Abstract
Ectopic Cushing syndrome is a rare clinical disorder resulting from excessive adrenocorticotrophic hormone (ACTH) produced by non-pituitary neoplasms, mainly neuroendocrine neoplasms (NENs) of the lung, pancreas, and gastrointestinal tract, and other less common sites. The genetic background of ACTH-producing NENs has not been well studied. Inspired by an index case of ACTH-producing pancreatic NEN carrying a gene fusion, we postulated that ACTH-producing NENs might be enriched for gene fusions. We herein examined 21 ACTH-secreting NENs of the pancreas (10), lung (9), thymus (1), and kidney (1) using targeted RNA sequencing. The tumors were classified according to the most recent WHO classification as NET-G1/typical carcinoid (n = 4), NETG-2/atypical carcinoid (n = 14), and NET-G3 (n = 3). Overall, targeted RNA sequencing was successful in 11 cases (4 of 10 pancreatic tumors, 5 of 9 pulmonary tumors, and in the one renal and one thymic tumor). All four successfully tested pancreatic tumors revealed a gene fusion: two had a EWSR1::BEND2 and one case each had a KMT2A::BCOR and a TFG::ADGRG7 fusion, respectively. EWSR1 rearrangements were confirmed in both tumors with a EWSR1::BEND2 by FISH. Gene fusions were mutually exclusive with ATRX, DAXX, and MEN1 mutations (the most frequently mutated genes in NETs) in all four cases. Using RNA-based variant assessment (n = 16) or via the TSO500 panel (n = 5), no pathogenic BCOR mutations were detected in any of the cases. Taken together, gene fusions were detected in 4/4 (100%) pancreatic versus 0/7 (0%) non-pancreatic tumors, respectively. These results suggest a potential role for gene fusions in triggering the ACTH production in pancreatic NENs presenting with ectopic Cushing syndrome. While the exact mechanisms responsible for the ectopic ACTH secretion are beyond the scope of this study, overexpressed fusion proteins might be involved in promoter-mediated overexpression of pre-ACTH precursors in analogy to the mechanisms postulated for EWSR1::CREB1-mediated paraneoplastic phenomena in certain mesenchymal neoplasms. The genetic background of the ACTH-producing non-pancreatic NENs remains to be further studied.
Keywords: ACTH; Neuroendocrine tumor; NET, ectopic Cushing; EWSR1, BEND2 fusion; KMT2A, BCOR fusion
Introduction
Ectopic Cushing syndrome is a diagnostically and therapeutically challenging rare clinical disorder resulting from chronic exposure to excessive adrenocorticotrophic hormone (ACTH) secreted by neoplasms of non-pituitary origin [1]. These ACTH-producing tumors are generally divided into two main categories: highly aggressive poorly differentiated carcinomas (small cell lung cancer being their prototype), and well-differentiated neuroendocrine tumors (NET) of diverse organs [1, 2]. Among the latter, bronchopulmonary carcinoids [3], gastroenteropancreatic NETs [4], pheochromocytoma/paraganglioma [5], and much less frequently, thymic NET [6], medullary thyroid carcinoma [7], NET of unknown origin [8], and rare anecdotal sites have been well documented as sources of ectopic Cushing syndrome. Moreover, rare non-neuroendocrine neoplasms may be associated with ectopic Cushing syndrome that resolves after tumor resection [4, 9, 10].
Pancreatic ACTH-producing neuroendocrine neoplasms (NENs) are rare with < 150 cases reported to date, mostly as single case reports or rare case series [4, 11, 12]. They are generally more aggressive with an overall 5-year survival of 35%, compared to 72–97% for most other hormone-secreting NETs [4].
To date, the pathogenetic mechanisms underlying the ectopic ACTH production by certain NENs and their genotypes remain obscure. Several theories have been postulated to explain the ectopic hormone production, but none could be proven. Based on an index pancreatic NEN that we have encountered to harbor a EWSR1::BEND2 gene fusion detected by targeted RNA sequencing, we examined a total of 21 ACTH-producing NENs from pancreatic, pulmonary, thymic, and renal origins to test the hypothesis that the EWSR1::BEND2 fusion or other alternate gene fusions might be enriched among these rare tumors.
Material and methods
The cases were identified from the consultation files of the authors and from the routine pathology files of the University Hospital Erlangen, Germany. The tissue specimens were fixed in formalin and processed routinely for histopathology. Due to the consultation nature of the cases, immunohistochemistry (IHC) was performed in different laboratories and the stains applied varied from case to case, based on tissue availability and initial differential diagnostic considerations (details of the staining protocols and antibody sources are available upon request).
A total of 21 ACTH-producing NENs from the pancreas (n = 10), the lung (n = 9), the kidney (n = 1), and the mediastinum/thymus (n = 1) have been collected. All tumors have been diagnosed, subtyped, and graded according to the most recent World Health Organization (WHO) classification of neuroendocrine neoplasms for the respective organ [12, 13]. ACTH production by the tumor cells was verified in all 21 cases, both clinically (manifested Cushing syndrome or elevated serum ACTH level) and histologically via ACTH immunohistochemistry. Based on a recent description of a BCOR mutation in a case of typical lung carcinoid with ectopic ACTH production [14] and the presence of a BCOR fusion in one of our cases, we also performed BCOR IHC on all cases.
Next-generation sequencing
RNA was isolated from formalin-fixed paraffin embedded (FFPE) tissue sections using RNeasy FFPE Kit of Qiagen (Hilden, Germany) and quantified spectrophotometrically using NanoDrop-1000 (Waltham, USA). Molecular analysis was performed using the TruSight RNA Fusion panel (Illumina, Inc., San Diego, CA, USA) with 500-ng RNA as input according to the manufacturer’s protocol. Libraries were sequenced on a MiSeq (Illumina, Inc., San Diego, CA, USA) with > 3 million reads per case, and sequences were analyzed using the RNA-Seq Alignment workflow, version 2.0.1 (Illumina, Inc., San Diego, CA, USA). The Integrative Genomics Viewer (IGV), version 2.2.13 (Broad Institute, REF), was used for data visualization [15]. In addition, DNA from cases 1, 2, 9, 10, and 20 was tested for mutations using the TruSight-Oncology500 Panel (Illumina, Inc., San Diego, CA, USA) according to the manufacturer’s protocol. The TruSight-Oncology500 Panel includes ATRX, DAXX, and MEN1, the three most frequently mutated genes in NETs.
FISH testing for EWSR1 rearrangements
In Case 1 and 9, fluorescence in situ hybridization (FISH) was performed on sections cut from formalin-fixed paraffin-embedded tissue blocks using the ZytoLight ® SPEC EWSR1 Dual Color Break Apart Probe which is designed to detect translocations involving the chromosomal region 22q12.2 harboring the EWSR1 gene (retrieved from ZytoVision, Bremerhaven, Germany) with standard protocols according to the manufacturer’s instructions.
Results
Clinical and demographic features
The major clinicopathological features are summarized in Table 1. The cohort included 12 females and 8 males (one of unspecified sex), aged 2 to 81 years (median, 49). The tumors originated in the pancreas (n = 10), lung (n = 9), thymus/mediastinum (n = 1), and kidney (n = 1).
Table 1.
Clinicopathological and molecular features of ACTH-producing neuroendocrine neoplasms (n = 21)
| No | Age/sex | Site | Histology | SSTR2A | BCOR IHC | RNA panel | Other molecular findings (TSO500 DNA panel) |
|---|---|---|---|---|---|---|---|
| 1 | 53/M | Pancreas | NETG3 (Ki67:55%) | + | + (wk.) |
EWSR1::BEND2 t(X;22)(p22;q12): chr22:29,688,158 (Exon 10; 3′-end) chrX:18,234,853 (Exon 2; 5′-end) |
No pathogenic mutations in MEN1, ATRX, DAXX, FUBP1, BCOR, VHL, or other genes |
| 2 | 29/M | Pancreas | NETG3 (Ki67:25%) | + | - |
KMT2A::BCOR t(11;X)(q23;p11): chr11:118,350,953 (Exon 6, 3′-end) chrX:39,923,852 BCOR (Exon 7, 5′-end) |
Likely pathogenic TSC1 mutation (p.S505LfsTer27; VAF: 3%) No pathogenic mutations in MEN1, ATRX, DAXX, FUBP1, BCOR, VHL, or other genes |
| 3 | NA | Pancreas | NETG2 (Ki67: 5%) | NA | - | Failed | No BCOR mutation* |
| 4 | 34/F | Pancreas | NETG2 (Ki67:10%) | + | + (wk.-moderate) | Failed | No BCOR mutation* |
| 5 | 15/M | Pancreas | NETG2 (Ki67:4%) | + | NA | Failed | No BCOR mutation* |
| 6 | 02/M | Pancreas | NETG1 (Ki67: < 2%) | NA | NA | Failed | No BCOR mutation* |
| 7 | 63/F | Pancreas | NETG2 (Ki67:20%) | + | + (wk.) | Failed | No BCOR mutation* |
| 8 | 44/F | Pancreas | NETG2 (Ki67:3%) | + | - | Failed | No BCOR mutation* |
| 9 | 36/F | Pancreas | NETG1 (Ki67: < 2%) | + | - |
EWSR1::BEND2 t(X;22)(p22;q12): chr22:29,688,595 (Exon 11; 3′-end) chrX:18,222,035 (Exon 5; 5′-end) |
No pathogenic mutations in MEN1, ATRX, DAXX, FUBP1, BCOR, VHL, or other genes |
| 10 | 48/F | Pancreas | NETG3 (> 20%) | + | NA |
TFG::ADGRG7 t(3;3) (q12.1; q12.1): Chr3: 100,438,902 (Exon 3; 3′-end) Chr3: 100,348,442 (Exon 2; 5′-end) |
FUBP1: c.289dupA, p.S97Kfs*25 (53%). VHL: c.331A > T, p.S111C (60%); no ATRX, DAXX, MEN1, or BCOR mutations |
| 11 | 21/M | Lung | AC (Ki67: 3%) | NA | - | Failed | No BCOR mutation* |
| 12 | 49/F | Lung | TC (Ki67: 1%) | NA | - | No fusion (poor quality) | No BCOR mutation* |
| 13 | 70/M | Lung | AC (Ki67: 24%) | - | NA | No fusion | No BCOR mutation* |
| 14 | 48/F | Lung | AC (Ki67: 30%) | NA | + (wk.) | No fusion (poor quality) | No BCOR mutation* |
| 15 | 60/F | Lung | AC (Ki67: 2.4%) | + | NA | Failed | No BCOR mutation* |
| 16 | 74/M | Lung | AC (Ki67: 2%) | - | - | Failed | No BCOR mutation* |
| 17 | 49/F | Lung | AC (Ki67: 2%) | + | - | Failed | No BCOR mutation* |
| 18 | 53/F | Lung | AC (Ki67: 5%) | + | - | No fusion | No BCOR mutation* |
| 19 | 34/F | Lung | TC (Ki67: 1%) | + | - | No fusion | No BCOR mutation* |
| 20 | 42/M | Mediastinal/thymus | AC (Ki67: 13%) | - | - | No fusion (poor quality) | No ATRX, DAXX, MEN1, or BCOR mutations |
| 21 | 54/F | Kidney | AC (Ki67: 12%) | - | - | No fusion | No BCOR mutation* |
AC, atypical carcinoid; ANED, alive with no evidence of disease; DOD, died of disease; mo, months; NA, not available; NET, neuroendocrine tumor; NEC, neuroendocrine carcinoma; post-op, postoperative; TC, typical carcinoid; wk., weak
*Verified using the RNA-based variant assessment
Histological and immunohistochemical findings
The tumor tissue was obtained from resection specimens. This also applies to the cases seen in consultation. The pancreatic tumors were graded as G1 (2 cases), G2 (5 cases), and G3 (3 cases). Of the pulmonary NENs, 7 tumors qualified as atypical carcinoid and two as typical carcinoid. The kidney and the thymic tumors were both graded as atypical carcinoids, corresponding to NET G2. All tumors were purely neuroendocrine; none of the pancreatic and the non-pancreatic tumors had a non-neuroendocrine component. Histologically, the tumors showed mainly a solid growth pattern and were composed of tumor cells with round nuclei and rich eosinophilic cytoplasm (Figs. 1, 2). Unequivocal strong cytoplasmic expression of ACTH was detected by immunohistochemistry in all 21 cases.
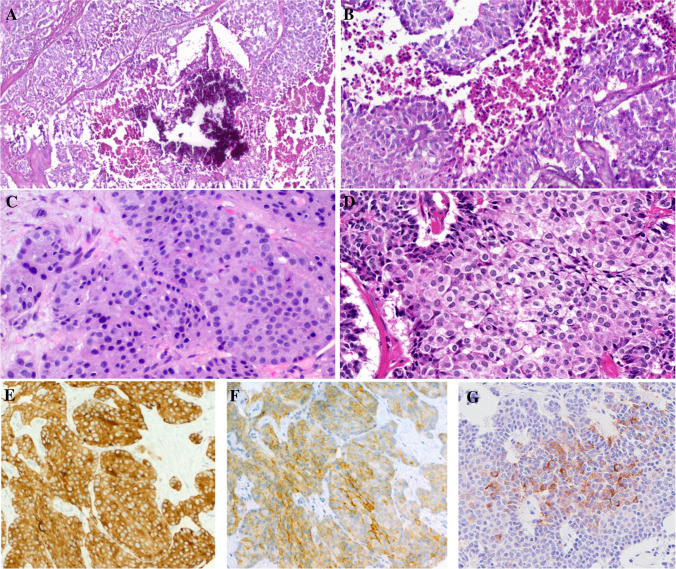
Fig. 1.
Representative examples of pancreatic ACTH-secreting neuroendocrine neoplasms. A + B This case (case 1 in Table 1 with EWSR1::BEND2 fusion) showed features of NETG3 with extensive foci of necrosis. C Liver metastasis from pancreatic NETG3 with KMT2A::BCOR fusion (case 2). D Primary pancreatic NETG3 (case 10 in Table 1 with TFG::ADGRG7 fusion). All tumors expressed synaptophysin (E), chromogranin-A (not shown), somatostatin receptor 2A (F), and ACTH (G)
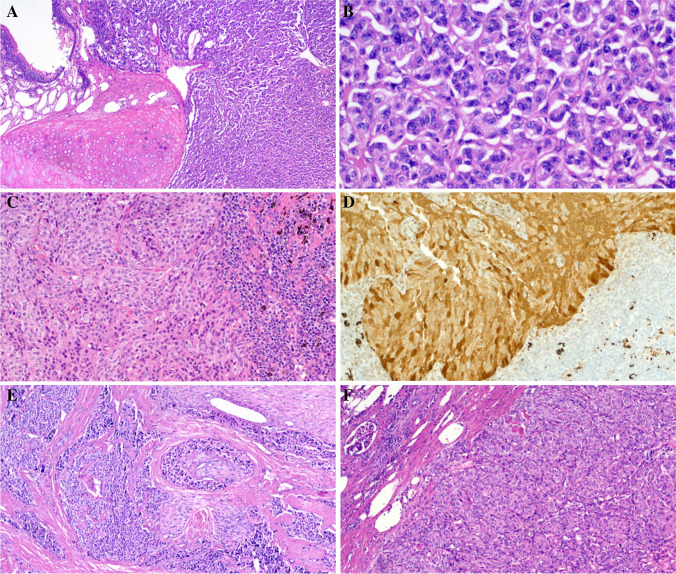
Fig. 2.
Representative examples of non-pancreatic ACTH-secreting neuroendocrine neoplasms. A + B Pulmonary typical carcinoid showing bronchocentric growth in A. C Pulmonary atypical carcinoid metastatic to peribronchial lymph node. D The same case shows strong diffuse ACTH expression in the nodal metastasis. E Thymic atypical carcinoid with extensive perineural invasion and stromal sclerosis. F Renal atypical carcinoid
The somatostatin receptor 2A showed a strong membranous expression in all pancreatic tumors, but only a weak and incomplete membranous reactivity in one of eight pulmonary tumors, and no expression in the mediastinal and renal tumors.
Results of BCOR immunohistochemistry
Overall, 16 cases revealed assessable results, with few cases showing suboptimal staining quality probably due to the old age of the tissue samples. BCOR expression was found in four tumors (25%), three of seven of pancreatic (Fig. 3A) and one of seven of pulmonary origin (Fig. 3B). Only one of three fusion-positive pancreatic NENs was BCOR-immunopositive. Notably, the BCOR-rearranged tumor showed no expression by immunohistochemistry.
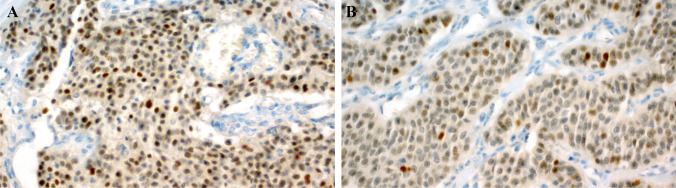
Fig. 3.
Representative examples of BCOR immunostaining. A This pancreatic tumor showed moderate expression in most of the tumor cell nuclei (case 4). B This pulmonary tumor revealed weak nuclear reactivity (case 14)
Molecular results
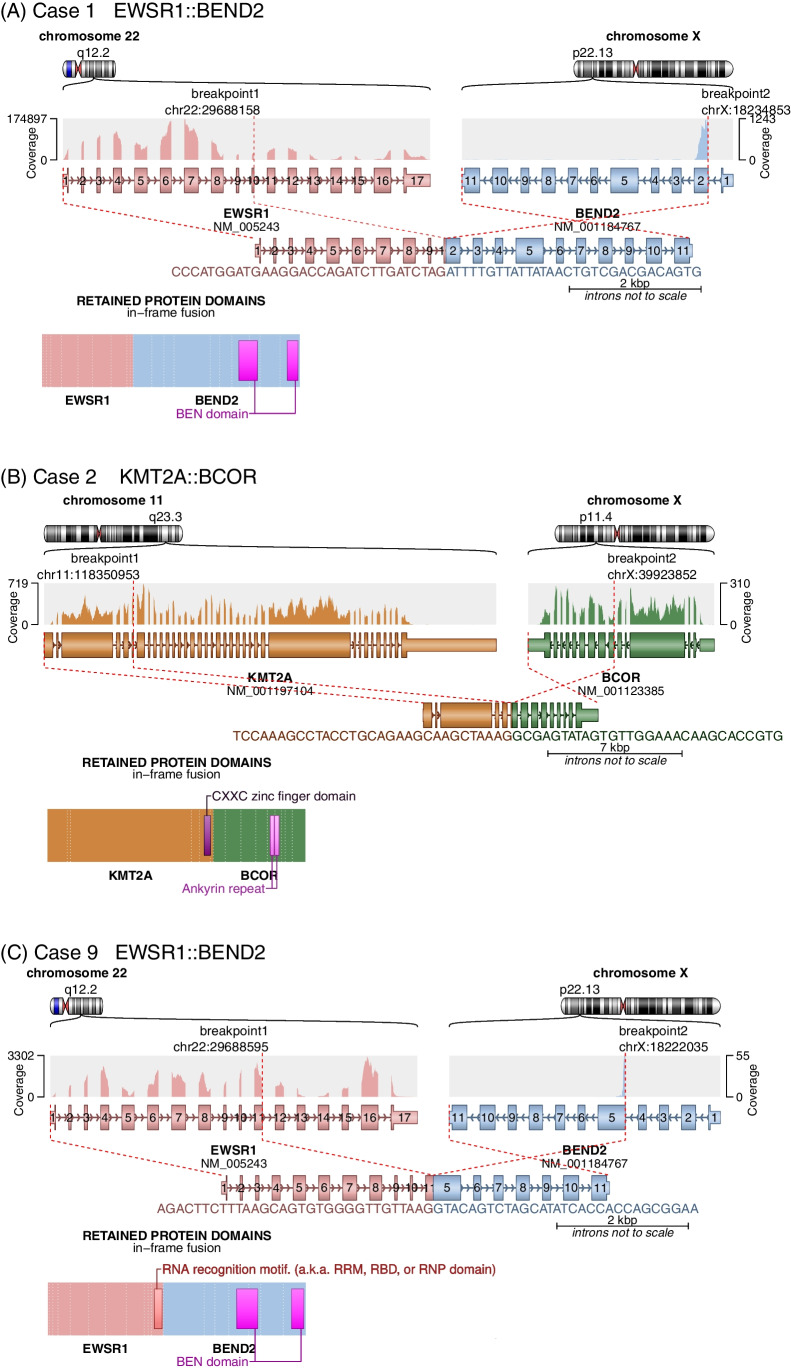
Overall, targeted RNA sequencing was successful in a total of 11 cases: 4 of 10 pancreatic tumors, 5 of 9 pulmonary tumors, and in the one renal and one thymic tumor (Table 1). All four successfully tested pancreatic tumors revealed a gene fusion: two had a EWSR1::BEND2 fusion and one case each had a KMT2A::BCOR and a TFG::ADGRG7 fusion, respectively (Fig. 4). A critical review of the histology of the two EWSR1-fused pancreatic cases revealed no evidence of neuroectodermal or mesenchymal differentiation. EWSR1 rearrangements were confirmed in both tumors using a EWSR1 FISH probe.
Fig. 4.
Schematic representation of gene fusions detected by panel sequencing. Illustrated is the gene structure of each fusion partner with the read coverage by panel sequencing, as well as the preserved exons resulting from the fusion event. The encoded chimeric protein including the retained protein domains is shown below. Depicted are gene fusions involving the genes EWSR1 and BEND2 (A+B = cases 1 and 9), as well as the KMT2A::BCOR fusion detected in case 2 (C). Arriba_v2.3.0 with RefSeq annotation was employed for visualization purpose (Uhrig et al., 2021) [44]
All five cases tested with the TruSight Oncology500 (TSO500) gene panel (cases 1, 2, 9, 10, and 20) revealed no pathogenic or likely pathogenic mutations in ATRX, DAXX and MEN1, BCOR, or any of the other genes included in the panel. However, case 10 harbored pathogenic mutations in FUBP1 and vhl in addition to the TFG::ADGRG7 fusion. This patient has no sign or family history of von Hippel-Lindau syndrome. BCOR was assessed in the remaining 16 cases using RNA-based variant assessment; none showed evidence of a pathogenic or likely pathogenic BCOR mutation.
Discussion
The genetic landscape of NENs has been emerging with several recently published comprehensive genetic studies. Inactivating somatic mutations in ATRX, DAXX, and MEN1 represent the major mutations encountered in pancreatic NETs, but are much less frequent in their thoracopulmonary counterparts, indicating distinct site-dependent genetic pathways driving these tumors [11, 16]. A recent whole-genome study on 102 primary pancreatic NETs uncovered four major molecular pathways altered in them. The major affected genes are those involved in chromatin remodelling, DNA damage repair, mTOR signaling activation, and telomere maintenance [17]. On the other hand, the genetic background of ACTH-producing NENs remains poorly studied.
In this study, we detected gene fusions in all four successfully tested ACTH-producing pancreatic NETs with associated Cushing syndrome. Two of the four gene fusions were EWSR1::BEND2 fusions which are exceptionally rare in solid cancers. They have been originally described in a subset of gliomas with astroblastoma-like morphology (eight cases reported to date) [18–20]. In these rare gliomas, EWSR1 represents an alternate fusion partner, which however became more frequently encountered than the originally described MN1::BEND2 fusion [18–20]. In decreasing order of frequency, EWSR1, MN1, and MAMLD1 are the reported fusion partners of BEND2 in astroblastoma-like gliomas [18–21]. It has been hypothesized that the fusion of MN1, EWSR1, or MAMLD1 with BEND2 enhances the expression of BEND2 downstream of the breakpoint and consequently drives oncogenesis [18–21]. Additional tumor types with BEND2 fusions include single-case reports of unclassified basal cell-like salivary gland adenocarcinoma with a EWSR1::BEND2 fusion [22] and one spindle cell soft tissue sarcoma with MN1::BEND2 fusion [23].
In pancreatic NETs, EWSR1::BEND2-fusions have only been once reported. Two tumors among the 102 primary pancreatic NETs analyzed by Scarpa et al. in the above cited whole-genome sequencing study carried the EWSR1::BEND2 fusion [17]. One of these reported EWSR1::BEND2 fusion positive NETs was an ACTH-secreting tumor, while the other was a nonfunctioning NET (personal communication given by Prof. Aldo Scarpa, University of Verona). A third case in that same study harbored a EWSR1::FLI1 fusion [17]. In addition, a CDH7::BEND2 fusion has been found in a case report of a hepatic metastasis of a pancreatic NET G3, but there was no mention of ectopic ACTH secretion by the tumor [24]. All fusion-positive pancreatic NENs were reportedly typical NETs without evidence of mesenchymal elements, and they lacked membranous CD99 expression [17], ruling out the possibility of mesenchymal mimics with neuroendocrine-like features [25].
In the remaining two fusion positive cases of our study, we detected a KMT2A::BCOR and a TFG::ADGRG7 fusion, respectively, but no fusion was detected in any of the 7 successfully analyzed ACTH-producing tumors of pulmonary (n = 5), renal (n = 1), or thymic (n = 1) origin. Although limited by the low number of successfully tested cases, our results indicate a potentially high frequency of recurrent gene fusions (mainly EWSR1::BEND2) in pancreatic ACTH-producing NENs. Inclusion of different tumor grades in our cohort argues against the possibility of a grade-dependent fusion association. Taken together (including the personal communication with Prof. Scarpa), three of the four pancreatic NETs with an EWSR1::BEND2 fusion were ACTH-producing, suggesting a significant association between this gene fusion and the ectopic ACTH production. Notably, the significant enrichment of our pancreatic ACTH-producing NETs for the EWSR1::BEND2 fusion (found in 2 of 4 successfully tested cases = 50%) strikingly contrasts with the very low overall frequency of this fusion in unselected pancreatic NETs (2% [17]).
The KMT2A::BCOR fusion which we detected in one tumor is novel. Although it is not clear, if the two different fusions EWSR1::BEND2 and KMT2A::BCOR might have functional relationship and hence represent two mutually exclusive alternate molecular pathomechanisms in ACTH-producing pancreatic NETs, it is noteworthy that two of the reported EWSR1::BEND2-rearranged astroblastoma-like gliomas expressed BCOR by immunohistochemistry, despite the absence of molecular BCOR alterations [20]. This suggests some oncogenic interactions between BEND2 and BCOR. Moreover, a recently reported case of ACTH-secreting pulmonary carcinoid revealed a BCOR mutation, representing another argument for a potential role of BCOR in the pathogenesis of ACTH-producing NENs lacking gene fusions [14]. The KTM2A::BCOR fusion we detected herein has been only recently reported in a single case of synovial chondromatosis [26], but it represents a novel fusion in the context of a NEN. Using RNA-based variant assessment, we detected no pathogenic BCOR mutations in our fusion-negative cases, but the RNA-based method is of limited reliability and direct DNA-based sequencing of BCOR is mandatory to rule out BCOR mutations. BCOR immunohistochemical analysis as surrogate for BCOR alterations revealed no significant overexpression across the different molecular subgroups of the NETs we tested, indicating the limited value of BCOR IHC in this context. The TFG::ADGRG7 fusion detected herein in our fourth fusion-positive case remains of unknown significance. TFG is a well-known fusion partner in a variety of mesenchymal and epithelial neoplasms of different organs, mostly fused to other receptor tyrosine kinases such as ALK, ROS1, and NTRK [27]. However, in a recent study, the TFG::ADGRG7 fusion was detected in a T-cell lymphoblastic leukemia sample and in normal tissue so it remains unclear, if it was present in the germline and, if yes, whether it is pathogenic or it represents a benign variant [28]. This remains an issue of future studies.
In light of the recurrent nature of the EWSR1::BEND2 fusions (detected in two of our cases and in the one ACTH-positive tumor in the Scarpa et al. study [17]), it seems that these fusions are likely driver events in these tumors as they proved to be mutually exclusive with ATRX, DAXX, and MEN1 mutations (as the most frequently mutated genes in NETs) in all four fusion-positive cases.
The occurrence of these unusual gene fusions in 3 of 4 ACTH-secreting pancreatic NENs makes us think that these findings are not a mere coincidence, but might be, in one way or another, related to the triggering of ectopic hormone production. It is well known that several mesenchymal entities driven by gene fusions including STAT6:NAB2 (hypoglycemia in 5% of solitary fibrous tumors), ETV6::NTRK3 (neonatal hypercalcemia in infantile fibrosarcoma/congenital mesoblastic nephroma), and EWSR1::CREB1 (hyper-Interleukin-6 in angiomatoid fibrous histiocytoma) are specifically associated with paraneoplastic phenomena [29]. Excessive production of IL-6 by the neoplastic cells of angiomatoid fibrous histiocytoma represents the major factor responsible for its associated paraneoplastic symptoms [30]. Notably, the presence of a CREB-binding site in the IL-6 promotor region together with the high expression of CREB triggered by the EWSR1::CREB1 fusion has been proposed to explain the IL-6 overexpression observed in this rare tumor type [30–32]. Moreover, paraneoplastic hypercalcemia, while being specifically associated with the ETV6::NTRK3 fusions, is also significantly associated with SMARCA4 inactivation in the majority of small cell carcinoma of the ovary, hypercalcemic type [29]. The same principle might apply to the observations that ectopic ACTH production can be associated with a variety of molecular genetic alterations including EWSR1::BEND2 and other gene fusions as in our cases, BCOR mutation as in one reported typical pulmonary carcinoid [14], and EWSR1::ETS fusions as in two recently reported Ewing sarcoma cases [33, 34].
ACTH-producing NENs express pro-opiomelanocortin (POMC), the precursor of ACTH, in addition to other pre-ACTH precursor molecules [1]. The gene encoding POMC is mapped to 2p23.3. The regulation of POMC expression seems highly complex and underlies different mechanisms, under both physiological and pathological conditions [35–42]. Hypomethylation of the POMC promoter, demethylation of the E2F1-binding site, and others have been suggested as a possible explanation for the ectopic secretion of POMC by non-corticotroph tumor cells [39, 42, 43]. In this study, we suggest possible additional mechanisms of overexpression of ACTH-precursors and hence ectopic ACTH production by the neoplastic cells of NEN via a yet unknown mechanism that is likely related to the underlying gene fusion, at least in a subset of cases. In this regard, molecular/structural homology between parts of the fusion transcript and the promoter of POMC or other pre-ACTH precursors might be responsible for the overexpression of the ACTH precursors, but this remains currently speculative and merits functional verification in future studies.
In summary, we herein have reported gene fusions involving BEND2, BCOR and TFG in four successfully tested ACTH-producing and Cushing syndrome-associated NENs of the pancreas, but not in those from non-pancreatic origin. This unusual genotype might be involved in the triggering of ectopic ACTH hormone production in pancreatic NENs, the mechanisms of which deserve future functional and genetic in-depth studies.
Acknowledgements
The authors would like to thank Petra Meyer (Technical University Munich, Munich, Germany) and Nina Oks (Institute of Pathology, University of Erlangen) for excellent technical support.
Author contribution
AA, GK: conception and design of the work, acquisition, analysis and interpretation of data, drafting the MS, and revising it critically for important intellectual content and scientific integrity.
AA, AK, RS, FH, CS, LT, NP, AvW, MEP, FS, SU, SLR, and GK: acquisition, analysis and interpretation of data, reading and revising the MS critically for important intellectual content and scientific integrity. All the authors have read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was financially supported by a grant of the Manfred Stolte Stiftung given to Dr. Atsuko Kasajima.
Samples were used in accordance with ethical guidelines for the use of retrospective tissue samples provided by the local ethics committee of the Friedrich-Alexander University Erlangen-Nuremberg (ethics committee statements 24.01.2005 and 18.01.2012).
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Isidori AM, Kaltsas GA, Pozza C, Frajese V, Newell-Price J, Reznek RH, Jenkins PJ, Monson JP, Grossman AB, Besser GM. The ectopic adrenocorticotropin syndrome: clinical features, diagnosis, management, and long-term follow-up. J Clin Endocrinol Metab. 2006;91:371–377. doi: 10.1210/jc.2005-1542. [DOI] [PubMed] [Google Scholar]
- 2.Coates PJ, Doniach I, Howlett TA, Rees LH, Besser GM. Immunocytochemical study of 18 tumours causing ectopic Cushing’s syndrome. J Clin Pathol. 1986;39:955–960. doi: 10.1136/jcp.39.9.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu TH, Liu HR, Lu ZL, Wang ZY, Cao Y, Chen J, Wang Y. Thoracic ectopic ACTH-producing tumors with Cushing’s syndrome. Zentralbl Pathol. 1993;139:131–139. [PubMed] [Google Scholar]
- 4.Maragliano R, Vanoli A, Albarello L, Milione M, Basturk O, Klimstra DS, Wachtel A, Uccella S, Vicari E, Milesi M, Davì MV, Scarpa A, Sessa F, Capella C, La Rosa S. ACTH-secreting pancreatic neoplasms associated with Cushing syndrome: clinicopathologic study of 11 cases and review of the literature. Am J Surg Pathol. 2015;39:374–382. doi: 10.1097/PAS.0000000000000340. [DOI] [PubMed] [Google Scholar]
- 5.Elliott PF, Berhane T, Ragnarsson O, Falhammar H. Ectopic ACTH- and/or CRH-producing pheochromocytomas. J Clin Endocrinol Metab. 2021;106:598–608. doi: 10.1210/clinem/dgaa488. [DOI] [PubMed] [Google Scholar]
- 6.Guerrero-Pérez F, Peiró I, Marengo AP, Teulé A, Ruffinelli JC, Llatjos R, Serrano T, Macia I, Vilarrasa N, Iglesias P, Villabona C. Rev Ectopic Cushing’s syndrome due to thymic neuroendocrine tumours: a systematic review. Endocr Metab Disord. 2021;22:1041–1056. doi: 10.1007/s11154-021-09660-2. [DOI] [PubMed] [Google Scholar]
- 7.Koehler VF, Fuss CT, Berr CM, Frank-Raue K, Raue F, Hoster E, Hepprich M, Christ E, Pusl T, Reincke M, Spitzweg C, Kroiss M, German Study Group for Rare Malignant Tumours of the Thyroid and Parathyroid Glands Medullary thyroid cancer with ectopic Cushing’s syndrome: a multicentre case series. Clin Endocrinol (Oxf) 2022;96:847–856. doi: 10.1111/cen.14617. [DOI] [PubMed] [Google Scholar]
- 8.Bostan H, Duger H, Akhanli P, Calapkulu M, Turkmenoglu TT, Erdol AK, Duru SA, Sencar ME, Kizilgul M, Ucan B, Ozbek M, Cakal E. Cushing’s syndrome due to adrenocorticotropic hormone-secreting metastatic neuroendocrine tumor of unknown primary origin: a case report and literature review. Hormones (Athens) 2022;21:147–154. doi: 10.1007/s42000-021-00316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Zakhari R, Aljammali S, Ataallah B, Bardarov S, Otterbeck P. Ectopic Cushing syndrome in adenocarcinoma of the lung: case report and literature review. Cureus. 2021;13:e14733. doi: 10.7759/cureus.14733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makhlouf HR, Abdul-Al HM, Wang G, Goodman ZD. Calcifying nested stromal-epithelial tumors of the liver: a clinicopathologic, immunohistochemical, and molecular genetic study of 9 cases with a long-term follow-up. Am J Surg Pathol. 2009;33:976–983. doi: 10.1097/PAS.0b013e31819c1ab3. [DOI] [PubMed] [Google Scholar]
- 11.Guilmette JM, Nosé V. Neoplasms of the neuroendocrine pancreas: an update in the classification, definition, and molecular genetic advances. Adv Anat Pathol. 2019;26:13–30. doi: 10.1097/PAP.0000000000000201. [DOI] [PubMed] [Google Scholar]
- 12.The WHO Classification of Tumours Editorial Board (2019) Tumours of the digestive system. Lyon: IARC Press, 5th Edition.
- 13.The WHO Classification of Tumours Editorial Board (2021) Thoracic tumours. Lyon: IARC Press, 5th Edition.
- 14.Wu Y, Yue L, Li J, Yuan M, Chai Y. Cushing’s syndrome secondary to typical pulmonary carcinoid with mutation in BCOR gene: a case report. Medicine (Baltimore) 2017;96:e7870. doi: 10.1097/MD.0000000000007870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP. Integrative Genomics Viewer. Nat Biotechnol. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volante M, Mete O, Pelosi G, Roden AC, Speel EJM, Uccella S. Molecular pathology of well-differentiated pulmonary and thymic neuroendocrine tumors: what do pathologists need to know? Endocr Pathol. 2021;32:154–168. doi: 10.1007/s12022-021-09668-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scarpa A, Chang DK, Nones K, et al. Whole-genome landscape of pancreatic neuroendocrine tumours. Nature. 2017;543:65–71. doi: 10.1038/nature21063. [DOI] [PubMed] [Google Scholar]
- 18.Burford A, Mackay A, Popov S, Vinci M, Carvalho D, Clarke M, Izquierdo E, Avery A, Jacques TS, Ingram WJ, Moore AS, Frawley K, Hassall TE, Robertson T, Jones C. The ten-year evolutionary trajectory of a highly recurrent paediatric high grade neuroepithelial tumour with MN1:BEND2 fusion. Sci Rep. 2018;8:1032. doi: 10.1038/s41598-018-19389-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsutsui T, Arakawa Y, Makino Y, Kataoka H, Mineharu Y, Naito K, Minamiguchi S, Hirose T, Nobusawa S, Nakano Y, Ichimura K, Haga H, Miyamoto S. Spinal cord astroblastoma with EWSR1-BEND2 fusion classified as HGNET-MN1 by methylation classification: a case report. Brain Tumor Pathol. 2021;38:283–289. doi: 10.1007/s10014-021-00412-3. [DOI] [PubMed] [Google Scholar]
- 20.Lucas CG, Gupta R, Wu J, Shah K, Ravindranathan A, Barreto J, Gener M, Ginn KF, Prall OWJ, Xu H, Kee D, Ko HS, Yaqoob N, Zia N, Florez A, Cha S, Perry A, Clarke JL, Chang SM, Berger MS, Solomon DA. EWSR1-BEND2 fusion defines an epigenetically distinct subtype of astroblastoma. Acta Neuropathol. 2022;143:109–113. doi: 10.1007/s00401-021-02388-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rossi S, Barresi S, Colafati GS, Giovannoni I, Miele E, Alesi V, Cacchione A, Diomedi-Camassei F, Macari G, Antonelli M, Carboni A, Carai A, Mastronuzzi A, Giangaspero F, Gessi M, Alaggio R. Paediatric astroblastoma-like neuroepithelial tumour of the spinal cord with a MAMLD1-BEND2 rearrangement. Neuropathol Appl Neurobiol. 2022;48:e12814. doi: 10.1111/nan.12814. [DOI] [PubMed] [Google Scholar]
- 22.Todorovic E, Dickson BC, Weinreb I. Salivary gland cancer in the era of routine next-generation sequencing. Head Neck Pathol. 2020;14:311–320. doi: 10.1007/s12105-020-01140-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshida A, Satomi K, Kobayashi E, Ryo E, Matsushita Y, Narita Y, Ichimura K, Kawai A, Mori T. Soft-tissue sarcoma with MN1-BEND2 fusion: a case report and comparison with astroblastoma. Genes Chromosome Cancer. 2022;61:427–431. doi: 10.1002/gcc.23028. [DOI] [PubMed] [Google Scholar]
- 24.Williamson LM, Steel M, Grewal JK, Thibodeau ML, Zhao EY, Loree JM, Yang KC, Gorski SM, Mungall AJ, Mungall KL, Moore RA, Marra MA, Laskin J, Renouf DJ, Schaeffer DF, Jones SJM. Genomic characterization of a well-differentiated grade 3 pancreatic neuroendocrine tumor. Cold Spring Harb Mol Case Stud. 2019;5:a003814. doi: 10.1101/mcs.a003814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasajima A, Konukiewitz B, Schlitter AM, Weichert W, Bräsen JH, Agaimy A, Klöppel G. Mesenchymal/non-epithelial mimickers of neuroendocrine neoplasms with a focus on fusion gene-associated and SWI/SNF-deficient tumors. Virchows Arch. 2021;479:1209–1219. doi: 10.1007/s00428-021-03156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agaram NP, Zhang L, Dickson BC, Swanson D, Sung YS, Panicek DM, Hameed M, Healey JH, Antonescu CR. A molecular study of synovial chondromatosis. Genes Chromosome Cancer. 2020;59:144–151. doi: 10.1002/gcc.22812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flucke U, van Noesel MM, Wijnen M, Zhang L, Chen CL, Sung YS, Antonescu CR. TFG-MET fusion in an infantile spindle cell sarcoma with neural features. Genes Chromosome Cancer. 2017;56:663–667. doi: 10.1002/gcc.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.López-Nieva P, Fernández-Navarro P, Graña-Castro O, Andrés-León E, Santos J, Villa-Morales M, Cobos-Fernández MÁ, González-Sánchez L, Malumbres M, Salazar-Roa M, Fernández-Piqueras J. Detection of novel fusion-transcripts by RNA-Seq in T-cell lymphoblastic lymphoma. Sci Rep. 2019;9:5179. doi: 10.1038/s41598-019-41675-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agaimy A. Paraneoplastic disorders associated with miscellaneous neoplasms with focus on selected soft tissue and undifferentiated/ rhabdoid malignancies. Semin Diagn Pathol. 2019;36:269–278. doi: 10.1053/j.semdp.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Akiyama M, Yamaoka M, Mikami-Terao Y, Yokoi K, Inoue T, Hiramatsu T, Ashizuka S, Yoshizawa J, Katagi H, Ikegami M, Ida H, Nakazawa A, Okita H, Matsumoto K. Paraneoplastic syndrome of angiomatoid fibrous histiocytoma may be caused by EWSR1-CREB1 fusion-induced excessive Interleukin-6 production. J Pediatr Hematol Oncol. 2015;37:554–559. doi: 10.1097/MPH.0000000000000390. [DOI] [PubMed] [Google Scholar]
- 31.Xiao W, Hodge DR, Wang L, Yang X, Zhang X, Farrar WL. Co-operative functions between nuclear factors NFkappaB and CCAT/enhancer-binding protein-beta (C/EBP-beta) regulate the IL-6 promoter in autocrine human prostate cancer cells. Prostate. 2004;61:354–370. doi: 10.1002/pros.20113. [DOI] [PubMed] [Google Scholar]
- 32.Siu YT, Jin DY. CREB–a real culprit in oncogenesis. FEBS J. 2007;274:3224–3232. doi: 10.1111/j.1742-4658.2007.05884.x. [DOI] [PubMed] [Google Scholar]
- 33.Shimizu N, Hasumi M, Hamano T, Iijima M, Yoshioka T, Yamazaki Y, Sasano H. Renal primitive neuroectodermal tumor with elevated plasma adrenocorticotropic hormone levels: a case report. IJU Case Rep. 2019;2:128–131. doi: 10.1002/iju5.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mao W, Xu J, Lu H, Wang Y, Zhang L, Chen M. A rare case report of renal Ewing sarcoma/primitive neuroectodermal tumor with ACTH production. BMC Urol. 2022;22:103. doi: 10.1186/s12894-022-01055-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White A, Clark AJ, Stewart MF. The synthesis of ACTH and related peptides by tumours. Baillieres Clin Endocrinol Metab. 1990;4:1–27. doi: 10.1016/S0950-351X(05)80313-1. [DOI] [PubMed] [Google Scholar]
- 36.Mönig H, Ali IU, Oldfield EH, Schulte HM. Structure of the POMC promoter region in pituitary and extrapituitary ACTH producing tumors. Exp Clin Endocrinol. 1993;101:36–38. doi: 10.1055/s-0029-1211205. [DOI] [PubMed] [Google Scholar]
- 37.Murphy EP, Conneely OM. Neuroendocrine regulation of the hypothalamic pituitary adrenal axis by the nurr1/nur77 subfamily of nuclear receptors. Mol Endocrinol. 1997;11:39–47. doi: 10.1210/mend.11.1.9874. [DOI] [PubMed] [Google Scholar]
- 38.Katahira M, Iwasaki Y, Aoki Y, Oiso Y, Saito H. Cytokine regulation of the rat proopiomelanocortin gene expression in AtT-20 cells. Endocrinology. 1998;139:2414–2422. doi: 10.1210/endo.139.5.6005. [DOI] [PubMed] [Google Scholar]
- 39.Newell-Price J. Proopiomelanocortin gene expression and DNA methylation: implications for Cushing's syndrome and beyond. J Endocrinol. 2003;177:365–372. doi: 10.1677/joe.0.1770365. [DOI] [PubMed] [Google Scholar]
- 40.Mizoguchi Y, Kajiume T, Miyagawa S, Okada S, Nishi Y, Kobayashi M. Steroid-dependent ACTH-produced thymic carcinoid: regulation of POMC gene expression by cortisol via methylation of its promoter region. Horm Res. 2007;67:257–262. doi: 10.1159/000098548. [DOI] [PubMed] [Google Scholar]
- 41.Araki T, Tone Y, Yamamoto M, Kameda H, Ben-Shlomo A, Yamada S, Takeshita A, Yamamoto M, Kawakami Y, Tone M, Melmed S. Two distinctive POMC promoters modify gene expression in cushing disease. J Clin Endocrinol Metab. 2021;106:e3346–e3363. doi: 10.1210/clinem/dgab387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Araki T, Liu NA, Tone Y, Cuevas-Ramos D, Heltsley R, Tone M, Melmed S. E2F1-mediated human POMC expression in ectopic Cushing’s syndrome. Endocr Relat Cancer. 2016;23:857–870. doi: 10.1530/ERC-16-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fukuoka H, Shichi H, Yamamoto M, Takahashi Y. The mechanisms underlying autonomous adrenocorticotropic hormone secretion in cushing’s disease. Int J Mol Sci. 2020;21:9132. doi: 10.3390/ijms21239132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uhrig S, Ellermann J, Walther T, Burkhardt P, Fröhlich M, Hutter B, Toprak UH, Neumann O, Stenzinger A, Scholl C, Fröhling S, Brors B. Accurate and efficient detection of gene fusions from RNA sequencing data. Genome Res. 2021;31:448–460. doi: 10.1101/gr.257246.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.