Abstract
INTRODUCTION:
We hypothesized that liver fibrosis is associated with worse cognitive performance and corresponding brain imaging changes.
METHODS:
We examined the association of liver fibrosis with cognition and brain imaging parameters in the UK Biobank study. Liver fibrosis was assessed using the Fibrosis-4 (FIB-4) score. The primary cognitive outcome was the Digit Symbol Substitution Test (DSST); secondary outcomes were additional executive function/processing speed and memory tests. Imaging outcomes were hippocampal, total brain, and white matter hyperintensity volumes.
RESULTS:
We included 105,313 participants with cognitive test data, and 41,982 with MRI. In adjusted models, liver fibrosis was associated with worse performance on the DSST and tests of executive function but not memory. Liver fibrosis was associated with lower hippocampal and total brain volumes, without compelling association with white matter hyperintensity volume.
DISCUSSION:
Liver fibrosis is associated with worse performance on select cognitive tests and lower hippocampal and total brain volumes.
Keywords: liver fibrosis, cognition, brain health, epidemiology
1. Background
The impact of systemic conditions on brain health is increasingly recognized. There is growing appreciation of the contributions of chronic liver conditions to neurological health.[1] Several epidemiological and mechanistic studies suggest that chronic liver conditions have a multifaceted impact on cognition besides the known relationship between liver cirrhosis and hepatic encephalopathy.[1–3] Chronic liver conditions can advance through progressive stages of fibrosis, which occurs in response to chronic liver injury of a variety of causes.[4] It is increasingly clear that liver fibrosis is a common condition and that it is often subclinical, without laboratory and clinical signs and symptoms typically associated with liver disease.[5–7] However, even when subclinical, liver fibrosis is not silent; it has been associated with cardiovascular and cerebrovascular disease.[8–12] We previously demonstrated an association between liver fibrosis and worse cognitive performance in a population-based sample.[13] Additionally, a cross-sectional analysis Framingham Study analysis found an association of liver fibrosis with worse executive function and abstract reasoning.[14] Mechanistically, liver fibrosis may impact cognitive function through aberrations in peripheral clearance of amyloid-β, vascular injury, and changes in neuroprotective hepatokine levels.[15–21] Clinically, an improved understanding of systemic determinants of cognitive impairment may yield new prevention and treatment strategies. In prior analyses, liver fibrosis and cognitive performance assessments were contemporaneous, and imaging data were limited.[13 ,14] In this analysis, our objective was to leverage data from a large, prospective cohort to test the hypothesis that liver fibrosis is associated with worse future cognitive test performance and corresponding imaging changes.
2. Methods
2.1. Design
This is a retrospective cohort study using data from the United Kingdom (UK) Biobank. The UK Biobank is a prospective cohort study that recruited approximately 500,000 participants between 40–69 years of age from across the UK.[22] Baseline assessments occurred from 2007 to 2010, and included detailed health questionnaires, physical measurements, laboratory testing, and genotyping. Laboratory tests included blood count and chemistries, such as those required for this analysis. Participants later completed cognitive tests, and some participants were invited for brain imaging for research purposes unrelated to clinical need. Participants provided signed, informed consent for UK Biobank participation. The anonymized data that support the findings of this study are available to qualified investigators upon application to the UK Biobank. Analytic methods are available upon reasonable request. The Weill Cornell Medicine institutional review board certified analyses of these deidentified data as exempt from review.
2.2. Population
From among approximately 500,000 participants in the UK Biobank, we included all participants who attended a baseline assessment center visit. We excluded people with missing exposure variable data (missing platelet count or aspartate/alanine aminotransferase), possible acute hepatitis (aspartate aminotransferase or alanine aminotransferase ≥250 international units/liter),[23] and people with severe thrombocytopenia (<50,000 per microliter).[24] These exclusions resulted in a study sample of 455,426 participants (Figure 1).
Figure 1.
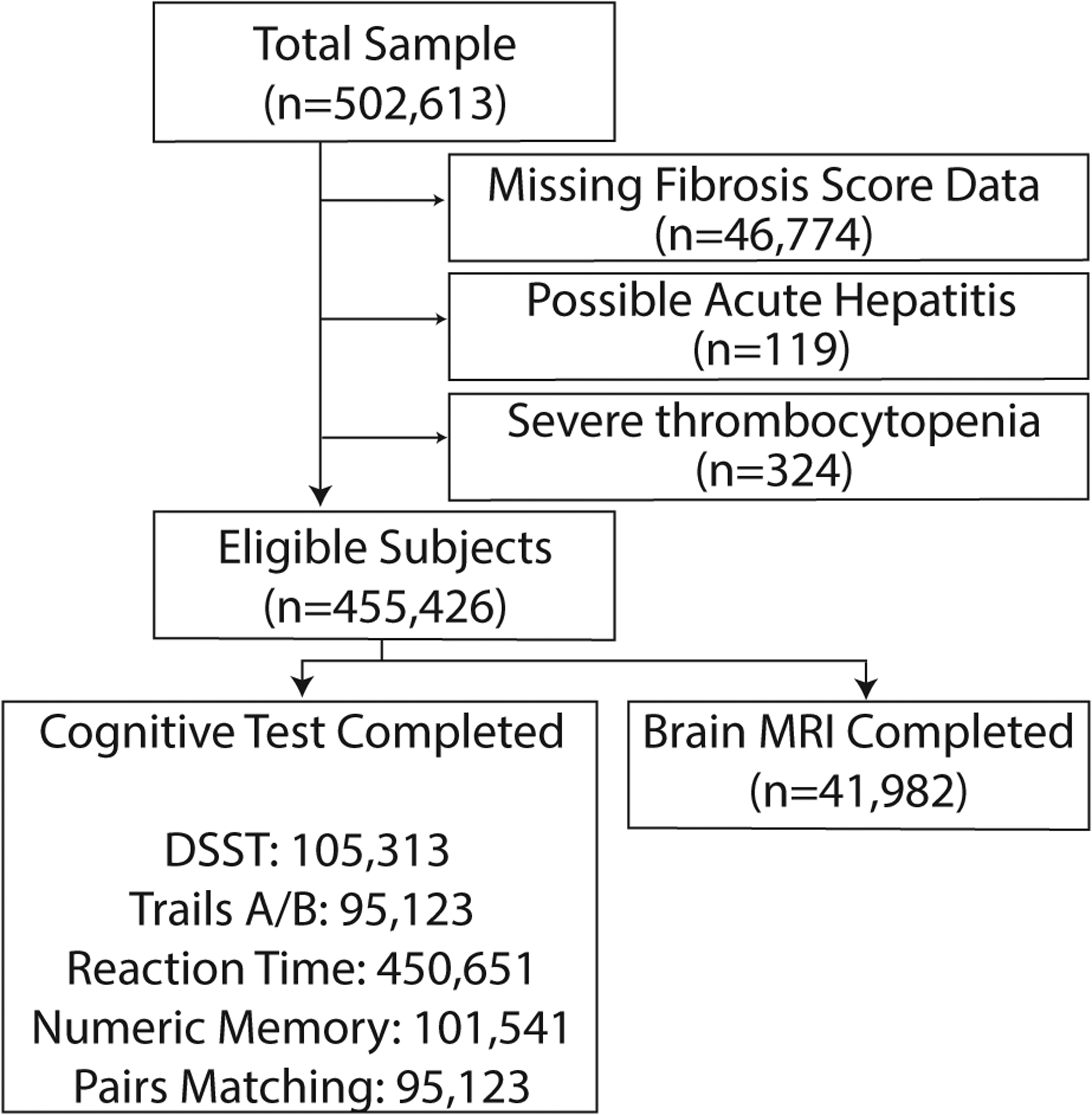
Study population flow chart.
We excluded participants with missing liver fibrosis score data, possible acute hepatitis (aspartate or alanine aminotransferase ≥250 international units per liter), and severe thrombocytopenia (platelet count <50,000 per microliter). Liver fibrosis score was missing because of missing platelet count in 24,488, aspartate aminotransferase in 34,860, or alanine aminotransferase in 33,255. Some participants had multiple reasons for exclusion.
2.3. Measurements
The exposure variable was liver fibrosis. We calculated the Fibrosis-4 (FIB-4) score according to its formula:
with age in years, aminotransferases in units/liter, and platelet count in 109/liter.[25] In the primary approach, we classified participants with FIB-4 score >2.67 as having liver fibrosis and those with FIB-4 ≤2.67 as not having liver fibrosis.[25 ,26] We prespecified the use of this categorical exposure variable to select participants with a high probability of liver fibrosis, mitigating the risk of misclassification by indeterminate range scores. A secondary approach treated the FIB-4 score as a continuous variable. Additional cutoffs were explored in a post-hoc analysis, below. The FIB-4 score has been validated to have good accuracy for liver fibrosis across common chronic liver conditions, including nonalcoholic fatty liver disease, alcoholic liver disease, and viral hepatitides.[26–29] Further, as evidence of validity in the general population, a high FIB-4 score, among people unselected for individual liver conditions, was associated with a 17-fold higher risk of future clinically apparent liver disease.[30] Our approach accounts for the real-world overlap between conditions such as alcoholic and presumed nonalcoholic fatty liver disease.[31]
The primary cognitive outcome was performance on the Digit Symbol Substitution Test (DSST), a multidomain test of processing speed, attention, and working memory. This was specified as the primary outcome based on a previously observed association with liver fibrosis.[13] We selected the following secondary cognitive measures: executive function/processing speed (Trail Making Test A, Trail Making Test B, Reaction Time) and memory (Numeric Memory, Pairs Matching). The Reaction Time test was completed in-person at baseline. Apart from this, tests were conducted using a web-based program upon e-mail invitation from the UK Biobank. The cognitive battery used in the UK Biobank has been validated.[32] Imaging outcomes were white matter hyperintensity (WMH), hippocampal, and total brain volumes. The UK Biobank performed brain magnetic resonance imaging (MRI) using 3-Tesla Siemens Skyra scanners starting 2014, and quality-checked these data.[33] In UK Biobank, imaging is acquired using identical hardware and software.[34] Additionally, an acquisition site variable is provided for model adjustment. We used imaging-derived phenotypes generated by an UK Biobank image-processing pipeline.[33 ,35] Total WMH volume was calculated based on T1 and T2 FLAIR sequence data, by UK Biobank using the Brain Intensity Abnormality Classification Algorithm.[35] Hippocampal volume was the mean of right and left hippocampal T1 structural volumes. MRI-derived measures accounted for total intracranial volume. MRI-derived measures accounted for total intracranial volume. WMH and hippocampal volumes were adjusted using the intracranial volume scaling factor recommended by UK Biobank.[36] Total brain volume was normalized for intracranial volume.[35 ,36] WMH volume was log transformed because of a positively-skewed distribution.
Covariates were sociodemographic factors (age, sex, race/ethnicity, education level, socioeconomic status) and cardiometabolic risk factors (hypertension, diabetes, dyslipidemia, tobacco use, metabolic syndrome, body mass index category), which were assessed at baseline using standard definitions based a combination of self-reported diagnoses, medication data, anthropometrics, and laboratory data (Appendix A.1). In addition, participants reporting prevalent hepatobiliary conditions as codified by UK Biobank (viral hepatitis, non-infective hepatitis, cirrhosis, alcoholic cirrhosis, primary biliary cirrhosis, bile duct obstruction/ascending cholangitis, sclerosing cholangitis, cholelithiasis, cholecystitis) during their nurse-led interview were categorized as having a known hepatobiliary condition; these participants were excluded in a sensitivity analysis to mitigate the possible impact of these heterogenous conditions on FIB-4 scores in the study population. A history of neurological disorder was defined, as coded by UK Biobank, as report of dementia or cognitive impairment of any etiology, Parkinson’s disease, epilepsy, multiple sclerosis, or stroke. Participants with at least one apolipoprotein E4 allele were categorized as carriers. We used standard definitions to assign genotypes based on rs429358 and rs7412 alleles, which were directly genotyped in UK Biobank.[37 ,38]
2.4. Statistical Analyses
The primary statistical approach entailed use of multivariable linear regression models to evaluate the association of liver fibrosis with cognitive and imaging measures, while adjusting for relevant confounders. In a secondary approach, the FIB-4 score was instead treated as a continuous variable to assess the association between 1-unit increase in FIB-4 and each outcome measure. We constructed the following models. The preliminary model was adjusted for age at time of cognitive/imaging measure, time from baseline to cognitive/imaging test, sex, race/ethnicity, educational attainment, and socioeconomic deprivation. The final model was additionally adjusted for hypertension, diabetes, dyslipidemia, body mass index, metabolic syndrome, smoking status, and alcohol consumption frequency. Models for MRI-derived measures were adjusted for acquisition site. We performed three sensitivity analyses. First, we replaced hypertension, diabetes, and dyslipidemia with systolic blood pressure, hemoglobin A1c, and total cholesterol, respectively. Second, we excluded all 2,653 participants with known hepatobiliary conditions at baseline to isolate the role of subclinical liver fibrosis and remove any possible influence of these conditions on baseline FIB-4 scores. Third, we excluded 11,793 participants with known neurological conditions at baseline to remove the possible influence of these conditions on outcome measures. We assessed four possible effect modifiers, which were metabolic syndrome (Appendix A.1), apolipoprotein E4 carrier status (carrier versus not), age (≥65 versus <65), and hypertension. Effect modification was evaluated based on the P-value of cross-product terms (liver fibrosis*effect modifier) on the multiplicative scale, and stratified analyses were performed where the interaction was significant. We performed several post-hoc analyses. First, to permit comparison of relative effect sizes across outcome measures, we performed additional analyses in which all outcome measures were treated uniformly: outcome measures were natural log transformed and then standardized (mean, 0; standard deviation, 1). Second, we categorized participants as having possible liver fibrosis based on more sensitive, age-specific FIB-4 cut-offs (age ≥65: FIB-4 score >2; age 35–64: FIB-4 score >1.3)[26] and compared them to the remaining participants without liver fibrosis. Third, we used an additional liver fibrosis score (Nonalcoholic Fatty Liver Disease Fibrosis Score [NFS]) to categorize participants as liver fibrosis (NFS>0.675) and without (NFS≤0.675).[39] The threshold of statistical significance was set at α = 0.05. Statistical analyses were performed using SAS Version 9.4 (Cary, NC).
3. Results
3.1. Population Characteristics
From among 502,613 participants in the UK Biobank, we included 455,426 participants after exclusions (Figure 1). Missing FIB-4 data was the most frequent reason for exclusion; participants with and without missing FIB-4 score data appeared similar (Appendix Table A.1). The mean age of participants was 56.5 (SD, 8.1), and 246,559 (54%) were women. Hepatobiliary conditions were prevalent in 2,653 (0.6%) participants. However, 9,907 (2.18%; 95% CI, 2.13–2.22%) participants had a FIB-4 score in the range validated to reflect a high probability of liver fibrosis. Participants with liver fibrosis were older, more likely men, had a higher prevalence of hypertension and diabetes, and more often reported daily alcohol consumption (Table 1).
Table 1.
Baseline characteristics* of participants in the UK Biobank study sample, stratified by liver fibrosis.
| With Liver Fibrosis | Without Liver Fibrosis | |
|---|---|---|
| Participants | N=9,907 | N=445,519 |
| Age, years (mean, SD) | 62.5 (5.9) | 56.4 (8.1) |
| Female | 3,338 (34%) | 243,221 (55%) |
| Race/ethnicity† | ||
| White | 9,255 (94%) | 420,309 (95%) |
| Black | 277 (3%) | 6,637 (2%) |
| Asian | 179 (2%) | 9,939 (2%) |
| Other | 122 (1%) | 6,584 (1%) |
| Education‡ | ||
| University graduate/Professional | 3,470 (47%) | 167,563 (46%) |
| Pre-university qualifications | 2,794 (38%) | 144,159 (39%) |
| Vocational school completion | 790 (11%) | 29,137 (8%) |
| Certificate of Secondary Education only | 280 (4%) | 24,252 (7%) |
| Socioeconomic deprivation§ | ||
| Quartile 1 (lowest deprivation) | 2,388 (24%) | 112,634 (25%) |
| Quartile 4 (highest deprivation) | 2,648 (27%) | 109,845 (25%) |
| Hypertension | 7,793 (79%) | 320,315 (72%) |
| Systolic blood pressure, mmHg (mean, SD) | 142 (20) | 138 (19) |
| Diabetes | 1,035 (10%) | 25,989 (6%) |
| Hemoglobin A1c, % (mean, SD) | 5.5 (0.7) | 5.5 (0.6) |
| Dyslipidemia | 7,1232 (72%) | 332,073 (75%) |
| Total cholesterol, mg/dL (mean, SD) | 5.3 (1.2) | 5.7 (1.1) |
| History of tobacco smoking | 6,247 (63%) | 265,129 (60%) |
| Metabolic syndrome | 2,922 (29%) | 125,705 (28%) |
| Body mass index, kg/m2 (mean, SD) | 27.3 (4.9) | 27.4 (4.8) |
| Known liver condition | 229 (2%) | 2,424 (1%) |
| History of neurological disorder | 410 (4%) | 11,383 (3%) |
| History of stroke | 244 (2%) | 5,757 (1%) |
| Alcohol consumption frequency | ||
| None | 971 (10%) | 35,902 (8%) |
| Less than once weekly | 1,912 (19%) | 100,806 (23%) |
| 1–4 times weekly | 4,246 (43%) | 218,367 (49%) |
| Daily or almost daily | 2,758 (28%) | 89,939 (20%) |
| Aspartate aminotransferase, IU/L (mean, SD) | 44 (30) | 26 (8) |
| Alanine aminotransferase, IU/L (mean, SD) | 30 (27) | 23 (13) |
| Platelet count, 109 cells/liter (mean, SD) | 155 (45) | 255 (58) |
| Albumin, g/dl (mean, SD) | 4.5 (0.3) | 4.5 (0.3) |
| Digit Symbol Substitution, no. correct (mean, SD) | 17.7 (4.8) | 19.9 (5.0) |
| Trail Making A, seconds (mean, SD) | 43.4 (22.8) | 39.1 (14.8) |
| Trail Making B, seconds (mean, SD) | 75.5 (28.6) | 66.6 (25.6) |
| Reaction time, milliseconds (mean, SD) | 586.7 (126.4) | 558.4 (117.2) |
| Numeric memory, no. digits recalled (mean, SD) | 6.8 (1.5) | 6.9 (1.5) |
| Pair matching, no. incorrect (mean, SD) | 0.9 (1.5) | 0.7 (1.3) |
| White matter hyperintensity volume, mm3 (mean, SD) | 8,632.4 (8,639.9) | 6,339.9 (8231.75) |
| Hippocampal volume, mm3 (mean, SD) | 4,659.0 (614.4) | 4,959.4 (573.4) |
| Total brain volume, mm3 (mean, SD) | 1,453,447 (72,527) | 1,495,223 (72,889) |
Abbreviations: SD, standard deviation; mmHg, millimeters mercury; mg/dL, milligrams per deciliter; kg/m2, kilograms per meter-squared; IU/L, international units per liter; g/dl, grams per deciliter.
Data are presented as n(%) unless otherwise specified. Percentages may not sum to 100% because of rounding.
Race/ethnicity groupings based on UK Biobank population composition.
UK education: A and O level certifications indicate higher educational attainment than Certificate of Secondary Education.
Socioeconomic deprivation measured using the Townsend deprivation index.
3.2. Association between liver fibrosis and cognitive test performance
Apart from the Reaction Time test, which was contemporaneous with the baseline visit as per UK Biobank study protocol, cognitive tests were completed a median of 5.8 years (IQR, 5.1-.6.5) after baseline. The primary cognitive measure was the DSST, which was available for 105,313 participants. These participants were more often White and had higher educational attainment and less socioeconomic deprivation than participants who did not complete the DSST, among other differences (Appendix Table A.2). The median score on the DSST was 20 correct matches (IQR, 17–23). Individuals with liver fibrosis had worse DSST performance than individuals without liver fibrosis, after adjusting for sociodemographic factors, cardiometabolic risk factors, and alcohol use (β, −0.46; 95% CI, −0.66 to −0.26; P<0.001). Similar associations were seen with executive function/processing tests, whereby individuals with liver fibrosis had worse (slower) performance on Trail A (β, 0.02; 95% CI, 0.01 to 0.04; P=0.002), Trail B (β, 0.03; 95% CI, 0.01 to 0.04; P<0.001), and Reaction Time Test (β, 4.40; 95% CI, 1.89 to 6.91; P=0.001), in adjusted models (Table 2). Trails A and B were log transformed; thus, for example, a β of 0.02 indicates a 2% change ((e0.02-1)*100) in the outcome measure for a 1.0 unit change in the exposure. However, liver fibrosis was not associated with memory test performance in adjusted models. This pattern of findings was also observed in a secondary approach that modeled the impact of each 1.0 unit change in FIB-4 score on cognitive measures (Table 2). Further, results were consistent in sensitivity analyses that adjusted for continuous measures of cardiometabolic risk factors, when excluding individuals with any clinically known liver condition at baseline, and when excluding individuals with neurological conditions at baseline (Table 2).
Table 2.
Association* of Liver Fibrosis with Cognitive Test Performance in the UK Biobank.
| Outcome and Model | Primary Approach: Liver Fibrosis versus no Liver Fibrosis | Secondary Approach: For each 1.0 unit increase in FIB-4 |
|---|---|---|
| Digit Symbol Substitution Test (no. correct) | ||
| Preliminary model† | −0.47 (−0.67, −0.27; P<0.001) | −0.16 (−0.22, −0.10; P<0.001) |
| Final adjusted model‡ | −0.46 (−0.66, −0.26; P<0.001) | −0.18 (−0.24, −0.11; P<0.001) |
| Sensitivity analysis 1§ | −0.40 (−0.61, −0.19; P<0.001) | −0.16 (−0.23, −0.09; P<0.001) |
| Sensitivity analysis 2¶ | −0.44 (−0.65, −0.23; P<0.001) | −0.17 (−0.23, −0.11; P<0.001) |
| Sensitivity analysis 3# | −0.44 (−0.64, −0.23; P<0.001) | −0.17 (−0.24, −0.11; P<0.001) |
| Trail Making Test A (time, seconds) || | ||
| Preliminary model† | 0.03 (0.01, 0.04; P<0.001) | 0.01 (0.005, 0.014; P<0.001) |
| Final adjusted model‡ | 0.02 (0.01, 0.04; P=0.002) | 0.01 (0.003, 0.012; P=0.001) |
| Sensitivity analysis 1§ | 0.02 (0.00, 0.04; P=0.011) | 0.01 (0.004, 0.013; P=0.001) |
| Sensitivity analysis 2¶ | 0.02 (0.01, 0.04; P=0.002) | 0.01 (0.003, 0.012; P=0.001) |
| Sensitivity analysis 3# | 0.02 (0.01, 0.04; P=0.003) | 0.01 (0.003, 0.012; P<0.001) |
| Trail Making Test B (time, seconds) || | ||
| Preliminary model† | 0.03 (0.02, 0.05; P<0.001) | 0.01 (0.002, 0.011; P=0.007) |
| Final adjusted model‡ | 0.03 (0.01, 0.04; P<0.001) | 0.01 (0.000, 0.010; P=0.008) |
| Sensitivity analysis 1§ | 0.03 (0.01, 0.04; P=0.001) | 0.01 (0.000, 0.010; P=0.009) |
| Sensitivity analysis 2¶ | 0.03 (0.01, 0.04; P<0.001) | 0.01 (0.000, 0.010; P=0.011) |
| Sensitivity analysis 3# | 0.03 (0.01, 0.04; P<0.001) | 0.01 (0.000, 0.010; P=0.012) |
| Reaction Time Test (time, milliseconds) | ||
| Preliminary model† | 5.05 (2.56, 7.54; P<0.001) | 1.94 (1.22, 2.65; P<0.001) |
| Final adjusted model‡ | 4.40 (1.89, 6.91; P=0.001) | 1.87 (1.14, 2.60; P<0.001) |
| Sensitivity analysis 1§ | 4.41 (1.77, 7.05; P=0.001) | 2.15 (1.38, 2.92; P<0.001) |
| Sensitivity analysis 2¶ | 3.78 (1.25, 6.31; P=0.003) | 1.61 (0.87, 2.35; P<0.001) |
| Sensitivity analysis 3# | 4.12 (1.59, 6.64; P=0.001) | 1.92 (1.18, 2.65; P<0.001) |
| Numeric Memory Test (no. digits recalled) | ||
| Preliminary model† | −0.01 (−0.08, 0.06; P=0.713) | 0.02 (−0.08, 0.12; P=0.141) |
| Final adjusted model‡ | −0.01 (−0.08, 0.06; P=0.739) | 0.00 (−0.02, 0.02; P=0.993) |
| Sensitivity analysis 1§ | 0.01 (−0.06, 0.08; P=0.804) | 0.00 (−0.02, 0.02; P=0.872) |
| Sensitivity analysis 2¶ | −0.01 (−0.08, 0.06; P=0.746) | 0.00 (−0.02, 0.02; P=0.996) |
| Sensitivity analysis 3# | −0.01 (−0.08, 0.06; P=0.724) | 0.00 (−0.02, 0.02; P=0.849) |
| Pair Matching Test (number incorrect) | ||
| Preliminary model† | 0.06 (0.00, 0.12; P=0.040) | 0.03 (0.01, 0.05; P=0.001) |
| Final adjusted model‡ | 0.06 (0.00, 0.12; P=0.062) | 0.02 (0.00, 0.03; P=0.020) |
| Sensitivity analysis 1§ | 0.06 (0.00, 0.12; P=0.055) | 0.02 (0.00, 0.04; P=0.013) |
| Sensitivity analysis 2¶ | 0.06 (0.00, 0.12; P=0.070) | 0.02 (0.00, 0.04; P=0.014) |
| Sensitivity analysis 3# | 0.06 (0.00, 0.12; P=0.069) | 0.02 (0.00, 0.04; P=0.024) |
Abbreviations: No., number; FIB-4, Fibrosis-4 score.
Results of multivariable linear regression reported as beta (95% CI; P value).
Adjusted for age at time of cognitive test, time from baseline to cognitive test, sex, race/ethnicity, educational attainment, and socioeconomic deprivation.
Additionally adjusted for hypertension, diabetes, dyslipidemia, body mass index, metabolic syndrome, smoking, and alcohol intake frequency.
Systolic blood pressure, hemoglobin A1c, and total cholesterol were used as continuous covariates in placed of hypertension, diabetes, and dyslipidemia, respectively, using final adjusted model covariates.
Excluded participants with any known liver condition at baseline, using final model covariates.
These outcome measures were natural log transformed; exponentiation of regression coefficients is required to interpret the percent change in the outcome measure for each 1.0 unit increase in the exposure [(eβ−1)*100].
Excluded participants with any known neurological disorder, using final model covariates.
The association of liver fibrosis with cognitive measures was not modified by metabolic syndrome or hypertension; however, effect modification by apolipoprotein E4 carrier status was observed for Trail A (P=0.030 for interaction), Trail B (P=0.051 for interaction), and Numeric Memory (P=0.015 for interaction) (Appendix Table A.3). Liver fibrosis appeared more strongly associated with worse performance on these among apolipoprotein E4 carriers than noncarriers, in stratified analyses (Appendix Table A.4) For numeric memory, divergent albeit nonsignificant associations between liver fibrosis and numeric memory were seen in apolipoprotein E4 noncarriers. Additionally, there was evidence of effect modification by age for DSST (P=0.016 for interaction) and Reaction Time Test (P=0.027 for interaction). For both tests, liver fibrosis was associated with worse performance among participants <65 years old but not participants ≥65 years old (Appendix Table A.4).
3.3. Association between liver fibrosis and brain imaging parameters
Brain MRI was completed for 41,982 participants, a median of 9.3 years (IQR, 7.7–10.3) after liver fibrosis measurements. These participants were more often White, better educated, less socioeconomically deprived, and less frequently had neurological disorders than those without MRI data (Appendix Table A.5). Individuals with liver fibrosis did not have higher WMH volume in adjusted models (β, 0.05; 95% CI, −0.02 to 0.12; P=0.196) (Table 3). However, FIB-4 score modeled as a continuous variable was associated with WMH volume in the final model (β, 0.03; 95% CI, 0.01 to 0.05; P=0.019), but this was not significant after adjusting for multiple comparisons. Liver fibrosis was associated with lower hippocampal volume (β, −86.8; 95% CI, −132.7 to −40.8; P<0.001) in adjusted models (Table 3). This finding was corroborated in a secondary approach that treated FIB-4 score as a continuous variable (Table 3). Last, while individuals with liver fibrosis did not have lower total brain volumes compared to individuals without liver fibrosis in the primary approach, higher FIB-4 score was associated with lower total brain volumes in the final adjusted model (β, −3768.0 per 1.0 unit increase in FIB-4; 95% CI, −5278.8 to −2257.2; P<0.001) (Table 3). The overall pattern of findings was unchanged in sensitivity analyses (Table 3). Effect modification by metabolic syndrome, apolipoprotein E4 carrier status, age, or hypertension was not observed (Appendix Table A.3).
Table 3.
Association* of Liver Fibrosis with Brain MRI Parameters in the UK Biobank.
| Primary Approach: Liver Fibrosis versus no Liver Fibrosis | Secondary Approach: For each 1.0 unit increase in FIB-4 | |
|---|---|---|
| White matter hyperintensity volume (mm3) † | ||
| Preliminary model‡ | 0.02 (−0.05, 0.09; P=0.594) | −0.02 (−0.04, 0.00; P=0.156) |
| Final adjusted model§ | 0.05 (−0.02, 0.12; P=0.196) | 0.03 (0.01, 0.05; P=0.019) |
| Sensitivity analysis 1¶ | 0.04 (−0.04, 0.12; P=0.268) | 0.03 (0.01, 0.05; P=0.011) |
| Sensitivity analysis 2# | 0.05 (−0.02, 0.12; P=0.227) | 0.03 (0.01, 0.05; P=0.020) |
| Sensitivity analysis 3** | 0.04 (−0.03, 0.12; P=0.248) | 0.03 (0.00, 0.05; P=0.021) |
| Hippocampal volume (mm3) † | ||
| Preliminary model‡ | −85.57 (−131.38, −39.76; P<0.001) | −20.55 (−33.79, −7.31; P=0.002) |
| Final adjusted model§ | −86.78 (−132.72, −40.84; P<0.001)* | −27.00 (−40.50, −13.50; P<0.001)* |
| Sensitivity analysis 1¶ | −86.46 (−134.36, −38.56; P<0.001) | −27.38 (−41.53, −13.23; P<0.001) |
| Sensitivity analysis 2# | −79.39 (−125.66, −33.12; P=0.001) | −26.19 (−39.75, −12.63; P<0.001) |
| Sensitivity analysis 3** | −79.39 (−125.66, −33.12; P=0.001) | −27.05 (−40.60, −13.50; P<0.001) |
| Total brain volume (mm3) † | ||
| Preliminary model‡ | −3679.6 (−8829.2, 1469.9; P=0.161) | −2329.8 (−3818.8, −840.7; P=0.002) |
| Final adjusted model§ | −4363.4 (−9581.0, 854.1; P=0.096) | −3768.0 (−5278.8, −2257.2; P<0.001)* |
| Sensitivity analysis 1¶ | −3996.9 (−9392.7, 1399.0; P=0.147) | −4013.1 (−5608.1, −2418.0; P<0.001) |
| Sensitivity analysis 2# | −3983.2 (−11096.4, 3130.1; P=0.131) | −3672.7 (−5189.6, −2155.7; P<0.001) |
| Sensitivity analysis 3** | −3649.6 (−8831.1, 1531.9; P=0.168) | −3658.8 (−5175.6, −2141.9; P<0.001) |
Abbreviations: FIB-4, Fibrosis-4 score; mm3, cubic millimeters.
Results of multivariable linear regression reported as beta (95% CI; P value). Bolded cells indicate that P value for final adjusted model met criteria for significance after applying multiple comparison penalty for 3 outcomes. 41,982 completed MRI: 34,730 had data for inclusion in final models for WMH analysis, 35,949 for hippocampal volume, and 35,964 for total brain volume.
All MRI-derived measure were adjusted for total intracranial volume. White matter hyperintensity volume was log-transformed; exponentiation of regression coefficients is required to interpret the percent change in the outcome measure for each 1.0 unit increase in the exposure [(eβ−1)*100].
Adjusted for age at time of cognitive test, time from baseline to cognitive test, MRI acquisition site, sex, race/ethnicity, educational attainment, and socioeconomic deprivation.
Additionally adjusted for hypertension, diabetes, dyslipidemia, body mass index, metabolic syndrome, smoking, and alcohol intake frequency.
Systolic blood pressure, hemoglobin A1c, and total cholesterol were used as continuous covariates in placed of hypertension, diabetes, and dyslipidemia, respectively, using final adjusted model covariates.
Excluded participants with any known liver condition at baseline, using final model covariates.
Excluded participants with any known neurological disorder, using final model covariates.
3.4. Post-hoc analyses of association of liver fibrosis with cognitive test performance and brain imaging parameters
First, results for cognitive and imaging measures were consistent in direction after standardizing outcome measures (Table 4, Figure 2). Second, we compared 155,853 (34%) participants with possible liver fibrosis based on more sensitive FIB-4 cutoffs to 299,573 participants without liver fibrosis; results were attenuated but overall consistent in direction as compared to results of primary analyses (Appendix Table A.6). Third, we used the NFS to categorize liver fibrosis in place of the FIB-4 score; results were overall consistent with primary analyses (Appendix Table A.7). As examples, full Model Results for one cognitive and one imaging outcome are shown in Appendix Table A.8.
Table 4.
Association* of Liver Fibrosis with Standardized Cognitive Test Performance and Brain MRI Parameters in the UK Biobank
| Outcome Measure | Primary Approach: Liver Fibrosis versus no Liver Fibrosis | Secondary Approach: For each 1.0 unit increase in FIB-4 |
|---|---|---|
| Digit Symbol Substitution Test | −0.06 (P<0.001) | −0.02 (P<0.001) |
| Trail Making Test A | 0.07 (P=0.002) | 0.05 (P<0.001) |
| Trail Making Test B | 0.09 (P<0.001) | 0.03 (P=0.008) |
| Reaction Time Test | 0.04 (P<0.001) | 0.03 (P<0.001) |
| Numeric Memory Test | −0.01 (P=0.602) | −0.00 (P=0.845) |
| Pair Matching Test | 0.02 (P=0.358) | 0.00 (P=0.896) |
| White matter hyperintensity volume | 0.05 (P=0.196) | 0.05 (P=0.019) |
| Hippocampal volume | −0.17 (P<0.001) | −0.09 (P<0.001) |
| Total brain volume | −0.07 (P=0.061) | −0.10 (P<0.001) |
Abbreviations: No., number; FIB-4, Fibrosis-4 score.
Results of multivariable linear regression reported as beta (P value). The outcome measures were natural log transformed and then standardized (mean=0, standard deviation=1) to permit comparison of effect size across outcomes. Models were adjusted for age at time of test/imaging, time from baseline to test/imaging, sex, race/ethnicity, educational attainment, socioeconomic deprivation, hypertension, diabetes, dyslipidemia, body mass index, metabolic syndrome, smoking, and alcohol intake frequency. MRI-derived measures were adjusted for total intracranial volume, and models for these measures were additionally adjusted for acquisition site.
Figure 2:
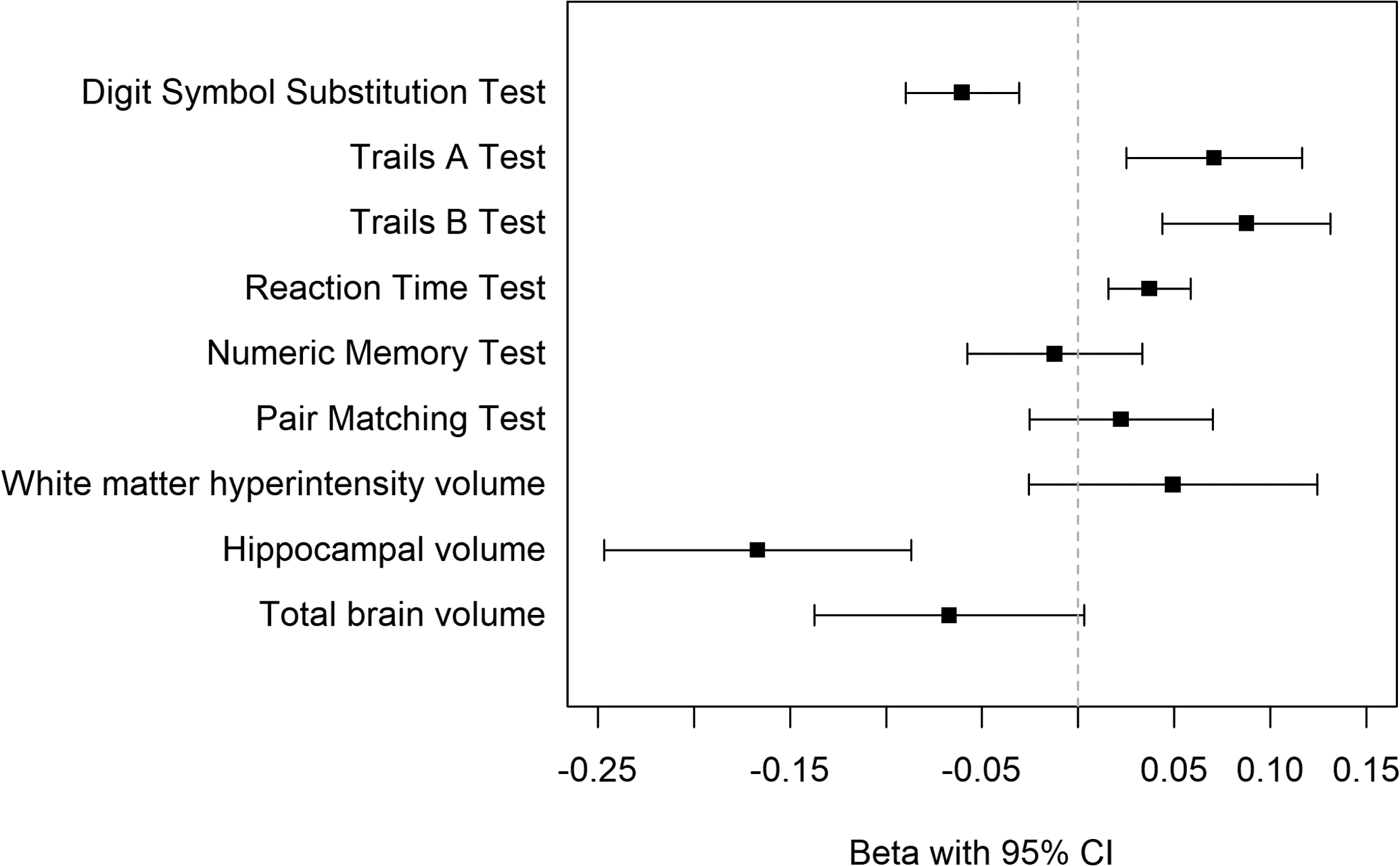
Association of Liver Fibrosis with Standardized Cognitive Test and Brain volume measures
Results of fully adjusted multivariable linear regression visualized as beta coefficient with 95% confidence interval bands. The outcome measures were natural log transformed and then standardized to permit comparison of effect size across outcomes. Positive beta values for Trails A and B, Reaction Time Test, and Pair Matching indicate worse performance.
4. Discussion
In this analysis using the UK Biobank cohort study, liver fibrosis was associated with worse future executive function/processing speed, but not memory test performance. In addition, liver fibrosis was associated with lower hippocampal and total brain volumes, with the latter finding only seen in secondary analysis.
Our findings advance our understanding of the liver-brain axis by using population-based data, critically including cognitive testing and imaging completed 5–9 years from baseline. In a prior population-based study of older Americans (mean age, 69), liver fibrosis and cognitive assessment were contemporaneous.[13] The pattern of findings was similar to our findings in the UK Biobank population: liver fibrosis was associated with worse DSST performance, but not memory tests. The large UK Biobank sample size permitted the detection of some associations that may not be clinically significant at the individual level, although the effect sizes were similar to that for cardiometabolic risk factors such as hypertension,[47 ,48] and similar to effect of liver fibrosis on Trail Making Test in the Framingham study.[14] The effect size for DSST, the primary cognitive outcome, was stronger in the prior analysis of NHANES participants; however, UK Biobank participants were younger and had 6-year interval between baseline and cognitive testing, during which associations may have attenuated.[13] Findings were similar in other cross-sectional cohorts of select populations, such as individuals with human immunodeficiency virus and hepatitis C virus infection or people with computed tomography evidence of hepatic steatosis.[14 ,40–42] Taken together, liver fibrosis is associated with worse performance on select cognitive tests, including when testing is years after liver fibrosis testing. This association has now been demonstrated in older and middle-aged population-based cohorts. Further, effect modification analyses suggest that some associations may be more pronounced for individuals under age 65 who may not yet have accrued a high burden of other risk factors, and for apolipoprotein E4 carriers. For the age interactions, another possible explanation is that FIB-4 scores may be less reliable in people ≥65 at baseline.[26] The absence of effect modification by metabolic syndrome and hypertension suggests that these conditions are not required for liver fibrosis to impact cognition.
There is growing data linking liver fibrosis to imaging markers associated with cognitive impairment and dementia. Several prior cross-sectional analyses identified a link between liver fibrosis and imaging markers of cerebral microvascular disease, including WMH volume and microbleeds.[15 ,16 ,43] These findings were not definitively corroborated in our analysis; an association with WMH volume was seen only in a secondary analysis, and the association was not significant after accounting for multiple comparisons. This may be because brain MRI was performed a median of 9 years after liver fibrosis assessment in UK Biobank, during which time other factors such as hypertension may have had a stronger influence on WMH accrual. Conversely, this 9-year interval allowed us to observe associations with smaller hippocampal and total brain volumes. Although imprecise and not necessarily specific, atrophy is an imaging marker of neurodegeneration.[44] Recent data also link liver health with other Alzheimer’s disease (AD) biomarkers. While not specifically implicating liver fibrosis, select liver enzymes were associated with increased cortical β-amyloid and reduced cerebral glucose metabolism in an Alzheimer’s Disease Neuroimaging Initiative analysis.[45] Additionally, a Framingham Study analysis reported associations of liver fibrosis markers with cortical tau in the rhinal cortex.[21] In that study, liver fibrosis was also associated with cortical β-amyloid in a subgroup analysis. These findings are supported by the observation that the liver may play a role in peripheral amyloid-β clearance.[18 ,19 ,46] Thus, using the prevailing biomarker paradigm,[44] liver fibrosis is associated with multiple, complementary imaging markers of AD. Further work in this area is required to determine whether these associations reflect causal relationships.
Whether liver fibrosis is a risk factor specifically for AD is not clear based on these data. The possibility of residual confounding by shared risk factors is possible; however, our results were robust to sensitivity analyses and approaches that sought to mitigate this risk. Apart from these concerns, we observed discordance between cognitive test data, which showed executive dysfunction without clear memory impairment, and the imaging data supportive of neurodegeneration. One possibility is that memory tests have ceiling effects, especially considering the good health and educational attainment in UK Biobank.[47] Alternatively, the association of liver fibrosis with executive dysfunction may be through additional mechanisms unrelated to neurodegeneration, such as covert hepatic encephalopathy, which is common in patients with cirrhosis (advanced liver disease).[48] However, our results were unchanged when excluding individuals with known hepatobiliary conditions, making it less likely that hepatic encephalopathy alone accounts for our findings. Vascular contributions are an additional possibility, although we did not find definitive evidence of increased WMH volumes. Last, emerging data suggests that liver derived hepatokines may be neuroprotective, although their role in human health remains unclear.[20] Future studies should investigate these pathways.
The key strengths of this analysis are large sample size, use of validated liver fibrosis scores, and temporal dispersion between fibrosis measurement and cognitive and imaging tests. There are several limitations. First, the FIB-4 score is an indirect marker, introducing the possibility of misclassification. The overall consistency of findings when using FIB-4 as a continuous variable, alternate FIB-4 cut-offs, and the NFS, is reassuring. Second, mediation relationships between liver fibrosis, cognition, and brain volumes could not be assessed because imaging followed, rather than preceded, cognitive testing. Third, UK Biobank lacks AD plasma biomarkers, ammonia levels, and neuropsychological testing. Fourth, UK Biobank has a healthy participant bias.[47] In this analysis, liver fibrosis prevalence was 2.2%, which is on the lower range of population-based estimates.[7] The UK Biobank population is also not representative of countries with different demographic compositions.
In conclusion, liver fibrosis was associated with worse performance on select cognitive tests and smaller hippocampal and total brain volumes. Our findings should not be interpreted as evidence of a direct, specific link between liver fibrosis and AD. Hippocampal and total brain volume loss may reflect neurodegeneration nonspecifically.[44] Additionally, a key limitation was the lack of neuropsychological testing and important fluid-based and imaging AD biomarkers. Further, because we lacked repeated cognitive and brain imaging measurements, our findings are not evidence of cognitive decline or neurodegeneration over time. Emerging evidence has linked liver fibrosis to cerebral β-amyloid and tau deposition[21]; however, more work is required before confidently concluding that liver fibrosis is associated specifically with AD rather than neurodegeneration broadly or cognitive impairment through systemic metabolic pathways. The fundamental outstanding task is to identify the mechanistic underpinnings of our findings and potential treatment targets therein. Accomplishing this will require leveraging metabolomics and proteomics in a longitudinal cohort of individuals that has undergone more direct liver fibrosis assessment (using transient hepatic elastography or magnetic resonance hepatic elastography), neuropsychological characterization, and AD biomarker analysis. In parallel, strategies to screen for liver fibrosis in people with or at risk of cognitive disorders should be evaluated, in order to identify individuals in whom to ultimately test whether targeting liver-related factors can preserve or improve cognitive health.
Supplementary Material
Acknowledgements
Funding:
Dr. Parikh is supported by the NIH/NIA (K23 AG073524), the Leon Levy Neuroscience Fellowship, and the Florence Gould Endowment for Discovery in Stroke. Dr. Gottesman is supported by the Intramural Research Program at the National Institute of Neurological Disorders and Stroke. This content is solely the responsibility of the authors and does not represent the official views of the National Institute of Health (NIH).
Conflicts of Interest and Financial Disclosures:
Dr. Parikh has received personal compensation for medicolegal consulting on stroke and research support from the New York State Empire Clinical Research Investigator Program, unrelated to this work. Dr. Kamel serves as a PI for the NIH-funded ARCADIA trial (NINDS U01NS095869) which receives in-kind study drug from the BMS-Pfizer Alliance for Eliquis® and ancillary study support from Roche Diagnostics, serves as Deputy Editor for JAMA Neurology, serves as a steering committee member of Medtronic’s Stroke AF trial, serves on a trial executive committee for Janssen, and serves on an endpoint adjudication committee for a trial of empagliflozin for Boehringer-Ingelheim. Dr. de Leon is supported by the NIH (RF1 AG057570, R56 AG058913). Dr. Iadecola serves on the Scientific Advisory Board of Broadview Ventures.
References
- [1].Chen TB, Yiao SY, Sun Y, et al. Comorbidity and dementia: A nationwide survey in Taiwan. PLoS One 2017;12:e0175475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bordet R, Deplanque D. Brain-liver axis: a new pathway for cognitive disorders related to hepatic fibrosis. Eur J Neurol 2020;27:2111–12. [DOI] [PubMed] [Google Scholar]
- [3].Bassendine MF, Taylor-Robinson SD, Fertleman M, et al. Is Alzheimer’s Disease a Liver Disease of the Brain? Journal of Alzheimer’s disease : JAD 2020;75:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Altamirano-Barrera A, Barranco-Fragoso B, Méndez-Sánchez N. Management strategies for liver fibrosis. Ann Hepatol 2017;16:48–56. [DOI] [PubMed] [Google Scholar]
- [5].Caballería L, Pera G, Arteaga I, et al. High Prevalence of Liver Fibrosis Among European Adults With Unknown Liver Disease: A Population-Based Study. Clin Gastroenterol Hepatol 2018;16:1138–45.e5. [DOI] [PubMed] [Google Scholar]
- [6].Calvaruso V, Craxì A. Implication of normal liver enzymes in liver disease. J Viral Hepat 2009;16:529–36. [DOI] [PubMed] [Google Scholar]
- [7].Harris R, Harman DJ, Card TR, et al. Prevalence of clinically significant liver disease within the general population, as defined by non-invasive markers of liver fibrosis: a systematic review. Lancet Gastroenterol Hepatol 2017;2:288–97. [DOI] [PubMed] [Google Scholar]
- [8].Ostovaneh MR, Ambale-Venkatesh B, Fuji T, et al. Association of Liver Fibrosis With Cardiovascular Diseases in the General Population: The Multi-Ethnic Study of Atherosclerosis (MESA). Circ Cardiovasc Imaging 2018;11:e007241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Long MT, Zhang X, Xu H, et al. Hepatic Fibrosis Associates With Multiple Cardiometabolic Disease Risk Factors: The Framingham Heart Study. Hepatology 2021;73:548–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Parikh NS, VanWagner LB, Elkind MSV, et al. Association between nonalcoholic fatty liver disease with advanced fibrosis and stroke. Journal of the neurological sciences 2019;407:116524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Park JG, Jung J, Verma KK, et al. Liver stiffness by magnetic resonance elastography is associated with increased risk of cardiovascular disease in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2021;53:1030–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Parikh NS, Kamel H, Navi BB, et al. Liver Fibrosis Indices and Outcomes After Primary Intracerebral Hemorrhage. Stroke 2020;51:830–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Parikh NS, Kumar S, Rosenblatt R, et al. Association between liver fibrosis and cognition in a nationally representative sample of older adults. Eur J Neurol 2020;27:1895–903. [DOI] [PubMed] [Google Scholar]
- [14].Weinstein G, Davis-Plourde K, Himali JJ, et al. Non-alcoholic fatty liver disease, liver fibrosis score and cognitive function in middle-aged adults: The Framingham Study. Liver Int 2019;39:1713–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Petta S, Tuttolomondo A, Gagliardo C, et al. The Presence of White Matter Lesions Is Associated With the Fibrosis Severity of Nonalcoholic Fatty Liver Disease. Medicine (Baltimore) 2016;95:e3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kim YD, Song D, Heo JH, et al. Relationship between Cerebral Microbleeds and Liver Stiffness Determined by Transient Elastography. PLoS One 2015;10:e0139227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bazerbachi F, Haffar S, Wang Z, et al. Range of Normal Liver Stiffness and Factors Associated With Increased Stiffness Measurements in Apparently Healthy Individuals. Clin Gastroenterol Hepatol 2019;17:54–64 e1. [DOI] [PubMed] [Google Scholar]
- [18].Xiang Y, Bu XL, Liu YH, et al. Physiological amyloid-beta clearance in the periphery and its therapeutic potential for Alzheimer’s disease. Acta Neuropathol 2015;130:487–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Maarouf CL, Walker JE, Sue LI, et al. Impaired hepatic amyloid-beta degradation in Alzheimer’s disease. PLOS ONE 2018;13:e0203659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Horowitz AM, Fan X, Bieri G, et al. Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain. Science 2020;369:167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Weinstein G, O’Donnell A, Davis-Plourde K, et al. Non-Alcoholic Fatty Liver Disease, Liver Fibrosis, and Regional Amyloid-beta and Tau Pathology in Middle-Aged Adults: The Framingham Study. Journal of Alzheimer’s disease : JAD 2022;86:1371–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Manolio TA, Weis BK, Cowie CC, et al. New models for large prospective studies: is there a better way? Am J Epidemiol 2012;175:859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kwo PY, Cohen SM, Lim JK. ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries. Am J Gastroenterol 2017;112:18–35. [DOI] [PubMed] [Google Scholar]
- [24].Williamson DR, Albert M, Heels-Ansdell D, et al. Thrombocytopenia in critically ill patients receiving thromboprophylaxis: frequency, risk factors, and outcomes. Chest 2013;144:1207–15. [DOI] [PubMed] [Google Scholar]
- [25].McPherson S, Stewart SF, Henderson E, et al. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010;59:1265–9. [DOI] [PubMed] [Google Scholar]
- [26].McPherson S, Hardy T, Dufour JF, et al. Age as a Confounding Factor for the Accurate Non-Invasive Diagnosis of Advanced NAFLD Fibrosis. Am J Gastroenterol 2017;112:740–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007;46:32–6. [DOI] [PubMed] [Google Scholar]
- [28].Kim BK, Kim DY, Park JY, et al. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int 2010;30:546–53. [DOI] [PubMed] [Google Scholar]
- [29].Xiao G, Zhu S, Xiao X, et al. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology 2017;66:1486–501. [DOI] [PubMed] [Google Scholar]
- [30].Hagström H, Talbäck M, Andreasson A, et al. Ability of Noninvasive Scoring Systems to Identify Individuals in the Population at Risk for Severe Liver Disease. Gastroenterology 2020;158:200–14. [DOI] [PubMed] [Google Scholar]
- [31].Long MT, Massaro JM, Hoffmann U, et al. Alcohol Use is Associated with Hepatic Steatosis Among Persons with Presumed Non-alcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol 2019;18:1831–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fawns-Ritchie C, Deary IJ. Reliability and validity of the UK Biobank cognitive tests. PLoS One 2020;15:e0231627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Alfaro-Almagro F, Jenkinson M, Bangerter NK, et al. Image processing and Quality Control for the first 10,000 brain imaging datasets from UK Biobank. Neuroimage 2018;166:400–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Littlejohns TJ, Holliday J, Gibson LM, et al. The UK Biobank imaging enhancement of 100,000 participants: rationale, data collection, management and future directions. Nature Communications 2020;11:2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Miller KL, Alfaro-Almagro F, Bangerter NK, et al. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat Neurosci 2016;19:1523–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Smith SM, Alfaro-Almargo F, Miller K. Brain Imaging Documentation. 2020. [Available from: https://biobank.ctsu.ox.ac.uk/crystal/ukb/docs/brain_mri.pdf accessed January 15, 2022.
- [37].Lumsden AL, Mulugeta A, Zhou A, et al. Apolipoprotein E (APOE) genotype-associated disease risks: a phenome-wide, registry-based, case-control study utilising the UK Biobank. EBioMedicine 2020;59:102954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bycroft C, Freeman C, Petkova D, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 2018;562:203–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007;45:846–54. [DOI] [PubMed] [Google Scholar]
- [40].Valcour VG, Rubin LH, Obasi MU, et al. Liver Fibrosis Linked to Cognitive Performance in HIV and Hepatitis C. J Acquir Immune Defic Syndr 2016;72:266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ciccarelli N, Fabbiani M, Grima P, et al. Liver fibrosis is associated with cognitive impairment in HIV-positive patients. J Int Aids Soc 2014;17:144–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Fabbiani M, Ciccarelli N, Castelli V, et al. Hepatitis C virus-related factors associated WITH cognitive performance in HIV-HCV-coinfected patients. J Neurovirol 2019;25:866–73. [DOI] [PubMed] [Google Scholar]
- [43].Jang H, Kang D, Chang Y, et al. Non-alcoholic fatty liver disease and cerebral small vessel disease in Korean cognitively normal individuals. Sci Rep 2019;9:1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jack CR, Bennett DA, Blennow K, et al. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 2016;87:539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Nho K, Kueider-Paisley A, Ahmad S, et al. Association of Altered Liver Enzymes With Alzheimer Disease Diagnosis, Cognition, Neuroimaging Measures, and Cerebrospinal Fluid Biomarkers. JAMA Netw Open 2019;2:e197978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wang YR, Wang QH, Zhang T, et al. Associations Between Hepatic Functions and Plasma Amyloid-Beta Levels-Implications for the Capacity of Liver in Peripheral Amyloid-Beta Clearance. Mol Neurobiol 2017;54:2338–44. [DOI] [PubMed] [Google Scholar]
- [47].Fry A, Littlejohns TJ, Sudlow C, et al. Comparison of Sociodemographic and Health-Related Characteristics of UK Biobank Participants With Those of the General Population. Am J Epidemiol 2017;186:1026–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wijdicks EF. Hepatic Encephalopathy. The New England Journal of Medicine 2016;375:1660–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


