Abstract
Satellite cells (SCs) are muscle stem cells responsible for muscle hypertrophic growth and the regeneration of damaged muscle. Proliferation and differentiation of the pectoralis major (p. major) muscle SCs are responsive to thermal stress in turkeys, which are, in part, regulated by mechanistic target of rapamycin (mTOR) and Frizzled7 (Fzd7)-mediated wingless-type mouse mammary tumor virus integration site family/planar cell polarity (Wnt/PCP) pathways in a growth dependent-manner. It is not known if chicken p. major SCs respond to thermal stress in a manner similar to that of turkey p. major SCs. The objective of the current study was to investigate the effects of thermal stress and mTOR and Wnt/PCP pathways on the proliferation, differentiation, and expression of myogenic transcriptional regulatory factors in SCs isolated from the p. major muscle of a current modern commercial (MC) broiler line as compared to that of a Cornish Rock (BPM8) and Randombred (RBch) chicken line in the 1990s. The MC line SCs had lower proliferation and differentiation rates and decreased expression of myoblast determination factor 1 (MyoD) and myogenin (MyoG) compared to the BPM8 and RBch lines. Heat stress (43°C) increased proliferation and MyoD expression in all the cell lines, while cold stress (33°C) showed a suppressive effect compared to the control temperature (38°C). Satellite cell differentiation was altered with heat and cold stress in a cell line-specific manner. In general, the differentiation of the MC SCs was less responsive to both heat and cold stress compared to the BPM8 and RBch lines. Knockdown of the expression of either mTOR or Fzd7 decreased the proliferation, differentiation, and the expression of MyoD and MyoG in all the cell lines. The MC line during proliferation was more dependent on the expression of mTOR and Fzd7 than during differentiation. Thus, modern commercial meat-type chickens have decreased myogenic activity and temperature sensitivity of SCs in an mTOR- and Fzd7-dependent manner. The decrease in muscle regeneration will make modern commercial broilers more susceptible to the negative effects of myopathies with muscle fiber necrosis requiring satellite cell-mediated repair.
Key words: chicken, mTOR, satellite cell, thermal stress, Wnt
INTRODUCTION
The quality of poultry breast meat is, in part, the direct result of the growth, structure, and cellular biology of the pectoralis major (p. major: breast) muscle (Dransfield and Sosnicki, 1999; Duclos et al., 2007). Thus, the cellular mechanisms regulating muscle development and growth, and repair or regeneration of damaged muscle will impact breast meat quality. One of the focuses of the poultry industry has been on the selection for increased growth performance, particularly on increased body weight and breast muscle yield at processing age (Havenstein et al., 2003,2004,2007; Collins et al., 2014). Muscle structural defects including reduced connective tissue spacing and decreased capillary density due to excessive muscle fiber hypertrophy have been observed in the p. major muscle of modern heavy-weight rapid-growing poultry lines (Velleman et al., 2003; Joiner et al., 2014). Loss of connective tissue spacing results in myofibers making direct contact with each other increasing the incidence of myofiber degeneration in the p. major muscle of faster-growing poultry (Wilson et al., 1990; Velleman et al., 2003). Inadequate capillary supply in the p. major muscle reduces the capacity to remove anaerobic respiration byproducts like lactic acid, which may further exacerbate muscle damage (Velleman et al., 2003; Joiner et al., 2014). Accompanying the structural defects, conditions like wooden breast (Sihvo et al., 2014; Papah et al., 2017), pale soft exudative (Barbut, 1997; Owens et al., 2000; Strasburg and Chiang, 2009), and white stripping (Soglia et al., 2018; Zampiga et al., 2019), which can have a negative impact on meat quality, have been identified in the p. major muscle of modern rapid-growing poultry lines.
The formation of myofibers occurs prior to hatch (Smith, 1963). Muscle growth after hatch is mainly dependent on the hypertrophy of existing myofibers, which occurs through the proliferation, differentiation, and donation of nuclei from muscle stem cells called satellite cells (SCs) (Moss and Leblond, 1971; Cardiasis and Cooper, 1975). Satellite cells have the peak mitotic activity during the first week after hatch (Mozdziak et al., 1994; Halevy et al., 2000), and then, become mitotically quiescent in mature muscle (Schultz et al., 1978; Schultz and Lipton, 1982). In response to extrinsic stimuli such as muscle injury (Bischoff, 1975; Snow, 1977) and exercise (Darr and Schultz, 1987; Bazgir et al., 2017), the quiescent SCs will re-enter the cell cycle and regenerate the damaged muscle. Given the critical role of SCs in posthatch muscle hypertrophy and muscle regeneration, muscle structural damage resulting in meat quality problems may, in part, be associated with changes in the myogenic activity of SCs. For example, wooden breast is a necrotic and fibrotic myopathy (Sihvo et al., 2014; Papah et al., 2017) having a negative impact on chicken breast meat quality (Mudalal et al., 2015; Soglia et al., 2016). A growing number of studies have indicated that SCs in wooden breast-affected chicken p. major muscle are not able to regenerate myofibers to the original size indicating impaired regeneration of myofiber structure mediated by the SCs (Velleman and Clark, 2015; Clark and Velleman, 2016; Velleman et al., 2018). The impaired SC-mediated myofiber regeneration is likely a key factor in the development of necrotic and fibrotic myopathies in the chicken p. major muscle.
Despite growth selection strategies being similar between chickens and turkeys (Havenstein et al., 2003,2004,2007; Collins et al., 2014), myofiber necrotic and fibrotic myopathies like wooden breast have not been reported to date in the p. major muscle of modern commercial meat-type turkeys. As reported by Xu et al. (2021), SCs isolated from the p. major muscle of modern commercial turkeys have increased proliferation and differentiation compared to that of slower-growing turkeys. The difference between chickens and turkeys in the incidence of necrotic and fibrotic myopathies may be associated with differences in the myogenic and regenerative potential of p. major SCs.
In poultry, SC myogenic activity is highly responsive to temperatures, particularly during the first week after hatch when SCs exhibit the highest mitotic activity (Halevy et al., 2001; Xu et al., 2021). In in vitro studies, heat stress increased both proliferation and differentiation of chicken (Halevy et al., 2001; Harding et al., 2016) and turkey (Clark et al., 2016; Xu et al., 2021) p. major muscle SCs while cold stress showed an inhibitory effect. As homeotherms, birds maintain the body temperature only in a small range (Yahav, 2000). Newly hatched chicks and poults have an immature thermal regulatory system (Dunnington and Siegel, 1984; Modrey and Nichelmann, 1992), and thus, are susceptible to the fluctuation of temperatures. Thermal stress-induced changes in satellite cell (SC) proliferation and differentiation at an early age will have long-lasting effects on the growth and morphological structure of the p. major muscle (Piestun et al., 2017; Patael et al., 2019).
Satellite cell myogenic activity is regulated by signal transduction pathways and gene expression networks. In the turkey p. major muscle, changes in SC proliferation and differentiation in response to growth selection and thermal stress are regulated by mechanistic target of rapamycin (mTOR) pathway (Xu et al., 2022b) and the Frizzled7 (Fzd7)-mediated wingless-type mouse mammary tumor virus integration site family/planar cell polarity (Wnt/PCP) pathway (Xu et al., 2022a). Downstream genes regulated by the mTOR and Wnt/PCP pathways in myogenic SCs including myoblast determination factor 1 (MyoD) and myogenin (MyoG) (Le Grand et al., 2009; Zhang et al., 2015; Xu et al., 2022a,b). As myogenic transcriptional regulatory factors, MyoD is required for SC activation and proliferation (Rudnicki et al., 1993; Yablonka-Reuveni and Rivera, 1994) while MyoG is required for SC differentiation and myotube formation (Brunetti and Goldfine, 1990; Hasty et al., 1993). In addition to regulating SC myogenic activity, the mTOR pathway is able to promote myofiber hypertrophy by stimulating protein synthesis (Bodine et al., 2001; Wang and Proud, 2006) while the Wnt/PCP pathway is involved in the migration of SCs during myogenesis (Fortier et al., 2008; Wang et al., 2018). Thus, any alterations in the activity of the mTOR and Wnt/PCP pathways in SCs will change muscle hypertrophic growth, regeneration potential, morphological structure, and impact meat quality.
Our previous studies have reported both hot and cold thermal stress affect the proliferation and differentiation of turkey p. major muscle SCs through the mTOR and Wnt/PCP pathways in a growth-dependent manner (Xu et al., 2021, 2022a,b). Although sharing similarities, chickens and turkeys are different species. Comparisons of SC myogenic activity, temperature sensitivity, and pathway responsiveness among a current commercial broiler line (MC), a Randombred broiler line (RBch) composed of all commercial parent stocks available in 1997 (Harford et al., 2014), and a Rock Cornish line (BPM8) from 1995 (McFarland et al., 1997) will provide valuable information as to how chicken SCs have changed with growth selection. Possible differences in SC function and mechanism between chickens and turkeys may be a major aspect affecting the incidence of degenerative or necrotic myopathies in the p. major muscle. Thus, the objective of the current study was to investigate the effects of thermal stress as well as the mTOR and Wnt/PCP pathways on the proliferation, differentiation, and expression of myogenic regulatory transcriptional factors in SCs isolated from the p. major muscle of a current MC meat-type chickens as compared to that of BPM8 and RBch chicken lines from the 1990s. Effects of the mTOR and Wnt/PCP pathways on the function of SCs were investigated through knocking down the expression of the critical genes mTOR and Fzd7 with small interfering RNA (siRNA). The myogenic transcriptional regulatory factor genes detected in this study were the proliferation marker, MyoD, and the differentiation marker, MyoG. Studying SC functional differences between older lines and a modern commercial chicken line is necessary, because changes in SC myogenic activity and thermal sensitivity will affect breast muscle growth and development, morphological structure, and ultimately meat quality. In addition, results of the current study will also provide cellular mechanistic information regarding the role of mTOR and Wnt signal transduction pathways in the regulation of chicken SC myogenic activity during thermal stress.
MATERIALS AND METHODS
Satellite Cells
Satellite cells used in this study were previously isolated from the p. major muscle of 5-wk female BPM8, 4-wk male RBch, and 4-wk male MC line chickens according to the method of Velleman et al. (2000). The BPM8 SCs were cultured to the sixth pass while the RBch and MC SCs were cultured to the fourth pass, and all the cells were stored in the lipid nitrogen until use.
Small Interfering RNA
Small interfering RNAs targeting mTOR (Gene bank ID: XM_417614.8) and Fzd7 (Gene bank ID: NM_204221.3) were designed using Invitrogen Block-iT software (https://rnaidesigner.thermofisher.com/rnaiexpress/setOption.do?designOption=stealth&pid). The mTOR siRNA and Fzd7 siRNA were synthesized stealth duplex siRNAs (Thermo Fisher Scientific, Waltham, MA). The mTOR siRNA targets the mTOR open reading frame from 4,427 to 4,450 with the following sequence: sense strand: 5′-CAA AGA UGA CUG GUU GGA AUG GUU A-3′; antisense strand: 5′-UUA CCA UUC CAA GUC AUC UUU G-3′. The Fzd7 siRNA targets the Fzd7 open reading frame from 2,069 to 2,080 with the following sequence: sense strand: 5′-CCG GAC UUC ACA GUC UUC AUG AUC A-3′; antisense strand: 5′-UGA UCA UGA AGA CUG UGA AGU CCG G-3′. A stealth siRNA with 48% guanine and cytosine content was used as the negative control siRNA (Thermo Fisher Scientific).
To determine the knockdown efficiency of the synthesized mTOR and Fzd7 siRNA, 18,000 of BPM8, RBch and MC line SCs were plated in each well of 24-well gelatin-coated plates in 500 μL of Dulbecco's modified Eagle's medium (DMEM, Sigma-Aldrich, St. Louis, MO) transfection medium supplemented with 10% chicken serum (Sigma-Aldrich) and 5% horse serum (Sigma-Aldrich) and incubated in a 95% air/5% CO2 incubator (Thermo Fisher Scientific) at 38°C allowing the cells attach to the plates. After 24 h attachment, cells were transfected with 20 pmol/μL of the negative control siRNA, mTOR siRNA, or Fzd7 siRNA with 1 μL of Lipofectamine 2000 (Thermo Fisher Scientific) in each well according to the manufacturer's protocol. After 6 h of transfection, the transfection medium was replaced with McCoy's 5A growth medium (Sigma-Aldrich) containing 10% chicken serum, 5% horse serum, 1% antibiotics-antimycotics (Corning, Corning, NY), and 0.1% gentamicin (Gemini Bio-Products, West Sacramento, CA) for 72 h of proliferation, and the growth medium was changed every 24 h. At 72 h of proliferation, cells were removed from the incubator and total RNA was extracted for gene expression analysis by real-time quantitative PCR (RT-qPCR) as described below in the Gene Expression Analysis section. The transfection experiment was repeated twice independently to confirm knockdown efficiency of mTOR and Fzd7 siRNAs.
Proliferation Assay
For the cell proliferation assay without a knockdown of mTOR or Fzd7, cells from each chicken line were plated in DMEM plating medium containing 10% chicken serum, 5% horse serum, 1% antibiotics-antimycotics, and 0.1% gentamicin in 24-well gelatin-coated plates with 15,000 cells per well, and incubated at 38°C in a 95% air/5% CO2 incubator. After 24 h of attachment, the plating medium was replaced with growth medium. Cells from each chicken line were randomly assigned to be incubated at 33°C, 38°C, or 43°C for 72 h of proliferation. During the 72 h of proliferation, 1 plate from each temperature group was removed from the incubator every 24 h and stored at −70°C until assayed.
For the knockdown study, cells from each chicken line were plated in 48-well gelatin-coated plates in the transfection medium with 18,000 cells per well, and incubated at 38°C in a 95% air/5% CO2 incubator. After 24 h of attachment, cells in each well were transfected with 20 pmol/μL of mTOR siRNA, Fzd7 siRNA, or the control siRNA with 1 μL of Lipofectamine 2000 for 6 h at 38°C. After 6 h of transfection, the transfection medium was replaced with growth medium, and cells were randomly assigned to be incubated at 38°C, 43°C, or 33°C for 72 h, with the growth medium changed every 24 h. At 24 h intervals, the culture plates were removed from the incubator, rinsed with phosphate buffered saline (PBS, 171 mM NaCl, 3.35 mM KCl, 1.84 mM KH2PO4, and 10 mM Na2HPO4, pH7.08), and stored at −70°C until assay.
The proliferation assay was conducted according to the method of McFarland et al. (1995). All plates were removed from −70°C and thawed at room temperature for 15 min, 200 µL of 0.05% trypsin-EDTA (Thermo Fisher Scientific) in 10 mM Tris, 2 M NaCl, and 1 mM EDTA (TNE) was added to each culture well and incubated at room temperature for 7 min. The plates were then returned to −70°C overnight. After thawing the plates for 15 min at room temperature, 1.8 mL of TNE buffer containing 0.2% (1 mg/mL) Hoechst dye (Sigma-Aldrich) was added to each well, and the plates were gently agitated for 2 h at room temperature. DNA-incorporated Hoechst dye was measured using a Fluoroskan Ascent FL plate reader (Thermal Fisher Scientific). A standard curve with double-stranded calf thymus DNA (Sigma-Aldrich) was used to determine sample DNA concentration. The proliferation assay was repeated in 2 independent cultures with 4 replicate wells per treatment per culture.
Differentiation Assay
For the cell differentiation assay without a knockdown of mTOR or Fzd7, 9,000 cells from each chicken line were plated in DMEM plating medium containing 10% chicken serum, 5% horse serum, 1% antibiotics-antimycotics, and 0.1% gentamicin in each well of 24-well gelatin-coated plates, and incubated at 38°C in a 95% air/5% CO2 incubator. After 24 h of attachment, the plating medium was replaced with growth medium. Cells from each chicken line were randomly assigned to be incubated at 33°C, 38°C, or 43°C for 72 h of proliferation with the growth medium changed daily. At 72 h of proliferation, the growth medium was replaced with DMEM differentiation medium containing 3% horse serum, 1% antibiotics-antimycotics, 0.1% gentamicin, 0.1% gelatin (Thermo Fisher Scientific), and 1 mg/mL bovine serum albumin (BSA, Sigma-Aldrich) for 72 h of differentiation with the media changed every day. Cells were removed from the incubator every 24 h, rinsed with PBS, and stored at −70°C until assay.
For the knockdown study, SCs (11,000 cells per cell line) were plated in each well of 48-well gelatin-coated plates in 500 μL of transfection medium and incubated at 38°C in a 95% air/5% CO2 incubator. After 24 h of attachment, cells in each well were transfected with 20 pmol/μL of mTOR siRNA, Fzd7 siRNA, or the control siRNA with 0.5 μL of Lipofectamine 2000. After 6 h of transfection at 38°C, the transfection medium was replaced with growth medium, and the cells were randomly assigned to be incubated at 38°C, 43°C, or 33°C for 72 h of proliferation with the growth medium changed daily. After 72 h of proliferation, the growth medium was replaced with the differentiation medium for 72 h of differentiation with the media changed every 24 h. Cells were collected at 48 h of differentiation, rinsed with PBS, and stored at −70°C for the differentiation assay.
Satellite cell differentiation was assayed by measuring creatine kinase activity using a modified method of Yun et al. (1997). All plates were removed from −70°C and thawed at room temperature for 15 min, and 500 μL of creatine kinase buffer [20 mM glucose (Thermo Fisher Scientific), 20 mM phosphocreatine (Calbiochem, San Diego, CA), 10 mM mg acetate (Thermo Fisher Scientific), 10 mM adenosine monophosphate (Sigma Aldrich), 1 mM adenosine diphosphate (Sigma Aldrich), 1 Unit/mL glucose-6-phosphate dehydrogenase (Worthington Biochemical, Lakewood, NJ), 0.5 Unit/mL hexokinase (Worthington Biochemical) 0.4 mM thio-nicotinamide adenine dinucleotide (Oriental Yeast Co., Tokyo, Japan), 1 mg/mL BSA, to 0.1 M glycylglycine, pH 7.5] was added to each well including the standard curve wells containing creatine phosphokinase with concentrations from 0 to 140 milliunits/well, Sigma-Aldrich). The optical density of each well was measured at a wavelength of 405 nm using a BioTek ELx800 (BioTek, Winooski, VT) plate reader. The differentiation assay was repeated in 2 independent cultures with 5 wells per treatment per culture.
Myotube Diameter Measurement
Cell culture and transfection procedures were the same as the procedures as described in the Differentiation Assay section. At 48 h of differentiation, photomicrographs of SCs and myotubes in each treatment group were taken with an Olympus IX70 fluorescence microscope equipped with a QImaging Retiga Exi Fast digital camera (Qimaging, Surrey, British Columbia, Canada) and CellSens software (Olympus America, Center Valley, PA). The diameter of the myotubes was measured using Image Pro Software (Media Cybernectics, Silver Spring, MD). Five to 10 different myotubes were randomly selected with 10 measurements were conducted per well. If the randomly selected myotube had branches in the photomicrograph, both main myotube and the branches were measured. The measurements were independently repeated in 2 cultures with 5 replicate wells per treatment per culture.
Gene Expression Analysis
For cell gene expression without knockdown of mTOR or Fzd7, cells from each cell line were plated in 24-well gelatin-coated plates with 15,000 cells per well in the plating medium, and were incubated at 38°C in a 95% air/5% CO2 incubator. After 24 h of attachment, the plating medium was replaced with growth medium for 72 h of proliferation with the medium changed every day. At 72 h of proliferation, the growth medium was replaced with differentiation medium for 48 h of differentiation with the medium changed at 24 h intervals. Cells from each group were removed from incubator at 72 h of proliferation and 48 h of differentiation and stored at −70°C until RNA isolation.
For the knockdown study, cells from each chicken line (18,000 cells/well) were plated in 24-well gelatin-coated plates in plating medium, and incubated at 38°C in a 95% air/5% CO2 incubator. After 24 h of attachment, cells in each well were transfected with 20 pmol/μL of mTOR siRNA, Fzd7 siRNA, or the control siRNA with 1 μL of Lipofectamine 2000 for 6 h at 38°C. After 6 h of transfection, the transfection medium was replaced with growth medium, and cells were randomly assigned to be incubated at 38°C, 43°C, or 33°C for 72 h, and the growth medium was changed every 24 h. At 72 h of proliferation, the growth medium was replaced with differentiation medium for 48 h of differentiation with the medium changed daily. Cells were collected at 72 h of proliferation and 48 h of differentiation and stored at −70°C until RNA isolation.
Total RNA from each sample was extracted using RNAzol (Molecular Research Center, Cincinnati, OH). A spectrophotometer (NanoDrop ND-1000, Thermo Fisher Scientific) was used to measure the concentration of each RNA sample. Reverse transcription was performed with Moloney Murine Leukemia Virus Reverse Transcriptase (M-MLV; Promega, Madison, WI) to produce cDNA from total RNA. RT-qPCR was conducted with a DyNAmo Hot Start SYBR Green qPCR kit (Thermo Fisher Scientific). Information for all the primers is listed in Table 1. Primers for MyoD, MyoG, and an internal control gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were previously designed and confirmed the specificity in this lab as reported by Harding et al. (2016). Primers for mTOR and Fzd7 were designed using primer-BLAST tool (https://www.ncbi.nlm.nih.gov/tools/primer-blast/), and the specificity of the mTOR and Fzd7 primers was confirmed by DNA sequencing the PCR product. The RT-qPCR was performed in a DNA Engine Opticon 2 real-time machine (Bio-Rad). Each RT-qPCR reaction included the following cycling conditions: 1) denaturation at 94°C for 15 min; 2) amplification for 35 cycles with each cycle including denaturation for 30 s at 94°C, annealing for 30 s at 55°C for GAPDH and mTOR, at 58°C for MyoD and MyoG, or at 60°C for Fzd7, and elongation for 30 s at 72°C; and 3) a final elongation at 72°C for 5 min. A standard curve of each gene was generated using serial dilutions of purified PCR products (Liu et al., 2006). Arbitrary concentrations from 1 to 100,000 were assigned to each serial dilution. An arbitrary molar concentration for each amplified sample was calculated according to the threshold cycle and was normalized by GAPDH. The RT-qPCR for each gene was independently repeated in 2 cultures with 12 wells per treatment per culture.
Table 1.
Primer sequences for real-time quantitative polymerase chain reaction.
| Primer | Sequence | Product size | GenBank accession number |
|---|---|---|---|
| MyoD1 | 5′-GAC GGC ATG ATG GAG TAC AG-3′ (forward) | 201 bp6 | AY641567.1 |
| 5′-AGC TTC AGC TGG AGG CAG TA-3′ (reverse) | |||
| MyoG2 | 5′-GGC TTT GGA GGA GAA GGA CT-3′ (forward) | 184 bp | D90157.1 |
| 5′-CAG AGT GCT GCG TTT CAG AG-3′ (reverse) | |||
| Fzd73 | 5′-TCT ACC CGC TGG TCA AGG T-3′ (forward) | 249 bp | NM204221.3 |
| 5′-GGG CAT CCG ATG TGT TCT G-3′ (reverse) | |||
| mTOR4 | 5′-GTC AAG GAC CTA ACC CAG GC-3′ (forward) | 261 bp | XM417614.8 |
| 5′-ATG CCC GTT GCT TCC CAT TA-3′ (reverse) | |||
| GAPDH5 | 5′-GAG GGT AGT GAA GGC TGC TG-3′ (forward) | 200 bp | U94327.1 |
| 5′-CCA CAA CAC GGT TGC TGT AT-3′ (reverse) |
MyoD, myogenic determination factor-1.
MyoG, myogenin.
Fzd7, frizzled-7.
mTOR, mechanistic of rapamycin.
GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
bp, number of base pairs.
Statistical Analysis
Data from proliferation, differentiation, myotube measurement, and gene expression analysis without knockdown of gene were analyzed as a mixed model at each sampling time in SAS (SAS 9.4, SAS Institute Inc., Cary, NC). Two fixed effects of temperature and cell line, an interaction effect between temperature and cell line, and a random effect of repeat experiment were included in this mixed model. The statement of lsmean in the MIXED procedure was used to determine each mean value and the standard error of the mean (SEM). Differences between each mean were separated with the pdiff option. In addition, for the proliferation and differentiation assays, the REG procedure was used to evaluate the linear relationship between sampling time and cellular response in each treatment group. Difference in the linear response was determined with the contrast statement. P ≤ 0.05 was considered as statistically significant.
Data from proliferation, differentiation, myotube measurement, and gene expression analysis with knockdown of mTOR or Fzd7 were analyzed as a mixed model at each sampling time in SAS. This model including 3 fixed effects (temperature, cell line, and knockdown of gene), 3 two-way interaction effects (temperature and cell line, temperature and knockdown of gene, and cell line and knockdown of gene), and a random effect of repeat experiment. The statement of lsmean in the MIXED procedure was used to determine each mean value and the SEM. Differences between each mean were separated with the pdiff option. P ≤ 0.05 was considered as statistically significant.
RESULTS
Effect of Thermal Stress on the Proliferation of Satellite Cells From Different Chicken Lines
The effect of hot (43°C) and cold (33°C) thermal stress on the proliferation of the BPM8, RBch, and MC SCs was measured at 0, 24, 48, and 72 h of proliferation (Table 2). A significant interaction effect between cell line and temperature was observed at 24 h (P = 0.031), 48 h (P < 0.001), and 72 h (P < 0.001). For the effect of cell line, the proliferation of BPM8 SCs was higher than that of the RBch line at all the temperatures at 24, 48, and 72 h (P < 0.001). The proliferation of the RBch line SCs was greater compared to the MC line only at 38°C and at 72 h (P < 0.001). For the temperature effect, heat stress (43°C) increased the proliferation of the BPM8, RBch, and MC line SCs compared to the control temperature (38°C) at 24, 48, and 72 h (P < 0.001). In contrast, with the cold stress (33°C), SC proliferation was decreased in all the 3 cell lines compared to the control temperature at 48 and 72 h (P < 0.001).
Table 2.
Effect of temperature on the proliferation of satellite cells isolated from Cornish Rock chickens (BPM8), Randombred chickens (RBch), and modern commercial chickens (MC)1.
| Sampling time |
|||||
|---|---|---|---|---|---|
| Line | Temperature2 | 0 h | 24 h | 48 h | 72 h |
| BPM8 | 33 | 82.63a,w ± 6.74 | 174.30c,x ± 7.06 | 225.10d,y ± 18.98 | 305.70e,z ± 35.61 |
| 38 | 69.05ab,w ± 4.92 | 215.40b,x ± 4.99 | 436.50b,y ± 13.42 | 880.10b,z ± 25.18 | |
| 43 | 58.24bc,w ± 7.21 | 283.00a,x ± 7.06 | 629.30a,y ± 18.98 | 1134.70a,z ± 35.61 | |
| RBch | 33 | 72.88ab,z ± 6.74 | 112.10de,z ± 7.06 | 120.90e,z ± 18.98 | 120.60f,z ± 35.61 |
| 38 | 57.17bc,w ± 5.10 | 118.90d,x ± 4.99 | 234.10d,y ± 13.42 | 460.60d,z± 25.18 | |
| 43 | 64.88abc,w ± 6.74 | 180.10c,x ± 7.06 | 379.40c,y ± 18.98 | 746.90c,z± 35.61 | |
| MC | 33 | 55.75bc,z ± 6.74 | 93.50e,z ± 7.06 | 109.10e,z ± 18.98 | 111.20f,z ± 35.61 |
| 38 | 55.11c,x ± 4.92 | 109.80de,x ± 4.99 | 211.30d,y ± 13.42 | 369.90e,z ± 25.18 | |
| 43 | 51.50c,w ± 6.74 | 170.90c,x ± 7.06 | 365.40c,y ± 18.98 | 701.80c,z ± 35.61 | |
| P value3 | L × T | 0.366 | 0.031 | < 0.001 | < 0.001 |
Mean DNA concentration (ng/well) ± standard error of mean (SEM).
Incubation temperature (°C).
Interaction effect between cell line and temperature (L × T).
Mean DNA concentration (ng/well ± SEM) within a column (sampling time) without a common letter are significantly different.
Mean DNA concentration (ng/well ± SEM) within a row (line and temperature) without a common letter are significantly different.
P ≤ 0.05 was considered as significantly different.
From 0 to 72 h, proliferation of SCs linearly increased as a function of sampling time in all the cell lines at all the temperatures (Table 2). Within each temperature group (38°C, 43°C, or 33°C), the slope of linear regression was greater (P < 0.001) in the BPM8 line SCs (38°C: slope 5.56, 43°C: slope 7.51, and 33°C: slope 1.50) compared to the RBch (38°C: slope 2.80, 43°C: slope 4.64, and 33°C: slope 0.32) and MC (38°C: slope 2.19, 43°C: slope 4.47, and 33°C: slope 0.38) lines, respectively. For the temperature effect, heat stress increased (P < 0.001) the slope of the linear regression while cold stress showed an inhibitory effect (P < 0.001) in each cell line.
Effect of Thermal Stress on the Differentiation of Satellite Cells From Different Chicken Lines
The effect of hot (43°C) and cold (33°C) thermal stress on the differentiation of the BPM8, RBch, and MC line SCs was measured at 0, 24, 48, and 72 h of differentiation (Table 3). An interaction effect between cell line and temperature was significant at 0 h (P < 0.001), 24 h (P < 0.001), 48 h (P < 0.001), and 72 h (P < 0.001). At the control temperature of 38°C, SC differentiation was higher in the BPM8 line compared to the RBch (P < 0.001) and MC line (P < 0.001), respectively, at 0 and 24 h. At 48 and 72 h, the differentiation of the RBch line SCs was greater compared to the BPM8 (P < 0.001), and the differentiation of the BPM8 line was higher than that of the MC line (P < 0.001) line at 38°C. At 43°C, SC differentiation was greater in both BPM8 (P < 0.001) and RBch (P ≤ 0.011) line SCs compared to the MC line at all the sampling times. At 33°C, the BPM8 cells had a greater differentiation level compared to the RBch (P < 0.001) and MC (P < 0.001) lines only at 0 h. For the temperature effect, the differentiation of the BPM8, RBch, and MC line SCs was lower at 43°C compared to the control temperature (38°C) at 0 and 24 h (P < 0.001). At 48 h, heat stress decreased the differentiation of RBch line SCs (P < 0.001), however, had no significant effect on the differentiation of either BPM8 (P = 0.315) or MC line (P = 0.121). At 72 h, heat stress increased (P < 0.001) the differentiation of the BPM8 SCs and decreased (P < 0.001) the differentiation of the RBch SCs. At all the sampling times, cold stress inhibited the differentiation in all the cell lines (P < 0.001).
Table 3.
Effect of temperature on the differentiation of satellite cells isolated from Cornish Rock chickens (BPM8), Randombred chickens (RBch), and modern commercial chickens (MC)1.
| Sampling time |
|||||
|---|---|---|---|---|---|
| Line | Temperature2 | 0 h | 24 h | 48 h | 72 h |
| BPM8 | 33 | 7.12c,z ± 0.38 | 1.86ef,y ± 0.60 | 1.85e,y ± 0.91 | 2.49e,y ± 0.98 |
| 38 | 12.10a,x ± 0.32 | 13.44a,x ± 0.38 | 25.29b,z ± 0.75 | 19.44c,y ± 0.78 | |
| 43 | 8.35b,x ± 0.40 | 8.30c,x ± 0.65 | 23.83bc,y ± 1.22 | 32.94b,z ± 1.50 | |
| RBch | 33 | 4.63de,z ± 0.40 | 0.88f,y ± 0.50 | 1.41e,y ± 1.04 | 3.76e,z± 1.07 |
| 38 | 8.19b,x ± 0.27 | 11.49b,x ± 0.42 | 31.73a,y ± 0.91 | 49.39a,z ± 0.78 | |
| 43 | 6.40c,w ± 0.40 | 8.67c,x ± 0.60 | 21.62c,y ± 1.36 | 29.15b,z ± 1.50 | |
| MC | 33 | 3.93e,z ± 0.36 | 0.87f,y ± 0.60 | 0.46e,y ± 1.12 | 1.18e,y ± 1.31 |
| 38 | 4.07e,y ± 0.27 | 3.13e,y ± 0.46 | 4.91d,y ± 0.83 | 9.20d,z ± 0.75 | |
| 43 | 5.03d,x ± 0.34 | 6.07d,y ± 0.56 | 7.22d,y ± 1.22 | 10.39d,z ± 1.30 | |
| P value3 | L × T | <0.001 | <0.001 | <0.001 | <0.001 |
Mean creatine kinase activity (Unit/well) ± standard error of mean (SEM).
Incubation temperature (°C).
Interaction effect between cell line and temperature (L × T).
Mean creatine kinase activity (Unit/well ± SEM) within a column (sampling time) without a common letter are significantly different.
Mean creatine kinase activity (Unit/well ± SEM) within a row (line and temperature) without a common letter are significantly different.
P ≤ 0.05 was considered as significantly different.
From 0 to 72 h, SC differentiation linearly increased with sampling time in all the cell lines at 38°C and 43°C (Table 3). At 38°C, the slope of linear regression was the highest in the RBch line SCs among the 3 cell lines (BPM8: slope 1.53, RBch: slope 5.84, and MC: slope: 0.67, P < 0.001). At 43°C, either the BPM8 (slope 3.56, P < 0.001) or the RBch (slope 3.28, P < 0.001) line had a greater slope in the linear regression compared to that of the MC line (slope 0.67). During cold stress (33°C), SC differentiation linearly decreased with sampling time in all the cell lines with the BPM8 (slope −0.62, P < 0.001) and MC (slope −0.50, P < 0.001) line SCs showed a greater linear reduction in differentiation than that of the RBch line (slope −0.14).
Effect of Thermal Stress on Myotube Diameter in Satellite Cells From Different Chicken Lines
In addition to measuring SC differentiation, the diameter of myotubes was measured at 48 h of differentiation (Figure S1). Myotube diameter was greater in the BPM8 line compared to the RBch line (P < 0.001), and the diameter of the RBch line was larger than that of the MC line (P < 0.001) at both 38°C and 43°C. Heat stress further increased the myotube diameter in all the cell lines (P < 0.001) whereas cold stress decreased the myotube diameter in the BPM8 and RBch lines (P < 0.001).
Effect of Thermal Stress on the Expression of Myogenic Transcriptional Regulatory Genes in Satellite Cells From Different Chicken Lines
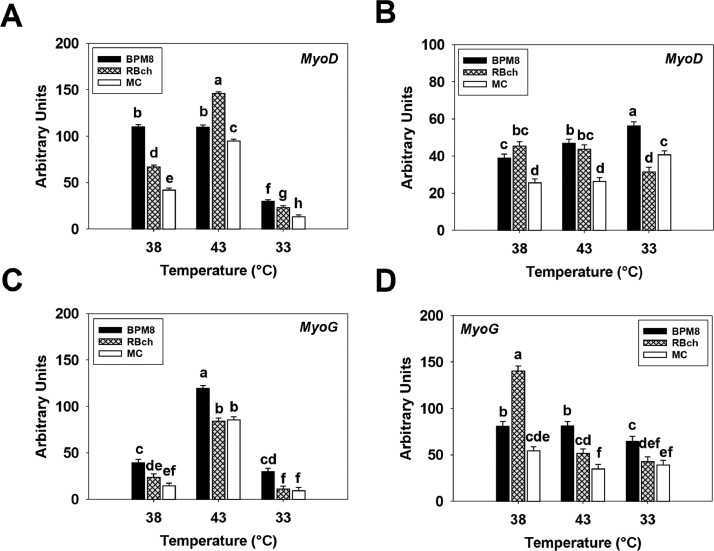
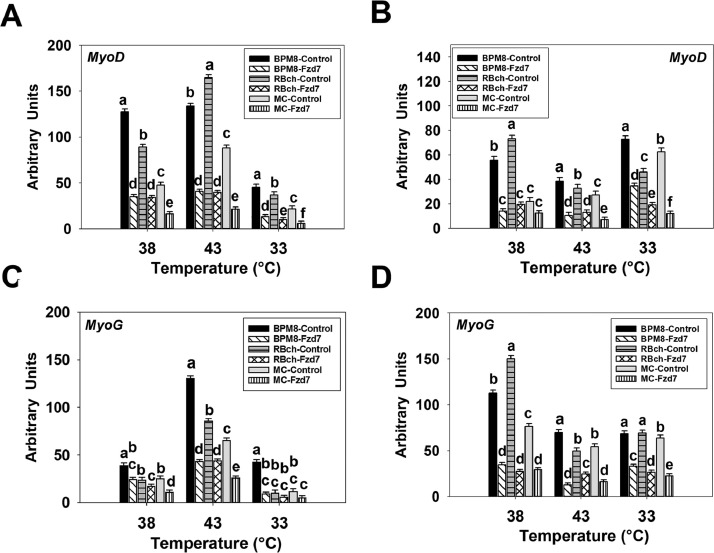
At 72 h of proliferation, an interaction effect between cell line and temperature was significant in affecting the expression of MyoD (P < 0.001, Figure 1A). At 38°C and 33°C, MyoD expression was higher in the BPM8 SCs compared to the RBch line (P ≤ 0.018), and was higher in the RBch line than that in the MC line (P < 0.001). At 43°C, the BPM8 SCs had a greater expression in MyoD compared to the RBch line (P < 0.001), while the RBch line showed a greater MyoD expression than that of the MC line (P < 0.001). For the temperature effect, heat stress increased the MyoD expression in the RBch and MC lines (P < 0.001) whereas the cold stress suppressed the expression of MyoD in all the cell lines (P < 0.001). At 48 h of differentiation, an interaction between line and temperature effects was significant (P < 0.001, Figure 1B). At 38°C and 43°C, both BPM8 (P < 0.001) and RBch (P < 0.001) line SCs had a greater MyoD expression compared to the MC line, respectively. At 33°C, MyoD expression was higher in the BPM8 line compared to the MC line (P < 0.001), and was higher in the MC line than that of the RBch line (P < 0.001). Both heat and cold stress increased the expression of MyoD in the BPM8 line (P < 0.001). The expression of MyoD was lower at 33°C compared to 38°C and 43°C only in the RBch line SCs (P < 0.001). For the MC line, MyoD expression had no significant change with heat stress (P = 0.086) whereas significantly decreased with cold stress (P < 0.001).
Figure 1.
Effect of temperature on the expression of myogenic determination factor-1 (MyoD) and myogenin (MyoG) in satellite cells isolated from Cornish Rock chickens (BPM8), Randombred chickens (RBch), and modern commercial chickens (MC). Satellite cells isolated from the BPM8, RBch, and MC chicken lines were incubated at 38°C, 43°C, or 33°C. Expression of MyoD was determined at 72 h of proliferation (72hP) (A) and 48 h of differentiation (48hD) (B). Expression of MyoG was determined at 72 h of proliferation (72hP) (C) and 48 h of differentiation (48hD) (D). Each graph bar represents a mean arbitrary unit, and each error bar represents the standard error of the mean. Within each temperature group, mean values with different letters are significantly different (P ≤ 0.05).
At 72 h of proliferation, a significant interaction effect between cell line and temperature was observed in affecting MyoG expression (P < 0.001, Figure 1C). The expression of MyoG was greater in the BPM8 SCs compared to the RBch (P ≤ 0.004) and MC line (P < 0.001) at all the temperatures. Heat stress increased the expression of MyoG in all the cell lines (P < 0.001) while cold stress suppressed MyoG expression only in the RBch line (P = 0.013). At 48 h of differentiation, there was a significant interaction between line and temperature effects (P < 0.001, Figure 1D). At 38°C, RBch SCs had a higher MyoG expression compared to the BPM8 line (P < 0.001), and the expression of MyoG was greater in the BPM8 line compared to the MC line (P < 0.001). At 43°C, MyoG expression was greater in the BPM8 SCs compared to the RBch line (P < 0.001), and was higher in the RBch line compared to the MC line (P = 0.021). At 33°C, the BPM8 cells showed higher expression in MyoG than that in the RBch (P = 0.007) and MC (P = 0.001) line, respectively. For the temperature effect, heat stress decreased the MyoG expression in both RBch (P < 0.001) and MC (P = 0.006) lines, however, had no significant effect on MyoG expression in the BPM8 line (P = 0.951). Cold stress decreased the expression of MyoG in both the BPM8 (P = 0.044) and RBch (P < 0.001) line SCs.
Effect of mTOR Knockdown on Chicken Satellite Cell Proliferation and Differentiation During Thermal Stress
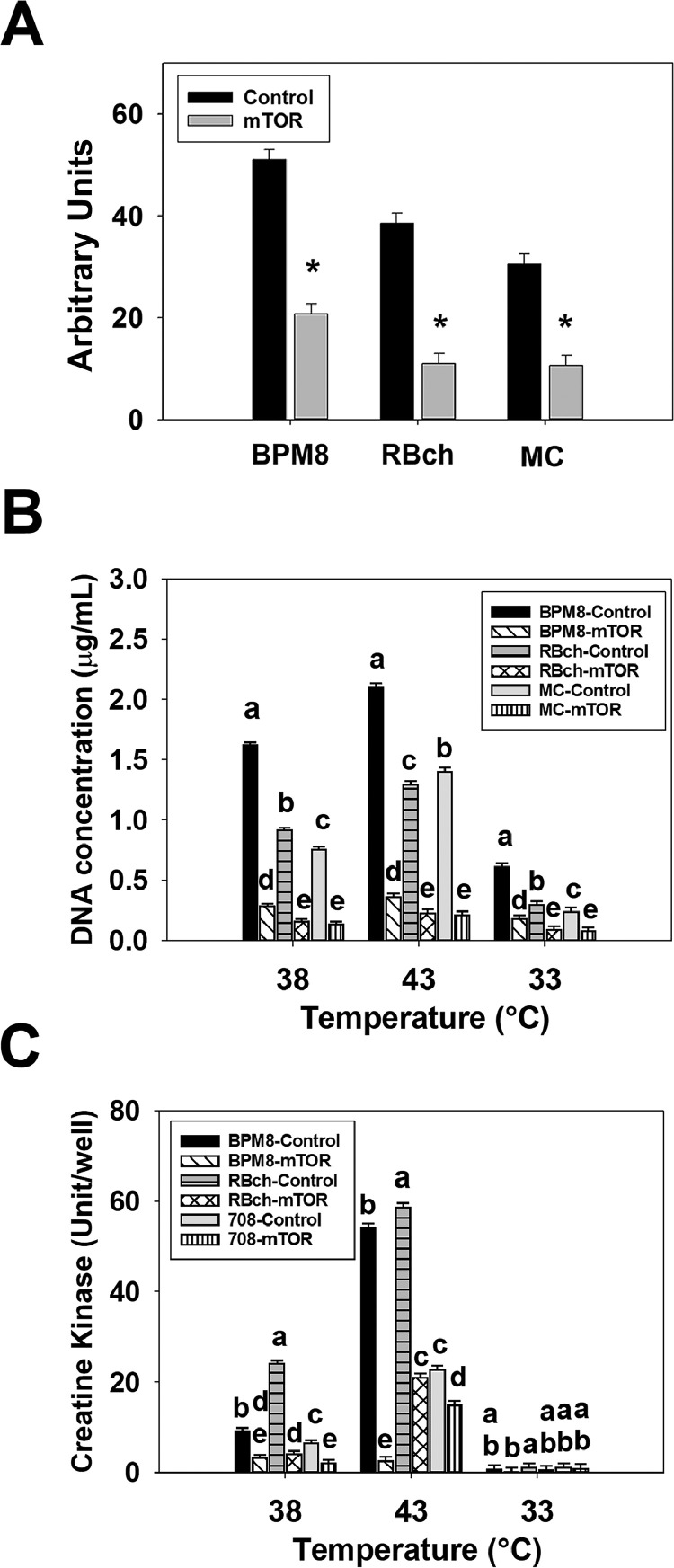
To confirm the knockdown of mTOR, expression of mTOR mRNA was determined with RT-qPCR at 72 h of proliferation in the BPM8, RBch, and MC line SCs (Figure 2A). At 72 h of proliferation, expression of mTOR decreased 2.47-fold (P < 0.001), 3.50-fold (P < 0.001), and 2.87-fold (P < 0.001) in the BPM8, RBch, and MC mTOR knockdown groups compared to the negative control groups, respectively (Figure 2A).
Figure 2.
Effect of temperature and knockdown of mechanistic target of rapamycin (mTOR) on the proliferation and differentiation of satellite cells isolated from Cornish Rock chickens (BPM8), Randombred chickens (RBch), and modern commercial chickens (MC). (A) Expression of mTOR was measured at 72 h of proliferation after knockdown of mTOR at the beginning of proliferation in the BPM8, RBch, and MC line SCs. Asterisk (*) above the bars represents a significant difference between the 2 adjacent groups (P ≤ 0.05). (B) Satellite cells isolated from the BPM8, RBch, and MC chicken lines were transfected with either a control small interfering RNA (siRNA) or an mTOR siRNA at the beginning of proliferation, and then incubated at 38°C, 43°C, or 33°C for 72 h of proliferation. Each bar represents mean DNA concentration (μg/well) with an error bar representing the standard error of the mean. (C) Satellite cells isolated from the BPM8, RBch, and MC chicken lines were transfected with either a control siRNA or a mTOR at the beginning of proliferation, and then incubated at 38°C, 43°C, or 33°C for 72 h of proliferation followed by 48 h of differentiation. Each bar represents mean creatine kinase activity (Unit/well) with an error bar representing the standard error of the mean. Within each temperature group in (B) and (C), mean values without a common letter are significantly different (P ≤ 0.05).
A significant interaction effect among the effects of cell line, temperature, and knockdown of mTOR was observed at 72 h of proliferation in affecting the proliferation of SCs (P < 0.001, Figure 2B). At all the temperatures, knockdown of mTOR decreased (P < 0.001) the proliferation of SCs in the BPM8 (38°C: 5.69-fold, 43°C: 5.85-fold, 33°C: 3.40-fold), RBch (38°C: 5.83-fold, 43°C: 5.72-fold, 33°C: 3.26-fold), and MC (38°C: 5.57-fold, 43°C: 6.64-fold, 33°C: 3.08-fold) line SCs, respectively.
For SC differentiation, an interaction effect among cell line, temperature, and knockdown of mTOR was significant at 48 h of differentiation (P < 0.001, Figure 2C). At 38°C and 43°C, SC differentiation was lower (P < 0.001) in the BPM8 (38°C: 2.85-fold, 43°C: 21.59-fold), RBch (38°C: 5.89-fold, 43°C: 2.79-fold), and MC (38°C: 3.08-fold, 43°C: 1.52-fold) mTOR knockdown groups compared to the control groups. At 33°C, knockdown of mTOR had no significant effect on the differentiation of SCs in all the cell lines (P ≥ 0.690).
For the myotubes, knockdown of mTOR significantly decreased the diameter of myotubes in all the cell lines at 38°C (P < 0.001, Figure S2) and 43°C (P < 0.001, Figure S3) [BPM8 (38°C: 1.51-fold, 43°C: 1.65-fold), RBch (38°C: 1.72-fold, 43°C: 1.59-fold), and MC (38°C: 1.20-fold, 43°C: 1.29-fold)]. At 33°C, the myotube diameter was smaller only in the BPM8 (1.08-fold, P = 0.005) and RBch (1.09-fold, P = 0.002) mTOR knockdown groups compared to the controls (Figure S4).
Effect of Fzd7 Knockdown on Chicken Satellite Cell Proliferation and Differentiation During Thermal Stress
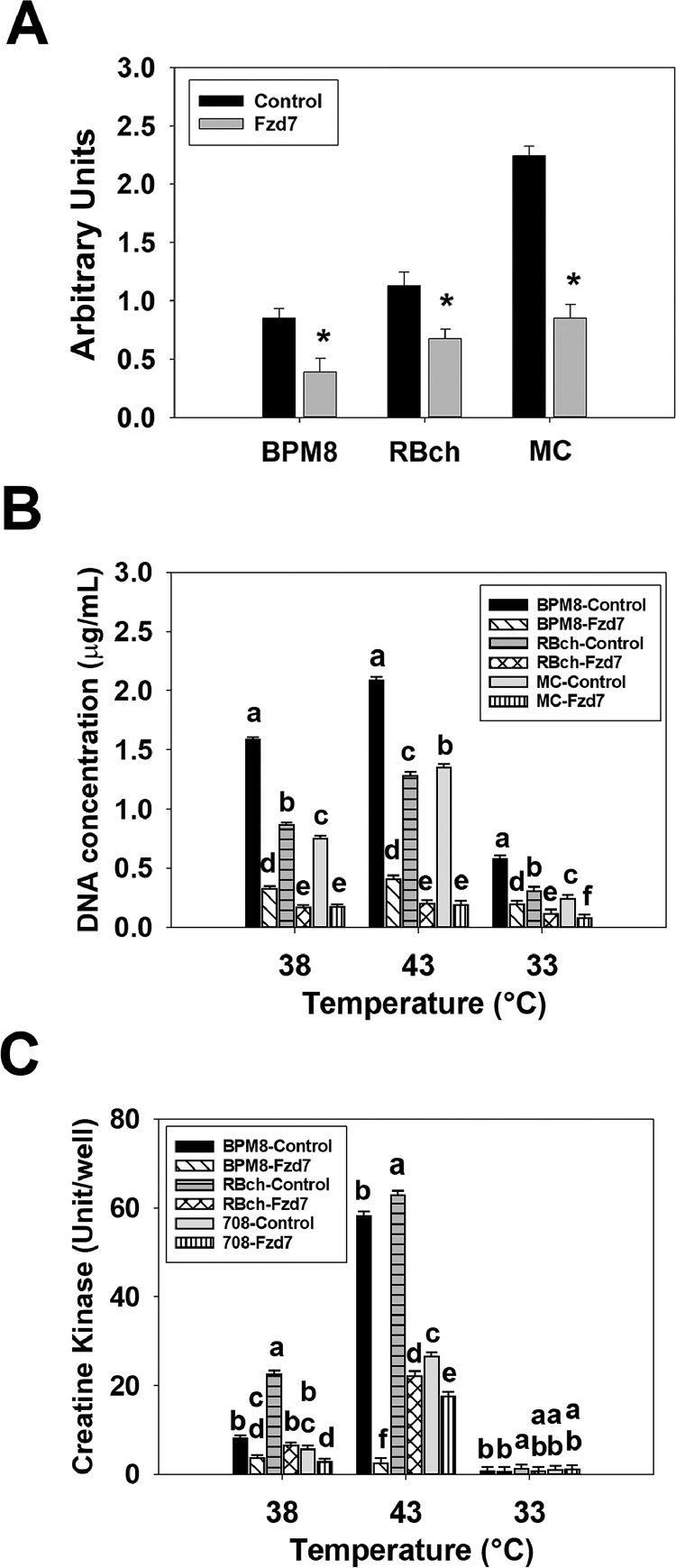
Expression of Fzd7 mRNA was quantified by RT-qPCR at 72 h of proliferation after knockdown the Fzd7 expression in the BPM8, RBch, and MC line SCs at the beginning of proliferation (Figure 3A). Expression of Fzd7 was 2.18-fold (P < 0.001), 1.68-fold (P < 0.001), and 2.63-fold (P < 0.001) lower in the BPM8, RBch, and MC Fzd7 knockdown groups compared to the control groups, respectively (Figure 3A).
Figure 3.
Effect of temperature and knockdown of mechanistic target of frizzled-7 (Fzd7) on the proliferation and differentiation of satellite cells isolated from Cornish Rock chickens (BPM8), Randombred chickens (RBch), and modern commercial chickens (MC). (A) Expression of Fzd7 was measured at 72 h of proliferation after knockdown of Fzd7 at the beginning of proliferation in the BPM8, RBch, and MC line SCs. Asterisk (*) above the bars represents a significant difference between the 2 adjacent groups (P ≤ 0.05). (B) Satellite cells isolated from the BPM8, RBch, and MC chicken lines were transfected with either a control small interfering RNA (siRNA) or an Fzd7 siRNA at the beginning of proliferation, and then incubated at 38°C, 43°C, or 33°C for 72 h of proliferation. Each bar represents mean DNA concentration (μg/well) with an error bar representing the standard error of the mean. (C) Satellite cells isolated from the BPM8, RBch, and MC chicken lines were transfected with either a control siRNA or a Fzd7 siRNA at the beginning of proliferation, and then incubated at 38°C, 43°C, or 33°C for 72 h of proliferation followed by 48 h of differentiation. Each bar represents mean creatine kinase activity (Unit/well) with an error bar representing the standard error of the mean. Within each temperature group in (B) and (C), mean values without a common letter are significantly different (P ≤ 0.05).
For SC proliferation, a significant interaction was observed among the effects of Fzd7 knockdown, temperature, and cell line at 72 h of proliferation (Figure 3B). In all the temperature groups, knockdown of Fzd7 decreased (P < 0.001) the proliferation of SCs in the BPM8 (38°C: 4.80-fold, 43°C: 5.10-fold, 33°C: 2.93-fold), RBch (38°C: 5.14-fold, 43°C: 6.33-fold, 33°C: 2.63-fold), and MC (38°C: 4.34-fold, 43°C: 7.02-fold, 33°C: 3.14-fold) line SCs, respectively.
An interaction effect among cell line, temperature, and knockdown of Fzd7 was significant (P < 0.001) in affecting the differentiation of SCs at 48 h of differentiation (Figure 3C). At 38° and 43°C, SC differentiation was lower (P < 0.001) in the BPM8 (38°C: 2.20-fold, 43°C: 22.89-fold), RBch (38°C: 3.49-fold, 43°C: 2.84-fold), and MC (38°C: 2.04-fold, 43°C: 1.51-fold) Fzd7 knockdown groups compared to the control groups. Knockdown of Fzd7 had no significant effect on the differentiation of SCs in all the cell lines at 33°C (P ≥ 0.706).
For the myotubes, knockdown of Fzd7 significantly decreased the diameter of myotubes in all the cell lines at all temperatures [(38°C: Figure S2, 43°C: Figure S3, and 33°C: Figure S4). [38°C (BPM8: 1.55-fold, RBch: 1.91-fold, MC: 1.62-fold, Figure S2, P < 0.001), 43°C (BPM8: 1.51-fold, RBch: 1.63-fold, MC: 1.21-fold, Figure S3, P < 0.001), 33°C (BPM8: 1.10-fold, RBch: 1.06-fold, MC: 1.08-fold, Figure S4, P ≤ 0.019)].
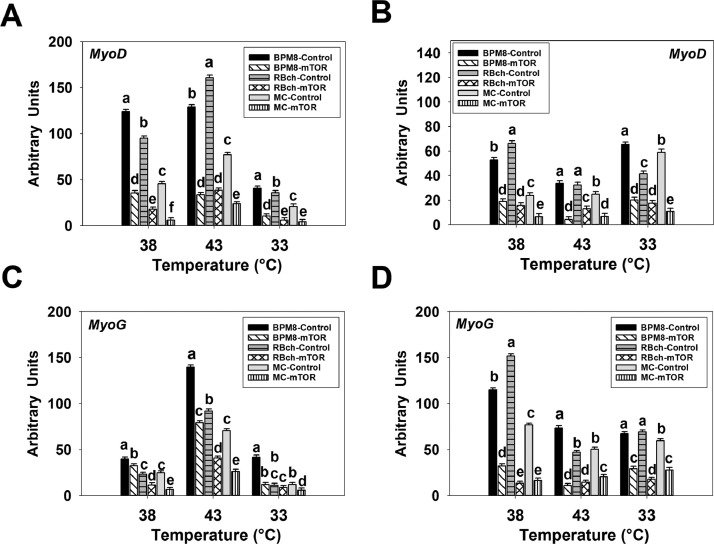
Effect of mTOR or Fzd7 Knockdown on the Expression of Myogenic Transcriptional Regulatory Genes in Chicken Satellite Cells During Thermal Stress
For MyoD expression, a significant interaction among the effects of cell line, temperature, and knockdown of mTOR was observed at both 72 h of proliferation (P < 0.001, Figure 4A) and 48 h of differentiation (P < 0.001, Figure 4B). At 72 h of proliferation, MyoD expression was lower (P < 0.001) in the BPM8 (38°C: 3.49-fold, 43°C: 3.85-fold, 33°C: 3.84-fold), RBch (38°C: 5.34-fold, 43°C: 4.17-fold, 33°C: 5.90-fold), and MC (38°C: 7.39-fold, 43°C: 3.25-fold, 33°C: 4.90-fold) mTOR knockdown groups compared to the control groups at all the temperatures (Figure 4A). At 48 h of differentiation, the expression of MyoD was decreased (P < 0.001) with the knockdown of mTOR in all the cell lines [BPM8 (38°C: 2.80-fold, 43°C: 8.19-fold, 33°C: 3.26-fold), RBch (38°C: 4.25-fold, 43°C: 2.51-fold, 33°C: 2.39-fold), and MC (38°C: 3.69-fold, 43°C: 3.74-fold, 33°C: 5.55-fold), Figure 4B].
Figure 4.
Effect of temperature and knockdown of mechanistic target of rapamycin (mTOR) on the expression of myogenic determination factor-1 (MyoD) and myogenin (MyoG) in satellite cells isolated from Cornish Rock chickens (BPM8), Randombred chickens (RBch), and modern commercial chickens (MC). Satellite cells isolated from the BPM8, RBch, and MC chicken lines were transfected with either a control small interfering RNA (siRNA) or an mTOR siRNA that can knockdown the expression of mTOR at the beginning of proliferation, and then incubated at 38°C, 43°C, or 33°C. Expression of MyoD was determined at 72 h of proliferation (72hP) (A) and 48 h of differentiation (48hD) (B). Expression of MyoG was determined at 72 h of proliferation (72hP) (C) and 48 h of differentiation (48hD) (D). Each graph bar represents a mean arbitrary unit, and each error bar represents the standard error of the mean. Within each temperature group, mean values with different letters are significantly different (P ≤ 0.05).
For MyoG expression, an interaction effect was significant among the effects of cell line, temperature, and knockdown of mTOR at both 72 h of proliferation (P < 0.001, Figure 4C) and 48 h of differentiation (P < 0.001, Figure 4D). At 72 h of proliferation, MyoG expression was decreased (P ≤ 0.018) with the knockdown of mTOR in the BPM8 (38°C: 1.23-fold, 43°C: 1.76-fold), RBch (38°C: 1.99-fold, 43°C: 2.24-fold), and MC (38°C: 3.78-fold, 43°C: 2.68-fold) line SCs at both 38° and 43°C (Figure 4C). At 33°C, the expression of MyoG was lower in the BPM8 (3.47-fold, P < 0.001) and MC (1.99-fold, P < 0.001) mTOR knockdown groups compared to the controls (Figure 4C). At 48 h of differentiation, the expression of MyoG was decreased (P < 0.001) with the knockdown of mTOR in BPM8 (38°C: 3.56-fold, 43°C: 6.74-fold, 33°C: 2.31-fold), RBch (38°C: 11.30-fold, 43°C: 3.25-fold, 33°C: 3.90-fold), and MC (38°C: 4.64-fold, 43°C: 2.47-fold, 33°C: 2.14-fold) line SCs (Figure 4D).
There was a significant interaction effect among the effects of cell line, temperature, and knockdown of Fzd7 in the determination of MyoD expression at 72 h of proliferation (P < 0.001, Figure 5A) and 48 h of differentiation (P < 0.001, Figure 5B). At 72 h of proliferation, knockdown of Fzd7 inhibited the expression of MyoD in the BPM8 (38°C: 3.64-fold, 43°C: 3.29-fold, 33°C: 3.43-fold), RBch (38°C: 2.61-fold, 43°C: 4.18-fold, 33°C: 3.75-fold), and MC (38°C: 2.92-fold, 43°C: 4.12-fold, 33°C: 3.70-fold) line SCs at all the temperatures (Figure 5A). At 48 h of differentiation, MyoD expression was lower in the BPM8 (3.98-fold, P < 0.001) and RBch (3.68-fold, P < 0.001) Fzd7 knockdown groups compared to the controls at 38°C (Figure 5B). Expression of MyoD decreased (P < 0.001) with the knockdown of Fzd7 in all the cell lines at 43° and 33°C [BPM8 (43°C: 3.68-fold, 33°C: 2.11-fold), RBch (43°C: 2.53-fold, 33°C: 2.42-fold), and MC (43°C: 3.89-fold, 33°C: 5.26-fold), Figure 5B].
Figure 5.
Effect of temperature and knockdown of frizzled 7 (Fzd7) on the expression of myogenic determination factor-1 (MyoD) and myogenin (MyoG) in satellite cells isolated from Cornish Rock chickens (BPM8), Randombred chickens (RBch), and modern commercial chickens (MC). Satellite cells isolated from the BPM8, RBch, and MC chicken lines were transfected with either a control small interfering RNA (siRNA) or a Fzd7 siRNA that can knockdown the expression of Fzd7 at the beginning of proliferation, and then incubated at 38°C, 43°C, or 33°C. Expression of MyoD was determined at 72 h of proliferation (72hP) (A) and 48 h of differentiation (48hD) (B). Expression of MyoG was determined at 72 h of proliferation (72hP) (C) and 48 h of differentiation (48hD) (D). Each graph bar represents a mean arbitrary unit, and each error bar represents the standard error of the mean. Within each temperature group, mean values with different letters are significantly different (P ≤ 0.05).
With respect to the expression of MyoG, an interaction effect was significant among the effects of cell line, temperature, and knockdown of Fzd7 at 72 h of proliferation (P < 0.001, Figure 5C) and 48 h of differentiation (P < 0.001, Figure 5D). At 72 h of proliferation, MyoG expression was decreased with the knockdown of Fzd7 in BPM8 (38°C: 1.61-fold, 43°C: 3.02-fold), RBch (38°C: 1.36-fold, 43°C: 1.96-fold), and MC (38°C: 2.28-fold, 43°C: 2.52-fold) line SCs at both 38°C (P ≤ 0.072) and 43°C (P < 0.001, Figure 5C). At 33°C, knockdown of Fzd7 decreased the expression of MyoG only in the BPM8 line SCs (P < 0.001, Figure 5C). At 48 h of differentiation, the expression of MyoG was lower in the BPM8 (38°C: 3.25-fold, 43°C: 5.55-fold, 33°C: 2.07-fold), RBch (38°C: 5.52-fold, 43°C: 2.03-fold, 33°C: 2.63-fold), and MC (38°C: 2.61-fold, 43°C: 3.38-fold, 33°C: 2.86-fold) Fzd7 knockdown groups compared to the controls at all the temperatures (Figure 5D).
DISCUSSION
Selection strategies to achieve increased muscle accretion have focused on the posthatch period during the hypertrophic phase of muscle growth mediated by the SCs. Changes in the morphological structure and cellular biology of the p. major muscle have resulted in muscle structural defects (Velleman et al., 2003) and meat quality issues (Kuttappan et al., 2012; Sihvo et al., 2014) particularly in the rapid-growing heavy-weight poultry lines. Satellite cells are the only reservoir of cells responsible for posthatch muscle hypertrophic growth (Moss and Leblond, 1971; Cardiasis and Cooper, 1975) and the regeneration of damaged muscle (Bischoff, 1975; Snow, 1977). In poultry, SC myogenic function is highly responsive to environmental temperatures (Halevy et al., 2001; Clark et al., 2016; Halevy, 2020). Xu et al. (2021) in an in vitro study showed that SCs from modern commercial meat-type turkeys have increased sensitivity to both heat and cold stress compared to that of a historic turkey line representative of commercial turkeys from the 1960s (Nestor et al., 1969). Transcriptome analysis also demonstrated the expression of genes that are involved in muscle regeneration, muscle atrophy, sarcomere assembly, and inflammatory processes associated with myopathies were greatly altered with both heat and cold stress, particularly in the p. major SCs isolated from the rapid-growing commercial line turkeys (Reed et al., 2022a,b). Thermal stress-induced changes in SC myogenic function and biochemistry may further affect the regeneration potential of chicken p. major muscle impacting breast meat quality.
Selection for increased growth and breast muscle yield greatly increases the proliferation, differentiation, and expression of MyoD and MyoG in SCs in the p. major muscle of growth selected faster-growing turkeys (Clark et al., 2016; Xu et al., 2021). Although the selection strategies are similar between chickens and turkeys, it has not been reported to date if chicken p. major muscle SCs have changed in a similar manner as turkey p. major SCs. Results of the current study showed that SC proliferation and differentiation was greatly reduced in the modern commercial MC line SCs compared to the BPM8 and RBch lines. Expression of MyoD and MyoG was also lower in the MC line SCs than that of the BPM8 and RBch lines during both proliferation and differentiation. These results suggest that modern commercial chickens have decreased regeneration of damaged myofibers as SC myogenic activity in the p. major muscle is reduced. Thus, the commercial chicken p. major muscle will be more susceptible to degenerative myopathies.
One of the frequently identified degenerative myopathies in modern commercial heavy-weight broilers is wooden breast (Sihvo et al., 2017), which is characterized by a palpably rigid p. major muscle with a viscous coating on the outside (Sihvo et al., 2014). Myofiber necrosis, fibrosis, lipidosis, and inflammatory cell infiltration were observed in wooden breast affected chicken p. major muscle changing the morphological structure of the p. major muscle and affecting breast meat yield (Sihvo et al., 2014; Soglia et al., 2016). In addition, meat quality problems such as decreased contractile protein levels, tough meat, reduced water holding capacity, and increased cooking loss have been identified in wooden breast-affected muscle (Mudalal et al., 2015; Soglia et al., 2016). Previous studies have linked the wooden breast myopathy to a decrease in blood vessel density in the p. major muscle of rapid-growing modern commercial chickens (Sihvo et al., 2018). Since proximity of blood supply to SCs is required for the SC-mediated muscle regeneration (Luque et al., 1995; Christov et al., 2007), SCs in wooden breast-affected muscle has been hypothesized to have decreased repair activity due, in part, to decreased circulatory supply (Velleman, 2019). Furthermore, wooden breast-affected chicken p. major muscle contains a greater percentage of smaller diameter myofibers (Velleman and Clark, 2015; Clark and Velleman, 2016). These smaller myofibers have poorly organized contractile sarcomeres and do not restore the myofiber to its original state (Clark and Velleman, 2016; Velleman et al., 2018) suggesting a reduction in SC-mediated regeneration of damaged muscle fibers. Muscle fibrosis with the replacement of damaged muscle fibers with connective tissue and fat will occur when SC-mediated repair is inhibited. Taken together, these findings support the results of the current study that SCs in modern commercial meat-type chickens have impaired myogenic function and are not able to regenerate the damaged muscle resulting in breast meat products that are downgraded and in severe cases condemned from human consumption. Notably, degenerative myopathies like wooden breast have not been reported in modern commercial meat-type turkeys, and this is likely associated with the high myogenic and regenerative potential of turkey p. major muscle SCs. Reed et al. (2022b) further showed in heavy-weight meat-type turkeys that the expression of genes involved in muscle degeneration (ubiquitin proteasome pathway) are downregulated, which may further diminish the possibility of degenerative myopathies in turkey breast muscle.
With regard to temperature effects, there are some similarities and differences in SC responses between chickens and turkeys. Previous studies have shown that heat stress greatly increased both proliferation and differentiation of turkey p. major muscle SCs, while cold stress showed a suppressive effect (Clark et al., 2016; Xu et al., 2021). For chicken SCs, proliferation was increased with heat stress and decreased with cold stress in all the cell lines which is similar to the response of turkey SCs. Expression of MyoD was highly upregulated during heat stress in the RBch and MC line SCs and was downregulated with cold stress in all the cell lines. During differentiation, SC differentiation was altered with heat and cold stress in a cell line-specific manner. In general, the differentiation of the MC line SCs was less responsive to both heat and cold stress compared to the BPM8 and RBch lines. These results suggest that chicken SC proliferation and differentiation are responsive to both heat and cold thermal stress in a cell line-specific manner. However, when compared to turkey SCs (Xu et al., 2021), the temperature sensitivity of chicken SCs was overall lower, particularly in the modern commercial broiler chickens.
Birds are homeotherms with the body temperature being maintained only in a narrow range (Yahav, 2000). As a result of climate change and extreme weather, modern commercial chickens may encounter both hot and cold temperature extremes. Exposure of chickens to heat and cold stress can significantly change the body temperature (Shinder et al., 2007; Maman et al., 2019) and affect the growth and development of the p. major muscle (Piestun et al., 2017; Patael et al., 2019). For example, posthatch heat stress has been reported to reduce muscle fiber diameter (Joiner et al., 2014; Piestun et al., 2017; Patael et al., 2019), decrease vascular density (Joiner et al., 2014), and stimulate collagen deposition (Patael et al., 2019) which will negatively impact breast muscle growth and structure. In the current study, the expression of MyoG during differentiation was significantly lower at both hot and cold temperatures compared to the control temperature in the MC line SCs. Thus, even though the p. major SCs in the modern commercial meat-type chickens have decreased proliferation and differentiation, extrinsic thermal stress both hot and cold will further inhibit the myogenic or regenerative potential of the p. major SCs, which may further suppress muscle growth and structure and increase the possibility of degenerative myopathies.
Satellite cell activity and temperature sensitivity are regulated by signal transduction pathways and downstream gene expression networks. The mTOR pathway is highly reactive to both heat and cold thermal stress in turkey p. major muscle SCs (Reed et al., 2017; Xu et al., 2022b). As reported by Xu et al. (2022b), the activity of the mTOR pathway was increased with growth selection and with heat stress in modern commercial turkey SCs. The mTOR pathway not only promotes myofiber hypertrophy through protein synthesis (Bodine et al., 2001; Ohanna et al., 2005) but also regulates the myogenic activity of SCs (Vignale et al., 2015; Xu et al., 2022b). For example, mTOR knockout mouse SCs had decreased proliferation and differentiation and lower MyoD and MyoG expression compared to that of the wild type mouse (Zhang et al., 2015). In turkeys SCs, knocking down the expression of mTOR decreased the proliferation, differentiation, and expression of both MyoD and MyoG (Xu et al., 2022b). In the present study, the mRNA expression of mTOR was lower in the MC line SCs compared to the BPM8 and RBch lines suggesting reduced hypertrophic and regenerative potential of the p. major muscle in modern commercial chickens. Knocking down mTOR expression greatly decreased both proliferation and differentiation of chicken SCs with the modern commercial MC line SCs having less sensitivity to the knockdown of mTOR compared to the BPM8 and RBch lines. Expression of MyoD and MyoG was also decreased with the knockdown of mTOR independent of cell lines. Furthermore, when compared to the cellular responses observed in the turkey SCs (Xu et al., 2022b), the proliferation and myogenic activity of chicken SCs were more sensitive to the knockdown of mTOR. These comparisons suggest that chicken SCs are more dependent on the mTOR pathway in the maintenance of myogenic or regenerative function compared to the turkey SCs.
With regard to the Wnt pathway, transcriptome analysis showed the expression of genes in the Fzd7-mediated Wnt/PCP pathway is highly upregulated with heat stress in proliferating commercial line turkey SCs (Reed et al., 2022a). Our previous study reported the Fzd7-mediated Wnt/PCP pathway is involved in the regulation of turkey SC proliferation and differentiation in a growth- and temperature-dependent manner (Xu et al., 2022a). In addition to regulating myogenic activity, the Wnt/PCP pathway also regulates SC migration (Wang et al., 2018), which is required for the alignment of SCs prior to subsequent fusion to existing myofibers (Chazaud et al., 1998). Thus, the Wnt/PCP pathway plays an essential role in SC migration and myogenesis promoting muscle mass accretion through SC-mediated muscle hypertrophy (Le Grand et al., 2009). Consistent with the previous findings (Xu et al., 2022a), results of the current study showed the knockdown of Fzd7 not only decreased chicken SC proliferation and differentiation but also inhibited the expression of MyoD and MyoG in all the cell lines. Since the proliferation and differentiation of MC line SCs was the lowest among the 3 cell lines, the MC line SCs were less responsive to the knockdown of Fzd7 compared to the BPM8 and RBch lines. In addition, within the modern commercial MC line, SC proliferation was more dependent on the Fzd7-mediated Wnt/PCP pathway than differentiation, and this may affect both hypertrophic potential and regeneration capacity of the p. major muscle.
In summary, SCs in the p. major muscle of current commercial MC line chickens have decreased proliferation and differentiation and are less responsive to both hot and cold thermal stress compared to the 1990s BPM8 and RBch lines. Satellite cell proliferation was increased with heat stress and decreased with cold stress in all the cell lines. Differentiation of SCs was affected by thermal stress in a cell line-specific manner. Specifically, both hot and cold thermal stress had a negative effect on the differentiation of the BPM8 and RBch SCs. The differentiation of the modern commercial MC line SCs was not changed with heat stress and was decreased with cold stress. Knockdown of the expression of either mTOR or Fzd7 reduced the proliferation and differentiation of SCs independent of cell line at the control and hot temperatures. In addition, for the modern commercial SCs, maintaining proliferation was more dependent on mTOR and Fzd7-mediated Wnt/PCP pathways than maintaining differentiation. Hence, modern commercial meat-type chickens have decreased myogenic potential due to reduced proliferation and differentiation rates and temperature sensitivity of SCs, in part, in an mTOR- and Fzd7-dependent manner. The decrease in muscle regeneration will make modern commercial broiler chickens more susceptible to necrotic and fibrotic degenerative myopathies. As an outcome of this study, selection strategies should include SC proliferation and differentiation, and responsiveness to extrinsic stimuli like temperature to maximize muscle mass accretion and maintain product quality.
ACKNOWLEDGMENTS
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
DISCLOSURES
No conflict of interest is declared.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2023.102608.
Appendix. Supplementary materials
REFERENCES
- Barbut S. Problem of pale soft exudative meat in broiler chickens. Br. Poult. Sci. 1997;38:355–358. doi: 10.1080/00071669708418002. [DOI] [PubMed] [Google Scholar]
- Bazgir B., Fathi R., Valojerdi M.R., Mozdziak P., Asgari A. Satellite cells contribution to exercise mediated muscle hypertrophy and repair. Cell J. 2017;18:473. doi: 10.22074/cellj.2016.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff R. Regeneration of single skeletal muscle fibers in vitro. Anat. Rec. 1975;182:215–235. doi: 10.1002/ar.1091820207. [DOI] [PubMed] [Google Scholar]
- Bodine S.C., Stitt T.N., Gonzalez M., Kline W.O., Stover G.L., Bauerlein R., Zlotchenko E., Scrimgeour A., Lawrence J.C., Glass D.J. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 2001;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- Brunetti A., Goldfine I.D. Role of myogenin in myoblast differentiation and its regulation by fibroblast growth factor. J. Biol. Chem. 1990;265:5960–5963. [PubMed] [Google Scholar]
- Cardiasis A., Cooper G. An analysis of nuclear number in individual muscle fiber during differentiation and growth: a satellite cell-muscle fiber growth unit. J. Exp. Zool. 1975;191:347–358. doi: 10.1002/jez.1401910305. [DOI] [PubMed] [Google Scholar]
- Chazaud B., Christov C., Gherardi R.K., Barlovatz-Meimon G. In vitro evaluation of human muscle satellite cell migration prior to fusion into myotubes. J. Muscle Res. Cell. Motil. 1998;19:931–936. doi: 10.1023/a:1005451725719. [DOI] [PubMed] [Google Scholar]
- Christov C., Chrétien F., Abou-Khalil R., Bassez G., Vallet G., Authier F.-J., Bassaglia Y., Shinin V., Tajbakhsh S., Chazaud B. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol. Biol. Cell. 2007;18:1397–1409. doi: 10.1091/mbc.E06-08-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D.L., Coy C.S., Strasburg G.M., Reed K.M., Velleman S.G. Temperature effect on proliferation and differentiation of satellite cells from turkeys with different growth rates. Poult. Sci. 2016;95:934–947. doi: 10.3382/ps/pev437. [DOI] [PubMed] [Google Scholar]
- Clark D., Velleman S. Spatial influence on breast muscle morphological structure, myofiber size, and gene expression associated with the wooden breast myopathy in broilers. Poult. Sci. 2016;95:2930–2945. doi: 10.3382/ps/pew243. [DOI] [PubMed] [Google Scholar]
- Collins K., Kiepper B., Ritz C., McLendon B., Wilson J. Growth, livability, feed consumption, and carcass composition of the Athens Canadian Random Bred 1955 meat-type chicken versus the 2012 high-yielding Cobb 500 broiler. Poult. Sci. 2014;93:2953–2962. doi: 10.3382/ps.2014-04224. [DOI] [PubMed] [Google Scholar]
- Darr K.C., Schultz E. Exercise-induced satellite cell activation in growing and mature skeletal muscle. J. Appl. Physiol. 1987;63:1816–1821. doi: 10.1152/jappl.1987.63.5.1816. [DOI] [PubMed] [Google Scholar]
- Dransfield E., Sosnicki A. Relationship between muscle growth and poultry meat quality. Poult. Sci. 1999;78:743–746. doi: 10.1093/ps/78.5.743. [DOI] [PubMed] [Google Scholar]
- Duclos M.J., Berri C., Le Bihan-Duval E. Muscle growth and meat quality. J. Appl. Poult. Res. 2007;16:107–112. [Google Scholar]
- Dunnington E.A., Siegel P.B. Thermoregulation in newly hatched chicks. Poult. Sci. 1984;63:1303–1313. doi: 10.3382/ps.0631303. [DOI] [PubMed] [Google Scholar]
- Fortier M., Comunale F., Kucharczak J., Blangy A., Charrasse S., Gauthier-Rouvière C. RhoE controls myoblast alignment prior fusion through RhoA and ROCK. Cell Death Differ. 2008;15:1221–1231. doi: 10.1038/cdd.2008.34. [DOI] [PubMed] [Google Scholar]
- Halevy O. Timing is everything—the high sensitivity of avian satellite cells to thermal conditions during embryonic and posthatch periods. Front. Physiol. 2020;11:235. doi: 10.3389/fphys.2020.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevy O., Geyra A., Barak M., Uni Z., Sklan D. Early posthatch starvation decreases satellite cell proliferation and skeletal muscle growth in chicks. J. Nutr. 2000;130:858–864. doi: 10.1093/jn/130.4.858. [DOI] [PubMed] [Google Scholar]
- Halevy O., Krispin A., Leshem Y., McMurtry J.P., Yahav S. Early-age heat exposure affects skeletal muscle satellite cell proliferation and differentiation in chicks. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;281:R302–R309. doi: 10.1152/ajpregu.2001.281.1.R302. [DOI] [PubMed] [Google Scholar]
- Harding R.L., Halevy O., Yahav S., Velleman S.G. The effect of temperature on proliferation and differentiation of chicken skeletal muscle satellite cells isolated from different muscle types. Physiol. Rep. 2016;4:e12770. doi: 10.14814/phy2.12770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harford I., Pavlidis H., Anthony N. Divergent selection for muscle color in broilers. Poult. Sci. 2014;93:1059–1066. doi: 10.3382/ps.2013-03446. [DOI] [PubMed] [Google Scholar]
- Hasty P., Bradley A., Morris J.H., Edmondson D.G., Venuti J.M., Olson E.N., Klein W.H. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature. 1993;364:501–506. doi: 10.1038/364501a0. [DOI] [PubMed] [Google Scholar]
- Havenstein G., Ferket P., Grimes J., Qureshi M., Nestor K. Performance of 1966 vs. 2003-type turkeys when fed representative 1966 and 2003 turkey diets. Proc. World's Poult. Congr; Istanbul, Turkey; 2004. [Google Scholar]
- Havenstein G., Ferket P., Grimes J., Qureshi M., Nestor K. Comparison of the performance of 1966-versus 2003-type turkeys when fed representative 1966 and 2003 turkey diets: growth rate, livability, and feed conversion. Poult. Sci. 2007;86:232–240. doi: 10.1093/ps/86.2.232. [DOI] [PubMed] [Google Scholar]
- Havenstein G., Ferket P., Qureshi M. Carcass composition and yield of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult. Sci. 2003;82:1509–1518. doi: 10.1093/ps/82.10.1509. [DOI] [PubMed] [Google Scholar]
- Joiner K.S., Hamlin G.A., Lien A.R., Bilgili S.F. Evaluation of capillary and myofiber density in the pectoralis major muscles of rapidly growing, high-yield broiler chickens during increased heat stress. Avian Dis. 2014;58:377–382. doi: 10.1637/10733-112513-Reg.1. [DOI] [PubMed] [Google Scholar]
- Kuttappan V., Brewer V., Apple J., Waldroup P., Owens C. Influence of growth rate on the occurrence of white striping in broiler breast fillets. Poult. Sci. 2012;91:2677–2685. doi: 10.3382/ps.2012-02259. [DOI] [PubMed] [Google Scholar]
- Le Grand F., Jones A.E., Seale V., Scimè A., Rudnicki M.A. Wnt7a activates the planar cell polarity pathway to drive the symmetric expansion of satellite stem cells. Cell Stem Cell. 2009;4:535–547. doi: 10.1016/j.stem.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., McFarland D.C., Nestor K.E., Velleman S.G. Differential expression of membrane-associated heparan sulfate proteoglycans in the skeletal muscle of turkeys with different growth rates. Poult. Sci. 2006;85:422–428. doi: 10.1093/ps/85.3.422. [DOI] [PubMed] [Google Scholar]
- Luque E., Pena J., Martin P., Jimena I., Vaamonde R. Capillary supply during development of individual regenerating muscle fibers. Anat. Histol. Embryol. 1995;24:87–89. doi: 10.1111/j.1439-0264.1995.tb00016.x. [DOI] [PubMed] [Google Scholar]
- Maman A.H., Özlü S., Uçar A., Elibol O. Effect of chick body temperature during post-hatch handling on broiler live performance. Poult. Sci. 2019;98:244–250. doi: 10.3382/ps/pey395. [DOI] [PubMed] [Google Scholar]
- McFarland D.C., Gilkerson K.K., Pesall J.E., Ferrin N.H., Wellenreiter R. In vitro characteristics of myogenic satellite cells derived from the pectoralis major and biceps femoris muscles of the chicken. Cytobios. 1997;91:45–52. [PubMed] [Google Scholar]
- McFarland D., Pesall J., Gilkerson K., Ferrin N. The response to growth factors of cultured satellite cells derived from turkeys having different growth rates. Cytobios. 1995;82:229–238. [PubMed] [Google Scholar]
- Modrey P., Nichelmann M. Development of autonomic and behavioural thermoregulation in turkeys (Meleagris gallopavo) J. Therm. Biol. 1992;17:287–292. [Google Scholar]
- Moss F., Leblond C. Satellite cells as the source of nuclei in muscles of growing rats. Anat. Rec. 1971;170:421–435. doi: 10.1002/ar.1091700405. [DOI] [PubMed] [Google Scholar]
- Mozdziak P.E., Schultz E., Cassens R.G. Satellite cell mitotic activity in posthatch turkey skeletal muscle growth. Poult. Sci. 1994;73:547–555. doi: 10.3382/ps.0730547. [DOI] [PubMed] [Google Scholar]
- Mudalal S., Lorenzi M., Soglia F., Cavani C., Petracci M. Implications of white striping and wooden breast abnormalities on quality traits of raw and marinated chicken meat. Animal. 2015;9:728–734. doi: 10.1017/S175173111400295X. [DOI] [PubMed] [Google Scholar]
- Nestor K.E., McCartney M.G., Bachev N. Relative contributions of genetics and environment to turkey improvement. Poult. Sci. 1969;48:1944–1949. doi: 10.3382/ps.0481944. [DOI] [PubMed] [Google Scholar]
- Ohanna M., Sobering A.K., Lapointe T., Lorenzo L., Praud C., Petroulakis E., Sonenberg N., Kelly P.A., Sotiropoulos A., Pende M. Atrophy of S6K1−/− skeletal muscle cells reveals distinct mTOR effectors for cell cycle and size control. Nat. Cell Biol. 2005;7:286–294. doi: 10.1038/ncb1231. [DOI] [PubMed] [Google Scholar]
- Owens C., Hirschler E., McKee S., Martinez-Dawson R., Sams A. The characterization and incidence of pale, soft, exudative turkey meat in a commercial plant. Poult. Sci. 2000;79:553–558. doi: 10.1093/ps/79.4.553. [DOI] [PubMed] [Google Scholar]
- Papah M.B., Brannick E.M., Schmidt C.J., Abasht B. Evidence and role of phlebitis and lipid infiltration in the onset and pathogenesis of wooden breast disease in modern broiler chickens. Avian Pathol. 2017;46:623–643. doi: 10.1080/03079457.2017.1339346. [DOI] [PubMed] [Google Scholar]
- Patael T., Piestun Y., Soffer A., Mordechay S., Yahav S., Velleman S.G., Halevy O. Early posthatch thermal stress causes long-term adverse effects on pectoralis muscle development in broilers. Poult. Sci. 2019;98:3268–3277. doi: 10.3382/ps/pez123. [DOI] [PubMed] [Google Scholar]
- Piestun Y., Patael T., Yahav S., Velleman S.G., Halevy O. Early posthatch thermal stress affects breast muscle development and satellite cell growth and characteristics in broilers. Poult. Sci. 2017;96:2877–2888. doi: 10.3382/ps/pex065. [DOI] [PubMed] [Google Scholar]
- Reed K.M., Mendoza K.M., Strasburg G.M., Velleman S.G. Response of turkey muscle satellite cells to thermal challenge. II. Transcriptome effects in differentiating cells. Front. Physiol. 2017;8:948. doi: 10.3389/fphys.2017.00948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed K.M., Mendoza K.M., Strasburg G.M., Velleman S.G. Transcriptome response of proliferating muscle satellite cells to thermal challenge in commercial turkey. Front. Physiol. 2022;13:1607. doi: 10.3389/fphys.2022.970243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed K.M., Mendoza K.M., Xu J., Strasburg G.M., Velleman S.G. Transcriptome response of differentiating muscle satellite cells to thermal challenge in commercial turkey. Genes. 2022;13:1857. doi: 10.3390/genes13101857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnicki M.A., Schnegelsberg P.N., Stead R.H., Braun T., Arnold H.-H., Jaenisch R. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell. 1993;75:1351–1359. doi: 10.1016/0092-8674(93)90621-v. [DOI] [PubMed] [Google Scholar]
- Schultz E., Gibson M.C., Champion T. Satellite cells are mitotically quiescent in mature mouse muscle: an EM and radioautographic study. J. Exp. Zool. 1978;206:451–456. doi: 10.1002/jez.1402060314. [DOI] [PubMed] [Google Scholar]
- Schultz E., Lipton B.H. Skeletal muscle satellite cells: changes in proliferation potential as a function of age. Mech. Ageing Dev. 1982;20:377–383. doi: 10.1016/0047-6374(82)90105-1. [DOI] [PubMed] [Google Scholar]
- Shinder D., Rusal M., Tanny J., Druyan S., Yahav S. Thermoregulatory responses of chicks (Gallus domesticus) to low ambient temperatures at an early age. Poult. Sci. 2007;86:2200–2209. doi: 10.1093/ps/86.10.2200. [DOI] [PubMed] [Google Scholar]
- Sihvo H.-K., Airas N., Lindén J., Puolanne E. Pectoral vessel density and early ultrastructural changes in broiler chicken wooden breast myopathy. J. Comp. Pathol. 2018;161:1–10. doi: 10.1016/j.jcpa.2018.04.002. [DOI] [PubMed] [Google Scholar]
- Sihvo H.-K., Immonen K., Puolanne E. Myodegeneration with fibrosis and regeneration in the pectoralis major muscle of broilers. Vet. Pathol. 2014;51:619–623. doi: 10.1177/0300985813497488. [DOI] [PubMed] [Google Scholar]
- Sihvo H.-K., Lindén J., Airas N., Immonen K., Valaja J., Puolanne E. Wooden breast myodegeneration of pectoralis major muscle over the growth period in broilers. Vet. Pathol. 2017;54:119–128. doi: 10.1177/0300985816658099. [DOI] [PubMed] [Google Scholar]
- Smith J.H. Relation of body size to muscle cell size and number in the chicken. Poult. Sci. 1963;42:283–290. [Google Scholar]
- Snow M.H. Myogenic cell formation in regenerating rat skeletal muscle injured by mincing. II. An autoradiographic study. Anat. Rec. 1977;188:201–217. doi: 10.1002/ar.1091880206. [DOI] [PubMed] [Google Scholar]
- Soglia F., Baldi G., Laghi L., Mudalal S., Cavani C., Petracci M. Effect of white striping on turkey breast meat quality. Animal. 2018;12:2198–2204. doi: 10.1017/S1751731117003469. [DOI] [PubMed] [Google Scholar]
- Soglia F., Mudalal S., Babini E., Di Nunzio M., Mazzoni M., Sirri F., Cavani C., Petracci M. Histology, composition, and quality traits of chicken pectoralis major muscle affected by wooden breast abnormality. Poult. Sci. 2016;95:651–659. doi: 10.3382/ps/pev353. [DOI] [PubMed] [Google Scholar]
- Strasburg G., Chiang W. Pale, soft, exudative turkey—the role of ryanodine receptor variation in meat quality. Poult. Sci. 2009;88:1497–1505. doi: 10.3382/ps.2009-00181. [DOI] [PubMed] [Google Scholar]
- Velleman S.G. Recent developments in breast muscle myopathies associated with growth in poultry. Annu. Rev. Anim. Biosci. 2019;7:289–308. doi: 10.1146/annurev-animal-020518-115311. [DOI] [PubMed] [Google Scholar]
- Velleman S.G., Anderson J.W., Coy C.S., Nestor K.E. Effect of selection for growth rate on muscle damage during turkey breast muscle development. Poult. Sci. 2003;82:1069–1074. doi: 10.1093/ps/82.7.1069. [DOI] [PubMed] [Google Scholar]
- Velleman S.G., Clark D.L. Histopathologic and myogenic gene expression changes associated with wooden breast in broiler breast muscles. Avian Dis. 2015;59:410–418. doi: 10.1637/11097-042015-Reg.1. [DOI] [PubMed] [Google Scholar]
- Velleman S.G., Clark D.L., Tonniges J.R. The effect of the wooden breast myopathy on sarcomere structure and organization. Avian Dis. 2018;62:28–35. doi: 10.1637/11766-110217-Reg.1. [DOI] [PubMed] [Google Scholar]
- Velleman S.G., Liu X., Nestor K.E., McFarland D.C. Heterogeneity in growth and differentiation characteristics in male and female satellite cells isolated from turkey lines with different growth rates. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2000;125:503–509. doi: 10.1016/s1095-6433(00)00178-1. [DOI] [PubMed] [Google Scholar]
- Vignale K., Greene E.S., Caldas J.V., England J.A., Boonsinchai N., Sodsee P., Pollock E.D., Dridi S., Coon C.N. 25-Hydroxycholecalciferol enhances male broiler breast meat yield through the mTOR pathway. J. Nutr. Sci. 2015;145:855–863. doi: 10.3945/jn.114.207936. [DOI] [PubMed] [Google Scholar]
- Wang W., Chen M., Gao Y., Song X., Zheng H., Zhang K., Zhang B., Chen D. P2Y6 regulates cytoskeleton reorganization and cell migration of C2C12 myoblasts via ROCK pathway. J. Cell. Biochem. 2018;119:1889–1898. doi: 10.1002/jcb.26350. [DOI] [PubMed] [Google Scholar]
- Wang X., Proud C.G. The mTOR pathway in the control of protein synthesis. Physiology. 2006;21:362–369. doi: 10.1152/physiol.00024.2006. [DOI] [PubMed] [Google Scholar]
- Wilson B.W., Nieberg P.S., Buhr R.J., Kelly B.J., Shultz F.T. Turkey muscle growth and focal myopathy. Poult. Sci. 1990;69:1553–1562. doi: 10.3382/ps.0691553. [DOI] [PubMed] [Google Scholar]
- Xu J., Strasburg G.M., Reed K.M., Velleman S.G. Response of turkey pectoralis major muscle satellite cells to hot and cold thermal stress: effect of growth selection on satellite cell proliferation and differentiation. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2021;252 doi: 10.1016/j.cbpa.2020.110823. [DOI] [PubMed] [Google Scholar]
- Xu J., Strasburg G.M., Reed K.M., Velleman S.G. Temperature and growth selection effects on proliferation, differentiation, and adipogenic potential of turkey myogenic satellite cells through Frizzled-7-mediated Wnt planar cell polarity pathway. Front. Physiol. 2022;13:889. doi: 10.3389/fphys.2022.892887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Strasburg G.M., Reed K.M., Velleman S.G. Thermal stress affects proliferation and differentiation of turkey satellite cells through the mTOR/S6K pathway in a growth-dependent manner. PLoS One. 2022;17 doi: 10.1371/journal.pone.0262576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z., Rivera A.J. Temporal expression of regulatory and structural muscle proteins during myogenesis of satellite cells on isolated adult rat fibers. Dev. Biol. 1994;164:588–603. doi: 10.1006/dbio.1994.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahav S. Domestic fowl-strategies to confront environmental conditions. Poult. Avian Biol. Rev. 2000;11:81–95. [Google Scholar]
- Yun Y., McFarland D.C., Pesall J.E., Gilkerson K.K., Vander Wal L.S., Ferrin N.H. Variation in response to growth factor stimuli in satellite cell populations. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1997;117:463–470. doi: 10.1016/s0300-9629(96)00404-5. [DOI] [PubMed] [Google Scholar]
- Zampiga M., Tavaniello S., Soglia F., Petracci M., Mazzoni M., Maiorano G., Meluzzi A., Clavenzani P., Sirri F. Comparison of 2 commercial turkey hybrids: productivity, occurrence of breast myopathies, and meat quality properties. Poult. Sci. 2019;98:2305–2315. doi: 10.3382/ps/pey607. [DOI] [PubMed] [Google Scholar]
- Zhang P., Liang X., Shan T., Jiang Q., Deng C., Zheng R., Kuang S. mTOR is necessary for proper satellite cell activity and skeletal muscle regeneration. Biochem. Biophys. Res. Commun. 2015;463:102–108. doi: 10.1016/j.bbrc.2015.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.