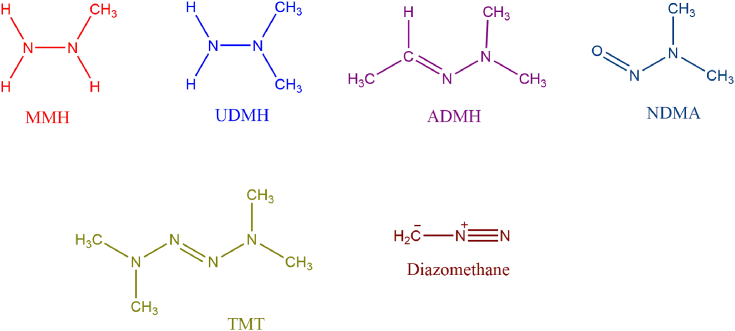
Abstract
N- Nitrosodimethyl amine, the simplest member of the N-Nitrosamine family, is a carcinogenic and mutagenic agent that has gained considerable research interest owing to its toxic nature. Ozonation of industrially important hydrazines, such as unsymmetrical dimethylhydrazine (UDMH) or monomethylhydrazine (MMH), has been associated with NDMA formation and accumulation in the environment. UDMH/MMH - ozonation also leads to several other transformation products such as acetaldehyde dimethyl hydrazine (ADMH), tetramethyl tetra azene (TMT), diazomethane, methyl diazene, etc, which can be either precursors or competitors for NDMA formation. However, the relevant chemistry detailing the formation of these transformation products from UDMH/MMH -ozone reaction and their subsequent conversion to NDMA is not well understood. In this work, we explored the formation mechanism of ADMH and TMT from UDMH-ozonation and their further oxidation to NDMA using the second-order Moller Plesset perturbation theory employing the 6-311G(d) basis set. We have also investigated how MMH selectively forms methyl diazene and diazomethane under normal conditions and NDMA in the presence of excess ozone. Our calculations indicate that the reactions proceed via an initial H abstraction from the hydrazine –NH2 group, followed by the oxidation of the generated N-radical species. The formation of ADMH from the UDMH-ozone reaction involves an acetaldehyde intermediate, which then reacts with a second UDMH molecule to generate ADMH. The preferable attack of ozone molecule on N=C bond of ADMH generates DMAN intermediate, which subsequently undergoes oxidation to form NDMA. Unlike other transformation products, TMT formation occurs via the dimerization of DMAN. 1Though there exists an N=N bond in the TMT, which are preferable attacking sites for ozone, experimental studies show the lower yields of NDMA formation, which corroborates with the high activation barrier required for the process (42 kcal/mol). Overall, our calculated results agree well with the experimental observations and rate constants. Computational calculations bring new insights into the electronic nature and kinetics of the elementary reactions of this pathway, enabled by computed energies of structures that are not possible to access experimentally.
Keywords: NDMA, Ozonation, Computational modeling, Activation barrier, Substituted hydrazine
1. Introduction
N-nitrosamines constitute the class of organic nitrogen (ON) compounds that gained significant research attention owing to their highly toxic nature as well as carcinogenic properties [[1], [2], [3], [4], [5]]. These toxicants are primarily produced by the oxidation or nitrosation of secondary amines [[6], [7], [8], [9], [10], [11], [12], [13]], and such reactions occur in the atmosphere [[14], [15], [16]], drinking water [[17], [18], [19], [20], [21]], organic chemical industry [5], rubber industry [22], food processing [23, 24], drug synthesis [[25], [26], [27], [28], [29], [30], [31], [32]], and so on. Correspondingly, nitrosamine compounds are ubiquitous, contaminating human tissues, food, water, air, soil, drugs etc, thereby posing severe health concerns [33]. Hence, numerous studies have been conducted on analyzing and removing N-nitrosamines.
N-Nitrosodimethylamine (NDMA), a disinfection by-product formed either during chlorination or ozonation of water, is the simplest and most prominent member of the nitrosamine family and is carcinogenic, genotoxic, and hepatotoxic in animals [[34], [35], [36], [37], [38]]. According to the Integrated Risk Information System of the US Environmental Protection Agency, NDMA is classified as a probable human carcinogen (B2) and is estimated to have a lifetime cancer risk level of 10−6 at a concentration of 0.7 ng/L in drinking water. In order to reduce excessive NDMA formation, identifying potential NDMA precursors and their reactivity are crucial [39, 40]. Organic hydrazine compounds such as unsymmetrical hydrazine (UDMH) and monomethylhydrazines (MMH), being the major NDMA precursors, upon ozonation form NDMA up to 90% in molar yields [41]. They act as eco-toxicants and can form several toxic transformation products, posing severe health risks [42, 43]. In wastewater treatment plants, UDMH and MMH undergoes ozonation to produce NDMA in high yields [17, 44]. In addition, these substituted hydrazines serve as rocket propellants and thus bring about a chance to leak into the environment during transportation, storage, filling, or improper disposal of excess quantities. Since these hydrazines do not undergo photolysis in the solar actinic region (>290nm), UDMH and MMH are more likely to react with ozone in the atmosphere to form NDMA along with other toxicants. Huang et al. [41] found that the conversion rate of UDMH and MMH to NDMA on ozonation depends on the O3/MMH or UDMH ratios. With excess ozone, UDMH and MMH produce 88% and 58% of NDMA, respectively, in molar yields, making them the foremost ozone-NDMA precursors.
Apart from NDMA, other notable UDMH-ozone transformation products include formaldehyde dimethyl hydrazone (FDMH), acetaldehyde dimethyl hydrazine (ADMH), dimethylamine (DMA), tetramethyl tetrazene (TMT),N, N-dimethylformamide (DMF), formaldehyde (HCHO), and more, all of which are highly toxic and potential NDMA precursors [43]. Thus, these UDMH/MMH-ozone transformation products can be either competitors or intermediates for NDMA formation. Based on Quantitative Structure-Activity/Toxicity ((Q)SAR/(Q)STR) modeling studies, Carlsen et al. [45] ranked ADMH and TMT, along with NDMA, among the three most toxic UDMH transformation products that require significant attention. Since ozone reacts strongly and rapidly with double bonds and ADMH possesses an "-NC-" double bond, the structure "-N=C-" is modified to "-N=O″ with an average yield of 85% [46]. Kosaka et al. [43] reported 55% conversion to NDMA when 1 M of TMT reacted with 0.5 mg/L of ozone in ultrapure water (pH = 7). Recent studies suggest TMT [47] as an alternative for hydrazines in rocket fuel. Nevertheless, its conversion to NDMA could pose serious concerns. Yet, NDMA formation mechanisms from TMT and ADMH on ozonation are poorly understood. In the case of monomethyl hydrazine (MMH), previous experimental studies suggest the formation of methyldiazene and diazomethane in its reaction with ozone [48]. Diazomethane formed is a potential carcinogen and a highly poisonous substance that causes acute toxicity if inhaled or comes in direct contact with skin or eyes. Despite the fact that diazomethane is the major product in MMH-O3 reaction, NDMA constitutes the prime product when MMH is made to react with excess ozone. Still, minimal knowledge is available on the formation mechanism of methyldiazene, diazomethane, and NDMA from the MMH - Ozone reaction.
NDMA formation mechanism from hydrazines on ozonation is understood to involve the oxidation of the NH2 group of hydrazine [49]. This can occur via two pathways: the first path initiates via an H-abstraction from the hydrazine by O3 molecule to form an N-oxide radical (for UDMH) or diazene (for MMH), which subsequently forms NDMA by another H-atom abstraction or O-atom addition. The second pathway involves an initial O-addition to the –NH2 group forming an N-oxide radical, which then undergoes an H atom rearrangement facilitated by a water molecule to form NDMA [46]. Recent studies by Huang et al. [49] and Dong et al. [50] suggested the H-abstraction pathway as the more feasible route for NDMA formation during UDMH -ozone reaction. Though the formation mechanism of NDMA by UDMH-ozonation is well studied, mechanistic insights into the formation of the UDMH-ozone transformation products such as ADMH or TMT are scarce. Since the UDMH/MMH-ozone reactions undergo rapidly, it is often difficult to determine the underlying mechanism for these reactions with experimental studies alone.
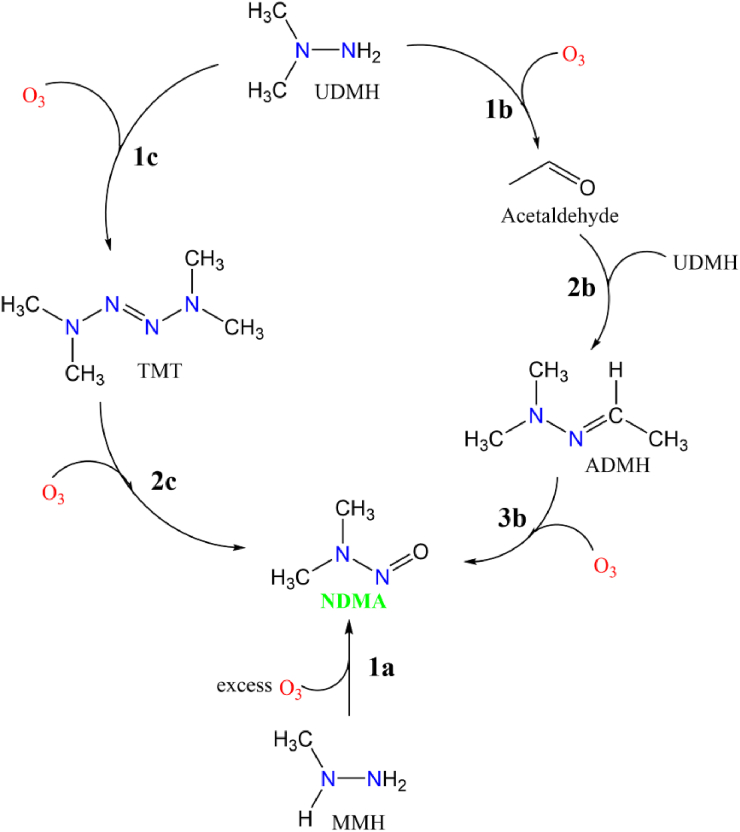
Computational modeling and electronic structure calculations complement experimental studies and are beneficial for key insights into such fast reactions. Moreover, quantum chemical calculations could provide necessary data on the reaction mechanism, plausible intermediates, and thermochemistry of these reactions, aiding in better interpretation of experimental observations. In this work, we used second-order Møller–Plesset perturbation (MP2) theory to investigate the viable formation mechanism of UDMH/MMH – ozonation to ADMH, TMT, or diazomethane and their subsequent oxidation to NDMA. Considering how frequently these substances are used annually, the atmospheric fate and environmental implications of the NDMA precursors are much significant. However little is known about the chemical processes involving these compounds. These considerations led to the objective and focus of the present study, namely to determine the initial abstraction mechanism from the parent molecule by ozone. We also aim to determine the elementary reaction mechanism of the decomposition of MMH, ADMH and TMT and thereby to estimate the thermochemical properties and rate parameters. The results are expected to deepen our comprehension of NDMA formation and aid to develop effective methods to prevent the carcinogenic NDMA generation. We explored both the hydrogen transfer and oxygen addition mechanisms to identify the most feasible mechanism for our system. Structures of the reactants and products involved in these reactions are shown in Figure 1. Scheme 1 depicts the overall mechanism of formation of NDMA from MMH, ADMH and TMT during ozonation. This was the first study conducted to assess the possible detailed mechanism for each of the three reactions by modeling both the thermodynamics and kinetics of elementary steps along the pathway. Our work aims to reveal the fundamental knowledge of specific reaction pathways, stable end products and the reaction energy profile surfaces that could aid to develop effective strategies to limit the creation of harmful transformation products during ozonation.
Figure 1.
Structures of monomethylhydrazine (MMH) 1,1- dimethylhydrazine (UDMH) and ozonation products investigated in this study.
Scheme 1.
Overall reaction mechanism of formation of NDMA from MMH, ADMH and TMT during ozonation.
2. Theoretical methods
All the geometries of the reactants, possible intermediates, products, and transition states in gaseous and solvated phase were optimized with Møller–Plesset second-order perturbation (MP2) [51] theory with the 6-311G(d) [52] level basis set. For comparison, calculations were done using other basis sets (See Supporting Information Table S1). Nonetheless, the combination of MP2 with 6-311G(d) method gives a good compromise between accuracy and computational cost. Harmonic vibrational frequency calculations were performed at the same level of theory to determine the nature of various stationary points. Open shell species were treated with an unrestricted spin manner. The effect of water as the solvent on the reaction is estimated using the implicit conductor-like polarizable continuum (CPCM) [53, 54] model. The minimum energy path (MEP) was determined using intrinsic reaction coordinate (IRC) [55] calculations to confirm transition states uniquely connected to the reactants and products. The Gibb's free reaction energy (ΔG) and activation free energy (ΔG≠) as shown in equation (i), (ii) is described below
| (i) |
| (ii) |
According to transition state theory [56, 57] (TST), the appropriate rate constant for the elementary reaction in which the reactants directly converted to the product through a concerted pathway was estimated based on Eyring- Polanyi equation as shown in equation (iii)
| (iii) |
where kB describes the Boltzmann constant, T is the temperature (unit: K), h is Planck's constant, R is the molar gas constant, and ΔG≠ (unit: kcal/mol) is the corrected activation free energy relative to the reactant complexes. All the calculations were carried out using the Gaussian 09[58] suite of programs.
3. Results and discussion
3.1. Ozonation of monomethyl hydrazine (MMH)
Ozone molecule in its ground state exists as singlet or in biradical state. The previous work shows [59] that ozone molecule bears 82% closed shell singlet structure. The electronic structure calculations of O3 reveal that the molecule has a regular closed shell singlet rather than an open singlet state [60]. In this study ozone molecule is treated as closed shell singlet molecule.
3.1.1. Formation of diazomethane from MMH
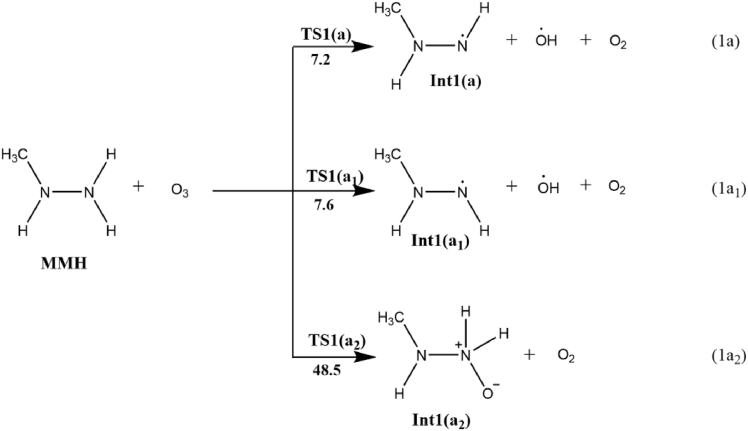
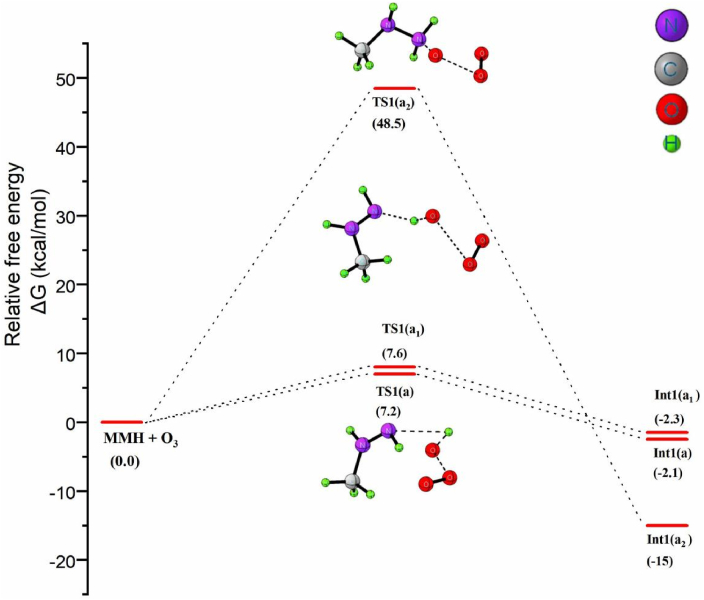
The reaction of ozone with MMH is considered to take place by hydrogen abstraction method or via oxygen addition on the –NH2 moiety as implied in Scheme 2. In the case of H abstraction the two possible pathways are either the trans H abstraction (1a) or the cis H (1a1) abstraction from the aminyl segment of MMH. As implied in Figure 2, the calculation results obtained seem that the hydrogen abstraction from NH2 moiety in methyl hydrazine is a rapid process with a potential barrier of 7 kcal/mol. It can be found that the estimated ΔG≠ value agrees well with the previous findings of ΔG≠ for the HA from –NH2 in the range of 7–15 kcal/mol50. On the other hand, an initial oxygen addition to the N atom of –NH2 moiety is unlikely to occur as it requires an energy barrier of 48.5 kcal/mol, which is significantly higher than that for the H-abstraction step.
Scheme 2.
The initial reaction between MMH and ozone (activation free energy ΔG≠, in kcal/mol).
Figure 2.
Free energy profile diagram for the initial reaction between MMH and ozone.
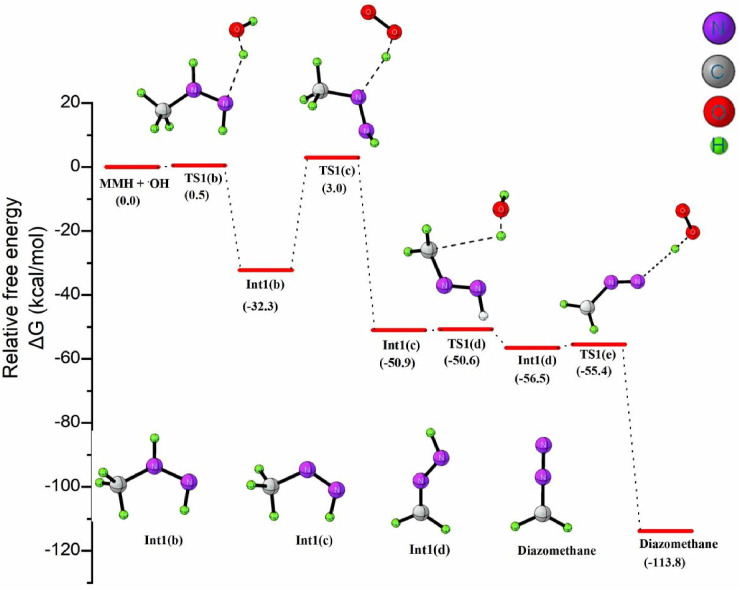
The formation of diazomethane during ozonation of MMH follows a three step route, as outlined in Scheme 3. The initial step, as previously suggested [46] involves H-atom abstraction from MMH by ozone to form N-radical species and hydroxyl radical, in the second step the reaction propagates by the oxidation of N-radical to form diazomethane and the final step presumes the recombination of active radical species. Change in enthalpy (at 298K and 1 atm) for each step of diazomethane formation pathway during the ozonation of MMH in gaseous phase and solvated phase are given in Table 1. In the initiation step, O3 abstracts an H atom from the NH2 moiety of MMH to generate Int1(a), OH radical, and triplet O2. The calculated relative free energy of singlet oxygen molecule is about 10 kcal/mol greater than that of triplet oxygen molecule. Out of the two hydrogen in –NH2 moiety, the one anti to –CH3 is easier to abstract due to less steric hindrance, and hence, the formation of Int1(a) via TS1(a) is the more feasible over Int1 (a1) in the initiation step. The OH radical abstracts the H atom from a second MMH molecule to generate Int1(b) in the propagation step (1b). The transition state (TS1(b)) for this step is only 0.5 kcal/mol above Int1(b), indicating the high feasibility of this step. TS1(b) is an actual first-order saddle point with an imaginary frequency of 206i. The active radical Int1(b) then reacts with an O2 to form the stable methyldiazene intermediate-Int1(c) and hydroperoxyl radical. Int1(c) formation is exothermic, and TS1(c) represents the rate determining transition state for the process, which has an energy of 35.3 kcal/mol. The inclusion of solvent molecule in the reaction step increases the activation barrier to 37.6 kcal/mol. On comparing equation (1c) with a similar H atom abstraction from unsubstituted N2H4, the activation barrier for both reactions is high and close, suggesting that the substituents on the diazyl group have minimal effect on the barrier, while the energy variation might be due to hyperconjugation.
Scheme 3.
Proposed reaction scheme for the overall reaction between MMH and ozone.
Table 1.
Calculated Thermodynamic Properties, gaseous and solvated Relative Change in Enthalpy (ΔH in kcal/mol) and solvated Gibbs Free Energy (ΔGsol in kcal/mol) for the optimized structures involved in the reaction mechanism of MMH + O3→Diazomethane, using MP2/6311G(d) at 298K.
| Species | ΔH | ΔGsol | ΔHsol |
|---|---|---|---|
| MMH + O3 | 0 | 0 | 0 |
| TS1(b) | −15.2 | 5.5 | −34.3 |
| Int 1(b) | 29.8 | −34.4 | −33.5 |
| TS1(c) | −3.1 | 1.2 | −1.1 |
| Int 1(c) | −48.5 | −27.9 | −37.6 |
| TS1(d) | −58.7 | −21.9 | −38.5 |
| Int 1(d) | −53.8 | −34.5 | −34.7 |
| TS1(e) | −16.8 | −35.2 | −17.1 |
| Diazomethane | −62.4 | −9.6 | −90.7 |
Further, an OH radical abstracts an H atom from Int1(c) to form the radical intermediate Int1(d), which oxidizes to diazomethane, releasing H2O and HO2 as side products as shown in equation 1d. The Int1(d) lies 56.5 kcal/mol below the reactant molecules. TS1(e) represents the transition state for the final N–H bond cleavage step, which results in CH2N2 formation. In TS1(e), the dissociating N–H bond length is 1.89 Å, and the newly formed O–H bond length of the HO2 radical is 0.98 Å. In TS1(e), homolytic cleavage of the N–H bond and the combination of H radical with oxygen molecule occur simultaneously. In this reaction step, the dissociating N–H bond length is increased by a value of 0.9 Å from the optimized equilibrium geometry. The low activation energy of 1 kcal/mol corroborates the experimentally high molar yield of diazomethane formed during the ozonation of MMH. The ΔGsol≠ in water for the reaction is negative, implying that the reaction could easily occur in water. The two hydroperoxide radicals formed during the propagation step (1f) will then combine in the termination step to form hydrogen peroxide and molecular oxygen to complete the reaction. This radical association occurs via the transition state TS1(f) (ΔG≠ = 10.4 kcal/mol). The transition state TS1(f) exhibits a simple structure of C1 symmetry and acquires an anti position to each other while approaching each other. The dissociating O–H bond length is elongated by 0.7 Å and can be easily broken. The newly formed O–H bond distance is 0.4 Å longer than the product H2O2.
Figure 3 shows that the overall reaction is exergonic with the hydrogen abstraction from Int1(b) by O2 molecule forming methyl diazene as the rate-limiting step with a calculated barrier of 35.3 kcal/mol at 298 K. The rate constant calculated for the reaction is k = 4.2 × 10−15 M−1 s−1, which is in close agreement with the experimentally determined value of 4.3 × 10-15 M−1 s−1 [61].
Figure 3.
Free energy profile diagram for the reaction between MMH and ozone.
3.1.2. Reaction of MMH with excess ozone (1a)
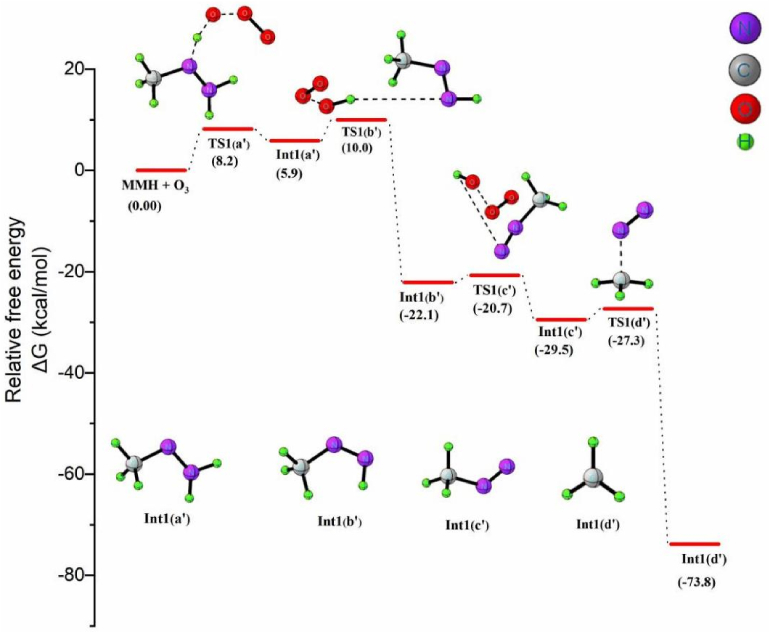
In the presence of excess ozone, monomethylhydrazine has a high potential to form NDMA because of the formation of a methyl radical in the reaction. The steps involved in this process are given in Scheme 4. The reaction begins with the H abstraction from the -NH- moiety of MMH by ozone to form Int1 (a’) as depicted in equation 1a. TS1 (a’) represents the transition state for this H-abstraction step, which requires an activation energy of 8.2 kcal/mol. Since the energy gradient is too small in the presence of excess ozone, the formation of NDMA will be more prominent than that of diazomethane production. Int1 (a’) then reacts with another O3 molecule to generate Int1 (b’), the methyl diazene molecule, which yields via the H abstraction (anti) from the terminal NH2 moiety. The activation barrier for this step that proceeds via TS1 (b’) is 4.1 kcal/mol and 5.3 kcal/mol in the gaseous and aqueous phases, respectively. Following this, another O3 molecule abstracts a second H atom from Int1 (b’) to generate the radical species Int1 (c’) via TS1 (c’). After the dissociation of the H atom, the adjacent N–N bond length in Int1 (c’) decreases to 1.15Å from 1.25Å. Int1 (c’) then undergoes a homolytic cleavage of the C–N bond to generate a methyl radical and nitrogen molecule via TS1 (d’) with an activation barrier of 2.2 kcal/mol.
Scheme 4.
The pathway of reaction between MMH and excess ozone.
As examined in Figure 4, a free energy change of −73.8 kcal/mol indicates that the formation of N2 molecule and methyl radical is highly exergonic. The methyl radical generated rapidly attacks Int1 (a’) to yield 1,2-dimethylhydrazine (UDMH) as the product. TS1 (e’) represents the transition state for the formation of UDMH from the Int1 (a’) -CH3 reaction and has an activation energy of 5.8 kcal/mol (Figure 4a in supplementary data). The methylation of Int1 (a') promotes the formation of more stable compounds such as NDMA when reacted with oxidizing agents like ozone, such that the products formed via the ozonation of MMH and UDMH are very similar. Thus the reaction of MMH with ozone initiates via the H-abstraction mechanism and proceeds to form diazomethane as the major product. Nonetheless, with excess ozone, the initial H-abstraction is followed by an oxygen addition mechanism forming NDMA as the major product. It is noticeable that the CH3 radical is the relevant reactive species for the formation of dimethylamine in MMH-O3 system.
Figure 4.
Free energy profile diagram for the reaction between MMH and excess ozone.
3.2. Ozonation of acetaldehyde dimethyl hydrazine (ADMH)
ADMH is formed during the reaction of UDMH with ozone. Initially, the UDMH ozonation generates an acetaldehyde intermediate, further reacting with UDMH to form ADMH. The generated ADMH then undergoes ozonation to yield NDMA.
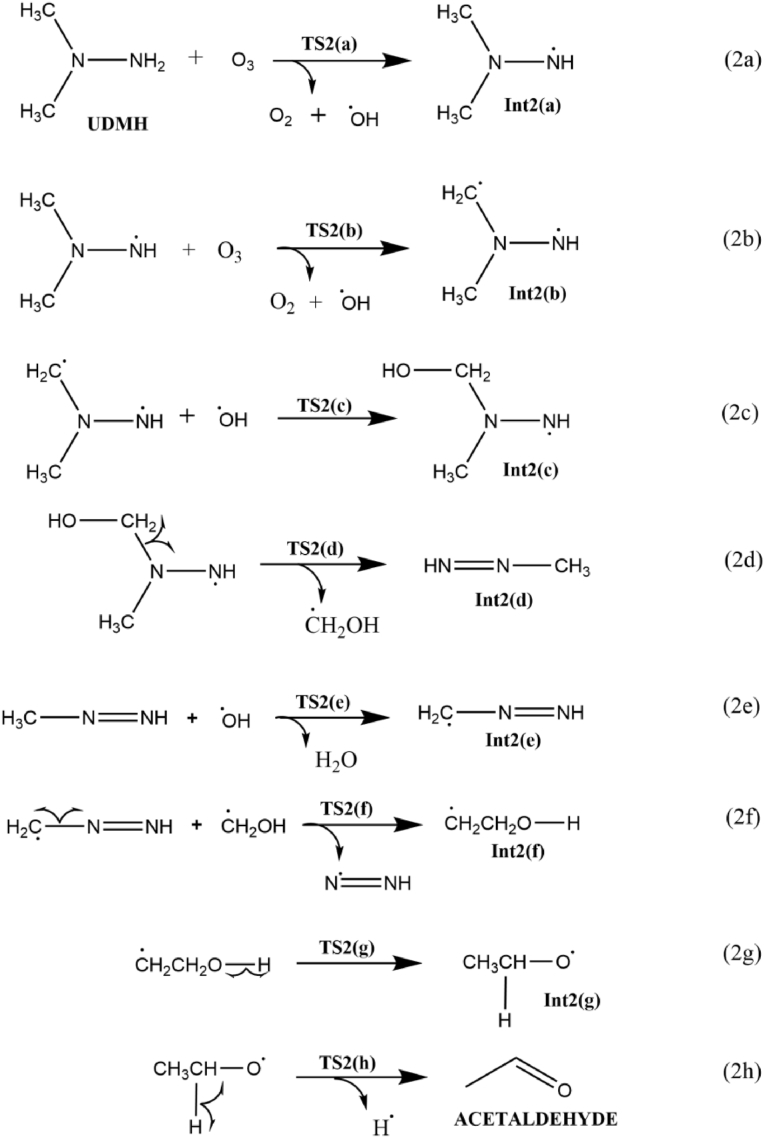
3.2.1. Acetaldehyde formation mechanisms from UDMH (1b)
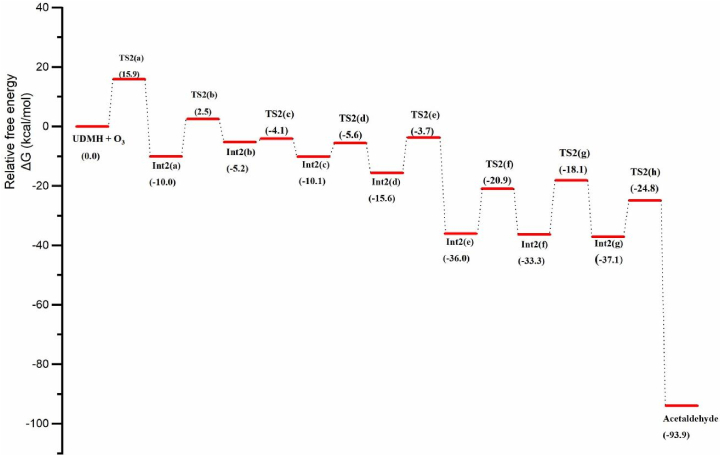
Scheme 5 represents the reaction pathway for acetaldehyde formation from UDMH-O3 reaction, Figure 5 shows the corresponding free energy profile and Figure 6 shows the optimized structures of all the transition states and intermediates involved in the reaction. During the ozonation of UDMH, the initial step (2a) begins with the abstraction of the H atom from the –NH2 moiety by ozone results in Int2(a) through the transition state TS2(a), along with the release of a oxygen molecule and hydroxyl radical. In the transition state TS2(a), the dissociating N–H bond length is 1.5Å, whereas the newly formed O–H bond length is about 1.02Å; the elementary step has an energy barrier of 15.9 kcal/mol, implies that the process is both thermodynamically and kinetically favorable. However, the inclusion of implicitly modeled water lowers the ΔGsol≠ value to 10 kcal/mol. It emphasizes the significance of water molecule in facilitating the hydrogen abstraction. Change in enthalpy (at 298K and 1 atm) for each step of the acetaldehyde formation pathway during the ozonation of UDMH is given in Table 2.
Scheme 5.
The pathway of acetaldehyde formation from UDMH - Ozonation.
Figure 5.
Free energy profile diagram for the reaction between UDMH and Ozone to form acetaldehyde.
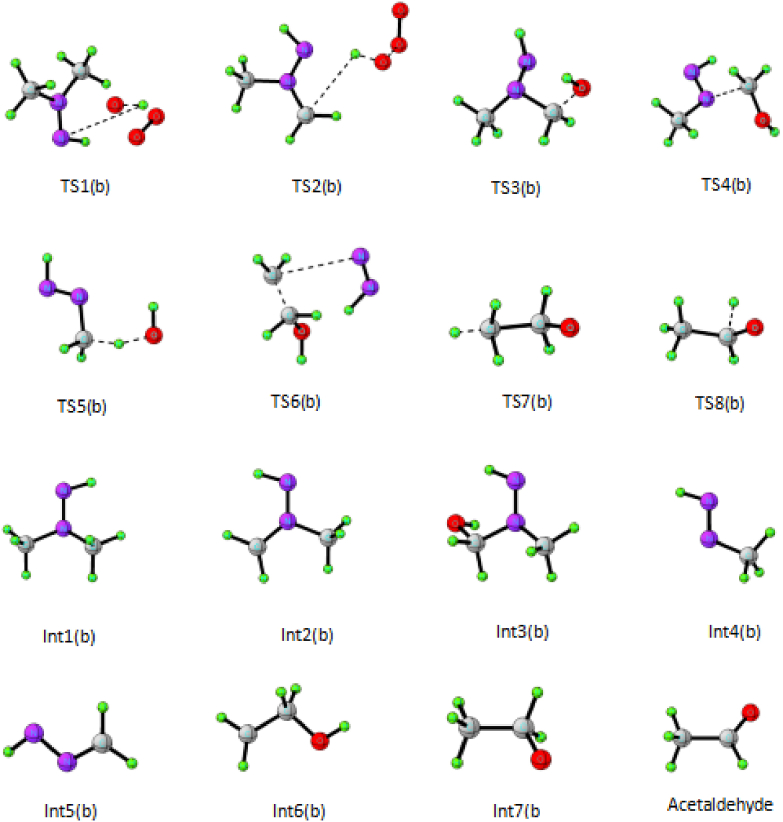
Figure 6.
Optimized geometry of transition state and intermediate involved in the reaction between UDMH and Ozone to form acetaldehyde.
Table 2.
Calculated Thermodynamic Properties, gaseous and solvated Relative Change in Enthalpy (ΔH in kcal/mol) and solvated Gibbs Free Energy (ΔGsol in kcal/mol) for the optimized structures involved in the reaction mechanism UDMH + O3 →Acetaldehyde, using MP2/6311G(d) at 298K.
| Species | ΔH | ΔGsol | ΔHsol |
|---|---|---|---|
| UDMH + O3 | 0 | 0 | 0 |
| TS2(a) | 26.5 | 10.1 | −1.7 |
| Int2(a) | −12.7 | −4.5 | 6.4 |
| TS2(b) | 37.6 | 8.7 | 11.9 |
| Int2(b) | −0.5 | −12.4 | −12.1 |
| TS2(c) | 11.3 | −11.9 | −48.5 |
| Int2(c) | −53.7 | −17.8 | −65.2 |
| TS2(d) | −10.0 | −12.2 | −19.0 |
| Int2(d) | −23.9 | −27.9 | −39.3 |
| TS2(e) | −6.1 | −16.8 | −15.5 |
| Int2(e) | −56.7 | −35.3 | −45.3 |
| TS2(f) | −42.8 | −24.2 | −81.4 |
| Int2(f) | −68.4 | −43.1 | −81.2 |
| TS2(g) | −9.6 | −39.1 | −77.9 |
| Int2(g) | −10.1 | −54.6 | −81.3 |
| TS2(h) | −46.6 | −49.4 | −59.3 |
| Acetaldehyde | −85.0 | −90.1 | −169.1 |
Subsequently, H abstraction by the O3 molecule from the methyl group of Int2(a) generates the biradical intermediate Int2(b). The ΔG value for the formation of Int2(b) in the gaseous and solvated phases are -5.2 and -12.4, respectively. It implies that the Int2(b) slightly gets stabilised in the solvated phase rather than in gas phase. The calculated energy barrier for the step 2b is 12.5 kcal/mol. The two radicals, Int2(b) and hydroxyl radical, then recombine to form intermediate Int2(c) via the transition state TS2(c). The homolytic cleavage of the C–N bond in Int2(c) generates the stable methyl diazene intermediate Int2(d) via TS2(d). The OH radical generated in the reaction medium abstracts an H radical from the methyldiazene to form the intermediate Int2(e), along with the release of a water molecule, and this step requires an activation energy of 11.9 kcal/mol. Further, the homolytic bond cleavage of the C–N bond in Int2(e) as shown in equation 2f results in the release of methylene radical. The formed CH2 radical readily combines with the hydroxyl methyl radical generating Int2(f) via the transition state TS2(f).
Following this, an intramolecular H transfer (resulting from the homolysis of the O–H bond) to the CH2 radical forms Int2(g) as illustrated in equation 2g. The transition state TS2(g) corresponding to this elementary reaction step is C1 symmetric with an O–H bond length of 1.61 Å. The removal of the H radical from Int2(g) forms acetaldehyde along with diazene via transition state TS2(h) and has an energy barrier of 12.3 kcal/mol. It should be noticed that the ΔGsol≠ and ΔGsol for the above-mentioned steps are 13 and 56 kcal/mol, indicating that the formation of acetaldehyde is favorable in both thermodynamic and kinetic aspects. Furthermore, Figure 5 shows that the energy barrier for all the steps involved in the formation of acetaldehyde is within 15 kcal/mol, which indicates the feasibility of the reaction under the general atmospheric condition. Hence, the low barrier explains why experimentally high amounts of acetaldehyde are formed during UDMH ozonation.
3.2.2. ADMH formation from acetaldehyde (2b)
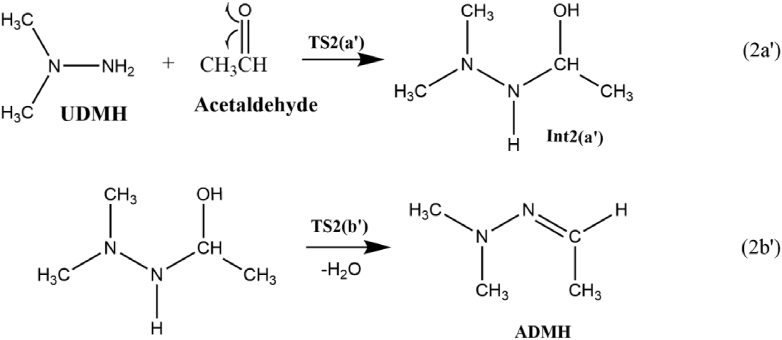
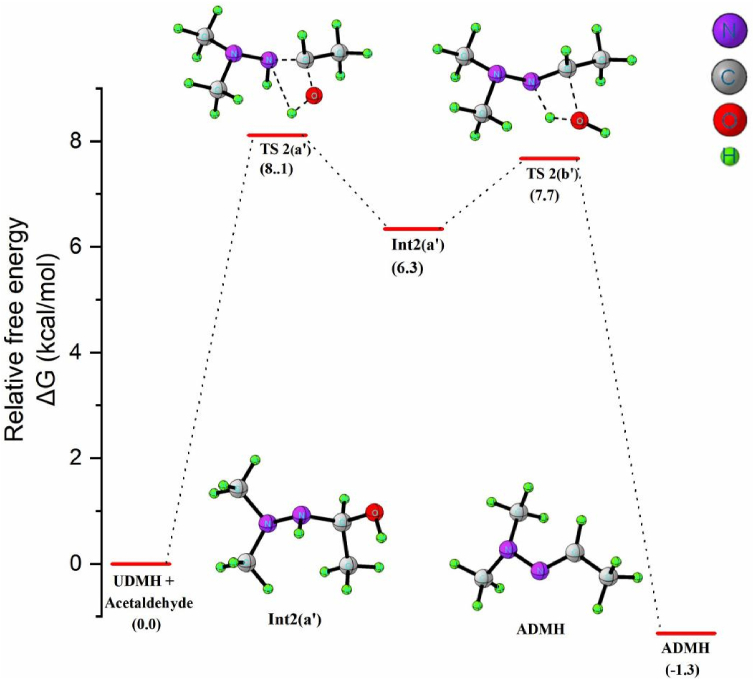
The acetaldehyde generated can react with UDMH to form ADMH. The proposed reaction mechanism is shown in Scheme 6. The reaction initiates by an intermolecular H radical transfer from –NH2 moiety of UDMH to the oxygen atom of carbonyl carbon in acetaldehyde by the simultaneous homolytic dissociation of N–H and C–O bond and generates Int2 (a'). The reaction step 2a’ proceeds via TS2 (a'). The change in the bond distance shows that TS2 (a') is a reactant-like transition state, and the process needs a potential barrier of 8.1 kcal/mol as demonstrated in Figure 7. Subsequently, the intramolecular condensation reaction within Int2 (a') releases a water molecule and forms ADMH as the product via TS2 (b'). TS2 (b') exhibits a simple structure of C1 symmetry, in which the C–N bond length is shortened to 1.33 Å from 1.5 Å and can be easily formed. The estimated ΔG≠ for the step is only 1.4 kcal/mol, which could be accounted as a barrier less rapid process. The incorporation of the water molecule as the solvent accelerates the formation of ADMH by decreasing the potential barrier to 1.1 kcal/mol. Relative to the reactants, the total energy of the products is -1.3 kcal/mol implying that the overall reaction is exergonic in nature.
Scheme 6.
The pathway of ADMH formation from the reaction between UDMH with Acetaldehyde.
Figure 7.
Free energy profile diagram for the reaction between UDMH and Acetaldehyde to form ADMH.
3.2.3. NDMA formation mechanisms from ADMH (3b)
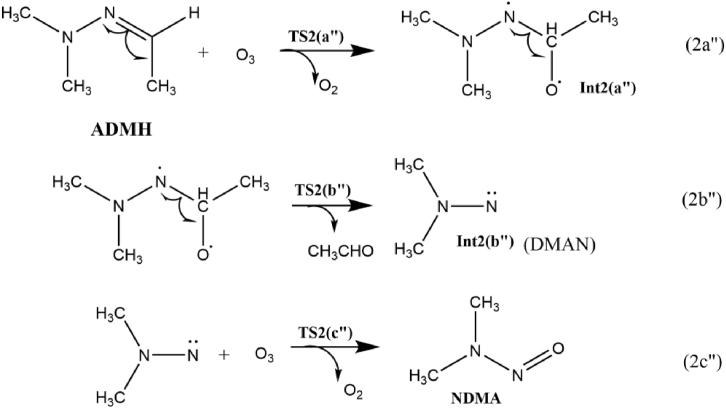
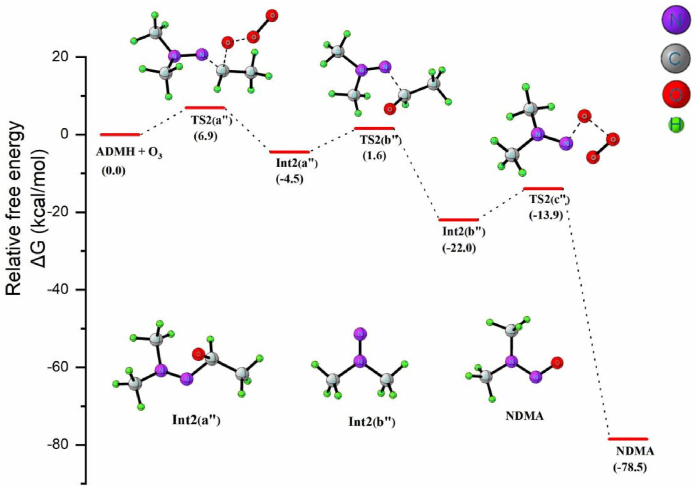
Scheme 7 represents NDMA formation mechanism from ADMH-O3 reaction, and the Figure 8 shows the corresponding free energy profile. As shown in Scheme7, the proposed NDMA formation pathway in the ozonation of ADMH is an oxygen addition mechanism (2a”). Unlike UDMH, the addition site of ADMH is the C atom rather than the N atom of –N=C. Calculated Mulliken charges are shown in Figure S1 (Supplementary Information). The determined Mulliken charges show that, in ADMH the C atom of the –N=C moiety especially in the transition states has lesser electron density than the N atom, which implies that the C atom is the more preferable attacking site for the ozone molecule and the reaction forms Int2 (a") as the product.
Scheme 7.
The pathway of NDMA formation from ADMH ozonation.
Figure 8.
Free energy profile diagram for the reaction between ADMH and Ozone to form NDMA.
Following the formation of Int2 (a”), as shown in equation 2b” the release of acetaldehyde along with the formation of highly reactive N,N-dimethylaminonitrene (DMAN) intermediate Int2 (b”) takes place via the transition state TS2 (b”). As depicted in Figure 8, the values of ΔG≠ for the above steps are only 6.9 and 6.1 kcal/mol, indicating that these steps are thermodynamically feasible and kinetically favorable. The reactive DMAN then gets oxidized by ozone to form NDMA (2c’’). The formation of NDMA is a highly exothermic reaction with ΔG of -78.5 kcal/mol. The estimated potential barrier is within the range of 6–14 kcal/mol. Therefore the reaction seems to occur under general atmospheric conditions.
Taking all the reaction steps from the ozone attack on UDMH to the formation of NDMA, the initial attack of O3 on UDMH to generate Int2(a) in the initial reaction serves as the rate-limiting step for the process. The activation energy for this step is 15.9 kcal/mol. The estimated rate constant for the reaction is 2.24×101 M−1 s−1. The formation of NDMA from ADMH is a three-step sequential process. The initial step is the generation of acetaldehyde via the H- abstraction mechanism from UDMH by the attack of ozone. The subsequent reaction of the generated acetaldehyde with UDMH forms the transformation product; ADMH. In the final reaction sequence, NDMA is formed from ADMH via the oxygen addition mechanism. For the entire steps, the inclusion of water molecule in the reaction step decreases the potential barrier.
3.3. NDMA formation mechanisms from TMT
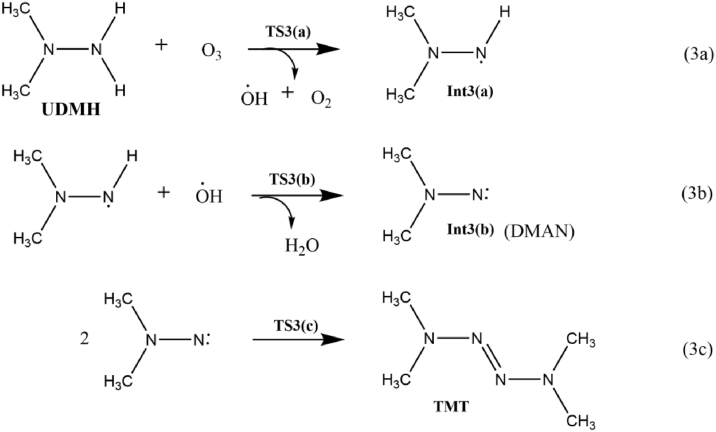
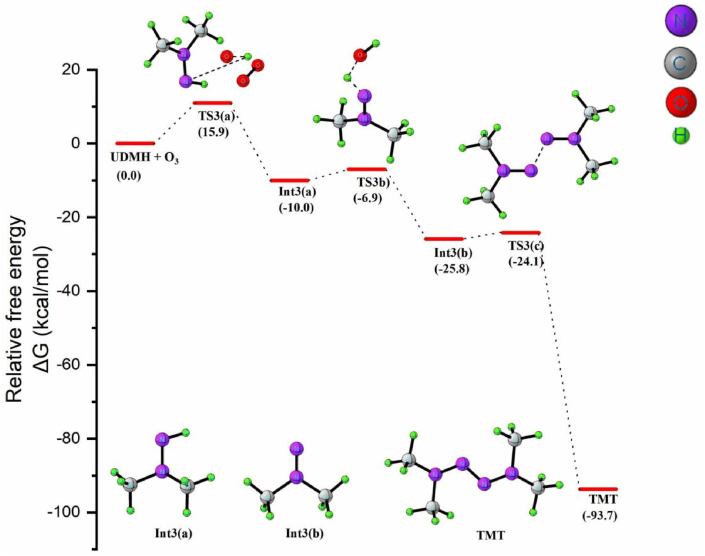
3.3.1. TMT formation from UDMH (1c)
As shown in equation 3a in Scheme 8, the transformation of UDMH to TMT begins by the attack of O3 molecule on the H atom of –NH2 moiety of UDMH. The reaction results in the formation of Int3(a) via transition state TS3(a). The second H abstraction is a rapid process with a low energy barrier (3.1 kcal/mol). In this reaction step 3b, the H atom of –NH moiety is oriented towards the OH radical or away from the hydrazine species. Such an orientation results in radical recombination and prompted to the formation of N,N-dimethylaminonitrene (DMAN) intermediate Int3(b) with the simultaneous elimination of water molecule. TS3(b) represents the transition state for the process. In TS3(b), the dissociating N–H bond length is 1.4Å, and the newly formed O–H bond distance is 1.1Å. The ΔGsol≠ and ΔGsol for the specific reaction is 2 and -50 kcal/mol, showing that the presence of water molecule expedites the formation of DMAN.
Scheme 8.
The formation of TMT from the ozonation of UDMH.
The DMAN intermediate could undergo different reactions. The oxygen addition mechanism in DMAN could generate an intermediate Me2NNOO, and the release of a triplet oxygen atom from this intermediate forms NDMA, while the stoichiometric dimerization of Int3(b) yields TMT. The reaction 3c proceeds via TS3(c), in which the associating molecules align in a trans position to each other. As shown in Figure 9, the calculated activation energy for the reaction is 1.7 kcal/mol, which can be regarded as a barrierless process. The low energy barrier clearly indicates that the reaction is thermodynamically feasible process. The overall reaction is highly exergonic by 93.7 kcal/mol and exothermic by 104.9 kcal/mol. Changes in enthalpy (at 298K and 1 atm) for each step of TMT formation pathway during the ozonation of UDMH are given in Table 3. The computed harmonic vibrational frequencies of the optimized TMT are in relatively good agreement with the experimental values [47]. The most notable band observed in the vibrational spectra of TMT is the N=N azo bridge stretch which is observed at 1468cm−1, and the symmetric stretch of C–N at 1302 cm−1.
Figure 9.
Free energy profile diagram for the formation of TMT by reaction between UDMH and Ozone.
Table 3.
Calculated Thermodynamic Properties, gaseous and solvated Relative Change in Enthalpy (ΔH in kcal/mol), and solvated Gibbs Free Energy (ΔGsol in kcal/mol) for the optimized structures involved in the reaction mechanism UDMH + O3 →TMT, using MP2/6311G(d) at 298 K.
| Species | ΔH | ΔGsol | ΔHsol |
|---|---|---|---|
| UDMH + O3 | 0 | 0 | 0 |
| TS3(a) | 26.5 | 10.1 | −1.7 |
| Int 3(a) | −12.7 | −4.5 | 6.4 |
| TS3(b) | −14.5 | −2.2 | −21.7 |
| Int 3(b) | −38.5 | −54.6 | −48.8 |
| TS3(c) | −32.8 | −39.8 | −36.7 |
| TMT | −104.9 | −105.9 | −105.2 |
3.3.2. NDMA formation from TMT (2c)
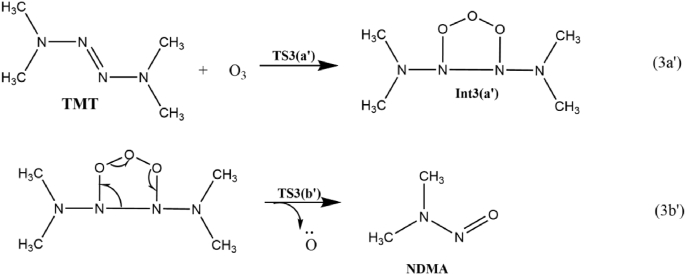
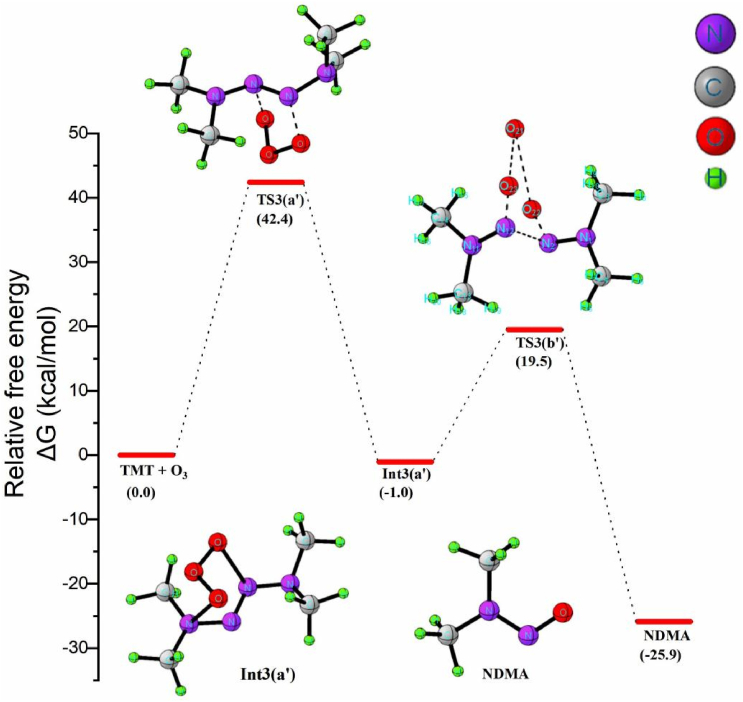
Little is known about the complete chemistry of NDMA formation from TMT-O3 reaction after the initial attack of ozone. The most important decay channel of symmetric tetramethylazene with ozone to form NDMA is shown in Scheme 9. The formation of nitrosamine from TMT involves two elementary steps:1) the addition of ozone to N=N bond followed by the cleavage of adduct intermediate to form NDMA. The ground state structure of TMT was found to be Ci symmetric, and of ozone is C2V. In the transition state structure TS1, the methyl groups become unsymmetric, achievingC1symmetry, and the central oxygen atom of O3 is out of the plane. As illustrated in equation 3a′, the TS3 (a') leads to the formation of a less stable ozonide intermediate where two new N–O bonds are formed. As shown in Figure 10, our calculations indicate that the reaction requires to cross a barrier of 42.4 kcal/mol to proceed, which suggests why NDMA yields are low during TMT ozonation. The high activation energy for the elementary step could be credited to the steric effects of the four bulky –CH3 groups placed at the two terminals of the azene molecule and the high bond dissociation energy of N=N. The energy of the ozonide intermediate is 1 kcal/mol lower than the total energy of the reactant molecules, and the reaction step is exothermic. The formation of NDMA takes place through the transition state TS3 (b') with the bifurcation of the reactive intermediate associated with the dissociation of the non-planar central oxygen atom and the azene covalent bond. In TS3 (b') the N–O bond is almost the same as the equilibrium structure of NDMA and is equal to a value of 1.2A0. The N2–N6,O3–O4 and O4–O5 bonds being broken to form the products are elongated by 33.2%, 45.1% and 42.5%, respectively. The TS3 (b') has a dihedral angle of 2° between the N2 and the O5 atom, where the atoms are almost in a plane. The potential barrier in the gas phase of the step is calculated to be 20.5 kcal/mol and the reaction is highly exothermic. The ΔGsol≠ estimated for the step is 17.3 kcal/mol. This insinuated that the reaction is relatively more easier to take place in the solvated phase. Taking all the steps of formation of NDMA from TMT, the formation of Int3 (a’) during the ozonation of TMT is determined as the rate limiting step with ΔG≠ of 42.4 kcal/mol. The estimated rate constant for the reaction is about 8.9 × 10−19 M−1 s−1. Relatively high energy barrier is consistent with an experimentally low yield of formation of NDMA during TMT ozonation. Therefore it seems that two consecutive H abstractions from –NH2 moiety in UDMH via ozonation leads to less stable DMAN intermediate. Dimerization of DMAN generates the TMT. The subsequent reaction of TMT with ozone forms NDMA via an ozonide intermediate. The formation of NDMA from TMT ozonation is unfavorable in the typical atmospheric condition due to high activation energy attributed from the steric hindrance of the bulky methyl groups on the terminals.
Scheme 9.
The formation of NDMA from the ozonation of TMT.
Figure 10.
Free energy profile diagram for the formation of NDMA from the ozonation of TMT.
4. Conclusion
In this study, NDMA formation mechanism during ozonation from the hydrazines like MMH and the transformation products of UDMH such as ADMH and TMT were investigated for the first time using MP2 theory with the 6-311G(d) basis set. The reaction of ozone with MMH results in the formation of highly toxic diazomethane and methyl diazene. The ozone-MMH reaction initiates with an H- abstraction step. The lower activation energy for the elementary steps indicates that the reaction could occur under the general atmospheric conditions and the calculated rate and experimentally determined values are in complete agreement. In the presence of excess ozone, the formation of NDMA from MMH occurs via a CH3 radical formation. In the subsequent reaction, the generated methyl radicals account for the high yields of the observed dimethylamine groups. The reaction is thermodynamically viable since the potential barriers of the reaction steps are low.
Ozonation of UDMH can produce several other N, N -dimethylamino compounds such as ADMH and TMT, which are also competitors for NDMA. These transformation products can, in turn, react with ozone to produce NDMA. UDMH ozonation produces acetaldehyde in the initial stage, which further reacts with UDMH to form ADMH. For ADMH, the C atom of the –N=C moiety in hydrazine is preferred to be attacked by ozone to generate DMAN, a key intermediate in NDMA formation during ozonation, and DMAN gets easily oxidized by ozone to form NDMA. The relatively low activation free energy (being around 6 kcal/mol) for NDMA formation from ADMH agrees well with the high experimental reaction rate and high NDMA conversion yield.
The two consecutive H abstractions from the NH2 moiety in UDMH via oxidation with ozone leads to the less stable reactive intermediate DMAN. The association of two molecules of DMAN intermediate generates the tetramethyl tetrazene as the major product. The thermochemistry of the reaction is studied, and the activation Gibbs free energy for the elementary step is in the range of 4–17 kcal/mol, which could take place in the atmospheric temperature range. Based on our study and previous experimental observations, the formation of NDMA from TMT is not favorable under general atmospheric conditions, even though there are two N, N-Dimethyl hydrazine functional groups that can undergo ozonation. This unfavorability can be attributed to the high activation energy required to form a five-membered ozonide intermediate in the process. The relatively high potential barrier arises due to the bulky methyl groups on the terminals and the high energy required for the dissociation of –N=N bonds. In general, MMH forms NDMA by hydrogen abstraction followed by an oxygen addition mechanism. The transformation products of UDMH-ozonation reaction; ADMH and TMT, are generated by the H- abstraction mechanism. The subsequent ozonation of these transformation products via the oxygen addition mechanism forms highly toxic NDMA.
Author contribution statement
Rehin Sulay: Conceptualization,Methodology, Formal analysis; Wrote the paper.
Jintumol Mathew: Writing-review and editing, Conceptualization, Methodology.
Anandhu Krishnan: Methodology
Vibin Ipe Thomas: Conceptualization, Visualization and Supervision.
Funding statement
This work was supported by University Grants Commission (Grant no. 2368-MRP/15-16/KLMG002/UGC-SWRO).
Data availability statement
Data will be made available on request.
Declaration of interest's statement
The authors declare no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e14511.
Abbreviations
MMH - Mono Methyl Hydrazine UDMH - Unsymmetrical Dimethyl Hydrazine ADMH - Acetaldehyde Dimethyl Hydrazine TMT - Tetra Methyl Tetrazene NDMA - N-Nitroso Dimethyl Amine DMAN - Dimethyl Amino Nitrene.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Barnes J.M., Magee P.N. 1954. Some Toxic Properties of Dimethylnitrosamine by. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heath D.F., Magee P.N. Toxic properties of dialkylnitrosamines and some related compounds. Occup. Environ. Med. 1962 doi: 10.1136/oem.19.4.276. [DOI] [Google Scholar]
- 3.Tricker A.R., Preussmann R. Carcinogenic N-nitrosamines in the diet: occurrence, formation. Mechanisms and Carcinogenic Potential. 1991;259:277–289. doi: 10.1016/0165-1218(91)90123-4. [DOI] [PubMed] [Google Scholar]
- 4.Shah A.D., Mitch W.A. 119–131. 2012. A Critical Review of Nitrogenous Disinfection Byproduct Formation Pathways. [DOI] [PubMed] [Google Scholar]
- 5.Beard J.C., Swager T.M. An organic chemist’s guide to N-nitrosamines: their structure, reactivity, and role as contaminants. J. Org. Chem. 2021 doi: 10.1021/acs.joc.0c02774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rawksworth G.M., Hill M.J. Bacteria and the N-nitrosation of secondary amines. Br. J. Cancer. 1971 doi: 10.1038/bjc.1971.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakajima M., Warner J.C., Anselme J.P. N-nitrosamines via the phase-transfer mediated nitrosation of secondary amines with sodium nitrite and n-haloamides. Tetrahedron Lett. 1984 doi: 10.1016/S0040-4039(01)81245-4. [DOI] [Google Scholar]
- 8.Zhao Y.L., Garrison S.L., Gonzalez C., Thweatt W.D., Marquez M. N-nitrosation of amines by NO2 and no: a theoretical study. J. Phys. Chem. A. 2007 doi: 10.1021/jp0677703. [DOI] [PubMed] [Google Scholar]
- 9.Andrzejewski P., Kasprzyk-Hordern B., Nawrocki J. N-nitrosodimethylamine (NDMA) formation during ozonation of dimethylamine-containing waters. Water Res. 2008 doi: 10.1016/j.watres.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 10.Padhye L., Wang P., Karanfil T., Huang C.H. Unexpected role of activated carbon in promoting transformation of secondary amines to N -nitrosamines. Environ. Sci. Technol. 2010 doi: 10.1021/es903916t. [DOI] [PubMed] [Google Scholar]
- 11.Bond T., Templeton M.R. 2011. Nitrosamine Formation from the Oxidation of Secondary Amines; pp. 259–265. [DOI] [Google Scholar]
- 12.Lim S., Lee W., Na S., Shin J., Lee Y. N-nitrosodimethylamine (NDMA) formation during ozonation of N,N-dimethylhydrazine compounds: reaction kinetics, mechanisms, and implications for NDMA formation control. Water Res. 2016 doi: 10.1016/j.watres.2016.08.054. [DOI] [PubMed] [Google Scholar]
- 13.Keeper L.K., Roller P.P. N -nitrosation by nitrite ion in neutral and basic medium. Science. 1973;181(4106):1245–1247. doi: 10.1126/science.181.4106.1245. [DOI] [PubMed] [Google Scholar]
- 14.Glasson W.A. Vol. 13. 1979. An Experimental Evaluation of Atmospheric Nitrosamine Formation; pp. 1145–1146. [Google Scholar]
- 15.Hutchings J.W., Ervens B., Straub D., Herckes P. N-nitrosodimethylamine occurrence, formation and cycling in clouds and fogs. Environ. Sci. Technol. 2010 doi: 10.1021/es101698q. [DOI] [PubMed] [Google Scholar]
- 16.Choi N.R., Ahn Y.G., Lee J.Y., Kim E., Kim S., Park S.M., Song I.H., Shin H.J., Kim Y.P. 2021. Particulate Nitrosamines and Nitramines in Seoul and Their Major Sources : Primary Emission versus Secondary Formation. [DOI] [PubMed] [Google Scholar]
- 17.Mitch W.A., Sharp J.O., Trussell R.R., Valentine R.L., Alvarez-Cohen L., Sedlak D.L. Environmental Engineering Science; 2003. N-Nitrosodimethylamine (NDMA) as a Drinking Water Contaminant: A Review. [DOI] [Google Scholar]
- 18.Nawrocki J., Andrzejewski P. Nitrosamines and water. J. Hazard Mater. 2011;189(1–2):1–18. doi: 10.1016/j.jhazmat.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Bond T., Templeton M.R., Graham N. Precursors of nitrogenous disinfection by-products in drinking water –– A critical review and analysis. J. Hazard Mater. 2012;235–236:1–16. doi: 10.1016/j.jhazmat.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 20.Krasner S.W., Mitch W.A., Mccurry D.L., Hanigan D., Westerhoff P. Formation , precursors , control , and occurrence of nitrosamines in drinking water : a review. Water Res. 2013;47(13):4433–4450. doi: 10.1016/j.watres.2013.04.050. [DOI] [PubMed] [Google Scholar]
- 21.Sgroi M., Vagliasindi F.G.A., Snyder S.A., Roccaro P. N-nitrosodimethylamine (NDMA) and its precursors in water and wastewater: a review on formation and removal. Chemosphere. 2018 doi: 10.1016/j.chemosphere.2017.10.089. [DOI] [PubMed] [Google Scholar]
- 22.Vocht F. De, Burstyn I., Straif K., Vermeulen R., Jakobsson K., Nichols L., Peplonska B., Taeger D., Kromhout H. 2007. Occupational Exposure to NDMA and NMor in the European Rubber Industry W. [DOI] [PubMed] [Google Scholar]
- 23.Crews C. 2010. The Determination of N- Nitrosamines in Food. No. 1975. [DOI] [Google Scholar]
- 24.Herrmann S.S., Duedahl-Olesen L., Granby K. Occurrence of volatile and non-volatile N-nitrosamines in processed meat products and the role of heat treatment. Food Control. 2015 doi: 10.1016/j.foodcont.2014.05.030. [DOI] [Google Scholar]
- 25.Eisenbrand G., Spiegelhalder B., Kann J., Klein R., Preussmann R. Arzneimittel-Forschung/Drug Res.; 1979. Carcinogenic N-Nitrosodimethylamine as a Contamination in Drugs Containing 4-Dimethylamino-2,3-Dimethyl-1-Phenyl-3-Pyrazolin-5-One (Aminopyrine, Aminophenazone) [DOI] [PubMed] [Google Scholar]
- 26.Parr M.K., Joseph J.F. NDMA impurity in valsartan and other pharmaceutical products: analytical methods for the determination of N-nitrosamines. J. Pharmaceut. Biomed. Anal. 2019 doi: 10.1016/j.jpba.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 27.Snodin D.J., Elder D.P. Short commentary on NDMA (N-nitrosodimethylamine) contamination of valsartan products. Regul. Toxicol. Pharmacol. 2019 doi: 10.1016/j.yrtph.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 28.White C.M. Understanding and preventing (N-nitrosodimethylamine) NDMA contamination of medications. Ann. Pharmacother. 2020 doi: 10.1177/1060028019892222. [DOI] [PubMed] [Google Scholar]
- 29.Zmysłowski A., Książek I., Szterk A. N-nitrosodimethylamine contamination in the metformin finished products. Molecules. 2020 doi: 10.3390/molecules25225304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogbede J.U., Giaever G., Nislow C. A genome-wide portrait of pervasive drug contaminants. Sci. Rep. 2021 doi: 10.1038/s41598-021-91792-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner J.A., Dinh J.C., Colombo J.M., Gold B.D. Is this the end for ranitidine. NDMA Presence Continues to Confound. 2021 doi: 10.1111/cts.12995. No. January, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fritzsche M., Blom G., Keitel J., Goettsche A., Seegel M., Leicht S., Guessregen B., Hickert S., Reifenberg P., Cimelli A., Baranowski R., Desmartin E., Barrau E., Harrison M., Bristow T., Neill N.O., Kirsch A., Krueger P., Saal C., Mouton B., Schlingemann J. European journal of pharmaceutical sciences NDMA analytics in metformin products : comparison of methods and pitfalls. Eur. J. Pharmaceut. Sci. 2022;168 doi: 10.1016/j.ejps.2021.106026. [DOI] [PubMed] [Google Scholar]
- 33.Richardson S.D., Plewa M.J., Wagner E.D., Schoeny R., Demarini D.M. Occurrence, genotoxicity, and carcinogenicity disinfection by-products a review and roadmap. Mutat. Res. 2007 doi: 10.1016/j.mrrev.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Berger M.R., Schmähl D., Zerban H. Combination experiments with very low doses of three genotoxic N-nitrosamines with similar organotropic carcinogenidty in rats. Carcinogenesis. 1987 doi: 10.1093/carcin/8.11.1635. [DOI] [PubMed] [Google Scholar]
- 35.Bartsch H., Hietanen E., Malaveille C. Carcinogenic nitrosamines: free radical aspects of their action. Free Radic. Biol. Med. 1989 doi: 10.1016/0891-5849(89)90144-5. [DOI] [PubMed] [Google Scholar]
- 36.Pool-Zobel B.L., Klein R.G., Liegibel U.M., Kuchenmeister F., Weber S., Schmezer P. Systemic genotoxic effects of tobacco-related nitrosamines following oral and inhalational administration to sprague-dawley rats. Clin. Invest. 1992 doi: 10.1007/BF00184666. [DOI] [PubMed] [Google Scholar]
- 37.Lin J.K. Nitrosamines as Potential Environmental Carcinogens in Man. 1990;23(1):67–71. doi: 10.1016/0009-9120(90)90489-h. [DOI] [PubMed] [Google Scholar]
- 38.Taniguchi M., Yasutake A., Takedomi K., Inoue K. Effects of N-nitrosodimethylamine (NDMA) on the oxidative status of rat liver. Arch. Toxicol. 1999 doi: 10.1007/s002040050598. [DOI] [PubMed] [Google Scholar]
- 39.Bates R. Organic Synthesis Using Transition Metals. 2012. Coupling reactions. [DOI] [Google Scholar]
- 40.Kadmi Y., Favier L., Wolbert D. 2015. N-nitrosamines , Emerging Disinfection By-Products of Health Concern : an Overview of Occurrence , Mechanisms of Formation , Control and Analysis in Water. [DOI] [Google Scholar]
- 41.Huang D., Liu X., Xie Z., Wang X., Gao X., Yang Y. Products and mechanistic investigations on the reactions of hydrazines with ozone in gas-phase. Symmetry (Basel) 2018;10(9) doi: 10.3390/sym10090394. [DOI] [Google Scholar]
- 42.Ul’yanovskii N.V., Lakhmanov D.E., Pikovskoi I.I., Falev D.I., Popov M.S., Kozhevnikov A.Y., Kosyakov D.S. Migration and transformation of 1,1-dimethylhydrazine in peat bog soil of rocket stage fall site in Russian north. Sci. Total Environ. 2020;726 doi: 10.1016/j.scitotenv.2020.138483. [DOI] [PubMed] [Google Scholar]
- 43.Kosaka K., Fukui K., Kayanuma Y., Asami M., Akiba M. N-nitrosodimethylamine formation from hydrazine compounds on ozonation. Ozone Sci. Eng. 2014;36(3):215–220. doi: 10.1080/01919512.2014.875765. [DOI] [Google Scholar]
- 44.Gunten U. Von, Salhi E., Schmidt C.K., Arnold W.A. Kinetics and mechanisms of N -nitrosodimethylamine formation upon ozonation of N, N -Dimethylsulfamide-Containing waters: bromide catalysis. Environ. Sci. Technol. 2010;44(15):5762–5768. doi: 10.1021/es1011862. [DOI] [PubMed] [Google Scholar]
- 45.Carlsen L., Kenessov B.N., Batyrbekova S.Y. A QSAR/QSTR study on the human health impact of the rocket fuel 1,1-dimethyl hydrazine and its transformation products. Multicriteria hazard ranking based on partial order methodologies. Environ. Toxicol. Pharmacol. 2009;27(3):415–423. doi: 10.1016/j.etap.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y.D., Zhong R. Comparison of N-nitrosodimethylamine formation mechanisms from dimethylamine during chloramination and ozonation: a computational study. J. Hazard Mater. 2017;321:362–370. doi: 10.1016/j.jhazmat.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 47.Dhenain A., Darwich C., Sabaté C.M., Le D.M., Bougrine A.J., Delalu H., Lacôte E., Payen L., Guitton J., Labarthe E., Jacob G.(E. -1,1,4,4-Tetramethyl-2-Tetrazene (tmtz): a prospective alternative to hydrazines in rocket propulsion. Chem. Eur J. 2017;23(41):9897–9907. doi: 10.1002/chem.201701468. [DOI] [PubMed] [Google Scholar]
- 48.Tuazon E.C., Carter W.P.L., Winer A.M., Pitts J.N. Reactions of hydrazines with ozone under simulated atmospheric conditions. Environ. Sci. Technol. 1981;15(7):823–828. doi: 10.1021/es00089a008. [DOI] [Google Scholar]
- 49.Huang D., Liu X., Wang X., Zuo C., Xie Z., Gao X. The competitive formation mechanism of N-nitrosodimethylamine and formaldehyde dimethylhydrazone from 1,1-dimethylhydrazine during ozonation in air: a combined theoretical and experimental study. Chem. Phys. 2019;522:220–227. doi: 10.1016/j.chemphys.2019.01.011. [DOI] [Google Scholar]
- 50.Dong M., Liu Y.D., Zhong R. NDMA formation mechanisms from typical hydrazines and hydrazones during ozonation: a computational study. J. Hazard Mater. 2019;366:370–377. doi: 10.1016/j.jhazmat.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 51.Pulay P., Saebø S. Orbital-invariant formulation and second-order gradient evaluation in møller-Plesset perturbation theory. Theor. Chim. Acta. 1986;69(5–6):357–368. doi: 10.1007/BF00526697. [DOI] [Google Scholar]
- 52.Krishnan R., Binkley J.S., Seeger R., Pople J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980;72(1):650–654. doi: 10.1063/1.438955. [DOI] [Google Scholar]
- 53.Tomasi J., Persico M. Molecular interactions in solution: an overview of methods based on continuous distributions of the solvent. Chem. Rev. 1994;94(7):2027–2094. doi: 10.1021/cr00031a013. [DOI] [Google Scholar]
- 54.Takano Y., Houk K.N. Benchmarking the conductor-like polarizable continuum model (CPCM) for aqueous solvation free energies of neutral and ionic organic molecules. J. Chem. Theor. Comput. 2005;1(1):70–77. doi: 10.1021/ct049977a. [DOI] [PubMed] [Google Scholar]
- 55.Gonzalez C., Bernhard Schlegel H. An improved algorithm for reaction path following. J. Chem. Phys. 1989;90(4):2154–2161. doi: 10.1063/1.456010. [DOI] [Google Scholar]
- 56.Prigogine I. Chemical kinetics and dynamics. Ann. N. Y. Acad. Sci. 2003;988:128–132. doi: 10.1111/j.1749-6632.2003.tb06091.x. [DOI] [PubMed] [Google Scholar]
- 57.Hill T.L. Dover Publication; Newyork: 1986. An Introduction to Statstical Thermodynamics. [Google Scholar]
- 58.27. Gaussian 09, Revision A.02. Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Scalmani G., Barone V., Petersson G.A., Nakatsuji H., Li X., Caricato M., Marenich A., Bloino J., Janesko B.G., Gomperts R., Menn B. 2009. No Title. [Google Scholar]
- 59.Miliordos E., Xantheas S.S. On the bonding nature of ozone (O 3 ) and its sulfur-substituted analogues SO 2 , OS 2 , and S 3 : correlation between their biradical character and molecular properties. J. Am. Chem. Soc. 2014;136(7):2808–2817. doi: 10.1021/ja410726u. [DOI] [PubMed] [Google Scholar]
- 60.Kalemos A., Mavridis A. Electronic structure and bonding of ozone. J. Chem. Phys. 2008;129(5) doi: 10.1063/1.2960629. [DOI] [PubMed] [Google Scholar]
- 61.Richters S., Berndt T. 2013. Gas-Phase Reaction of Monomethylhydrazine with Ozone : Kinetics and OH Radical Formation. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.