascular Spaces and CVR to Hypocapnia
Mean CVR to hypocapnia in gray matter was 11.3% ΔCBF/ Δ mmHg etCO2. We observed no association between perivascular spaces and CVR to hypocapnia, after adjusting for age and sex (Table III, Figure IV). Sensitivity analysis revealed the same results when listwise deletion was employed and when accounting for number of vascular risk factors. Pearson’s correlation also revealed no association with perivascular spaces specifically in the basal ganglia or centrum semiovale. We observed no associations between perivascular spaces in the basal ganglia or centrum semiovale and regional CVR to hypocapnia.
Table III.
Association Between Perivascular Spaces and CVR to Hypocapnia
| Variable | Unstandardized Coefficients | t | Sig. | 95% Confidence Interval for B | ||
|---|---|---|---|---|---|---|
| B | Std. Error | Lower Bound | Upper Bound | |||
| Age (Years) | 0.04 | 0.21 | 0.21 | 0.832 | −0.36 | 0.45 |
| Sex (Male) | 1.33 | 2.80 | 0.48 | 0.635 | −4.16 | 6.83 |
| Perivascular Spaces Score | −0.33 | 2.07 | −0.16 | 0.871 | −4.38 | 3.72 |
Dependent Variable: CVR to Hypocapnia
Figure IV.
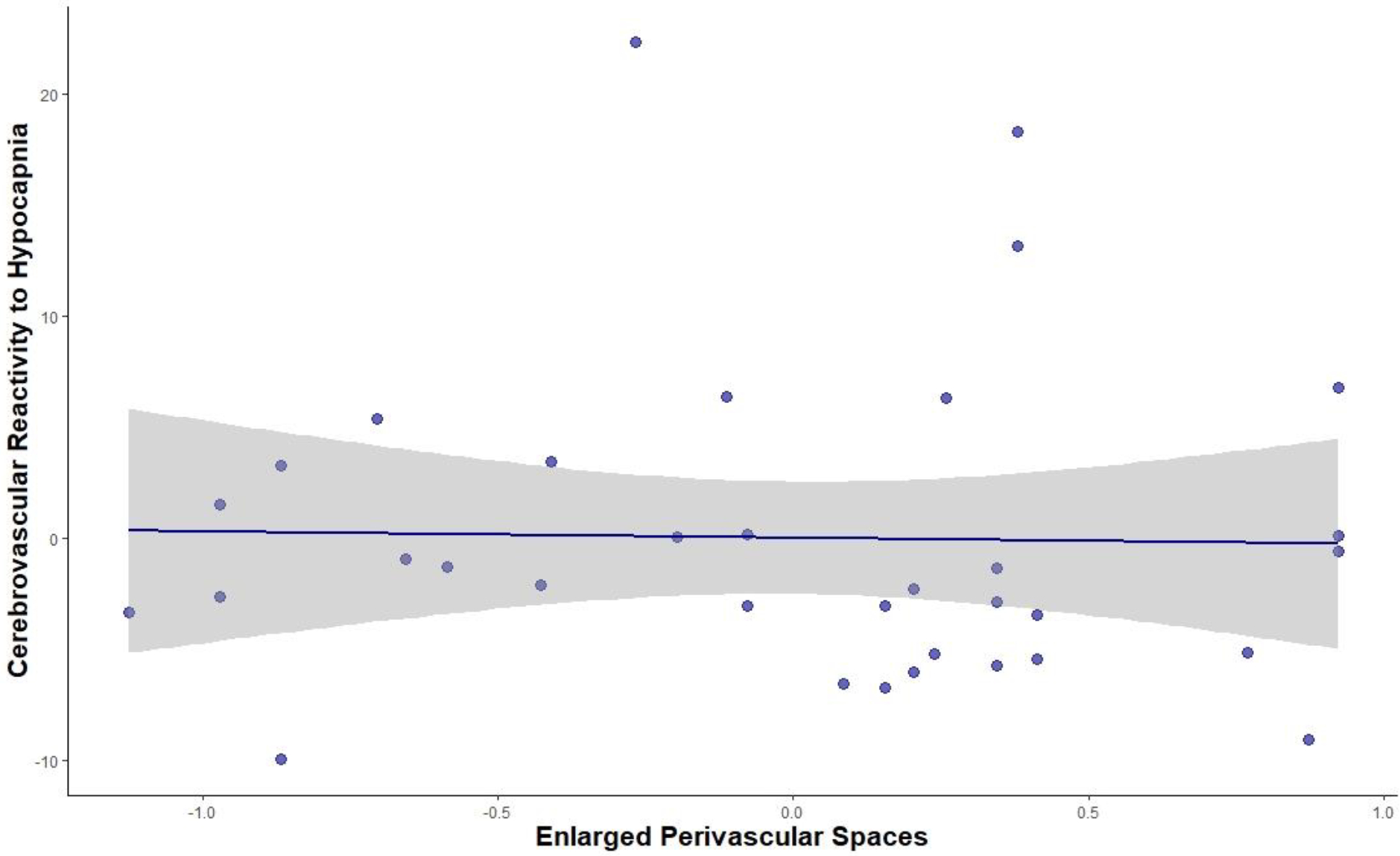
Partial Regression Plot of Association between Perivascular Spaces and CVR to Hypocapnia
Note: CVR to hypocapnia was measured as %ΔCBF/ΔmmHg etCO2 and enlarged perivascular spaces was measured using the perivascular spaces visual rating scale. This partial regression plot accounts for age and sex, as adjusted in the reported regression models.
We also conducted a site-specific sensitivity analysis which revealed the same findings, with significant relationship between PVS and CVR to hypercapnia at both USC (p = .014) and UCI (p = .042) and no relationship between PVS and CVR to hypocapnia at either site
Discussion
Enlarged perivascular spaces are increasingly known to be involved in numerous cerebrovascular and neurodegenerative conditions (Gouveia-Freitas and Bastos-Leite, 2021). Prior studies have demonstrated the perivascular space may widen due to waste and protein accumulation and that clearance of the perivascular space is primarily driven by CSF flow and cerebrovascular pulsatility (Hablitz and Nedergaard, 2021). Few prior studies have examined how perivascular spaces may alter the ability of cerebral blood vessels to regulate blood flow in response to vasoactive stimuli (i.e., CVR; Blair et al., 2016, 2020; Hund-Georgiadis et al., 2001), with only one prior study reporting the specific relationship between perivascular spaces and CVR (Blair et al., 2020). The current study found that higher burden of perivascular spaces is associated with a diminished CVR to hypercapnia (vasodilation) response. Our findings are consistent with Blair et al. (2020), where higher basal ganglia perivascular spaces were associated with lower white matter CVR using BOLD-fMRI. However, this study was one of the first to utilize pCASL and examine vasoconstriction. No association was observed between perivascular spaces and CVR to hypocapnia (vasoconstriction).
Prior studies have demonstrated that the vasodilatory response declines more rapidly during midlife (Peng et al., 2018) and is more vulnerable to decline in old age compared to the vasoconstriction response. The CVR values we obtained and the burden of perivascular spaces observed in this study is similar to other studies in older adults (Doubal et al., 2010; Lara et al., 2022; Yew et al., 2022) although studies establishing prevalence and norms are limited. Higher burden of perivascular spaces could potentially contribute to decline in the vasodilatory response in older adults. Dilation of the blood vessel in response to increased CO2 or other vasodilatory stimuli requires expansion of blood vessels into the perivascular space (; Hablitz and Nedergaard, 2021). Accumulation of waste, protein and debris along the perivascular space—which leads to enlargement of the space—may impede with the extent to which the blood vessel is able to dilate. In contrast, vasoconstriction leads to a reduction the amount of space occupied by the blood vessel and a larger perivascular space (Mestre et al., 2020). Enlarged perivascular spaces may indicate decrease glymphatic flow and diminish movement of CSF (Hablitz and Nedergaard, 2021; Kress et al., 2014; Mestre et al., 2017). Moreover, vasodilation is expected to decrease flow along the perivascular space (Hablitz and Nedergaard, 2021). It is therefore possible that blockage along the perivascular space limits the extent to which vasodilation can occur and may especially impact the vasodilatory response (i.e., CVR to hypercapnia). While additional studies are needed to determine directionality, it is also possible that both these processes occur at the same time, with blockage of the perivascular space causing reduced vasodilation and diminished vasodilation further impairing interstitial fluid flow and causing perivascular space expansion. Future studies are needed to address this hypothesis directly.
Regional analysis revealed that perivascular spaces in the basal ganglia were associated with CVR to hypercapnia in basal ganglia regions (i.e., caudate) as well as posterior regions such as the posterior cingulate cortex and precuneus. Given our sample size, future studies may reveal additional areas where CVR to hypercapnia may be related to perivascular spaces. Moreover, associations between perivascular spaces in the basal ganglia and CVR to hypercapnia were primarily in the right hemisphere, and additional studies are warranted to determine whether CVR to hypercapnia may be more vulnerable to small vessel damage in this hemisphere. Perivascular spaces in the centrum semiovale were associated with CVR to hypercapnia in anterior and frontal brain regions as well as the hippocampus. Higher burden of perivascular spaces in the centrum semiovale has previously been reported in amyloid-PET positive individuals with AD-related cognitive impairment (Charidimou et al., 2015). The hippocampus is key structure impacted in AD (Braak & Braak, 1990). Our findings are consistent with these associations, with perivascular spaces in centrum semiovale being associated with CVR to hypercapnia in both the left and right hippocampus. The mechanism for this remains unknown and warrants further investigation.
Some of the limitations of this study include the cross-sectional design and limited sample size. The present study also only evaluated CBF in gray matter due to the low signal-to-noise ratio of white matter pCASL. However, we observed a strong association between perivascular spaces and CVR despite these limitations. Moreover, in this study, we were able to assess CVR to both the hypercapnia and hypocapnia and directly evaluate CBF using pCASL. Prior studies have primarily examined only the hypercapnia response and utilized transcranial doppler and blood oxygen level-dependent (BOLD) functional MRI. While both TCD and BOLD-fMRI provide unique information, pCASL perfusion MRI allows direct examination of CBF in each voxel in the brain (Liu et al., 2019; Yew, Jang, Dutt et al., 2022). However, low signal-to-noise ratio and temporal resolution of pCASL also remain limitations. It is also currently accepted that perivascular spaces extend to the capillary level, however current T2-weighted imaging may not be able to capture para-capillary space (Gouveia-Freitas and Bastos-Leite, 2021). Future studies and advances to visualize para-capillary space at higher resolutions will provide further insight into this glymphatic clearance system. Given that the participants in this study had no history of stroke or dementia, additional studies are needed to determine if these findings generalize to those with more severe cerebrovascular and neurodegenerative conditions. Longitudinal studies are also warranted to examine the predictive value of CVR and perivascular spaces as pre-clinical biomarkers of cerebrovascular disease and cognitive decline. We also utilized a breathing paradigm as opposed to gas inhalation, which may introduce inter-subject and inter-breath variations. Our findings should further be validated in future studies utilizing other methods to induce hypercapnia. In addition, prior studies have shown differences in labeling efficiency during hypocapnia and hypercapnia due to changes in arterial flow velocity during vascular challenge (Aslan et al., 2010). In this study, we used the same post-labeling delay for both conditions, which is a limitation. Future studies with multiple post-labeling delays would be valuable in determining how this parameter influences CBF and CVR in different breathing conditions.
These findings suggest that perivascular spaces are associated with diminished CVR, specifically for the vasodilatory response. It remains unclear whether widening of perivascular spaces is a cause, consequence or co-occurring phenomenon to deficits in CVR. Future longitudinal studies are warranted to examine how this dysfunction may be related to the development of cerebrovascular and neurodegenerative conditions.
Supplementary Material
Acknowledgements:
This research was supported by National Institutes of Health grants (DAN: R01AG064228, R01AG060049, P50AG016573, P01AG052350), the Alzheimer’s Association (DAN: AARG-17-532905), and the Canadian Institutes of Health Research (AK: DFD-170763).
Footnotes
Conflict of Interest/Disclosures: None
References
- Aslan S, Xu F, Wang PL, Uh J, Yezhuvath US, van Osch M, Lu H, 2010. Estimation of labeling efficiency in pseudocontinuous arterial spin labeling. Magn. Reson. Med 63, 765–771. 10.1002/mrm.22245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker ENTP, Bacskai BJ, Arbel-Ornath M, Aldea R, Bedussi B, Morris AWJ, Weller RO, Carare RO, 2016. Lymphatic Clearance of the Brain: Perivascular, Paravascular and Significance for Neurodegenerative Diseases. Cell. Mol. Neurobiol 36, 181–194. 10.1007/s10571-015-0273-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudin AE, McCreary CR, Mazerolle EL, Gee M, Sharma B, Subotic A, Zwiers AM, Cox E, Nelles K, Charlton A, Frayne R, Ismail Z, Beaulieu C, Jickling GC, Camicioli RM, Pike GB, Smith EE, 2022. Cerebrovascular Reactivity Across the Entire Brain in Cerebral Amyloid Angiopathy. Neurology 98, e1716–e1728. 10.1212/WNL.0000000000200136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair GW, Thrippleton MJ, Shi Y, Hamilton I, Stringer M, Chappell F, Dickie DA, Andrews P, Marshall I, Doubal FN, Wardlaw JM, 2020. Intracranial hemodynamic relationships in patients with cerebral small vessel disease. Neurology 94, e2258–e2269. 10.1212/WNL.0000000000009483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AL, Weigand AJ, Bangen KJ, Merritt VC, Bondi MW, Delano-Wood L, 2021. Repetitive mTBI is associated with age-related reductions in cerebral blood flow but not cortical thickness. J. Cereb. Blood Flow Metab 41, 431–444. 10.1177/0271678X19897443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debette S, Schilling S, Duperron M-G, Larsson SC, Markus HS, 2019. Clinical Significance of Magnetic Resonance Imaging Markers of Vascular Brain Injury. JAMA Neurol 76, 81. 10.1001/jamaneurol.2018.3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis F, Ballerini L, Wardlaw JM, 2019. Perivascular spaces and their associations with risk factors, clinical disorders and neuroimaging features: A systematic review and meta-analysis. Int. J. Stroke 14, 359–371. 10.1177/1747493019830321 [DOI] [PubMed] [Google Scholar]
- Gouveia-Freitas K, Bastos-Leite AJ, 2021. Perivascular spaces and brain waste clearance systems: relevance for neurodegenerative and cerebrovascular pathology. Neuroradiology 63, 1581–1597. 10.1007/s00234-021-02718-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hablitz LM, Nedergaard M, 2021. The Glymphatic System: A Novel Component of Fundamental Neurobiology. J. Neurosci 41, 7698–7711. 10.1523/JNEUROSCI.0619-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Zeppenfeld DM, Venkataraman A, Plog BA, Liao Y, Deane R, Nedergaard M, 2013. Cerebral Arterial Pulsation Drives Paravascular CSF-Interstitial Fluid Exchange in the Murine Brain. J. Neurosci 33, 18190–18199. 10.1523/JNEUROSCI.1592-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen NA, Munk ASF, Lundgaard I, Nedergaard M, 2015. The Glymphatic System: A Beginner’s Guide. Neurochem. Res 40, 2583–99. 10.1007/s11064-015-1581-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, Xie L, Kang H, Xu Q, Liew JA, Plog BA, Ding F, Deane R, Nedergaard M, 2014. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol 76, 845–861. 10.1002/ana.24271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwee RM, Kwee TC, 2007. Virchow-Robin Spaces at MR Imaging. RadioGraphics 27, 1071–1086. 10.1148/rg.274065722 [DOI] [PubMed] [Google Scholar]
- Liem MK, Lesnik Oberstein SAJ, Haan J, Boom R. v. d., Ferrari MD, Buchem M.A. v., Grond J. v. d., 2009. Cerebrovascular Reactivity Is a Main Determinant of White Matter Hyperintensity Progression in CADASIL. Am. J. Neuroradiol 30, 1244–1247. 10.3174/ajnr.A1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, De Vis JB, Lu H, 2019. Cerebrovascular reactivity (CVR) MRI with CO2 challenge: A technical review. Neuroimage 187, 104–115. 10.1016/j.neuroimage.2018.03.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Hou B, You H, Zhang Y, Zhu Y, Ma C, Zuo Z, Feng F, 2021. The Association Between Perivascular Spaces and Cerebral Blood Flow, Brain Volume, and Cardiovascular Risk. Front. Aging Neurosci 13. 10.3389/fnagi.2021.599724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestre H, Du T, Sweeney AM, Liu G, Samson AJ, Peng W, Mortensen KN, Stæger FF, Bork PAR, Bashford L, Toro ER, Tithof J, Kelley DH, Thomas JH, Hjorth PG, Martens EA, Mehta RI, Solis O, Blinder P, Kleinfeld D, Hirase H, Mori Y, Nedergaard M, 2020. Cerebrospinal fluid influx drives acute ischemic tissue swelling. Science (80-.) 367. 10.1126/science.aax7171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestre H, Kostrikov S, Mehta RI, Nedergaard M, 2017. Perivascular spaces, glymphatic dysfunction, and small vessel disease. Clin. Sci 131, 2257–2274. 10.1042/CS20160381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestre H, Tithof J, Du T, Song W, Peng W, Sweeney AM, Olveda G, Thomas JH, Nedergaard M, Kelley DH, 2018. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat. Commun 9, 4878. 10.1038/s41467-018-07318-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina C, Sabín JA, Montaner J, Rovira A, Abilleira S, Codina A, 1999. Impaired Cerebrovascular Reactivity as a Risk Marker for First-Ever Lacunar Infarction. Stroke 30, 2296–2301. 10.1161/01.STR.30.11.2296 [DOI] [PubMed] [Google Scholar]
- Nation DA, Tan A, Dutt S, McIntosh EC, Yew B, Ho JK, Blanken AE, Jang JY, Rodgers KE, Gaubert A, 2018. Circulating Progenitor Cells Correlate with Memory, Posterior Cortical Thickness, and Hippocampal Perfusion. J. Alzheimers. Dis 61, 91–101. 10.3233/JAD-170587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nation DA, Wierenga CE, Clark LR, Dev SI, Stricker NH, Jak AJ, Salmon DP, Delano-Wood L, Bangen KJ, Rissman RA, Liu TT, Bondi MW, 2013. Cortical and Subcortical Cerebrovascular Resistance Index in Mild Cognitive Impairment and Alzheimer’s Disease. J. Alzheimer’s Dis 36, 689–698. 10.3233/JAD-130086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradise M, Crawford JD, Lam BCP, Wen W, Kochan NA, Makkar S, Dawes L, Trollor J, Draper B, Brodaty H, Sachdev PS, 2021. Association of Dilated Perivascular Spaces With Cognitive Decline and Incident Dementia. Neurology 96, e1501–e1511. 10.1212/WNL.0000000000011537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S-L, Chen X, Li Y, Rodrigue KM, Park DC, Lu H, 2018. Age-related changes in cerebrovascular reactivity and their relationship to cognition: A four-year longitudinal study. Neuroimage 174, 257–262. 10.1016/j.neuroimage.2018.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petr J, Mutsaerts HJMM, De Vita E, Steketee RME, Smits M, Nederveen AJ, Hofheinz F, van den Hoff J, Asllani I, 2018. Effects of systematic partial volume errors on the estimation of gray matter cerebral blood flow with arterial spin labeling MRI. Magn. Reson. Mater. Physics, Biol. Med 31, 725–734. 10.1007/s10334-018-0691-y [DOI] [PubMed] [Google Scholar]
- Rasmussen MK, Mestre H, Nedergaard M, 2022. Fluid transport in the brain. Physiol. Rev 102, 1025–1151. 10.1152/physrev.00031.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Liu S, Lian C, Li H, Li K, Li L, Zhao G, 2021. Dysfunction of the Glymphatic System as a Potential Mechanism of Perioperative Neurocognitive Disorders. Front. Aging Neurosci 13. 10.3389/fnagi.2021.659457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Biessels GJ, De Guio F, de Leeuw FE, Duchesne S, Düring M, Frayne R, Ikram MA, Jouvent E, MacIntosh BJ, Thrippleton MJ, Vernooij MW, Adams H, Backes WH, Ballerini L, Black SE, Chen C, Corriveau R, DeCarli C, Greenberg SM, Gurol ME, Ingrisch M, Job D, Lam BYK, Launer LJ, Linn J, McCreary CR, Mok VCT, Pantoni L, Pike GB, Ramirez J, Reijmer YD, Romero JR, Ropele S, Rost NS, Sachdev PS, Scott CJM, Seshadri S, Sharma M, Sourbron S, Steketee RME, Swartz RH, van Oostenbrugge R, van Osch M, van Rooden S, Viswanathan A, Werring D, Dichgans M, Wardlaw JM, 2019. Harmonizing brain magnetic resonance imaging methods for vascular contributions to neurodegeneration. Alzheimer’s Dement. (Amsterdam, Netherlands) 11, 191–204. 10.1016/j.dadm.2019.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urback AL, MacIntosh BJ, Goldstein BI, 2017. Cerebrovascular reactivity measured by functional magnetic resonance imaging during breath-hold challenge: A systematic review. Neurosci. Biobehav. Rev 79, 27–47. 10.1016/j.neubiorev.2017.05.003 [DOI] [PubMed] [Google Scholar]
- Wang Z, 2012. Improving cerebral blood flow quantification for arterial spin labeled perfusion MRI by removing residual motion artifacts and global signal fluctuations. Magn. Reson. Imaging 30, 1409–1415. 10.1016/j.mri.2012.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Aguirre GK, Rao H, Wang J, Fernández-Seara MA, Childress AR, Detre JA, 2008. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn. Reson. Imaging 26, 261–269. 10.1016/j.mri.2007.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw JM, Benveniste H, Nedergaard M, Zlokovic BV, Mestre H, Lee H, Doubal FN, Brown R, Ramirez J, MacIntosh BJ, Tannenbaum A, Ballerini L, Rungta RL, Boido D, Sweeney M, Montagne A, Charpak S, Joutel A, Smith KJ, Black SE, 2020. Perivascular spaces in the brain: anatomy, physiology and pathology. Nat. Rev. Neurol 16, 137–153. 10.1038/s41582-020-0312-z [DOI] [PubMed] [Google Scholar]
- Wuerfel J, Haertle M, Waiczies H, Tysiak E, Bechmann I, Wernecke KD, Zipp F, Paul F, 2008. Perivascular spaces--MRI marker of inflammatory activity in the brain? Brain 131, 2332–2340. 10.1093/brain/awn171 [DOI] [PubMed] [Google Scholar]
- Yew B, Jang JY, Dutt S, Li Y, Sible IJ, Gaubert A, Ho JK, Blanken AE, Marshall A, Shao X, Wang DJJ, Nation DA, 2022. Cerebrovascular reactivity deficits in cognitively unimpaired older adults: vasodilatory versus vasoconstrictive responses. Neurobiol. Aging 113, 55–62. 10.1016/j.neurobiolaging.2022.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


