Abstract
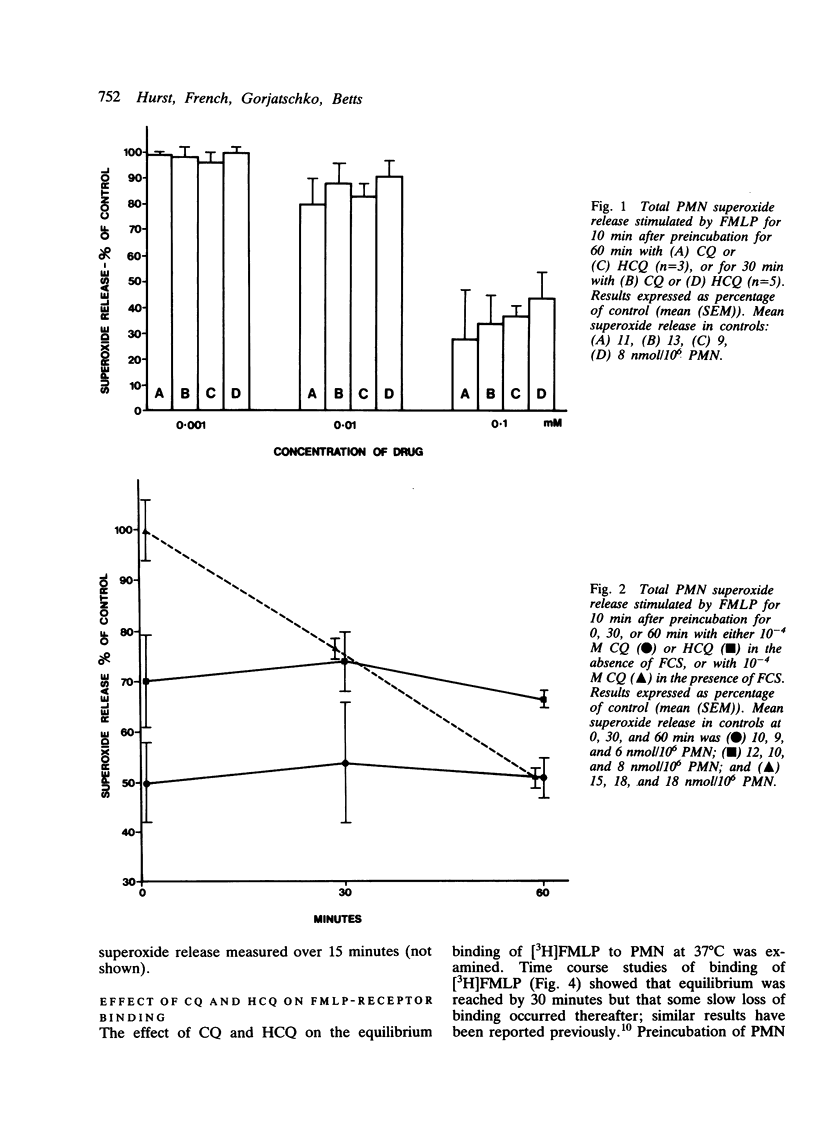
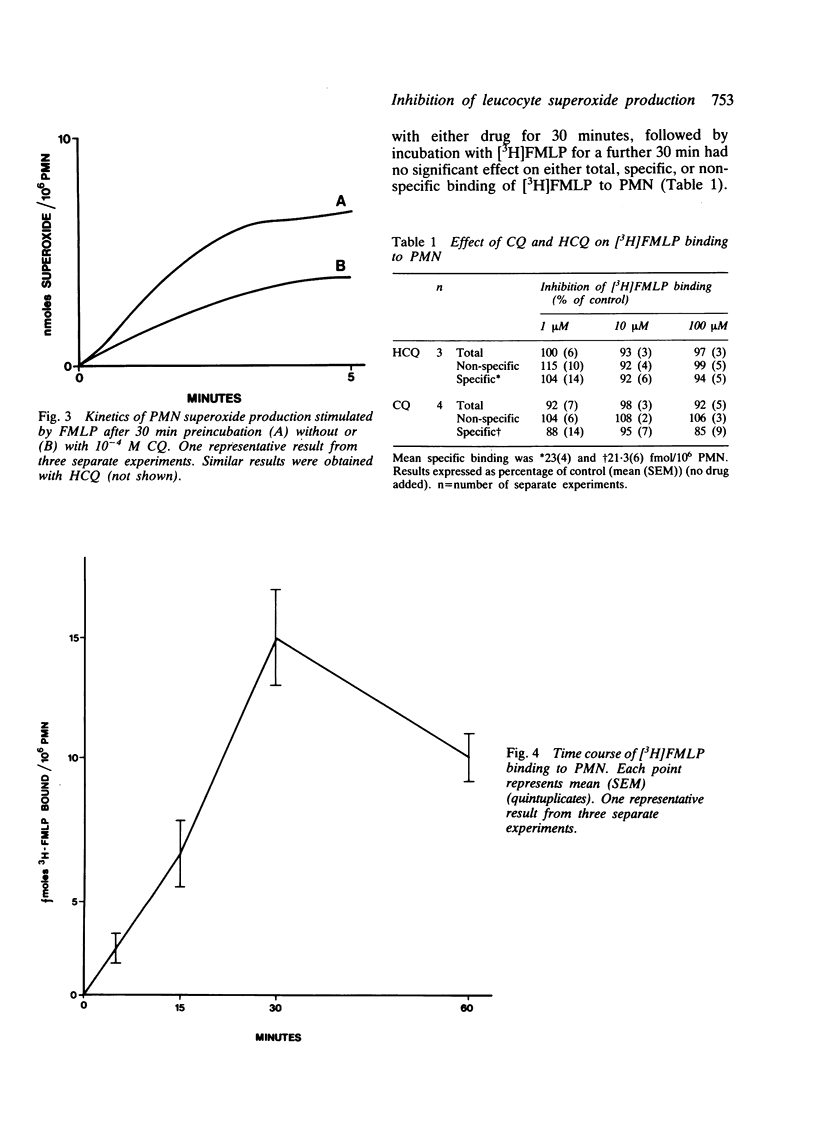
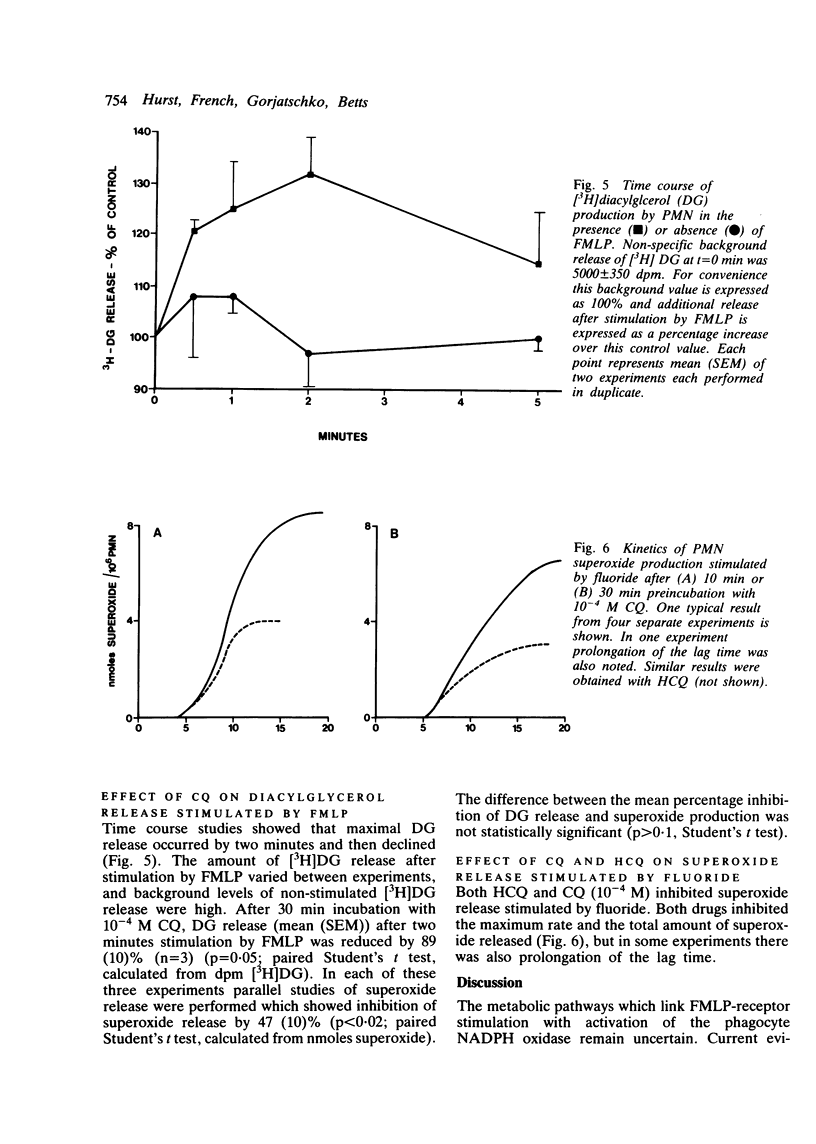
The effect of chloroquine and hydroxychloroquine on neutrophil superoxide release stimulated by the chemotactic tripeptide N-formyl-methionyl-leucyl-phenylalanine (FMLP) was examined. Both drugs caused time and dose dependent inhibition of superoxide release but had no effect on equilibrium binding of [3H]FMLP to its receptor. Preliminary experiments suggest that these drugs may exert their inhibitory effect on superoxide release by inhibiting the FMLP stimulated hydrolysis of phosphoinositides.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balazovich K. J., Smolen J. E., Boxer L. A. Ca2+ and phospholipid-dependent protein kinase (protein kinase C) activity is not necessarily required for secretion by human neutrophils. Blood. 1986 Oct;68(4):810–817. [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G., Cuatrecasas P. Phospholipase A2 and phospholipase C activities of platelets. Differential substrate specificity, Ca2+ requirement, pH dependence, and cellular localization. J Biol Chem. 1980 Nov 10;255(21):10227–10231. [PubMed] [Google Scholar]
- Burgess G. M., McKinney J. S., Irvine R. F., Berridge M. J., Hoyle P. C., Putney J. W., Jr Inositol 1,4,5-trisphosphate may be a signal for f-Met-Leu-Phe-induced intracellular Ca mobilisation in human leucocytes (HL-60 cells). FEBS Lett. 1984 Oct 15;176(1):193–196. doi: 10.1016/0014-5793(84)80939-4. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Barrowman M. M., Gomperts B. D. Breakdown and synthesis of polyphosphoinositides in fMetLeuPhe-stimulated neutrophils. FEBS Lett. 1985 Feb 25;181(2):259–263. doi: 10.1016/0014-5793(85)80271-4. [DOI] [PubMed] [Google Scholar]
- Cross A. R., Jones O. T. The effect of the inhibitor diphenylene iodonium on the superoxide-generating system of neutrophils. Specific labelling of a component polypeptide of the oxidase. Biochem J. 1986 Jul 1;237(1):111–116. doi: 10.1042/bj2370111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French J. K., Hurst N. P., O'Donnell M. L., Betts W. H. Uptake of chloroquine and hydroxychloroquine by human blood leucocytes in vitro: relation to cellular concentrations during antirheumatic therapy. Ann Rheum Dis. 1987 Jan;46(1):42–45. doi: 10.1136/ard.46.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French J. K., Hurst N. P., Zalewski P. D., Valente L., Forbes I. J. Calcium ionophore A23187 enhances human neutrophil superoxide release, stimulated by phorbol dibutyrate, by converting phorbol ester receptors from a low- to high-affinity state. FEBS Lett. 1987 Feb 23;212(2):242–246. doi: 10.1016/0014-5793(87)81353-4. [DOI] [PubMed] [Google Scholar]
- Hurst N. P., French J. K., Bell A. L., Nuki G., O'Donnell M. L., Betts W. H., Cleland L. G. Differential effects of mepacrine, chloroquine and hydroxychloroquine on superoxide anion generation, phospholipid methylation and arachidonic acid release by human blood monocytes. Biochem Pharmacol. 1986 Sep 15;35(18):3083–3089. doi: 10.1016/0006-2952(86)90390-4. [DOI] [PubMed] [Google Scholar]
- Hurst N. P. Molecular basis of activation and regulation of the phagocyte respiratory burst. Ann Rheum Dis. 1987 Apr;46(4):265–272. doi: 10.1136/ard.46.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie A. H. Pharmacologic actions of 4-aminoquinoline compounds. Am J Med. 1983 Jul 18;75(1A):5–10. doi: 10.1016/0002-9343(83)91264-0. [DOI] [PubMed] [Google Scholar]
- Miyachi Y., Yoshioka A., Imamura S., Niwa Y. Antioxidant action of antimalarials. Ann Rheum Dis. 1986 Mar;45(3):244–248. doi: 10.1136/ard.45.3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedel J., Wilkinson S., Cuatrecasas P. Receptor-mediated uptake and degradation of 125I-chemotactic peptide by human neutrophils. J Biol Chem. 1979 Nov 10;254(21):10700–10706. [PubMed] [Google Scholar]
- Niwa Y., Sakane T., Taniguchi S. Phospholipid transmethylation in the membrane of human neutrophils and lymphocytes. Arch Biochem Biophys. 1984 Oct;234(1):7–14. doi: 10.1016/0003-9861(84)90318-7. [DOI] [PubMed] [Google Scholar]
- Salmeron G., Lipsky P. E. Immunosuppressive potential of antimalarials. Am J Med. 1983 Jul 18;75(1A):19–24. doi: 10.1016/0002-9343(83)91266-4. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Heyworth P. G., Cockcroft S., Barrowman M. M. Stimulated neutrophils from patients with autosomal recessive chronic granulomatous disease fail to phosphorylate a Mr-44,000 protein. Nature. 1985 Aug 8;316(6028):547–549. doi: 10.1038/316547a0. [DOI] [PubMed] [Google Scholar]
- Ward P. A. The chemosuppression of chemotaxis. J Exp Med. 1966 Aug 1;124(2):209–226. doi: 10.1084/jem.124.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weening R. S., Wever R., Roos D. Quantitative aspects of the production of superoxide radicals by phagocytizing human granulocytes. J Lab Clin Med. 1975 Feb;85(2):245–252. [PubMed] [Google Scholar]
- Wright C. D., Hoffman M. D. The protein kinase C inhibitors H-7 and H-9 fail to inhibit human neutrophil activation. Biochem Biophys Res Commun. 1986 Mar 28;135(3):749–755. doi: 10.1016/0006-291x(86)90992-7. [DOI] [PubMed] [Google Scholar]


